Search
- Page Path
- HOME > Search
- Clinicopathological and molecular mechanisms of CLDN18.2 in gastric cancer aggressiveness: a high-risk population study with multi-omics profiling
- Hengquan Wu, Mei Li, Gang Wang, Peiqing Liao, Peng Zhang, Luxi Yang, Yumin Li, Tao Liu, Wenting He
- J Pathol Transl Med. 2026;60(1):47-57. Published online January 5, 2026
- DOI: https://doi.org/10.4132/jptm.2025.09.11
- 625 View
- 67 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
The tight junction protein claudin18.2 (CLDN18.2) has been implicated in poor prognosis and suboptimal immunotherapy response in gastric cancer (GC). This study investigates the clinicopathological relevance of CLDN18.2 expression and its association with molecular subtypes in GC patients from a high-incidence region, combining transcriptomic and proteomic approaches to explore how CLDN18.2 contributes to progression and metastasis.
Methods
A retrospective cohort of 494 GC patients (2019–2024) underwent immunohistochemical analysis for CLDN18.2, Epstein-Barr virus (Epstein–Barr virus–encoded RNA), p53, human epidermal growth factor receptor 2 (HER2), and mismatch repair proteins (MLH1, MSH2, PMS2, and MSH6). CLDN18.2 positivity was defined as moderate to strong (2+/3+) membranous staining in ≥75% of tumor cells. Clinicopathological correlations, biomarker associations, and survival outcomes were evaluated. Transcriptomic and proteomic sequencing was performed to explore molecular mechanisms.
Results
CLDN18.2 positivity was observed in 26.9% (133/494) of gastric adenocarcinomas. CLDN18.2-positive tumors correlated with TNM stage (p = .003) and shorter overall survival (p = .018). No associations were identified with age, sex, HER2 status, microsatellite instability, or Epstein-Barr virus infection. Transcriptomic profiling revealed CLDN18.2-high tumors enriched in pathways involving cell junction disruption, signaling regulation, and immune modulation. Proteomic profiling showed that tumors with high CLDN18.2 were enriched in multiple mechanism-related pathways such as integrated metabolic reprogramming, cytoskeletal recombination, immune microenvironment dysregulation, and pro-survival signaling. These mechanisms may collectively contribute to tumor progression and metastasis.
Conclusions
CLDN18.2 overexpression is associated with poor prognosis in GC patients. Transcriptomic and proteomic analyses demonstrate that CLDN18.2 promotes tumor progression and metastasis, underscoring its potential as an independent prognostic factor in regions with a high incidence of GC.
- Cytological features of atypical adenomatous hyperplasia and adenocarcinoma in situ of the lung: a case report
- Misa Takahashi, Seiya Homma, Chisato Setoguchi, Yoko Umezawa, Atsuhiko Sakamoto
- J Pathol Transl Med. 2025;59(3):195-200. Published online May 9, 2025
- DOI: https://doi.org/10.4132/jptm.2025.04.09
- 3,934 View
- 128 Download
-
 Abstract
Abstract
 PDF
PDF - Atypical adenomatous hyperplasia (AAH) and adenocarcinoma in situ (AIS) are generally treated as different lesions, depending on the differences in lesion size and histological findings. However, these differences are not absolute; thus, AAH and AIS are often difficult to distinguish. Moreover, whether AAH and AIS can be regarded as different lesions remains unknown because cytological specimens, especially those of AAH, are rare. In this study, we examined these uncommon cytological specimens and compared the cytological findings between AAH and AIS. We observed many common cytological features with no obvious differences between AAH and AIS. These findings suggest that these two distinct lesions can be grouped into a single category. Therefore, we propose creating a new cytological category.
- Fine needle aspiration cytology diagnoses of follicular thyroid carcinoma: results from a multicenter study in Asia
- Hee Young Na, Miyoko Higuchi, Shinya Satoh, Kaori Kameyama, Chan Kwon Jung, Su-Jin Shin, Shipra Agarwal, Jen-Fan Hang, Yun Zhu, Zhiyan Liu, Andrey Bychkov, Kennichi Kakudo, So Yeon Park
- J Pathol Transl Med. 2024;58(6):331-340. Published online November 7, 2024
- DOI: https://doi.org/10.4132/jptm.2024.10.12
- 5,729 View
- 265 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
This study was designed to compare diagnostic categories of thyroid fine needle aspiration cytology (FNAC) and incidence of thyroid tumors in the multi-institutional Asian series with a special focus on diagnostic category IV (suspicious for a follicular neoplasm) and follicular thyroid carcinomas (FTCs). Methods: Distribution of FNAC categories, incidence of thyroid tumors in resection specimens and cytologic diagnoses of surgically confirmed follicular adenomas (FAs) and FTCs were collected from 10 institutes from five Asian countries and were compared among countries and between FAs and FTCs. Results: The frequency of category IV diagnoses (3.0%) in preoperative FNAC were significantly lower compared to those in Western countries (10.1%). When comparing diagnostic categories among Asian countries, category IV was more frequent in Japan (4.6%) and India (7.9%) than in Taiwan (1.4%), Korea (1.4%), and China (3.6%). Similarly, incidence of FAs and FTCs in surgical resection specimens was significantly higher in Japan (10.9%) and India (10.1%) than in Taiwan (5.5%), Korea (3.0%), and China (2.5%). FTCs were more commonly diagnosed as category IV in Japan (77.5%) than in Korea (33.3%) and China (35.0%). Nuclear pleomorphism, nuclear crowding, microfollicular pattern, and dyshesive cell pattern were more common in FTCs compared with FAs. Conclusions: Our study highlighted the difference in FNAC diagnostic categories of FTCs among Asian countries, which is likely related to different reporting systems and thyroid cancer incidence. Cytologic features such as nuclear pleomorphism, nuclear crowding, microfollicular pattern, and dyshesive cell pattern were found to be useful in diagnosing FTCs more effectively. -
Citations
Citations to this article as recorded by- Misdiagnosed follicular adenoma with 11 year postoperative liver and lung metastases a case report and literature review
Kai-Li Yang, Heng-Tong Han, Shou-Hua Li, Xiao-Xiao Li, Ze Yang, Li-Bin Ma, Yong-Xun Zhao
Discover Oncology.2025;[Epub] CrossRef
- Misdiagnosed follicular adenoma with 11 year postoperative liver and lung metastases a case report and literature review
- A rare goblet cell adenocarcinoma arising from Barrett’s esophagus: the first reported case in the esophagus
- Chi Eun Oh, Sung Eun Kim, Sun-Ju Oh
- J Pathol Transl Med. 2024;58(2):81-86. Published online January 8, 2024
- DOI: https://doi.org/10.4132/jptm.2023.12.26
- 4,580 View
- 324 Download
-
 Abstract
Abstract
 PDF
PDF - Goblet cell adenocarcinoma (GCA) is a rare and distinctive amphicrine tumor comprised of goblet-like mucinous cells and neuroendocrine cells. It is believed to originate from pluripotent stem cells located at the base of crypts. GCA predominantly arises from the appendix, with a few reported cases in extra-appendiceal locations such as the colorectum, small intestine, and stomach. In this case report, we present a unique instance of a 64-year-old male who initially received a diagnosis of neuroendocrine carcinoma in the distal esophagus based on biopsy but, following resection, was subsequently re-diagnosed with GCA arising from Barrett’s esophagus.
- Usefulness of BRAF VE1 immunohistochemistry in non–small cell lung cancers: a multi-institutional study by 15 pathologists in Korea
- Sunhee Chang, Yoon-La Choi, Hyo Sup Shim, Geon Kook Lee, Seung Yeon Ha
- J Pathol Transl Med. 2022;56(6):334-341. Published online October 27, 2022
- DOI: https://doi.org/10.4132/jptm.2022.08.22
- 7,409 View
- 163 Download
- 11 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Next-generation sequencing (NGS) is an approved test to select patients for BRAF V600E targeted therapy in Korea. However, the high cost, long turnaround times, and the need for sophisticated equipment and skilled personnel limit the use of NGS in daily practice. Immunohistochemistry (IHC) is a rapid and relatively inexpensive assay available in most laboratories. Therefore, in this study, we evaluate the usefulness of BRAF VE1 IHC in terms of predictive value and interobserver agreement in non–small cell lung cancers (NSCLCs).
Methods
A total of 30 cases with known BRAF mutation status were selected, including 20 cases of lung adenocarcinomas, six cases of colorectal adenocarcinomas, and four cases of papillary thyroid carcinomas. IHC for BRAF V600E was carried out using the VE1 antibody. Fifteen pathologists independently scored both the staining intensity and the percentage of tumor cell staining on whole slide images.
Results
In the lung adenocarcinoma subset, interobserver agreement for the percentage of tumor cell staining and staining intensity was good (percentage of tumor cell staining, intraclass correlation coefficient = 0.869; staining intensity, kappa = 0.849). The interobserver agreement for the interpretation using the cutoff of 40% was almost perfect in the entire study group and the lung adenocarcinoma subset (kappa = 0.815). Sensitivity, specificity, positive predictive value, and negative predictive value of BRAF VE1 IHC were 80.0%, 90.0%, 88.9%, and 81.8%, respectively.
Conclusions
BRAF VE1 IHC could be a screening test for the detection of BRAF V600E mutation in NSCLC. However, further studies are needed to optimize the protocol and to establish and validate interpretation criteria for BRAF VE1 IHC. -
Citations
Citations to this article as recorded by- Dedifferentiated Leiomyosarcoma of the Uterine Corpus with Heterologous Component: Clinicopathological Analysis of Five Consecutive Cases from a Single Institution and Comprehensive Literature Review
Suyeon Kim, Hyunsik Bae, Hyun-Soo Kim
Diagnostics.2024; 14(2): 160. CrossRef - Differentiating BRAF V600E- and RAS-like alterations in encapsulated follicular patterned tumors through histologic features: a validation study
Chankyung Kim, Shipra Agarwal, Andrey Bychkov, Jen-Fan Hang, Agnes Stephanie Harahap, Mitsuyoshi Hirokawa, Kennichi Kakudo, Somboon Keelawat, Chih-Yi Liu, Zhiyan Liu, Truong Phan-Xuan Nguyen, Chanchal Rana, Huy Gia Vuong, Yun Zhu, Chan Kwon Jung
Virchows Archiv.2024; 484(4): 645. CrossRef - BRAF V600E Mutation of Non-Small Cell Lung Cancer in Korean Patients
Hyo Yeong Ahn, Chang Hun Lee, Min Ki Lee, Jung Seop Eom, Yeon Joo Jeong, Yeong Dae Kim, Jeong Su Cho, Jonggeun Lee, So Jeong Lee, Dong Hoon Shin, Ahrong Kim
Medicina.2023; 59(6): 1085. CrossRef - Reevaluating diagnostic categories and associated malignancy risks in thyroid core needle biopsy
Chan Kwon Jung
Journal of Pathology and Translational Medicine.2023; 57(4): 208. CrossRef
- Dedifferentiated Leiomyosarcoma of the Uterine Corpus with Heterologous Component: Clinicopathological Analysis of Five Consecutive Cases from a Single Institution and Comprehensive Literature Review
- Cytopathologic features of human papillomavirus–independent, gastric-type endocervical adenocarcinoma
- Min-Kyung Yeo, Go Eun Bae, Dong-Hyun Kim, In-Ock Seong, Kwang-Sun Suh
- J Pathol Transl Med. 2022;56(5):260-269. Published online September 13, 2022
- DOI: https://doi.org/10.4132/jptm.2022.07.05
- 6,007 View
- 163 Download
- 5 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Gastric-type endocervical adenocarcinoma (GEA) is unrelated to human papillomavirus (HPV) infection and is clinically aggressive compared with HPV-associated usual-type endocervical adenocarcinoma (UEA). The cytological diagnosis falls short of a definitive diagnosis of GEA and is often categorized as atypical glandular cells (AGCs). To improve cytologic recognition, cytological findings of HPV-independent GEA were analyzed and the results compared with HPV-associated UEA.
Methods
Cervical Papanicolaou (Pap) smears from eight patients with a histopathologic diagnosis of GEA and 12 control cases of UEA were reviewed. All slides were conventionally prepared and/or liquid-based prepared (ThinPrep) and stained following the Pap method. A mucinous background, architectural, nuclear, and cytoplasmic features were analyzed and compared with UEA.
Results
Preoperative cytologic diagnoses of the eight GEA cases were AGCs, favor neoplastic in three cases, adenocarcinoma in situ in one case, and adenocarcinoma in four cases. Cytologically, monolayered honeycomb-like sheets (p = .002) of atypical endocervical cells with vacuolar granular cytoplasm (p = .001) were extensive in GEA, and three-dimensional clusters (p = .010) were extensive in UEA. Although the differences were not statistically significant, background mucin (p = .058), vesicular nuclei (p = .057), and golden-brown intracytoplasmic mucin (p = .089) were also discriminatory findings for GEA versus UEA.
Conclusions
Although GEA is difficult to diagnose on cytologic screening, GEA can be recognized based on cytologic features of monolayered honeycomb sheets of atypical endocervical cells with abundant vacuolar cytoplasm and some golden-brown intracytoplasmic mucin. UEA cases are characterized by three-dimensional clusters. -
Citations
Citations to this article as recorded by- A Comparative Analysis of Usual- and Gastric-Type Cervical Adenocarcinoma in a Japanese Population Reveals Distinct Clinicopathological and Molecular Features with Prognostic and Therapeutic Insights
Umme Farzana Zahan, Hasibul Islam Sohel, Kentaro Nakayama, Masako Ishikawa, Mamiko Nagase, Sultana Razia, Kosuke Kanno, Hitomi Yamashita, Shahataj Begum Sonia, Satoru Kyo
International Journal of Molecular Sciences.2025; 26(15): 7469. CrossRef - Diagnostic value of cytology in detecting human papillomavirus–independent cervical malignancies: a nation-wide study in Korea
Hye-Ra Jung, Junyoung Shin, Chong Woo Yoo, Eun Na Kim, Cheol Lee, Kyeongmin Kim, Ho-chang Lee, Yonghee Lee, Ji Hye Kim, Soo Jin Jung, Yumin Chung, Joo Yeon Kim, Hye Eun Park, Tae Hoen Kim, Wonae Lee, Min-Sun Cho, Ran Hong, Yoon Jung Choi, Younghee Choi, Y
Journal of Pathology and Translational Medicine.2025; 59(6): 444. CrossRef - Risk Factors Affecting Clinical Outcomes of Low-risk Early-stage Human Papillomavirus–Associated Endocervical Adenocarcinoma Treated by Surgery Alone: Application of Silva Pattern
Bong Kyung Bae, Hyunsik Bae, Won Kyung Cho, Byoung-Gie Kim, Chel Hun Choi, Tae-Joong Kim, Yoo-Young Lee, Jeong-Won Lee, Hyun-Soo Kim, Won Park
International Journal of Gynecological Pathology.2024; 43(5): 447. CrossRef - Tall‐columnar glandular cells in SurePath™ liquid‐based cytology Pap sample: Learning from mimics/pitfalls
Nalini Gupta, Vanita Jain, Radhika Srinivasan, Tulika Singh
Cytopathology.2024; 35(4): 510. CrossRef
- A Comparative Analysis of Usual- and Gastric-Type Cervical Adenocarcinoma in a Japanese Population Reveals Distinct Clinicopathological and Molecular Features with Prognostic and Therapeutic Insights
- Landscape of EGFR mutations in lung adenocarcinoma: a single institute experience with comparison of PANAMutyper testing and targeted next-generation sequencing
- Jeonghyo Lee, Yeon Bi Han, Hyun Jung Kwon, Song Kook Lee, Hyojin Kim, Jin-Haeng Chung
- J Pathol Transl Med. 2022;56(5):249-259. Published online September 13, 2022
- DOI: https://doi.org/10.4132/jptm.2022.06.11
- 8,732 View
- 144 Download
- 7 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Activating mutations in the tyrosine kinase domain of epidermal growth factor receptor (EGFR) are predictive biomarkers for response to EGFR–tyrosine kinase inhibitor (TKI) therapy in lung adenocarcinoma (LUAD). Here, we characterized the clinicopathologic features associated with EGFR mutations via peptide nucleic acid clamping-assisted fluorescence melting curve analysis (PANAMutyper) and evaluated the feasibility of targeted deep sequencing for detecting the mutations.
Methods
We examined EGFR mutations in exons 18 through 21 for 2,088 LUADs from July 2017 to April 2020 using PANAMutyper. Of these, we performed targeted deep sequencing in 73 patients and evaluated EGFR-mutation status and TKI clinical response.
Results
EGFR mutation was identified in 55.7% of LUADs by PANAMutyper, with mutation rates higher in females (69.3%) and never smokers (67.1%) and highest in the age range of 50 to 59 years (64.9%). For the 73 patients evaluated using both methods, next-generation sequencing (NGS) identified EGFR mutation–positive results in 14 of 61 patients (23.0%) who were EGFR-negative according to PANAMutyper testing. Of the 10 patients reportedly harboring a sensitizing mutation according to NGS, seven received TKI treatment, with all showing partial response or stable disease. In the 12 PANAMutyper-positive cases, NGS identified two additional mutations in exon 18, whereas a discordant negative result was observed in two cases.
Conclusions
Although PANAMutyper identified high frequencies of EGFR mutations, targeted deep sequencing revealed additional uncommon EGFR mutations. These findings suggested that appropriate use of NGS may benefit LUAD patients with otherwise negative screening test results. -
Citations
Citations to this article as recorded by- Comparison of tissue-based and plasma-based testing for EGFR mutation in non–small cell lung cancer patients
Yoon Kyung Kang, Dong Hoon Shin, Joon Young Park, Chung Su Hwang, Hyun Jung Lee, Jung Hee Lee, Jee Yeon Kim, JooYoung Na
Journal of Pathology and Translational Medicine.2025; 59(1): 60. CrossRef - Localization of epidermal growth factor receptor-mutations using PNA:DNA probes in clinical specimens from patients with non-small cell lung cancer
Haruo Miyata, Hajime Shigeto, Tomoatsu Ikeya, Tadashi Ashizawa, Akira Iizuka, Yasufumi Kikuchi, Chie Maeda, Akari Kanematsu, Kazue Yamashita, Kenichi Urakami, Yuji Shimoda, Takeshi Nagashima, Keiichi Ohshima, Yasuhisa Ohde, Mitsuhiro Isaka, Takashi Sugino
Scientific Reports.2025;[Epub] CrossRef - Molecular characteristics and responses to EGFR tyrosine kinase inhibitors in non-small cell lung cancer patients with EGFR exon 19 insertions
Yang Li, Yunfeng Ni, Feng Lv, Yan Shi, Yedan Chen, Xiaoying Wu, Jiaohui Pang, Long Huang, Yang Shao, Tao Wang, Jie Min, Yang Song
BMC Medicine.2025;[Epub] CrossRef - Detection of EGFR exon 20 insertion mutations in non-small cell lung cancer: implications for consistent nomenclature in precision medicine
Jieun Park, Boram Lee, Ji-Young Song, Minjung Sung, Mi Jeong Kwon, Chae Rin Kim, Sangjin Lee, Young Kee Shin, Yoon-La Choi
Pathology.2024; 56(5): 653. CrossRef - Histo-pillar strip for optimal histogel block construction and biomarker analysis in 3D-lung cancer patient-derived organoids
Sang-Yun Lee, Eunyoung Lee, Ji-O Ryu, Kyuhwan Kim, Yongki Hwang, Bosung Ku, Seok Whan Moon, Mi Hyoung Moon, Kyung Soo Kim, Kwanyong Hyun, Jeong Uk Lim, Chan Kwon Park, Sung Won Kim, Chang Dong Yeo, Dong Woo Lee, Seung Joon Kim
Biofabrication.2024; 16(4): 045017. CrossRef
- Comparison of tissue-based and plasma-based testing for EGFR mutation in non–small cell lung cancer patients
- Colorectal adenocarcinoma with enteroblastic differentiation: diagnostic challenges of a rare case encountered in clinical practice
- Evi Abada, Ifeoma C. Anaya, Othuke Abada, Anthony Lebbos, Rafic Beydoun
- J Pathol Transl Med. 2022;56(2):97-102. Published online January 21, 2022
- DOI: https://doi.org/10.4132/jptm.2021.10.28
- 8,155 View
- 207 Download
- 11 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF - Colorectal adenocarcinoma with enteroblastic differentiation (CAED) is a rare subtype of colonic adenocarcinoma characterized by increased α-fetoprotein (AFP) production and the expression of at least one enteroblastic marker including AFP, glypican 3 (GPC3), or Spalt like transcription factor 4 (SALL4). We report a case of a 26-year-old female who presented with low back pain and constipation which persisted despite supportive measures. Imaging revealed multiple liver lesions and enlarged retroperitoneal nodes. Tumor markers including AFP were markedly elevated. On biopsy, samples from the liver revealed infiltrating glands lined by columnar-type epithelium with mostly eosinophilic granular to focally clear cytoplasm. By immunohistochemistry, the tumor showed immunoreactivity with AFP, hepatocyte antigen, GPC3, SALL4, CDX2, SATB2, and cytokeratin 20. A colonoscopy performed subsequently revealed a mass in the sigmoid colon and biopsy of this mass revealed a similar histology as that seen in the liver. A diagnosis of CAED was made, following the results of gene expression profiling by the tumor with next-generation sequencing which identified pathogenic variants in MUTYH, TP53, and KDM6A genes and therefore supported its colonic origin. Cases such as this underscores the use of ancillary diagnostic techniques in arriving at the correct diagnosis in lesions with overlapping clinicopathologic characteristics.
-
Citations
Citations to this article as recorded by- Exploring the Multifunctional Role of Alpha-Fetoprotein in Cancer Progression: Implications for Targeted Therapy in Hepatocellular Carcinoma and Beyond
Hyunjung Kim, Minji Jang, Eunmi Kim
International Journal of Molecular Sciences.2025; 26(10): 4863. CrossRef - Rectal adenocarcinoma with a yolk sac tumor component: A rare case report and review of the literature
Sato Nishida, Tomohiro Takeda, Tatsuya Shonaka, Shoichiro Mizukami, Masahide Otani, Mizuho Ohara, Chikayoshi Tani, Kimiharu Hasegawa, Yuki Kamikokura, Mishie Tanino, Hideki Yokoo
International Cancer Conference Journal.2025;[Epub] CrossRef - SALL4 in gastrointestinal tract cancers: upstream and downstream regulatory mechanisms
Tairan Wang, Yan Jin, Mengyao Wang, Boya Chen, Jinyu Sun, Jiaying Zhang, Hui Yang, Xinyao Deng, Xingyue Cao, Lidong Wang, Yuanyuan Tang
Molecular Medicine.2024;[Epub] CrossRef - Gastric adenocarcinoma with enteroblastic differentiation in a
67-year-old man in Korea: a case report
Hae Rin Lee, Gwang Ha Kim, Dong Chan Joo, Moon Won Lee, Bong Eun Lee, Kyung Bin Kim
The Ewha Medical Journal.2024;[Epub] CrossRef - Colorectal adenocarcinoma with clear cell changes: immunohistological and molecular findings in three cases
Andreas Gocht, Carsten Heidel, Jutta Kirfel, Rita Vesce, Pamela Lazar-Karsten, Helen Pasternack, Madelaine Melzer, Phillip Hildebrand, Nicole Warkentin, Hendrik Schimmelpenning, Verena-Wilbeth Sailer
Virchows Archiv.2024; 485(3): 569. CrossRef - Ureteral Metastasis of Colonic Adenocarcinoma with Enteroblastic Differentiation: A Rare Case to be Distinguished from Clear Cell Adenocarcinoma of the Urinary Tract
Hiroshi Minato, Akane Yoshikawa, Sho Tsuyama, Kazuyoshi Katayanagi, Kengo Hayashi, Yusuke Sakimura, Hiroyuki Bando, Tomohiro Hori, Yosuke Kito
International Journal of Surgical Pathology.2023; 31(8): 1553. CrossRef - Beyond liver cancer, more application scenarios for alpha-fetoprotein in clinical practice
Chenyu Ma, Yuexinzi Jin, Yuhan Wang, Huaguo Xu, Jiexin Zhang
Frontiers in Oncology.2023;[Epub] CrossRef - AIEgens assisted label free DNA supersandwich immunoassay for ultrasensitive α-fetoprotein detection
Xiaowen Ou, Jingman Dai, Yiting Huang, Xiaoqin Xiong, Zhi Zheng, Xiaoding Lou, Fan Xia
Giant.2022; 11: 100110. CrossRef - Rectal carcinoma with dual differentiation toward enteroblastic and neuroendocrine features arising in a patient with ulcerative colitis: a case report
Takako Kihara, Ryuichi Kuwahara, Kurando Kusunoki, Tomohiro Minagawa, Yuki Horio, Motoi Uchino, Hiroki Ikeuchi, Seiichi Hirota
World Journal of Surgical Oncology.2022;[Epub] CrossRef
- Exploring the Multifunctional Role of Alpha-Fetoprotein in Cancer Progression: Implications for Targeted Therapy in Hepatocellular Carcinoma and Beyond
- Extremely well-differentiated adenocarcinoma of the stomach: diagnostic pitfalls in endoscopic biopsy
- Jongwon Lee, In-Seob Lee, Ji Yong Ahn, Young Soo Park, Jihun Kim
- J Pathol Transl Med. 2022;56(2):63-72. Published online November 16, 2021
- DOI: https://doi.org/10.4132/jptm.2021.10.12
- 8,448 View
- 469 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Extremely well-differentiated adenocarcinoma (EWDA) is a deceptively bland-looking adenocarcinoma of the stomach. It often causes diagnostic problems, especially in endoscopic biopsy samples. To better recognize this deceptively bland lesion, we carefully reviewed a series of EWDAs treated at our institution.
Methods
A total of 55 specimens from 19 patients were obtained. Endoscopic, gross and microscopic features defining EWDA were described and documented. For comparison, hyperplastic polyp specimens were randomly selected and analyzed.
Results
Most cases (18 of 19, 94.7%) were advanced gastric cancer (AGC) and primarily located in the body of the stomach (15 of 19, 79.0%). The majority of AGCs were non-ulcerated (11 of 18, 61.1%) with an undermining growth pattern and a relatively small mucosal involvement. Specific histologic features included an irregular glandular shape, an undulating apical cytoplasmic border, disproportionately large glands, a variably distended mucinous cytoplasm. Classical features, such as small infiltrating glands or desmoplastic reactions, were barely observed. Identification of irregularly spaced nuclei and disruption of the foveolar epithelial structure, along with atypical features described above were helpful in making a diagnosis especially in gastric forceps biopsies.
Conclusions
Awareness of the histomorphologic characteristics described in this report would lead to timely diagnosis and prevent repeated endoscopic procedures. -
Citations
Citations to this article as recorded by- Artificial intelligence-assisted diagnosis of early gastric cancer: present practice and future prospects
Changda Lei, Wenqiang Sun, Kun Wang, Ruixia Weng, Xiuji Kan, Rui Li
Annals of Medicine.2025;[Epub] CrossRef - Unusual or Uncommon Histology of Gastric Cancer
Jinho Shin, Young Soo Park
Journal of Gastric Cancer.2024; 24(1): 69. CrossRef - A case of gastric adenocarcinoma with pyloric gland-type infiltrating submucosa
Kaiho Hirata, Shusuke Yagi, Hideki Miyazaki, Kazuhiko Yamada, Naoki Akazawa, Naoki Enomoto, Kyoko Nohara, Chizu Yokoi, Toru Igari, Norihiro Kokudo
Surgical Case Reports.2024;[Epub] CrossRef - Gastric-type extremely well-differentiated adenocarcinoma of the stomach: A rare tumor with diagnostic difficulties and high inter-observer variation in endoscopic pinch biopsies
Soomin Ahn, Sujin Park, Hyun Hee Koh, Han Gyeol Kim, Hyunjin Kim, Jae Yeong Son, Boram Lee, Hyunwoo Lee, Soohyun Hwang, Junhun Cho, Yun Kyung Lee, Ryoji Kushima, Amitabh Srivastava, Kyoung-Mee Kim
Pathology - Research and Practice.2024; 263: 155599. CrossRef
- Artificial intelligence-assisted diagnosis of early gastric cancer: present practice and future prospects
- Correlation of TTF-1 immunoexpression and EGFR mutation spectrum in non–small cell lung carcinoma
- Tripti Nakra, Varsha Singh, Aruna Nambirajan, Prabhat Singh Malik, Anant Mohan, Deepali Jain
- J Pathol Transl Med. 2021;55(4):279-288. Published online July 8, 2021
- DOI: https://doi.org/10.4132/jptm.2021.05.10
- 7,984 View
- 186 Download
- 10 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Thyroid transcription factor (TTF-1) is a diagnostic marker expressed in 75%–85% of primary lung adenocarcinomas (ACs). Activating mutations in the tyrosine kinase domain of the epidermal growth factor receptor (EGFR) gene is the most common targetable driver alteration in lung AC. Previous studies have shown a positive correlation between TTF-1 and EGFR mutation status. We aimed to determine the predictive value of TTF-1 immunoexpression for underlying EGFR mutation status in a large Indian cohort.
Methods
This retrospective designed study was conducted with medical record data from 2011 to 2020. All cases of primary lung AC and non–small cell lung carcinoma not otherwise specified (NSCLC, NOS) with known TTF-1 expression diagnosed by immunohistochemistry using 8G7G3/1 antibodies and EGFR mutation status diagnosed by quantitative polymerase chain reaction were retrieved, reviewed, and the
results
were analyzed. Results: Among 909 patient samples diagnosed as lung AC and NSCLC, NOS, TTF-1 was positive in 76.8% cases (698/909) and EGFR mutations were detected in 29.6% (269/909). A strong positive correlation was present between TTF-1 positivity and EGFR mutation status (odds ratio, 3.61; p < .001), with TTF-1 positivity showing high sensitivity (90%) and negative predictive value (87%) for EGFR mutation. TTF-1 immunoexpression did not show significant correlation with uncommon/dual EGFR mutations (odds ratio, 1.69; p = .098). EGFR–tyrosine kinase inhibitor therapy was significantly superior to chemotherapy among EGFR mutant cases irrespective of TTF-1 status; however, no significant differences among survival outcomes were observed.
Conclusions
Our study confirms a strong positive correlation between TTF-1 expression and common EGFR mutations (exon 19 deletion and exon 21 L858R) in advanced lung AC with significantly high negative predictive value of TTF-1 for EGFR mutations. -
Citations
Citations to this article as recorded by- Baseline retinoblastoma transcriptional corepressor 1 (Rb1) functional inactivation is a pre-requisite but not sufficient for small-cell histological transformation in epidermal growth factor receptor (EGFR) mutant lung adenocarcinomas post-tyrosine kinas
Aruna Nambirajan, Amber Rathor, Hemavathi Baskarane, Anju GS, Sachin Khurana, Somagattu Sushmitha, Aparna Sharma, Prabhat Singh Malik, Deepali Jain
Virchows Archiv.2025; 487(3): 639. CrossRef - Lung Carcinoids in Adolescents and Young Adults (AYAs): A Still Overlooked Clinical Entity
Alice Laffi, Laura Pala, Chiara Catania, Marzia Locatelli, Priscilla Cascetta, Emilia Cocorocchio, Giovanni Luca Ceresoli, Daniele Laszlo, Flaminia Facella, Emily Governini, Marzia Bendoni, Giuseppe Pelosi, Fabio Conforti, Tommaso Martino De Pas
Current Oncology.2025; 32(8): 458. CrossRef - Correlation between TTF-1 expression and EGFR mutations in moroccan lung adenocarcinoma: A prospective six-year study
Sara Boukansa, Ismail Mouhrach, Fatima El Agy, Mokhtar El Mekhtoume, Laila Bouguenouch, Mounia Serraj, Bouchra Amara, Yassine Ouadnouni, Mohamed Smahi, Badreeddine Alami, Nawfel Mellas, Zineb Benbrahim, Hinde El Fatemi
Cancer Treatment and Research Communications.2025; 45: 101012. CrossRef - Mutation profile and programmed death ligand 1 status of patients with non‐small cell lung cancer diagnosed with “adenocarcinoma” and “non‐small cell carcinoma favor adenocarcinoma”
Naoko Shigeta, Tomoyuki Yokose, Shuji Murakami, Tetsuya Isaka, Kanako Shinada, Emi Yoshioka, Atsuya Narita, Kengo Katakura, Tetsuro Kondo, Terufumi Kato, Takuya Nagashima, Haruhiro Saito, Hiroyuki Ito
Thoracic Cancer.2024; 15(6): 458. CrossRef - Significance of NKX2-1 as a biomarker for clinical prognosis, immune infiltration, and drug therapy in lung squamous cell carcinoma
Huiyue Lin, Juyong Wang, Qing Shi, Minmin Wu
PeerJ.2024; 12: e17338. CrossRef - TTF-1 is a highly sensitive but not fully specific marker for pulmonary and thyroidal cancer: a tissue microarray study evaluating more than 17,000 tumors from 152 different tumor entities
Katharina Möller, Tayyaba Gulzar, Maximilian Lennartz, Florian Viehweger, Martina Kluth, Claudia Hube-Magg, Christian Bernreuther, Ahmed Abdulwahab Bawahab, Ronald Simon, Till S. Clauditz, Guido Sauter, Ria Schlichter, Andrea Hinsch, Simon Kind, Frank Jac
Virchows Archiv.2024; 485(5): 815. CrossRef - Identifying immunohistochemical biomarkers panel for non-small cell lung cancer in optimizing treatment and forecasting efficacy
Xiaoya Zhang, Junhong Meng, Mingyue Gao, Cheng Gong, Cong Peng, Duxian Liu
BMC Cancer.2024;[Epub] CrossRef - Expression landscapes in non-small cell lung cancer shaped by the thyroid transcription factor 1
Herdee Gloriane C. Luna, Marcelo Severino Imasa, Necy Juat, Katherine V. Hernandez, Treah May Sayo, Gloria Cristal-Luna, Sheena Marie Asur-Galang, Mirasol Bellengan, Kent John Duga, Bien Brian Buenaobra, Marvin I. De los Santos, Daniel Medina, Jamirah Sam
Lung Cancer.2023; 176: 121. CrossRef - Malignant pleural effusion cell blocks are reliable resources for PD-L1 analysis in advanced lung adenocarcinomas: a concordance study with matched histologic samples
Swati Mahajan, Aruna Nambirajan, Ishan Gupta, Nalini Gupta, Parikshaa Gupta, Deepali Jain
Journal of the American Society of Cytopathology.2022; 11(5): 253. CrossRef - Clinicopathologic Features and Molecular Biomarkers as Predictors of Epidermal Growth Factor Receptor Gene Mutation in Non-Small Cell Lung Cancer Patients
Lanlan Liu, Xianzhi Xiong
Current Oncology.2021; 29(1): 77. CrossRef
- Baseline retinoblastoma transcriptional corepressor 1 (Rb1) functional inactivation is a pre-requisite but not sufficient for small-cell histological transformation in epidermal growth factor receptor (EGFR) mutant lung adenocarcinomas post-tyrosine kinas
- SMARCA4/BRG1 protein-deficient thoracic tumors dictate re-examination of small biopsy reporting in non–small cell lung cancer
- Anurag Mehta, Divya Bansal, Rupal Tripathi, Ankush Jajodia
- J Pathol Transl Med. 2021;55(5):307-316. Published online June 21, 2021
- DOI: https://doi.org/10.4132/jptm.2021.05.11
- 11,645 View
- 336 Download
- 12 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
SMARCA4/BRG1 protein–deficient lung adenocarcinomas and thoracic sarcoma are recently described entities that lack distinctive histological features, transcription termination factor 1 (TTF1) reactivity, and actionable driver mutations. The current diagnostic path for small lung biopsies as recommended by the World Health Organization (WHO, 2015) is likely to categorize these as non– small cell carcinoma–not otherwise specified (NSCC-NOS). The present study attempts to define the subtle but distinctive clinicopathologic features of SMARCA4/BRG1 protein-deficient thoracic tumors; highlight their unique biology; and addresses the unmet need to segregate these using a new, tissue-proficient diagnostic pathway.
Methods
All lung biopsies and those from metastatic sites in patients with suspected advanced lung cancer and classified as NSCC-NOS as per WHO (2015) guidelines were subjected to BRG1 testing by immunohistochemistry. SMARCA4/BRG1 protein–deficient thoracic tumors were evaluated by an extended immunohistochemistry panel. Predictive biomarker and programmed death–ligand 1 testing was conducted in all cases.
Results
Of 110 cases, nine were found to be SMARCA4/BRG1 protein-deficient; six were identified as SMARCA4/BRG1 protein–deficient lung adenocarcinomas, and three were SMARCA4/BRG1 protein-deficient thoracic sarcomas. The histology ranged from poorly differentiated to undifferentiated to rhabdoid. None of the cases showed significant expression of TTF1 or p40, and no actionable mutation was identified.
Conclusions
It is difficult to separate BRG1-deficient lung adenocarcinomas and thoracic sarcomas based on morphology alone. We propose a diagnostic pathway for small biopsies of thoracic tumors to segregate these distinct entities so that they can be studied more efficaciously for new biomarkers and therapeutic options. -
Citations
Citations to this article as recorded by- Unravelling switch/sucrose non-fermentable (SWI-SNF) complex-deficient thoracic tumours: a clinicopathological comparative on undifferentiated tumours and non-small cell lung carcinomas with BRG1 and BRM deficiency
Ridhi Sood, Arshi Tandon, Warisa Khatoon, Jayashimman Vasanthraman, Aruna Nambirajan, Anant Mohan, Prabhat Singh Malik, Deepali Jain
Journal of Clinical Pathology.2025; 78(6): 370. CrossRef - Clinicopathologic and genomic analyses of SMARCA4-mutated non-small cell lung carcinoma implicate the needs for tailored treatment strategies
Bokyung Ahn, Deokhoon Kim, Wonjun Ji, Sung-Min Chun, Goeun Lee, Se Jin Jang, Hee Sang Hwang
Lung Cancer.2025; 201: 108445. CrossRef - SMARCA4-deficient non-small cell lung cancer with metastasis to the sigmoid colon: a case report
Rong Xiao, Guang Fu, Xinglan Li, Tao Lu
World Journal of Surgical Oncology.2025;[Epub] CrossRef - Clinicopathological and molecular perspectives on thoracic SMARCA4-deficient undifferentiated tumors and SMARCA4-deficient non-small cell lung carcinomas
Sumanta Das, Pallavi Mishra, Sunita Ahlawat
Pathologica.2025; 117(5): 455. CrossRef - Case report: The first account of undifferentiated sarcoma with epithelioid features originating in the pleura
Ling-Xi Xiao, Li Liu, Wang Deng
Frontiers in Medicine.2024;[Epub] CrossRef - SMARCA4-deficient central nervous system metastases: A case series and systematic review
Meaghan Morris, Kerime Ararat, Hannah Cutshall, Murat Gokden, Analiz Rodriguez, Lisa Rooper, Matthew Lindberg, James Stephen Nix
Journal of Neuropathology & Experimental Neurology.2024; 83(8): 638. CrossRef - Chemotherapy and Immune Checkpoint Inhibitors in a Case of SMARCA4-dUT: A Case Report and Review of Literature
Akriti Pokhrel, Ruchi Yadav, Kapil Kumar Manvar, Richard Wu, Vijay Jaswani, Carrie Brooke Wasserman, Jen C. Wang
Journal of Investigative Medicine High Impact Case Reports.2023;[Epub] CrossRef - TTF1-positive SMARCA4/BRG1 deficient lung adenocarcinoma
Anurag Mehta, Himanshi Diwan, Divya Bansal, Manoj Gupta
Journal of Pathology and Translational Medicine.2022; 56(1): 53. CrossRef - Delineation of a SMARCA4-specific competing endogenous RNA network and its function in hepatocellular carcinoma
Lei Zhang, Ting Sun, Xiao-Ye Wu, Fa-Ming Fei, Zhen-Zhen Gao
World Journal of Clinical Cases.2022; 10(29): 10501. CrossRef - Artificial intelligence platform, RADR®, aids in the discovery of DNA damaging agent for the ultra-rare cancer Atypical Teratoid Rhabdoid Tumors
Joseph McDermott, Drew Sturtevant, Umesh Kathad, Sudhir Varma, Jianli Zhou, Aditya Kulkarni, Neha Biyani, Caleb Schimke, William C. Reinhold, Fathi Elloumi, Peter Carr, Yves Pommier, Kishor Bhatia
Frontiers in Drug Discovery.2022;[Epub] CrossRef
- Unravelling switch/sucrose non-fermentable (SWI-SNF) complex-deficient thoracic tumours: a clinicopathological comparative on undifferentiated tumours and non-small cell lung carcinomas with BRG1 and BRM deficiency
- A case of concomitant EGFR/ALK alteration against a mutated EGFR background in early-stage lung adenocarcinoma
- Ki-Chang Lee, Jiwon Koh, Doo Hyun Chung, Yoon Kyung Jeon
- J Pathol Transl Med. 2021;55(2):139-144. Published online January 22, 2021
- DOI: https://doi.org/10.4132/jptm.2020.12.16
- 5,194 View
- 111 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - Rare cases of lung adenocarcinoma (LUAD) with concomitant epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) translocation have been reported. However, their clonal and evolutional relationship remains unclear. We report a case of early-stage EGFR-mutated LUAD with a focal concomitant EGFR/ALK alteration. A 63-year-old male underwent lobectomy to remove a 1.9-cm-sized lung nodule, which was diagnosed with EGFR-mutated LUAD. ALK immunohistochemistry (IHC) showed focal positivity within the part of the tumor characterized by lepidic pattern, also confirmed by fluorescence in-situ hybridization (FISH). Targeted next-generation sequencing was performed separately on the ALK IHC/FISH-positive and -negative areas. EGFR L833V/L858R mutations were detected in both areas, whereas EML4 (echinoderm microtubule-associated protein-like 4)-ALK translocations was confirmed only in the ALK IHC/FISH-positive area, suggesting the divergence of an EGFR/ALK co-altered subclone from the original EGFR-mutant clone. Our study suggests that concurrent alterations of EGFR and ALK can arise via divergent tumor evolution, even in the relatively early phases of tumorigenesis.
