Search
- Page Path
- HOME > Search
- PSMA expression in hepatic colorectal cancer metastasis
- Eundong Park, Michel Kmeid, Xin Wang, Haiyan Qiu, Clifton G. Fulmer, Marcello P. Toscano, Nusret Bekir Subasi, Maciej Gracz, Hwajeong Lee
- J Pathol Transl Med. 2026;60(1):107-123. Published online January 14, 2026
- DOI: https://doi.org/10.4132/jptm.2025.10.20
- 358 View
- 32 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Prostate-specific membrane antigen (PSMA) is expressed in the neovasculature of various malignancies, such as colorectal cancer (CRC) and hepatocellular carcinoma (HCC). However, PSMA expression in hepatic CRC metastasis has not been studied in detail. Methods: The PSMA expression in primary CRC and corresponding hepatic metastasis was evaluated by immunohistochemistry in a metastatic CRC cohort (n = 56), which was divided into subgroups according to treatment history and timing of metastasis. Demographic and histological characteristics of primary CRC were collected and their relationships with PSMA expression were examined. Additionally, the PSMA expression in resected HCC (n = 76) was compared with that of hepatic CRC metastasis. Results: In primary CRC, PSMA level showed a positive association with tumor size. Lower PSMA expression in hepatic metastasis was associated with higher primary CRC grade, advanced pTNM stage at the time of CRC resection, presence of tumor deposit, and unresectability of metastatic lesion. PSMA expression in primary CRC correlated with that in hepatic metastasis only in concurrent and untreated metastasis subgroup. PSMA expression in primary CRC and hepatic metastasis, regardless of treatment history and timing of metastasis, was not significantly different from that of HCC. Conclusions: Several adverse pathological features of primary CRC were associated with a lower PSMA expression in hepatic metastasis. PSMA expression in hepatic metastasis correlated with that of primary CRC only in concurrent and untreated subgroup. Primary HCC and hepatic CRC metastasis show comparable levels of PSMA expression.
- The combination of CDX2 expression status and tumor-infiltrating lymphocyte density as a prognostic factor in adjuvant FOLFOX-treated patients with stage III colorectal cancers
- Ji-Ae Lee, Hye Eun Park, Hye-Yeong Jin, Lingyan Jin, Seung Yeon Yoo, Nam-Yun Cho, Jeong Mo Bae, Jung Ho Kim, Gyeong Hoon Kang
- J Pathol Transl Med. 2025;59(1):50-59. Published online October 24, 2024
- DOI: https://doi.org/10.4132/jptm.2024.09.26
- 3,605 View
- 279 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Colorectal carcinomas (CRCs) with caudal-type homeobox 2 (CDX2) loss are recognized to pursue an aggressive behavior but tend to be accompanied by a high density of tumor-infiltrating lymphocytes (TILs). However, little is known about whether there is an interplay between CDX2 loss and TIL density in the survival of patients with CRC.
Methods
Stage III CRC tissues were assessed for CDX2 loss using immunohistochemistry and analyzed for their densities of CD8 TILs in both intraepithelial (iTILs) and stromal areas using a machine learning-based analytic method.
Results
CDX2 loss was significantly associated with a higher density of CD8 TILs in both intraepithelial and stromal areas. Both CDX2 loss and a high CD8 iTIL density were found to be prognostic parameters and showed hazard ratios of 2.314 (1.050–5.100) and 0.378 (0.175–0.817), respectively, for cancer-specific survival. A subset of CRCs with retained CDX2 expression and a high density of CD8 iTILs showed the best clinical outcome (hazard ratio of 0.138 [0.023–0.826]), whereas a subset with CDX2 loss and a high density of CD8 iTILs exhibited the worst clinical outcome (15.781 [3.939–63.230]).
Conclusions
Altogether, a high density of CD8 iTILs did not make a difference in the survival of patients with CRC with CDX2 loss. The combination of CDX2 expression and intraepithelial CD8 TIL density was an independent prognostic marker in adjuvant chemotherapy-treated patients with stage III CRC.
- Senescent tumor cells in colorectal cancer are characterized by elevated enzymatic activity of complexes 1 and 2 in oxidative phosphorylation
- Jun Sang Shin, Tae-Gyu Kim, Young Hwa Kim, So Yeong Eom, So Hyun Park, Dong Hyun Lee, Tae Jun Park, Soon Sang Park, Jang-Hee Kim
- J Pathol Transl Med. 2023;57(6):305-314. Published online November 7, 2023
- DOI: https://doi.org/10.4132/jptm.2023.10.09
- 6,924 View
- 315 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Cellular senescence is defined as an irreversible cell cycle arrest caused by various internal and external insults. While the metabolic dysfunction of senescent cells in normal tissue is relatively well-established, there is a lack of information regarding the metabolic features of senescent tumor cells.
Methods
Publicly available single-cell RNA-sequencing data from the GSE166555 and GSE178341 datasets were utilized to investigate the metabolic features of senescent tumor cells. To validate the single-cell RNA-sequencing data, we performed senescence-associated β-galactosidase (SA-β-Gal) staining to identify senescent tumor cells in fresh frozen colorectal cancer tissue. We also evaluated nicotinamide adenine dinucleotide dehydrogenase–tetrazolium reductase (NADH-TR) and succinate dehydrogenase (SDH) activity using enzyme histochemical methods and compared the staining with SA-β-Gal staining. MTT assay was performed to reveal the complex 1 activity of the respiratory chain in in-vitro senescence model.
Results
Single-cell RNA-sequencing data revealed an upregulation in the activity of complexes 1 and 2 in oxidative phosphorylation, despite overall mitochondrial dysfunction in senescent tumor cells. Both SA-β-Gal and enzyme histochemical staining using fresh frozen colorectal cancer tissues indicated a high correlation between SA-β-Gal positivity and NADH-TR/SDH staining positivity. MTT assay showed that senescent colorectal cancer cells exhibit higher absorbance in 600 nm wavelength.
Conclusions
Senescent tumor cells exhibit distinct metabolic features, characterized by upregulation of complexes 1 and 2 in the oxidative phosphorylation pathway. NADH-TR and SDH staining represent efficient methods for detecting senescent tumor cells in colorectal cancer. -
Citations
Citations to this article as recorded by- Senescence, Aging and Disease Throughout the Gastrointestinal System
Sofia Ferreira-Gonzalez, Tomonori Matsumoto, Eiji Hara, Stuart J. Forbes
Gastroenterology.2025; 169(7): 1357. CrossRef - Cellular Aging and Senescence in Cancer: A Holistic Review of Cellular Fate Determinants
Muhammad Tufail, Yu-Qi Huang, Jia-Ju Hu, Jie Liang, Cai-Yun He, Wen-Dong Wan, Can-Hua Jiang, Hong Wu, Ning Li
Aging and disease.2024;[Epub] CrossRef - Real-time assessment of relative mitochondrial ATP synthesis response against inhibiting and stimulating substrates (MitoRAISE)
Eun Sol Chang, Kyoung Song, Ji-Young Song, Minjung Sung, Mi-Sook Lee, Jung Han Oh, Ji-Yeon Kim, Yeon Hee Park, Kyungsoo Jung, Yoon-La Choi
Cancer & Metabolism.2024;[Epub] CrossRef
- Senescence, Aging and Disease Throughout the Gastrointestinal System
- Prognostic and clinicopathological significance of Fusobacterium nucleatum in colorectal cancer: a systemic review and meta-analysis
- Younghoon Kim, Nam Yun Cho, Gyeong Hoon Kang
- J Pathol Transl Med. 2022;56(3):144-151. Published online May 15, 2022
- DOI: https://doi.org/10.4132/jptm.2022.03.13
- 8,645 View
- 144 Download
- 12 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Fusobacterium nucleatum has been identified to promote tumor progression in colorectal cancer (CRC). However, association between F. nucleatum and prognostic or clinicopathological features has been diverse among studies, which could be affected by type of biospecimen (formalin-fixed paraffin-embedded or fresh frozen [FF]).
Methods
Articles were systemically reviewed for studies that included the correlation between F. nucleatum and prognosis or clinicopathological features in CRC.
Results
Ten articles, eight studies with survival-related features involving 3,199 patients and nine studies with clinical features involving 2,655 patients, were eligible for the meta-analysis. Overall survival, disease-free survival, and cancer-specific survival were all associated with worse prognosis in F. nucleatum–high patients (p<.05). In subgroup analysis, only studies with FF tissues retained prognostic significance with F. nucleatum. In meta-analysis of clinicopathological variables, F. nucleatum level was associated with location within colon, pT category, MLH1 hypermethylation, microsatellite instability status, and BRAF mutation regardless of type of biospecimen. However, lymph node metastasis and KRAS mutation was only associated with F. nucleatum level in FF-based studies.
Conclusions
In conclusion, type of biospecimen could affect the role of F. nucleatum as a biomarker associated with clinicopathological features and prognosis. -
Citations
Citations to this article as recorded by- Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights
Linda Galasso, Fabrizio Termite, Irene Mignini, Giorgio Esposto, Raffaele Borriello, Federica Vitale, Alberto Nicoletti, Mattia Paratore, Maria Elena Ainora, Antonio Gasbarrini, Maria Assunta Zocco
Cancers.2025; 17(3): 368. CrossRef - Intratumoural pks Escherichia coli is associated with risk of metachronous colorectal cancer and adenoma development in people with Lynch syndrome
Yen Lin Chu, Peter Georgeson, Mark Clendenning, Khalid Mahmood, Romy Walker, Julia Como, Sharelle Joseland, Susan G. Preston, Toni Rice, Brigid M. Lynch, Roger L. Milne, Melissa C. Southey, Graham G. Giles, Amanda I. Phipps, John L. Hopper, Aung K. Win, C
eBioMedicine.2025; 114: 105661. CrossRef - Fusobacterium nucleatum Enrichment in Colorectal Tumor Tissue: Associations With Tumor Characteristics and Survival Outcomes
Amanda I. Phipps, Courtney M. Hill, Genevieve Lin, Rachel C. Malen, Adriana M. Reedy, Orsalem Kahsai, Hamza Ammar, Keith Curtis, Ningxin Ma, Timothy W. Randolph, Jing Ma, Shuji Ogino, Polly A. Newcomb, Meredith AJ. Hullar
Gastro Hep Advances.2025; 4(6): 100644. CrossRef - Enhancing fibroblast–epithelial cell communications: Serpine2 as a key molecule in Fusobacterium nucleatum–promoted colon cancer
Xueke Li, Simin Luo, Yifang Jiang, Qiong Ma, Fengming You, Qixuan Kuang, Xi Fu, Chuan Zheng
Frontiers in Immunology.2025;[Epub] CrossRef - The presence and relative abundance of salivary Fusobacterium nucleatum are not associated with colorectal cancer: a systematic review and meta-analysis
Ellay Gutmacher, Bálint Zsombor Sárai, Petrana Martineková, Szilvia Kiss-Dala, Gergely Agócs, Péter Hegyi, Andrea Bródy, Ákos Zsembery
Scientific Reports.2025;[Epub] CrossRef - The role of oral microbiota in digestive system diseases: current advances and perspectives
Yaqi Li, Yiping Xin, Wenlu Zong, Xiaoyu Li
Journal of Oral Microbiology.2025;[Epub] CrossRef - Fusobacterium Nucleatum in Colorectal Cancer: Relationship Among Immune Modulation, Potential Biomarkers and Therapeutic Implications
Dalila Incognito, Giuliana Ciappina, Claudia Gelsomino, Antonio Picone, Pierluigi Consolo, Alessandra Scano, Tindara Franchina, Nicola Maurea, Vincenzo Quagliariello, Salvatore Berretta, Alessandro Ottaiano, Massimiliano Berretta
International Journal of Molecular Sciences.2025; 26(19): 9710. CrossRef - Intratumoral presence of the genotoxic gut bacteria pks+ E. coli, Enterotoxigenic Bacteroides fragilis, and Fusobacterium nucleatum and their association with clinicopathological and molecular features of colorectal cancer
Jihoon E. Joo, Yen Lin Chu, Peter Georgeson, Romy Walker, Khalid Mahmood, Mark Clendenning, Aaron L. Meyers, Julia Como, Sharelle Joseland, Susan G. Preston, Natalie Diepenhorst, Julie Toner, Danielle J. Ingle, Norelle L. Sherry, Andrew Metz, Brigid M. Ly
British Journal of Cancer.2024; 130(5): 728. CrossRef - The role of Fusobacterium nucleatum in cancer and its implications for clinical applications
Wanyi Luo, Juxi Han, Xian Peng, Xuedong Zhou, Tao Gong, Xin Zheng
Molecular Oral Microbiology.2024; 39(6): 417. CrossRef -
Fusobacterium nucleatum Load Correlates with KRAS Mutation and Sessile Serrated Pathogenesis in Colorectal Adenocarcinoma
Koki Takeda, Minoru Koi, Yoshiki Okita, Sija Sajibu, Temitope O. Keku, John M. Carethers
Cancer Research Communications.2023; 3(9): 1940. CrossRef - Tumour Colonisation of Parvimonas micra Is Associated with Decreased Survival in Colorectal Cancer Patients
Thyra Löwenmark, Anna Löfgren-Burström, Carl Zingmark, Ingrid Ljuslinder, Michael Dahlberg, Sofia Edin, Richard Palmqvist
Cancers.2022; 14(23): 5937. CrossRef
- Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights
- Colorectal adenocarcinoma with enteroblastic differentiation: diagnostic challenges of a rare case encountered in clinical practice
- Evi Abada, Ifeoma C. Anaya, Othuke Abada, Anthony Lebbos, Rafic Beydoun
- J Pathol Transl Med. 2022;56(2):97-102. Published online January 21, 2022
- DOI: https://doi.org/10.4132/jptm.2021.10.28
- 8,165 View
- 207 Download
- 11 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF - Colorectal adenocarcinoma with enteroblastic differentiation (CAED) is a rare subtype of colonic adenocarcinoma characterized by increased α-fetoprotein (AFP) production and the expression of at least one enteroblastic marker including AFP, glypican 3 (GPC3), or Spalt like transcription factor 4 (SALL4). We report a case of a 26-year-old female who presented with low back pain and constipation which persisted despite supportive measures. Imaging revealed multiple liver lesions and enlarged retroperitoneal nodes. Tumor markers including AFP were markedly elevated. On biopsy, samples from the liver revealed infiltrating glands lined by columnar-type epithelium with mostly eosinophilic granular to focally clear cytoplasm. By immunohistochemistry, the tumor showed immunoreactivity with AFP, hepatocyte antigen, GPC3, SALL4, CDX2, SATB2, and cytokeratin 20. A colonoscopy performed subsequently revealed a mass in the sigmoid colon and biopsy of this mass revealed a similar histology as that seen in the liver. A diagnosis of CAED was made, following the results of gene expression profiling by the tumor with next-generation sequencing which identified pathogenic variants in MUTYH, TP53, and KDM6A genes and therefore supported its colonic origin. Cases such as this underscores the use of ancillary diagnostic techniques in arriving at the correct diagnosis in lesions with overlapping clinicopathologic characteristics.
-
Citations
Citations to this article as recorded by- Exploring the Multifunctional Role of Alpha-Fetoprotein in Cancer Progression: Implications for Targeted Therapy in Hepatocellular Carcinoma and Beyond
Hyunjung Kim, Minji Jang, Eunmi Kim
International Journal of Molecular Sciences.2025; 26(10): 4863. CrossRef - Rectal adenocarcinoma with a yolk sac tumor component: A rare case report and review of the literature
Sato Nishida, Tomohiro Takeda, Tatsuya Shonaka, Shoichiro Mizukami, Masahide Otani, Mizuho Ohara, Chikayoshi Tani, Kimiharu Hasegawa, Yuki Kamikokura, Mishie Tanino, Hideki Yokoo
International Cancer Conference Journal.2025;[Epub] CrossRef - SALL4 in gastrointestinal tract cancers: upstream and downstream regulatory mechanisms
Tairan Wang, Yan Jin, Mengyao Wang, Boya Chen, Jinyu Sun, Jiaying Zhang, Hui Yang, Xinyao Deng, Xingyue Cao, Lidong Wang, Yuanyuan Tang
Molecular Medicine.2024;[Epub] CrossRef - Gastric adenocarcinoma with enteroblastic differentiation in a
67-year-old man in Korea: a case report
Hae Rin Lee, Gwang Ha Kim, Dong Chan Joo, Moon Won Lee, Bong Eun Lee, Kyung Bin Kim
The Ewha Medical Journal.2024;[Epub] CrossRef - Colorectal adenocarcinoma with clear cell changes: immunohistological and molecular findings in three cases
Andreas Gocht, Carsten Heidel, Jutta Kirfel, Rita Vesce, Pamela Lazar-Karsten, Helen Pasternack, Madelaine Melzer, Phillip Hildebrand, Nicole Warkentin, Hendrik Schimmelpenning, Verena-Wilbeth Sailer
Virchows Archiv.2024; 485(3): 569. CrossRef - Ureteral Metastasis of Colonic Adenocarcinoma with Enteroblastic Differentiation: A Rare Case to be Distinguished from Clear Cell Adenocarcinoma of the Urinary Tract
Hiroshi Minato, Akane Yoshikawa, Sho Tsuyama, Kazuyoshi Katayanagi, Kengo Hayashi, Yusuke Sakimura, Hiroyuki Bando, Tomohiro Hori, Yosuke Kito
International Journal of Surgical Pathology.2023; 31(8): 1553. CrossRef - Beyond liver cancer, more application scenarios for alpha-fetoprotein in clinical practice
Chenyu Ma, Yuexinzi Jin, Yuhan Wang, Huaguo Xu, Jiexin Zhang
Frontiers in Oncology.2023;[Epub] CrossRef - AIEgens assisted label free DNA supersandwich immunoassay for ultrasensitive α-fetoprotein detection
Xiaowen Ou, Jingman Dai, Yiting Huang, Xiaoqin Xiong, Zhi Zheng, Xiaoding Lou, Fan Xia
Giant.2022; 11: 100110. CrossRef - Rectal carcinoma with dual differentiation toward enteroblastic and neuroendocrine features arising in a patient with ulcerative colitis: a case report
Takako Kihara, Ryuichi Kuwahara, Kurando Kusunoki, Tomohiro Minagawa, Yuki Horio, Motoi Uchino, Hiroki Ikeuchi, Seiichi Hirota
World Journal of Surgical Oncology.2022;[Epub] CrossRef
- Exploring the Multifunctional Role of Alpha-Fetoprotein in Cancer Progression: Implications for Targeted Therapy in Hepatocellular Carcinoma and Beyond
- Polo-like kinase 4 as a potential predictive biomarker of chemoradioresistance in locally advanced rectal cancer
- Hyunseung Oh, Soon Gu Kim, Sung Uk Bae, Sang Jun Byun, Shin Kim, Jae-Ho Lee, Ilseon Hwang, Sun Young Kwon, Hye Won Lee
- J Pathol Transl Med. 2022;56(1):40-47. Published online November 16, 2021
- DOI: https://doi.org/10.4132/jptm.2021.10.07
- 5,653 View
- 163 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Polo-like kinase 4 (PLK4) is a serine/threonine protein kinase located in the centriole of the chromosome during the cell cycle. PLK4 overexpression has been described in a variety of many common human epithelial tumors. Conversely, PLK4 acts as a haploinsufficient tumor suppressor in some situations, highlighting the importance of strict regulation of PLK4 expression, activity, and function. Meanwhile, the importance of chemoradiation resistance in rectal cancer is being emphasized more than ever. We aimed to analyze PLK4 expression and the tumor regression grade (TRG) in patients with rectal cancer, treated with chemoradiotherapy (CRT).
Methods
A retrospective study was conducted on 102 patients with rectal cancer who received preoperative CRT. Immunohistochemistry for PLK4 in paraffin-embedded tissue was performed from the biopsy and surgical specimens.
Results
We found significant association between high expression of PLK4 and poor response to neoadjuvant CRT (according to both Mandard and The Korean Society of Pathologists TRG systems) in the pre-CRT specimens. Other clinicopathologic parameters did not reveal any correlation with PLK4 expression.
Conclusions
This study revealed an association between high expression of PLK4 in the pre-CRT specimens and TRG. Our results indicated that PLK4 could potentially be a new predictor for CRT effect in patients with rectal cancer. -
Citations
Citations to this article as recorded by- Polo-like kinase 4 (PLK4) as a therapeutic target in breast cancer
Armen Parsyan, Harjot Athwal, Vasudeva Bhat, Alison L Allan
Carcinogenesis.2025;[Epub] CrossRef - Polo-like kinase 4: A molecular linchpin in cancer and its management
Durdana Muntaqua, Gagan Chhabra, Karla B. Anaya Aldrete, Nihal Ahmad
iScience.2025; 28(12): 114186. CrossRef
- Polo-like kinase 4 (PLK4) as a therapeutic target in breast cancer
- MicroRNA-552 expression in colorectal cancer and its clinicopathological significance
- Joon Im, Soo Kyung Nam, Hye Seung Lee
- J Pathol Transl Med. 2021;55(2):125-131. Published online February 19, 2021
- DOI: https://doi.org/10.4132/jptm.2021.01.17
- 5,583 View
- 128 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
MicroRNA-552 (miR-552) has been reported to correlate with the development and progression of various cancers, including colorectal cancer (CRC). This study aimed to investigate miR-552 expression in cancer tissue samples compared to normal mucosal tissue and its role as a diagnostic or prognostic marker in CRC patients.
Methods
Normal mucosal tissues and primary cancer tissues from 80 surgically resected CRC specimens were used. Quantitative real-time polymerase chain reaction was performed for miR-552 and U6 small nuclear RNA to analyze miR-552 expression and its clinicopathological significance. Immunohistochemistry for p53 and phosphatase and tension homolog (PTEN) was performed to evaluate their association with miR-552 expression.
Results
miR-552 expression was significantly higher in primary cancer tissues compared to normal mucosal tissues (p<.001). The expression level of miR552 was inversely correlated with that of PTEN (p=.068) and p53 (p=.004). Survival analysis showed that high miR-552 expression was associated with worse prognosis but this was not statistically significant (p=.255). However, patients with CRC having high miR-552 expression and loss of PTEN expression had significantly worse prognosis than others (p=.029).
Conclusions
Our results suggest that high miR-552 expression might be a potential diagnostic biomarker for CRC, and its combined analysis with PTEN expression can possibly be used as a prognostic marker. -
Citations
Citations to this article as recorded by- MicroRNAs involved in colorectal cancer, a rapid mini-systematic review
Sogol Shirzad, Majid Eterafi, Zeinab Karimi, Mahdi Barazesh
BMC Cancer.2025;[Epub] CrossRef - Diagnostic and Therapeutic Potential of Selected microRNAs in Colorectal Cancer: A Literature Review
Grzegorz Sychowski, Hanna Romanowicz, Wojciech Ciesielski, Piotr Hogendorf, Adam Durczyński, Beata Smolarz
Cancers.2025; 17(13): 2135. CrossRef - Blood miRNAs miR-549a, miR-552, and miR-592 serve as potential disease-specific panels to diagnose colorectal cancer
Soroush Akbar, Samaneh Mashreghi, Mohammad Reza Kalani, Akram Valanik, Farzaneh Ahmadi, Mahdi Aalikhani, Zahra Bazi
Heliyon.2024; 10(7): e28492. CrossRef - Integration of TE Induces Cancer Specific Alternative Splicing Events
Woo Ryung Kim, Eun Gyung Park, Yun Ju Lee, Woo Hyeon Bae, Du Hyeong Lee, Heui-Soo Kim
International Journal of Molecular Sciences.2022; 23(18): 10918. CrossRef
- MicroRNAs involved in colorectal cancer, a rapid mini-systematic review
- A comparative prognostic performance of definitions of Crohn-like lymphoid reaction in colorectal carcinoma
- Younghoon Kim, Jeong Mo Bae, Jung Ho Kim, Nam-Yun Cho, Gyeong Hoon Kang
- J Pathol Transl Med. 2021;55(1):53-59. Published online November 27, 2020
- DOI: https://doi.org/10.4132/jptm.2020.10.06
- 6,334 View
- 148 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
The prognostic potential of Crohn-like lymphoid reaction (CLR) in colorectal carcinoma (CRC) has been investigated through the assessment of different criteria.
Methods
The prognostic impact of CLR was investigated in 636 CRC patients to compare methods from previously published articles. These methods included CLR measured by number of lymphoid aggregates (LAs) (CLR count), LA size greater than or equal to 1 mm (CLR size), CLR density with a cutoff value of 0.38, and subjective criteria as defined by intense CLR.
Results
In univariate survival analysis, CLR-positive CRC as defined by the four aforementioned methods was associated with better overall survival (OS) (hazard ratio [HR], 0.463; 95% confidence interval [CI], 0.305 to 0.702; p <.001; HR, 0.656; 95% CI, 0.411 to 1.046; p=.077; HR, 0.363; 95% CI, 0.197 to 0.669; p=.001; and HR, 0.433; 95% CI, 0.271 to 0.690; p<.001, respectively) and disease-free survival (DFS) (HR, 0.411; 95% CI, 0.304 to 0.639; p<.001; HR, 0.528; 95% CI, 0.340 to 0.821; p=.004; HR, 0.382; 95% CI, 0.226 to 0.645, p=.004; and HR, 0.501; 95% CI, 0.339 to 0.741; p<.001, respectively) than CLR-negative CRC, regardless of criteria with the exception of OS for CLR density. In multivariate analysis, two objective criteria (CLR count and CLR density) and one subjective criterion (intense CLR) for defining CLR were considered independent prognostic factors of OS and DFS in CRC patients.
Conclusions
CLR has similar traits regardless of criteria, but CLR-positivity should be defined by objective criteria for better reproducibility and prognostic value. -
Citations
Citations to this article as recorded by- Prognostic Significance of Immune and Stromal Components in Colorectal Cancer
Mi Jang, Yongki Hong, Soojung Hong, Eun Kyung Kim
Archives of Pathology & Laboratory Medicine.2025; 149(11): 982. CrossRef
- Prognostic Significance of Immune and Stromal Components in Colorectal Cancer
- Immune landscape and biomarkers for immuno-oncology in colorectal cancers
- Jeong Mo Bae, Seung-Yeon Yoo, Jung Ho Kim, Gyeong Hoon Kang
- J Pathol Transl Med. 2020;54(5):351-360. Published online June 26, 2020
- DOI: https://doi.org/10.4132/jptm.2020.05.15
- 10,263 View
- 330 Download
- 13 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF - Recent advances in immuno-oncology have increased understanding of the tumor immune microenvironment (TIME), and clinical trials for immune checkpoint inhibitor treatment have shown remission and/or durable response in certain proportions of patients stratified by predictive biomarkers. The TIME in colorectal cancer (CRC) was initially evaluated several decades ago. The prognostic value of the immune response to tumors, including tumor-infiltrating lymphocytes, peritumoral lymphoid reaction, and Crohn’s-like lymphoid reaction, has been well demonstrated. In this review, we describe the chronology of TIME research and review the up-to-date high-dimensional TIME landscape of CRC. We also summarize the clinical relevance of several biomarkers associated with immunotherapy in CRC, such as microsatellite instability, tumor mutational burden, POLE/POLD mutation, consensus molecular subtype, and programmed death-ligand 1 expression.
-
Citations
Citations to this article as recorded by- Aptasensors in colorectal Cancer: Emerging tools for precision diagnostics
Qamar Abuhassan, Ali M. Atoom, R. Roopashree, Jaya Bhanu Kanwar, T. Sudhakar, Vipasha Sharma, Ashish Singh Chauhan, Oybek Ruziyev
Clinica Chimica Acta.2026; 581: 120751. CrossRef - Five decades of colorectal cancer pathology: The World and China
Maode Lai
Chinese Science Bulletin.2025; 70(31): 5256. CrossRef - Recent research progress and clinical status of immunotherapy for colorectal cancer
Zhikui Huo, Guoliang Liu, Jiannan Li
Journal of Advanced Research.2025;[Epub] CrossRef - Real world effectiveness of chemotherapy plus bevacizumab with immunotherapy in colorectal cancer
Zhao Gao, Xiaoyan Wang, Tao Song, Shikai Wu, Xuan Jin
Scientific Reports.2025;[Epub] CrossRef - Targeting the “tumor microenvironment”: RNA-binding proteins in the spotlight in colorectal cancer therapy
Yiwei Zhang, Yujun Zhang, Jingjing Song, Xifu Cheng, Chulin Zhou, Shuo Huang, Wentao Zhao, Zhen Zong, Lingling Yang
International Immunopharmacology.2024; 131: 111876. CrossRef - T cell receptor clonotype in tumor microenvironment contributes to intratumoral signaling network in patients with colorectal cancer
In Hye Song, Seung-been Lee, Byung-Kwan Jeong, Jungwook Park, Honggeun Kim, GunHee Lee, Su Min Cha, Heejae Lee, Gyungyub Gong, Nak-Jung Kwon, Hee Jin Lee
Immunologic Research.2024; 72(5): 921. CrossRef - Identification of Key Immune and Cell Cycle Modules and Prognostic Genes for Glioma Patients through Transcriptome Analysis
Kaimin Guo, Jinna Yang, Ruonan Jiang, Xiaxia Ren, Peng Liu, Wenjia Wang, Shuiping Zhou, Xiaoguang Wang, Li Ma, Yunhui Hu
Pharmaceuticals.2024; 17(10): 1295. CrossRef - Unraveling the Role of Molecular Profiling in Predicting Treatment Response in Stage III Colorectal Cancer Patients: Insights from the IDEA International Study
Ippokratis Messaritakis, Eleni Psaroudaki, Konstantinos Vogiatzoglou, Maria Sfakianaki, Pantelis Topalis, Ioannis Iliopoulos, Dimitrios Mavroudis, John Tsiaoussis, Nikolaos Gouvas, Maria Tzardi, John Souglakos
Cancers.2023; 15(19): 4819. CrossRef - Biomarkers for Predicting Response to Personalized Immunotherapy in Gastric Cancer
Moonsik Kim, Ji Yun Jeong, An Na Seo
Diagnostics.2023; 13(17): 2782. CrossRef - Evaluation on Braking Stability of Autonomous Vehicles Running along Curved Sections Based on Asphalt Pavement Adhesion Properties
Binshuang Zheng, Xiaoming Huang, Junyao Tang, Jiaying Chen, Runmin Zhao, Zhengqiang Hong, Tao Tang, Meiling Han, Yang Yang
Journal of Advanced Transportation.2022; 2022: 1. CrossRef - Intratumoral spatial heterogeneity of tumor-infiltrating lymphocytes is a significant factor for precisely stratifying prognostic immune subgroups of microsatellite instability-high colorectal carcinomas
Minsun Jung, Ji Ae Lee, Seung-Yeon Yoo, Jeong Mo Bae, Gyeong Hoon Kang, Jung Ho Kim
Modern Pathology.2022; 35(12): 2011. CrossRef - Association of Tumor-Infiltrating Lymphocytes With Survival in Stages II and III Colorectal Cancer
Marina Vitorino, Inês Eiriz, Tiago C Tomás, Rodrigo Vicente, Ana Mendes, Ana Rita Freitas, Sofia Braga, Catarina Alves-Vale, Paula Borralho, André Ferreira, Luisa Leal da Costa
Cureus.2022;[Epub] CrossRef - Tumor Mutational Burden Predicting the Efficacy of Immune Checkpoint Inhibitors in Colorectal Cancer: A Systematic Review and Meta-Analysis
Yan Li, Yiqi Ma, Zijun Wu, Fanxin Zeng, Bin Song, Yanrong Zhang, Jinxing Li, Su Lui, Min Wu
Frontiers in Immunology.2021;[Epub] CrossRef - Genomic and transcriptomic characterization of heterogeneous immune subgroups of microsatellite instability-high colorectal cancers
Jung Ho Kim, Mi-Kyoung Seo, Ji Ae Lee, Seung-Yeon Yoo, Hyeon Jeong Oh, Hyundeok Kang, Nam-Yun Cho, Jeong Mo Bae, Gyeong Hoon Kang, Sangwoo Kim
Journal for ImmunoTherapy of Cancer.2021; 9(12): e003414. CrossRef
- Aptasensors in colorectal Cancer: Emerging tools for precision diagnostics
- Evolving pathologic concepts of serrated lesions of the colorectum
- Jung Ho Kim, Gyeong Hoon Kang
- J Pathol Transl Med. 2020;54(4):276-289. Published online June 26, 2020
- DOI: https://doi.org/10.4132/jptm.2020.04.15
- 18,112 View
- 841 Download
- 36 Web of Science
- 34 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Here, we provide an up-to-date review of the histopathology and molecular pathology of serrated colorectal lesions. First, we introduce the updated contents of the 2019 World Health Organization classification for serrated lesions. The sessile serrated lesion (SSL) is a new diagnostic terminology that replaces sessile serrated adenoma and sessile serrated polyp. The diagnostic criteria for SSL were revised to require only one unequivocal distorted serrated crypt, which is sufficient for diagnosis. Unclassified serrated adenomas have been included as a new category of serrated lesions. Second, we review ongoing issues concerning the morphology of serrated lesions. Minor morphologic variants with distinct molecular features were recently defined, including serrated tubulovillous adenoma, mucin-rich variant of traditional serrated adenoma (TSA), and superficially serrated adenoma. In addition to intestinal dysplasia and serrated dysplasia, minimal deviation dysplasia and not otherwise specified dysplasia were newly suggested as dysplasia subtypes of SSLs. Third, we summarize the molecular features of serrated lesions. The critical determinant of CpG island methylation development in SSLs is patient age. Interestingly, there may be ethnic differences in BRAF/KRAS mutation frequencies in SSLs. The molecular pathogenesis of TSAs is divided into KRAS and BRAF mutation pathways. SSLs with MLH1 methylation can progress into favorable prognostic microsatellite instability-positive (MSI+)/CpG island methylator phenotype-positive (CIMP+) carcinomas, whereas MLH1-unmethylated SSLs and BRAF-mutated TSAs can be precursors of poor-prognostic MSI−/CIMP+ carcinomas. Finally, based on our recent data, we propose an algorithm for stratifying risk subgroups of non-dysplastic SSLs.
-
Citations
Citations to this article as recorded by- Predominant Serrated Molecular Signature in Postcolonoscopy Colorectal Cancer: A Systematic Review and Meta-Analysis
Jen-Hao Yeh, Sin-Hua Moi, Chia-Chi Chen, Chao-Wen Hsu, Wen-Shuo Yeh, Tzu-Ning Tseng, Chuan-Pin Lin, Yu-Peng Liu, Jaw-Yuan Wang
American Journal of Gastroenterology.2026; 121(1): 122. CrossRef - Clinical and endoscopic characteristics of colorectal traditional serrated adenomas with dysplasia/adenocarcinoma in a Korean population
Ki-Hyun Kim, Eun Myung, Hyung Hoon Oh, Chan-Muk Im, Young-Eun Seo, Je-Seong Kim, Chae-June Lim, Ga-Ram You, Sung-Bum Cho, Wan-Sik Lee, Myung-Giun Noh, Kyung-Hwa Lee, Young-Eun Joo
World Journal of Gastrointestinal Oncology.2025;[Epub] CrossRef - MicroRNA: role in macrophage polarisation and colorectal cancer pathogenesis
Haihong Lin, Jun Zhou, Ying He, Yifan Zhu, Puwen Chen, Hongwei Yan, Junyun Huang, Ersheng Gong, Xiaoling Wang
Frontiers in Cell and Developmental Biology.2025;[Epub] CrossRef - Submucosal fibrosis in large colorectal serrated lesions in cases receiving endoscopic submucosal dissection
Erik Manriquez-Alegria, Naohisa Yoshida, Reo Kobayashi, Naoto Iwai, Ken Inoue, Osamu Dohi, Lucas Cardoso, Hideyuki Konishi
Therapeutic Advances in Gastroenterology.2025;[Epub] CrossRef - Navigating the Colorectal Cancer Maze: Unveiling Pathways To Diagnosis, Management, Pathophysiology and Prevention
Khalid Ali Mohammed Al Kamzari, Constantina Constantinou
Current Oncology Reports.2025; 27(10): 1115. CrossRef - Fosl1 is a transcriptional effector of BRAFV600E-driven intestinal tumorigenesis
Zakia Alam, Rebecca Nightingale, Analia Lesmana, Cheng Liu, Laura J. Jenkins, Mark F. Richardson, Lawrence Croft, Ian Y. Luk, Camilla M. Reehorst, Fiona Chionh, Natalia Vukelic, Faiza Basheer, Eugene Tulchinsky, Joshua Badshah, Troy Dumenil, Latifa Bakiri
iScience.2025; 28(11): 113875. CrossRef - Sessile Serrated Lesions in Inflammatory Bowel Disease: Hidden Players in Colitis-Associated Colorectal Cancer?
Roberto de Sire, Diletta De Deo, Miriana Mercurio, Gianluca Franchellucci, Giulio Calabrese, Livio Bonacci, Mauro Sollai Pinna, Cristina Bezzio, Alessandro Armuzzi, Cesare Hassan, Alessandro Repici, Fabiana Castiglione, Sandro Ardizzone, Roberta Maselli
Journal of Clinical Medicine.2025; 14(22): 8042. CrossRef - Histologic Reappraisal and Evaluation of MLH1 Protein Expression in Sessile Serrated Lesions of the Proximal Colon
Priscilla de Sene Portel Oliveira, Miriam Aparecida da Silva Trevisan, Rita Barbosa de Carvalho, Rita de Cássia Perina Martins, João José Fagundes, Claudio Saddy Rodrigues Coy, Ashwini Esnakula
Gastroenterology Research and Practice.2025;[Epub] CrossRef - Impact of AI-aided colonoscopy in clinical practice: a prospective randomised controlled trial
Johanna Schöler, Marko Alavanja, Thomas de Lange, Shunsuke Yamamoto, Per Hedenström, Jonas Varkey
BMJ Open Gastroenterology.2024; 11(1): e001247. CrossRef - The histologic features, molecular features, detection and management of serrated polyps: a review
Jin-Dong Wang, Guo-Shuai Xu, Xin-Long Hu, Wen-Qiang Li, Nan Yao, Fu-Zhou Han, Yin Zhang, Jun Qu
Frontiers in Oncology.2024;[Epub] CrossRef - Serrated polyps <10 mm cannot reliably be characterized by i-Scan without magnification at routine colonoscopy
Sabrina G.G. TESTONI, Chiara NOTARISTEFANO, Giuliano F. BONURA, Maria NAPOLITANO, Dario ESPOSITO, Edi VIALE, Lorella FANTI, Francesco AZZOLINI, Giulia M. CAVESTRO, PierAlberto TESTONI
Minerva Gastroenterology.2024;[Epub] CrossRef - Interobserver variability in the histopathological classification and grading of dysplasia in elevated colon lesions in the city of Lima
Guido Gallegos-Serruto, Aldo Gutiérrez, César Chian García, Isthvan Torres Perez
Revista de Gastroenterología del Perú.2024; 44(3): 239. CrossRef - Comparison of adenoma detection rate and proximal serrated polyp detection rate and their effect on post-colonoscopy colorectal cancer mortality in screening patients
Jasmin Zessner-Spitzenberg, Elisabeth Waldmann, Lena Jiricka, Lisa-Maria Rockenbauer, Anna Hinterberger, Jeremy Cook, Arno Asaturi, Aleksandra Szymanska, Barbara Majcher, Michael Trauner, Monika Ferlitsch
Endoscopy.2023; 55(05): 434. CrossRef - The yield of dysplasia and serrated lesions in a single-centre tertiary inflammatory bowel disease cohort
Fiona Yeaman, Lena Thin
Therapeutic Advances in Gastroenterology.2023;[Epub] CrossRef -
The BEETS (JACCRO CC-18) Trial: An Observational and Translational Study of
BRAF
-Mutated Metastatic Colorectal Cancer
Chiaki Inagaki, Ryo Matoba, Satoshi Yuki, Manabu Shiozawa, Akihito Tsuji, Eisuke Inoue, Kei Muro, Wataru Ichikawa, Masashi Fujii, Yu Sunakawa
Future Oncology.2023; 19(17): 1165. CrossRef - A retrospective analysis of the histology of resected polyps and colonoscopy quality parameters in Belgium
E Macken, S Van Dongen, G Van Hal
Acta Gastro Enterologica Belgica.2023; 86(2): 277. CrossRef - Prognostic Biomarkers of Cell Proliferation in Colorectal Cancer (CRC): From Immunohistochemistry to Molecular Biology Techniques
Aldona Kasprzak
Cancers.2023; 15(18): 4570. CrossRef - Assimilating Epigenetics and Transcriptomics for the Identification
of Prognostic Novel Biomarkers and Imminent Targets in
Colorectal Carcinoma with Therapeutic Potential
Suman Kumar Ray, Sukhes Mukherjee
Current Molecular Medicine.2023; 23(8): 784. CrossRef - Multitarget Stool RNA Test for Colorectal Cancer Screening
Erica K. Barnell, Elizabeth M. Wurtzler, Julie La Rocca, Thomas Fitzgerald, Jessica Petrone, Yansheng Hao, Yiming Kang, Faith L. Holmes, David A. Lieberman
JAMA.2023; 330(18): 1760. CrossRef - Microbiome in Colonic Carcinogenesis
Jun Sun, Yinglin Xia
Comprehensive Physiology.2023; 13(3): 4685. CrossRef - Impact of comprehensive optical diagnosis training using Workgroup serrAted polypS and Polyposis classification on detection of adenoma and sessile serrated lesion
Jooyoung Lee, Jung Ho Bae, Su Jin Chung, Hae Yeon Kang, Seung Joo Kang, Min‐Sun Kwak, Ji Yeon Seo, Ji Hyun Song, Sun Young Yang, Jong In Yang, Seon Hee Lim, Jeong Yoon Yim, Joo Hyun Lim, Goh Eun Chung, Eun Hyo Jin, Ji Min Choi, Yoo Min Han, Joo Sung Kim
Digestive Endoscopy.2022; 34(1): 180. CrossRef - Clinicopathological and molecular analyses of hyperplastic lesions including microvesicular variant and goblet cell rich variant hyperplastic polyps and hyperplastic nodules—Hyperplastic nodule is an independent histological entity
Noriyuki Uesugi, Yoichi Ajioka, Tomio Arai, Yoshihito Tanaka, Tamotsu Sugai
Pathology International.2022; 72(2): 128. CrossRef - Comprehensive clinicopathologic, molecular, and immunologic characterization of colorectal carcinomas with loss of three intestinal markers, CDX2, SATB2, and KRT20
Ji Ae Lee, Mi-Kyoung Seo, Seung-Yeon Yoo, Nam-Yun Cho, Yoonjin Kwak, Kyoungbun Lee, Jung Ho Kim, Gyeong Hoon Kang
Virchows Archiv.2022; 480(3): 543. CrossRef - Serrated Colorectal Lesions: An Up-to-Date Review from Histological Pattern to Molecular Pathogenesis
Martino Mezzapesa, Giuseppe Losurdo, Francesca Celiberto, Salvatore Rizzi, Antonio d’Amati, Domenico Piscitelli, Enzo Ierardi, Alfredo Di Leo
International Journal of Molecular Sciences.2022; 23(8): 4461. CrossRef - Arterial stiffness is associated with high-risk colorectal adenomas and serrated lesions: A cross-sectional study in a Taiwanese population
Hung-Yu Chen, Wen-Huang Lee, Hung-Lung Hsu, Yu-Tsung Chou, Fei-Lin Su, I-Hsuan Wu, Ting-Hsing Chao
Journal of Cardiology.2022; 80(2): 139. CrossRef - Morphological and molecular characterization of colorectal sessile serrated lesions with dysplasia
Filippo Cappello, Valentina Angerilli, Luca Dal Santo, Giada Munari, Marianna Sabbadin, Marcello Lo Mele, Gianmaria Pennelli, Claudio Luchini, Paola Parente, Stefano Lazzi, Matteo Fassan
Pathology - Research and Practice.2022; 240: 154214. CrossRef - Serrated polyposis: an overview
Jonathan Fawkes
Gastrointestinal Nursing.2022; 20(9): 24. CrossRef - Sessile serrated lesion presenting as large pedunculated polyp in the rectum: A case report
Shin Ju Oh, Jung-Wook Kim, Chi Hyuk Oh
Medicine.2022; 101(51): e32287. CrossRef - WHICH LESIONS ARE AT HIGHER RISK OF DEVELOPING COLORECTAL CARCINOMAS: SUPERFICIALLY ELEVATED SERRATED LESIONS OR DEPRESSED LESIONS?
Artur Adolfo PARADA, Filadelfio Euclydes VENCO, Miguel Reynaldo VARCA-NETO, Roberto EL IBRAHIM, Paula Bechara POLETTI, Helcio Pedrosa BRITO, Heloisa de Fátima SARE, Osvaldo MALAFAIA
ABCD. Arquivos Brasileiros de Cirurgia Digestiva (São Paulo).2022;[Epub] CrossRef - WNT5a in Colorectal Cancer: Research Progress and Challenges
Guangshun Sun, Liangliang Wu, Guoqiang Sun, Xuesong Shi, Hongyong Cao, Weiwei Tang
Cancer Management and Research.2021; Volume 13: 2483. CrossRef - Endoscopic diagnosis for colorectal sessile serrated lesions
Toshihiro Nishizawa, Shuntaro Yoshida, Akira Toyoshima, Tomoharu Yamada, Yoshiki Sakaguchi, Taiga Irako, Hirotoshi Ebinuma, Takanori Kanai, Kazuhiko Koike, Osamu Toyoshima
World Journal of Gastroenterology.2021; 27(13): 1321. CrossRef - NTRK oncogenic fusions are exclusively associated with the serrated neoplasia pathway in the colorectum and begin to occur in sessile serrated lesions
Jung Ho Kim, Jeong Hoon Hong, Yoon‐La Choi, Ji Ae Lee, Mi‐kyoung Seo, Mi‐Sook Lee, Sung Bin An, Min Jung Sung, Nam‐Yun Cho, Sung‐Su Kim, Young Kee Shin, Sangwoo Kim, Gyeong Hoon Kang
The Journal of Pathology.2021; 255(4): 399. CrossRef - Differential pre-malignant programs and microenvironment chart distinct paths to malignancy in human colorectal polyps
Bob Chen, Cherie’ R. Scurrah, Eliot T. McKinley, Alan J. Simmons, Marisol A. Ramirez-Solano, Xiangzhu Zhu, Nicholas O. Markham, Cody N. Heiser, Paige N. Vega, Andrea Rolong, Hyeyon Kim, Quanhu Sheng, Julia L. Drewes, Yuan Zhou, Austin N. Southard-Smith, Y
Cell.2021; 184(26): 6262. CrossRef - Molecular Insights Into Colorectal Carcinoma
Domenika Ortiz Requena, Monica Garcia-Buitrago
Archives of Medical Research.2020; 51(8): 839. CrossRef
- Predominant Serrated Molecular Signature in Postcolonoscopy Colorectal Cancer: A Systematic Review and Meta-Analysis
- Colorectal epithelial neoplasm associated with gut-associated lymphoid tissue
- Yo Han Jeon, Ji Hyun Ahn, Hee Kyung Chang
- J Pathol Transl Med. 2020;54(2):135-145. Published online January 29, 2020
- DOI: https://doi.org/10.4132/jptm.2019.11.06
- 9,692 View
- 255 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Colorectal epithelial neoplasm extending into the submucosal gut-associated lymphoid tissue (GALT) can cause difficulties in the differential diagnosis. Regarding GALT-associated epithelial neoplasms, a few studies favor the term “GALT carcinoma” while other studies have mentioned the term “GALT-associated pseudoinvasion/epithelial misplacement (PEM)”.
Methods
The clinicopathologic characteristics of 11 cases of colorectal epithelial neoplasm associated with submucosal GALT diagnosed via endoscopic submucosal dissection were studied.
Results
Eight cases (72.7%) were in males. The median age was 59 years, and age ranged from 53 to 73. All cases had a submucosal tumor component more compatible with GALT-associated PEM. Eight cases (72.7%) were located in the right colon. Ten cases (90.9%) had a non-protruding endoscopic appearance. Nine cases (81.8%) showed continuity between the submucosal and surface adenomatous components. Nine cases showed (81.8%) focal defects or discontinuation of the muscularis mucosae adjacent to the submucosal GALT. No case showed hemosiderin deposits in the submucosa or desmoplastic reaction. No case showed single tumor cells or small clusters of tumor cells in the submucosal GALT. Seven cases (63.6%) showed goblet cells in the submucosa. No cases showed oncocytic columnar cells lining submucosal glands.
Conclusions
Our experience suggests that pathologists should be aware of the differential diagnosis of GALT-associated submucosal extension by colorectal adenomatous neoplasm. Further studies are needed to validate classification of GALT-associated epithelial neoplasms. -
Citations
Citations to this article as recorded by- Family adenomatous polyposis come across dome type adenocarcinoma: a case report and literature review
Ying-Ying Chang, Xiao-Long Zhang, Yao-Hui Wang, Ting-Sheng Ling
Diagnostic Pathology.2025;[Epub] CrossRef - Radiation-induced injury and the gut microbiota: insights from a microbial perspective
Qiaoli Wang, Guoqiang Xu, Ouying Yan, Shang Wang, Xin Wang
Therapeutic Advances in Gastroenterology.2025;[Epub] CrossRef
- Family adenomatous polyposis come across dome type adenocarcinoma: a case report and literature review
- Standardized Pathology Report for Colorectal Cancer, 2nd Edition
- Baek-hui Kim, Joon Mee Kim, Gyeong Hoon Kang, Hee Jin Chang, Dong Wook Kang, Jung Ho Kim, Jeong Mo Bae, An Na Seo, Ho Sung Park, Yun Kyung Kang, Kyung-Hwa Lee, Mee Yon Cho, In-Gu Do, Hye Seung Lee, Hee Kyung Chang, Do Youn Park, Hyo Jeong Kang, Jin Hee Sohn, Mee Soo Chang, Eun Sun Jung, So-Young Jin, Eunsil Yu, Hye Seung Han, Youn Wha Kim
- J Pathol Transl Med. 2020;54(1):1-19. Published online November 13, 2019
- DOI: https://doi.org/10.4132/jptm.2019.09.28
- 28,857 View
- 1,308 Download
- 44 Web of Science
- 38 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - The first edition of the ‘Standardized Pathology Report for Colorectal Cancer,’ which was developed by the Gastrointestinal Pathology Study Group (GIP) of the Korean Society of Pathologists, was published 13 years ago. Meanwhile, there have been many changes in the pathologic diagnosis of colorectal cancer (CRC), pathologic findings included in the pathology report, and immunohistochemical and molecular pathology required for the diagnosis and treatment of colorectal cancer. In order to reflect these changes, we (GIP) decided to make the second edition of the report. The purpose of this standardized pathology report is to provide a practical protocol for Korean pathologists, which could help diagnose and treat CRC patients. This report consists of “standard data elements” and “conditional data elements.” Basic pathologic findings and parts necessary for prognostication of CRC patients are classified as “standard data elements,” while other prognostic factors and factors related to adjuvant therapy are classified as “conditional data elements” so that each institution could select the contents according to the characteristics of the institution. The Korean version is also provided separately so that Korean pathologists can easily understand and use this report. We hope that this report will be helpful in the daily practice of CRC diagnosis.
-
Citations
Citations to this article as recorded by- Proteogenomic profiling predicts outcomes of adjuvant chemotherapy in extrahepatic cholangiocarcinoma
Hyehyun Jeong, Ji-Hye Oh, Hee-Sung Ahn, Baek-Yeol Ryoo, Kyu-pyo Kim, Jae Ho Jeong, Inkeun Park, Dae Wook Hwang, Jae Hoon Lee, Ki Byung Song, Woohyung Lee, Ki-Hun Kim, Deog-Bog Moon, Gi Won Song, Dong-Hwan Jung, Seung-Mo Hong, Chae Won Park, In-Pyo Baek, Y
Journal of Hepatology.2026; 84(1): 122. CrossRef - Diagnostic accuracy and pitfalls of MRI for restaging locally advanced rectal cancer in patients following anti-PD1 therapy plus neoadjuvant chemoradiotherapy: a multicenter study
Lixue Xu, Liting Sun, Yuhuan Fu, Guangyong Chen, Hongwei Yao, Zhenchang Wang, Zhenghan Yang, Jie Zhang
Abdominal Radiology.2025;[Epub] CrossRef - Unraveling the role of perineural invasion in cancer progression across multiple tumor types
Muqtada Shaikh, Sanket Shirodkar, Gaurav Doshi
Medical Oncology.2025;[Epub] CrossRef - MALT lymphoma affecting the oral cavity: a clinical, pathologic and genetic study of MALT1 gene translocation
Juan Manuel Arteaga Legarrea, Mauro Lima dos Santos, Nathalia Gomes Rodrigues, Ricardo Santiago Gomez, Ricardo Alves Mesquita, Silvia Ferreira de Sousa, Cinthia Verónica Bardález López de Cáceres, Hélder Antônio Rebelo Pontes, Pablo Agustin Vargas, Luiz A
Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology.2025; 140(6): 740. CrossRef - Additional staining for lymphovascular invasion is associated with increased estimation of lymph node metastasis in patients with T1 colorectal cancer: Systematic review and meta‐analysis
Jun Watanabe, Katsuro Ichimasa, Yuki Kataoka, Atsushi Miki, Hidehiro Someko, Munenori Honda, Makiko Tahara, Takeshi Yamashina, Khay Guan Yeoh, Shigeo Kawai, Kazuhiko Kotani, Naohiro Sata
Digestive Endoscopy.2024; 36(5): 533. CrossRef - The use of core descriptors from the ENiGMA code study in recent literature: a systematic review
Saher‐Zahra Khan, Andrea Arline, Kate M. Williams, Matthew J. Lee, Emily Steinhagen, Sharon L. Stein
Colorectal Disease.2024; 26(3): 428. CrossRef - Efficacy and safety of PD-1 blockade plus long-course chemoradiotherapy in locally advanced rectal cancer (NECTAR): a multi-center phase 2 study
Zhengyang Yang, Jiale Gao, Jianyong Zheng, Jiagang Han, Ang Li, Gang Liu, Yi Sun, Jie Zhang, Guangyong Chen, Rui Xu, Xiao Zhang, Yishan Liu, Zhigang Bai, Wei Deng, Wei He, Hongwei Yao, Zhongtao Zhang
Signal Transduction and Targeted Therapy.2024;[Epub] CrossRef - Diagnostic Accuracy of Highest-Grade or Predominant Histological Differentiation of T1 Colorectal Cancer in Predicting Lymph Node Metastasis: A Systematic Review and Meta-Analysis
Jun Watanabe, Katsuro Ichimasa, Yuki Kataoka, Shoko Miyahara, Atsushi Miki, Khay Guan Yeoh, Shigeo Kawai, Fernando Martínez de Juan, Isidro Machado, Kazuhiko Kotani, Naohiro Sata
Clinical and Translational Gastroenterology.2024; 15(3): e00673. CrossRef - Comparative evaluation of CT and MRI in the preoperative staging of colon cancer
Effrosyni Bompou, Aikaterini Vassiou, Ioannis Baloyiannis, Konstantinos Perivoliotis, Ioannis Fezoulidis, George Tzovaras
Scientific Reports.2024;[Epub] CrossRef - Pathologic Implications of Magnetic Resonance Imaging-detected Extramural Venous Invasion of Rectal Cancer
Hyun Gu Lee, Chan Wook Kim, Jong Keon Jang, Seong Ho Park, Young Il Kim, Jong Lyul Lee, Yong Sik Yoon, In Ja Park, Seok-Byung Lim, Chang Sik Yu, Jin Cheon Kim
Clinical Colorectal Cancer.2023; 22(1): 129. CrossRef - A standardized pathology report for gastric cancer: 2nd edition
Young Soo Park, Myeong-Cherl Kook, Baek-hui Kim, Hye Seung Lee, Dong-Wook Kang, Mi-Jin Gu, Ok Ran Shin, Younghee Choi, Wonae Lee, Hyunki Kim, In Hye Song, Kyoung-Mee Kim, Hee Sung Kim, Guhyun Kang, Do Youn Park, So-Young Jin, Joon Mee Kim, Yoon Jung Choi,
Journal of Pathology and Translational Medicine.2023; 57(1): 1. CrossRef - IGFL2-AS1, a Long Non-Coding RNA, Is Associated with Radioresistance in Colorectal Cancer
Jeeyong Lee, Da Yeon Kim, Younjoo Kim, Ui Sup Shin, Kwang Seok Kim, Eun Ju Kim
International Journal of Molecular Sciences.2023; 24(2): 978. CrossRef - A Standardized Pathology Report for Gastric Cancer: 2nd Edition
Young Soo Park, Myeong-Cherl Kook, Baek-hui Kim, Hye Seung Lee, Dong-Wook Kang, Mi-Jin Gu, Ok Ran Shin, Younghee Choi, Wonae Lee, Hyunki Kim, In Hye Song, Kyoung-Mee Kim, Hee Sung Kim, Guhyun Kang, Do Youn Park, So-Young Jin, Joon Mee Kim, Yoon Jung Choi,
Journal of Gastric Cancer.2023; 23(1): 107. CrossRef - Incremental Detection Rate of Dysplasia and Sessile Serrated Polyps/Adenomas Using Narrow-Band Imaging and Dye Spray Chromoendoscopy in Addition to High-Definition Endoscopy in Patients with Long-Standing Extensive Ulcerative Colitis: Segmental Tandem End
Ji Eun Kim, Chang Wan Choi, Sung Noh Hong, Joo Hye Song, Eun Ran Kim, Dong Kyung Chang, Young-Ho Kim
Diagnostics.2023; 13(3): 516. CrossRef - Prognostic Impact of Extramural Lymphatic, Vascular, and Perineural Invasion in Stage II Colon Cancer: A Comparison With Intramural Invasion
Sang Sik Cho, Ji Won Park, Gyeong Hoon Kang, Jung Ho Kim, Jeong Mo Bae, Sae-Won Han, Tae-You Kim, Min Jung Kim, Seung-Bum Ryoo, Seung-Yong Jeong, Kyu Joo Park
Diseases of the Colon & Rectum.2023; 66(3): 366. CrossRef - Towards targeted colorectal cancer biopsy based on tissue morphology assessment by compression optical coherence elastography
Anton A. Plekhanov, Marina A. Sirotkina, Ekaterina V. Gubarkova, Elena B. Kiseleva, Alexander A. Sovetsky, Maria M. Karabut, Vladimir E. Zagainov, Sergey S. Kuznetsov, Anna V. Maslennikova, Elena V. Zagaynova, Vladimir Y. Zaitsev, Natalia D. Gladkova
Frontiers in Oncology.2023;[Epub] CrossRef - Is High-Grade Tumor Budding an Independent Prognostic Factor in Stage II Colon Cancer?
Jung Kyong Shin, Yoon Ah Park, Jung Wook Huh, Seong Hyeon Yun, Hee Cheol Kim, Woo Yong Lee, Seok Hyung Kim, Sang Yun Ha, Yong Beom Cho
Diseases of the Colon & Rectum.2023; 66(8): e801. CrossRef - Detection of Human cytomegalovirus UL55 Gene and IE/E Protein Expression in Colorectal Cancer Patients in Egypt
May Raouf, Ahmed A. Sabry, Mahinour A. Ragab, Samar El Achy, Amira Amer
BMC Cancer.2023;[Epub] CrossRef - Polo-like kinase 4 as a potential predictive biomarker of chemoradioresistance in locally advanced rectal cancer
Hyunseung Oh, Soon Gu Kim, Sung Uk Bae, Sang Jun Byun, Shin Kim, Jae-Ho Lee, Ilseon Hwang, Sun Young Kwon, Hye Won Lee
Journal of Pathology and Translational Medicine.2022; 56(1): 40. CrossRef - A Prediction Model for Tumor Recurrence in Stage II–III Colorectal Cancer Patients: From a Machine Learning Model to Genomic Profiling
Po-Chuan Chen, Yu-Min Yeh, Bo-Wen Lin, Ren-Hao Chan, Pei-Fang Su, Yi-Chia Liu, Chung-Ta Lee, Shang-Hung Chen, Peng-Chan Lin
Biomedicines.2022; 10(2): 340. CrossRef - Rationale and design of a prospective, multicenter, phase II clinical trial of safety and efficacy evaluation of long course neoadjuvant chemoradiotherapy plus tislelizumab followed by total mesorectal excision for locally advanced rectal cancer (NCRT-PD1
Zhengyang Yang, Xiao Zhang, Jie Zhang, Jiale Gao, Zhigang Bai, Wei Deng, Guangyong Chen, Yongbo An, Yishan Liu, Qi Wei, Jiagang Han, Ang Li, Gang Liu, Yi Sun, Dalu Kong, Hongwei Yao, Zhongtao Zhang
BMC Cancer.2022;[Epub] CrossRef - Potential of DEK proto‑oncogene as a prognostic biomarker for colorectal cancer: An evidence‑based review
Muhammad Habiburrahman, Muhammad Wardoyo, Stefanus Sutopo, Nur Rahadiani
Molecular and Clinical Oncology.2022;[Epub] CrossRef - Reproducibility and Feasibility of Classification and National Guidelines for Histological Diagnosis of Canine Mammary Gland Tumours: A Multi-Institutional Ring Study
Serenella Papparella, Maria Crescio, Valeria Baldassarre, Barbara Brunetti, Giovanni Burrai, Cristiano Cocumelli, Valeria Grieco, Selina Iussich, Lorella Maniscalco, Francesca Mariotti, Francesca Millanta, Orlando Paciello, Roberta Rasotto, Mariarita Roma
Veterinary Sciences.2022; 9(7): 357. CrossRef - Composite scoring system and optimal tumor budding cut-off number for estimating lymph node metastasis in submucosal colorectal cancer
Jeong-ki Kim, Ye-Young Rhee, Jeong Mo Bae, Jung Ho Kim, Seong-Joon Koh, Hyun Jung Lee, Jong Pil Im, Min Jung Kim, Seung-Bum Ryoo, Seung-Yong Jeong, Kyu Joo Park, Ji Won Park, Gyeong Hoon Kang
BMC Cancer.2022;[Epub] CrossRef - Automated Hybrid Model for Detecting Perineural Invasion in the Histology of Colorectal Cancer
Jiyoon Jung, Eunsu Kim, Hyeseong Lee, Sung Hak Lee, Sangjeong Ahn
Applied Sciences.2022; 12(18): 9159. CrossRef - Clinical Implication of Perineural and Lymphovascular Invasion in Rectal Cancer Patients Who Underwent Surgery After Preoperative Chemoradiotherapy
Young Il Kim, Chan Wook Kim, Jong Hoon Kim, Jihun Kim, Jun-Soo Ro, Jong Lyul Lee, Yong Sik Yoon, In Ja Park, Seok-Byung Lim, Chang Sik Yu, Jin Cheon Kim
Diseases of the Colon & Rectum.2022; 65(11): 1325. CrossRef - Molecular Pathology of Gastric Cancer
Moonsik Kim, An Na Seo
Journal of Gastric Cancer.2022; 22(4): 264. CrossRef - Selective approach to arterial ligation in radical sigmoid colon cancer surgery with D3 lymph node dissection: A multicenter comparative study
Sergey Efetov, Albina Zubayraeva, Cüneyt Kayaalp, Alisa Minenkova, Yusuf Bağ, Aftandil Alekberzade, Petr Tsarkov
Turkish Journal of Surgery.2022; 38(4): 382. CrossRef - Evaluation of lncRNA FOXD2-AS1 Expression as a Diagnostic Biomarker in Colorectal Cancer
Hooman Shalmashi, Sahar Safaei, Dariush Shanehbandi, Milad Asadi, Soghra Bornehdeli, Abdolreza Mehdi Navaz
Reports of Biochemistry and Molecular Biology.2022; 11(3): 471. CrossRef - Improvement in the Assessment of Response to Preoperative Chemoradiotherapy for Rectal Cancer Using Magnetic Resonance Imaging and a Multigene Biomarker
Eunhae Cho, Sung Woo Jung, In Ja Park, Jong Keon Jang, Seong Ho Park, Seung-Mo Hong, Jong Lyul Lee, Chan Wook Kim, Yong Sik Yoon, Seok-Byung Lim, Chang Sik Yu, Jin Cheon Kim
Cancers.2021; 13(14): 3480. CrossRef - Addition of V-Stage to Conventional TNM Staging to Create the TNVM Staging System for Accurate Prediction of Prognosis in Colon Cancer: A Multi-Institutional Retrospective Cohort Study
Jung Hoon Bae, Ji Hoon Kim, Jaeim Lee, Bong-Hyeon Kye, Sang Chul Lee, In Kyu Lee, Won Kyung Kang, Hyeon-Min Cho, Yoon Suk Lee
Biomedicines.2021; 9(8): 888. CrossRef - Gene Expression Profiles Associated with Radio-Responsiveness in Locally Advanced Rectal Cancer
Jeeyong Lee, Junhye Kwon, DaYeon Kim, Misun Park, KwangSeok Kim, InHwa Bae, Hyunkyung Kim, JoonSeog Kong, Younjoo Kim, UiSup Shin, EunJu Kim
Biology.2021; 10(6): 500. CrossRef - A Patient-Derived Organoid-Based Radiosensitivity Model for the Prediction of Radiation Responses in Patients with Rectal Cancer
Misun Park, Junhye Kwon, Joonseog Kong, Sun Mi Moon, Sangsik Cho, Ki Young Yang, Won Il Jang, Mi Sook Kim, Younjoo Kim, Ui Sup Shin
Cancers.2021; 13(15): 3760. CrossRef - Comparison between Local Excision and Radical Resection for the Treatment of Rectal Cancer in ypT0-1 Patients: An Analysis of the Clinicopathological Factors and Survival Rates
Soo Young Oh, In Ja Park, Young IL Kim, Jong-Lyul Lee, Chan Wook Kim, Yong Sik Yoon, Seok-Byung Lim, Chang Sik Yu, Jin Cheon Kim
Cancers.2021; 13(19): 4823. CrossRef - Comparison of Vascular Invasion With Lymph Node Metastasis as a Prognostic Factor in Stage I-III Colon Cancer: An Observational Cohort Study
Jung Hoon Bae, Ji Hoon Kim, Bong-Hyeon Kye, Abdullah Al-Sawat, Chul Seung Lee, Seung-Rim Han, In Kyu Lee, Sung Hak Lee, Yoon Suk Lee
Frontiers in Surgery.2021;[Epub] CrossRef - Clinicopathological significance of Ki67 expression in colorectal cancer
Jing Li, Zhi-ye Liu, Hai-bo Yu, Qing Xue, Wen-jie He, Hai-tao Yu
Medicine.2020; 99(20): e20136. CrossRef - Lateral lymph node and its association with distant recurrence in rectal cancer: A clue of systemic disease
Young Il Kim, Jong Keon Jang, In Ja Park, Seong Ho Park, Jong Beom Kim, Jin-Hong Park, Tae Won Kim, Jun-Soo Ro, Seok-Byung Lim, Chang Sik Yu, Jin Cheon Kim
Surgical Oncology.2020; 35: 174. CrossRef - Transformation of Pathology Reports Into the Common Data Model With Oncology Module: Use Case for Colon Cancer
Borim Ryu, Eunsil Yoon, Seok Kim, Sejoon Lee, Hyunyoung Baek, Soyoung Yi, Hee Young Na, Ji-Won Kim, Rong-Min Baek, Hee Hwang, Sooyoung Yoo
Journal of Medical Internet Research.2020; 22(12): e18526. CrossRef
- Proteogenomic profiling predicts outcomes of adjuvant chemotherapy in extrahepatic cholangiocarcinoma
- Clinicopathological Characterization and Prognostic Implication of SMAD4 Expression in Colorectal Carcinoma
- Seung-Yeon Yoo, Ji-Ae Lee, Yunjoo Shin, Nam-Yun Cho, Jeong Mo Bae, Gyeong Hoon Kang
- J Pathol Transl Med. 2019;53(5):289-297. Published online June 24, 2019
- DOI: https://doi.org/10.4132/jptm.2019.06.07
- 9,515 View
- 170 Download
- 9 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
SMAD family member 4 (SMAD4) has gained attention as a promising prognostic factor of colorectal cancer (CRC) as well as a key molecule to understand the tumorigenesis and progression of CRC.
Methods
We retrospectively analyzed 1,281 CRC cases immunohistochemically for their expression status of SMAD4, and correlated this status with clinicopathologic and molecular features of CRCs.
Results
A loss of nuclear SMAD4 was significantly associated with frequent lymphovascular and perineural invasion, tumor budding, fewer tumor-infiltrating lymphocytes, higher pT and pN category, and frequent distant metastasis. In contrast, tumors overexpressing SMAD4 showed a significant association with sporadic microsatellite instability. After adjustment for TNM stage, tumor differentiation, adjuvant chemotherapy, and lymphovascular invasion, the loss of SMAD4 was found to be an independent prognostic factor for worse 5-year progression-free survival (hazard ratio [HR], 1.27; 95% confidence interval [CI], 1.01 to 1.60; p=.042) and 7-year cancerspecific survival (HR, 1.45; 95% CI, 1.06 to 1.99; p=.022).
Conclusions
We confirmed the value of determining the loss of SMAD4 immunohistochemically as an independent prognostic factor for CRC in general. In addition, we identified some histologic and molecular features that might be clues to elucidate the role of SMAD4 in colorectal tumorigenesis and progression. -
Citations
Citations to this article as recorded by- Механизмы резистентности к иммунотерапии при MSI фенотипе
М. Ю. Федянин
Malignant tumours.2025; 15(3s1): 11. CrossRef - A set cover algorithm identifies minimal circulating tumour DNA sequencing targets for colorectal cancer detection
Kit Moloney-Geany, Michael A. Black, Robert C. Day, Parry Guilford, Michael J. Dunnet
Scientific Reports.2025;[Epub] CrossRef - Association between the expression of epithelial–mesenchymal transition (EMT)-related markers and oncologic outcomes of colorectal cancer
Mona Hany Emile, Sameh Hany Emile, Amr Awad El-Karef, Mohamed Awad Ebrahim, Ibrahim Eldosoky Mohammed, Dina Abdallah Ibrahim
Updates in Surgery.2024; 76(6): 2181. CrossRef - TGF-β and SMAD2/4 Expression in Nonmetastatic and Metastatic Colorectal Cancer Patients
Ainul Mardiah, Hendra Susanto, Sri Rahayu Lestari, A. Taufiq, H. Susanto, H. Nur, M. Diantoro, M. Aziz, N.A.N.N. Malek
BIO Web of Conferences.2024; 117: 01001. CrossRef - Unraveling Resistance to Immunotherapy in MSI-High Colorectal Cancer
Ronald Heregger, Florian Huemer, Markus Steiner, Alejandra Gonzalez-Martinez, Richard Greil, Lukas Weiss
Cancers.2023; 15(20): 5090. CrossRef - Association of β-Catenin, APC, SMAD3/4, Tp53, and Cyclin D1 Genes in Colorectal Cancer: A Systematic Review and Meta-Analysis
Hongfeng Yan, Fuquan Jiang, Jianwu Yang, Ying-Kun Xu
Genetics Research.2022; 2022: 1. CrossRef - Comprehensive genetic features of gastric mixed adenoneuroendocrine carcinomas and pure neuroendocrine carcinomas
Jiwon Koh, Soo Kyung Nam, Yoonjin Kwak, Gilhyang Kim, Ka‐Kyung Kim, Byung‐Chul Lee, Sang‐Hoon Ahn, Do Joong Park, Hyung‐Ho Kim, Kyoung Un Park, Woo Ho Kim, Hye Seung Lee
The Journal of Pathology.2021; 253(1): 94. CrossRef - Alterations of PTEN and SMAD4 methylation in diagnosis of breast cancer: implications of methyl II PCR assay
Menha Swellam, Entsar A. Saad, Shimaa Sabry, Adel Denewer, Camelia Abdel Malak, Amr Abouzid
Journal of Genetic Engineering and Biotechnology.2021; 19(1): 54. CrossRef - Molecular Characterization and Functional Analysis of Two Steroidogenic Genes TSPO and SMAD4 in Yellow Catfish
Fang Chen, Chong-Chao Zhong, Chang-Chun Song, Shu-Wei Chen, Yang He, Xiao-Ying Tan
International Journal of Molecular Sciences.2021; 22(9): 4505. CrossRef - SMAD7 and SMAD4 expression in colorectal cancer progression and therapy response
Jovana Rosic, Sandra Dragicevic, Marko Miladinov, Jovana Despotovic, Aleksandar Bogdanovic, Zoran Krivokapic, Aleksandra Nikolic
Experimental and Molecular Pathology.2021; 123: 104714. CrossRef - Actionable Potentials of Less Frequently Mutated Genes in Colorectal Cancer and Their Roles in Precision Medicine
Ryia Illani Mohd Yunos, Nurul Syakima Ab Mutalib, Francis Yew Fu Tieng, Nadiah Abu, Rahman Jamal
Biomolecules.2020; 10(3): 476. CrossRef
- Механизмы резистентности к иммунотерапии при MSI фенотипе
- CpG Island Methylation in Sessile Serrated Adenoma/Polyp of the Colorectum: Implications for Differential Diagnosis of Molecularly High-Risk Lesions among Non-dysplastic Sessile Serrated Adenomas/Polyps
- Ji Ae Lee, Hye Eun Park, Seung-Yeon Yoo, Seorin Jeong, Nam-Yun Cho, Gyeong Hoon Kang, Jung Ho Kim
- J Pathol Transl Med. 2019;53(4):225-235. Published online March 19, 2019
- DOI: https://doi.org/10.4132/jptm.2019.03.12
- 9,607 View
- 245 Download
- 8 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Although colorectal sessile serrated adenomas/polyps (SSA/Ps) with morphologic dysplasia are regarded as definite high-risk premalignant lesions, no reliable grading or risk-stratifying system exists for non-dysplastic SSA/Ps. The accumulation of CpG island methylation is a molecular hallmark of progression of SSA/Ps. Thus, we decided to classify non-dysplastic SSA/Ps into risk subgroups based on the extent of CpG island methylation.
Methods
The CpG island methylator phenotype (CIMP) status of 132 non-dysplastic SSA/Ps was determined using eight CIMP-specific promoter markers. SSA/Ps with CIMP-high and/or MLH1 promoter methylation were regarded as a high-risk subgroup.
Results
Based on the CIMP analysis results, methylation frequency of each CIMP marker suggested a sequential pattern of CpG island methylation during progression of SSA/P, indicating MLH1 as a late-methylated marker. Among the 132 non-dysplastic SSA/Ps, 34 (26%) were determined to be high-risk lesions (33 CIMP-high and 8 MLH1-methylated cases; seven cases overlapped). All 34 high-risk SSA/Ps were located exclusively in the proximal colon (100%, p = .001) and were significantly associated with older age (≥ 50 years, 100%; p = .003) and a larger histologically measured lesion size (> 5 mm, 100%; p = .004). In addition, the high-risk SSA/Ps were characterized by a relatively higher number of typical base-dilated serrated crypts.
Conclusions
Both CIMP-high and MLH1 methylation are late-step molecular events during progression of SSA/Ps and rarely occur in SSA/Ps of young patients. Comprehensive consideration of age (≥ 50), location (proximal colon), and histologic size (> 5 mm) may be important for the prediction of high-risk lesions among non-dysplastic SSA/Ps. -
Citations
Citations to this article as recorded by- MLH1 Methylation Status and Microsatellite Instability in Patients with Colorectal Cancer
Manuel Alejandro Rico-Méndez, Miguel Angel Trujillo-Rojas, María de la Luz Ayala-Madrigal, Jesús Arturo Hernández-Sandoval, Anahí González-Mercado, Melva Gutiérrez-Angulo, José Geovanni Romero-Quintana, Jesús Alonso Valenzuela-Pérez, Ruth Ramírez-Ramírez,
Genes.2025; 16(2): 182. CrossRef - Histologic Reappraisal and Evaluation of MLH1 Protein Expression in Sessile Serrated Lesions of the Proximal Colon
Priscilla de Sene Portel Oliveira, Miriam Aparecida da Silva Trevisan, Rita Barbosa de Carvalho, Rita de Cássia Perina Martins, João José Fagundes, Claudio Saddy Rodrigues Coy, Ashwini Esnakula
Gastroenterology Research and Practice.2025;[Epub] CrossRef - Immune microenvironmental heterogeneity according to tumor DNA methylation phenotypes in microsatellite instability-high colorectal cancers
Jung Ho Kim, Jiyun Hong, Ji Ae Lee, Minsun Jung, Eunwoo Choi, Nam-Yun Cho, Gyeong Hoon Kang, Sangwoo Kim
Cancer Immunology, Immunotherapy.2024;[Epub] CrossRef - How to "pick up" colorectal serrated lesions and polyps in daily histopathology practice: From terminologies to diagnostic pitfalls
Thai H Tran, Vinh H Nguyen, Diem TN Vo
World Journal of Clinical Oncology.2024; 15(9): 1157. CrossRef - Serrated Colorectal Lesions: An Up-to-Date Review from Histological Pattern to Molecular Pathogenesis
Martino Mezzapesa, Giuseppe Losurdo, Francesca Celiberto, Salvatore Rizzi, Antonio d’Amati, Domenico Piscitelli, Enzo Ierardi, Alfredo Di Leo
International Journal of Molecular Sciences.2022; 23(8): 4461. CrossRef - NTRK oncogenic fusions are exclusively associated with the serrated neoplasia pathway in the colorectum and begin to occur in sessile serrated lesions
Jung Ho Kim, Jeong Hoon Hong, Yoon‐La Choi, Ji Ae Lee, Mi‐kyoung Seo, Mi‐Sook Lee, Sung Bin An, Min Jung Sung, Nam‐Yun Cho, Sung‐Su Kim, Young Kee Shin, Sangwoo Kim, Gyeong Hoon Kang
The Journal of Pathology.2021; 255(4): 399. CrossRef - Evolving pathologic concepts of serrated lesions of the colorectum
Jung Ho Kim, Gyeong Hoon Kang
Journal of Pathology and Translational Medicine.2020; 54(4): 276. CrossRef
- MLH1 Methylation Status and Microsatellite Instability in Patients with Colorectal Cancer
- Prognostic Impact of Fusobacterium nucleatum Depends on Combined Tumor Location and Microsatellite Instability Status in Stage II/III Colorectal Cancers Treated with Adjuvant Chemotherapy
- Hyeon Jeong Oh, Jung Ho Kim, Jeong Mo Bae, Hyun Jung Kim, Nam-Yun Cho, Gyeong Hoon Kang
- J Pathol Transl Med. 2019;53(1):40-49. Published online December 26, 2018
- DOI: https://doi.org/10.4132/jptm.2018.11.29
- 19,598 View
- 252 Download
- 50 Web of Science
- 53 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
This study aimed to investigate the prognostic impact of intratumoral Fusobacterium nucleatum in colorectal cancer (CRC) treated with adjuvant chemotherapy.
Methods
F. nucleatumDNA was quantitatively measured in a total of 593 CRC tissues retrospectively collectedfrom surgically resected specimens of stage III or high-risk stage II CRC patients who had receivedcurative surgery and subsequent oxaliplatin-based adjuvant chemotherapy (either FOLFOXor CAPOX). Each case was classified into one of the three categories: F. nucleatum–high, –low, or –negative.
Results
No significant differences in survival were observed between the F.nucleatum–high and –low/negative groups in the 593 CRCs (p = .671). Subgroup analyses accordingto tumor location demonstrated that disease-free survival was significantly better in F.nucleatum–high than in –low/negative patients with non-sigmoid colon cancer (including cecal,ascending, transverse, and descending colon cancers; n = 219; log-rank p = .026). In multivariateanalysis, F. nucleatum was determined to be an independent prognostic factor in non-sigmoidcolon cancers (hazard ratio, 0.42; 95% confidence interval, 0.18 to 0.97; p = .043). Furthermore,the favorable prognostic effect of F. nucleatum–high was observed only in a non-microsatellite instability-high (non-MSI-high) subset of non-sigmoid colon cancers (log-rank p = 0.014), but not ina MSI-high subset (log-rank p = 0.844), suggesting that the combined status of tumor locationand MSI may be a critical factor for different prognostic impacts of F. nucleatum in CRCs treatedwith adjuvant chemotherapy.
Conclusions
Intratumoral F. nucleatum load is a potential prognosticfactor in a non-MSI-high/non-sigmoid/non-rectal cancer subset of stage II/III CRCs treatedwith oxaliplatin-based adjuvant chemotherapy. -
Citations
Citations to this article as recorded by- Microbiota and tumor epigenetics: deep interconnections and emerging therapeutic perspectives
Lei Duan, Dan Hu, Haoling Zhang, Yan Liao
Critical Reviews in Clinical Laboratory Sciences.2026; : 1. CrossRef - Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights
Linda Galasso, Fabrizio Termite, Irene Mignini, Giorgio Esposto, Raffaele Borriello, Federica Vitale, Alberto Nicoletti, Mattia Paratore, Maria Elena Ainora, Antonio Gasbarrini, Maria Assunta Zocco
Cancers.2025; 17(3): 368. CrossRef - Emerging roles of intratumoral microbiota: a key to novel cancer therapies
Pengzhong Fang, Jing Yang, Huiyun Zhang, Diankui Shuai, Min Li, Lin Chen, Liping Liu
Frontiers in Oncology.2025;[Epub] CrossRef - Association of Fusobacterium nucleatum with colorectal cancer molecular subtypes and its outcome: a systematic review
Luana Greco, Federica Rubbino, Clarissa Ferrari, Michela Cameletti, Fabio Grizzi, Fabrizio Bonelli, Alberto Malesci, Massimiliano Mazzone, Luigi Ricciardiello, Luigi Laghi
Gut Microbiome.2025;[Epub] CrossRef - The Interplay Between the Gut Microbiota and Colorectal Cancer: A Review of the Literature
Marco Cintoni, Marta Palombaro, Eleonora Zoli, Giuseppe D’Agostino, Gabriele Pulcini, Elena Leonardi, Pauline Raoul, Emanuele Rinninella, Flavio De Maio, Esmeralda Capristo, Antonio Gasbarrini, Maria Cristina Mele
Microorganisms.2025; 13(6): 1410. CrossRef - Harnessing intratumoral microbiota: new horizons in immune microenvironment and immunotherapy
Jinhe Zhang, Zinan You, Xinqiao Li, Jinpeng Hu, Jiamu Li, Zhitao Jing
Journal of Translational Medicine.2025;[Epub] CrossRef - Prognostic impact of Fusobacterium nucleatum on survival in colorectal cancer: A systematic review and meta-analysis
Tianyu Wang, Shengcheng Lin, Yong Ji, Ciren Puqiong, Jidong Gao, Shuluan Li
Journal of Cancer Research and Therapeutics.2025; 21(4): 796. CrossRef - Fusobacterium nucleatum in Health and Disease
Xinyi Yang, Shutian Zhang, Tingting Ning, Jing Wu
MedComm.2025;[Epub] CrossRef - Periodontitis and Oral Pathogens in Colorectal Cancer: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis
Luis Chauca-Bajaña, Andrea Ordoñez Balladares, Alejandro Ismael Lorenzo-Pouso, Rosangela Caicedo-Quiroz, Rafael Xavier Erazo Vaca, Rolando Fabricio Dau Villafuerte, Yajaira Vanessa Avila-Granizo, Carlos Hans Salazar Minda, Miguel Amador Salavarria Vélez,
Dentistry Journal.2025; 13(12): 595. CrossRef - Composition of the colon microbiota in the individuals with inflammatory bowel disease and colon cancer
Ceren Acar, Sibel Kucukyildirim Celik, H. Ozgur Ozdemirel, Beril Erdem Tuncdemir, Saadet Alan, Hatice Mergen
Folia Microbiologica.2024; 69(2): 333. CrossRef - Intratumoral microorganisms in tumors of the digestive system
Mengjuan Xuan, Xinyu Gu, Yingru Liu, Li Yang, Yi Li, Di Huang, Juan Li, Chen Xue
Cell Communication and Signaling.2024;[Epub] CrossRef - Prognostic impact of oral microbiome on survival of malignancies: a systematic review and meta-analysis
Shuluan Li, Tianyu Wang, Ya Ren, Zhou Liu, Jidong Gao, Zhi Guo
Systematic Reviews.2024;[Epub] CrossRef - Exploring the Potential of Humoral Immune Response to Commensal Bifidobacterium as a Biomarker for Human Health, including Both Malignant and Non-Malignant Diseases: A Perspective on Detection Strategies and Future Directions
Kyogo Itoh, Satoko Matsueda
Biomedicines.2024; 12(4): 803. CrossRef - Unveiling intratumoral microbiota: An emerging force for colorectal cancer diagnosis and therapy
Jinjing Zhang, Penghui Wang, Jiafeng Wang, Xiaojie Wei, Mengchuan Wang
Pharmacological Research.2024; 203: 107185. CrossRef - Spatial transcriptomic analysis reveals local effects of intratumoral fusobacterial infection on DNA damage and immune signaling in rectal cancer
William P. Duggan, Batuhan Kisakol, Ina Woods, Mohammedreza Azimi, Heiko Dussmann, Joanna Fay, Tony O’Grady, Barry Maguire, Ian S. Reynolds, Manuela Salvucci, Daniel J. Slade, Deborah A. McNamara, John P. Burke, Jochen H.M. Prehn
Gut Microbes.2024;[Epub] CrossRef - Gut microbiota characteristics of colorectal cancer patients in Hubei, China, and differences with cohorts from other Chinese regions
Jianguo Shi, Hexiao Shen, Hui Huang, Lifang Zhan, Wei Chen, Zhuohui Zhou, Yongling Lv, Kai Xiong, Zhiwei Jiang, Qiyi Chen, Lei Liu
Frontiers in Microbiology.2024;[Epub] CrossRef - The role of Fusobacterium nucleatum in cancer and its implications for clinical applications
Wanyi Luo, Juxi Han, Xian Peng, Xuedong Zhou, Tao Gong, Xin Zheng
Molecular Oral Microbiology.2024; 39(6): 417. CrossRef - Gut Microbiome and colorectal cancer: discovery of bacterial changes with metagenomics application in Turkısh population
Yakup Ulger, Anıl Delik, Hikmet Akkız
Genes & Genomics.2024; 46(9): 1059. CrossRef - Intratumoral Microbiota: Metabolic Influences and Biomarker Potential in Gastrointestinal Cancer
Xueyuan Bi, Jihan Wang, Cuicui Liu
Biomolecules.2024; 14(8): 917. CrossRef - Intratumoral Microbiota: Insights from Anatomical, Molecular, and Clinical Perspectives
Claudia Lombardo, Rosanna Fazio, Marta Sinagra, Giuseppe Gattuso, Federica Longo, Cinzia Lombardo, Mario Salmeri, Guido Nicola Zanghì, Carla Agata Erika Loreto
Journal of Personalized Medicine.2024; 14(11): 1083. CrossRef - Exploring the role of Fusobacterium nucleatum in colorectal cancer: implications for tumor proliferation and chemoresistance
Leila Dadgar-Zankbar, Zahra Elahi, Aref Shariati, Azad Khaledi, Shabnam Razavi, Amin Khoshbayan
Cell Communication and Signaling.2024;[Epub] CrossRef - Fusobacterium nucleatum Abundance is Associated with Cachexia in Colorectal Cancer Patients: The ColoCare Study
Mmadili N. Ilozumba, Tengda Lin, Sheetal Hardikar, Doratha A. Byrd, June L. Round, W. Zac Stephens, Andreana N. Holowatyj, Christy A. Warby, Victoria Damerell, Christopher I. Li, Jane C. Figueiredo, Adetunji T. Toriola, David Shibata, Gary C. Fillmore, Ba
Cancer Medicine.2024;[Epub] CrossRef - Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy
Li Yang, Aitian Li, Ying Wang, Yi Zhang
Signal Transduction and Targeted Therapy.2023;[Epub] CrossRef - Increased Fusobacterium tumoural abundance affects immunogenicity in mucinous colorectal cancer and may be associated with improved clinical outcome
William P. Duggan, Manuela Salvucci, Batuhan Kisakol, Andreas U. Lindner, Ian S. Reynolds, Heiko Dussmann, Joanna Fay, Tony O’Grady, Daniel B. Longley, Fiona Ginty, Elizabeth Mc Donough, Daniel J. Slade, John P. Burke, Jochen H. M. Prehn
Journal of Molecular Medicine.2023; 101(7): 829. CrossRef -
Fusobacterium nucleatum Load Correlates with KRAS Mutation and Sessile Serrated Pathogenesis in Colorectal Adenocarcinoma
Koki Takeda, Minoru Koi, Yoshiki Okita, Sija Sajibu, Temitope O. Keku, John M. Carethers
Cancer Research Communications.2023; 3(9): 1940. CrossRef - La asociación entre Fusobacterium nucleatum y el cáncer colorrectal: una revisión sistemática y metaanálisis
Paola Villar-Ortega, Manuela Expósito-Ruiz, Miguel Gutiérrez-Soto, Miguel Ruiz-Cabello Jiménez, José María Navarro-Marí, José Gutiérrez-Fernández
Enfermedades Infecciosas y Microbiología Clínica.2022; 40(5): 224. CrossRef - The association between Fusobacterium nucleatum and cancer colorectal: A systematic review and meta-analysis
Paola Villar-Ortega, Manuela Expósito-Ruiz, Miguel Gutiérrez-Soto, Miguel Ruiz-Cabello Jiménez, José María Navarro-Marí, José Gutiérrez-Fernández
Enfermedades infecciosas y microbiologia clinica (English ed.).2022; 40(5): 224. CrossRef - Suppression of Berberine and Probiotics (in vitro and in vivo) on the Growth of Colon Cancer With Modulation of Gut Microbiota and Butyrate Production
Chao Huang, Ying Sun, Sheng-rong Liao, Zhao-xin Chen, Han-feng Lin, Wei-zeng Shen
Frontiers in Microbiology.2022;[Epub] CrossRef - Prognostic and clinicopathological significance of Fusobacterium nucleatum in colorectal cancer: a systemic review and meta-analysis
Younghoon Kim, Nam Yun Cho, Gyeong Hoon Kang
Journal of Pathology and Translational Medicine.2022; 56(3): 144. CrossRef - Iron accelerates Fusobacterium nucleatum–induced CCL8 expression in macrophages and is associated with colorectal cancer progression
Taishi Yamane, Yohei Kanamori, Hiroshi Sawayama, Hiromu Yano, Akihiro Nita, Yudai Ohta, Hironori Hinokuma, Ayato Maeda, Akiko Iwai, Takashi Matsumoto, Mayuko Shimoda, Mayumi Niimura, Shingo Usuki, Noriko Yasuda-Yoshihara, Masato Niwa, Yoshifumi Baba, Taka
JCI Insight.2022;[Epub] CrossRef - Clinicopathological differences of high Fusobacterium nucleatum levels in colorectal cancer: A review and meta-analysis
Yi Wang, Yuting Wen, Jiayin Wang, Xin Lai, Ying Xu, Xuanping Zhang, Xiaoyan Zhu, Chenglin Ruan, Yao Huang
Frontiers in Microbiology.2022;[Epub] CrossRef - Clinical Significance of Fusobacterium nucleatum and Microsatellite Instability in Evaluating Colorectal Cancer Prognosis
Yanxuan Xie, Xiaoyang Jiao, Mi Zeng, Zhiqiang Fan, Xin Li, Yumeng Yuan, Qiaoxin Zhang, Yong Xia
Cancer Management and Research.2022; Volume 14: 3021. CrossRef - Influence of the Microbiome Metagenomics and Epigenomics on Gastric Cancer
Precious Mathebela, Botle Precious Damane, Thanyani Victor Mulaudzi, Zilungile Lynette Mkhize-Khwitshana, Guy Roger Gaudji, Zodwa Dlamini
International Journal of Molecular Sciences.2022; 23(22): 13750. CrossRef - Circulating IgA Antibodies Against Fusobacterium nucleatum Amyloid Adhesin FadA are a Potential Biomarker for Colorectal Neoplasia
Jung Eun Baik, Li Li, Manish A. Shah, Daniel E. Freedberg, Zhezhen Jin, Timothy C. Wang, Yiping W. Han
Cancer Research Communications.2022; 2(11): 1497. CrossRef - Differential immune microenvironmental features of microsatellite-unstable colorectal cancers according to Fusobacterium nucleatum status
Ji Ae Lee, Seung-Yeon Yoo, Hyeon Jeong Oh, Seorin Jeong, Nam-Yun Cho, Gyeong Hoon Kang, Jung Ho Kim
Cancer Immunology, Immunotherapy.2021; 70(1): 47. CrossRef - Fusobacterium nucleatum and Clinicopathologic Features of Colorectal Cancer: Results From the ColoCare Study
Yannick Eisele, Patrick M. Mallea, Biljana Gigic, W. Zac Stephens, Christy A. Warby, Kate Buhrke, Tengda Lin, Juergen Boehm, Petra Schrotz-King, Sheetal Hardikar, Lyen C. Huang, T. Bartley Pickron, Courtney L. Scaife, Richard Viskochil, Torsten Koelsch, A
Clinical Colorectal Cancer.2021; 20(3): e165. CrossRef - Role of gut microbiota in epigenetic regulation of colorectal Cancer
Yinghui Zhao, Chuanxin Wang, Ajay Goel
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer.2021; 1875(1): 188490. CrossRef - Fusobacterium nucleatum: caution with interpreting historical patient sample cohort
Kate L. F. Johnstone, Sinead Toomey, Stephen Madden, Brian D. P. O’Neill, Bryan T Hennessy
Journal of Pathology and Translational Medicine.2021; 55(6): 415. CrossRef -
Fusobacterium nucleatum colonization is associated with decreased survival of helicobacter pylori-positive gastric cancer patients
Yung-Yu Hsieh, Shui-Yi Tung, Hung-Yu Pan, Te-Sheng Chang, Kuo-Liang Wei, Wei-Ming Chen, Yi-Fang Deng, Chung-Kuang Lu, Yu-Hsuan Lai, Cheng-Shyong Wu, Chin Li
World Journal of Gastroenterology.2021; 27(42): 7311. CrossRef - Analysis of changes in microbiome compositions related to the prognosis of colorectal cancer patients based on tissue-derived 16S rRNA sequences
Sukjung Choi, Jongsuk Chung, Mi-La Cho, Donghyun Park, Sun Shim Choi
Journal of Translational Medicine.2021;[Epub] CrossRef - Gastrointestinal tumors and infectious agents: A wide field to explore
Miriam López-Gómez, Belén García de Santiago, Pedro-David Delgado-López, Eduardo Malmierca, Jesús González-Olmedo, César Gómez-Raposo, Carmen Sandoval, Pilar Ruiz-Seco, Nora Escribano, Jorge Francisco Gómez-Cerezo, Enrique Casado
World Journal of Meta-Analysis.2021; 9(6): 505. CrossRef - Gut Microbiota Profiles in Early- and Late-Onset Colorectal Cancer: A Potential Diagnostic Biomarker in the Future
Murdani Abdullah, Ninik Sukartini, Saskia Aziza Nursyirwan, Rabbinu Rangga Pribadi, Hasan Maulahela, Amanda Pitarini Utari, Virly Nanda Muzellina, Agustinus Wiraatmadja, Kaka Renaldi
Digestion.2021; 102(6): 823. CrossRef - The effect of periodontal bacteria infection on incidence and prognosis of cancer
Li Xiao, Qianyu Zhang, Yanshuang Peng, Daqing Wang, Ying Liu
Medicine.2020; 99(15): e19698. CrossRef - The impact of the gut microbiota on prognosis after surgery for colorectal cancer – a systematic review and meta‐analysis
Emilie Palmgren Colov, Thea Helene Degett, Hans Raskov, Ismail Gögenur
APMIS.2020; 128(2): 162. CrossRef - Can the microbiota predict response to systemic cancer therapy, surgical outcomes, and survival? The answer is in the gut
Khalid El Bairi, Rachid Jabi, Dario Trapani, Hanae Boutallaka, Bouchra Ouled Amar Bencheikh, Mohammed Bouziane, Mariam Amrani, Said Afqir, Adil Maleb
Expert Review of Clinical Pharmacology.2020; 13(4): 403. CrossRef - Predictive Values of Colon Microbiota in the Treatment Response to Colorectal Cancer
Jorge Galan-Ros, Verónica Ramos-Arenas, Pablo Conesa-Zamora
Pharmacogenomics.2020; 21(14): 1045. CrossRef - The gut microbiome and potential implications for early-onset colorectal cancer
Reetu Mukherji, Benjamin A Weinberg
Colorectal Cancer.2020;[Epub] CrossRef -
Fusobacterium nucleatum in the Colorectum and Its Association with Cancer Risk and Survival: A Systematic Review and Meta-analysis
Christian Gethings-Behncke, Helen G. Coleman, Haydee W.T. Jordao, Daniel B. Longley, Nyree Crawford, Liam J. Murray, Andrew T. Kunzmann
Cancer Epidemiology, Biomarkers & Prevention.2020; 29(3): 539. CrossRef - CpG Island Methylation in Sessile Serrated Adenoma/Polyp of the Colorectum: Implications for Differential Diagnosis of Molecularly High-Risk Lesions among Non-dysplastic Sessile Serrated Adenomas/Polyps
Ji Ae Lee, Hye Eun Park, Seung-Yeon Yoo, Seorin Jeong, Nam-Yun Cho, Gyeong Hoon Kang, Jung Ho Kim
Journal of Pathology and Translational Medicine.2019; 53(4): 225. CrossRef - Fusobacterium nucleatum tumor DNA levels are associated with survival in colorectal cancer patients
Andrew T. Kunzmann, Marcela Alcântara Proença, Haydee WT Jordao, Katerina Jiraskova, Michaela Schneiderova, Miroslav Levy, Václav Liska, Tomas Buchler, Ludmila Vodickova, Veronika Vymetalkova, Ana Elizabete Silva, Pavel Vodicka, David J. Hughes
European Journal of Clinical Microbiology & Infectious Diseases.2019; 38(10): 1891. CrossRef - Gut Microbiome: A Promising Biomarker for Immunotherapy in Colorectal Cancer
Sally Temraz, Farah Nassar, Rihab Nasr, Maya Charafeddine, Deborah Mukherji, Ali Shamseddine
International Journal of Molecular Sciences.2019; 20(17): 4155. CrossRef - The Four Horsemen in Colon Cancer
Marco Antonio Hernández-Luna, Sergio López-Briones, Rosendo Luria-Pérez
Journal of Oncology.2019; 2019: 1. CrossRef - The role of Fusobacterium nucleatum in colorectal cancer: from carcinogenesis to clinical management
Chun‐Hui Sun, Bin‐Bin Li, Bo Wang, Jing Zhao, Xiao‐Ying Zhang, Ting‐Ting Li, Wen‐Bing Li, Di Tang, Miao‐Juan Qiu, Xin‐Cheng Wang, Cheng‐Ming Zhu, Zhi‐Rong Qian
Chronic Diseases and Translational Medicine.2019; 5(3): 178. CrossRef
- Microbiota and tumor epigenetics: deep interconnections and emerging therapeutic perspectives
- Prognostic Significance of EPHB2 Expression in Colorectal Cancer Progression
- Bo Gun Jang, Hye Sung Kim, Weon Young Chang, Jeong Mo Bae, Gyeong Hoon Kang
- J Pathol Transl Med. 2018;52(5):298-306. Published online July 18, 2018
- DOI: https://doi.org/10.4132/jptm.2018.06.29
- 8,648 View
- 161 Download
- 18 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
A receptor tyrosine kinase for ephrin ligands, EPHB2, is expressed in normal colorectal tissues and colorectal cancers (CRCs). The aim of this study was to investigate EPHB2 expression over CRC progression and determine its prognostic significance in CRC.
Methods
To measure EPHB2 mRNA and protein expression, real-time polymerase chain reaction and immunohistochemistry were performed in 32 fresh-frozen and 567 formalin-fixed paraffin-embedded CRC samples, respectively. We further investigated clinicopathological features and overall and recurrence-free survival according to EPHB2 protein expression.
Results
The EPHB2 level was upregulated in CRC samples compared to non-cancerous tissue in most samples and showed a strong positive correlation with AXIN2. Notably, CD44 had a positive association with both mRNA and protein levels of EPHB2. Immunohistochemical analysis revealed no difference in EPHB2 expression between adenoma and carcinoma areas. Although EPHB2 expression was slightly lower in invasive fronts compared to surface area (p < .05), there was no difference between superficial and metastatic areas. EPHB2 positivity was associated with lymphatic (p < .001) and venous (p = .001) invasion, TNM stage (p < .001), and microsatellite instability (p = .036). Kaplan–Meier analysis demonstrated that CRC patients with EPHB2 positivity showed better clinical outcomes in both overall (p = .049) and recurrence-free survival (p = .015). However, multivariate analysis failed to show that EPHB2 is an independent prognostic marker in CRCs (hazard ratio, 0.692; p = .692).
Conclusions
Our results suggest that EPHB2 is overexpressed in a subset of CRCs and is a significant prognostic marker. -
Citations
Citations to this article as recorded by- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
Yanlai Xiao, Jian Wang, Xiangzhai Zhao, Jie Xu, Huan Zhao, Zhaojun Guo, Jun Zhao, Yajing Zhang, Ruoxi Wang, Yiwei Zhang
Open Life Sciences.2025;[Epub] CrossRef - Potential role of the Eph/ephrin system in colorectal cancer: emerging druggable molecular targets
João Figueira Scarini, Moisés Willian Aparecido Gonçalves, Reydson Alcides de Lima-Souza, Luccas Lavareze, Talita de Carvalho Kimura, Ching-Chu Yang, Albina Altemani, Fernanda Viviane Mariano, Heloisa Prado Soares, Gary Chris Fillmore, Erika Said Abu Egal
Frontiers in Oncology.2024;[Epub] CrossRef - Comprehensive pan-cancer analysis reveals EPHB2 is a novel predictive biomarker for prognosis and immunotherapy response
Shengshan Xu, Youbin Zheng, Min Ye, Tao Shen, Dongxi Zhang, Zumei Li, Zhuming Lu
BMC Cancer.2024;[Epub] CrossRef - Therapeutic effects of ephrin B receptor 2 inhibitors screened by molecular docking on cutaneous squamous cell carcinoma
Yan Li, Xuanfen Zhang
Journal of Dermatological Treatment.2022; 33(1): 373. CrossRef - The EPH/Ephrin System in Colorectal Cancer
Stavros P. Papadakos, Leonidas Petrogiannopoulos, Alexandros Pergaris, Stamatios Theocharis
International Journal of Molecular Sciences.2022; 23(5): 2761. CrossRef - Prognostic Significance of Autophagy-Relevant Gene Markers in Colorectal Cancer
Qinglian He, Ziqi Li, Jinbao Yin, Yuling Li, Yuting Yin, Xue Lei, Wei Zhu
Frontiers in Oncology.2021;[Epub] CrossRef - Genetic Predisposition to Numerous Large Ulcerating Basal Cell Carcinomas and Response to Immune Therapy
Bahar Dasgeb, Leila Youssefian, Amir Hossein Saeidian, Jun Kang, Wenyin Shi, Elizabeth Shoenberg, Adam Ertel, Paolo Fortina, Hassan Vahidnezhad, Jouni Uitto
International Journal of Dermatology and Venereology.2021; 4(2): 70. CrossRef - Delineation of colorectal cancer ligand-receptor interactions and their roles in the tumor microenvironment and prognosis
Hexin Lin, Lu Xia, Jiabian Lian, Yinan Chen, Yiyi Zhang, Zhicheng Zhuang, HuaJun Cai, Jun You, Guoxian Guan
Journal of Translational Medicine.2021;[Epub] CrossRef - Downregulation of hsa-microRNA-204-5p and identification of its potential regulatory network in non-small cell lung cancer: RT-qPCR, bioinformatic- and meta-analyses
Chang-Yu Liang, Zu-Yun Li, Ting-Qing Gan, Ye-Ying Fang, Bin-Liang Gan, Wen-Jie Chen, Yi-Wu Dang, Ke Shi, Zhen-Bo Feng, Gang Chen
Respiratory Research.2020;[Epub] CrossRef - The role of Eph receptors in cancer and how to target them: novel approaches in cancer treatment
Oscar J Buckens, Btissame El Hassouni, Elisa Giovannetti, Godefridus J Peters
Expert Opinion on Investigational Drugs.2020; 29(6): 567. CrossRef - Expression Profile and Prognostic Significance of EPHB3 in Colorectal Cancer
Bo Jang, Hye Kim, Jeong Bae, Woo Kim, Chang Hyun, Gyeong Kang
Biomolecules.2020; 10(4): 602. CrossRef Gene Expression Signature to Predict Prognosis and Adjuvant Chemosensitivity of Colorectal Cancer Patients
Jianxia Li, Jianwei Zhang, Huabin Hu, Yue Cai, Jiayu Ling, Zehua Wu, Yanhong Deng
Cancer Management and Research.2020; Volume 12: 3301. CrossRef- SMOC2, an intestinal stem cell marker, is an independent prognostic marker associated with better survival in colorectal cancers
Bo Gun Jang, Hye Sung Kim, Jeong Mo Bae, Woo Ho Kim, Heung Up Kim, Gyeong Hoon Kang
Scientific Reports.2020;[Epub] CrossRef
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Utility of BRAF VE1 Immunohistochemistry as a Screening Tool for Colorectal Cancer Harboring BRAF V600E Mutation
- Jeong-Hwa Kwon, Byung-Kwan Jeong, Yong Sik Yoon, Chang Sik Yu, Jihun Kim
- J Pathol Transl Med. 2018;52(3):157-163. Published online March 29, 2018
- DOI: https://doi.org/10.4132/jptm.2018.03.28
- 9,183 View
- 206 Download
- 12 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
BRAF mutation has been recognized as an important biomarker of colorectal cancer (CRC) for targeted therapy and prognosis prediction. However, sequencing for every CRC case is not cost-effective. An antibody specific for BRAF V600E mutant protein has been introduced, and we thus examined the utility of BRAF VE1 immunohistochemistry for evaluating BRAF mutations in CRC.
Methods
Fifty-one BRAF-mutated CRCs and 100 age and sexmatched BRAF wild-type CRCs between 2005 and 2015 were selected from the archives of Asan Medical Center. Tissue microarrays were constructed and stained with BRAF VE1 antibody.
Results
Forty-nine of the 51 BRAF-mutant CRCs (96.1%) showed more than moderate cytoplasmic staining, except for two weakly stained cases. Six of 100 BRAF wild-type cases also stained positive with BRAF VE1 antibody; four stained weakly and two stained moderately. Normal colonic crypts showed nonspecific weak staining, and a few CRC cases exhibited moderate nuclear reactivity (3 BRAF-mutant and 10 BRAF wild-type cases). BRAF-mutated CRC patients had higher pathologic stages and worse survival than BRAF wild-type patients.
Conclusions
BRAF VE1 immunohistochemistry showed high sensitivity and specificity, but occasional nonspecific staining in tumor cell nuclei and normal colonic crypts may limit their routine clinical use. Thus, BRAF VE1 immunohistochemistry may be a useful screening tool for BRAF V600E mutation in CRCs, provided that additional sequencing studies can be done to confirm the mutation in BRAF VE1 antibody-positive cases. -
Citations
Citations to this article as recorded by- Next-Generation Sequencing in Oncology—A Guiding Compass for Targeted Therapy and Emerging Applications
Laurenția Nicoleta Galeș, Mihai-Andrei Păun, Ioana Butnariu, Laurentiu Simion, Loredana Sabina Cornelia Manolescu, Oana Gabriela Trifănescu, Rodica Maricela Anghel
International Journal of Molecular Sciences.2025; 26(7): 3123. CrossRef - A multi-omic analysis reveals a predictive value of tertiary lymphoid structures in improving the prognosis of colorectal cancer patients with BRAF mutation
Chao Qin, Shumin Cheng, Jingyun Ma, Lujing Li, Yun Leng, Lei Zheng, Huiying Chen, Hui Mo, Shi Li, Yuhong Liang, Yi Zhang, Wenxia Li, Jing Liang, Yuxuan Liu, Junxuan Mai, Linlin Hou, Di Wang, Ke Zhu, Bihui Huang
Frontiers in Immunology.2025;[Epub] CrossRef - The evolving landscape of tissue‐agnostic therapies in precision oncology
Vivek Subbiah, Mohamed A. Gouda, Bettina Ryll, Howard A. Burris, Razelle Kurzrock
CA: A Cancer Journal for Clinicians.2024; 74(5): 433. CrossRef - Deciphering the Role of BRAFV600E Immunohistochemistry in Breast Lesions: A Comprehensive Review
Simran Khan, Arvind Bhake, Shakti Sagar
Cureus.2024;[Epub] CrossRef - Immunohistochemistry as a Surrogate Marker of Underlying Molecular Derangements in Sporadic Colorectal Carcinoma in Children – A Series of Three Cases
Priyanka Maity, Aniket Halder, Ranajoy Ghosh, Uttara Chatterjee, Shibsankar Barman, Ruchirendra Sarkar
Fetal and Pediatric Pathology.2022; 41(1): 98. CrossRef - Risk assessment and genetic counseling for Lynch syndrome – Practice resource of the National Society of Genetic Counselors and the Collaborative Group of the Americas on Inherited Gastrointestinal Cancer
Spring Holter, Michael J. Hall, Heather Hampel, Kory Jasperson, Sonia S. Kupfer, Joy Larsen Haidle, Maureen E. Mork, Selvi Palaniapppan, Leigha Senter, Elena M. Stoffel, Scott M. Weissman, Matthew B. Yurgelun
Journal of Genetic Counseling.2022; 31(3): 568. CrossRef - Current concepts in ameloblastoma-targeted therapies in B-raf proto-oncogene serine/threonine kinase V600E mutation: Systematic review
Rogelio González-González, Sandra López-Verdín, Jesús Lavalle-Carrasco, Nelly Molina-Frechero, Mario Isiordia-Espinoza, Ramón G Carreón-Burciaga, Ronell Bologna-Molina
World Journal of Clinical Oncology.2020; 11(1): 31. CrossRef - Genetic and histopathological analysis of a case of primary intraosseous carcinoma, NOS with features of both ameloblastic carcinoma and squamous cell carcinoma
Akane Yukimori, Maiko Tsuchiya, Akane Wada, Yasuyuki Michi, Kou Kayamori, Kei Sakamoto, Tohru Ikeda
World Journal of Surgical Oncology.2020;[Epub] CrossRef
- Next-Generation Sequencing in Oncology—A Guiding Compass for Targeted Therapy and Emerging Applications
- Prognostic Utility of Histological Growth Patterns of Colorectal Lung Oligometastasis
- Son Jae Yeong, Min Gyoung Pak, Hyoun Wook Lee, Seung Yeon Ha, Mee Sook Roh
- J Pathol Transl Med. 2018;52(2):98-104. Published online February 12, 2018
- DOI: https://doi.org/10.4132/jptm.2017.12.27
- 7,861 View
- 127 Download
- 3 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Patients with resectable colorectal lung oligometastasis (CLOM) demonstrate a heterogeneous oncological outcome. However, the parameters for predicting tumor aggressiveness have not yet been fully investigated in CLOM. This study was performed to determine the prognostic value of histological growth patterns in patients who underwent surgery for CLOM.
Methods
The study included 92 patients who were diagnosed with CLOM among the first resection cases. CLOMs grow according to three histological patterns: aerogenous, pushing, and desmoplastic patterns. The growth patterns were evaluated on archival hematoxylin and eosin–stained tissue sections.
Results
The aerogenous pattern was found in 29.4% (n=27) of patients, the pushing pattern in 34.7% (n=32), the desmoplastic pattern in 6.5% (n=6), and a mix of two growth patterns in 29.4% (n=27). The size of the aerogenous pattern was significantly smaller than that of metastases with other patterns (p=.033). Kaplan-Meier analysis demonstrated that patients showing an aerogenous pattern appeared to have a poorer prognosis, which was calculated from the time of diagnosis of the CLOM (p=.044). The 5-year survival rate from the diagnosis of colorectal cancer tended to be lower in patients with an aerogenous pattern than in those who had a non-aerogenous pattern; however, the difference was marginally significant (p=.051). In the multivariate Cox analysis, the aerogenous pattern appeared as an independent predictor of poor overall survival (hazard ratio, 3.122; 95% confidence interval, 1.196 to 8.145; p=.020).
Conclusions
These results suggest that the growth patterns may play a part as a histology-based prognostic parameter for patients with CLOM. -
Citations
Citations to this article as recorded by- Predicting liver metastases growth patterns: Current status and future possibilities
Rui Caetano Oliveira, Henrique Alexandrino, Maria Augusta Cipriano, Filipe Caseiro Alves, José Guilherme Tralhão
Seminars in Cancer Biology.2021; 71: 42. CrossRef - Histological growth patterns and molecular analysis of resected colorectal lung metastases
Emanuela Pilozzi, Damiano Fedele, Andrea Montori, Laura Lorenzon, Valentina Peritore, Giorgia Mannocchi, Nikta Bagheri, Chiara Leone, Antonio Palumbo, Michela Roberto, Giulio Ranazzi, Erino Rendina, Genoveffa Balducci, Mohsen Ibrahim
Pathology - Research and Practice.2021; 222: 153414. CrossRef
- Predicting liver metastases growth patterns: Current status and future possibilities
- Overexpression of POSTN in Tumor Stroma Is a Poor Prognostic Indicator of Colorectal Cancer
- Hyeon Jeong Oh, Jeong Mo Bae, Xian-Yu Wen, Nam-Yun Cho, Jung Ho Kim, Gyeong Hoon Kang
- J Pathol Transl Med. 2017;51(3):306-313. Published online April 12, 2017
- DOI: https://doi.org/10.4132/jptm.2017.01.19
- 15,928 View
- 258 Download
- 48 Web of Science
- 45 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Tumor microenvironment has recently drawn attention in that it is related with tumor prognosis. Cancer-associated fibroblast also plays a critical role in cancer invasiveness and progression in colorectal cancers. Periostin (POSTN), originally identified to be expressed in osteoblasts and osteoblast-derived cells, is expressed in cancer-associated fibroblasts in several tissue types of cancer. Recent studies suggest an association between stromal overexpression of POSTN and poor prognosis of cancer patients.
Methods
We analyzed colorectal cancer cases for their expression status of POSTN in tumor stroma using immunohistochemistry and correlated the expression status with clinicopathological and molecular features.
Results
High level of POSTN expression in tumor stroma was closely associated with tumor location in proximal colon, infiltrative growth pattern, undifferentiated histology, tumor budding, luminal necrosis, and higher TNM stage. High expression status of POSTN in tumor stroma was found to be an independent prognostic parameter implicating poor 5-year cancer-specific survival and 5-year progression-free survival.
Conclusions
Our findings suggest that POSTN overexpression in tumor stroma of colorectal cancers could be a possible candidate marker for predicting poor prognosis in patients with colorectal cancers. -
Citations
Citations to this article as recorded by- Unmasking a Recessive Allele by a Rare Interstitial Deletion at 10q26.13q26.2: Prenatal Diagnosis of MMP21 ‐Related Disorder and Further Refine INSYN2A Involvement in the Postnatal Cognitive Phenotype
Jiasun Su, Shujie Zhang, Wei Li, Yuan Wei, Fei Lin, Chaofan Zhou, Xianglian Tang, Yueyun Lan, Minpan Huang, Qiang Zhang, Shang Yi, Qi Yang, Sheng Yi, Xunzhao Zhou, Zailong Qin, Peng Huang
Molecular Genetics & Genomic Medicine.2025;[Epub] CrossRef - Periostin from Tumor Stromal Cells Might Be Associated with Malignant Progression of Colorectal Cancer via Smad2/3
Canfeng Fan, Qiang Wang, Saki Kanei, Kyoka Kawabata, Hinano Nishikubo, Rika Aoyama, Zhonglin Zhu, Daiki Imanishi, Takashi Sakuma, Koji Maruo, Gen Tsujio, Yurie Yamamoto, Tatsunari Fukuoka, Masakazu Yashiro
Cancers.2025; 17(3): 551. CrossRef - Attributes of HPV associated cancers
Ashi Robert Thobias, Mrugdha Patel, Chirag Vaghela, Prabhudas Shankarbhai Patel
Clinical and Translational Oncology.2025; 27(11): 4131. CrossRef - The association between periostin and tumor microenvironment: a promising cancer prognostic biomarker and therapeutic target to combat tumor progression and chemoresistance
Mohsen Nabi-Afjadi, Farnoosh Farzam, Sina Soltani, Nafiseh Golestani, Shiva Sarani Asl, Fatemeh Aziziyan, Hamidreza Zalpoor, Fatemeh Ghasemi, Bahareh Dabirmanesh, Khosro Khajeh
Cancer Cell International.2025;[Epub] CrossRef - Cancer-associated fibroblasts and their prognostic role in colorectal cancer: review and meta-analysis
Silvia Mihaela Ilie, Laurentia Gales, Ana Maria Musina, Kevin Rouet, Alexandra Elena Stefan, Mara Simona Belceanu, Anda Stefania Trandafir, Constantin Guillaume, Aurélie Bertaut, Ikram Charifi, Adel Cueff, Valentin Derangère, Francois Ghiringhelli
Frontiers in Oncology.2025;[Epub] CrossRef - Electroanalytical Immunotool to Determine Matricellular Protein Periostin, a Stromal Biomarker of Prognosis in Colorectal Cancer
Marina Blázquez‐García, Jennifer Quinchia, Víctor Ruiz‐Valdepeñas Montiel, Rebeca M. Torrente‐Rodríguez, Verónica Serafín, María Garranzo‐Asensio, Ana García‐Romero, Jahir Orozco, Rodrigo Barderas, José M. Pingarrón, Susana Campuzano
ChemElectroChem.2024;[Epub] CrossRef - Simultaneous Expression of CD70 and POSTN in Cancer-Associated Fibroblasts Predicts Worse Survival of Colorectal Cancer Patients
Masayuki Komura, Chengbo Wang, Sunao Ito, Shunsuke Kato, Akane Ueki, Masahide Ebi, Naotaka Ogasawara, Toyonori Tsuzuki, Kenji Kasai, Kunio Kasugai, Shuji Takiguchi, Satoru Takahashi, Shingo Inaguma
International Journal of Molecular Sciences.2024; 25(5): 2537. CrossRef - The combined tumour-based Fascin/Snail and stromal periostin reveals the effective prognosis prediction in colorectal cancer patients
Niphat Jirapongwattana, Suyanee Thongchot, Ananya Pongpaibul, Atthaphorn Trakarnsanga, Jean Quinn, Peti Thuwajit, Chanitra Thuwajit, Joanne Edwards, Peng Zhang
PLOS ONE.2024; 19(6): e0304666. CrossRef - SPOCK1 and POSTN are valuable prognostic biomarkers and correlate with tumor immune infiltrates in colorectal cancer
Caiqin Gan, Mengting Li, Yuanyuan Lu, Ganjing Peng, Wenjie Li, Haizhou Wang, Yanan Peng, Qian Hu, Wanhui Wei, Fan Wang, Lan Liu, Qiu Zhao
BMC Gastroenterology.2023;[Epub] CrossRef - Stromal POSTN Enhances Motility of Both Cancer and Stromal Cells and Predicts Poor Survival in Colorectal Cancer
Akane Ueki, Masayuki Komura, Akira Koshino, Chengbo Wang, Kazuhiro Nagao, Mai Homochi, Yuki Tsukada, Masahide Ebi, Naotaka Ogasawara, Toyonori Tsuzuki, Kenji Kasai, Kunio Kasugai, Satoru Takahashi, Shingo Inaguma
Cancers.2023; 15(3): 606. CrossRef - POSTN Secretion by Extracellular Matrix Cancer-Associated Fibroblasts (eCAFs) Correlates with Poor ICB Response via Macrophage Chemotaxis Activation of Akt Signaling Pathway in Gastric Cancer
Tingting You, Hui Tang, Wenjing Wu, Jingxi Gao, Xuechun Li, Ningning Li, Xiuxiu Xu, Jiazhang Xing, Hui Ge, Yi Xiao, Junchao Guo, Bin Wu, Xiaoyi Li, Liangrui Zhou, Lin Zhao, Chunmei Bai, Qin Han, Zhao Sun, Robert Chunhua Zhao
Aging and disease.2023; 14(6): 2177. CrossRef - A Pan-cancer Analysis Reveals the Tissue Specificity and Prognostic Impact
of Angiogenesis-associated Genes in Human Cancers
Zhenshen Bao, Minzhen Liao, Wanqi Dong, Yanhao Huo, Xianbin Li, Peng Xu, Wenbin Liu
Current Bioinformatics.2023; 18(8): 670. CrossRef - Cancer‐associated stroma reveals prognostic biomarkers and novel insights into the tumour microenvironment of colorectal cancer and colorectal liver metastases
Kai M. Brown, Aiqun Xue, Ross C. Smith, Jaswinder S. Samra, Anthony J. Gill, Thomas J. Hugh
Cancer Medicine.2022; 11(2): 492. CrossRef - Periostin in Angiogenesis and Inflammation in CRC—A Preliminary Observational Study
Agnieszka Kula, Miriam Dawidowicz, Sylwia Mielcarska, Paweł Kiczmer, Magdalena Chrabańska, Magdalena Rynkiewicz, Elżbieta Świętochowska, Dariusz Waniczek
Medicina.2022; 58(1): 96. CrossRef - Periostin promotes the proliferation and metastasis of osteosarcoma by increasing cell survival and activates the PI3K/Akt pathway
Chaojian Xu, Ziyue Wang, Long Zhang, Yi Feng, Jia Lv, Zhuangzhuang Wu, Rong Yang, Taiyong Wu, Jian Li, Ruhao Zhou, Zhi Tian, Junjun Bai, Huadong Zhang, Yanping Lan, Zhi Lv
Cancer Cell International.2022;[Epub] CrossRef - Periostin in lymph node pre-metastatic niches governs lymphatic endothelial cell functions and metastatic colonization
Lionel Gillot, Alizée Lebeau, Louis Baudin, Charles Pottier, Thomas Louis, Tania Durré, Rémi Longuespée, Gabriel Mazzucchelli, Christophe Nizet, Silvia Blacher, Frédéric Kridelka, Agnès Noël
Cellular and Molecular Life Sciences.2022;[Epub] CrossRef - Prognostic impact of stromal periostin expression in upper urinary tract urothelial carcinoma
Kosuke Miyai, Kazuki Kawamura, Keiichi Ito, Susumu Matsukuma, Hitoshi Tsuda
BMC Cancer.2022;[Epub] CrossRef - Periostin‐ and podoplanin‐positive cancer‐associated fibroblast subtypes cooperate to shape the inflamed tumor microenvironment in aggressive pancreatic adenocarcinoma
Cindy Neuzillet, Rémy Nicolle, Jérôme Raffenne, Annemilaï Tijeras‐Raballand, Alexia Brunel, Lucile Astorgues‐Xerri, Sophie Vacher, Floriane Arbateraz, Marjorie Fanjul, Marc Hilmi, Rémi Samain, Christophe Klein, Aurélie Perraud, Vinciane Rebours, Muriel Ma
The Journal of Pathology.2022; 258(4): 408. CrossRef - Periostin: biology and function in cancer
Shima Dorafshan, Mahdieh Razmi, Sadegh Safaei, Erica Gentilin, Zahra Madjd, Roya Ghods
Cancer Cell International.2022;[Epub] CrossRef - Periostin as a key molecule defining desmoplastic environment in colorectal cancer
Takahiro Sueyama, Yoshiki Kajiwara, Satsuki Mochizuki, Hideyuki Shimazaki, Eiji Shinto, Kazuo Hase, Hideki Ueno
Virchows Archiv.2021; 478(5): 865. CrossRef - Expression Patterns of Microenvironmental Factors and Tenascin-C at the Invasive Front of Stage II and III Colorectal Cancer: Novel Tumor Prognostic Markers
Mai Hashimoto, Noriyuki Uesugi, Mitsumasa Osakabe, Naoki Yanagawa, Koki Otsuka, Yoshiki Kajiwara, Hideki Ueno, Akira Sasaki, Tamotsu Sugai
Frontiers in Oncology.2021;[Epub] CrossRef - Deregulation of extracellular matrix modeling with molecular prognostic markers revealed by transcriptome sequencing and validations in Oral Tongue squamous cell carcinoma
Soundara Viveka Thangaraj, Vidyarani Shyamsundar, Arvind Krishnamurthy, Vijayalakshmi Ramshankar
Scientific Reports.2021;[Epub] CrossRef - Inhibition of Postn Rescues Myogenesis Defects in Myotonic Dystrophy Type 1 Myoblast Model
Xiaopeng Shen, Zhongxian Liu, Chunguang Wang, Feng Xu, Jingyi Zhang, Meng Li, Yang Lei, Ao Wang, Chao Bi, Guoping Zhu
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - Radiographical assessment of tumour stroma and treatment outcomes using deep learning: a retrospective, multicohort study
Yuming Jiang, Xiaokun Liang, Zhen Han, Wei Wang, Sujuan Xi, Tuanjie Li, Chuanli Chen, Qingyu Yuan, Na Li, Jiang Yu, Yaoqin Xie, Yikai Xu, Zhiwei Zhou, George A Poultsides, Guoxin Li, Ruijiang Li
The Lancet Digital Health.2021; 3(6): e371. CrossRef - Periostin expression and its supposed roles in benign and malignant thyroid nodules: an immunohistochemical study of 105 cases
Kimihide Kusafuka, Masaru Yamashita, Tomohiro Iwasaki, Chinatsu Tsuchiya, Aki Kubota, Kazuki Hirata, Akinori Murakami, Aya Muramatsu, Kazumori Arai, Makoto Suzuki
Diagnostic Pathology.2021;[Epub] CrossRef - Cancer-associated fibroblasts in colorectal cancer
S. Kamali Zonouzi, P. S. Pezeshki, S. Razi, N. Rezaei
Clinical and Translational Oncology.2021; 24(5): 757. CrossRef - Prognostic value of periostin in multiple solid cancers: A systematic review with meta‐analysis
Tao Yang, Zhengdong Deng, Zhongya Pan, Yawei Qian, Wei Yao, Jianming Wang
Journal of Cellular Physiology.2020; 235(3): 2800. CrossRef - Serum periostin is associated with cancer mortality but not cancer risk in older home-dwelling men: A 8-year prospective analysis of the STRAMBO study
Jean-Charles Rousseau, Cindy Bertholon, Roland Chapurlat, Pawel Szulc
Bone.2020; 132: 115184. CrossRef - Systematic prediction of key genes for ovarian cancer by co‐expression network analysis
Mingyuan Wang, Jinjin Wang, Jinglan Liu, Lili Zhu, Heng Ma, Jiang Zou, Wei Wu, Kangkai Wang
Journal of Cellular and Molecular Medicine.2020; 24(11): 6298. CrossRef - Upregulation of adipocyte enhancer‐binding protein 1 in endothelial cells promotes tumor angiogenesis in colorectal cancer
Akira Yorozu, Eiichiro Yamamoto, Takeshi Niinuma, Akihiro Tsuyada, Reo Maruyama, Hiroshi Kitajima, Yuto Numata, Masahiro Kai, Gota Sudo, Toshiyuki Kubo, Toshihiko Nishidate, Kenji Okita, Ichiro Takemasa, Hiroshi Nakase, Tamotsu Sugai, Kenichi Takano, Hiro
Cancer Science.2020; 111(5): 1631. CrossRef - Periostin aggravates NLRP3 inflammasome-mediated pyroptosis in myocardial ischemia-reperfusion injury
Lei Yao, Jie Song, Xiao wen Meng, Jian yun Ge, Bo xiang Du, Jun Yu, Fu hai Ji
Molecular and Cellular Probes.2020; 53: 101596. CrossRef - Vitamin K and Kidney Transplantation
Maria Fusaro, Laura Cosmai, Pieter Evenepoel, Thomas L. Nickolas, Angela M. Cheung, Andrea Aghi, Giovanni Tripepi, Mario Plebani, Giorgio Iervasi, Roberto Vettor, Martina Zaninotto, Maura Ravera, Marina Foramitti, Sandro Giannini, Stefania Sella, Maurizio
Nutrients.2020; 12(9): 2717. CrossRef - Periostin regulates autophagy through integrin α5β1 or α6β4 and an AKT‐dependent pathway in colorectal cancer cell migration
Suyanee Thongchot, Ekapot Singsuksawat, Nuttavut Sumransub, Ananya Pongpaibul, Attaporn Trakarnsanga, Peti Thuwajit, Chanitra Thuwajit
Journal of Cellular and Molecular Medicine.2020; 24(21): 12421. CrossRef - Genomic, transcriptomic, and viral integration profiles associated with recurrent/metastatic progression in high‐risk human papillomavirus cervical carcinomas
Jing Jing Liu, Jung Yoon Ho, Jung Eum Lee, Soo Young Hur, Jinseon Yoo, Kyu Ryung Kim, Daeun Ryu, Tae Min Kim, Youn Jin Choi
Cancer Medicine.2020; 9(21): 8243. CrossRef - Periostin Secreted by Carcinoma-Associated Fibroblasts Promotes Ovarian Cancer Cell Platinum Resistance Through the PI3K/Akt Signaling Pathway
Lei Chu, Fangce Wang, Wenjun Zhang, Huai-fang Li, Jun Xu, Xiao-wen Tong
Technology in Cancer Research & Treatment.2020;[Epub] CrossRef - Overexpression of periostin is positively associated with gastric cancer metastasis through promoting tumor metastasis and invasion
Hai Zhong, Xiang Li, Junhua Zhang, Xu Wu
Journal of Cellular Biochemistry.2019; 120(6): 9927. CrossRef - Genes associated with bowel metastases in ovarian cancer
Andrea Mariani, Chen Wang, Ann L. Oberg, Shaun M. Riska, Michelle Torres, Joseph Kumka, Francesco Multinu, Gunisha Sagar, Debarshi Roy, Deok–Beom Jung, Qing Zhang, Tommaso Grassi, Daniel W. Visscher, Vatsal P. Patel, Ling Jin, Julie K. Staub, William A. C
Gynecologic Oncology.2019; 154(3): 495. CrossRef - Periostin: A Matricellular Protein With Multiple Functions in Cancer Development and Progression
Laura González-González, Javier Alonso
Frontiers in Oncology.2018;[Epub] CrossRef - Upregulation of Periostin expression in the pathogenesis of ameloblastoma
Yuanyuan Kang, Jie Liu, Ying Zhang, Yan Sun, Junting Wang, Biying Huang, Ming Zhong
Pathology - Research and Practice.2018; 214(12): 1959. CrossRef - Periostin expression in neoplastic and non-neoplastic diseases of bone and joint
Jennifer M. Brown, Akiro Mantoku, Afsie Sabokbar, Udo Oppermann, A. Bass Hassan, Akiro Kudo, Nick Athanasou
Clinical Sarcoma Research.2018;[Epub] CrossRef - Molecular patterns of cancer colonisation in lymph nodes of breast cancer patients
Gaurav Chatterjee, Trupti Pai, Thomas Hardiman, Kelly Avery-Kiejda, Rodney J. Scott, Jo Spencer, Sarah E. Pinder, Anita Grigoriadis
Breast Cancer Research.2018;[Epub] CrossRef - Multiplicity of Advanced T Category–Tumors Is a Risk Factor for Survival in Patients with Colorectal Carcinoma
Hye Eun Park, Seungyeon Yoo, Jeong Mo Bae, Seorin Jeong, Nam-Yun Cho, Gyeong Hoon Kang
Journal of Pathology and Translational Medicine.2018; 52(6): 386. CrossRef - Periostin attenuates tumor growth by inducing apoptosis in colitis-related colorectal cancer
Yusuke Shimoyama, Keiichi Tamai, Rie Shibuya, Mao Nakamura, Mai Mochizuki, Kazunori Yamaguchi, Yoichi Kakuta, Yoshitaka Kinouchi, Ikuro Sato, Akira Kudo, Tooru Shimosegawa, Kennichi Satoh
Oncotarget.2018; 9(28): 20008. CrossRef - Periostin serves an important role in the pathogenesis of oral squamous cell carcinoma
Yuanyuan Kang, Xue Wang, Ying Zhang, Yan Sun
Oncology Letters.2018;[Epub] CrossRef - The prognostic significance of cancer-associated fibroblasts in pancreatic ductal adenocarcinoma
Hyunjin Park, Yangkyu Lee, Hyejung Lee, Jin-Won Kim, Jin-Hyeok Hwang, Jaihwan Kim, Yoo-Seok Yoon, Ho-Seong Han, Haeryoung Kim
Tumor Biology.2017; 39(10): 101042831771840. CrossRef
- Unmasking a Recessive Allele by a Rare Interstitial Deletion at 10q26.13q26.2: Prenatal Diagnosis of MMP21 ‐Related Disorder and Further Refine INSYN2A Involvement in the Postnatal Cognitive Phenotype
- Molecular Testing for Gastrointestinal Cancer
- Hye Seung Lee, Woo Ho Kim, Yoonjin Kwak, Jiwon Koh, Jeong Mo Bae, Kyoung-Mee Kim, Mee Soo Chang, Hye Seung Han, Joon Mee Kim, Hwal Woong Kim, Hee Kyung Chang, Young Hee Choi, Ji Y. Park, Mi Jin Gu, Min Jin Lhee, Jung Yeon Kim, Hee Sung Kim, Mee-Yon Cho
- J Pathol Transl Med. 2017;51(2):103-121. Published online February 19, 2017
- DOI: https://doi.org/10.4132/jptm.2017.01.24
- 22,764 View
- 910 Download
- 62 Web of Science
- 54 Crossref
-
 Abstract
Abstract
 PDF
PDF - With recent advances in molecular diagnostic methods and targeted cancer therapies, several molecular tests have been recommended for gastric cancer (GC) and colorectal cancer (CRC). Microsatellite instability analysis of gastrointestinal cancers is performed to screen for Lynch syndrome, predict favorable prognosis, and screen patients for immunotherapy. The epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor has been approved in metastatic CRCs with wildtype RAS (KRAS and NRAS exon 2–4). A BRAF mutation is required for predicting poor prognosis. Additionally, amplification of human epidermal growth factor receptor 2 (HER2) and MET is also associated with resistance to EGFR inhibitor in metastatic CRC patients. The BRAF V600E mutation is found in sporadic microsatellite unstable CRCs, and thus is helpful for ruling out Lynch syndrome. In addition, the KRAS mutation is a prognostic biomarker and the PIK3CA mutation is a molecular biomarker predicting response to phosphoinositide 3-kinase/AKT/mammalian target of rapamycin inhibitors and response to aspirin therapy in CRC patients. Additionally, HER2 testing should be performed in all recurrent or metastatic GCs. If the results of HER2 immunohistochemistry are equivocal, HER2 silver or fluorescence in situ hybridization testing are essential for confirmative determination of HER2 status. Epstein-Barr virus–positive GCs have distinct characteristics, including heavy lymphoid stroma, hypermethylation phenotype, and high expression of immune modulators. Recent advances in next-generation sequencing technologies enable us to examine various genetic alterations using a single test. Pathologists play a crucial role in ensuring reliable molecular testing and they should also take an integral role between molecular laboratories and clinicians.
-
Citations
Citations to this article as recorded by- Spatial and Temporal Tumor Heterogeneity in Gastric Cancer: Discordance of Predictive Biomarkers
Hye Seung Lee
Journal of Gastric Cancer.2025; 25(1): 192. CrossRef - Korean Practice Guidelines for Gastric Cancer 2024: An Evidence-based, Multidisciplinary Approach (Update of 2022 Guideline)
In-Ho Kim, Seung Joo Kang, Wonyoung Choi, An Na Seo, Bang Wool Eom, Beodeul Kang, Bum Jun Kim, Byung-Hoon Min, Chung Hyun Tae, Chang In Choi, Choong-kun Lee, Ho Jung An, Hwa Kyung Byun, Hyeon-Su Im, Hyung-Don Kim, Jang Ho Cho, Kyoungjune Pak, Jae-Joon Kim
Journal of Gastric Cancer.2025; 25(1): 5. CrossRef - Elucidating the Role of KRAS, NRAS, and BRAF Mutations and Microsatellite Instability in Colorectal Cancer via Next-Generation Sequencing
Marta Rada Rodríguez, Bárbara Angulo Biedma, Irene Rodríguez Pérez, Javier Azúa Romeo
Cancers.2025; 17(13): 2071. CrossRef - Chitosan and Its Derivative‐Based Nanoparticles in Gastrointestinal Cancers: Molecular Mechanisms of Action and Promising Anticancer Strategies
Zahra Shokati Eshkiki, Fatemeh Mansouri, Amir Reza Karamzadeh, Abolfazl Namazi, Hafez Heydari, Javad Akhtari, Seidamir Pasha Tabaeian, Abolfazl Akbari, Hongda Liu
Journal of Clinical Pharmacy and Therapeutics.2024;[Epub] CrossRef - Colorectal Cancer: Genetic Underpinning and Molecular Therapeutics for Precision Medicine
Gideon T. Dosunmu, Ardaman Shergill
Genes.2024; 15(5): 538. CrossRef - Effector Function Characteristics of Exhausted CD8+ T-Cell in Microsatellite Stable and Unstable Gastric Cancer
Dong-Seok Han, Yoonjin Kwak, Seungho Lee, Soo Kyung Nam, Seong-Ho Kong, Do Joong Park, Hyuk-Joon Lee, Nak-Jung Kwon, Hye Seung Lee, Han-Kwang Yang
Cancer Research and Treatment.2024; 56(4): 1146. CrossRef - A Standardized Pathology Report for Gastric Cancer: 2nd Edition
Young Soo Park, Myeong-Cherl Kook, Baek-hui Kim, Hye Seung Lee, Dong-Wook Kang, Mi-Jin Gu, Ok Ran Shin, Younghee Choi, Wonae Lee, Hyunki Kim, In Hye Song, Kyoung-Mee Kim, Hee Sung Kim, Guhyun Kang, Do Youn Park, So-Young Jin, Joon Mee Kim, Yoon Jung Choi,
Journal of Gastric Cancer.2023; 23(1): 107. CrossRef - A standardized pathology report for gastric cancer: 2nd edition
Young Soo Park, Myeong-Cherl Kook, Baek-hui Kim, Hye Seung Lee, Dong-Wook Kang, Mi-Jin Gu, Ok Ran Shin, Younghee Choi, Wonae Lee, Hyunki Kim, In Hye Song, Kyoung-Mee Kim, Hee Sung Kim, Guhyun Kang, Do Youn Park, So-Young Jin, Joon Mee Kim, Yoon Jung Choi,
Journal of Pathology and Translational Medicine.2023; 57(1): 1. CrossRef - Korean Practice Guidelines for Gastric Cancer 2022: An Evidence-based, Multidisciplinary Approach
Tae-Han Kim, In-Ho Kim, Seung Joo Kang, Miyoung Choi, Baek-Hui Kim, Bang Wool Eom, Bum Jun Kim, Byung-Hoon Min, Chang In Choi, Cheol Min Shin, Chung Hyun Tae, Chung sik Gong, Dong Jin Kim, Arthur Eung-Hyuck Cho, Eun Jeong Gong, Geum Jong Song, Hyeon-Su Im
Journal of Gastric Cancer.2023; 23(1): 3. CrossRef - Influence of location-dependent sex difference on PD-L1, MMR/MSI, and EGFR in colorectal carcinogenesis
Jina Choi, Nayoung Kim, Ryoung Hee Nam, Jin Won Kim, Chin-Hee Song, Hee Young Na, Gyeong Hoon Kang, Alvaro Galli
PLOS ONE.2023; 18(2): e0282017. CrossRef - Comprehensive Analysis of Epigenetic Associated Genes with Differential
Gene Expression and Prognosis in Gastric Cancer
Songlin An, Xinbao Li, Bing Li, Yan Li
Combinatorial Chemistry & High Throughput Screening.2023; 26(3): 527. CrossRef - Liquid Biopsy in Advanced Colorectal Cancer: Clinical Applications of Different Analytes
Marco Donatello Delcuratolo, Andrea Modrego-Sánchez, Maristella Bungaro, Beatriz Antón-Pascual, Santiago Teran, Valentina Dipace, Silvia Novello, Rocio Garcia-Carbonero, Francesco Passiglia, Cristina Graválos-Castro
Journal of Molecular Pathology.2023; 4(3): 128. CrossRef - Exosomal circ_0001190 Regulates the Progression of Gastric Cancer via miR-586/SOSTDC1 Axis
Chao Liu, Jing Yang, Fengchi Zhu, Zhiying Zhao, Lixue Gao
Biochemical Genetics.2022; 60(6): 1895. CrossRef - Optimization of pre‐analytical and analytical steps for DNA and RNA analysis of fresh cytology samples
Ana Dolinar, Gašper Grubelnik, Irena Srebotnik‐Kirbiš, Margareta Strojan Fležar, Margareta Žlajpah
Cancer Medicine.2022; 11(21): 4021. CrossRef - Retracted: Connexin 43 upregulation by dioscin‐inhibited gastric cancer metastasis by suppressing PI3K/Akt pathway
Food Science & Nutrition.2022;[Epub] CrossRef - Molecular Pathology of Gastric Cancer
Moonsik Kim, An Na Seo
Journal of Gastric Cancer.2022; 22(4): 264. CrossRef - Case report: Undifferentiated sarcoma with multiple tumors involved in Lynch syndrome: Unexpected favorable outcome to sintilimab combined with chemotherapy
Jiaying Liu, Xiaona Chang, Guixiang Xiao, Jingmin Zhong, Bo Huang, Jiwei Zhang, Beibei Gao, Gang Peng, Xiu Nie
Frontiers in Oncology.2022;[Epub] CrossRef - The SUMO E3 ligase CBX4 is identified as a poor prognostic marker of gastric cancer through multipronged OMIC analyses
Yi Pan, Qingshang Li, Zhijun Cao, Shuliang Zhao
Genes & Diseases.2021; 8(6): 827. CrossRef - Worldwide variation in lynch syndrome screening: case for universal screening in low colorectal cancer prevalence areas
George Kunnackal John, Vipin Das Villgran, Christine Caufield-Noll, Francis Giardiello
Familial Cancer.2021; 20(2): 145. CrossRef - Tamoxifen Downregulates the Expression of Notch1 and DLL1 Genes in MKN-45 Gastric Cancer Cells
Faranak Khanipouyani, Hassan Akrami
Journal of Gastrointestinal Cancer.2021; 52(3): 922. CrossRef - Kallikrein-11, in Association with Coiled-Coil Domain Containing 25, as a Potential Prognostic Marker for Cholangiocarcinoma with Lymph Node Metastasis
Saeranee Siriphak, Ravinnipa Chanakankun, Tanakorn Proungvitaya, Sittiruk Roytrakul, Doungdean Tummanatsakun, Wunchana Seubwai, Molin Wongwattanakul, Siriporn Proungvitaya
Molecules.2021; 26(11): 3105. CrossRef - ISH-based HER2 diagnostics
Josef Rüschoff, Iris Nagelmeier, Bharat Jasani, Oliver Stoss
Der Pathologe.2021; 42(S1): 62. CrossRef - Identification and Analysis of Key Genes Driving Gastric Cancer Through Bioinformatics
Zhao Liu, Shihai Liu, Jing Guo, Libin Sun, Shasha Wang, Yixuan Wang, Wensheng Qiu, Jing Lv
Genetic Testing and Molecular Biomarkers.2021; 25(1): 1. CrossRef - Microsatellite Instability in Colorectal Cancer Liquid Biopsy—Current Updates on Its Potential in Non-Invasive Detection, Prognosis and as a Predictive Marker
Francis Yew Fu Tieng, Nadiah Abu, Learn-Han Lee, Nurul-Syakima Ab Mutalib
Diagnostics.2021; 11(3): 544. CrossRef - Metformin attenuates synergic effect of diabetes mellitus and Helicobacter pylori infection on gastric cancer cells proliferation by suppressing PTEN expression
Huibin Lu, Xinwei Han, Jianzhuang Ren, Kewei Ren, Zongming Li, Quanhui Zhang
Journal of Cellular and Molecular Medicine.2021; 25(10): 4534. CrossRef - Recent Advances in the Diagnosis, Staging, Treatment, and Prognosis of Advanced Gastric Cancer: A Literature Review
Zhi-da Chen, Peng-fei Zhang, Hong-qing Xi, Bo Wei, Lin Chen, Yun Tang
Frontiers in Medicine.2021;[Epub] CrossRef - Tumor immune response and immunotherapy in gastric cancer
Yoonjin Kwak, An Na Seo, Hee Eun Lee, Hye Seung Lee
Journal of Pathology and Translational Medicine.2020; 54(1): 20. CrossRef - Comparative analysis of HER2 copy number between plasma and tissue samples in gastric cancer using droplet digital PCR
Boram Kim, Soo Kyung Nam, Soo Hyun Seo, Kyoung Un Park, Sang-Hoon Ahn, Do Joong Park, Hyung-Ho Kim, Woo Ho Kim, Hye Seung Lee
Scientific Reports.2020;[Epub] CrossRef - Differential prognostic impact of CD8+ T cells based on human leucocyte antigen I and PD-L1 expression in microsatellite-unstable gastric cancer
Yoonjin Kwak, Jiwon Koh, Yujun Park, Yun Ji Hong, Kyoung Un Park, Hyung-Ho Kim, Do Joong Park, Sang-Hoon Ahn, Woo Ho Kim, Hye Seung Lee
British Journal of Cancer.2020; 122(9): 1399. CrossRef - High-Accuracy Determination of Microsatellite Instability Compatible with Liquid Biopsies
Amanda Bortolini Silveira, François-Clément Bidard, Amélie Kasperek, Samia Melaabi, Marie-Laure Tanguy, Manuel Rodrigues, Guillaume Bataillon, Luc Cabel, Bruno Buecher, Jean-Yves Pierga, Charlotte Proudhon, Marc-Henri Stern
Clinical Chemistry.2020; 66(4): 606. CrossRef - Chitosan: A compound for drug delivery system in gastric cancer-a review
Rana Shafabakhsh, Bahman Yousefi, Zatollah Asemi, Banafsheh Nikfar, Mohammad Ali Mansournia, Jamal Hallajzadeh
Carbohydrate Polymers.2020; 242: 116403. CrossRef - MSI and EBV Positive Gastric Cancer’s Subgroups and Their Link with Novel Immunotherapy
Maria Grazia Rodriquenz, Giandomenico Roviello, Alberto D’Angelo, Daniele Lavacchi, Franco Roviello, Karol Polom
Journal of Clinical Medicine.2020; 9(5): 1427. CrossRef - Theoretical calculations of molecular descriptors for anticancer activities of 1, 2, 3-triazole-pyrimidine derivatives against gastric cancer cell line (MGC-803): DFT, QSAR and docking approaches
Rhoda Oyeladun Oyewole, Abel Kolawole Oyebamiji, Banjo Semire
Heliyon.2020; 6(5): e03926. CrossRef - Identification of a Clinical Cutoff Value for Multiplex KRASG12/G13 Mutation Detection in Colorectal Adenocarcinoma Patients Using Digital Droplet PCR, and Comparison with Sanger Sequencing and PNA Clamping Assay
Kyung Ha Lee, Tae Hee Lee, Min Kyung Choi, In Sun Kwon, Go Eun Bae, Min-Kyung Yeo
Journal of Clinical Medicine.2020; 9(7): 2283. CrossRef - PD-L1 Testing in Gastric Cancer by the Combined Positive Score of the 22C3 PharmDx and SP263 Assay with Clinically Relevant Cut-offs
Yujun Park, Jiwon Koh, Hee Young Na, Yoonjin Kwak, Keun-Wook Lee, Sang-Hoon Ahn, Do Joong Park, Hyung-Ho Kim, Hye Seung Lee
Cancer Research and Treatment.2020; 52(3): 661. CrossRef - Clinical and Molecular Assessment of Patients with Lynch Syndrome and Sarcomas Underpinning the Association with MSH2 Germline Pathogenic Variants
Nathália de Angelis de Carvalho, Bianca Naomi Niitsuma, Vanessa Nascimento Kozak, Felipe D’almeida Costa, Mariana Petaccia de Macedo, Bruna Elisa Catin Kupper, Maria Letícia Gobo Silva, Maria Nirvana Formiga, Sahlua Miguel Volc, Samuel Aguiar Junior, Eden
Cancers.2020; 12(7): 1848. CrossRef - Farnesoid X receptor antagonizes Wnt/β-catenin signaling in colorectal tumorigenesis
Junhui Yu, Shan Li, Jing Guo, Zhengshui Xu, Jianbao Zheng, Xuejun Sun
Cell Death & Disease.2020;[Epub] CrossRef - YAP promotes self-renewal of gastric cancer cells by inhibiting expression of L-PTGDS and PTGDR2
Qingli Bie, Xiaozhe Li, Shiqi Liu, Xiao Yang, Zhenwen Qian, Rou Zhao, Xiaobei Zhang, Bin Zhang
International Journal of Clinical Oncology.2020; 25(12): 2055. CrossRef - ISH-basierte HER2-Diagnostik
Josef Rüschoff, Iris Nagelmeier, Bharat Jasani, Oliver Stoss
Der Pathologe.2020; 41(6): 606. CrossRef - Histone Deacetylase Inhibitor Trichostatin A Suppresses Cell Proliferation and Induces Apoptosis by Regulating the PI3K/AKT Signalling Pathway in Gastric Cancer Cells
Xinli An, Zekun Wei, Botian Ran, Hao Tian, Hongyu Gu, Yan Liu, Hongjuan Cui, Shunqin Zhu
Anti-Cancer Agents in Medicinal Chemistry.2020; 20(17): 2114. CrossRef - Role of Her-2 in Gastrointestinal Tumours beyond Gastric Cancer: A Tool for Precision Medicine
Csongor G. Lengyel, Baker Habeeb, Shah Z. Khan, Khalid El Bairi, Sara C. Altuna, Sadaqat Hussain, Syed Ayub Mazher, Dario Trapani, Angelica Petrillo
Gastrointestinal Disorders.2020; 3(1): 1. CrossRef - Next-generation Sequencing in the Management of Gastric and Esophageal Cancers
Jill C. Rubinstein, Norman G. Nicolson, Nita Ahuja
Surgical Clinics of North America.2019; 99(3): 511. CrossRef - Molecular profile in Paraguayan colorectal cancer patients, towards to a precision medicine strategy
Tania Fleitas-Kanonnikoff, Carolina Martinez‐Ciarpaglini, Josefina Ayala, Cinthia Gauna, Rita Denis, Ita Yoffe, Silvia Sforza, María Teresa Martínez, Alicia Pomata, Maider Ibarrola‐Villava, Sipan Arevshatyan, Verónica Burriel, Diego Boscá, Oscar Pastor, A
Cancer Medicine.2019; 8(6): 3120. CrossRef - Human epidermal growth factor receptor 2-positive digestive tumors
Anna D. Wagner, Berna C. Özdemir, Josef Rüschoff
Current Opinion in Oncology.2019; 31(4): 354. CrossRef - Assessing molecular subtypes of gastric cancer: microsatellite unstable and Epstein-Barr virus subtypes. Methods for detection and clinical and pathological implications
Carolina Martinez-Ciarpaglini, Tania Fleitas-Kanonnikoff, Valentina Gambardella, Marta Llorca, Cristina Mongort, Regina Mengual, Gema Nieto, Lara Navarro, Marisol Huerta, Susana Rosello, Desamparados Roda, Noelia Tarazona, Samuel Navarro, Gloria Ribas, An
ESMO Open.2019; 4(3): e000470. CrossRef - Current and future molecular diagnostics of gastric cancer
Rachel Sin-Yu Choi, Wing Yin Xenia Lai, Lok Ting Claire Lee, Wing Lam Christa Wong, Xiao Meng Pei, Hin Fung Tsang, Joel Johnson Leung, William Chi Shing Cho, Man Kee Maggie Chu, Elaine Yue Ling Wong, Sze Chuen Cesar Wong
Expert Review of Molecular Diagnostics.2019; 19(10): 863. CrossRef - Clinicopathologic significance of human leukocyte antigen class I expression in patients with stage II and III gastric cancer
Yujun Park, Jiwon Koh, Yoonjin Kwak, Sang-Hoon Ahn, Do Joong Park, Hyung-Ho Kim, Woo Ho Kim, Hye Seung Lee
Cancer Immunology, Immunotherapy.2019; 68(11): 1779. CrossRef - Development and Validation of an Easy-to-Implement, Practical Algorithm for the Identification of Molecular Subtypes of Gastric Cancer: Prognostic and Therapeutic Implications
Jiwon Koh, Keun-Wook Lee, Soo Kyung Nam, An Na Seo, Ji-Won Kim, Jin Won Kim, Do Joong Park, Hyung-Ho Kim, Woo Ho Kim, Hye Seung Lee
The Oncologist.2019; 24(12): e1321. CrossRef - Mechanisms and Therapy for Cancer Metastasis to the Brain
Federica Franchino, Roberta Rudà, Riccardo Soffietti
Frontiers in Oncology.2018;[Epub] CrossRef - Status of programmed death-ligand 1 expression in sarcomas
Hyung Kyu Park, Mingi Kim, Minjung Sung, Seung Eun Lee, Yu Jin Kim, Yoon-La Choi
Journal of Translational Medicine.2018;[Epub] CrossRef - Design and synthesis of near-infrared fluorescence-enhancement probes for the cancer-specific enzyme hNQO1
Changyu Zhang, Bei-Bei Zhai, Tao Peng, Zelin Zhong, Lianbin Xu, Qiang-Zhe Zhang, Lu-Yuan Li, Long Yi, Zhen Xi
Dyes and Pigments.2017; 143: 245. CrossRef - Progress in the treatment of advanced gastric cancer
Zheyu Song, Yuanyu Wu, Jiebing Yang, Dingquan Yang, Xuedong Fang
Tumor Biology.2017; 39(7): 101042831771462. CrossRef - Pathologische Einteilung und Diagnostik des Ösophagus- und Magenkarzinoms
S. Förster, A. Tannapfel
Der Gastroenterologe.2017; 12(5): 394. CrossRef - NR4A1-induced increase in the sensitivity of a human gastric cancer line to TNFα-mediated apoptosis is associated with the inhibition of JNK/Parkin-dependent mitophagy
Hongzhu Yan, Feng Xiao, Jue Zou, Chengmin Qiu, Weiwei Sun, Minmin Gu, Li Zhang
International Journal of Oncology.2017;[Epub] CrossRef
- Spatial and Temporal Tumor Heterogeneity in Gastric Cancer: Discordance of Predictive Biomarkers
- Comparison of the Mismatch Repair System between Primary and Metastatic Colorectal Cancers Using Immunohistochemistry
- Jiyoon Jung, Youngjin Kang, Yoo Jin Lee, Eojin Kim, Bokyung Ahn, Eunjung Lee, Joo Young Kim, Jeong Hyeon Lee, Youngseok Lee, Chul Hwan Kim, Yang-Seok Chae
- J Pathol Transl Med. 2017;51(2):129-136. Published online February 14, 2017
- DOI: https://doi.org/10.4132/jptm.2016.12.09
- 12,442 View
- 330 Download
- 31 Web of Science
- 28 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Colorectal cancer (CRC) is one of the most common malignancies worldwide. Approximately 10%–15% of the CRC cases have defective DNA mismatch repair (MMR) genes. Although the high level of microsatellite instability status is a predictor of favorable outcome in primary CRC, little is known about its frequency and importance in secondary CRC. Immunohistochemical staining (IHC) for MMR proteins (e.g., MLH1, MSH2, MSH6, and PMS2) has emerged as a useful technique to complement polymerase chain reaction (PCR) analyses. Methods: In this study, comparison between the MMR system of primary CRCs and paired liver and lung metastatic lesions was done using IHC and the correlation with clinical outcomes was also examined. Results: Based on IHC, 7/61 primary tumors (11.4%) showed deficient MMR systems, while 13/61 secondary tumors (21.3%) showed deficiencies. In total, 44 cases showed proficient expression in both the primary and metastatic lesions. Three cases showed deficiencies in both the primary and paired metastatic lesions. In 10 cases, proficient expression was found only in the primary lesions, and not in the corresponding metastatic lesions. In four cases, proficient expression was detected in the secondary tumor, but not in the primary tumor. Conclusions: Although each IHC result and the likely defective genes were not exactly matched between the primary and the metastatic tumors, identical results for primary and metastatic lesions were obtained in 77% of the cases (47/61). These data are in agreement with the previous microsatellite detection studies that used PCR and IHC. -
Citations
Citations to this article as recorded by- Immunotherapy-induced microsatellite instability status shift in recurrent perihilar cholangiocarcinoma: A case report
Hailing Yu, Tan Deng, Hongbing Liu
Human Vaccines & Immunotherapeutics.2025;[Epub] CrossRef - Prognostic and Predictive Value of Microsatellite Instability Analysis in Circulating Tumor DNA Using Digital Droplet PCR for Patients With Microsatellite Instability Colorectal Cancers
Camille Evrard, Tristan Rochelle, Marine Martel, Anis Al Achkar, Aurélie Ferru, Violaine Randrian, Lucie Karayan-Tapon, David Tougeron
Laboratory Investigation.2025; 105(8): 104176. CrossRef - HER2, HER3, and Mismatch Repair Protein Expression in Stage IV Small Bowel Adenocarcinoma: Results From a Multicenter Series
Alessandro Vanoli, Tommaso Colella, Paola Parente, Giuseppe De Lisi, Federica Grillo, Erica Quaquarini, Salvatore Corallo, Rondell Patrell Graham, Marc Ferrante, Annick Moens, Gert De Hertogh, Camilla Guerini, Roberta Riboni, Paola Alberizzi, Luca Mastrac
Modern Pathology.2025; 38(11): 100825. CrossRef - Tumor Immune Microenvironment and Current Status of Immune Checkpoint Inhibitor Therapy in Colorectal Cancer Liver Metastasis
Dandan Cao, Aiping Zhou
Current Oncology.2025; 32(9): 493. CrossRef - MMR profile and microsatellite instability status in colorectal mucinous adenocarcinoma with synchronous metastasis: a new clue for the clinical practice
Paola Parente, Umberto Malapelle, Valentina Angerilli, Mariangela Balistreri, Sara Lonardi, Salvatore Pucciarelli, Caterina De Luca, Francesco Pepe, Gianluca Russo, Elena Vigliar, Angela Danza, Fabio Scaramuzzi, Giancarlo Troncone, Paolo Graziano, Matteo
Journal of Clinical Pathology.2023; 76(7): 492. CrossRef - Histomorphological and molecular genetic characterization of different intratumoral regions and matched metastatic lymph nodes of colorectal cancer with heterogenous mismatch repair protein expression
Jing Zhang, Xin Zhang, Qian Wang, Yu-yin Xu, Qian-lan Yao, Dan Huang, Wei-qi Sheng, Xiao-li Zhu, Xiao-yan Zhou, Qian-ming Bai
Journal of Cancer Research and Clinical Oncology.2023; 149(7): 3423. CrossRef - Intraindividual Tumor Heterogeneity of Mismatch Repair Status in Metastatic Colorectal Cancer
Qianpeng Huang, Tao Yu, Lei Li, Qi Zhang, Shiyao Zhang, Baosong Li, Xiaoping Li, Wanyi Xiao, Gang Liu
Applied Immunohistochemistry & Molecular Morphology.2023; 31(2): 84. CrossRef - Patterns of DNA mismatch repair protein expression for primary and recurrent colorectal cancer at an advanced surgical unit: A retrospective audit
Charles Risbey, Timothy Fielder, Daniel Steffens, Joo‐Shik Shin, Michael Solomon
Colorectal Disease.2023; 25(3): 369. CrossRef - Mesonephric-like Adenocarcinoma of the Uterine Corpus: Genomic and Immunohistochemical Profiling with Comprehensive Clinicopathological Analysis of 17 Consecutive Cases from a Single Institution
Hyun-Hee Koh, Eunhyang Park, Hyun-Soo Kim
Biomedicines.2023; 11(8): 2269. CrossRef - Multilevel Heterogeneity of Colorectal Cancer Liver Metastasis
Hao Chen, Chongya Zhai, Xian Xu, Haidong Wang, Weidong Han, Jiaying Shen
Cancers.2023; 16(1): 59. CrossRef - Heterogeneity of Mismatch Repair Status and Microsatellite Instability between Primary Tumour and Metastasis and Its Implications for Immunotherapy in Colorectal Cancers
Camille Evrard, Stéphane Messina, David Sefrioui, Éric Frouin, Marie-Luce Auriault, Romain Chautard, Aziz Zaanan, Marion Jaffrelot, Christelle De La Fouchardière, Thomas Aparicio, Romain Coriat, Julie Godet, Christine Silvain, Violaine Randrian, Jean-Chri
International Journal of Molecular Sciences.2022; 23(8): 4427. CrossRef - Clinicopathologic Factors Associated with Mismatch Repair Status Among Filipino Patients with Young-Onset Colorectal Cancer
Dennis Lee Sacdalan, Reynaldo L Garcia, Michele H Diwa, Danielle Benedict Sacdalan
Cancer Management and Research.2021; Volume 13: 2105. CrossRef - Recommendations for Specimen and Therapy Selection in Colorectal Cancer
Snehal B. Patel, Robert Bookstein, Navid Farahani, Myriam Chevarie-Davis, Andy Pao, Angela Aguiluz, Christian Riley, Jennelle C. Hodge, Serhan Alkan, Zhenqui Liu, Nan Deng, Jean R. Lopategui
Oncology and Therapy.2021; 9(2): 451. CrossRef - Evaluating Mismatch Repair/Microsatellite Instability Status Using Cytology Effusion Specimens to Determine Eligibility for Immunotherapy
Elizabeth M. Jacobi, Gene Landon, Russell R. Broaddus, Sinchita Roy-Chowdhuri
Archives of Pathology & Laboratory Medicine.2021; 145(1): 46. CrossRef - Médecine de précision et immunoradiothérapie
C. Chargari, C. Robert, C. Genestie, E. Deutsch
Cancer/Radiothérapie.2021; 25(6-7): 570. CrossRef - Identificación del fenotipo de inestabilidad microsatelital en carcinoma colorrectal mediante el análisis de la expresión de proteínas reparadoras del ADN: Revisión narrativa
Orlando Rodas-Pernillo, Edith Oregón
Ciencia, Tecnología y Salud.2021; 8(2): 232. CrossRef - Japan Society of Clinical Oncology provisional clinical opinion for the diagnosis and use of immunotherapy in patients with deficient DNA mismatch repair tumors, cooperated by Japanese Society of Medical Oncology, First Edition
Saori Mishima, Hiroya Taniguchi, Kiwamu Akagi, Eishi Baba, Yutaka Fujiwara, Akira Hirasawa, Masafumi Ikeda, Osamu Maeda, Kei Muro, Hiroshi Nishihara, Hiroyki Nishiyama, Tadao Takano, Katsuya Tsuchihara, Yasushi Yatabe, Yasuhiro Kodera, Takayuki Yoshino
International Journal of Clinical Oncology.2020; 25(2): 217. CrossRef - Microsatellite Stable Colorectal Cancer With an Immunogenic Phenotype: Challenges in Diagnosis and Treatment
James Saller, Dahui Qin, Seth Felder, Domenico Coppola
Clinical Colorectal Cancer.2020; 19(2): 123. CrossRef - Should you repeat mismatch repair testing in cases of tumour recurrence? An evaluation of repeat mismatch repair testing by the use of immunohistochemistry in recurrent tumours of the gastrointestinal and gynaecological tracts
John J Aird, Michael J Steel, Christine Chow, Julie Ho, Robert Wolber, C Blake Gilks, Lynn N Hoang, David F Schaeffer
Histopathology.2020; 76(4): 521. CrossRef - Microsatellite instability as a unique characteristic of tumors and a predictor of response to immune therapy
A. A. Tryakin, M. Yu. Fedyanin, A. S. Tsukanov, Yu. A. Shelygin, I. A. Pokataev, E. O. Ignatova, G. G. Khakimova, M. A. Frolova, S. A. Tjulandin
Malignant tumours.2020; 9(4): 59. CrossRef - Spontaneous regression of transverse colon cancer with high-frequency microsatellite instability: a case report and literature review
Nozomi Karakuchi, Manabu Shimomura, Kazuhiro Toyota, Takao Hinoi, Hideki Yamamoto, Seiji Sadamoto, Koichi Mandai, Hiroyuki Egi, Hideki Ohdan, Tadateru Takahashi
World Journal of Surgical Oncology.2019;[Epub] CrossRef - Biomarker concordance between primary colorectal cancer and its metastases
D.S. Bhullar, J. Barriuso, S. Mullamitha, M.P. Saunders, S.T. O'Dwyer, O. Aziz
EBioMedicine.2019; 40: 363. CrossRef - Identification of novel pathogenic MSH2 mutation and new DNA repair genes variants: investigation of a Tunisian Lynch syndrome family with discordant twins
Amira Jaballah-Gabteni, Haifa Tounsi, Maria Kabbage, Yosr Hamdi, Sahar Elouej, Ines Ben Ayed, Mouna Medhioub, Moufida Mahmoudi, Hamza Dallali, Hamza Yaiche, Nadia Ben Jemii, Afifa Maaloul, Najla Mezghani, Sonia Abdelhak, Lamine Hamzaoui, Mousaddak Azzouz,
Journal of Translational Medicine.2019;[Epub] CrossRef - Mismatch repair status between primary colorectal tumor and metastatic tumor, a retrospective consistent study
Zheng Wang, Xiaoli Tang, Xiaoqing Wu, Meiyuan Yang, Daorong Wang
Bioscience Reports.2019;[Epub] CrossRef - Heterogeneity of mismatch repair defect in colorectal cancer and its implications in clinical practice
Gaelle Tachon, Eric Frouin, Lucie Karayan-Tapon, Marie-Luce Auriault, Julie Godet, Valerie Moulin, Qing Wang, David Tougeron
European Journal of Cancer.2018; 95: 112. CrossRef - DNA mismatch repair in cancer
Marina Baretti, Dung T. Le
Pharmacology & Therapeutics.2018; 189: 45. CrossRef - Discordant loss of mismatch repair proteins in advanced endometrial endometrioid carcinoma compared to paired primary uterine tumors
Robert M. Ta, Jonathan L. Hecht, Douglas I. Lin
Gynecologic Oncology.2018; 151(3): 401. CrossRef - The CpG island methylator phenotype is concordant between primary colorectal carcinoma and matched distant metastases
Stacey A. Cohen, Ming Yu, Kelsey Baker, Mary Redman, Chen Wu, Tai J. Heinzerling, Ralph M. Wirtz, Elpida Charalambous, George Pentheroudakis, Vassiliki Kotoula, Konstantine T. Kalogeras, George Fountzilas, William M. Grady
Clinical Epigenetics.2017;[Epub] CrossRef
- Immunotherapy-induced microsatellite instability status shift in recurrent perihilar cholangiocarcinoma: A case report
- Clinicopathologic Significance of Survivin Expression in Relation to CD133 Expression in Surgically Resected Stage II or III Colorectal Cancer
- Wanlu Li, Mi-Ra Lee, EunHee Choi, Mee-Yon Cho
- J Pathol Transl Med. 2017;51(1):17-23. Published online December 15, 2016
- DOI: https://doi.org/10.4132/jptm.2016.09.23
- 11,272 View
- 176 Download
- 20 Web of Science
- 19 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Cancer stem cells have been investigated as new targets for colorectal cancer (CRC) treatment. We recently reported that CD133+ colon cancer cells showed chemoresistance to 5-fluorouracil through increased survivin expression and proposed the survivin inhibitor YM155 as an effective therapy for colon cancer in an in vitro study. Here, we investigate the relationship between survivin and CD133 expression in surgically resected CRC to identify whether the results obtained in our in vitro study are applicable to clinical samples.
Methods
We performed immunohistochemical staining for survivin and CD133 in surgically resected tissue from 187 stage II or III CRC patients. We also comparatively analyzed apoptosis according to survivin and CD133 expression using terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling.
Results
The results of the Mantel-Haenszel test established a linear association between nuclear survivin and CD133 expression (p = .018), although neither had prognostic significance, according to immunohistochemical expression level. No correlation was found between survivin expression and the following pathological parameters: invasion depth, lymph node metastasis, or histologic differentiation (p > .05). The mean apoptotic index in survivin+ and CD133+ tumors was higher than that in negative tumors: 5.116 ± 4.894 in survivin+ versus 4.103 ± 3.691 in survivin– (p = .044); 5.165 ± 4.961 in CD133+ versus 4.231 ± 3.812 in CD133– (p = .034).
Conclusions
As observed in our in vitro study, survivin expression is significantly related to CD133 expression. Survivin may be considered as a new therapeutic target for chemoresistant CRC. -
Citations
Citations to this article as recorded by- A comprehensive review and in silico analysis of the role of survivin (BIRC5) in hepatocellular carcinoma hallmarks: A step toward precision
Nermin M. Mohamed, Rania Hassan Mohamed, John F. Kennedy, Mahmoud M. Elhefnawi, Nadia M. Hamdy
International Journal of Biological Macromolecules.2025; 311: 143616. CrossRef - Genetic Variants in BIRC5 (rs8073069, rs17878467, and rs9904341) Are Associated with Susceptibility in Mexican Patients with Breast Cancer: Clinical Associations and Their Analysis In Silico
María Renee Jiménez-López, César de Jesús Tovar-Jácome, Alejandra Palacios-Ramírez, Martha Patricia Gallegos-Arreola, Teresa Giovanna María Aguilar-Macedo, Rubria Alicia González-Sánchez, Efraín Salas-González, José Elías García-Ortiz, Clara Ibet Juárez-V
Genes.2025; 16(7): 786. CrossRef - Upregulation of EMR1 (ADGRE1) by Tumor-Associated Macrophages Promotes Colon Cancer Progression by Activating the JAK2/STAT1,3 Signaling Pathway in Tumor Cells
Rokeya Akter, Rackhyun Park, Soo Kyung Lee, Eun ju Han, Kyu-Sang Park, Junsoo Park, Mee-Yon Cho
International Journal of Molecular Sciences.2024; 25(8): 4388. CrossRef - Antiapoptotic Gene Genotype and Allele Variations and the Risk of Lymphoma
Osama M. Al-Amer, Rashid Mir, Abdullah Hamadi, Mohammed I. Alasseiri, Malik A. Altayar, Waseem AlZamzami, Mamdoh Moawadh, Sael Alatawi, Hanan A. Niaz, Atif Abdulwahab A. Oyouni, Othman R. Alzahrani, Hanan E. Alatwi, Aishah E. Albalawi, Khalaf F. Alsharif,
Cancers.2023; 15(4): 1012. CrossRef - The Prognostic and Therapeutic Implications of the Chemoresistance Gene BIRC5 in Triple-Negative Breast Cancer
Getinet M. Adinew, Samia Messeha, Equar Taka, Karam F. A. Soliman
Cancers.2022; 14(21): 5180. CrossRef - EMR1/ADGRE1 Expression in Cancer Cells Upregulated by Tumor-Associated Macrophages Is Related to Poor Prognosis in Colorectal Cancer
Rokeya Akter, Kwangmin Kim, Hye Youn Kwon, Youngwan Kim, Young Woo Eom, Hye-mi Cho, Mee-Yon Cho
Biomedicines.2022; 10(12): 3121. CrossRef - Prognostic Significance of BIRC5/Survivin in Breast Cancer: Results from Three Independent Cohorts
Nina Oparina, Malin C. Erlandsson, Anna Fäldt Beding, Toshima Parris, Khalil Helou, Per Karlsson, Zakaria Einbeigi, Maria I. Bokarewa
Cancers.2021; 13(9): 2209. CrossRef - Obatoclax, a Pan-BCL-2 Inhibitor, Downregulates Survivin to Induce Apoptosis in Human Colorectal Carcinoma Cells Via Suppressing WNT/β-catenin Signaling
Chi-Hung R. Or, Chiao-Wen Huang, Ching-Chin Chang, You-Chen Lai, Yi-Ju Chen, Chia-Che Chang
International Journal of Molecular Sciences.2020; 21(5): 1773. CrossRef - M1 Macrophages Promote TRAIL Expression in Adipose Tissue-Derived Stem Cells, Which Suppresses Colitis-Associated Colon Cancer by Increasing Apoptosis of CD133+ Cancer Stem Cells and Decreasing M2 Macrophage Population
Young Woo Eom, Rokeya Akter, Wanlu Li, Suji Lee, Soonjae Hwang, Jiye Kim, Mee-Yon Cho
International Journal of Molecular Sciences.2020; 21(11): 3887. CrossRef - Emerging Importance of Survivin in Stem Cells and Cancer: the Development of New Cancer Therapeutics
Neerada Meenakshi Warrier, Prasoon Agarwal, Praveen Kumar
Stem Cell Reviews and Reports.2020; 16(5): 828. CrossRef - MMR-proficient and MMR-deficient colorectal cancer cells: 5-Fluorouracil treatment response and correlation to CD133 and MGMT expression
Jaime A. Oliver, Raúl Ortiz, Cristina Jiménez-Luna, Laura Cabeza, Gloria Perazzoli, Octavio Caba, Cristina Mesas, Consolación Melguizo, Jose Prados
Journal of Biosciences.2020;[Epub] CrossRef - Survivin rs9904341 polymorphism significantly increased the risk of cancer: evidence from an updated meta-analysis of case–control studies
Abdolkarim Moazeni-Roodi, Saeid Ghavami, Mohammad Hashemi
International Journal of Clinical Oncology.2019; 24(4): 335. CrossRef - CRISPR-Cas9 mediated CD133 knockout inhibits colon cancer invasion through reduced epithelial-mesenchymal transition
Wanlu Li, Mee-Yon Cho, Suji Lee, Mirae Jang, Junsoo Park, Rackhyun Park, Aamir Ahmad
PLOS ONE.2019; 14(8): e0220860. CrossRef - Diagnostic Approaches for Salivary Gland Tumors with Secretory and Microcystic Features
Ha Young Woo, Eun Chang Choi, Sun Och Yoon
Head and Neck Pathology.2018; 12(2): 237. CrossRef - MUC1‐ and Survivin‐based DNA Vaccine Combining Immunoadjuvants CpG and interleukin‐2 in a Bicistronic Expression Plasmid Generates Specific Immune Responses and Antitumour Effects in a Murine Colorectal Carcinoma Model
C. Liu, Y. Xie, B. Sun, F. Geng, F. Zhang, Q. Guo, H. Wu, B. Yu, J. Wu, X. Yu, W. Kong, H. Zhang
Scandinavian Journal of Immunology.2018; 87(2): 63. CrossRef - Activated STAT3 may participate in tumor progression through increasing CD133/survivin expression in early stage of colon cancer
Wanlu Li, Mi-Ra Lee, Taeyeong Kim, Young Wan Kim, Mee-Yon Cho
Biochemical and Biophysical Research Communications.2018; 497(1): 354. CrossRef - MiRNA-142-3p increases radiosensitivity in human umbilical cord blood mononuclear cells by inhibiting the expression of CD133
Fang Yuan, Lu Liu, Yonghong Lei, Yi Hu
Scientific Reports.2018;[Epub] CrossRef - MLH1 enhances the sensitivity of human endometrial carcinoma cells to cisplatin by activating the MLH1/c-Abl apoptosis signaling pathway
Yue Li, Shihong Zhang, Yuanjian Wang, Jin Peng, Fang Fang, Xingsheng Yang
BMC Cancer.2018;[Epub] CrossRef - Establishment of CMab-43, a Sensitive and Specific Anti-CD133 Monoclonal Antibody, for Immunohistochemistry
Shunsuke Itai, Yuki Fujii, Takuro Nakamura, Yao-Wen Chang, Miyuki Yanaka, Noriko Saidoh, Saori Handa, Hiroyoshi Suzuki, Hiroyuki Harada, Shinji Yamada, Mika K. Kaneko, Yukinari Kato
Monoclonal Antibodies in Immunodiagnosis and Immunotherapy.2017; 36(5): 231. CrossRef
- A comprehensive review and in silico analysis of the role of survivin (BIRC5) in hepatocellular carcinoma hallmarks: A step toward precision
- CD9 Expression in Colorectal Carcinomas and Its Prognostic Significance
- Kyung-Ju Kim, Hee Jung Kwon, Min Chong Kim, Young Kyung Bae
- J Pathol Transl Med. 2016;50(6):459-468. Published online October 25, 2016
- DOI: https://doi.org/10.4132/jptm.2016.10.02
- 10,368 View
- 155 Download
- 19 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
CD9, a member of the tetraspanin superfamily, is a tumor suppressor in many malignancies. The aim of this study was to evaluate the immunohistochemical expression of CD9 in colorectal carcinomas (CRCs) and determine clinicopathological and prognostic significance of its expression.
Methods
The CD9 expression status of 305 CRCs was evaluated using a semi-quantitative scoring system in tumor cells (T-CD9) and immune cells (I-CD9) by classifying the results as high and low expression.
Results
High T-CD9 (T-CD9 [+]) expression was detected in 175 samples (57.6%) and high I-CD9 (I-CD9 [+]) expression was detected in 265 samples (86.9%). Using Kaplan- Meier survival analysis, the T-CD9 (+) group showed a tendency for better disease-free survival (DFS) (p = .057). In left-sided tumors, DFS was significantly longer in the T-CD9 (+) group (p = .021) but no statistical significance was observed with right-sided tumors (p = .453). I-CD9 (+) CRCs significantly correlated with well/moderately differentiation (p = .014). In Kaplan-Meier analysis, the I-CD9 (+) group had a tendency towards worse DFS compared to the I-CD9 (–) group (p = .156). In combined survival analysis of T-CD9 and I-CD9, we found that the longest DFS was among patients in the T-CD9 (+)/I-CD9 (–) group, whereas the T-CD9 (–)/I-CD9 (+) group showed the shortest DFS (p = .054).
Conclusions
High expression of T-CD9 was associated with a favorable DFS, especially in left-sided CRCs. Combined evaluation of T-CD9 and I-CD9 is required to determine the comprehensive prognostic effect of CD9 in CRCs. -
Citations
Citations to this article as recorded by- Predicting patient outcomes with gene-expression biomarkers from colorectal cancer organoids and cell lines
Alexandra Razumovskaya, Mariia Silkina, Andrey Poloznikov, Timur Kulagin, Maria Raigorodskaya, Nina Gorban, Anna Kudryavtseva, Maria Fedorova, Boris Alekseev, Alexander Tonevitsky, Sergey Nikulin
Frontiers in Molecular Biosciences.2025;[Epub] CrossRef - Intestinal estrogen receptor beta modulates the murine colon tumor immune microenvironment
Madeleine Birgersson, Matilda Holm, Carlos J. Gallardo-Dodd, Baizhen Chen, Lina Stepanauskaitė, Linnea Hases, Claudia Kutter, Amena Archer, Cecilia Williams
Cancer Letters.2025; 622: 217661. CrossRef - Establishment of Coala: a novel 3D and 2D cancer cell line derived from colorectal cancer liver metastasis
Sandra Mersakova, Juraj Marcinek, Dusan Brany, Olga Chodelkova, Martin Vorcak, Martin Cermak, Martina Poturnajova, Zuzana Kozovska, Henrieta Skovierova, Slavomira Novakova, Barbora Mitruskova, Dusan Loderer, Marian Grendar, Veronika Holubekova, Andrea Hor
Human Cell.2025;[Epub] CrossRef - CD9, a tetraspanin in cancer: biology and therapeutic promise in acute leukemia
Océane Guého, Elie Cousin, Jérémie Rouger-Gaudichon, Anne-Gaëlle Rio, Sébastien Corre, Virginie Gandemer, Frédéric Mazurier
Oncogenesis.2025;[Epub] CrossRef - Proteomic analysis of plasma exosomes in patients with metastatic colorectal cancer
Zhaoyue Zhong, Jiayin Ji, Hongxia Li, Ling Kang, Haipeng Zhu
Clinical Proteomics.2024;[Epub] CrossRef - Prognostic value and multifaceted roles of tetraspanin CD9 in cancer
Róbert Ondruššek, Barbora Kvokačková, Karolína Kryštofová, Světlana Brychtová, Karel Souček, Jan Bouchal
Frontiers in Oncology.2023;[Epub] CrossRef - Proteomic Signature of Extracellular Vesicles Associated with Colorectal Cancer
Natalia Soloveva, Svetlana Novikova, Tatiana Farafonova, Olga Tikhonova, Victor Zgoda
Molecules.2023; 28(10): 4227. CrossRef - Anti-Human CD9 Fab Fragment Antibody Blocks the Extracellular Vesicle-Mediated Increase in Malignancy of Colon Cancer Cells
Mark F. Santos, Germana Rappa, Simona Fontana, Jana Karbanová, Feryal Aalam, Derek Tai, Zhiyin Li, Marzia Pucci, Riccardo Alessandro, Chikao Morimoto, Denis Corbeil, Aurelio Lorico
Cells.2022; 11(16): 2474. CrossRef - The Study of the Extracellular Matrix in Chronic Inflammation: A Way to Prevent Cancer Initiation?
Asia Marangio, Andrea Biccari, Edoardo D’Angelo, Francesca Sensi, Gaya Spolverato, Salvatore Pucciarelli, Marco Agostini
Cancers.2022; 14(23): 5903. CrossRef - In vivo expansion of a CD9+ decidual-like NK cell subset following autologous hematopoietic stem cell transplantation
Ane Orrantia, Enrique Vázquez-De Luis, Gabirel Astarloa-Pando, Iñigo Terrén, Ainhoa Amarilla-Irusta, Diego Polanco-Alonso, Carmen González, Alasne Uranga, Tomás Carrascosa, Juan J. Mateos-Mazón, Juan C. García-Ruiz, Sergio Callejas, Ana Quintas, Ana Dopaz
iScience.2022; 25(10): 105235. CrossRef - Inhibition of cancer-cell migration by tetraspanin CD9-binding peptide
Thanawat Suwatthanarak, Masayoshi Tanaka, Yoshitaka Miyamoto, Kenji Miyado, Mina Okochi
Chemical Communications.2021; 57(40): 4906. CrossRef - High expression of tumor susceptibility gene 101 (TSG101) is associated with more aggressive behavior in colorectal carcinoma
Elmira Gheytanchi, Leili Saeednejad Zanjani, Roya Ghods, Maryam Abolhasani, Marzieh Shahin, Somayeh Vafaei, Marzieh Naseri, Fahimeh Fattahi, Zahra Madjd
Journal of Cancer Research and Clinical Oncology.2021; 147(6): 1631. CrossRef - Increased CD9 expression predicts favorable prognosis in human cancers: a systematic review and meta-analysis
Hyun Min Koh, Bo Gun Jang, Dong Hui Lee, Chang Lim Hyun
Cancer Cell International.2021;[Epub] CrossRef - Prognostic Value of CD9 in Solid Tumor: A Systematic Review and Meta-Analysis
Ping Zeng, Meng Si, Rui-xia Sun, Xu Cheng, Xiao-yang Li, Min-bin Chen
Frontiers in Oncology.2021;[Epub] CrossRef - Matrix Effect in the Isolation of Breast Cancer-Derived Nanovesicles by Immunomagnetic Separation and Electrochemical Immunosensing—A Comparative Study
Silio Lima Moura, Mercè Martì, María Isabel Pividori
Sensors.2020; 20(4): 965. CrossRef - CD9 expression indicates a poor outcome in acute lymphoblastic leukemia
Peiqi Liang, Miao Miao, Zhuogang Liu, Hongtao Wang, Wei Jiang, Shiyu Ma, Chuan Li, Rong Hu
Cancer Biomarkers.2018; 21(4): 781. CrossRef
- Predicting patient outcomes with gene-expression biomarkers from colorectal cancer organoids and cell lines
- Stromal Expression of MicroRNA-21 in Advanced Colorectal Cancer Patients with Distant Metastases
- Kyu Sang Lee, Soo Kyung Nam, Jiwon Koh, Duck-Woo Kim, Sung-Bum Kang, Gheeyoung Choe, Woo Ho Kim, Hye Seung Lee
- J Pathol Transl Med. 2016;50(4):270-277. Published online May 31, 2016
- DOI: https://doi.org/10.4132/jptm.2016.03.19
- 10,624 View
- 99 Download
- 21 Web of Science
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
The aim of this study was to determine the regional heterogeneity and clinicopathological significance of microRNA-21 (miR-21) in advanced colorectal cancer (CRC) patients with distant metastasis.
Methods
miR-21 expression was investigated by using locked nucleic acid– fluorescence in situ hybridization in the center and periphery of the primary cancer and in distant metastasis from 170 patients with advanced CRC. In addition, α-smooth muscle actin and desmin were evaluated to identify cancer-associated fibroblasts (CAFs) by using immunohistochemistry.
Results
The miR-21 signal was observed in the cancer stroma. The expression of miR-21 (a score of 1–4) in the center and periphery of the primary cancer and in distant metastasis was observed in specimens from 133 (78.2%), 105 (61.8%), and 91 (53.5%) patients, respectively. miR-21 expression was heterogeneous in advanced CRC. Discordance between miR-21 expression in the center of the primary cancer and either the periphery of the primary cancer or distant metastasis was 31.7% or 44.7%, respectively. miR-21 stromal expression in the periphery of the primary cancer was significantly associated with a better prognosis (p=.004). miR-21 expression was significantly associated with CAFs in the center of the primary cancer (p=.001) and distant metastases (p=.041).
Conclusions
miR-21 expression is observed in cancer stroma related to the CAF quantity and frequently presents regional heterogeneity in CRC. Our findings indicate that the role of miR-21 in predicting prognosis may be controversial but provide a new perspective of miR-21 level measurement in cancer specimens. -
Citations
Citations to this article as recorded by- Heterogeneity of primary and metastatic CAFs: From differential treatment outcomes to treatment opportunities (Review)
Zixing Kou, Cun Liu, Wenfeng Zhang, Changgang Sun, Lijuan Liu, Qiming Zhang
International Journal of Oncology.2024;[Epub] CrossRef - Expression of Selected miRNAs in Normal and Cancer-Associated Fibroblasts and in BxPc3 and MIA PaCa-2 Cell Lines of Pancreatic Ductal Adenocarcinoma
Václav Mandys, Alexey Popov, Robert Gürlich, Jan Havránek, Lucie Pfeiferová, Michal Kolář, Jana Vránová, Karel Smetana, Lukáš Lacina, Pavol Szabo
International Journal of Molecular Sciences.2023; 24(4): 3617. CrossRef - MicroRNAs and colorectal cancer: clinical potential and regulatory networks
George Yiadom Osei, Joseph Adu-Amankwaah, Selina Koomson, Solomon Beletaa, Emmanuel Akomanin Asiamah, Cecilia Smith-Togobo, Siti Razila Abdul Razak
Molecular Biology Reports.2023; 50(11): 9575. CrossRef - MicroRNA-552 expression in colorectal cancer and its clinicopathological significance
Joon Im, Soo Kyung Nam, Hye Seung Lee
Journal of Pathology and Translational Medicine.2021; 55(2): 125. CrossRef - Postoperative changes in plasma miR21‐5p as a novel biomarker for colorectal cancer recurrence: A prospective study
Masahiro Fukada, Nobuhisa Matsuhashi, Takao Takahashi, Nobuhiko Sugito, Kazuki Heishima, Kazuhiro Yoshida, Yukihiro Akao
Cancer Science.2021; 112(10): 4270. CrossRef - Differential expression of microRNAs in colorectal cancer: Different patterns between isolated cancer gland and stromal cells
Ayaka Sato, Yasuko Fujita, Koki Otsuka, Akira Sasaki, Hiromu Suzuki, Takayuki Matsumoto, Tamotsu Sugai
Pathology International.2020; 70(1): 21. CrossRef - High-Throughput Multiplex Immunohistochemical Imaging of the Tumor and Its Microenvironment
Jiwon Koh, Yoonjin Kwak, Jin Kim, Woo Ho Kim
Cancer Research and Treatment.2020; 52(1): 98. CrossRef - Tumor Tissue MIR92a and Plasma MIRs21 and 29a as Predictive Biomarkers Associated with Clinicopathological Features and Surgical Resection in a Prospective Study on Colorectal Cancer Patients
Masahiro Fukada, Nobuhisa Matsuhashi, Takao Takahashi, Nobuhiko Sugito, Kazuki Heishima, Yukihiro Akao, Kazuhiro Yoshida
Journal of Clinical Medicine.2020; 9(8): 2509. CrossRef - The promising role of noncoding RNAs in cancer-associated fibroblasts: an overview of current status and future perspectives
Zengli Fang, Jin Xu, Bo Zhang, Wei Wang, Jiang Liu, Chen Liang, Jie Hua, Qingcai Meng, Xianjun Yu, Si Shi
Journal of Hematology & Oncology.2020;[Epub] CrossRef - Altered miR-21, miRNA-148a Expression in Relation to KRAS Mutation Status as Indicator of Adenoma-Carcinoma Transitional Pattern in Colorectal Adenoma and Carcinoma Lesions
Somayeh Igder, Javad Mohammadiasl, Pooneh Mokarram
Biochemical Genetics.2019; 57(6): 767. CrossRef - Development and Validation of an Easy-to-Implement, Practical Algorithm for the Identification of Molecular Subtypes of Gastric Cancer: Prognostic and Therapeutic Implications
Jiwon Koh, Keun-Wook Lee, Soo Kyung Nam, An Na Seo, Ji-Won Kim, Jin Won Kim, Do Joong Park, Hyung-Ho Kim, Woo Ho Kim, Hye Seung Lee
The Oncologist.2019; 24(12): e1321. CrossRef - Prognostic Utility of Histological Growth Patterns of Colorectal Lung Oligometastasis
Son Jae Yeong, Min Gyoung Pak, Hyoun Wook Lee, Seung Yeon Ha, Mee Sook Roh
Journal of Pathology and Translational Medicine.2018; 52(2): 98. CrossRef - Effect of dietary components on miRNA and colorectal carcinogenesis
Adewale Oluwaseun Fadaka, Babajide A. Ojo, Olusola Bolaji Adewale, Temitope Esho, Ashley Pretorius
Cancer Cell International.2018;[Epub] CrossRef - Ligand-Independent Epidermal Growth Factor Receptor Overexpression Correlates with Poor Prognosis in Colorectal Cancer
Sumi Yun, Yoonjin Kwak, Soo Kyung Nam, An Na Seo, Heung-Kwon Oh, Duck-Woo Kim, Sung-Bum Kang, Hye Seung Lee
Cancer Research and Treatment.2018; 50(4): 1351. CrossRef - Mutation analysis of CTNNB1 gene and the ras pathway genes KRAS , NRAS , BRAF , and PIK3CA in eyelid sebaceous carcinomas
Mi Jung Kwon, Eun Sook Nam, Seong Jin Cho, Hye-Rim Park, Soo Kee Min, Jinwon Seo, Ji-Young Choe
Pathology - Research and Practice.2017; 213(6): 654. CrossRef - Exosomal miRNAs and miRNA dysregulation in cancer-associated fibroblasts
Fengming Yang, Zhiqiang Ning, Ling Ma, Weitao Liu, Chuchu Shao, Yongqian Shu, Hua Shen
Molecular Cancer.2017;[Epub] CrossRef - Clinicopathologic implications of immune classification by PD-L1 expression and CD8-positive tumor-infiltrating lymphocytes in stage II and III gastric cancer patients
Jiwon Koh, Chan-Young Ock, Jin Won Kim, Soo Kyung Nam, Yoonjin Kwak, Sumi Yun, Sang-Hoon Ahn, Do Joong Park, Hyung-Ho Kim, Woo Ho Kim, Hye Seung Lee
Oncotarget.2017; 8(16): 26356. CrossRef
- Heterogeneity of primary and metastatic CAFs: From differential treatment outcomes to treatment opportunities (Review)
- Eosinophils in Colorectal Neoplasms Associated with Expression of CCL11 and CCL24
- Hyuck Cho, Sung-Jig Lim, Kyu Yeoun Won, Go Eun Bae, Gou Young Kim, Ji Won Min, Byeong-joo Noh
- J Pathol Transl Med. 2016;50(1):45-51. Published online December 14, 2015
- DOI: https://doi.org/10.4132/jptm.2015.10.16
- 12,438 View
- 139 Download
- 48 Web of Science
- 47 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
A decrease in the number of tissue eosinophils is known to reflect the malignancy potential of neoplastic lesions and even prognosis. Increased levels of the chemokines CCL11 and CCL24 in serum and tissue are also known to have diagnostic value as serum tumor markers or prognostic factors. The aim of this study was to evaluate the correlation between the degree of tissue eosinophilia and the expression of these chemokines in the glandular and stromal cells of colorectal neoplastic lesions ranging from benign to malignant tumors. Methods: We counted the number of infiltrating eosinophils in neoplastic lesion tissue and we evaluated the expression of CCL11 and CCL24 in glandular cells and stromal cells by immunohistochemical staining. Results: The results showed that the number of eosinophils decreased significantly and the expression of CCL11 and CCL24 in glandular cells decreased with tumor progression, whereas the stromal expression of CCL11 and CCL24 appeared to increase. Conclusions: The discrepancy in CCL11 and CCL24 expression between glandular cells and stromal cells might shed light on how colorectal cancer evades the immune system, which would enable further development of immunotherapies that target these chemokines. Further research on eosinophil biology and the expression pattern of chemokines in tumor cells is needed. -
Citations
Citations to this article as recorded by- Fibroblast Activation Protein-α Expression in Cancer-Associated Fibroblasts Shows the Poor Survival of Colorectal Cancer via Immune-Mediated Pathways
Yubo Qin, Toru Miyake, Keiji Muramoto, Takeru Maekawa, Yusuke Nishina, Ying Wang, Tomoharu Shimizu, Masaji Tani
Annals of Surgical Oncology.2025; 32(3): 1941. CrossRef - Harnessing myeloid cells in cancer
Su-Yeon Park, Ekaterina Pylaeva, Vikas Bhuria, Adriana Rosa Gambardella, Giovanna Schiavoni, Dimitrios Mougiakakos, Sung-Hoon Kim, Jadwiga Jablonska
Molecular Cancer.2025;[Epub] CrossRef - The Immune Environment in Colorectal Adenoma: A Systematic Review
Ugne Silinskaite, Jurate Valciukiene, Matas Jakubauskas, Tomas Poskus
Biomedicines.2025; 13(3): 699. CrossRef - Emerging Roles of C-C Motif Ligand 11 (CCL11) in Cancers and Liver Diseases: Mechanisms and Therapeutic Implications
Jiaqi Wang, Kwan Man, Kevin Tak-Pan Ng
International Journal of Molecular Sciences.2025; 26(10): 4662. CrossRef - Evolving role for eosinophils in cancer: from bench to bedside
S. Grisaru-Tal, E.A. Jacobsen, A. Munitz
Trends in Cancer.2025; 11(9): 862. CrossRef - Eosinophil extracellular traps formation is correlated with cancer prognosis by tumor microenvironment remodeling
Jingdai Zhang, Yu Qiu, Yifan Liu, Shengwei Mo, Muwen Nie, Hui Zhang, Xiaohang Liu, Wei Chen
Scientific Reports.2025;[Epub] CrossRef - A Novel Gene Signature Associated with Protein Post-translational Modification to Predict Clinical Outcomes and Therapeutic Responses of Colorectal Cancer
Jun Liu, Peng Zhu
Molecular Biotechnology.2024; 66(8): 2106. CrossRef - Genetic fusion of CCL11 to antigens enhances antigenicity in nucleic acid vaccines and eradicates tumor mass through optimizing T-cell response
Hailong Qi, Zhongjie Sun, Tianle Gao, Yanling Yao, Yu Wang, Weiwei Li, Xudong Wang, Xiaofang Wang, Defang Liu, Jian-Dong Jiang
Molecular Cancer.2024;[Epub] CrossRef - Eosinophils in Colorectal Cancer: Emerging Insights into Anti-Tumoral Mechanisms and Clinical Implications
David Lopez-Perez, Belen Prados-Lopez, Julio Galvez, Josefa Leon, Angel Carazo
International Journal of Molecular Sciences.2024; 25(11): 6098. CrossRef - Comprehensive evaluation of immunological attributes and immunotherapy responses of positive T cell function regulators in colorectal cancer
Ke Pu, Jingyuan Gao, Yang Feng, Jian Hu, Shunli Tang, Guodong Yang, Chuan Xu
BMC Gastroenterology.2024;[Epub] CrossRef - Eosinophilia in cancer and its regulation by sex hormones
Sandeep Artham, Ching-Yi Chang, Donald P. McDonnell
Trends in Endocrinology & Metabolism.2023; 34(1): 5. CrossRef - A novel inflammation-related signature for predicting prognosis and characterizing the tumor microenvironment in colorectal cancer
Jinna Li, Jiapeng Yang, Rui Xing, Ying Wang
Aging.2023; 15(7): 2554. CrossRef - Potential Mechanisms of Melatonin in Osteosarcoma and Bone-Related
Neoplasms: Updated Review
Parisa Maleki Dana, Fatemeh Sadoughi, Russel J. Reiter, Bahman Yousefi, Zatollah Asemi
Mini-Reviews in Medicinal Chemistry.2023; 23(3): 290. CrossRef -
Epitope Mapping of Anti-Mouse CCR3 Monoclonal Antibodies (C
3
Mab-6 and C
3
Mab-7)
Nami Tateyama, Teizo Asano, Tomohiro Tanaka, Yu Isoda, Yuki Okada, Hiyori Kobayashi, Guanjie Li, Ren Nanamiya, Takeo Yoshikawa, Mika K. Kaneko, Hiroyuki Suzuki, Yukinari Kato
Monoclonal Antibodies in Immunodiagnosis & Immunotherapy.2023; 42(2): 68. CrossRef - Improved colorectal cancer screening by adding noninvasive serum-based biomarkers
Ayman M. Farouk, Mona K. ElDeeb, Mona H. Kandil, Noha A. ElBanna, Mohamed M. Shamseya, Amel S. Elsedafy, Nevine L. Micheal, Mohamed A. Selimah
The Egyptian Journal of Surgery.2023; 42(1): 1. CrossRef - Eosinophils in the tumor microenvironment: implications for cancer immunotherapy
Sasan Ghaffari, Nima Rezaei
Journal of Translational Medicine.2023;[Epub] CrossRef - Assessing serum cytokine profiles in inflammatory breast cancer patients using Luminex® technology
Maryem Bessaad, Azza Habel, Mariem Hadj Ahmed, Weili Xu, Mouna Stayoussef, Hanen Bouaziz, Monia Hachiche, Amel Mezlini, Anis Larbi, Besma Yaacoubi-Loueslati
Cytokine.2023; 172: 156409. CrossRef - Systemic Evaluation of the Effect of Diabetes Mellitus on Breast Cancer in a Mouse Model
Nana Wei, Jinmiao Lu, Zhibing Lin, Xiaoyu Wang, Mengmeng Cai, Shengyao Jiang, Xiaoyu Chen, Shilan Zhu, Dong Zhang, Li Cui
Frontiers in Oncology.2022;[Epub] CrossRef - Extracellular DNA Traps: Origin, Function and Implications for Anti-Cancer Therapies
Medina Mamtimin, Akif Pinarci, Chao Han, Attila Braun, Hans-Joachim Anders, Thomas Gudermann, Elmina Mammadova-Bach
Frontiers in Oncology.2022;[Epub] CrossRef - Immune-Related Biomarkers Associated with Lung Metastasis from the Colorectal Cancer Microenvironment
Wang Da, Wu Yinhang, Zhuang Jing, Xu Jiamin, Gao Xinyi, Song Yongmao, Pan Yuefen
Journal of Interferon & Cytokine Research.2022; 42(5): 220. CrossRef - Cancer-Associated Fibroblasts Promote Tumor Aggressiveness in Head and Neck Cancer through Chemokine Ligand 11 and C-C Motif Chemokine Receptor 3 Signaling Circuit
Wen-Yen Huang, Yaoh-Shiang Lin, Yu-Chun Lin, Shin Nieh, Yi-Ming Chang, Tsai-Yu Lee, Su-Feng Chen, Kuender D. Yang
Cancers.2022; 14(13): 3141. CrossRef - Eosinophils Decrease Pulmonary Metastatic Mammary Tumor Growth
Rachel A. Cederberg, Sarah Elizabeth Franks, Brennan J. Wadsworth, Alvina So, Lisa R. Decotret, Michael G. Hall, Rocky Shi, Michael R. Hughes, Kelly M. McNagny, Kevin L. Bennewith
Frontiers in Oncology.2022;[Epub] CrossRef - An Immune-Related Prognostic Risk Model in Colon Cancer by Bioinformatics Analysis
Qing Lai, Haifei Feng, Shuli Yang
Evidence-Based Complementary and Alternative Medicine.2022; 2022: 1. CrossRef - Epitope Mapping of Anti-Mouse CCR3 Monoclonal Antibodies Using Flow Cytometry
Nami Tateyama, Teizo Asano, Hiroyuki Suzuki, Guanjie Li, Takeo Yoshikawa, Tomohiro Tanaka, Mika K. Kaneko, Yukinari Kato
Antibodies.2022; 11(4): 75. CrossRef - Granulocytes and Cells of Granulocyte Origin—The Relevant Players in Colorectal Cancer
Izabela Siemińska, Ewa Poljańska, Jarek Baran
International Journal of Molecular Sciences.2021; 22(7): 3801. CrossRef - CCL24 Protects Renal Function by Controlling Inflammation in Podocytes
Youdi Wang, Xue Wu, Mengya Geng, Jiamin Ding, Kangjia Lv, Hui Du, Jiahui Ding, Wenjun Pei, Xin Hu, Jing Gu, Lizhuo Wang, Yao Zhang, Jialin Gao, Roberta Rizzo
Disease Markers.2021; 2021: 1. CrossRef - Primary tumors from mucosal barrier organs drive unique eosinophil infiltration patterns and clinical associations
Sharon Grisaru-Tal, Michal Itan, Daniel G Grass, Javier Torres-Roca, Steven A Eschrich, Yaara Gordon, Avishay Dolitzky, Inbal Hazut, Shmuel Avlas, Elizabeth A Jacobsen, Tomer Ziv-Baran, Ariel Munitz
OncoImmunology.2021;[Epub] CrossRef - Discovery of core gene families associated with liver metastasis in colorectal cancer and regulatory roles in tumor cell immune infiltration
Wei-Qing Liu, Wen-Liang Li, Shu-Min Ma, Lei Liang, Zhi-Yong Kou, Jun Yang
Translational Oncology.2021; 14(3): 101011. CrossRef - Metastasis-Entrained Eosinophils Enhance Lymphocyte-Mediated Antitumor Immunity
Sharon Grisaru-Tal, Shai Dulberg, Lir Beck, Chunyan Zhang, Michal Itan, Soroor Hediyeh-zadeh, Julie Caldwell, Perri Rozenberg, Avishay Dolitzky, Shmuel Avlas, Inbal Hazut, Yaara Gordon, Ophir Shani, Shlomo Tsuriel, Motti Gerlic, Neta Erez, Nicolas Jacquel
Cancer Research.2021; 81(21): 5555. CrossRef - Tumor Immunology and Tumor Evolution: Intertwined Histories
Jérôme Galon, Daniela Bruni
Immunity.2020; 52(1): 55. CrossRef - A new dawn for eosinophils in the tumour microenvironment
Sharon Grisaru-Tal, Michal Itan, Amy D. Klion, Ariel Munitz
Nature Reviews Cancer.2020; 20(10): 594. CrossRef - Eotaxins and Their Receptor in Colorectal Cancer—A Literature Review
Monika Zajkowska, Barbara Mroczko
Cancers.2020; 12(6): 1383. CrossRef - Eosinophilic peritonitis with colon cancer: a case report
Ryo Ataka, Hirokazu Tanaka, Shintaro Yagi, Kei Yamane, Kenji Yoshino, Tomoyuki Miyauchi, Tomoaki Yoh, Keiichi Arafuka, Shinichi Fujita, Akihiko Hamada, Bunji Endo, Shinji Uemoto
BMC Gastroenterology.2020;[Epub] CrossRef - Hypoxia Alters the Expression of CC Chemokines and CC Chemokine Receptors in a Tumor–A Literature Review
Jan Korbecki, Klaudyna Kojder, Katarzyna Barczak, Donata Simińska, Izabela Gutowska, Dariusz Chlubek, Irena Baranowska-Bosiacka
International Journal of Molecular Sciences.2020; 21(16): 5647. CrossRef - CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of the Ligands of Receptors CCR1, CCR2, CCR3, and CCR4
Jan Korbecki, Klaudyna Kojder, Donata Simińska, Romuald Bohatyrewicz, Izabela Gutowska, Dariusz Chlubek, Irena Baranowska-Bosiacka
International Journal of Molecular Sciences.2020; 21(21): 8412. CrossRef - Opposing roles of eosinophils in cancer
Sonja C. S. Simon, Jochen Utikal, Viktor Umansky
Cancer Immunology, Immunotherapy.2019; 68(5): 823. CrossRef - Prognostic significance of inflammatory cell response in patients with colorectal cancer
Katarzyna Jakubowska, Mariusz Koda, Wojciech Kisielewski, Luiza Kańczuga‑Koda, Waldemar Famulski
Oncology Letters.2019;[Epub] CrossRef - Activated Eosinophils Exert Antitumorigenic Activities in Colorectal Cancer
Hadar Reichman, Michal Itan, Perri Rozenberg, Tal Yarmolovski, Eli Brazowski, Chen Varol, Nathan Gluck, Shiran Shapira, Nadir Arber, Udi Qimron, Danielle Karo-Atar, James J. Lee, Ariel Munitz
Cancer Immunology Research.2019; 7(3): 388. CrossRef - Developmental endothelial locus‐1 (Del‐1) antagonizes Interleukin‐17‐mediated allergic asthma
Shu Yan, Li Chen, Qi Zhao, Ya‐Nan Liu, Rui Hou, Jing Yu, Hong Zhang
Immunology & Cell Biology.2018; 96(5): 526. CrossRef - Expression of Galectins-1 and Galectin-3 in Stomach and Colorectal Cancer with Tissue Eosinophilia
Yu. V. Kolobovnikova, A. I. Dmitrieva, K. I. Yankovich, O. A. Vasil’eva, I. L. Purlik, V. S. