Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 58(2); 2024 > Article
-
Case Study
A rare goblet cell adenocarcinoma arising from Barrett’s esophagus: the first reported case in the esophagus -
Chi Eun Oh1
 , Sung Eun Kim2
, Sung Eun Kim2 , Sun-Ju Oh1
, Sun-Ju Oh1
-
Journal of Pathology and Translational Medicine 2024;58(2):81-86.
DOI: https://doi.org/10.4132/jptm.2023.12.26
Published online: January 8, 2024
1Department of Pathology, Kosin University College of Medicine, Busan, Korea
2Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea
- Corresponding Author: Sun-Ju Oh, MD, Department of Pathology, Kosin University College of Medicine, 262 Gamcheon-ro, Seo-gu, Busan 49267, Korea Tel: +82-51-990-6744, Fax: +82-51-990-3080, E-mail: 10highpowerfield@gmail.com
© 2024 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,847 Views
- 310 Download
Abstract
- Goblet cell adenocarcinoma (GCA) is a rare and distinctive amphicrine tumor comprised of goblet-like mucinous cells and neuroendocrine cells. It is believed to originate from pluripotent stem cells located at the base of crypts. GCA predominantly arises from the appendix, with a few reported cases in extra-appendiceal locations such as the colorectum, small intestine, and stomach. In this case report, we present a unique instance of a 64-year-old male who initially received a diagnosis of neuroendocrine carcinoma in the distal esophagus based on biopsy but, following resection, was subsequently re-diagnosed with GCA arising from Barrett’s esophagus.
- A 64-year-old man presented with epigastric pain of several months’ duration and was diagnosed with carcinoma on biopsy after endoscopy at a local clinic. He was referred to our clinic for subsequent treatment. In addition to the epigastric pain, the patient recently reported a sensation of food getting stuck in his in his throat but had no other abnormalities such as weight loss. The patient also reported that he had undergone a gastroscopy during a routine checkup 2 years prior to his visit, and there were no abnormal findings at that time.