-
Citations
Citations to this article as recorded by- Machine learning-based characterization of a PANoptosis-associated model for enhancing prognosis and immunotherapy response in lung adenocarcinoma patients
Ziqiao Fu, Jia Zeng, Xiaomin Xiong, Weimin Zhong
Discover Oncology.2025;[Epub] CrossRef - Identification and validation of molecular subtype and prognostic signature for lung adenocarcinoma based on neutrophil extracellular traps
Yanhua Zuo, Guangyi Leng, Ping Leng
Pathology and Oncology Research.2023;[Epub] CrossRef - Machine Learning-Based Integration Develops a Macrophage-Related Index for Predicting Prognosis and Immunotherapy Response in Lung Adenocarcinoma
Zuwei Li, Minzhang Guo, Wanli Lin, Peiyuan Huang
Archives of Medical Research.2023; 54(7): 102897. CrossRef - Big data analysis identified a telomere-related signature predicting the prognosis and drug sensitivity in lung adenocarcinoma
Weiyi Zhang
Medicine.2023; 102(46): e35526. CrossRef
- Machine learning-based characterization of a PANoptosis-associated model for enhancing prognosis and immunotherapy response in lung adenocarcinoma patients
- Liquid biopsy using extracellular vesicle–derived DNA in lung adenocarcinoma
- In Ae Kim, Jae Young Hur, Hee Joung Kim, Seung Eun Lee, Wan Seop Kim, Kye Young Lee
- J Pathol Transl Med. 2020;54(6):453-461. Published online October 8, 2020
- DOI: https://doi.org/10.4132/jptm.2020.08.13
- 8,697 View
- 175 Download
- 20 Web of Science
- 18 Crossref
-
 Abstract
Abstract
 PDF
PDF - Blood liquid biopsy has emerged as a way of overcoming the clinical limitations of repeat biopsy by testing for the presence of acquired resistance mutations to therapeutic agents. Despite its merits of repeatability and non-invasiveness, this method is currently only used as a supplemental test due to a relatively low sensitivity rate of 50%–60%, and cannot replace tissue biopsy. The circulating tumor DNAs used in blood liquid biopsies are passive products of fragmented DNA with a short half-life released following tumor cell death; the low sensitivity seen with liquid blood biopsy results from this instability, which makes increasing the sensitivity of this test fundamentally difficult. Extracellular vesicles (EVs) are ideal carriers of cancer biomarkers, as cancer cells secret an abundance of EVs, and the contents of tumor cell-originated EVs reflect the molecular and genetic composition of parental cells. In addition, EV-derived DNAs (EV DNAs) consist of large-sized genomic DNAs and tumor-specific oncogenic mutant DNAs. For these reasons, liquid biopsy using EV DNA has the potential to overcome issues arising from tissue shortages associated with small biopsies, which are often seen in lung cancer patients, and the biopsy product can be used in other diagnostic methods, such as epidermal growth factor receptor (EGFR) mutation testing and next-generation sequencing (NGS). A higher sensitivity can be achieved when EV DNAs obtained from bronchoalveolar lavage fluid (BALF) are used rather than those from blood. BALF, when obtained close to the tumor site, is a promising liquid biopsy tool, as it enables the gathering of both cellular and non-cellular fractions of the tumor microenvironment, and provides increased diagnostic sensitivity when compared to blood.
-
Citations
Citations to this article as recorded by- Circulating Extracellular Vesicles as Promising Biomarkers for Precession Diagnostics: A Perspective on Lung Cancer
Sunil Vasu, Vinith Johnson, Archana M, K. Anki Reddy, Uday Kumar Sukumar
ACS Biomaterials Science & Engineering.2025; 11(1): 95. CrossRef - Diagnostic performance of metagenomic next-generation sequencing among hematological malignancy patients with bloodstream infections after antimicrobial therapy
Yueyi Xu, Miaoxin Peng, Tong Zhou, Yonggong Yang, Peipei Xu, Ting Xie, Xuefang Cao, Bing Chen, Jian Ouyang
Journal of Infection.2025; 90(2): 106395. CrossRef - Application of extracellular vesicles in tumor liquid biopsy
Shuai Li, Yating Liu, Dayong Yang
Chinese Science Bulletin.2025;[Epub] CrossRef - Unraveling extracellular vesicle DNA: Biogenesis, functions, and clinical implications
Mehraneh Nouri, Fateme Nasiri, Samaneh Sharif, Mohammad Reza Abbaszadegan
Pathology - Research and Practice.2025; 269: 155937. CrossRef - Nanobiotechnology: A smart platform of the future transform liquid biopsy era
Srijan Goswami, Palas Samanta, Manab Deb Adhikari
The Journal of Liquid Biopsy.2024; 3: 100137. CrossRef - Mesenchymal stem/stromal cells: dedicator to maintain tumor homeostasis
Juncun Yao, Li Sun, Feng Gao, Wei Zhu
Human Cell.2024;[Epub] CrossRef - Extracellular Vesicle-DNA: The Next Liquid Biopsy Biomarker for Early Cancer Diagnosis?
Irène Tatischeff
Cancers.2023; 15(5): 1456. CrossRef - Isolation of extracellular vesicles from human plasma samples: The importance of controls
Migmar Tsamchoe, Stephanie Petrillo, Anthoula Lazaris, Peter Metrakos
Biotechnology Journal.2023;[Epub] CrossRef - The role of extracellular vesicles in non-small-cell lung cancer, the unknowns, and how new approach methodologies can support new knowledge generation in the field
Sive Mullen, Dania Movia
European Journal of Pharmaceutical Sciences.2023; 188: 106516. CrossRef - Silicon microfabrication technologies for biology integrated advance devices and interfaces
Vuslat B. Juska, Graeme Maxwell, Pedro Estrela, Martyn E. Pemble, Alan O'Riordan
Biosensors and Bioelectronics.2023; 237: 115503. CrossRef - Bronchoalveolar Lavage as Potential Diagnostic Specimens to Genetic Testing in Advanced Nonsmall Cell Lung Cancer
Xuwen Lin, Yazhou Cai, Chenyu Zong, Binbin Chen, Di Shao, Hao Cui, Zheng Li, Ping Xu
Technology in Cancer Research & Treatment.2023;[Epub] CrossRef - In-Cell Labeling Coupled to Direct Analysis of Extracellular Vesicles in the Conditioned Medium to Study Extracellular Vesicles Secretion with Minimum Sample Processing and Particle Loss
Anissa Viveiros, Vaibhavi Kadam, John Monyror, Luis Carlos Morales, Desmond Pink, Aja M. Rieger, Simonetta Sipione, Elena Posse de Chaves
Cells.2022; 11(3): 351. CrossRef - Recent advances in liquid biopsy in cancers: Diagnosis, disease state and treatment response monitoring
Zhixian Chen, Judy Wai Ping Yam
Clinical and Translational Discovery.2022;[Epub] CrossRef - Cell-Secreted Vesicles: Novel Opportunities in Cancer Diagnosis, Monitoring and Treatment
Cristina Catoni, Veronica Di Paolo, Elisabetta Rossi, Luigi Quintieri, Rita Zamarchi
Diagnostics.2021; 11(6): 1118. CrossRef - DNA-Loaded Extracellular Vesicles in Liquid Biopsy: Tiny Players With Big Potential?
Susana García-Silva, Miguel Gallardo, Héctor Peinado
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - Characteristics and Clinical Application of Extracellular Vesicle-Derived DNA
Jae Young Hur, Kye Young Lee
Cancers.2021; 13(15): 3827. CrossRef - Bronchoalveolar Lavage as a Potential Diagnostic Specimens to Genetic Testing in Advanced Lung Cancer
Xuwen Lin, Xueying Wang, Yazhou Cai, Chenyu Zong, Dawei Liu, Jiming Yu, Chenxin Zhou, Jing Yao, Zheng Li, ping xu
SSRN Electronic Journal .2021;[Epub] CrossRef - Multi-Omics Data Integration in Extracellular Vesicle Biology—Utopia or Future Reality?
Leona Chitoiu, Alexandra Dobranici, Mihaela Gherghiceanu, Sorina Dinescu, Marieta Costache
International Journal of Molecular Sciences.2020; 21(22): 8550. CrossRef
- Circulating Extracellular Vesicles as Promising Biomarkers for Precession Diagnostics: A Perspective on Lung Cancer
- Evaluation of human papillomavirus (HPV) prediction using the International Endocervical Adenocarcinoma Criteria and Classification system, compared to p16 immunohistochemistry and HPV RNA in-situ hybridization
- Hezhen Ren, Jennifer Pors, Christine Chow, Monica Ta, Simona Stolnicu, Robert Soslow, David Huntsman, Lynn Hoang
- J Pathol Transl Med. 2020;54(6):480-488. Published online August 31, 2020
- DOI: https://doi.org/10.4132/jptm.2020.07.18
- 8,382 View
- 175 Download
- 11 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
The International Endocervical Adenocarcinoma Criteria and Classification (IECC) separated endocervical adenocarcinomas into human papillomavirus (HPV) associated (HPVA) and non–HPV-associated (NHPVA) categories by morphology alone. Our primary objective was to assess the accuracy of HPV prediction by the IECC system compared to p16 immunohistochemistry and HPV RNA in-situ hybridization (RISH). Our secondary goal was to directly compare p16 and HPV RISH concordance.
Methods
Cases were classified by IECC and stained for p16 and HPV RISH on tissue microarray, with discordant p16/HPV RISH cases re-stained on whole tissue sections. Remaining discordant cases (p16/HPV, IECC/p16, IECC/HPV discordances) were re-reviewed by the original pathologists (n = 3) and external expert pathologists (n = 2) blinded to the p16 and HPV RISH results. Final IECC diagnosis was assigned upon independent agreement between all reviewers.
Results
One hundred and eleven endocervical adenocarcinomas were classified originally into 94 HPVA and 17 NHPVA cases. p16 and HPV RISH was concordant in 108/111 cases (97%) independent of the IECC. HPV RISH and p16 was concordant with IECC in 103/111 (93%) and 106/111 (95%), respectively. After expert review, concordance improved to 107/111 (96%) for HPV RISH. After review of the eight discordant cases, one remained as HPVA, four were reclassified to NHPVA from HPVA, two were unclassifiable, and one possibly represented a mixed usual and gastric-type adenocarcinoma.
Conclusions
p16 and HPV RISH have excellent concordance in endocervical adenocarcinomas, and IECC can predict HPV status in most cases. Focal apical mitoses and apoptotic debris on original review led to the misclassification of several NHPVA as HPVA. -
Citations
Citations to this article as recorded by- EdgeNeXt-SEDP for cervical adenocarcinoma HPV-associated and non-HPV-associated diagnosis and decision support
Qi Chen, Hao Wang, Hao Zhang, Zhenkun Zhu, Xi Wei
Life Sciences.2025; 380: 123931. CrossRef - Cytology and histology of endocervical glandular lesions: a review with emphasis on recent developments
Natalie Banet, Karen L. Talia
Pathology.2025; 57(7): 817. CrossRef - Role of human papillomavirus status in the classification, diagnosis, and prognosis of malignant cervical epithelial tumors and precursor lesions
Simona Stolnicu
Die Pathologie.2025;[Epub] CrossRef - Joint detection of multiple HPV-testing technologies and evaluation of clinicopathological characteristics discriminate between HPV-independent and low-copy HPV-associated cervical squamous cell carcinoma (CSCC) -an analysis of 3869 cases
Linghui Lu, Tianqi Liu, Shunni Wang, Jing Li, Feiran Zhang, Yan Ning, Yiqin Wang
Gynecologic Oncology.2023; 170: 59. CrossRef - Incidence and Clinicopathologic Characteristics of Human Papillomavirus–independent Invasive Squamous Cell Carcinomas of the Cervix
Simona Stolnicu, Douglas Allison, Aaron M. Praiss, Basile Tessier-Cloutier, Amir Momeni Boroujeni, Jessica Flynn, Alexia Iasonos, Rene Serrette, Lien Hoang, Andrei Patrichi, Cristina Terinte, Anna Pesci, Claudia Mateoiu, Ricardo R. Lastra, Takako Kiyokawa
American Journal of Surgical Pathology.2023; 47(12): 1376. CrossRef - Testing Algorithms for the Diagnosis of Malignant Glandular Tumors of the Uterine Cervix Histotyped per the International Endocervical Adenocarcinoma Criteria and Classification (IECC) System
Máire A. Duggan, Qiuli Duan, Ruth M. Pfeiffer, Mary Anne Brett, Sandra Lee, Mustapha Abubakar, Martin Köbel, Monica Rodriguez, Aylin Sar
Applied Immunohistochemistry & Molecular Morphology.2022; 30(2): 91. CrossRef - Local and Metastatic Relapses in a Young Woman with Papillary Squamous Cell Carcinoma of the Uterine Cervix
Ha Young Woo, Hyun-Soo Kim
Diagnostics.2022; 12(3): 599. CrossRef - Clinical correlation of lymphovascular invasion and Silva pattern of invasion in early-stage endocervical adenocarcinoma: proposed binary Silva classification system
Simona Stolnicu, Lien Hoang, Noorah Almadani, Louise De Brot, Glauco Baiocchi, Graziele Bovolim, Maria Jose Brito, Georgia Karpathiou, Antonio Ieni, Esther Guerra, Takako Kiyokawa, Pavel Dundr, Carlos Parra-Herran, Sofia Lérias, Ana Felix, Andres Roma, An
Pathology.2022; 54(5): 548. CrossRef - Reproducibility of Morphologic Parameters of the International Endocervical Adenocarcinoma Criteria and Classification System and Correlation With Clinicopathologic Parameters: A Multi-Institutional Study
Pinar Bulutay, Nihan Haberal, Özlem Özen, Özlem Erdem, Emine H. Zeren, İbrahim Kulac, Çagatay Taskiran, Dogan Vatansever, Ali Ayhan, Nilgün Kapucuoğlu
International Journal of Gynecological Pathology.2022; 41(5): 447. CrossRef - HPV-Negative Cervical Cancer: A Narrative Review
Francesca Arezzo, Gennaro Cormio, Vera Loizzi, Gerardo Cazzato, Viviana Cataldo, Claudio Lombardi, Giuseppe Ingravallo, Leonardo Resta, Ettore Cicinelli
Diagnostics.2021; 11(6): 952. CrossRef - International Endocervical Adenocarcinoma Criteria and Classification (IECC): An Independent Cohort With Clinical and Molecular Findings
Hezhen Ren, Noorah Almadani, Jennifer Pors, Samuel Leung, Julie Ho, Christine Chow, Monica Ta, Kay J. Park, Simona Stolnicu, Robert Soslow, David Huntsman, Blake C. Gilks, Lynn Hoang
International Journal of Gynecological Pathology.2021; 40(6): 533. CrossRef
- EdgeNeXt-SEDP for cervical adenocarcinoma HPV-associated and non-HPV-associated diagnosis and decision support
- Clinicopathologic characteristics of HER2-positive pure mucinous carcinoma of the breast
- Yunjeong Jang, Hera Jung, Han-Na Kim, Youjeong Seo, Emad Alsharif, Seok Jin Nam, Seok Won Kim, Jeong Eon Lee, Yeon Hee Park, Eun Yoon Cho, Soo Youn Cho
- J Pathol Transl Med. 2020;54(1):95-102. Published online November 13, 2019
- DOI: https://doi.org/10.4132/jptm.2019.10.24
- 10,690 View
- 293 Download
- 22 Web of Science
- 19 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Pure mucinous carcinoma (PMC) is a rare type of breast cancer, estimated to represent 2% of invasive breast cancer. PMC is typically positive for estrogen receptors (ER) and progesterone receptors (PR) and negative for human epidermal growth factor receptor 2 (HER2). The clinicopathologic characteristics of HER2-positive PMC have not been investigated.
Methods
Pathology archives were searched for PMC diagnosed from January 1999 to April 2018. Clinicopathologic data and microscopic findings were reviewed and compared between HER2-positive PMC and HER2-negative PMC. We also analyzed the differences in disease-free survival (DFS) and overall survival according to clinicopathologic parameters including HER2 status in overall PMC cases.
Results
There were 21 HER2-positive cases (4.8%) in 438 PMCs. The average tumor size of HER2-positive PMC was 32.21 mm (± 26.55). Lymph node metastasis was present in seven cases. Compared to HER2-negative PMC, HER2-positive PMC presented with a more advanced T category (p < .001), more frequent lymph node metastasis (p = .009), and a higher nuclear and histologic grade (p < .001). Microscopically, signet ring cells were frequently observed in HER2-positive PMC (p < .001), whereas a micropapillary pattern was more frequent in HER2-negative PMC (p = .012). HER2-positive PMC was more frequently negative for ER (33.3% vs. 1.2%) and PR (28.6% vs. 7.2%) than HER2-negative PMC and showed a high Ki-67 labeling index. During follow-up, distant metastasis and recurrence developed in three HER2-positive PMC patients. Multivariate analysis revealed that only HER2-positivity and lymph node status were significantly associated with DFS.
Conclusions
Our results suggest that HER2-positive PMC is a more aggressive subgroup of PMC. HER2 positivity should be considered for adequate management of PMC. -
Citations
Citations to this article as recorded by- Mucin‐producing breast lesions: a practical approach to diagnosis
Sunayana Misra, Mihir Gudi, Kimberly H Allison, Edi Brogi, Cecily Quinn, Hannah Y Wen, Puay Hoon Tan
Histopathology.2026;[Epub] CrossRef - Clinicopathological characteristics of mucinous breast cancer: a retrospective analysis of a 6-years study from national cancer center in Vietnam
Thi Huyen Phung, Thanh Tung Pham, Huu Thang Nguyen, Dinh Thach Nguyen, Thanh Long Nguyen, Thi Hoai Hoang
Breast Cancer Research and Treatment.2025; 209(3): 667. CrossRef - Poor response of HER2-positive mucinous carcinomas of breast to neoadjuvant HER2-targeted therapy: A study of four cases
Min Han, Daniel Schmolze, Javier A. Arias-Stella, Christina H. Wei, Joanne Mortimer, Fang Fan
Annals of Diagnostic Pathology.2025; 74: 152396. CrossRef - Comprehensive Immunohistochemical Analysis of Mesonephric Marker Expression in Low-grade Endometrial Endometrioid Carcinoma
Yurimi Lee, Sangjoon Choi, Hyun-Soo Kim
International Journal of Gynecological Pathology.2024; 43(3): 221. CrossRef - Clinicopathological features and prognosis of mucinous breast carcinoma with a micropapillary structure
Beibei Yang, Menglu Shen, Bo Sun, Jing Zhao, Meng Wang
Thoracic Cancer.2024; 15(36): 2530. CrossRef - Pure Mucinous Carcinoma of the Breast: Radiologic-Pathologic Correlation
Cherie M Kuzmiak, Benjamin C Calhoun
Journal of Breast Imaging.2023;[Epub] CrossRef - Role of circ-FOXO3 and miR-23a in radiosensitivity of breast cancer
Elahe Abdollahi, Hossein Mozdarani, Behrooz Z. Alizadeh
Breast Cancer.2023; 30(5): 714. CrossRef - On Ultrasonographic Features of Mucinous Carcinoma with Micropapillary Pattern
Wei-Sen Yang, Yang Li, Ya Gao
Breast Cancer: Targets and Therapy.2023; Volume 15: 473. CrossRef - Spectrum of Mucin-containing Lesions of the Breast: Multimodality Imaging Review with Pathologic Correlation
Janice N. Thai, Melinda F. Lerwill, Shinn-Huey S. Chou
RadioGraphics.2023;[Epub] CrossRef - Mesonephric-like Adenocarcinoma of the Ovary: Clinicopathological and Molecular Characteristics
Hyun Hee Koh, Eunhyang Park, Hyun-Soo Kim
Diagnostics.2022; 12(2): 326. CrossRef - Alveolar Soft Part Sarcoma of the Uterus: Clinicopathological and Molecular Characteristics
Yurimi Lee, Kiyong Na, Ha Young Woo, Hyun-Soo Kim
Diagnostics.2022; 12(5): 1102. CrossRef - Metastasis of the Mucionous adenocarcinoma of breast to the mandibular gingiva: Rare case report
Ivana Mijatov, Aleksandra Fejsa Levakov, Aleksandar Spasić, Jelena Nikolić, Saša Mijatov
Medicine.2022; 101(38): e30732. CrossRef - Endometrioid Carcinomas of the Ovaries and Endometrium Involving Endocervical Polyps: Comprehensive Clinicopathological Analyses
Jihee Sohn, Yurimi Lee, Hyun-Soo Kim
Diagnostics.2022; 12(10): 2339. CrossRef - Serous Carcinoma of the Endometrium with Mesonephric-Like Differentiation Initially Misdiagnosed as Uterine Mesonephric-Like Adenocarcinoma: A Case Report with Emphasis on the Immunostaining and the Identification of Splice Site TP53 Mutation
Sangjoon Choi, Yoon Yang Jung, Hyun-Soo Kim
Diagnostics.2021; 11(4): 717. CrossRef - HER2 positive mucinous carcinoma of breast with micropapillary features: Report of a case and review of literature
Dinesh Chandra Doval, Rupal Tripathi, Sunil Pasricha, Pankaj Goyal, Chaturbhuj Agrawal, Anurag Mehta
Human Pathology: Case Reports.2021; 25: 200531. CrossRef - Carcinoma mucosecretor de mama HER2-positivo, un caso clínico
A.M. González Aranda, E. Martínez Gómez, A. Santana Costa, F. Arnanz Velasco, M.H. González de Diego, A. Zapico Goñi
Clínica e Investigación en Ginecología y Obstetricia.2021; 48(4): 100685. CrossRef - Clinicopathologic features of unexpectedly HER2 positive breast carcinomas: An institutional experience
Carissa LaBoy, Kalliopi P. Siziopikou, Lauren Rosen, Luis Z. Blanco, Jennifer L. Pincus
Pathology - Research and Practice.2021; 222: 153441. CrossRef - Mesonephric-like Differentiation of Endometrial Endometrioid Carcinoma: Clinicopathological and Molecular Characteristics Distinct from Those of Uterine Mesonephric-like Adenocarcinoma
Sujin Park, Go Eun Bae, Jiyoung Kim, Hyun-Soo Kim
Diagnostics.2021; 11(8): 1450. CrossRef - Mesonephric-like Adenocarcinoma of the Uterine Corpus: Comprehensive Immunohistochemical Analyses Using Markers for Mesonephric, Endometrioid and Serous Tumors
Hyunjin Kim, Kiyong Na, Go Eun Bae, Hyun-Soo Kim
Diagnostics.2021; 11(11): 2042. CrossRef
- Mucin‐producing breast lesions: a practical approach to diagnosis
- Expression of female sex hormone receptors and its relation to clinicopathological characteristics and prognosis of lung adenocarcinoma
- Jin Hwan Lee, Han Kyeom Kim, Bong Kyung Shin
- J Pathol Transl Med. 2020;54(1):103-111. Published online November 13, 2019
- DOI: https://doi.org/10.4132/jptm.2019.10.12
- 7,955 View
- 141 Download
- 6 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Adenocarcinoma (ADC) of the lung exhibits different clinicopathological characteristics in men and women. Recent studies have suggested that these differences originate from the expression of female sex hormone receptors in tumor cells. The aim of the present study was to evaluate the immunohistochemical expression of female sex hormone receptors in lung ADC and determine the expression patterns in patients with different clinicopathological characteristics.
Methods
A total of 84 patients with lung ADC who underwent surgical resection and/or core biopsy were recruited for the present study. Immunohistochemical staining was performed for estrogen receptor α (ERα), estrogen receptor β (ERβ), progesterone receptor (PR), epidermal growth factor receptor (EGFR), EGFR E746- A750 del, and EGFR L858R using tissue microarray.
Results
A total of 39 (46.4%) ERα-positive, 71 (84.5%) ERβ-positive, and 46 (54.8%) PR-positive lung ADCs were identified. In addition, there were 81 (96.4%) EGFR-positive, 14 (16.7%) EGFR E746-A750 del–positive, and 34 (40.5%) EGFR L858R–positive cases. The expression of female sex hormone receptors was not significantly different in clinicopathologically different subsets of lung ADC.
Conclusions
Expression of female sex hormone receptors is not associated with the prognosis and clinicopathological characteristics of patients with lung ADC. -
Citations
Citations to this article as recorded by- Molecular characteristics of non-small cell lung cancer tissue based on quantitative indicators of progesterone receptors expression
I. P. Romanov, T. A. Bogush, A. M. Scherbakov, A. A. Alimov, E. A. Bogush, A. B. Ravcheeva, A. Lee, V. S. Kosorukov
Antibiot Khimioter = Antibiotics and Chemotherapy.2024; 69(1-2): 29. CrossRef - Genes Co-Expressed with ESR2 Influence Clinical Outcomes in Cancer Patients: TCGA Data Analysis
Julia Maria Lipowicz, Agnieszka Malińska, Michał Nowicki, Agnieszka Anna Rawłuszko-Wieczorek
International Journal of Molecular Sciences.2024; 25(16): 8707. CrossRef - Complex Differential Diagnosis between Primary Breast Cancer and Breast Metastasis from EGFR-Mutated Lung Adenocarcinoma: Case Report and Literature Review
Carmine Valenza, Francesca Maria Porta, Alessandra Rappa, Elena Guerini-Rocco, Giuseppe Viale, Massimo Barberis, Filippo de Marinis, Giuseppe Curigliano, Chiara Catania
Current Oncology.2021; 28(5): 3384. CrossRef - Development of a 15‐Gene Signature Model as a Prognostic Tool in Sex Hormone‐Dependent Cancers
Zhi Xia, Jian Xiao, Aibin Liu, Qiong Chen, Arumugam R. Jayakumar
BioMed Research International.2021;[Epub] CrossRef - Gender-specific aspects of epidemiology, molecular genetics and outcome: lung cancer
Nuria Mederos, Alex Friedlaender, Solange Peters, Alfredo Addeo
ESMO Open.2020; 5(Suppl 4): e000796. CrossRef
- Molecular characteristics of non-small cell lung cancer tissue based on quantitative indicators of progesterone receptors expression
- Morule-like features in pulmonary adenocarcinoma associated with epidermal growth factor receptor mutations: two case reports with targeted next-generation sequencing analysis
- Yoo Jin Lee, Harim Oh, Eojin Kim, Bokyung Ahn, Jeong Hyeon Lee, Youngseok Lee, Yang Seok Chae, Chul Hwan Kim
- J Pathol Transl Med. 2020;54(1):119-122. Published online November 1, 2019
- DOI: https://doi.org/10.4132/jptm.2019.09.30
- 6,546 View
- 137 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - Morules, or morule-like features, can be identified in benign and malignant lesions in various organs. Morular features are unusual in pulmonary adenocarcinoma cases with only 26 cases reported to date. Here, we describe two cases of pulmonary adenocarcinoma with morule-like features in Korean women. One patient had a non-mucinous-type adenocarcinoma in situ and the other had an acinarpredominant adenocarcinoma with a micropapillary component. Both patients showed multiple intra-alveolar, nodular, whorled proliferative foci composed of atypical spindle cells with eosinophilic cytoplasm. Targeted next-generation sequencing was performed on DNA extracted from formalin-fixed paraffin-embedded samples of the tumors. Results showed unusual epidermal growth factor receptor (EGFR) mutations, which are associated with drug resistance to EGFR tyrosine kinase inhibitors, revealing the importance of identifying morule-like features in pulmonary adenocarcinoma and the need for additional study, since there are few reported cases.
-
Citations
Citations to this article as recorded by- Pulmonary adenocarcinoma in situ with morule - like components: A surgical case report
Mitsuteru Yosida, Mitsuru Tomita, Naoya Kawakita, Teruki Shimizu, Ryou Yamada, Hiromitsu Takizawa, Hisanori Uehara
Respiratory Medicine Case Reports.2024; 48: 102008. CrossRef - Clinicopathological, Radiological, and Molecular Features of Primary Lung Adenocarcinoma with Morule-Like Components
Li-Li Wang, Li Ding, Peng Zhao, Jing-Jing Guan, Xiao-Bin Ji, Xiao-Li Zhou, Shi-Hong Shao, Yu-Wei Zou, Wei-Wei Fu, Dong-Liang Lin, Dong Pan
Disease Markers.2021; 2021: 1. CrossRef
- Pulmonary adenocarcinoma in situ with morule - like components: A surgical case report
- MicroRNA-374a Expression as a Prognostic Biomarker in Lung Adenocarcinoma
- Yeseul Kim, Jongmin Sim, Hyunsung Kim, Seong Sik Bang, Seungyun Jee, Sungeon Park, Kiseok Jang
- J Pathol Transl Med. 2019;53(6):354-360. Published online October 24, 2019
- DOI: https://doi.org/10.4132/jptm.2019.10.01
- 6,674 View
- 130 Download
- 5 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Lung cancer is the most common cause of cancer-related death, and adenocarcinoma is the most common histologic subtype. MicroRNA is a small non-coding RNA that inhibits multiple target gene expression at the post-transcriptional level and is commonly dysregulated in malignant tumors. The purpose of this study was to analyze the expression of microRNA-374a (miR-374a) in lung adenocarcinoma and correlate its expression with various clinicopathological characteristics.
Methods
The expression level of miR-374a was measured in 111 formalin-fixed paraffin-embedded lung adenocarcinoma tissues using reverse transcription-quantitative polymerase chain reaction assays. The correlation between miR-374a expression and clinicopathological parameters, including clinical outcome, was further analyzed.
Results
High miR-374 expression was correlated with advanced pT category (chi-square test, p=.004) and pleural invasion (chi-square test, p=.034). Survival analysis revealed that patients with high miR-374a expression had significantly shorter disease-free survival relative to those with low miR-374a expression (log-rank test, p=.032).
Conclusions
miR-374a expression may serve as a potential prognostic biomarker for predicting recurrence in early stage lung adenocarcinoma after curative surgery. -
Citations
Citations to this article as recorded by- Upregulated miR-374a-5p drives psoriasis pathogenesis through WIF1 downregulation and Wnt5a/NF-κB activation
Jing Ma, Lu Gan, Hongying Chen, Lihao Chen, Yu Hu, Chao Luan, Kun Chen, Jiaan Zhang
Cellular Signalling.2024; 119: 111171. CrossRef - Association between the expression level of miRNA‑374a and TGF‑β1 in patients with colorectal cancer
Noha El Din, Reem El‑Shenawy, Rehab Moustafa, Ahmed Khairy, Sally Farouk
World Academy of Sciences Journal.2024;[Epub] CrossRef - Cell-free plasma miRNAs analysis for low invasive lung cancer diagnostics
M. Yu. Konoshenko, P. P. Laktionov, Yu. A. Lancuhaj, S. V. Pak, S. E. Krasilnikov, O. E. Bryzgunova
Advances in Molecular Oncology.2023; 10(2): 78. CrossRef - MicroRNA‑mediated regulation in lung adenocarcinoma: Signaling pathways and potential therapeutic implications (Review)
Jiye Liu, Fei Zhang, Jiahe Wang, Yibing Wang
Oncology Reports.2023;[Epub] CrossRef - Dysregulation of miR-374a is involved in the progression of diabetic retinopathy and regulates the proliferation and migration of retinal microvascular endothelial cells
Zhanhong Wang, Xiao Zhang, Yanjun Wang, Dailing Xiao
Clinical and Experimental Optometry.2022; 105(3): 287. CrossRef - MicroRNA Profile for Diagnostic and Prognostic Biomarkers in Thyroid Cancer
Jong-Lyul Park, Seon-Kyu Kim, Sora Jeon, Chan-Kwon Jung, Yong-Sung Kim
Cancers.2021; 13(4): 632. CrossRef
- Upregulated miR-374a-5p drives psoriasis pathogenesis through WIF1 downregulation and Wnt5a/NF-κB activation
- Rectal Invasion by Prostatic Adenocarcinoma That Was Initially Diagnosed in a Rectal Polyp on Colonoscopy
- Ghilsuk Yoon, Man-Hoon Han, An Na Seo
- J Pathol Transl Med. 2019;53(4):266-269. Published online April 11, 2019
- DOI: https://doi.org/10.4132/jptm.2019.03.25
- 9,229 View
- 129 Download
- 9 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF - Despite anatomical proximity, prostatic adenocarcinoma with rectal invasion is extremely rare. We present a case of rectal invasion by prostatic adenocarcinoma that was initially diagnosed from a rectal polyp biopsied on colonoscopy in a 69-year-old Korean man. He presented with dull anal pain and voiding discomfort for several days. Computed tomography revealed either prostatic adenocarcinoma with rectal invasion or rectal adenocarcinoma with prostatic invasion. His tumor marker profile showed normal prostate specific antigen (PSA) level and significantly elevated carcinoembryonic antigen level. Colonoscopy was performed, and a specimen was obtained from a round, 1.5 cm, sessile polyp that was 1.5 cm above the anal verge. Microscopically, glandular tumor structures infiltrated into the rectal mucosa and submucosa. Immunohistochemically, the tumor cells showed alpha-methylacyl-CoA-racemase positivity, PSA positivity, and caudal-related homeobox 2 negativity. The final diagnosis of the rectal polyp was consistent with prostatic adenocarcinoma. Here, we present a rare case that could have been misdiagnosed as rectal adenocarcinoma.
-
Citations
Citations to this article as recorded by- An Extremely Rare Metastatic Prostate Tumor From Rectal Cancer With Characteristic MRI Findings Due to Necrosis
Sohei Iwagami, Shoji Oura, Haruka Miyai, Naoki Kataoka, Masaya Nishihata
Cureus.2025;[Epub] CrossRef - Prostate cancer invading rectal serosa and anal sphincter treated with definitive radiation therapy: Case report and review of the literature
Mi-Jo Lee
Journal of Cancer Research and Therapeutics.2024; 20(3): 1081. CrossRef - Metastatic Adenocarcinoma of the Prostate Masquerading as a Splenic Flexure Colonic Polyp: A Diagnostic Conundrum
Zakaria W Shkoukani, Alaa Chamsin, Mohamed I Abdulmajed
Cureus.2024;[Epub] CrossRef - An Interesting Case of Prostate Cancer Presenting With Colonic Metastasis
Shawn Keating, Ayesha Imtiaz, Kenneth Nahum, Ankita Prasad, Pramil Cheriyath
Cureus.2023;[Epub] CrossRef - Metastase d’un adenocarcinome prostatique au sein d’un polype colique. À propos d’un cas et revue de la littérature
Guillaume Abitbol, Clémence Barthomeuf, Olivier Varennes, Marine Clement, Sami Hakim, Denis Chatelain
Annales de Pathologie.2023; 43(4): 342. CrossRef - Isolated Rectal Metastases from Locally Advanced Carcinoma Prostate Detected by 18F-PSMA-1007 PET/CT
Shashank Shekhar Singh, Rani Kunti Randhir Singh, Narvesh Kumar, Harshvardhan Atrey
World Journal of Nuclear Medicine.2022; 21(03): 248. CrossRef - Rectal Invasion by Metastatic Prostate Adenocarcinoma
Anshu Wadehra, Samer Alkassis, Aliza Rizwan, Omid Yazdanpanah
Cureus.2021;[Epub] CrossRef - Metastatic Prostate Cancer Presenting as a Rectal Polyp: A Rare Occurrence
Ese Uwagbale, Ifeanyichukwu Onukogu, Vimal Bodiwala, Solomon Agbroko, Niket Sonpal
Cureus.2021;[Epub] CrossRef - Local Staging of Prostate Cancer with Multiparametric MRI
Nandan Keshav, Mark D. Ehrhart, Steven C. Eberhardt, Martha F. Terrazas
Seminars in Roentgenology.2021; 56(4): 366. CrossRef
- An Extremely Rare Metastatic Prostate Tumor From Rectal Cancer With Characteristic MRI Findings Due to Necrosis
- The Expression of Adipophilin Is Frequently Found in Solid Subtype Adenocarcinoma and Is Associated with Adverse Outcomes in Lung Adenocarcinoma
- Sun Ah Shin, Hee Young Na, Ji Young Choe, Doohyun Chung, Mira Park, Sohee Oh, Ji Eun Kim
- J Pathol Transl Med. 2018;52(6):357-362. Published online October 4, 2018
- DOI: https://doi.org/10.4132/jptm.2018.09.13
- 6,935 View
- 111 Download
- 7 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
The up-regulation of the lipogenic pathway has been reported in many types of malignant tumors. However, its pathogenic role or clinical significance is not fully understood. The objective of this study was to examine the expression levels of adipophilin and related hypoxic signaling proteins and to determine their prognostic impacts and associations with the pathologic characteristics of lung adenocarcinoma.
Methods
Expression levels of adipophilin, heat shock protein 27 (HSP27), carbonic anhydrase IX, and hypoxia-inducible factor 1α were examined by immunohistochemical staining using tissue microarray blocks. Correlations between protein expression levels and various clinicopathologic features were analyzed.
Results
A total of 230 cases of primary adenocarcinoma of the lung were enrolled in this study. Adipophilin expression was more frequent in males and with the solid histologic type. It was correlated with HSP27 expression. Patients with adipophilin-positive adenocarcinoma showed a shorter progression-free survival (PFS) (median PFS, 17.2 months vs 18.4 months) in a univariable survival analysis, whereas HSP27 positivity correlated with favorable overall survival (OS) and PFS. In a multivariable analysis, adipophilin and HSP27 were independent prognostic markers of both OS and PFS.
Conclusions
Activated lipid metabolism and the hypoxic signaling pathway might play a major role in the progression of lung adenocarcinoma, especially in the solid histologic type. -
Citations
Citations to this article as recorded by- Perilipin 2 Mediates Progression of Lung Adenocarcinoma by Modulating Lipid Metabolism
Kana Miyata-Morita, Akira Kawashima, Mitsuo Kiriya, Hitoshi Dejima, Koji Saito, Yukinori Sakao, Koichi Suzuki, Yuko Sasajima, Shigeki Morita
The American Journal of Pathology.2025; 195(9): 1588. CrossRef - Prognostic implications of the immunohistochemical expression of perilipin 1 and adipophilin in high-grade liposarcoma
Kengo Kawaguchi, Kenichi Kohashi, Taro Mori, Hidetaka Yamamoto, Takeshi Iwasaki, Izumi Kinoshita, Yosuke Susuki, Hiroshi Furukawa, Makoto Endo, Yoshihiro Matsumoto, Yasuharu Nakashima, Yoshinao Oda
Journal of Clinical Pathology.2024; 77(10): 676. CrossRef - Prognostic and clinicopathologic significance of PLIN2 in cancers: A systematic review with meta-analysis
Ming-Lin Li, Han-Yong Luo, Zi-Wei Quan, Le-Tian Huang, Jia-He Wang
The International Journal of Biological Markers.2023; 38(1): 3. CrossRef - Novel prognostication biomarker adipophilin reveals a metabolic shift in uveal melanoma and new therapeutic opportunities
Maisoon Matareed, Eleftheria Maranou, Saara A Koskela, Arfa Mehmood, Helen Kalirai, Sarah E Coupland, Carlos R Figueiredo
The Journal of Pathology.2023; 260(2): 203. CrossRef - Adipophilin expression in cutaneous malignant melanoma is associated with high proliferation and poor clinical prognosis
Masakazu Fujimoto, Ibu Matsuzaki, Kazuchika Nishitsuji, Yuki Yamamoto, Daisuke Murakami, Takanori Yoshikawa, Ayaka Fukui, Yuuki Mori, Masaru Nishino, Yuichi Takahashi, Yoshifumi Iwahashi, Kenji Warigaya, Fumiyoshi Kojima, Masatoshi Jinnin, Shin-ichi Murat
Laboratory Investigation.2020; 100(5): 727. CrossRef - The clinicopathological significance of the adipophilin and fatty acid synthase expression in salivary duct carcinoma
Hideaki Hirai, Yuichiro Tada, Masato Nakaguro, Daisuke Kawakita, Yukiko Sato, Tomotaka Shimura, Kiyoaki Tsukahara, Satoshi Kano, Hiroyuki Ozawa, Kenji Okami, Yuichiro Sato, Chihiro Fushimi, Akira Shimizu, Isaku Okamoto, Soichiro Takase, Takuro Okada, Hiro
Virchows Archiv.2020; 477(2): 291. CrossRef
- Perilipin 2 Mediates Progression of Lung Adenocarcinoma by Modulating Lipid Metabolism
- Duodenal Adenocarcinoma of Brunner Gland Origin: A Case Report
- Ji Hye Moon, Kyoungbun Lee, Han-Kwang Yang, Woo Ho Kim
- J Pathol Transl Med. 2018;52(3):179-182. Published online December 27, 2017
- DOI: https://doi.org/10.4132/jptm.2017.10.09
- 9,639 View
- 172 Download
- 7 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF - We report a case of adenocarcinoma originating from the duodenal Brunner glands in a 47-year-old female patient. The lesion was 0.8 cm in extent and located at the posterior wall of the first part of the duodenum. Histologically, the tumor showed transition from non-neoplastic Brunner glands through dysplastic epithelium into adenocarcinoma. The carcinoma cells were strongly positive for MUC6 protein, which is an epithelial marker for the Brunner glands. Tumor protein p53 was overexpressed in the carcinoma cells, but not in the non-neoplastic or dysplastic epithelium. Dystrophic calcification was predominant. This is the first case report of duodenal adenocarcinoma of Brunner gland origin in Korea.
