Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 56(5); 2022 > Article
-
Original Article
Cytopathologic features of human papillomavirus–independent, gastric-type endocervical adenocarcinoma -
Min-Kyung Yeo
 , Go Eun Bae
, Go Eun Bae , Dong-Hyun Kim
, Dong-Hyun Kim , In-Ock Seong
, In-Ock Seong , Kwang-Sun Suh
, Kwang-Sun Suh
-
Journal of Pathology and Translational Medicine 2022;56(5):260-269.
DOI: https://doi.org/10.4132/jptm.2022.07.05
Published online: September 13, 2022
Department of Pathology, Chungnam National University School of Medicine, Daejeon, Korea
- Corresponding Author: Kwang-Sun Suh, MD, Department of Pathology, Chungnam National University School of Medicine, 266 Munwha-ro, Jung-gu, Daejeon 35015, Korea, Tel: +82-42-280-7199, Fax: +82-42-280-7189, E-mail: kssuh@cnu.ac.kr
© 2022 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Gastric-type endocervical adenocarcinoma (GEA) is unrelated to human papillomavirus (HPV) infection and is clinically aggressive compared with HPV-associated usual-type endocervical adenocarcinoma (UEA). The cytological diagnosis falls short of a definitive diagnosis of GEA and is often categorized as atypical glandular cells (AGCs). To improve cytologic recognition, cytological findings of HPV-independent GEA were analyzed and the results compared with HPV-associated UEA.
-
Methods
- Cervical Papanicolaou (Pap) smears from eight patients with a histopathologic diagnosis of GEA and 12 control cases of UEA were reviewed. All slides were conventionally prepared and/or liquid-based prepared (ThinPrep) and stained following the Pap method. A mucinous background, architectural, nuclear, and cytoplasmic features were analyzed and compared with UEA.
-
Results
- Preoperative cytologic diagnoses of the eight GEA cases were AGCs, favor neoplastic in three cases, adenocarcinoma in situ in one case, and adenocarcinoma in four cases. Cytologically, monolayered honeycomb-like sheets (p = .002) of atypical endocervical cells with vacuolar granular cytoplasm (p = .001) were extensive in GEA, and three-dimensional clusters (p = .010) were extensive in UEA. Although the differences were not statistically significant, background mucin (p = .058), vesicular nuclei (p = .057), and golden-brown intracytoplasmic mucin (p = .089) were also discriminatory findings for GEA versus UEA.
-
Conclusions
- Although GEA is difficult to diagnose on cytologic screening, GEA can be recognized based on cytologic features of monolayered honeycomb sheets of atypical endocervical cells with abundant vacuolar cytoplasm and some golden-brown intracytoplasmic mucin. UEA cases are characterized by three-dimensional clusters.
- Case selection and HPV testing
- After obtaining Institutional Review Board approval (CNUH-IRB 2021-12-001) of the Chungnam National University Hospital (CNUH) (Daejeon, Republic of Korea), the pathology database was searched for GEA and UEA diagnoses in cytological and/or surgical pathology specimens at CNUH from July 2016 to June 2021. Clinicopathologic findings and follow-up results were obtained from the electronic medical records (Table 1).
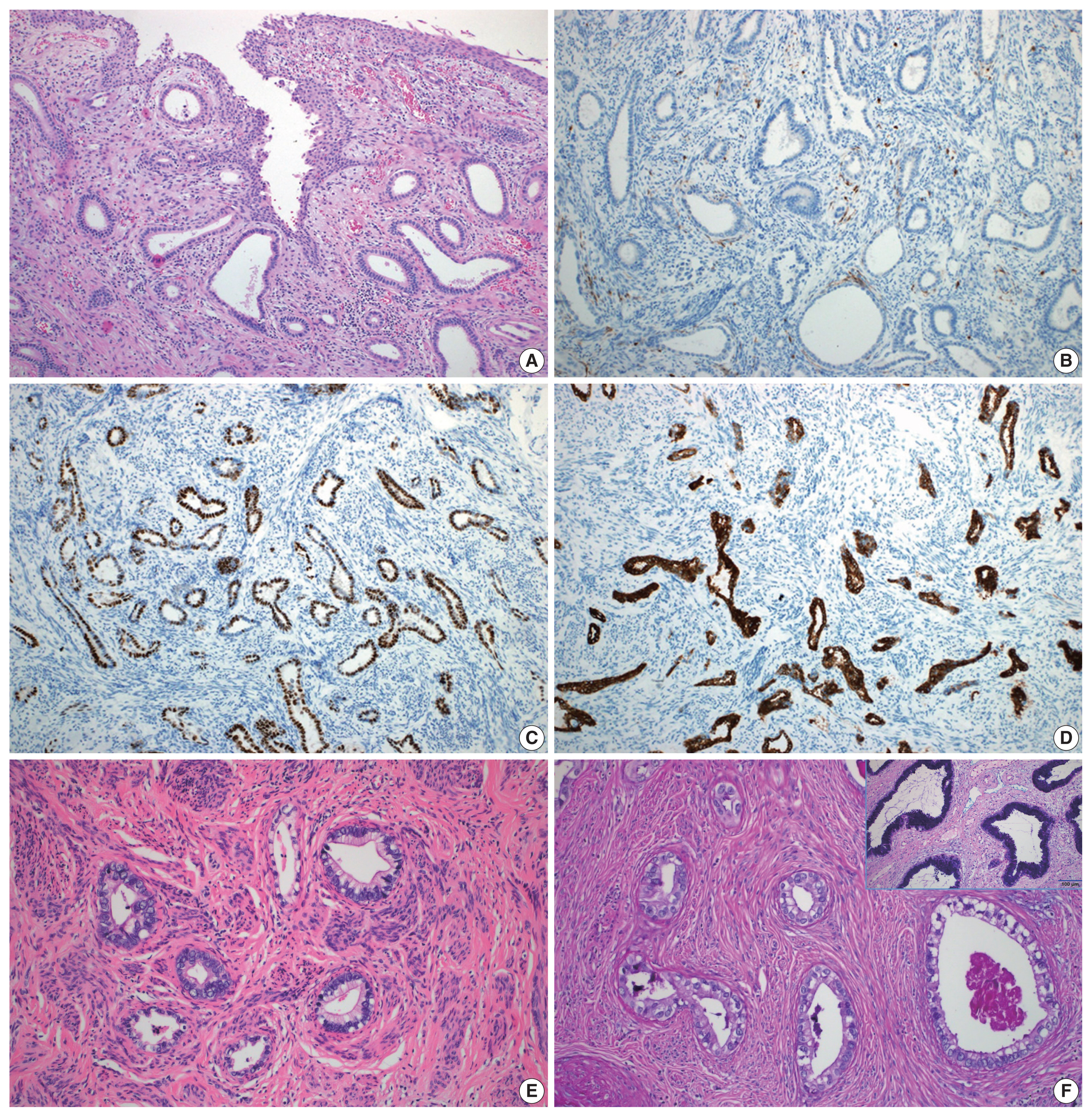
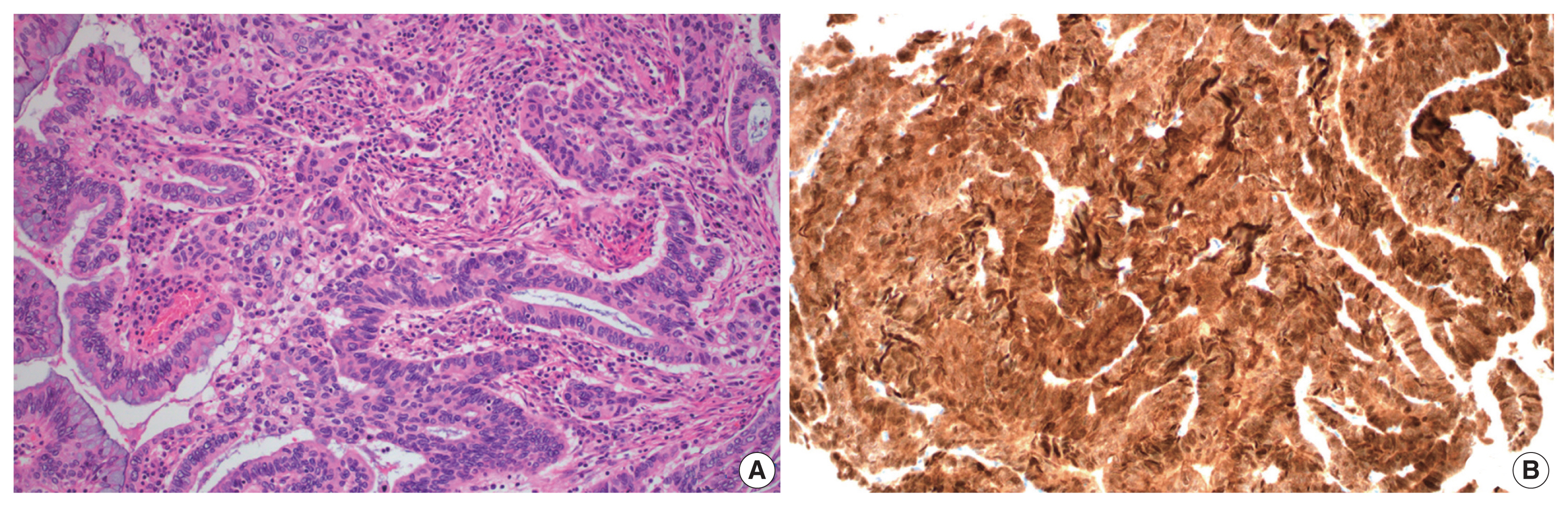
- A total of 10 GEA cases were included in the study (Table 2). Diagnosis of GEA was based on the morphologic criteria of GEA [1,2] (Fig. 1A–F) and negative HPV test results. For comparison, 12 control cases of UEA from the same pathology file were also reviewed. These cases were histopathologically diagnosed based on the morphologic criteria of UEA (Fig. 2A), a positive high-risk HPV test, and block-type positivity for p16 (Fig. 2B). Immunostained slides were retrieved from the pathology archives and the authors reviewed the slides or pathology reports.