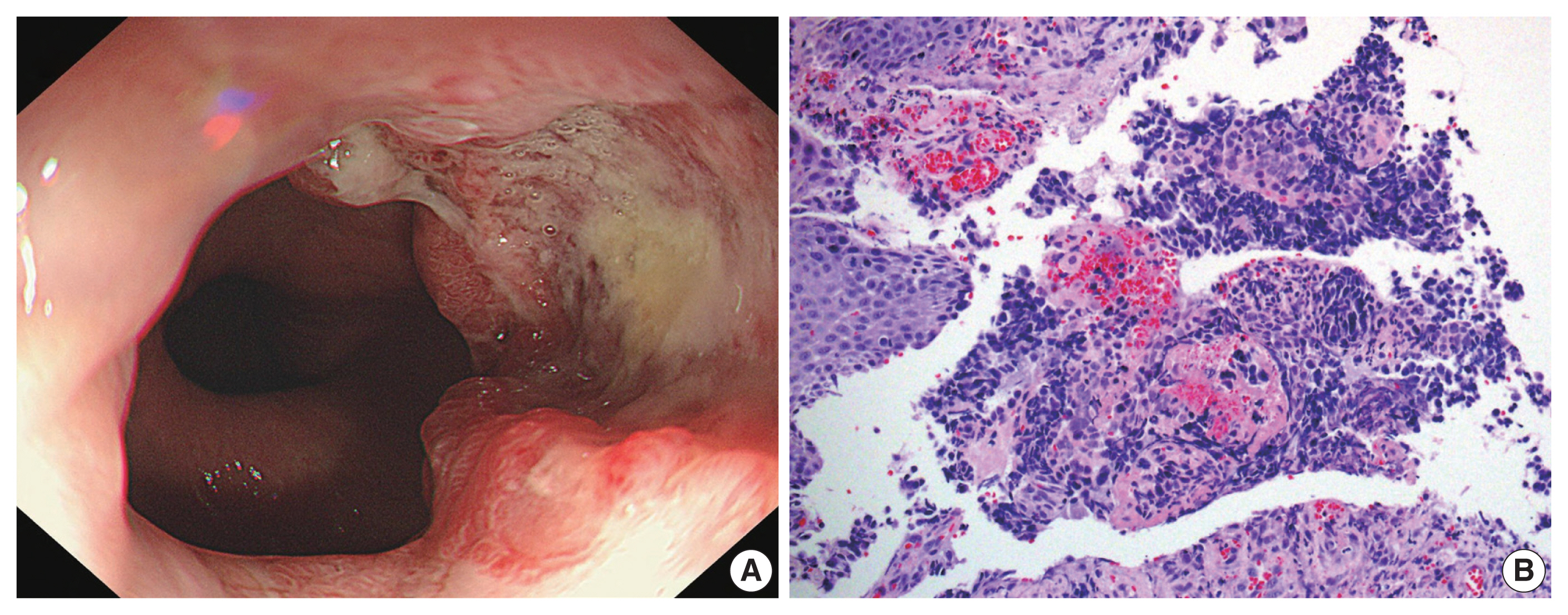
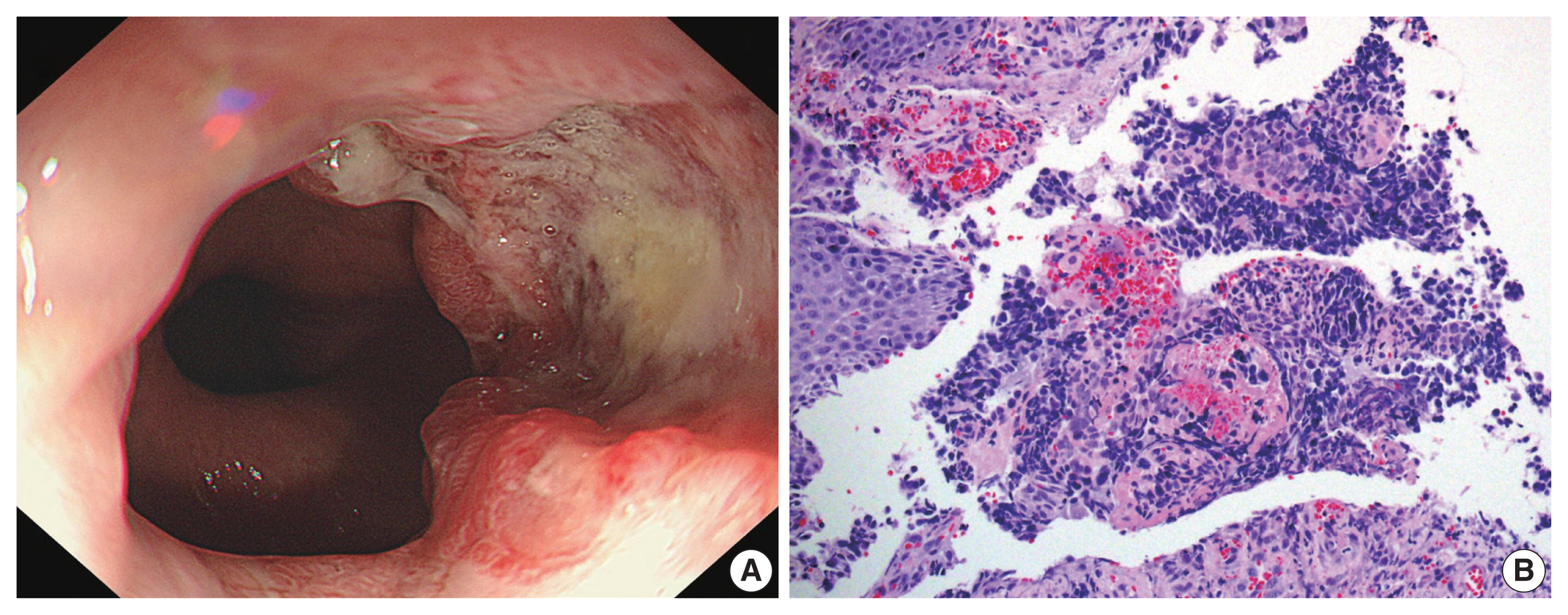
- A current gastroscopy revealed a mass measuring approximately 3.0 × 3.0 cm, displaying a central irregular ulcer and a raised, reddish nodular edge without clear boundaries (Fig. 1A). This mass encircled an area located 38–40 cm from the upper incisor. The biopsy showed sheets of small hyperchromatic cells with minimal cytoplasm and stippled chromatin (Fig. 1B). Immunochemical staining showed that the tumor cells were positive for cytokeratin, CD56, and synaptophysin and negative for chromogranin A. Initially, the patient was diagnosed with neuroendocrine carcinoma.
- Subsequently, a comprehensive evaluation for surgical intervention was conducted, encompassing diagnostic modalities such as chest and abdominal computed tomography (CT) as well as positron emission tomography–CT (PET-CT). These examinations revealed a tumor situated in the lower esophagus along with lymph nodes suspected to be indicative of metastatic involvement within the lower paraesophageal region and the left gastric region. Given the infeasibility of immediate surgical intervention due to the concurrent presence of severe hypothyroidism identified during the examination and the generally unfavorable prognosis associated with neuroendocrine carcinoma in the gastrointestinal tract, neoadjuvant chemotherapy was planned as a preparatory measure preceding the surgical procedure. Consequently, a regimen comprising a four-cycle administration of etoposide and cisplatin combination therapy over a span of 2 months was implemented prior to the surgical intervention.