-
Citations
Citations to this article as recorded by- Duodenal Adenocarcinoma with a Gastric Phenotype Demonstrating a Rapidly Progressive Course
Sho Matsuyama, Akihisa Fukuda, Nobukazu Agatsuma, Masahito Hoki, Takahiro Utsumi, Hiroshi Seno
Internal Medicine.2025; 64(11): 1684. CrossRef - Relationship Between Immunophenotypes, Genetic Profiles, and Clinicopathologic Characteristics in Small Bowel Adenocarcinoma
Aitoshi Hoshimoto, Atsushi Tatsuguchi, Takeshi Yamada, Sho Kuriyama, Ryohei Hamakubo, Takayoshi Nishimoto, Jun Omori, Naohiko Akimoto, Katya Gudis, Keigo Mitsui, Shu Tanaka, Shunji Fujimori, Tsutomu Hatori, Akira Shimizu, Katsuhiko Iwakiri
American Journal of Surgical Pathology.2024; 48(2): 127. CrossRef - Recurrence of Brunner Gland Adenocarcinoma After Duodenectomy and Gastrojejunostomy
Paul Hong, Marcel Ghanim, Abdul Haseeb, Xianzhong Ding, Ayokunle T. Abegunde
ACG Case Reports Journal.2023; 10(6): e01060. CrossRef - HER2-positive adenocarcinoma arising from heterotopic pancreas tissue in the duodenum: A case report
Yoshifumi S Hirokawa, Takashi Iwata, Yoshinaga Okugawa, Koji Tanaka, Hiroyuki Sakurai, Masatoshi Watanabe
World Journal of Gastroenterology.2021; 27(28): 4738. CrossRef - Brunner’s gland adenoma is a rare cause of upper gastrointestinal bleeding: A case report and literature review
Nader Bakheet, Ahmed Cordie, Mohamed Nabil alkady, Ibrahim Naguib
Arab Journal of Gastroenterology.2020; 21(2): 122. CrossRef - Brunner's Gland Adenocarcinoma in an Aged Western Gorilla (Gorilla gorilla)
Jun Sasaki, Ippei Muneuchi, Kanako Ushio, Chihiro Sochi, Youichi Irie, Kazunori Yoshizumi, Waturu Hashimoto
Journal of Comparative Pathology.2020; 181: 47. CrossRef - Adenocarcinoma of the duodenum arising from Brunner’s gland resected by partial duodenectomy: a case report
Tetsuya Mochizuki, Nobuaki Fujikuni, Koichi Nakadoi, Masahiro Nakahara, Kazuaki Tanabe, Shuji Yonehara, Toshio Noriyuki
Surgical Case Reports.2019;[Epub] CrossRef
- Duodenal Adenocarcinoma with a Gastric Phenotype Demonstrating a Rapidly Progressive Course
- Cytologic Characteristics of Thymic Adenocarcinoma with Enteric Differentiation: A Study of Four Fine-Needle Aspiration Specimens
- Ah-Young Kwon, Joungho Han, Hae-yon Cho, Seokhwi Kim, Heejin Bang, Jiyeon Hyeon
- J Pathol Transl Med. 2017;51(5):509-512. Published online August 4, 2017
- DOI: https://doi.org/10.4132/jptm.2017.03.22
- 8,700 View
- 126 Download
- 6 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF - Thymic adenocarcinoma is extremely rare. Although its histologic features have been occasionally reported, a lack of description of the cytologic features has hampered the prompt and accurate diagnosis of this condition. Herein, we describe the cytologic findings and histology of four aspiration cytology specimens of thymic adenocarcinoma. The specimens were obtained from primary tumors, metastatic lymph nodes, and pericardial effusions. All four specimens showed three-dimensional glandular clusters with a loss of polarity and nuclear overlapping. One specimen had extensive extracellular mucinous material. Three specimens contained tumor cells with intracytoplasmic vacuoles. While the specimen with extracellular mucin showed relatively mild cytologic atypia, other specimens exhibited more atypical cytologic changes: irregular nuclear membranes, a coarse chromatin pattern, and prominent nucleoli. The cytologic features were correlated with the histologic features in each case of enteric type thymic adenocarcinoma. The differential diagnosis included other thymic carcinomas, yolk sac tumors, and metastatic adenocarcinoma from the lung or colorectum.
-
Citations
Citations to this article as recorded by- Primary thymic adenocarcinoma with signet ring cell-like features: A case report and literature review
Ya Jiang, Ziran Gao, Wenmang Xu, Yuanyuan Wang
Asian Journal of Surgery.2025;[Epub] CrossRef - Case report: Primary adenocarcinoma NOS of the thymus and cytological features
Jonathan Willner, Osvaldo Hernandez, Lea Azour, Andre L. Moreira
Diagnostic Cytopathology.2023;[Epub] CrossRef - Systemic chemotherapy for unresectable or recurrent primary thymic adenocarcinoma of enteric type
Xiaofang Gao
International Cancer Conference Journal.2022; 12(1): 46. CrossRef - Thymic adenocarcinoma accompanied by type A thymoma and pulmonary minimally invasive adenocarcinoma and harboring distinct gene alterations
Yi-Wen Zheng, Lin-Lin Bai, Gui-Yang Jiang, Xu-Yong Lin, Yang Liu, Hong-Tao Xu
Medicine.2021; 100(15): e25254. CrossRef - A case report: primary thymic adenocarcinoma with enteric differentiation
Yuuki Kou, Hirokazu Tanaka, Nobuhisa Yamazaki, Hiroyoshi Watanabe, Makoto Sonobe
The Journal of the Japanese Association for Chest Surgery.2020; 34(2): 107. CrossRef - Primary thymic adenocarcinoma with an aggressive clinical course: An autopsy case showing signet ring cell‐like features
Ayako Shiono, Takashi Fujino, Kyoichi Kaira, Tomomi Kato, Masanori Yasuda, Kunihiko Kobayashi, Hiroshi Kagamu
Thoracic Cancer.2020; 11(12): 3609. CrossRef - Primary Thymic Signet Ring Cell Adenocarcinoma: A Currently Unrecognized Variant
Richard Benedict Supan Roxas, Marie Christine Fajatin Bernardo, Araceli Pacis Jacoba, Janet Lim-Dy, Anarose Cariaga Alvarado, Jasna Metovic, Laura Annaratone, Mauro Papotti
International Journal of Surgical Pathology.2019; 27(3): 315. CrossRef - Disseminated and massive tumor burden in a case of primary thymic mucinous adenocarcinoma
Hui-Wen Liu, Chih-Yi Liu, Yi-Chen Yeh
Journal of Cancer Research and Practice.2019; 6(3): 151. CrossRef
- Primary thymic adenocarcinoma with signet ring cell-like features: A case report and literature review
- Yes-Associated Protein Expression Is Correlated to the Differentiation of Prostate Adenocarcinoma
- Myung-Giun Noh, Sung Sun Kim, Eu Chang Hwang, Dong Deuk Kwon, Chan Choi
- J Pathol Transl Med. 2017;51(4):365-373. Published online June 9, 2017
- DOI: https://doi.org/10.4132/jptm.2017.05.04
- 9,024 View
- 189 Download
- 10 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Yes-associated protein (YAP) in the Hippo signaling pathway is a growth control pathway that regulates cell proliferation and stem cell functions. Abnormal regulation of YAP was reported in human cancers including liver, lung, breast, skin, colon, and ovarian cancer. However, the function of YAP is not known in prostate adenocarcinoma. The purpose of this study was to investigate the role of YAP in tumorigenesis, differentiation, and prognosis of prostate adenocarcinoma.
Methods
The nuclear and cytoplasmic expression of YAP was examined in 188 cases of prostate adenocarcinoma using immunohistochemistry. YAP expression levels were evaluated in the nucleus and cytoplasm of the prostate adenocarcinoma and the adjacent normal prostate tissue. The presence of immunopositive tumor cells was evaluated and interpreted in comparison with the patients’ clinicopathologic data.
Results
YAP expression levels were not significantly different between normal epithelial cells and prostate adenocarcinoma. However, YAP expression level was significantly higher in carcinomas with a high Gleason grades (8–10) than in carcinomas with a low Gleason grades (6–7) (p < .01). There was no statistical correlation between YAP expression and stage, age, prostate-specific antigen level, and tumor volume. Biochemical recurrence (BCR)–free survival was significantly lower in patients with high YAP expressing cancers (p = .02). However high YAP expression was not an independent prognostic factor for BCR in the Cox proportional hazards model.
Conclusions
The results suggested that YAP is not associated with prostate adenocarcinoma development, but it may be associated with the differentiation of the adenocarcinoma. YAP was not associated with BCR. -
Citations
Citations to this article as recorded by- Deep learning uncovers histological patterns of YAP1/TEAD activity related to disease aggressiveness in cancer patients
Benoit Schmauch, Vincent Cabeli, Omar Darwiche Domingues, Jean-Eudes Le Douget, Alexandra Hardy, Reda Belbahri, Charles Maussion, Alberto Romagnoni, Markus Eckstein, Florian Fuchs, Aurélie Swalduz, Sylvie Lantuejoul, Hugo Crochet, François Ghiringhelli, V
iScience.2025; 28(1): 111638. CrossRef - Connecting Hippo Pathway and Cytoophidia in Drosophila Posterior Follicle Cells
Rui-Yu Weng, Lei Zhang, Ji-Long Liu
International Journal of Molecular Sciences.2024; 25(3): 1453. CrossRef - TLK1>Nek1 Axis Promotes Nuclear Retention and Activation of YAP with Implications for Castration-Resistant Prostate Cancer
Damilola Olatunde, Arrigo De Benedetti
Cancers.2024; 16(16): 2918. CrossRef - NEK1-Mediated Phosphorylation of YAP1 Is Key to Prostate Cancer Progression
Ishita Ghosh, Md Imtiaz Khalil, Rusella Mirza, Judy King, Damilola Olatunde, Arrigo De Benedetti
Biomedicines.2023; 11(3): 734. CrossRef - Epigenetic Inheritance From Normal Origin Cells Can Determine the Aggressive Biology of Tumor-Initiating Cells and Tumor Heterogeneity
Jiliang Feng, Dawei Zhao, Fudong Lv, Zhongyu Yuan
Cancer Control.2022;[Epub] CrossRef - A Yes-Associated Protein (YAP) and Insulin-Like Growth Factor 1 Receptor (IGF-1R) Signaling Loop Is Involved in Sorafenib Resistance in Hepatocellular Carcinoma
Mai-Huong T. Ngo, Sue-Wei Peng, Yung-Che Kuo, Chun-Yen Lin, Ming-Heng Wu, Chia-Hsien Chuang, Cheng-Xiang Kao, Han-Yin Jeng, Gee-Way Lin, Thai-Yen Ling, Te-Sheng Chang, Yen-Hua Huang
Cancers.2021; 13(15): 3812. CrossRef - Evidence for discrete modes of YAP1 signaling via mRNA splice isoforms in development and diseases
Jan Vrbský, Vladimir Vinarský, Ana Rubina Perestrelo, Jorge Oliver De La Cruz, Fabiana Martino, Antonio Pompeiano, Valerio Izzi, Ota Hlinomaz, Vladimir Rotrekl, Marius Sudol, Stefania Pagliari, Giancarlo Forte
Genomics.2021; 113(3): 1349. CrossRef - Up regulation of the Hippo signalling effector YAP1 is linked to early biochemical recurrence in prostate cancers
Andreas Marx, Aljoscha Schumann, Doris Höflmayer, Elena Bady, Claudia Hube-Magg, Katharina Möller, Maria Christina Tsourlakis, Stefan Steurer, Franziska Büscheck, Till Eichenauer, Till S. Clauditz, Markus Graefen, Ronald Simon, Guido Sauter, Jakob R. Izbi
Scientific Reports.2020;[Epub] CrossRef - NEK1 Phosphorylation of YAP Promotes Its Stabilization and Transcriptional Output
Md Imtiaz Khalil, Ishita Ghosh, Vibha Singh, Jing Chen, Haining Zhu, Arrigo De Benedetti
Cancers.2020; 12(12): 3666. CrossRef
- Deep learning uncovers histological patterns of YAP1/TEAD activity related to disease aggressiveness in cancer patients
- Mesothelin Expression in Gastric Adenocarcinoma and Its Relation to Clinical Outcomes
- Song-Hee Han, Mee Joo, Hanseong Kim, Sunhee Chang
- J Pathol Transl Med. 2017;51(2):122-128. Published online February 15, 2017
- DOI: https://doi.org/10.4132/jptm.2016.11.18
- 9,780 View
- 171 Download
- 22 Web of Science
- 20 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Although surgical resection with chemotherapy is considered effective for patients with advanced gastric cancer, it remains the third leading cause of cancer-related death in South Korea. Several studies have reported that mesothelial markers including mesothelin, calretinin, and Wilms tumor protein 1 (WT1) were positive in variable carcinomas, associated with prognosis, and were evaluated as potential markers for targeted therapy. The aim of this study was to assess the immunohistochemical expression of mesothelial markers (mesothelin, calretinin, and WT1) in gastric adenocarcinoma and their relations to clinocopathological features and prognosis. Methods: We evaluated calretinin, WT1, and mesothelin expression by immunohistochemical staining in 117 gastric adenocarcinomas. Results: Mesothelin was positively stained in 30 cases (25.6%). Mesothelin expression was related to increased depth of invasion (p = .002), lymph node metastasis (p = .013), and presence of lymphovascular (p = .015) and perineural invasion (p = .004). Patients with mesothelin expression had significantly worse disease-free survival rate compared with that of nonmesothelin expression group (p = .024). Univariate analysis showed that mesothelin expression is related to short-term survival. None of the 117 gastric adenocarcinomas stained for calretinin or WT1. Conclusions: Mesothelin expression was associated with poor prognosis. Our results suggest that mesothelin-targeted therapy should be considered as an important therapeutic alternative for gastric adenocarcinoma patients with mesothelin expression. -
Citations
Citations to this article as recorded by- Mesothelin as a diagnostic biomarker in endometriosis: A cross-sectional study
Kamil Kiecka, Aleksandra Zygula, Anna Sankiewicz, Mariusz Kuzmicki, Slawomir Lawicki, Michal Ciebiera, Tadeusz Issat, Jan Blaszczyk, Krzysztof Cendrowski, Ewa Gorodkiewicz, Piotr Laudanski
Advances in Medical Sciences.2026; 71(1): 1. CrossRef - High mesothelin expression is associated with low cytotoxic T cell infiltration in pancreatic cancer
Oliver Liang, Amy L. White, Timothy Fielder, Joo-Shik Shin, Sharon M. Sagnella, Ulf Schmitz, Dannel Yeo
Frontiers in Immunology.2025;[Epub] CrossRef - On-target off-tumor toxicity of claudin18.2-directed CAR-T cells in preclinical models
Filippo Birocchi, Antonio J. Almazan, Aiyana Parker, Amanda A. Bouffard, Sadie Goncalves, Christopher Kelly, Jessica Frank, Mark B. Leick, Nicholas J. Haradhvala, Shaw Kagawa, Gad Getz, Giulia Escobar, Diego Salas-Benito, Adele Mucci, Trisha R. Berger, Ma
Nature Communications.2025;[Epub] CrossRef - Novel perspectives on MSLN-targeted cancer therapy: from molecular mechanisms to clinical translation
Zhendong Wu, Xuefei Fu, Yuan Feng, Rong Zeng, Huan Qin, Kai Yao
Cancer Biology & Therapy.2025;[Epub] CrossRef - Persistence of activated anti‐mesothelin hYP218 chimeric antigen receptor T cells in the tumour is associated with efficacy in gastric and colorectal carcinomas
Sameer Mir, Abhilash Venugopalan, Jingli Zhang, Nishanth Ulhas Nair, Manjistha Sengupta, Manakamana Khanal, Chaido Stathopoulou, Qun Jiang, Raffit Hassan
Clinical and Translational Medicine.2024;[Epub] CrossRef - Targeting Mesothelin in Solid Tumours: Anti-mesothelin Antibody and Drug Conjugates
Quincy Chu
Current Oncology Reports.2023; 25(4): 309. CrossRef - High expression of mesothelin in plasma and tissue is associated with poor prognosis and promotes invasion and metastasis in gastric cancer
Suryendu Saha, Chitranjan Mukherjee, Dipjit Basak, Prasun Panja, Pronoy Kanti Mondal, Ranajoy Ghosh, Aniket Halder, Abhijit Chowdhury, Gopal Krishna Dhali, Bitan Kumar Chattopadhyay, Saurabh Ghosh, Somsubhra Nath, Shalini Datta
Advances in Cancer Biology - Metastasis.2023; 7: 100098. CrossRef - Novel Anti-Mesothelin Nanobodies and Recombinant Immunotoxins with Pseudomonas Exotoxin Catalytic Domain for Cancer Therapeutics
Minh Quan Nguyen, Do Hyung Kim, Hye Ji Shim, Huynh Kim Khanh Ta, Thi Luong Vu, Thi Kieu Oanh Nguyen, Jung Chae Lim, Han Choe
Molecules and Cells.2023; 46(12): 764. CrossRef - Immunotherapy for lung cancer: Focusing on chimeric antigen receptor (CAR)-T cell therapy
Tongqing Xue, Xiang Zhao, Kun Zhao, Yan Lu, Juan Yao, Xianguo Ji
Current Problems in Cancer.2022; 46(1): 100791. CrossRef - Cellular Immunotherapy in Gastric Cancer: Adoptive Cell Therapy and Dendritic Cell-Based Vaccination
Elnaz Faghfuri, Mahdi Abdoli Shadbad, Amir Hossein Faghfouri, Narges Soozangar
Immunotherapy.2022; 14(6): 475. CrossRef - Mesothelin Expression in Human Tumors: A Tissue Microarray Study on 12,679 Tumors
Sören Weidemann, Pauline Gagelmann, Natalia Gorbokon, Maximilian Lennartz, Anne Menz, Andreas M. Luebke, Martina Kluth, Claudia Hube-Magg, Niclas C. Blessin, Christoph Fraune, Katharina Möller, Christian Bernreuther, Patrick Lebok, Till S. Clauditz, Frank
Biomedicines.2021; 9(4): 397. CrossRef - Host Mesothelin Expression Increases Ovarian Cancer Metastasis in the Peritoneal Microenvironment
Tyvette S. Hilliard, Brooke Kowalski, Kyle Iwamoto, Elizabeth A. Agadi, Yueying Liu, Jing Yang, Marwa Asem, Yuliya Klymenko, Jeff Johnson, Zonggao Shi, Gifty Marfowaa, Madeleine G. Yemc, Phillip Petrasko, M. Sharon Stack
International Journal of Molecular Sciences.2021; 22(22): 12443. CrossRef - CAR-T Cell Therapy—An Overview of Targets in Gastric Cancer
Dominika Bębnowska, Ewelina Grywalska, Paulina Niedźwiedzka-Rystwej, Barbara Sosnowska-Pasiarska, Jolanta Smok-Kalwat, Marcin Pasiarski, Stanisław Góźdź, Jacek Roliński, Wojciech Polkowski
Journal of Clinical Medicine.2020; 9(6): 1894. CrossRef - Mesothelin-Targeted Recombinant Immunotoxins for Solid Tumors
Brendan L. Hagerty, Guillaume J. Pegna, Jian Xu, Chin-Hsien Tai, Christine Alewine
Biomolecules.2020; 10(7): 973. CrossRef - Phase I/II clinical trial of a Wilms’ tumor 1-targeted dendritic cell vaccination-based immunotherapy in patients with advanced cancer
Wen Zhang, Xu Lu, Peilin Cui, Chunmei Piao, Man Xiao, Xuesong Liu, Yue Wang, Xuan Wu, Jingwei Liu, Lin Yang
Cancer Immunology, Immunotherapy.2019; 68(1): 121. CrossRef - Mesothelin as a target for cervical cancer therapy
Korinna Jöhrens, Lea Lazzerini, Jana Barinoff, Jalid Sehouli, Guenter Cichon
Archives of Gynecology and Obstetrics.2019; 299(1): 211. CrossRef - A targeted proteomics approach reveals a serum protein signature as diagnostic biomarker for resectable gastric cancer
Qiujin Shen, Karol Polom, Coralie Williams, Felipe Marques Souza de Oliveira, Mariana Guergova-Kuras, Frederique Lisacek, Niclas G. Karlsson, Franco Roviello, Masood Kamali-Moghaddam
eBioMedicine.2019; 44: 322. CrossRef - Mesothelin as a biomarker for targeted therapy
Jiang Lv, Peng Li
Biomarker Research.2019;[Epub] CrossRef - Antibody drug conjugates under investigation in phase I and phase II clinical trials for gastrointestinal cancer
Alexis D. Leal, Anuradha Krishnamurthy, Lia Head, Wells A. Messersmith
Expert Opinion on Investigational Drugs.2018; 27(11): 901. CrossRef - Mesothelin‑targeted second generation CAR‑T cells inhibit growth of mesothelin‑expressing tumors in�vivo
Lin Ye, Yuqing Lou, Liming Lu, Xiaohong Fan
Experimental and Therapeutic Medicine.2018;[Epub] CrossRef
- Mesothelin as a diagnostic biomarker in endometriosis: A cross-sectional study
- Aurora Kinase A Is a Prognostic Marker in Colorectal Adenocarcinoma
- Hyun Min Koh, Bo Geun Jang, Chang Lim Hyun, Young Sill Kim, Jin Won Hyun, Weon Young Chang, Young Hee Maeng
- J Pathol Transl Med. 2017;51(1):32-39. Published online December 25, 2016
- DOI: https://doi.org/10.4132/jptm.2016.10.17
- 11,093 View
- 189 Download
- 28 Web of Science
- 29 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Aurora kinase A (AURKA), or STK15/BTAK, is a member of the serine/threonine kinase family and plays important roles in mitosis and chromosome stability. This study investigated the clinical significance of AURKA expression in colorectal cancer patients in Korea.
Methods
AURKA protein expression was evaluated by immunohistochemistry in 151 patients with colorectal adenocarcinoma using tissue microarray blocks. We analyzed the relationship between clinicopathological characteristics and AURKA expression. In addition, the prognostic significance of various clinicopathological data for progression-free survival (PFS) was assessed. Also we evaluated copy number variations by array comparative genomic hybridization and AURKA gene amplification using fluorescence in situ hybridization in colorectal carcinoma tissues.
Results
AURKA gene amplification was found more frequently in the 20q13.2–13.33 gain-positive group than the group with no significant gain on the AURKA-containing locus. AURKA protein expression was detected in 45% of the cases (68/151). Positive staining for AURKA was observed more often in male patients (p = .035) and distally located tumors (p = .021). PFS was shorter in patients with AURKA expression compared to those with low-level AURKA expression (p < .001). Univariate analysis revealed that AURKA expression (p = .001), age (p = .034), lymphatic invasion (p = .001), perineural invasion (p = .002), and TNM stage (p = .013) significantly affected PFS. In a multivariate analysis of PFS, a Cox proportional hazard model confirmed that AURKA expression was an independent and significant prognostic factor in colorectal adenocarcinoma (hazard ratio, 3.944; p < .001).
Conclusions
AURKA could serve as an independent factor to predict a poor prognosis in Korean colorectal adenocarcinoma patients. -
Citations
Citations to this article as recorded by- Exploring pyrimidine scaffolds for cancer therapy: A triad of synthetic chemistry, computational studies, and in-vitro biological testing
Pooja Kumari, Anandkumar Tengli
Journal of Molecular Structure.2026; 1350: 144013. CrossRef - First-in-class dual inhibitors of MASTL and Aurora A kinase: Discovery of selective cyclohexa[b]thiophenes with potent anticancer activity
Somaya A. Abdel-Rahman, Hossam Nada, Moustafa T. Gabr
European Journal of Medicinal Chemistry.2025; 293: 117729. CrossRef - RNAi delivery mediated by milk extracellular vesicles in colon cancer
Jessie Santoro, Silvia Nuzzo, Andrea Soricelli, Marco Salvatore, Anna Maria Grimaldi
Molecular Therapy Nucleic Acids.2025; 36(3): 102644. CrossRef - Potential Signature Therapeutic Biomarkers TOP2A, MAD2L1, and CDK1 in Colorectal Cancer: A Systems Biomedicine-Based Approach
P. Priyamvada, Sudha Ramaiah
Biochemical Genetics.2024; 62(3): 2166. CrossRef - Neutralizing IL-16 enhances the efficacy of targeting Aurora-A therapy in colorectal cancer with high lymphocyte infiltration through restoring anti-tumor immunity
Shiang-Jie Yang, Sheng-Tsung Chang, Kung-Chao Chang, Bo-Wen Lin, Kwang-Yu Chang, Yao-Wen Liu, Ming-Derg Lai, Liang-Yi Hung
Cell Death & Disease.2024;[Epub] CrossRef - Development and validation of epigenetic modification-related signals for the diagnosis and prognosis of colorectal cancer
Xia Li, Jingjing Li, Jie Li, Nannan Liu, Liwei Zhuang
BMC Genomics.2024;[Epub] CrossRef - Recent Updates on Oncogenic Signaling of Aurora Kinases in
Chemosensitive, Chemoresistant Cancers: Novel Medicinal Chemistry
Approaches for Targeting Aurora Kinases
Pooja Kumari, Narasimha Murthy Beeraka, Anandkumar Tengli, Gurupadayya Bannimath, Ramandeep Kaur Baath, Mayuri Patil
Current Medicinal Chemistry.2024; 31(23): 3502. CrossRef - Identification of Aurora A kinase allosteric inhibitors: A comprehensive virtual screening through fingerprint-based similarity search, molecular docking, machine learning and molecular dynamics simulation
Mahima Sudhir Kolpe, Surbhi Pravin Pawar, Vikramsinh Sardarsinh Suryawanshi, Heba Taha M. Abdelghani, Pritee Chunarkar Patil, Shovonlal Bhowmick
Journal of Molecular Liquids.2024; 414: 126115. CrossRef - Exploring Core Genes by Comparative Transcriptomics Analysis for Early Diagnosis, Prognosis, and Therapies of Colorectal Cancer
Md. Ariful Islam, Md. Bayazid Hossen, Md. Abu Horaira, Md. Alim Hossen, Md. Kaderi Kibria, Md. Selim Reza, Khanis Farhana Tuly, Md. Omar Faruqe, Firoz Kabir, Rashidul Alam Mahumud, Md. Nurul Haque Mollah
Cancers.2023; 15(5): 1369. CrossRef - The Oncology Biomarker Discovery framework reveals cetuximab and bevacizumab response patterns in metastatic colorectal cancer
Alexander J. Ohnmacht, Arndt Stahler, Sebastian Stintzing, Dominik P. Modest, Julian W. Holch, C. Benedikt Westphalen, Linus Hölzel, Marisa K. Schübel, Ana Galhoz, Ali Farnoud, Minhaz Ud-Dean, Ursula Vehling-Kaiser, Thomas Decker, Markus Moehler, Matthias
Nature Communications.2023;[Epub] CrossRef - Disease Modeling on Tumor Organoids Implicates AURKA as a Therapeutic Target in Liver Metastatic Colorectal Cancer
Sophie L. Boos, Leon P. Loevenich, Sebastian Vosberg, Thomas Engleitner, Rupert Öllinger, Jörg Kumbrink, Matjaz Rokavec, Marlies Michl, Philipp A. Greif, Andreas Jung, Heiko Hermeking, Jens Neumann, Thomas Kirchner, Roland Rad, Peter Jung
Cellular and Molecular Gastroenterology and Hepatology.2022; 13(2): 517. CrossRef - Colorectal cancer on a dish: exploring the 3D-sphere culture of primary colorectal cancer cells from an Indonesian perspective
Murdani Abdullah, DR Noor, Amanda Pitarini Utari, Virly Nanda Muzellina, Nur Rahadiani, Radiana Dhewayani Antarianto
F1000Research.2022; 11: 182. CrossRef - Mitotic protein kinase-driven crosstalk of machineries for mitosis and metastasis
Chang-Hyeon Kim, Da-Eun Kim, Dae-Hoon Kim, Ga-Hong Min, Jung-Won Park, Yeo-Bin Kim, Chang K. Sung, Hyungshin Yim
Experimental & Molecular Medicine.2022; 54(4): 414. CrossRef - AURKA is a prognostic biomarker for good overall survival in stage II colorectal cancer patients
Peter Jung, David Horst, Thomas Kirchner, Frederick Klauschen, Jens Neumann
Pathology - Research and Practice.2022; 235: 153936. CrossRef - Therapeutic Potential of Mitotic Kinases’ Inhibitors in Cancers of the Gastrointestinal System
Aadil Javed, Gianluca Malagraba, Mahdieh Yarmohammadi, Catalina M. Perelló-Reus, Carles Barceló, Teresa Rubio-Tomás
Future Pharmacology.2022; 2(3): 214. CrossRef - Bioinformatics Analysis of RNA-seq Data Reveals Genes Related to Cancer Stem Cells in Colorectal Cancerogenesis
Kristian Urh, Nina Zidar, Emanuela Boštjančič
International Journal of Molecular Sciences.2022; 23(21): 13252. CrossRef - Unweaving the mitotic spindle: A focus on Aurora kinase inhibitors in lung cancer
Alessio Stefani, Geny Piro, Francesco Schietroma, Alessandro Strusi, Emanuele Vita, Simone Fiorani, Diletta Barone, Federico Monaca, Ileana Sparagna, Giustina Valente, Miriam Grazia Ferrara, Ettore D’Argento, Mariantonietta Di Salvatore, Carmine Carbone,
Frontiers in Oncology.2022;[Epub] CrossRef - Increased expression levels of AURKA and KIFC1 are promising predictors of progression and poor survival associated with gastric cancer
Jiyoon Jung, Hoiseon Jeong, Jung-Woo Choi, Hye-Sun Kim, Hwa Eun Oh, Eung Seok Lee, Young-Sik Kim, Ju-Han Lee
Pathology - Research and Practice.2021; 224: 153524. CrossRef - SALL Proteins; Common and Antagonistic Roles in Cancer
Claudia Álvarez, Aracelly Quiroz, Diego Benítez-Riquelme, Elizabeth Riffo, Ariel F. Castro, Roxana Pincheira
Cancers.2021; 13(24): 6292. CrossRef - AURKA gene polymorphisms and central nervous system tumor susceptibility in Chinese children
Yong-Ping Chen, Li Yuan, Hui-Ran Lin, Xiao-Kai Huang, Ji-Chen Ruan, Zhen-Jian Zhuo
Discover Oncology.2021;[Epub] CrossRef - Palmatine induces G2/M phase arrest and mitochondrial-associated pathway apoptosis in colon cancer cells by targeting AURKA
Xiaojiang Liu, Yaru Zhang, Siqi Wu, Minmin Xu, Youfeng Shen, Min Yu, Jinhua Fan, Sijia Wei, Chaohang Xu, Lu Huang, Han Zhao, Xuegang Li, Xiaoli Ye
Biochemical Pharmacology.2020; 175: 113933. CrossRef - New landscapes and horizons in hepatocellular carcinoma therapy
Melchiorre Cervello, Maria R. Emma, Giuseppa Augello, Antonella Cusimano, Lydia Giannitrapani, Maurizio Soresi, Shaw M. Akula, Stephen L. Abrams, Linda S. Steelman, Alessandro Gulino, Beatrice Belmonte, Giuseppe Montalto, James A. McCubrey
Aging.2020; 12(3): 3053. CrossRef - The New Paradigm of Network Medicine to Analyze Breast Cancer Phenotypes
Anna Maria Grimaldi, Federica Conte, Katia Pane, Giulia Fiscon, Peppino Mirabelli, Simona Baselice, Rosa Giannatiempo, Francesco Messina, Monica Franzese, Marco Salvatore, Paola Paci, Mariarosaria Incoronato
International Journal of Molecular Sciences.2020; 21(18): 6690. CrossRef - The GTEx Consortium atlas of genetic regulatory effects across human tissues
François Aguet, Shankara Anand, Kristin G. Ardlie, Stacey Gabriel, Gad A. Getz, Aaron Graubert, Kane Hadley, Robert E. Handsaker, Katherine H. Huang, Seva Kashin, Xiao Li, Daniel G. MacArthur, Samuel R. Meier, Jared L. Nedzel, Duyen T. Nguyen, Ayellet V.
Science.2020; 369(6509): 1318. CrossRef - Upregulation of aurora kinase A promotes vascular smooth muscle cell proliferation and migration by activating the GSK-3β/β-catenin pathway in aortic-dissecting aneurysms
Jia Meng, He-Liang Liu, Dong Ma, Hong-Yan Wang, Yue Peng, Hong-Li Wang
Life Sciences.2020; 262: 118491. CrossRef - Inhibition of AURKA Reduces Proliferation and Survival of Gastrointestinal Cancer Cells With Activated KRAS by Preventing Activation of RPS6KB1
Lihong Wang-Bishop, Zheng Chen, Ahmed Gomaa, Albert Craig Lockhart, Safia Salaria, Jialiang Wang, Keeli B. Lewis, Jeffrey Ecsedy, Kay Washington, Robert Daniel Beauchamp, Wael El-Rifai
Gastroenterology.2019; 156(3): 662. CrossRef - Discovery and Validation of Novel Biomarkers for Detection of Epithelial Ovarian Cancer
Hagen Kulbe, Raik Otto, Silvia Darb-Esfahani, Hedwig Lammert, Salem Abobaker, Gabriele Welsch, Radoslav Chekerov, Reinhold Schäfer, Duska Dragun, Michael Hummel, Ulf Leser, Jalid Sehouli, Elena Ioana Braicu
Cells.2019; 8(7): 713. CrossRef - Epigenetic regulation of AURKA by miR-4715-3p in upper gastrointestinal cancers
Ahmed Gomaa, Dunfa Peng, Zheng Chen, Mohammed Soutto, Khaled Abouelezz, Alejandro Corvalan, Wael El-Rifai
Scientific Reports.2019;[Epub] CrossRef - The functional diversity of Aurora kinases: a comprehensive review
Estelle Willems, Matthias Dedobbeleer, Marina Digregorio, Arnaud Lombard, Paul Noel Lumapat, Bernard Rogister
Cell Division.2018;[Epub] CrossRef
- Exploring pyrimidine scaffolds for cancer therapy: A triad of synthetic chemistry, computational studies, and in-vitro biological testing
- Size of Non-lepidic Invasive Pattern Predicts Recurrence in Pulmonary Mucinous Adenocarcinoma: Morphologic Analysis of 188 Resected Cases with Reappraisal of Invasion Criteria
- Soohyun Hwang, Joungho Han, Misun Choi, Myung-Ju Ahn, Yong Soo Choi
- J Pathol Transl Med. 2017;51(1):56-68. Published online October 16, 2016
- DOI: https://doi.org/10.4132/jptm.2016.09.17
- 12,282 View
- 237 Download
- 8 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
We reviewed a series of 188 resected pulmonary mucinous adenocarcinomas (MAs) to clarify the prognostic significance of lepidic and non-lepidic patterns.
Methods
Non-lepidic patterns were divided into bland, non-distorted acini with uncertain invasiveness (pattern 1), unequivocal invasion into stroma (pattern 2), or invasion into alveolar spaces (pattern 3).
Results
The mean proportion of invasive patterns (patterns 2 and 3) was lowest in small (≤ 3 cm) tumors, and gradually increased in intermediate (> 3 cm and ≤ 7 cm) and large (> 7 cm) tumors (8.4%, 34.3%, and 50.1%, respectively). Adjusted T (aT) stage, as determined by the size of invasive patterns, was positively correlated with adverse histologic and clinical features including older age, male sex, and ever smokers. aTis tumors, which were exclusively composed of lepidic pattern (n = 9), or a mixture of lepidic and pattern 1 (n = 40) without any invasive patterns, showed 100% disease- free survival (DFS). The aT1mi tumors, with minimal (≤ 5 mm) invasive patterns (n = 63), showed a 95.2% 5-year DFS, with recurrences (n = 2) limited to tumors greater than 3 cm in total size (n = 23). Both T and aT stage were significantly associated with DFS; however, survival within the separate T-stage subgroups was stratified according to the aT stage, most notably in the intermediatestage subgroups. In multivariate analysis, the size of invasive patterns (p = .020), pleural invasion (p < .001), and vascular invasion (p = .048) were independent predictors of recurrence, whereas total size failed to achieve statistical significance (p = .121).
Conclusions
This study provides a rationale for histologic risk stratification in pulmonary MA based on the extent of invasive growth patterns with refined criteria for invasion. -
Citations
Citations to this article as recorded by- Distinct Recurrence Pattern and Survival Outcomes of Invasive Mucinous Adenocarcinoma of the Lung: The Potential Role of Local Therapy in Intrapulmonary Spread
Dong Woog Yoon, Soohyun Hwang, Tae Hee Hong, Yoon-La Choi, Hong Kwan Kim, Yong Soo Choi, Jhingook Kim, Young Mog Shim, Jong Ho Cho
Annals of Surgical Oncology.2024; 31(1): 201. CrossRef - Pulmonary invasive mucinous adenocarcinoma
Wei‐Chin Chang, Yu Zhi Zhang, Andrew G Nicholson
Histopathology.2024; 84(1): 18. CrossRef - Micropapillary Pattern in Invasive Mucinous Adenocarcinoma of the Lung: Comparison With Invasive Non-Mucinous Adenocarcinoma
Hui He, Lue Li, Yuan-yuan Wen, Li-yong Qian, Zhi-qiang Yang
International Journal of Surgical Pathology.2024; 32(5): 926. CrossRef - Radiological and clinical features of screening-detected pulmonary invasive mucinous adenocarcinoma
Dae Hyeon Kim, So Young Bae, Kwon Joong Na, Samina Park, In Kyu Park, Chang Hyun Kang, Young Tae Kim
Interactive CardioVascular and Thoracic Surgery.2022; 34(2): 229. CrossRef - Micropapillary Pattern in Invasive Mucinous Adenocarcinoma of the Lung: Comparison with Invasive Non-Mucinous Adenocarcinoma
Hui He, Yuanyuan Wen, Liyong Qian, Zhiqiang Yang
SSRN Electronic Journal .2022;[Epub] CrossRef - Optimal method for measuring invasive size that predicts survival in invasive mucinous adenocarcinoma of the lung
Tomonari Oki, Keiju Aokage, Shogo Nomura, Kenta Tane, Tomohiro Miyoshi, Norihiko Shiiya, Kazuhito Funai, Masahiro Tsuboi, Genichiro Ishii
Journal of Cancer Research and Clinical Oncology.2020; 146(5): 1291. CrossRef - Prognostic Impact of Histopathologic Features in Pulmonary Invasive Mucinous Adenocarcinomas
Wei-Chin Chang, Yu Zhi Zhang, Eric Lim, Andrew G Nicholson
American Journal of Clinical Pathology.2020; 154(1): 88. CrossRef
- Distinct Recurrence Pattern and Survival Outcomes of Invasive Mucinous Adenocarcinoma of the Lung: The Potential Role of Local Therapy in Intrapulmonary Spread
- Differential Immunohistochemical Profiles for Distinguishing Prostate Carcinoma and Urothelial Carcinoma
- Woo Jin Oh, Arthur Minwoo Chung, Jee Soon Kim, Ji Heun Han, Sung Hoo Hong, Ji Yeol Lee, Yeong Jin Choi
- J Pathol Transl Med. 2016;50(5):345-354. Published online August 7, 2016
- DOI: https://doi.org/10.4132/jptm.2016.06.14
- 15,026 View
- 351 Download
- 32 Web of Science
- 33 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
The pathologic distinction between high-grade prostate adenocarcinoma (PAC) involving the urinary bladder and high-grade urothelial carcinoma (UC) infiltrating the prostate can be difficult. However, making this distinction is clinically important because of the different treatment modalities for these two entities.
Methods
A total of 249 patient cases (PAC, 111 cases; UC, 138 cases) collected between June 1995 and July 2009 at Seoul St. Mary’s Hospital were studied. An immunohistochemical evaluation of prostatic markers (prostate-specific antigen [PSA], prostate-specific membrane antigen [PSMA], prostate acid phosphatase [PAP], P501s, NKX3.1, and α-methylacyl coenzyme A racemase [AMACR]) and urothelial markers (CK34βE12, p63, thrombomodulin, S100P, and GATA binding protein 3 [GATA3]) was performed using tissue microarrays from each tumor.
Results
The sensitivities of prostatic markers in PAC were 100% for PSA, 83.8% for PSMA, 91.9% for PAP, 93.7% for P501s, 88.3% for NKX 3.1, and 66.7% for AMACR. However, the urothelial markers CK34βE12, p63, thrombomodulin, S100P, and GATA3 were also positive in 1.8%, 0%, 0%, 3.6%, and 0% of PAC, respectively. The sensitivities of urothelial markers in UC were 75.4% for CK34βE12, 73.9% for p63, 45.7% for thrombomodulin, 22.5% for S100P, and 84.8% for GATA3. Conversely, the prostatic markers PSA, PSMA, PAP, P501s, NKX3.1, and AMACR were also positive in 9.4%, 0.7%, 18.8%, 0.7%, 0%, and 8.7% of UCs, respectively.
