Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 59(6); 2025 > Article
-
Original Article
Clinicopathological implications of miR-3127 in melanoma -
Truong Phan-Xuan Nguyen1
 , Minh-Khang Le2
, Minh-Khang Le2 , Chau M. Bui3
, Chau M. Bui3 , Vuong Gia Huy4
, Vuong Gia Huy4
-
Journal of Pathology and Translational Medicine 2025;59(6):371-381.
DOI: https://doi.org/10.4132/jptm.2025.07.08
Published online: October 16, 2025
1Department of Pathology, School of Medicine, University of Medicine & Pharmacy at Ho Chi Minh City, Ho Chi Minh, Vietnam
2Department of Pathology, University of Yamanashi, Yamanashi, Japan
3Department of Pathology, University of Rochester Medical Center, Rochester, NY, USA
4Department of Pathology, University of Iowa Hospitals and Clinics, Iowa City, IA, USA
- Corresponding author Vuong Gia Huy, MD, PhD Department of Pathology, University of Iowa Hospitals and Clinics, Iowa City, IA 52242, USA Tel: +1-4057193776, E-mail: huyvuong@hotmail.com
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,923 Views
- 137 Download
Abstract
-
Background
- Cutaneous melanoma is the most lethal of all skin cancers. Recent studies suggested that miR-3127 is dysregulated in multiple tumor types and has important roles in tumorigenesis and cancer progression, giving it potential as a prognostic biomarker. The aim of this study was to use bioinformatic analysis to assess miR-3127 expression and correlate expression patterns with disease course in patients with cutaneous melanoma.
-
Methods
- miRNA, mRNA sequencing, DNA methylation data, and clinical information of cutaneous melanoma cases were downloaded from the Human Cancer Atlas – Skin Cutaneous Melanoma (TCGA-SKCM). miR-3127 expression was classified into miR-3127–low and miR-3127–high clusters using maximally selected rank statistics.
-
Results
- Clustering analysis showed that high expression of miR-3127 (≥20.3 reads per million) was associated with worse progression-free (p < .001) and overall (p = .011) survival compared to low miR-3127 expression. More than five thousand differentially expressed genes between the two miR-3127 sample groups encoded cell differentiation markers, cytokines, growth factors, translocated cancer genes, and oncogenes. Pathway analysis revealed that miR-3127–high samples related to activity of proliferation, DNA repair, and ultraviolet response.
-
Conclusions
- The expression level of miR-3127 could act as a prognostic indicator for patients with melanoma.
- Cutaneous melanoma, which frequently develops from the epidermal-dermal junction where melanocytes undergo malignant transformation, is known for its rapid progression, high invasiveness, metastatic potential, and limited responsiveness to treatment [1]. There were an estimated 324,000 new cases and approximately 57,000 deaths worldwide in 2020 of this primary skin cancer [2]. Surgical removal of the primary melanoma typically yields favorable outcomes, resulting in a relatively high survival rate. However, the 3-year survival rate drops to only 5%–32% when melanoma spreads to lymph nodes or distant sites [3]. Therefore, it is crucial to identify effective biomarkers for melanoma to improve prognostic accuracy and guide targeted treatments.
- MicroRNAs (miRNAs) are a class of small non-coding RNAs that regulate gene expression post-transcription by binding to the 3′-untranslated regions (UTRs) of target mRNAs. miRNAs are crucial in the regulation of tumor growth and metastasis [4]. Recent studies suggested that miR-3127 functions in a variety of malignancies as either a tumor suppressor or an oncogene [5]. For instance, there is evidence to suggest that downregulation of miR-3127-5p could facilitate the transition from epithelial to mesenchymal by activating the WNT/FZD4/β-catenin signaling pathway, thereby promoting tumor progression and metastasis [6]. Furthermore, miR-3127 represses the growth and invasion of bladder cancer cells by directly targeting the 3′-UTR of RAP2A, whereas in hepatocellular carcinoma (HCC), it stimulates proliferation and tumorigenesis via the AKT/FOXO1 signaling pathway [5-7]. At present, the precise function of miR-3127 in melanoma and its role in disease progression are not well understood.
- In this study, we aimed to investigate the association between expression levels of miR-3127, clinicopathological characteristics, and overall survival (OS) in cases of melanoma. We also worked on establishing miR-3127 as a prognostic biomarker to help guide the management and treatment of patients with melanoma.
INTRODUCTION
- Data preprocessing
- We accessed The Human Cancer Atlas – Skin Cutaneous Melanoma (TCGA-SKCM) (https://portal.gdc.cancer.gov/projects/TCGA-SKCM) and retrieved data regarding miRNA expression (n = 450 samples), mRNA expression (n = 472 samples), and DNA methylation (n = 472 samples) of melanoma patients. We first filtered out normal samples and included primary (n=237) and metastatic (n=209) tumor samples. We categorized samples from stages I and II, as well as tumors from stages III and IV with code "01," as primary samples. Meanwhile, samples from stages III and IV with code "06" were classified as metastatic samples. Metastatic melanomas included tumors from regional sites (such as regional lymph nodes and regional skin/soft tissue) as well as distant sites. We then eliminated duplicated samples (same sample type in a patient). Finally, the numbers of samples available for miRNA, mRNA, and DNA methylation were 446, 468, and 467, respectively. Included clinical characteristics were age, sex, race, Breslow’s depth, TNM stages, radiation, chemotherapy, OS, and disease-free survival (DFS).
- Clustering of miR-3127 expression
- Maximally selected rank statistics (MSRS) is a method that selects the cut-off point, whose rank metric is maximum [8]. Since our purpose was to investigate the clinicopathological significance of miR-3127, we employed standardized log-rank statistics as the ranking metric. This metric usually evaluates the association between genes and/or miRNAs and survival outcomes [9]. We used the expression of miR-3127 and OS to compute the rank metric. We then divided the studied samples into tumors with low (miR-3127–low) and high (miR-3127–high) expressions of miR-3127.
- mRNA and miRNA expression analyses
- We employed the DESeq2 pipeline to perform differential expression analysis (DEA) [10]. DEA was conducted to compare each miRNA and mRNA expression between miR-3127–high and miR-3127–low samples. In pathway analysis, we used the gene set enrichment analysis (GSEA) method to examine the difference in the pathway activity between these two sample groups [11]. The Benjamini-Hochberg multiple corrections were applied to reduce false-positive results.
- DNA methylation analysis
- We followed the ChAMP pipeline to perform differential methylation position (DMP), differential methylation region (DMR), and methylation GSEA [12]. We focused on the difference in methylation statuses between miR-3127–low and miR-3127–high tumors.
- Analysis platform
- We used R software programming language ver. 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) for all the analyses. For clinicopathological analyses, we performed Wilcoxon and chi-squared tests for continuous and categorical variables, respectively. For survival analyses, Kaplan-Meier, univariate, and multivariate Cox analyses were conducted. All the hypothesis tests were considered significant when p < .05 or adjusted p < .5.
