Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 59(3); 2025 > Article
-
Case Study
Cytological features of atypical adenomatous hyperplasia and adenocarcinoma in situ of the lung: a case report -
Misa Takahashi,1
 , Seiya Homma1
, Seiya Homma1 , Chisato Setoguchi1
, Chisato Setoguchi1 , Yoko Umezawa2
, Yoko Umezawa2 , Atsuhiko Sakamoto1
, Atsuhiko Sakamoto1
-
Journal of Pathology and Translational Medicine 2025;59(3):195-200.
DOI: https://doi.org/10.4132/jptm.2025.04.09
Published online: May 9, 2025
1Department of Pathology and Laboratory Medicine, Omori Red Cross Hospital, Tokyo, Japan
2Department of Pathology, Fukushima Medical University Hospital, Fukushima, Japan
- Corresponding Author: Misa Takahashi, CT Department of Pathology and Laboratory Medicine, Omori Red Cross Hospital, 4-30-1, Chuo, Ota-ku, Tokyo 143-8527, Japan Tel: +81-3-3775-3111, Fax: +81-3-3776-0004, E-mail: mishaaa000.t@gmail.com
- *This article was presented at the 20th Korea-Japan Joint Meeting for Diagnostic Cytopathology, September 2, 2023, in Gunsan, Korea.
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,986 Views
- 121 Download
Abstract
- Atypical adenomatous hyperplasia (AAH) and adenocarcinoma in situ (AIS) are generally treated as different lesions, depending on the differences in lesion size and histological findings. However, these differences are not absolute; thus, AAH and AIS are often difficult to distinguish. Moreover, whether AAH and AIS can be regarded as different lesions remains unknown because cytological specimens, especially those of AAH, are rare. In this study, we examined these uncommon cytological specimens and compared the cytological findings between AAH and AIS. We observed many common cytological features with no obvious differences between AAH and AIS. These findings suggest that these two distinct lesions can be grouped into a single category. Therefore, we propose creating a new cytological category.
- Atypical adenomatous hyperplasia (AAH) is generally defined as lesions ≤0.5 cm in diameter [1,2]. These lesions are difficult to detect with preoperative computed tomography (CT) scans owing to the 0.5 cm detection limit of thin-slice CT [3,4]. Therefore, most AAHs are incidentally discovered during visual examination of the cut surfaces of resected lung cancer specimens or through the investigation of pathological specimens using microscopy. Thus, cytological specimens of AAH are rare and their details remain unclear [4]. The present study aimed to address this gap by preparing cytological specimens of lesions diagnosed as AAH or adenocarcinoma in situ (AIS), respectively. We examined the cytological features of AAH and compared them with those of AIS. We then investigated the differences between AAH and AIS, and whether they could be distinguished based on cytological findings. We also compared the definitional, histological, and genetic differences and similarities between AAH and AIS to investigate the need to consider them as different lesions.
INTRODUCTION
- Case 1 involved a 53-year-old Japanese non-smoking female. The patient’s comorbidities included chronic cough due to gastroesophageal reflux disease, cough variant asthma, hyperlipidemia, hypertension, and leiomyoma. The patient's uncle had tongue cancer. Chest CT was performed to investigate the chronic cough and revealed ground-glass opacity (GGO) 0.7 cm in diameter in the left upper lobe of the lung. Subsequently, as the GGO could have been lung cancer, the patient underwent thoracoscopic partial resection of the left upper lobe.
- Case 2 involved a 78-year-old Japanese female exposed to secondhand smoke. The patient’s comorbidities included severe mitral stenosis and atrial fibrillation. The patient’s father and brother had pancreatic cancer. Chest CT was performed for preoperative evaluation of severe mitral stenosis and revealed multiple GGOs in both lungs. These GGOs were followed up with CT. During follow-up, one of the multiple GGOs at the apex of the right upper lobe increased from 0.6 cm to 0.8 cm in diameter and its density also increased. These CT findings suggested that the precancerous lesion may have transformed into invasive cancer. Therefore, thoracoscopic partial resection of the right upper lobe was performed.
- Cytological findings
- In both cases, we rubbed the respective lesions on isolated lung specimens with Orcellex Brush RT (Rovers Medical Devices BV, Oss, Netherlands) and collected the lesions in BD Cytorich red preservation solution (Becton Dickinson and Company, Sparks, MD, USA). Then, we performed liquid-based cytological examination of the Papanicolaou-stained specimens using the BD Cytorich method.
- The low-power view showed sheet-like, mildly overlapping cell aggregates (Fig. 1A). The high-power view revealed that the atypical cells comprising these aggregates had pale cytoplasm, nuclei with finely granular chromatin, and tiny, prominent nucleoli (Fig. 1B). Nuclear irregularities, including nuclear wrinkles, were often observed (Fig. 1C), while intranuclear cytoplasmic inclusion bodies (Fig. 1B) and nuclear grooves appearing similar to coffee beans, were sometimes observed (Fig. 1D). Binuclear cells were rarely identified.
- At low-magnification, atypical cell clusters appeared sheet-like and showed mild overlap, with several binuclear cells observed in a single cell cluster (Fig. 1E). Binuclear cells were often observed on the cytological slides. At high-magnification, the atypical cells were small and had high nucleus-to-cytoplasm (N/C) ratios, pale cytoplasm, nuclei with finely granular chromatin, small prominent nucleoli, and often clear nuclear wrinkles (Fig. 1F). Intranuclear cytoplasmic inclusion bodies and nuclear grooves were occasionally observed (Fig. 1G). The atypical cells were usually uniform but showed mild size variation (Fig. 1H).
- Histological findings
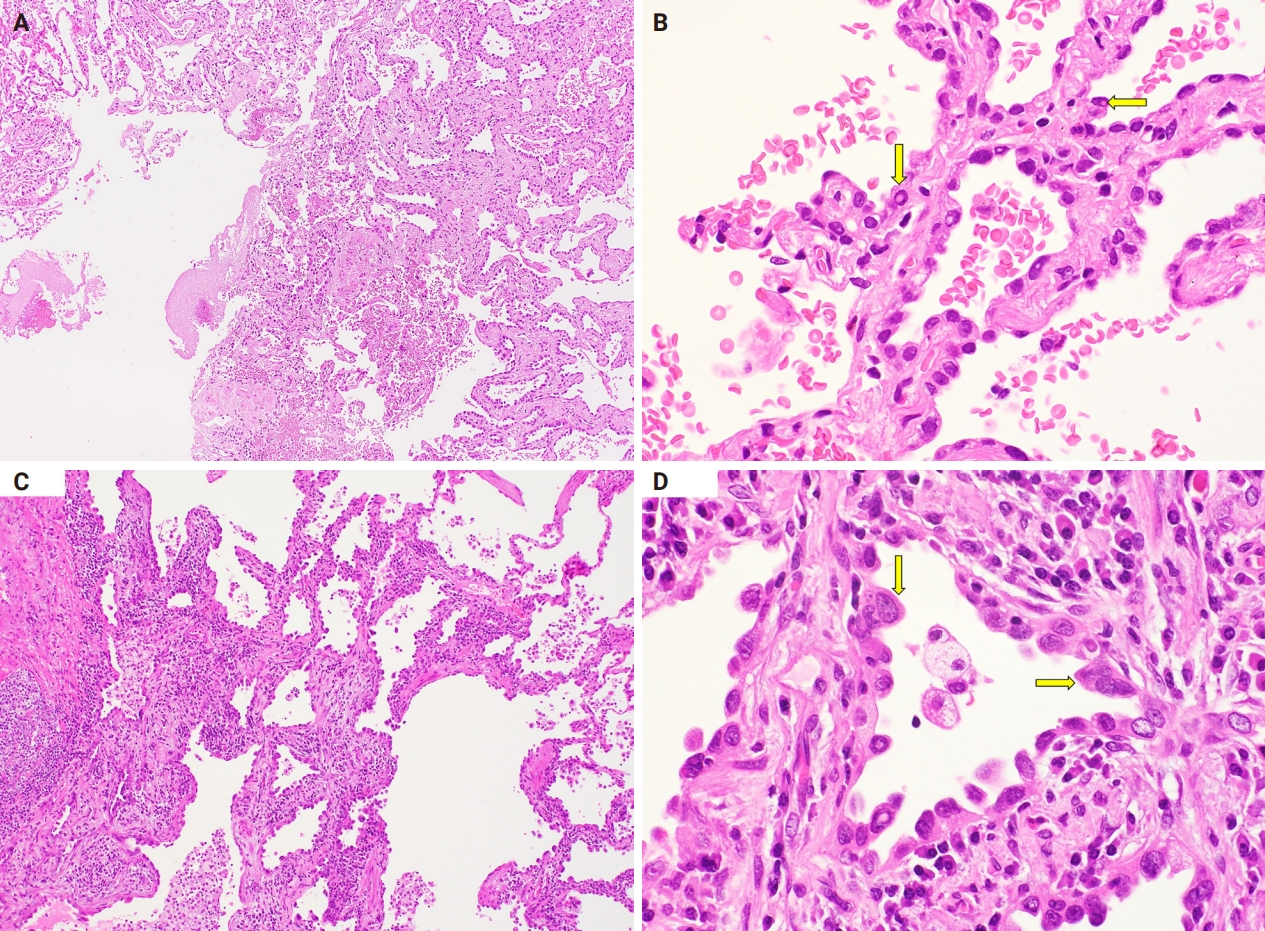
- The alveolar septal walls were thickened (Fig. 2A). Atypical cells resembling type II alveolar epithelial cells and Clara cells had proliferated and replaced the existing alveolar epithelium (Fig. 2B). These atypical cells were small, uniform, and hyperchromatic. Moreover, they also had high N/C ratios and sometimes showed intranuclear cytoplasmic inclusion bodies (Fig. 2B). Based on these histological findings and the lesion size (0.5 cm in diameter), we diagnosed the lesion as AAH.