Conclusions
Prostatic and urothelial markers, including PSA, NKX3.1, p63, thrombomodulin, and GATA3 are very useful for differentiating PAC from UC. The optimal combination of prostatic and urothelial markers could improve the ability to differentiate PAC from UC pathologically. -
Citations
Citations to this article as recorded by- Comparative histologic survey and transcriptomic investigation into canine prostate carcinoma
Nathan K. Hoggard, Said M. Elshafae, Nigel A. Daniels, Jonathan A. Young, Chris Premanandan, John B. Echols, Darshan S. Chandrashekar, Blake E. Hildreth, Michael C. Haffner, Thomas J. Rosol
Research in Veterinary Science.2026; 198: 105981. CrossRef - Impact of hormone sensitivity status on aberrant expression of CK7, CK20, CDX2, GATA3 and TTF1 in prostate cancer
Qing Yin Wang, Nazim Benzerdjeb, Samuel Jaquet, Andreea Stepanov, Mame-Kany Diop, Mirela Birlea, Fred Saad, Dominique Trudel
Human Pathology.2025; 163: 105877. CrossRef - Unusual Perineal Metastasis in a Case of Prostate Cancer on 68Ga-PSMA-11 PET/CT
Ritanshu Solanki, Bhagwant Rai Mittal, Rajender Kumar, Aravindh Sekar, Narender Kumar
Clinical Nuclear Medicine.2024; 49(2): e73. CrossRef - NKX3.1 Expression in Non-Prostatic Tumors and Characterizing its Expression in Esophageal/Gastroesophageal Adenocarcinoma
Ansa Mehreen, Kiran G. Manjee, Divyangi Paralkar, Gladell P. Paner, Thanh Lan
Advances in Anatomic Pathology.2024; 31(3): 202. CrossRef - Clinical Management of Intraductal Carcinoma of the Prostate
Gabriel Wasinger, Olivier Cussenot, Eva Compérat
Cancers.2024; 16(9): 1650. CrossRef - Adenocarcinomas of the Gynecologic Tract Involving the Urinary Bladder: A Series of 16 Cases Potentially Mimicking Urothelial Malignancy
Daniel H. Russell, Jonathan I. Epstein, Oleksandr N. Kryvenko, Matthew Schlumbrecht, Merce Jorda, Andre Pinto
Archives of Pathology & Laboratory Medicine.2024; 148(6): 705. CrossRef - Assessing the diagnostic impact of P63, PSA and BCL-2 proteins in premalignant and malignant prostate tissues
Aderonke C. Ogunlayi, Victor O. Ekundina, Adedapo O. Kehinde, Linus A. Enye, Adegoke O. Aremu
International Journal of Scientific Reports.2024; 10(6): 188. CrossRef - Concurrent occurrence of adenocarcinoma and urothelial carcinoma of the prostate gland: A case report
Jhe Yuan Hsu, Yi Sheng Lin, Li Hua Huang, Tang Yi Tsao, Chao Yu Hsu, Yen Chuan Ou, Min Che Tung
World Journal of Clinical Cases.2024; 12(26): 5952. CrossRef - Metastatic prostate cancer presenting as a posterior mediastinal mass: A rare presentation
Muhammad Haider, Arun Umesh Mahtani, Bachar Botrus, Foma Munoh Kenne, Madiha Fatima Master
Clinical Case Reports.2023;[Epub] CrossRef - Diagnostic and Prognostic Roles of GATA3 Immunohistochemistry in Urothelial Carcinoma
Daeseon Yoo, Kyueng-Whan Min, Jung-Soo Pyo, Nae Yu Kim
Medicina.2023; 59(8): 1452. CrossRef - Primary high-grade urothelial carcinoma of prostate with prostatic hyperplasia: a rare case report and review of the literature
Liang Liu, Fu-zhen Sun, Pan-ying Zhang, Yu Xiao, Xiao Yue, Dong-Ming Wang, Qiang Wang
The Aging Male.2023;[Epub] CrossRef - Expression of Gata Binding Protein 3 as a Prognostic Factor in Urogenital Lesions and Its Association With Morphology
T Govardhan, Debahuti Mohapatra, Sujata Naik, Prateek Das, Pranita Mohanty, Ankita Pal
Cureus.2023;[Epub] CrossRef - Histological and immunohistochemical investigation of canine prostate carcinoma with identification of common intraductal carcinoma component
Simone de Brot, Jennifer Lothion‐Roy, Llorenç Grau‐Roma, Emily White, Franco Guscetti, Mark A. Rubin, Nigel P. Mongan
Veterinary and Comparative Oncology.2022; 20(1): 38. CrossRef - Urothelial Carcinoma and Prostate-specific Membrane Antigen: Cellular, Imaging, and Prognostic Implications
Arsalan Tariq, Amy E. McCart Reed, Andrew Morton, Sima Porten, Ian Vela, Elizabeth D. Williams, John W. Yaxley, Peter C. Black, Matthew J. Roberts
European Urology Focus.2022; 8(5): 1256. CrossRef - Immunohistochemical Reactivity of Prostate-Specific Membrane Antigen in Salivary Gland Tumors
Haruto Nishida, Yoshihiko Kondo, Takahiro Kusaba, Hiroko Kadowaki, Tsutomu Daa
Head and Neck Pathology.2022; 16(2): 427. CrossRef - Weak NKX3.1 expression in a urothelial carcinoma: A diagnostic pitfall
Maryam Abdo, Robert Hoyt, Ashley Highfill, Daniel Mettman
Human Pathology Reports.2022; 27: 300599. CrossRef - Gene of the month: NKX3.1
Jon Griffin, Yuqing Chen, James W F Catto, Sherif El-Khamisy
Journal of Clinical Pathology.2022; 75(6): 361. CrossRef - Diagnostic Value of GATA3 and Uroplakin 3 in Differentiating Urothelial Carcinoma from Prostatic and Colorectal Carcinoma
Maha Salama, Dina A. Khairy
Open Access Macedonian Journal of Medical Sciences.2022; 10(A): 514. CrossRef - Diagnostic challenges for the distinction of high-grade prostatic adenocarcinoma and high-grade urothelial carcinoma of simultaneous occurrences - A literature review
Shreyas Bhushan Jayade, Manana Jikurashvili
GEORGIAN SCIENTISTS.2022;[Epub] CrossRef - Cytomorphology, immunoprofile, and clinicopathologic correlation of metastatic prostatic carcinoma
Xiaoqi Lin, Qiuying Shi, Ximing J. Yang
Human Pathology.2022; 130: 36. CrossRef - Cutaneous Metastasis of Prostate Adenocarcinoma: A Rare Presentation of a Common Disease
Alexander Dills, Okechukwu Obi, Kevin Bustos, Jesse Jiang, Shweta Gupta
Journal of Investigative Medicine High Impact Case Reports.2021;[Epub] CrossRef - Mining The Cancer Genome Atlas gene expression data for lineage markers in distinguishing bladder urothelial carcinoma and prostate adenocarcinoma
Ewe Seng Ch’ng
Scientific Reports.2021;[Epub] CrossRef - Immunohistochemical analysis of thrombomodulin expression in myocardial tissue from autopsy cases of ischemic heart disease
Takeshi Kondo, Motonori Takahashi, Gentaro Yamasaki, Marie Sugimoto, Azumi Kuse, Mai Morichika, Kanako Nakagawa, Makoto Sakurada, Migiwa Asano, Yasuhiro Ueno
Legal Medicine.2021; 51: 101897. CrossRef - Application and Pitfalls of Immunohistochemistry in Diagnosis of Challenging Genitourinary Cases
Jenny Ross, Guangyuan Li, Ximing J. Yang
Archives of Pathology & Laboratory Medicine.2020; 144(3): 290. CrossRef - New Screening Test Improves Detection of Prostate Cancer Using Circulating Tumor Cells and Prostate-Specific Markers
Karin Ried, Tasnuva Tamanna, Sonja Matthews, Peter Eng, Avni Sali
Frontiers in Oncology.2020;[Epub] CrossRef - An Unlikely Culprit: Gastric Metastasis from Primary Prostatic Adenocarcinoma
Eric Omar Then, Spoorthi Nutakki, Andrew Ofosu, Saad Saleem, Vijay Gayam, Tagore Sunkara, Vinaya Gaduputi
Journal of Gastrointestinal Cancer.2020; 51(3): 1081. CrossRef - MRI of prostatic urethral mucinous urothelial carcinoma: Expanding the differential diagnosis for T2 hyperintense prostatic masses
Neel Patel, Bryan R. Foster, Elena K. Korngold, Kyle Jensen, Kevin R. Turner, Fergus V. Coakley
Clinical Imaging.2020; 68: 68. CrossRef - Morphological and Immunohistochemical Biomarkers in Distinguishing Prostate Carcinoma and Urothelial Carcinoma: A Comprehensive Review
Francesca Sanguedolce, Davide Russo, Vito Mancini, Oscar Selvaggio, Beppe Calò, Giuseppe Carrieri, Luigi Cormio
International Journal of Surgical Pathology.2019; 27(2): 120. CrossRef - A Case of Metastatic Prostate Cancer to the Urethra That Resolved After Androgen Deprivation Therapy
Darren J. Bryk, Kenneth W. Angermeier, Eric A. Klein
Urology.2019; 129: e4. CrossRef - The Homeodomain Transcription Factor NKX3.1 Modulates Bladder Outlet Obstruction Induced Fibrosis in Mice
Mehul S. Patel, Diana K. Bowen, Nicholas M. Tassone, Andrew D. Gould, Kirsten S. Kochan, Paula R. Firmiss, Natalie A. Kukulka, Megan Y. Devine, Belinda Li, Edward M. Gong, Robert W. Dettman
Frontiers in Pediatrics.2019;[Epub] CrossRef - Cancer of unknown primary: Ancillary testing of cytologic and small biopsy specimens in the era of targeted therapy
Morgan L. Cowan, Christopher J. VandenBussche
Cancer Cytopathology.2018; 126(S8): 724. CrossRef - Glandular Tumors of the Urachus and Urinary Bladder: A Practical Overview of a Broad Differential Diagnosis
Alexander S. Taylor, Rohit Mehra, Aaron M. Udager
Archives of Pathology & Laboratory Medicine.2018; 142(10): 1164. CrossRef - S100P as a Marker for Urothelial Histogenesis: A Critical Review and Comparison With Novel and Traditional Urothelial Immunohistochemical Markers
Moushumi Suryavanshi, Julian Sanz-Ortega, Deepika Sirohi, Mukul K. Divatia, Chisato Ohe, Claudia Zampini, Daniel Luthringer, Steven C. Smith, Mahul B. Amin
Advances in Anatomic Pathology.2017; 24(3): 151. CrossRef
- Comparative histologic survey and transcriptomic investigation into canine prostate carcinoma
- Aquaporin 1 Is an Independent Marker of Poor Prognosis in Lung Adenocarcinoma
- Sumi Yun, Ping-Li Sun, Yan Jin, Hyojin Kim, Eunhyang Park, Soo Young Park, Kyuho Lee, Kyoungyul Lee, Jin-Haeng Chung
- J Pathol Transl Med. 2016;50(4):251-257. Published online June 7, 2016
- DOI: https://doi.org/10.4132/jptm.2016.03.30
- 11,679 View
- 123 Download
- 25 Web of Science
- 25 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Aquaporin 1 (AQP1) overexpression has been shown to be associated with uncontrolled cell replication, invasion, migration, and tumor metastasis. We aimed to evaluate AQP1 expression in lung adenocarcinomas and to examine its association with clinicopathological features and prognostic significance. We also investigated the association between AQP1 overexpression and epithelial-mesenchymal transition (EMT) markers.
Methods
We examined AQP1 expression in 505 cases of surgically resected lung adenocarcinomas acquired at the Seoul National University Bundang Hospital from 2003 to 2012. Expression of AQP1 and EMT-related markers, including Ecadherin and vimentin, were analyzed by immunohistochemistry and tissue microarray.
Results
AQP1 overexpression was associated with several aggressive pathological parameters, including venous invasion, lymphatic invasion, and tumor recurrence. AQP1 overexpression tended to be associated with higher histological grade, advanced pathological stage, and anaplastic lymphoma kinase (ALK) translocation; however, these differences were not statistically significant. In addition, AQP1 overexpression positively correlated with loss of E-cadherin expression and acquired expression of vimentin. Lung adenocarcinoma patients with AQP1 overexpression showed shorter progression- free survival (PFS, 46.1 months vs. 56.2 months) compared to patients without AQP1 overexpression. Multivariate analysis confirmed that AQP1 overexpression was significantly associated with shorter PFS (hazard ratio, 1.429; 95% confidence interval, 1.033 to 1.977; p=.031).
Conclusions
AQP1 overexpression was thereby concluded to be an independent factor of poor prognosis associated with shorter PFS in lung adenocarcinoma. These results suggested that AQP1 overexpression might be considered as a prognostic biomarker of lung adenocarcinoma. -
Citations
Citations to this article as recorded by- The Expanding Role of Aquaporin-1, Aquaporin-3 and Aquaporin-5 as Transceptors: Involvement in Cancer Development and Potential Druggability
Catarina Pimpão, Inês V. da Silva, Graça Soveral
International Journal of Molecular Sciences.2025; 26(3): 1330. CrossRef - The comprehensive potential of AQP1 as a tumor biomarker: evidence from kidney neoplasm cohorts, cell experiments and pan-cancer analysis
Yifan Liu, Donghao Lyu, Yuntao Yao, Jinming Cui, Jiangui Liu, Zikuan Bai, Zihui Zhao, Yuanan Li, Bingnan Lu, Keqin Dong, Xiuwu Pan
Human Genomics.2025;[Epub] CrossRef - The Association of Aquaporins with MAPK Signaling Pathway Unveils Potential Prognostic Biomarkers for Pancreatic Cancer: A Transcriptomics Approach
Inês V. da Silva, Paula A. Lopes, Elisabete Fonseca, Emanuel Vigia, Jorge Paulino, Graça Soveral
Biomolecules.2025; 15(4): 488. CrossRef - Obesity Impacts Post‐Myocardial Infarction Neovascularization by Downregulating AQP1 Expression via the TRPC5‐NFATc3 Signaling Pathway
Mengru Gao, Jing Han, Yifei Zhu, Xin Wen, Lei Feng, Tingting Zhou
Comprehensive Physiology.2025;[Epub] CrossRef - Aquaporin‐1, aquaporin‐3 and aquaporin‐5 differentially modulate cell biophysical and biomechanical properties, impacting cell stiffness and cell–cell adhesion
Catarina Pimpão, Filomena A. Carvalho, Inês Vieira da Silva, Andreia Barateiro, Nuno C. Santos, Graça Soveral
The FEBS Journal.2025;[Epub] CrossRef - Prognostic Assessment of Aquaporins in Pancreatic Adenocarcinoma: An In Silico Analysis
Vignesh Krishnasamy, Lalhmingliana, Nachimuthu Senthil Kumar
Current Biotechnology.2025; 14(2): 130. CrossRef - Clinical application of cold atmospheric-pressure plasma: mechanisms and irradiation conditions
Eun Ji Jeong, Hyun Min Park, Dong Jae Lee, Jun Lee, Jun Yeong Cho, Kyung Deok Seo, Seokjun Je, Min Hyung Jung, Woo Yeon Hwang, Kyung Sook Kim
Journal of Physics D: Applied Physics.2024; 57(37): 373001. CrossRef - Aquaporins in Cancer Biology
Chul So Moon, David Moon, Sung Koo Kang
Frontiers in Oncology.2022;[Epub] CrossRef - A Comprehensive Prognostic Analysis of Tumor-Related Blood Group Antigens in Pan-Cancers Suggests That SEMA7A as a Novel Biomarker in Kidney Renal Clear Cell Carcinoma
Yange Wang, Chenyang Li, Xinlei Qi, Yafei Yao, Lu Zhang, Guosen Zhang, Longxiang Xie, Qiang Wang, Wan Zhu, Xiangqian Guo
International Journal of Molecular Sciences.2022; 23(15): 8799. CrossRef - Differential modulation of lung aquaporins among other pathophysiological markers in acute (Cl2 gas) and chronic (carbon nanoparticles, cigarette smoke) respiratory toxicity mouse models
Sukanta S. Bhattacharya, Brijesh Yadav, Ekta Yadav, Ariel Hus, Niket Yadav, Perminder Kaur, Lauren Rosen, Roman Jandarov, Jagjit S. Yadav
Frontiers in Physiology.2022;[Epub] CrossRef - Aquaporin water channels as regulators of cell-cell adhesion proteins
Sarannya Edamana, Frédéric H. Login, Soichiro Yamada, Tae-Hwan Kwon, Lene N. Nejsum
American Journal of Physiology-Cell Physiology.2021; 320(5): C771. CrossRef - Targeting Aquaporins in Novel Therapies for Male and Female Breast and Reproductive Cancers
Sidra Khan, Carmela Ricciardelli, Andrea J. Yool
Cells.2021; 10(2): 215. CrossRef - Targeting ion channels for the treatment of lung cancer
Liqin Zhang, Shuya Bing, Mo Dong, Xiaoqiu Lu, Yuancheng Xiong
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer.2021; 1876(2): 188629. CrossRef - Comprehensive Analysis of Aquaporin Superfamily in Lung Adenocarcinoma
Guofu Lin, Luyang Chen, Lanlan Lin, Hai Lin, Zhifeng Guo, Yingxuan Xu, Chanchan Hu, Jinglan Fu, Qinhui Lin, Wenhan Chen, Yiming Zeng, Yuan Xu
Frontiers in Molecular Biosciences.2021;[Epub] CrossRef - Diagnostic accuracy of urinary aquaporin-1 as a biomarker for renal cell carcinoma
Abhilash Cheriyan, Arun Jose Nellickal, Nirmal Thampi John, Lakshmanan Jeyaseelan, Santosh Kumar, Antony Devasia, Nitin Kekre
Indian Journal of Urology.2021; 37(1): 59. CrossRef - Aquaporin 1, 3, and 5 Patterns in Salivary Gland Mucoepidermoid Carcinoma: Expression in Surgical Specimens and an In Vitro Pilot Study
Mérin Barbara Stamboni, Ágatha Nagli de Mello Gomes, Milena Monteiro de Souza, Katia Klug Oliveira, Claudia Fabiana Joca Arruda, Fernanda de Paula, Barbara Beltrame Bettim, Márcia Martins Marques, Luiz Paulo Kowalski, Clóvis Antônio Lopes Pinto, Victor El
International Journal of Molecular Sciences.2020; 21(4): 1287. CrossRef - Combined Systematic Review and Transcriptomic Analyses of Mammalian Aquaporin Classes 1 to 10 as Biomarkers and Prognostic Indicators in Diverse Cancers
Pak Hin Chow, Joanne Bowen, Andrea J Yool
Cancers.2020; 12(7): 1911. CrossRef - Aquaporins in lung health and disease: Emerging roles, regulation, and clinical implications
Ekta Yadav, Niket Yadav, Ariel Hus, Jagjit S. Yadav
Respiratory Medicine.2020; 174: 106193. CrossRef - Dissecting gene‐environment interactions: A penalized robust approach accounting for hierarchical structures
Cen Wu, Yu Jiang, Jie Ren, Yuehua Cui, Shuangge Ma
Statistics in Medicine.2018; 37(3): 437. CrossRef - Immunohistochemical Expression of Aquaporin-1 in Fluoro-Edenite-Induced Malignant Mesothelioma: A Preliminary Report
Giuseppe Angelico, Rosario Caltabiano, Carla Loreto, Antonio Ieni, Giovanni Tuccari, Caterina Ledda, Venerando Rapisarda
International Journal of Molecular Sciences.2018; 19(3): 685. CrossRef - Mechanisms of Aquaporin-Facilitated Cancer Invasion and Metastasis
Michael L. De Ieso, Andrea J. Yool
Frontiers in Chemistry.2018;[Epub] CrossRef - Aquaporin 1 suppresses apoptosis and affects prognosis in esophageal squamous cell carcinoma
Yuzo Yamazato, Atsushi Shiozaki, Daisuke Ichikawa, Toshiyuki Kosuga, Katsutoshi Shoda, Tomohiro Arita, Hirotaka Konishi, Shuhei Komatsu, Takeshi Kubota, Hitoshi Fujiwara, Kazuma Okamoto, Mitsuo Kishimoto, Eiichi Konishi, Yoshinori Marunaka, Eigo Otsuji
Oncotarget.2018; 9(52): 29957. CrossRef - Aquaporin 1 expression is associated with response to adjuvant chemotherapy in stage�II and III colorectal cancer
Hideko Imaizumi, Keiichiro Ishibashi, Seiichi Takenoshita, Hideyuki Ishida
Oncology Letters.2018;[Epub] CrossRef - Aquaporin 3 facilitates tumor growth in pancreatic cancer by modulating mTOR signaling
Xunwei Huang, Li Huang, Minhua Shao
Biochemical and Biophysical Research Communications.2017; 486(4): 1097. CrossRef - Prognostic implication of aquaporin 1 overexpression in resected lung adenocarcinoma†
Guido Bellezza, Jacopo Vannucci, Fortunato Bianconi, Giulio Metro, Rachele Del Sordo, Marco Andolfi, Ivana Ferri, Paola Siccu, Vienna Ludovini, Francesco Puma, Angelo Sidoni, Lucio Cagini
Interactive CardioVascular and Thoracic Surgery.2017; 25(6): 856. CrossRef
- The Expanding Role of Aquaporin-1, Aquaporin-3 and Aquaporin-5 as Transceptors: Involvement in Cancer Development and Potential Druggability
- Transformation to Small Cell Lung Cancer of Pulmonary Adenocarcinoma: Clinicopathologic Analysis of Six Cases
- Soomin Ahn, Soo Hyun Hwang, Joungho Han, Yoon-La Choi, Se-Hoon Lee, Jin Seok Ahn, Keunchil Park, Myung-Ju Ahn, Woong-Yang Park
- J Pathol Transl Med. 2016;50(4):258-263. Published online May 10, 2016
- DOI: https://doi.org/10.4132/jptm.2016.04.19
- 14,093 View
- 253 Download
- 53 Web of Science
- 49 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are considered the first line treatment for a subset of EGFR-mutated non-small cell lung cancer (NSCLC) patients. Although transformation to small cell lung cancer (SCLC) is one of the known mechanisms of resistance to EGFR TKIs, it is not certain whether transformation to SCLC is exclusively found as a mechanism of TKI resistance in EGFR-mutant tumors.
Methods
We identified six patients with primary lung adenocarcinoma that showed transformation to SCLC on second biopsy (n = 401) during a 6-year period. Clinicopathologic information was analyzed and EGFR mutation results were compared between initial and second biopsy samples.
Results
Six patients showed transformation from adenocarcinoma to SCLC, of which four were pure SCLCs and two were combined adenocarcinoma and SCLCs. Clinically, four cases were EGFR-mutant tumors from non-smoking females who underwent TKI treatment, and the EGFR mutation was retained in the transformed SCLC tumors. The remaining two adenocarcinomas were EGFR wild-type, and one of these patients received EGFR TKI treatment.
Conclusions
NSCLC can acquire a neuroendocrine phenotype with or without EGFR TKI treatment. -
Citations
Citations to this article as recorded by- Patients outcomes in lung adenocarcinoma transforming to small-cell lung cancer after tyrosine kinase inhibitor therapy
Shuai Wang, Yongsen Wang, Xuan Wu, Li Yang, Xiaoju Zhang
World Journal of Surgical Oncology.2025;[Epub] CrossRef - Baseline retinoblastoma transcriptional corepressor 1 (Rb1) functional inactivation is a pre-requisite but not sufficient for small-cell histological transformation in epidermal growth factor receptor (EGFR) mutant lung adenocarcinomas post-tyrosine kinas
Aruna Nambirajan, Amber Rathor, Hemavathi Baskarane, Anju GS, Sachin Khurana, Somagattu Sushmitha, Aparna Sharma, Prabhat Singh Malik, Deepali Jain
Virchows Archiv.2025; 487(3): 639. CrossRef - Case Report: Transforming small cell lung cancer: two cases report and literature review
Jinlong Liu, Jing Ai, Lize Zhao, Yimeng Qian, Qingxin Zhao, Chunling Ma, Yu Zhao, Jing Zhao
Frontiers in Oncology.2025;[Epub] CrossRef - Exploration of CT-based discrimination and diagnosis of various pathological types of ground glass nodules in the lungs
Haihui Wu, Xiong Zhang, Zheng Zhong
BMC Medical Imaging.2025;[Epub] CrossRef - Correlation between treatments and outcomes of patients with EGFR-mutated non-small-cell lung cancer that transitioned into small-cell lung cancer: an international retrospective study
C. Catania, S.V. Liu, M. Garassino, A. Delmonte, V. Scotti, F. Cappuzzo, C. Genova, A. Russo, M. Russano, C. Bennati, I. Colantonio, S. Martini, M. Pino, F. Conforti, L. Pala, G. Minuti, F. Citarella, E. Olmetto, A. Esposito, P. Cascetta, A. Di Lello, T.
ESMO Open.2025; 10(7): 105326. CrossRef - TPM3–NTRK1 fusion confers resistance to osimertinib in lung adenocarcinoma: a model in a continuous cell line
Fang Cao, Jiayin Dai, Kun Dong, Zhenli Yang, Yanli Zhu, Changsong Qi, Dongmei Lin, Xiaocui Bian, Yuqin Liu
Human Cell.2025;[Epub] CrossRef - Small cell lung cancer transdifferentiation: not a negligible phenomenon
Lilla Horvath, Kristiina Boettiger, Zsolt Megyesfalvi, Balázs Döme, Clemens Aigner, Anita Horváth-Rózsás, Judit Berta
Current Opinion in Oncology.2025;[Epub] CrossRef - Transformation to small cell lung cancer is irrespective of EGFR and accelerated by SMAD4-mediated ASCL1 transcription independently of RB1 in non-small cell lung cancer
Xi Ding, Min-xing Shi, Di Liu, Jing-xue Cao, Kai-xuan Zhang, Run-dong Zhang, Li-ping Zhang, Kai-xing Ai, Bo Su, Jie Zhang
Cell Communication and Signaling.2024;[Epub] CrossRef - The study of primary and acquired resistance to first-line osimertinib to improve the outcome of EGFR-mutated advanced Non-small cell lung cancer patients: the challenge is open for new therapeutic strategies
Alessandra Ferro, Gian Marco Marinato, Cristiana Mulargiu, Monica Marino, Giulia Pasello, Valentina Guarneri, Laura Bonanno
Critical Reviews in Oncology/Hematology.2024; 196: 104295. CrossRef - Comprehensive molecular and clinical insights into non-small cell lung cancer transformation to small cell lung cancer with an illustrative case report
Kresimir Tomic, Kristina Krpina, Lara Baticic, Miroslav Samarzija, Semir Vranic
Journal of Drug Targeting.2024; 32(5): 499. CrossRef - Transcriptomic Heterogeneity of EGFR-Mutant Non–Small Cell Lung Cancer Evolution Toward Small-Cell Lung Cancer
Songji Oh, Jaemoon Koh, Tae Min Kim, Soyeon Kim, Jeonghwan Youk, Miso Kim, Bhumsuk Keam, Yoon Kyung Jeon, Ja-Lok Ku, Dong-Wan Kim, Doo Hyun Chung, Dae Seog Heo
Clinical Cancer Research.2024; 30(20): 4729. CrossRef - Transformation of lung adenocarcinoma into small cell lung cancer after treatment with epidermal growth factor receptor tyrosine kinase inhibitors
Linwu Kuang, Yangkai Li
Oncology and Translational Medicine.2024; 10(6): 286. CrossRef - Exon-18-EGFR Mutated Transformed Small-Cell Lung Cancer: A Case Report and Literature Review
Nunzio Digiacomo, Tommaso De Pas, Giovanna Rossi, Paola Bossi, Erika Stucchi, Fabio Conforti, Emilia Cocorocchio, Daniele Laszlo, Laura Pala, Emma Zattarin, Chiara Catania
Current Oncology.2023; 30(3): 3494. CrossRef - Current knowledge of small cell lung cancer transformation from non-small cell lung cancer
Giuseppe Giaccone, Yongfeng He
Seminars in Cancer Biology.2023; 94: 1. CrossRef - Targeting the EGFR signaling pathway in cancer therapy: What’s new in 2023?
Sushanta Halder, Soumi Basu, Shobhit P. Lall, Apar K. Ganti, Surinder K. Batra, Parthasarathy Seshacharyulu
Expert Opinion on Therapeutic Targets.2023; 27(4-5): 305. CrossRef - Outcomes in Patients With Lung Adenocarcinoma With Transformation to Small Cell Lung Cancer After EGFR Tyrosine Kinase Inhibitors Resistance: A Systematic Review and Pooled Analysis
Jinhe Xu, Lihuan Xu, Baoshan Wang, Wencui Kong, Ying Chen, Zongyang Yu
Frontiers in Oncology.2022;[Epub] CrossRef - Small cell lung cancer transformation: From pathogenesis to treatment
Xiaomeng Yin, Yueyi Li, Hang Wang, Tingting Jia, Enli Wang, Yuling Luo, Yuhao Wei, Zeyi Qin, Xuelei Ma
Seminars in Cancer Biology.2022; 86: 595. CrossRef - Small Cell Lung Cancer Transformation following Treatment in EGFR-Mutated Non-Small Cell Lung Cancer
Isa Mambetsariev, Leonidas Arvanitis, Jeremy Fricke, Rebecca Pharaon, Angel R. Baroz, Michelle Afkhami, Marianna Koczywas, Erminia Massarelli, Ravi Salgia
Journal of Clinical Medicine.2022; 11(5): 1429. CrossRef - Morphologic-Molecular Transformation of Oncogene Addicted Non-Small Cell Lung Cancer
Fiorella Calabrese, Federica Pezzuto, Francesca Lunardi, Francesco Fortarezza, Sofia-Eleni Tzorakoleftheraki, Maria Vittoria Resi, Mariaenrica Tiné, Giulia Pasello, Paul Hofman
International Journal of Molecular Sciences.2022; 23(8): 4164. CrossRef - Genomic and Gene Expression Studies Helped to Define the Heterogeneity of Small-Cell Lung Cancer and Other Lung Neuroendocrine Tumors and to Identify New Therapeutic Targets
Ugo Testa, Elvira Pelosi, Germana Castelli
Onco.2022; 2(3): 186. CrossRef - Neuroendocrine transformation from EGFR/ALK-wild type or TKI-naïve non-small cell lung cancer: An under-recognized phenomenon
Xiao Chu, Yuyin Xu, Ye Li, Yue Zhou, Li Chu, Xi Yang, Jianjiao Ni, Yida Li, Tiantian Guo, Zhiqin Zheng, Qiang Zheng, Qianlan Yao, Yuan Li, Xiaoyan Zhou, Zhengfei Zhu
Lung Cancer.2022; 169: 22. CrossRef - Three Third-Generation Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer: Similarities and Differences
Ling Chen, Yangqingqing Zhou, Chaosheng Gan, XiaoLi Wang, Yihui Liu, Chunhui Dong, Ruiyuan He, Jin Yang
Cancer Investigation.2022; 40(7): 590. CrossRef - Histomorphological transformation from non-small cell lung carcinoma to small cell lung carcinoma after targeted therapy or immunotherapy: A report of two cases
Hao Liu, Li-Hong Chen, Zhi-Hui Zhang, Ning Wang, Si-Hui Zhuang, Hao Chen, Jin Du, Li-Juan Pang, Yan Qi
Frontiers in Oncology.2022;[Epub] CrossRef - Impressive response to dabrafenib, trametinib, and osimertinib in a metastatic EGFR-mutant/BRAF V600E lung adenocarcinoma patient
Maurício Fernando Silva Almeida Ribeiro, Franciele Hinterholz Knebel, Fabiana Bettoni, Rodrigo Saddi, Karina Perez Sacardo, Felipe Sales Nogueira Amorim Canedo, João Victor Machado Alessi, Andrea Kazumi Shimada, José Flávio Gomes Marin, Anamaria Aranha Ca
npj Precision Oncology.2021;[Epub] CrossRef - Histological transformation of non-small cell lung cancer: Clinical analysis of nine cases
Cai-Bao Jin, Ling Yang
World Journal of Clinical Cases.2021; 9(18): 4617. CrossRef - Lamellarin 14, a derivative of marine alkaloids, inhibits the T790M/C797S mutant epidermal growth factor receptor
Naoyuki Nishiya, Yusuke Oku, Chie Ishikawa, Tsutomu Fukuda, Shingo Dan, Tetsuo Mashima, Masaru Ushijima, Yoko Furukawa, Yuka Sasaki, Keishi Otsu, Tomoko Sakyo, Masanori Abe, Honami Yonezawa, Fumito Ishibashi, Masaaki Matsuura, Akihiro Tomida, Hiroyuki Sei
Cancer Science.2021; 112(5): 1963. CrossRef - Case Report: Transformation From Non-Small Cell Lung Cancer to Small Cell Lung Cancer During Anti-PD-1 Therapy: A Report of Two Cases
Qian Shen, Jingjing Qu, Lingyan Sheng, Qiqi Gao, Jianying Zhou
Frontiers in Oncology.2021;[Epub] CrossRef - Exploring the resistance mechanisms of second-line osimertinib and their prognostic implications using next-generation sequencing in patients with non-small-cell lung cancer
Kyoungmin Lee, Deokhoon Kim, Shinkyo Yoon, Dae Ho Lee, Sang-We Kim
European Journal of Cancer.2021; 148: 202. CrossRef - Comprehensive analysis of treatment modes and clinical outcomes of small cell lung cancer transformed from epidermal growth factor receptor mutant lung adenocarcinoma
Shouzheng Wang, Tongji Xie, Xuezhi Hao, Yan Wang, Xingsheng Hu, Lin Wang, Yan Li, Junling Li, Puyuan Xing
Thoracic Cancer.2021; 12(19): 2585. CrossRef - EGFR-Mutant SCLC Exhibits Heterogeneous Phenotypes and Resistance to Common Antineoplastic Drugs
Chih-An Lin, Sung-Liang Yu, Hsuan-Yu Chen, Huei-Wen Chen, Shr-Uen Lin, Chia-Ching Chang, Chong-Jen Yu, Pan-Chyr Yang, Chao-Chi Ho
Journal of Thoracic Oncology.2019; 14(3): 513. CrossRef - The clinicopathologic of pulmonary adenocarcinoma transformation to small cell lung cancer
Haiyan Yang, Li Liu, Chunhua Zhou, Yi Xiong, Yijuan Hu, Nong Yang, Jingjing Qu
Medicine.2019; 98(12): e14893. CrossRef - Chemistry and pharmacological diversity of quinoxaline motifs as anticancer agents
Olayinka O. Ajani, Martins T. Nlebemuo, Joseph A. Adekoya, Kehinde O. Ogunniran, Tolutope O. Siyanbola, Christiana O. Ajanaku
Acta Pharmaceutica.2019; 69(2): 177. CrossRef - Resistance to EGFR inhibitors in non-small cell lung cancer: Clinical management and future perspectives
Chiara Tomasello, Cinzia Baldessari, Martina Napolitano, Giulia Orsi, Giulia Grizzi, Federica Bertolini, Fausto Barbieri, Stefano Cascinu
Critical Reviews in Oncology/Hematology.2018; 123: 149. CrossRef - Small cell lung cancer transformation from EGFR-mutated lung adenocarcinoma: A case report and literatures review
Yangyang Liu
Cancer Biology & Therapy.2018; 19(6): 445. CrossRef - Anaplastic lymphoma kinase (ALK)-expressing Lung Adenocarcinoma with Combined Neuroendocrine Component or Neuroendocrine Transformation: Implications for Neuroendocrine Transformation and Response to ALK-tyrosine Kinase Inhibitors
Jongmin Sim, Hyunjin Kim, Jiyeon Hyeon, Yoon-La Choi, Joungho Han
Journal of Korean Medical Science.2018;[Epub] CrossRef - Assessment of Resistance Mechanisms and Clinical Implications in Patients WithEGFRT790M–Positive Lung Cancer and Acquired Resistance to Osimertinib
Geoffrey R. Oxnard, Yuebi Hu, Kathryn F. Mileham, Hatim Husain, Daniel B. Costa, Philip Tracy, Nora Feeney, Lynette M. Sholl, Suzanne E. Dahlberg, Amanda J. Redig, David J. Kwiatkowski, Michael S. Rabin, Cloud P. Paweletz, Kenneth S. Thress, Pasi A. Jänne
JAMA Oncology.2018; 4(11): 1527. CrossRef - Clinicopathological and genomic comparisons between different histologic components in combined small cell lung cancer and non-small cell lung cancer
Mong-Wei Lin, Kang-Yi Su, Te-Jen Su, Chia-Ching Chang, Jing-Wei Lin, Yi-Hsuan Lee, Sung-Liang Yu, Jin-Shing Chen, Min-Shu Hsieh
Lung Cancer.2018; 125: 282. CrossRef - Transformation to small‑cell lung cancer following treatment with icotinib in a patient with lung adenocarcinoma
Hongyang Lu, Bo Chen, Jing Qin, Fajun Xie, Na Han, Zhiyu Huang
Oncology Letters.2018;[Epub] CrossRef - Histological transformation of adenocarcinoma to small cell carcinoma lung as a rare mechanism of resistance to epidermal growth factor receptor-tyrosine kinase inhibitors: Report of a case with review of literature
Monalisa Hui, ShantveerG Uppin, BalaJoseph Stalin, G Sadashivudu
Lung India.2018; 35(2): 160. CrossRef - A rare case of squamous cell carcinoma lung with multiple locoregional recurrences and histological transformation
Ram Niwas, Shibdas Chakrabarti, Viswesvaran Balasubramanian, ManasKamal Sen, JagdishChander Suri
Lung India.2018; 35(6): 511. CrossRef - Small cell lung cancer transformation during immunotherapy with nivolumab: A case report
Takuma Imakita, Kohei Fujita, Osamu Kanai, Tsuyoshi Terashima, Tadashi Mio
Respiratory Medicine Case Reports.2017; 21: 52. CrossRef - Pulmonary neuroendocrine tumor in a female wolf (Canis lupus lupus)
Ayako SHIRAKI, Toshinori YOSHIDA, Masahi KAWASHIMA, Hirotada MURAYAMA, Rei NAGAHARA, Nanao ITO, Makoto SHIBUTANI
Journal of Veterinary Medical Science.2017; 79(3): 588. CrossRef - Secondary biopsy of non‐oncogenic‐driven lung cancer may reveal a clinically sensible histologic change. A brief report of two paradigmatic cases
Maria C. Mengoli, Giulia Orsi, Filippo Lococo, Giulia Grizzi, Fausto Barbieri, Federica Bertolini, Giulio Rossi, Silvia Novello
Thoracic Cancer.2017; 8(4): 359. CrossRef - Small-cell lung cancer in never smokers: The clinicopathological features including the prognosis
Masahiro Yamasaki, Naomi Saito, Wakako Daido, Sayaka Ishiyama, Naoko Deguchi, Masaya Taniwaki, Akio Sakatani, Megumu Fujihara, Nobuyuki Ohashi, Ken-ichi Arita
Cancer Treatment and Research Communications.2017; 12: 1. CrossRef - Clonal History and Genetic Predictors of Transformation Into Small-Cell Carcinomas From Lung Adenocarcinomas
June-Koo Lee, Junehawk Lee, Sehui Kim, Soyeon Kim, Jeonghwan Youk, Seongyeol Park, Yohan An, Bhumsuk Keam, Dong-Wan Kim, Dae Seog Heo, Young Tae Kim, Jin-Soo Kim, Se Hyun Kim, Jong Seok Lee, Se-Hoon Lee, Keunchil Park, Ja-Lok Ku, Yoon Kyung Jeon, Doo Hyun
Journal of Clinical Oncology.2017; 35(26): 3065. CrossRef - Australian recommendations for EGFR T790M testing in advanced non-small cell lung cancer
Thomas John, Jeffrey J Bowden, Stephen Clarke, Stephen B Fox, Kerryn Garrett, Keith Horwood, Christos S Karapetis
Asia-Pacific Journal of Clinical Oncology.2017; 13(4): 296. CrossRef - Metastatic Squamous Cell Carcinoma from Lung Adenocarcinoma after Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy
Hyung Kyu Park, Youjeong Seo, Yoon-La Choi, Myung-Ju Ahn, Joungho Han
Journal of Pathology and Translational Medicine.2017; 51(4): 441. CrossRef - Sequential occurrence of small cell and non-small lung cancer in a male patient: Is it a transformation?
Ahsan Wahab, Kavitha Kesari, Siddique Chaudhary, Mahin Khan, Hafiz Khan, Susan Smith, Yanis Boumber
Cancer Biology & Therapy.2017; 18(12): 940. CrossRef - The expression of S100B protein in serum of patients with brain metastases from small-cell lung cancer and its clinical significance
Shanling Mu, Hong Ma, Jun Shi, Dezhi Zhen
Oncology Letters.2017;[Epub] CrossRef
- Patients outcomes in lung adenocarcinoma transforming to small-cell lung cancer after tyrosine kinase inhibitor therapy
- Prognostic Significance of Aquaporin 5 Expression in Non-small Cell Lung Cancer
- Young Min Jo, Tae In Park, Hwa Young Lee, Ji Yun Jeong, Won Kee Lee
- J Pathol Transl Med. 2016;50(2):122-128. Published online February 8, 2016
- DOI: https://doi.org/10.4132/jptm.2015.10.31
- 13,478 View
- 85 Download
- 15 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Aquaporins are water channel proteins that play a major role in the movement of water in various human tissues. Recently, it has been found that aquaporins have influence in the carcinogenesis of human malignancies. We analyzed the prognostic impact of aquaporin 5 (AQP5) in non-small lung cancer (NSCLC). Methods: Seventy-six cases of NSCLC were studied, including 44 cases of adenocarcinoma (ADC) and 32 cases of squamous cell carcinoma (SQCC). Tissue microarray was constructed and immunohistochemical staining for AQP5 was performed. Results: AQP5 was positive in 59.2% of the total enrolled NSCLCs (63.7% in ADC and 53.1% in SQCC). The difference in expression of AQP5 according to the histologic grade of the tumor was significant (p<.047), but not in a serial order. When ADC and SQCC were separately evaluated, no significant difference was observed according to the histologic grade of the tumor (p=.076 in ADC and p=.631 in SQCC). No difference was observed between AQP5 expression and other demographic data and tumor characteristics. Disease-free survival (DFS) was higher in AQP5 negative cases than positive cases in ADC (p=.047), but no significance was found in SQCC (p=.068). We were unable to find a significance between AQP5 overexpression and overall survival in either ADC (p=.210) or SQCC (p=.533). Conclusions: AQP5 expression is associated with DFS in ADC of the lung and tumor grade of NSCLC. The present study suggests that AQP5 can be a prognostic factor of NSCLC. -
Citations
Citations to this article as recorded by- AQP5 promotes epithelial-mesenchymal transition and tumor growth through activating the Wnt/β-catenin pathway in triple-negative breast cancer
Zhengcai Zhu, Tao Li, Honggang Wang, Lianghe Jiao
Mutation Research - Fundamental and Molecular Mechanisms of Mutagenesis.2024; 829: 111868. CrossRef - Aquaporin-mediated dysregulation of cell migration in disease states
Ian M. Smith, Shohini Banerjee, Allison K. Moses, Kimberly M. Stroka
Cellular and Molecular Life Sciences.2023;[Epub] CrossRef - The Role of Aquaporin 5 (AQP5) in Lung Adenocarcinoma: A Review Article
Lukasz Jaskiewicz, Anna Romaszko-Wojtowicz, Anna Doboszynska, Agnieszka Skowronska
Cells.2023; 12(3): 468. CrossRef - Aquaporins 1, 3 and 5 in Different Tumors, their Expression, Prognosis Value and Role as New Therapeutic Targets
Mahdieh-Sadat Moosavi, Yalda Elham
Pathology & Oncology Research.2020; 26(2): 615. CrossRef - Aquaporins in lung health and disease: Emerging roles, regulation, and clinical implications
Ekta Yadav, Niket Yadav, Ariel Hus, Jagjit S. Yadav
Respiratory Medicine.2020; 174: 106193. CrossRef - Combined Systematic Review and Transcriptomic Analyses of Mammalian Aquaporin Classes 1 to 10 as Biomarkers and Prognostic Indicators in Diverse Cancers
Pak Hin Chow, Joanne Bowen, Andrea J Yool
Cancers.2020; 12(7): 1911. CrossRef - Prognostic Role of S100A8 and S100A9 Protein Expressions in Non-small Cell Carcinoma of the Lung
Hyun Min Koh, Hyo Jung An, Gyung Hyuck Ko, Jeong Hee Lee, Jong Sil Lee, Dong Chul Kim, Jung Wook Yang, Min Hye Kim, Sung Hwan Kim, Kyung Nyeo Jeon, Gyeong-Won Lee, Se Min Jang, Dae Hyun Song
Journal of Pathology and Translational Medicine.2019; 53(1): 13. CrossRef - Effect of FGF/FGFR pathway blocking on lung adenocarcinoma and its cancer‐associated fibroblasts
Ahmed E Hegab, Mari Ozaki, Naofumi Kameyama, Jingtao Gao, Shizuko Kagawa, Hiroyuki Yasuda, Kenzo Soejima, Yongjun Yin, Robert D Guzy, Yoshikazu Nakamura, David M Ornitz, Tomoko Betsuyaku
The Journal of Pathology.2019; 249(2): 193. CrossRef - Anti-cancer effect of Aquaporin 5 silencing in colorectal cancer cells in association with inhibition of Wnt/β-catenin pathway
Wei Wang, Qing Li, Tao Yang, Dongsheng Li, Feng Ding, Hongzhi Sun, Guang Bai
Cytotechnology.2018; 70(2): 615. CrossRef - Knockdown of aquaporin-5 sensitizes colorectal cancer cells to 5-fluorouracil via inhibition of the Wnt–β-catenin signaling pathway
Qing Li, Tao Yang, Dongsheng Li, Feng Ding, Guang Bai, Wei Wang, Hongzhi Sun
Biochemistry and Cell Biology.2018; 96(5): 572. CrossRef - Implications of KRAS mutations in acquired resistance to treatment in NSCLC
Marzia Del Re, Eleonora Rofi, Giuliana Restante, Stefania Crucitta, Elena Arrigoni, Stefano Fogli, Massimo Di Maio, Iacopo Petrini, Romano Danesi
Oncotarget.2018; 9(5): 6630. CrossRef
- AQP5 promotes epithelial-mesenchymal transition and tumor growth through activating the Wnt/β-catenin pathway in triple-negative breast cancer
- Clear Cell Adenocarcinoma Arising from Adenofibroma in a Patient with Endometriosis of the Ovary
- Inju Cho, Sung-Chul Lim
- J Pathol Transl Med. 2016;50(2):155-159. Published online October 26, 2015
- DOI: https://doi.org/10.4132/jptm.2015.08.07
- 11,302 View
- 134 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - Ovarian clear cell adenocarcinomas (CCACs) are frequently associated with endometriosis and, less often with clear cell adenofibromas (CCAFs). We encountered a case of ovarian CCAC arising from benign and borderline adenofibromas of the clear cell and endometrioid types with endometriosis in a 53-year-old woman. Regions of the adenofibromas showed transformation to CCAC and regions of the endometriosis showed atypical endometriotic cysts. This case demonstrates that CCAC can arise from CCAF or endometriosis.
-
Citations
Citations to this article as recorded by- Ovarian Clear Cell Adenofibroma of Low Malignant Potential Developing Into Clear Cell Adenocarcinoma
Zhiwei Yin, Stephen Peters, Ravi Chokshi, Debra Heller
International Journal of Surgical Pathology.2018; 26(6): 578. CrossRef - Origins based clinical and molecular complexities of epithelial ovarian cancer
Thingreila Muinao, Mintu Pal, Hari Prasanna Deka Boruah
International Journal of Biological Macromolecules.2018; 118: 1326. CrossRef
- Ovarian Clear Cell Adenofibroma of Low Malignant Potential Developing Into Clear Cell Adenocarcinoma
- Gastric-Type Extremely Well-Differentiated Adenocarcinoma of the Stomach: A Challenge for Preoperative Diagnosis
- Mee Joo, Song Hee Han
- J Pathol Transl Med. 2016;50(1):71-74. Published online September 30, 2015
- DOI: https://doi.org/10.4132/jptm.2015.07.14
- 13,496 View
- 179 Download
- 10 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF - Gastric-type extremely well-differentiated adenocarcinoma (EWDA) is a rare type of gastric adenocarcinoma characterized by infiltration of well-formed mucinous glands with little or no nuclear atypia, which resemble foveolar epithelium or pyloric glands. Because of its high degree of differentiation, preoperative biopsy diagnosis of gastric-type EWDA is very difficult. We encountered a case of gastric-type EWDA, manifesting as a Borrmann type 4 lesion, in a 47-year-old man. Despite four repeated biopsies, the preoperative biopsy diagnosis was not conclusive due to the scarcity of diagnostic tumor cells and lack of knowledge regarding the unusual histologic findings of gastric-type EWDA. We herein describe the histologic findings of gastric-type EWDA in detail, with the aim of facilitating a preoperative biopsy diagnosis and understanding of this rare type of gastric adenocarcinoma.
-
Citations
Citations to this article as recorded by- Unusual or Uncommon Histology of Gastric Cancer
Jinho Shin, Young Soo Park
Journal of Gastric Cancer.2024; 24(1): 69. CrossRef - Clinical pathological characteristics of “crawling-type” gastric adenocarcinoma cancer: A case report
Yong-Wei Xu, Yan Song, Jun Tian, Ba-Cui Zhang, Yu-Sheng Yang, Jing Wang
World Journal of Gastrointestinal Oncology.2024; 16(4): 1660. CrossRef - Gastric-type extremely well-differentiated adenocarcinoma of the stomach: A rare tumor with diagnostic difficulties and high inter-observer variation in endoscopic pinch biopsies
Soomin Ahn, Sujin Park, Hyun Hee Koh, Han Gyeol Kim, Hyunjin Kim, Jae Yeong Son, Boram Lee, Hyunwoo Lee, Soohyun Hwang, Junhun Cho, Yun Kyung Lee, Ryoji Kushima, Amitabh Srivastava, Kyoung-Mee Kim
Pathology - Research and Practice.2024; 263: 155599. CrossRef - Clinicopathological features of gastric adenocarcinoma of fundic gland type
Bao-Zhen Guo, Zhen-Zhen Liu, Gao-Fei Shen, Fei Zhu, Hui-Fen Lian, Xin Li, Jun-Yi Zheng, Jin-Peng Li, Shui-Miao Deng, Rui Huang
World Chinese Journal of Digestology.2023; 31(6): 244. CrossRef - Characterization of pathological stomach tissue using polarization-sensitive second harmonic generation microscopy
Hwanhee Jeon, MacAulay Harvey, Richard Cisek, Elisha Bennett, Danielle Tokarz
Biomedical Optics Express.2023; 14(10): 5376. CrossRef - Extremely well-differentiated adenocarcinoma of the stomach: diagnostic pitfalls in endoscopic biopsy
Jongwon Lee, In-Seob Lee, Ji Yong Ahn, Young Soo Park, Jihun Kim
Journal of Pathology and Translational Medicine.2022; 56(2): 63. CrossRef - Helicobacter pylori-negative Gastric Cancer
Sun-Young Lee
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2021; 21(1): 10. CrossRef - Preoperative diagnosis of a gastric extremely well-differentiated adenocarcinoma
Katsushi Suenaga, Shiro Matsumoto, Alan Kawarai Lefor, Yoshimasa Miura, Yoshinori Hosoya, Daigo Kuboki, Hidenori Haruta, Kentaro Kurashina, Atsushi Kihara, Daisuke Matsubara, Yasunari Sakuma, Joji Kitayama, Naohiro Sata
International Journal of Surgery Case Reports.2020; 73(C): 319. CrossRef - Gastric adenocarcinoma of the fundic gland type: clinicopathological features of eight patients treated with endoscopic submucosal dissection
Chengfang Li, Xinglong Wu, Shuang Yang, Xiaorong Yang, Jin Yao, Hong Zheng
Diagnostic Pathology.2020;[Epub] CrossRef - Gastric Adenocarcinoma of the Fundic Gland Type
Mark A Benedict, Gregory Y Lauwers, Dhanpat Jain
American Journal of Clinical Pathology.2018; 149(6): 461. CrossRef
- Unusual or Uncommon Histology of Gastric Cancer
- Analysis of Histologic Features Suspecting Anaplastic Lymphoma Kinase (ALK)-Expressing Pulmonary Adenocarcinoma
- In Ho Choi, Dong Won Kim, Sang Yun Ha, Yoon-La Choi, Hee Jeong Lee, Joungho Han
- J Pathol Transl Med. 2015;49(4):310-317. Published online June 22, 2015
- DOI: https://doi.org/10.4132/jptm.2015.05.13
- 12,135 View
- 93 Download
- 20 Web of Science
- 20 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Since 2007 when anaplastic lymphoma kinase (ALK) rearrangements were discovered in non-small cell lung cancer, the ALK gene has received attention due to ALK-targeted therapy, and a notable treatment advantage has been observed in patients harboring the EML4/ALK translocation. However, using ALK-fluorescence in situ hybridization (FISH) as the standard method has demerits such as high cost, a time-consuming process, dependency on interpretation skill, and tissue preparation. We analyzed the histologic findings which could complement the limitation of ALK-FISH test for pulmonary adenocarcinoma. Methods: Two hundred five cases of ALK-positive and 101 of ALK-negative pulmonary adenocarcinoma from January 2007 to May 2013 were enrolled in this study. The histologic findings and ALK immunohistochemistry results were reviewed and compared with the results of ALK-FISH and EGFR/KRAS mutation status. Results: Acinar, cribriform, and solid growth patterns, extracellular and intracellular mucin production, and presence of signet-ring-cell element, and psammoma body were significantly more often present in ALK-positive cancer. In addition, the presence of goblet cell-like cells and presence of nuclear inclusion and groove resembling papillary thyroid carcinoma were common in the ALK-positive group. Conclusions: The above histologic parameters can be helpful in predicting ALK rearranged pulmonary adenocarcinoma, leading to rapid FISH analysis and timely treatment. -
Citations
Citations to this article as recorded by- Evidential deep learning-based ALK-expression screening using H&E-stained histopathological images
Sai Chandra Kosaraju, Sai Phani Parsa, Dae Hyun Song, Hyo Jung An, Yoon-La Choi, Joungho Han, Jung Wook Yang, Mingon Kang
npj Digital Medicine.2025;[Epub] CrossRef - Clinicopathological significances of cribriform pattern in lung adenocarcinoma
Jung-Soo Pyo, Byoung-Hoon Lee, Kyueng-Whan Min, Nae Yu Kim
Pathology - Research and Practice.2024; 253: 155035. CrossRef - Clinicopathological features and prognostic significance of pulmonary adenocarcinoma with signet ring cell components: meta-analysis and SEER analysis
Yang Tan, Ying-he Huang, Jia-wen Xue, Rui Zhang, Run Liu, Yan Wang, Zhen-Bo Feng
Clinical and Experimental Medicine.2023; 23(8): 4341. CrossRef - Lung-Cancer Risk in Mice after Exposure to Gamma Rays, Carbon Ions or Neutrons: Egfr Pathway Activation and Frequent Nuclear Abnormality
Kenshi Suzuki, Shunsuke Yamazaki, Ken-ichi Iwata, Yutaka Yamada, Takamitsu Morioka, Kazuhiro Daino, Mutsumi Kaminishi, Mari Ogawa, Yoshiya Shimada, Shizuko Kakinuma
Radiation Research.2022;[Epub] CrossRef - Pathological cytomorphologic features and the percentage of ALK FISH-positive cells predict pulmonary adenocarcinoma prognosis: a prospective cohort study
Fenge Jiang, Congcong Wang, Ping Yang, Ping Sun, Jiannan Liu
World Journal of Surgical Oncology.2021;[Epub] CrossRef - Cribriform pattern in lung invasive adenocarcinoma correlates with poor prognosis in a Chinese cohort
Yang Qu, Haifeng Lin, Chen Zhang, Kun Li, Haiqing Zhang
Pathology - Research and Practice.2019; 215(2): 347. CrossRef - Incidence of brain metastasis in lung adenocarcinoma at initial diagnosis on the basis of stage and genetic alterations
Bumhee Yang, Hyun Lee, Sang-Won Um, Kyunga Kim, Jae Il Zo, Young Mog Shim, O Jung Kwon, Kyung Soo Lee, Myung-Ju Ahn, Hojoong Kim
Lung Cancer.2019; 129: 28. CrossRef - Qualitative and quantitative cytomorphological features of primary anaplastic lymphoma kinase‐positive lung cancer
Ryuko Tsukamoto, Hiroyuki Ohsaki, Sho Hosokawa, Yasunori Tokuhara, Shingo Kamoshida, Toshiko Sakuma, Tomoo Itoh, Chiho Ohbayashi
Cytopathology.2019; 30(3): 295. CrossRef - Double Trouble: A Case Series on Concomitant Genetic Aberrations in NSCLC
Nele Van Der Steen, Yves Mentens, Marc Ramael, Leticia G. Leon, Paul Germonpré, Jose Ferri, David R. Gandara, Elisa Giovannetti, Godefridus J. Peters, Patrick Pauwels, Christian Rolfo
Clinical Lung Cancer.2018; 19(1): 35. CrossRef - Update on the potential significance of psammoma bodies in lung adenocarcinoma from a modern perspective
Akio Miyake, Koji Okudela, Mai Matsumura, Mitsui Hideaki, Hiromasa Arai, Shigeaki Umeda, Shoji Yamanaka, Yoshihiro Ishikawa, Michihiko Tajiri, Kenichi Ohashi
Histopathology.2018; 72(4): 609. CrossRef - Integrin β3 Inhibition Enhances the Antitumor Activity of ALK Inhibitor in ALK-Rearranged NSCLC
Ka-Won Noh, Insuk Sohn, Ji-Young Song, Hyun-Tae Shin, Yu-Jin Kim, Kyungsoo Jung, Minjung Sung, Mingi Kim, Sungbin An, Joungho Han, Se-Hoon Lee, Mi-Sook Lee, Yoon-La Choi
Clinical Cancer Research.2018; 24(17): 4162. CrossRef - An anaplastic lymphoma kinase-positive lung cancer microlesion: A case report
Tetsuo Kon, Youichiro Baba, Ichiro Fukai, Gen Watanabe, Tomoko Uchiyama, Tetsuya Murata
Human Pathology: Case Reports.2017; 7: 11. CrossRef - The prevalence of ALK rearrangement in pulmonary adenocarcinomas in an unselected Caucasian population from a defined catchment area: impact of smoking
Birgit G Skov, Paul Clementsen, Klaus R Larsen, Jens B Sørensen, Anders Mellemgaard
Histopathology.2017; 70(6): 889. CrossRef - Ciliated muconodular papillary tumor of the lung harboring ALK gene rearrangement: Case report and review of the literature
Yan Jin, Xuxia Shen, Lei Shen, Yihua Sun, Haiquan Chen, Yuan Li
Pathology International.2017; 67(3): 171. CrossRef - Molecular breakdown: a comprehensive view of anaplastic lymphoma kinase (ALK)‐rearranged non‐small cell lung cancer
Ka‐Won Noh, Mi‐Sook Lee, Seung Eun Lee, Ji‐Young Song, Hyun‐Tae Shin, Yu Jin Kim, Doo Yi Oh, Kyungsoo Jung, Minjung Sung, Mingi Kim, Sungbin An, Joungho Han, Young Mog Shim, Jae Ill Zo, Jhingook Kim, Woong‐Yang Park, Se‐Hoon Lee, Yoon‐La Choi
The Journal of Pathology.2017; 243(3): 307. CrossRef - Anaplastic lymphoma kinase immunohistochemistry in lung adenocarcinomas: Evaluation of performance of standard manual method using D5F3 antibody
D Jain, K Jangra, PS Malik, S Arulselvi, K Madan, S Mathur, MC Sharma
Indian Journal of Cancer.2017; 54(1): 209. CrossRef - Clinicopathological Features and Therapeutic Responses of Chinese Patients with Advanced Lung Adenocarcinoma Harboring an Anaplastic Lymphoma Kinase Rearrangement
Danxia Lin, De Zeng, Chen Chen, Xiao Wu, Miaojun Wang, Jiongyu Chen, Hui Lin, Xihui Qiu
Oncology Research and Treatment.2017; 40(1-2): 27. CrossRef - A Validation Study for the Use of ROS1 Immunohistochemical Staining in Screening for ROS1 Translocations in Lung Cancer
Patrizia Viola, Manisha Maurya, James Croud, Jana Gazdova, Nadia Suleman, Eric Lim, Tom Newsom-Davis, Nick Plowman, Alexandra Rice, M. Angeles Montero, David Gonzalez de Castro, Sanjay Popat, Andrew G. Nicholson
Journal of Thoracic Oncology.2016; 11(7): 1029. CrossRef - Non-small Cell Lung Cancer with Concomitant EGFR, KRAS, and ALK Mutation: Clinicopathologic Features of 12 Cases
Taebum Lee, Boram Lee, Yoon-La Choi, Joungho Han, Myung-Ju Ahn, Sang-Won Um
Journal of Pathology and Translational Medicine.2016; 50(3): 197. CrossRef - ALK gene rearranged lung adenocarcinomas: molecular genetics and morphology in cohort of patients from North India
Amanjit Bal, Navneet Singh, Parimal Agarwal, Ashim Das, Digambar Behera
APMIS.2016; 124(10): 832. CrossRef
- Evidential deep learning-based ALK-expression screening using H&E-stained histopathological images
- A Rare Case of Primary Tubular Adenocarcinoma of the Thymus, Enteric Immunophenotype: A Case Study and Review of the Literature
- Hae Yoen Jung, Hyundeuk Cho, Jin-Haeng Chung, Sang Byoung Bae, Ji-Hye Lee, Hyun Ju Lee, Si-Hyong Jang, Mee-Hye Oh
- J Pathol Transl Med. 2015;49(4):331-334. Published online June 1, 2015
- DOI: https://doi.org/10.4132/jptm.2015.04.16
- 11,002 View
- 84 Download
- 13 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF - Thymic carcinomas are uncommon malignant tumors, and thymic adenocarcinomas are extremely rare. Here, we describe a case of primary thymic adenocarcinoma in a 59-year-old woman. Histological examination of the tumor revealed tubular morphology with expression of cytokeratin 20 and caudal-type homeobox 2 according to immunohistochemistry, suggesting enteric features. Extensive clinical and radiological studies excluded the possibility of an extrathymic primary tumor. A review of the literature revealed only two global cases of primary tubular adenocarcinomas of the thymus with enteric immunophenotype.
-
Citations
Citations to this article as recorded by- Unresectable Primary Enteric‐Type Thymic Adenocarcinoma Treated With FOLFOX Chemotherapy: A Case Report
Carl He, Georgia Bentick, Patrick Hosking, Andrew Mant
Cancer Reports.2025;[Epub] CrossRef - Enteric thymic adenocarcinoma: Understanding a unique pathology in the mediastinum
Raja Chhabra, Kartik Mittal, Anmol Tufchi, Sajjan Rajpurohit, Deepak Kumar Mittal, Aditya Vidushi, Swati Saxena, Md Ali Osama
Indian Journal of Pathology and Microbiology.2025; 68(4): 820. CrossRef - Clinical characteristics and prognosis of primary thymic adenocarcinoma: A single‐center retrospective analysis
Qian Hong, Rui Han, Chen Chen, Fuquan Wang, Sining Zhang, Chenguang Zhao, Fang Li, Juwei Mu, Jiagen Li
Thoracic Cancer.2024; 15(24): 1815. CrossRef - Case report: Primary adenocarcinoma NOS of the thymus and cytological features
Jonathan Willner, Osvaldo Hernandez, Lea Azour, Andre L. Moreira
Diagnostic Cytopathology.2023;[Epub] CrossRef - Enteric-type thymic adenocarcinoma: a case report and literature review focusing on prognosis based on histological subtypes
Rurika Hamanaka, Kei Nakano, Takaaki Tsuboi, Kazuhito Hatanaka, Mitsutomo Kohno, Ryota Masuda, Masayuki Iwazaki
General Thoracic and Cardiovascular Surgery.2022; 70(5): 501. CrossRef - Metastatic thymic-enteric adenocarcinoma responding to chemoradiation plus anti-angiogenic therapy: A case report
Man Li, Xiao-Yu Pu, Li-Hua Dong, Peng-Yu Chang
World Journal of Clinical Cases.2021; 9(7): 1676. CrossRef - A case report: primary thymic adenocarcinoma with enteric differentiation
Yuuki Kou, Hirokazu Tanaka, Nobuhisa Yamazaki, Hiroyoshi Watanabe, Makoto Sonobe
The Journal of the Japanese Association for Chest Surgery.2020; 34(2): 107. CrossRef - Thymic enteric type adenocarcinoma: A case report with cytological features
Marie Tamai, Mitsuaki Ishida, Yusuke Ebisu, Hisashi Okamoto, Chika Miyasaka, Chisato Ohe, Yoshiko Uemura, Tomohito Saito, Tomohiro Murakawa, Koji Tsuta
Diagnostic Cytopathology.2018; 46(1): 92. CrossRef - Histologic characteristics of thymic adenocarcinomas: Clinicopathologic study of a nine-case series and a review of the literature
Ah-Young Kwon, Joungho Han, Jinah Chu, Yong Soo Choi, Byeong-Ho Jeong, Myung-Ju Ahn, Yong Chan Ahn
Pathology - Research and Practice.2017; 213(2): 106. CrossRef - Characterization of genetic aberrations in a single case of metastatic thymic adenocarcinoma
Yeonghun Lee, Sehhoon Park, Se-Hoon Lee, Hyunju Lee
BMC Cancer.2017;[Epub] CrossRef - Cytologic Characteristics of Thymic Adenocarcinoma with Enteric Differentiation: A Study of Four Fine-Needle Aspiration Specimens
Ah-Young Kwon, Joungho Han, Hae-yon Cho, Seokhwi Kim, Heejin Bang, Jiyeon Hyeon
Journal of Pathology and Translational Medicine.2017; 51(5): 509. CrossRef - Mucinous cystic tumor with CK20 and CDX2 expression of the thymus: Is this a benign counterpart of adenocarcinoma of the thymus, enteric type?
Jun Akiba, Hiroshi Harada, Shintaro Yokoyama, Toshihiro Hashiguchi, Akihiko Kawahara, Masahiro Mitsuoka, Shinzo Takamori, Hirohisa Yano
Pathology International.2016; 66(1): 29. CrossRef - Colon cancer chemotherapy for a patient with CDX2-expressing metastatic thymic adenocarcinoma: a case report and literature review
Akihiko Sawaki, Mikiya Ishihara, Yuji Kozuka, Hiroyasu Oda, Satoshi Tamaru, Yumiko Sugawara, Yoshiki Yamashita, Toshiro Mizuno, Taizo Shiraishi, Naoyuki Katayama
International Cancer Conference Journal.2016; 5(2): 113. CrossRef - Metastatic Thymic Adenocarcinoma from Colorectal Cancer
Mina Lee, Suk Jin Choi, Yong Han Yoon, Joung-Taek Kim, Wan Ki Baek, Young Sam Kim
The Korean Journal of Thoracic and Cardiovascular Surgery.2015; 48(6): 447. CrossRef
- Unresectable Primary Enteric‐Type Thymic Adenocarcinoma Treated With FOLFOX Chemotherapy: A Case Report
- Follicular Proliferative Lesion Arising in Struma Ovarii
- Min Jee Park, Min A Kim, Mi Kyung Shin, Hye Sook Min
- J Pathol Transl Med. 2015;49(3):262-266. Published online May 15, 2015
- DOI: https://doi.org/10.4132/jptm.2015.03.26
- 10,303 View
- 149 Download
- 5 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - Malignant struma ovarii is extremely rare and difficult to diagnose histologically, particularly in cases of follicular carcinoma. This case study is intended to describe three cases of follicular proliferative lesion arising in struma ovarii that we experienced. The first case was clearly malignant given the clinical picture of multiple recurrences, but there was little histological evidence of malignancy. Our second case featured architectural and cellular atypia and necrosis and was diagnosed as malignant despite the absence of vascular and stromal invasion. Our third case exhibit-ed solid microfollicular proliferation without any definite evidence of malignancy (even the molecular data was negative); however, we could not completely exclude malignant potential after conducting a literature review. In cases such as our third case, it has been previously suggested that a diagnostic term recognizing the low-grade malignant potential, such as “proliferative stromal ovarii” or “follicular proliferative lesion arising in the stromal ovarii” would be appropriate.
-
Citations
Citations to this article as recorded by- Role of gene sequencing in classifying struma ovarii: BRAF p.G469A mutation and TERT promoter alterations favour malignant struma ovarii
Sophie Neyrand, Alexis Trecourt, Jonathan Lopez, Pierre Alexandre Just, Françoise Descotes, Françoise Borson‐Chazot, Isabelle Ray‐Coquard, Myriam Decaussin‐Petrucci, Mojgan Devouassoux‐Shisheboran
Histopathology.2024; 84(2): 291. CrossRef - Malignant struma ovarii: next-generation sequencing of six cases revealed Nras, Braf, and Jak3 mutations
Roberta Poli, Maria Scatolini, Enrico Grosso, Francesca Maletta, Marco Gallo, Daniele Liscia, Anna Nelva, Flora Cesario, Giuseppe Forte, Jasna Metovic, Marco Volante, Emanuela Arvat, Mauro Papotti
Endocrine.2021; 71(1): 216. CrossRef - Proliferative struma ovarii: A rare case report
Shankhanila Mazumdar, GaganKumar Rangari, Neeraj Dhameja, NishaRani Agrawal
International Journal of Clinicopathological Correlation.2021; 5(2): 85. CrossRef - Malignant struma ovarii presenting with follicular carcinoma: A case report with molecular analysis
Takafumi Tsukada, Hiroshi Yoshida, Mitsuya Ishikawa, Yuka Asami, Kouya Shiraishi, Tomoyasu Kato
Gynecologic Oncology Reports.2019; 30: 100498. CrossRef - A Rare Case: Struma Ovarii in a 14-Year-Old Girl
Elif Iltar, Isin Ureyen, Tayfun Toptas, Melike Savas, Sema Çekiç, Aysel Uysal
Journal of Adolescent and Young Adult Oncology.2018; 7(1): 134. CrossRef - Proliferative Highly Differentiated Follicular Carcinoma Of Ovarian Origin (Hdfco) Presenting Long After Bilateral Oophorectomy
Natalie M. Liu, Neda Moatamed, Racquel S. Bueno, Wendy L. Sacks
AACE Clinical Case Reports.2017; 3(3): e264. CrossRef
- Role of gene sequencing in classifying struma ovarii: BRAF p.G469A mutation and TERT promoter alterations favour malignant struma ovarii
- The Diagnostic Usefulness of HMGA2, Survivin, CEACAM6, and SFN/14-3-3 δ in Follicular Thyroid Carcinoma
- Min Hye Jang, Kyeong Cheon Jung, Hye Sook Min
- J Pathol Transl Med. 2015;49(2):112-117. Published online March 12, 2015
- DOI: https://doi.org/10.4132/jptm.2015.01.31
- 10,068 View
- 75 Download
- 13 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Follicular thyroid carcinoma (FTC) is the second most common thyroid malignancy and its differential diagnosis includes follicular adenoma (FA) and adenomatous goiter (AG). Several ancillary markers have been suggested to aid in the diagnosis of FTC, but the successful use of these methods still needs to be validated. Methods: In the present study, we verified the immunoexpression of HMGA2, CEACAM6, survivin, and SFN/14-3-3 δ in lesions including 41 AGs, 72 FAs, and 79 FTCs. We evaluated their diagnostic usefulness, combined with galectin 3, Hector Battifora mesothelial 1 (HBME1), cytokeratin 19, and cyclin D1, in diagnosing FTC. Results: The expressions of HBME1 (65.8%) and HMGA2 (55.7%) were significantly higher in FTCs than in FAs and AGs (p<.001 and p=.005, respectively). HBME1 was the only marker that was more frequently expressed in FTCs than in FAs (p=.021) and it was more frequently expressed in follicular neoplasms than in AGs (p<.001). Among the novel markers, the combination of HMGA2 and HBME1 showed the highest sensitivity (72.2%) and specificity (76.1%) for diagnosing FTC. CEACAM6, survivin, and SFN/14-3-3 δ were barely expressed in most cases. Conclusions: Our present results show that only HMGA2 can be beneficial in differentiating FTC using the novel markers. -
Citations
Citations to this article as recorded by- High mobility group protein 2 (HMGA2) is highly expressed in a broad range of benign and malignant tumors
Viktoria Chirico, Fatih Baybars Ergüven, Katharina Möller, Florian Lutz, Florian Viehweger, Martina Kluth, Claudia Hube-Magg, Christian Bernreuther, Guido Sauter, Andreas H. Marx, Ronald Simon, Frank Jacobsen, Patrick Lebok, Till S. Clauditz, Waldemar Wil
Virchows Archiv.2025; 487(1): 183. CrossRef - Assessing the potential of high-mobility group AT-hook 2 immunohistochemical staining as a prognostic marker of metastatic recurrence in follicular thyroid cancer: a retrospective cohort study
Yuka Ito, Junko Sakumoto, Hideki Hirabayashi, Shinichi Haruna, Wataru Konno, Itsuo Nakajima, Kazuyuki Ishida, Yasuo Haruyama, Toshimi Sairenchi, Eijun Nishihara, Shuji Fukata, Akira Hishinuma, Takahiko Kogai
Endocrine Journal.2025; 72(5): 535. CrossRef - HMGA2 promotes nasopharyngeal carcinoma progression and is associated with tumor resistance and poor prognosis
Xinting Ouyang, Kangxin Li, Jiaqi Wang, Weijian Zhu, Qiang Yi, Jinghua Zhong
Frontiers in Oncology.2024;[Epub] CrossRef - miR‐98‐5p promotes apoptosis and inhibits migration and cell growth in papillary thyroid carcinoma through Bax/Caspase‐3 by HMGA2
Kai Qiu, QingJi Xie, Shan Jiang, Ting Lin
Journal of Clinical Laboratory Analysis.2020;[Epub] CrossRef - High mobility group A protein-2 as a tumor cancer diagnostic and prognostic marker: a systematic review and meta-analysis
Yen Thi-Hai Pham, Ovie Utuama, Claire E. Thomas, Jong A. Park, Carlo La Vecchia, Harvey A. Risch, Chi Thi-Du Tran, Thanh V. Le, Paolo Boffetta, Leon Raskin, Hung N. Luu
European Journal of Cancer Prevention.2020; 29(6): 565. CrossRef - Diagnostic performance of HMGA2 gene expression for differentiation of malignant thyroid nodules: A systematic review and meta‐analysis
Bo Hyun Kim, Seong Jang Kim, Mijin Kim, Sang‐Woo Lee, Shin Young Jeong, Kyoungjune Pak, Keunyoung Kim, In Joo Kim
Clinical Endocrinology.2018; 89(6): 856. CrossRef - Thyroid follicular adenomas and carcinomas: molecular profiling provides evidence for a continuous evolution
Geneviève Dom, Sandra Frank, Sebastien Floor, Pashalina Kehagias, Frederick Libert, Catherine Hoang, Guy Andry, Alex Spinette, Ligia Craciun, Nicolas de Saint Aubin, Christophe Tresallet, Frederique Tissier, Frederique Savagner, Samira Majjaj, Ilse Gutier
Oncotarget.2018; 9(12): 10343. CrossRef - APLP2, RRM2, and PRC1: New Putative Markers for the Differential Diagnosis of Thyroid Follicular Lesions
Esmeralda Castelblanco, Carles Zafon, Javier Maravall, Pilar Gallel, Montserrat Martinez, Ismael Capel, Maria Rosa Bella, Irene Halperin, Jordi Temprana, Carmela Iglesias, Manel Puig-Domingo, Mercedes Robledo, Xavier Matias-Guiu, Didac Mauricio
Thyroid.2017; 27(1): 59. CrossRef - Prognostic implication of histological features associated with EHD2 expression in papillary thyroid carcinoma
Yourha Kim, Min-Hee Kim, Sora Jeon, Jeeyoon Kim, Chankyung Kim, Ja Seong Bae, Chan Kwon Jung, Yves St-Pierre
PLOS ONE.2017; 12(3): e0174737. CrossRef - Survivin DEx3 as a biomarker of thyroid cancers: A study at the mRNA and protein level
Joanna Waligórska-Stachura, Nadia Sawicka-Gutaj, Maciej Zabel, Mirosław Andrusiewicz, Paweł Gut, Agata Czarnywojtek, Marek Ruchała
Oncology Letters.2017; 13(4): 2437. CrossRef - High-Frequency Ultrasound-Guided Injection for the Generation of a Novel Orthotopic Mouse Model of Human Thyroid Carcinoma
Adelaide Greco, Sandra Albanese, Luigi Auletta, Peppino Mirabelli, Antonella Zannetti, Crescenzo D'Alterio, Gennaro Di Maro, Francesca Maria Orlandella, Giuliana Salvatore, Andrea Soricelli, Marco Salvatore
Thyroid.2016; 26(4): 552. CrossRef - The study of galectin-3, Ki-67, ubiquitin, HMGA-2 by polymerase chain reaction in real time (RT-PCR) in the puncture specimens of nodular goiter
Irina S. Berjozkina, Tat'jana V. Saprina, Anastasija P. Zima, Anna V. Isaeva, Venera N. Latipova, Marat R. Muhamedov, Leonid R. Bazilevich, Oleg S. Popov, Dar'ja A. Skuratovskaja, Kristina A. Jurova, Larisa C. Litvinova
Clinical and experimental thyroidology.2016; 12(2): 19. CrossRef - High-mobility group A2 overexpression is an unfavorable prognostic biomarker for nasopharyngeal carcinoma patients
Zhuoxing Liu, Kunpeng Wu, Zhixiong Yang, Aibing Wu
Molecular and Cellular Biochemistry.2015; 409(1-2): 155. CrossRef - Defining the value of CD56, CK19, Galectin 3 and HBME-1 in diagnosis of follicular cell derived lesions of thyroid with systematic review of literature
Duško Dunđerović, Jasmina Marković Lipkovski, Ivan Boričic, Ivan Soldatović, Vesna Božic, Dubravka Cvejić, Svetislav Tatić
Diagnostic Pathology.2015;[Epub] CrossRef
- High mobility group protein 2 (HMGA2) is highly expressed in a broad range of benign and malignant tumors
- The Limitations of Endoscopic Ultrasound-Guided Fine Needle Aspiration Cytology in the Diagnosis of Pancreatic Serous Cystadenoma: A Brief Case Report
- Heae Surng Park, Sun Och Yoon, Beom Jin Lim, Joo Hee Kim, Soon Won Hong
- Korean J Pathol. 2014;48(5):405-408. Published online October 27, 2014
- DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.5.405
- 8,299 View
- 63 Download
- Detection of Human Papillomavirus Type 39 in a Seborrheic Inclusion Cyst of the Buttock
- Dae Hyun Song, Sang-Guk Lee, Dong Chul Kim, Jeong Hee Lee, Gyung Hyuck Ko, Jong Sil Lee
- Korean J Pathol. 2014;48(5):398-400. Published online October 27, 2014
- DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.5.398
- 8,073 View
- 49 Download
- Cytomorphological Findings and Histological Correlation of Low-Grade Cribriform Cystadenocarcinoma of Salivary Gland in Fine-Needle Aspiration: A Case Study
- Young Sin Ko, Ja Seung Koo
- Korean J Pathol. 2013;47(6):592-595. Published online December 24, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.6.592
- 9,087 View
- 70 Download
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF Low-grade cribriform cystadenocarcinoma (LGCCC) of the salivary gland is a rare tumor. We report the cytologic features and histologic correlation of a patient with LGCCC. A 57-year-old man had a hardly palpable, nontender mass in the right cheek area followed over nine months. Radiologic analysis revealed a 1.2 cm multiseptated, cystic, solid nodule in an anterior superficial lobe of the right parotid gland. Fine-needle aspiration cytology revealed many irregular overlapping sheets or clusters of ductal epithelial cells forming solid, pseudopapillary, and cribriform architectures. Nuclei of the tumor cells revealed inconspicuous atypia with minimal size variation. On the basis of these findings, we confirmed a diagnosis of ductal epithelial proliferative lesion, favoring neoplasm, with uncertain malignant potential. Tumor excision was performed, revealing a tiny multicystic nodule (0.7 cm). Histopathologically, this tumor showed the characteristic morphology of LGCCC. This is the first report of cytomorphological findings of LGCCC in Korea.
-
Citations
Citations to this article as recorded by- Duct tales of a parotid gland swelling
Swati Raj, Monika Singh, Mamta Gupta, Naveen Thapliyal
Cytojournal.2023; 20: 22. CrossRef - Salivary Gland Intraductal Carcinoma: How Do 183 Reported Cases Fit Into a Developing Classification
Lester D.R. Thompson, Justin A. Bishop
Advances in Anatomic Pathology.2023; 30(2): 112. CrossRef - Intraductal carcinoma of the parotid gland
Yukiya HIRATA, Kayoko HIGUCHI, Toshitaka NAGAO, Yoko ZUKERAN, Takao KINJO, Naoki WADA
The Journal of the Japanese Society of Clinical Cytology.2022; 61(6): 431. CrossRef - Intraductal carcinomas of the salivary glands: systematic review and classification of 93 published cases
Andrea Palicelli
APMIS.2020; 128(3): 191. CrossRef - What do we know about the cytological features of pure intraductal carcinomas of the salivary glands?
Andrea Palicelli
Cytopathology.2020; 31(3): 185. CrossRef - Diagnosing Recently Defined and Uncommon Salivary Gland Lesions in Limited Cellularity Specimens: Cytomorphology and Ancillary Studies
Esther Diana Rossi, Zubair Baloch, William Faquin, Liron Pantanowitz
AJSP: Reviews and Reports.2020; 25(5): 210. CrossRef - Low-grade intraductal carcinoma of salivary glands: A systematic review of this rare entity
Francesco Giovacchini, Caterina Bensi, Stefano Belli, Maria Elena Laurenti, Martina Mandarano, Daniele Paradiso, Michele Giansanti, Antonio Tullio
Journal of Oral Biology and Craniofacial Research.2019; 9(1): 96. CrossRef - The rare entity of cystadenocarcinoma (CAC) in parotid gland: A single-center experience
Bing Guo, Yu-an Cao, Xingjun Qin, Chunyue Ma
Journal of Cranio-Maxillofacial Surgery.2019; 47(5): 826. CrossRef - Cytopathology approach to rare salivary gland lesions with oncocytic features
Siba El Hussein, Samer N. Khader
Diagnostic Cytopathology.2019; 47(10): 1090. CrossRef - Unicystic high‐grade intraductal carcinoma of the parotid gland: cytological and histological description with clinic–pathologic review of the literature
Andrea Palicelli, Paola Barbieri, Narciso Mariani, Paola Re, Stefania Galla, Raffaele Sorrentino, Francesca Locatelli, Nunzio Salfi, Guido Valente
APMIS.2018; 126(9): 771. CrossRef - Low-grade cribriform cystadenocarcinoma arising from a minor salivary gland: a case report
Masashi Kimura, Shinji Mii, Shinichi Sugimoto, Kosuke Saida, Shojiroh Morinaga, Masahiro Umemura
Journal of Oral Science.2016; 58(1): 145. CrossRef - A Case of Cystadenocarcinoma Arising from Parotid Gland
Jong Chul Hong, Tae Kyoung Koh, Min Gyoung Pak, Heon Soo Park
Korean Journal of Otorhinolaryngology-Head and Neck Surgery.2016; 59(4): 300. CrossRef - Mammary analogue secretory carcinoma of parotid gland
Atsuko NASU, Sakae HATA, Masaru FUJITA, Toyoko YAMAUCHI, Satoko NAKAMURA, Takehiro TANAKA, Kouichi ICHIMURA, Hiroyuki YANAI
The Journal of the Japanese Society of Clinical Cytology.2016; 55(2): 112. CrossRef
- Duct tales of a parotid gland swelling
- Fine-Needle Aspiration Cytology of Low-Grade Cribriform Cystadenocarcinoma with Many Psammoma Bodies of the Salivary Gland
- Ji Yun Jeong, Dongbin Ahn, Ji Young Park
- Korean J Pathol. 2013;47(5):481-485. Published online October 25, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.5.481
- 8,375 View
- 49 Download
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF Low-grade cribriform cystadenocarcinoma (LGCCC) is a rare salivary gland tumor that was recently defined as a variant of cystadenocarcinoma by the 2005 World Health Orgazniation (WHO) classification system. We report cytologic findings of an unusual case of LGCCC with many psammoma bodies. A 90-year-old man presented a palpable mass on his left parotid gland. Fine-needle aspiration (FNA) cytology showed tumor cells that were arranged in clusters and dispersed individually. The tumor cells showed mild atypia and had clear or dense cytoplasm with some vacuoles. Numerous psammoma bodies were noted. After surgical resection, the histologic examination revealed a mixed solid and cystic mass showing intraductal growth with focal stromal invasion. The S-100 protein expressed in the tumor cells, but smooth muscle actin and p63 were positive only in myoepithelial cells. Although LGCCCs resemble other salivary gland tumors, differentiating LGCCC during preoperative FNA is important to avoid unnecessary overtreatment.