- A total of 19 patients (9 GEA and 10 UEA) had HPV based on PANA RealTyper HPV kit (PANA RealTyper, PANAGENE, Daejeon, Korea) results. The remaining one GEA case and two UEA cases had in situ hybridization test results for high-risk HPV using paraffin blocks.
- Cytologic examination
- Among 10 GEA patients, two patients (cases Nos. 6 and 10) had no Papanicolaou (Pap) test results. Pathologists with expertise in cytopathology and gynecologic pathology (M.K.Y., G.E.B., and K.S.S.) reviewed preoperative cervical smears from the eight GEA patients and 12 UEA patients. All cytologic slides were prepared using the conventional method and/or the liquid-based preparation (LBP; ThinPrep Pap test, Hologic, Bedford, MA, USA), and Pap staining was performed. Both conventional smear and LBP were performed in five GEA cases and nine UEA cases. Only LBP was performed in three GEA cases and one UEA case. Only a conventional smear was performed in two UEA cases. All cytologic results were reported according to The Bethesda System terminology [7].
- Among cytologic features, a mucinous background, architectural (monolayered honeycomb-like sheets, 3-dimensional clusters, picket fence-like feathering), nuclear (vesicular nuclei, hyperchromasia, prominent nucleoli, grooves, intranuclear cytoplasmic pseudoinclusions [INCIs]), and cytoplasmic features (finely vacuolated cytoplasm, cytoplasmic golden-brown mucin, intracytoplasmic neutrophils) were analyzed.
- Cytologic findings of the 20 cases (eight GEA and 12 UEA) were scored. A mucinous background and architectural findings were scored as absent, focal (rare or mild degree; 1–2 fields, × 100/slide) or extensive (moderate to high degree; > 3 fields, × 100/slide). Nuclear and cytoplasmic findings were scored as absent, focal (rare or mild degree; < 30% of tumor cells/slide) or extensive (moderate to high degree; > 30% of tumor cells/slide). Cytologic scores were analyzed.
- Statistical analysis
- All statistical analyses were performed using Statistical Package for Social Sciences Statistics for Windows (SPSS ver. 26.0, IBM Corp., Armonk, NY, USA) software. Groups were compared using Pearson’s χ2 test for categorical variables and p < .05 were considered statistically significant.
MATERIALS AND METHODS
- Clinical features
- The mean age of the 10 GEA patients and 12 UEA patients was 61.7 ± 11.9 years and 45.3 ± 10.9 years, respectively (p = .003). Among the 10 patients with histologically diagnosed GEA, a radial hysterectomy was performed in seven, a trachelectomy in one, and conization in two patients. Among the 12 UEA patients, eight (8/12. 66.7%) underwent radical hysterectomy, three (3/12, 25.0%) total hysterectomy, and one (1/12, 8.3%) conization. Based on Revised International Federation of Gynecology and Obstetrics 2018 staging [8], nine GEA patients (9/10, 90.0%) were diagnosed with stage II or higher and nine UEA patients (9/12, 75.0%) with stage I tumor. Follow-up information was available in 9 GEA and 10 UEA patients with follow-up times ranging from 10–45 months (mean, 22.3 months) and 15–49 months (mean, 24.9 months), respectively. One GEA patient and two UEA patients were lost to follow-up. The three GEA patients who underwent radical hysterectomy and 1 GEA patient who had conization received chemoradiation therapy. Four GEA patients died of the disease 17–38 months postoperatively. Nine UEA patients (9/12, 75.0%) had no evidence of disease and 1 UEA patient died of stomach cancer 11 months after conization (Tables 1, 2).
- HPV testing
- HPV tests were performed on cytologic or surgical specimens from patients. Among 10 GEA patients, high-risk HPV was not detected in nine subjects. High-risk HPV DNA detection based on in situ hybridization was negative in one patient (case No. 4). Among 12 UEA cases, HPV 18 was positive in nine patients and HPV 16 in one patient. High-risk HPV DNA was detected based on in situ hybridization using a paraffin block in the remaining two UEA cases (Tables 1, 2). Detection of high-risk HPV based on in situ hybridization showed punctate nuclear signals in tumor nuclei indicating the presence of high-risk HPV DNA.
- Histopathological and immunohistochemical findings
- Histologically, the GEA case (case No. 4) consisted of irregular angulated glands invading the cervical stroma (Fig. 1A). Nine GEA cases exhibited a negative or patchy immunohistochemical reaction for p16 (Fig. 1B) but one case showed block-type positivity for p16. All UEA cases showed block-type positivity for p16. Aberrant p53 (overexpression or null pattern) expression was observed in six GEA cases (60%): overexpression in five cases (Fig. 1C) and a null pattern in one case. All UEA cases showed patchy positive reactions for p53. Eight GEA cases showed diffuse or focal positive reactions for MUC6 (Fig. 1D) but two cases were negative (cases Nos. 2 and 7). The 2 MUC6 negative cases (Fig. 1E) showed pale pinkish-red cytoplasmic neutral mucin on Alcian blue/PAS special staining (Tables 1, 2, Fig. 1F).
- Cytologic features
- Preoperative cytologic diagnoses of the eight GEA cases were as follows: atypical glandular cells (AGCs), favor neoplastic in three cases, adenocarcinoma in situ (AIS) in one case, and adenocarcinoma in four cases.
- Cytologic findings of GEA and UEA cases are summarized in Table 3.
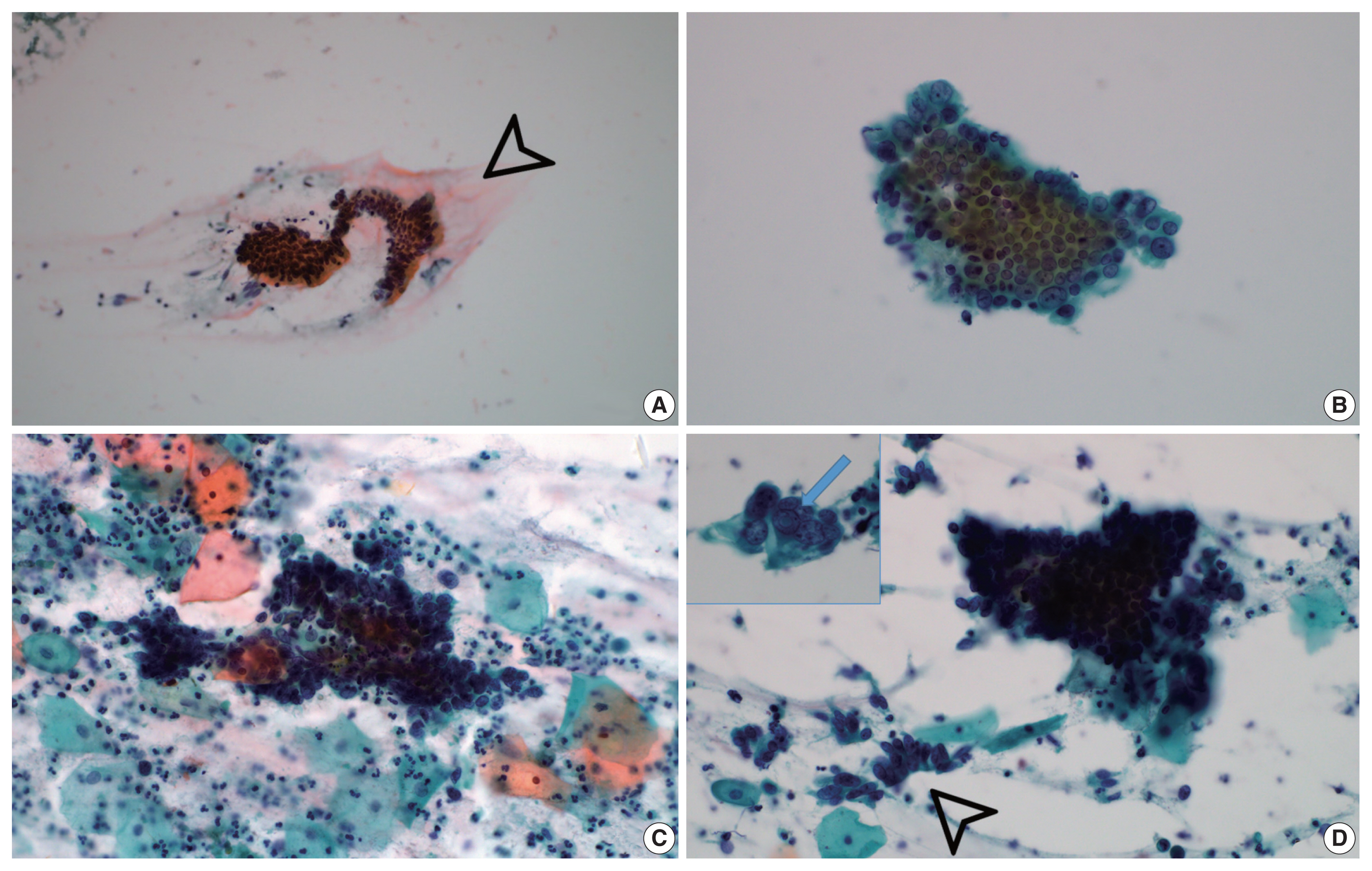
- Background extracellular mucin (Fig. 3A) was identified in six GEA and 10 UEA cases. This finding was more extensive in GEA (4/8) than UEA patients (1/12). However, the difference was not statistically significant (p = .058).
- Monolayered honeycomb sheets of atypical endocervical cells (Fig. 3B) were identified in eight cases and were extensive in 87.5% (7/8) of GEA patients; however, these were identified in only 50.0% (6/12) of UEA patients and were extensive in only one UEA case (8.3%) (p = .002). Three-dimensional clusters of hyperchromatic nuclei were identified in 8 GEA and 12 UEA cases (Fig. 3C) and were more extensive in UEA (11/12) than in GEA patients (3/8; p = .010). Feathering clusters were also observed in 5/8 GEA cases and 9/12 UEA cases but were extensive in only 4/12 UEA cases (p = .189) (Fig. 3D).
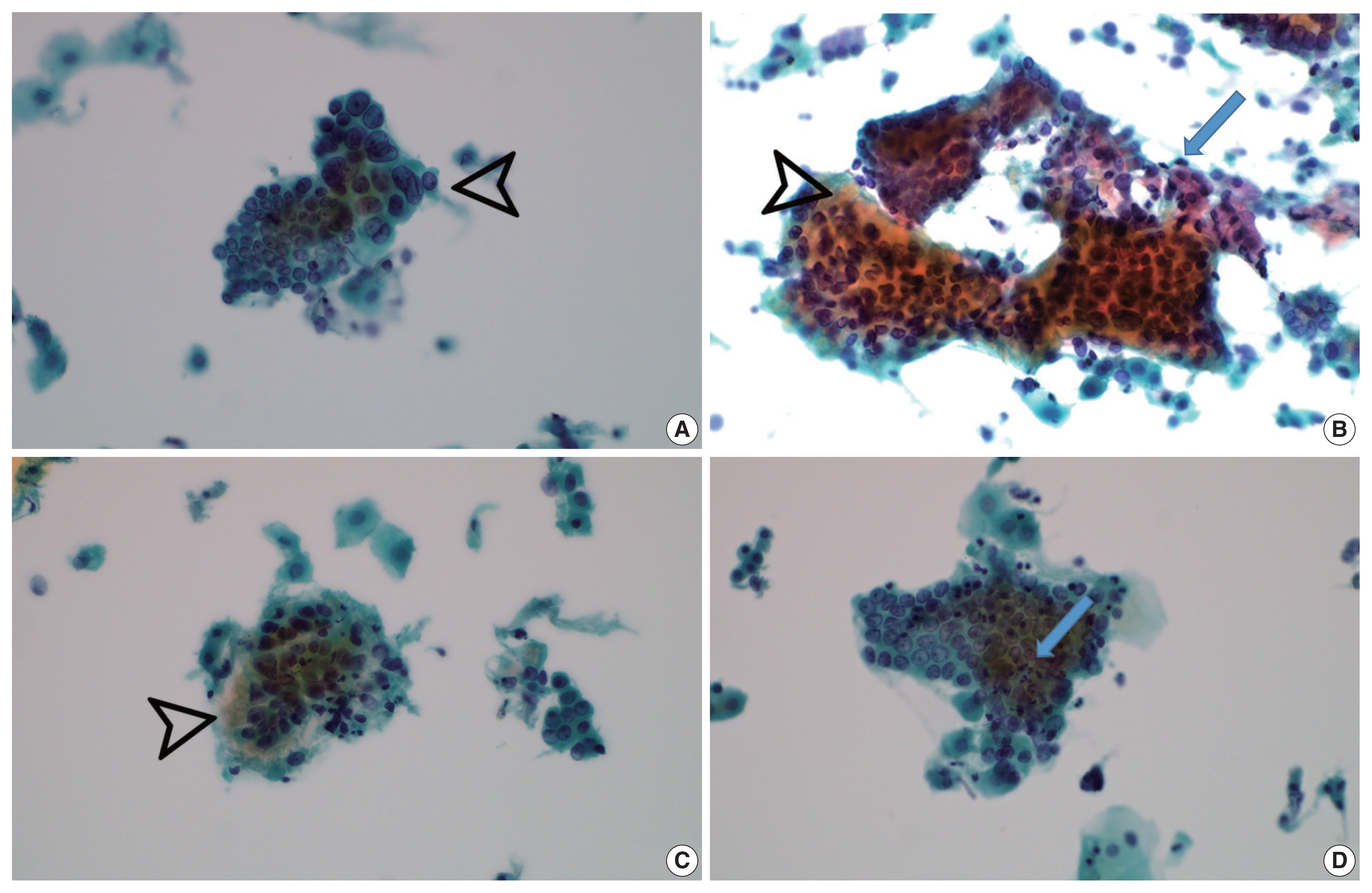
- Vesicular tumor cell nuclei were extensive in all GEA patients (8/8) (Fig. 4A) but were also identified in 11/12 UEA patients and were extensive in six subjects (p = .057). Nuclear hyperchromasia was present in all UEA patients (12/12) and extensive in three patients. Nuclear hyperchromasia was also present in six GEA cases (6/8) but not extensive in any GEA case (p = .082). Nuclear grooves were present in all GEA patients (8/8) and extensive in two patients (Fig. 4A). Among UEA cases, nuclear grooves were also present in 9/12 patients and extensive in one patient (p = .230). Prominent nucleoli were present in 8/8 GEA patients and extensive in five patients. Among UEA cases, nucleoli were present in 9/12 cases and extensive in three patients (p = .146). INCIs were found in one GEA case and one UEA case (p = .761). Among nuclear features, vesicular nuclei tended to be extensive in GEA cases but without statistical significance. Nuclear hyperchromasia was more extensive in UEA cases but also without statistical significance.
- Vacuolar granular cytoplasm was extensive in 8/8 GEA patients (Fig. 4B). Vacuolar cytoplasm was also identified in 10/12 UEA cases but extensive in only two patients (p = .0.001). Cytoplasmic golden-brown mucin was identified in 5/8 GEA patients but extensive in only one patient (Fig. 4C). Among UEA cases, cytoplasmic golden-brown mucin was identified in only 2/12 cases and not extensive in any patient (p = .089). Intracytoplasmic neutrophils were identified in 8/8 GEA patients and prominent in six patients (Fig. 4D). Among UEA cases, intracytoplasmic neutrophils were also identified in 11 patients (11/12) and extensive in six patients (p = .461). Among cytoplasmic features, vacuolar granular cytoplasm was extensive in GEA cases (Table 3).