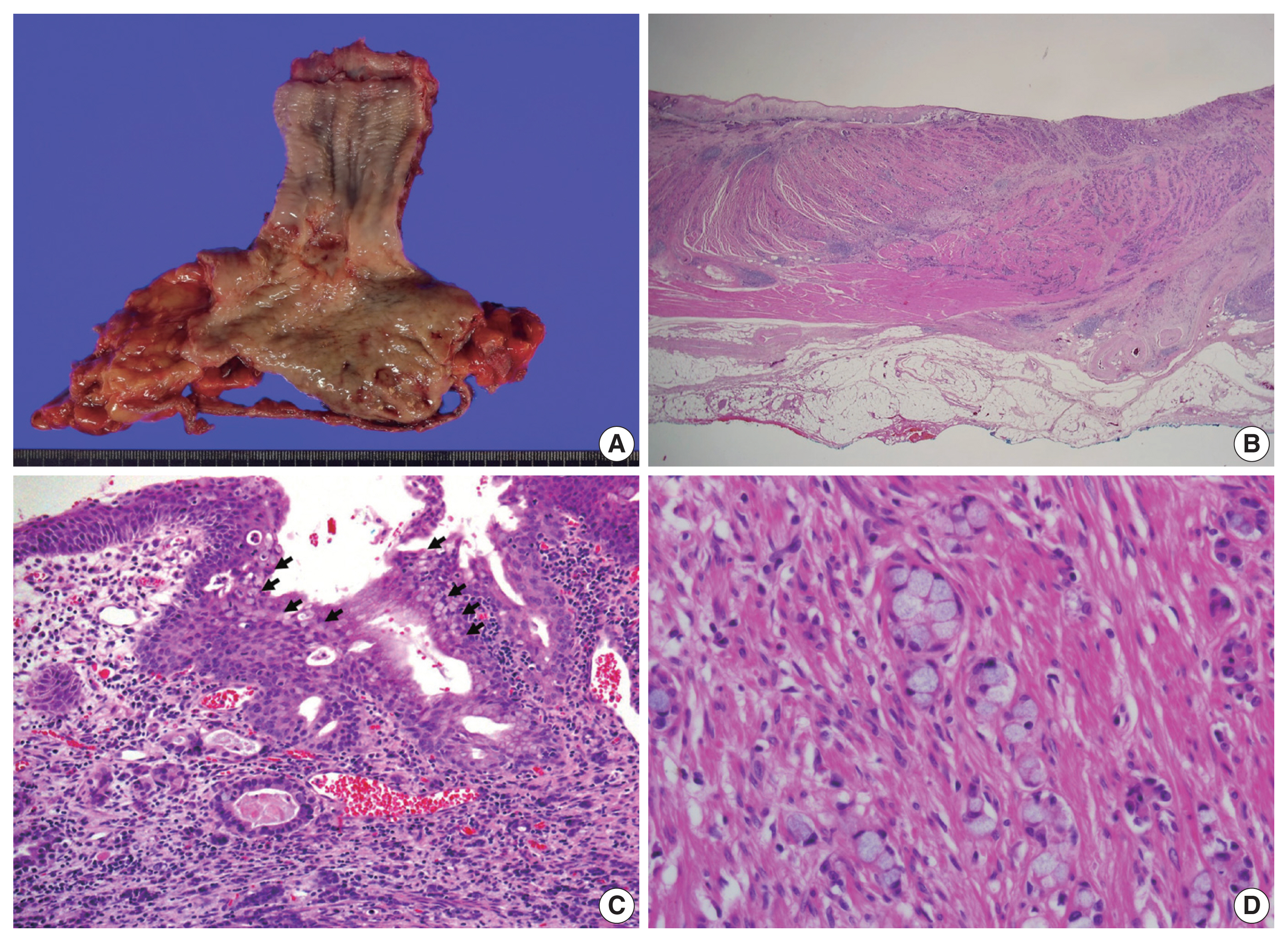
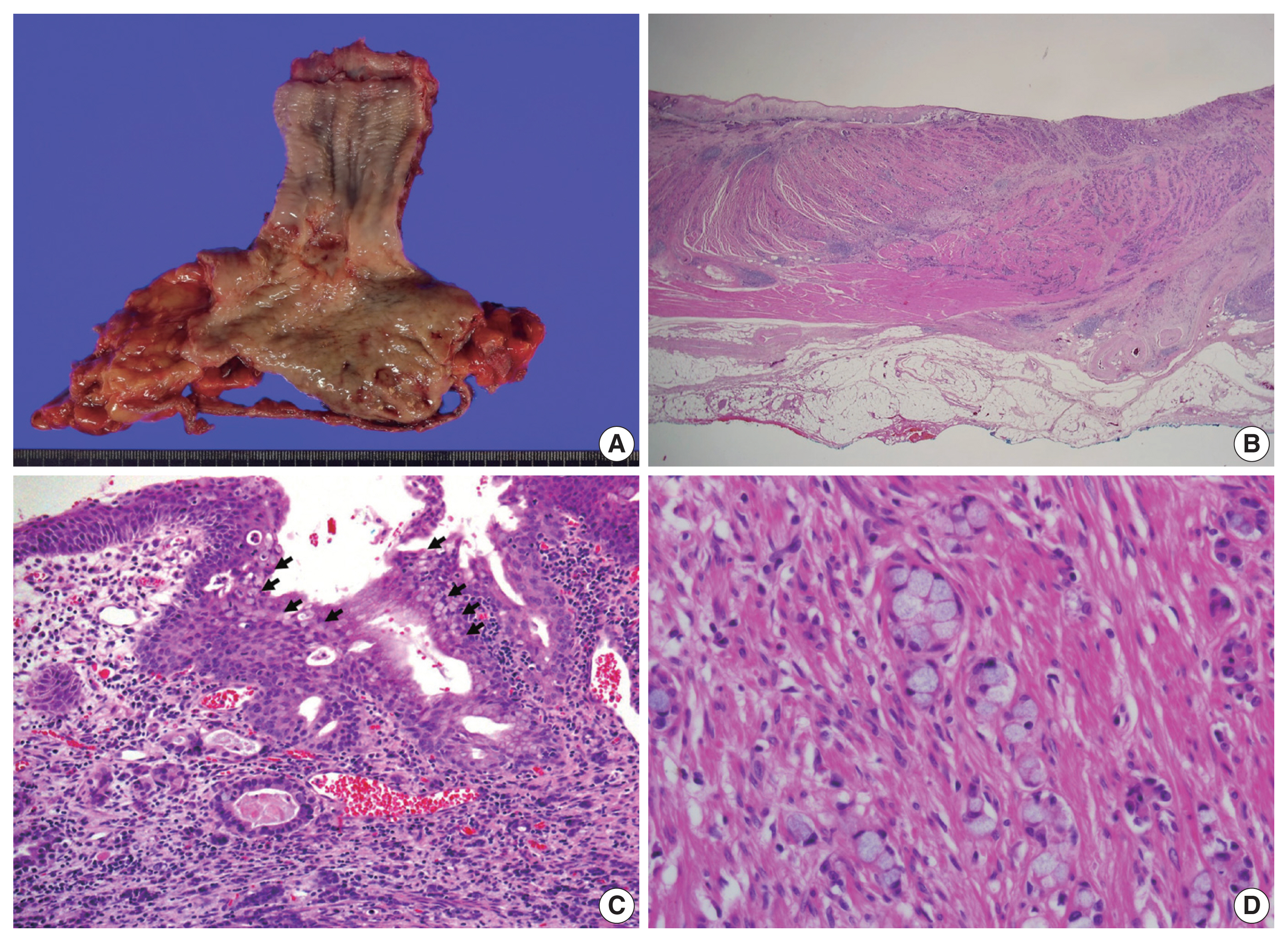
- Surgery was performed using an Ivor-Lewis operation to remove the distal esophagus and proximal stomach, including the lymph nodes that were suspected to harbor metastatic involvement. The resected tumor was a firm mass located in the distal esophagus with a poorly demarcated central ulcer, measuring 4.2 × 2.7 × 0.7 cm (Fig. 2A). Its distal end closely adjoined the gastroesophageal junction.
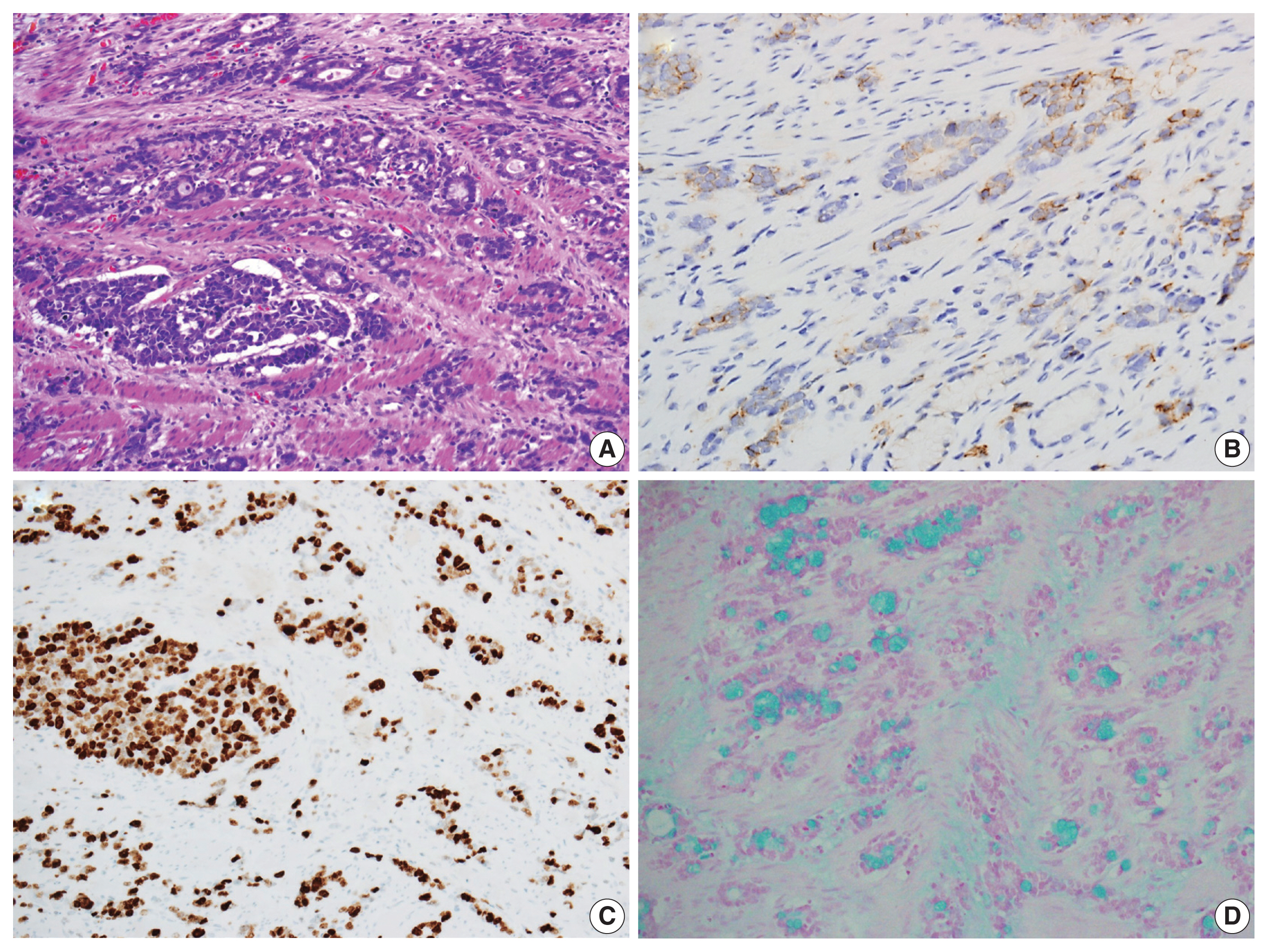
- Upon cross-sectional examination, the tumor displayed a solid, gray-white appearance and exhibited infiltrative growth extending into the adventitia (Fig. 2B). Microscopically, tumor cells exhibited a goblet cell morphology with intracytoplasmic mucin and formed the characteristic small tight clusters with or without lumens (Fig. 2C, D). Nuclear atypia was mild, and mitosis was infrequent. The adjacent non-neoplastic esophageal squamous epithelium did not show precancerous lesions such as dysplasia but instead showed intestinal metaplasia, a finding of Barrett’s esophagus (Fig. 2C). In other areas, higher-grade tumor components such as complex anastomosing tubules, cribriform architecture, and single-file growth patterns were also observed (Fig. 3A). The cells in this area displayed hyperchromatic nuclei and limited cytoplasm rather than goblet cell morphology, presenting histological features like the neuroendocrine carcinoma that were diagnosed in the initial biopsy. The tumor cells also showed lymphatic and perineural invasion. The absence of necrosis, fibrosis, or histiocytic infiltration, indicative of neoadjuvant chemotherapy, led to the conclusion that there was no tumor regression resulting from the chemotherapy.