-
Citations
Citations to this article as recorded by- Salivary Gland Intraductal Carcinoma: How Do 183 Reported Cases Fit Into a Developing Classification
Lester D.R. Thompson, Justin A. Bishop
Advances in Anatomic Pathology.2023; 30(2): 112. CrossRef - Duct tales of a parotid gland swelling
Swati Raj, Monika Singh, Mamta Gupta, Naveen Thapliyal
Cytojournal.2023; 20: 22. CrossRef - Intraductal carcinoma of the parotid gland
Yukiya HIRATA, Kayoko HIGUCHI, Toshitaka NAGAO, Yoko ZUKERAN, Takao KINJO, Naoki WADA
The Journal of the Japanese Society of Clinical Cytology.2022; 61(6): 431. CrossRef - Intraductal carcinoma of the retromolar trigone found with elevated serum CEA and CA19-9 levels: a case report
Mao KAWAKAMI, Nobuhiro UEDA, Yuka TAKAHASHI, Sho ARIKAWA, Nobuhiro YAMAKAWA, Tadaaki KIRITA
Japanese Journal of Oral and Maxillofacial Surgery.2021; 67(5): 292. CrossRef - Endoscopic trans‐pterygoid resection of a low‐grade cribriform cystadenocarcinoma of the infratemporal fossa
Vikram G. Ramjee, Landon J. Massoth, John P. Richards, Kibwei A. McKinney
World Journal of Otorhinolaryngology - Head and Neck Surgery.2020; 6(2): 115. CrossRef - Psammoma Bodies in a Large Myoepithelioma
Marcela Pessoa de Melo, Diego Filipe Bezerra Silva, Rodrigo Alves Ribeiro, Tony Santos Peixoto, Daliana Queiroga de Castro Gomes, Pollianna Muniz Alves, Cassiano Francisco Weege Nonaka, Bárbara Vanessa de Brito Monteiro
Journal of Craniofacial Surgery.2020; 31(4): e326. CrossRef - Low-grade intraductal carcinoma of salivary glands: A systematic review of this rare entity
Francesco Giovacchini, Caterina Bensi, Stefano Belli, Maria Elena Laurenti, Martina Mandarano, Daniele Paradiso, Michele Giansanti, Antonio Tullio
Journal of Oral Biology and Craniofacial Research.2019; 9(1): 96. CrossRef - What is your diagnosis? Submandibular mass in a dog
Julie Allen, Ashley M. Talley, Carol B. Grindem, Jennifer A. Neel
Veterinary Clinical Pathology.2018; 47(4): 676. CrossRef - Primary acinic cell carcinoma of the lung with psammoma bodies: A case report and review of literature
Xiu-Peng Zhang, Gui-Yang Jiang, Qing-Fu Zhang, Hong-Tao Xu, Qing-Chang Li, En-Hua Wang
Pathology - Research and Practice.2017; 213(4): 405. CrossRef - Cytology of low‐grade cribriform cystadenocarcinoma in salivary glands: Cytological and immunohistochemical distinctions from other salivary gland neoplasms
Yoshiki Ohta, Yuko Hirota, Yohko Kohno, Koji Kishimoto, Tomoko Norose, Nobuyuki Ohike, Masafumi Takimoto, Akira Shiokawa, Hidekazu Ota
Diagnostic Cytopathology.2016; 44(3): 241. CrossRef - Low-grade cribriform cystadenocarcinoma arising from a minor salivary gland: a case report
Masashi Kimura, Shinji Mii, Shinichi Sugimoto, Kosuke Saida, Shojiroh Morinaga, Masahiro Umemura
Journal of Oral Science.2016; 58(1): 145. CrossRef - A Case of Cystadenocarcinoma Arising from Parotid Gland
Jong Chul Hong, Tae Kyoung Koh, Min Gyoung Pak, Heon Soo Park
Korean Journal of Otorhinolaryngology-Head and Neck Surgery.2016; 59(4): 300. CrossRef
- Salivary Gland Intraductal Carcinoma: How Do 183 Reported Cases Fit Into a Developing Classification
- Rhabdoid Colorectal Carcinomas: Reports of Two Cases
- Sang Hwa Lee, Hyesil Seol, Wook Youn Kim, So Dug Lim, Wan Seop Kim, Tae Sook Hwang, Hye Seung Han
- Korean J Pathol. 2013;47(4):372-377. Published online August 26, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.4.372
- 9,353 View
- 54 Download
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF Rhabdoid colorectal carcinomas are very rare and only 10 cases have been previously reported. We report two cases of rhabdoid colorectal carcinoma, one arising in the sigmoid colon of a 62-year-old man and another in the rectum of an 83-year-old woman. In both cases, the patients had advanced tumors with lymph node metastases. The tumors mostly showed a diffuse arrangement with rhabdoid features and small glandular regions were combined. Transitional areas from the adenocarcinomas to the rhabdoid tumors were also noted. Adenocarcinoma cells were positive for mixed cytokeratin (CK), CK20 and epithelial membranous antigen (EMA), but focal positive for vimentin. The rhabdoid tumor cells were positive for mixed CK, but focal positive or negative for CK20 and EMA. In addition, they were diffusely positive for vimentin, but negative for desmin. The histological and immunohistologial findings of these two cases suggest that the rhabodid tumor cells originated from dedifferentiated adenocarcinomas.
-
Citations
Citations to this article as recorded by- Undifferentiated Rhabdoid Carcinoma of the Gastrointestinal Tract: A Rare and Aggressive Malignancy
Justin M Hsieh, Zara Summers, Shinn Yeung
Cureus.2025;[Epub] CrossRef - SMARCB1/INI1-Deficient Poorly Differentiated Carcinoma of the Colon With Rhabdoid Features—A Rare Tumor With Serrated Phenotype: Case Report and Review of Literature
Shivali Maurya, Sujata Yadav, Subham Bhowmik, Jasmine Dhal, Lalita Mehra, Raju Sharma, Asuri Krishna, Atul Sharma, Adarsh Barwad, Prasenjit Das
International Journal of Surgical Pathology.2024; 32(1): 187. CrossRef - Emerging and under-recognised patterns of colorectal carcinoma morphologies: a comprehensive review
Yuho Ono, Osman Yilmaz
Journal of Clinical Pathology.2024; 77(7): 439. CrossRef - A Case of Ascending Colon Carcinoma with Rhabdoid Features
Masanari YAMADA, Masanori ICHINOSE, Atsushi HIRATA, Yoshihiro KURATA, Kimiaki FUKASAWA, Hisahiro MATSUBARA
Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association).2024; 85(6): 755. CrossRef - A Rare Case of Undifferentiated Rhabdoid Carcinoma of the Colon
Syed Alishan Nasir, Ronak Patel, Lalaine Ruiz, Michael Bush
Cureus.2022;[Epub] CrossRef - INI1-negative colorectal undifferentiated carcinoma with rhabdoid features and postoperative rapidly growing liver metastases: a case report and review of the literature
Masatsugu Kojima, Toru Miyake, Tomoyuki Ueki, Hiroyuki Ohta, Ryoji Kushima, Masanori Shiohara, Hiroo Mizuta, Hiroya Iida, Tsuyoshi Yamaguchi, Sachiko Kaida, Katsushi Takebayashi, Hiromitsu Maehira, Yusuke Nishina, Tomoharu Shimizu, Eiji Mekata, Masaji Tan
Surgical Case Reports.2021;[Epub] CrossRef - Undifferentiated carcinoma of the transverse colon with rhabdoid features that developed during treatment of non-small cell lung carcinoma with pembrolizumab: a case report
Yuya Ashitomi, Mitsuhiro Yano, Michihisa Kono, Takefumi Suzuki, Ichiro Kawamura, Shinji Okazaki, Yukinori Kamio, Osamu Hachiya, Yuka Urano, Fuyuhiko Motoi
Surgical Case Reports.2020;[Epub] CrossRef - BRAF Mutation in Colorectal Rhabdoid and Poorly Differentiated Medullary Carcinomas
Elena Bolzacchini, Nunzio Digiacomo, Cristina Marrazzo, Nora Sahnane, Roberta Maragliano, Anthony Gill, Luca Albarello, Fausto Sessa, Daniela Furlan, Carlo Capella
Cancers.2019; 11(9): 1252. CrossRef - Pathologic complete response to bevacizumab-FOLFIRI in metastatic colonic undifferentiated carcinoma with rhabdoid features
Tien-Chan Hsieh, Hung-Wei Liu, Chao-Wen Hsu
Journal of Cancer Research and Practice.2019; 6(3): 140. CrossRef - Extraordinary disease-free survival in a rare malignant extrarenal rhabdoid tumor: a case report and review of the literature
Francesco D’Amico, Alessandra Bertacco, Maurizio Cesari, Claudia Mescoli, Giorgio Caturegli, Gabriel Gondolesi, Umberto Cillo
Journal of Medical Case Reports.2018;[Epub] CrossRef - Tumor rabdoide extrarrenal maligno de colon: presentación de 3 casos y revisión de la literatura
María José Sánchez-de las Matas Garre, José García Solano, Pablo Conesa Zamora, Fidel Fernández Fernández, Miguel Pérez-Guillermo
Revista Española de Patología.2016; 49(2): 119. CrossRef - Poorly differentiated cecal adenocarcinoma showing prominent rhabdoid feature combined with appendiceal mucinous cystadenoma: A case report and review of the literature
IN-JU CHO, SUNG-SOO KIM, YOUNG-DON MIN, MUN-WHAN NOH, RAN HONG
Oncology Letters.2015; 9(4): 1527. CrossRef - A Rare Case of Undifferentiated Carcinoma of the Colon with Rhabdoid Features: A Case Report and Review of the Literature
E. Moussaly, J. P. Atallah
Case Reports in Oncological Medicine.2015; 2015: 1. CrossRef - Case Report of Rhabdoid Colon Cancer and Review of Literature
Aparna Kalyan, Gurleen Pasricha, Dulabh Monga, Aatur Singhi, Nathan Bahary
Clinical Colorectal Cancer.2015; 14(1): e5. CrossRef - Malignant Rhabdoid Tumor of the Colon: A Case Report
Elena Romera Barba, Ainhoa Sánchez Pérez, Carlos Duque Pérez, José Antonio García Marcilla, José Luis Vázquez Rojas
Cirugía Española (English Edition).2014; 92(9): 638. CrossRef - Tumor rabdoide maligno de colon: a propósito de un caso☆
Elena Romera Barba, Ainhoa Sánchez Pérez, Carlos Duque Pérez, José Antonio García Marcilla, José Luis Vázquez Rojas
Cirugía Española.2014; 92(9): 638. CrossRef
- Undifferentiated Rhabdoid Carcinoma of the Gastrointestinal Tract: A Rare and Aggressive Malignancy
- SIRT1 Expression Is Associated with Good Prognosis in Colorectal Cancer
- Wonkyung Jung, Kwang Dae Hong, Woon Yong Jung, Eunjung Lee, Bong Kyung Shin, Han Kyeom Kim, Aeree Kim, Baek-hui Kim
- Korean J Pathol. 2013;47(4):332-339. Published online August 26, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.4.332
- 12,212 View
- 81 Download
- 46 Crossref
-
 Abstract
Abstract
 PDF
PDF Background Silent mating type information regulation 2 homolog 1 (SIRT1), an NAD+-dependent deacetylase, might act as a tumor promoter by inhibiting p53, but may also as a tumor suppressor by inhibiting several oncogenes such as β-catenin and survivin. Deleted in breast cancer 1 (DBC1) is known as a negative regulator of SIRT1.
Methods Immunohistochemical expressions of SIRT1, DBC1, β-catenin, surviving, and p53 were evaluated using 2 mm tumor cores from 349 colorectal cancer patients for tissue microarray.
Results Overexpression of SIRT1, DBC1, survivin, and p53 was seen in 235 (67%), 183 (52%), 193 (55%), and 190 (54%) patients, respectively. Altered expression of β-catenin was identified in 246 (70%) patients. On univariate analysis, overexpression of SIRT1 (p=0.029) and altered expression of β-catenin (p=0.008) were significantly associated with longer overall survival. Expression of SIRT1 was significantly related to DBC1 (p=0.001), β-catenin (p=0.001), and survivin (p=0.002), but not with p53. On multivariate analysis, age, tumor stage, differentiation, and expression of SIRT1 were independent prognostic factors significantly associated with overall survival.
Conclusions SIRT1 overexpression is a good prognostic factor for colorectal cancer, and SIRT1 may interact with β-catenin and survivin rather than p53.
-
Citations
Citations to this article as recorded by- Ocular surface squamous neoplasia: Update on genetics, epigenetics and opportunities for targeted therapy
Nefeli Eleni Kounatidou, Evangelos Vitkos, Sotiria Palioura
The Ocular Surface.2025; 35: 1. CrossRef - The Prognostic Impact of SIRT1, STAT3, and YAP1 in Colorectal Carcinoma
Shimaa Elkholy, Aya Abdelbary, Dina Elazab, Mohamed Elkablawy, Asmaa G. Abdou
Applied Immunohistochemistry & Molecular Morphology.2025; 33(1): 29. CrossRef - The NR3C2-SIRT1 signaling axis promotes autophagy and inhibits epithelial mesenchymal transition in colorectal cancer
Feng Li, Xing Wan, Zhigui Li, Liming Zhou
Cell Death & Disease.2025;[Epub] CrossRef - Prognostic and clinicopathological value of dbc1 expression in human cancers: a systematic review and meta-analysis
Haojia Wang, Xinhong Cheng, Bruce Xianzhuo Zhang, Yong Wang, Shuo Gao, Fanghui Ding, Xiaojing Song, Dandan Li, Haixu Ni, Yang Luo, Xun Li
Frontiers in Oncology.2025;[Epub] CrossRef - Glutamine signaling specifically activates c-Myc and Mcl-1 to facilitate cancer cell proliferation and survival
Meng Wang, Fu-Shen Guo, Dai-Sen Hou, Hui-Lu Zhang, Xiang-Tian Chen, Yan-Xin Shen, Zi-Fan Guo, Zhi-Fang Zheng, Yu-Peng Hu, Pei-Zhun Du, Chen-Ji Wang, Yan Lin, Yi-Yuan Yuan, Shi-Min Zhao, Wei Xu
Protein & Cell.2025; 16(11): 968. CrossRef - Targeting TGF-β–Smad2/3–JNK1-mediated SIRT1 activity overcomes the chemoresistance of KRAS mutation lung cancer
Dong Hoon Shin, Minyoung Choi, Chungyong Han, Sang Soo Kim
Experimental & Molecular Medicine.2025; 57(9): 2022. CrossRef - The integrated analysis of SIRT family expression, prognostic value, and potential implications in childhood acute lymphoblastic leukemia
Xusan Xu, Zhendong Wang, Xiaoxia Wang, Wensen Zhang, Zhengqiang Luo, Xiaomei Zheng, Ronghua Pan, Ying Fu, Yajun Wang, Guochun Huang, Riling Chen, Guoda Ma
Frontiers in Oncology.2025;[Epub] CrossRef - ZMIZ1 Regulates Proliferation, Autophagy and Apoptosis of Colon Cancer Cells by Mediating Ubiquitin–Proteasome Degradation of SIRT1
Min Huang, Junfeng Wang, Zhengrong Zhang, Xueliang Zuo
Biochemical Genetics.2024; 62(4): 3245. CrossRef - Research Progress of Biological Function and Prognosis of Colorectal Cancer in Sirtuins Family
瑞阳 李
Journal of Clinical Personalized Medicine.2024; 03(04): 1805. CrossRef - Oncogenic KRAS mutation confers chemoresistance by upregulating SIRT1 in non-small cell lung cancer
Dong Hoon Shin, Jeong Yeon Jo, Minyoung Choi, Kyung-Hee Kim, Young-Ki Bae, Sang Soo Kim
Experimental & Molecular Medicine.2023; 55(10): 2220. CrossRef - Association of β-Catenin, APC, SMAD3/4, Tp53, and Cyclin D1 Genes in Colorectal Cancer: A Systematic Review and Meta-Analysis
Hongfeng Yan, Fuquan Jiang, Jianwu Yang, Ying-Kun Xu
Genetics Research.2022; 2022: 1. CrossRef - Resveratrol-related compounds: Potential for cancer and beyond
MONICA SAVIO, VALENTINA MINOIA, PAOLA FULGHIERI, LUCIA ANNA STIVALA, VIRGINIE SOTTILE
BIOCELL.2022; 46(12): 2525. CrossRef - The relationship between β-catenin and patient survival in colorectal cancer systematic review and meta-analysis
Amna Matly, Jean A. Quinn, Donald C. McMillan, James H. Park, Joanne Edwards
Critical Reviews in Oncology/Hematology.2021; 163: 103337. CrossRef - Trending topics of SIRT1 in tumorigenicity
Liz M. Garcia-Peterson, Xiaoling Li
Biochimica et Biophysica Acta (BBA) - General Subjects.2021; 1865(9): 129952. CrossRef - Surtuin 1 as a potential prognostic biomarker in very elderly patients with colorectal cancer
Guk Jin Lee, Yun Hwa Jung, Tae-Jung Kim, Yosep Chong, Seo-Won Jeong, In Kyu Lee, In Sook Woo
The Korean Journal of Internal Medicine.2021; 36(Suppl 1): S235. CrossRef - Survival and Clinicopathological Significance of SIRT1 Expression in Cancers: A Meta-Analysis
Min Sun, Mengyu Du, Wenhua Zhang, Sisi Xiong, Xingrui Gong, Peijie Lei, Jin Zha, Hongrui Zhu, Heng Li, Dong Huang, Xinsheng Gu
Frontiers in Endocrinology.2019;[Epub] CrossRef - SIRT1: a potential tumour biomarker and therapeutic target
Bin Zhao, Xin Li, Liangfu Zhou, Ye Wang, Peng Shang
Journal of Drug Targeting.2019; 27(10): 1046. CrossRef - The clinicopathological significance of SIRT1 expression in colon cancer: An immunohistochemical study and meta-analysis
Won Gi Hong, Jung-Soo Pyo
Pathology - Research and Practice.2018; 214(10): 1550. CrossRef - Sirtuin 1 and oral cancer (Review)
Shajedul Islam, Yoshihiro Abiko, Osamu Uehara, Itsuo Chiba
Oncology Letters.2018;[Epub] CrossRef - A novel SIRT1 inhibitor, 4bb induces apoptosis in HCT116 human colon carcinoma cells partially by activating p53
Ananga Ghosh, Amrita Sengupta, Guru Pavan Kumar Seerapu, Ali Nakhi, E.V. Venkat Shivaji Ramarao, Navneet Bung, Gopalakrishnan Bulusu, Manojit Pal, Devyani Haldar
Biochemical and Biophysical Research Communications.2017; 488(3): 562. CrossRef - SIRT1 gene polymorphisms and its protein level in colorectal cancer
Olfat Gamil Shaker, Miriam Safwat Wadie, Reham Maher Mohamed Ali, Ayman Yosry
Gene Reports.2017; 7: 164. CrossRef - Overexpression of SIRT1 is Associated With Poor Outcomes in Patients With Ovarian Carcinoma
David H. Mvunta, Tsutomu Miyamoto, Ryoichi Asaka, Yasushi Yamada, Hirofumi Ando, Shotaro Higuchi, Koichi Ida, Hiroyasu Kashima, Tanri Shiozawa
Applied Immunohistochemistry & Molecular Morphology.2017; 25(6): 415. CrossRef - SIRT1 suppresses colorectal cancer metastasis by transcriptional repression of miR-15b-5p
Li-Na Sun, Zheng Zhi, Liang-Yan Chen, Qun Zhou, Xiu-Ming Li, Wen-Juan Gan, Shu Chen, Meng Yang, Yao Liu, Tong Shen, Yong Xu, Jian-Ming Li
Cancer Letters.2017; 409: 104. CrossRef - TrpC5 regulates differentiation through the Ca2+/Wnt5a signalling pathway in colorectal cancer
Zhen Chen, Chunlei Tang, Yaodan Zhu, Mingxu Xie, Dongxu He, Qiongxi Pan, Peng Zhang, Dong Hua, Teng Wang, Linfang Jin, Xiaowei Qi, Yifei Zhu, Xiaoqiang Yao, Jian Jin, Xin Ma
Clinical Science.2017; 131(3): 227. CrossRef - Meta-analysis of SIRT1 expression as a prognostic marker for overall survival in gastrointestinal cancer
Shuangjie Wu, Jinghui Jiang, Jun Liu, Xinhai Wang, Yu Gan, Yifan Tang
Oncotarget.2017; 8(37): 62589. CrossRef - Prognostic and clinicopathological significance of SIRT1 expression in NSCLC: a meta-analysis
Yifei Chen, Tao Wang, Wei Wang, Jiahao Hu, Ruiting Li, Shaojun He, Jiong Yang
Oncotarget.2017; 8(37): 62537. CrossRef - The prognostic role of Sirt1 expression in solid malignancies: a meta-analysis
Changwen Wang, Wen Yang, Fang Dong, Yawen Guo, Jie Tan, Shengnan Ruan, Tao Huang
Oncotarget.2017; 8(39): 66343. CrossRef - SIRT1 induces tumor invasion by targeting epithelial mesenchymal transition-related pathway and is a prognostic marker in triple negative breast cancer
Min-Sun Jin, Chang Lim Hyun, In Ae Park, Ji Young Kim, Yul Ri Chung, Seock-Ah Im, Kyung-Hun Lee, Hyeong-Gon Moon, Han Suk Ryu
Tumor Biology.2016; 37(4): 4743. CrossRef - Survivin and SIRT1: can be two prognostic factors in chronic myeloid leukemia?
Fatemeh Salari, Javad Mohammdai-asl, Amal Saki Malehi, Ahmad Ahmadzadeh, Mohammad Ali Jalali far, Zari Tahannejad Asadi, Najmaldin Saki
Comparative Clinical Pathology.2016; 25(2): 415. CrossRef - Clinicopathological significance of SIRT1 expression in colorectal cancer: A systematic review and meta analysis
Guo Zu, Anlong Ji, Tingting Zhou, Ningwei Che
International Journal of Surgery.2016; 26: 32. CrossRef - The small molecule survivin inhibitor YM155 may be an effective treatment modality for colon cancer through increasing apoptosis
Wan Lu Li, Mi-Ra Lee, Mee-Yon Cho
Biochemical and Biophysical Research Communications.2016; 471(2): 309. CrossRef - Nuclear expression and/or reduced membranous expression of β-catenin correlate with poor prognosis in colorectal carcinoma
Shizhen Zhang, Zhen Wang, Jinlan Shan, Xiuyan Yu, Ling Li, Rui Lei, Daozhe Lin, Siqi Guan, Xiaochen Wang
Medicine.2016; 95(49): e5546. CrossRef - Association of SIRT1 and HMGA1 expression in non-small cell lung cancer
SHUANG-YAN LIN, FANG PENG
Oncology Letters.2016; 11(1): 782. CrossRef - SIRT1 is a regulator of autophagy: Implications in gastric cancer progression and treatment
Guanglin Qiu, Xuqi Li, Xiangming Che, Chao Wei, Shicai He, Jing Lu, Zongliang Jia, Ke Pang, Lin Fan
FEBS Letters.2015; 589(16): 2034. CrossRef - Stromal expression of miR-21 in T3-4a colorectal cancer is an independent predictor of early tumor relapse
Won Kyung Kang, Jin Kwon Lee, Seong Taek Oh, Sung Hak Lee, Chan Kwon Jung
BMC Gastroenterology.2015;[Epub] CrossRef - Association of SIRT1 and tumor suppressor gene TAp63 expression in head and neck squamous cell carcinoma
Keiji Kikuchi, Akira Noguchi, Rika Kasajima, Yohei Miyagi, Daisuke Hoshino, Naohiko Koshikawa, Akira Kubota, Tomoyuki Yokose, Yasuo Takano
Tumor Biology.2015; 36(10): 7865. CrossRef - Differential expressions of cancer-associated genes and their regulatory miRNAs in colorectal carcinoma
Murat Kara, Onder Yumrutas, Onder Ozcan, Ozgur Ilhan Celik, Esra Bozgeyik, Ibrahim Bozgeyik, Sener Tasdemir
Gene.2015; 567(1): 81. CrossRef - Distinctive role of SIRT1 expression on tumor invasion and metastasis in breast cancer by molecular subtype
Yul Ri Chung, Hyojin Kim, Soo Young Park, In Ae Park, Ja June Jang, Ji-Young Choe, Yoon Yang Jung, Seock-Ah Im, Hyeong-Gon Moon, Kyung-Hun Lee, Koung Jin Suh, Tae-Yong Kim, Dong-Young Noh, Wonshik Han, Han Suk Ryu
Human Pathology.2015; 46(7): 1027. CrossRef - Expression of ROR1, pAkt, and pCREB in gastric adenocarcinoma
Hyeyoon Chang, Woon Yong Jung, Youngran Kang, Hyunjoo Lee, Aeree Kim, Baek-hui Kim
Annals of Diagnostic Pathology.2015; 19(5): 330. CrossRef - miR-34a inhibits cell proliferation in prostate cancer by downregulation of SIRT1 expression
KUN DUAN, YONG-CHAO GE, XUE-PEI ZHANG, SHU-YI WU, JIN-SHUN FENG, SHI-LIN CHEN, LI ZHANG, ZHI-HAO YUAN, CHAO-HONG FU
Oncology Letters.2015; 10(5): 3223. CrossRef - Immunohistochemical Characterization of Large Intestinal Adenocarcinoma in the Rhesus Macaque (Macaca mulatta)
C. E. Harbison, F. Taheri, H. Knight, A. D. Miller
Veterinary Pathology.2015; 52(4): 732. CrossRef - Correlation and prognostic value of SIRT1 and Notch1 signaling in breast cancer
Yu-Wen Cao, Wen-Qin Li, Guo-Xing Wan, Yi-Xiao Li, Xiao-Ming Du, Yu-Cong Li, Feng Li
Journal of Experimental & Clinical Cancer Research.2014;[Epub] CrossRef - Fentanyl Increases Colorectal Carcinoma Cell Apoptosis by Inhibition of NF-κB in a Sirt1-dependent Manner
Xiu-Lai Zhang, Min-Li Chen, Sheng-Li Zhou
Asian Pacific Journal of Cancer Prevention.2014; 15(22): 10015. CrossRef - Elevated HOXB9 expression promotes differentiation and predicts a favourable outcome in colon adenocarcinoma patients
J Zhan, M Niu, P Wang, X Zhu, S Li, J Song, H He, Y Wang, L Xue, W Fang, H Zhang
British Journal of Cancer.2014; 111(5): 883. CrossRef - Prognostic Factors for Metastatic Colorectal Cancer after First-line Chemotherapy with FOLFOX-4 or FOLFIRI Regimen
Jae Hyun Kim, Pyoung Rak Choi, Seun Ja Park, Moo In Park, Won Moon, Sung Eun Kim, Gyu Won Lee
The Korean Journal of Gastroenterology.2014; 63(4): 209. CrossRef - Down-Regulation of mir-221 and mir-222 Restrain Prostate Cancer Cell Proliferation and Migration That Is Partly Mediated by Activation of SIRT1
Xiao Yang, Yingmei Yang, Rong Gan, Lingxu Zhao, Wei Li, Huaibin Zhou, Xiaojuan Wang, Jianxin Lu, Qing H. Meng, George Calin
PLoS ONE.2014; 9(6): e98833. CrossRef
- Ocular surface squamous neoplasia: Update on genetics, epigenetics and opportunities for targeted therapy
- The New 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification of Lung Adenocarcinoma in Resected Specimens: Clinicopathologic Relevance and Emerging Issues
- Seung Yeon Ha, Mee Sook Roh
- Korean J Pathol. 2013;47(4):316-325. Published online August 26, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.4.316
- 12,024 View
- 78 Download
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF Pathologists play an increasingly important role in personalized medicine for patients with lung cancer as a result of the newly recognized relationship between histologic classification and molecular change. In 2011, the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) proposed a new architectural classification for invasive lung adenocarcinomas to provide uniform terminology and diagnostic criteria. This review highlighted the evolution of the classification of lung adenocarcinomas in resected specimens with special respect to both histologic subtyping and invasion. Histologic subtyping of lung adenocarcinoma has been updated based on five major predominant patterns. New concepts of adenocarcinoma
in situ and minimally invasive adenocarcinomas have been introduced to define the condition of patients who are expected to have excellent survival. Although the new IASLC/ATS/ERS classification has promising clinical relevance, significant clarification remains necessary for the definitions of subtyping and invasion. More precise definitions and subsequent better education on the interpretation of terminology will be helpful for future studies.-
Citations
Citations to this article as recorded by- Radiomics for Classifying Histological Subtypes of Lung Cancer Based on Multiphasic Contrast-Enhanced Computed Tomography
Linning E, Lin Lu, Li Li, Hao Yang, Lawrence H. Schwartz, Binsheng Zhao
Journal of Computer Assisted Tomography.2019; 43(2): 300. CrossRef - Tumor heterogeneity assessed by texture analysis on contrast-enhanced CT in lung adenocarcinoma: association with pathologic grade
Ying Liu, Shichang Liu, Fangyuan Qu, Qian Li, Runfen Cheng, Zhaoxiang Ye
Oncotarget.2017; 8(32): 53664. CrossRef - Clinicopathological and prognostic significance of metastasis-associated protein 1 expression and its correlation with angiogenesis in lung invasive adenocarcinomas, based on the 2011 IASLC/ATS/ERS classification
SHUHAI LI, HUI TIAN, WEIMING YUE, LIN LI, CUN GAO, LIBO SI, WENSI HU, LEI QI, MING LU, CHUANLE CHENG, JINGJING CUI, GUANQING CHEN
Oncology Letters.2016; 11(1): 224. CrossRef - Myoferlin expression in non-small cell lung cancer: Prognostic role and correlation with VEGFR-2 expression
DAE HYUN SONG, GYUNG HYUCK KO, JEONG HEE LEE, JONG SIL LEE, GYEONG-WON LEE, HYEON CHEOL KIM, JUNG WOOK YANG, ROK WON HEO, GU SEOB ROH, SUN-YOUNG HAN, DONG CHUL KIM
Oncology Letters.2016; 11(2): 998. CrossRef - ROS1 gene rearrangement and copy number gain in non-small cell lung cancer
Yan Jin, Ping-Li Sun, Hyojin Kim, Eunhyang Park, Hyo Sup Shim, Sanghoon Jheon, Kwhanmien Kim, Choon-Taek Lee, Jin-Haeng Chung
Virchows Archiv.2015; 466(1): 45. CrossRef - The Demise of the Term Bronchioloalveolar Carcinoma
Yasmeen M. Butt, Timothy Craig Allen
Archives of Pathology & Laboratory Medicine.2015; 139(8): 981. CrossRef - Expression of EGFR and Molecules Downstream to PI3K/Akt, Raf-1-MEK-1-MAP (Erk1/2), and JAK (STAT3) Pathways in Invasive Lung Adenocarcinomas Resected at a Single Institution
Alba Fabiola Torres, Cleto Nogueira, Juliana Magalhaes, Igor Santos Costa, Alessa Aragao, Antero Gomes Neto, Filadelfia Martins, Fabio Tavora
Analytical Cellular Pathology.2014; 2014: 1. CrossRef - Usual Interstitial Pneumonia with Lung Cancer: Clinicopathological Analysis of 43 Cases
Dae Hyun Song, In Ho Choi, Sang Yun Ha, Kang Min Han, Jae Jun Lee, Min Eui Hong, Kyeongman Jeon, Man Pyo Chung, Jhingook Kim, Joungho Han
Korean Journal of Pathology.2014; 48(1): 10. CrossRef - Cytoplasmic YAP Expression is Associated with Prolonged Survival in Patients with Lung Adenocarcinomas and Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Treatment
Ping-Li Sun, Ji Eun Kim, Seol Bong Yoo, Hyojin Kim, Yan Jin, Sanghoon Jheon, Kwhanmien Kim, Choon Taek Lee, Jin-Haeng Chung
Annals of Surgical Oncology.2014; 21(S4): 610. CrossRef
- Radiomics for Classifying Histological Subtypes of Lung Cancer Based on Multiphasic Contrast-Enhanced Computed Tomography
- Colonic Adenocarcinoma Arising from Gastric Heterotopia: A Case Study
- Hyoungsuk Ko, Shin Young Park, Eun Jung Cha, Jang Sihn Sohn
- Korean J Pathol. 2013;47(3):289-292. Published online June 25, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.3.289
- 9,900 View
- 53 Download
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF Heterotopic gastric mucosa occurs in all areas of the gastrointestinal tract including the nasopharynx, tongue, esophagus, small intestine, colon, and rectum. Gastric heterotopia of the large bowel is infrequent, and most cases have been reported in the rectum. Review of the literature has revealed only eight cases involving the colon proximal to the rectum. Little is known of the natural history of gastric heterotopias, except that. It usually presents with gastrointestinal bleeding, though other serious complications such as bowel perforation, intussusceptions, and fistula formation, are possible. Further, it is unclear whether heterotopic gastric mucosa progresses to malignancy. Herein, we describe a case of adenocarcinoma of the transverse colon arising from gastric heterotopia. To the best of our knowledge, this is the first report of adenocarcinoma arising from heterotopic gastric mucosa in the colon.
-
Citations
Citations to this article as recorded by- Anorectal gastric heterotopia as a rare cause of constipation: Case report and review of pediatric literature
Kathryn M. Stephenson, Raj P. Kapur, Jeffrey R. Avansino, Lusine Ambartsumyan
JPGN Reports.2025; 6(4): 407. CrossRef - Intussusception of Heterotopic Gastric Mucosa in the Transverse Colon: A Rare Cause of Perforation and Bleeding
Sho Fujiwara, Ryuichi Nishimura, Nozomi Koyamada
Cureus.2024;[Epub] CrossRef - Gastric heterotopia of colon found cancer workup in liver abscess: A case report
Jun Gi Park, Jeong Ill Suh, Yeo Un Kim
World Journal of Clinical Cases.2022; 10(15): 5012. CrossRef - Gastric heterotopia in the ileum mimicking Meckel's diverticulum
Reza Shojaeian, Negar Nekooei, Paria Dehghanian
Journal of Pediatric Surgery Case Reports.2022; 84: 102361. CrossRef - Sometimes Things Are Not Where They Are Supposed to Be: A Case Report of Gastric Heterotopia in the Rectum
Asher Lippe, Scott Lippe
Physician's Journal of Medicine.2022;[Epub] CrossRef - Gastric heterotopia of the rectum
Eduardo Dantas, Diva Yamaguti, Kendi Yamazaki
Gastroenterología y Hepatología.2021; 44(8): 579. CrossRef - Bleeding Gastric Heterotopia of Cecal Diverticulum in an Adolescent: A Case Report
Hyun-Il Seo, Jae-Young Kwak
Advances in Pediatric Surgery.2021; 27(1): 32. CrossRef - Gastric heterotopia of the rectum
Eduardo Dantas, Diva Yamaguti, Kendi Yamazaki
Gastroenterología y Hepatología (English Edition).2021; 44(8): 579. CrossRef - Polypoid Gastric Heterotopia of Colon
Marcela Adriana Duran Alvarez, Carla Noemi Tafur Sanchez
GE - Portuguese Journal of Gastroenterology.2020; 27(1): 65. CrossRef - Heterotopic Respiratory Mucosa in the Rectum: An Unusual Type and Site of Heterotopia in the Gastrointestinal Tract
Caroline Bsirini, Pratyusha Tirumanisetty, Joseph N. Dytoc, Diana Agostini-Vulaj, Christopher Steevens, Asad Ullah, Aaron R. Huber
International Journal of Surgical Pathology.2019; 27(2): 221. CrossRef - Perforation of Heterotopic Gastric Mucosa in ileal duplication in an adult: A case report
Vaanathi Paulvannan, Seshukumar Bylapudi, Mithun Kumar Ramesh Kumar, Mahesh Nachimuthu, Paulvannan Subramanian
Journal of Surgical Case Reports.2019;[Epub] CrossRef - Mixed adenoneuroendocrine carcinoma of the tongue arising within a congenital enteric cyst
Louis J. Ligthelm, Belinda K. Bunn, Erich J. Raubenheimer, Willie F. P. van Heerden
Head & Neck.2018;[Epub] CrossRef - The outlet patch: gastric heterotopia of the colorectum and anus
Abul A S R Mannan, Michael Vieth, Armen Khararjian, Binny Khandakar, Dora Lam‐Himlin, David Heydt, Feriyl Bhaijee, Henry J Venbrux, Kathleen Byrnes, Lysandra Voltaggio, Norman Barker, Songyang Yuan, Elizabeth A Montgomery
Histopathology.2018; 73(2): 220. CrossRef - Large heterotopic gastric mucosa and a concomitant diverticulum in the rectum: Clinical experience and endoscopic management
Wen-Guo Chen, Hua-Tuo Zhu, Ming Yang, Guo-Qiang Xu, Li-Hua Chen, Hong-Tan Chen
World Journal of Gastroenterology.2018; 24(30): 3462. CrossRef - Gastric heterotopia in the rectum. A rare cause of ectopic gastric tissue
George A. Salem, Javid Fazili, Tauseef Ali
Arab Journal of Gastroenterology.2017; 18(1): 42. CrossRef - Gastric heterotopia in rectum: A literature review and its diagnostic pitfall
Peyman Dinarvand, Ashley A. Vareedayah, Nancy J Phillips, Christine Hachem, Jinping Lai
SAGE Open Medical Case Reports.2017;[Epub] CrossRef - Heterotopic gastric mucosa in the anus and rectum: first case report of endoscopic submucosal dissection and systematic review
Federico Iacopini, Takuji Gotoda, Walter Elisei, Patrizia Rigato, Fabrizio Montagnese, Yutaka Saito, Guido Costamagna, Giampaolo Iacopini
Gastroenterology Report.2016; 4(3): 196. CrossRef
- Anorectal gastric heterotopia as a rare cause of constipation: Case report and review of pediatric literature
- Aspiration Cytopathology of Peripancreatic Space: A Clinicoradiologic and Cytopathologic Analyses of 42 Cases
- Justin Bishop, Wei Zhang, Olga B. Ioffe, Syed Z. Ali
- Korean J Pathol. 2013;47(3):258-264. Published online June 25, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.3.258
- 9,563 View
- 41 Download
-
 Abstract
Abstract
 PDF
PDF Background The pancreas is surrounded by soft tissue known as the peripancreatic space (PPS). Pathologic lesions of the PPS are infrequent and have only rarely been reported in the cytopathology literature.
Methods A retrospective review of cytopathology files at two large institutions revealed 42 cases of PPS lesions obtained by transabdominal fine needle aspiration (FNA) or endoscopic ultrasound-guided FNA over a 16-year period. Clinicoradiologic findings and follow-up information were also reviewed.
Results Patients ranged in age from 23-83 years (mean, 60 years) with an equal gender distribution. The major clinical presentations included pain, jaundice, nausea/vomiting, and abnormal liver enzymes. Radiographic characteristics included lymphadenopathy and cystic/solid soft tissue masses with a size range of 1.5 to 8 cm. Cytologically, 4 (9.5%) cases were nondiagnostic, 9 (21.5%) were diagnosed as benign, 4 (9.5%) were atypical or suspicious for cancer, and 25 (59.5%) were malignant. Six of 25 (24%) patients had metastasis of a prior known malignancy.
Conclusions FNA of PPS masses is a rare occurrence. The majority of lesions are metastatic carcinomas from a variety of primary sites. Flow cytometry and immunoperoxidase studies are useful adjuncts to determine the tumor origin. The sensitivity of PPS aspiration for a malignant diagnosis is 90% with a positive predictive value of 100%.
- Cytologic Features of
ALK -Positive Pulmonary Adenocarcinoma - Seung Yeon Ha, Jungsuk Ahn, Mee Sook Roh, Joungho Han, Jae Jun Lee, Boin Lee, Jun Yim
- Korean J Pathol. 2013;47(3):252-257. Published online June 25, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.3.252
- 9,870 View
- 33 Download
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF Background The aim of this study was to determine the cytologic features of anaplastic lymphoma kinase (
ALK ) expressing pulmonary adenocarcinoma.Methods We analyzed the cytopathological findings of 15 cases of endobronchial ultrasound guided aspiration and a case of bronchial washing. These cases were selected based on the histomorphology of
ALK -rearranged lung adenocarcinoma.Results Cytology showed mucinous (81.3%) and hemorrhagic (50%) backgrounds. The cells were arranged in tubulopapillary or tubulocribriform patterns (93.8%), and clusters (56.3%) admixed with signet ring cell features (87.5%). The tumor cells were monotonous and uniform with vesicular nuclei and a small nucleolus.