- Both conventional smear and LBP were performed in only 5 GEA cases. The cytologic findings of GEA in LBP compared with conventional smears included cleaner background with reduced background mucin and smaller cellular sheets or clusters. On conventional smears, two-toned mucin (golden-brown gastric-type and pink normal endocervical) was identified in GEA cases (Fig. 4B).
RESULTS
Mucinous background
Architectural findings
Nucleus
Cytoplasm
- According to a new World Health Organization classification, adenocarcinomas of the uterine cervix are divided into HPV-associated and HPV-independent types with different implications for prognosis [1]. The HPV-independent category includes gastric, clear cell, mesonephric, and endometrioid types [1]. GEA, representing the most common HPV-independent adenocarcinoma, is an aggressive tumor type with a poor prognosis regardless of the degree of differentiation [9]. GEA should not be graded but should be considered high-grade regardless of morphology [10].
- Due to widespread HPV vaccination, the proportion of HPV-independent GEA may increase, rendering recognition of GEA at an early stage even more important [11]. In the present study, cytological findings of HPV-independent GEA were analyzed and compared with HPV-associated UEA. Monolayered sheets (p = .002) of atypical endocervical cells with vacuolar granular cytoplasm (p = .001) were extensive in GEA; however, three-dimensional clusters (p = .010) were characteristic of UEA.
- Kawakami et al. [12] reviewed the cytologic features of 14 GEA cases compared with 20 UEA control cases based on conventional Pap smear preparations. The characteristic cytologic findings of GEA included monolayered and honeycomb sheets, vacuolar and/or foamy cytoplasm, intracytoplasmic neutrophil entrapment, and vesicular nuclei with prominent nucleoli. Using LBP, tall columnar epithelial cells with pale, foamy or vacuolated cytoplasm were the most common cytologic findings of GEA, followed by well-defined cytoplasmic borders [11]. Recently, Schwock et al. [13] reported cytomorphologic features of GEA based on LBP. The most discriminatory findings for GEA versus UEA were microvesicular cytoplasm (100% vs. 17%), honeycomb-like sheets (87% vs. 8%), prominent nucleoli (93% vs. 25%), and anisonucleosis (93% vs. 50%). UEA is cytologically characterized by syncytial aggregates and 3-dimensional clusters of malignant cells with enlarged pleomorphic nuclei, coarse chromatin, macronucleoli, and a finely vacuolated cytoplasm [7].
- In the present study, monolayered and honeycomb sheets of atypical endocervical cells were characteristic findings of GEA cases compared with UEA cases (p = .002). Three-dimensional clusters were significantly more extensive in UEA (11/12) than in GEA patients (3/8, p = .010).
- According to Kawakami et al. [12], a mucinous background was significantly more common in GEA than in UEA cases (p = .024) using the conventional method. In contrast, the so-called “background” extracellular mucin may not be easily detectable in LBP versus direct smears due to preparation-related, technical causes [13]. Background mucin may clump and cling to tumor cells in LBP instead of the diffuse distribution observed in direct smears [14]. In general, the diagnostic usefulness of extracellular mucin is doubtful [13]. In the present study, a background extracellular mucin was identified in 6 GEA and 10 UEA cases. This finding was more extensive in GEA (4/8) than in UEA patients (1/12); however, the difference was not statistically significant (p = .058). The cytologic findings of GEA in LBP compared with conventional smears include cleaner background with reduced background mucin and smaller cellular sheets or clusters. Recently, the diagnostic characteristics to detect GEA using the conventional approach, namely distinct cell borders and prominent nucleoli, were reportedly not useful for excluding UEA in LBP samples. The conventional direct smear provides diagnostic indicators of GEA compared with LBP [15].
- Golden-brown mucin on Pap smears appears to represent a gastric phenotype of endocervical glandular cells that is a unique characteristic shared by MDA and pyloric gland metaplasia. Golden-brown mucin is also present in the context of benign gastric-type glandular proliferations [16]. On conventional Pap smears, identification of two-toned mucin (yellow gastric-type and pink normal endocervical) has been proposed a diagnostic indicator for lesions with gastric differentiation. However, recognizing yellow mucin on LBP is difficult because the mucin color becomes paler [17]. Golden-brown mucin may not be a particularly sensitive feature for GEA detection, at least in LBP [11]. In the present study, two-toned mucin was identified on conventional smears of GEA cases.
- Golden-brown intracytoplasmic mucin was occasionally observed in GEA patients (6/14, 42.9%) but observed in only 1 UEA patient (1/20, 5%; p = .034) [12]. Golden-brown mucin (20%), INCIs (20%), and goblet/Paneth-like cells (20%), although uncommon, represented unique features identified only in GEA [13]. In the present study, cytoplasmic golden-brown mucin was identified in 5/8 GEA cases and only 2/12 UEA cases; however, the difference was not statistically significant (p = .089).
- Intracytoplasmic neutrophil entrapment/phagocytosis was identified in 93% of GEA and 70% of UEA cases (which included endometrial cytology in 12/20 cases). Marked intracytoplasmic neutrophil entrapment was more common in GEA (7/14, 50%) than in UEA cases (2/20, 10%; p = .038) [12]. In contrast, neutrophil entrapment/phagocytosis was identified in a minority (33%) of both GEA and UEA cases [13]. In the present study, intracytoplasmic neutrophils among GEA and UEA cases were not statistically significant (p = .461), indicating this feature is of minimal utility for distinguishing between GEA and UEA.
- Lobular endocervical glandular hyperplasia (LEGH) is a cervical lesion with pyloric gland metaplasia. Abundant yellow mucin was frequently present in both LEGH and MDA; however, INCIs were found in 22/24 LEGH cases and not found in either MDA or adenocarcinoma cells associated with LEGH [18]. INCIs were identified in a minority (20%) of GEA cases and not in any UEA case [13]. In the present study, INCIs were identified in 1 GEA case and 1 UEA case (p = .761) (Fig. 3D).
- Cytologic diagnosis of GEA is problematic and may be difficult to recognize in cytologic specimens. In the present study, preoperative cytologic diagnoses included AGCs, favor neoplastic in three cases, AIS in one case, and adenocarcinoma in four cases. Preoperative cytologic diagnoses of the previously reported GEA cases were reviewed [11–13,15] including the eight cases used in this study (Table 4). Among a total of 56 cases, the original cytologic interpretations were the following: negative for intraepithelial lesion or malignancy (n = 10, 17.9%); AGC, not otherwise specified/favor neoplastic (n = 16, 28.6%); AIS (n = 3, 5.4%); high-grade squamous intraepithelial lesion (n = 1, 1.8%); adenocarcinoma (n = 25, 44.6%); unsatisfactory for evaluation (n = 1, 1.8%) (Table 4).
- In cytologic differential diagnosis of GEA, non-neoplastic atypical glandular changes and syncytial aggregates/hyperchromatic crowded groups (HCGs) should also be considered. In patients with an intrauterine device, the endocervical cells are large and have smooth nuclei with smudged chromatin, prominent nucleoli, and occasional nuclear clefts. Large degenerative cytoplasmic vacuoles push the nucleus toward the periphery [7]. In a reparative process, sheets of atypical endocervical cells have enlarged nuclei with increased nuclear to cytoplasmic ratios, sometimes multiple nucleoli, and mitotic activity. A regular honeycomb arrangement of reactive endocervical cells is also a cytologic feature of non-neoplastic HCGs [19]. Endocervical or endometrial cells presenting as HCGs may mimic glandular high-grade precancers. Atypical endocervical cells associated with tubal metaplasia can be challenging due to nuclear overlapping and crowding of enlarged, variably sized nuclei; however, identification of cilia and pseudostratified nuclei can be helpful [7].
- In conclusion, GEA can be recognized based on cytologic features of monolayered honeycomb sheets of atypical endocervical cells with abundant vacuolar cytoplasm and some golden-brown intracytoplasmic mucin. In contrast, three-dimensional clusters, feathering, and nuclear hyperchromasia tended to be extensive in UEA. Awareness of the cytomorphologic features of GEA will allow pathologists to recognize and accurately diagnose this rare and aggressive entity.
DISCUSSION
Ethics Statement
Ethical approval for the study was obtained from the ethics committee of Chungnam National University Hospital (CNUH-IRB 2021-12-001) (Daejeon, Republic of Korea). An exemption from informed consent was also approved.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Author Contributions
Conceptualization: MKY, KSS. Data curation: DHK, IOS. Funding acquisition: KSS. Investigation: DHK, IOS. Methodology: MKY, GEB, KSS. Project administration: KSS. Resources: KSS, IOS. Supervision: KSS. Validation: MKY, GEB. Visualization: DHK, KSS. Writing—original draft: MKY, KSS. Writing—review & editing: MKY, KSS. Approval of final manuscript: all authors.
