Search
- Page Path
- HOME > Search
- Correlations and prognostic impacts of tumor spread through airspaces in surgically resected non–small cell lung cancer: a retrospective study from Jordan
- Ola Abu Al Karsaneh, Amani Al-Rousan, Sofian Al Shboul, Mohammed El-Sadoni, Anas Hayajneh, Moath Alrjoub, Sura Al-Rawabdeh, Tareq Saleh
- J Pathol Transl Med. 2026;60(1):92-106. Published online January 9, 2026
- DOI: https://doi.org/10.4132/jptm.2025.10.15
- 567 View
- 43 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Spread through air spaces (STAS) has been identified as an invasion pattern in non–small cell lung cancer (NSCLC). This study evaluated the association between tumor STAS and various clinicopathological parameters of NSCLC, with emphasis on the prognostic role of STAS. Methods: We evaluated 96 cases of NSCLC for STAS. STAS-positive cases were graded according to the distance between the edge of the primary tumor and the furthest STAS, in millimeters, or the number of alveoli separating STAS from the tumor. Results: STAS was observed in 33 patients (34.4%). In 28 cases, STAS was located in airspaces >3 alveoli away from the primary tumor. In 18 cases, STAS was found in airspaces > 2.5 mm away from the edge of the primary tumor. Morphologically, 18 cases of STAS demonstrated a solid nest pattern, eight showed a micropapillary cluster pattern, and seven exhibited a single-cell pattern. In multivariate analysis, only high tumor grade (p = .001) was independently associated with STAS in NSCLC. The presence of STAS (p = .047), lymphovascular invasion (p = .001), positive surgical margin (p = .021), adenocarcinoma histology (p = .020), and postoperative therapy (p = .049) showed a statistically significant lower overall survival (OS). However, multivariate analyses showed that STAS is not an independent predictor of OS in NSCLC. In addition, STAS-positive cases with an extension of >2.5 mm had significantly lower disease-free survival (DFS) (p = .018). Conclusions: The findings demonstrated that STAS is independently associated with a higher tumor grade and appears to have an adverse impact on OS and DFS in the examined subpopulation.
- Clinicopathological implications of miR-3127 in melanoma
- Truong Phan-Xuan Nguyen, Minh-Khang Le, Chau M. Bui, Vuong Gia Huy
- J Pathol Transl Med. 2025;59(6):371-381. Published online October 16, 2025
- DOI: https://doi.org/10.4132/jptm.2025.07.08
- 2,923 View
- 137 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Cutaneous melanoma is the most lethal of all skin cancers. Recent studies suggested that miR-3127 is dysregulated in multiple tumor types and has important roles in tumorigenesis and cancer progression, giving it potential as a prognostic biomarker. The aim of this study was to use bioinformatic analysis to assess miR-3127 expression and correlate expression patterns with disease course in patients with cutaneous melanoma. Methods: miRNA, mRNA sequencing, DNA methylation data, and clinical information of cutaneous melanoma cases were downloaded from the Human Cancer Atlas – Skin Cutaneous Melanoma (TCGA-SKCM). miR-3127 expression was classified into miR-3127–low and miR-3127–high clusters using maximally selected rank statistics. Results: Clustering analysis showed that high expression of miR-3127 (≥20.3 reads per million) was associated with worse progression-free (p < .001) and overall (p = .011) survival compared to low miR-3127 expression. More than five thousand differentially expressed genes between the two miR-3127 sample groups encoded cell differentiation markers, cytokines, growth factors, translocated cancer genes, and oncogenes. Pathway analysis revealed that miR-3127–high samples related to activity of proliferation, DNA repair, and ultraviolet response. Conclusions: The expression level of miR-3127 could act as a prognostic indicator for patients with melanoma.
- Unraveling the crucial role of CCL3 in nasopharyngeal carcinoma: bioinformatics and immunohistochemical insights
- Xiaopeng Guo, Zhen Sun, Ya Liang, Aoshuang Chang, Junjun Ling, Houyu Zhao, Xianlu Zhuo
- J Pathol Transl Med. 2025;59(5):281-290. Published online September 8, 2025
- DOI: https://doi.org/10.4132/jptm.2025.05.23
- 1,499 View
- 135 Download
-
 Abstract
Abstract
 PDF
PDF - Background
C-C motif chemokine ligand 3 (CCL3) is a crucial chemokine that plays a fundamental role in the immune microenvironment and is closely linked to the development of various cancers. Despite its importance, there is limited research regarding the expression and function of CCL3 in nasopharyngeal carcinoma (NPC). Therefore, this study seeks to examine the expression of CCL3 and assess its clinical significance in NPC using bioinformatics analysis and experiments. Methods: The bioinformatics approach was employed to assess the expression and function of CCL3 in NPC. Subsequently, protein expression of CCL3 was detected in an NPC cohort using immunohistochemistry based on a tissue microarray. The relationship between CCL3 expression and clinical features was then investigated. Results: A total of 20 CCL3-related genes and 14 possible target genes were identified through bioinformatics analysis, many of which play crucial roles in pathways such as chemokine signaling pathway and transcriptional misregulation in cancer signaling pathways. CCL3 was found to be associated with drug resistance and various immune cell infiltrations. In NPC, CCL3 expression was significantly higher than normal controls, and high expression of CCL3 correlated with cervical lymph node metastasis, tumor recurrence, advanced clinical stage, and poor prognosis. Conclusions: CCL3 may be a key gene in the initiation and progression of NPC. It has the potential to serve as both a diagnostic biomarker and a therapeutic target for NPC.
- Central nervous system tumors with BCOR internal tandem duplications: a systematic review of clinical, radiological, and pathological features in 69 cases
- Ji Young Lee, Sung Sun Kim, Hee Jo Baek, Tae-Young Jung, Kyung-Sub Moon, Jae-Hyuk Lee, Kyung-Hwa Lee
- J Pathol Transl Med. 2025;59(5):273-280. Published online September 1, 2025
- DOI: https://doi.org/10.4132/jptm.2025.07.23
- 3,208 View
- 174 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Central nervous system tumors with BCL6 corepressor (BCOR) internal tandem duplications (ITDs) constitute a rare, recently characterized pediatric neoplasm with distinct molecular and histopathological features. To date, 69 cases have been documented in the literature, including our institutional case. These neoplasms predominantly occur in young children, with the cerebellum representing the most frequent anatomical location. Radiologically, these tumors present as large, well-circumscribed masses frequently demonstrating necrosis, hemorrhage, and heterogeneous enhancement. Histologically, they are characterized by a monomorphic cellular population featuring ependymoma-like perivascular pseudorosettes, myxoid stroma, and elevated mitotic activity. Immunohistochemically, these tumors exhibit sparse glial fibrillary acidic protein expression while consistently demonstrating positive staining for vimentin and CD56. The defining molecular hallmark is a heterozygous ITD within exon 15 of the BCOR gene, with insertions ranging from 9 to 42 amino acids in length. BCOR immunohistochemistry reveals nuclear positivity in 97.9% of examined cases, although this finding is not pathognomonic for BCOR ITDs. This comprehensive review synthesizes data from all published cases of this novel tumor entity, providing a detailed analysis of clinical presentation, neuroimaging findings, histopathological features with differential diagnostic considerations, therapeutic approaches, and prognostic outcomes.
- Cytologic hallmarks and differential diagnosis of papillary thyroid carcinoma subtypes
- Agnes Stephanie Harahap, Chan Kwon Jung
- J Pathol Transl Med. 2024;58(6):265-282. Published online November 7, 2024
- DOI: https://doi.org/10.4132/jptm.2024.10.11
- 13,713 View
- 593 Download
- 9 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF - Papillary thyroid carcinoma (PTC) is the most common thyroid malignancy, characterized by a range of subtypes that differ in their cytologic features, clinical behavior, and prognosis. Accurate cytologic evaluation of PTC using fine-needle aspiration is essential but can be challenging due to the morphologic diversity among subtypes. This review focuses on the distinct cytologic characteristics of various PTC subtypes, including the classic type, follicular variant, tall cell, columnar cell, hobnail, diffuse sclerosing, Warthin-like, solid/trabecular, and oncocytic PTCs. Each subtype demonstrates unique nuclear features, architectural patterns, and background elements essential for diagnosis and differentiation from other thyroid lesions. Recognizing these distinct cytologic patterns is essential for identifying aggressive subtypes like tall cell, hobnail, and columnar cell PTCs, which have a higher risk of recurrence, metastasis, and poorer clinical outcomes. Additionally, rare subtypes such as diffuse sclerosing and Warthin-like PTCs present unique cytologic profiles that must be carefully interpreted to avoid diagnostic errors. The review also highlights the cytologic indicators of lymph node metastasis and high-grade features, such as differentiated high-grade thyroid carcinoma. The integration of molecular testing can further refine subtype diagnosis by identifying specific genetic mutations. A thorough understanding of these subtype-specific cytologic features and molecular profiles is vital for accurate diagnosis, risk stratification, and personalized management of PTC patients. Future improvements in diagnostic techniques and standardization are needed to enhance cytologic evaluation and clinical decision-making in thyroid cancer.
-
Citations
Citations to this article as recorded by- Oncocytic Thyroid Tumours With Pathogenic FLCN Mutations Mimic Oncocytic Papillary Thyroid Carcinoma on Fine‐Needle Aspiration
Adeel M. Ashraf, Faisal Hassan, Adrian A. Dawkins, Julie C. Dueber, Derek B. Allison, Thèrése J. Bocklage
Cytopathology.2026; 37(1): 108. CrossRef - Using a new type of visible light-based emission fluorescence microscope to identify the benign and malignant nature of thyroid tissue during the surgical process: Analysis of diagnostic results
Yu Miao, Liu Xiaowei, Li Muyang, Gao Jian, Chen Lu
Photodiagnosis and Photodynamic Therapy.2026; 57: 105324. CrossRef - Nuclear pseudoinclusion is associated with BRAFV600E mutation: Analysis of nuclear features in papillary thyroid carcinoma
Agnes Stephanie Harahap, Dina Khoirunnisa, Salinah, Maria Francisca Ham
Annals of Diagnostic Pathology.2025; 75: 152434. CrossRef - 2025 Korean Thyroid Association Clinical Management Guideline on Active Surveillance for Low-Risk Papillary Thyroid Carcinoma
Eun Kyung Lee, Min Joo Kim, Seung Heon Kang, Bon Seok Koo, Kyungsik Kim, Mijin Kim, Bo Hyun Kim, Ji-hoon Kim, Shin Je Moon, Kyorim Back, Young Shin Song, Jong-hyuk Ahn, Hwa Young Ahn, Ho-Ryun Won, Won Sang Yoo, Min Kyoung Lee, Jeongmin Lee, Ji Ye Lee, Kyo
International Journal of Thyroidology.2025; 18(1): 30. CrossRef - Structure-based molecular screening and dynamic simulation of phytocompounds targeting VEGFR-2: a novel therapeutic approach for papillary thyroid carcinoma
Shuai Wang, Lingqian Zhang, Wenjun Zhang, Xiong Zeng, Jie Mei, Weidong Xiao, Lijie Yang
Frontiers in Pharmacology.2025;[Epub] CrossRef - 2025 Korean Thyroid Association Clinical Management Guideline on Active Surveillance for Low-Risk Papillary Thyroid Carcinoma
Eun Kyung Lee, Min Joo Kim, Seung Heon Kang, Bon Seok Koo, Kyungsik Kim, Mijin Kim, Bo Hyun Kim, Ji-hoon Kim, Shinje Moon, Kyorim Back, Young Shin Song, Jong-hyuk Ahn, Hwa Young Ahn, Ho-Ryun Won, Won Sang Yoo, Min Kyoung Lee, Jeongmin Lee, Ji Ye Lee, Kyon
Endocrinology and Metabolism.2025; 40(3): 307. CrossRef - A Case of Warthin-Like Variant of Papillary Thyroid Cancer
Amy Chow, Israa Laklouk
Cureus.2025;[Epub] CrossRef - Propensity score-matched analysis of the ‘2+2’ parathyroid strategy in total thyroidectomy with central neck dissection
Hao Gong, Simei Yao, Tianyuchen Jiang, Yi Yang, Yuhan Jiang, Zhujuan Wu, Anping Su
Frontiers in Endocrinology.2025;[Epub] CrossRef
- Oncocytic Thyroid Tumours With Pathogenic FLCN Mutations Mimic Oncocytic Papillary Thyroid Carcinoma on Fine‐Needle Aspiration
- The combination of CDX2 expression status and tumor-infiltrating lymphocyte density as a prognostic factor in adjuvant FOLFOX-treated patients with stage III colorectal cancers
- Ji-Ae Lee, Hye Eun Park, Hye-Yeong Jin, Lingyan Jin, Seung Yeon Yoo, Nam-Yun Cho, Jeong Mo Bae, Jung Ho Kim, Gyeong Hoon Kang
- J Pathol Transl Med. 2025;59(1):50-59. Published online October 24, 2024
- DOI: https://doi.org/10.4132/jptm.2024.09.26
- 3,597 View
- 279 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Colorectal carcinomas (CRCs) with caudal-type homeobox 2 (CDX2) loss are recognized to pursue an aggressive behavior but tend to be accompanied by a high density of tumor-infiltrating lymphocytes (TILs). However, little is known about whether there is an interplay between CDX2 loss and TIL density in the survival of patients with CRC.
Methods
Stage III CRC tissues were assessed for CDX2 loss using immunohistochemistry and analyzed for their densities of CD8 TILs in both intraepithelial (iTILs) and stromal areas using a machine learning-based analytic method.
Results
CDX2 loss was significantly associated with a higher density of CD8 TILs in both intraepithelial and stromal areas. Both CDX2 loss and a high CD8 iTIL density were found to be prognostic parameters and showed hazard ratios of 2.314 (1.050–5.100) and 0.378 (0.175–0.817), respectively, for cancer-specific survival. A subset of CRCs with retained CDX2 expression and a high density of CD8 iTILs showed the best clinical outcome (hazard ratio of 0.138 [0.023–0.826]), whereas a subset with CDX2 loss and a high density of CD8 iTILs exhibited the worst clinical outcome (15.781 [3.939–63.230]).
Conclusions
Altogether, a high density of CD8 iTILs did not make a difference in the survival of patients with CRC with CDX2 loss. The combination of CDX2 expression and intraepithelial CD8 TIL density was an independent prognostic marker in adjuvant chemotherapy-treated patients with stage III CRC.
- EWSR1 rearranged primary renal myoepithelial carcinoma: a diagnostic conundrum
- Nilay Nishith, Zachariah Chowdhury
- J Pathol Transl Med. 2023;57(5):284-288. Published online September 15, 2023
- DOI: https://doi.org/10.4132/jptm.2023.08.08
- 4,150 View
- 212 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - Primary renal myoepithelial carcinoma is an exceedingly rare neoplasm with an aggressive phenotype and Ewing sarcoma breakpoint region 1 (EWSR1) rearrangement in a small fraction of cases. In addition to its rarity, the diagnosis can be challenging for the pathologist due to morphologic heterogeneity, particularly on the biopsy specimen. At times, immunohistochemistry may be indecisive; therefore, molecular studies should be undertaken for clinching the diagnosis. We aim to illustrate a case of primary myoepithelial carcinoma of the kidney with EWSR1-rearrangement in a 67-year-old male patient who presented with right supraclavicular mass, which was clinically diagnosed as carcinoma of an unknown primary. An elaborate immunohistochemical work-up aided by fluorescent in-situ hybridization allowed us to reach a conclusive diagnosis. This unusual case report advocates that one should be aware of the histological mimickers and begin with broad differential diagnoses alongside sporadic ones and then narrow them down with appropriate ancillary studies.
-
Citations
Citations to this article as recorded by- Primary Ewing Sarcoma of the Kidney
João Lobo, Huiying He, Raheel Ahmed, Bassel Zein-Sabatto, Thomas Winokur, Shi Wei, Shuko Harada, Jesse K. McKenney, Jonathan L. Myles, Jane K. Nguyen, Christopher G. Przybycin, Sean R. Williamson, Cristina Magi-Galluzzi, Reza Alaghehbandan
American Journal of Surgical Pathology.2025; 49(10): 1078. CrossRef
- Primary Ewing Sarcoma of the Kidney
- Loss of aquaporin-1 expression is associated with worse clinical outcomes in clear cell renal cell carcinoma: an immunohistochemical study
- Seokhyeon Lee, Bohyun Kim, Minsun Jung, Kyung Chul Moon
- J Pathol Transl Med. 2023;57(4):232-237. Published online July 11, 2023
- DOI: https://doi.org/10.4132/jptm.2023.06.17
- 4,676 View
- 171 Download
- 2 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Aquaporin (AQP) expression has been investigated in various malignant neoplasms, and the overexpression of AQP is related to poor prognosis in some malignancies. However, the expression of AQP protein in clear cell renal cell carcinoma (ccRCC) has not been extensively investigated by immunohistochemistry with large sample size.
Methods
We evaluated the AQP expression in 827 ccRCC with immunohistochemical staining in tissue microarray blocks and classified the cases into two categories, high and low expression.
Results
High expression of aquaporin-1 (AQP1) was found in 320 cases (38.7%), but aquaporin-3 was not expressed in ccRCC. High AQP1 expression was significantly related to younger age, low TNM stage, low World Health Organization/International Society of Urologic Pathology nuclear grade, and absence of distant metastasis. Furthermore, high AQP1 expression was also significantly associated with longer overall survival (OS; p<.001) and progression-specific survival (PFS; p<.001) and was an independent predictor of OS and PFS in ccRCC.
Conclusions
Our study revealed the prognostic significance of AQP1 protein expression in ccRCC. These findings could be applied to predict the prognosis of ccRCC. -
Citations
Citations to this article as recorded by- Loss of Aquaporin-1 in Tumor Cells Fosters Intrahepatic Cholangiocarcinoma Progression
César I. Gaspari, Carine Beaupere, Seth Richard, Estanislao Peixoto, Bouchra Lekbaby, Mirko Minini, Branko Dubravcic, Javier Vaquero, Marie Vallette, Ander Arbelaiz, Marion Janona, Corentin Louis, Pauline Le Gall, Cédric Coulouarn, Julieta Marrone, Juan E
The American Journal of Pathology.2026; 196(2): 428. CrossRef - Construction and validation of renal cell carcinoma tumor cell differentiation-related prognostic classification (RCC-TCDC): an integrated bioinformatic analysis and clinical study
Yifan Liu, Keqin Dong, Yuntao Yao, Bingnan Lu, Lei Wang, Guo Ji, Haoyu Zhang, Zihui Zhao, Xinyue Yang, Runzhi Huang, Wang Zhou, Xiuwu Pan, Xingang Cui
Annals of Medicine.2025;[Epub] CrossRef - Prognostic Assessment of Aquaporins in Pancreatic Adenocarcinoma: An In Silico Analysis
Vignesh Krishnasamy, Lalhmingliana, Nachimuthu Senthil Kumar
Current Biotechnology.2025; 14(2): 130. CrossRef - Targeting PLOD2 induces epithelioid differentiation and improves therapeutic response in sarcomatoid renal cell carcinoma
Xiangyu Chen, Dongkui Xu, Yu Ji, Xichen Dong, Xiaomei Dong, Zihan Li, Jingyu Tan, Qianqian Sun, Huixian Xin, Ziwei Liu, Qing Deng, Tao Wen, Yanjun Jia, Xuhui Zhu, Jian Liu
Journal of Advanced Research.2025;[Epub] CrossRef - Serum Exosomal MiR-874 as a Potential Biomarker for Nonsmall Cell Lung Cancer Diagnosis and Prognosis
Amal F. Gharib, Saad S. Al-Shehri, Abdulraheem Almalki, Ayman Alhazmi, Mamdouh Allahyani, Ahmed Alghamdi, Amani A. Alrehaili, Maha M. Bakhuraysah, Althobaiti Naif Saad M., Weal H. Elsawy
Indian Journal of Medical and Paediatric Oncology.2024;[Epub] CrossRef
- Loss of Aquaporin-1 in Tumor Cells Fosters Intrahepatic Cholangiocarcinoma Progression
- Unusual biclonal IgA plasma cell myeloma with aberrant expression of high-risk immunophenotypes: first report of a new diagnostic and clinical challenge
- Carlos A. Monroig-Rivera, Clara N. Finch Cruz
- J Pathol Transl Med. 2023;57(2):132-137. Published online March 14, 2023
- DOI: https://doi.org/10.4132/jptm.2023.02.07
- 4,978 View
- 140 Download
-
 Abstract
Abstract
 PDF
PDF - IgA plasma cell myeloma (PCM) has been linked to molecular abnormalities that confer a higher risk for adverse patient outcomes. However, since IgA PCM only accounts for approximately 20% of all PCM, there are very few reports on high-risk IgA PCM. Moreover, no such reports are found on the more infrequent biclonal IgA PCM. Hence, we present a 65-year-old Puerto Rican female with acute abdominal pain, concomitant hypercalcemia, and acute renal failure. Protein electrophoresis with immunofixation found high IgA levels and detected a biclonal IgA gammopathy with kappa specificity. Histomorphologically, bone marrow showed numerous abnormal plasma cells (32%) replacing over 50% of the marrow stroma. Immunophenotyping analysis detected CD45-negative plasma cells aberrantly expressing CD33, CD43, OCT-2, and c-MYC. Chromosomal analysis revealed multiple abnormalities including the gain of chromosome 1q. Thus, we report on an unusual biclonal IgA PCM and the importance of timely diagnosing aggressive plasma cell neoplasms.
- Prognostic and clinicopathological significance of Fusobacterium nucleatum in colorectal cancer: a systemic review and meta-analysis
- Younghoon Kim, Nam Yun Cho, Gyeong Hoon Kang
- J Pathol Transl Med. 2022;56(3):144-151. Published online May 15, 2022
- DOI: https://doi.org/10.4132/jptm.2022.03.13
- 8,581 View
- 144 Download
- 12 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Fusobacterium nucleatum has been identified to promote tumor progression in colorectal cancer (CRC). However, association between F. nucleatum and prognostic or clinicopathological features has been diverse among studies, which could be affected by type of biospecimen (formalin-fixed paraffin-embedded or fresh frozen [FF]).
Methods
Articles were systemically reviewed for studies that included the correlation between F. nucleatum and prognosis or clinicopathological features in CRC.
Results
Ten articles, eight studies with survival-related features involving 3,199 patients and nine studies with clinical features involving 2,655 patients, were eligible for the meta-analysis. Overall survival, disease-free survival, and cancer-specific survival were all associated with worse prognosis in F. nucleatum–high patients (p<.05). In subgroup analysis, only studies with FF tissues retained prognostic significance with F. nucleatum. In meta-analysis of clinicopathological variables, F. nucleatum level was associated with location within colon, pT category, MLH1 hypermethylation, microsatellite instability status, and BRAF mutation regardless of type of biospecimen. However, lymph node metastasis and KRAS mutation was only associated with F. nucleatum level in FF-based studies.
Conclusions
In conclusion, type of biospecimen could affect the role of F. nucleatum as a biomarker associated with clinicopathological features and prognosis. -
Citations
Citations to this article as recorded by- Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights
Linda Galasso, Fabrizio Termite, Irene Mignini, Giorgio Esposto, Raffaele Borriello, Federica Vitale, Alberto Nicoletti, Mattia Paratore, Maria Elena Ainora, Antonio Gasbarrini, Maria Assunta Zocco
Cancers.2025; 17(3): 368. CrossRef - Intratumoural pks Escherichia coli is associated with risk of metachronous colorectal cancer and adenoma development in people with Lynch syndrome
Yen Lin Chu, Peter Georgeson, Mark Clendenning, Khalid Mahmood, Romy Walker, Julia Como, Sharelle Joseland, Susan G. Preston, Toni Rice, Brigid M. Lynch, Roger L. Milne, Melissa C. Southey, Graham G. Giles, Amanda I. Phipps, John L. Hopper, Aung K. Win, C
eBioMedicine.2025; 114: 105661. CrossRef - Fusobacterium nucleatum Enrichment in Colorectal Tumor Tissue: Associations With Tumor Characteristics and Survival Outcomes
Amanda I. Phipps, Courtney M. Hill, Genevieve Lin, Rachel C. Malen, Adriana M. Reedy, Orsalem Kahsai, Hamza Ammar, Keith Curtis, Ningxin Ma, Timothy W. Randolph, Jing Ma, Shuji Ogino, Polly A. Newcomb, Meredith AJ. Hullar
Gastro Hep Advances.2025; 4(6): 100644. CrossRef - Enhancing fibroblast–epithelial cell communications: Serpine2 as a key molecule in Fusobacterium nucleatum–promoted colon cancer
Xueke Li, Simin Luo, Yifang Jiang, Qiong Ma, Fengming You, Qixuan Kuang, Xi Fu, Chuan Zheng
Frontiers in Immunology.2025;[Epub] CrossRef - The presence and relative abundance of salivary Fusobacterium nucleatum are not associated with colorectal cancer: a systematic review and meta-analysis
Ellay Gutmacher, Bálint Zsombor Sárai, Petrana Martineková, Szilvia Kiss-Dala, Gergely Agócs, Péter Hegyi, Andrea Bródy, Ákos Zsembery
Scientific Reports.2025;[Epub] CrossRef - The role of oral microbiota in digestive system diseases: current advances and perspectives
Yaqi Li, Yiping Xin, Wenlu Zong, Xiaoyu Li
Journal of Oral Microbiology.2025;[Epub] CrossRef - Fusobacterium Nucleatum in Colorectal Cancer: Relationship Among Immune Modulation, Potential Biomarkers and Therapeutic Implications
Dalila Incognito, Giuliana Ciappina, Claudia Gelsomino, Antonio Picone, Pierluigi Consolo, Alessandra Scano, Tindara Franchina, Nicola Maurea, Vincenzo Quagliariello, Salvatore Berretta, Alessandro Ottaiano, Massimiliano Berretta
International Journal of Molecular Sciences.2025; 26(19): 9710. CrossRef - Intratumoral presence of the genotoxic gut bacteria pks+ E. coli, Enterotoxigenic Bacteroides fragilis, and Fusobacterium nucleatum and their association with clinicopathological and molecular features of colorectal cancer
Jihoon E. Joo, Yen Lin Chu, Peter Georgeson, Romy Walker, Khalid Mahmood, Mark Clendenning, Aaron L. Meyers, Julia Como, Sharelle Joseland, Susan G. Preston, Natalie Diepenhorst, Julie Toner, Danielle J. Ingle, Norelle L. Sherry, Andrew Metz, Brigid M. Ly
British Journal of Cancer.2024; 130(5): 728. CrossRef - The role of Fusobacterium nucleatum in cancer and its implications for clinical applications
Wanyi Luo, Juxi Han, Xian Peng, Xuedong Zhou, Tao Gong, Xin Zheng
Molecular Oral Microbiology.2024; 39(6): 417. CrossRef -
Fusobacterium nucleatum Load Correlates with KRAS Mutation and Sessile Serrated Pathogenesis in Colorectal Adenocarcinoma
Koki Takeda, Minoru Koi, Yoshiki Okita, Sija Sajibu, Temitope O. Keku, John M. Carethers
Cancer Research Communications.2023; 3(9): 1940. CrossRef - Tumour Colonisation of Parvimonas micra Is Associated with Decreased Survival in Colorectal Cancer Patients
Thyra Löwenmark, Anna Löfgren-Burström, Carl Zingmark, Ingrid Ljuslinder, Michael Dahlberg, Sofia Edin, Richard Palmqvist
Cancers.2022; 14(23): 5937. CrossRef
- Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights
- Follicular lymphoma: updates for pathologists
- Mahsa Khanlari, Jennifer R. Chapman
- J Pathol Transl Med. 2022;56(1):1-15. Published online December 27, 2021
- DOI: https://doi.org/10.4132/jptm.2021.09.29
- 30,745 View
- 1,032 Download
- 21 Web of Science
- 19 Crossref
-
 Abstract
Abstract
 PDF
PDF - Follicular lymphoma (FL) is the most common indolent B-cell lymphoma and originates from germinal center B-cells (centrocytes and centroblasts) of the lymphoid follicle. Tumorigenesis is believed to initiate early in precursor B-cells in the bone marrow (BM) that acquire the t(14;18)(q32;q21). These cells later migrate to lymph nodes to continue their maturation through the germinal center reaction, at which time they acquire additional genetic and epigeneticabnormalities that promote lymphomagenesis. FLs are heterogeneous in terms of their clinicopathologic features. Most FLs are indolent and clinically characterized by peripheral lymphadenopathy with involvement of the spleen, BM, and peripheral blood in a substantial subset of patients, sometimes accompanied by constitutional symptoms and laboratory abnormalities. Diagnosis is established by the histopathologic identification of a B-cell proliferation usually distributed in an at least partially follicular pattern, typically, but not always, in a lymph node biopsy. The B-cell proliferation is biologically of germinal center cell origin, thus shows an expression of germinal center-associated antigens as detected by immunophenotyping. Although many cases of FLs are typical and histopathologic features are straightforward, the biologic and histopathologic variability of FL is wide, and an accurate diagnosis of FL over this disease spectrum requires knowledge of morphologic variants that can mimic other lymphomas, and rarely non-hematologic malignancies, clinically unique variants, and pitfalls in the interpretation of ancillary studies. The overall survival for most patients is prolonged, but relapses are frequent. The treatment landscape in FL now includes the application of immunotherapy and targeted therapy in addition to chemotherapy.
-
Citations
Citations to this article as recorded by- Follicular Cholecystitis: A Case Report Highlighting the Diagnostic Challenges and Management Implications
Ativitch Asavachaisuvikom, Burana Khiankaew, Narongsak Rungsakulkij
Gastro Hep Advances.2026; 5(2): 100833. CrossRef - Relapsed/Refractory Follicular Lymphoma: Current Advances and Emerging Perspectives
Giulio Caridà, Enrica Antonia Martino, Antonella Bruzzese, Daniele Caracciolo, Caterina Labanca, Francesco Mendicino, Eugenio Lucia, Virginia Olivito, Teresa Rossi, Antonino Neri, Ernesto Vigna, Pierfrancesco Tassone, Pierosandro Tagliaferri, Fortunato Mo
European Journal of Haematology.2025; 114(5): 775. CrossRef - Frequency and Distribution of Lymphomas in Northwestern India: A Retrospective Analysis of 923 Cases Using the Latest World Health Organization Classification 5th Edition
Immanuel Paul Thayakaran, Biren Parikh
Indian Journal of Hematology and Blood Transfusion.2025;[Epub] CrossRef - IGH/IGK gene rearrangement in the diagnosis of B-cell non-Hodgkin lymphoma: experience from three centers
Ke Yang, Zhizhong Wang, Beibei Xin, Yunhang Li, Jiuzhou Zhao, Rui Sun, Weizhen Wang, Dongxu Chen, Chengzhi Zhao, Yongjun Guo, Jie Ma, Bing Wei
Annals of Hematology.2025; 104(7): 3779. CrossRef - Imaging Evaluation of Periarticular Soft Tissue Masses in the Appendicular Skeleton: A Pictorial Review
Francesco Pucciarelli, Maria Carla Faugno, Daniela Valanzuolo, Edoardo Massaro, Lorenzo Maria De Sanctis, Elisa Zaccaria, Marta Zerunian, Domenico De Santis, Michela Polici, Tiziano Polidori, Andrea Laghi, Damiano Caruso
Journal of Imaging.2025; 11(7): 217. CrossRef - Understanding the clinical approach to “pathologically ambiguous follicular lymphoma” through a Real-World cohort
Sarah Matarasso Greenfeld, Svetlana Dmitrienko, Ian Shrier, Jean Luc Deschenes, Sarit Assouline
Leukemia & Lymphoma.2025; 66(12): 2332. CrossRef - Deciphering and targeting oncogenic pathways through integrated approaches and amino acid metabolism in hematologic malignancies
Farhan Ikhtiar, Adil Jamal, Syed M. Safeer Mehdi Bokhari
Discover Oncology.2025;[Epub] CrossRef - Transformation of low-grade follicular lymphoma to a high-grade follicular lymphoma with the histopathological diagnosis from oral biopsy: a case report
Gabriela Silveira de Araujo, Leandro Dorigan de Macedo, Alfredo Ribeiro-Silva, Hilton Marcos Alves Ricz, Lara Maria Alencar Ramos Innocentini
Hematology, Transfusion and Cell Therapy.2024; 46: S380. CrossRef - The follicular lymphoma tumor microenvironment at single-cell and spatial resolution
Andrea J. Radtke, Mark Roschewski
Blood.2024; 143(12): 1069. CrossRef - Chronic pancreatitis for the clinician: complications and special forms of the disease. Interdisciplinary position paper of the Catalan Society of Digestology (SCD) and the Catalan Pancreatic Society (SCPanc)
Xavier MOLERO, Juan R. AYUSO, Joaquim BALSELLS, Jaume BOADAS, Juli BUSQUETS, Anna CASTERÀS, Mar CONCEPCIÓN, Míriam CUATRECASAS, Gloria FERNÀNDEZ ESPARRACH, Esther FORT, Francisco GARCIA BOROBIA, Àngels GINÈS, Lucas ILZARBE, Carme LORAS, Miquel MASACHS, Xa
Minerva Gastroenterology.2024;[Epub] CrossRef - Concurrent identification of follicular lymphoma and papillary thyroid carcinoma
Lama A. Alzelfawi, Norah ALhumaidan, Abrar H. Alageel, Buthaina J. Yahya, Saud D. Alrasheedi, Adel S. Alqahtani
International Journal of Surgery Case Reports.2024; 122: 110009. CrossRef - Impact of Primary Disease Site of Involvement by Early-Stage Follicular Lymphoma on Patient Outcomes
Olivia Davis, Carmen Lessani, Rana Kasht, Andrew Cohoon, Sami Ibrahimi, Adam Asch, Silas Day, Taha Al-Juhaishi
Clinical Lymphoma Myeloma and Leukemia.2024; 24(12): 837. CrossRef - Recent developments in CD19-targeted therapies for follicular lymphoma
Aditi Saha, Julio C. Chavez
Expert Opinion on Biological Therapy.2024; 24(10): 1049. CrossRef - Unraveling the complexity of follicular lymphoma: insights and innovations
Xijing Li
American Journal of Cancer Research.2024; 14(12): 5573. CrossRef - Clinical features and prognostic factors in 49 patients with follicular lymphoma at a single center: A retrospective analysis
Hao Wu, Hui-Cong Sun, Gui-Fang Ouyang
World Journal of Clinical Cases.2023; 11(14): 3176. CrossRef - A rare case of follicular lymphoma of the bladder
Matthew DeSanto, Robert Strait, Jared Zopp, Kevin Brown, Samuel Deem
Urology Case Reports.2023; 51: 102542. CrossRef - Analysis of immunophenotypic features in hyaline vascular type Castleman disease
Yu Chang, Yu Ma, Chen Chang, Wensheng Li
Diagnostic Pathology.2023;[Epub] CrossRef - Leg Edema Unveiled: The Uncommon Culprit of Follicular Lymphoma
Syed Muhammad IbnE Ali Jaffari, Samaha Nisar, Narjis Malik, Syed Muhammad Aun Ali Jaffari, Omar Nisar
Journal of Shalamar Medical & Dental College - JSHMDC.2023; 4(2): 125. CrossRef - A Review of the Totality of Evidence in the Development of ABP 798, A Rituximab Biosimilar
Patrick Cobb, Dietger Niederwieser, Stanley Cohen, Caroline Hamm, Gerd Burmester, Neungseon Seo, Sonya G Lehto, Vladimir Hanes
Immunotherapy.2022; 14(9): 727. CrossRef
- Follicular Cholecystitis: A Case Report Highlighting the Diagnostic Challenges and Management Implications
- Prognostic significance of viable tumor size measurement in hepatocellular carcinomas after preoperative locoregional treatment
- Yoon Jung Hwang, Youngeun Lee, Hyunjin Park, Yangkyu Lee, Kyoungbun Lee, Haeryoung Kim
- J Pathol Transl Med. 2021;55(5):338-348. Published online September 2, 2021
- DOI: https://doi.org/10.4132/jptm.2021.07.26
- 6,387 View
- 122 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Preoperative locoregional treatment (LRT) for hepatocellular carcinoma (HCC) often induces intratumoral necrosis without affecting the overall tumor size, and residual viable tumor size (VTS) on imaging is an important clinical parameter for assessing post-treatment response. However, for surgical specimens, it is unclear whether the VTS would be more relevant to prognosis compared to total tumor size (TTS).
Methods
A total of 142 surgically resected solitary HCC cases were retrospectively reviewed. The TTS and VTS were assessed by applying the modified Response Evaluation Criteria in Solid Tumors method to the resected specimens, and correlated with the clinicopathological features and survival.
Results
As applying VTS, 13/142 cases (9.2%) were down-staged to ypT1a. Although the survival analysis results for overall survival according to TTS or VTS were similar, VTS was superior to predict disease-free survival (DFS; p = .023) compared to TTS (p = .08). In addition, multivariate analysis demonstrated VTS > 2 cm to be an independent predictive factor for decreased DFS (p = .001). In the subpopulation of patients with LRT (n = 54), DFS in HCCs with TTS or VTS > 2 cm were significantly shorter than those with TTS or VTS ≤ 2 cm (p = .047 and p = .001, respectively). Interestingly, HCCs with TTS > 2 cm but down-staged to VTS ≤ 2 cm after preoperative LRT had similar survival to those with TTS ≤ 2 cm.
Conclusions
Although the prognostic impact of tumor size was similar regardless of whether TTS or VTS was applied, reporting VTS may help to increase the number of candidates for surgery in HCC patients with preoperative LRT. -
Citations
Citations to this article as recorded by- PET-Assessed Metabolic Tumor Volume Across the Spectrum of Solid-Organ Malignancies: A Review of the Literature
Anusha Agarwal, Chase J. Wehrle, Sangeeta Satish, Paresh Mahajan, Suneel Kamath, Shlomo Koyfman, Wen Wee Ma, Maureen Linganna, Jamak Modaresi Esfeh, Charles Miller, David C. H. Kwon, Andrea Schlegel, Federico Aucejo
Biomedicines.2025; 13(1): 123. CrossRef - Measures for response assessment in HCC treatment
Fereshteh Yazdanpanah, Omar Al-Daoud, Moein Moradpour, Stephen Hunt
Hepatoma Research.2024;[Epub] CrossRef - Machine Learning for Dynamic Prognostication of Patients With Hepatocellular Carcinoma Using Time-Series Data: Survival Path Versus Dynamic-DeepHit HCC Model
Lujun Shen, Yiquan Jiang, Tao Zhang, Fei Cao, Liangru Ke, Chen Li, Gulijiayina Nuerhashi, Wang Li, Peihong Wu, Chaofeng Li, Qi Zeng, Weijun Fan
Cancer Informatics.2024;[Epub] CrossRef - Construction and validation of a novel signature based on epithelial-mesenchymal transition–related genes to predict prognosis and immunotherapy response in hepatocellular carcinoma by comprehensive analysis of the tumor microenvironment
Biao Gao, Yafei Wang, Shichun Lu
Functional & Integrative Genomics.2023;[Epub] CrossRef - Cellular senescence affects energy metabolism, immune infiltration and immunotherapeutic response in hepatocellular carcinoma
Biao Gao, Yafei Wang, Shichun Lu
Scientific Reports.2023;[Epub] CrossRef
- PET-Assessed Metabolic Tumor Volume Across the Spectrum of Solid-Organ Malignancies: A Review of the Literature
- Prognostic role of ALK-1 and h-TERT expression in glioblastoma multiforme: correlation with ALK gene alterations
- Dalia Elsers, Doaa F. Temerik, Alia M. Attia, A. Hadia, Marwa T. Hussien
- J Pathol Transl Med. 2021;55(3):212-224. Published online May 11, 2021
- DOI: https://doi.org/10.4132/jptm.2021.03.15
- 6,336 View
- 132 Download
- 5 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase that is expressed in the developing central and peripheral nervous systems during embryogenesis. Human telomerase reverse transcriptase (h-TERT) protein resumption is the main process of preservation of telomeres that maintains DNA integrity. The present study aims to evaluate the prognostic role of ALK-1 and h-TERT protein expression and their correlation with ALK gene alterations in glioblastoma multiforme (GBM).
Methods
The current study is a retrospective study on a cohort of patients with GBM (n = 53) that attempted to detect ALK gene alterations using fluorescence in situ hybridization. ALK-1 and h-TERT proteins were evaluated using immunohistochemistry.
Results
Score 3 ALK-1 expression was significantly associated with male sex, tumor multiplicity, Ki labeling index (Ki LI), and type of therapeutic modality. Score 3 h-TERT expression exhibited a significant association with Ki LI. ALK gene amplifications (ALK-A) were significantly associated with increased Ki LI and therapeutic modalities. Score 3 ALK-1 protein expression, score 3 h-TERT protein expression, and ALK-A were associated with poor overall survival (OS) and progression-free survival (PFS). Multivariate analysis for OS revealed that ALK gene alterations were an independent prognostic factor for OS and PFS.
Conclusions
High protein expression of both ALK-1 and h-TERT, as well as ALK-A had a poor impact on the prognosis of GBM. Further studies are needed to establish the underlying mechanisms. -
Citations
Citations to this article as recorded by- TERT Gene Mutation in Gliomas Cross‐Linked With (NTRK, PDL1, ALK, IDH, P53, EGFR, HER2): A Integrative Review TERT Gene Mutation in Gliomas
Gunter Gerson Santos, Guilherme Nobre Nogueira, Iasmin Maria Rodrigues Saldanha, Ana Gabriela Ponte Farias, Cauan Miranda Mateus, Osvaldo Mariano Viana Neto, Maria Jânia Teixeira
Journal of Surgical Oncology.2025; 131(6): 1202. CrossRef - Mapping chromatin remodelling in glioblastoma identifies epigenetic regulation of key molecular pathways and novel druggable targets
Claire Vinel, James Boot, Weiwei Jin, Nicola Pomella, Alexandra Hadaway, Charles Mein, Nicolae Radu Zabet, Silvia Marino
BMC Biology.2025;[Epub] CrossRef - Association of human telomerase reverse transcriptase promoter mutation with unfavorable prognosis in glioma: A systematic review and meta-analysis
Rongxuan Hua, Qiuxuan Li, Han Gao, Boya Wang, Chengwei He, Ying Wang, Sitian Zhang, Lei Gao, Hongwei Shang, Wen Wang, Jingdong Xu
Journal of Research in Medical Sciences.2023;[Epub] CrossRef - Immunohistochemical surrogates for molecular alterations for the classification and grading of gliomas
Viharkumar Patel, Sanda Alexandrescu
Seminars in Diagnostic Pathology.2022; 39(1): 78. CrossRef - Meme Kanseri Hastalarında hTERT Gen Ekspresyonunun Klinikopatolojik Önemi
Ebubekir DİRİCAN, Burak KANKAYA, Zeynep TATAR
Sağlık Bilimlerinde Değer.2022; 12(1): 22. CrossRef - Prognostic and predictive markers in glioblastoma and ALK overexpression
Jang-Hee Kim
Journal of Pathology and Translational Medicine.2021; 55(3): 236. CrossRef
- TERT Gene Mutation in Gliomas Cross‐Linked With (NTRK, PDL1, ALK, IDH, P53, EGFR, HER2): A Integrative Review TERT Gene Mutation in Gliomas
- MicroRNA-552 expression in colorectal cancer and its clinicopathological significance
- Joon Im, Soo Kyung Nam, Hye Seung Lee
- J Pathol Transl Med. 2021;55(2):125-131. Published online February 19, 2021
- DOI: https://doi.org/10.4132/jptm.2021.01.17
- 5,576 View
- 128 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
MicroRNA-552 (miR-552) has been reported to correlate with the development and progression of various cancers, including colorectal cancer (CRC). This study aimed to investigate miR-552 expression in cancer tissue samples compared to normal mucosal tissue and its role as a diagnostic or prognostic marker in CRC patients.
Methods
Normal mucosal tissues and primary cancer tissues from 80 surgically resected CRC specimens were used. Quantitative real-time polymerase chain reaction was performed for miR-552 and U6 small nuclear RNA to analyze miR-552 expression and its clinicopathological significance. Immunohistochemistry for p53 and phosphatase and tension homolog (PTEN) was performed to evaluate their association with miR-552 expression.
Results
miR-552 expression was significantly higher in primary cancer tissues compared to normal mucosal tissues (p<.001). The expression level of miR552 was inversely correlated with that of PTEN (p=.068) and p53 (p=.004). Survival analysis showed that high miR-552 expression was associated with worse prognosis but this was not statistically significant (p=.255). However, patients with CRC having high miR-552 expression and loss of PTEN expression had significantly worse prognosis than others (p=.029).
Conclusions
Our results suggest that high miR-552 expression might be a potential diagnostic biomarker for CRC, and its combined analysis with PTEN expression can possibly be used as a prognostic marker. -
Citations
Citations to this article as recorded by- MicroRNAs involved in colorectal cancer, a rapid mini-systematic review
Sogol Shirzad, Majid Eterafi, Zeinab Karimi, Mahdi Barazesh
BMC Cancer.2025;[Epub] CrossRef - Diagnostic and Therapeutic Potential of Selected microRNAs in Colorectal Cancer: A Literature Review
Grzegorz Sychowski, Hanna Romanowicz, Wojciech Ciesielski, Piotr Hogendorf, Adam Durczyński, Beata Smolarz
Cancers.2025; 17(13): 2135. CrossRef - Blood miRNAs miR-549a, miR-552, and miR-592 serve as potential disease-specific panels to diagnose colorectal cancer
Soroush Akbar, Samaneh Mashreghi, Mohammad Reza Kalani, Akram Valanik, Farzaneh Ahmadi, Mahdi Aalikhani, Zahra Bazi
Heliyon.2024; 10(7): e28492. CrossRef - Integration of TE Induces Cancer Specific Alternative Splicing Events
Woo Ryung Kim, Eun Gyung Park, Yun Ju Lee, Woo Hyeon Bae, Du Hyeong Lee, Heui-Soo Kim
International Journal of Molecular Sciences.2022; 23(18): 10918. CrossRef
- MicroRNAs involved in colorectal cancer, a rapid mini-systematic review
- The prognostic significance of p16 expression pattern in diffuse gliomas
- Jin Woo Park, Jeongwan Kang, Ka Young Lim, Hyunhee Kim, Seong-Ik Kim, Jae Kyung Won, Chul-Kee Park, Sung-Hye Park
- J Pathol Transl Med. 2021;55(2):102-111. Published online December 23, 2020
- DOI: https://doi.org/10.4132/jptm.2020.10.22
- 9,777 View
- 309 Download
- 22 Web of Science
- 19 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
CDKN2A is a tumor suppressor gene that encodes the cell cycle inhibitor protein p16. Homozygous deletion of the CDKN2A gene has been associated with shortened survival in isocitrate dehydrogenase (IDH)–mutant gliomas. This study aimed to analyze the prognostic value of p16 and to evaluate whether p16 immunohistochemical staining could be used as a prognostic marker to replace CDKN2A genotyping in diffuse gliomas.
Methods
p16 immunohistochemistry was performed on tissue microarrays of 326 diffuse gliomas with diagnoses that reflected IDH-mutations and 1p/19q codeletion status. The results were divided into three groups (negative, focal expression, overexpression) according to the presence and degree of p16 expression. Survival analysis was performed to assess the prognostic value of p16 expression.
Results
A loss of p16 expression predicted a significantly worse outcome in all glioma patients (n=326, p<.001), in the IDH-mutant glioma patients (n=103, p=.010), and in the IDH-mutant astrocytoma patients (n=73, p=.032). However, loss of p16 expression did not predict the outcome in the IDH-wildtype glioma patients (n=223, p=.121) or in the oligodendroglial tumor patients with the IDH-mutation and 1p/19q codeletion (n=30, p=.457). Multivariate analysis showed the association was still significant in the IDH-mutant glioma patients (p=.008; hazard ratio [HR], 2.637; 95% confidence interval [CI], 1.295 to 5.372) and in the IDH-mutant astrocytoma patients (p=.001; HR, 3.586; 95% CI, 1.649 to 7.801). Interestingly, patients who presented with tumors with p16 overexpression also had shorter survival times than did patients with tumors with p16 focal expression in the whole glioma (p< .001) and in IDH-mutant glioma groups. (p=.046).
Conclusions
This study suggests that detection of p16 expression by immunohistochemistry can be used as a useful surrogate test to predict prognosis, especially in IDH-mutant astrocytoma patients. -
Citations
Citations to this article as recorded by- A comparison of CDKN2A status in gliomas using different techniques: The loss of p16 as a surrogate marker
Arnault Tauziède-Espariat, Audrey Rousseau, Laetitia Basset, Raphaël Saffroy, Ana Cavillon, Amélie Lusque, Lauren Hasty, Alice Métais, Yvan Nicaise, Emmanuelle Uro-Coste, Pascale Varlet, Pascale Varlet, Arnault Tauziède-Espariat
Journal of Neuropathology & Experimental Neurology.2025; 84(10): 847. CrossRef - Cell-Specific Vulnerability of Human Glioblastoma and Astrocytoma Cells to Mephedrone—An In Vitro Study
Marta Marszalek-Grabska, Marta Kinga Lemieszek, Michal Chojnacki, Sylwia Winiarczyk, Joanna Jakubowicz-Gil, Barbara Zarzyka, Jarosław Pawelec, Jolanta H. Kotlinska, Wojciech Rzeski, Waldemar A. Turski
Molecules.2025; 30(11): 2277. CrossRef - Clinical value and mechanism of CDKN2A in clear cell renal cell carcinoma
Yan Li, Songsong Wang, Yilong Lin, Junting Li, Xin Lan, Anqi Lv, Junwei Chen, Ziming Liu
Discover Oncology.2025;[Epub] CrossRef - Revealing the role of necroptosis microenvironment: FCGBP + tumor-associated macrophages drive primary liver cancer differentiation towards cHCC-CCA or iCCA
Chun Wang, Cuimin Chen, Wenting Hu, Lili Tao, Jiakang Chen
Apoptosis.2024; 29(3-4): 460. CrossRef - FISH analysis reveals CDKN2A and IFNA14 co-deletion is heterogeneous and is a prominent feature of glioblastoma
Sofian Al Shboul, Shelagh Boyle, Ashita Singh, Tareq Saleh, Moath Alrjoub, Ola Abu Al Karsaneh, Amel Mryyian, Rand Dawoud, Sinem Gul, Shaden Abu Baker, Kathryn Ball, Ted Hupp, Paul M. Brennan
Brain Tumor Pathology.2024; 41(1): 4. CrossRef - p16 Expression in Laryngeal Squamous Cell Carcinoma: A Surrogate or Independent Prognostic Marker?
Roberto Gallus, Davide Rizzo, Giorgia Rossi, Luca Mureddu, Jacopo Galli, Alberto Artuso, Francesco Bussu
Pathogens.2024; 13(2): 100. CrossRef - CDKN2A/B deletion in IDH-mutant astrocytomas: An evaluation by Fluorescence in-situ hybridization
Manali Ranade, Sridhar Epari, Omshree Shetty, Sandeep Dhanavade, Sheetal Chavan, Ayushi Sahay, Arpita Sahu, Prakash Shetty, Aliasgar Moiyadi, Vikash Singh, Archya Dasgupta, Abhishek Chatterjee, Sadhana Kannan, Tejpal Gupta
Journal of Neuro-Oncology.2024; 167(1): 189. CrossRef - Molecular prognostication in grade 3 meningiomas and p16/MTAP immunohistochemistry for predicting CDKN2A/B status
Kira Tosefsky, Karina Chornenka Martin, Alexander D Rebchuk, Justin Z Wang, Farshad Nassiri, Amy Lum, Gelareh Zadeh, Serge Makarenko, Stephen Yip
Neuro-Oncology Advances.2024;[Epub] CrossRef - Insights into brain tumor diagnosis: exploring in situ hybridization techniques
E. D. Namiot, G. M. Zembatov, P. P. Tregub
Frontiers in Neurology.2024;[Epub] CrossRef - CDKN2A Homozygous Deletion Is a Stronger Predictor of Outcome than IDH1/2-Mutation in CNS WHO Grade 4 Gliomas
Sang Hyuk Lee, Tae Gyu Kim, Kyeong Hwa Ryu, Seok Hyun Kim, Young Zoon Kim
Biomedicines.2024; 12(10): 2256. CrossRef - Homozygous CDKN2A/B deletions in low- and high-grade glioma: a meta-analysis of individual patient data and predictive values of p16 immunohistochemistry testing
Darius Noack, Johannes Wach, Alonso Barrantes-Freer, Nils H. Nicolay, Erdem Güresir, Clemens Seidel
Acta Neuropathologica Communications.2024;[Epub] CrossRef - p16 Immunohistochemical Expression as a Surrogate Assessment of CDKN2A Alteration in Gliomas Leading to Prognostic Significances
Lucas Geyer, Thibaut Wolf, Marie-Pierre Chenard, Helene Cebula, Roland Schott, Georges Noel, Eric Guerin, Erwan Pencreach, Damien Reita, Natacha Entz-Werlé, Benoît Lhermitte
Cancers.2023; 15(5): 1512. CrossRef - P16 immunohistochemistry is a sensitive and specific surrogate marker for CDKN2A homozygous deletion in gliomas
Meenakshi Vij, Benjamin B. Cho, Raquel T. Yokoda, Omid Rashidipour, Melissa Umphlett, Timothy E. Richardson, Nadejda M. Tsankova
Acta Neuropathologica Communications.2023;[Epub] CrossRef -
CDKN2A mutations have equivalent prognostic significance to homozygous deletion in IDH-mutant astrocytoma
Raquel T Yokoda, William S Cobb, Raymund L Yong, John F Crary, Mariano S Viapiano, Jamie M Walker, Melissa Umphlett, Nadejda M Tsankova, Timothy E Richardson
Journal of Neuropathology & Experimental Neurology.2023; 82(10): 845. CrossRef - Efficient diagnosis of IDH-mutant gliomas: 1p/19qNET assesses 1p/19q codeletion status using weakly-supervised learning
Gi Jeong Kim, Tonghyun Lee, Sangjeong Ahn, Youngjung Uh, Se Hoon Kim
npj Precision Oncology.2023;[Epub] CrossRef - The Prognostic Significance of P16 Immunohistochemical Expression Pattern in Women with Invasive Ductal Breast Carcinoma
Alireza Rezaei, Navidreza Shayan, Saman Shirazinia, Sara Mollazadeh, Negin Ghiyasi-Moghaddam
Reports of Biochemistry and Molecular Biology.2023; 12(1): 83. CrossRef - Sporadic and Lynch syndrome-associated mismatch repair-deficient brain tumors
Hyunhee Kim, Ka Young Lim, Jin Woo Park, Jeongwan Kang, Jae Kyung Won, Kwanghoon Lee, Yumi Shim, Chul-Kee Park, Seung-Ki Kim, Seung-Hong Choi, Tae Min Kim, Hongseok Yun, Sung-Hye Park
Laboratory Investigation.2022; 102(2): 160. CrossRef - Simple approach for the histomolecular diagnosis of central nervous system gliomas based on 2021 World Health Organization Classification
Maher Kurdi, Rana H Moshref, Yousef Katib, Eyad Faizo, Ahmed A Najjar, Basem Bahakeem, Ahmed K Bamaga
World Journal of Clinical Oncology.2022; 13(7): 567. CrossRef - P16INK4A—More Than a Senescence Marker
Hasan Safwan-Zaiter, Nicole Wagner, Kay-Dietrich Wagner
Life.2022; 12(9): 1332. CrossRef
- A comparison of CDKN2A status in gliomas using different techniques: The loss of p16 as a surrogate marker
- A comparative prognostic performance of definitions of Crohn-like lymphoid reaction in colorectal carcinoma
- Younghoon Kim, Jeong Mo Bae, Jung Ho Kim, Nam-Yun Cho, Gyeong Hoon Kang
- J Pathol Transl Med. 2021;55(1):53-59. Published online November 27, 2020
- DOI: https://doi.org/10.4132/jptm.2020.10.06
- 6,326 View
- 147 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
The prognostic potential of Crohn-like lymphoid reaction (CLR) in colorectal carcinoma (CRC) has been investigated through the assessment of different criteria.
Methods
The prognostic impact of CLR was investigated in 636 CRC patients to compare methods from previously published articles. These methods included CLR measured by number of lymphoid aggregates (LAs) (CLR count), LA size greater than or equal to 1 mm (CLR size), CLR density with a cutoff value of 0.38, and subjective criteria as defined by intense CLR.
Results
In univariate survival analysis, CLR-positive CRC as defined by the four aforementioned methods was associated with better overall survival (OS) (hazard ratio [HR], 0.463; 95% confidence interval [CI], 0.305 to 0.702; p <.001; HR, 0.656; 95% CI, 0.411 to 1.046; p=.077; HR, 0.363; 95% CI, 0.197 to 0.669; p=.001; and HR, 0.433; 95% CI, 0.271 to 0.690; p<.001, respectively) and disease-free survival (DFS) (HR, 0.411; 95% CI, 0.304 to 0.639; p<.001; HR, 0.528; 95% CI, 0.340 to 0.821; p=.004; HR, 0.382; 95% CI, 0.226 to 0.645, p=.004; and HR, 0.501; 95% CI, 0.339 to 0.741; p<.001, respectively) than CLR-negative CRC, regardless of criteria with the exception of OS for CLR density. In multivariate analysis, two objective criteria (CLR count and CLR density) and one subjective criterion (intense CLR) for defining CLR were considered independent prognostic factors of OS and DFS in CRC patients.
Conclusions
CLR has similar traits regardless of criteria, but CLR-positivity should be defined by objective criteria for better reproducibility and prognostic value. -
Citations
Citations to this article as recorded by- Prognostic Significance of Immune and Stromal Components in Colorectal Cancer
Mi Jang, Yongki Hong, Soojung Hong, Eun Kyung Kim
Archives of Pathology & Laboratory Medicine.2025; 149(11): 982. CrossRef
- Prognostic Significance of Immune and Stromal Components in Colorectal Cancer
- Programmed death-ligand 1 expression and its correlation with clinicopathological parameters in gallbladder cancer
- Ji Hye Kim, Kyungbin Kim, Misung Kim, Young Min Kim, Jae Hee Suh, Hee Jeong Cha, Hye Jeong Choi
- J Pathol Transl Med. 2020;54(2):154-164. Published online February 10, 2020
- DOI: https://doi.org/10.4132/jptm.2019.11.13
- 9,585 View
- 172 Download
- 16 Web of Science
- 15 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Immunomodulatory therapies targeting the interaction between programmed cell death protein 1 and programmed death-ligand 1 (PD-L1) have become increasingly important in anticancer treatment. Previous research on the subject of this immune response has established an association with tumor aggressiveness and a poor prognosis in certain cancers. Currently, scant information is available on the relationship between PD-L1 expression and gallbladder cancer (GBC).
Methods
We investigated the expression of PD-L1 in 101 primary GBC cases to determine the potential association with prognostic impact. PD-L1 expression was immunohistochemically assessed using a single PD-L1 antibody (clone SP263). Correlations with clinicopathological parameters, overall survival (OS), or progression- free survival (PFS) were analyzed.
Results
PD-L1 expression in tumor cells at cutoff levels of 1%, 10%, and 50% was present in 18.8%, 13.8%, and 7.9% of cases. Our study showed that positive PD-L1 expression at any cutoff was significantly correlated with poorly differentiated histologic grade and the presence of lymphovascular invasion (p < .05). PD-L1 expression at cutoff levels of 10% and 50% was significantly positive in patients with perineural invasion, higher T categories, and higher pathologic stages (p < .05). Additionally, there was a significant association noted between PD-L1 expression at a cutoff level of 50% and worse OS or PFS (p = .049 for OS, p = .028 for PFS). Other poor prognostic factors included histologic grade, T category, N category, pathologic stage, lymphovascular invasion, perineural invasion, growth pattern, and margin of resection (p < .05).
Conclusions
The expression of PD-L1 in GBC varies according to cutoff level but is valuably associated with poor prognostic parameters and survival. Our study indicates that the overexpression of PD-L1 in GBC had a negative prognostic impact. -
Citations
Citations to this article as recorded by- PD-L1 Expression in Biliary Tract Cancer: Comparison Across Antibody Clones and Role as a Predictor of Response to Chemoimmunotherapy: A Meta-Analysis
Juan J. Juarez-Vignon Whaley, Soravis Osataphan, Ben Ponvilawan, Nipith Charoenngam, Mary Linton Peters
JCO Precision Oncology.2025;[Epub] CrossRef - An MRI-based model for preoperative prediction of tertiary lymphoid structures in patients with gallbladder cancer
Ying Xu, Zhuo Li, Weihua Zhi, Yi Yang, Jingzhong Ouyang, Yanzhao Zhou, Zeliang Ma, Sicong Wang, Lizhi Xie, Jianming Ying, Jinxue Zhou, Xinming Zhao, Feng Ye
Insights into Imaging.2025;[Epub] CrossRef - Lacking Immunotherapy Biomarkers for Biliary Tract Cancer: A Comprehensive Systematic Literature Review and Meta-Analysis
Giorgio Frega, Fernando P. Cossio, Jesus M. Banales, Vincenzo Cardinale, Rocio I. R. Macias, Chiara Braconi, Angela Lamarca
Cells.2023; 12(16): 2098. CrossRef - Gallbladder carcinomas: review and updates on morphology, immunohistochemistry, and staging
Whayoung Lee, Vishal S. Chandan
Human Pathology.2023; 132: 149. CrossRef - Prognostic Relevance of PDL1 and CA19-9 Expression in Gallbladder Cancer vs. Inflammatory Lesions
Neetu Rawal, Supriya Awasthi, Nihar Ranjan Dash, Sunil Kumar, Prasenjit Das, Amar Ranjan, Anita Chopra, Maroof Ahmad Khan, Sundeep Saluja, Showket Hussain, Pranay Tanwar
Current Oncology.2023; 30(2): 1571. CrossRef - Identification of genes associated with gall bladder cell carcinogenesis: Implications in targeted therapy of gall bladder cancer
Ishita Ghosh, Ruma Dey Ghosh, Soma Mukhopadhyay
World Journal of Gastrointestinal Oncology.2023; 15(12): 2053. CrossRef - CD73 and PD-L1 as Potential Therapeutic Targets in Gallbladder Cancer
Lu Cao, Kim R. Bridle, Ritu Shrestha, Prashanth Prithviraj, Darrell H. G. Crawford, Aparna Jayachandran
International Journal of Molecular Sciences.2022; 23(3): 1565. CrossRef - Evolving Role of Immunotherapy in Advanced Biliary Tract Cancers
Sandra Kang, Bassel F. El-Rayes, Mehmet Akce
Cancers.2022; 14(7): 1748. CrossRef - Novel immune scoring dynamic nomograms based on B7-H3, B7-H4, and HHLA2: Potential prediction in survival and immunotherapeutic efficacy for gallbladder cancer
Chao Lv, Shukun Han, Baokang Wu, Zhiyun Liang, Yang Li, Yizhou Zhang, Qi Lang, Chongli Zhong, Lei Fu, Yang Yu, Feng Xu, Yu Tian
Frontiers in Immunology.2022;[Epub] CrossRef - PD-1 inhibitors plus nab-paclitaxel-containing chemotherapy for advanced gallbladder cancer in a second-line setting: A retrospective analysis of a case series
Sirui Tan, Jing Yu, Qiyue Huang, Nan Zhou, Hongfeng Gou
Frontiers in Oncology.2022;[Epub] CrossRef - Expression of HER2 and Mismatch Repair Proteins in Surgically Resected Gallbladder Adenocarcinoma
You-Na Sung, Sung Joo Kim, Sun-Young Jun, Changhoon Yoo, Kyu-Pyo Kim, Jae Hoon Lee, Dae Wook Hwang, Shin Hwang, Sang Soo Lee, Seung-Mo Hong
Frontiers in Oncology.2021;[Epub] CrossRef - Programmed Death Ligand-1 (PD-L1) Is an Independent Negative Prognosticator in Western-World Gallbladder Cancer
Thomas Albrecht, Fritz Brinkmann, Michael Albrecht, Anke S. Lonsdorf, Arianeb Mehrabi, Katrin Hoffmann, Yakup Kulu, Alphonse Charbel, Monika N. Vogel, Christian Rupp, Bruno Köhler, Christoph Springfeld, Peter Schirmacher, Stephanie Roessler, Benjamin Goep
Cancers.2021; 13(7): 1682. CrossRef - Immune Microenvironment in Gallbladder Adenocarcinomas
Pallavi A. Patil, Kara Lombardo, Weibiao Cao
Applied Immunohistochemistry & Molecular Morphology.2021; 29(8): 557. CrossRef - Molecular Targets and Emerging Therapies for Advanced Gallbladder Cancer
Matteo Canale, Manlio Monti, Ilario Giovanni Rapposelli, Paola Ulivi, Francesco Giulio Sullo, Giulia Bartolini, Elisa Tiberi, Giovanni Luca Frassineti
Cancers.2021; 13(22): 5671. CrossRef - Overview of current targeted therapy in gallbladder cancer
Xiaoling Song, Yunping Hu, Yongsheng Li, Rong Shao, Fatao Liu, Yingbin Liu
Signal Transduction and Targeted Therapy.2020;[Epub] CrossRef
- PD-L1 Expression in Biliary Tract Cancer: Comparison Across Antibody Clones and Role as a Predictor of Response to Chemoimmunotherapy: A Meta-Analysis
- Expression of female sex hormone receptors and its relation to clinicopathological characteristics and prognosis of lung adenocarcinoma
- Jin Hwan Lee, Han Kyeom Kim, Bong Kyung Shin
- J Pathol Transl Med. 2020;54(1):103-111. Published online November 13, 2019
- DOI: https://doi.org/10.4132/jptm.2019.10.12
- 7,953 View
- 141 Download
- 6 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Adenocarcinoma (ADC) of the lung exhibits different clinicopathological characteristics in men and women. Recent studies have suggested that these differences originate from the expression of female sex hormone receptors in tumor cells. The aim of the present study was to evaluate the immunohistochemical expression of female sex hormone receptors in lung ADC and determine the expression patterns in patients with different clinicopathological characteristics.
Methods
A total of 84 patients with lung ADC who underwent surgical resection and/or core biopsy were recruited for the present study. Immunohistochemical staining was performed for estrogen receptor α (ERα), estrogen receptor β (ERβ), progesterone receptor (PR), epidermal growth factor receptor (EGFR), EGFR E746- A750 del, and EGFR L858R using tissue microarray.
Results
A total of 39 (46.4%) ERα-positive, 71 (84.5%) ERβ-positive, and 46 (54.8%) PR-positive lung ADCs were identified. In addition, there were 81 (96.4%) EGFR-positive, 14 (16.7%) EGFR E746-A750 del–positive, and 34 (40.5%) EGFR L858R–positive cases. The expression of female sex hormone receptors was not significantly different in clinicopathologically different subsets of lung ADC.
Conclusions
Expression of female sex hormone receptors is not associated with the prognosis and clinicopathological characteristics of patients with lung ADC. -
Citations
Citations to this article as recorded by- Molecular characteristics of non-small cell lung cancer tissue based on quantitative indicators of progesterone receptors expression
I. P. Romanov, T. A. Bogush, A. M. Scherbakov, A. A. Alimov, E. A. Bogush, A. B. Ravcheeva, A. Lee, V. S. Kosorukov
Antibiot Khimioter = Antibiotics and Chemotherapy.2024; 69(1-2): 29. CrossRef - Genes Co-Expressed with ESR2 Influence Clinical Outcomes in Cancer Patients: TCGA Data Analysis
Julia Maria Lipowicz, Agnieszka Malińska, Michał Nowicki, Agnieszka Anna Rawłuszko-Wieczorek
International Journal of Molecular Sciences.2024; 25(16): 8707. CrossRef - Complex Differential Diagnosis between Primary Breast Cancer and Breast Metastasis from EGFR-Mutated Lung Adenocarcinoma: Case Report and Literature Review
Carmine Valenza, Francesca Maria Porta, Alessandra Rappa, Elena Guerini-Rocco, Giuseppe Viale, Massimo Barberis, Filippo de Marinis, Giuseppe Curigliano, Chiara Catania
Current Oncology.2021; 28(5): 3384. CrossRef - Development of a 15‐Gene Signature Model as a Prognostic Tool in Sex Hormone‐Dependent Cancers
Zhi Xia, Jian Xiao, Aibin Liu, Qiong Chen, Arumugam R. Jayakumar
BioMed Research International.2021;[Epub] CrossRef - Gender-specific aspects of epidemiology, molecular genetics and outcome: lung cancer
Nuria Mederos, Alex Friedlaender, Solange Peters, Alfredo Addeo
ESMO Open.2020; 5(Suppl 4): e000796. CrossRef
- Molecular characteristics of non-small cell lung cancer tissue based on quantitative indicators of progesterone receptors expression
- Clinicopathological Characterization and Prognostic Implication of SMAD4 Expression in Colorectal Carcinoma
- Seung-Yeon Yoo, Ji-Ae Lee, Yunjoo Shin, Nam-Yun Cho, Jeong Mo Bae, Gyeong Hoon Kang
- J Pathol Transl Med. 2019;53(5):289-297. Published online June 24, 2019
- DOI: https://doi.org/10.4132/jptm.2019.06.07
- 9,497 View
- 170 Download
- 9 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
SMAD family member 4 (SMAD4) has gained attention as a promising prognostic factor of colorectal cancer (CRC) as well as a key molecule to understand the tumorigenesis and progression of CRC.
Methods
We retrospectively analyzed 1,281 CRC cases immunohistochemically for their expression status of SMAD4, and correlated this status with clinicopathologic and molecular features of CRCs.
Results
A loss of nuclear SMAD4 was significantly associated with frequent lymphovascular and perineural invasion, tumor budding, fewer tumor-infiltrating lymphocytes, higher pT and pN category, and frequent distant metastasis. In contrast, tumors overexpressing SMAD4 showed a significant association with sporadic microsatellite instability. After adjustment for TNM stage, tumor differentiation, adjuvant chemotherapy, and lymphovascular invasion, the loss of SMAD4 was found to be an independent prognostic factor for worse 5-year progression-free survival (hazard ratio [HR], 1.27; 95% confidence interval [CI], 1.01 to 1.60; p=.042) and 7-year cancerspecific survival (HR, 1.45; 95% CI, 1.06 to 1.99; p=.022).
Conclusions
We confirmed the value of determining the loss of SMAD4 immunohistochemically as an independent prognostic factor for CRC in general. In addition, we identified some histologic and molecular features that might be clues to elucidate the role of SMAD4 in colorectal tumorigenesis and progression. -
Citations
Citations to this article as recorded by- Механизмы резистентности к иммунотерапии при MSI фенотипе
М. Ю. Федянин
Malignant tumours.2025; 15(3s1): 11. CrossRef - A set cover algorithm identifies minimal circulating tumour DNA sequencing targets for colorectal cancer detection
Kit Moloney-Geany, Michael A. Black, Robert C. Day, Parry Guilford, Michael J. Dunnet
Scientific Reports.2025;[Epub] CrossRef - Association between the expression of epithelial–mesenchymal transition (EMT)-related markers and oncologic outcomes of colorectal cancer
Mona Hany Emile, Sameh Hany Emile, Amr Awad El-Karef, Mohamed Awad Ebrahim, Ibrahim Eldosoky Mohammed, Dina Abdallah Ibrahim
Updates in Surgery.2024; 76(6): 2181. CrossRef - TGF-β and SMAD2/4 Expression in Nonmetastatic and Metastatic Colorectal Cancer Patients
Ainul Mardiah, Hendra Susanto, Sri Rahayu Lestari, A. Taufiq, H. Susanto, H. Nur, M. Diantoro, M. Aziz, N.A.N.N. Malek
BIO Web of Conferences.2024; 117: 01001. CrossRef - Unraveling Resistance to Immunotherapy in MSI-High Colorectal Cancer
Ronald Heregger, Florian Huemer, Markus Steiner, Alejandra Gonzalez-Martinez, Richard Greil, Lukas Weiss
Cancers.2023; 15(20): 5090. CrossRef - Association of β-Catenin, APC, SMAD3/4, Tp53, and Cyclin D1 Genes in Colorectal Cancer: A Systematic Review and Meta-Analysis
Hongfeng Yan, Fuquan Jiang, Jianwu Yang, Ying-Kun Xu
Genetics Research.2022; 2022: 1. CrossRef - Comprehensive genetic features of gastric mixed adenoneuroendocrine carcinomas and pure neuroendocrine carcinomas
Jiwon Koh, Soo Kyung Nam, Yoonjin Kwak, Gilhyang Kim, Ka‐Kyung Kim, Byung‐Chul Lee, Sang‐Hoon Ahn, Do Joong Park, Hyung‐Ho Kim, Kyoung Un Park, Woo Ho Kim, Hye Seung Lee
The Journal of Pathology.2021; 253(1): 94. CrossRef - Alterations of PTEN and SMAD4 methylation in diagnosis of breast cancer: implications of methyl II PCR assay
Menha Swellam, Entsar A. Saad, Shimaa Sabry, Adel Denewer, Camelia Abdel Malak, Amr Abouzid
Journal of Genetic Engineering and Biotechnology.2021; 19(1): 54. CrossRef - Molecular Characterization and Functional Analysis of Two Steroidogenic Genes TSPO and SMAD4 in Yellow Catfish
Fang Chen, Chong-Chao Zhong, Chang-Chun Song, Shu-Wei Chen, Yang He, Xiao-Ying Tan
International Journal of Molecular Sciences.2021; 22(9): 4505. CrossRef - SMAD7 and SMAD4 expression in colorectal cancer progression and therapy response
Jovana Rosic, Sandra Dragicevic, Marko Miladinov, Jovana Despotovic, Aleksandar Bogdanovic, Zoran Krivokapic, Aleksandra Nikolic
Experimental and Molecular Pathology.2021; 123: 104714. CrossRef - Actionable Potentials of Less Frequently Mutated Genes in Colorectal Cancer and Their Roles in Precision Medicine
Ryia Illani Mohd Yunos, Nurul Syakima Ab Mutalib, Francis Yew Fu Tieng, Nadiah Abu, Rahman Jamal
Biomolecules.2020; 10(3): 476. CrossRef
- Механизмы резистентности к иммунотерапии при MSI фенотипе
- Prognostic Significance of CD109 Expression in Patients with Ovarian Epithelial Cancer
- So Young Kim, Kyung Un Choi, Chungsu Hwang, Hyung Jung Lee, Jung Hee Lee, Dong Hoon Shin, Jee Yeon Kim, Mee Young Sol, Jae Ho Kim, Ki Hyung Kim, Dong Soo Suh, Byung Su Kwon
- J Pathol Transl Med. 2019;53(4):244-252. Published online May 2, 2019
- DOI: https://doi.org/10.4132/jptm.2019.04.16
- 8,510 View
- 129 Download
- 9 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Ovarian epithelial cancer (OEC) is the second-most common gynecologic malignancy. CD109 expression is elevated in human tumor cell lines and carcinomas. A previous study showed that CD109 expression is elevated in human tumor cell lines and CD109 plays a role in cancer progression. Therefore, this study aimed to determine whether CD109 is expressed in OEC and can be useful in predicting the prognosis.
Methods
Immunohistochemical staining for CD109 and reverse transcription-quantitative polymerase chain reaction was performed. Then we compared CD109 expression and chemoresistance, overall survival, and recurrence-free survival of OEC patients. Chemoresistance was evaluated by dividing into good-response group and poor-response group by the time to recurrence after chemotherapy.
Results
CD109 expression was associated with overall survival (p = .020), but not recurrence-free survival (p = .290). CD109 expression was not an independent risk factor for overall survival due to its reliability (hazard ratio, 1.58; p = .160; 95% confidence interval, 0.82 to 3.05), although we found that CD109 positivity was related to chemoresistance. The poor-response group showed higher rates of CD109 expression than the good-response group (93.8% vs 66.7%, p = .047). Also, the CD109 mRNA expression level was 2.88 times higher in the poor-response group as compared to the good-response group (p = .001).
Conclusions
Examining the CD109 expression in patients with OEC may be helpful in predicting survival and chemotherapeutic effect. -
Citations
Citations to this article as recorded by- Advances in the Study of CD109 in Tumors
平慧 周
Medical Diagnosis.2024; 14(02): 167. CrossRef - Identification of CD109 in the extracellular vesicles derived from ovarian cancer stem-like cells
Ye Eun Kim, Jun Se Kim, Min Joo Shin, Seo Yul Lee, Dae Kyoung Kim, Nam-Kyung Lee, Yang Woo Kwon, Kyung-Un Choi, Dong-Soo Suh, Byoung Soo Kim, Sanghwa Jeong, Jae Ho Kim
BMB Reports.2024; 57(12): 527. CrossRef - CD109 Promotes Drug Resistance in A2780 Ovarian Cancer Cells by Regulating the STAT3-NOTCH1 Signaling Axis
Jun Se Kim, Min Joo Shin, Seo Yul Lee, Dae Kyoung Kim, Kyung-Un Choi, Dong-Soo Suh, Dayea Kim, Jae Ho Kim
International Journal of Molecular Sciences.2023; 24(12): 10306. CrossRef - CD109 facilitates progression and 5-fluorouracil resistance of nasopharyngeal carcinoma
Zhenwei Zhu, Fang Zhou, Cheng Mao
Materials Express.2022; 12(9): 1189. CrossRef - Usefulness of CD109 expression as a prognostic biomarker in patients with cancer
Hyun Min Koh, Hyun Ju Lee, Dong Chul Kim
Medicine.2021; 100(11): e25006. CrossRef - Serum CD109 levels reflect the node metastasis status in head and neck squamous cell carcinoma
Sumitaka Hagiwara, Eiichi Sasaki, Yasuhisa Hasegawa, Hidenori Suzuki, Daisuke Nishikawa, Shintaro Beppu, Hoshino Terada, Michi Sawabe, Masahide Takahashi, Nobuhiro Hanai
Cancer Medicine.2021; 10(4): 1335. CrossRef
- Advances in the Study of CD109 in Tumors
- Prognostic Role of Claudin-1 Immunohistochemistry in Malignant Solid Tumors: A Meta-Analysis
- Jung-Soo Pyo, Nae Yu Kim, Won Jin Cho
- J Pathol Transl Med. 2019;53(3):173-179. Published online March 5, 2019
- DOI: https://doi.org/10.4132/jptm.2019.02.03
- 8,531 View
- 167 Download
- 6 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Although the correlation between low claudin-1 expression and worse prognosis has been reported, details on the prognostic implications of claudin-1 expression in various malignant tumors remain unclear. The present study aimed to elucidate the prognostic roles of claudin- 1 immunohistochemistry (IHC) in various malignant tumors through a meta-analysis.
Methods
The study included 2,792 patients from 22 eligible studies for assessment of the correlation between claudin-1 expression and survival rate in various malignant tumors. A subgroup analysis based on the specific tumor and evaluation criteria of claudin-1 IHC was conducted.
Results
Low claudin-1 expression was significantly correlated with worse overall survival (OS) (hazard ratio [HR], 1.851; 95% confidence interval [CI], 1.506 to 2.274) and disease-free survival (DFS) (HR, 2.028; 95% CI, 1.313 to 3.134) compared to high claudin-1 expression. Breast, colorectal, esophageal, gallbladder, head and neck, and lung cancers, but not cervical, liver or stomach cancers, were significantly correlated with worse OS. Breast, colorectal, esophageal, and thyroid cancers with low claudin-1 expression were associated with poorer DFS. In the lower cut-off subgroup (< 25.0%) with respect to claudin-1 IHC, low claudin-1 expression was significantly correlated with worse OS and DFS.
Conclusions
Taken together, low claudin-1 IHC expression is significantly correlated with worse survival in various malignant tumors. More detailed criteria for claudin-1 IHC expression in various malignant tumors are needed for application in daily practice. -
Citations
Citations to this article as recorded by- Expression and Targeted Application of Claudins Family in Hepatobiliary and Pancreatic Diseases
Fangqian Du, Yuwei Xie, Shengze Wu, Mengling Ji, Bingzi Dong, Chengzhan Zhu
Journal of Hepatocellular Carcinoma.2024; Volume 11: 1801. CrossRef - The Significance of Relative Claudin Expression in Odontogenic Tumors
Ekarat Phattarataratip, Kraisorn Sappayatosok
Head and Neck Pathology.2020; 14(2): 480. CrossRef - Claudin-1 upregulation is associated with favorable tumor features and a reduced risk for biochemical recurrence in ERG-positive prostate cancer
Simon Kind, Franziska Büscheck, Doris Höflmayer, Claudia Hube-Magg, Martina Kluth, Maria Christina Tsourlakis, Stefan Steurer, Till S. Clauditz, Andreas M. Luebke, Eike Burandt, Waldemar Wilczak, Andrea Hinsch, David Dum, Sören Weidemann, Christoph Fraune
World Journal of Urology.2020; 38(9): 2185. CrossRef - Characterisation of endogenous Claudin‐1 expression, motility and susceptibility to hepatitis C virus in CRISPR knock‐in cells
Camille M.H. Clément, Maika S. Deffieu, Cristina M. Dorobantu, Thomas F. Baumert, Nilda Vanesa Ayala‐Nunez, Yves Mély, Philippe Ronde, Raphael Gaudin
Biology of the Cell.2020; 112(5): 140. CrossRef - Comment on “Prognostic Role of Claudin-1 Immunohistochemistry in Malignant Solid Tumors: A Meta-Analysis”
Bolin Wang, Yan Huang
Journal of Pathology and Translational Medicine.2019; 53(6): 411. CrossRef
- Expression and Targeted Application of Claudins Family in Hepatobiliary and Pancreatic Diseases
- Potential Role for a Panel of Immunohistochemical Markers in the Management of Endometrial Carcinoma
- Amany Salama, Mohammad Arafa, Eman ElZahaf, Abdelhadi Mohamed Shebl, Azmy Abd El-Hameed Awad, Sylvia A. Ashamallah, Reda Hemida, Anas Gamal, Abd AlRahman Foda, Khaled Zalata, El-Said M. Abdel-Hady
- J Pathol Transl Med. 2019;53(3):164-172. Published online February 28, 2019
- DOI: https://doi.org/10.4132/jptm.2019.02.12
- 11,603 View
- 383 Download
- 13 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
In order to improve the efficacy of endometrial carcinoma (EC) treatment, identifying prognostic factors for high risk patients is a high research priority. This study aimed to assess the relationships among the expression of estrogen receptors (ER), progesterone receptors (PR), human epidermal growth factor receptor 2 (HER2), Ki-67, and the different histopathological prognostic parameters in EC and to assess the value of these in the management of EC.
Methods
We examined 109 cases of EC. Immunohistochemistry for ER, PR, HER2, and Ki-67 were evaluated in relation to age, tumor size, International Federation of Gynecology and Obstetrics (FIGO) stage and grade, depth of infiltration, cervical and ovarian involvement, lymphovascular space invasion (LVSI), and lymph node (LN) metastasis.
Results
The mean age of patients in this study was 59.8 ± 8.2 years. Low ER and PR expression scores and high Ki-67 expression showed highly significant associations with non-endometrioid histology (p = .007, p < .001, and p < .001, respectively) and poor differentiation (p = .007, p < .001, and p <. 001, respectively). Low PR score showed a significant association with advanced stage (p = .009). Low ER score was highly associated with LVSI (p = .006), and low PR scores were associated significantly with LN metastasis (p = .026). HER2 expression was significantly related to advanced stages (p = .04), increased depth of infiltration (p = .02), LVSI (p = .017), ovarian involvement (p = .038), and LN metastasis (p = .038). There was a close relationship between HER2 expression and uterine cervical involvement (p = .009). Higher Ki-67 values were associated with LN involvement (p = .012).
Conclusions
The over-expression of HER2 and Ki-67 and low expression of ER and PR indicate a more malignant EC behavior. An immunohistochemical panel for the identification of high risk tumors can contribute significantly to prognostic assessments. -
Citations
Citations to this article as recorded by- Tissue Microarray for Gynecological Pathology Studies: A Mini-Review
Mohammad Arafa, Abd AlRahman Foda, Amany Salama, Ola Shalaby, Muna Al-Jabri, Fatma Al Hinai, Afrah Al-Rashdi, Samya Al-Husaini, Suaad Al-Badi
Journal of Microscopy and Ultrastructure.2026;[Epub] CrossRef - Clinicopathological Correlation of Hormone Receptors, Angiogenesis, and Tumor Budding in Endometrial Carcinoma: A Tertiary Care Center Study
Senjuti Dasgupta, Arpita Das, Ujjwal Bandyopadhyay
The Journal of Obstetrics and Gynecology of India.2025;[Epub] CrossRef - Multiparameter MRI-based radiomics analysis for preoperative prediction of type II endometrial cancer
Yingying Cao, Wei Zhang, Xiaorong Wang, Xiaojing Lv, Yaping Zhang, Kai Guo, Shuai Ren, Yuan Li, Zhongqiu Wang, Jingya Chen
Heliyon.2024; 10(12): e32940. CrossRef - Correlation of PD-L1 expression with different clinico-pathological and immunohistochemical features of ovarian surface epithelial tumors
Asem Shalaby, Ola Shalaby, Hazem Abdullah, Mohamed Rachid Boulassel, Mohammad Arafa
Clinical and Translational Oncology.2024; 27(2): 699. CrossRef - Estrogen/Progesterone Receptor Loss, CTNNB1 and KRAS Mutations Are Associated With Local Recurrence or Distant Metastasis in Low-Grade Endometrial Endometrioid Carcinoma
Rajni Chibbar, Sabrina Foerstner, Janarathnee Suresh, Richa Chibbar, Alexandre Piche, Deeksha Kundapur, Rani Kanthan, Vijayanand Kundapur, Cheng Han Lee, Anita Agrawal, Raymond Lai
Applied Immunohistochemistry & Molecular Morphology.2023; 31(3): 181. CrossRef - Exploring the Prognostic and Predictive Roles of Ki-67 in Endometrial Cancer
Laura Paleari, Mariangela Rutigliani, Oriana D’Ecclesiis, Sara Gandini, Irene Maria Briata, Tania Buttiron Webber, Nicoletta Provinciali, Andrea DeCensi
International Journal of Translational Medicine.2023; 3(4): 479. CrossRef - Analysis of human epidermal growth factor receptor 2 immunohistochemical expression in high-grade endometrial carcinomas and its association with variable clinical outcomes
Malames M. Faisal, Marwa M. Shakweer, Ghada Refaat, Khaled S. Mohammed, Tarek I. ElMallawy, Magda H. Nasreldin, Laila M. Farid, Mariam B. Abouelkhair
Egyptian Journal of Pathology.2023; 43(2): 119. CrossRef - Correlation of PD-L1 immunohistochemical expression with microsatellite instability and p53 status in endometrial carcinoma
Mohammad Arafa, Abdelhadi Mohamed Shebl, Amany Salama, Eman ElZahaf, Sylvia A. Ashamallah, Abd AlRahman Foda, AzmyAbd El-Hameed Awad, Asem Shalaby
European Journal of Obstetrics & Gynecology and Reproductive Biology: X.2022; 16: 100172. CrossRef - Immunohistochemical Expression of Oestrogen and Epidermal Growth Factor Receptors in Endometrial Cancerous in Sudanese Patients
Salwa Abdalraheem Abubaker, Mohamed Elfatih Abdelwadoud, Mutaz Mohamed Ali, Hadia Alhaj Ahmad, Abuobieda Mohamed Khlafalla, Osman Mohammed Elmahi, Hisham Ali Waggiallah
Journal Of Biochemical Technology.2021; 12(1): 58. CrossRef - Expression of ER/PR Receptor, Her-2/neu, Ki67 and p53 in Endometrial Carcinoma: Clinicopathological Implication and Prognostic Value
V. B. Shivkumar, Manisha A. Atram, Nitin M. Gangane
Indian Journal of Gynecologic Oncology.2020;[Epub] CrossRef - Immunohistochemical study of ER, PR, p53 and Ki67 expression in patients with endometrial adenocarcinoma and atypical endometrial hyperplasia
Rachana Lakhe, Ravi M Swami, Preeti Doshi, Manjiri N Karandikar, Ravindra Nimbargi
IP Archives of Cytology and Histopathology Research.2020; 5(4): 274. CrossRef
- Tissue Microarray for Gynecological Pathology Studies: A Mini-Review
- Prognostic Impact of Fusobacterium nucleatum Depends on Combined Tumor Location and Microsatellite Instability Status in Stage II/III Colorectal Cancers Treated with Adjuvant Chemotherapy
- Hyeon Jeong Oh, Jung Ho Kim, Jeong Mo Bae, Hyun Jung Kim, Nam-Yun Cho, Gyeong Hoon Kang
- J Pathol Transl Med. 2019;53(1):40-49. Published online December 26, 2018
- DOI: https://doi.org/10.4132/jptm.2018.11.29
- 19,572 View
- 252 Download
- 50 Web of Science
- 53 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
This study aimed to investigate the prognostic impact of intratumoral Fusobacterium nucleatum in colorectal cancer (CRC) treated with adjuvant chemotherapy.
Methods
F. nucleatumDNA was quantitatively measured in a total of 593 CRC tissues retrospectively collectedfrom surgically resected specimens of stage III or high-risk stage II CRC patients who had receivedcurative surgery and subsequent oxaliplatin-based adjuvant chemotherapy (either FOLFOXor CAPOX). Each case was classified into one of the three categories: F. nucleatum–high, –low, or –negative.
Results
No significant differences in survival were observed between the F.nucleatum–high and –low/negative groups in the 593 CRCs (p = .671). Subgroup analyses accordingto tumor location demonstrated that disease-free survival was significantly better in F.nucleatum–high than in –low/negative patients with non-sigmoid colon cancer (including cecal,ascending, transverse, and descending colon cancers; n = 219; log-rank p = .026). In multivariateanalysis, F. nucleatum was determined to be an independent prognostic factor in non-sigmoidcolon cancers (hazard ratio, 0.42; 95% confidence interval, 0.18 to 0.97; p = .043). Furthermore,the favorable prognostic effect of F. nucleatum–high was observed only in a non-microsatellite instability-high (non-MSI-high) subset of non-sigmoid colon cancers (log-rank p = 0.014), but not ina MSI-high subset (log-rank p = 0.844), suggesting that the combined status of tumor locationand MSI may be a critical factor for different prognostic impacts of F. nucleatum in CRCs treatedwith adjuvant chemotherapy.
Conclusions
Intratumoral F. nucleatum load is a potential prognosticfactor in a non-MSI-high/non-sigmoid/non-rectal cancer subset of stage II/III CRCs treatedwith oxaliplatin-based adjuvant chemotherapy. -
Citations
Citations to this article as recorded by- Microbiota and tumor epigenetics: deep interconnections and emerging therapeutic perspectives
Lei Duan, Dan Hu, Haoling Zhang, Yan Liao
Critical Reviews in Clinical Laboratory Sciences.2026; : 1. CrossRef - Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights
Linda Galasso, Fabrizio Termite, Irene Mignini, Giorgio Esposto, Raffaele Borriello, Federica Vitale, Alberto Nicoletti, Mattia Paratore, Maria Elena Ainora, Antonio Gasbarrini, Maria Assunta Zocco
Cancers.2025; 17(3): 368. CrossRef - Emerging roles of intratumoral microbiota: a key to novel cancer therapies
Pengzhong Fang, Jing Yang, Huiyun Zhang, Diankui Shuai, Min Li, Lin Chen, Liping Liu
Frontiers in Oncology.2025;[Epub] CrossRef - Association of Fusobacterium nucleatum with colorectal cancer molecular subtypes and its outcome: a systematic review
Luana Greco, Federica Rubbino, Clarissa Ferrari, Michela Cameletti, Fabio Grizzi, Fabrizio Bonelli, Alberto Malesci, Massimiliano Mazzone, Luigi Ricciardiello, Luigi Laghi
Gut Microbiome.2025;[Epub] CrossRef - The Interplay Between the Gut Microbiota and Colorectal Cancer: A Review of the Literature
Marco Cintoni, Marta Palombaro, Eleonora Zoli, Giuseppe D’Agostino, Gabriele Pulcini, Elena Leonardi, Pauline Raoul, Emanuele Rinninella, Flavio De Maio, Esmeralda Capristo, Antonio Gasbarrini, Maria Cristina Mele
Microorganisms.2025; 13(6): 1410. CrossRef - Harnessing intratumoral microbiota: new horizons in immune microenvironment and immunotherapy
Jinhe Zhang, Zinan You, Xinqiao Li, Jinpeng Hu, Jiamu Li, Zhitao Jing
Journal of Translational Medicine.2025;[Epub] CrossRef - Prognostic impact of Fusobacterium nucleatum on survival in colorectal cancer: A systematic review and meta-analysis
Tianyu Wang, Shengcheng Lin, Yong Ji, Ciren Puqiong, Jidong Gao, Shuluan Li
Journal of Cancer Research and Therapeutics.2025; 21(4): 796. CrossRef - Fusobacterium nucleatum in Health and Disease
Xinyi Yang, Shutian Zhang, Tingting Ning, Jing Wu
MedComm.2025;[Epub] CrossRef - Periodontitis and Oral Pathogens in Colorectal Cancer: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis
Luis Chauca-Bajaña, Andrea Ordoñez Balladares, Alejandro Ismael Lorenzo-Pouso, Rosangela Caicedo-Quiroz, Rafael Xavier Erazo Vaca, Rolando Fabricio Dau Villafuerte, Yajaira Vanessa Avila-Granizo, Carlos Hans Salazar Minda, Miguel Amador Salavarria Vélez,
Dentistry Journal.2025; 13(12): 595. CrossRef - Composition of the colon microbiota in the individuals with inflammatory bowel disease and colon cancer
Ceren Acar, Sibel Kucukyildirim Celik, H. Ozgur Ozdemirel, Beril Erdem Tuncdemir, Saadet Alan, Hatice Mergen
Folia Microbiologica.2024; 69(2): 333. CrossRef - Intratumoral microorganisms in tumors of the digestive system
Mengjuan Xuan, Xinyu Gu, Yingru Liu, Li Yang, Yi Li, Di Huang, Juan Li, Chen Xue
Cell Communication and Signaling.2024;[Epub] CrossRef - Prognostic impact of oral microbiome on survival of malignancies: a systematic review and meta-analysis
Shuluan Li, Tianyu Wang, Ya Ren, Zhou Liu, Jidong Gao, Zhi Guo
Systematic Reviews.2024;[Epub] CrossRef - Exploring the Potential of Humoral Immune Response to Commensal Bifidobacterium as a Biomarker for Human Health, including Both Malignant and Non-Malignant Diseases: A Perspective on Detection Strategies and Future Directions
Kyogo Itoh, Satoko Matsueda
Biomedicines.2024; 12(4): 803. CrossRef - Unveiling intratumoral microbiota: An emerging force for colorectal cancer diagnosis and therapy
Jinjing Zhang, Penghui Wang, Jiafeng Wang, Xiaojie Wei, Mengchuan Wang
Pharmacological Research.2024; 203: 107185. CrossRef - Spatial transcriptomic analysis reveals local effects of intratumoral fusobacterial infection on DNA damage and immune signaling in rectal cancer
William P. Duggan, Batuhan Kisakol, Ina Woods, Mohammedreza Azimi, Heiko Dussmann, Joanna Fay, Tony O’Grady, Barry Maguire, Ian S. Reynolds, Manuela Salvucci, Daniel J. Slade, Deborah A. McNamara, John P. Burke, Jochen H.M. Prehn
Gut Microbes.2024;[Epub] CrossRef - Gut microbiota characteristics of colorectal cancer patients in Hubei, China, and differences with cohorts from other Chinese regions
Jianguo Shi, Hexiao Shen, Hui Huang, Lifang Zhan, Wei Chen, Zhuohui Zhou, Yongling Lv, Kai Xiong, Zhiwei Jiang, Qiyi Chen, Lei Liu
Frontiers in Microbiology.2024;[Epub] CrossRef - The role of Fusobacterium nucleatum in cancer and its implications for clinical applications
Wanyi Luo, Juxi Han, Xian Peng, Xuedong Zhou, Tao Gong, Xin Zheng
Molecular Oral Microbiology.2024; 39(6): 417. CrossRef - Gut Microbiome and colorectal cancer: discovery of bacterial changes with metagenomics application in Turkısh population
Yakup Ulger, Anıl Delik, Hikmet Akkız
Genes & Genomics.2024; 46(9): 1059. CrossRef - Intratumoral Microbiota: Metabolic Influences and Biomarker Potential in Gastrointestinal Cancer
Xueyuan Bi, Jihan Wang, Cuicui Liu
Biomolecules.2024; 14(8): 917. CrossRef - Intratumoral Microbiota: Insights from Anatomical, Molecular, and Clinical Perspectives
Claudia Lombardo, Rosanna Fazio, Marta Sinagra, Giuseppe Gattuso, Federica Longo, Cinzia Lombardo, Mario Salmeri, Guido Nicola Zanghì, Carla Agata Erika Loreto
Journal of Personalized Medicine.2024; 14(11): 1083. CrossRef - Exploring the role of Fusobacterium nucleatum in colorectal cancer: implications for tumor proliferation and chemoresistance
Leila Dadgar-Zankbar, Zahra Elahi, Aref Shariati, Azad Khaledi, Shabnam Razavi, Amin Khoshbayan
Cell Communication and Signaling.2024;[Epub] CrossRef - Fusobacterium nucleatum Abundance is Associated with Cachexia in Colorectal Cancer Patients: The ColoCare Study
Mmadili N. Ilozumba, Tengda Lin, Sheetal Hardikar, Doratha A. Byrd, June L. Round, W. Zac Stephens, Andreana N. Holowatyj, Christy A. Warby, Victoria Damerell, Christopher I. Li, Jane C. Figueiredo, Adetunji T. Toriola, David Shibata, Gary C. Fillmore, Ba
Cancer Medicine.2024;[Epub] CrossRef - Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy
Li Yang, Aitian Li, Ying Wang, Yi Zhang
Signal Transduction and Targeted Therapy.2023;[Epub] CrossRef - Increased Fusobacterium tumoural abundance affects immunogenicity in mucinous colorectal cancer and may be associated with improved clinical outcome
William P. Duggan, Manuela Salvucci, Batuhan Kisakol, Andreas U. Lindner, Ian S. Reynolds, Heiko Dussmann, Joanna Fay, Tony O’Grady, Daniel B. Longley, Fiona Ginty, Elizabeth Mc Donough, Daniel J. Slade, John P. Burke, Jochen H. M. Prehn
Journal of Molecular Medicine.2023; 101(7): 829. CrossRef -
Fusobacterium nucleatum Load Correlates with KRAS Mutation and Sessile Serrated Pathogenesis in Colorectal Adenocarcinoma
Koki Takeda, Minoru Koi, Yoshiki Okita, Sija Sajibu, Temitope O. Keku, John M. Carethers
Cancer Research Communications.2023; 3(9): 1940. CrossRef - La asociación entre Fusobacterium nucleatum y el cáncer colorrectal: una revisión sistemática y metaanálisis
Paola Villar-Ortega, Manuela Expósito-Ruiz, Miguel Gutiérrez-Soto, Miguel Ruiz-Cabello Jiménez, José María Navarro-Marí, José Gutiérrez-Fernández
Enfermedades Infecciosas y Microbiología Clínica.2022; 40(5): 224. CrossRef - The association between Fusobacterium nucleatum and cancer colorectal: A systematic review and meta-analysis
Paola Villar-Ortega, Manuela Expósito-Ruiz, Miguel Gutiérrez-Soto, Miguel Ruiz-Cabello Jiménez, José María Navarro-Marí, José Gutiérrez-Fernández
Enfermedades infecciosas y microbiologia clinica (English ed.).2022; 40(5): 224. CrossRef - Suppression of Berberine and Probiotics (in vitro and in vivo) on the Growth of Colon Cancer With Modulation of Gut Microbiota and Butyrate Production
Chao Huang, Ying Sun, Sheng-rong Liao, Zhao-xin Chen, Han-feng Lin, Wei-zeng Shen
Frontiers in Microbiology.2022;[Epub] CrossRef - Prognostic and clinicopathological significance of Fusobacterium nucleatum in colorectal cancer: a systemic review and meta-analysis
Younghoon Kim, Nam Yun Cho, Gyeong Hoon Kang
Journal of Pathology and Translational Medicine.2022; 56(3): 144. CrossRef - Iron accelerates Fusobacterium nucleatum–induced CCL8 expression in macrophages and is associated with colorectal cancer progression
Taishi Yamane, Yohei Kanamori, Hiroshi Sawayama, Hiromu Yano, Akihiro Nita, Yudai Ohta, Hironori Hinokuma, Ayato Maeda, Akiko Iwai, Takashi Matsumoto, Mayuko Shimoda, Mayumi Niimura, Shingo Usuki, Noriko Yasuda-Yoshihara, Masato Niwa, Yoshifumi Baba, Taka
JCI Insight.2022;[Epub] CrossRef - Clinicopathological differences of high Fusobacterium nucleatum levels in colorectal cancer: A review and meta-analysis
Yi Wang, Yuting Wen, Jiayin Wang, Xin Lai, Ying Xu, Xuanping Zhang, Xiaoyan Zhu, Chenglin Ruan, Yao Huang
Frontiers in Microbiology.2022;[Epub] CrossRef - Clinical Significance of Fusobacterium nucleatum and Microsatellite Instability in Evaluating Colorectal Cancer Prognosis
Yanxuan Xie, Xiaoyang Jiao, Mi Zeng, Zhiqiang Fan, Xin Li, Yumeng Yuan, Qiaoxin Zhang, Yong Xia
Cancer Management and Research.2022; Volume 14: 3021. CrossRef - Influence of the Microbiome Metagenomics and Epigenomics on Gastric Cancer
Precious Mathebela, Botle Precious Damane, Thanyani Victor Mulaudzi, Zilungile Lynette Mkhize-Khwitshana, Guy Roger Gaudji, Zodwa Dlamini
International Journal of Molecular Sciences.2022; 23(22): 13750. CrossRef - Circulating IgA Antibodies Against Fusobacterium nucleatum Amyloid Adhesin FadA are a Potential Biomarker for Colorectal Neoplasia
Jung Eun Baik, Li Li, Manish A. Shah, Daniel E. Freedberg, Zhezhen Jin, Timothy C. Wang, Yiping W. Han
Cancer Research Communications.2022; 2(11): 1497. CrossRef - Differential immune microenvironmental features of microsatellite-unstable colorectal cancers according to Fusobacterium nucleatum status
Ji Ae Lee, Seung-Yeon Yoo, Hyeon Jeong Oh, Seorin Jeong, Nam-Yun Cho, Gyeong Hoon Kang, Jung Ho Kim
Cancer Immunology, Immunotherapy.2021; 70(1): 47. CrossRef - Fusobacterium nucleatum and Clinicopathologic Features of Colorectal Cancer: Results From the ColoCare Study
Yannick Eisele, Patrick M. Mallea, Biljana Gigic, W. Zac Stephens, Christy A. Warby, Kate Buhrke, Tengda Lin, Juergen Boehm, Petra Schrotz-King, Sheetal Hardikar, Lyen C. Huang, T. Bartley Pickron, Courtney L. Scaife, Richard Viskochil, Torsten Koelsch, A
Clinical Colorectal Cancer.2021; 20(3): e165. CrossRef - Role of gut microbiota in epigenetic regulation of colorectal Cancer
Yinghui Zhao, Chuanxin Wang, Ajay Goel
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer.2021; 1875(1): 188490. CrossRef - Fusobacterium nucleatum: caution with interpreting historical patient sample cohort
Kate L. F. Johnstone, Sinead Toomey, Stephen Madden, Brian D. P. O’Neill, Bryan T Hennessy
Journal of Pathology and Translational Medicine.2021; 55(6): 415. CrossRef -
Fusobacterium nucleatum colonization is associated with decreased survival of helicobacter pylori-positive gastric cancer patients
Yung-Yu Hsieh, Shui-Yi Tung, Hung-Yu Pan, Te-Sheng Chang, Kuo-Liang Wei, Wei-Ming Chen, Yi-Fang Deng, Chung-Kuang Lu, Yu-Hsuan Lai, Cheng-Shyong Wu, Chin Li
World Journal of Gastroenterology.2021; 27(42): 7311. CrossRef - Analysis of changes in microbiome compositions related to the prognosis of colorectal cancer patients based on tissue-derived 16S rRNA sequences
Sukjung Choi, Jongsuk Chung, Mi-La Cho, Donghyun Park, Sun Shim Choi
Journal of Translational Medicine.2021;[Epub] CrossRef - Gastrointestinal tumors and infectious agents: A wide field to explore
Miriam López-Gómez, Belén García de Santiago, Pedro-David Delgado-López, Eduardo Malmierca, Jesús González-Olmedo, César Gómez-Raposo, Carmen Sandoval, Pilar Ruiz-Seco, Nora Escribano, Jorge Francisco Gómez-Cerezo, Enrique Casado
World Journal of Meta-Analysis.2021; 9(6): 505. CrossRef - Gut Microbiota Profiles in Early- and Late-Onset Colorectal Cancer: A Potential Diagnostic Biomarker in the Future
Murdani Abdullah, Ninik Sukartini, Saskia Aziza Nursyirwan, Rabbinu Rangga Pribadi, Hasan Maulahela, Amanda Pitarini Utari, Virly Nanda Muzellina, Agustinus Wiraatmadja, Kaka Renaldi
Digestion.2021; 102(6): 823. CrossRef - The effect of periodontal bacteria infection on incidence and prognosis of cancer
Li Xiao, Qianyu Zhang, Yanshuang Peng, Daqing Wang, Ying Liu
Medicine.2020; 99(15): e19698. CrossRef - The impact of the gut microbiota on prognosis after surgery for colorectal cancer – a systematic review and meta‐analysis
Emilie Palmgren Colov, Thea Helene Degett, Hans Raskov, Ismail Gögenur
APMIS.2020; 128(2): 162. CrossRef - Can the microbiota predict response to systemic cancer therapy, surgical outcomes, and survival? The answer is in the gut
Khalid El Bairi, Rachid Jabi, Dario Trapani, Hanae Boutallaka, Bouchra Ouled Amar Bencheikh, Mohammed Bouziane, Mariam Amrani, Said Afqir, Adil Maleb
Expert Review of Clinical Pharmacology.2020; 13(4): 403. CrossRef - Predictive Values of Colon Microbiota in the Treatment Response to Colorectal Cancer
Jorge Galan-Ros, Verónica Ramos-Arenas, Pablo Conesa-Zamora
Pharmacogenomics.2020; 21(14): 1045. CrossRef - The gut microbiome and potential implications for early-onset colorectal cancer
Reetu Mukherji, Benjamin A Weinberg
Colorectal Cancer.2020;[Epub] CrossRef -
Fusobacterium nucleatum in the Colorectum and Its Association with Cancer Risk and Survival: A Systematic Review and Meta-analysis
Christian Gethings-Behncke, Helen G. Coleman, Haydee W.T. Jordao, Daniel B. Longley, Nyree Crawford, Liam J. Murray, Andrew T. Kunzmann
Cancer Epidemiology, Biomarkers & Prevention.2020; 29(3): 539. CrossRef - CpG Island Methylation in Sessile Serrated Adenoma/Polyp of the Colorectum: Implications for Differential Diagnosis of Molecularly High-Risk Lesions among Non-dysplastic Sessile Serrated Adenomas/Polyps
Ji Ae Lee, Hye Eun Park, Seung-Yeon Yoo, Seorin Jeong, Nam-Yun Cho, Gyeong Hoon Kang, Jung Ho Kim
Journal of Pathology and Translational Medicine.2019; 53(4): 225. CrossRef - Fusobacterium nucleatum tumor DNA levels are associated with survival in colorectal cancer patients
Andrew T. Kunzmann, Marcela Alcântara Proença, Haydee WT Jordao, Katerina Jiraskova, Michaela Schneiderova, Miroslav Levy, Václav Liska, Tomas Buchler, Ludmila Vodickova, Veronika Vymetalkova, Ana Elizabete Silva, Pavel Vodicka, David J. Hughes
European Journal of Clinical Microbiology & Infectious Diseases.2019; 38(10): 1891. CrossRef - Gut Microbiome: A Promising Biomarker for Immunotherapy in Colorectal Cancer
Sally Temraz, Farah Nassar, Rihab Nasr, Maya Charafeddine, Deborah Mukherji, Ali Shamseddine
International Journal of Molecular Sciences.2019; 20(17): 4155. CrossRef - The Four Horsemen in Colon Cancer
Marco Antonio Hernández-Luna, Sergio López-Briones, Rosendo Luria-Pérez
Journal of Oncology.2019; 2019: 1. CrossRef - The role of Fusobacterium nucleatum in colorectal cancer: from carcinogenesis to clinical management
Chun‐Hui Sun, Bin‐Bin Li, Bo Wang, Jing Zhao, Xiao‐Ying Zhang, Ting‐Ting Li, Wen‐Bing Li, Di Tang, Miao‐Juan Qiu, Xin‐Cheng Wang, Cheng‐Ming Zhu, Zhi‐Rong Qian
Chronic Diseases and Translational Medicine.2019; 5(3): 178. CrossRef
- Microbiota and tumor epigenetics: deep interconnections and emerging therapeutic perspectives
- Prognostic Role of S100A8 and S100A9 Protein Expressions in Non-small Cell Carcinoma of the Lung
- Hyun Min Koh, Hyo Jung An, Gyung Hyuck Ko, Jeong Hee Lee, Jong Sil Lee, Dong Chul Kim, Jung Wook Yang, Min Hye Kim, Sung Hwan Kim, Kyung Nyeo Jeon, Gyeong-Won Lee, Se Min Jang, Dae Hyun Song
- J Pathol Transl Med. 2019;53(1):13-22. Published online November 26, 2018
- DOI: https://doi.org/10.4132/jptm.2018.11.12
- 9,537 View
- 249 Download
- 17 Web of Science
- 15 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
S100A8 and S100A9 have been gaining recognition for modulating tumor growthand metastasis. This study aimed at evaluating the clinical significance of S100A8 and S100A9 innon-small cell lung cancer (NSCLC).
Methods
We analyzed the relationship between S100A8and S100A9 expressions, clinicopathological characteristics, and prognostic significance in tumorcells and peritumoral inflammatory cells.
Results
The positive staining of S100A8 in tumorcells was significantly increased in male (p < .001), smoker (p = .034), surgical method other thanlobectomy (p = .024), squamous cell carcinoma (SQCC) (p < .001) and higher TNM stage (p = .022)compared with female, non-smoker, lobectomy, adenocarcinoma (ADC), and lower stage. Theproportion of tumor cells stained for S100A8 was related to histologic type (p < .001) and patientsex (p = .027). The proportion of inflammatory cells stained for S100A8 was correlated with patientage (p = .022), whereas the proportion of inflammatory cells stained for S100A9 was correlatedwith patient sex (p < .001) and smoking history (p = .031). Moreover, positive staining in tumorcells, more than 50% of the tumor cells stained and less than 30% of the inflammatory cellsstained for S100A8 and S100A9 suggested a tendency towards increased survivability in SQCCbut towards decreased survivability in ADC.
Conclusions
S100A8 and S100A9 expressions might be potential prognostic markers in patients with NSCLC. -
Citations
Citations to this article as recorded by- S100A8 as a potential therapeutic target for cancer metastasis
Atsuko Deguchi, Yoshiro Maru
Cancer Science.2025; 116(2): 322. CrossRef - Identifying candidate biomarkers for detecting bronchogenic carcinoma stages using metaheuristic algorithms based on information fusion theory
Bagher Khalvati, Kaveh Kavousi, Amir Hosein Keyhanipour, Masoud Arabfard
Discover Oncology.2025;[Epub] CrossRef - A review on clinical implications of S100 proteins in lung diseases
Vineesh V. Raveendran, Somaya AlQattan, Eid AlMutairy
Frontiers in Medicine.2025;[Epub] CrossRef - Differential Expression of S100A8 in Tumor and Immune Compartments of Endometrial Carcinoma and Its Clinical Relevance
Dae Hyun Song, Min Hye Kim, Juseok Yang, Hyen Chul Jo, Ji Eun Park, Jong Chul Baek
Medicina.2025; 61(11): 1918. CrossRef - The role of S100A8 and S100A9 in external auditory canal cholesteatoma
Guanwen He, Weijing Han, Zhongshou Zhu, Rifu Wei, Chang Lin
Frontiers in Immunology.2024;[Epub] CrossRef - Gene expression related to lung cancer altered by PHMG-p treatment in PBTE cells
Yoon Hee Park, Sang Hoon Jeong, Hyejin Lee, Cherry Kim, Yoon Jeong Nam, Ja Young Kang, Jin Young Choi, Yu-Seon Lee, Su A. Park, Jaeyoung Kim, Eun-Kee Park, Yong-Wook Baek, Hong Lee, Ju-Han Lee
Molecular & Cellular Toxicology.2023; 19(1): 205. CrossRef - Discovery of protein biomarkers for venous thromboembolism in non-small cell lung cancer patients through data-independent acquisition mass spectrometry
Yanhong Liu, Lan Gao, Yanru Fan, Rufei Ma, Yunxia An, Guanghui Chen, Yan Xie
Frontiers in Oncology.2023;[Epub] CrossRef - S100A8 and S100A9 in Cancer
Yu Chen, Yuzhen Ouyang, Zhixin Li, Xiufang Wang, Jian Ma
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer.2023; 1878(3): 188891. CrossRef - Gene expression of S100a8/a9 predicts Staphylococcus aureus-induced septic arthritis in mice
Meghshree Deshmukh, Santhilal Subhash, Zhicheng Hu, Majd Mohammad, Anders Jarneborn, Rille Pullerits, Tao Jin, Pradeep Kumar Kopparapu
Frontiers in Microbiology.2023;[Epub] CrossRef - Single-cell immunophenotyping revealed the association of CD4+ central and CD4+ effector memory T cells linking exacerbating chronic obstructive pulmonary disease and NSCLC
Nikolett Gémes, József Á. Balog, Patrícia Neuperger, Erzsébet Schlegl, Imre Barta, János Fillinger, Balázs Antus, Ágnes Zvara, Zoltán Hegedűs, Zsolt Czimmerer, Máté Manczinger, Gergő Mihály Balogh, József Tóvári, László G. Puskás, Gábor J. Szebeni
Frontiers in Immunology.2023;[Epub] CrossRef - A Prognostic Gene Signature for Hepatocellular Carcinoma
Rong Chen, Meng Zhao, Yanli An, Dongfang Liu, Qiusha Tang, Gaojun Teng
Frontiers in Oncology.2022;[Epub] CrossRef - The S100 protein family in lung cancer
Ting Wang, Ge Du, Dong Wang
Clinica Chimica Acta.2021; 520: 67. CrossRef - The associations of serum S100A9 with the severity and prognosis in patients with community-acquired pneumonia: a prospective cohort study
Hong-Yan Liu, Hui-Xian Xiang, Ying Xiang, Zheng Xu, Chun-Mei Feng, Jun Fei, Lin Fu, Hui Zhao
BMC Infectious Diseases.2021;[Epub] CrossRef - Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
Xiao Li Zhang, Zheng Zhi Wu, Yun Xu, Ji Guo Wang, Yong Qiang Wang, Mei Qun Cao, Chang Hao Wang
Open Chemistry.2020; 18(1): 918. CrossRef - Prognostic Role of S100A8 in Human Solid Cancers: A Systematic Review and Validation
An Huang, Wei Fan, Jiacui Liu, Ben Huang, Qingyuan Cheng, Ping Wang, Yiping Duan, Tiantian Ma, Liangyue Chen, Yanping Wang, Mingxia Yu
Frontiers in Oncology.2020;[Epub] CrossRef
- S100A8 as a potential therapeutic target for cancer metastasis
- The Prognostic Impact of Synchronous Ipsilateral Multiple Breast Cancer: Survival Outcomes according to the Eighth American Joint Committee on Cancer Staging and Molecular Subtype
- Jinah Chu, Hyunsik Bae, Youjeong Seo, Soo Youn Cho, Seok-Hyung Kim, Eun Yoon Cho
- J Pathol Transl Med. 2018;52(6):396-403. Published online October 23, 2018
- DOI: https://doi.org/10.4132/jptm.2018.10.03
- 8,132 View
- 102 Download
- 8 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
In the current American Joint Committee on Cancer staging system of breast cancer, only tumor size determines T-category regardless of whether the tumor is single or multiple. This study evaluated if tumor multiplicity has prognostic value and can be used to subclassify breast cancer.
Methods
We included 5,758 patients with invasive breast cancer who underwent surgery at Samsung Medical Center, Seoul, Korea, from 1995 to 2012.
Results
Patients were divided into two groups according to multiplicity (single, n = 4,744; multiple, n = 1,014). Statistically significant differences in lymph node involvement and lymphatic invasion were found between the two groups (p < .001). Patients with multiple masses tended to have luminal A molecular subtype (p < .001). On Kaplan-Meier survival analysis, patients with multiple masses had significantly poorer disease-free survival (DFS) (p = .016). The prognostic significance of multiplicity was seen in patients with anatomic staging group I and prognostic staging group IA (p = .019 and p = .032, respectively). When targeting patients with T1-2 N0 M0, hormone receptor–positive, and human epidermal growth factor receptor 2 (HER2)–negative cancer, Kaplan-Meier survival analysis also revealed significantly reduced DFS with multiple cancer (p = .031). The multivariate analysis indicated that multiplicity was independently correlated with worse DFS (hazard ratio, 1.23; 95% confidence interval, 1.03 to 1.47; p = .025). The results of this study indicate that tumor multiplicity is frequently found in luminal A subtype, is associated with frequent lymph node metastasis, and is correlated with worse DFS.
Conclusions
Tumor multiplicity has prognostic value and could be used to subclassify invasive breast cancer at early stages. Adjuvant chemotherapy would be necessary for multiple masses of T1–2 N0 M0, hormone-receptor-positive, and HER2-negative cancer. -
Citations
Citations to this article as recorded by- The Role of Serum Beta-Human Chorionic Gonadotropin (β-hCG) in Differentiating Benign and Malignant Breast Lesions at a Tertiary Care Center in Jharkhand
Neyaz Ahmad, Khushboo Rani, Zenith Kerketta, Krishna Murari, Anish Baxla, Ujala Murmu, Amit Nishant, Shreya .
Cureus.2025;[Epub] CrossRef - Role of Large Format Histology in Diagnosis of Breast Carcinoma
Hari Shankar Pandey, Sanya Bhasin, Suman Kumari Pandey
NMO Journal.2025; 19(2): 189. CrossRef - Prognostic Impact of Multiple Synchronous T1 Breast Cancer
Hongki Gwak, Sung Hoo Jung, Young Jin Suh, Seok Jin Nam, Jai Hong Han, Se Jeong Oh, Eun Hwa Park, Seong Hwan Kim
Cancers.2024; 16(23): 4019. CrossRef - Deep learning-based system for automatic prediction of triple-negative breast cancer from ultrasound images
Alexandre Boulenger, Yanwen Luo, Chenhui Zhang, Chenyang Zhao, Yuanjing Gao, Mengsu Xiao, Qingli Zhu, Jie Tang
Medical & Biological Engineering & Computing.2023; 61(2): 567. CrossRef - Multicentre prospective cohort study of unmet supportive care needs among patients with breast cancer throughout their cancer treatment trajectory in Penang: a PenBCNeeds Study protocol
Noorsuzana Mohd Shariff, Nizuwan Azman, Rohayu Hami, Noor Mastura Mohd Mujar, Mohammad Farris Iman Leong Bin Abdullah
BMJ Open.2021; 11(3): e044746. CrossRef - The subgross morphology of breast carcinomas: a single-institution series of 2033 consecutive cases documented in large-format histology slides
Tibor Tot, Maria Gere, Syster Hofmeyer, Annette Bauer, Ulrika Pellas
Virchows Archiv.2020; 476(3): 373. CrossRef - Editorial for “Synchronous Breast Cancer: Phenotypic Similarities on MRI”
Uma Sharma
Journal of Magnetic Resonance Imaging.2020; 52(1): 309. CrossRef - Synchronous Multiple Breast Cancers—Do We Need to Reshape Staging?
Minodora Onisâi, Adrian Dumitru, Iuliana Iordan, Cătălin Aliuș, Oana Teodor, Adrian Alexandru, Daniela Gheorghiță, Iulian Antoniac, Adriana Nica, Alexandra-Ana Mihăilescu, Sebastian Grădinaru
Medicina.2020; 56(5): 230. CrossRef - Molecular mechanism of triple‑negative breast cancer‑associated BRCA1 and the identification of signaling pathways
Feng Qi, Wen‑Xing Qin, Yuan‑Sheng Zang
Oncology Letters.2019;[Epub] CrossRef
- The Role of Serum Beta-Human Chorionic Gonadotropin (β-hCG) in Differentiating Benign and Malignant Breast Lesions at a Tertiary Care Center in Jharkhand
- The Expression of Adipophilin Is Frequently Found in Solid Subtype Adenocarcinoma and Is Associated with Adverse Outcomes in Lung Adenocarcinoma
- Sun Ah Shin, Hee Young Na, Ji Young Choe, Doohyun Chung, Mira Park, Sohee Oh, Ji Eun Kim
- J Pathol Transl Med. 2018;52(6):357-362. Published online October 4, 2018
- DOI: https://doi.org/10.4132/jptm.2018.09.13
- 6,925 View
- 111 Download
- 7 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
The up-regulation of the lipogenic pathway has been reported in many types of malignant tumors. However, its pathogenic role or clinical significance is not fully understood. The objective of this study was to examine the expression levels of adipophilin and related hypoxic signaling proteins and to determine their prognostic impacts and associations with the pathologic characteristics of lung adenocarcinoma.
Methods
Expression levels of adipophilin, heat shock protein 27 (HSP27), carbonic anhydrase IX, and hypoxia-inducible factor 1α were examined by immunohistochemical staining using tissue microarray blocks. Correlations between protein expression levels and various clinicopathologic features were analyzed.
Results
A total of 230 cases of primary adenocarcinoma of the lung were enrolled in this study. Adipophilin expression was more frequent in males and with the solid histologic type. It was correlated with HSP27 expression. Patients with adipophilin-positive adenocarcinoma showed a shorter progression-free survival (PFS) (median PFS, 17.2 months vs 18.4 months) in a univariable survival analysis, whereas HSP27 positivity correlated with favorable overall survival (OS) and PFS. In a multivariable analysis, adipophilin and HSP27 were independent prognostic markers of both OS and PFS.
Conclusions
Activated lipid metabolism and the hypoxic signaling pathway might play a major role in the progression of lung adenocarcinoma, especially in the solid histologic type. -
Citations
Citations to this article as recorded by- Perilipin 2 Mediates Progression of Lung Adenocarcinoma by Modulating Lipid Metabolism
Kana Miyata-Morita, Akira Kawashima, Mitsuo Kiriya, Hitoshi Dejima, Koji Saito, Yukinori Sakao, Koichi Suzuki, Yuko Sasajima, Shigeki Morita
The American Journal of Pathology.2025; 195(9): 1588. CrossRef - Prognostic implications of the immunohistochemical expression of perilipin 1 and adipophilin in high-grade liposarcoma
Kengo Kawaguchi, Kenichi Kohashi, Taro Mori, Hidetaka Yamamoto, Takeshi Iwasaki, Izumi Kinoshita, Yosuke Susuki, Hiroshi Furukawa, Makoto Endo, Yoshihiro Matsumoto, Yasuharu Nakashima, Yoshinao Oda
Journal of Clinical Pathology.2024; 77(10): 676. CrossRef - Prognostic and clinicopathologic significance of PLIN2 in cancers: A systematic review with meta-analysis
Ming-Lin Li, Han-Yong Luo, Zi-Wei Quan, Le-Tian Huang, Jia-He Wang
The International Journal of Biological Markers.2023; 38(1): 3. CrossRef - Novel prognostication biomarker adipophilin reveals a metabolic shift in uveal melanoma and new therapeutic opportunities
Maisoon Matareed, Eleftheria Maranou, Saara A Koskela, Arfa Mehmood, Helen Kalirai, Sarah E Coupland, Carlos R Figueiredo
The Journal of Pathology.2023; 260(2): 203. CrossRef - Adipophilin expression in cutaneous malignant melanoma is associated with high proliferation and poor clinical prognosis
Masakazu Fujimoto, Ibu Matsuzaki, Kazuchika Nishitsuji, Yuki Yamamoto, Daisuke Murakami, Takanori Yoshikawa, Ayaka Fukui, Yuuki Mori, Masaru Nishino, Yuichi Takahashi, Yoshifumi Iwahashi, Kenji Warigaya, Fumiyoshi Kojima, Masatoshi Jinnin, Shin-ichi Murat
Laboratory Investigation.2020; 100(5): 727. CrossRef - The clinicopathological significance of the adipophilin and fatty acid synthase expression in salivary duct carcinoma
Hideaki Hirai, Yuichiro Tada, Masato Nakaguro, Daisuke Kawakita, Yukiko Sato, Tomotaka Shimura, Kiyoaki Tsukahara, Satoshi Kano, Hiroyuki Ozawa, Kenji Okami, Yuichiro Sato, Chihiro Fushimi, Akira Shimizu, Isaku Okamoto, Soichiro Takase, Takuro Okada, Hiro
Virchows Archiv.2020; 477(2): 291. CrossRef
- Perilipin 2 Mediates Progression of Lung Adenocarcinoma by Modulating Lipid Metabolism
- Prognostic Role of Metastatic Lymph Node Ratio in Papillary Thyroid Carcinoma
- Jung-Soo Pyo, Jin Hee Sohn, Kyungseek Chang
- J Pathol Transl Med. 2018;52(5):331-338. Published online August 30, 2018
- DOI: https://doi.org/10.4132/jptm.2018.08.07
- 9,164 View
- 131 Download
- 16 Web of Science
- 15 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
The aim of this study is to elucidate the clinicopathological significances, including the prognostic role, of metastatic lymph node ratio (mLNR) and tumor deposit diameter in papillary thyroid carcinoma (PTC) through a retrospective review and meta-analysis.
Methods
We categorized the cases into high (≥ 0.44) and low mLNR (< 0.44) and investigated the correlations with clinicopathological parameters in 64 PTCs with neck level VI lymph node (LN) metastasis. In addition, meta-analysis of seven eligible studies was used to investigate the correlation between mLNR and survival.
Results
Among 64 PTCs with neck level VI LN metastasis, high mLNR was found in 34 PTCs (53.1%). High mLNR was significantly correlated with macrometastasis (tumor deposit diameter ≥ 0.2 cm), extracapsular spread, and number of metastatic LNs. Based on linear regression test, mLNR was significantly increased by the largest LN size but not the largest metastatic LN (mLN) size. High mLNR was not correlated with nuclear factor κB or cyclin D1 immunohistochemical expression, Ki-67 labeling index, or other pathological parameters of primary tumor. Based on meta-analysis, high mLNR significantly correlated with worse disease-free survival at the 5-year and 10-year follow-up (hazard ratio [HR], 4.866; 95% confidence interval [CI], 3.527 to 6.714 and HR, 5.769; 95% CI, 2.951 to 11.275, respectively).
Conclusions
Our data showed that high mLNR significantly correlated with worse survival, macrometastasis, and extracapsular spread of mLNs. Further cumulative studies for more detailed criteria of mLNR are needed before application in daily practice. -
Citations
Citations to this article as recorded by- The application of a clinical-multimodal ultrasound radiomics model for predicting cervical lymph node metastasis of thyroid papillary carcinoma
Chang Liu, Shangjie Yang, Tian Xue, Qian Zhang, Yanjing Zhang, Yufang Zhao, Guolin Yin, Xiaohui Yan, Ping Liang, Liping Liu
Frontiers in Oncology.2025;[Epub] CrossRef - The Predictive Value of a Nomogram Based on Ultrasound Radiomics, Clinical Factors, and Enhanced Ultrasound Features for Central Lymph Node Metastasis in Papillary Thyroid Microcarcinoma
Lei Gao, Xiuli Wen, Guanghui Yue, Hui Wang, Ziqing Lu, Beibei Wu, Zhihong Liu, Yuming Wu, Dongmei Lin, Shijian Yi, Wei Jiang, Yi Hao
Ultrasonic Imaging.2025; 47(2): 93. CrossRef - Lymph Node Metastasis Ratio: Prognostic Significance in Papillary Thyroid Cancer
Ana Rita Ferreira, Diogo Ramalho, Daniela Martins, Andreia Amado, Susana Graça, Carlos Soares, Bela Pereira, Maria João Oliveira, Manuel Oliveira, Antónia Póvoa
Indian Journal of Surgery.2025; 87(6): 1047. CrossRef - CD105 (Endoglin) Expression as a Prognostic Marker in Aggressive Papillary Thyroid Carcinoma
İlker Çordan, Tuğba Günler
Clinical Endocrinology.2025; 103(4): 596. CrossRef - Application and subgroup analysis of competing risks model based on different lymph node staging systems in differentiated thyroid cancer
Zhe Xu Cao, Jiang Sheng Huang, Ming Ming Wang
Updates in Surgery.2024; 76(5): 1927. CrossRef - Цитологічне прогнозування агресії раку щитоподібної залози як новий перспективний напрямок у клінічній тиреоїдології
H.V. Zelinska
Endokrynologia.2024; 29(4): 363. CrossRef - Thyroglobulin expression, Ki-67 index, and lymph node ratio in the prognostic assessment of papillary thyroid cancer
Helene Lindfors, Marie Karlsen, Ellinor Karlton, Jan Zedenius, Catharina Larsson, Catharina Ihre Lundgren, C. Christofer Juhlin, Ivan Shabo
Scientific Reports.2023;[Epub] CrossRef - Incidental Node Metastasis as an Independent Factor of Worse Disease-Free Survival in Patients with Papillary Thyroid Carcinoma
Renan Aguera Pinheiro, Ana Kober Leite, Beatriz Godoi Cavalheiro, Evandro Sobroza de Mello, Luiz Paulo Kowalski, Leandro Luongo Matos
Cancers.2023; 15(3): 943. CrossRef - A High-Quality Nomogram for Predicting Lung Metastasis in Newly Diagnosed Stage IV Thyroid Cancer: A Population-Based Study
WenYi Wang, JiaJing Liu, XiaoFan Xu, LiQun Huo, XuLin Wang, Jun Gu
Technology in Cancer Research & Treatment.2023;[Epub] CrossRef - Lymph Node Ratio Predicts Recurrence in Patients with Papillary Thyroid Carcinoma with Low Lymph Node Yield
Il Ku Kang, Joonseon Park, Ja Seong Bae, Jeong Soo Kim, Kwangsoon Kim
Cancers.2023; 15(11): 2947. CrossRef - Superiority of metastatic lymph node ratio over number of node metastases and TNM/AJCC N classification in predicting cancer‐specific survival in medullary thyroid cancer
Andreas Machens, Kerstin Lorenz, Frank Weber, Henning Dralle
Head & Neck.2022; 44(12): 2717. CrossRef - Value of Combining Clinical Factors, Conventional Ultrasound, and Contrast-Enhanced Ultrasound Features in Preoperative Prediction of Central Lymph Node Metastases of Different Sized Papillary Thyroid Carcinomas
Yanfang Wang, Fang Nie, Guojuan Wang, Ting Liu, Tiantian Dong, Yamin Sun
Cancer Management and Research.2021; Volume 13: 3403. CrossRef - Atypical Histiocytoid Cells and Multinucleated Giant Cells in Fine-Needle Aspiration Cytology of the Thyroid Predict Lymph Node Metastasis of Papillary Thyroid Carcinoma
Ji Eun Choi, Ja Seong Bae, Dong-Jun Lim, So Lyung Jung, Chan Kwon Jung
Cancers.2019; 11(6): 816. CrossRef - Patients Aged ≥55 Years With Stage T1-2N1M1 Differentiated Thyroid Cancer Should Be Downstaged in the Eighth Edition AJCC/TNM Cancer Staging System
Zeming Liu, Sichao Chen, Yihui Huang, Di Hu, Min Wang, Wei Wei, Chao Zhang, Wen Zeng, Liang Guo
Frontiers in Oncology.2019;[Epub] CrossRef - Prognostic Implication of Metastatic Lymph Node Ratio in Colorectal Cancers: Comparison Depending on Tumor Location
Jung-Soo Pyo, Young-Min Shin, Dong-Wook Kang
Journal of Clinical Medicine.2019; 8(11): 1812. CrossRef
- The application of a clinical-multimodal ultrasound radiomics model for predicting cervical lymph node metastasis of thyroid papillary carcinoma
- C-reactive Protein Overexpression in the Background Liver of Hepatitis B Virus–Associated Hepatocellular Carcinoma Is a Prognostic Biomarker
- Jin Ho Shin, Eunsil Yu, Eun Na Kim, Chong Jai Kim
- J Pathol Transl Med. 2018;52(5):267-274. Published online July 27, 2018
- DOI: https://doi.org/10.4132/jptm.2018.07.14
- 8,863 View
- 179 Download
- 7 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Chronic hepatitis B virus (HBV) infection is a leading cause of hepatocellular carcinoma (HCC). Peripheral blood C-reactive protein (CRP) concentration and CRP overexpression in HCC cells are proven to be prognostic markers for HCC, but the significance of CRP expression in non-neoplastic hepatocytes, which are the primary origin of CRP, has not been studied. This study was conducted to determine the clinicopathologic significance of CRP immunoreactivity in the background liver of HBV-associated HCC.
Methods
CRP immunostaining was done on tissue microarrays of non-neoplastic liver tissues obtained from surgically resected, treatment-naïve HBV-associated HCCs (n = 156). The relationship between CRP immunoreactivity and other clinicopathologic parameters including cancer-specific survival was analyzed. CRP immunoreactivity was determined using a 4-tier grading system: grades 0, 1, 2, and 3.
Results
CRP was positive in 139 of 156 cases (89.1%) of non-neoplastic liver in patients with HCCs: grade 1 in 83 cases (53.2%); grade 2 in 50 cases (32.1%); and grade 3 in six cases (3.8%). The patients with diffuse CRP immunoreactivity (grade 3) had decreased cancer-specific survival (p = .031) and a tendency for shorter interval before early recurrence (p = .050). The degree of CRP immunoreactivity correlated with serum CRP concentration (p < .001).
Conclusions
CRP immunoreactivity in non-neoplastic liver is a novel biomarker for poor cancer-specific survival of HBV-associated HCC and correlates with serum CRP concentration. -
Citations
Citations to this article as recorded by- Plasma thrombomodulin is a valuable biomarker to predict the severity of hemorrhagic fever with renal syndrome caused by the Hantaan virus
Han-Dong Zhao, Yan Zhang, Xiao-Hong Wang, Hong-Bo Qian, Tong-Bo Yu, Peng Li, Kang-Xiao Ma, Hong-Li Liu
Frontiers in Cellular and Infection Microbiology.2025;[Epub] CrossRef - Multivariate Assessment of Thyroid, Lipid, and Inflammatory Profiles by HBV Status and Viral Load: Age- and Sex-Specific Findings
Hyeokjun Yun, Jong Wan Kim, Jae Kyung Kim
Viruses.2025; 17(9): 1208. CrossRef - Analysis of Inflammatory and Thyroid Hormone Levels Based on Hepatitis A and B Virus Immunity Status: Age and Sex Stratification
Hyeokjun Yun, Jae-Sik Jeon, Jae Kyung Kim
Viruses.2024; 16(8): 1329. CrossRef - Ferritin and procalcitonin serve as discriminative inflammatory biomarkers and can predict the prognosis of severe fever with thrombocytopenia syndrome in its early stages
Keping Chen, Huidi Sun, Yu Geng, Chuankun Yang, Chun Shan, Yuxin Chen
Frontiers in Microbiology.2023;[Epub] CrossRef - Evaluation of Serum Ferritin, Procalcitonin, and C-Reactive Protein for the Prediction of Severity and Mortality in Hemorrhagic Fever With Renal Syndrome
Lihe Che, Zedong Wang, Na Du, Liang Li, Yinghua Zhao, Kaiyu Zhang, Quan Liu
Frontiers in Microbiology.2022;[Epub] CrossRef - Hepatocellular adenomas: recent updates
Haeryoung Kim, Young Nyun Park
Journal of Pathology and Translational Medicine.2021; 55(3): 171. CrossRef - A prospective follow-up study of the relationship between high-sensitivity C-reactive protein and primary liver cancer
Sarah Tan Siyin, Tong Liu, Wenqiang Li, Nan Yao, Guoshuai Xu, Jun Qu, Yajun Chen
BMC Cancer.2020;[Epub] CrossRef - CRP Levels in Viral Hepatitis: A Meta-Analysis Study
Sukhpal Singh, Abhishek Bansal, Pardeep Kumar
International Journal of Infection.2020;[Epub] CrossRef
- Plasma thrombomodulin is a valuable biomarker to predict the severity of hemorrhagic fever with renal syndrome caused by the Hantaan virus
- Prognostic Significance of EPHB2 Expression in Colorectal Cancer Progression
- Bo Gun Jang, Hye Sung Kim, Weon Young Chang, Jeong Mo Bae, Gyeong Hoon Kang
- J Pathol Transl Med. 2018;52(5):298-306. Published online July 18, 2018
- DOI: https://doi.org/10.4132/jptm.2018.06.29
- 8,640 View
- 161 Download
- 18 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
A receptor tyrosine kinase for ephrin ligands, EPHB2, is expressed in normal colorectal tissues and colorectal cancers (CRCs). The aim of this study was to investigate EPHB2 expression over CRC progression and determine its prognostic significance in CRC.
Methods
To measure EPHB2 mRNA and protein expression, real-time polymerase chain reaction and immunohistochemistry were performed in 32 fresh-frozen and 567 formalin-fixed paraffin-embedded CRC samples, respectively. We further investigated clinicopathological features and overall and recurrence-free survival according to EPHB2 protein expression.
Results
The EPHB2 level was upregulated in CRC samples compared to non-cancerous tissue in most samples and showed a strong positive correlation with AXIN2. Notably, CD44 had a positive association with both mRNA and protein levels of EPHB2. Immunohistochemical analysis revealed no difference in EPHB2 expression between adenoma and carcinoma areas. Although EPHB2 expression was slightly lower in invasive fronts compared to surface area (p < .05), there was no difference between superficial and metastatic areas. EPHB2 positivity was associated with lymphatic (p < .001) and venous (p = .001) invasion, TNM stage (p < .001), and microsatellite instability (p = .036). Kaplan–Meier analysis demonstrated that CRC patients with EPHB2 positivity showed better clinical outcomes in both overall (p = .049) and recurrence-free survival (p = .015). However, multivariate analysis failed to show that EPHB2 is an independent prognostic marker in CRCs (hazard ratio, 0.692; p = .692).
Conclusions
Our results suggest that EPHB2 is overexpressed in a subset of CRCs and is a significant prognostic marker. -
Citations
Citations to this article as recorded by- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
Yanlai Xiao, Jian Wang, Xiangzhai Zhao, Jie Xu, Huan Zhao, Zhaojun Guo, Jun Zhao, Yajing Zhang, Ruoxi Wang, Yiwei Zhang
Open Life Sciences.2025;[Epub] CrossRef - Potential role of the Eph/ephrin system in colorectal cancer: emerging druggable molecular targets
João Figueira Scarini, Moisés Willian Aparecido Gonçalves, Reydson Alcides de Lima-Souza, Luccas Lavareze, Talita de Carvalho Kimura, Ching-Chu Yang, Albina Altemani, Fernanda Viviane Mariano, Heloisa Prado Soares, Gary Chris Fillmore, Erika Said Abu Egal
Frontiers in Oncology.2024;[Epub] CrossRef - Comprehensive pan-cancer analysis reveals EPHB2 is a novel predictive biomarker for prognosis and immunotherapy response
Shengshan Xu, Youbin Zheng, Min Ye, Tao Shen, Dongxi Zhang, Zumei Li, Zhuming Lu
BMC Cancer.2024;[Epub] CrossRef - Therapeutic effects of ephrin B receptor 2 inhibitors screened by molecular docking on cutaneous squamous cell carcinoma
Yan Li, Xuanfen Zhang
Journal of Dermatological Treatment.2022; 33(1): 373. CrossRef - The EPH/Ephrin System in Colorectal Cancer
Stavros P. Papadakos, Leonidas Petrogiannopoulos, Alexandros Pergaris, Stamatios Theocharis
International Journal of Molecular Sciences.2022; 23(5): 2761. CrossRef - Prognostic Significance of Autophagy-Relevant Gene Markers in Colorectal Cancer
Qinglian He, Ziqi Li, Jinbao Yin, Yuling Li, Yuting Yin, Xue Lei, Wei Zhu
Frontiers in Oncology.2021;[Epub] CrossRef - Genetic Predisposition to Numerous Large Ulcerating Basal Cell Carcinomas and Response to Immune Therapy
Bahar Dasgeb, Leila Youssefian, Amir Hossein Saeidian, Jun Kang, Wenyin Shi, Elizabeth Shoenberg, Adam Ertel, Paolo Fortina, Hassan Vahidnezhad, Jouni Uitto
International Journal of Dermatology and Venereology.2021; 4(2): 70. CrossRef - Delineation of colorectal cancer ligand-receptor interactions and their roles in the tumor microenvironment and prognosis
Hexin Lin, Lu Xia, Jiabian Lian, Yinan Chen, Yiyi Zhang, Zhicheng Zhuang, HuaJun Cai, Jun You, Guoxian Guan
Journal of Translational Medicine.2021;[Epub] CrossRef - Downregulation of hsa-microRNA-204-5p and identification of its potential regulatory network in non-small cell lung cancer: RT-qPCR, bioinformatic- and meta-analyses
Chang-Yu Liang, Zu-Yun Li, Ting-Qing Gan, Ye-Ying Fang, Bin-Liang Gan, Wen-Jie Chen, Yi-Wu Dang, Ke Shi, Zhen-Bo Feng, Gang Chen
Respiratory Research.2020;[Epub] CrossRef - The role of Eph receptors in cancer and how to target them: novel approaches in cancer treatment
Oscar J Buckens, Btissame El Hassouni, Elisa Giovannetti, Godefridus J Peters
Expert Opinion on Investigational Drugs.2020; 29(6): 567. CrossRef - Expression Profile and Prognostic Significance of EPHB3 in Colorectal Cancer
Bo Jang, Hye Kim, Jeong Bae, Woo Kim, Chang Hyun, Gyeong Kang
Biomolecules.2020; 10(4): 602. CrossRef Gene Expression Signature to Predict Prognosis and Adjuvant Chemosensitivity of Colorectal Cancer Patients
Jianxia Li, Jianwei Zhang, Huabin Hu, Yue Cai, Jiayu Ling, Zehua Wu, Yanhong Deng
Cancer Management and Research.2020; Volume 12: 3301. CrossRef- SMOC2, an intestinal stem cell marker, is an independent prognostic marker associated with better survival in colorectal cancers
Bo Gun Jang, Hye Sung Kim, Jeong Mo Bae, Woo Ho Kim, Heung Up Kim, Gyeong Hoon Kang
Scientific Reports.2020;[Epub] CrossRef
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Prognostic Utility of Histological Growth Patterns of Colorectal Lung Oligometastasis
- Son Jae Yeong, Min Gyoung Pak, Hyoun Wook Lee, Seung Yeon Ha, Mee Sook Roh
- J Pathol Transl Med. 2018;52(2):98-104. Published online February 12, 2018
- DOI: https://doi.org/10.4132/jptm.2017.12.27
- 7,853 View
- 127 Download
- 3 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Patients with resectable colorectal lung oligometastasis (CLOM) demonstrate a heterogeneous oncological outcome. However, the parameters for predicting tumor aggressiveness have not yet been fully investigated in CLOM. This study was performed to determine the prognostic value of histological growth patterns in patients who underwent surgery for CLOM.
Methods
The study included 92 patients who were diagnosed with CLOM among the first resection cases. CLOMs grow according to three histological patterns: aerogenous, pushing, and desmoplastic patterns. The growth patterns were evaluated on archival hematoxylin and eosin–stained tissue sections.
Results
The aerogenous pattern was found in 29.4% (n=27) of patients, the pushing pattern in 34.7% (n=32), the desmoplastic pattern in 6.5% (n=6), and a mix of two growth patterns in 29.4% (n=27). The size of the aerogenous pattern was significantly smaller than that of metastases with other patterns (p=.033). Kaplan-Meier analysis demonstrated that patients showing an aerogenous pattern appeared to have a poorer prognosis, which was calculated from the time of diagnosis of the CLOM (p=.044). The 5-year survival rate from the diagnosis of colorectal cancer tended to be lower in patients with an aerogenous pattern than in those who had a non-aerogenous pattern; however, the difference was marginally significant (p=.051). In the multivariate Cox analysis, the aerogenous pattern appeared as an independent predictor of poor overall survival (hazard ratio, 3.122; 95% confidence interval, 1.196 to 8.145; p=.020).
Conclusions
These results suggest that the growth patterns may play a part as a histology-based prognostic parameter for patients with CLOM. -
Citations
Citations to this article as recorded by- Predicting liver metastases growth patterns: Current status and future possibilities
Rui Caetano Oliveira, Henrique Alexandrino, Maria Augusta Cipriano, Filipe Caseiro Alves, José Guilherme Tralhão
Seminars in Cancer Biology.2021; 71: 42. CrossRef - Histological growth patterns and molecular analysis of resected colorectal lung metastases
Emanuela Pilozzi, Damiano Fedele, Andrea Montori, Laura Lorenzon, Valentina Peritore, Giorgia Mannocchi, Nikta Bagheri, Chiara Leone, Antonio Palumbo, Michela Roberto, Giulio Ranazzi, Erino Rendina, Genoveffa Balducci, Mohsen Ibrahim
Pathology - Research and Practice.2021; 222: 153414. CrossRef
- Predicting liver metastases growth patterns: Current status and future possibilities
- The Clinicopathological and Prognostic Significance of the Gross Classification of Hepatocellular Carcinoma
- Yangkyu Lee, Hyunjin Park, Hyejung Lee, Jai Young Cho, Yoo-Seok Yoon, Young-Rok Choi, Ho-Seong Han, Eun Sun Jang, Jin-Wook Kim, Sook-Hyang Jeong, Soomin Ahn, Haeryoung Kim
- J Pathol Transl Med. 2018;52(2):85-92. Published online November 24, 2017
- DOI: https://doi.org/10.4132/jptm.2017.11.13
- 14,687 View
- 389 Download
- 27 Web of Science
- 24 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
We aimed to determine the clinicopathological significance of the gross classification of hepatocellular carcinoma (HCC) according to the Korean Liver Cancer Association (KLCA) guidelines.
Methods
A retrospective analysis was performed on 242 cases of consecutively resected solitary primary HCC between 2003 and 2012 at Seoul National University Bundang Hospital. The gross classification (vaguely nodular [VN], expanding nodular [EN], multinodular confluent [MC], nodular with perinodular extension [NP], and infiltrative [INF]) was reviewed for all cases, and were correlated with various clinicopathological features and the expression status of “stemness”-related (cytokeratin 19 [CK19], epithelial cell adhesion molecule [EpCAM]), and epithelial-mesenchymal transition (EMT)–related (urokinase plasminogen activator receptor [uPAR] and Ezrin) markers.
Results
Significant differences were seen in overall survival (p=.015) and disease-free survival (p = .034) according to the gross classification; INF type showed the worst prognosis while VN and EN types were more favorable. When the gross types were simplified into two groups, type 2 HCCs (MC/NP/INF) were more frequently larger and poorly differentiated, and showed more frequent microvascular and portal venous invasion, intratumoral fibrous stroma and higher pT stages compared to type 1 HCCs (EN/VN) (p<.05, all). CK19, EpCAM, uPAR, and ezrin expression was more frequently seen in type 2 HCCs (p<.05, all). Gross classification was an independent predictor of both overall and disease-free survival by multivariate analysis (overall survival: p=.030; hazard ratio, 4.118; 95% confidence interval, 1.142 to 14.844; disease-free survival: p=.016; hazard ratio, 1.617; 95% confidence interval, 1.092 to 2.394).
Conclusions
The gross classification of HCC had significant prognostic value and type 2 HCCs were associated with clinicopathological features of aggressive behavior, increased expression of “stemness”- and EMT-related markers, and decreased survival. -
Citations
Citations to this article as recorded by- Long-Term Outcomes of Transarterial Chemoembolization plus Ablation versus Surgical Resection in Patients with Large BCLC Stage A/B HCC
Ying-Wen Hou, Tian-Qi Zhang, Li-Di Ma, Yi-Quan Jiang, Xue Han, Tian Di, Lu Tang, Rong-Ping Guo, Min-Shan Chen, Jin-Xin Zhang, Zhi-Mei Huang, Jin-Hua Huang
Academic Radiology.2025; 32(7): 3960. CrossRef - Membranous Overexpression of Fibronectin Predicts Microvascular Invasion and Poor Survival Outcomes in Patients with Hepatocellular Carcinoma
Yoon Jung Hwang, Hyejung Lee, Suk Kyun Hong, Su Jong Yu, Haeryoung Kim
Gut and Liver.2025; 19(2): 275. CrossRef - Association between tumor morphology and efficacy of atezolizumab plus bevacizumab for advanced hepatocellular carcinoma
Nobuaki Ishihara, Shohei Komatsu, Keitaro Sofue, Eisuke Ueshima, Yoshihiko Yano, Yoshimi Fujishima, Jun Ishida, Masahiro Kido, Hidetoshi Gon, Kenji Fukushima, Takeshi Urade, Hiroaki Yanagimoto, Hirochika Toyama, Yoshihide Ueda, Yuzo Kodama, Takamichi Mura
Hepatology Research.2024; 54(8): 773. CrossRef - Macroscopic Characterization of Hepatocellular Carcinoma: An Underexploited Source of Prognostic Factors
Stéphanie Gonvers, Sebastiao Martins-Filho, André Hirayama, Julien Calderaro, Rebecca Phillips, Emilie Uldry, Nicolas Demartines, Emmanuel Melloul, Young Nyun Park, Valérie Paradis, Swan Thung, Venancio Alves, Christine Sempoux, Ismail Labgaa
Journal of Hepatocellular Carcinoma.2024; Volume 11: 707. CrossRef - Serum Total Superoxide Dismutase Activity as a Predictive Factor in Patients with Hepatocellular Carcinoma
Yanqiu Xu, Bin Liu, Shiqing Cheng, Junguo Zhang, Xiue Cao, Yong Wang, Fang Luan
Hepatitis Monthly.2024;[Epub] CrossRef - Diagnostic value of expressions of cancer stem cell markers for adverse outcomes of hepatocellular carcinoma and their associations with prognosis: A Bayesian network meta‑analysis
Zhengrong Ou, Shoushuo Fu, Jian Yi, Jingxuan Huang, Weidong Zhu
Oncology Letters.2024;[Epub] CrossRef - Recurrence of hepatocellular carcinoma in noncirrhotic patients with nonalcoholic fatty liver disease versus hepatitis B infection
Jungnam Lee, Jong-In Chang, Young-Joo Jin, Jeong-Hoon Lee, Ju Yeon Kim, Dong Hyun Sinn, Soon Sun Kim, Hyun Woong Lee, Sun Hong Yoo, Jung Hwan Yu, Jin-Woo Lee
European Journal of Gastroenterology & Hepatology.2023; 35(4): 431. CrossRef - Comprehensive investigating of MMR gene in hepatocellular carcinoma with chronic hepatitis B virus infection in Han Chinese population
Ning Ma, Ao Jin, Yitong Sun, Yiyao Jin, Yucheng Sun, Qian Xiao, XuanYi Sha, Fengxue Yu, Lei Yang, Wenxuan Liu, Xia Gao, Xiaolin Zhang, Lu Li
Frontiers in Oncology.2023;[Epub] CrossRef - LI-RADS Morphological Type Predicts Prognosis of Patients with Hepatocellular Carcinoma After Radical Resection
Chunhui Zhang, Rui Yang, Xinxin Wang, Yuqing Tao, Shuli Tang, Zhennan Tian, Yang Zhou
Annals of Surgical Oncology.2023; 30(8): 4876. CrossRef - From clinical variables to multiomics analysis: a margin morphology-based gross classification system for hepatocellular carcinoma stratification
Zhongqi Fan, Meishan Jin, Lei Zhang, Nanya Wang, Mingyue Li, Chuanlei Wang, Feng Wei, Ping Zhang, Xiaohong Du, Xiaodong Sun, Wei Qiu, Meng Wang, Hongbin Wang, Xiaoju Shi, Junfeng Ye, Chao Jiang, Jianpeng Zhou, Wengang Chai, Jun Qi, Ting Li, Ruoyan Zhang,
Gut.2023; 72(11): 2149. CrossRef - A clinical and pathological update on hepatocellular carcinoma
Salvatore Lorenzo Renne, Luca Di Tommaso
Journal of Liver Cancer.2022; 22(1): 14. CrossRef - An approach to grossing of hepatectomy specimens
Archana Rastogi
Indian Journal of Pathology and Microbiology.2021; 64(5): 121. CrossRef - Hepatocellular carcinoma: a clinical and pathological overview
Salvatore Lorenzo Renne, Samantha Sarcognato, Diana Sacchi, Maria Guido, Massimo Roncalli, Luigi Terracciano, Luca Di Tommaso
Pathologica.2021; 113(3): 203. CrossRef - The Clinicopathological Significance of YAP/TAZ Expression in Hepatocellular Carcinoma with Relation to Hypoxia and Stemness
Hyunjin Park, Yangkyu Lee, Kiryang Lee, Hyejung Lee, Jeong Eun Yoo, Soomin Ahn, Young Nyun Park, Haeryoung Kim
Pathology and Oncology Research.2021;[Epub] CrossRef - Adjuvant versus Neoadjuvant Immunotherapy for Hepatocellular Carcinoma: Clinical and Immunologic Perspectives
Yung-Yeh Su, Chia-Chen Li, Yih-Jyh Lin, Chiun Hsu
Seminars in Liver Disease.2021; 41(03): 263. CrossRef - Prognostic significance of viable tumor size measurement in hepatocellular carcinomas after preoperative locoregional treatment
Yoon Jung Hwang, Youngeun Lee, Hyunjin Park, Yangkyu Lee, Kyoungbun Lee, Haeryoung Kim
Journal of Pathology and Translational Medicine.2021; 55(5): 338. CrossRef - Survival according to recurrence patterns after resection for transplantable hepatocellular carcinoma in HBV endemic area: Appraisal of liver transplantation strategy
Chung Gyo Seo, Sun Young Yim, Soon Ho Um, Yoo Ra Lee, Yoo Jin Lee, Tae Hyung Kim, Hyun Gil Goh, Young Sun Lee, Sang Jun Suh, Na Yeon Han, Hyuk Soon Choi, Eun Sun Kim, Bora Keum, Yeon Seok Seo, Hyung Joon Yim, Ji Hoon Kim, Dong Sik Kim, Yoon Tae Jeen, Hoon
Clinics and Research in Hepatology and Gastroenterology.2020; 44(4): 532. CrossRef - A radiomics-based biomarker for cytokeratin 19 status of hepatocellular carcinoma with gadoxetic acid–enhanced MRI
Wentao Wang, Dongsheng Gu, Jingwei Wei, Ying Ding, Li Yang, Kai Zhu, Rongkui Luo, Sheng-Xiang Rao, Jie Tian, Mengsu Zeng
European Radiology.2020; 30(5): 3004. CrossRef Paeonol Inhibits Cell Proliferation, Migration and Invasion and Induces Apoptosis in Hepatocellular Carcinoma by Regulating miR-21-5p/KLF6 Axis
Miaoguo Cai, Wei Shao, Huijun Yu, Ye Hong, Lili Shi
Cancer Management and Research.2020; Volume 12: 5931. CrossRef- Update on Hepatocellular Carcinoma: a Brief Review from Pathologist Standpoint
Nese Karadag Soylu
Journal of Gastrointestinal Cancer.2020; 51(4): 1176. CrossRef - Clinico-Radio-Pathological and Molecular Features of Hepatocellular Carcinomas with Keratin 19 Expression
Hyungjin Rhee, Haeryoung Kim, Young Nyun Park
Liver Cancer.2020; 9(6): 663. CrossRef - Histopathological characteristics of needle core biopsy and surgical specimens from patients with solitary hepatocellular carcinoma or intrahepatic cholangiocarcinoma
Ju-Shan Wu, Ji-Liang Feng, Rui-Dong Zhu, San-Guang Liu, Da-Wei Zhao, Ning Li
World Journal of Gastrointestinal Oncology.2019; 11(5): 404. CrossRef - The strengths and weaknesses of gross and histopathological evaluation in hepatocellular carcinoma: a brief review
Sebastião N. Martins-Filho, Venâncio Avancini Ferreira Alves
Surgical and Experimental Pathology.2019;[Epub] CrossRef - Changing role of histopathology in the diagnosis and management of hepatocellular carcinoma
Archana Rastogi
World Journal of Gastroenterology.2018; 24(35): 4000. CrossRef
- Long-Term Outcomes of Transarterial Chemoembolization plus Ablation versus Surgical Resection in Patients with Large BCLC Stage A/B HCC
- The Smad4/PTEN Expression Pattern Predicts Clinical Outcomes in Colorectal Adenocarcinoma
- Yumin Chung, Young Chan Wi, Yeseul Kim, Seong Sik Bang, Jung-Ho Yang, Kiseok Jang, Kyueng-Whan Min, Seung Sam Paik
- J Pathol Transl Med. 2018;52(1):37-44. Published online October 23, 2017
- DOI: https://doi.org/10.4132/jptm.2017.10.20
- 12,755 View
- 234 Download
- 13 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Smad4 and PTEN are prognostic indicators for various tumor types. Smad4 regulates tumor suppression, whereas PTEN inhibits cell proliferation. We analyzed and compared the performance of Smad4 and PTEN for predicting the prognosis of patients with colorectal adenocarcinoma.
Methods
Combined expression patterns based on Smad4+/– and PTEN+/– status were evaluated by immunostaining using a tissue microarray of colorectal adenocarcinoma. The relationships between the protein expression and clinicopathological variables were analyzed.
Results
Smad4–/PTEN– status was most frequently observed in metastatic adenocarcinoma, followed by primary adenocarcinoma and tubular adenoma (p<.001). When Smad4–/PTEN– and Smad4+/PTEN+ groups were compared, Smad4–/PTEN– status was associated with high N stage (p=.018) and defective mismatch repair proteins (p=.006). Significant differences in diseasefree survival and overall survival were observed among the three groups (Smad4+/PTEN+, Smad4–/PTEN+ or Smad4+/PTEN–, and Smad4–/PTEN–) (all p<.05).
Conclusions
Concurrent loss of Smad4 and PTEN may lead to more aggressive disease and poor prognosis in patients with colorectal adenocarcinoma compared to the loss of Smad4 or PTEN alone. -
Citations
Citations to this article as recorded by- Precision oncolytic viral therapy in colorectal cancer: Genetic targeting and immune modulation for personalized treatment (Review)
Muhammad Haris Sultan, Qi Zhan, Yigang Wang, Yulong Xia, Xiaoyuan Jia
International Journal of Molecular Medicine.2025; 56(1): 1. CrossRef - Focal Adhesion Kinase Intersects With the BRD4‐MYC Axis and YAP1 to Drive Tumor Cell Growth, Phenotypic Plasticity, Stemness, and Metastatic Potential in Colorectal Cancer
Rongbo Han, Junfeng Shi, Kai Cheng, Zian Wang, Yecang Chen, Orion Spellecy, Abu Saleh Mosa Faisal, Isha Aryal, Jinfei Chen, Rolf Craven, Olivier Thibault, Lauren Baldwin, Lawrence D. Brewer, Sonia Erfani, Chi Wang, Zhenheng Guo, Eric Chen, Burton Yang, Fr
Cancer Medicine.2025;[Epub] CrossRef - Association between the expression of epithelial–mesenchymal transition (EMT)-related markers and oncologic outcomes of colorectal cancer
Mona Hany Emile, Sameh Hany Emile, Amr Awad El-Karef, Mohamed Awad Ebrahim, Ibrahim Eldosoky Mohammed, Dina Abdallah Ibrahim
Updates in Surgery.2024; 76(6): 2181. CrossRef - The Potential Role of Genomic Signature in Stage II Relapsed Colorectal Cancer (CRC) Patients: A Mono-Institutional Study
Michela Roberto, Giulia Arrivi, Emanuela Pilozzi, Andrea Montori, Genoveffa Balducci, Paolo Mercantini, Andrea Laghi, Debora Ierinò, Martina Panebianco, Daniele Marinelli, Silverio Tomao, Paolo Marchetti, Federica Mazzuca
Cancer Management and Research.2022; Volume 14: 1353. CrossRef - Alterations of PTEN and SMAD4 methylation in diagnosis of breast cancer: implications of methyl II PCR assay
Menha Swellam, Entsar A. Saad, Shimaa Sabry, Adel Denewer, Camelia Abdel Malak, Amr Abouzid
Journal of Genetic Engineering and Biotechnology.2021; 19(1): 54. CrossRef - E3 ubiquitin ligase HECW1 promotes the metastasis of non-small cell lung cancer cells through mediating the ubiquitination of Smad4
Chen Lu, Guangyao Ning, Panpan Si, Chunsheng Zhang, Wenjian Liu, Wei Ge, Kai Cui, Renquan Zhang, Shenglin Ge
Biochemistry and Cell Biology.2021; 99(5): 675. CrossRef - Computational quantification of global effects induced by mutations and drugs in signaling networks of colorectal cancer cells
Sara Sommariva, Giacomo Caviglia, Silvia Ravera, Francesco Frassoni, Federico Benvenuto, Lorenzo Tortolina, Nicoletta Castagnino, Silvio Parodi, Michele Piana
Scientific Reports.2021;[Epub] CrossRef - Clinicopathological characterization of SMAD4-mutated intestinal adenocarcinomas: A case-control study
Xiaoyan Liao, Yansheng Hao, Xiaofei Zhang, Stephen Ward, Jane Houldsworth, Alexandros D. Polydorides, Noam Harpaz, Aldo Scarpa
PLOS ONE.2019; 14(2): e0212142. CrossRef - Clinicopathological Characterization and Prognostic Implication of SMAD4 Expression in Colorectal Carcinoma
Seung-Yeon Yoo, Ji-Ae Lee, Yunjoo Shin, Nam-Yun Cho, Jeong Mo Bae, Gyeong Hoon Kang
Journal of Pathology and Translational Medicine.2019; 53(5): 289. CrossRef - Dissecting the therapeutic implications of the complex SMAD4 regulatory network in metastatic colorectal cancer
Ion Cristóbal, Blanca Torrejón, Andrea Santos, Melani Luque, Marta Sanz-Alvarez, Federico Rojo, Jesús García-Foncillas
European Journal of Surgical Oncology.2018; 44(8): 1283. CrossRef - Reply to: Dissecting the therapeutic implications of the complex SMAD4 regulatory network in metastatic colorectal cancer
Jordan M. Cloyd, Takashi Mizuno, Jean-Nicolas Vauthey
European Journal of Surgical Oncology.2018; 44(8): 1285. CrossRef
- Precision oncolytic viral therapy in colorectal cancer: Genetic targeting and immune modulation for personalized treatment (Review)
- Combined Adenosquamous and Large Cell Neuroendocrine Carcinoma of the Gallbladder
- Jiyoon Jung, Yang-Seok Chae, Chul Hwan Kim, Youngseok Lee, Jeong Hyeon Lee, Dong-Sik Kim, Young-Dong Yu, Joo Young Kim
- J Pathol Transl Med. 2018;52(2):121-125. Published online October 5, 2017
- DOI: https://doi.org/10.4132/jptm.2017.08.20
- 8,853 View
- 155 Download
- 12 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF - Large cell neuroendocrine carcinoma (LCNEC) of the gallbladder is extremely rare and usually combined with other type of malignancy, mostly adenocarcinoma. We report an unusual case of combined adenosquamous carcinoma and LCNEC of the gallbladder in a 54-year-old woman. A radical cholecystectomy specimen revealed a 4.3×4.0 cm polypoid mass in the fundus with infiltration of adjacent liver parenchyma. Microscopically, the tumor consisted of two distinct components. Adenosquamous carcinoma was predominant and abrupt transition from adenocarcinoma to squamous cell carcinoma was observed. LCNEC showed round cells with large, vesicular nuclei, abundant mitotic figures, and occasional pseudorosette formation. The patient received adjuvant chemotherapy. However, multiple liver metastases were identified at 3-month follow-up. Metastatic nodules were composed of LCNEC and squamous cell carcinoma components. Detecting LCNEC component is important in gallbladder cancer, because the tumor may require a different chemotherapy regimen and show early metastasis and poor prognosis.
-
Citations
Citations to this article as recorded by- Postoperative gastric cancer accompanied by large-cell neuroendocrine carcinoma: A case report
Zhiqin Chen, Jiang Liu, Jin Liu, Yinhang Wu, Jian Liu
Medicine.2025; 104(41): e44367. CrossRef - Does the size of the neuroendocrine-carcinoma component determine the prognosis of gallbladder cancer?
Ya-Fei Hu, Jun-Ke Wang, Wen-Jie Ma, Hai-Jie Hu, Han-Fei Gu, Fei Liu, Tian-Run Lv, Si-Qi Yang, Yu-Shi Dai, Rui-Qi Zou, Yan-Wen Jin, Fu-Yu Li
Frontiers in Endocrinology.2024;[Epub] CrossRef - Az epehólyag adenosquamosus daganata
Fanni Hegedűs, Anita Sejben
Orvosi Hetilap.2024; 165(49): 1945. CrossRef - Comparison of Metastatic Patterns Among Neuroendocrine Tumors, Neuroendocrine Carcinomas, and Nonneuroendocrine Carcinomas of Various Primary Organs
Hyung Kyu Park, Ghee Young Kwon
Journal of Korean Medical Science.2023;[Epub] CrossRef - Clinical features and outcomes analysis of Gallbladder neuroendocrine carcinoma
Man Jiang, Yijing Zhang
Journal of Cancer Research and Therapeutics.2023; 19(4): 910. CrossRef - Primary mixed large cell neuroendocrine carcinoma and adenocarcinoma of the gallbladder: A case report and literature review
Tingting Yu, Shike Li, Zhuo Zhang
Asian Journal of Surgery.2022; 45(11): 2336. CrossRef - Mixed neuroendocrine-non-neuroendocrine neoplasm of the gallbladder: case report and literature review
Xu Ren, Hong Jiang, Kan Sun, Xufu Qin, Yongping Qu, Tian Xia, Yan Chen
Diagnostic Pathology.2022;[Epub] CrossRef - Neuroendocrine Neoplasms of the Gallbladder: A Clinicopathological Analysis of 13 Patients and a Review of the Literature
Pengyan Wang, Jingci Chen, Ying Jiang, Congwei Jia, Junyi Pang, Shan Wang, Xiaoyan Chang, Oronzo Brunetti
Gastroenterology Research and Practice.2021; 2021: 1. CrossRef - Gallbladder Mixed Neuroendocrine-Non-neuroendocrine Neoplasm (MiNEN) Arising in Intracholecystic Papillary Neoplasm: Clinicopathologic and Molecular Analysis of a Case and Review of the Literature
Amedeo Sciarra, Edoardo Missiaglia, Mounir Trimech, Emmanuel Melloul, Jean-Philippe Brouland, Christine Sempoux, Stefano La Rosa
Endocrine Pathology.2020; 31(1): 84. CrossRef - Mixed neuroendocrine-non-neuroendocrine carcinoma of gallbladder: case report
Adam Skalický, Lucie Vištejnová, Magdaléna Dubová, Tomáš Malkus, Tomáš Skalický, Ondřej Troup
World Journal of Surgical Oncology.2019;[Epub] CrossRef
- Postoperative gastric cancer accompanied by large-cell neuroendocrine carcinoma: A case report
- The Potential Roles of MELF-Pattern, Microvessel Density, and VEGF Expression in Survival of Patients with Endometrioid Endometrial Carcinoma: A Morphometrical and Immunohistochemical Analysis of 100 Cases
- Dmitry Aleksandrovich Zinovkin, Md Zahidul Islam Pranjol, Daniil Rudolfovich Petrenyov, Eldar Arkadievich Nadyrov, Oleg Gennadievich Savchenko
- J Pathol Transl Med. 2017;51(5):456-462. Published online September 14, 2017
- DOI: https://doi.org/10.4132/jptm.2017.07.19
- 10,169 View
- 193 Download
- 24 Web of Science
- 20 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
In this study, we hypothesized that microcystic, elongated, fragmented (MELF)-pattern, vascular endothelial growth factor (VEGF) expression by cancer cells and microvessel density of cancer stroma may be associated with progression of endometrioid adenocarcinoma. Methods: The study used data from the Belarus Cancer Registry and archival histological material of 100 patients with retrospectively known good (survival) and poor (disease progression and death) outcomes. All cases were immunohistochemically stained for CD34 and VEGF. Two independent samples were compared for the characteristics of signs, and obtained results were analyzed by receiver operating characteristic analysis, Mann-Whitney U test, χ2 test (Yates correction), and Mantel-Cox test. Multivariate Cox hazard analysis and Spearman correlation test were used. A p-value of less than .05 was considered statistically significant. Results: The observed survival rate of patients with endometrioid adenocarcinoma was significantly lower (p = .002) in MELF-pattern positive patients when compared with MELF-pattern negative patients. The overall survival rate of patients whose tumors had more than 114 vessels/mm2 of tissue was significantly low (p < .001). Interestingly, a similar observation was found in patients with increased vessel area, evidenced by VEGF expression in the glandular tumor component. Conclusions: Our study suggests, for the first time, that these criteria may be used as risk factors of endometrioid adenocarcinoma progression during 5 years after radical surgical treatment. However, a large independent cohort of samples should be considered in the future to validate our findings. -
Citations
Citations to this article as recorded by- Association of Local and Distant Organ Metastases With MELF Pattern in Endometrial Cancer
Varol Gülseren, Ertuğrul Şen, Mehmet Dolanbay, Fulya Çağli, Nahit Topaloğlu, Figen Öztürk, Bülent Özçelik, Serdar Serin, Kemal Güngördük
International Journal of Gynecological Pathology.2025; 44(3): 237. CrossRef - Molecular Classification of Endometrial Endometrioid Carcinoma With Microcystic Elongated and Fragmented Pattern
Baohui Ju, Jianghua Wu, Lin Sun, Chunrui Yang, Hu Yu, Quan Hao, Jianmei Wang, Huiying Zhang
International Journal of Gynecological Pathology.2024; 43(3): 233. CrossRef -
The vasculogenic mimicry, CD146
+
and CD105
+
microvessel density in the prognosis of endometrioid endometrial adenocarcinoma: a single-centre immunohistochemical study
Dmitry A. Zinovkin, Hongbo Wang, Zhicheng Yu, Qian Zhang, Yang Zhang, Sitian Wei, Ting Zhou, Qi Zhang, Jun Zhang, Eldar A. Nadyrov, Abdullah Farooq, Yulia Lyzikova, Ilya V. Vejalkin, Irina I. Slepokurova, Md Zahidul Islam Pranjol
Biomarkers.2024; 29(7): 459. CrossRef - Dynamic contrast-enhanced MR imaging of uterine endometrial carcinoma with/without squamous differentiation
Mayumi Takeuchi, Kenji Matsuzaki, Yoshimi Bando, Masafumi Harada
Abdominal Radiology.2023; 48(8): 2494. CrossRef - Role of adipocytokines in endometrial cancer progression
Ran Li, Fang Dong, Ling Zhang, Xiuqin Ni, Guozhi Lin
Frontiers in Pharmacology.2022;[Epub] CrossRef - Endometrial carcinoma: use of tracer kinetic modeling of dynamic contrast-enhanced MRI for preoperative risk assessment
Zhijun Ye, Gang Ning, Xuesheng Li, Tong San Koh, Huizhu Chen, Wanjing Bai, Haibo Qu
Cancer Imaging.2022;[Epub] CrossRef - Microcystic elongated and fragmented (MELF) pattern of invasion: Molecular features and prognostic significance in the PORTEC-1 and -2 trials
A.S.V.M. van den Heerik, K.T.S. Aiyer, E. Stelloo, I.M. Jürgenliemk-Schulz, L.C.H.W. Lutgens, J.J. Jobsen, J.W.M. Mens, E.M. van der Steen-Banasik, C.L. Creutzberg, V.T.H.B.M. Smit, N. Horeweg, T. Bosse
Gynecologic Oncology.2022; 166(3): 530. CrossRef - Pathological features, immunoprofile and mismatch repair protein expression status in uterine endometrioid carcinoma: focus on MELF pattern of myoinvasion
Angela Santoro, Giuseppe Angelico, Frediano Inzani, Saveria Spadola, Damiano Arciuolo, Michele Valente, Teresa Musarra, Giovanni Capelli, Francesco Fanfani, Valerio Gallotta, Giovanni Scambia, Gian Franco Zannoni
European Journal of Surgical Oncology.2021; 47(2): 338. CrossRef - Prognostic impact of tumor budding in endometrial carcinoma within distinct molecular subgroups
Tilman T. Rau, Eva Bettschen, Carol Büchi, Lucine Christe, Amanda Rohner, Michael D. Müller, Joseph W. Carlson, Sara Imboden, Inti Zlobec
Modern Pathology.2021; 34(1): 222. CrossRef - Sentinel Nodal Metastasis Detection in Endometrial Carcinoma With Microcystic, Elongated, and Fragmented (MELF) Pattern by Cytokeratin Immunostaining
Kimmie M Rabe, Molly E Klein, Sayak Ghatak, Irina Stout, Alexandra Schefter, Britt K Erickson, Mahmoud A Khalifa
American Journal of Clinical Pathology.2021; 156(5): 846. CrossRef - Patterns of Myometrial Invasion in Endometrial Adenocarcinoma with Emphasizing on Microcystic, Elongated and Fragmented (MELF) Glands Pattern: A Narrative Review of the Literature
Svetlana Mateva, Margarita Nikolova, Angel Yordanov
Diagnostics.2021; 11(9): 1707. CrossRef - Increase in FoxP3, CD56 immune cells and decrease in glands PGRMC1 expression in the endometrium are associated with recurrent miscarriages
Yulia Anatolievna Lyzikova, Dmitry Aleksandrovich Zinovkin, Md Zahidul Islam Pranjol
European Journal of Obstetrics & Gynecology and Reproductive Biology.2020; 245: 121. CrossRef - Clinicopathologic Association and Prognostic Value of MELF Pattern in Invasive Endocervical Adenocarcinoma (ECA) as Classified by IECC
Sheila E. Segura, Lien Hoang, Monica Boros, Cristina Terinte, Anna Pesci, Sarit Aviel-Ronen, Takako Kiyokawa, Isabel Alvarado-Cabrero, Esther Oliva, Kay J. Park, Robert A. Soslow, Simona Stolnicu
International Journal of Gynecological Pathology.2020; 39(5): 436. CrossRef - Usual-Type Endocervical Adenocarcinoma with a Microcystic, Elongated, and Fragmented Pattern of Stromal Invasion: A Case Report with Emphasis on Ki-67 Immunostaining and Targeted Sequencing Results
Sangjoon Choi, Soohyun Hwang, Sung-Im Do, Hyun-Soo Kim
Case Reports in Oncology.2020; 13(3): 1421. CrossRef - High Expression of Galectin-1, VEGF and Increased Microvessel Density Are Associated with MELF Pattern in Stage I-III Endometrioid Endometrial Adenocarcinoma
Dmitry Aleksandrovich Zinovkin, Sergey Leonidovich Achinovich, Mikhail Grigoryevich Zubritskiy, Jacqueline Linda Whatmore, Md Zahidul Islam Pranjol
Journal of Pathology and Translational Medicine.2019; 53(5): 280. CrossRef - The Roles of Melf Patterns, the Depth of Invasion and Number of Tumor Emboli as the Predictive Factors of the Survival Rate Among Patients with Endometrioid Adenocarcinoma of the Corpus Uterus
D. A. Zinovkin
Health and Ecology Issues.2019; (1): 49. CrossRef - Non-endometrioid and high-grade endometrioid endometrial cancers show DNA fragmentation factor 40 (DFF40) and B-cell lymphoma 2 protein (BCL2) underexpression, which predicts disease-free and overall survival, but not DNA fragmentation factor 45 (DFF45) u
Tomasz Banas, Kazimierz Pitynski, Krzysztof Okon, Aleksandra Winiarska
BMC Cancer.2018;[Epub] CrossRef - Tumor-Associated T-Lymphocytes and Macrophages are Decreased in Endometrioid Endometrial Carcinoma with MELF-Pattern Stromal Changes
Dmitry Aleksandrovich Zinovkin, Md Zahidul Islam Pranjol, Il’ya Andreevich Bilsky, Valeriya Alexandrovna Zmushko
Cancer Microenvironment.2018; 11(2-3): 107. CrossRef - MELF pattern of myometrial invasion and role in possible endometrial cancer diagnostic pathway: A systematic review of the literature
Anastasia Prodromidou, George Vorgias, Konstantinos Bakogiannis, Nikolaos Kalinoglou, Christos Iavazzo
European Journal of Obstetrics & Gynecology and Reproductive Biology.2018; 230: 147. CrossRef - CORRELATIVE INTERRELATIONS OF THE TUMOR MICROENVIRONMENT AND RELATIVE RISK OF UNFAVOURABLE OUTCOME OF ENDOMETRIOID ADENOCARCINOMA OF THE CORPUS UTERI
D. A. Zinovkin
Health and Ecology Issues.2018; (3): 48. CrossRef
- Association of Local and Distant Organ Metastases With MELF Pattern in Endometrial Cancer
- Diverse Immunoprofile of Ductal Adenocarcinoma of the Prostate with an Emphasis on the Prognostic Factors
- Se Un Jeong, Anuja Kashikar Kekatpure, Ja-Min Park, Minkyu Han, Hee Sang Hwang, Hui Jeong Jeong, Heounjeong Go, Yong Mee Cho
- J Pathol Transl Med. 2017;51(5):471-481. Published online August 9, 2017
- DOI: https://doi.org/10.4132/jptm.2017.06.02
- 10,705 View
- 208 Download
- 14 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Ductal adenocarcinoma (DAC) of the prostate is an uncommon histologic subtype whose prognostic factors and immunoprofile have not been fully defined. Methods: To define its prognostic factors and immunoprofile, the clinicopathological features, including biochemical recurrence (BCR), of 61 cases of DAC were analyzed. Immunohistochemistry was performed on tissue microarray constructs to assess the expression of prostate cancer-related and mammalian target of rapamycin (mTOR) signaling-related proteins. Results: During the median follow-up period of 19.3 months, BCR occurred in 26 cases (42.6%). DAC demonstrated a wide expression range of prostate cancer-related proteins, including nine cases (14.8%) that were totally negative for pan-cytokeratin (PanCK) immunostaining. The mTOR signaling-related proteins also showed diverse expression. On univariate analysis, BCR was associated with high preoperative serum levels of prostate-specific antigen (PSA), large tumor volume, predominant ductal component, high Gleason score (GS), comedo-necrosis, high tumor stage (pT), lymphovascular invasion, and positive surgical margin. High expressions of phospho-mTOR (p-mTOR) as well as low expressions of PSA, phospho-S6 ribosomal protein (pS6) and PanCK were associated with BCR. On multivariable analysis, GS, pT, and immunohistochemical expressions of PanCK and p-mTOR remained independent prognostic factors for BCR. Conclusions: These results suggest GS, pT, and immunohistochemical expressions of PanCK and p-mTOR as independent prognostic factors for BCR in DAC. Since DAC showed diverse expression of prostate cancer–related proteins, this should be recognized in interpreting the immunoprofile of DAC. The diverse expression of mTOR-related proteins implicates their potential utility as predictive markers for mTOR targeted therapy. -
Citations
Citations to this article as recorded by- Intermediate risk prostate tumors contain lethal subtypes
William L. Harryman, James P. Hinton, Rafael Sainz, Jaime M. C. Gard, John M. Ryniawec, Gregory C. Rogers, Noel A. Warfel, Beatrice S. Knudsen, Raymond B. Nagle, Juan J. Chipollini, Benjamin R. Lee, Belinda L. Sun, Anne E. Cress
Frontiers in Urology.2025;[Epub] CrossRef - High GLUT1 membrane expression and low PSMA membrane expression in Ductal Adenocarcinoma and Intraductal Carcinoma of the prostate
Xingming Wang, Li Zhou, Lin Qi, Ye Zhang, Hongling Yin, Yu Gan, Xiaomei Gao, Yi Cai
Prostate Cancer and Prostatic Diseases.2024; 27(4): 720. CrossRef - Association of Lymphovascular Invasion with Biochemical Recurrence and Adverse Pathological Characteristics of Prostate Cancer: A Systematic Review and Meta-analysis
Jakub Karwacki, Marcel Stodolak, Andrzej Dłubak, Łukasz Nowak, Adam Gurwin, Kamil Kowalczyk, Paweł Kiełb, Nazar Holdun, Wojciech Szlasa, Wojciech Krajewski, Agnieszka Hałoń, Anna Karwacka, Tomasz Szydełko, Bartosz Małkiewicz
European Urology Open Science.2024; 69: 112. CrossRef - Impact of Epithelial Histological Types, Subtypes, and Growth Patterns on Oncological Outcomes for Patients with Nonmetastatic Prostate Cancer Treated with Curative Intent: A Systematic Review
Giancarlo Marra, Geert J.L.H. van Leenders, Fabio Zattoni, Claudia Kesch, Pawel Rajwa, Philip Cornford, Theodorus van der Kwast, Roderick C.N. van den Bergh, Erik Briers, Thomas Van den Broeck, Gert De Meerleer, Maria De Santis, Daniel Eberli, Andrea Faro
European Urology.2023; 84(1): 65. CrossRef - Impact of comedonecrosis on prostate cancer outcome: a systematic review
Kaveri T S Aiyer, Lisa J Kroon, Geert J L H van Leenders
Histopathology.2023; 83(3): 339. CrossRef - Survival after radical prostatectomy vs. radiation therapy in ductal carcinoma of the prostate
Francesco Chierigo, Marco Borghesi, Christoph Würnschimmel, Rocco Simone Flammia, Benedikt Horlemann, Gabriele Sorce, Benedikt Höh, Zhe Tian, Fred Saad, Markus Graefen, Michele Gallucci, Alberto Briganti, Francesco Montorsi, Felix K. H. Chun, Shahrokh F.
International Urology and Nephrology.2022; 54(1): 89. CrossRef - Defining Diagnostic Criteria for Prostatic Ductal Adenocarcinoma at Multiparametric MRI
Weranja K. B. Ranasinghe, Patricia Troncoso, Devaki Shilpa Surasi, Juan José Ibarra Rovira, Priya Bhosale, Janio Szklaruk, Andrea Kokorovic, Xuemei Wang, Mohamed Elsheshtawi, Miao Zhang, Ana Aparicio, Brian F. Chapin, Tharakeswara K. Bathala
Radiology.2022; 303(1): 110. CrossRef - Oncological outcomes of patients with ductal adenocarcinoma of the prostate receiving radical prostatectomy or radiotherapy
Mengzhu Liu, Kun Jin, Shi Qiu, Pengyong Xu, Mingming Zhang, Wufeng Cai, Xiaonan Zheng, Lu Yang, Qiang Wei
Asian Journal of Urology.2021; 8(2): 227. CrossRef - Ductal Prostate Cancers Demonstrate Poor Outcomes with Conventional Therapies
Weranja Ranasinghe, Daniel D. Shapiro, Hyunsoo Hwang, Xuemei Wang, Chad A. Reichard, Mohamed Elsheshtawi, Mary F. Achim, Tharakeswara Bathala, Chad Tang, Ana Aparicio, Shi-Ming Tu, Nora Navone, Timothy C. Thompson, Louis Pisters, Patricia Troncoso, John W
European Urology.2021; 79(2): 298. CrossRef - Optimizing the diagnosis and management of ductal prostate cancer
Weranja Ranasinghe, Daniel D. Shapiro, Miao Zhang, Tharakeswara Bathala, Nora Navone, Timothy C. Thompson, Bradley Broom, Ana Aparicio, Shi-Ming Tu, Chad Tang, John W. Davis, Louis Pisters, Brian F. Chapin
Nature Reviews Urology.2021; 18(6): 337. CrossRef - A first case of ductal adenocarcinoma of the prostate having characteristics of neuroendocrine phenotype with PTEN, RB1 and TP53 alterations
Hiroaki Kobayashi, Takeo Kosaka, Kohei Nakamura, Kazunori Shojo, Hiroshi Hongo, Shuji Mikami, Hiroshi Nishihara, Mototsugu Oya
BMC Medical Genomics.2021;[Epub] CrossRef - Knowing what’s growing: Why ductal and intraductal prostate cancer matter
Mitchell G. Lawrence, Laura H. Porter, David Clouston, Declan G. Murphy, Mark Frydenberg, Renea A. Taylor, Gail P. Risbridger
Science Translational Medicine.2020;[Epub] CrossRef - Integrative Genomic Analysis of Coincident Cancer Foci Implicates CTNNB1 and PTEN Alterations in Ductal Prostate Cancer
Marc Gillard, Justin Lack, Andrea Pontier, Divya Gandla, David Hatcher, Adam G. Sowalsky, Jose Rodriguez-Nieves, Donald Vander Griend, Gladell Paner, David VanderWeele
European Urology Focus.2019; 5(3): 433. CrossRef - Genomic Characterization of Prostatic Ductal Adenocarcinoma Identifies a High Prevalence of DNA Repair Gene Mutations
Michael T. Schweizer, Emmanuel S. Antonarakis, Tarek A. Bismar, Liana B. Guedes, Heather H. Cheng, Maria S. Tretiakova, Funda Vakar-Lopez, Nola Klemfuss, Eric Q. Konnick, Elahe A. Mostaghel, Andrew C. Hsieh, Peter S. Nelson, Evan Y. Yu, R. Bruce Montgomer
JCO Precision Oncology.2019; (3): 1. CrossRef
- Intermediate risk prostate tumors contain lethal subtypes
- Prognostic Significance of a Micropapillary Pattern in Pure Mucinous Carcinoma of the Breast: Comparative Analysis with Micropapillary Carcinoma
- Hyun-Jung Kim, Kyeongmee Park, Jung Yeon Kim, Guhyun Kang, Geumhee Gwak, Inseok Park
- J Pathol Transl Med. 2017;51(4):403-409. Published online June 9, 2017
- DOI: https://doi.org/10.4132/jptm.2017.03.18
- 9,244 View
- 201 Download
- 17 Web of Science
- 18 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Mucinous carcinoma of the breast is an indolent tumors with a favorable prognosis; however, micropapillary features tend to lead to aggressive behavior. Thus, mucinous carcinoma and micropapillary carcinoma exhibit contrasting biologic behaviors. Here, we review invasive mucinous carcinoma with a focus on micropapillary features and correlations with clinicopathological factors.
Methods
A total of 64 patients with invasive breast cancer with mucinous or micropapillary features were enrolled in the study. Of 36 pure mucinous carcinomas, 17 (47.2%) had micropapillary features and were termed mucinous carcinoma with micropapillary features (MUMPC), and 19 (52.8%) had no micropapillary features and were termed mucinous carcinoma without micropapillary features. MUMPC were compared with 15 invasive micropapillary carcinomas (IMPC) and 13 invasive ductal and micropapillary carcinomas (IDMPC).
Results
The clinicopathological factors of pure mucinous carcinoma and MUMPC were not significantly different. In contrast to IMPC and IDMPC, MUMPC had a low nuclear grade, lower mitotic rate, higher expression of hormone receptors, negative human epidermal growth factor receptor 2 (HER2) status, lower Ki-67 proliferating index, and less frequent lymph node metastasis (p < .05). According to univariate analyses, progesterone receptor, HER2, T-stage, and lymph node metastasis were significant risk factors for overall survival; however, only T-stage remained significant in a multivariate analysis (p < .05).
Conclusions
In contrast to IMPC and IDMPC, the micropapillary pattern in mucinous carcinoma does not contribute to aggressive behavior. However, further analysis of a larger series of patients is required to clarify the prognostic significance of micropapillary patterns in mucinous carcinoma of the breast. -
Citations
Citations to this article as recorded by- Pure mucinous adenocarcinoma of the breast with the rare lymphoplasmacytic infiltration: A case report with review of literature
Yash Hasmukhbhai Prajapati, Vishal Bhabhor, Kahan Samirkumar Mehta, Mithoon Barot, Husen Boriwala, Mohamed Omar
Clinical Case Reports.2024;[Epub] CrossRef - Clinicopathological features and prognosis of mucinous breast carcinoma with a micropapillary structure
Beibei Yang, Menglu Shen, Bo Sun, Jing Zhao, Meng Wang
Thoracic Cancer.2024; 15(36): 2530. CrossRef - Expression of autocrine motility factor receptor (AMFR) in human breast and lung invasive micropapillary carcinomas
Jing Xu, Hongfei Ma, Qi Wang, Hui Zhang
International Journal of Experimental Pathology.2023; 104(1): 43. CrossRef - The Spectrum of Mucinous Lesions of the Breast
Upasana Joneja, Juan Palazzo
Archives of Pathology & Laboratory Medicine.2023; 147(1): 19. CrossRef - Pure Mucinous Carcinoma of the Breast: Radiologic-Pathologic Correlation
Cherie M Kuzmiak, Benjamin C Calhoun
Journal of Breast Imaging.2023; 5(2): 180. CrossRef - On Ultrasonographic Features of Mucinous Carcinoma with Micropapillary Pattern
Wei-Sen Yang, Yang Li, Ya Gao
Breast Cancer: Targets and Therapy.2023; Volume 15: 473. CrossRef - Micropapillary Breast Carcinoma: From Molecular Pathogenesis to Prognosis
Georgios-Ioannis Verras, Levan Tchabashvili, Francesk Mulita, Ioanna Maria Grypari, Sofia Sourouni, Evangelia Panagodimou, Maria-Ioanna Argentou
Breast Cancer: Targets and Therapy.2022; Volume 14: 41. CrossRef - Mucinous carcinoma of the breast: distinctive histopathologic and genetic characteristics
Minjung Jung
Kosin Medical Journal.2022; 37(3): 176. CrossRef - Triple-Positive Breast Carcinoma: Histopathologic Features and Response to Neoadjuvant Chemotherapy
Jennifer Zeng, Marcia Edelweiss, Dara S. Ross, Bin Xu, Tracy-Ann Moo, Edi Brogi, Timothy M. D'Alfonso
Archives of Pathology & Laboratory Medicine.2021; 145(6): 728. CrossRef - HER2 positive mucinous carcinoma of breast with micropapillary features: Report of a case and review of literature
Dinesh Chandra Doval, Rupal Tripathi, Sunil Pasricha, Pankaj Goyal, Chaturbhuj Agrawal, Anurag Mehta
Human Pathology: Case Reports.2021; 25: 200531. CrossRef - Sonographic Features of Pure Mucinous Breast Carcinoma With Micropapillary Pattern
Wu Zhou, Yong-Zhong Li, Li-Min Gao, Di-Ming Cai
Frontiers in Oncology.2021;[Epub] CrossRef - Clinicopathologic characteristics of HER2-positive pure mucinous carcinoma of the breast
Yunjeong Jang, Hera Jung, Han-Na Kim, Youjeong Seo, Emad Alsharif, Seok Jin Nam, Seok Won Kim, Jeong Eon Lee, Yeon Hee Park, Eun Yoon Cho, Soo Youn Cho
Journal of Pathology and Translational Medicine.2020; 54(1): 95. CrossRef - Mucinous carcinoma with micropapillary features is morphologically, clinically and genetically distinct from pure mucinous carcinoma of breast
Peng Sun, Zaixuan Zhong, Qianyi Lu, Mei Li, Xue Chao, Dan Chen, Wenyan Hu, Rongzhen Luo, Jiehua He
Modern Pathology.2020; 33(10): 1945. CrossRef - Micropapillary pattern in pure mucinous carcinoma of the breast – does it matter or not?
Xiaoli Xu, Rui Bi, Ruohong Shui, Baohua Yu, Yufan Cheng, Xiaoyu Tu, Wentao Yang
Histopathology.2019; 74(2): 248. CrossRef - An Update of Mucinous Lesions of the Breast
Beth T. Harrison, Deborah A. Dillon
Surgical Pathology Clinics.2018; 11(1): 61. CrossRef - The clinicopathological significance of micropapillary pattern in colorectal cancers
Jung-Soo Pyo, Mee Ja Park, Dong-Wook Kang
Human Pathology.2018; 77: 159. CrossRef - The sonographic findings of micropapillary pattern in pure mucinous carcinoma of the breast
Heqing Zhang, Li Qiu, Yulan Peng
World Journal of Surgical Oncology.2018;[Epub] CrossRef - Diagnostic dilemma of micropapillary variant of mucinous breast cancer
Geok Hoon Lim, Zhiyan Yan, Mihir Gudi
BMJ Case Reports.2018; 2018: bcr-2018-225775. CrossRef
- Pure mucinous adenocarcinoma of the breast with the rare lymphoplasmacytic infiltration: A case report with review of literature
- Overexpression of POSTN in Tumor Stroma Is a Poor Prognostic Indicator of Colorectal Cancer
- Hyeon Jeong Oh, Jeong Mo Bae, Xian-Yu Wen, Nam-Yun Cho, Jung Ho Kim, Gyeong Hoon Kang
- J Pathol Transl Med. 2017;51(3):306-313. Published online April 12, 2017
- DOI: https://doi.org/10.4132/jptm.2017.01.19
- 15,891 View
- 258 Download
- 48 Web of Science
- 45 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Tumor microenvironment has recently drawn attention in that it is related with tumor prognosis. Cancer-associated fibroblast also plays a critical role in cancer invasiveness and progression in colorectal cancers. Periostin (POSTN), originally identified to be expressed in osteoblasts and osteoblast-derived cells, is expressed in cancer-associated fibroblasts in several tissue types of cancer. Recent studies suggest an association between stromal overexpression of POSTN and poor prognosis of cancer patients.
Methods
We analyzed colorectal cancer cases for their expression status of POSTN in tumor stroma using immunohistochemistry and correlated the expression status with clinicopathological and molecular features.
Results
High level of POSTN expression in tumor stroma was closely associated with tumor location in proximal colon, infiltrative growth pattern, undifferentiated histology, tumor budding, luminal necrosis, and higher TNM stage. High expression status of POSTN in tumor stroma was found to be an independent prognostic parameter implicating poor 5-year cancer-specific survival and 5-year progression-free survival.