MATERIALS AND METHODS
- Clustering analysis showed high expression of miR-3127 conferred worse outcomes
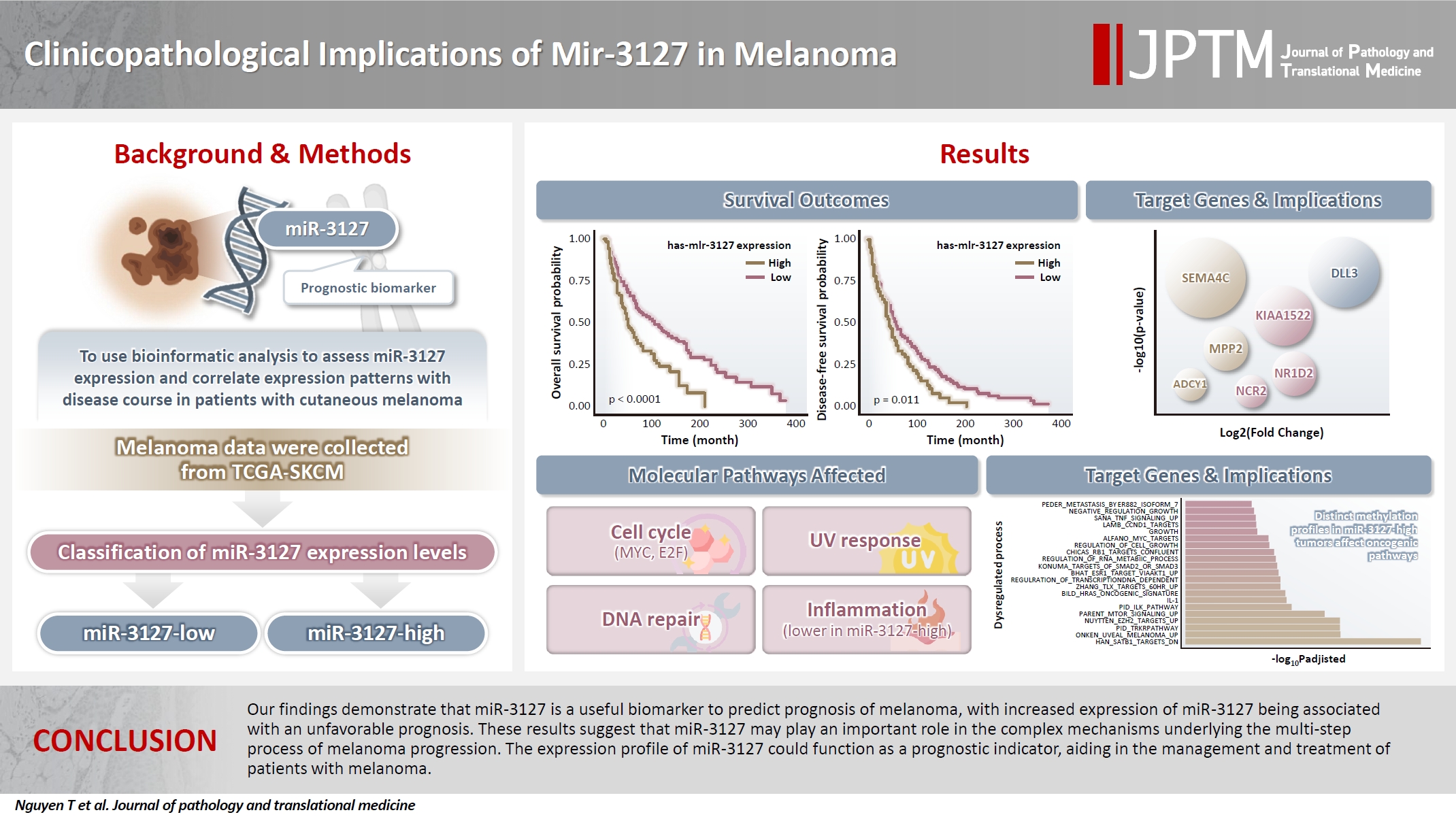
- Using MSRS, the optimal miR-3127 expression threshold was 20.3 read per million (RPM) (Fig. 1A, D). Therefore, samples were classified as miR-3127–low (n = 317) and miR-3127–high (n = 129) based on miR-3127 expression of <20.3 and ≥20.3 RPM, respectively. Table 1 presented a summary of the study cohort's characteristics, divided into miR-3127–high and miR-3127–low groups. Among the samples, 48.8% of metastatic and 51.2% of non-metastatic patients exhibited high miR-3127 expression, with no statistically significant difference observed. This indicates that miR-3127 expression was not associated with metastatic status.
- Overall, patients with miR-3127–high samples showed significantly poorer DFS (p < .001) and OS (p = .011) (Table 2, Fig. 1B, C). When stratified into primary and metastatic groups, those with miR-3127–high metastatic melanoma exhibited the poorest prognosis. Notably, patients with miR-3127–high non-metastatic melanoma had both worse OS and DFS compared to those with miR-3127–low metastatic melanoma (Supplementary Fig. S1).
- Fig. 1E illustrated the even distributions of miR-3127–low and miR-3127–high in miRNA “space,” suggesting limited relationships between miR-3127 and other miRNAs. DEA of miRNA expression found that 165 (8.8%) and 150 (8.0%) miRNAs of 1,881 miRNA probes were respectively upregulated and downregulated in miR-3127–high samples (Supplementary Table S1). DEA revealed that the top 5 differentially expressed miRNAs (excluding miR-3127) were miR-1301, miR-3691, miR-6509, miR-3150b, and miR-3680-1 (Fig. 1F).
- Large portions of genes in cell differentiation markers, cytokines, growth factors, translocated cancer genes, and oncogenes are differentially expressed by miR-3127 groups
- The DEA of mRNA expression indicated 5,484 differentially expressed genes (DEGs), accounting for 9.1% of genes analyzed (Supplementary Table S2). Fig. 2A shows the dimension reduction of only DEGs, where miR-3127–high samples are more densely distributed in the lower area of the graph. To investigate the expression pattern of miR-3127 targets, we first collected the target genes of miR-3127 by accessing collection “C3” of the MSigDB database and retrieving genes in MIR3127-3P and MIR3127-5P (Supplementary Table S3). We then examined the DEGs within these genes (Fig. 2B). Among the predicted target genes, the expressions of PAFAH1B2, FAM172A, GOLM2, NKAPD1, and DDX6 were lower in miR-3127–high samples while SEMA4C, KIAA1522, DLL3, SEMA4F, and MPP2 were highly expressed by miR-3127–high tumors. In the analysis of the overall mRNA dataset, the top 10 most significant DEGs (Fig. 2C) included two downregulated genes (IGFBP3 and KIAA0040) and eight upregulated genes (RTN4R, PAK4, SNTA1, CNNM3, NAXE, SMYD5, PPARD, and EPHX1).
- We then asked which gene functions were largely different between the two miR-3127 sample groups. We collected the genes belonging to eight important function families, including cell differentiation markers (CDM), cytokine and growth factor (CGF), homeodomain proteins (HP), oncogenes (O), protein kinase (PK), translocated cancer gene (TCG), transcription factor (TF), and tumor suppressor (TS). The detail information can be found in the website of the MSigDB databse (https://www.gsea-msigdb.org/gsea/msigdb/human/gene_families.jsp). We used genes that only belong to CDM (n = 367), CGF (n = 452), HP (n = 291), O (n = 328), PK (n = 513), TCG (n = 290), TF (n = 1536), and TS (n = 82). Genes with mutual functions were excluded. Fig. 2D shows the number of genes in each function family, color-coded by DEGs. We found that large proportions of CDM (48.3%), CGF (47.1%), TCG (42.1%), and oncogenes (41.8%) were DEGs.
- Pathway analysis revealed that miR-3127–high samples related to activity of proliferation, DNA repair, and ultraviolet response and lower inflammation-related processes
- We employed collection H (hallmark gene sets) of the MSigDB database, including 50 hallmark pathways. Next, we performed the classic GSEA to compare the pathway activity between miR-3127–low and miR-3127–high samples (Fig. 2E). The full results are shown in Supplementary Table S4. Overall, there were 15 upregulated and 16 downregulated pathways while 19 pathways showed no significant difference. We found that cell cycle-related (MYC targets, E2F targets, and G2M checkpoint), DNA repair, upregulated genes of ultraviolet (UV) response, and metabolism-related (glycolysis and reactive oxygen species) pathways were enriched in miR-3127–high melanomas. On the other hand, miR-3127–low samples showed the enrichment of inflammation-related (interferon-γ and α response, and inflammatory responses) processes.
- Methylation analysis
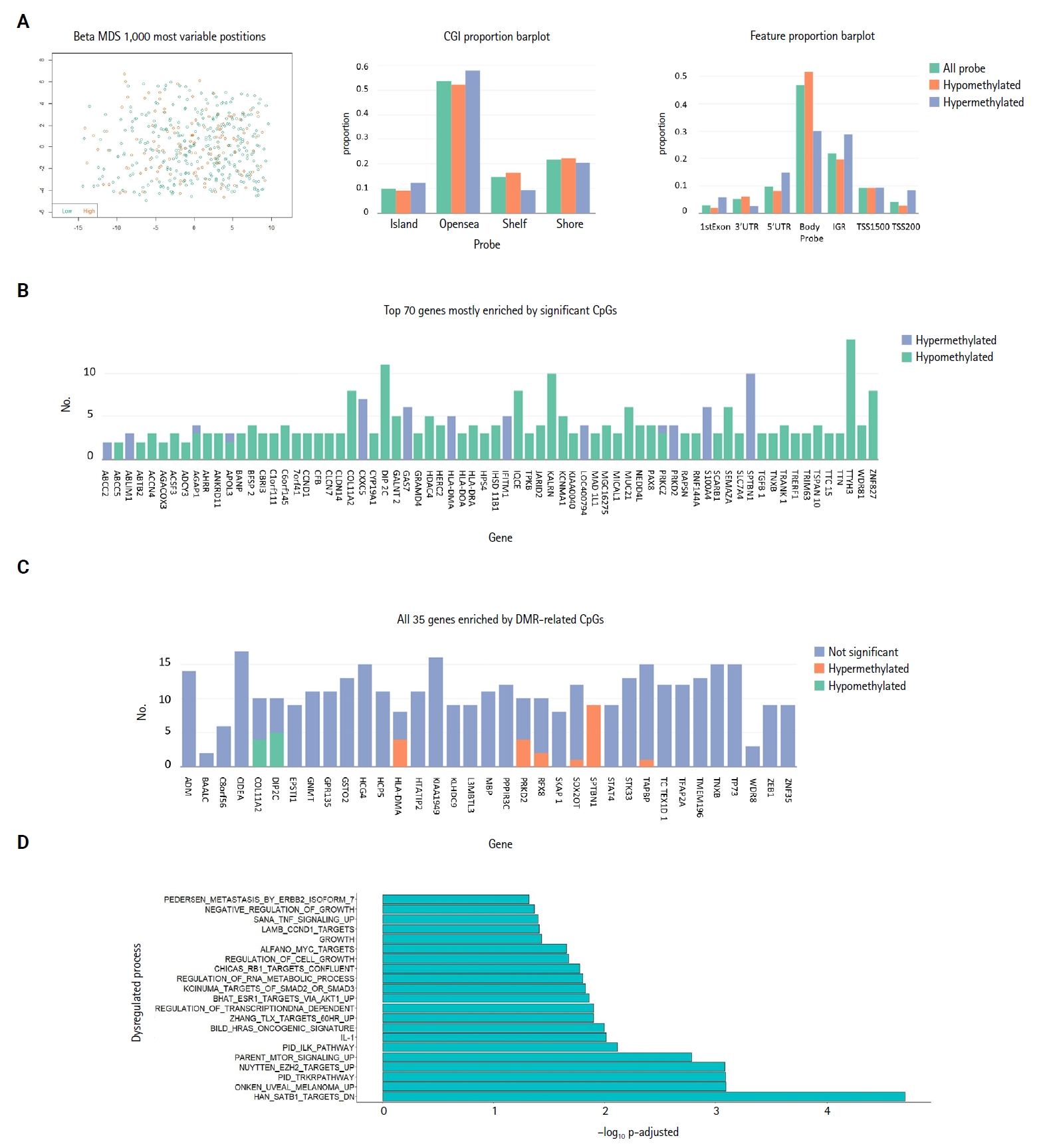
- Investigation of the 1,000 most variable methylation positions (Fig. 3A, left) showed no clear difference in the distribution between miR-3127–low and miR-3127–high samples. Fig. 3A illustrates the proportion of hypomethylated and hypermethylated CpG probes (in miR-3127–high samples) by CpG island (Fig. 3A, middle) and features (Fig. 3A, right). In DMP, there were 57 hypomethylated, 10 hypermethylated, and three mixed CpG positions that exhibited both hypermethylation and hypomethylation in miR-3127–high melanomas (Fig. 3B) while six and two DNA regions of these tumors were hypermethylated and hypomethylated, respectively, in DMR (Fig. 3C) when compare to miR-3127–low melanomas. In summary, ABCC2, ABLIM1, CXXC5, GAS7, HLA-DMA, IFITIM1, LOC400794, PRKD2, S100A4, SPTBN1, and TAPBP were found to be hypermethylated in miR-3127–high melanomas compared to miR-3127–low melanomas. In methylation GSEA, there were a total of 122 biological processes that were significantly altered. Epigenetic dysregulation took place in several cancer-related processes, including SATB1 targets (adjusted p < .001), uveal melanoma signal (adjusted p < .001), tropomyosin receptor kinase receptor (TRKR) pathway (adjusted p < .001), EZH2 targets (adjusted p < .001), and mammalian target of rapamycin (mTOR) pathway (adjusted p < .001) (Fig. 3D). The full result is shown in Supplementary Table S5.
RESULTS
- Melanoma, the most prevalent malignant skin cancer, is marked by rising incidence, mortality rates, and significant disease burden [1,2]. Despite advancements in diagnosis and treatment, the three-year survival rate is still poor, particularly for metastatic melanoma [3]. The identification of appropriate biomarkers is needed to enhance prognostication and overall outcomes for patients with melanoma.