- The alveolar septal walls were thickened with inflammatory cell infiltration and papillary architecture (Fig. 2C). Atypical cells that resembled type II alveolar epithelial cells and Clara cells had proliferated, replacing the existing alveolar epithelium (Fig. 2D). These atypical cells were small, hyperchromatic, and had high N/C ratios (Fig. 2D). Mild size variations were observed, with partial nuclei overlap (Fig. 2D). Intranuclear cytoplasmic inclusion bodies and binuclear cells were also observed. Based on these histological findings and the lesion size (0.8 cm in diameter), we diagnosed the lesion as AIS.
CASE REPORT
Case 1
Case 2
Case 1
Case 2
- We initially compared the histological findings of AAH and AIS. Both AAH and AIS cells resemble type II alveolar epithelial cells and Clara cells, and proliferate and replace existing alveolar epithelium. However, AAH and AIS differ in lesion size, overall lesion structure, and the presence of mild variations in cell size. In addition to lesion size, the cell density and chromatin condition [5,6] can be used to differentiate AAH from AIS, as in the present study. However, distinguishing these conditions remains challenging [4]. Thus, differentiating AAH from AIS should consider both histological findings and lesion size.
- Second, we compared the cytological findings between AAH and AIS. Many cytological features were shared between AAH and AIS, including sheet-like and mildly overlapping cell aggregates; small cells; high N/C ratio; pale cytoplasm; fine granular chromatin; small prominent nucleoli; and cell irregularities such as intranuclear cytoplasmic inclusion bodies, nuclear wrinkles, and some nuclear grooves. Slight differences were observed between the two conditions. The first was the size variation in atypical cells. While the AAH cells were generally uniform in size, some AIS cells exhibited mild size variation. The second was the frequency of binuclear cells, as more binuclear cells were observed on AIS slides than on AAH slides, and more than one binuclear cell was identified in each AIS cell cluster. In contrast, binuclear cells were barely observed on the AAH slides. Despite these two slight differences, they did not differentiate AAH from AIS as the mild size variations were not seen on the entire AIS specimen, and binuclear cells are also observed in AAH [6]. In addition, the overall lesion structures and lesion sizes could not be determined in the cytological specimens. Therefore, our cases suggest that cytological specimens alone cannot be used to differentiate AAH from AIS.
- These slight cytological differences between AAH and AIS are also observed in severe dysplasia and carcinoma in situ in the gynecological region. These types show slight cytological differences, including the N/C ratio and nuclear findings [7]. Severe dysplasia shows an N/C ratio ≤80% and nuclear irregularities [7]. In contrast, carcinoma in situ has an N/C ratio >80% and smooth and taut nuclear edges [7]. Both severe dysplasia and carcinoma in situ are atypical parabasal cells of approximately the same size, with fine granular chromatin. Despite these differences, they are often difficult to distinguish; therefore, they are treated as a single category (CIN3) in the World Health Organization (WHO) classification [7]. Thus, the same categorization might be considered for cytological findings in AAH and AIS.
- Finally, we summarize the histological, genetic, and definitional differences and similarities between AAH and AIS. One difference was lesion size. The “General Rule for Clinical and Pathological Record of Lung Cancer” defines AAH as a localized precancerous lesion of peripheral adenocarcinoma of the lung with a diameter ≤0.5 cm [1]. It defines AIS as a localized adenocarcinoma ≤3.0 cm in diameter, with tumor cells resembling type II alveolar epithelial and Clara cells and dense proliferation [1]. AIS is further defined as replacement growth without stromal, vascular, or pleural invasion [1]. However, AAH measuring >0.5 cm has been reported [3,8,9]; in such cases, lesion sizes cannot inform their differentiation. Other differences between AAH and AIS include the histological type and findings. AIS rarely has a mucinous type, whereas AAH only has a nonmucinous type [1,6]. While some histological findings may also help to differentiate AAH and AIS [5,6], the potential for strong cell atypia in AAH [9] can make this challenging. The histological similarities between AAH and AIS include type II alveolar epithelial cells and Clara cells and proliferation displacing the alveolar epithelium [1,4,6]. Additionally, both have thick alveolar septal walls [6] and show immunohistochemical positivity for thyroid transcription factor-1 [1,6]. In addition, if surgically removed, the survival rate is almost 100% [4,10]. Peripheral-type lung adenocarcinoma is thought to occur through a multistage process involving AAH, AIS, and invasive adenocarcinoma with gradual accumulation of genetic mutations [6,9,11,12]. KRAS mutations have been detected as early as the AAH stage [11], with comparable detection rates between AAH and AIS [11,13].