Conclusions The characteristic findings were sheets showing a tubulopapillary or tubulocribriform appearance, with vesicular nuclei and a bland chromatin pattern (p<0.001). Scattered signet ring cells were helpful in suggesting
ALK -positive adenocarcinoma (p<0.001).-
Citations
Citations to this article as recorded by- Cytomorphological and histomorphological features of lung adenocarcinoma with epidermal growth factor receptor mutation and anaplastic lymphoma kinase gene rearrangement
Nikola Gardić, Aleksandra Lovrenski, Vanesa Sekeruš, Svetlana Lečić, Milorad Bijelović, Tanja Lakić, Aleksandra Ilić, Bojan Zarić, Sofija Glumac
Oncology Letters.2024;[Epub] CrossRef - Machine learning‐based gene alteration prediction model for primary lung cancer using cytologic images
Shuhei Ishii, Manabu Takamatsu, Hironori Ninomiya, Kentaro Inamura, Takeshi Horai, Akira Iyoda, Naoko Honma, Rira Hoshi, Yuko Sugiyama, Noriko Yanagitani, Mingyon Mun, Hitoshi Abe, Tetuo Mikami, Kengo Takeuchi
Cancer Cytopathology.2022; 130(10): 812. CrossRef - Fine‐needle aspiration cytology of non‐small cell lung carcinoma: A paradigm shift
Pranab Dey, Ratan Kumar Ghosh
Diagnostic Cytopathology.2019; 47(4): 351. CrossRef - Qualitative and quantitative cytomorphological features of primary anaplastic lymphoma kinase‐positive lung cancer
Ryuko Tsukamoto, Hiroyuki Ohsaki, Sho Hosokawa, Yasunori Tokuhara, Shingo Kamoshida, Toshiko Sakuma, Tomoo Itoh, Chiho Ohbayashi
Cytopathology.2019; 30(3): 295. CrossRef - Primary signet-ring adenocarcinoma of the lung: A rare lung tumor
Varun Rajpal, Rahul Kumar Sharma, Charul Dabral, Deepak Talwar
South Asian Journal of Cancer.2019; 08(04): 257. CrossRef - Cytological features in eight patients with ALK‐rearranged lung cancer
Naoto Kuroda, Masahiko Ohara, Yukari Wada, Kaori Yasuoka, Keiko Mizuno, Kenji Yorita, Chiho Obayashi, Kengo Takeuchi
Diagnostic Cytopathology.2018; 46(6): 516. CrossRef - Cytological markers for predicting ALK‐positive pulmonary adenocarcinoma
K. Miyata, S. Morita, H. Dejima, N. Seki, N. Matsutani, M. Mieno, F. Kondo, Y. Soejima, F. Tanaka, M. Sawabe
Diagnostic Cytopathology.2017; 45(11): 963. CrossRef - ALK-rearranged adenocarcinoma with extensive mucin production can mimic mucinous adenocarcinoma: clinicopathological analysis and comprehensive histological comparison with KRAS-mutated mucinous adenocarcinoma
Yoon Jin Cha, Joungho Han, Soo Hyun Hwang, Tae Bum Lee, Hojoong Kim, Jea Ill Zo
Pathology.2016; 48(4): 325. CrossRef - Cytomorphological identification of advanced pulmonary adenocarcinoma harboring KRAS mutation in lymph node fine‐needle aspiration specimens: Comparative investigation of adenocarcinoma with KRAS and EGFR mutations
Dae Hyun Song, Boram Lee, Yooju Shin, In Ho Choi, Sang Yun Ha, Jae Jun Lee, Min Eui Hong, Yoon‐La Choi, Joungho Han, Sang‐Won Um
Diagnostic Cytopathology.2015; 43(7): 539. CrossRef - Comprehensive analysis of RET and ROS1 rearrangement in lung adenocarcinoma
Seung Eun Lee, Boram Lee, Mineui Hong, Ji-Young Song, Kyungsoo Jung, Maruja E Lira, Mao Mao, Joungho Han, Jhingook Kim, Yoon-La Choi
Modern Pathology.2015; 28(4): 468. CrossRef
- Cytomorphological and histomorphological features of lung adenocarcinoma with epidermal growth factor receptor mutation and anaplastic lymphoma kinase gene rearrangement
- Naked Cuticle Drosophila 1 Expression in Histologic Subtypes of Small Adenocarcinoma of the Lung
- Sangjeong Ahn, Won Hwangbo, Hyunchul Kim, Chul Hwan Kim
- Korean J Pathol. 2013;47(3):211-218. Published online June 25, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.3.211
- 7,982 View
- 28 Download
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF Background Naked cuticle Drosophila 1 (NKD1) has been related to non-small cell lung cancer in that decreased NKD1 levels have been associated with both poor prognosis and increased invasive quality.
Methods Forty cases of lung adenocarcinoma staged as Tis or T1a were selected. Cases were subclassified into adenocarcinoma
in situ (AIS), minimally invasive adenocarcinoma (MIA), and small adenocarcinoma (SAD). Immunohistochemical studies for NKD1 were performed.Results Forty samples comprised five cases of AIS (12.5%), eight of MIA (20.0%), and 27 of SAD (67.5%). AIS and MIA showed no lymph node metastasis and 100% disease-free survival, whereas among 27 patients with SAD, 2 (7.4%) had lymph node metastasis, and 3 (11.1%) died from the disease. Among the 40 cases, NKD1-reduced expression was detected in 8 (20%) samples, whereas normal expression was found in 15 (37.5%) and overexpression in 17 (42.5%). Loss of NKD1 expression was significantly associated with lymph node metastasis (p=0.001). All cases with predominant papillary pattern showed overexpression of NKD1 (p=0.026).
Conclusions Among MIA and SAD, MIA had better outcomes than SAD. Down-regulated NKD1 expression was closely associated with nodal metastasis, and overexpression was associated with papillary predominant adenocarcinoma.
-
Citations
Citations to this article as recorded by- Challenges of the eighth edition of the American Joint Committee on Cancer staging system for pathologists focusing on early stage lung adenocarcinoma
Yu‐Ting Wang, Il‐Chi Chang, Chih‐Yi Chen, Jiun‐Yi Hsia, Frank Cheau‐Feng Lin, Wan‐Ru Chao, Tuan‐Ying Ke, Ya‐Ting Chen, Chih‐Jung Chen, Min‐Shu Hsieh, Shiu‐Feng Huang
Thoracic Cancer.2023; 14(6): 592. CrossRef - Clinical Significance of NKD Inhibitor of WNT Signaling Pathway 1 (NKD1) in Glioblastoma
Lijun Li, Ruiying Gao, Weizhong Huangfu, Fang Zhang, Ruixia Wang, Hongda Liu
Genetics Research.2023; 2023: 1. CrossRef - Naked cuticle homolog 1 prevents mouse pulmonary arterial hypertension via inhibition of Wnt/β-catenin and oxidative stress
Shanwu Wei, Lu Lin, Wen Jiang, Jie Chen, Gu Gong, Daming Sui
Aging.2023;[Epub] CrossRef - MicroRNA‐195‐5p suppresses osteosarcoma cell proliferation and invasion by suppressing naked cuticle homolog 1
Qiang Qu, Xiangdong Chu, Peng Wang
Cell Biology International.2017; 41(3): 287. CrossRef - Downregulation of NKD1 in human osteosarcoma and its clinical significance
Xiang Chen, Ping Xu, Jianwei Zhu, Fan Liu
Molecular Medicine Reports.2017;[Epub] CrossRef - Lung Adenocarcinoma Staging Using the 2011 IASLC/ATS/ERS Classification: A Pooled Analysis of Adenocarcinoma In Situ and Minimally Invasive Adenocarcinoma
Madhusmita Behera, Taofeek K. Owonikoko, Anthony A. Gal, Conor E. Steuer, Sungjin Kim, Rathi N. Pillai, Fadlo R. Khuri, Suresh S. Ramalingam, Gabriel L. Sica
Clinical Lung Cancer.2016; 17(5): e57. CrossRef - The NKD1/Rac1 feedback loop regulates the invasion and migration ability of hepatocarcinoma cells
Jie Li, Sheng Zhang, Qing Hu, Kang Zhang, Jianbin Jin, Xuqing Zheng, Zhenyu Yin, Xiaomin Wang
Scientific Reports.2016;[Epub] CrossRef - NKD1 correlates with a poor prognosis and inhibits cell proliferation by inducing p53 expression in hepatocellular carcinoma
Sheng Zhang, Jie Li, Xiaomin Wang
Tumor Biology.2016; 37(10): 14059. CrossRef - Expression pattern and clinicopathologic significance of NKD1 in human primary hepatocellular carcinoma
Sheng Zhang, Jie Li, Zhen‐Yu Yin, Ping‐Guo Liu, Wen‐Xiu Zhao, Cheng‐Rong Xie, Bi‐Xin Zhao, Xiao‐Min Wang
APMIS.2015; 123(4): 315. CrossRef - Early lung cancer with lepidic pattern
Wilko Weichert, Arne Warth
Current Opinion in Pulmonary Medicine.2014; 20(4): 309. CrossRef - Altered Expression of PTEN and Its Major Regulator MicroRNA-21 in Pulmonary Neuroendocrine Tumors
Hyoun Wook Lee, Seung Yeon Ha, Mee Sook Roh
Korean Journal of Pathology.2014; 48(1): 17. CrossRef - The New 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification of Lung Adenocarcinoma in Resected Specimens: Clinicopathologic Relevance and Emerging Issues
Seung Yeon Ha, Mee Sook Roh
Korean Journal of Pathology.2013; 47(4): 316. CrossRef
- Challenges of the eighth edition of the American Joint Committee on Cancer staging system for pathologists focusing on early stage lung adenocarcinoma
- Microsatellite Instability Status in Gastric Cancer: A Reappraisal of Its Clinical Significance and Relationship with Mucin Phenotypes
- Joo-Yeun Kim, Na Ri Shin, Ahrong Kim, Hyun-Jeong Lee, Won-young Park, Jee-Yeon Kim, Chang-Hun Lee, Gi-Young Huh, Do Youn Park
- Korean J Pathol. 2013;47(1):28-35. Published online February 25, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.1.28
- 12,535 View
- 126 Download
- 76 Crossref
-
 Abstract
Abstract
 PDF
PDF Background Gastric cancers with microsatellite instabilities (MSI) have been reported to be associated with favorable prognosis. However, the significance of the effect of MSI on the clinicopathological features, as well as its association with mucin phenotype, remains unclear.
Methods MSI status was assessed in 414 cases of gastric cancer using polymerase chain reaction analysis of five microsatellite loci, as recommended by National Cancer Institution criteria. The expression of mucins (MUC5AC, MUC6, MUC2, and CD10) was assessed.
Results Out of 414 total cases of gastric cancer, 380 (91.7%), 11 (2.7%), and 23 (5.6%) were microsatellite stable (MSS), low-level MSI (MSI-L), and high-level MSI (MSI-H), respectively. Compared to MSS/MSI-L, MSI-H gastric cancers were associated with older age (p=0.010), tumor size (p=0.014), excavated gross (p=0.042), intestinal type (p=0.028), aggressive behaviors (increase of T stage [p=0.009]), perineural invasion [p=0.022], and lymphovascular emboli [p=0.027]). MSI-H gastric cancers were associated with tumor necrosis (p=0.041), tumor-infiltrating lymphocytes (≥2/high power field, p<0.001), expanding growth patterns (p=0.038), gastric predominant mucin phenotypes (p=0.028), and MUC6 expression (p=0.016). Tumor necrosis (≥10% of mass, p=0.031), tumor-infiltrating lymphocytes (p<0.001), intestinal type (p=0.014), and gastric mucin phenotypes (p=0.020) could represent independent features associated with MSI-H gastric cancers. MSI-H intestinal type gastric cancers had a tendency for poor prognosis in univariate analysis (p=0.054) but no association in Cox multivariate analysis (p=0.197).
Conclusions Our data suggest that MSI-H gastric cancers exhibit distinct aggressive biologic behaviors and a gastric mucin phenotype. This contradicts previous reports that describe MSI-H gastric cancer as being associated with favorable prognosis.
-
Citations
Citations to this article as recorded by- Intestinal Subtype as a Biomarker of Response to Neoadjuvant Immunochemotherapy in Locally Advanced Gastric Adenocarcinoma: Insights from a Prospective Phase II Trial
Lei Wang, Mengting Sun, Jinyang Li, Linghong Wan, Yuting Tan, Shuoran Tian, Yongying Hou, Linyu Wu, Ziyi Peng, Xiao Hu, Qihua Zhang, Zening Huang, Mengyi Han, Shiyin Peng, Yuwei Pan, Yuanfeng Ren, Mengsi Zhang, Dongfeng Chen, Qin Liu, Xianfeng Li, Zhong-y
Clinical Cancer Research.2025; 31(1): 74. CrossRef - How do I treat dMMR/MSI gastro-oesophageal adenocarcinoma in 2025? A position paper from the EORTC-GITCG gastro-esophageal task force
Christelle de la Fouchardière, Antonella Cammarota, Magali Svrcek, Maria Alsina, Tania Fleitas-Kanonnikoff, Radka Lordick Obermannová, Anna Dorothea Wagner, Dominic Yap Wei Ting, Diana Enea, Angelica Petrillo, Elizabeth C. Smyth
Cancer Treatment Reviews.2025; 134: 102890. CrossRef - T-bet+CD8+ T cells govern anti-PD-1 responses in microsatellite-stable gastric cancers
Shiying Tang, Xiaofang Che, Jinyan Wang, Ce Li, Xin He, Kezuo Hou, Xiaojie Zhang, Jia Guo, Bowen Yang, Danni Li, Lili Cao, Xiujuan Qu, Zhenning Wang, Yunpeng Liu
Nature Communications.2025;[Epub] CrossRef - Prediction of a Panel of Programmed Cell Death Protein-1 (PD-1) Inhibitor–Sensitive Biomarkers Using Multiphase Computed Tomography Imaging Textural Features: Retrospective Cohort Analysis
Shiqi Wang, Na Chai, Jingji Xu, Pengfei Yu, Luguang Huang, Quan Wang, Zhifeng Zhao, Bin Yang, Jiangpeng Wei, Xiangjie Wang, Gang Ji, Minwen Zheng
JMIR Cancer.2025; 11: e67379. CrossRef - Isolated tumor cell clusters (ITC) in lymph nodes and PD-L1 expression on tumor-associated immune cells are prognostic factors for microsatellite instable-high gastric cancers
Menghan Cui, Yangli Zhou, Yin Han, Nannan Chen, Min Zhao, Yan Wang, Fengxia He
Translational Oncology.2025; 59: 102465. CrossRef - Microsatellite Instability and BAT-26 Marker Expression in a Mexican Prostate Cancer Population with Different Gleason Scores
Ana K. Flores-Islas, Manuel A. Rico-Méndez, Marisol Godínez-Rubí, Martha Arisbeth Villanueva-Pérez, Erick Sierra-Díaz, Ana Laura Pereira-Suárez, Saul A. Beltrán-Ontiveros, Perla Y. Gutiérrez-Arzapalo, José M. Moreno-Ortiz, Adrián Ramírez-de-Arellano
Diseases.2025; 13(7): 202. CrossRef - Prognostic implications of ERBB2 amplification and mismatch repair in gastric adenocarcinoma: a single-center study
Han Xia, Zekang Li, Minyi Wang, Dachuan Zhang, Xiao Zheng, Jun Wu, Chen Wu
Gastrointestinal Tumors.2025;[Epub] CrossRef - Microsatellite Instability in Chronic Gastritis Associated with Gastric Cancer
A. V. Kononov, V. A. Rubtsov, E. V. Demidova, M. N. Parygina, A. G. Shimanskaya, S. I. Mozgovoi, E. G. Pomorgailo, М. V. Markelova
Russian Journal of Gastroenterology, Hepatology, Coloproctology.2025; 35(4): 48. CrossRef - Non–Pure Intestinal Phenotype as an Indicator of Progression in Sporadic Nonampullary Duodenal Adenomas: A Multicenter Retrospective Cohort Study
Ryotaro Uema, Yoshito Hayashi, Masato Komori, Narihiro Shibukawa, Noriko Hayashi, Masayoshi Horimoto, Takuya Yamada, Masashi Yamamoto, Satoshi Hiyama, Kazuo Kinoshita, Hideharu Ogiyama, Shinjiro Yamaguchi, Satoshi Egawa, Takashi Kanesaka, Minoru Kato, Shu
Clinical and Translational Gastroenterology.2024; 15(1): e00649. CrossRef - Intratumoral and peritumoral CT-based radiomics for predicting the microsatellite instability in gastric cancer
Xingchi Chen, Zijian Zhuang, Lin Pen, Jing Xue, Haitao Zhu, Lirong Zhang, Dongqing Wang
Abdominal Radiology.2024; 49(5): 1363. CrossRef - The tumor immune composition of mismatch repair deficient and Epstein-Barr virus-positive gastric cancer: A systematic review
J. Bos, T.S. Groen-van Schooten, C.P. Brugman, F.S. Jamaludin, H.W.M. van Laarhoven, S. Derks
Cancer Treatment Reviews.2024; 127: 102737. CrossRef - Potent therapeutic strategy in gastric cancer with microsatellite instability-high and/or deficient mismatch repair
Akira Ooki, Hiroki Osumi, Koichiro Yoshino, Kensei Yamaguchi
Gastric Cancer.2024; 27(5): 907. CrossRef - The mechanism of RGS5 regulating gastric cancer mismatch repair protein
Zhenwei Yang, Ranran Zhang, Jialong Liu, Sufang Tian, Hailin Zhang, Lingxiu Zeng, Yangyang Zhang, Liping Gao, Meng Wang, Wenqing Shan, Jing Liu
Molecular Carcinogenesis.2024; 63(9): 1750. CrossRef - Prognostic significance of microsatellite instability in patients with resectable gastric cancer
Marina Alessandra Pereira, Marcus Fernando Kodama Pertille Ramos, Leonardo Cardili, André Roncon Dias, Venancio Avancini Ferreira Alves, Evandro Sobroza de Mello, Ulysses Ribeiro
Journal of Gastrointestinal Surgery.2024; 28(10): 1687. CrossRef - Access to radiotherapy in improving gastric cancer care quality and equality
Minmin Wang, Kepei Huang, Xiaohan Fan, Jia Wang, Yinzi Jin, Zhi-Jie Zheng
Communications Medicine.2024;[Epub] CrossRef - Deep learning captures selective features for discrimination of microsatellite instability from pathologic tissue slides of gastric cancer
Sung Hak Lee, Yujin Lee, Hyun‐Jong Jang
International Journal of Cancer.2023; 152(2): 298. CrossRef - Novel Biomarkers of Gastric Cancer: Current Research and Future Perspectives
Yasushi Sato, Koichi Okamoto, Yutaka Kawano, Akinari Kasai, Tomoyuki Kawaguchi, Tamotsu Sagawa, Masahiro Sogabe, Hiroshi Miyamoto, Tetsuji Takayama
Journal of Clinical Medicine.2023; 12(14): 4646. CrossRef - The results of treatment for resectable gastric cancer with microsatellite instability
H. Sun, S. N. Nered, A. A. Tryakin, E. V. Artamonova, A. E. Kalinin, V. E. Bugaev, A. M. Stroganova, N. S. Besova, P. P. Arkhiri, V. I. Marshall, R. Sh. Abdulaeva, I. S. Stilidi
Pelvic Surgery and Oncology.2023; 13(2): 17. CrossRef - Heterogeneity and Adjuvant Therapeutic Approaches in MSI-H/dMMR Resectable Gastric Cancer: Emerging Trends in Immunotherapy
Hui Wu, Wenyuan Ma, Congfa Jiang, Ning Li, Xin Xu, Yongfeng Ding, Haiping Jiang
Annals of Surgical Oncology.2023; 30(13): 8572. CrossRef - Dual-layer spectral-detector CT for predicting microsatellite instability status and prognosis in locally advanced gastric cancer
Yongjian Zhu, Peng Wang, Bingzhi Wang, Zhichao Jiang, Ying Li, Jun Jiang, Yuxin Zhong, Liyan Xue, Liming Jiang
Insights into Imaging.2023;[Epub] CrossRef - Concordance between microsatellite instability testing and immunohistochemistry for mismatch repair proteins and efficient screening of mismatch repair deficient gastric cancer
Gou Yamamoto, Tetsuya Ito, Okihide Suzuki, Nao Kamae, Miho Kakuta, Akemi Takahashi, Katsuya Iuchi, Tomio Arai, Hideyuki Ishida, Kiwamu Akagi
Oncology Letters.2023;[Epub] CrossRef - Low incidence of microsatellite instability in gastric cancers and its association with the clinicopathological characteristics: a comparative study
Fateme Fooladi Talari, Ali Bozorg, Sirous Zeinali, Mohammadreza Zali, Zhale Mohsenifar, Hamid Asadzadeh Aghdaei, Kaveh Baghaei
Scientific Reports.2023;[Epub] CrossRef - Mutational separation and clinical outcomes of TP53 and CDH1 in gastric cancer
He-Li Liu, Huan Peng, Chang-Hao Huang, Hai-Yan Zhou, Jie Ge
World Journal of Gastrointestinal Surgery.2023; 15(12): 2855. CrossRef - Genomic and Immunologic Markers of Intrinsic Resistance to Pembrolizumab Monotherapy in Microsatellite Instability-High Gastric Cancer: Observations from a Prospective Phase II Study
Haibo Qiu
Global Medical Genetics.2022; 09(02): 060. CrossRef - Clinicopathological features of PD-L1 protein expression, EBV positivity, and MSI status in patients with advanced gastric and esophagogastric junction adenocarcinoma in Japan
Tsutomu Yoshida, Go Ogura, Mikiko Tanabe, Takuo Hayashi, Chiho Ohbayashi, Mizutomo Azuma, Chikara Kunisaki, Yoichi Akazawa, Soji Ozawa, Sohei Matsumoto, Takayoshi Suzuki, Akira Mitoro, Tetsu Fukunaga, Akiko Shimizu, Go Fujimoto, Takashi Yao
Cancer Biology & Therapy.2022; 23(1): 191. CrossRef - Development of Tissue-Agnostic Treatments for Patients with Cancer
Steven Lemery, Lola Fashoyin-Aje, Leigh Marcus, Sandra Casak, Julie Schneider, Marc Theoret, Paul Kluetz, Richard Pazdur, Julia A. Beaver
Annual Review of Cancer Biology.2022; 6(1): 147. CrossRef - A multicenter study on the preoperative prediction of gastric cancer microsatellite instability status based on computed tomography radiomics
Xiuqun Liang, Yinbo Wu, Ying Liu, Danping Yu, Chencui Huang, Zhi Li
Abdominal Radiology.2022; 47(6): 2036. CrossRef - Combination of AKT1 and CDH1 mutations predicts primary resistance to immunotherapy in dMMR/MSI-H gastrointestinal cancer
Zhenghang Wang, Qi Zhang, Changsong Qi, Yuezong Bai, Feilong Zhao, Hui Chen, Zhongwu Li, Xicheng Wang, Mifen Chen, Jifang Gong, Zhi Peng, Xiaotian Zhang, Jinping Cai, Shiqing Chen, Xiaochen Zhao, Lin Shen, Jian Li
Journal for ImmunoTherapy of Cancer.2022; 10(6): e004703. CrossRef - Eldest gastric cancer patient with high microsatellite instability responding to pembrolizumab
Akinobu Wakasugi, Akinori Sasaki, Risa Okamoto, Yasuaki Motomura
International Cancer Conference Journal.2022; 12(1): 59. CrossRef - Baseline lesion number as an efficacy predictive and independent prognostic factor and its joint utility with TMB for PD-1 inhibitor treatment in advanced gastric cancer
Xiao-Li Wei, Jian-Ying Xu, De-Shen Wang, Dong-Liang Chen, Chao Ren, Jia-Ning Li, Feng Wang, Feng-Hua Wang, Rui-Hua Xu
Therapeutic Advances in Medical Oncology.2021;[Epub] CrossRef - Clinical and morphological portrait of tumors with microsatellite instability
A. A. Musaelyan, V. D. Nazarov, A. S. Budnikova, S. V. Lapin, S. L. Vorobyev, V. L. Emanuel, A. A. Zakharenko, S. V. Orlov
Advances in Molecular Oncology.2021; 8(2): 52. CrossRef - How to Best Exploit Immunotherapeutics in Advanced Gastric Cancer: Between Biomarkers and Novel Cell-Based Approaches
Michele Ghidini, Angelica Petrillo, Andrea Botticelli, Dario Trapani, Alessandro Parisi, Anna La Salvia, Elham Sajjadi, Roberto Piciotti, Nicola Fusco, Shelize Khakoo
Journal of Clinical Medicine.2021; 10(7): 1412. CrossRef - Microsatellite instability in Gastric Cancer: Between lights and shadows
Elisabetta Puliga, Simona Corso, Filippo Pietrantonio, Silvia Giordano
Cancer Treatment Reviews.2021; 95: 102175. CrossRef - Impact of microsatellite status on negative lymph node count and prognostic relevance after curative gastrectomy
Zhenghao Cai, Junjun Ma, Shuchun Li, Abe Fingerhut, Jing Sun, Lu Zang, Chao Yan, Wentao Liu, Zhenggang Zhu, Minhua Zheng
Journal of Surgical Oncology.2021;[Epub] CrossRef - A greater lymph node yield is required during pathological examination in microsatellite instability-high gastric cancer
Zhenghao Cai, Haiqin Song, Abe Fingerhut, Jing Sun, Junjun Ma, Luyang Zhang, Shuchun Li, Chaoran Yu, Minhua Zheng, Lu Zang
BMC Cancer.2021;[Epub] CrossRef - Determinants of Response and Intrinsic Resistance to PD-1 Blockade in Microsatellite Instability–High Gastric Cancer
Minsuk Kwon, Minae An, Samuel J. Klempner, Hyuk Lee, Kyoung-Mee Kim, Jason K. Sa, Hee Jin Cho, Jung Yong Hong, Taehyang Lee, Yang Won Min, Tae Jun Kim, Byung-Hoon Min, Woong-Yang Park, Won Ki Kang, Kyu-Tae Kim, Seung Tae Kim, Jeeyun Lee
Cancer Discovery.2021; 11(9): 2168. CrossRef - Advanced Gastric Cancer: Current Treatment Landscape and a Future Outlook for Sequential and Personalized Guide: Swiss Expert Statement Article
Alexander R. Siebenhüner, Sara De Dosso, Daniel Helbling, Christoforos Astaras, Petr Szturz, Peter Moosmann, Stefanie Pederiva, Thomas Winder, Philippe Von Burg, Markus Borner
Oncology Research and Treatment.2021; 44(9): 485. CrossRef - High homogeneity of mismatch repair deficiency in advanced prostate cancer
Christoph Fraune, Ronald Simon, Doris Höflmayer, Katharina Möller, David Dum, Franziska Büscheck, Claudia Hube-Magg, Georgia Makrypidi-Fraune, Martina Kluth, Andrea Hinsch, Eike Burandt, Till Sebastian Clauditz, Waldemar Wilczak, Guido Sauter, Stefan Steu
Virchows Archiv.2020; 476(5): 745. CrossRef - High homogeneity of MMR deficiency in ovarian cancer
Christoph Fraune, Janina Rosebrock, Ronald Simon, Claudia Hube-Magg, Georgia Makrypidi-Fraune, Martina Kluth, Franziska Büscheck, Doris Höflmayer, Barbara Schmalfeldt, Volkmar Müller, Linn Wölber, Isabell Witzel, Peter Paluchowski, Christian Wilke, Uwe He
Gynecologic Oncology.2020; 156(3): 669. CrossRef - Molecular Classification of Gastric Cancer among Alaska Native People
Holly Martinson, Dominic Mallari, Christine Richter, Tsung-Teh Wu, James Tiesinga, Steven Alberts, Matthew Olnes
Cancers.2020; 12(1): 198. CrossRef - Tumor immune response and immunotherapy in gastric cancer
Yoonjin Kwak, An Na Seo, Hee Eun Lee, Hye Seung Lee
Journal of Pathology and Translational Medicine.2020; 54(1): 20. CrossRef - MMR deficiency in urothelial carcinoma of the bladder presents with temporal and spatial homogeneity throughout the tumor mass
Christoph Fraune, Ronald Simon, Claudia Hube-Magg, Georgia Makrypidi-Fraune, Christian Kähler, Martina Kluth, Doris Höflmayer, Franziska Büscheck, David Dum, Andreas M. Luebke, Eike Burandt, Till Sebastian Clauditz, Waldemar Wilczak, Guido Sauter, Stefan
Urologic Oncology: Seminars and Original Investigations.2020; 38(5): 488. CrossRef - MMR Deficiency is Homogeneous in Pancreatic Carcinoma and Associated with High Density of Cd8-Positive Lymphocytes
Christoph Fraune, Eike Burandt, Ronald Simon, Claudia Hube-Magg, Georgia Makrypidi-Fraune, Martina Kluth, Franziska Büscheck, Doris Höflmayer, Niclas Ch. Blessin, Tim Mandelkow, Wenchao Li, Daniel Perez, Jakob R. Izbicki, Waldemar Wilczak, Guido Sauter, J
Annals of Surgical Oncology.2020; 27(10): 3997. CrossRef - CD73's Potential as an Immunotherapy Target in Gastrointestinal Cancers
Jerry B. Harvey, Luan H. Phan, Oscar E. Villarreal, Jessica L. Bowser
Frontiers in Immunology.2020;[Epub] CrossRef - Tumor copy-number alterations predict response to immune-checkpoint-blockade in gastrointestinal cancer
Zhihao Lu, Huan Chen, Shuang Li, Jifang Gong, Jian Li, Jianling Zou, Lihong Wu, Jianing Yu, Wenbo Han, Huaibo Sun, Xi Jiao, Xiaotian Zhang, Zhi Peng, Ming Lu, Zhenghang Wang, Henghui Zhang, Lin Shen
Journal for ImmunoTherapy of Cancer.2020; 8(2): e000374. CrossRef - Protein expression-based classification of gastric cancer by immunohistochemistry of tissue microarray
Chong Zhao, Zhiqiang Feng, Hongzhen He, Dan Zang, Hong Du, Hongli Huang, Yanlei Du, Jie He, Yongjian Zhou, Yuqiang Nie, Girijesh Kumar Patel
PLOS ONE.2020; 15(10): e0238836. CrossRef - Clinicopathologic Characteristics and Long-Term Outcome of Gastric Cancer Patients with Family History: Seven-Year Follow-Up Study for Korean Health Check-Up Subjects
Jooyoung Lee, Su Jin Chung, Ji Min Choi, Yoo Min Han, Joo Sung Kim, Greger Lindberg
Gastroenterology Research and Practice.2020; 2020: 1. CrossRef - Implication of expression of MMR proteins and clinicopathological characteristics in gastric cancer
Renu Verma, Puja Sakhuja, Ritu Srivastava, Prakash Chand Sharma
Asia-Pacific Journal of Oncology.2020; : 1. CrossRef - Prognostic significance of microsatellite‐instability in gastric and gastroesophageal junction cancer patients undergoing neoadjuvant chemotherapy
Georg Martin Haag, Elena Czink, Aysel Ahadova, Thomas Schmidt, Leila Sisic, Susanne Blank, Ulrike Heger, Leonidas Apostolidis, Anne Katrin Berger, Christoph Springfeld, Felix Lasitschka, Dirk Jäger, Magnus von Knebel Doeberitz, Matthias Kloor
International Journal of Cancer.2019; 144(7): 1697. CrossRef - Serological Markers Associated With Response to Immune Checkpoint Blockade in Metastatic Gastrointestinal Tract Cancer
Zhihao Lu, Jianling Zou, Ying Hu, Shuang Li, Tao Zhou, Jifang Gong, Jian Li, Xiaotian Zhang, Jun Zhou, Ming Lu, Xicheng Wang, Zhi Peng, Changsong Qi, Yanyan Li, Jie Li, Yan Li, Jianyin Zou, Xiao Du, Henghui Zhang, Lin Shen
JAMA Network Open.2019; 2(7): e197621. CrossRef - Assessing molecular subtypes of gastric cancer: microsatellite unstable and Epstein-Barr virus subtypes. Methods for detection and clinical and pathological implications
Carolina Martinez-Ciarpaglini, Tania Fleitas-Kanonnikoff, Valentina Gambardella, Marta Llorca, Cristina Mongort, Regina Mengual, Gema Nieto, Lara Navarro, Marisol Huerta, Susana Rosello, Desamparados Roda, Noelia Tarazona, Samuel Navarro, Gloria Ribas, An
ESMO Open.2019; 4(3): e000470. CrossRef - The role of pembrolizumab in the treatment of PD-L1 expressing gastric and gastroesophageal junction adenocarcinoma
Gagandeep Brar, Manish A. Shah
Therapeutic Advances in Gastroenterology.2019;[Epub] CrossRef - Novel Biomarkers for Prediction of Response to Preoperative Systemic Therapies in Gastric Cancer
Alessandro Cavaliere, Valeria Merz, Simona Casalino, Camilla Zecchetto, Francesca Simionato, Hayley Louise Salt, Serena Contarelli, Raffaela Santoro, Davide Melisi
Journal of Gastric Cancer.2019; 19(4): 375. CrossRef - MICROSATELLITE INSTABILITY AND GASTRIC CARCINOMA. REVIEW OF THELITERATURE
D. L. Rotin, O. V. Paklina, I. O. Tin’kova, D. N. Grekov
Russian Journal of Biotherapy.2019; 18(4): 17. CrossRef - Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer
K Polom, L Marano, D Marrelli, R De Luca, G Roviello, V Savelli, P Tan, F Roviello
Journal of British Surgery.2018; 105(3): 159. CrossRef - Gastric poorly cohesive carcinoma: a correlative study of mutational signatures and prognostic significance based on histopathological subtypes
Chae H Kwon, Young K Kim, Sojeong Lee, Ahrong Kim, Hye J Park, Yuri Choi, Yeo J Won, Do Y Park, Gregory Y Lauwers
Histopathology.2018; 72(4): 556. CrossRef - Microsatellite instability in gastric cancer: molecular bases, clinical perspectives, and new treatment approaches
Margherita Ratti, Andrea Lampis, Jens C. Hahne, Rodolfo Passalacqua, Nicola Valeri
Cellular and Molecular Life Sciences.2018; 75(22): 4151. CrossRef - High-throughput Protein and mRNA Expression–based Classification of Gastric Cancers Can Identify Clinically Distinct Subtypes, Concordant With Recent Molecular Classifications
Sangjeong Ahn, So-Jeong Lee, Yonugkeum Kim, Ahrong Kim, Nari Shin, Kyung Un Choi, Chang-Hun Lee, Gi Yeong Huh, Kyong-Mee Kim, Namrata Setia, Gregory Y. Lauwers, Do Youn Park
American Journal of Surgical Pathology.2017; 41(1): 106. CrossRef - Molecular Testing for Gastrointestinal Cancer
Hye Seung Lee, Woo Ho Kim, Yoonjin Kwak, Jiwon Koh, Jeong Mo Bae, Kyoung-Mee Kim, Mee Soo Chang, Hye Seung Han, Joon Mee Kim, Hwal Woong Kim, Hee Kyung Chang, Young Hee Choi, Ji Y. Park, Mi Jin Gu, Min Jin Lhee, Jung Yeon Kim, Hee Sung Kim, Mee-Yon Cho
Journal of Pathology and Translational Medicine.2017; 51(2): 103. CrossRef - Molecular testing of gastrointestinal tumours
Matthew Evans, Matthew Smith, Brendan O'Sullivan, Philippe Taniere
Diagnostic Histopathology.2017; 23(10): 442. CrossRef - Gastric Carcinomas With Lymphoid Stroma
Raul S Gonzalez, Justin M M Cates, Frank Revetta, Loralee A McMahon, Kay Washington
American Journal of Clinical Pathology.2017; 148(6): 477. CrossRef - Meta-Analysis of Prognostic Role of Ki-67 Labeling Index in Gastric Carcinoma
Jung-Soo Pyo, Nae Yu Kim
The International Journal of Biological Markers.2017; 32(4): 447. CrossRef - Tissue-Agnostic Drug Development
Keith T. Flaherty, Dung T. Le, Steven Lemery
American Society of Clinical Oncology Educational Book.2017; (37): 222. CrossRef - Programmed death ligand-1 and MET co-expression is a poor prognostic factor in gastric cancers after resection
Mi Jung Kwon, Kab-Choong Kim, Eun Sook Nam, Seong Jin Cho, Hye-Rim Park, Soo Kee Min, Jinwon Seo, Ji-Young Choe, Hye Kyung Lee, Ho Suk Kang, Kyueng-Whan Min
Oncotarget.2017; 8(47): 82399. CrossRef - Hypermutation and microsatellite instability in gastrointestinal cancers
Kizuki Yuza, Masayuki Nagahashi, Satoshi Watanabe, Kazuaki Takabe, Toshifumi Wakai
Oncotarget.2017; 8(67): 112103. CrossRef - The Emerging Role of Immunotherapy in Gastric and Esophageal Adenocarcinoma
Bruno Bockorny, Eirini Pectasides
Future Oncology.2016; 12(15): 1833. CrossRef - Expression of Mismatch Repair Proteins in Early and Advanced Gastric Cancer in Poland
Katarzyna Karpińska-Kaczmarczyk, Magdalena Lewandowska, Małgorzata Ławniczak, Andrzej Białek, Elżbieta Urasińska
Medical Science Monitor.2016; 22: 2886. CrossRef - Immunotherapy for Gastroesophageal Cancer
Emily Goode, Elizabeth Smyth
Journal of Clinical Medicine.2016; 5(10): 84. CrossRef - Lauren classification and individualized chemotherapy in gastric cancer
JUNLI MA, HONG SHEN, LINDA KAPESA, SHAN ZENG
Oncology Letters.2016; 11(5): 2959. CrossRef - High-risk and low-risk gastric cancer areas in Italy and its association with microsatellite instability
Karol Polom, Daniele Marrelli, Valeria Pascale, Giandomenico Roviello, Costantino Voglino, Henry Rho, Carla Vindigni, Mario Marini, Raffaele Macchiarelli, Franco Roviello
Journal of Cancer Research and Clinical Oncology.2016; 142(8): 1817. CrossRef - MUC2 Expression Is Correlated with Tumor Differentiation and Inhibits Tumor Invasion in Gastric Carcinomas: A Systematic Review and Meta-analysis
Jung-Soo Pyo, Jin Hee Sohn, Guhyun Kang, Dong-Hoon Kim, Kyungeun Kim, In-Gu Do, Dong Hyun Kim
Journal of Pathology and Translational Medicine.2015; 49(3): 249. CrossRef - Correlation between microsatellite instability-high phenotype and occult lymph node metastasis in gastric carcinoma
Jiwoon Choi, Soo Kyung Nam, Do Joong Park, Hwal Woong Kim, Hyung-Ho Kim, Woo Ho Kim, Hye Seung Lee
APMIS.2015; 123(3): 215. CrossRef - Clinicopathologic and molecular features associated with patient age in gastric cancer
Ji Yeon Seo, Eun Hyo Jin, Hyun Jin Jo, Hyuk Yoon, Cheol Min Shin, Young Soo Park, Nayoung Kim, Hyun Chae Jung, Dong Ho Lee
World Journal of Gastroenterology.2015; 21(22): 6905. CrossRef - Molecular classification of gastric cancer: Towards a pathway-driven targeted therapy
Ismael Riquelme, Kathleen Saavedra, Jaime A. Espinoza, Helga Weber, Patricia García, Bruno Nervi, Marcelo Garrido, Alejandro H. Corvalán, Juan Carlos Roa, Carolina Bizama
Oncotarget.2015; 6(28): 24750. CrossRef - A phylogenetic model for understanding the effect of gene duplication on cancer progression
Qin Ma, Jaxk H. Reeves, David A. Liberles, Lili Yu, Zheng Chang, Jing Zhao, Juan Cui, Ying Xu, Liang Liu
Nucleic Acids Research.2014; 42(5): 2870. CrossRef - The analysis of microsatellite instability in extracolonic gastrointestinal malignancy
Andrew S. Williams, Weei-Yuarn Huang
Pathology.2013; 45(6): 540. CrossRef
- Intestinal Subtype as a Biomarker of Response to Neoadjuvant Immunochemotherapy in Locally Advanced Gastric Adenocarcinoma: Insights from a Prospective Phase II Trial
- Primary Mucinous Cystadenocarcinoma of the Breast: Cytologic Finding and Expression of MUC5 Are Different from Mucinous Carcinoma
- Sung Eun Kim, Ji Hye Park, SoonWon Hong, Ja Seung Koo, Joon Jeong, Woo-Hee Jung
- Korean J Pathol. 2012;46(6):611-616. Published online December 26, 2012
- DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.6.611
- 9,629 View
- 66 Download
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF Mucinous cystadenocarcinoma (MCA) in the breast is a rare neoplasm. There have been 13 cases of primary breast MCA reported. The MCA presents as a large, partially cystic mass in postmenopausal woman with a good prognosis. The microscopic findings resemble those of ovarian, pancreatic, or appendiceal MCA. The aspiration findings showed mucin-containing cell clusters in the background of mucin and necrotic material. The cell clusters had intracytoplasmic mucin displacing atypical nuclei to the periphery. Histologically, the tumor revealed an abundant mucin pool with small floating clusters of mucin-containing tumor cells. There were also small cysts lined by a single layer of tall columnar mucinous cells, resembling those of the uterine endocervix. The cancer cells were positive for mucin (MUC) 5 and negative for MUC2 and MUC6. This mucin profile is different from ordinary mucinous carcinoma and may be a unique characteristic of breast MCA.