Conclusions
Our findings suggest that POSTN overexpression in tumor stroma of colorectal cancers could be a possible candidate marker for predicting poor prognosis in patients with colorectal cancers. -
Citations
Citations to this article as recorded by- Unmasking a Recessive Allele by a Rare Interstitial Deletion at 10q26.13q26.2: Prenatal Diagnosis of MMP21 ‐Related Disorder and Further Refine INSYN2A Involvement in the Postnatal Cognitive Phenotype
Jiasun Su, Shujie Zhang, Wei Li, Yuan Wei, Fei Lin, Chaofan Zhou, Xianglian Tang, Yueyun Lan, Minpan Huang, Qiang Zhang, Shang Yi, Qi Yang, Sheng Yi, Xunzhao Zhou, Zailong Qin, Peng Huang
Molecular Genetics & Genomic Medicine.2025;[Epub] CrossRef - Periostin from Tumor Stromal Cells Might Be Associated with Malignant Progression of Colorectal Cancer via Smad2/3
Canfeng Fan, Qiang Wang, Saki Kanei, Kyoka Kawabata, Hinano Nishikubo, Rika Aoyama, Zhonglin Zhu, Daiki Imanishi, Takashi Sakuma, Koji Maruo, Gen Tsujio, Yurie Yamamoto, Tatsunari Fukuoka, Masakazu Yashiro
Cancers.2025; 17(3): 551. CrossRef - Attributes of HPV associated cancers
Ashi Robert Thobias, Mrugdha Patel, Chirag Vaghela, Prabhudas Shankarbhai Patel
Clinical and Translational Oncology.2025; 27(11): 4131. CrossRef - The association between periostin and tumor microenvironment: a promising cancer prognostic biomarker and therapeutic target to combat tumor progression and chemoresistance
Mohsen Nabi-Afjadi, Farnoosh Farzam, Sina Soltani, Nafiseh Golestani, Shiva Sarani Asl, Fatemeh Aziziyan, Hamidreza Zalpoor, Fatemeh Ghasemi, Bahareh Dabirmanesh, Khosro Khajeh
Cancer Cell International.2025;[Epub] CrossRef - Cancer-associated fibroblasts and their prognostic role in colorectal cancer: review and meta-analysis
Silvia Mihaela Ilie, Laurentia Gales, Ana Maria Musina, Kevin Rouet, Alexandra Elena Stefan, Mara Simona Belceanu, Anda Stefania Trandafir, Constantin Guillaume, Aurélie Bertaut, Ikram Charifi, Adel Cueff, Valentin Derangère, Francois Ghiringhelli
Frontiers in Oncology.2025;[Epub] CrossRef - Electroanalytical Immunotool to Determine Matricellular Protein Periostin, a Stromal Biomarker of Prognosis in Colorectal Cancer
Marina Blázquez‐García, Jennifer Quinchia, Víctor Ruiz‐Valdepeñas Montiel, Rebeca M. Torrente‐Rodríguez, Verónica Serafín, María Garranzo‐Asensio, Ana García‐Romero, Jahir Orozco, Rodrigo Barderas, José M. Pingarrón, Susana Campuzano
ChemElectroChem.2024;[Epub] CrossRef - Simultaneous Expression of CD70 and POSTN in Cancer-Associated Fibroblasts Predicts Worse Survival of Colorectal Cancer Patients
Masayuki Komura, Chengbo Wang, Sunao Ito, Shunsuke Kato, Akane Ueki, Masahide Ebi, Naotaka Ogasawara, Toyonori Tsuzuki, Kenji Kasai, Kunio Kasugai, Shuji Takiguchi, Satoru Takahashi, Shingo Inaguma
International Journal of Molecular Sciences.2024; 25(5): 2537. CrossRef - The combined tumour-based Fascin/Snail and stromal periostin reveals the effective prognosis prediction in colorectal cancer patients
Niphat Jirapongwattana, Suyanee Thongchot, Ananya Pongpaibul, Atthaphorn Trakarnsanga, Jean Quinn, Peti Thuwajit, Chanitra Thuwajit, Joanne Edwards, Peng Zhang
PLOS ONE.2024; 19(6): e0304666. CrossRef - SPOCK1 and POSTN are valuable prognostic biomarkers and correlate with tumor immune infiltrates in colorectal cancer
Caiqin Gan, Mengting Li, Yuanyuan Lu, Ganjing Peng, Wenjie Li, Haizhou Wang, Yanan Peng, Qian Hu, Wanhui Wei, Fan Wang, Lan Liu, Qiu Zhao
BMC Gastroenterology.2023;[Epub] CrossRef - Stromal POSTN Enhances Motility of Both Cancer and Stromal Cells and Predicts Poor Survival in Colorectal Cancer
Akane Ueki, Masayuki Komura, Akira Koshino, Chengbo Wang, Kazuhiro Nagao, Mai Homochi, Yuki Tsukada, Masahide Ebi, Naotaka Ogasawara, Toyonori Tsuzuki, Kenji Kasai, Kunio Kasugai, Satoru Takahashi, Shingo Inaguma
Cancers.2023; 15(3): 606. CrossRef - POSTN Secretion by Extracellular Matrix Cancer-Associated Fibroblasts (eCAFs) Correlates with Poor ICB Response via Macrophage Chemotaxis Activation of Akt Signaling Pathway in Gastric Cancer
Tingting You, Hui Tang, Wenjing Wu, Jingxi Gao, Xuechun Li, Ningning Li, Xiuxiu Xu, Jiazhang Xing, Hui Ge, Yi Xiao, Junchao Guo, Bin Wu, Xiaoyi Li, Liangrui Zhou, Lin Zhao, Chunmei Bai, Qin Han, Zhao Sun, Robert Chunhua Zhao
Aging and disease.2023; 14(6): 2177. CrossRef - A Pan-cancer Analysis Reveals the Tissue Specificity and Prognostic Impact
of Angiogenesis-associated Genes in Human Cancers
Zhenshen Bao, Minzhen Liao, Wanqi Dong, Yanhao Huo, Xianbin Li, Peng Xu, Wenbin Liu
Current Bioinformatics.2023; 18(8): 670. CrossRef - Cancer‐associated stroma reveals prognostic biomarkers and novel insights into the tumour microenvironment of colorectal cancer and colorectal liver metastases
Kai M. Brown, Aiqun Xue, Ross C. Smith, Jaswinder S. Samra, Anthony J. Gill, Thomas J. Hugh
Cancer Medicine.2022; 11(2): 492. CrossRef - Periostin in Angiogenesis and Inflammation in CRC—A Preliminary Observational Study
Agnieszka Kula, Miriam Dawidowicz, Sylwia Mielcarska, Paweł Kiczmer, Magdalena Chrabańska, Magdalena Rynkiewicz, Elżbieta Świętochowska, Dariusz Waniczek
Medicina.2022; 58(1): 96. CrossRef - Periostin promotes the proliferation and metastasis of osteosarcoma by increasing cell survival and activates the PI3K/Akt pathway
Chaojian Xu, Ziyue Wang, Long Zhang, Yi Feng, Jia Lv, Zhuangzhuang Wu, Rong Yang, Taiyong Wu, Jian Li, Ruhao Zhou, Zhi Tian, Junjun Bai, Huadong Zhang, Yanping Lan, Zhi Lv
Cancer Cell International.2022;[Epub] CrossRef - Periostin in lymph node pre-metastatic niches governs lymphatic endothelial cell functions and metastatic colonization
Lionel Gillot, Alizée Lebeau, Louis Baudin, Charles Pottier, Thomas Louis, Tania Durré, Rémi Longuespée, Gabriel Mazzucchelli, Christophe Nizet, Silvia Blacher, Frédéric Kridelka, Agnès Noël
Cellular and Molecular Life Sciences.2022;[Epub] CrossRef - Prognostic impact of stromal periostin expression in upper urinary tract urothelial carcinoma
Kosuke Miyai, Kazuki Kawamura, Keiichi Ito, Susumu Matsukuma, Hitoshi Tsuda
BMC Cancer.2022;[Epub] CrossRef - Periostin‐ and podoplanin‐positive cancer‐associated fibroblast subtypes cooperate to shape the inflamed tumor microenvironment in aggressive pancreatic adenocarcinoma
Cindy Neuzillet, Rémy Nicolle, Jérôme Raffenne, Annemilaï Tijeras‐Raballand, Alexia Brunel, Lucile Astorgues‐Xerri, Sophie Vacher, Floriane Arbateraz, Marjorie Fanjul, Marc Hilmi, Rémi Samain, Christophe Klein, Aurélie Perraud, Vinciane Rebours, Muriel Ma
The Journal of Pathology.2022; 258(4): 408. CrossRef - Periostin: biology and function in cancer
Shima Dorafshan, Mahdieh Razmi, Sadegh Safaei, Erica Gentilin, Zahra Madjd, Roya Ghods
Cancer Cell International.2022;[Epub] CrossRef - Periostin as a key molecule defining desmoplastic environment in colorectal cancer
Takahiro Sueyama, Yoshiki Kajiwara, Satsuki Mochizuki, Hideyuki Shimazaki, Eiji Shinto, Kazuo Hase, Hideki Ueno
Virchows Archiv.2021; 478(5): 865. CrossRef - Expression Patterns of Microenvironmental Factors and Tenascin-C at the Invasive Front of Stage II and III Colorectal Cancer: Novel Tumor Prognostic Markers
Mai Hashimoto, Noriyuki Uesugi, Mitsumasa Osakabe, Naoki Yanagawa, Koki Otsuka, Yoshiki Kajiwara, Hideki Ueno, Akira Sasaki, Tamotsu Sugai
Frontiers in Oncology.2021;[Epub] CrossRef - Deregulation of extracellular matrix modeling with molecular prognostic markers revealed by transcriptome sequencing and validations in Oral Tongue squamous cell carcinoma
Soundara Viveka Thangaraj, Vidyarani Shyamsundar, Arvind Krishnamurthy, Vijayalakshmi Ramshankar
Scientific Reports.2021;[Epub] CrossRef - Inhibition of Postn Rescues Myogenesis Defects in Myotonic Dystrophy Type 1 Myoblast Model
Xiaopeng Shen, Zhongxian Liu, Chunguang Wang, Feng Xu, Jingyi Zhang, Meng Li, Yang Lei, Ao Wang, Chao Bi, Guoping Zhu
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - Radiographical assessment of tumour stroma and treatment outcomes using deep learning: a retrospective, multicohort study
Yuming Jiang, Xiaokun Liang, Zhen Han, Wei Wang, Sujuan Xi, Tuanjie Li, Chuanli Chen, Qingyu Yuan, Na Li, Jiang Yu, Yaoqin Xie, Yikai Xu, Zhiwei Zhou, George A Poultsides, Guoxin Li, Ruijiang Li
The Lancet Digital Health.2021; 3(6): e371. CrossRef - Periostin expression and its supposed roles in benign and malignant thyroid nodules: an immunohistochemical study of 105 cases
Kimihide Kusafuka, Masaru Yamashita, Tomohiro Iwasaki, Chinatsu Tsuchiya, Aki Kubota, Kazuki Hirata, Akinori Murakami, Aya Muramatsu, Kazumori Arai, Makoto Suzuki
Diagnostic Pathology.2021;[Epub] CrossRef - Cancer-associated fibroblasts in colorectal cancer
S. Kamali Zonouzi, P. S. Pezeshki, S. Razi, N. Rezaei
Clinical and Translational Oncology.2021; 24(5): 757. CrossRef - Prognostic value of periostin in multiple solid cancers: A systematic review with meta‐analysis
Tao Yang, Zhengdong Deng, Zhongya Pan, Yawei Qian, Wei Yao, Jianming Wang
Journal of Cellular Physiology.2020; 235(3): 2800. CrossRef - Serum periostin is associated with cancer mortality but not cancer risk in older home-dwelling men: A 8-year prospective analysis of the STRAMBO study
Jean-Charles Rousseau, Cindy Bertholon, Roland Chapurlat, Pawel Szulc
Bone.2020; 132: 115184. CrossRef - Systematic prediction of key genes for ovarian cancer by co‐expression network analysis
Mingyuan Wang, Jinjin Wang, Jinglan Liu, Lili Zhu, Heng Ma, Jiang Zou, Wei Wu, Kangkai Wang
Journal of Cellular and Molecular Medicine.2020; 24(11): 6298. CrossRef - Upregulation of adipocyte enhancer‐binding protein 1 in endothelial cells promotes tumor angiogenesis in colorectal cancer
Akira Yorozu, Eiichiro Yamamoto, Takeshi Niinuma, Akihiro Tsuyada, Reo Maruyama, Hiroshi Kitajima, Yuto Numata, Masahiro Kai, Gota Sudo, Toshiyuki Kubo, Toshihiko Nishidate, Kenji Okita, Ichiro Takemasa, Hiroshi Nakase, Tamotsu Sugai, Kenichi Takano, Hiro
Cancer Science.2020; 111(5): 1631. CrossRef - Periostin aggravates NLRP3 inflammasome-mediated pyroptosis in myocardial ischemia-reperfusion injury
Lei Yao, Jie Song, Xiao wen Meng, Jian yun Ge, Bo xiang Du, Jun Yu, Fu hai Ji
Molecular and Cellular Probes.2020; 53: 101596. CrossRef - Vitamin K and Kidney Transplantation
Maria Fusaro, Laura Cosmai, Pieter Evenepoel, Thomas L. Nickolas, Angela M. Cheung, Andrea Aghi, Giovanni Tripepi, Mario Plebani, Giorgio Iervasi, Roberto Vettor, Martina Zaninotto, Maura Ravera, Marina Foramitti, Sandro Giannini, Stefania Sella, Maurizio
Nutrients.2020; 12(9): 2717. CrossRef - Periostin regulates autophagy through integrin α5β1 or α6β4 and an AKT‐dependent pathway in colorectal cancer cell migration
Suyanee Thongchot, Ekapot Singsuksawat, Nuttavut Sumransub, Ananya Pongpaibul, Attaporn Trakarnsanga, Peti Thuwajit, Chanitra Thuwajit
Journal of Cellular and Molecular Medicine.2020; 24(21): 12421. CrossRef - Genomic, transcriptomic, and viral integration profiles associated with recurrent/metastatic progression in high‐risk human papillomavirus cervical carcinomas
Jing Jing Liu, Jung Yoon Ho, Jung Eum Lee, Soo Young Hur, Jinseon Yoo, Kyu Ryung Kim, Daeun Ryu, Tae Min Kim, Youn Jin Choi
Cancer Medicine.2020; 9(21): 8243. CrossRef - Periostin Secreted by Carcinoma-Associated Fibroblasts Promotes Ovarian Cancer Cell Platinum Resistance Through the PI3K/Akt Signaling Pathway
Lei Chu, Fangce Wang, Wenjun Zhang, Huai-fang Li, Jun Xu, Xiao-wen Tong
Technology in Cancer Research & Treatment.2020;[Epub] CrossRef - Overexpression of periostin is positively associated with gastric cancer metastasis through promoting tumor metastasis and invasion
Hai Zhong, Xiang Li, Junhua Zhang, Xu Wu
Journal of Cellular Biochemistry.2019; 120(6): 9927. CrossRef - Genes associated with bowel metastases in ovarian cancer
Andrea Mariani, Chen Wang, Ann L. Oberg, Shaun M. Riska, Michelle Torres, Joseph Kumka, Francesco Multinu, Gunisha Sagar, Debarshi Roy, Deok–Beom Jung, Qing Zhang, Tommaso Grassi, Daniel W. Visscher, Vatsal P. Patel, Ling Jin, Julie K. Staub, William A. C
Gynecologic Oncology.2019; 154(3): 495. CrossRef - Periostin: A Matricellular Protein With Multiple Functions in Cancer Development and Progression
Laura González-González, Javier Alonso
Frontiers in Oncology.2018;[Epub] CrossRef - Upregulation of Periostin expression in the pathogenesis of ameloblastoma
Yuanyuan Kang, Jie Liu, Ying Zhang, Yan Sun, Junting Wang, Biying Huang, Ming Zhong
Pathology - Research and Practice.2018; 214(12): 1959. CrossRef - Periostin expression in neoplastic and non-neoplastic diseases of bone and joint
Jennifer M. Brown, Akiro Mantoku, Afsie Sabokbar, Udo Oppermann, A. Bass Hassan, Akiro Kudo, Nick Athanasou
Clinical Sarcoma Research.2018;[Epub] CrossRef - Molecular patterns of cancer colonisation in lymph nodes of breast cancer patients
Gaurav Chatterjee, Trupti Pai, Thomas Hardiman, Kelly Avery-Kiejda, Rodney J. Scott, Jo Spencer, Sarah E. Pinder, Anita Grigoriadis
Breast Cancer Research.2018;[Epub] CrossRef - Multiplicity of Advanced T Category–Tumors Is a Risk Factor for Survival in Patients with Colorectal Carcinoma
Hye Eun Park, Seungyeon Yoo, Jeong Mo Bae, Seorin Jeong, Nam-Yun Cho, Gyeong Hoon Kang
Journal of Pathology and Translational Medicine.2018; 52(6): 386. CrossRef - Periostin attenuates tumor growth by inducing apoptosis in colitis-related colorectal cancer
Yusuke Shimoyama, Keiichi Tamai, Rie Shibuya, Mao Nakamura, Mai Mochizuki, Kazunori Yamaguchi, Yoichi Kakuta, Yoshitaka Kinouchi, Ikuro Sato, Akira Kudo, Tooru Shimosegawa, Kennichi Satoh
Oncotarget.2018; 9(28): 20008. CrossRef - Periostin serves an important role in the pathogenesis of oral squamous cell carcinoma
Yuanyuan Kang, Xue Wang, Ying Zhang, Yan Sun
Oncology Letters.2018;[Epub] CrossRef - The prognostic significance of cancer-associated fibroblasts in pancreatic ductal adenocarcinoma
Hyunjin Park, Yangkyu Lee, Hyejung Lee, Jin-Won Kim, Jin-Hyeok Hwang, Jaihwan Kim, Yoo-Seok Yoon, Ho-Seong Han, Haeryoung Kim
Tumor Biology.2017; 39(10): 101042831771840. CrossRef
- Unmasking a Recessive Allele by a Rare Interstitial Deletion at 10q26.13q26.2: Prenatal Diagnosis of MMP21 ‐Related Disorder and Further Refine INSYN2A Involvement in the Postnatal Cognitive Phenotype
- Molecular Testing for Gastrointestinal Cancer
- Hye Seung Lee, Woo Ho Kim, Yoonjin Kwak, Jiwon Koh, Jeong Mo Bae, Kyoung-Mee Kim, Mee Soo Chang, Hye Seung Han, Joon Mee Kim, Hwal Woong Kim, Hee Kyung Chang, Young Hee Choi, Ji Y. Park, Mi Jin Gu, Min Jin Lhee, Jung Yeon Kim, Hee Sung Kim, Mee-Yon Cho
- J Pathol Transl Med. 2017;51(2):103-121. Published online February 19, 2017
- DOI: https://doi.org/10.4132/jptm.2017.01.24
- 22,747 View
- 910 Download
- 62 Web of Science
- 54 Crossref
-
 Abstract
Abstract
 PDF
PDF - With recent advances in molecular diagnostic methods and targeted cancer therapies, several molecular tests have been recommended for gastric cancer (GC) and colorectal cancer (CRC). Microsatellite instability analysis of gastrointestinal cancers is performed to screen for Lynch syndrome, predict favorable prognosis, and screen patients for immunotherapy. The epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor has been approved in metastatic CRCs with wildtype RAS (KRAS and NRAS exon 2–4). A BRAF mutation is required for predicting poor prognosis. Additionally, amplification of human epidermal growth factor receptor 2 (HER2) and MET is also associated with resistance to EGFR inhibitor in metastatic CRC patients. The BRAF V600E mutation is found in sporadic microsatellite unstable CRCs, and thus is helpful for ruling out Lynch syndrome. In addition, the KRAS mutation is a prognostic biomarker and the PIK3CA mutation is a molecular biomarker predicting response to phosphoinositide 3-kinase/AKT/mammalian target of rapamycin inhibitors and response to aspirin therapy in CRC patients. Additionally, HER2 testing should be performed in all recurrent or metastatic GCs. If the results of HER2 immunohistochemistry are equivocal, HER2 silver or fluorescence in situ hybridization testing are essential for confirmative determination of HER2 status. Epstein-Barr virus–positive GCs have distinct characteristics, including heavy lymphoid stroma, hypermethylation phenotype, and high expression of immune modulators. Recent advances in next-generation sequencing technologies enable us to examine various genetic alterations using a single test. Pathologists play a crucial role in ensuring reliable molecular testing and they should also take an integral role between molecular laboratories and clinicians.
-
Citations
Citations to this article as recorded by- Spatial and Temporal Tumor Heterogeneity in Gastric Cancer: Discordance of Predictive Biomarkers
Hye Seung Lee
Journal of Gastric Cancer.2025; 25(1): 192. CrossRef - Korean Practice Guidelines for Gastric Cancer 2024: An Evidence-based, Multidisciplinary Approach (Update of 2022 Guideline)
In-Ho Kim, Seung Joo Kang, Wonyoung Choi, An Na Seo, Bang Wool Eom, Beodeul Kang, Bum Jun Kim, Byung-Hoon Min, Chung Hyun Tae, Chang In Choi, Choong-kun Lee, Ho Jung An, Hwa Kyung Byun, Hyeon-Su Im, Hyung-Don Kim, Jang Ho Cho, Kyoungjune Pak, Jae-Joon Kim
Journal of Gastric Cancer.2025; 25(1): 5. CrossRef - Elucidating the Role of KRAS, NRAS, and BRAF Mutations and Microsatellite Instability in Colorectal Cancer via Next-Generation Sequencing
Marta Rada Rodríguez, Bárbara Angulo Biedma, Irene Rodríguez Pérez, Javier Azúa Romeo
Cancers.2025; 17(13): 2071. CrossRef - Chitosan and Its Derivative‐Based Nanoparticles in Gastrointestinal Cancers: Molecular Mechanisms of Action and Promising Anticancer Strategies
Zahra Shokati Eshkiki, Fatemeh Mansouri, Amir Reza Karamzadeh, Abolfazl Namazi, Hafez Heydari, Javad Akhtari, Seidamir Pasha Tabaeian, Abolfazl Akbari, Hongda Liu
Journal of Clinical Pharmacy and Therapeutics.2024;[Epub] CrossRef - Colorectal Cancer: Genetic Underpinning and Molecular Therapeutics for Precision Medicine
Gideon T. Dosunmu, Ardaman Shergill
Genes.2024; 15(5): 538. CrossRef - Effector Function Characteristics of Exhausted CD8+ T-Cell in Microsatellite Stable and Unstable Gastric Cancer
Dong-Seok Han, Yoonjin Kwak, Seungho Lee, Soo Kyung Nam, Seong-Ho Kong, Do Joong Park, Hyuk-Joon Lee, Nak-Jung Kwon, Hye Seung Lee, Han-Kwang Yang
Cancer Research and Treatment.2024; 56(4): 1146. CrossRef - A Standardized Pathology Report for Gastric Cancer: 2nd Edition
Young Soo Park, Myeong-Cherl Kook, Baek-hui Kim, Hye Seung Lee, Dong-Wook Kang, Mi-Jin Gu, Ok Ran Shin, Younghee Choi, Wonae Lee, Hyunki Kim, In Hye Song, Kyoung-Mee Kim, Hee Sung Kim, Guhyun Kang, Do Youn Park, So-Young Jin, Joon Mee Kim, Yoon Jung Choi,
Journal of Gastric Cancer.2023; 23(1): 107. CrossRef - A standardized pathology report for gastric cancer: 2nd edition
Young Soo Park, Myeong-Cherl Kook, Baek-hui Kim, Hye Seung Lee, Dong-Wook Kang, Mi-Jin Gu, Ok Ran Shin, Younghee Choi, Wonae Lee, Hyunki Kim, In Hye Song, Kyoung-Mee Kim, Hee Sung Kim, Guhyun Kang, Do Youn Park, So-Young Jin, Joon Mee Kim, Yoon Jung Choi,
Journal of Pathology and Translational Medicine.2023; 57(1): 1. CrossRef - Korean Practice Guidelines for Gastric Cancer 2022: An Evidence-based, Multidisciplinary Approach
Tae-Han Kim, In-Ho Kim, Seung Joo Kang, Miyoung Choi, Baek-Hui Kim, Bang Wool Eom, Bum Jun Kim, Byung-Hoon Min, Chang In Choi, Cheol Min Shin, Chung Hyun Tae, Chung sik Gong, Dong Jin Kim, Arthur Eung-Hyuck Cho, Eun Jeong Gong, Geum Jong Song, Hyeon-Su Im
Journal of Gastric Cancer.2023; 23(1): 3. CrossRef - Influence of location-dependent sex difference on PD-L1, MMR/MSI, and EGFR in colorectal carcinogenesis
Jina Choi, Nayoung Kim, Ryoung Hee Nam, Jin Won Kim, Chin-Hee Song, Hee Young Na, Gyeong Hoon Kang, Alvaro Galli
PLOS ONE.2023; 18(2): e0282017. CrossRef - Comprehensive Analysis of Epigenetic Associated Genes with Differential
Gene Expression and Prognosis in Gastric Cancer
Songlin An, Xinbao Li, Bing Li, Yan Li
Combinatorial Chemistry & High Throughput Screening.2023; 26(3): 527. CrossRef - Liquid Biopsy in Advanced Colorectal Cancer: Clinical Applications of Different Analytes
Marco Donatello Delcuratolo, Andrea Modrego-Sánchez, Maristella Bungaro, Beatriz Antón-Pascual, Santiago Teran, Valentina Dipace, Silvia Novello, Rocio Garcia-Carbonero, Francesco Passiglia, Cristina Graválos-Castro
Journal of Molecular Pathology.2023; 4(3): 128. CrossRef - Exosomal circ_0001190 Regulates the Progression of Gastric Cancer via miR-586/SOSTDC1 Axis
Chao Liu, Jing Yang, Fengchi Zhu, Zhiying Zhao, Lixue Gao
Biochemical Genetics.2022; 60(6): 1895. CrossRef - Optimization of pre‐analytical and analytical steps for DNA and RNA analysis of fresh cytology samples
Ana Dolinar, Gašper Grubelnik, Irena Srebotnik‐Kirbiš, Margareta Strojan Fležar, Margareta Žlajpah
Cancer Medicine.2022; 11(21): 4021. CrossRef - Retracted: Connexin 43 upregulation by dioscin‐inhibited gastric cancer metastasis by suppressing PI3K/Akt pathway
Food Science & Nutrition.2022;[Epub] CrossRef - Molecular Pathology of Gastric Cancer
Moonsik Kim, An Na Seo
Journal of Gastric Cancer.2022; 22(4): 264. CrossRef - Case report: Undifferentiated sarcoma with multiple tumors involved in Lynch syndrome: Unexpected favorable outcome to sintilimab combined with chemotherapy
Jiaying Liu, Xiaona Chang, Guixiang Xiao, Jingmin Zhong, Bo Huang, Jiwei Zhang, Beibei Gao, Gang Peng, Xiu Nie
Frontiers in Oncology.2022;[Epub] CrossRef - The SUMO E3 ligase CBX4 is identified as a poor prognostic marker of gastric cancer through multipronged OMIC analyses
Yi Pan, Qingshang Li, Zhijun Cao, Shuliang Zhao
Genes & Diseases.2021; 8(6): 827. CrossRef - Worldwide variation in lynch syndrome screening: case for universal screening in low colorectal cancer prevalence areas
George Kunnackal John, Vipin Das Villgran, Christine Caufield-Noll, Francis Giardiello
Familial Cancer.2021; 20(2): 145. CrossRef - Tamoxifen Downregulates the Expression of Notch1 and DLL1 Genes in MKN-45 Gastric Cancer Cells
Faranak Khanipouyani, Hassan Akrami
Journal of Gastrointestinal Cancer.2021; 52(3): 922. CrossRef - Kallikrein-11, in Association with Coiled-Coil Domain Containing 25, as a Potential Prognostic Marker for Cholangiocarcinoma with Lymph Node Metastasis
Saeranee Siriphak, Ravinnipa Chanakankun, Tanakorn Proungvitaya, Sittiruk Roytrakul, Doungdean Tummanatsakun, Wunchana Seubwai, Molin Wongwattanakul, Siriporn Proungvitaya
Molecules.2021; 26(11): 3105. CrossRef - ISH-based HER2 diagnostics
Josef Rüschoff, Iris Nagelmeier, Bharat Jasani, Oliver Stoss
Der Pathologe.2021; 42(S1): 62. CrossRef - Identification and Analysis of Key Genes Driving Gastric Cancer Through Bioinformatics
Zhao Liu, Shihai Liu, Jing Guo, Libin Sun, Shasha Wang, Yixuan Wang, Wensheng Qiu, Jing Lv
Genetic Testing and Molecular Biomarkers.2021; 25(1): 1. CrossRef - Microsatellite Instability in Colorectal Cancer Liquid Biopsy—Current Updates on Its Potential in Non-Invasive Detection, Prognosis and as a Predictive Marker
Francis Yew Fu Tieng, Nadiah Abu, Learn-Han Lee, Nurul-Syakima Ab Mutalib
Diagnostics.2021; 11(3): 544. CrossRef - Metformin attenuates synergic effect of diabetes mellitus and Helicobacter pylori infection on gastric cancer cells proliferation by suppressing PTEN expression
Huibin Lu, Xinwei Han, Jianzhuang Ren, Kewei Ren, Zongming Li, Quanhui Zhang
Journal of Cellular and Molecular Medicine.2021; 25(10): 4534. CrossRef - Recent Advances in the Diagnosis, Staging, Treatment, and Prognosis of Advanced Gastric Cancer: A Literature Review
Zhi-da Chen, Peng-fei Zhang, Hong-qing Xi, Bo Wei, Lin Chen, Yun Tang
Frontiers in Medicine.2021;[Epub] CrossRef - Tumor immune response and immunotherapy in gastric cancer
Yoonjin Kwak, An Na Seo, Hee Eun Lee, Hye Seung Lee
Journal of Pathology and Translational Medicine.2020; 54(1): 20. CrossRef - Comparative analysis of HER2 copy number between plasma and tissue samples in gastric cancer using droplet digital PCR
Boram Kim, Soo Kyung Nam, Soo Hyun Seo, Kyoung Un Park, Sang-Hoon Ahn, Do Joong Park, Hyung-Ho Kim, Woo Ho Kim, Hye Seung Lee
Scientific Reports.2020;[Epub] CrossRef - Differential prognostic impact of CD8+ T cells based on human leucocyte antigen I and PD-L1 expression in microsatellite-unstable gastric cancer
Yoonjin Kwak, Jiwon Koh, Yujun Park, Yun Ji Hong, Kyoung Un Park, Hyung-Ho Kim, Do Joong Park, Sang-Hoon Ahn, Woo Ho Kim, Hye Seung Lee
British Journal of Cancer.2020; 122(9): 1399. CrossRef - High-Accuracy Determination of Microsatellite Instability Compatible with Liquid Biopsies
Amanda Bortolini Silveira, François-Clément Bidard, Amélie Kasperek, Samia Melaabi, Marie-Laure Tanguy, Manuel Rodrigues, Guillaume Bataillon, Luc Cabel, Bruno Buecher, Jean-Yves Pierga, Charlotte Proudhon, Marc-Henri Stern
Clinical Chemistry.2020; 66(4): 606. CrossRef - Chitosan: A compound for drug delivery system in gastric cancer-a review
Rana Shafabakhsh, Bahman Yousefi, Zatollah Asemi, Banafsheh Nikfar, Mohammad Ali Mansournia, Jamal Hallajzadeh
Carbohydrate Polymers.2020; 242: 116403. CrossRef - MSI and EBV Positive Gastric Cancer’s Subgroups and Their Link with Novel Immunotherapy
Maria Grazia Rodriquenz, Giandomenico Roviello, Alberto D’Angelo, Daniele Lavacchi, Franco Roviello, Karol Polom
Journal of Clinical Medicine.2020; 9(5): 1427. CrossRef - Theoretical calculations of molecular descriptors for anticancer activities of 1, 2, 3-triazole-pyrimidine derivatives against gastric cancer cell line (MGC-803): DFT, QSAR and docking approaches
Rhoda Oyeladun Oyewole, Abel Kolawole Oyebamiji, Banjo Semire
Heliyon.2020; 6(5): e03926. CrossRef - Identification of a Clinical Cutoff Value for Multiplex KRASG12/G13 Mutation Detection in Colorectal Adenocarcinoma Patients Using Digital Droplet PCR, and Comparison with Sanger Sequencing and PNA Clamping Assay
Kyung Ha Lee, Tae Hee Lee, Min Kyung Choi, In Sun Kwon, Go Eun Bae, Min-Kyung Yeo
Journal of Clinical Medicine.2020; 9(7): 2283. CrossRef - PD-L1 Testing in Gastric Cancer by the Combined Positive Score of the 22C3 PharmDx and SP263 Assay with Clinically Relevant Cut-offs
Yujun Park, Jiwon Koh, Hee Young Na, Yoonjin Kwak, Keun-Wook Lee, Sang-Hoon Ahn, Do Joong Park, Hyung-Ho Kim, Hye Seung Lee
Cancer Research and Treatment.2020; 52(3): 661. CrossRef - Clinical and Molecular Assessment of Patients with Lynch Syndrome and Sarcomas Underpinning the Association with MSH2 Germline Pathogenic Variants
Nathália de Angelis de Carvalho, Bianca Naomi Niitsuma, Vanessa Nascimento Kozak, Felipe D’almeida Costa, Mariana Petaccia de Macedo, Bruna Elisa Catin Kupper, Maria Letícia Gobo Silva, Maria Nirvana Formiga, Sahlua Miguel Volc, Samuel Aguiar Junior, Eden
Cancers.2020; 12(7): 1848. CrossRef - Farnesoid X receptor antagonizes Wnt/β-catenin signaling in colorectal tumorigenesis
Junhui Yu, Shan Li, Jing Guo, Zhengshui Xu, Jianbao Zheng, Xuejun Sun
Cell Death & Disease.2020;[Epub] CrossRef - YAP promotes self-renewal of gastric cancer cells by inhibiting expression of L-PTGDS and PTGDR2
Qingli Bie, Xiaozhe Li, Shiqi Liu, Xiao Yang, Zhenwen Qian, Rou Zhao, Xiaobei Zhang, Bin Zhang
International Journal of Clinical Oncology.2020; 25(12): 2055. CrossRef - ISH-basierte HER2-Diagnostik
Josef Rüschoff, Iris Nagelmeier, Bharat Jasani, Oliver Stoss
Der Pathologe.2020; 41(6): 606. CrossRef - Histone Deacetylase Inhibitor Trichostatin A Suppresses Cell Proliferation and Induces Apoptosis by Regulating the PI3K/AKT Signalling Pathway in Gastric Cancer Cells
Xinli An, Zekun Wei, Botian Ran, Hao Tian, Hongyu Gu, Yan Liu, Hongjuan Cui, Shunqin Zhu
Anti-Cancer Agents in Medicinal Chemistry.2020; 20(17): 2114. CrossRef - Role of Her-2 in Gastrointestinal Tumours beyond Gastric Cancer: A Tool for Precision Medicine
Csongor G. Lengyel, Baker Habeeb, Shah Z. Khan, Khalid El Bairi, Sara C. Altuna, Sadaqat Hussain, Syed Ayub Mazher, Dario Trapani, Angelica Petrillo
Gastrointestinal Disorders.2020; 3(1): 1. CrossRef - Next-generation Sequencing in the Management of Gastric and Esophageal Cancers
Jill C. Rubinstein, Norman G. Nicolson, Nita Ahuja
Surgical Clinics of North America.2019; 99(3): 511. CrossRef - Molecular profile in Paraguayan colorectal cancer patients, towards to a precision medicine strategy
Tania Fleitas-Kanonnikoff, Carolina Martinez‐Ciarpaglini, Josefina Ayala, Cinthia Gauna, Rita Denis, Ita Yoffe, Silvia Sforza, María Teresa Martínez, Alicia Pomata, Maider Ibarrola‐Villava, Sipan Arevshatyan, Verónica Burriel, Diego Boscá, Oscar Pastor, A
Cancer Medicine.2019; 8(6): 3120. CrossRef - Human epidermal growth factor receptor 2-positive digestive tumors
Anna D. Wagner, Berna C. Özdemir, Josef Rüschoff
Current Opinion in Oncology.2019; 31(4): 354. CrossRef - Assessing molecular subtypes of gastric cancer: microsatellite unstable and Epstein-Barr virus subtypes. Methods for detection and clinical and pathological implications
Carolina Martinez-Ciarpaglini, Tania Fleitas-Kanonnikoff, Valentina Gambardella, Marta Llorca, Cristina Mongort, Regina Mengual, Gema Nieto, Lara Navarro, Marisol Huerta, Susana Rosello, Desamparados Roda, Noelia Tarazona, Samuel Navarro, Gloria Ribas, An
ESMO Open.2019; 4(3): e000470. CrossRef - Current and future molecular diagnostics of gastric cancer
Rachel Sin-Yu Choi, Wing Yin Xenia Lai, Lok Ting Claire Lee, Wing Lam Christa Wong, Xiao Meng Pei, Hin Fung Tsang, Joel Johnson Leung, William Chi Shing Cho, Man Kee Maggie Chu, Elaine Yue Ling Wong, Sze Chuen Cesar Wong
Expert Review of Molecular Diagnostics.2019; 19(10): 863. CrossRef - Clinicopathologic significance of human leukocyte antigen class I expression in patients with stage II and III gastric cancer
Yujun Park, Jiwon Koh, Yoonjin Kwak, Sang-Hoon Ahn, Do Joong Park, Hyung-Ho Kim, Woo Ho Kim, Hye Seung Lee
Cancer Immunology, Immunotherapy.2019; 68(11): 1779. CrossRef - Development and Validation of an Easy-to-Implement, Practical Algorithm for the Identification of Molecular Subtypes of Gastric Cancer: Prognostic and Therapeutic Implications
Jiwon Koh, Keun-Wook Lee, Soo Kyung Nam, An Na Seo, Ji-Won Kim, Jin Won Kim, Do Joong Park, Hyung-Ho Kim, Woo Ho Kim, Hye Seung Lee
The Oncologist.2019; 24(12): e1321. CrossRef - Mechanisms and Therapy for Cancer Metastasis to the Brain
Federica Franchino, Roberta Rudà, Riccardo Soffietti
Frontiers in Oncology.2018;[Epub] CrossRef - Status of programmed death-ligand 1 expression in sarcomas
Hyung Kyu Park, Mingi Kim, Minjung Sung, Seung Eun Lee, Yu Jin Kim, Yoon-La Choi
Journal of Translational Medicine.2018;[Epub] CrossRef - Design and synthesis of near-infrared fluorescence-enhancement probes for the cancer-specific enzyme hNQO1
Changyu Zhang, Bei-Bei Zhai, Tao Peng, Zelin Zhong, Lianbin Xu, Qiang-Zhe Zhang, Lu-Yuan Li, Long Yi, Zhen Xi
Dyes and Pigments.2017; 143: 245. CrossRef - Progress in the treatment of advanced gastric cancer
Zheyu Song, Yuanyu Wu, Jiebing Yang, Dingquan Yang, Xuedong Fang
Tumor Biology.2017; 39(7): 101042831771462. CrossRef - Pathologische Einteilung und Diagnostik des Ösophagus- und Magenkarzinoms
S. Förster, A. Tannapfel
Der Gastroenterologe.2017; 12(5): 394. CrossRef - NR4A1-induced increase in the sensitivity of a human gastric cancer line to TNFα-mediated apoptosis is associated with the inhibition of JNK/Parkin-dependent mitophagy
Hongzhu Yan, Feng Xiao, Jue Zou, Chengmin Qiu, Weiwei Sun, Minmin Gu, Li Zhang
International Journal of Oncology.2017;[Epub] CrossRef
- Spatial and Temporal Tumor Heterogeneity in Gastric Cancer: Discordance of Predictive Biomarkers
- Mesothelin Expression in Gastric Adenocarcinoma and Its Relation to Clinical Outcomes
- Song-Hee Han, Mee Joo, Hanseong Kim, Sunhee Chang
- J Pathol Transl Med. 2017;51(2):122-128. Published online February 15, 2017
- DOI: https://doi.org/10.4132/jptm.2016.11.18
- 9,770 View
- 171 Download
- 22 Web of Science
- 20 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Although surgical resection with chemotherapy is considered effective for patients with advanced gastric cancer, it remains the third leading cause of cancer-related death in South Korea. Several studies have reported that mesothelial markers including mesothelin, calretinin, and Wilms tumor protein 1 (WT1) were positive in variable carcinomas, associated with prognosis, and were evaluated as potential markers for targeted therapy. The aim of this study was to assess the immunohistochemical expression of mesothelial markers (mesothelin, calretinin, and WT1) in gastric adenocarcinoma and their relations to clinocopathological features and prognosis. Methods: We evaluated calretinin, WT1, and mesothelin expression by immunohistochemical staining in 117 gastric adenocarcinomas. Results: Mesothelin was positively stained in 30 cases (25.6%). Mesothelin expression was related to increased depth of invasion (p = .002), lymph node metastasis (p = .013), and presence of lymphovascular (p = .015) and perineural invasion (p = .004). Patients with mesothelin expression had significantly worse disease-free survival rate compared with that of nonmesothelin expression group (p = .024). Univariate analysis showed that mesothelin expression is related to short-term survival. None of the 117 gastric adenocarcinomas stained for calretinin or WT1. Conclusions: Mesothelin expression was associated with poor prognosis. Our results suggest that mesothelin-targeted therapy should be considered as an important therapeutic alternative for gastric adenocarcinoma patients with mesothelin expression. -
Citations
Citations to this article as recorded by- Mesothelin as a diagnostic biomarker in endometriosis: A cross-sectional study
Kamil Kiecka, Aleksandra Zygula, Anna Sankiewicz, Mariusz Kuzmicki, Slawomir Lawicki, Michal Ciebiera, Tadeusz Issat, Jan Blaszczyk, Krzysztof Cendrowski, Ewa Gorodkiewicz, Piotr Laudanski
Advances in Medical Sciences.2026; 71(1): 1. CrossRef - High mesothelin expression is associated with low cytotoxic T cell infiltration in pancreatic cancer
Oliver Liang, Amy L. White, Timothy Fielder, Joo-Shik Shin, Sharon M. Sagnella, Ulf Schmitz, Dannel Yeo
Frontiers in Immunology.2025;[Epub] CrossRef - On-target off-tumor toxicity of claudin18.2-directed CAR-T cells in preclinical models
Filippo Birocchi, Antonio J. Almazan, Aiyana Parker, Amanda A. Bouffard, Sadie Goncalves, Christopher Kelly, Jessica Frank, Mark B. Leick, Nicholas J. Haradhvala, Shaw Kagawa, Gad Getz, Giulia Escobar, Diego Salas-Benito, Adele Mucci, Trisha R. Berger, Ma
Nature Communications.2025;[Epub] CrossRef - Novel perspectives on MSLN-targeted cancer therapy: from molecular mechanisms to clinical translation
Zhendong Wu, Xuefei Fu, Yuan Feng, Rong Zeng, Huan Qin, Kai Yao
Cancer Biology & Therapy.2025;[Epub] CrossRef - Persistence of activated anti‐mesothelin hYP218 chimeric antigen receptor T cells in the tumour is associated with efficacy in gastric and colorectal carcinomas
Sameer Mir, Abhilash Venugopalan, Jingli Zhang, Nishanth Ulhas Nair, Manjistha Sengupta, Manakamana Khanal, Chaido Stathopoulou, Qun Jiang, Raffit Hassan
Clinical and Translational Medicine.2024;[Epub] CrossRef - Targeting Mesothelin in Solid Tumours: Anti-mesothelin Antibody and Drug Conjugates
Quincy Chu
Current Oncology Reports.2023; 25(4): 309. CrossRef - High expression of mesothelin in plasma and tissue is associated with poor prognosis and promotes invasion and metastasis in gastric cancer
Suryendu Saha, Chitranjan Mukherjee, Dipjit Basak, Prasun Panja, Pronoy Kanti Mondal, Ranajoy Ghosh, Aniket Halder, Abhijit Chowdhury, Gopal Krishna Dhali, Bitan Kumar Chattopadhyay, Saurabh Ghosh, Somsubhra Nath, Shalini Datta
Advances in Cancer Biology - Metastasis.2023; 7: 100098. CrossRef - Novel Anti-Mesothelin Nanobodies and Recombinant Immunotoxins with Pseudomonas Exotoxin Catalytic Domain for Cancer Therapeutics
Minh Quan Nguyen, Do Hyung Kim, Hye Ji Shim, Huynh Kim Khanh Ta, Thi Luong Vu, Thi Kieu Oanh Nguyen, Jung Chae Lim, Han Choe
Molecules and Cells.2023; 46(12): 764. CrossRef - Immunotherapy for lung cancer: Focusing on chimeric antigen receptor (CAR)-T cell therapy
Tongqing Xue, Xiang Zhao, Kun Zhao, Yan Lu, Juan Yao, Xianguo Ji
Current Problems in Cancer.2022; 46(1): 100791. CrossRef - Cellular Immunotherapy in Gastric Cancer: Adoptive Cell Therapy and Dendritic Cell-Based Vaccination
Elnaz Faghfuri, Mahdi Abdoli Shadbad, Amir Hossein Faghfouri, Narges Soozangar
Immunotherapy.2022; 14(6): 475. CrossRef - Mesothelin Expression in Human Tumors: A Tissue Microarray Study on 12,679 Tumors
Sören Weidemann, Pauline Gagelmann, Natalia Gorbokon, Maximilian Lennartz, Anne Menz, Andreas M. Luebke, Martina Kluth, Claudia Hube-Magg, Niclas C. Blessin, Christoph Fraune, Katharina Möller, Christian Bernreuther, Patrick Lebok, Till S. Clauditz, Frank
Biomedicines.2021; 9(4): 397. CrossRef - Host Mesothelin Expression Increases Ovarian Cancer Metastasis in the Peritoneal Microenvironment
Tyvette S. Hilliard, Brooke Kowalski, Kyle Iwamoto, Elizabeth A. Agadi, Yueying Liu, Jing Yang, Marwa Asem, Yuliya Klymenko, Jeff Johnson, Zonggao Shi, Gifty Marfowaa, Madeleine G. Yemc, Phillip Petrasko, M. Sharon Stack
International Journal of Molecular Sciences.2021; 22(22): 12443. CrossRef - CAR-T Cell Therapy—An Overview of Targets in Gastric Cancer
Dominika Bębnowska, Ewelina Grywalska, Paulina Niedźwiedzka-Rystwej, Barbara Sosnowska-Pasiarska, Jolanta Smok-Kalwat, Marcin Pasiarski, Stanisław Góźdź, Jacek Roliński, Wojciech Polkowski
Journal of Clinical Medicine.2020; 9(6): 1894. CrossRef - Mesothelin-Targeted Recombinant Immunotoxins for Solid Tumors
Brendan L. Hagerty, Guillaume J. Pegna, Jian Xu, Chin-Hsien Tai, Christine Alewine
Biomolecules.2020; 10(7): 973. CrossRef - Phase I/II clinical trial of a Wilms’ tumor 1-targeted dendritic cell vaccination-based immunotherapy in patients with advanced cancer
Wen Zhang, Xu Lu, Peilin Cui, Chunmei Piao, Man Xiao, Xuesong Liu, Yue Wang, Xuan Wu, Jingwei Liu, Lin Yang
Cancer Immunology, Immunotherapy.2019; 68(1): 121. CrossRef - Mesothelin as a target for cervical cancer therapy
Korinna Jöhrens, Lea Lazzerini, Jana Barinoff, Jalid Sehouli, Guenter Cichon
Archives of Gynecology and Obstetrics.2019; 299(1): 211. CrossRef - A targeted proteomics approach reveals a serum protein signature as diagnostic biomarker for resectable gastric cancer
Qiujin Shen, Karol Polom, Coralie Williams, Felipe Marques Souza de Oliveira, Mariana Guergova-Kuras, Frederique Lisacek, Niclas G. Karlsson, Franco Roviello, Masood Kamali-Moghaddam
eBioMedicine.2019; 44: 322. CrossRef - Mesothelin as a biomarker for targeted therapy
Jiang Lv, Peng Li
Biomarker Research.2019;[Epub] CrossRef - Antibody drug conjugates under investigation in phase I and phase II clinical trials for gastrointestinal cancer
Alexis D. Leal, Anuradha Krishnamurthy, Lia Head, Wells A. Messersmith
Expert Opinion on Investigational Drugs.2018; 27(11): 901. CrossRef - Mesothelin‑targeted second generation CAR‑T cells inhibit growth of mesothelin‑expressing tumors in�vivo
Lin Ye, Yuqing Lou, Liming Lu, Xiaohong Fan
Experimental and Therapeutic Medicine.2018;[Epub] CrossRef
- Mesothelin as a diagnostic biomarker in endometriosis: A cross-sectional study
- Aurora Kinase A Is a Prognostic Marker in Colorectal Adenocarcinoma
- Hyun Min Koh, Bo Geun Jang, Chang Lim Hyun, Young Sill Kim, Jin Won Hyun, Weon Young Chang, Young Hee Maeng
- J Pathol Transl Med. 2017;51(1):32-39. Published online December 25, 2016
- DOI: https://doi.org/10.4132/jptm.2016.10.17
- 11,089 View
- 189 Download
- 28 Web of Science
- 29 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Aurora kinase A (AURKA), or STK15/BTAK, is a member of the serine/threonine kinase family and plays important roles in mitosis and chromosome stability. This study investigated the clinical significance of AURKA expression in colorectal cancer patients in Korea.
Methods
AURKA protein expression was evaluated by immunohistochemistry in 151 patients with colorectal adenocarcinoma using tissue microarray blocks. We analyzed the relationship between clinicopathological characteristics and AURKA expression. In addition, the prognostic significance of various clinicopathological data for progression-free survival (PFS) was assessed. Also we evaluated copy number variations by array comparative genomic hybridization and AURKA gene amplification using fluorescence in situ hybridization in colorectal carcinoma tissues.
Results
AURKA gene amplification was found more frequently in the 20q13.2–13.33 gain-positive group than the group with no significant gain on the AURKA-containing locus. AURKA protein expression was detected in 45% of the cases (68/151). Positive staining for AURKA was observed more often in male patients (p = .035) and distally located tumors (p = .021). PFS was shorter in patients with AURKA expression compared to those with low-level AURKA expression (p < .001). Univariate analysis revealed that AURKA expression (p = .001), age (p = .034), lymphatic invasion (p = .001), perineural invasion (p = .002), and TNM stage (p = .013) significantly affected PFS. In a multivariate analysis of PFS, a Cox proportional hazard model confirmed that AURKA expression was an independent and significant prognostic factor in colorectal adenocarcinoma (hazard ratio, 3.944; p < .001).
Conclusions
AURKA could serve as an independent factor to predict a poor prognosis in Korean colorectal adenocarcinoma patients. -
Citations
Citations to this article as recorded by- Exploring pyrimidine scaffolds for cancer therapy: A triad of synthetic chemistry, computational studies, and in-vitro biological testing
Pooja Kumari, Anandkumar Tengli
Journal of Molecular Structure.2026; 1350: 144013. CrossRef - First-in-class dual inhibitors of MASTL and Aurora A kinase: Discovery of selective cyclohexa[b]thiophenes with potent anticancer activity
Somaya A. Abdel-Rahman, Hossam Nada, Moustafa T. Gabr
European Journal of Medicinal Chemistry.2025; 293: 117729. CrossRef - RNAi delivery mediated by milk extracellular vesicles in colon cancer
Jessie Santoro, Silvia Nuzzo, Andrea Soricelli, Marco Salvatore, Anna Maria Grimaldi
Molecular Therapy Nucleic Acids.2025; 36(3): 102644. CrossRef - Potential Signature Therapeutic Biomarkers TOP2A, MAD2L1, and CDK1 in Colorectal Cancer: A Systems Biomedicine-Based Approach
P. Priyamvada, Sudha Ramaiah
Biochemical Genetics.2024; 62(3): 2166. CrossRef - Neutralizing IL-16 enhances the efficacy of targeting Aurora-A therapy in colorectal cancer with high lymphocyte infiltration through restoring anti-tumor immunity
Shiang-Jie Yang, Sheng-Tsung Chang, Kung-Chao Chang, Bo-Wen Lin, Kwang-Yu Chang, Yao-Wen Liu, Ming-Derg Lai, Liang-Yi Hung
Cell Death & Disease.2024;[Epub] CrossRef - Development and validation of epigenetic modification-related signals for the diagnosis and prognosis of colorectal cancer
Xia Li, Jingjing Li, Jie Li, Nannan Liu, Liwei Zhuang
BMC Genomics.2024;[Epub] CrossRef - Recent Updates on Oncogenic Signaling of Aurora Kinases in
Chemosensitive, Chemoresistant Cancers: Novel Medicinal Chemistry
Approaches for Targeting Aurora Kinases
Pooja Kumari, Narasimha Murthy Beeraka, Anandkumar Tengli, Gurupadayya Bannimath, Ramandeep Kaur Baath, Mayuri Patil
Current Medicinal Chemistry.2024; 31(23): 3502. CrossRef - Identification of Aurora A kinase allosteric inhibitors: A comprehensive virtual screening through fingerprint-based similarity search, molecular docking, machine learning and molecular dynamics simulation
Mahima Sudhir Kolpe, Surbhi Pravin Pawar, Vikramsinh Sardarsinh Suryawanshi, Heba Taha M. Abdelghani, Pritee Chunarkar Patil, Shovonlal Bhowmick
Journal of Molecular Liquids.2024; 414: 126115. CrossRef - Exploring Core Genes by Comparative Transcriptomics Analysis for Early Diagnosis, Prognosis, and Therapies of Colorectal Cancer
Md. Ariful Islam, Md. Bayazid Hossen, Md. Abu Horaira, Md. Alim Hossen, Md. Kaderi Kibria, Md. Selim Reza, Khanis Farhana Tuly, Md. Omar Faruqe, Firoz Kabir, Rashidul Alam Mahumud, Md. Nurul Haque Mollah
Cancers.2023; 15(5): 1369. CrossRef - The Oncology Biomarker Discovery framework reveals cetuximab and bevacizumab response patterns in metastatic colorectal cancer
Alexander J. Ohnmacht, Arndt Stahler, Sebastian Stintzing, Dominik P. Modest, Julian W. Holch, C. Benedikt Westphalen, Linus Hölzel, Marisa K. Schübel, Ana Galhoz, Ali Farnoud, Minhaz Ud-Dean, Ursula Vehling-Kaiser, Thomas Decker, Markus Moehler, Matthias
Nature Communications.2023;[Epub] CrossRef - Disease Modeling on Tumor Organoids Implicates AURKA as a Therapeutic Target in Liver Metastatic Colorectal Cancer
Sophie L. Boos, Leon P. Loevenich, Sebastian Vosberg, Thomas Engleitner, Rupert Öllinger, Jörg Kumbrink, Matjaz Rokavec, Marlies Michl, Philipp A. Greif, Andreas Jung, Heiko Hermeking, Jens Neumann, Thomas Kirchner, Roland Rad, Peter Jung
Cellular and Molecular Gastroenterology and Hepatology.2022; 13(2): 517. CrossRef - Colorectal cancer on a dish: exploring the 3D-sphere culture of primary colorectal cancer cells from an Indonesian perspective
Murdani Abdullah, DR Noor, Amanda Pitarini Utari, Virly Nanda Muzellina, Nur Rahadiani, Radiana Dhewayani Antarianto
F1000Research.2022; 11: 182. CrossRef - Mitotic protein kinase-driven crosstalk of machineries for mitosis and metastasis
Chang-Hyeon Kim, Da-Eun Kim, Dae-Hoon Kim, Ga-Hong Min, Jung-Won Park, Yeo-Bin Kim, Chang K. Sung, Hyungshin Yim
Experimental & Molecular Medicine.2022; 54(4): 414. CrossRef - AURKA is a prognostic biomarker for good overall survival in stage II colorectal cancer patients
Peter Jung, David Horst, Thomas Kirchner, Frederick Klauschen, Jens Neumann
Pathology - Research and Practice.2022; 235: 153936. CrossRef - Therapeutic Potential of Mitotic Kinases’ Inhibitors in Cancers of the Gastrointestinal System
Aadil Javed, Gianluca Malagraba, Mahdieh Yarmohammadi, Catalina M. Perelló-Reus, Carles Barceló, Teresa Rubio-Tomás
Future Pharmacology.2022; 2(3): 214. CrossRef - Bioinformatics Analysis of RNA-seq Data Reveals Genes Related to Cancer Stem Cells in Colorectal Cancerogenesis
Kristian Urh, Nina Zidar, Emanuela Boštjančič
International Journal of Molecular Sciences.2022; 23(21): 13252. CrossRef - Unweaving the mitotic spindle: A focus on Aurora kinase inhibitors in lung cancer
Alessio Stefani, Geny Piro, Francesco Schietroma, Alessandro Strusi, Emanuele Vita, Simone Fiorani, Diletta Barone, Federico Monaca, Ileana Sparagna, Giustina Valente, Miriam Grazia Ferrara, Ettore D’Argento, Mariantonietta Di Salvatore, Carmine Carbone,
Frontiers in Oncology.2022;[Epub] CrossRef - Increased expression levels of AURKA and KIFC1 are promising predictors of progression and poor survival associated with gastric cancer
Jiyoon Jung, Hoiseon Jeong, Jung-Woo Choi, Hye-Sun Kim, Hwa Eun Oh, Eung Seok Lee, Young-Sik Kim, Ju-Han Lee
Pathology - Research and Practice.2021; 224: 153524. CrossRef - SALL Proteins; Common and Antagonistic Roles in Cancer
Claudia Álvarez, Aracelly Quiroz, Diego Benítez-Riquelme, Elizabeth Riffo, Ariel F. Castro, Roxana Pincheira
Cancers.2021; 13(24): 6292. CrossRef - AURKA gene polymorphisms and central nervous system tumor susceptibility in Chinese children
Yong-Ping Chen, Li Yuan, Hui-Ran Lin, Xiao-Kai Huang, Ji-Chen Ruan, Zhen-Jian Zhuo
Discover Oncology.2021;[Epub] CrossRef - Palmatine induces G2/M phase arrest and mitochondrial-associated pathway apoptosis in colon cancer cells by targeting AURKA
Xiaojiang Liu, Yaru Zhang, Siqi Wu, Minmin Xu, Youfeng Shen, Min Yu, Jinhua Fan, Sijia Wei, Chaohang Xu, Lu Huang, Han Zhao, Xuegang Li, Xiaoli Ye
Biochemical Pharmacology.2020; 175: 113933. CrossRef - New landscapes and horizons in hepatocellular carcinoma therapy
Melchiorre Cervello, Maria R. Emma, Giuseppa Augello, Antonella Cusimano, Lydia Giannitrapani, Maurizio Soresi, Shaw M. Akula, Stephen L. Abrams, Linda S. Steelman, Alessandro Gulino, Beatrice Belmonte, Giuseppe Montalto, James A. McCubrey
Aging.2020; 12(3): 3053. CrossRef - The New Paradigm of Network Medicine to Analyze Breast Cancer Phenotypes
Anna Maria Grimaldi, Federica Conte, Katia Pane, Giulia Fiscon, Peppino Mirabelli, Simona Baselice, Rosa Giannatiempo, Francesco Messina, Monica Franzese, Marco Salvatore, Paola Paci, Mariarosaria Incoronato
International Journal of Molecular Sciences.2020; 21(18): 6690. CrossRef - The GTEx Consortium atlas of genetic regulatory effects across human tissues
François Aguet, Shankara Anand, Kristin G. Ardlie, Stacey Gabriel, Gad A. Getz, Aaron Graubert, Kane Hadley, Robert E. Handsaker, Katherine H. Huang, Seva Kashin, Xiao Li, Daniel G. MacArthur, Samuel R. Meier, Jared L. Nedzel, Duyen T. Nguyen, Ayellet V.
Science.2020; 369(6509): 1318. CrossRef - Upregulation of aurora kinase A promotes vascular smooth muscle cell proliferation and migration by activating the GSK-3β/β-catenin pathway in aortic-dissecting aneurysms
Jia Meng, He-Liang Liu, Dong Ma, Hong-Yan Wang, Yue Peng, Hong-Li Wang
Life Sciences.2020; 262: 118491. CrossRef - Inhibition of AURKA Reduces Proliferation and Survival of Gastrointestinal Cancer Cells With Activated KRAS by Preventing Activation of RPS6KB1
Lihong Wang-Bishop, Zheng Chen, Ahmed Gomaa, Albert Craig Lockhart, Safia Salaria, Jialiang Wang, Keeli B. Lewis, Jeffrey Ecsedy, Kay Washington, Robert Daniel Beauchamp, Wael El-Rifai
Gastroenterology.2019; 156(3): 662. CrossRef - Discovery and Validation of Novel Biomarkers for Detection of Epithelial Ovarian Cancer
Hagen Kulbe, Raik Otto, Silvia Darb-Esfahani, Hedwig Lammert, Salem Abobaker, Gabriele Welsch, Radoslav Chekerov, Reinhold Schäfer, Duska Dragun, Michael Hummel, Ulf Leser, Jalid Sehouli, Elena Ioana Braicu
Cells.2019; 8(7): 713. CrossRef - Epigenetic regulation of AURKA by miR-4715-3p in upper gastrointestinal cancers
Ahmed Gomaa, Dunfa Peng, Zheng Chen, Mohammed Soutto, Khaled Abouelezz, Alejandro Corvalan, Wael El-Rifai
Scientific Reports.2019;[Epub] CrossRef - The functional diversity of Aurora kinases: a comprehensive review
Estelle Willems, Matthias Dedobbeleer, Marina Digregorio, Arnaud Lombard, Paul Noel Lumapat, Bernard Rogister
Cell Division.2018;[Epub] CrossRef
- Exploring pyrimidine scaffolds for cancer therapy: A triad of synthetic chemistry, computational studies, and in-vitro biological testing
- Prognosis of Hepatocellular Carcinoma after Liver Transplantation: Comparative Analysis with Partial Hepatectomy
- Kyuho Lee, Kyoung-Bun Lee, Nam-Joon Yi, Kyung-Suk Suh, Ja-June Jang
- J Pathol Transl Med. 2017;51(1):79-86. Published online December 25, 2016
- DOI: https://doi.org/10.4132/jptm.2016.10.13
- 9,321 View
- 150 Download
- 6 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Liver transplantation (LT) is the treatment of choice for hepatocellular carcinoma (HCC). The aim of this study was to investigate the recurrence rate of HCC after LT and prognostic factors for recurrence by comparing LT with non-transplanted resection. Methods: The participants were 338 patients who underwent LT between 1996 and 2012 at Seoul National University Hospital (LT group) and 520 HCC patients who underwent partial hepatectomy between 1995 and 2006 (control group, non-LT group). Results: In the LT group, 68 of 338 patients (19.8%) showed relapse, and the recurrence rate was lower than that in the non-LT group (64.9%, 357/520, p < .001). Stratification analysis by American Joint Committee on Cancer (AJCC) stage showed that the stage I-II LT group had a lower recurrence rate than the non-LT group. Univariate comparative analysis demonstrated that multiplicity of tumor, tumor size, gross type, Edmondson- Steiner (ES) nuclear grade, extent of tumor, angioinvasion, AJCC stage, Milan criteria, University of California at San Francisco criteria on explant pathology (all p < .001), positive expression of cytokeratin 19 (p = .002), and preoperative α-fetoprotein (AFP) (p < .001) were predictors of tumor recurrence. In multivariate analysis, LT, preoperative AFP, multiplicity of tumor, extent of tumor, size of tumor, and ES nuclear grade were independent prognostic factors. Conclusions: LT might have a protective effect against the late recurrence of stage I-II HCC compared to non-LT, and the prognostic factors for recurrence were similar to previously well-known prognostic factors for HCC. -
Citations
Citations to this article as recorded by- Locoregional and Surgical Treatment of Single-Nodule Hepatocellular Carcinoma Recurrence After Liver Transplantation: A Systematic Review and a Meta-Analysis
Marco Maria Pascale, Camilla Marandola, Francesco Frongillo, Erida Nure, Salvatore Agnes
Cancers.2025; 17(9): 1501. CrossRef - Risk Factor Analysis of Death in Patients With Hepatic Cellular Carcinoma After Radical Operation: A Consecutive Cohort of 433 Patients
Zhengyang He, Wenfeng Lu, Dongze Qiu, Weimin She
Health Science Reports.2025;[Epub] CrossRef - Related Factors of Hepatocellular Carcinoma Recurrence Associated With Hyperglycemia After Liver Transplantation
Yujian Zheng, Qing Cai, Lishan Peng, Shibo Sun, Shaoping Wang, Jie Zhou
Transplantation Proceedings.2021; 53(1): 177. CrossRef - Oncological Outcomes of Hepatic Resection vs Transplantation for Localized Hepatocellular Carcinoma
A.T. Akcam, A.G. Saritas, A. Ulku, A. Rencuzogullari
Transplantation Proceedings.2019; 51(4): 1147. CrossRef - Clustering Asian Countries According to the Trend of liver cancer Mortality Rates: an Application of Growth Mixture Models
Maryam Salari, Anoshirvan Kazemnejad, Farid Zayeri
Iranian Red Crescent Medical Journal.2017;[Epub] CrossRef
- Locoregional and Surgical Treatment of Single-Nodule Hepatocellular Carcinoma Recurrence After Liver Transplantation: A Systematic Review and a Meta-Analysis
- CD9 Expression in Colorectal Carcinomas and Its Prognostic Significance
- Kyung-Ju Kim, Hee Jung Kwon, Min Chong Kim, Young Kyung Bae
- J Pathol Transl Med. 2016;50(6):459-468. Published online October 25, 2016
- DOI: https://doi.org/10.4132/jptm.2016.10.02
- 10,363 View
- 155 Download
- 19 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
CD9, a member of the tetraspanin superfamily, is a tumor suppressor in many malignancies. The aim of this study was to evaluate the immunohistochemical expression of CD9 in colorectal carcinomas (CRCs) and determine clinicopathological and prognostic significance of its expression.
Methods
The CD9 expression status of 305 CRCs was evaluated using a semi-quantitative scoring system in tumor cells (T-CD9) and immune cells (I-CD9) by classifying the results as high and low expression.
Results
High T-CD9 (T-CD9 [+]) expression was detected in 175 samples (57.6%) and high I-CD9 (I-CD9 [+]) expression was detected in 265 samples (86.9%). Using Kaplan- Meier survival analysis, the T-CD9 (+) group showed a tendency for better disease-free survival (DFS) (p = .057). In left-sided tumors, DFS was significantly longer in the T-CD9 (+) group (p = .021) but no statistical significance was observed with right-sided tumors (p = .453). I-CD9 (+) CRCs significantly correlated with well/moderately differentiation (p = .014). In Kaplan-Meier analysis, the I-CD9 (+) group had a tendency towards worse DFS compared to the I-CD9 (–) group (p = .156). In combined survival analysis of T-CD9 and I-CD9, we found that the longest DFS was among patients in the T-CD9 (+)/I-CD9 (–) group, whereas the T-CD9 (–)/I-CD9 (+) group showed the shortest DFS (p = .054).
Conclusions
High expression of T-CD9 was associated with a favorable DFS, especially in left-sided CRCs. Combined evaluation of T-CD9 and I-CD9 is required to determine the comprehensive prognostic effect of CD9 in CRCs. -
Citations
Citations to this article as recorded by- Predicting patient outcomes with gene-expression biomarkers from colorectal cancer organoids and cell lines
Alexandra Razumovskaya, Mariia Silkina, Andrey Poloznikov, Timur Kulagin, Maria Raigorodskaya, Nina Gorban, Anna Kudryavtseva, Maria Fedorova, Boris Alekseev, Alexander Tonevitsky, Sergey Nikulin
Frontiers in Molecular Biosciences.2025;[Epub] CrossRef - Intestinal estrogen receptor beta modulates the murine colon tumor immune microenvironment
Madeleine Birgersson, Matilda Holm, Carlos J. Gallardo-Dodd, Baizhen Chen, Lina Stepanauskaitė, Linnea Hases, Claudia Kutter, Amena Archer, Cecilia Williams
Cancer Letters.2025; 622: 217661. CrossRef - Establishment of Coala: a novel 3D and 2D cancer cell line derived from colorectal cancer liver metastasis
Sandra Mersakova, Juraj Marcinek, Dusan Brany, Olga Chodelkova, Martin Vorcak, Martin Cermak, Martina Poturnajova, Zuzana Kozovska, Henrieta Skovierova, Slavomira Novakova, Barbora Mitruskova, Dusan Loderer, Marian Grendar, Veronika Holubekova, Andrea Hor
Human Cell.2025;[Epub] CrossRef - CD9, a tetraspanin in cancer: biology and therapeutic promise in acute leukemia
Océane Guého, Elie Cousin, Jérémie Rouger-Gaudichon, Anne-Gaëlle Rio, Sébastien Corre, Virginie Gandemer, Frédéric Mazurier
Oncogenesis.2025;[Epub] CrossRef - Proteomic analysis of plasma exosomes in patients with metastatic colorectal cancer
Zhaoyue Zhong, Jiayin Ji, Hongxia Li, Ling Kang, Haipeng Zhu
Clinical Proteomics.2024;[Epub] CrossRef - Prognostic value and multifaceted roles of tetraspanin CD9 in cancer
Róbert Ondruššek, Barbora Kvokačková, Karolína Kryštofová, Světlana Brychtová, Karel Souček, Jan Bouchal
Frontiers in Oncology.2023;[Epub] CrossRef - Proteomic Signature of Extracellular Vesicles Associated with Colorectal Cancer
Natalia Soloveva, Svetlana Novikova, Tatiana Farafonova, Olga Tikhonova, Victor Zgoda
Molecules.2023; 28(10): 4227. CrossRef - Anti-Human CD9 Fab Fragment Antibody Blocks the Extracellular Vesicle-Mediated Increase in Malignancy of Colon Cancer Cells
Mark F. Santos, Germana Rappa, Simona Fontana, Jana Karbanová, Feryal Aalam, Derek Tai, Zhiyin Li, Marzia Pucci, Riccardo Alessandro, Chikao Morimoto, Denis Corbeil, Aurelio Lorico
Cells.2022; 11(16): 2474. CrossRef - The Study of the Extracellular Matrix in Chronic Inflammation: A Way to Prevent Cancer Initiation?
Asia Marangio, Andrea Biccari, Edoardo D’Angelo, Francesca Sensi, Gaya Spolverato, Salvatore Pucciarelli, Marco Agostini
Cancers.2022; 14(23): 5903. CrossRef - In vivo expansion of a CD9+ decidual-like NK cell subset following autologous hematopoietic stem cell transplantation
Ane Orrantia, Enrique Vázquez-De Luis, Gabirel Astarloa-Pando, Iñigo Terrén, Ainhoa Amarilla-Irusta, Diego Polanco-Alonso, Carmen González, Alasne Uranga, Tomás Carrascosa, Juan J. Mateos-Mazón, Juan C. García-Ruiz, Sergio Callejas, Ana Quintas, Ana Dopaz
iScience.2022; 25(10): 105235. CrossRef - Inhibition of cancer-cell migration by tetraspanin CD9-binding peptide
Thanawat Suwatthanarak, Masayoshi Tanaka, Yoshitaka Miyamoto, Kenji Miyado, Mina Okochi
Chemical Communications.2021; 57(40): 4906. CrossRef - High expression of tumor susceptibility gene 101 (TSG101) is associated with more aggressive behavior in colorectal carcinoma
Elmira Gheytanchi, Leili Saeednejad Zanjani, Roya Ghods, Maryam Abolhasani, Marzieh Shahin, Somayeh Vafaei, Marzieh Naseri, Fahimeh Fattahi, Zahra Madjd
Journal of Cancer Research and Clinical Oncology.2021; 147(6): 1631. CrossRef - Increased CD9 expression predicts favorable prognosis in human cancers: a systematic review and meta-analysis
Hyun Min Koh, Bo Gun Jang, Dong Hui Lee, Chang Lim Hyun
Cancer Cell International.2021;[Epub] CrossRef - Prognostic Value of CD9 in Solid Tumor: A Systematic Review and Meta-Analysis
Ping Zeng, Meng Si, Rui-xia Sun, Xu Cheng, Xiao-yang Li, Min-bin Chen
Frontiers in Oncology.2021;[Epub] CrossRef - Matrix Effect in the Isolation of Breast Cancer-Derived Nanovesicles by Immunomagnetic Separation and Electrochemical Immunosensing—A Comparative Study
Silio Lima Moura, Mercè Martì, María Isabel Pividori
Sensors.2020; 20(4): 965. CrossRef - CD9 expression indicates a poor outcome in acute lymphoblastic leukemia
Peiqi Liang, Miao Miao, Zhuogang Liu, Hongtao Wang, Wei Jiang, Shiyu Ma, Chuan Li, Rong Hu
Cancer Biomarkers.2018; 21(4): 781. CrossRef
- Predicting patient outcomes with gene-expression biomarkers from colorectal cancer organoids and cell lines
- SALL4 Expression in Hepatocellular Carcinomas Is Associated with EpCAM-Positivity and a Poor Prognosis
- Hyunjin Park, Hyejung Lee, An Na Seo, Jai Young Cho, Young Rok Choi, Yoo-Seok Yoon, Ho-Seong Han, Young Nyun Park, Haeryoung Kim
- J Pathol Transl Med. 2015;49(5):373-381. Published online August 10, 2015
- DOI: https://doi.org/10.4132/jptm.2015.07.09
- 12,547 View
- 97 Download
- 23 Web of Science
- 22 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
There is increasing interest in hepatocellular carcinomas (HCC) expressing “stemness”-related markers, as they have been associated with aggressive behavior and poor prognosis. In this study, we investigated the usefulness of Sal-like protein 4 (SALL4), a recently proposed candidate marker of “stemness.” Methods: Immunohistochemical stains were performed for SALL4, K19, and epithelial cellular adhesion molecule (EpCAM) on tissue microarrays constructed from 190 surgically resected HCCs, and the results were correlated with the clinicopathological features and patient survival data. Results: Nuclear SALL4 expression was observed in 39/190 HCCs (20.5%), while K19 and EpCAM were expressed in 30 (15.9%) and 92 (48.7%) HCCs, respectively. The nuclear expression was generally weak, punctate or clumped. SALL4 expression was significantly associated with a poor overall survival compared to SALL4-negative HCCs (p = .014) compared to SALL4-negative HCCs. On multivariate analysis adjusted for tumor size, multiplicity, vascular invasion, and pathological tumor stage, SALL4 remained as a significant independent predictor of decreased overall survival (p= .004). SALL4 expression was positively correlated with EpCAM expression (p = .013) but not with K19 expression. HCCs that expressed both SALL4 and EpCAM were associated with significantly decreased overall survival, compared to those cases which were negative for both of these markers (p = .031). Conclusions: Although SALL4 expression was not significantly correlated with other clinicopathological parameters suggestive of tumor aggressiveness, SALL4 expression was an independent predictor of poor overall survival in human HCCs, and was also positively correlated with EpCAM expression. -
Citations
Citations to this article as recorded by- Cytoplasmic SALL4-A isoform expression as a diagnostic marker of less aggressive tumor behavior in gastric cancer
Saeed Rahmani, Amirhesam Babajani, Maryam Abolhasani, Roya Ghods, Elham Kalantari, Zahra Madjd
World Journal of Surgical Oncology.2025;[Epub] CrossRef - Cancer stem cells in hepatocellular carcinoma: therapy resistance and emerging treatments
Dipesh Kumar Yadav, Rajesh Kumar Yadav, Alina Singh, Yi Huang, Dandan Bao, Zhangwei Yang, Hanzhang Huang, Yin Jiang, Pengwei Wang, Sisi Lin, Yongfei Hua, Yiren Hu
Frontiers in Immunology.2025;[Epub] CrossRef - Stemness markers in hepatocellular carcinoma of Eastern vs. Western population: Etiology matters?
Caecilia HC Sukowati, Korri El-Khobar, Chyntia Olivia Maurine Jasirwan, Juferdy Kurniawan, Rino Alvani Gani
Annals of Hepatology.2024; 29(1): 101153. CrossRef - Research progress and prospects of AFP-positive gastric cancer
Long Zhao, Changjiang Yang, Yilin Lin, Shan Wang, Yingjiang Ye, Zhanlong Shen
Foregut Surgery.2022; 2(1): 29. CrossRef - SALL4 and microRNA: The Role of Let-7
Jun Liu, Madeline A. Sauer, Shaza G. Hussein, Junyu Yang, Daniel G. Tenen, Li Chai
Genes.2021; 12(9): 1301. CrossRef - Hepatoid Teratoma, Hepatoid Yolk Sac Tumor, and Hepatocellular Carcinoma
Khaleel I. Al-Obaidy, Sean R. Williamson, Nathan Shelman, Muhammad T. Idrees, Thomas M. Ulbright
American Journal of Surgical Pathology.2021; 45(1): 127. CrossRef - Targeting an Inducible SALL4-Mediated Cancer Vulnerability with Sequential Therapy
Junyu Yang, Chong Gao, Miao Liu, Yao-Chung Liu, Junsu Kwon, Jun Qi, Xi Tian, Alicia Stein, Yanjing V. Liu, Nikki R. Kong, Yue Wu, Shenyi Yin, Jianzhong Xi, Zhiyuan Chen, Kalpana Kumari, Hannan Wong, Hongbo Luo, Leslie E. Silberstein, Julie A.I. Thoms, Ash
Cancer Research.2021; 81(23): 6018. CrossRef - Lipoprotein‐Like Nanoparticle Carrying Small Interfering RNA Against Spalt‐Like Transcription Factor 4 Effectively Targets Hepatocellular Carcinoma Cells and Decreases Tumor Burden
William Cruz, Huang Huang, Brian Barber, Elisa Pasini, Lili Ding, Gang Zheng, Juan Chen, Mamatha Bhat
Hepatology Communications.2020; 4(5): 769. CrossRef - Hepatoid adenocarcinoma of the stomach: a unique subgroup with distinct clinicopathological and molecular features
Yakun Wang, Li Sun, Zhongwu Li, Jing Gao, Sai Ge, Cheng Zhang, Jiajia Yuan, Xicheng Wang, Jian Li, Zhihao Lu, Jifang Gong, Ming Lu, Jun Zhou, Zhi Peng, Lin Shen, Xiaotian Zhang
Gastric Cancer.2019; 22(6): 1183. CrossRef - Gynecologic Serous Carcinoma: An Immunohistochemical Analysis of Malignant Body Fluid Specimens
Shuyue Ren, William Klump
Archives of Pathology & Laboratory Medicine.2019; 143(6): 677. CrossRef - The Pluripotency Network in Colorectal Cancer Pathogenesis and Prognosis: an Update
Ioannis A Voutsadakis
Biomarkers in Medicine.2018; 12(6): 653. CrossRef - Cancer stem cells in hepatocellular carcinoma: an overview and promising therapeutic strategies
Nuozhou Wang, Shanshan Wang, Ming-Yue Li, Bao-guang Hu, Li-ping Liu, Sheng-li Yang, Shucai Yang, Zhongqin Gong, Paul B. S. Lai, George G. Chen
Therapeutic Advances in Medical Oncology.2018;[Epub] CrossRef - DNA demethylation induces SALL4 gene re-expression in subgroups of hepatocellular carcinoma associated with Hepatitis B or C virus infection
H Fan, Z Cui, H Zhang, S K Mani, A Diab, L Lefrancois, N Fares, P Merle, O Andrisani
Oncogene.2017; 36(17): 2435. CrossRef - Higher expression of SALL4 predicts poor cancer prognosis: A meta-analysis
Hongyu Shen, Liangpeng Li, Dandan Wang, Sujin Yang, Xiu Chen, Siying Zhou, Shanliang Zhong, Jianhua Zhao, Jinhai Tang
Cancer Biomarkers.2017; 19(4): 365. CrossRef - SALL4 suppresses PTEN expression to promote glioma cell proliferation via PI3K/AKT signaling pathway
Chuanjin Liu, Haibin Wu, Yanyan Li, Liang Shen, Renchun Yu, Hongwei Yin, Ting Sun, Chunming Sun, Youxin Zhou, Ziwei Du
Journal of Neuro-Oncology.2017; 135(2): 263. CrossRef - Liver Cancer: Molecular Characterization, Clonal Evolution and Cancer Stem Cells
Germana Castelli, Elvira Pelosi, Ugo Testa
Cancers.2017; 9(9): 127. CrossRef - Oncofetal gene SALL4 and prognosis in cancer: A systematic review with meta-analysis
Lorenzo Nicolè, Tiziana Sanavia, Nicola Veronese, Rocco Cappellesso, Claudio Luchini, Paolo Dabrilli, Ambrogio Fassina
Oncotarget.2017; 8(14): 22968. CrossRef - SALL4, the missing link between stem cells, development and cancer
Hiro Tatetsu, Nikki R. Kong, Gao Chong, Giovanni Amabile, Daniel G. Tenen, Li Chai
Gene.2016; 584(2): 111. CrossRef - A New Cell Block Method for Multiple Immunohistochemical Analysis of Circulating Tumor Cells in Patients with Liver Cancer
Soo Jeong Nam, Hyun Yang Yeo, Hee Jin Chang, Bo Hyun Kim, Eun Kyung Hong, Joong-Won Park
Cancer Research and Treatment.2016; 48(4): 1229. CrossRef - Functional and clinical significance of SALL4 in breast cancer
Ebubekir Dirican, Mustafa Akkiprik
Tumor Biology.2016; 37(9): 11701. CrossRef - MicroRNA-33b suppresses the proliferation and metastasis of hepatocellular carcinoma cells through the inhibition of Sal-like protein 4 expression
Qinggang Tian, Yao Xiao, Yanting Wu, Yun Liu, Zhiqing Song, Wenfeng Gao, Jing Zhang, Jingling Yang, Yuguo Zhang, Tuankui Guo, Furong Dai, Zhigang Sun
International Journal of Molecular Medicine.2016; 38(5): 1587. CrossRef - Oncogenic protein SALL4 and ZNF217 as prognostic indicators in solid cancers: a meta-analysis of individual studies
Ji Cheng, Jinbo Gao, Xiaoming Shuai, Kaixiong Tao
Oncotarget.2016; 7(17): 24314. CrossRef
- Cytoplasmic SALL4-A isoform expression as a diagnostic marker of less aggressive tumor behavior in gastric cancer
- Cancers with Higher Density of Tumor-Associated Macrophages Were Associated with Poor Survival Rates
- Kyong Yeun Jung, Sun Wook Cho, Young A Kim, Daein Kim, Byung-Chul Oh, Do Joon Park, Young Joo Park
- J Pathol Transl Med. 2015;49(4):318-324. Published online June 17, 2015
- DOI: https://doi.org/10.4132/jptm.2015.06.01
- 17,100 View
- 252 Download
- 157 Web of Science
- 148 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Macrophages are a component of a tumor’s microenvironment and have various roles in tumor progression and metastasis. This study evaluated the relationships between tumor-associated macrophage (TAM) density and clinical outcomes in 14 different types of human cancers. Methods: We investigated TAM density in human tissue microarray sections from 14 different types of human cancers (n = 266) and normal thyroid, lung, and breast tissues (n = 22). The five-year survival rates of each cancer were obtained from the 2011 Korea Central Cancer Registry. Results: Among 13 human cancers, excluding thyroid cancer, pancreas, lung, and gallbladder cancers had the highest density of CD163-positive macrophages (7.0±3.5%, 6.9±7.4%, and 6.9 ± 5.5%, respectively). The five-year relative survival rates of these cancers (pancreas, 8.7%; lung, 20.7%; gallbladder, 27.5%) were lower than those of other cancers. The histological subtypes in thyroid cancer exhibited significantly different CD163-positive macrophages densities (papillary, 1.8 ± 1.6% vs anaplastic, 22.9 ± 17.1%; p < .001), but no significant difference between histological subtypes was detected in lung and breast cancers. Moreover, there was no significant difference in CD163-positive macrophages densities among the TNM stages in lung, breast, and thyroid cancers. Conclusions: Cancers with higher TAM densities (pancreas, lung, anaplastic thyroid, and gallbladder) were associated with poor survival rate. -
Citations
Citations to this article as recorded by- Immune cells in thyroid adenoma and carcinoma: uncovering a hidden value of assessing tumor-host interplay and its potential application in thyroid cytopathology
Iryna Omelianenko, Nazarii Kobyliak, Tetyana Falalyeyeva, Oleksii Seleznov, Pavlina Botsun, Lyudmila Ostapchenko, Oleksandr Korotkyi, Liudmyla Domylivska, Olena Tsyryuk, Galyna Mykhalchyshyn, Tetiana Shapochka, Oksana Sulaieva
Frontiers in Molecular Biosciences.2025;[Epub] CrossRef - Research Progress on the Immunological Correlation Between Papillary Thyroid Carcinoma and Hashimoto’s Thyroiditis
Digui Fang, Limei Zhou, Biao Zheng, Ran Wang
Journal of Immunology Research.2025;[Epub] CrossRef - Tumour-associated macrophages in human meningiomas
Rahmina Meta, Henrik Sahlin Pettersen, Sofie Eline Tollefsen, Borgny Ytterhus, Øyvind Olav Salvesen, Wenche Sjursen, Sverre Helge Torp, Jianhong Zhou
PLOS One.2025; 20(5): e0319960. CrossRef - Turning tumor microenvironmental foes to friends: A new opportunity for thyroid cancer therapy and redifferentiation
Liya Zhu, Xiuli Jing, Byeong-Cheol Ahn
Oral Oncology.2025; 168: 107513. CrossRef - Unveiling Macrophage Content as a Predictive Biomarker for Intraoperative ICG Imaging Efficacy in Lung Cancer
Yue Yan, Jiahui Mi, Yun Li, Guanchao Jiang, Yimeng Zhang, Yanguo Liu, Hui Zhao, Jianfeng Li, Xing Yang, Jun Wang, Fan Yang, Kezhong Chen, Yiguang Wang, Jian Zhou
Advanced Science.2025;[Epub] CrossRef - Imaging Through Scattering Tissue Based on NIR Multispectral Image Fusion Technique
Nisan Atiya, Amir Shemer, Ariel Schwarz, Yevgeny Beiderman, Yossef Danan
Sensors.2025; 25(16): 4977. CrossRef - Molecular landscape of colorectal cancer liver metastasis: Tumor microenvironment heterogeneity and driver inference
Ailing Zhang, Yi Zhang, Jiayue Xu, Rui Zhu, Tingming Liang, Li Guo
Critical Reviews in Oncology/Hematology.2025; 216: 104946. CrossRef - Precision engineering of macrophage reprogramming with RNA interference-loaded lipid nanoparticles: a game-changer in cancer immunotherapy
Sezen Gül, Juliette Vergnaud, François Fay, Elias Fattal
Drug Delivery and Translational Research.2025;[Epub] CrossRef - Oncogenic mutation-driven metabolism-immunity regulatory axis: Potential prospects for thyroid cancer precision therapy
Tingting Zhang, Hengtong Han, Tianying Zhang, Yating Zhang, Libin Ma, Ze Yang, Yong-xun Zhao
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer.2025; 1880(6): 189459. CrossRef - Impact of the tumor microenvironment on survival in anaplastic thyroid carcinoma
Chanchal Rana, Prabhakar Mishra, Kulranjan Singh, Pooja Ramakant, Anand Mishra, Sumaira Qayoom, Gulshan Kumar, Jyoti Mishra, Komal Verma
Scientific Reports.2025;[Epub] CrossRef - Chimeric antigen receptor cells as a tool for localized delivery of TNFα in solid cancer treatment
Zhenqiang Yao, Chutamath Sittplangkoon
Human Vaccines & Immunotherapeutics.2025;[Epub] CrossRef - Defining Gene Signature of Tumor-Associated Macrophages in Intrahepatic Cholangiocarcinoma as Target for Immunotherapy Using Single Cell and Bulk RNA Sequencing
Joshua S. Badshah, Ryan M. Lee, Andrea Reitsma, Marc L. Melcher, Olivia M. Martinez, Sheri M. Krams, Daniel J. Delitto, Varvara A. Kirchner
Livers.2025; 5(4): 53. CrossRef - Therapeutic Strategies Targeting CD163 and CD169 in Macrophages for Cancer
Yukio Fujiwara, Yoshihiro Komohara
Pathology International.2025; 75(11): 551. CrossRef - Circulating immunophenotypes are potentially prognostic in follicular cell-derived thyroid cancer
Anupam Kotwal, Michael P. Gustafson, Svetlana Bornschlegl, Allan B. Dietz, Danae Delivanis, Mabel Ryder
Frontiers in Immunology.2024;[Epub] CrossRef - Nano-enhanced immunotherapy: Targeting the immunosuppressive tumor microenvironment
Yuzhi Jin, Yangyue Huang, Hui Ren, Huanhuan Huang, Chunyu Lai, Wenjun Wang, Zhou Tong, Hangyu Zhang, Wei Wu, Chuan Liu, Xuanwen Bao, Weijia Fang, Hongjun Li, Peng Zhao, Xiaomeng Dai
Biomaterials.2024; 305: 122463. CrossRef - Tumor microenvironment in thyroid cancer: Immune cells, patterns, and novel treatments
Beatriz Febrero, Juan José Ruiz‐Manzanera, Inmaculada Ros‐Madrid, Antonio Miguel Hernández, Esteban Orenes‐Piñero, José Manuel Rodríguez
Head & Neck.2024; 46(6): 1486. CrossRef - Research progress of immunotherapy against anaplastic thyroid cancer
Jiaqian Chen, Zuixuan Xiao, Hongyan Wu
Frontiers in Oncology.2024;[Epub] CrossRef - Tumor‐associated macrophages impair NK cell IFN‐γ production and contribute to tumor progression in clear cell renal cell carcinoma
Sol Yanel Núñez, Aldana Trotta, María Victoria Regge, María Sofía Amarilla, Florencia Secchiari, Jessica Mariel Sierra, María Cecilia Santilli, Mariana Gantov, Agustín Rovegno, Nicolás Richards, Carlos Ameri, Hernando Ríos Pita, Luis Rico, Mauro Mieggi, G
European Journal of Immunology.2024;[Epub] CrossRef - Monocytes in leukapheresis products affect the outcome of CD19–targeted CAR T-cell therapy in patients with lymphoma
Cristiana Carniti, Nicole M. Caldarelli, Luca Agnelli, Tommaso Torelli, Silva Ljevar, Sadhana Jonnalagadda, Giada Zanirato, Eugenio Fardella, Federico Stella, Daniele Lorenzini, Silvia Brich, Flavio Arienti, Anna Dodero, Annalisa Chiappella, Martina Magni
Blood Advances.2024; 8(8): 1968. CrossRef - Clinicopathological Features and Molecular Signatures of Lateral Neck Lymph Node Metastasis in Papillary Thyroid Microcarcinoma
Jinsun Lim, Han Sai Lee, Jin-Hyung Heo, Young Shin Song
Endocrinology and Metabolism.2024; 39(2): 324. CrossRef - Relaxin-2 is a novel biomarker for differentiated thyroid carcinoma in humans
Anupam Kotwal, Ronda Simpson, Nicholas Whiteman, Benjamin Swanson, Ana Yuil-Valdes, Madelyn Fitch, Joshua Nguyen, Salma Elhag, Oleg Shats, Whitney Goldner, Robert Bennett
Biochemical Pharmacology.2024; 225: 116323. CrossRef - Emerging therapeutic options for follicular-derived thyroid cancer in the era of immunotherapy
Naimah Turner, Sarah Hamidi, Rim Ouni, Rene Rico, Ying C. Henderson, Maria Puche, Sayan Alekseev, Jocelynn G. Colunga-Minutti, Mark E. Zafereo, Stephen Y. Lai, Sang T. Kim, Maria E. Cabanillas, Roza Nurieva
Frontiers in Immunology.2024;[Epub] CrossRef - Deciphering the performance of macrophages in tumour microenvironment: a call for precision immunotherapy
Belén Toledo, Linrui Zhu Chen, María Paniagua-Sancho, Juan Antonio Marchal, Macarena Perán, Elisa Giovannetti
Journal of Hematology & Oncology.2024;[Epub] CrossRef - Characterization of Immune Infiltrate Along the Leading Edge of Differentiated Thyroid Cancer
Anupam Kotwal, Krysten Vance, Kemal Hajric, Ana Yuil-Valdes, Benjamin Swanson, Ernesto Martinez Duarte, Oleg Shats, Michael Hollingsworth, Hamid Band, Whitney Goldner
Thyroid®.2024; 34(8): 999. CrossRef - Leveraging molecular targeted drugs and immune checkpoint inhibitors treat advanced thyroid carcinoma to achieve thyroid carcinoma redifferentiation
Jing-Yang Su
American Journal of Cancer Research.2024; 14(2): 407. CrossRef - Harnessing the tumor microenvironment to boost adoptive T cell therapy with engineered lymphocytes for solid tumors
Martina Spiga, Elisa Martini, Maria Chiara Maffia, Fabio Ciceri, Eliana Ruggiero, Alessia Potenza, Chiara Bonini
Seminars in Immunopathology.2024;[Epub] CrossRef - Anaplastic thyroid cancer cell-secreted TGFβ1 plays a key role in inducing macrophage polarization of human monocytes
Agustina Jaroszewski
American Journal of Cancer Research.2024; 14(7): 3626. CrossRef - Therapeutic treatments targeting communication between angiogenic and immune microenvironments in thyroid cancers
Alessandro Prete, Carmelo Nucera
Current Opinion in Endocrine and Metabolic Research.2024; 37: 100544. CrossRef - Immune microenvironment in papillary thyroid carcinoma: roles of immune cells and checkpoints in disease progression and therapeutic implications
Xun Zheng, Ruonan Sun, Tao Wei
Frontiers in Immunology.2024;[Epub] CrossRef - Impact of Hashimoto’s thyroiditis on the tumor microenvironment in papillary thyroid cancer: insights from single-cell analysis
Hongzhe Ma, Guoqi Li, Diwei Huo, Yangguang Su, Qing Jin, Yangxu Lu, Yanyan Sun, Denan Zhang, Xiujie Chen
Frontiers in Endocrinology.2024;[Epub] CrossRef - Unconventional p65/p52 NF-κB module regulates key tumor microenvironment-related genes in breast tumor-associated macrophages (TAMs)
Veronica De Paolis, Virginia Troisi, Antonella Bordin, Francesca Pagano, Viviana Caputo, Chiara Parisi
Life Sciences.2024; 357: 123059. CrossRef - Revolutionizing prognostic predictions in colorectal cancer: Macrophage‑driven transcriptional insights from single‑cell RNA sequencing and gene co‑expression network analysis
Yang Feng, Zhuo Cheng, Jingyuan Gao, Tao Huang, Jun Wang, Qian Tang, Ke Pu, Chang Liu
Oncology Letters.2024;[Epub] CrossRef - Aggressive Types of Malignant Thyroid Neoplasms
Maria Boudina, Eleana Zisimopoulou, Persefoni Xirou, Alexandra Chrisoulidou
Journal of Clinical Medicine.2024; 13(20): 6119. CrossRef - Unravelling the Reasons Behind Limited Response to Anti-PD Therapy in ATC: A Comprehensive Evaluation of Tumor-Infiltrating Immune Cells and Checkpoints
Monikongkona Boruah, Shipra Agarwal, Riyaz Ahmad Mir, Saumitra Dey Choudhury, Kapil Sikka, Sameer Rastogi, Nishikant Damle, Mehar C. Sharma
Endocrine Pathology.2024; 35(4): 419. CrossRef - Imaging tumor and ascites-associated macrophages in a mouse model of metastatic ovarian cancer
Catherine A. Foss, Flonné Wildes, Delia Mezzanzanica, Franca Podo, Chien-Fu Hung, Santosh Yadav, Marie-France Penet Vidaver
EJNMMI Research.2024;[Epub] CrossRef - From ductal carcinoma in situ to invasive breast cancer: the prognostic value of the extracellular microenvironment
Taylor S. Hulahan, Peggi M. Angel
Journal of Experimental & Clinical Cancer Research.2024;[Epub] CrossRef - Combinatory actions of cytokines induce M2-like macrophages in anaplastic thyroid cancer
Takahito Kimura
American Journal of Cancer Research.2024; 14(12): 5812. CrossRef - The ratio of adaptive to innate immune cells differs between genders and associates with improved prognosis and response to immunotherapy
Johanne Ahrenfeldt, Ditte S. Christensen, Andreas B. Østergaard, Judit Kisistók, Mateo Sokač, Nicolai J. Birkbak, Albert Rübben
PLOS ONE.2023; 18(2): e0281375. CrossRef - Current Landscape of Immunotherapy for Advanced Sarcoma
Víctor Albarrán, María Luisa Villamayor, Javier Pozas, Jesús Chamorro, Diana Isabel Rosero, María San Román, Patricia Guerrero, Patricia Pérez de Aguado, Juan Carlos Calvo, Coral García de Quevedo, Carlos González, María Ángeles Vaz
Cancers.2023; 15(8): 2287. CrossRef - Iron-mediated oxidative stress induces PD-L1 expression via activation of c-Myc in lung adenocarcinoma
Anna Martina Battaglia, Alessandro Sacco, Ilenia Aversa, Gianluca Santamaria, Camillo Palmieri, Cirino Botta, Roberto De Stefano, Maurizio Bitetto, Lavinia Petriaggi, Emanuele Giorgio, Concetta Maria Faniello, Francesco Costanzo, Flavia Biamonte
Frontiers in Cell and Developmental Biology.2023;[Epub] CrossRef - Molecular mechanisms of immunotherapy resistance in triple-negative breast cancer
Yiwen Zheng, Shujin Li, Hongchao Tang, Xuli Meng, Qinghui Zheng
Frontiers in Immunology.2023;[Epub] CrossRef - Modeling the tumor microenvironment of anaplastic thyroid cancer: an orthotopic tumor model in C57BL/6 mice
Zhen Xu, Hyo Shik Shin, Yoo Hyung Kim, Seong Yun Ha, Jae-Kyung Won, Su-jin Kim, Young Joo Park, Sareh Parangi, Sun Wook Cho, Kyu Eun Lee
Frontiers in Immunology.2023;[Epub] CrossRef - A new approach to overcoming resistance to immunotherapy: nanotechnology
Jiangbo Shao, Ying Jin, Chunxiang Jin
Frontiers in Oncology.2023;[Epub] CrossRef - Integrative single-cell transcriptome analysis reveals immune suppressive landscape in the anaplastic thyroid cancer
Chao Feng, Yujia Tao, Chao Yu, Lirui Wang, Xiao Liu, Yuan Cao
Cancer Gene Therapy.2023; 30(12): 1598. CrossRef - Tumor-associated macrophages as a potential therapeutic target in thyroid cancers
Liya Zhu, Xiu Juan Li, Prakash Gangadaran, Xiuli Jing, Byeong-Cheol Ahn
Cancer Immunology, Immunotherapy.2023; 72(12): 3895. CrossRef - Influence of Macrophages on Vascular Invasion of Inflammatory Breast Cancer Emboli Measured Using an In Vitro Microfluidic Multi-Cellular Platform
Manasa Gadde, Melika Mehrabi-Dehdezi, Bisrat G. Debeb, Wendy A. Woodward, Marissa Nichole Rylander
Cancers.2023; 15(19): 4883. CrossRef - The Prognosis of Cancer Depends on the Interplay of Autophagy, Apoptosis, and Anoikis within the Tumor Microenvironment
Shweta Gulia, Prakash Chandra, Asmita Das
Cell Biochemistry and Biophysics.2023; 81(4): 621. CrossRef - Comprehensive analysis of BTNL9 as a prognostic biomarker correlated with immune infiltrations in thyroid cancer
Luyao Zhang, Shuang Yu, Shubin Hong, Xi Xiao, Zhihong Liao, Yanbing Li, Haipeng Xiao
BMC Medical Genomics.2023;[Epub] CrossRef - FF-10850, a Novel Liposomal Topotecan Achieves Superior Antitumor Activity via Macrophage- and Ammonia-Mediated Payload Release in the Tumor Microenvironment
Susumu Shimoyama, Ken Okada, Toshifumi Kimura, Yasushi Morohashi, Shinji Nakayama, Sayaka Kemmochi, Keiko Makita-Suzuki, Ursula A. Matulonis, Mikinaga Mori
Molecular Cancer Therapeutics.2023; 22(12): 1454. CrossRef - Estrogen-related genes for thyroid cancer prognosis, immune infiltration, staging, and drug sensitivity
Leiying Zhang, Man Zhou, Xiaoni Gao, Yang Xie, Junqi Xiao, Tao Liu, Xiangtai Zeng
BMC Cancer.2023;[Epub] CrossRef - Harnessing Immunity to Treat Advanced Thyroid Cancer
Hiroki Komatsuda, Michihisa Kono, Risa Wakisaka, Ryosuke Sato, Takahiro Inoue, Takumi Kumai, Miki Takahara
Vaccines.2023; 12(1): 45. CrossRef - Are third-generation active-targeting nanoformulations definitely the best? In vitro and in vivo comparisons of pixantrone-loaded liposomes modified with different sialic acid derivatives
Yanzhi Song, Zhennan She, Zhenjun Huang, Shuo Wang, Xinrong Liu, Qi Zhang, Jing Sun, Donghua Di, Yihui Deng
Drug Delivery and Translational Research.2022; 12(3): 647. CrossRef - Synergistic nanoassemblies constructed from a STAT3 inhibitor and a cabazitaxel prodrug with enhanced cancer chemo-immunotherapy
X. Shi, L. Shu, Y. Qiao, J. Yao, H. Xie, L. Zhou, H. Wang, S. Zheng
Materials Today Nano.2022; 17: 100155. CrossRef - Polypharmacologic Reprogramming of Tumor-Associated Macrophages toward an Inflammatory Phenotype
Nao Nishida-Aoki, Taranjit S. Gujral
Cancer Research.2022; 82(3): 433. CrossRef - Role of Suprabasin in the Dedifferentiation of Follicular Epithelial Cell-Derived Thyroid Cancer and Identification of Related Immune Markers
Hao Tan, Lidong Wang, Zhen Liu
Frontiers in Genetics.2022;[Epub] CrossRef - Macrophage C/EBPδ Drives Gemcitabine, but Not 5-FU or Paclitaxel, Resistance of Pancreatic Cancer Cells in a Deoxycytidine-Dependent Manner
C. Arnold Spek, Hella L. Aberson, JanWillem Duitman
Biomedicines.2022; 10(2): 219. CrossRef - Anaplastic Thyroid Carcinoma: An Update
Arnaud Jannin, Alexandre Escande, Abir Al Ghuzlan, Pierre Blanchard, Dana Hartl, Benjamin Chevalier, Frédéric Deschamps, Livia Lamartina, Ludovic Lacroix, Corinne Dupuy, Eric Baudin, Christine Do Cao, Julien Hadoux
Cancers.2022; 14(4): 1061. CrossRef - LC3-associated phagocytosis in bone marrow macrophages suppresses acute myeloid leukemia progression through STING activation
Jamie A. Moore, Jayna J. Mistry, Charlotte Hellmich, Rebecca H. Horton, Edyta E. Wojtowicz, Aisha Jibril, Matthew Jefferson, Thomas Wileman, Naiara Beraza, Kristian M. Bowles, Stuart A. Rushworth
Journal of Clinical Investigation.2022;[Epub] CrossRef - Integration of chemokine signaling with non-coding RNAs in tumor microenvironment and heterogeneity in different cancers
Shweta Arora, Salman Khan, Almaz Zaki, Gulnaz Tabassum, Mohd Mohsin, Humaira Naaz Bhutto, Tanveer Ahmad, Tasneem Fatma, Mansoor Ali Syed
Seminars in Cancer Biology.2022; 86: 720. CrossRef - BRAFV600E Induction in Thyrocytes Triggers Important Changes in the miRNAs Content and the Populations of Extracellular Vesicles Released in Thyroid Tumor Microenvironment
Ophélie Delcorte, Catherine Spourquet, Pascale Lemoine, Jonathan Degosserie, Patrick Van Der Smissen, Nicolas Dauguet, Axelle Loriot, Jeffrey A. Knauf, Laurent Gatto, Etienne Marbaix, James A. Fagin, Christophe E. Pierreux
Biomedicines.2022; 10(4): 755. CrossRef - Inflammatory Tumor Microenvironment in Cranial Meningiomas: Clinical Implications and Intraindividual Reproducibility
Johannes Wach, Tim Lampmann, Ági Güresir, Hartmut Vatter, Ulrich Herrlinger, Albert Becker, Marieta Toma, Michael Hölzel, Erdem Güresir
Diagnostics.2022; 12(4): 853. CrossRef - Roles and new Insights of Macrophages in the Tumor Microenvironment of Thyroid Cancer
Qi Liu, Wei Sun, Hao Zhang
Frontiers in Pharmacology.2022;[Epub] CrossRef - Fucoxanthin Is a Potential Therapeutic Agent for the Treatment of Breast Cancer
Tsz-Ying Lau, Hiu-Yee Kwan
Marine Drugs.2022; 20(6): 370. CrossRef - Dissecting Immunosuppressive Cell Communication Patterns Reveals JunB Proto-Oncogene (JUNB) Shaping a Non-Inflamed Tumor Microenvironment
Hualin Chen, Gang Chen
Frontiers in Genetics.2022;[Epub] CrossRef - LIMK1: A promising prognostic and immune infiltration indicator in colorectal cancer
Xin Liu, Qiang Song, Daohan Wang, Yubiao Liu, Zhixiang Zhang, Weihua Fu
Oncology Letters.2022;[Epub] CrossRef - Unusual Association of NF-κB Components in Tumor-Associated Macrophages (TAMs) Promotes HSPG2-Mediated Immune-Escaping Mechanism in Breast Cancer
Veronica De Paolis, Fabio Maiullari, Maila Chirivì, Marika Milan, Chiara Cordiglieri, Francesca Pagano, Alessandra Rita La Manna, Elena De Falco, Claudia Bearzi, Roberto Rizzi, Chiara Parisi
International Journal of Molecular Sciences.2022; 23(14): 7902. CrossRef - Crosstalk between angiogenesis and immune regulation in the tumor microenvironment
Hei Jung Kim, Young Rae Ji, You Mie Lee
Archives of Pharmacal Research.2022; 45(6): 401. CrossRef - Cancer Resistance to Immunotherapy: Molecular Mechanisms and Tackling Strategies
Son Hai Vu, Preethi Vetrivel, Jongmin Kim, Myeong-Sok Lee
International Journal of Molecular Sciences.2022; 23(18): 10906. CrossRef - Transcription Factor MAFB as a Prognostic Biomarker for the Lung Adenocarcinoma
Omar Samir, Naohiro Kobayashi, Teppei Nishino, Mennatullah Siyam, Manoj Kumar Yadav, Yuri Inoue, Satoru Takahashi, Michito Hamada
International Journal of Molecular Sciences.2022; 23(17): 9945. CrossRef - Construction and Validation of a CNV-Driven Ferroptosis-Related Gene Signature for Predicting the Prognosis of Lung Adenocarcinoma
Yanqing Wang, Yi Zhao, Yong Li, Zemin Luo, Hongzhu Chen, Yuan Li
Journal of Sensors.2022; 2022: 1. CrossRef - CXCL12 derived from CD248-expressing cancer-associated fibroblasts mediates M2-polarized macrophages to promote nonsmall cell lung cancer progression
Jieheng Wu, Xinlei Liu, Jiangwei Wu, Chunju Lou, Qiaoling Zhang, Huiping Chen, Zeyang Yang, Shiqi Long, Yun Wang, Zhenling Shang, Zuquan Hu, Rui Zhang, Jian Zhang, Zhu Zeng
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2022; 1868(11): 166521. CrossRef - Cell Component and Function of Tumor Microenvironment in Thyroid Cancer
Eunah Shin, Ja Seung Koo
International Journal of Molecular Sciences.2022; 23(20): 12578. CrossRef - TIM3 Expression in Anaplastic-Thyroid-Cancer-Infiltrating Macrophages: An Emerging Immunotherapeutic Target
Luz Maria Palacios, Victoria Peyret, María Estefania Viano, Romina Celeste Geysels, Yair Aron Chocobar, Ximena Volpini, Claudia Gabriela Pellizas, Juan Pablo Nicola, Claudia Cristina Motran, María Cecilia Rodriguez-Galan, Laura Fozzatti
Biology.2022; 11(11): 1609. CrossRef - Potential diagnostic of lymph node metastasis and prognostic values of TM4SFs in papillary thyroid carcinoma patients
Kun Wang, Haomin Li, Junyu Zhao, Jinming Yao, Yiran Lu, Jianjun Dong, Jie Bai, Lin Liao
Frontiers in Cell and Developmental Biology.2022;[Epub] CrossRef - A distinct M2 macrophage infiltrate and transcriptomic profile decisively influence adipocyte differentiation in lipedema
Stefan Wolf, Jenna H. Rannikko, Reetta Virtakoivu, Paolo Cinelli, Gunther Felmerer, Anna Burger, Pietro Giovanoli, Michael Detmar, Nicole Lindenblatt, Maija Hollmén, Epameinondas Gousopoulos
Frontiers in Immunology.2022;[Epub] CrossRef - Macrophage targeting in cancer
Martha Lopez‐Yrigoyen, Luca Cassetta, Jeffrey W. Pollard
Annals of the New York Academy of Sciences.2021; 1499(1): 18. CrossRef - Immune Landscape of Thyroid Cancers: New Insights
Elisa Menicali, Martina Guzzetti, Silvia Morelli, Sonia Moretti, Efisio Puxeddu
Frontiers in Endocrinology.2021;[Epub] CrossRef - Targeting tumor-associated macrophages to synergize tumor immunotherapy
Xiaonan Xiang, Jianguo Wang, Di Lu, Xiao Xu
Signal Transduction and Targeted Therapy.2021;[Epub] CrossRef - FOXE1-Dependent Regulation of Macrophage Chemotaxis by Thyroid Cells In Vitro and In Vivo
Sara Credendino, Marta De Menna, Irene Cantone, Carmen Moccia, Matteo Esposito, Luigi Di Guida, Mario De Felice, Gabriella De Vita
International Journal of Molecular Sciences.2021; 22(14): 7666. CrossRef - Hidden Treasures: Macrophage Long Non-Coding RNAs in Lung Cancer Progression
Annika Karger, Rajender Nandigama, Albrecht Stenzinger, Friedrich Grimminger, Soni Savai Pullamsetti, Werner Seeger, Rajkumar Savai
Cancers.2021; 13(16): 4127. CrossRef - A Prognostic Role for Circulating microRNAs Involved in Macrophage Polarization in Advanced Non-Small Cell Lung Cancer
Alexia Monastirioti, Chara Papadaki, Konstantinos Rounis, Despoina Kalapanida, Dimitrios Mavroudis, Sofia Agelaki
Cells.2021; 10(8): 1988. CrossRef - Hyperglycemia-Induced miR-467 Drives Tumor Inflammation and Growth in Breast Cancer
Jasmine Gajeton, Irene Krukovets, Santoshi Muppala, Dmitriy Verbovetskiy, Jessica Zhang, Olga Stenina-Adognravi
Cancers.2021; 13(6): 1346. CrossRef - Targeting MARCO and IL37R on Immunosuppressive Macrophages in Lung Cancer Blocks Regulatory T Cells and Supports Cytotoxic Lymphocyte Function
Linnéa La Fleur, Johan Botling, Fei He, Catarina Pelicano, Chikai Zhou, Chenfei He, Giorgia Palano, Artur Mezheyeuski, Patrick Micke, Jeffrey V. Ravetch, Mikael C. I. Karlsson, Dhifaf Sarhan
Cancer Research.2021; 81(4): 956. CrossRef - Absent in melanoma 2-mediating M1 macrophages facilitate tumor rejection in renal carcinoma
Dafei Chai, Zichun Zhang, Shang yuchen Shi, Dong Qiu, Chen Zhang, Gang Wang, Lin Fang, Huizhong Li, Hui Tian, Hailong Li, Junnian Zheng
Translational Oncology.2021; 14(4): 101018. CrossRef - Hampering Stromal Cells in the Tumor Microenvironment as a Therapeutic Strategy to Destem Cancer Stem Cells
Katherine Po Sin Chung, Rainbow Wing Hei Leung, Terence Kin Wah Lee
Cancers.2021; 13(13): 3191. CrossRef - Thyroid Cancer Stem-Like Cells: From Microenvironmental Niches to Therapeutic Strategies
Elisa Stellaria Grassi, Viola Ghiandai, Luca Persani
Journal of Clinical Medicine.2021; 10(7): 1455. CrossRef - Enhanced Drug Delivery to Solid Tumors via Drug-Loaded Nanocarriers: An Image-Based Computational Framework
Farshad Moradi Kashkooli, M. Soltani, Mohammad Masoud Momeni, Arman Rahmim
Frontiers in Oncology.2021;[Epub] CrossRef - Mechanisms of primary and acquired resistance to PD-1/PD-L1 blockade and the emerging role of gut microbiome
R. Zou, Y. Wang, F. Ye, X. Zhang, M. Wang, S. Cui
Clinical and Translational Oncology.2021; 23(11): 2237. CrossRef - Role of Tumor-Associated Macrophages in Sarcomas
Tomohiro Fujiwara, John Healey, Koichi Ogura, Aki Yoshida, Hiroya Kondo, Toshiaki Hata, Miho Kure, Hiroshi Tazawa, Eiji Nakata, Toshiyuki Kunisada, Toshiyoshi Fujiwara, Toshifumi Ozaki
Cancers.2021; 13(5): 1086. CrossRef - Secreted Factors by Anaplastic Thyroid Cancer Cells Induce Tumor-Promoting M2-like Macrophage Polarization through a TIM3-Dependent Mechanism
Cinthia Carolina Stempin, Romina Celeste Geysels, Sunmi Park, Luz Maria Palacios, Ximena Volpini, Claudia Cristina Motran, Eva Virginia Acosta Rodríguez, Juan Pablo Nicola, Sheue-yann Cheng, Claudia Gabriela Pellizas, Laura Fozzatti
Cancers.2021; 13(19): 4821. CrossRef - Papillary Thyroid Carcinoma Landscape and Its Immunological Link With Hashimoto Thyroiditis at Single-Cell Resolution
Jun Pan, Fang Ye, Chengxuan Yu, Qinsheng Zhu, Jiaqi Li, Yaohui Zhang, Hedi Tian, Yunjin Yao, Minjie Zhu, Yibin Shen, Feng Zhu, Yingying Wang, Xinhui Zhou, Guoji Guo, Yijun Wu
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - Evaluation of solid tumor response to sequential treatment cycles via a new computational hybrid approach
Farshad Moradi Kashkooli, M. Soltani
Scientific Reports.2021;[Epub] CrossRef - Assessment of outcomes and novel immune biomarkers in metaplastic breast cancer: a single institution retrospective study
Evan Morgan, Anupama Suresh, Akaansha Ganju, Daniel G. Stover, Robert Wesolowski, Sagar Sardesai, Anne Noonan, Raquel Reinbolt, Jeffrey VanDeusen, Nicole Williams, Mathew A. Cherian, Zaibo Li, Gregory Young, Marilly Palettas, Julie Stephens, Joseph Liu, A
World Journal of Surgical Oncology.2020;[Epub] CrossRef - Differentiated agonistic antibody targeting CD137 eradicates large tumors without hepatotoxicity
Ugur Eskiocak, Wilson Guzman, Benjamin Wolf, Christine Cummings, Lauren Milling, Hsin-Jung Wu, Michael Ophir, Conner Lambden, Pearl Bakhru, Dana C. Gilmore, Samantha Ottinger, Lucy Liu, William K. McConaughy, Sunny Q. He, Chao Wang, Cheuk Lun Leung, Jason
JCI Insight.2020;[Epub] CrossRef - The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses
Alireza Labani-Motlagh, Mehrnoush Ashja-Mahdavi, Angelica Loskog
Frontiers in Immunology.2020;[Epub] CrossRef - Sevoflurane depletes macrophages from the melanoma microenvironment
Isabella Sztwiertnia, Judith Schenz, Katharina Bomans, Dominik Schaack, Johanna Ohnesorge, Sandra Tamulyte, Markus A. Weigand, Florian Uhle, Roger Chammas
PLOS ONE.2020; 15(5): e0233789. CrossRef - Lung cancer aggressiveness in an intermittent hypoxia murine model of postmenopausal sleep apnea
Marta Torres, Miguel Ángel Martinez-Garcia, Francisco Campos-Rodriguez, David Gozal, Josep M. Montserrat, Daniel Navajas, Ramon Farré, Isaac Almendros
Menopause.2020; 27(6): 706. CrossRef - Neck Disability and Swallowing Function in Posttreatment Head and Neck Cancer Patients
Alexandria Harris, Lingyun Lyu, Tamara Wasserman‐Winko, Susan George, Jonas T. Johnson, Marci Lee Nilsen
Otolaryngology–Head and Neck Surgery.2020; 163(4): 763. CrossRef - Metabolic modulation via mTOR pathway and anti-angiogenesis remodels tumor microenvironment using PD-L1-targeting codelivery
Binfan Chen, Ang Gao, Bin Tu, Yonghui Wang, Xiaolu Yu, Yingshu Wang, Yanfeng Xiu, Bing Wang, Yakun Wan, Yongzhuo Huang
Biomaterials.2020; 255: 120187. CrossRef - The Thyroid Tumor Microenvironment: Potential Targets for Therapeutic Intervention and Prognostication
Laura MacDonald, Jonathan Jenkins, Grace Purvis, Joshua Lee, Aime T. Franco
Hormones and Cancer.2020; 11(5-6): 205. CrossRef - The Contribution of Race to Breast Tumor Microenvironment Composition and Disease Progression
Gina Kim, Jessica M. Pastoriza, John S. Condeelis, Joseph A. Sparano, Panagiota S. Filippou, George S. Karagiannis, Maja H. Oktay
Frontiers in Oncology.2020;[Epub] CrossRef - Prognosis of Macrophage Density in the Absence of Neutrophils in Differentiated Thyroid Cancer
Amblessed E. Onuma, Lynn Schoenfield, Chengli Shen, Charity Edwards, John E. Phay, Lawrence A. Shirley, Allan Tsung
Journal of Surgical Research.2020; 256: 458. CrossRef - Targeting of CD163+ Macrophages in Inflammatory and Malignant Diseases
Maria K. Skytthe, Jonas Heilskov Graversen, Søren K. Moestrup
International Journal of Molecular Sciences.2020; 21(15): 5497. CrossRef - Synthetic chlorin derivative self-prevented from aggregation: Behavior in homogeneous medium for PDT applications
Bianca Martins Estevão, Camila Fabiano de Freitas, Douglas Santana Franciscato, Francisco Fávaro de Assis, Kleber Thiago de Oliveira, Noboru Hioka, Wilker Caetano, Edvani Curti Muniz
Journal of Molecular Liquids.2020; 320: 114363. CrossRef - Tumor‑associated macrophages in lung cancer: Friendly or evil? (Review)
Fei Xu, Ying Wei, Zhao Tang, Baojun Liu, Jingcheng Dong
Molecular Medicine Reports.2020;[Epub] CrossRef - Macrophage-secreted MMP9 induces mesenchymal transition in pancreatic cancer cells via PAR1 activation
Cansu Tekin, Hella L Aberson, Cynthia Waasdorp, Gerrit K J Hooijer, Onno J de Boer, Frederike Dijk, Maarten F Bijlsma, C Arnold Spek
Cellular Oncology.2020; 43(6): 1161. CrossRef - Cancer Stem Cells in Thyroid Tumors: From the Origin to Metastasis
Veronica Veschi, Francesco Verona, Melania Lo Iacono, Caterina D'Accardo, Gaetana Porcelli, Alice Turdo, Miriam Gaggianesi, Stefano Forte, Dario Giuffrida, Lorenzo Memeo, Matilde Todaro
Frontiers in Endocrinology.2020;[Epub] CrossRef - The Crosstalk Between Tumor-Associated Macrophages (TAMs) and Tumor Cells and the Corresponding Targeted Therapy
Zhe Ge, Shuzhe Ding
Frontiers in Oncology.2020;[Epub] CrossRef - Immune Cell Confrontation in the Papillary Thyroid Carcinoma Microenvironment
Zhenyu Xie, Xin Li, Yuzhen He, Song Wu, Shiyue Wang, Jianjian Sun, Yuchen He, Yu Lun, Jian Zhang
Frontiers in Endocrinology.2020;[Epub] CrossRef - Early macrophage infiltrates impair pancreatic cancer cell growth by TNF-α secretion
Cansu Tekin, Hella L. Aberson, Maarten F. Bijlsma, C. Arnold Spek
BMC Cancer.2020;[Epub] CrossRef - Attenuation of CD47-SIRPα Signal in Cholangiocarcinoma Potentiates Tumor-Associated Macrophage-Mediated Phagocytosis and Suppresses Intrahepatic Metastasis
Kulthida Vaeteewoottacharn, Ryusho Kariya, Phattarin Pothipan, Sawako Fujikawa, Chawalit Pairojkul, Sakda Waraasawapati, Kazuhiko Kuwahara, Chaisiri Wongkham, Sopit Wongkham, Seiji Okada
Translational Oncology.2019; 12(2): 217. CrossRef - Circulating CEA‐dNLR score predicts clinical outcome of metastatic gallbladder cancer patient
Jing‐Hui Du, Jun Lu
Journal of Clinical Laboratory Analysis.2019;[Epub] CrossRef - The Interplay between MicroRNAs and Cellular Components of Tumour Microenvironment (TME) on Non-Small-Cell Lung Cancer (NSCLC) Progression
Sook Shien Lee, Yoke Kqueen Cheah
Journal of Immunology Research.2019; 2019: 1. CrossRef - Papillary thyroid carcinoma behavior: clues in the tumor microenvironment
Kensey Bergdorf, Donna C Ferguson, Mitra Mehrad, Kim Ely, Thomas Stricker, Vivian L Weiss
Endocrine-Related Cancer.2019; 26(6): 601. CrossRef - Interplay between thyroid cancer cells and macrophages: effects on IL-32 mediated cell death and thyroid cancer cell migration
Yvette J. E. Sloot, Katrin Rabold, Thomas Ulas, Dennis M. De Graaf, Bas Heinhuis, Kristian Händler, Joachim L. Schultze, Mihai G. Netea, Johannes W. A. Smit, Leo A. B. Joosten, Romana T. Netea-Maier
Cellular Oncology.2019; 42(5): 691. CrossRef - Iron accumulation in tumor-associated macrophages marks an improved overall survival in patients with lung adenocarcinoma
Carl Maximilian Thielmann, Milene Costa da Silva, Thomas Muley, Michael Meister, Esther Herpel, Martina U. Muckenthaler
Scientific Reports.2019;[Epub] CrossRef - The Immune Landscape of Thyroid Cancer in the Context of Immune Checkpoint Inhibition
Gilda Varricchi, Stefania Loffredo, Giancarlo Marone, Luca Modestino, Poupak Fallahi, Silvia Martina Ferrari, Amato de Paulis, Alessandro Antonelli, Maria Rosaria Galdiero
International Journal of Molecular Sciences.2019; 20(16): 3934. CrossRef - A review on the role of M2 macrophages in bladder cancer; pathophysiology and targeting
Laleh Sharifi, Mohammad Reza Nowroozi, Erfan Amini, Masoumeh Kourosh Arami, Mohsen Ayati, Monireh Mohsenzadegan
International Immunopharmacology.2019; 76: 105880. CrossRef - Tumor-associated macrophage infiltration in meningioma
Dustin T Proctor, Jordan Huang, Sanju Lama, Abdulrahman Albakr, Guido Van Marle, Garnette R Sutherland
Neuro-Oncology Advances.2019;[Epub] CrossRef - Immune and Inflammatory Cells in Thyroid Cancer Microenvironment
Silvia Martina Ferrari, Poupak Fallahi, Maria Rosaria Galdiero, Ilaria Ruffilli, Giusy Elia, Francesca Ragusa, Sabrina Rosaria Paparo, Armando Patrizio, Valeria Mazzi, Gilda Varricchi, Gianni Marone, Alessandro Antonelli
International Journal of Molecular Sciences.2019; 20(18): 4413. CrossRef - Caveolin-2 deficiency induces a rapid anti-tumor immune response prior to regression of implanted murine lung carcinoma tumors
Yajun Liu, Xiaoqiang Qi, Guangfu Li, Grzegorz Sowa
Scientific Reports.2019;[Epub] CrossRef - Paclitaxel Treatment and Proprotein Convertase 1/3 (PC1/3) Knockdown in Macrophages is a Promising Antiglioma Strategy as Revealed by Proteomics and Cytotoxicity Studies
Marie Duhamel, Mélanie Rose, Franck Rodet, Adriana Natalia Murgoci, Lea Zografidou, Anne Régnier-Vigouroux, Fabien Vanden Abeele, Firas Kobeissy, Serge Nataf, Laurent Pays, Maxence Wisztorski, Dasa Cizkova, Isabelle Fournier, Michel Salzet
Molecular & Cellular Proteomics.2018; 17(6): 1126. CrossRef - Chloroquine and nanoparticle drug delivery: A promising combination
Joe Pelt, Sara Busatto, Mauro Ferrari, E. Aubrey Thompson, Kabir Mody, Joy Wolfram
Pharmacology & Therapeutics.2018; 191: 43. CrossRef - Conditioned medium from stimulated macrophages inhibits growth but induces an inflammatory phenotype in breast cancer cells
Wenzhe Song, Parth Thakor, David A. Vesey, Glenda C. Gobe, Christudas Morais
Biomedicine & Pharmacotherapy.2018; 106: 247. CrossRef - Potential involvement of neutrophils in human thyroid cancer
Maria Rosaria Galdiero, Gilda Varricchi, Stefania Loffredo, Claudio Bellevicine, Tiziana Lansione, Anne Lise Ferrara, Raffaella Iannone, Sarah di Somma, Francesco Borriello, Eduardo Clery, Maria Triassi, Giancarlo Troncone, Gianni Marone, Fabrizio Mattei
PLOS ONE.2018; 13(6): e0199740. CrossRef - CSF1R-Expressing Tumor-Associated Macrophages, Smoking and Survival in Lung Adenocarcinoma: Analyses Using Quantitative Phosphor-Integrated Dot Staining
Kentaro Inamura, Yasuyuki Shigematsu, Hironori Ninomiya, Yasuhiro Nakashima, Maki Kobayashi, Haruyuki Saito, Katsuhiro Takahashi, Etsuko Futaya, Sakae Okumura, Yuichi Ishikawa, Hiroaki Kanda
Cancers.2018; 10(8): 252. CrossRef - Tumor heterogeneity and nanoparticle-mediated tumor targeting: the importance of delivery system personalization
K. Laxmi Swetha, Aniruddha Roy
Drug Delivery and Translational Research.2018; 8(5): 1508. CrossRef - Immune Gene Signature Delineates a Subclass of Papillary Thyroid Cancer with Unfavorable Clinical Outcomes
Kyuryung Kim, Sora Jeon, Tae-Min Kim, Chan Jung
Cancers.2018; 10(12): 494. CrossRef - Immunohistochemical Expression of TGF-Β1, SMAD4, SMAD7, TGFβRII and CD68-Positive TAM Densities in Papillary Thyroid Cancer
Koni Ivanova, Irena Manolova, Maria-Magdalena Ignatova, Maya Gulubova
Open Access Macedonian Journal of Medical Sciences.2018; 6(3): 435. CrossRef - Bone marrow-derived cells are recruited by the melanoma tumor with endothelial cells contributing to tumor vasculature
R. Bonfim-Silva, L. E. B. Souza, F. U. F. Melo, V. C. Oliveira, D. A. R. Magalhães, H. F. Oliveira, D. T. Covas, A. M. Fontes
Clinical and Translational Oncology.2017; 19(1): 125. CrossRef - Stromal contributions to the carcinogenic process
Mark Spaw, Shrikant Anant, Sufi Mary Thomas
Molecular Carcinogenesis.2017; 56(4): 1199. CrossRef - The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer
Silvia Affo, Le-Xing Yu, Robert F. Schwabe
Annual Review of Pathology: Mechanisms of Disease.2017; 12(1): 153. CrossRef - PEDF increases the tumoricidal activity of macrophages towards prostate cancer cells in vitro
Dalia Martinez-Marin, Courtney Jarvis, Thomas Nelius, Werner de Riese, Olga V. Volpert, Stéphanie Filleur, Chih-Hsin Tang
PLOS ONE.2017; 12(4): e0174968. CrossRef - Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies
Eleonora Molinaro, Cristina Romei, Agnese Biagini, Elena Sabini, Laura Agate, Salvatore Mazzeo, Gabriele Materazzi, Stefano Sellari-Franceschini, Alessandro Ribechini, Liborio Torregrossa, Fulvio Basolo, Paolo Vitti, Rossella Elisei
Nature Reviews Endocrinology.2017; 13(11): 644. CrossRef - Iron Induces Anti-tumor Activity in Tumor-Associated Macrophages
Milene Costa da Silva, Michael O. Breckwoldt, Francesca Vinchi, Margareta P. Correia, Ana Stojanovic, Carl Maximilian Thielmann, Michael Meister, Thomas Muley, Arne Warth, Michael Platten, Matthias W. Hentze, Adelheid Cerwenka, Martina U. Muckenthaler
Frontiers in Immunology.2017;[Epub] CrossRef - Reprogramming Tumor-Associated Macrophages To Reverse EGFRT790M Resistance by Dual-Targeting Codelivery of Gefitinib/Vorinostat
Huige Peng, Binfan Chen, Wei Huang, Yubo Tang, Yifan Jiang, Wenyuan Zhang, Yongzhuo Huang
Nano Letters.2017; 17(12): 7684. CrossRef - Numerical modeling of nanodrug distribution in tumors with heterogeneous vasculature
Cheng-Ying Chou, Wan-I Chang, Tzyy-Leng Horng, Win-Li Lin, Han-Chung Wu
PLOS ONE.2017; 12(12): e0189802. CrossRef - HPMA–Copolymer Nanocarrier Targets Tumor-Associated Macrophages in Primary and Metastatic Breast Cancer
Melissa N. Zimel, Chloe B. Horowitz, Vinagolu K. Rajasekhar, Alexander B. Christ, Xin Wei, Jianbo Wu, Paulina M. Wojnarowicz, Dong Wang, Steven R. Goldring, P. Edward Purdue, John H. Healey
Molecular Cancer Therapeutics.2017; 16(12): 2701. CrossRef - Correlation of tumor-associated macrophages and NK cells with bladder cancer size and T stage in patients with solitary low-grade urothelial carcinoma
Kristian Krpina, Emina Babarović, Josip Španjol, Gordana Đorđević, Tobias Maurer, Nives Jonjić
Wiener klinische Wochenschrift.2016; 128(7-8): 248. CrossRef - The lymphatic system and pancreatic cancer
Darci M. Fink, Maria M. Steele, Michael A. Hollingsworth
Cancer Letters.2016; 381(1): 217. CrossRef - Jacalin-Activated Macrophages Exhibit an Antitumor Phenotype
Cláudia Danella Polli, Luciana Pereira Ruas, Luciana Chain Veronez, Thais Herrero Geraldino, Fabiana Rossetto de Morais, Maria Cristina Roque-Barreira, Gabriela Pereira-da-Silva
BioMed Research International.2016; 2016: 1. CrossRef - The immune network in thyroid cancer
Maria Rosaria Galdiero, Gilda Varricchi, Gianni Marone
OncoImmunology.2016; 5(6): e1168556. CrossRef - The Expression and Relationship of CD68-Tumor-Associated Macrophages and Microvascular Density With the Prognosis of Patients With Laryngeal Squamous Cell Carcinoma
Shujun Sun, Xinliang Pan, Limin Zhao, Jianming Zhou, Hongzeng Wang, Yonghong Sun
Clinical and Experimental Otorhinolaryngology.2016; 9(3): 270. CrossRef - Role of pulmonary macrophages in initiation of lung metastasis in anaplastic thyroid cancer
Xiu Juan Li, Prakash Gangadaran, Senthilkumar Kalimuthu, Ji Min Oh, Liya Zhu, Shin Young Jeong, Sang‐Woo Lee, Jaetae Lee, Byeong‐Cheol Ahn
International Journal of Cancer.2016; 139(11): 2583. CrossRef - Perspectives for immunotherapy in endocrine cancer
S Latteyer, V Tiedje, B Schilling, D Führer
Endocrine-Related Cancer.2016; 23(10): R469. CrossRef - Macrophage Densities Correlated with CXC Chemokine Receptor 4 Expression and Related with Poor Survival in Anaplastic Thyroid Cancer
Dae In Kim, Eunyoung Kim, Young A Kim, Sun Wook Cho, Jung Ah Lim, Young Joo Park
Endocrinology and Metabolism.2016; 31(3): 469. CrossRef - High expression of C-C chemokine receptor 2 associates with poor overall survival in gastric cancer patients after surgical resection
Ruochen Li, Heng Zhang, Hao Liu, Chao Lin, Yifan Cao, Weijuan Zhang, Zhenbin Shen, Jiejie Xu
Oncotarget.2016; 7(17): 23909. CrossRef - Polycationic carbosilane dendrimer decreases angiogenesis and tumor-associated macrophages in tumor-bearing mice
Ana Judith Perisé-Barrios, María Jesús Serramia, Javier de la Mata, Rafael Gomez, Angel Luis Corbí, Ángeles Domínguez-Soto, María Ángeles Muñoz-Fernandez
RSC Advances.2015; 5(126): 104110. CrossRef
- Immune cells in thyroid adenoma and carcinoma: uncovering a hidden value of assessing tumor-host interplay and its potential application in thyroid cytopathology
- Pathologic Factors Associated with Prognosis after Adjuvant Chemotherapy in Stage II/III Microsatellite-Unstable Colorectal Cancers
- Jung Ho Kim, Jeong Mo Bae, Hyeon Jeong Oh, Hye Seung Lee, Gyeong Hoon Kang
- J Pathol Transl Med. 2015;49(2):118-128. Published online March 12, 2015
- DOI: https://doi.org/10.4132/jptm.2015.02.05
- 13,002 View
- 120 Download
- 20 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Although there are controversies regarding the benefit of fluoropyrimidine-based adjuvant chemotherapy in patients with microsatellite instability–high (MSI-H) colorectal cancer (CRC), the pathologic features affecting postchemotherapeutic prognosis in these patients have not been fully identified yet. Methods: A total of 26 histopathologic and immunohistochemical factors were comprehensively evaluated in 125 stage II or III MSI-H CRC patients who underwent curative resection followed by fluoropyrimidine-based adjuvant chemotherapy. We statistically analyzed the associations of these factors with disease-free survival (DFS). Results: Using a Kaplan- Meier analysis with log-rank test, we determined that ulceroinfiltrative gross type (p=.003), pT4 (p<.001), pN2 (p=.002), perineural invasion (p=.001), absence of peritumoral lymphoid reaction (p=.041), signet ring cell component (p=.006), and cribriform comedo component (p=.004) were significantly associated with worse DFS in patients receiving oxaliplatin-based adjuvant chemotherapy (n=45). By contrast, pT4 (p<.001) and tumor budding-positivity (p=.032) were significant predictors of poor survival in patients receiving non-oxaliplatin–based adjuvant chemotherapy (n=80). In Cox proportional hazards regression model-based univariate and multivariate analyses, pT category (pT1-3 vs pT4) was the only significant prognostic factor in patients receiving non-oxaliplatin–based adjuvant chemotherapy, whereas pT category, signet ring cell histology and cribriform comedo histology remained independent prognostic factors in patients receiving oxaliplatin-based adjuvant chemotherapy. Conclusions: pT4 status is the most significant pathologic determinant of poor outcome after fluoropyrimidine-based adjuvant chemotherapy in patients with stage II/III MSI-H CRC. -
Citations
Citations to this article as recorded by- Evaluation of D-Mannoheptulose and Doxorubicin as Potential Therapeutic Agents for Breast Cancer by Targeting Glycolysis and Inducing Apoptosis
Ahmed Ghdhban Al-Ziaydi
Indian Journal of Clinical Biochemistry.2025; 40(3): 412. CrossRef - Clinicopathological features and evaluation of microsatellite stability of colorectal carcinoma with cribriform comedo pattern
Tuğba Günler, Pinar Karabağli, Hicret Tiyek, Özge Keskin, Muslu K. Körez
Indian Journal of Pathology and Microbiology.2024; 67(2): 275. CrossRef - Cribriform colon cancer: a morphological growth pattern associated with extramural venous invasion, nodal metastases and microsatellite stability
Alexander S Taylor, Natalia Liu, Jiayun M Fang, Nicole Panarelli, Lili Zhao, Jerome Cheng, Purva Gopal, Suntrea Hammer, Jing Sun, Henry Appelman, Maria Westerhoff
Journal of Clinical Pathology.2022; 75(7): 483. CrossRef - HSP110 as a Diagnostic but Not a Prognostic Biomarker in Colorectal Cancer With Microsatellite Instability
Gaelle Tachon, Arnaud Chong-Si-Tsaon, Thierry Lecomte, Audelaure Junca, Éric Frouin, Elodie Miquelestorena-Standley, Julie Godet, Camille Evrard, Violaine Randrian, Romain Chautard, Marie-Luce Auriault, Valérie Moulin, Serge Guyetant, Gaelle Fromont, Luci
Frontiers in Genetics.2022;[Epub] CrossRef - Comparative expression of immunohistochemical biomarkers in cribriform and pattern 4 non-cribriform prostatic adenocarcinoma
Guang-Qian Xiao, Elise Nguyen, Pamela D. Unger, Andy E. Sherrod
Experimental and Molecular Pathology.2020; 114: 104400. CrossRef - Prognostic Predictability of American Joint Committee on Cancer 8th Staging System for Perihilar Cholangiocarcinoma: Limited Improvement Compared with the 7th Staging System
Jong Woo Lee, Jae Hoon Lee, Yejong Park, Woohyung Lee, Jaewoo Kwon, Ki Byung Song, Dae Wook Hwang, Song Cheol Kim
Cancer Research and Treatment.2020; 52(3): 886. CrossRef - Prognostic predictability of the new American Joint Committee on Cancer 8th staging system for distal bile duct cancer: limited usefulness compared with the 7th staging system
Jae Seung Kang, Seungyeoun Lee, Donghee Son, Youngmin Han, Kyung Bun Lee, Jae Ri Kim, Wooil Kwon, Sun‐Whe Kim, Jin‐Young Jang
Journal of Hepato-Biliary-Pancreatic Sciences.2018; 25(2): 124. CrossRef - Invasion Depth Measured in Millimeters is a Predictor of Survival in Patients with Distal Bile Duct Cancer: Decision Tree Approach
Kyueng‐Whan Min, Dong‐Hoon Kim, Byoung Kwan Son, Eun‐Kyung Kim, Sang Bong Ahn, Seong Hwan Kim, Yun Ju Jo, Young Sook Park, Jinwon Seo, Young Ha Oh, Sukjoong Oh, Ho Young Kim, Mi Jung Kwon, Soo Kee Min, Hye‐Rim Park, Ji‐Young Choe, Jang Yong Jeon, Hong Il
World Journal of Surgery.2017; 41(1): 232. CrossRef - BRAF-Mutated Colorectal Cancer Exhibits Distinct Clinicopathological Features from Wild-TypeBRAF-Expressing Cancer Independent of the Microsatellite Instability Status
Min Hye Jang, Sehun Kim, Dae Yong Hwang, Wook Youn Kim, So Dug Lim, Wan Seop Kim, Tea Sook Hwang, Hye Seung Han
Journal of Korean Medical Science.2017; 32(1): 38. CrossRef - Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma
Hye Eun Park, Jung Ho Kim, Nam-Yun Cho, Hye Seung Lee, Gyeong Hoon Kang
Virchows Archiv.2017; 471(3): 329. CrossRef - Dominant high expression of wild‐type HSP110 defines a poor prognostic subgroup of colorectal carcinomas with microsatellite instability: a whole‐section immunohistochemical analysis
Hyeon Jeong Oh, Jung Ho Kim, Tae Hun Lee, Hye Eun Park, Jeong Mo Bae, Hye Seung Lee, Gyeong Hoon Kang
APMIS.2017; 125(12): 1076. CrossRef - TNM Staging of Colorectal Cancer Should be Reconsidered According to Weighting of the T Stage
Jun Li, Cheng-Hao Yi, Ye-Ting Hu, Jin-Song Li, Ying Yuan, Su-Zhan Zhang, Shu Zheng, Ke-Feng Ding
Medicine.2016; 95(6): e2711. CrossRef - Molecular genetics of colorectal cancer
James Church
Seminars in Colon and Rectal Surgery.2016; 27(4): 172. CrossRef - Characterisation of PD-L1-positive subsets of microsatellite-unstable colorectal cancers
Jung Ho Kim, Hye Eun Park, Nam-Yun Cho, Hye Seung Lee, Gyeong Hoon Kang
British Journal of Cancer.2016; 115(4): 490. CrossRef - Distinct features betweenMLH1-methylated and unmethylated colorectal carcinomas with the CpG island methylator phenotype: implications in the serrated neoplasia pathway
Jung Ho Kim, Jeong Mo Bae, Nam-Yun Cho, Gyeong Hoon Kang
Oncotarget.2016; 7(12): 14095. CrossRef - Tumor deposits: markers of poor prognosis in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy
Lu-Ning Zhang, Wei-Wei Xiao, Shao-Yan Xi, Pu-Yun OuYang, Kai-Yun You, Zhi-Fan Zeng, Pei-Rong Ding, Hui-Zhong Zhang, Zhi-Zhong Pan, Rui-Hua Xu, Yuan-Hong Gao
Oncotarget.2016; 7(5): 6335. CrossRef
- Evaluation of D-Mannoheptulose and Doxorubicin as Potential Therapeutic Agents for Breast Cancer by Targeting Glycolysis and Inducing Apoptosis
- Overexpression of C-reactive Protein as a Poor Prognostic Marker of Resectable Hepatocellular Carcinomas
- Jin Ho Shin, Chong Jai Kim, Eun Jeong Jeon, Chang Ohk Sung, Hwa Jeong Shin, Jene Choi, Eunsil Yu
- J Pathol Transl Med. 2015;49(2):105-111. Published online March 12, 2015
- DOI: https://doi.org/10.4132/jptm.2015.01.19
- 13,166 View
- 83 Download
- 22 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
C-reactive protein (CRP) is an acute phase reactant synthesized in the liver. CRP immunoreactivity is a feature of inflammatory hepatocellular adenomas with a higher risk of malignant transformation. A high serum CRP level denotes poor prognosis in hepatocellular carcinoma (HCC) patients. This study was conducted to determine whether CRP is produced in HCC and to assess the clinicopathologic significance of CRP expression in cancer cells. Methods: CRP immunoreactivity was examined in treatment-naïve HCCs (n=224) using tissue microarrays and was correlated with clinicopathologic parameters. The expression of CRP mRNA and protein was also assessed in 12 HCC cases by quantitative real-time polymerase chain reaction and immunoblotting. Hep3B and SNU-449 HCC cell lines were used for the analysis of CRP mRNA regulation by interleukin 6 (IL-6). Results: CRP was expressed in 133 of 224 HCCs (59.4%) with a variable degree of immunoreactivity (grade 1 in 25.9%; grade 2 in 20.1%; grade 3 in 13.4%). There was an inverse relationship between grade 3 CRP immunoreactivity and cancer-specific survival (p=.0047), while no associations were found with other parameters, including recurrence-free survival. The CRP mRNA expression level was significantly higher in CRP immunopositive cases than in immunonegative cases (p<.05). CRP mRNA expression was increased in Hep3B cells, but was not detected in SNU-449 cells even after IL-6 treatment. Conclusions: We report the expression of CRP in HCC for the first time. CRP expression was associated with poor cancer-specific survival in patients with resectable HCC. -
Citations
Citations to this article as recorded by- Diagnostic and prognostic relevance of inflammatory markers in surgically treated thymic epithelial tumors: An international multicenter study
Evelyn Megyesfalvi, Aron Ghimessy, Jonas Bauer, Orsolya Pipek, Kevin Saghi, Aron Gellert, Janos Fillinger, Ozlem Okumus, Vivien Teglas, Erna Ganofszky, Krisztina Bogos, Ferenc Renyi-Vamos, Zsolt Megyesfalvi, Clemens Aigner, Balazs Hegedus, Balazs Dome, Be
Lung Cancer.2025; 200: 108111. CrossRef - The Role of IL‐6, IL‐10 and CRP in Gastrointestinal Cancers
Reyhaneh Yarmohammadi, Kazem Najafi, Mina Noroozbeygi, Kimia Didehvar, Aria Rastin, Faezeh Ataei, Mohammad Reza Atashzar, Sahar Shomeil Shushtari
Cell Biology International.2025; 49(9): 1061. CrossRef - Peritumoral portal enhancement during transarterial chemoembolization: a potential prognostic factor for patients with hepatocellular carcinoma
Sofi Sennefelt Nyman, Angeliki Dimopoulou Creusen, Ulf Johnsson, Fredrik Rorsman, Johan Vessby, Charlotte Ebeling Barbier
Acta Radiologica.2022; 63(10): 1323. CrossRef - The quest for precision oncology with immune checkpoint inhibitors for hepatocellular carcinoma
Giuseppe Cabibbo, Amit G. Singal
Journal of Hepatology.2022; 76(2): 262. CrossRef - HNF-1β is a More Sensitive and Specific Marker Than C-Reactive Protein for Identifying Biliary Differentiation in Primary Hepatic Carcinomas
Pallavi A. Patil, Tamar Taddei, Dhanpat Jain, Xuchen Zhang
Archives of Pathology & Laboratory Medicine.2022; 146(2): 220. CrossRef - Malignant transformation of hepatocellular adenoma
Céline Julien, Brigitte Le Bail, Laurence Chiche, Charles Balabaud, Paulette Bioulac-Sage
JHEP Reports.2022; 4(3): 100430. CrossRef - Effect of Treatment with Colchicine after Acute Coronary Syndrome on Major Cardiovascular Events: A Systematic Review and Meta-Analysis of Clinical Trials
Erfan Razavi, Akam Ramezani, Asma Kazemi, Armin Attar, Baohui Xu
Cardiovascular Therapeutics.2022; 2022: 1. CrossRef - Steatotic and Steatohepatitic Hepatocellular Carcinomas
Umut Aykutlu, Asuman Argon, Mehmet Orman, Sezgin Ulukaya, Murat Zeytunlu, Zeki Karasu, Fulya Günşar, Deniz Nart, Ulus Akarca, Funda Yilmaz
American Journal of Surgical Pathology.2021; 45(9): 1252. CrossRef - Cytochrome P450 4A11 expression in tumor cells: A favorable prognostic factor for hepatocellular carcinoma patients
Hyuk Soo Eun, Sang Yeon Cho, Byung Seok Lee, Sup Kim, In‐Sang Song, Kwangsik Chun, Cheong‐Hae Oh, Min‐Kyung Yeo, Seok Hyun Kim, Kyung‐Hee Kim
Journal of Gastroenterology and Hepatology.2019; 34(1): 224. CrossRef - Investigating Trk Protein Expression between Oropharyngeal and Non-oropharyngeal Squamous Cell Carcinoma: Clinical Implications and Possible Roles of Human Papillomavirus Infection
Yoon Ah Cho, Ji Myung Chung, Hyunmi Ryu, Eun Kyung Kim, Byoung Chul Cho, Sun Och Yoon
Cancer Research and Treatment.2019; 51(3): 1052. CrossRef - Increased systemic zonula occludens 1 associated with inflammation and independent biomarker in patients with hepatocellular carcinoma
Amit Kumar Ram, Biju Pottakat, Balasubramaniyan Vairappan
BMC Cancer.2018;[Epub] CrossRef - C-reactive Protein Overexpression in the Background Liver of Hepatitis B Virus–Associated Hepatocellular Carcinoma Is a Prognostic Biomarker
Jin Ho Shin, Eunsil Yu, Eun Na Kim, Chong Jai Kim
Journal of Pathology and Translational Medicine.2018; 52(5): 267. CrossRef - Efficacy of vasopressin V2 receptor antagonist tolvaptan in treatment of hepatic edema
Yasunari Hiramine, Hirofumi Uto, Yasushi Imamura, Takuya Hiwaki, Takeshi Kure, Sho Ijuin, Kohei Oda, Seiichi Mawatari, Kotaro Kumagai, Koki Tokunaga, Hirofumi Higashi, Ichiro Kanetsuki, Osamu Kubozono, Shigeho Maenohara, Akio Ido
Hepatology Research.2017; 47(6): 542. CrossRef - Elevated CRP levels predict poor outcome and tumor recurrence in patients with thymic epithelial tumors: A pro- and retrospective analysis
Stefan Janik, Christine Bekos, Philipp Hacker, Thomas Raunegger, Bahil Ghanim, Elisa Einwallner, Lucian Beer, Walter Klepetko, Leonhard Müllauer, Hendrik J. Ankersmit, Bernhard Moser
Oncotarget.2017; 8(29): 47090. CrossRef - Pretreatment neutrophil-to-lymphocyte ratio predicts prognosis in patients with metastatic renal cell carcinoma receiving targeted therapy
Gui-Ming Zhang, Yao Zhu, Wei-Jie Gu, Hai-Liang Zhang, Guo-Hai Shi, Ding-Wei Ye
International Journal of Clinical Oncology.2016; 21(2): 373. CrossRef - Current Proceedings in the Molecular Dissection of Hepatocellular Adenomas: Review and Hands-on Guide for Diagnosis
Diane Goltz, Hans-Peter Fischer
International Journal of Molecular Sciences.2015; 16(9): 20994. CrossRef
- Diagnostic and prognostic relevance of inflammatory markers in surgically treated thymic epithelial tumors: An international multicenter study
- Transglutaminase 2 Expression and Its Prognostic Significance in Clear Cell Renal Cell Carcinoma
- Min Jee Park, Hae Woon Baek, Ye-Young Rhee, Cheol Lee, Jeong Whan Park, Hwal Woong Kim, Kyung Chul Moon
- J Pathol Transl Med. 2015;49(1):37-43. Published online January 15, 2015
- DOI: https://doi.org/10.4132/jptm.2014.10.25
- 11,171 View
- 81 Download
- 16 Web of Science
- 18 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
A few recent studies have demonstrated a possible role of transglutaminase 2 (TG2) in tumorigenesis or progression of renal cell carcinoma (RCC). The aim of this study was to examine TG2 expression and its clinicopathologic significance in a large number of human clear cell RCCs (CCRCCs). Methods: We analyzed 638 CCRCC patients who underwent partial or radical nephrectomy between 1995 and 2005. The expression of TG2 was determined by immunohistochemistry and categorized into four groups, according to staining intensity: negative (0), mild (1+), moderate (2+), and strong (3+). Results: TG2 staining intensity was negative in 8.5% of CCRCC (n=54), 1+ in 32.6% (n=208), 2+ in 50.5% (n=322), and 3+ in 8.5% (n=54). Strong TG2 expression was correlated with high Fuhrman nuclear grade (p=.011), high T category (p=.049), metastasis (p=.043) and male sex (p<.001) but not with N category.The survival analysis showed a significant association between strong TG2 expression and worse overall and cancer-specific survival (p=.027 and p=.010, respectively). On multivariate analysis, strong TG2 expression was a marginally significant prognostic indicator for Fuhrman nuclear grade and TNM staging (p=.054). Conclusions: Our study is the first to demonstrate the clinicopathologic significance of TG2 expression in a large number of human CCRCC samples. Strong TG2 expression was associated with high nuclear grade and poor prognosis. -
Citations
Citations to this article as recorded by- Sex-mediated effects of transglutaminase 2 inhibition on endothelial function in human resistance arteries from diabetic and non-diabetic patients
Khatera Saii, Judit Prat-Duran, Ulf Simonsen, Anders Riegels Knudsen, Jonas Amstrup Funder, Niels Henrik Buus, Estéfano Pinilla
Clinical Science.2025; 139(1): 1. CrossRef - Discovery of novel 1H-benzo[d]imidazole-4,7-dione based transglutaminase 2 inhibitors as p53 stabilizing anticancer agents in renal cell carcinoma
Ga-Ram Kim, Joon Hee Kang, Hyeon Joo Kim, Eunji Im, Jinsu Bae, Woo Sun Kwon, Sun Young Rha, Hyun Cheol Chung, Eun Yi Cho, Soo-Youl Kim, Yong-Chul Kim
Bioorganic Chemistry.2024; 143: 107061. CrossRef - Transglutaminase 2 is associated with adverse colorectal cancer survival and represents a therapeutic target
Patrizia Malkomes, Ilaria Lunger, Elsie Oppermann, Johannes Lorenz, Sara Fatima Faqar-Uz-Zaman, Jiaoyan Han, Sabrina Bothur, Paul Ziegler, Katrin Bankov, Peter Wild, Wolf Otto Bechstein, Michael A. Rieger
Cancer Gene Therapy.2023; 30(10): 1346. CrossRef - Transglutaminase Type 2-MITF axis regulates phenotype switching in skin cutaneous melanoma
Silvia Muccioli, Valentina Brillo, Tatiana Varanita, Federica Rossin, Elisabetta Zaltron, Angelo Velle, Giorgia Alessio, Beatrice Angi, Filippo Severin, Anna Tosi, Manuela D’Eletto, Luca Occhigrossi, Laura Falasca, Vanessa Checchetto, Roberto Ciaccio, Ame
Cell Death & Disease.2023;[Epub] CrossRef - The role of transglutaminase 2 in regulation of the balance between autophagy and apoptosis in tumor cells
Yu. A. Gnennaya, O. M. Semenov, N. A. Barlev
Advances in Molecular Oncology.2023; 10(4): 31. CrossRef - Application of a Fluorescence Anisotropy-Based Assay to Quantify Transglutaminase 2 Activity in Cell Lysates
Sandra Hauser, Paul Sommerfeld, Johanna Wodtke, Christoph Hauser, Paul Schlitterlau, Jens Pietzsch, Reik Löser, Markus Pietsch, Robert Wodtke
International Journal of Molecular Sciences.2022; 23(9): 4475. CrossRef - The Biological and Biomechanical Role of Transglutaminase-2 in the Tumour Microenvironment
Robert Tempest, Sonia Guarnerio, Rawan Maani, Jamie Cooper, Nicholas Peake
Cancers.2021; 13(11): 2788. CrossRef - A Precision Strategy to Cure Renal Cell Carcinoma by Targeting Transglutaminase 2
Soo-Youl Kim, Jeffrey W. Keillor
International Journal of Molecular Sciences.2020; 21(7): 2493. CrossRef - Evaluation of nuclear NF-κB, transglutaminase2, and ERCC1 as predictors of platinum resistance in testicular tumors
Alan A. Azambuja, Paula Engroff, Bruna T. Silva, Roberta C. S. Zorzetti, Fernanda B. Morrone
International braz j urol.2020; 46(3): 353. CrossRef - Transglutaminase 2-Mediated p53 Depletion Promotes Angiogenesis by Increasing HIF-1α-p300 Binding in Renal Cell Carcinoma
Seon-Hyeong Lee, Joon Hee Kang, Ji Sun Ha, Jae-Seon Lee, Su-Jin Oh, Hyun-Jung Choi, Jaewhan Song, Soo-Youl Kim
International Journal of Molecular Sciences.2020; 21(14): 5042. CrossRef - Role of Tissue Transglutaminase Catalytic and Guanosine Triphosphate-Binding Domains in Renal Cell Carcinoma Progression
Burge Ulukan, Ajna Bihorac, Tarik Sipahioglu, Robert Kiraly, Laszlo Fesus, Dilek Telci
ACS Omega.2020; 5(43): 28273. CrossRef - Transglutaminase 2: The Maestro of the Oncogenic Mediators in Renal Cell Carcinoma
Ayca Ece Nezir, Burge Ulukan, Dilek Telci
Medical Sciences.2019; 7(2): 24. CrossRef - Transglutaminase 2 takes center stage as a cancer cell survival factor and therapy target
Richard L. Eckert
Molecular Carcinogenesis.2019; 58(6): 837. CrossRef - Allosteric inhibition site of transglutaminase 2 is unveiled in the N terminus
Nayeon Kim, Joon Hee Kang, Won-Kyu Lee, Seul-Gi Kim, Jae-Seon Lee, Seon-Hyeong Lee, Jong Bae Park, Kyung-Hee Kim, Young-Dae Gong, Kwang Yeon Hwang, Soo-Youl Kim
Amino Acids.2018; 50(11): 1583. CrossRef - Renal Cell Carcinoma Is Abrogated by p53 Stabilization through Transglutaminase 2 Inhibition
Seon-Hyeong Lee, Won-Kyu Lee, Nayeon Kim, Joon Hee Kang, Kyung-Hee Kim, Seul-Gi Kim, Jae-Seon Lee, Soohyun Lee, Jongkook Lee, Jungnam Joo, Woo Sun Kwon, Sun Young Rha, Soo-Youl Kim
Cancers.2018; 10(11): 455. CrossRef - Tissue transglutaminase expression is necessary for adhesion, metastatic potential and cancer stemness of renal cell carcinoma
Yesim Bagatur, Ayca Zeynep Ilter Akulke, Ajna Bihorac, Merve Erdem, Dilek Telci
Cell Adhesion & Migration.2017; : 1. CrossRef - Characterization of clear cell renal cell carcinoma by gene expression profiling
Bryan J. Thibodeau, Matthew Fulton, Laura E. Fortier, Timothy J. Geddes, Barbara L. Pruetz, Samreen Ahmed, Amy Banes-Berceli, Ping L. Zhang, George D. Wilson, Jason Hafron
Urologic Oncology: Seminars and Original Investigations.2016; 34(4): 168.e1. CrossRef - Prognostic role of tissue transglutaminase 2 in colon carcinoma
María Jesús Fernández-Aceñero, Sofía Torres, Irene Garcia-Palmero, Cristina Díaz del Arco, J. Ignacio Casal
Virchows Archiv.2016; 469(6): 611. CrossRef
- Sex-mediated effects of transglutaminase 2 inhibition on endothelial function in human resistance arteries from diabetic and non-diabetic patients
- Prognostic Significance of Absolute Lymphocyte Count/Absolute Monocyte Count Ratio at Diagnosis in Patients with Multiple Myeloma
- Su-Jin Shin, Jin Roh, Misung Kim, Min Jung Jung, Young Wha Koh, Chan-Sik Park, Dok Hyun Yoon, Cheolwon Suh, Chan-Jeong Park, Hyun Sook Chi, Jooryung Huh
- Korean J Pathol. 2013;47(6):526-533. Published online December 24, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.6.526
- 13,539 View
- 88 Download
- 28 Crossref
-
 Abstract
Abstract
 PDF
PDF Background Absolute lymphocyte count (ALC) in peripheral blood has recently been reported to be an independent prognostic factor in multiple myeloma (MM). Previous studies indicated that the absolute monocyte count (AMC) in peripheral blood reflects the state of the tumor microenvironment in lymphomas. Neither the utility of the AMC nor its relationship with ALC has been studied in MM.
Methods The prognostic value of ALC, AMC, and the ALC/AMC ratio at the time of diagnosis was retrospectively examined in 189 patients with MM.
Results On univariate analysis, low ALC (<1,400 cells/µL), high AMC (≥490 cells/µL), and low ALC/AMC ratio (<2.9) were correlated with worse overall survival (OS) (p=.002, p=.038, and p=.001, respectively). On multivariate analysis, the ALC/AMC ratio was an independent prognostic factor (p=.047), whereas ALC and AMC were no longer statistical significant. Low ALC, high AMC, and low ALC/AMC ratio were associated with poor prognostic factors such as high International Staging System stage, plasmablastic morphology, hypoalbuminemia, and high β2-microglobulin.
Conclusions Univariate analysis demonstrated that changes in ALC, AMC, and the ALC/AMC ratio are associated with patient survival in MM. Multivariate analysis showed that, of these factors, the ALC/AMC ratio was an independent prognostic factor for OS.
-
Citations
Citations to this article as recorded by- Association between systemic immune-inflammatory markers and monoclonal gammopathy of undetermined significance in the elderly population: findings from the National health and nutrition examination survey
Chengpeng Zhang, Kangyan Hou, Dongjun Lin, Cong Xu
Clinical and Experimental Medicine.2026;[Epub] CrossRef - Low peripheral blood B lymphocyte count predicts poor outcome in patients with multiple myeloma
Yuqi Wang, Zhongxin Zheng, Qiaoxi Kang, Linjing Cai, Shanshan Zhang, Huan Chen, Youhai Yuan, Hanzhen Zhang, Xiaolei Wei, Ru Feng, Yongqiang Wei
Immunobiology.2025; 230(4): 153096. CrossRef - Inflammatory markers from routine blood tests predict survival in multiple myeloma: a Systematic Review and meta-analysis
Mengjiao Luo, Ling Qin, Yujie Li, Qianru Mei, Qiaoping Wu, Xudong Feng
Frontiers in Immunology.2025;[Epub] CrossRef - Variation of peripheral blood-based biomarkers for response of anti-PD-1 immunotherapy in non-small-cell lung cancer
Xiaoming Wang, Dayu Chen, Yuyan Ma, Dongping Mo, Feng Yan
Clinical and Translational Oncology.2024; 26(8): 1934. CrossRef - Descriptive analysis and prognostic factors in cats with myeloma‐related disorders: A multicenter retrospective study of 50 cases
Lorris Lecot, Isabelle Desmas‐Bazelle, Sarah Benjamin, Pauline De Fornel, Frédérique Ponce, Matthew Kornya, Loïc Desquilbet, Claire Beaudu‐Lange, Catherine Ibisch, David Sayag, Ghita Benchekroun, Jérémy Béguin
Journal of Veterinary Internal Medicine.2024; 38(3): 1693. CrossRef - Clinical features and treatment of newly diagnosed multiple myeloma with secondary myelofibrosis: a retrospective study
Han Xu, Yujie Xu, Mengying Wang, Chunxia Mao, Junxia Huang, Tianlan Li, Yan Gao, Shanshan Liu, Jingjing Zhou, Yi Zhang, Xianqi Feng
Therapeutic Advances in Hematology.2024;[Epub] CrossRef - Definers and drivers of functional high-risk multiple myeloma: insights from genomic, transcriptomic, and immune profiling
Rahul Banerjee, Kara I. Cicero, Sarah S. Lee, Andrew J. Cowan
Frontiers in Oncology.2023;[Epub] CrossRef - Normal Absolute Monocyte Count in Combination with Normal/High Absolute Lymphocyte Count at the Time of Relapse is Associated with Improved Survival in Patients with Early Relapsed Acute Myeloid Leukemia
Yu Zhang, Kanchun Dai, Qianying Zhang, Yisha Huang, Yiyun Feng, Deeksha Bhardwaj, Kang Yu, Jianhua Feng
Cancer Investigation.2021; 39(6-7): 550. CrossRef - Real World Experience of Daratumumab: Evaluating Lymphopenia and Adverse Events in Multiple Myeloma Patients
Francesca Cottini, Ying Huang, Nita Williams, Naresh Bumma, Abdullah M. Khan, Maria Chaudhry, Srinivas Devarakonda, Yvonne A. Efebera, Don M. Benson, Ashley E. Rosko
Frontiers in Oncology.2021;[Epub] CrossRef - Are the Derived Indexes of Peripheral Whole Blood Cell Counts (NLR, PLR, LMR/MLR) Clinically Significant Prognostic Biomarkers in Multiple Myeloma? A Systematic Review And Meta-Analysis
Xinwen Zhang, Jialin Duan, Zhenyu Wen, Hao Xiong, Xiaomin Chen, Yang Liu, Kunyu Liao, Chunlan Huang
Frontiers in Oncology.2021;[Epub] CrossRef - Combined immune score of lymphocyte to monocyte ratio and immunoglobulin levels predicts treatment-free survival of multiple myeloma patients after autologous stem cell transplant
Karen Sweiss, Jonathan Lee, Nadim Mahmud, Gregory S. Calip, Youngmin Park, Dolores Mahmud, Damiano Rondelli, Pritesh R. Patel
Bone Marrow Transplantation.2020; 55(1): 199. CrossRef - Low absolute CD4+ T cell counts in peripheral blood predict poor prognosis in patients with newly diagnosed multiple myeloma
Yan Gu, Yuanyuan Jin, Jie Ding, Wu Yujie, Qinglin Shi, Xiaoyan Qu, Sishu Zhao, Jianyong Li, Chen Lijuan
Leukemia & Lymphoma.2020; 61(8): 1869. CrossRef Normal Absolute Monocyte Count at the Time of Relapse is Associated with Improved Survival After First Salvage Therapy in Adult Patients with Early Relapsed B-Lineage Acute Lymphoblastic Leukemia
Yi-fen Shi, Na Wang, Zi-yang Huang, Rong-rong Chen, Yi-sha Huang, Yi-yi Zhu, Chong-yun Xing, Bin Liang, Kang Yu, Jian-hua Feng
Cancer Management and Research.2020; Volume 12: 7097. CrossRef- Peripheral blood biomarkers of early immune reconstitution in newly diagnosed multiple myeloma
Moritz Binder, S. Vincent Rajkumar, Martha Q. Lacy, Morie A. Gertz, Francis K. Buadi, Angela Dispenzieri, Yi L. Hwa, Amie Fonder, Miriam Hobbs, Suzanne R. Hayman, Steven R. Zeldenrust, John A. Lust, Stephen J. Russell, Nelson Leung, Prashant Kapoor, Ronal
American Journal of Hematology.2019; 94(3): 306. CrossRef - Effect of absolute monocyte count post-transplant on the outcome of patients with acute myeloid leukemia undergoing myeloablative allogeneic hematopoietic stem cell transplant with busulfan and cyclophosphamide conditioning
Liyuan Tang, Na Wang, Chongyun Xing, Qiang Zhuang, Bin Liang, Lan Sun, Yi Chen, Yan Qian, Zhijian Shen, Songfu Jiang, Kang Yu, Jianhua Feng
Leukemia Research.2018; 69: 60. CrossRef - A lower ALC/AMC ratio is associated with poor prognosis of peripheral T-cell lymphoma-not otherwise specified
Qian Li, Shuang Gao, Jing Ma, Su Liu, Yuanfang Yue, Lin Chen, Han Li, Xue Wang, Dongying Li, Zeng Cao, Zhigang Zhao, Xiaofang Wang, Yong Yu, Yizhuo Zhang, Yafei Wang
Leukemia Research.2018; 73: 5. CrossRef - Peripheral Blood Lymphocyte-to-Monocyte Ratio as a Useful Prognostic Factor in Newly Diagnosed Multiple Myeloma
Ying Tian, Yue Zhang, Wan-Qiu Zhu, Xiao-Lei Chen, He-Bing Zhou, Wen-Ming Chen
BioMed Research International.2018; 2018: 1. CrossRef - Significance of the absolute lymphocyte/monocyte ratio as a prognostic immune biomarker in newly diagnosed multiple myeloma
T Dosani, F Covut, R Beck, J J Driscoll, M de Lima, E Malek
Blood Cancer Journal.2017; 7(6): e579. CrossRef - Lymphocyte-to-monocyte ratio can predict mortality in pancreatic adenocarcinoma
Gurshawn Singh, Ammar Nassri, David Kim, Hong Zhu, Zeeshan Ramzan
World Journal of Gastrointestinal Pharmacology and Therapeutics.2017; 8(1): 60. CrossRef - Bone marrow microenvironmental CD4 + and CD8 + lymphocyte infiltration patterns define overall- and progression free survival in standard risk multiple myeloma – an analysis from the Austrian Myeloma Registry
Wolfgang Willenbacher, Ella Willenbacher, Claudia Zelle-Rieser, Rainer Biedermann, Roman Weger, Karin Jöhrer, Andrea Brunner
Leukemia & Lymphoma.2016; 57(6): 1478. CrossRef - Absolute lymphocyte count as a prognostic marker in newly diagnosed multiple myeloma patients
C. Suriu, L. Akria, D. Azoulay, E. Shaoul, M. Barhoum, A. Braester
International Journal of Laboratory Hematology.2016;[Epub] CrossRef - The Peripheral Blood Mononuclear Cell Count Is Associated With Bone Health in Elderly Men
Xianfeng Lin, Hejun Yu, Chenchen Zhao, Yu Qian, Dun Hong, Kangmao Huang, Jian Mo, An Qin, Xiangqian Fang, Shunwu Fan
Medicine.2016; 95(15): e3357. CrossRef - Prognostic role of lymphocyte to monocyte ratio for patients with cancer: evidence from a systematic review and meta-analysis
Liangyou Gu, Hongzhao Li, Luyao Chen, Xin Ma, Xintao Li, Yu Gao, Yu Zhang, Yongpeng Xie, Xu Zhang
Oncotarget.2016; 7(22): 31926. CrossRef - Do lymphocytes count in myeloma? Are we absolutely sure?
Tamar Tadmor
Leukemia & Lymphoma.2015; 56(5): 1193. CrossRef - Absolute lymphocyte count is unrelated to overall survival in newly diagnosed elderly patients with multiple myeloma treated with immunomodulatory drugs
Mariasanta Napolitano, Giorgia Saccullo, Roberto Bono, Antonio Branca, Clotilde Cangialosi, Salvatrice Mancuso, Simona Raso, Gerlando Quintini, Maria Grazia Lipari, Francesco Fabbiano, Giorgina Specchia, Alberto Dolce, Francesco Di Raimondo, Sergio Siragu
Leukemia & Lymphoma.2015; 56(5): 1507. CrossRef - Distinct Transcriptional and Anti-Mycobacterial Profiles of Peripheral Blood Monocytes Dependent on the Ratio of Monocytes: Lymphocytes
Vivek Naranbhai, Helen A. Fletcher, Rachel Tanner, Matthew K. O'Shea, Helen McShane, Benjamin P. Fairfax, Julian C. Knight, Adrian V.S. Hill
EBioMedicine.2015; 2(11): 1619. CrossRef - Prognostic value of absolute monocyte count in chronic lymphocytic leukaemia
László Szerafin, János Jakó, Ferenc Riskó
Orvosi Hetilap.2015; 156(15): 592. CrossRef - The lymphocyte to monocyte ratio in peripheral blood represents a novel prognostic marker in patients with pancreatic cancer
Michael Stotz, Joanna Szkandera, Tatjana Stojakovic, Julia Seidel, Hellmut Samonigg, Peter Kornprat, Renate Schaberl-Moser, Fridericke Seggewies, Gerald Hoefler, Armin Gerger, Martin Pichler
Clinical Chemistry and Laboratory Medicine (CCLM).2015;[Epub] CrossRef
- Association between systemic immune-inflammatory markers and monoclonal gammopathy of undetermined significance in the elderly population: findings from the National health and nutrition examination survey
- Proposal for a Standardized Pathology Report of Gastroenteropancreatic Neuroendocrine Tumors: Prognostic Significance of Pathological Parameters
- Mee-Yon Cho, Jin Hee Sohn, So Young Jin, Hyunki Kim, Eun Sun Jung, Mi-Jung Kim, Kyoung-Mee Kim, Woo Ho Kim, Joon Mee Kim, Yun Kyung Kang, Joon Hyuk Choi, Dae Young Kang, Youn Wha Kim, Eun Hee Choi
- Korean J Pathol. 2013;47(3):227-237. Published online June 25, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.3.227
- 15,319 View
- 148 Download
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF Background There is confusion in the diagnosis and biological behaviors of gastroenteropancreatic neuroendocrine tumors (GEP-NETs), because of independently proposed nomenclatures and classifications. A standardized form of pathology report is required for the proper management of patients.
Methods We discussed the proper pathological evaluation of GEP-NET at the consensus conference of the subcommittee meeting for the Gastrointestinal Pathology Study Group of the Korean Society of Pathologists. We then verified the prognostic significance of pathological parameters from our previous nationwide collection of pathological data from 28 hospitals in Korea to determine the essential data set for a pathology report.
Results Histological classification, grading (mitosis and/or Ki-67 labeling index), T staging (extent, size), lymph node metastasis, and lymphovascular and perineural invasion were significant prognostic factors and essential for the pathology report of GEP-NET, while immunostaining such as synaptophysin and chromogranin may be optional. Furthermore, the staging system, either that of the 2010 American Joint Cancer Committee (AJCC) or the European Neuroendocrine Tumor Society (ENETS), should be specified, especially for pancreatic neuroendocrine neoplasms.
Conclusions A standardized pathology report is crucial for the proper management and prediction of prognosis of patients with GEP-NET.
-
Citations
Citations to this article as recorded by- Analysis of Prognostic Risk Factors of Endoscopic Submucosal Dissection (ESD) and Curative Resection of Gastrointestinal Neuroendocrine Neoplasms
Yuan Si, ChaoKang Huang, JingBin Yuan, XianHui Zhang, QingQiang He, ZhiJin Lin, Ling He, ZhongXin Liu, Yuvaraja Teekaraman
Contrast Media & Molecular Imaging.2022;[Epub] CrossRef - Standardization of the pathologic diagnosis of appendiceal mucinous neoplasms
Dong-Wook Kang, Baek-hui Kim, Joon Mee Kim, Jihun Kim, Hee Jin Chang, Mee Soo Chang, Jin-Hee Sohn, Mee-Yon Cho, So-Young Jin, Hee Kyung Chang, Hye Seung Han, Jung Yeon Kim, Hee Sung Kim, Do Youn Park, Ha Young Park, So Jeong Lee, Wonae Lee, Hye Seung Lee,
Journal of Pathology and Translational Medicine.2021; 55(4): 247. CrossRef - Preoperative diagnosis of well‐differentiated neuroendocrine tumor in common hepatic duct by brush cytology: A case report
Jiwoon Choi, Kyong Joo Lee, Sung Hoon Kim, Mee‐Yon Cho
Diagnostic Cytopathology.2019; 47(7): 720. CrossRef - Primary renal well-differentiated neuroendocrine tumors: report of six cases with an emphasis on the Ki-67 index and mitosis
Bohyun Kim, Han-Seong Kim, Kyung Chul Moon
Diagnostic Pathology.2019;[Epub] CrossRef - Primary low‐grade neuroendocrine carcinoma of the skin: An exceedingly rare entity
Tiffany Y. Chen, Annie O. Morrison, Joe Susa, Clay J. Cockerell
Journal of Cutaneous Pathology.2017; 44(11): 978. CrossRef - Prognostic Validity of the American Joint Committee on Cancer and the European Neuroendocrine Tumors Staging Classifications for Pancreatic Neuroendocrine Tumors
Jae Hee Cho, Ji Kon Ryu, Si Young Song, Jin-Hyeok Hwang, Dong Ki Lee, Sang Myung Woo, Young-Eun Joo, Seok Jeong, Seung-Ok Lee, Byung Kyu Park, Young Koog Cheon, Jimin Han, Tae Nyeun Kim, Jun Kyu Lee, Sung-Hoon Moon, Hyunjin Kim, Eun Taek Park, Jae Chul Hw
Pancreas.2016; 45(7): 941. CrossRef - Early diagnosis and treatment of gastrointestinal neuroendocrine tumors
Hong Shen, Zhuo Yu, Jing Zhao, Xiu-Zhen Li, Wen-Sheng Pan
Oncology Letters.2016; 12(5): 3385. CrossRef - Recent Updates on Neuroendocrine Tumors From the Gastrointestinal and Pancreatobiliary Tracts
Joo Young Kim, Seung-Mo Hong
Archives of Pathology & Laboratory Medicine.2016; 140(5): 437. CrossRef - Pancreatic neuroendocrine tumors: Correlation between the contrast-enhanced computed tomography features and the pathological tumor grade
Koji Takumi, Yoshihiko Fukukura, Michiyo Higashi, Junnichi Ideue, Tomokazu Umanodan, Hiroto Hakamada, Ichiro Kanetsuki, Takashi Yoshiura
European Journal of Radiology.2015; 84(8): 1436. CrossRef - Tumeurs neuroendocrines du tube digestif et du pancréas : ce que le pathologiste doit savoir et doit faire en 2014
Jean-Yves Scoazec, Anne Couvelard
Annales de Pathologie.2014; 34(1): 40. CrossRef - Spectrum of Gastroenteropancreatic NENs in Routine Histological Examinations of Bioptic and Surgical Specimen: A Study of 161 Cases Collected from 17 Departments of Pathology in the Czech Republic
Václav Mandys, Tomáš Jirásek
Gastroenterology Research and Practice.2014; 2014: 1. CrossRef - p27 Loss Is Associated with Poor Prognosis in Gastroenteropancreatic Neuroendocrine Tumors
Hee Sung Kim, Hye Seung Lee, Kyung Han Nam, Jiwoon Choi, Woo Ho Kim
Cancer Research and Treatment.2014; 46(4): 383. CrossRef
- Analysis of Prognostic Risk Factors of Endoscopic Submucosal Dissection (ESD) and Curative Resection of Gastrointestinal Neuroendocrine Neoplasms
- Histopathologic Predictors of Lymph Node Metastasis and Prognosis in Tonsillar Squamous Cell Carcinoma
- Dong Jin Lee, Mi Jung Kwon, Eun Sook Nam, Ji Hyun Kwon, Jin Hwan Kim, Young-Soo Rho, Hyung Sik Shin, Seong Jin Cho
- Korean J Pathol. 2013;47(3):203-210. Published online June 25, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.3.203
- 11,359 View
- 67 Download
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF Background Risk factors for lymph node metastasis in tonsillar squamous cell carcinoma (TSCC) need to be established to determine the degree of surgery required to achieve high curative rates. However, little is known currently about the histopathological features predicting prognosis, specifically in TSCC.
Methods This study included 53 patients who underwent surgical resection with neck dissection. Clinicopathological factors investigated included age, gender, alcohol use, tobacco consumption, tumor stage, adjacent structure involvement, cell differentiation, squamous dysplasia,
in situ carcinoma associated with primary invasive cancer, carcinomain situ skip lesions, necrosis, invasive front, depth of invasion, and lymphatic, muscle, or perineural invasion.Results Contralateral cervical metastasis was associated with higher T stages and soft palate invasion. Lymphatic and muscle invasion were associated with ipsilateral cervical metastasis. Advanced T stage, invasion to the base of tongue, and skip lesions were associated with decreased disease-free survival. Advanced T stage and skip lesions were associated with worse overall survival.
Conclusions Advanced T stage and soft palate invasion may predict a high risk of contralateral nodal metastasis. T stage and skip lesion are worse prognostic factors in TSCC and should be commented in pathology reports.
-
Citations
Citations to this article as recorded by- A Systematic Review of Occult Contralateral Neck Metastasis in Tonsillar Squamous Cell Carcinoma
Nihal Punjabi, Arjun Sharma, Jamie Park, Kari Kennedy, Jared C. Inman
The Laryngoscope.2025; 135(1): 27. CrossRef - OpenAi’s ChatGPT-4, BARD and YOU.com (AI) and the Cancer Patient, for Now, Caveat Emptor, but Stay Tuned
Glenn Tisman, Raju Seetharam
Digital Medicine and Healthcare Technology.2023;[Epub] CrossRef - Comprehensive Transcriptome Analysis Reveals the Distinct Gene Expression Patterns of Tumor Microenvironment in HPV-Associated and HPV-Non Associated Tonsillar Squamous Cell Carcinoma
Reham M. Alahmadi, Najat Marraiki, Mohammed Alswayyed, Hatim A. Khoja, Abdullah E. Al-Anazi, Rawan M. Alahmadi, Meshael M. Alkusayer, Bandar Alosaimi, Maaweya Awadalla
Cancers.2023; 15(23): 5548. CrossRef - Predictors of contralateral‐bilateral nodal disease in oropharyngeal cancer: A National Cancer Data Base Study
Masanari G. Kato, Mark A. Ellis, Shaun A. Nguyen, Terry A. Day
Head & Neck.2018; 40(2): 338. CrossRef - Clinical implication of programmed cell death-1 ligand-1 expression in tonsillar squamous cell carcinoma in association with intratumoral heterogeneity, human papillomavirus, and epithelial-to-mesenchymal transition
Mi Jung Kwon, Young-Soo Rho, Eun Sook Nam, Seong Jin Cho, Hye-Rim Park, Soo Kee Min, Jinwon Seo, Ji-Young Choe, Eun Soo Kim, Bumjung Park, Mineui Hong, Kyueng-Whan Min
Human Pathology.2018; 80: 28. CrossRef - Comparison of the eighth version of the American Joint Committee on Cancer manual to the seventh version for colorectal cancer: A retrospective review of our data
Guo-Jun Tong, Gui-Yang Zhang, Jian Liu, Zhao-Zheng Zheng, Yan Chen, Ping-Ping Niu, Xu-Ting Xu
World Journal of Clinical Oncology.2018; 9(7): 148. CrossRef - HIPK2 Overexpression and Its Prognostic Role in Human Papillomavirus-Positive Tonsillar Squamous Cell Carcinoma
Mi Jung Kwon, So Young Kang, Eun Sook Nam, Seong Jin Cho, Young-Soo Rho
BioMed Research International.2017; 2017: 1. CrossRef - Frequent hepatocyte growth factor overexpression and low frequency of c-Met gene amplification in human papillomavirus–negative tonsillar squamous cell carcinoma and their prognostic significances
Mi Jung Kwon, Dong Hoon Kim, Hye-Rim Park, Hyung Sik Shin, Ji Hyun Kwon, Dong Jin Lee, Jin Hwan Kim, Seong Jin Cho, Eun Sook Nam
Human Pathology.2014; 45(7): 1327. CrossRef - CT and MR imaging findings of palatal tumors
Hiroki Kato, Masayuki Kanematsu, Hiroki Makita, Keizo Kato, Daijiro Hatakeyama, Toshiyuki Shibata, Keisuke Mizuta, Mitsuhiro Aoki
European Journal of Radiology.2014; 83(3): e137. CrossRef
- A Systematic Review of Occult Contralateral Neck Metastasis in Tonsillar Squamous Cell Carcinoma

 E-submission
E-submission

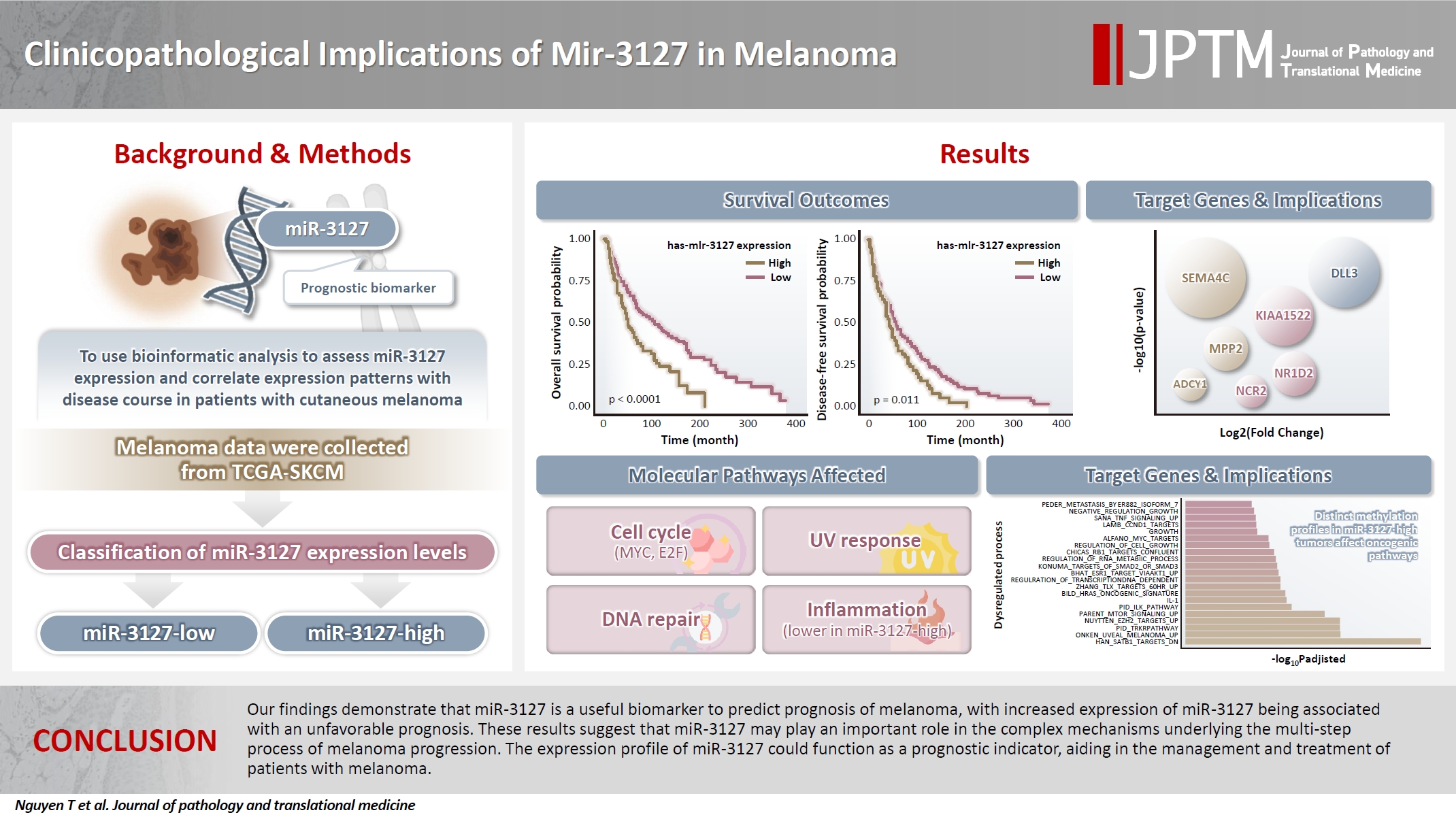
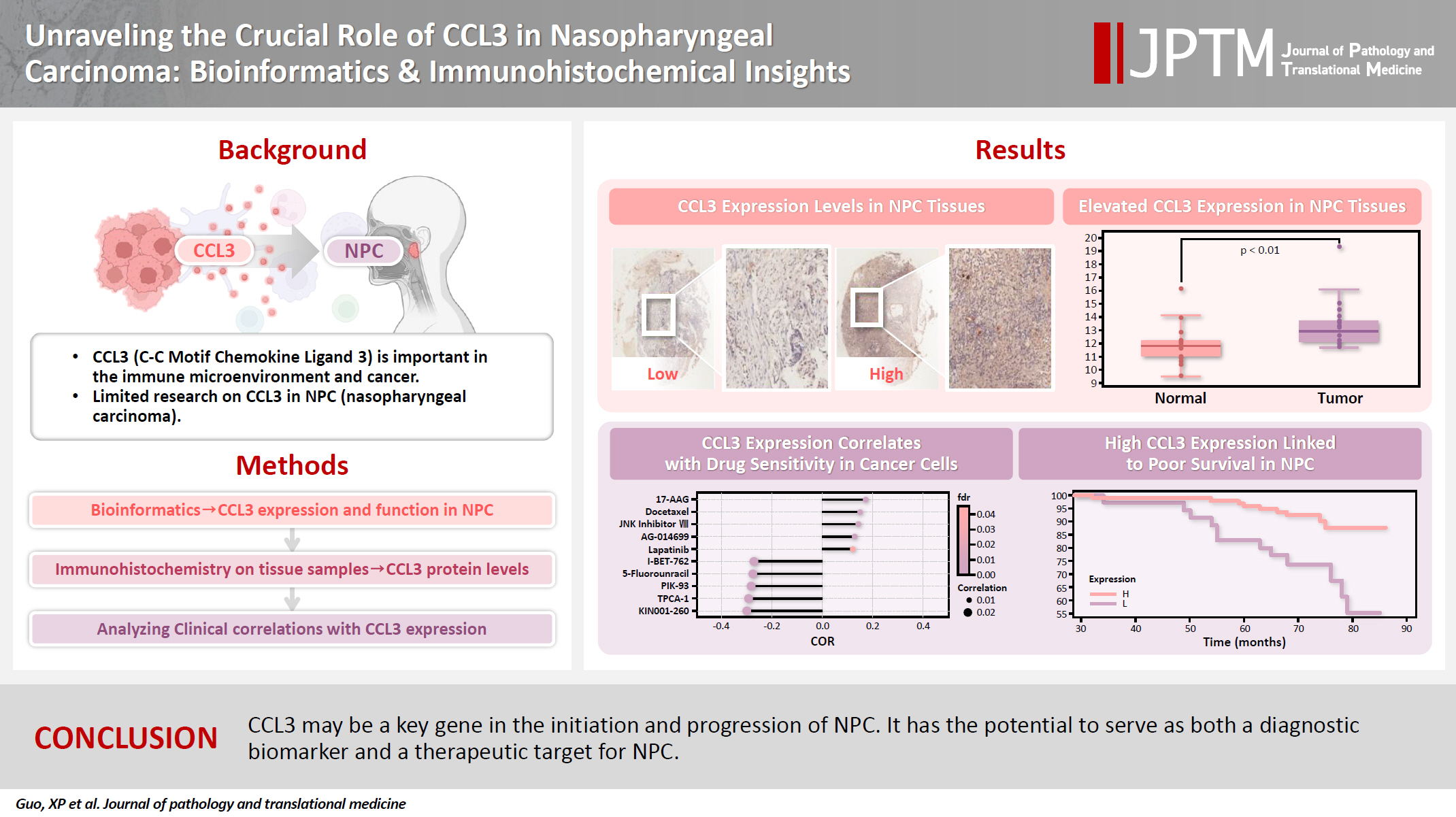

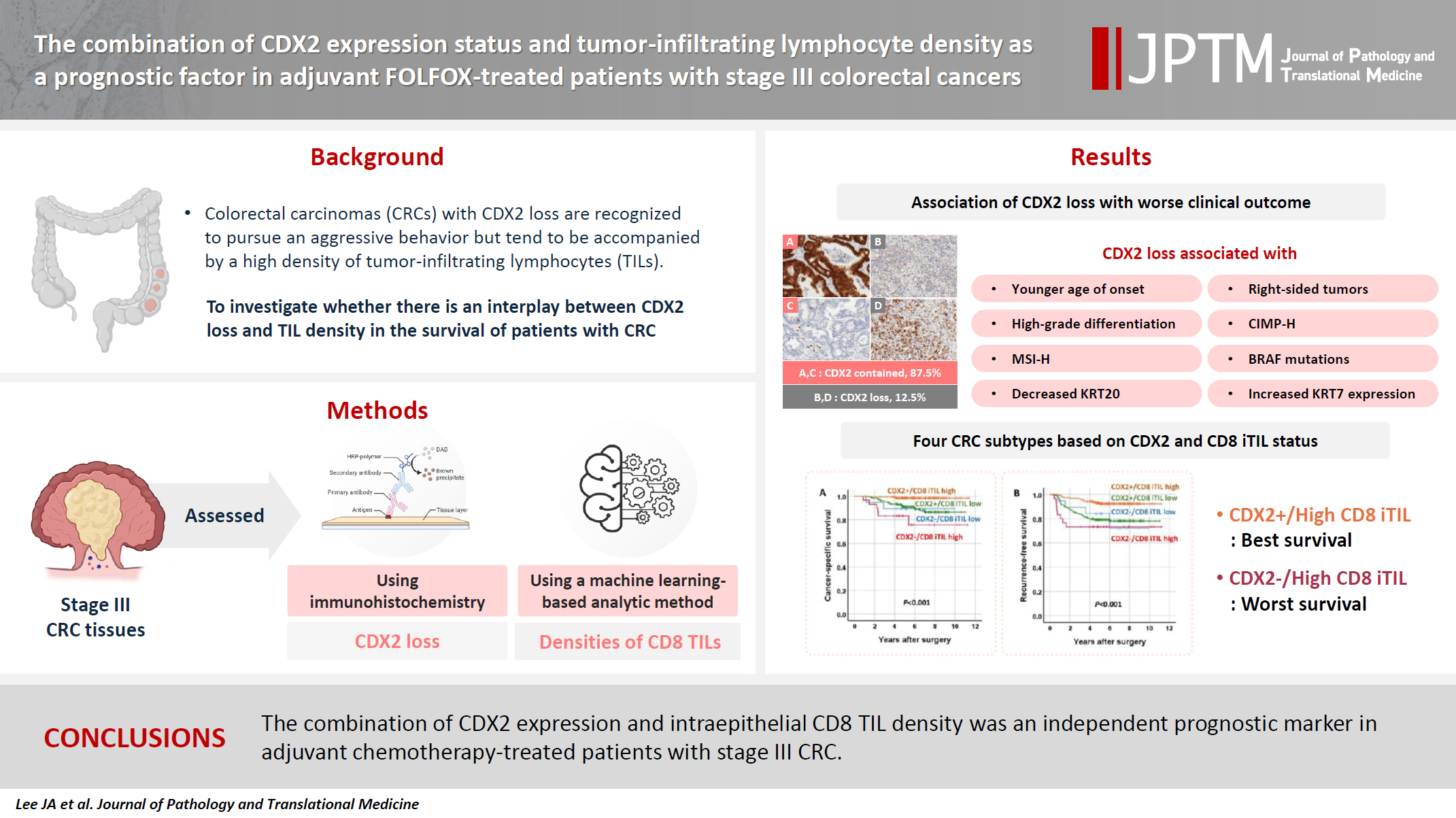


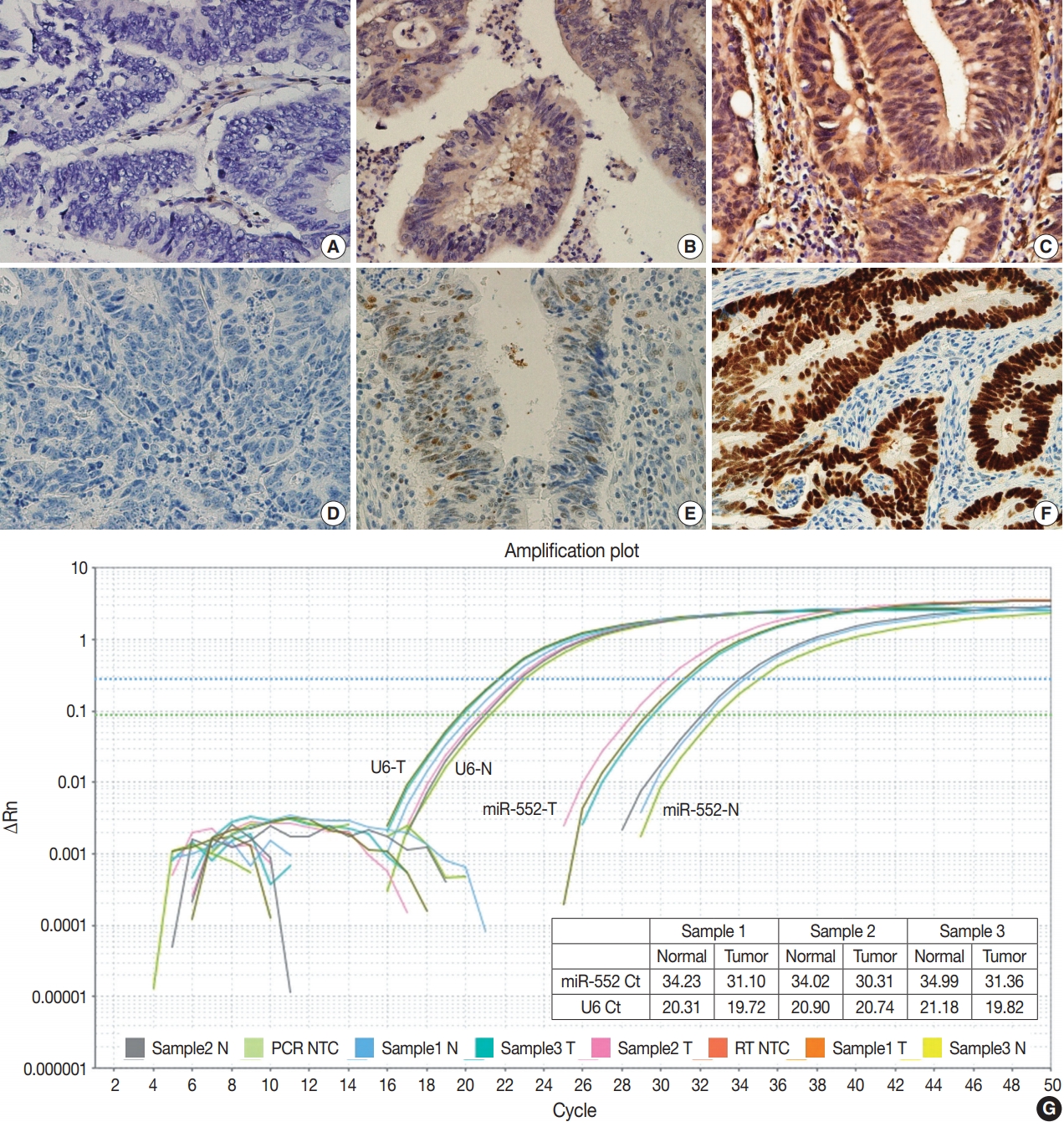
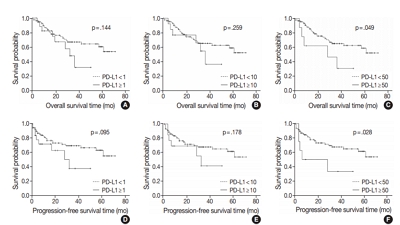
 First
First Prev
Prev