- Accumulating evidence suggests that miRNAs play crucial roles in various stages of cancer development, including melanoma [4,13]. Some studies have highlighted miRNAs as potential biomarkers for cancer diagnosis, targeted therapy, and prognosis [14,15]. Whether miR-3127 functions as an oncogene or TS depends on its target genes. For instance, miR-3127-5p has been shown to be a TS in non–small-cell lung cancer (NSCLC), wherein a decrease in miR-3127-5p levels encourages epithelial-mesenchymal transition by activating the WNT/FZD4/β-catenin signaling pathway, thereby promoting tumor progression and metastasis. [6]. Another study revealed that miR-3127 suppresses NSCLC cell proliferation and invasion by regulating the oncogene ABL1, leading to the inhibition of the RAS/ERK pathway [16]. Interestingly, in HCC, miR-3127 acts as an oncogene by activating AKT/FOXO1 signaling through direct targeting of the 3'-UTR of PHLPP1/2 [7]. However, the mechanisms and target genes of miR-3127 in melanoma remain unclear. To our knowledge, this study is the first to explore the expression patterns of miR-3127 and its association with patient survival in a melanoma patient cohort.
- In this study, we investigated the expression of miR-3127 in melanoma samples by utilizing The Cancer Genome Atlas (TCGA) miRNAs, mRNA-sequencing, and DNA methylation data for subsequent analysis. Our findings revealed that patients with elevated miR-3127 levels (≥20.3 RPM) exhibited significantly poorer PFS and OS compared to those with lower miR-3127 levels. miR-3127 expression was found to be independent of metastatic status. In metastatic melanoma, elevated miR-3127 levels were linked to the worst clinical outcomes. Interestingly, non-metastatic patients with high miR-3127 expression demonstrated even lower OS and DFS than those with low miR-3127 expression in metastatic cases. These findings indicate that elevated miR-3127 expression may be a useful prognostic marker in melanoma, both in early-stage disease and in metastatic settings.
- Moreover, we uncovered evidence implicating miR-3127 in several biological pathways pertinent to the pathogenesis of melanoma. In miR-3127–high melanoma, five other upregulated miRNAs were identified: miR-1301, miR-3691, miR-6509, miR-3150b, and miR-3680-1. Most of these miRNAs have established roles in tumor cell proliferation, invasion, or metastasis. miR-1301 is a recently discovered miRNA that exhibits abnormal expression across various tumors types, and dysregulated expression of miR-1301 is often linked to a poor prognosis. Studies have shown its involvement in promoting invasion, migration, and epithelial-mesenchymal transition progression by downregulating NBL1 expression in esophageal cancer and facilitating cell proliferation via targeting SIRT1 in gastric cancer [17-19]. Similarly, miR-3691-5p promotes HCC cell migration and invasion by activating phosphoinositide 3-kinase (PI3K)/Akt signaling through targeting PTEN [20]. miR-6509 has been associated with the progression of HCC in SNHG6/miR-6509-5p/HIF1A axis function and gastric cancer by binding to the long non-coding RNA HOXA10-AS to upregulate Y-box binding protein 1 [21,22]. Meanwhile, miR-3150b-3p is thought to act as a TS in colorectal cancer and HCC by inhibiting cancer cell proliferation, migration, and invasion through targeting GOLPH3 via the GOLPH3-mediated JAK2/STAT3 pathway [23,24]. Prior research on miR-3680-1 is limited. Although there are no prior studies investigating the association between these miRNAs and melanoma, their active expression in other cancer types suggests that they may be worth exploring as new therapeutic targets.
- To further elucidate the active mechanisms of miR-3127, we conducted target gene predictions and observed elevated expression of SEMA4C, KIAA1522, DLL3, SEMA4F, and MPP2 in miR-3127–high tumors. SEMA4C was an important regulator of axonal guidance linked to tumor development. High SEMA4C expression is associated with the promotion of epithelial-mesenchymal transition and predicts a poor prognosis in colorectal carcinoma [25]. KIAA1522 is involved in oncogenic KRAS pathways, and its high expression serves as an independent biomarker predicting unfavorable survival and platinum resistance in NSCLC patients [26]. KIAA1522 also promotes the in vitro proliferation, invasion, and migration of tumor cells, as well as distant metastasis of colorectal cancer in vivo. Additionally, KIAA1522 upregulates the Notch signaling pathway in colorectal cancer cell lines in vitro and lung metastatic nodes in vivo [27]. DLL3 plays a pivotal role in Notch signaling, influencing various cellular processes such as differentiation, proliferation, survival, and apoptosis [28]. SEMA4F acts as a key regulator of tumorigenesis and is associated with the activity-dependent progression of glioblastoma [29]. The elevated expression of these genes supports the notion of a poor prognosis in the miR-3127–high tumors group.
- We identified numerous DEGs between miR-3127–high and miR-3127–low melanoma samples. Among the upregulated genes in the miR-3127–high group were RTN4R, PAK4, SNTA1, CNNM3, NAXE, SMYD5, PPARD, and EPHX1, while IGFBP3 and KIAA0040 were downregulated. These genes are associated with various functions including as CDM, cytokines, growth factors, TCG, and oncogenes. For instance, PAK4 is a member of the PAK family and plays a crucial role in regulating cell adhesion, migration, proliferation, and survival. Its expression is frequently dysregulated in cancer [30,31]. SNTA1/P66shc-mediated Rac1 activation has been linked to increased reactive oxygen species production and migratory potential in breast cancer cells [32]. The protein tyrosine phosphatase PRL-2 forms a complex with the magnesium transporter CNNM3 to promote tumorigenesis [33]. Conversely, IGFBP-3, which was downregulated in tumors with elevated miR-3127 levels, binds to insulin-like growth factors to inhibit cell proliferation [34].
- Pathway analysis demonstrated that genes predominantly associated with cell cycle functions, DNA repair, response to UV exposure, and metabolic were enriched in melanomas with high miR-3127 levels. Conversely, miR-3127–low samples exhibited enrichment in inflammation-related genes, such as elevated expression of PAFAH1B2, a key player in inflammation and anaphylaxis [35]. The impact of inflammation on cancer is double-edged: it can either promote or suppress tumor progression. Chronic inflammation tends to facilitate tumor development and resistance to treatment, while acute inflammatory reactions can stimulate dendritic cell maturation, antigen presentation, and anti-tumor immune responses [36,37], which could account for the favorable prognosis seen in patients with low miR-3127 levels. Additionally, in methylation GSEA, a total of 122 biological processes were significantly altered, indicating epigenetic dysregulation in various cancer-related processes such as those mediated by SATB1 targets, uveal melanoma signal, the TRKR pathway, EZH2 targets, and the mTOR pathway.
- While miRNA signatures have shown promise as prognostic markers for melanoma, there are challenges associated with their application. A key limitation of this research is inherent to performing bioinformatic analysis from the TCGA database. There is a need to establish standardized methodologies to conduct larger studies with independent validation sets to ensure the reliability of miRNA signatures detected in these analyses. Addressing these challenges will contribute to the effective utilization of miR-3127 signatures in clinical settings.
- Our findings demonstrate that miR-3127 is a useful biomarker to predict prognosis of melanoma, with increased expression of miR-3127 being associated with an unfavorable prognosis. These results suggest that miR-3127 may play an important role in the complex mechanisms underlying the multi-step process of melanoma progression. The expression profile of miR-3127 could function as a prognostic indicator, aiding in the management and treatment of patients with melanoma.
DISCUSSION
Supplementary Information
Ethics Statement
Not applicable.
Availability of Data and Material
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: TPXN, MKL, CMB, VGH. Data curation: TPXN, MKL. Formal analysis: TPXN, MKL. Investigation: TPXN, MKL. Methodology: TPXN, MKL, CMB, VGH. Project administration: TPXN, VGH. Resources: TPXN, MKL. Software: MKL. Supervision: VGH. Validation: TPXN, MKL. Visualization: MKL. Writing—original draft: TPXN. Writing—review & editing: MKL, CMB, VGH. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.


- 1. Karimi K, Lindgren TH, Koch CA, Brodell RT. Obesity as a risk factor for malignant melanoma and non-melanoma skin cancer. Rev Endocr Metab Disord 2016; 17: 389-403. ArticlePubMedPDF
- 2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209-49. ArticlePubMedPDF
- 3. Song X, Zhao Z, Barber B, Farr AM, Ivanov B, Novich M. Overall survival in patients with metastatic melanoma. Curr Med Res Opin 2015; 31: 987-91. ArticlePubMed
- 4. Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer 2015; 15: 321-33. ArticlePubMedPMCPDF
- 5. Wang X, Meng R, Hu QM. LINC00319-mediated miR-3127 repression enhances bladder cancer progression through upregulation of RAP2A. Front Genet 2020; 11: 180.ArticlePubMedPMC
- 6. Yang Y, Sun Y, Wu Y, et al. Downregulation of miR-3127-5p promotes epithelial-mesenchymal transition via FZD4 regulation of Wnt/beta-catenin signaling in non-small-cell lung cancer. Mol Carcinog 2018; 57: 842-53. ArticlePubMed
- 7. Jiang J, Zhang Y, Guo Y, et al. MicroRNA-3127 promotes cell proliferation and tumorigenicity in hepatocellular carcinoma by disrupting of PI3K/AKT negative regulation. Oncotarget 2015; 6: 6359-72. ArticlePubMedPMC
- 8. Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput Stat Data Anal 2003; 43: 121-37. Article
- 9. Peace KE. Design and analysis of clinical trials with time-to-event endpoints. Boca Raton: CRC Press, 2018.
- 10. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014; 15: 550.ArticlePubMedPMCPDF
- 11. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005; 102: 15545-50. ArticlePubMedPMC
- 12. Tian Y, Morris TJ, Webster AP, et al. ChAMP: updated methylation analysis pipeline for Illumina BeadChips. Bioinformatics 2017; 33: 3982-4. ArticlePubMedPMCPDF
- 13. Gulyaeva LF, Kushlinskiy NE. Regulatory mechanisms of microRNA expression. J Transl Med 2016; 14: 143.ArticlePubMed
- 14. Lu T, Chen S, Qu L, Wang Y, Chen HD, He C. Identification of a five-miRNA signature predicting survival in cutaneous melanoma cancer patients. PeerJ 2019; 7: e7831. ArticlePubMedPMCPDF
- 15. Yerukala Sathipati S, Ho SY. Survival associated miRNA signature in patients with head and neck carcinomas. Heliyon 2023; 9: e17218. ArticlePubMedPMC
- 16. Sun Y, Chen C, Zhang P, et al. Reduced miR-3127-5p expression promotes NSCLC proliferation/invasion and contributes to dasatinib sensitivity via the c-Abl/Ras/ERK pathway. Sci Rep 2014; 4: 6527.ArticlePubMedPMCPDF
- 17. Zhong C, Dong Y, Zhang Q, Yuan C, Duan S. Aberrant expression of miR-1301 in human cancer. Front Oncol 2021; 11: 789626.ArticlePubMed
- 18. Du J, Zhang S, Zhang X, et al. miR-1301-3p promotes invasion and migration and EMT progression in esophageal cancer by downregulating NBL1 expression. Thorac Cancer 2023; 14: 3032-41. ArticlePubMedPMC
- 19. Luo D, Fan H, Ma X, et al. miR-1301-3p promotes cell proliferation and facilitates cell cycle progression via targeting SIRT1 in gastric cancer. Front Oncol 2021; 11: 664242.ArticlePubMedPMC
- 20. Du W, Zhang X, Wan Z. miR-3691-5p promotes hepatocellular carcinoma cell migration and invasion through activating PI3K/Akt signaling by targeting PTEN. Onco Targets Ther 2019; 12: 4897-906. ArticlePubMedPMC
- 21. Fan X, Zhao Z, Song J, et al. LncRNA-SNHG6 promotes the progression of hepatocellular carcinoma by targeting miR-6509-5p and HIF1A. Cancer Cell Int 2021; 21: 150.ArticlePubMedPMCPDF
- 22. Li S, Lu C, Li X, et al. LncRNA HOXA10-AS functions as an oncogene by binding miR-6509-5p to upregulate Y-box binding protein 1 in gastric cancer. Bioengineered 2022; 13: 11373-87. ArticlePubMedPMC
- 23. Zhang W, Chen X, Jia J. MiR-3150b-3p inhibits the progression of colorectal cancer cells via targeting GOLPH3. J Investig Med 2020; 68: 425-9. ArticlePubMedPDF
- 24. Zhang Y, Wang J, Su H. MiR-3150b inhibits hepatocellular carcinoma cell proliferation, migration and invasion by targeting GOLPH3. J Investig Med 2020; 68: 770-5. ArticlePubMedPDF
- 25. Hou Y, Wang W, Zeng Z, et al. High SEMA4C expression promotes the epithelial-mesenchymal transition and predicts poor prognosis in colorectal carcinoma. Aging (Albany NY) 2020; 12: 21992-2018. ArticlePubMedPMC
- 26. Liu YZ, Yang H, Cao J, et al. KIAA1522 is a novel prognostic biomarker in patients with non-small cell lung cancer. Sci Rep 2016; 6: 24786.ArticlePubMedPMCPDF
- 27. Yi X, Hu C, Zhang C, et al. KIAA1522 is a new biomarker of promoting the tumorigenesis and distant metastasis of colorectal carcinoma. Cell Signal 2022; 90: 110202.ArticlePubMed
- 28. Bulman MP, Kusumi K, Frayling TM, et al. Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis. Nat Genet 2000; 24: 438-41. ArticlePubMedPDF
- 29. Huang-Hobbs E, Cheng YT, Ko Y, et al. Remote neuronal activity drives glioma progression through SEMA4F. Nature 2023; 619: 844-50. ArticlePubMedPMCPDF
- 30. Won SY, Park JJ, Shin EY, Kim EG. PAK4 signaling in health and disease: defining the PAK4-CREB axis. Exp Mol Med 2019; 51: 1-9. ArticlePDF
- 31. Costa TD, Stromblad S. Why is PAK4 overexpressed in cancer? Int J Biochem Cell Biol 2021; 138: 106041.ArticlePubMed
- 32. Bhat HF, Baba RA, Adams ME, Khanday FA. Role of SNTA1 in Rac1 activation, modulation of ROS generation, and migratory potential of human breast cancer cells. Br J Cancer 2014; 110: 706-14. ArticlePubMedPMCPDF
- 33. Hardy S, Uetani N, Wong N, et al. The protein tyrosine phosphatase PRL-2 interacts with the magnesium transporter CNNM3 to promote oncogenesis. Oncogene 2015; 34: 986-95. ArticlePubMedPDF
- 34. Kim HS. Role of insulin-like growth factor binding protein-3 in glucose and lipid metabolism. Ann Pediatr Endocrinol Metab 2013; 18: 9-12. ArticlePubMedPMC
- 35. Ma C, Guo Y, Zhang Y, et al. PAFAH1B2 is a HIF1a target gene and promotes metastasis in pancreatic cancer. Biochem Biophys Res Commun 2018; 501: 654-60. ArticlePubMed
- 36. Zhao H, Wu L, Yan G, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther 2021; 6: 263.ArticlePubMedPMCPDF
- 37. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331: 1565-70. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
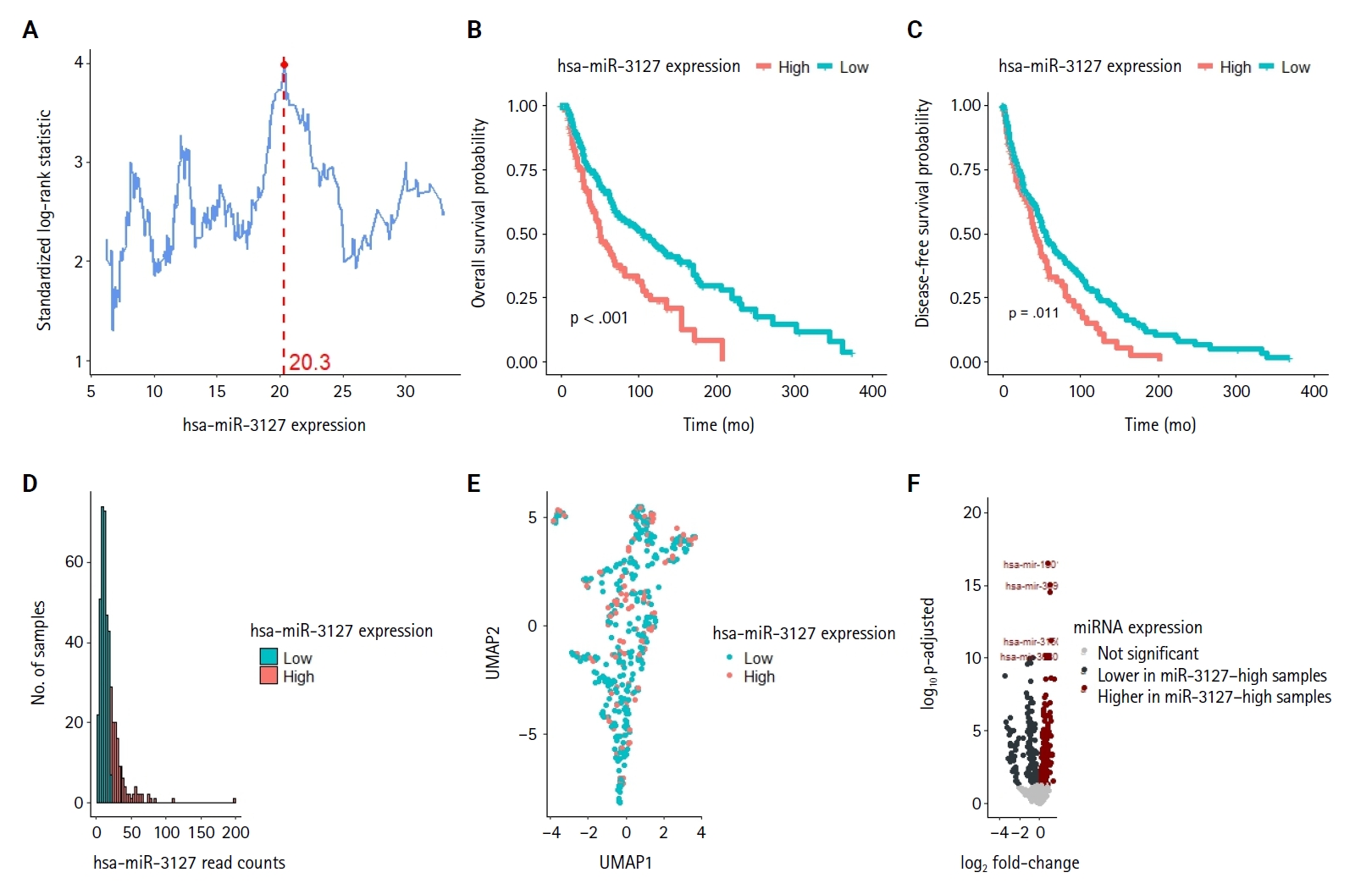
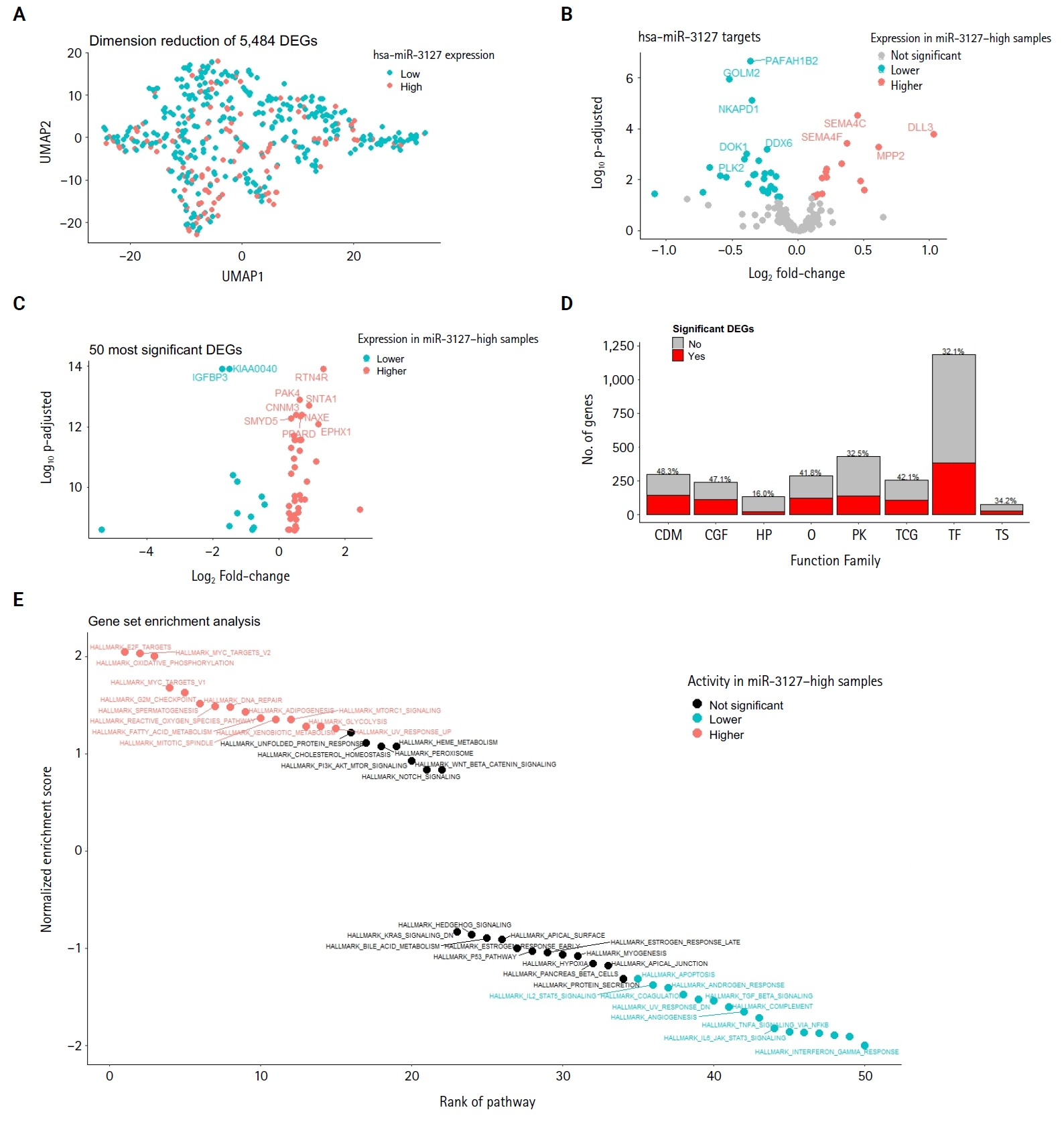
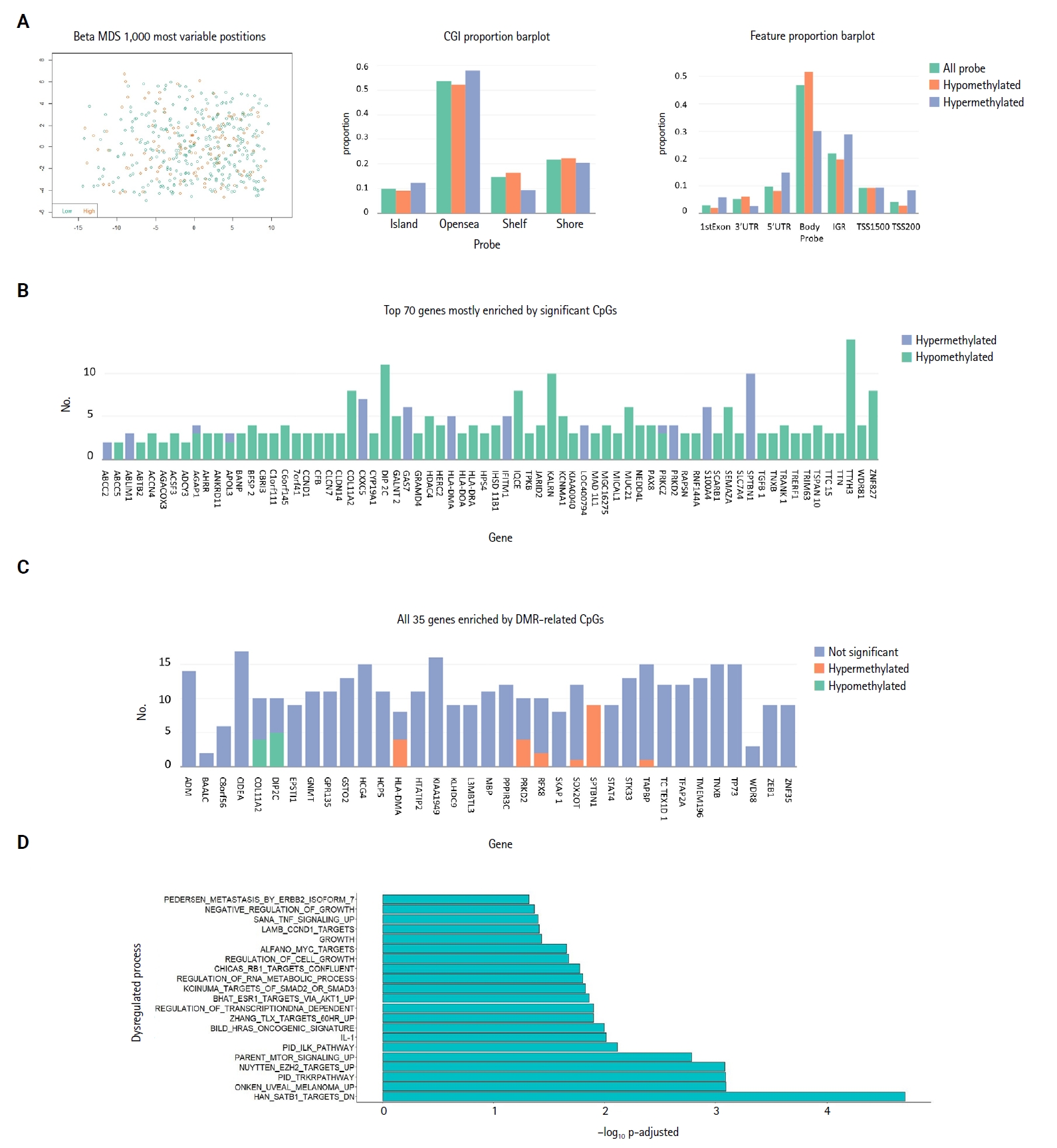
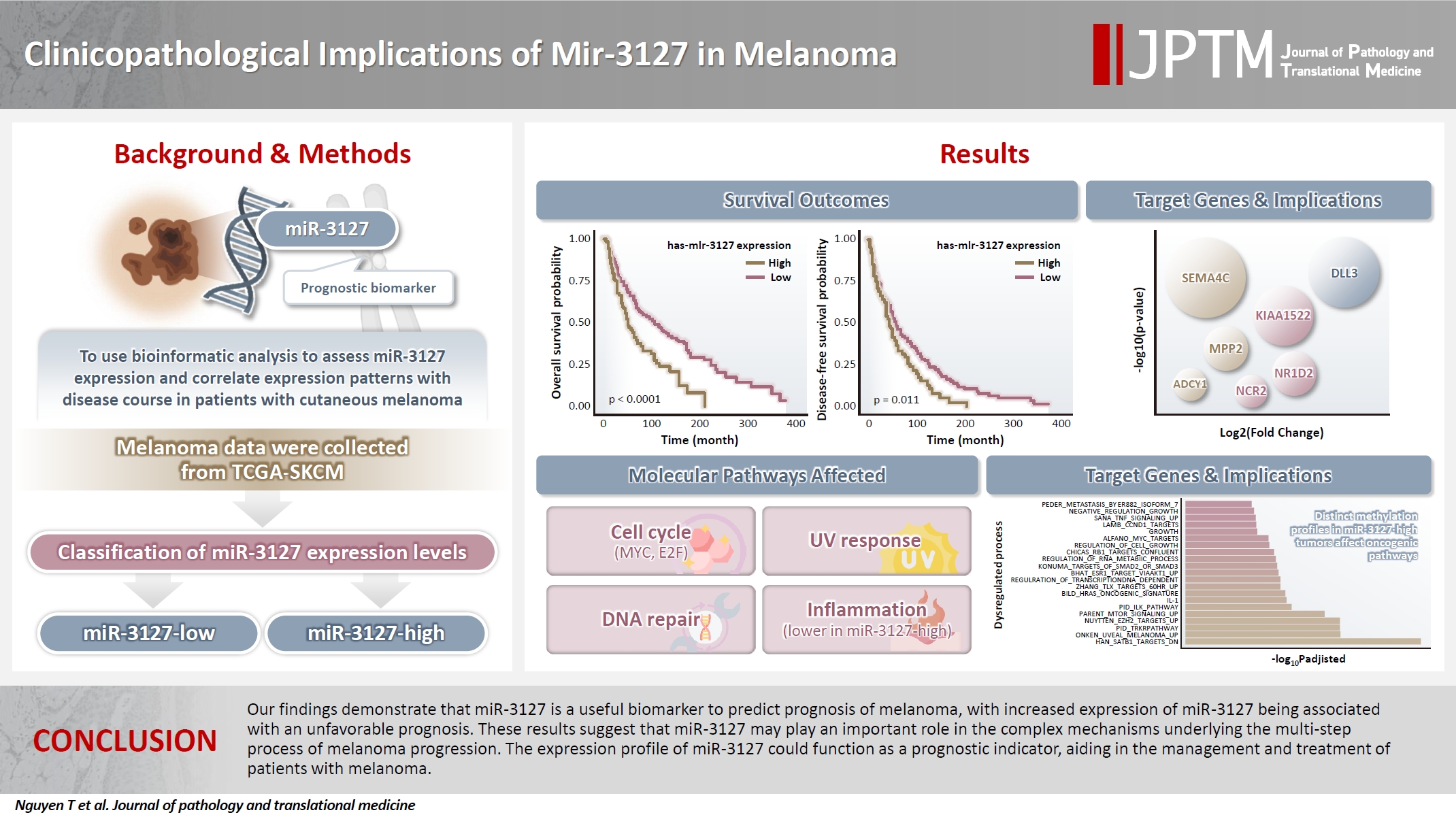
Fig. 1.
Fig. 2.
Fig. 3.
Graphical abstract
| Variable | miR-3127–low (n = 317) | miR-3127–high (n = 129) | p-value |
|---|---|---|---|
| Age (yr) | 58 (15–90) | 60 (23–87) | .547 |
| Sex | .062 | ||
| Female | 130 (41.0) | 40 (31.0) | |
| Male | 187 (59.0) | 89 (69.0) | |
| Race | .503 | ||
| Asian | 7 (2.3) | 5 (4.0) | |
| Black | 1 (0.3) | 0 (0.0) | |
| White | 309 (97.4) | 124 (96.0) | |
| Breslow depth (mm) | 2.9 (0.0–75.0) | 3.2 (0.0–74.0) | .391 |
| Sample | .668 | ||
| Primary | 171 (53.9) | 66 (51.2) | |
| Metastatic | 146 (46.1) | 63 (48.8) | |
| T categorya | .765 | ||
| T0 | 19 (7.2) | 4 (3.7) | |
| Tis | 5 (1.9) | 3 (2.8) | |
| T1 | 28 (10.6) | 9 (8.3) | |
| T2 | 56 (21.1) | 22 (20.4) | |
| T3 | 58 (21.9) | 26 (24.1) | |
| T4 | 99 (37.4) | 44 (40.7) | |
| N categorya | .974 | ||
| N0 | 160 (56.7) | 65 (57.0) | |
| N1 | 48 (17.0) | 21 (18.4) | |
| N2 | 36 (12.8) | 13 (11.4) | |
| N3 | 38 (13.5) | 15 (13.2) | |
| M categorya | >.99 | ||
| M0 | 282 (94.3) | 114 (94.2) | |
| M1 | 17 (5.7) | 7 (5.8) | |
| AJCC stagea | .865 | ||
| I | 51 (18.6) | 23 (20.2) | |
| II | 94 (34.3) | 34 (29.8) | |
| III | 113 (41.2) | 50 (43.9) | |
| IV | 16 (5.8) | 7 (6.1) | |
| Radiationa | .491 | ||
| No | 297 (95.8) | 119 (93.7) | |
| Yes | 13 (4.2) | 8 (6.3) | |
| Chemotherapya | .600 | ||
| No | 225 (78.7) | 87 (75.7) | |
| Yes | 61 (21.3) | 28 (24.3) | |
| Overall survival (mo) | 42 (0–374) | 33 (1–208) | .056 |
| Disease-free survival (mo) | 28 (0–368) | 27 (0–201) | .056 |
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| miR-3127 | ||||||
| Low | 1 | 1 | ||||
| High | 1.8 | 1.4–2.5 | <.001 | 1.5 | 1.0–2.1 | .033 |
| Age (10 yr) | 1.3 | 1.1–1.4 | <.001 | 1.2 | 1.0–1.3 | .015 |
| Race | ||||||
| Asian | 1 | 1 | ||||
| Black | 0.2 | 0.0–1.5 | .106 | n/a | n/a | n/a |
| White | 0.2 | 0.1–0.5 | <.001 | 0.2 | 0.1–0.6 | .004 |
| Breslow’s depth (cm) | 1.3 | 1.1–1.5 | <.001 | 1 | 0.8–1.3 | .889 |
| T category | ||||||
| T1 | 1 | 1 | ||||
| T2 | 1.6 | 0.8–3.0 | .171 | 1.7 | 0.8–3.4 | .151 |
| T3 | 2.2 | 1.1–4.1 | .017 | 2.4 | 1.0–6.0 | .059 |
| T4 | 3.8 | 2.1–7.2 | <.001 | 4.7 | 1.8–12.2 | .001 |
| N category | ||||||
| N0 | 1 | 1 | ||||
| N1 | 1.6 | 1.1–2.3 | .025 | 2.7 | 0.9–8.0 | .080 |
| N2 | 1.6 | 1.0–2.4 | .051 | 3.6 | 1.1–11.4 | .028 |
| N3 | 2.6 | 1.7–4.1 | <.001 | 7.4 | 2.4–23.2 | <.001 |
| M category | ||||||
| M0 | 1 | |||||
| M1 | 1.7 | 0.9–3.3 | .094 | |||
| Radiation | ||||||
| No | 1 | |||||
| Yes | 1 | 0.5–1.9 | .994 | |||
| Chemotherapy | ||||||
| No | 1 | |||||
| Yes | 0.9 | 0.7–1.3 | .658 | |||
AJCC, American Joint Committee on Cancer. aVariable could not be fully obtained because certain data were unavailable.
HR, hazard ratio; CI, confidence interval; n/a, not available.

 E-submission
E-submission