Funding Statement
This work was supported by research fund of Chungnam National University.
| Clinicopathologic findings | GEA (n = 10) | UEA (n = 12) |
|---|---|---|
| Age (yr) | ||
| Mean ± SD | 61.7 ± 11.9 | 45.3 ± 10.9 |
| Range | 48–80 | 31–68 |
| Type of surgical treatment | ||
| Radical hysterectomy | 7 (70.0) | 8 (66.7) |
| Total hysterectomy | 0 | 3 (25.0) |
| Trachelectomy | 1 (10.0) | 0 |
| Conization | 2 (20.0) | 1 (8.3) |
| FIGO stage | ||
| I | 1 (10.0) | 9 (75.0) |
| II | 4 (40.0) | 1 (8.3) |
| III | 3 (30.0) | 2 (16.7) |
| IV | 2 (20.0) | 0 |
| Human papillomavirus status | ||
| High-risk HPV | 0 | 12 (100) |
| Not detected | 10 (100) | 0 |
| p16 expression | ||
| Block positive | 1 (10.0) | 12 (100) |
| Patchy positive/negative | 9 (90.0) | 0 |
| p53 expression | ||
| Diffuse strong positive | 5 (50.0) | 0 |
| Complete loss (null) | 1 (10.0) | 0 |
| Patchy positive | 4 (40.0) | 12 (100) |
| Follow-up results | ||
| No evidence of disease | 2 (20.0) | 9 (75.0) |
| Alive with disease | 3 (30.0) | 0 (0.0) |
| Died of disease | 4 (40.0) | 0 (0.0) |
| Died of other disease | 0 | 1a (8.3) |
| Not available | 1 (10.0) | 2 (16.7) |
| Case No. | Age (yr) | Preparation type | Cytologic diagnosis | Type of surgery | FIGO stage | HPV | p16 | p53 | MUC-6 | Adjuvant treatment | Follow-up (mo) | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| CS | LBP | ||||||||||||
| 1 | 48 | + | + | AIS | Trachelectomy | IVA | − | − | + | + | CC | 20 | DOD |
| 2 | 80 | + | + | Adenocarcinoma | RH | IIB | − | − | Wild | −a | RT | 38 | DOD |
| 3 | 52 | + | + | AGC | RH | IIIC1 | − | − | Wild | + | CCRT | 48 | AWD |
| 4 | 58 | + | + | Adenocarcinoma | RH | IVA | −b | − | + | + | CC | 23 | DOD |
| 5 | 54 | + | + | Adenocarcinoma | RH | IIA1 | − | + | + | + | NA | FU loss | NA |
| 6 | 54 | NA | NA | NA | Conization | IIIC1 | − | − | Wild | + | CCRT | 17 | DOD |
| 7 | 78 | NA | + | Adenocarcinoma | Conization | IIA2 | − | − | + | −a | RT | 21 | NED |
| 8 | 63 | NA | + | AGC | RH | IB1 | − | − | Null | + | CCRT | 21 | NED |
| 9 | 76 | NA | + | AGC | RH | IIB | − | − | + | + | RT | 10 | AWD |
| 10 | 54 | NA | NA | NA | RH | IIIC1 | − | − | Wild | + | CCRT | 10 | AWD |
CS, conventional smear; LBP, liquid-based preparation; FIGO, International Federation of Gynecology and Obstetrics; HPV, human papillomavirus; AIS, adenocarcinoma in situ; CC, combined chemotherapy; DOD, died of disease; RH, radical hysterectomy; RT, radiotherapy; AGC, atypical glandular cells, favor neoplastic; CCRT, combined chemotherapy and radiotherapy; AWD, alive with disease; NA, not available; FU, follow-up; NED, no evidence of disease.
aPale pinkish-red cytoplasmic neutral mucin on Alcian blue/PAS special staining;
bHigh risk HPV DNA in situ hybridization; +, p16, block-type positivity; +, p53, overexpression.
| Kawakami et al. [12] | Lu et al. [11] | Schwock et al. [13] | Ryu et al. [15] | This study | Total, n (%) | |
|---|---|---|---|---|---|---|
| No. of cases | 14 | 11 | 15 | 8 | 8 | 56 (100) |
| Preparation type | CS | LBC | CS and LBC | CS and LBC | CS and/or LBC | CS and/or LBC |
| TBS classification | ||||||
| Unsatisfactory | 0 | 1 | 0 | 0 | 0 | 1 (1.8) |
| NILM | 0 | 5 | 5 | 0 | 0 | 10 (17.9) |
| AGC | 3 | 4 | 2 | 4 | 3 | 16 (28.6) |
| AIS | 0 | 0 | 1 | 1 | 1 | 3 (5.4) |
| HSIL | 0 | 1 | 0 | 0 | 0 | 1 (1.8) |
| Adenocarcinoma | 11 | 0 | 7 | 3 | 4 | 25 (44.6) |
GEA, gastric-type endocervical adenocarcinoma; CS, conventional smear; LBC, liquid-based cytology; TBS, The Bethesda System; NILM, negative for intraepithelial lesion or malignancy; AGC, atypical glandular cells, not otherwise specified and favor adenocarcinoma; AIS, adenocarcinoma in situ; HSIL, high-grade squamous intraepithelial lesion.