-
Citations
Citations to this article as recorded by- BMP2 alterations in mucinous cystadenocarcinoma of the breast: insights from whole-exome sequencing
Zhenyu Li, Yi Gong, Guangxin Li, Qingming Jiang, Juanhui Dong, Rui Chen
PeerJ.2025; 13: e19948. CrossRef - Primary mucinous cystadenocarcinoma of the breast commonly harbours TP53 mutations and PI3K/AKT pathway alterations
Cheng Xu, Jing Wu, Ying Ding, Zhihong Zhang, Cong Wang
Virchows Archiv.2025; 487(6): 1235. CrossRef - Mucinous cystadenocarcinoma of the breast harbours TRPS1 expressions and PIK3CA alterations
Wei‐Yu Chen, Yu‐Hsuan Hu, Yu‐Hsin Tsai, Jen‐Fan Hang, Puay Hoon Tan, Chih‐Jung Chen
Histopathology.2024; 84(3): 550. CrossRef - Pure mucinous adenocarcinoma of the breast with the rare lymphoplasmacytic infiltration: A case report with review of literature
Yash Hasmukhbhai Prajapati, Vishal Bhabhor, Kahan Samirkumar Mehta, Mithoon Barot, Husen Boriwala, Mohamed Omar
Clinical Case Reports.2024;[Epub] CrossRef - HER2‐positive mucinous cystadenocarcinoma of the breast coexisting with invasive lobular carcinoma: A case report and review of the literature
Ismail Guzelis, Betul Bolat Kucukzeybek, Mehmet Ali Uyaroglu, Melek Bekler Gokova, Gulten Sezgin, Yuksel Kucukzeybek
Diagnostic Cytopathology.2024;[Epub] CrossRef - Primary mucinous cystadenocarcinoma of the breast: A case report and literature review
Xi Cao, Yongchao Luo, Songjie Shen, Xinyu Ren
Oncology Letters.2024;[Epub] CrossRef - Mammary mucinous cystadenocarcinoma with long-term follow-up: molecular information and literature review
Ting Lei, Yong Qiang Shi, Tong Bing Chen
Diagnostic Pathology.2023;[Epub] CrossRef - Primary Mucinous Cystadenocarcinoma of the Breast Intermixed with Pleomorphic Invasive Lobular Carcinoma: The First Report of This Rare Association
Federica Vegni, Nicoletta D’Alessandris, Angela Santoro, Giuseppe Angelico, Giulia Scaglione, Angela Carlino, Damiano Arciuolo, Michele Valente, Stefania Sfregola, Maria Natale, Alejandro Martin Sanchez, Valeria Masciullo, Gian Franco Zannoni, Antonino Mu
Journal of Personalized Medicine.2023; 13(6): 948. CrossRef - Special Histologic Type and Rare Breast Tumors – Diagnostic Review and Clinico-Pathological Implications
Benjamin Yongcheng Tan, Elaine Hsuen Lim, Puay Hoon Tan
Surgical Pathology Clinics.2022; 15(1): 29. CrossRef - Mucinous cystadenocarcinoma of the breast: a new entity with broad differentials—a case report
Kanwalpreet Kaur, Ashini Shah, Jahnvi Gandhi, Priti Trivedi
Journal of the Egyptian National Cancer Institute.2022;[Epub] CrossRef - Mucinous carcinoma of the breast: distinctive histopathologic and genetic characteristics
Minjung Jung
Kosin Medical Journal.2022; 37(3): 176. CrossRef - Primary Mucinous Cystadenocarcinoma of the Breast: A Rare Case Report With Review of Literature
Ekta Jain, Abhishek Kumar, Raajul Jain, Shivani Sharma
International Journal of Surgical Pathology.2021; 29(7): 740. CrossRef - Mucinous Cystadenocarcinoma of the Breast: Report of 2 Cases Including One With Long-Term Local Recurrence
Anupma Nayak, Ira J. Bleiweiss, Kimberly Dumoff, Tawfiqul A. Bhuiya
International Journal of Surgical Pathology.2018; 26(8): 749. CrossRef - Mucinous breast carcinoma with tall columnar cells
N Tsoukalas, M Kiakou, M Tolia, ID Kostakis, M Galanopoulos, G Nakos, D Tryfonopoulos, G Kyrgias, G Koumakis
The Annals of The Royal College of Surgeons of England.2018; 100(5): e132. CrossRef - Radiologic Findings of Primary Mucinous Cystadenocarcinoma of the Breast: A Report of Two Cases and a Literature Review
Minjung Seong, Eun Young Ko, Boo-Kyung Han, Soo Youn Cho, Eun Yoon Cho, Se Kyung Lee, Jeong Eon Lee
Journal of Breast Cancer.2016; 19(3): 330. CrossRef - Primary Mucinous Cystadenocarcinoma of the Breast with Endocervical-Like Mucinous Epithelium
Dong-Liang Lin, Ji-Lin Hu, Shi-Hong Shao, Dong-Mei Sun, Ji-Gang Wang
Breast Care.2013; 8(6): 445. CrossRef
- BMP2 alterations in mucinous cystadenocarcinoma of the breast: insights from whole-exome sequencing
- Expression of SIRT1 and DBC1 in Gastric Adenocarcinoma
- Youngran Kang, Woon Yong Jung, Hyunjoo Lee, Eunjung Lee, Aeree Kim, Baek-hui Kim
- Korean J Pathol. 2012;46(6):523-531. Published online December 26, 2012
- DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.6.523
- 10,297 View
- 50 Download
- 31 Crossref
-
 Abstract
Abstract
 PDF
PDF Background Sirtuin 1 (SIRT1) and deleted in breast cancer 1 (DBC1) are known as tumor suppressor or promoter genes. This may be due to their diverse functions and interaction with other proteins. Gastric adenocarcinoma is one of the most common malignancies, but little is known about its carcinogenesis. Therefore, we investigated the association of immunohistochemical expression of SIRT1, DBC1, p53, and β-catenin and their variable clinicopathological characteristics.
Methods We obtained samples from 452 patients who underwent gastrectomy. Tissue microarray blocks were constructed and immonohistochemical staining was performed.
Results Expression of DBC1 and SIRT1 was associated with lower histologic grade, intestinal type of Lauren classification, and lower pT (p<0.001) and pN stage (DBC1, p=0.002; SIRT1, p<0.001). Association between absence of lymphatic invasion, and SIRT1 (p=0.001) and DBC1 (p=0.004) was observed. Cytoplasmic β-catenin expression was associated with lower histologic grade, pT, pN, tumor-node-metastasis (TNM) stage, DBC1 (p<0.001), and SIRT1 (p=0.001). Expression of SIRT1 and DBC1 was not associated with p53 (p=0.063 and p=0.060). DBC1 was an independent good prognostic factor in multivariate analysis (p=0.012).
Conclusions SIRC1 and DBC1 can be considered to be good prognostic factors in gastric adenocarcinoma.
-
Citations
Citations to this article as recorded by- Regulatory role of DBC1 in inflammation and autoimmune diseases
Jinzhi Wu, Fan Yang, Guanhua Xu, Xinlei Ma, Jin Lin, Weiqian Chen
Rheumatology & Autoimmunity.2025; 5(1): 15. CrossRef - Prognostic and clinicopathological value of dbc1 expression in human cancers: a systematic review and meta-analysis
Haojia Wang, Xinhong Cheng, Bruce Xianzhuo Zhang, Yong Wang, Shuo Gao, Fanghui Ding, Xiaojing Song, Dandan Li, Haixu Ni, Yang Luo, Xun Li
Frontiers in Oncology.2025;[Epub] CrossRef - Mechanistic insights into the dual role of CCAR2/DBC1 in cancer
Hwa Jin Kim, Sue Jin Moon, Jeong Hoon Kim
Experimental & Molecular Medicine.2023; 55(8): 1691. CrossRef - Sirtuins (SIRTs) As a Novel Target in Gastric Cancer
Agata Poniewierska-Baran, Paulina Warias, Katarzyna Zgutka
International Journal of Molecular Sciences.2022; 23(23): 15119. CrossRef - Histone Deacetylase Functions in Gastric Cancer: Therapeutic Target?
Amandine Badie, Christian Gaiddon, Georg Mellitzer
Cancers.2022; 14(21): 5472. CrossRef - Advances on the role of the deleted in breast cancer (DBC1) in cancer and autoimmune diseases
Qiannan Fang, Joseph A Bellanti, Song Guo Zheng
Journal of Leukocyte Biology.2021; 109(2): 449. CrossRef - miR-1301-3p Promotes Cell Proliferation and Facilitates Cell Cycle Progression via Targeting SIRT1 in Gastric Cancer
Dakui Luo, Hao Fan, Xiang Ma, Chao Yang, Yu He, Yugang Ge, Mingkun Jiang, Zekuan Xu, Li Yang
Frontiers in Oncology.2021;[Epub] CrossRef - CCAR1 and CCAR2 as gene chameleons with antagonistic duality: Preclinical, human translational, and mechanistic basis
Gavin S. Johnson, Praveen Rajendran, Roderick H. Dashwood
Cancer Science.2020; 111(10): 3416. CrossRef - Role of Histone Acetylation in Gastric Cancer: Implications of Dietetic Compounds and Clinical Perspectives
Danielle Q Calcagno, Fernanda Wisnieski, Elizangela R da Silva Mota, Stefanie B Maia de Sousa, Jéssica M Costa da Silva, Mariana F Leal, Carolina O Gigek, Leonardo C Santos, Lucas T Rasmussen, Paulo P Assumpção, Rommel R Burbano, Marília AC Smith
Epigenomics.2019; 11(3): 349. CrossRef - Survival and Clinicopathological Significance of SIRT1 Expression in Cancers: A Meta-Analysis
Min Sun, Mengyu Du, Wenhua Zhang, Sisi Xiong, Xingrui Gong, Peijie Lei, Jin Zha, Hongrui Zhu, Heng Li, Dong Huang, Xinsheng Gu
Frontiers in Endocrinology.2019;[Epub] CrossRef - Cell cycle and apoptosis regulator 2 at the interface between DNA damage response and cell physiology
Martina Magni, Giacomo Buscemi, Laura Zannini
Mutation Research/Reviews in Mutation Research.2018; 776: 1. CrossRef - NANOGP8 expression regulates gastric cancer cell progression by transactivating DBC1 in gastric cancer MKN‑45 cells
Li Li, Ru Feng, Sujuan Fei, Jiang Cao, Qinqin Zhu, Guozhong Ji, Jianwei Zhou
Oncology Letters.2018;[Epub] CrossRef - Overexpression of DBC1, correlated with poor prognosis, is a potential therapeutic target for hepatocellular carcinoma
Changcan Li, Jianhua Liao, Shaohan Wu, Junwei Fan, Zhihai Peng, Zhaowen Wang
Biochemical and Biophysical Research Communications.2017; 494(3-4): 511. CrossRef - Association of sirtuins with clinicopathological parameters and overall survival in gastric cancer
Xiaobing Shen, Pengfei Li, Yuchao Xu, Xiaowei Chen, Haixiang Sun, Ying Zhao, Mengqi Liu, Wenwen Zhang
Oncotarget.2017; 8(43): 74359. CrossRef - Co-ordinated overexpression of SIRT1 and STAT3 is associated with poor survival outcome in gastric cancer patients
Shu Zhang, Shuling Huang, Chao Deng, Yu Cao, Jun Yang, Guangxia Chen, Bin Zhang, Chaoqin Duan, Jiong Shi, Bo Kong, Helmut Friess, Nanyi Zhao, Chen Huang, Xiaoli Huang, Lei Wang, Xiaoping Zou
Oncotarget.2017; 8(12): 18848. CrossRef - The prognostic role of Sirt1 expression in solid malignancies: a meta-analysis
Changwen Wang, Wen Yang, Fang Dong, Yawen Guo, Jie Tan, Shengnan Ruan, Tao Huang
Oncotarget.2017; 8(39): 66343. CrossRef - SIRT1 induces tumor invasion by targeting epithelial mesenchymal transition-related pathway and is a prognostic marker in triple negative breast cancer
Min-Sun Jin, Chang Lim Hyun, In Ae Park, Ji Young Kim, Yul Ri Chung, Seock-Ah Im, Kyung-Hun Lee, Hyeong-Gon Moon, Han Suk Ryu
Tumor Biology.2016; 37(4): 4743. CrossRef - Prognostic and clinical value of Sirt1 expression in gastric cancer: A systematic meta-analysis
Bin Jiang, Jin-huang Chen, Wen-zheng Yuan, Jin-tong Ji, Zheng-yi Liu, Liang Wu, Qiang Tang, Xiao-gang Shu
Journal of Huazhong University of Science and Technology [Medical Sciences].2016; 36(2): 278. CrossRef - Significance of silent mating type information regulation 2 homolog 1 and claudin 4 expression in gastric carcinoma and precursor lesions
Nashwa M. Emara, Ranih Z. Amer, Khaled M. Elsadek Attia, Heba M. Rashad, Adel Z. Elseady, Abd El-Latif M. Elbalshy
Egyptian Journal of Pathology.2016; 36(2): 158. CrossRef - Distinctive role of SIRT1 expression on tumor invasion and metastasis in breast cancer by molecular subtype
Yul Ri Chung, Hyojin Kim, Soo Young Park, In Ae Park, Ja June Jang, Ji-Young Choe, Yoon Yang Jung, Seock-Ah Im, Hyeong-Gon Moon, Kyung-Hun Lee, Koung Jin Suh, Tae-Yong Kim, Dong-Young Noh, Wonshik Han, Han Suk Ryu
Human Pathology.2015; 46(7): 1027. CrossRef - SIRT1 is a regulator of autophagy: Implications in gastric cancer progression and treatment
Guanglin Qiu, Xuqi Li, Xiangming Che, Chao Wei, Shicai He, Jing Lu, Zongliang Jia, Ke Pang, Lin Fan
FEBS Letters.2015; 589(16): 2034. CrossRef - DBC1 Functions as a Tumor Suppressor by Regulating p53 Stability
Bo Qin, Katherine Minter-Dykhouse, Jia Yu, Jun Zhang, Tongzheng Liu, Haoxing Zhang, SeungBaek Lee, JungJin Kim, Liewei Wang, Zhenkun Lou
Cell Reports.2015; 10(8): 1324. CrossRef - DBC1 promotes anoikis resistance of gastric cancer cells by regulating NF-κB activity
YONGWEI HUAN, DEPING WU, DAYONG ZHOU, BO SUN, GUOXIN LI
Oncology Reports.2015; 34(2): 843. CrossRef - Resveratrol relieves ischemia‑induced oxidative stress in the hippocampus by activating SIRT1
Zhuangzhi Meng, Jianguo Li, Honglin Zhao, Haiying Liu, Guowei Zhang, Lingzhan Wang, He Hu, Di Li, Mingjing Liu, Fulong Bi, Xiaoping Wang, Geng Tian, Qiang Liu, Batu Buren
Experimental and Therapeutic Medicine.2015;[Epub] CrossRef - CCAR2 deficiency augments genotoxic stress-induced apoptosis in the presence of melatonin in non-small cell lung cancer cells
Wootae Kim, Joo-Won Jeong, Ja-Eun Kim
Tumor Biology.2014; 35(11): 10919. CrossRef - Radioprotective and Antioxidant Effect of Resveratrol in Hippocampus by Activating Sirt1
Jianguo Li, Li Feng, Yonghua Xing, Yan Wang, Liqing Du, Chang Xu, Jia Cao, Qin Wang, Saijun Fan, Qiang Liu, Feiyue Fan
International Journal of Molecular Sciences.2014; 15(4): 5928. CrossRef - SIRT1 expression is associated with a poor prognosis, whereas DBC1 is associated with favorable outcomes in gastric cancer
Akira Noguchi, Keiji Kikuchi, Huachuan Zheng, Hiroyuki Takahashi, Yohei Miyagi, Ichiro Aoki, Yasuo Takano
Cancer Medicine.2014; 3(6): 1553. CrossRef - Sirtuins and Cancer: New Insights and Cell Signaling
Marcos Vinícius Macedo de Oliveira, João Marcus Oliveira Andrade, Alanna Fernandes Paraíso, Sérgio Henrique Sousa Santos
Cancer Investigation.2013; 31(10): 645. CrossRef - Deleted in breast cancer-1 (DBC-1) in the interface between metabolism, aging and cancer
Eduardo Nunes Chini, Claudia C. S. Chini, Veronica Nin, Carlos Escande
Bioscience Reports.2013;[Epub] CrossRef - SIRT1 Expression Is Associated with Good Prognosis in Colorectal Cancer
Wonkyung Jung, Kwang Dae Hong, Woon Yong Jung, Eunjung Lee, Bong Kyung Shin, Han Kyeom Kim, Aeree Kim, Baek-hui Kim
Korean Journal of Pathology.2013; 47(4): 332. CrossRef - Clinicopathological significance of SIRT1 and p300/CBP expression in gastroesophageal junction (GEJ) cancer and the correlation with E-cadherin and MLH1
Li-Hua Zhang, Qin Huang, Xiang-Shan Fan, Hong-Yan Wu, Jun Yang, An-Ning Feng
Pathology - Research and Practice.2013; 209(10): 611. CrossRef
- Regulatory role of DBC1 in inflammation and autoimmune diseases

 E-submission
E-submission
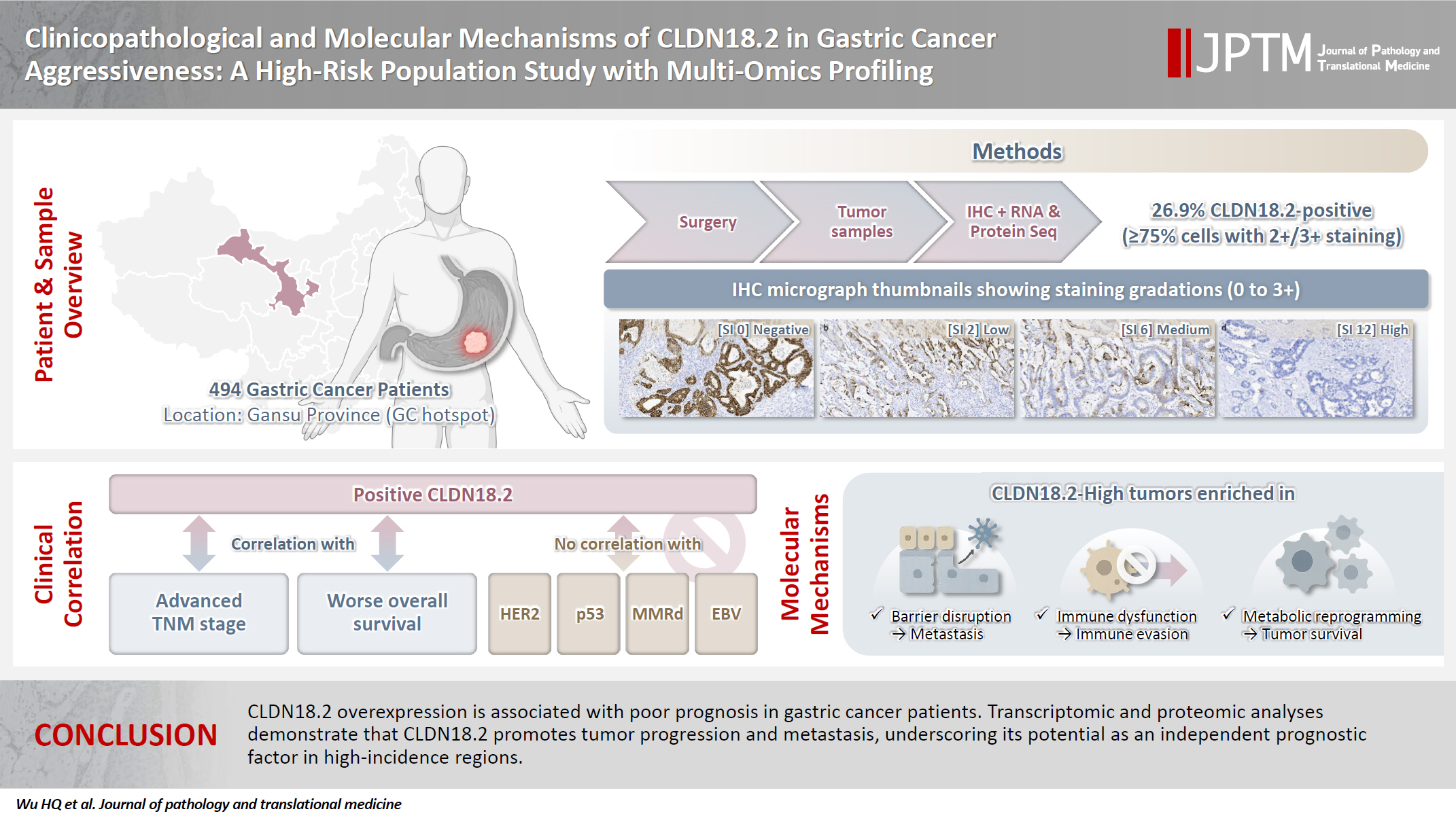

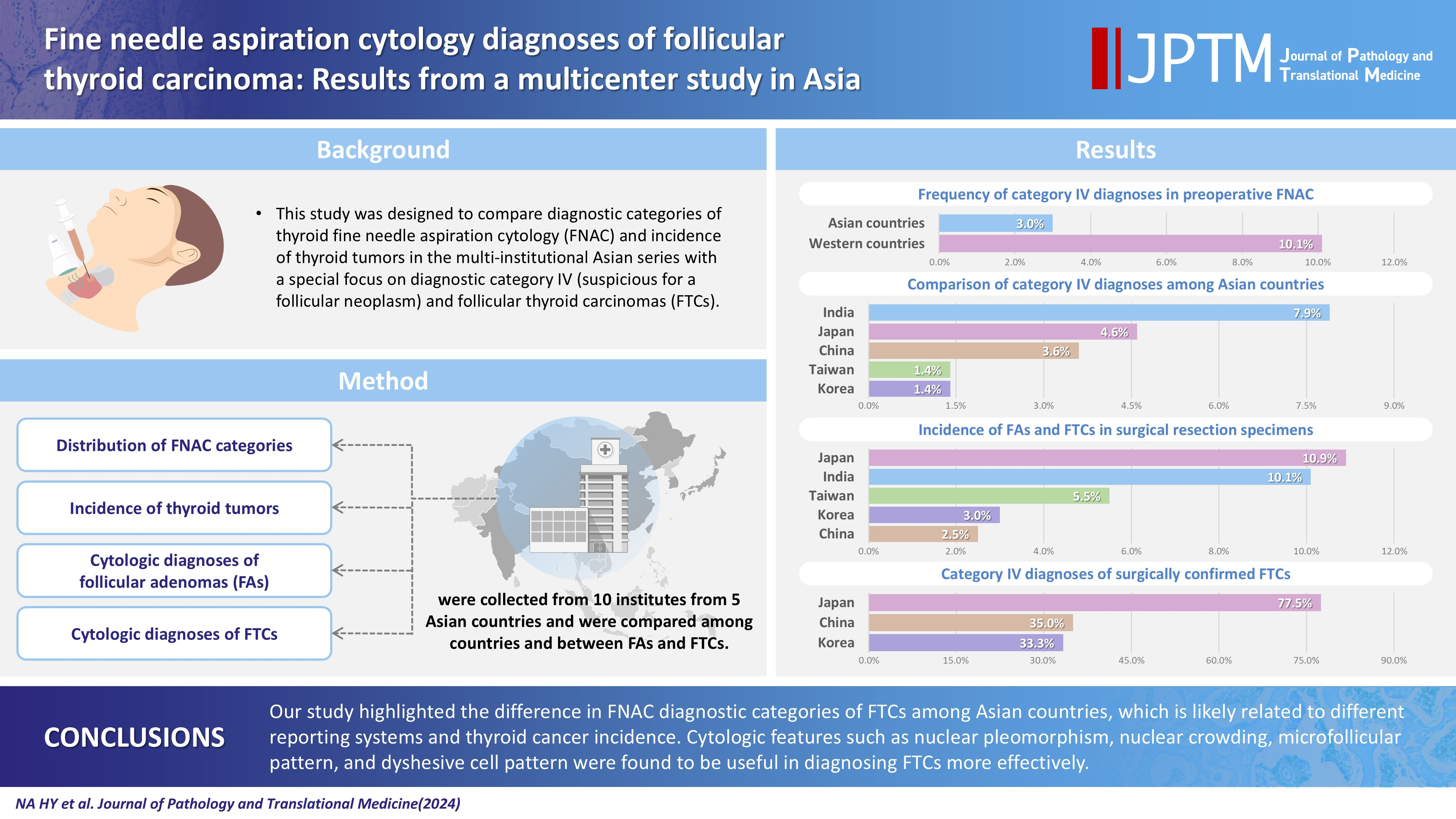

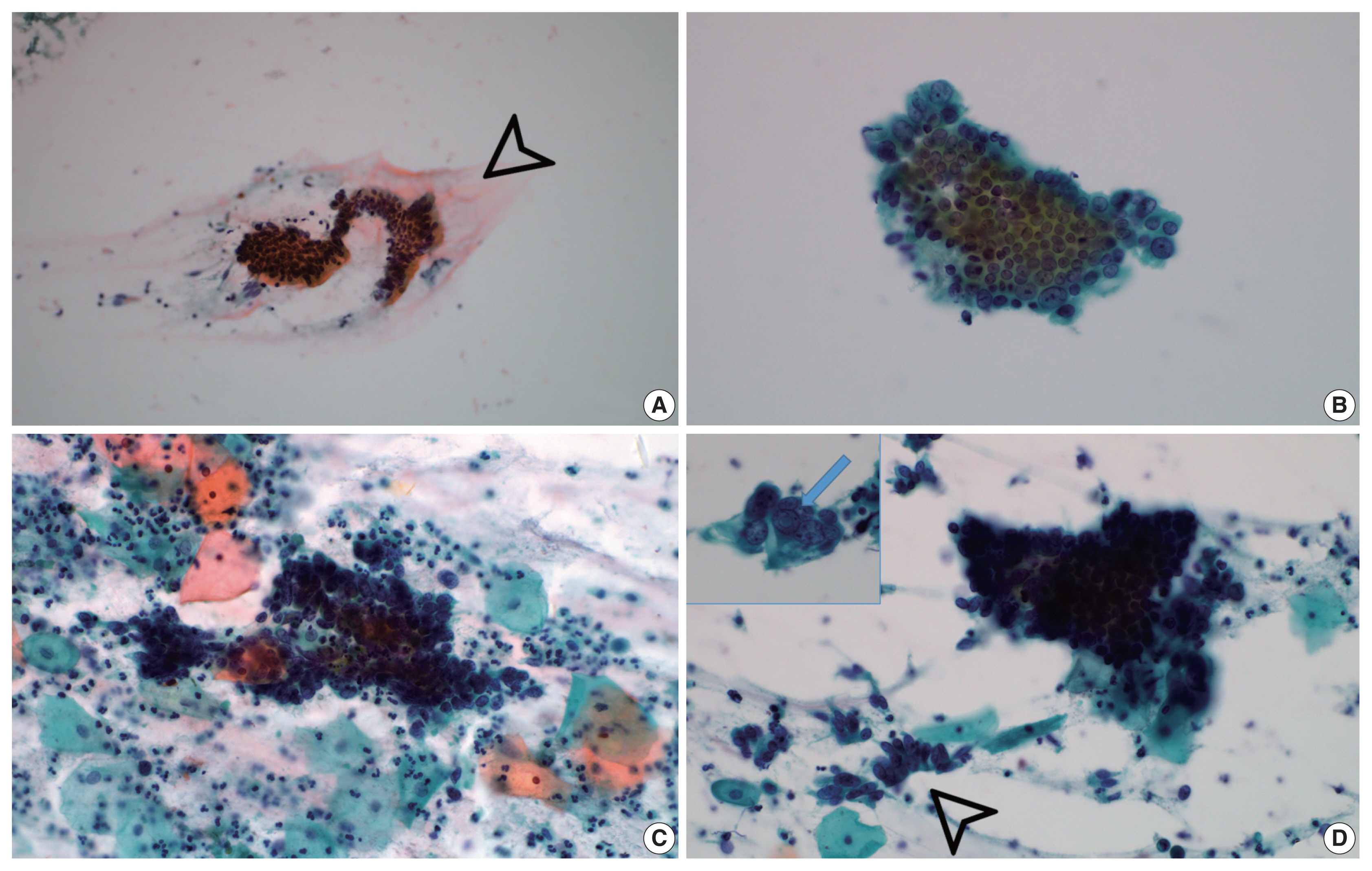
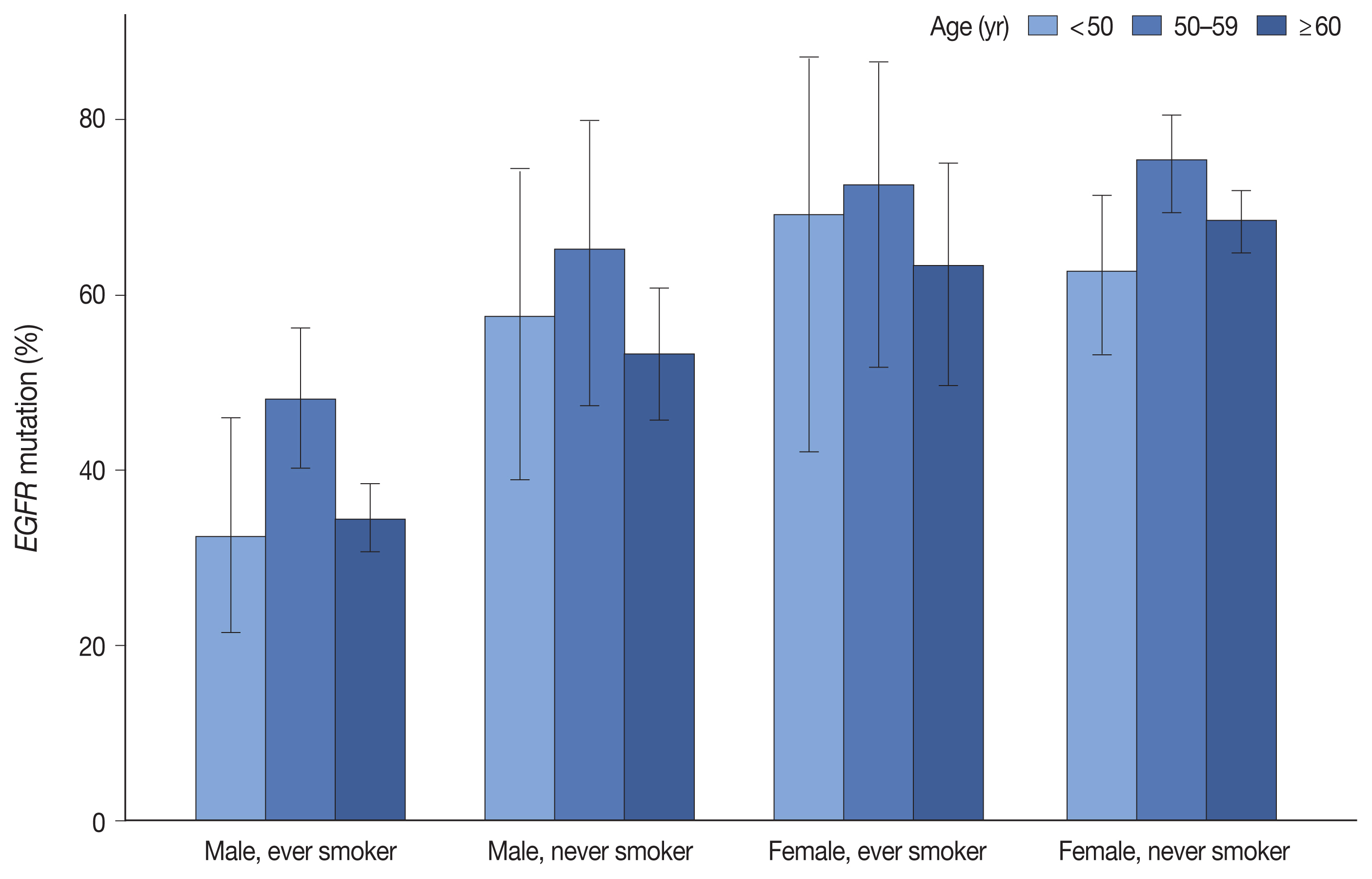
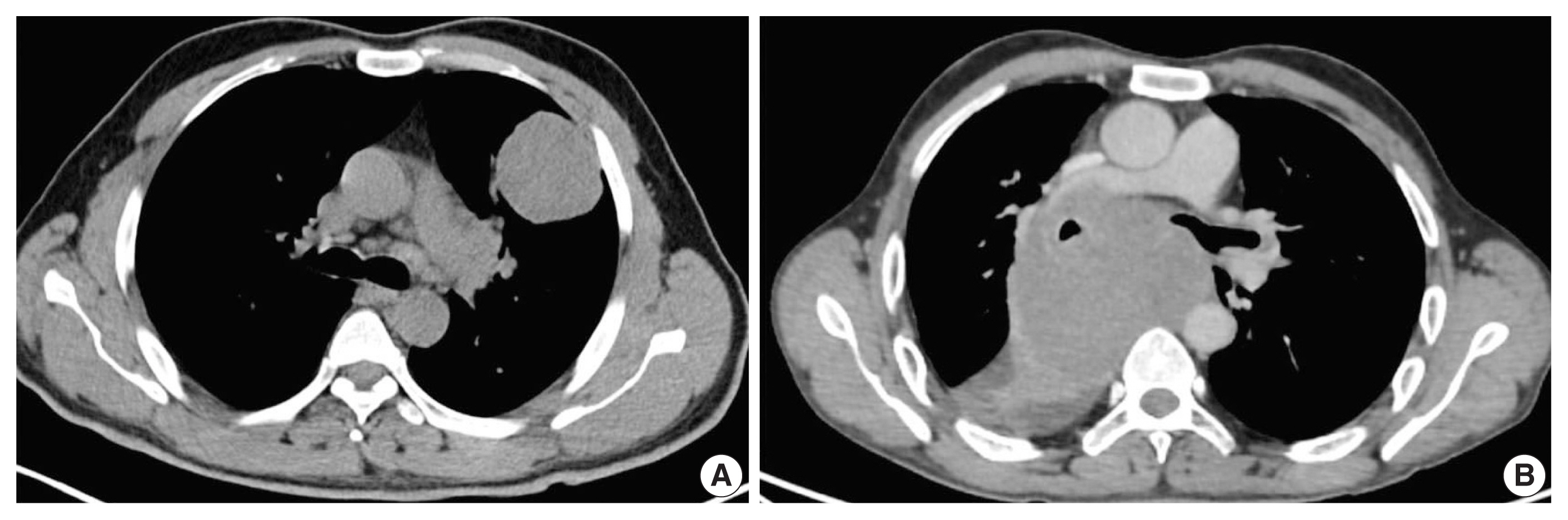
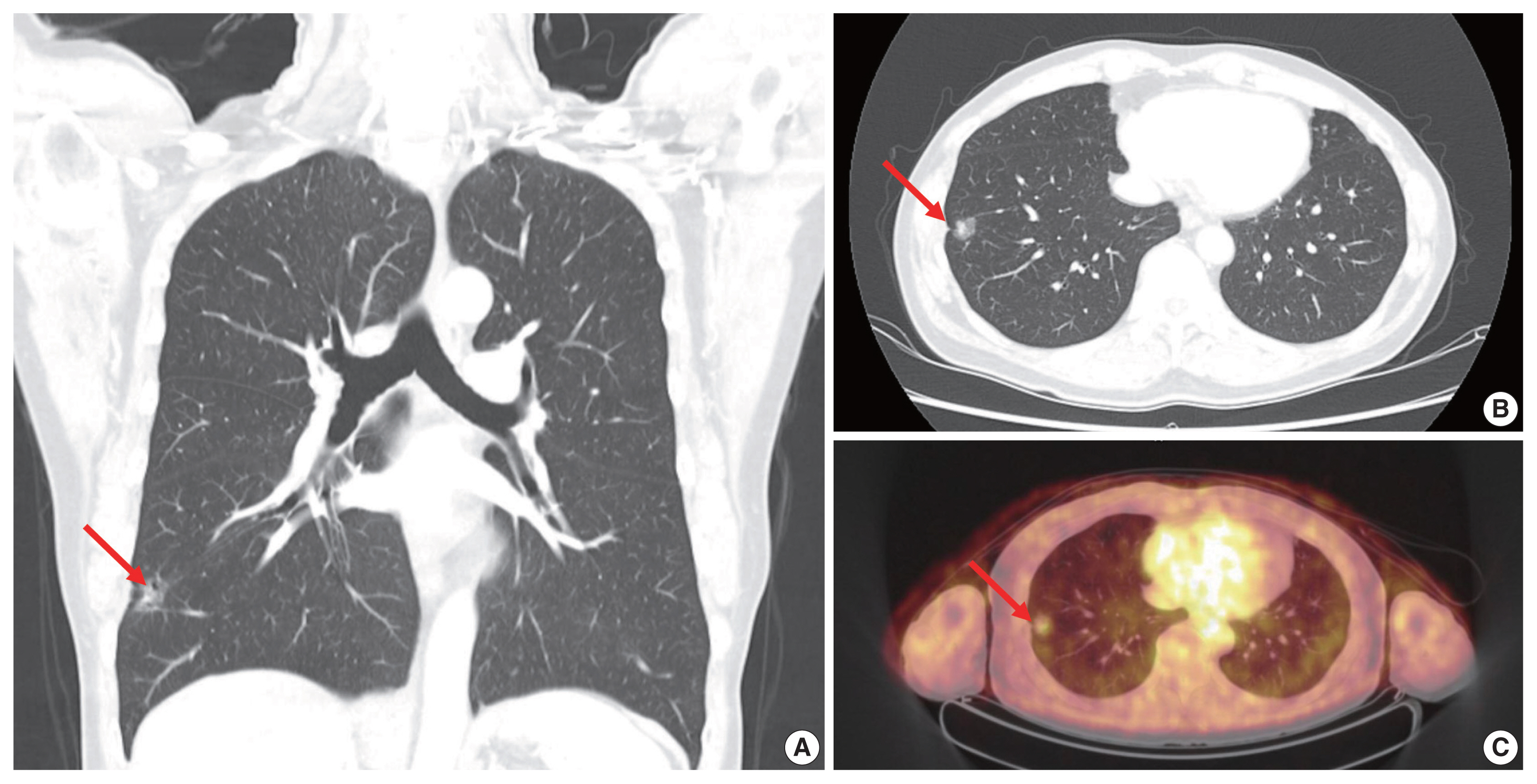
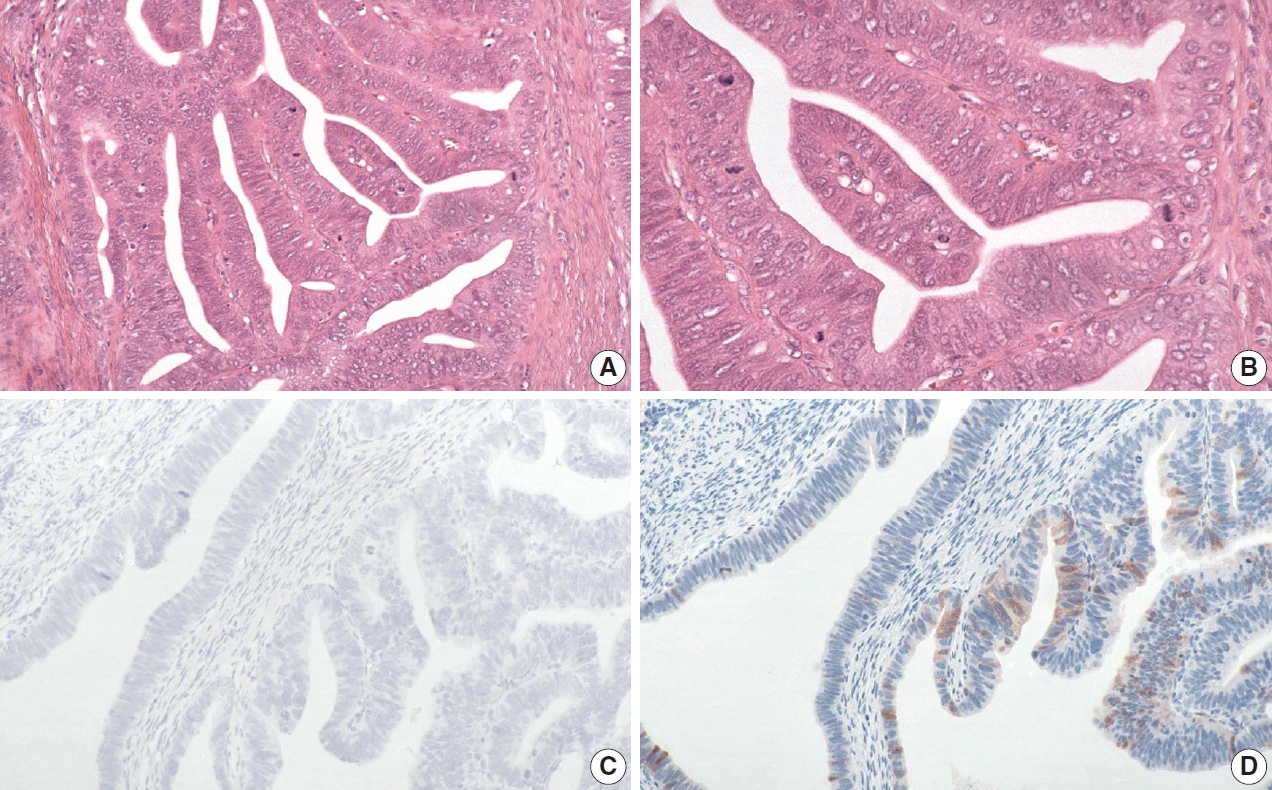
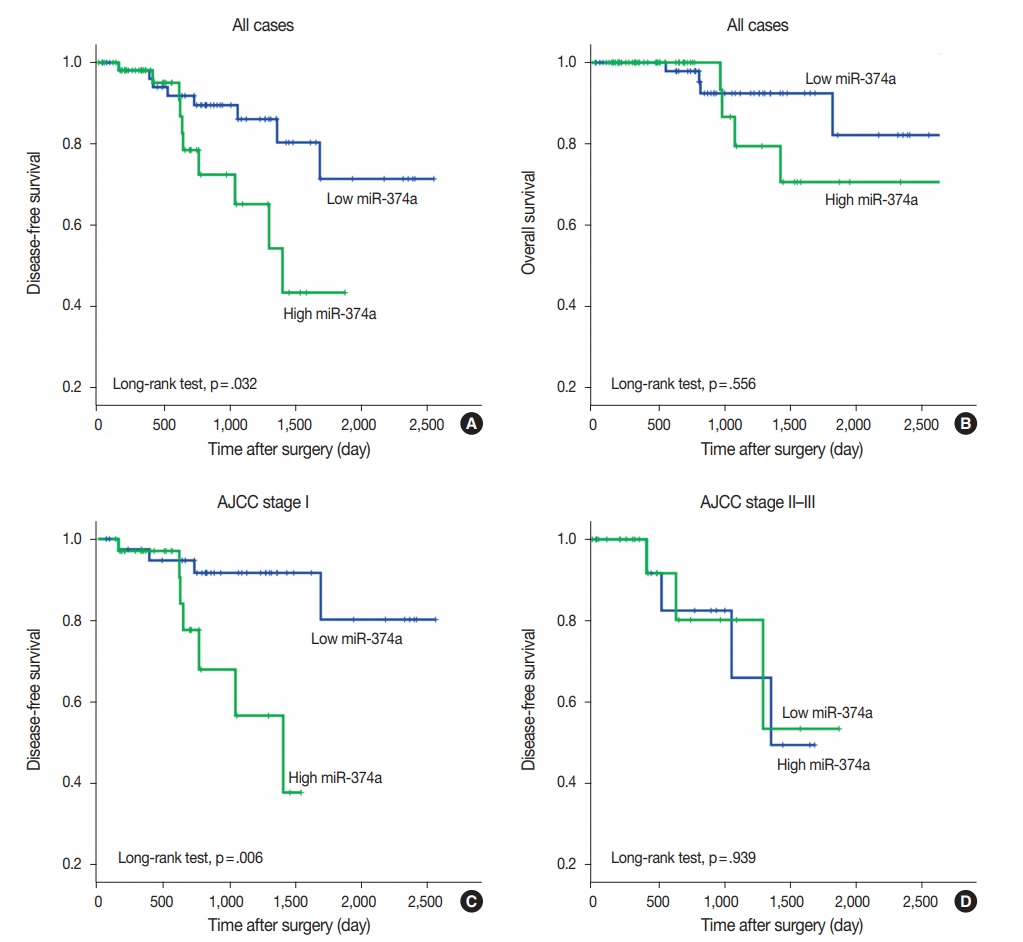
 First
First Prev
Prev