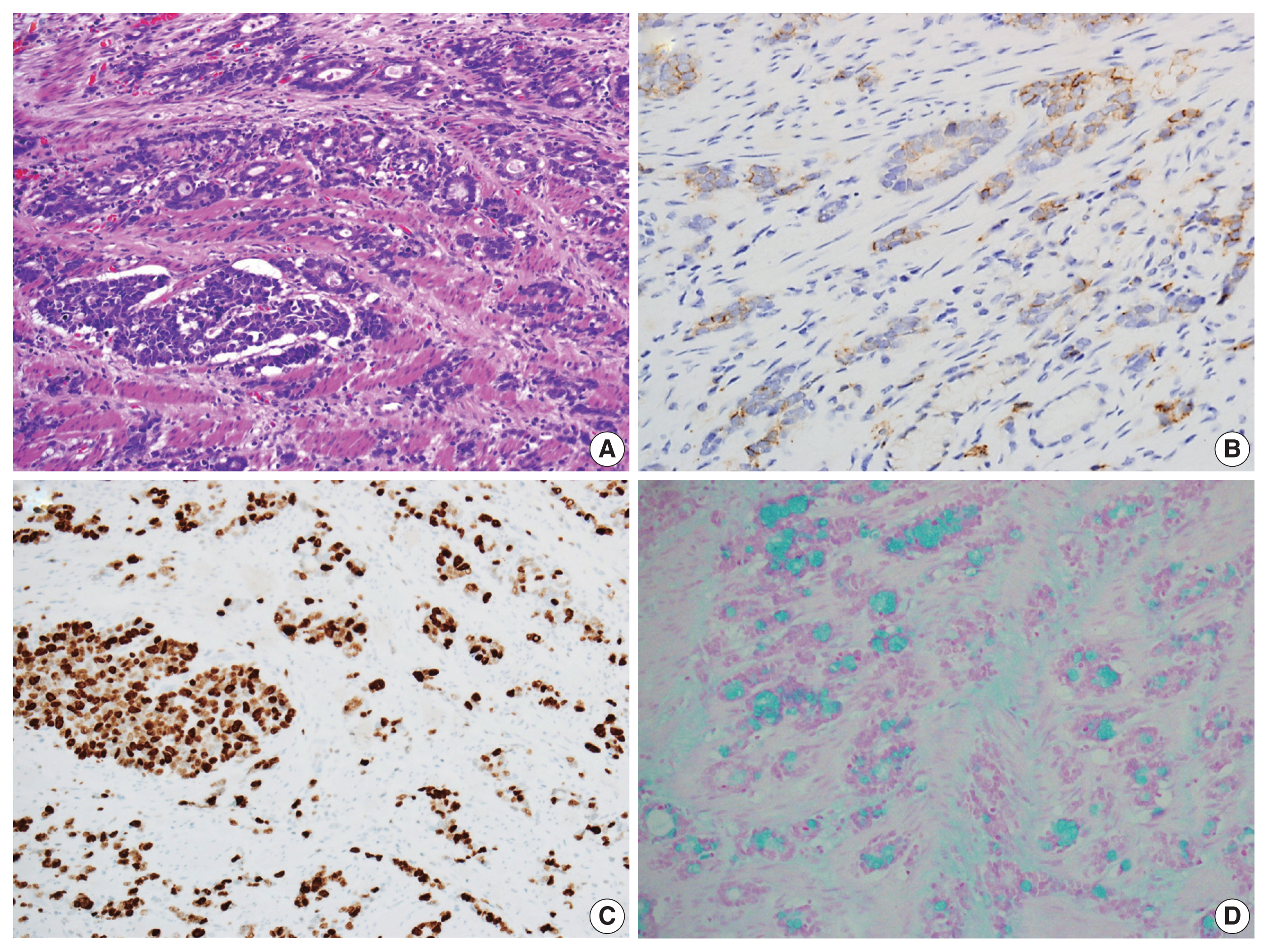
- Immunohistochemical analysis revealed that the tumor cells exhibited a positive reactivity to neuroendocrine markers, including synaptophysin and CD56, while testing negative for chromogranin A (Fig. 3B). Additionally, they displayed positive staining for cytokeratin 7, cytokeratin 20, and CDX2, while not staining for mucin stains MUC2, MUC5AC, and MUC6. The Ki-67 labeling index was greater than 90% positive (Fig. 3C). In addition, intracytoplasmic mucin in the goblet cells was well stained by mucin stains such as mucicarmine and alcian blue pH 2.5 (Fig. 3D).
- Based on the above histological observations, the tumor was diagnosed as GCA and was staged as pT3N1, corresponding to stage III with infiltration to the adventitia and the presence of metastasis in one gastric lymph node. Considering the three-tiered grading system of GCA provided by the 2019 World Health Organization (WHO) classification of tumors of the digestive system, the tumor’s low-grade pattern, characterized by tubular or clustered growth, constitutes a range of 50%–75%, which falls within the intermediate grade category.
- Given that GCA occurs almost exclusively in the appendix, specialized radiologists thoroughly reviewed the abdominal CT and PET-CT scans of the patient to exclude the possibility of metastasis from the appendix after the pathologic diagnosis had been rendered. The appendix was unremarkable, and the tumor was determined to be primary.
CASE REPORT
- GCA is a distinct tumor type that occurs almost exclusively in the appendix and consists of an admixed population of signet ring-like cells that resemble intestinal goblet cells in small, rounded nests or cords and neuroendocrine cells arranged in organoid patterns. GCA is characterized as an amphicrine tumor, exhibiting both exocrine and neuroendocrine components within the same cell. This stands in contrast to mixed (composite) glandular-endocrine cell carcinomas or collision tumors, where two distinct cellular components are either mixed or juxtaposed [7].
- GCA has been referred to by various terms, including goblet cell carcinoid, crypt cell carcinoma, microglandular carcinoma, adenocarcinoid, and adenocarcinoma ex goblet cell carcinoid. However, the term “goblet cell carcinoid” has traditionally been favored in the literature because GCA has been regarded as a type of neuroendocrine tumor (NET) due to its organoid growth pattern, the presence of scattered neuroendocrine cells, the existence of neurosecretory granules, and the absence of a precursor mucosal lesion [8].