- 1. WHO. Classification of Tumours Editorial Board WHO classification of tumours, 5th ed Female genital tumours. Lyon: International Agency for Research on Cancer, 2020.
- 2. Kojima A, Mikami Y, Sudo T, et al. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol 2007; 31: 664-72. ArticlePubMed
- 3. Kawakami F, Mikami Y, Kojima A, Ito M, Nishimura R, Manabe T. Diagnostic reproducibility in gastric-type mucinous adenocarcinoma of the uterine cervix: validation of novel diagnostic criteria. Histopathology 2010; 56: 551-3. ArticlePubMed
- 4. Kondo T, Hashi A, Murata SI, et al. Gastric mucin is expressed in a subset of endocervical tunnel clusters: type A tunnel clusters of gastric phenotype. Histopathology 2007; 50: 843-50. ArticlePubMed
- 5. Hodgson A, Parra-Herran C, Mirkovic J. Immunohistochemical expression of HIK1083 and MUC6 in endometrial carcinomas. Histopathology 2019; 75: 552-8. ArticlePubMedPDF
- 6. Castanon A, Landy R, Sasieni PD. Is cervical screening preventing adenocarcinoma and adenosquamous carcinoma of the cervix? Int J Cancer 2016; 139: 1040-5. ArticlePubMedPMCPDF
- 7. Wilbur DC, Chhieng DC, Guidos B, Mody DR. Epithelial abnormalities: glandular. In: Nayar R, Wilbur DC, eds. The Bethesda system for reporting cervical cytology: definitions, criteria, and explanatory notes. 3rd ed. Heidelberg: Springer, 2015; 193-240. Article
- 8. Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet 2019; 145: 129-35. ArticlePubMedPDF
- 9. Stolnicu S, Barsan I, Hoang L, et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC): a new pathogenetic classification for invasive adenocarcinomas of the endocervix. Am J Surg Pathol 2018; 42: 214-26. PubMedPMC
- 10. Talia KL, Oliva E, Rabban JT, Singh N, Stolnicu S, McCluggage WG. Grading of endocervical adenocarcinomas: review of the literature and recommendations from the International Society of Gynecological Pathologists. Int J Gynecol Pathol 2021; 40(Suppl 1):S66-74. ArticlePubMedPMC
- 11. Lu S, Shen D, Zhao Y, Kang N, Wang X. Primary endocervical gastric-type adenocarcinoma: a clinicopathologic and immunohistochemical analysis of 23 cases. Diagn Pathol 2019; 14: 72.ArticlePubMedPMCPDF
- 12. Kawakami F, Mikami Y, Sudo T, Fujiwara K, Hirose T, Itoh T. Cytologic features of gastric-type adenocarcinoma of the uterine cervix. Diagn Cytopathol 2015; 43: 791-6. ArticlePubMed
- 13. Schwock J, Starova B, Khan ZF, et al. Cytomorphologic features of gastric-type endocervical adenocarcinoma in liquid-based preparations. Acta Cytol 2021; 65: 56-66. ArticlePubMedPMCPDF
- 14. Hissong E, Yoxtheimer LM, Pacecca A, Hoda RS. Cytology of minimal deviation endocervical adenocarcinoma (adenoma malignum) on a ThinPrep Pap test. Diagn Cytopathol 2016; 44: 552-5. ArticlePubMed
- 15. Ryu A, Nagata S, Kubo C, et al. Conventional direct smear yields diagnostic indicators of gastric-type mucinous carcinoma compared with cytomorphological features identified by liquid-based cervical cytology. Acta Cytol 2021; 65: 150-7. ArticlePubMedPDF
- 16. Hata S, Mikami Y, Manabe T. Diagnostic significance of endocervical glandular cells with “golden-yellow” mucin on pap smear. Diagn Cytopathol 2002; 27: 80-4. ArticlePubMed
- 17. Omori M, Kondo T, Nakazawa K, et al. Interpretation of endocervical cells with gastric-type mucin on Pap smears. Am J Clin Pathol 2018; 150: 259-66. ArticlePubMed
- 18. Hashi A, Yuminamochi T, Xu JY, Kondo T, Katoh R, Hoshi K. Intranuclear cytoplasmic inclusion is a significant diagnostic feature for the differentiation of lobular endocervical glandular hyperplasia from minimal deviation adenocarcinoma of the cervix. Diagn Cytopathol 2008; 36: 535-44. ArticlePubMed
- 19. Lee Y, Lee C, Park IA, An HJ, Kim H. Cytomorphological features of hyperchromatic crowded groups in liquid-based cervicovaginal cytology: a single institutional experience. J Pathol Transl Med 2019; 53: 393-8. ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations

- A Comparative Analysis of Usual- and Gastric-Type Cervical Adenocarcinoma in a Japanese Population Reveals Distinct Clinicopathological and Molecular Features with Prognostic and Therapeutic Insights
Umme Farzana Zahan, Hasibul Islam Sohel, Kentaro Nakayama, Masako Ishikawa, Mamiko Nagase, Sultana Razia, Kosuke Kanno, Hitomi Yamashita, Shahataj Begum Sonia, Satoru Kyo
International Journal of Molecular Sciences.2025; 26(15): 7469. CrossRef - Diagnostic value of cytology in detecting human papillomavirus–independent cervical malignancies: a nation-wide study in Korea
Hye-Ra Jung, Junyoung Shin, Chong Woo Yoo, Eun Na Kim, Cheol Lee, Kyeongmin Kim, Ho-chang Lee, Yonghee Lee, Ji Hye Kim, Soo Jin Jung, Yumin Chung, Joo Yeon Kim, Hye Eun Park, Tae Hoen Kim, Wonae Lee, Min-Sun Cho, Ran Hong, Yoon Jung Choi, Younghee Choi, Y
Journal of Pathology and Translational Medicine.2025; 59(6): 444. CrossRef - Risk Factors Affecting Clinical Outcomes of Low-risk Early-stage Human Papillomavirus–Associated Endocervical Adenocarcinoma Treated by Surgery Alone: Application of Silva Pattern
Bong Kyung Bae, Hyunsik Bae, Won Kyung Cho, Byoung-Gie Kim, Chel Hun Choi, Tae-Joong Kim, Yoo-Young Lee, Jeong-Won Lee, Hyun-Soo Kim, Won Park
International Journal of Gynecological Pathology.2024; 43(5): 447. CrossRef - Tall‐columnar glandular cells in SurePath™ liquid‐based cytology Pap sample: Learning from mimics/pitfalls
Nalini Gupta, Vanita Jain, Radhika Srinivasan, Tulika Singh
Cytopathology.2024; 35(4): 510. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
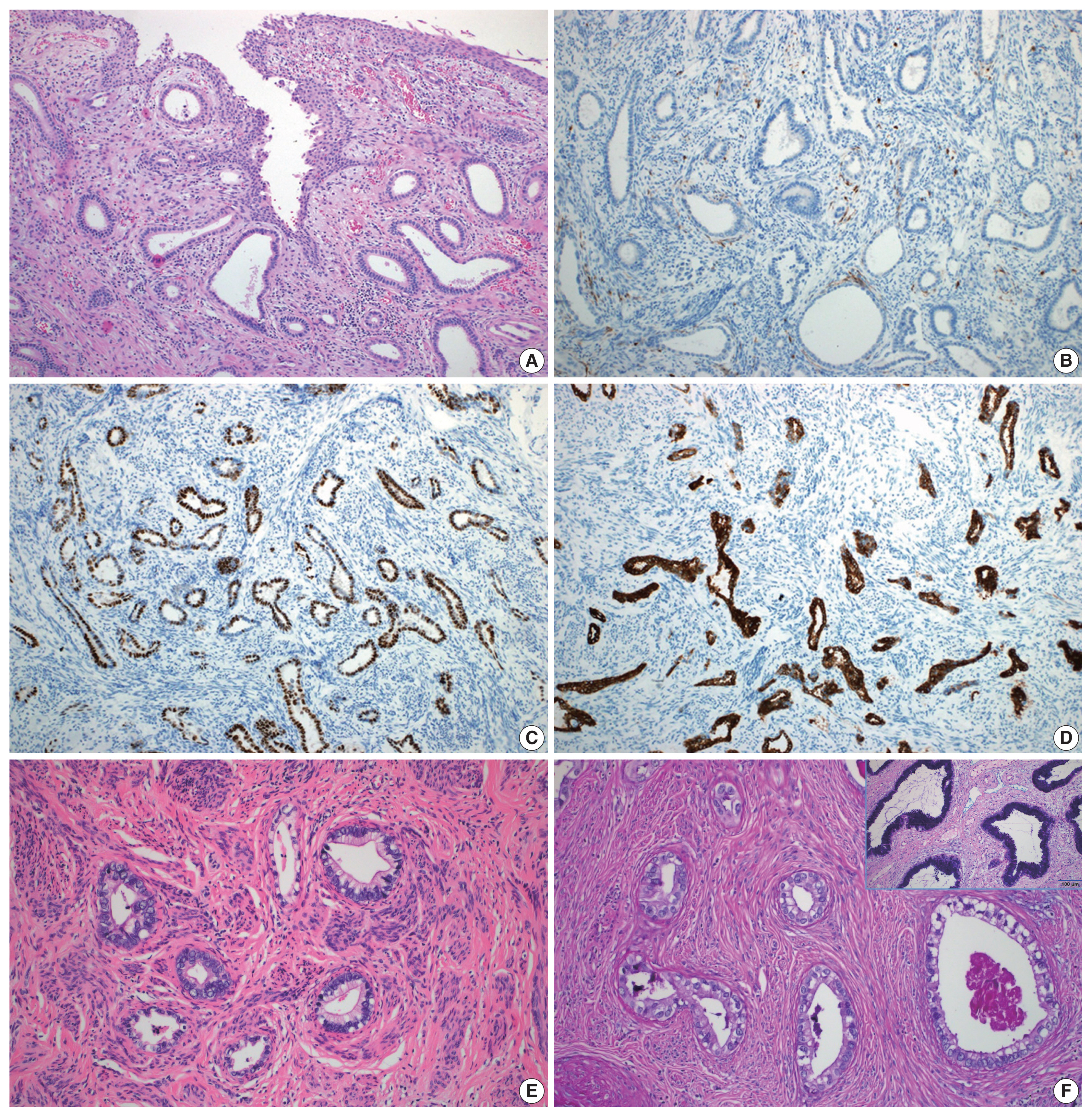
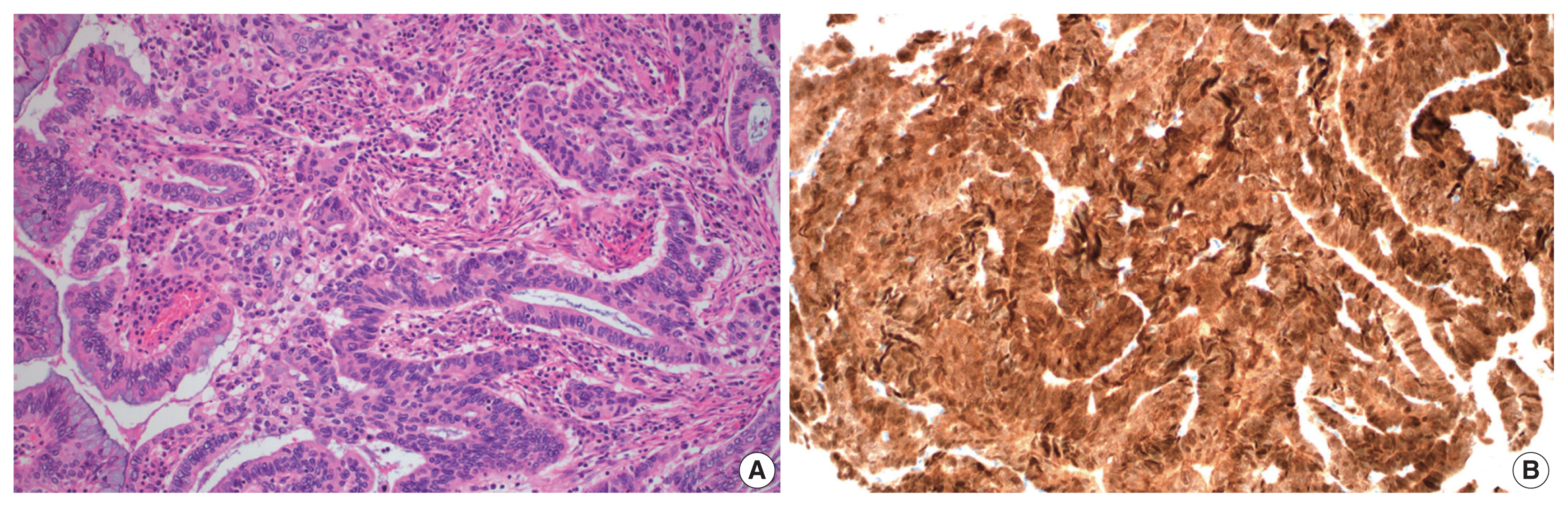
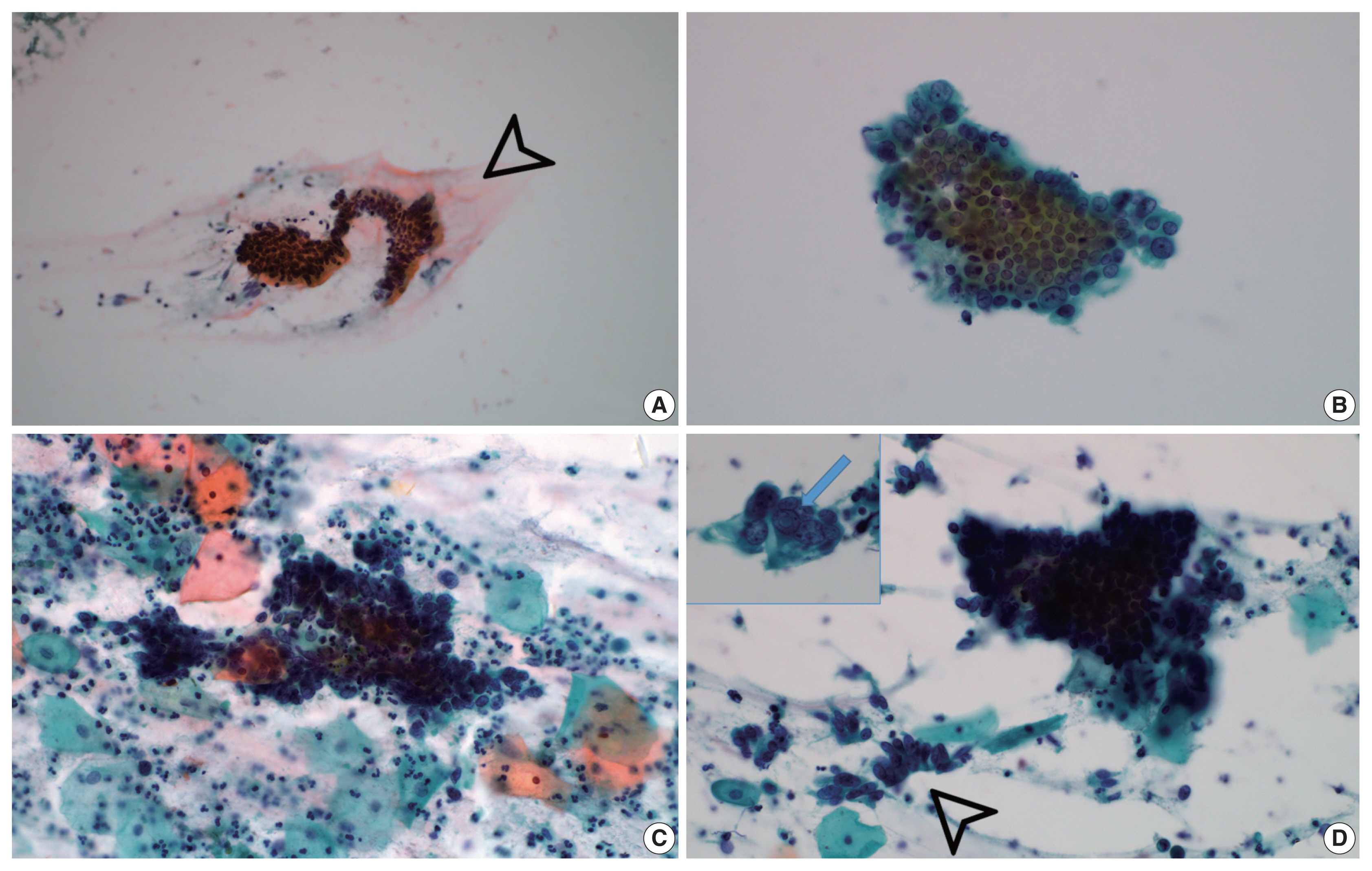
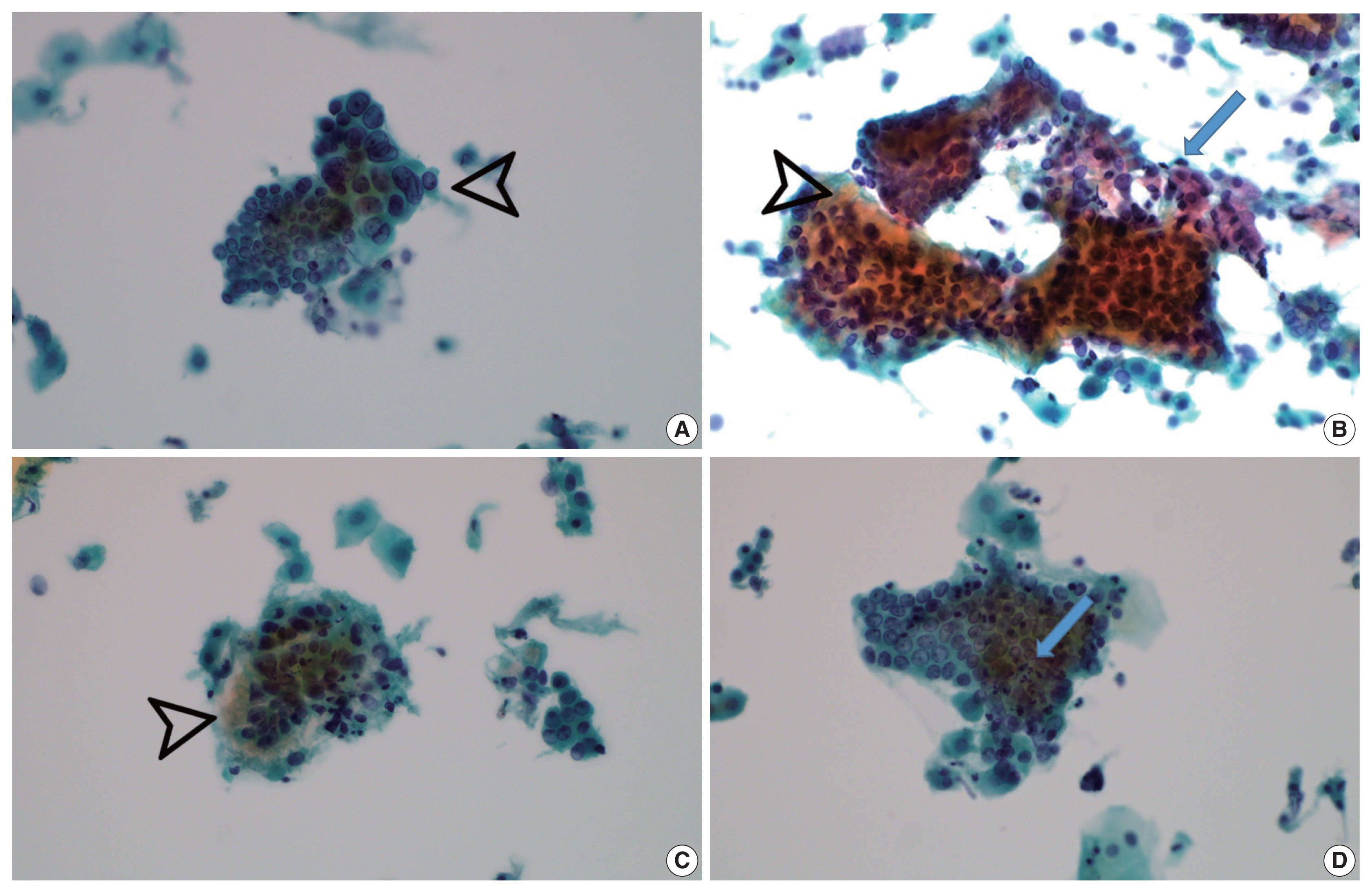
Fig. 1
Fig. 2
Fig. 3
Fig. 4
| Clinicopathologic findings | GEA (n = 10) | UEA (n = 12) |
|---|---|---|
| Age (yr) | ||
| Mean ± SD | 61.7 ± 11.9 | 45.3 ± 10.9 |
| Range | 48–80 | 31–68 |
| Type of surgical treatment | ||
| Radical hysterectomy | 7 (70.0) | 8 (66.7) |
| Total hysterectomy | 0 | 3 (25.0) |
| Trachelectomy | 1 (10.0) | 0 |
| Conization | 2 (20.0) | 1 (8.3) |
| FIGO stage | ||
| I | 1 (10.0) | 9 (75.0) |
| II | 4 (40.0) | 1 (8.3) |
| III | 3 (30.0) | 2 (16.7) |
| IV | 2 (20.0) | 0 |
| Human papillomavirus status | ||
| High-risk HPV | 0 | 12 (100) |
| Not detected | 10 (100) | 0 |
| p16 expression | ||
| Block positive | 1 (10.0) | 12 (100) |
| Patchy positive/negative | 9 (90.0) | 0 |
| p53 expression | ||
| Diffuse strong positive | 5 (50.0) | 0 |
| Complete loss (null) | 1 (10.0) | 0 |
| Patchy positive | 4 (40.0) | 12 (100) |
| Follow-up results | ||
| No evidence of disease | 2 (20.0) | 9 (75.0) |
| Alive with disease | 3 (30.0) | 0 (0.0) |
| Died of disease | 4 (40.0) | 0 (0.0) |
| Died of other disease | 0 | 1 |
| Not available | 1 (10.0) | 2 (16.7) |
| Case No. | Age (yr) | Preparation type | Cytologic diagnosis | Type of surgery | FIGO stage | HPV | p16 | p53 | MUC-6 | Adjuvant treatment | Follow-up (mo) | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| CS | LBP | ||||||||||||
| 1 | 48 | + | + | AIS | Trachelectomy | IVA | − | − | + | + | CC | 20 | DOD |
| 2 | 80 | + | + | Adenocarcinoma | RH | IIB | − | − | Wild | − |
RT | 38 | DOD |
| 3 | 52 | + | + | AGC | RH | IIIC1 | − | − | Wild | + | CCRT | 48 | AWD |
| 4 | 58 | + | + | Adenocarcinoma | RH | IVA | − |
− | + | + | CC | 23 | DOD |
| 5 | 54 | + | + | Adenocarcinoma | RH | IIA1 | − | + | + | + | NA | FU loss | NA |
| 6 | 54 | NA | NA | NA | Conization | IIIC1 | − | − | Wild | + | CCRT | 17 | DOD |
| 7 | 78 | NA | + | Adenocarcinoma | Conization | IIA2 | − | − | + | − |
RT | 21 | NED |
| 8 | 63 | NA | + | AGC | RH | IB1 | − | − | Null | + | CCRT | 21 | NED |
| 9 | 76 | NA | + | AGC | RH | IIB | − | − | + | + | RT | 10 | AWD |
| 10 | 54 | NA | NA | NA | RH | IIIC1 | − | − | Wild | + | CCRT | 10 | AWD |
| Cytologic finding | GEA (n=8) | UEA (n=12) | p-value |
|---|---|---|---|
| Mucinous background | .058 | ||
| Absent | 2 (25.0) | 2 (16.7) | |
| Focal | 2 (25.0) | 9 (75.0) | |
| Extensive | 4 (50.0) | 1 (8.3) | |
| Architecture | |||
| Monolayered honeycomb-like sheet | .002 | ||
| Absent | 0 | 6 (50.0) | |
| Focal | 1 (12.5) | 5 (41.7) | |
| Extensive | 7 (87.5) | 1 (8.3) | |
| 3-dimensional clusters | .010 | ||
| Absent | 0 | 0 | |
| Focal | 5 (62.5) | 1 (8.3) | |
| Extensive | 3 (37.5) | 11 (91.7) | |
| Feathering | .189 | ||
| Absent | 3 (37.5) | 3 (25.0) | |
| Focal | 5 (62.5) | 5 (41.7) | |
| Extensive | 0 | 4 (33.3) | |
| Nuclei | |||
| Vesicular nuclei | .057 | ||
| Absent | 0 (0.0) | 1 (8.3) | |
| Focal | 0 (0.0) | 5 (41.7) | |
| Extensive | 8 (100.0) | 6 (50.0) | |
| Hyperchromasia | .082 | ||
| Absent | 2 (25.0) | 0 | |
| Focal | 6 (75.0) | 9 (75.0) | |
| Extensive | 0 (0.0) | 3 (25.0) | |
| Nuclear groove | .230 | ||
| Absent | 0 (0.0) | 3 (25.0) | |
| Focal | 6 (75.0) | 8 (66.7) | |
| Extensive | 2 (25.0) | 1 (8.3) | |
| Nucleoli | .146 | ||
| Absent | 0 (0.0) | 3 (25.0) | |
| Focal | 3 (37.5) | 6 (50.0) | |
| Extensive | 5 (62.5) | 3 (25.0) | |
| Intranuclear pseudoinclusion | .761 | ||
| Absent | 7 (87.5) | 11 (91.7) | |
| Focal | 1 (12.5) | 1 (8.3) | |
| Extensive | 0 | 0 | |
| Cytoplasm | |||
| Vacuolar/granular | .001 | ||
| Absent | 0 | 2 (16.7) | |
| Focal | 0 | 8 (66.7) | |
| Extensive | 8 (100) | 2 (16.7) | |
| Golden-brown mucin | .089 | ||
| Absent | 3 (37.5) | 10 (83.3) | |
| Focal | 4 (50.0) | 2 (16.7) | |
| Extensive | 1 (12.5) | 0 | |
| Neutrophils | .461 | ||
| Absent | 0 | 1 (8.3) | |
| Focal | 2 (25.0) | 5 (41.7) | |
| Extensive | 6 (75.0) | 6 (50.0) | |
| Kawakami et al. [ |
Lu et al. [ |
Schwock et al. [ |
Ryu et al. [ |
This study | Total, n (%) | |
|---|---|---|---|---|---|---|
| No. of cases | 14 | 11 | 15 | 8 | 8 | 56 (100) |
| Preparation type | CS | LBC | CS and LBC | CS and LBC | CS and/or LBC | CS and/or LBC |
| TBS classification | ||||||
| Unsatisfactory | 0 | 1 | 0 | 0 | 0 | 1 (1.8) |
| NILM | 0 | 5 | 5 | 0 | 0 | 10 (17.9) |
| AGC | 3 | 4 | 2 | 4 | 3 | 16 (28.6) |
| AIS | 0 | 0 | 1 | 1 | 1 | 3 (5.4) |
| HSIL | 0 | 1 | 0 | 0 | 0 | 1 (1.8) |
| Adenocarcinoma | 11 | 0 | 7 | 3 | 4 | 25 (44.6) |
Values are presented as number (%) unless otherwise indicated. GEA, gastric-type endocervical adenocarcinoma; UEA, usual-type endocervical adenocarcinoma; SD, standard deviation; FIGO, International Federation of Gynecology and Obstetrics; HPV, human papillomavirus. Died of stomach cancer.
CS, conventional smear; LBP, liquid-based preparation; FIGO, International Federation of Gynecology and Obstetrics; HPV, human papillomavirus; AIS, adenocarcinoma in situ; CC, combined chemotherapy; DOD, died of disease; RH, radical hysterectomy; RT, radiotherapy; AGC, atypical glandular cells, favor neoplastic; CCRT, combined chemotherapy and radiotherapy; AWD, alive with disease; NA, not available; FU, follow-up; NED, no evidence of disease. Pale pinkish-red cytoplasmic neutral mucin on Alcian blue/PAS special staining; High risk HPV DNA in situ hybridization; +, p16, block-type positivity; +, p53, overexpression.
GEA, gastric-type endocervical adenocarcinoma; CS, conventional smear; LBC, liquid-based cytology; TBS, The Bethesda System; NILM, negative for intraepithelial lesion or malignancy; AGC, atypical glandular cells, not otherwise specified and favor adenocarcinoma; AIS, adenocarcinoma in situ; HSIL, high-grade squamous intraepithelial lesion.

 E-submission
E-submission