- Although AAH and AIS differ in lesion size and histological findings, the criteria for their differentiation are not always clear. However, as they share cytological, histological, genetic, and definition similarities, AAH and AIS may not need to be treated as different lesions. The WHO Reporting System for Lung Cytopathology does not differentiate AAH from AIS in the diagnosis of cytopathology specimens [14]. The monograph does not use AIS as cytodiagnosic terminology, and AAH is not mentioned [14]. Additionally, new criteria for assessing tumor invasion from the International Association for the Study of Lung Cancer Pathology Committee suggests the precise classification of AIS as low-risk lesions [10], supporting the findings of the present case report. Therefore, at least in the cytological field, where lesion sizes are not relevant, we suggest that AAH and AIS be regarded as a single category.
- The conclusions of this study require validation in future studies including additional cytology specimens from patients with AAH. However, as discussed above, such cases are rare [4], which complicates the collection of such specimens. In our experience, cytology specimens of AIS are easier to collect than those of AAH. Therefore, we intend to substantiate our conclusions by comparing the cytological images of AAH presented in this report with those of AIS we will collect in the future.
DISCUSSION
Ethics Statement
This report was approved by the Institutional Review Board (IRB) of Omori Red Cross Hospital (No 24-61, 2025.1.10) and complied with the principles of the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from all individual participants included in this study.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: MT, SH, CS, AS. Investigation: MT. Methodology: MT, YU, AS. Project administration: MT. Resources: MT, SH, CS. Supervision: MT, AS. Visualization: MT. Writing—original draft preparation: MT, YU, AS. Writing—review & editing: MT, YU, AS. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.


- 1. Japan Lung Cancer Society. General rule for clinical and pathological record of lung cancer. 8th ed. Tokyo: Kanehara, 2021; 89-90.
- 2. Thoracic tumor histology based on WHO 5th ed vol 1.3 ed [Internet]. Tokyo: Japan Lung Cancer Society, 2022 [cited 2024 Dec 15]. Available from: https://www.haigan.gr.jp/publication/guidance/who-histopathology/.
- 3. Sakaguchi M. A case of atypical adenomatous hyperplasia incidentally detected in resected specimen of spontaneous pneumothorax in a young adult. J Jpn Assoc Chest Surg 2022; 36: 75-9. Article
- 4. Kitamura A, Yatabe Y. Atypical adenomatous hyperplasia. Pathol Clin 2019; 37: 113-5.
- 5. Kunugi S, Kawamoto M. Pathology of lung cancer WHO classification and future revisions. Clin Images 2010; 26: 124-33.
- 6. Minami Y. Differentiation of preinvasive lesions of the glandular types (atypical adenomatous hyperplasia, adenocarcinoma in situ, minimally invasive adenocarcinoma). Pathol Clin 2016; 34: 256-9.
- 7. Aozasa K, Kinjo M, Kamei T, Higuchi K. Color atlas of differential diagnosis in cytology. Tokyo: Ishiyaku, 2021; 70-3.
- 8. Koizumi N, Sonoyama Y, Oi H, Asatani M, Ozaki T, Seki H. Ground-glass opacity nodule/subsolid nodules on thin-section CT of the lung. J Niigata Cancer Center Hosp 2019; 57: 48-52.
- 9. Kitaguchi S, Iwamoto Y, Inata J, Oyakawa T, Fujiwara T. Recent overview of atypical adenomatous hyperplasia of the lung. Med J Hiroshima City Hosp 2010; 26: 10-4.
- 10. Hong TH, Hwang S, Cho J, et al. Clinical significance of the proposed pathologic criteria for invasion by the International Association for the Study of Lung Cancer in resected nonmucinous lung adenocarcinoma. J Thorac Oncol 2024; 19: 425-33. ArticlePubMed
- 11. Minami Y, Noguchi M. Atypical adenomatous hyperplasia and lung cancer. Nippon Rinsho 2013; 71: 140-5.
- 12. Kitamura H, Hayashi H, Nozawa A, Ito T, Kanisawa M. Developmental mechanism of lung adenocarcinoma. Sougo Rinsho 2001; 50: 2229-35.
- 13. Yoshida Y, Shibata T, Kokubu A, et al. Mutations of the epidermal growth factor receptor gene in atypical adenomatous hyperplasia and bronchioalveolar carcinoma of the lung. Lung Cancer 2005; 50: 1-8. ArticlePubMed
- 14. IAC-IARC-WHO Joint Editorial Board. WHO Reporting System for Lung Cytopathology. Lyon: International Agency for Research on Cancer, 2022; 2-4, 111.
REFERENCES
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


 E-submission
E-submission