- Nonetheless, several researchers have found that GCA possesses distinct morphological and biological characteristics that set it apart from classical NETs. They believe it is a form of adenocarcinoma composed of mucin-secreting cells, and therefore, it should be reclassified as GCA [9]. This reclassification was later adopted by the 2019 WHO classification of tumors of the digestive system.
- Extra-appendiceal GCAs are extremely uncommon. A few isolated examples of extra-appendiceal GCA involving various segments of the gastrointestinal tract including the colorectum, small intestine, and stomach, have been published in the literature as case reports [2–6]. Nonetheless, this is the first reported case of GCA arising in the esophagus. Gui et al. [2] reported that among 16 cases of extra-appendiceal GCA, only one case (6.2%) was truly extra-appendiceal. In four of the 16 cases (25%) initially presumed to be of extra-appendiceal origin, a primary appendiceal GCA was diagnosed later. In 10 of the 11 remaining cases (68.7%), the appendix was absent or surgical specimens were not accessible for comprehensive review. In one case the examination was incomplete as only a single tissue section of the appendix was taken rather than the entire appendix. They concluded that GCAs found in locations other than the appendix are most likely extra-appendiceal presentations of a primary occult appendiceal tumor and a thorough review of the pathologic status of appendix should be a mandatory diagnostic criterion.
- Taking these considerations into account, some investigators proposed the use of the term ‘amphicrine carcinoma’ for primary GCA originating in the gastrointestinal tract excluding the appendix. They argued that the use of the term ‘extra-appendiceal GCA’ necessitates discrimination between primary GCA and extra-appendiceal metastasis of appendiceal GCA [10].
- The histogenesis of GCA is not yet fully understood. Nevertheless, it is generally believed that GCA may originate from pluripotent intestinal stem cells located at the base of crypts, which have the capacity for both mucinous and neuroendocrine differentiation [11]. Our patient had Barrett’s esophagus with intestinal metaplasia, a condition in which the squamous epithelium of the esophagus transforms into intestinal goblet cells. It is believed that the cancerous changes originated from the altered intestinal crypt within Barrett’s esophagus.
- Histologically, typical GCA is characterized by a concentric growth pattern that infiltrates the appendiceal wall, resulting in the formation of an ill-defined tumor mass. Notably, the mucosa is typically spared, except in regions where the tumor nests connect with the base of the crypts, revealing predominantly submucosal growth.
- The classic low-grade form of this tumor exhibits tubular growth composed of goblet-like mucinous cells, along with variable numbers of endocrine cells and Paneth-like cells that have granular eosinophilic cytoplasm. Glandular lumina are infrequently observed. The cells display mild to moderate atypia with low mitotic activity, and extracellular mucin is often present, sometimes in substantial quantities.
- High-grade histological characteristics in GCA include the infiltration of tumor cells as either individual mucinous or non-mucinous cells, the presence of complex anastomosing tubules, cribriform masses, sheets of tumor cells, and large clusters of goblet-like or signet-ring-like cells. Additionally, high-grade areas may exhibit a desmoplastic stromal response, high-grade cytological features, numerous mitoses with atypical mitotic figures, and necrosis.
- In immunohistochemical staining, GCA exhibits positivity for neuroendocrine markers like chromogranins and synaptophysin. However, these markers are often observed as scattered positive expressions, contrasting with the strong positives typically seen in classic NETs. The expression of neuroendocrine markers, however, is not considered a mandatory diagnostic criterion. In addition, the tumor cells show positive immunoreactivity for carcinoembryonic antigen, cytokeratin (CK) 7, CK20, CDX2, with a substantial portion also exhibiting positivity for MUC2. They also demonstrate staining with mucin-specific stains such as mucicarmine and alcian blue pH 2.5.
- GCA is graded using a three-tiered system proposed by Yozu et al. [9], which places a strong emphasis on quantifying the low-grade component by determining the extent of tubular or clustered growth within the tumor. Tumors exhibiting over 75% low-grade characteristics are categorized as low grade (grade 1), those with 50% to 75% fall into the intermediate grade (grade 2), and those with less than 50% are classified as high grade (grade 3).
- Due to its rarity, there are currently no established guidelines for the assessment or treatment of extra-appendiceal GCA, and limited information is available regarding the best approach to adjuvant treatment following resection. Appendiceal GCAs are typically managed in accordance with the guidelines for appendiceal adenocarcinomas. For stage III or IV GCAs arising in extra-appendiceal locations like the colorectal region, employing an adjuvant regimen based on 5-fluorouracil designed for colorectal adenocarcinoma is recommended [6]. Consequently, it is believed that employing a regimen designed for adenocarcinoma originating in the esophagus would be beneficial for this patient.
- While GCA has traditionally been considered as a low-grade malignancy associated with a more favorable prognosis compared with de novo adenocarcinoma, over 50% of patients with GCAs present with metastases at the time of their initial diagnosis [12]. In the study by Yozu et al. [9], histologic grade was a significant independent prognostic factor, and there was a significant difference in overall patient survival among three histologic grades. The median overall survival stands at 204, 86, and 29 months for grades 1, 2, and 3 tumors, respectively. They also indicated that tumor stage was a strong predictor of the outcome. However, other factors, including lymphovascular invasion, perineural invasion, perforation, and the type of surgical resection, did not exhibit a significant association with overall patient survival. In other studies, only stage remained a statistically significant prognostic factor [13].
- In summary, the current patient is the first reported case of esophageal GCA thought to have originated from the intestinal goblet cells of Barrett’s esophagus. In this case, the patient was initially diagnosed with neuroendocrine carcinoma on preoperative biopsy and subsequently received treatment with an etoposide and cisplatin combination therapy, which unfortunately did not yield significant benefit. Due to the importance of recognizing this uncommon entity in the esophagus and its pathological classification based on histological components for determining patient prognosis, we present this case along with a literature review.
DISCUSSION
Ethics Statement
This study was approved by the Institutional Review Board of Kosin University Gospel Hospital with a waiver of informed consent (IRB No. 2023-10-007).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: SJO. Data curation: CEO, SJO, SEK. Investigation: CEO, SEK. Formal analysis: CEO, SJO. Supervision: SJO. Writing—original draft: SJO, CEO. Writing—review & editing: SJO. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
- 1. Zhang K, Meyerson C, Kassardjian A, Westbrook LM, Zheng W, Wang HL. Goblet cell carcinoid/carcinoma: an update. Adv Anat Pathol 2019; 26: 75-83. ArticlePubMed
- 2. Gui X, Qin L, Gao ZH, Falck V, Harpaz N. Goblet cell carcinoids at extraappendiceal locations of gastrointestinal tract: an under-recognized diagnostic pitfall. J Surg Oncol 2011; 103: 790-5. ArticlePubMedPDF
- 3. Kang Y, Choi JW, Kim Y, Oh HE, Lee JH, Kim YS. Goblet cell carcinoid of the rectum in a patient with neurofibromatosis type 1. J Pathol Transl Med 2016; 50: 482-5. ArticlePubMedPMCPDF
- 4. Lenti MV, Peri A, D’Ambrosio G, Gallo V, Di Sabatino A, Vanoli A. Primary appendiceal-type goblet cell adenocarcinoma of the ileum mimicking an inflammatory stricture in a woman with Crohn’s disease. Dig Liver Dis 2021; 53: 506-8. ArticlePubMed
- 5. Murickan T, Onyemkpa C, Flynn C, Kavuturu S. Extra-appendiceal goblet cell carcinoid of the ascending colon. ACS Case Rev Surg 2020; 2: 66-70.
- 6. Inoue Y, Horie H, Homma Y, et al. Goblet cell carcinoid of the rectum: a case report. Surg Case Rep 2020; 6: 174.ArticlePubMedPMCPDF
- 7. Lewin K. Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol 1987; 11: Suppl 1. 71-86. ArticlePubMed
- 8. Toumpanakis C, Standish RA, Baishnab E, Winslet MC, Caplin ME. Goblet cell carcinoid tumors (adenocarcinoid) of the appendix. Dis Colon Rectum 2007; 50: 315-22. ArticlePubMed
- 9. Yozu M, Johncilla ME, Srivastava A, et al. Histologic and outcome study supports reclassifying appendiceal goblet cell carcinoids as goblet cell adenocarcinomas, and grading and staging similarly to colonic adenocarcinomas. Am J Surg Pathol 2018; 42: 898-910. ArticlePubMed
- 10. Huang D, Ren F, Ni S, et al. Amphicrine carcinoma of the stomach and intestine: a clinicopathologic and pan-cancer transcriptome analysis of a distinct entity. Cancer Cell Int 2019; 19: 310.ArticlePubMedPMCPDF
- 11. Roy P, Chetty R. Goblet cell carcinoid tumors of the appendix: an overview. World J Gastrointest Oncol 2010; 2: 251-8. ArticlePubMedPMC
- 12. Tang LH, Shia J, Soslow RA, et al. Pathologic classification and clinical behavior of the spectrum of goblet cell carcinoid tumors of the appendix. Am J Surg Pathol 2008; 32: 1429-43. ArticlePubMed
- 13. Palmer K, Weerasuriya S, Chandrakumaran K, et al. Goblet cell adenocarcinoma of the appendix: a systematic review and incidence and survival of 1,225 cases from an English cancer registry. Front Oncol 2022; 12: 915028.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

 E-submission
E-submission





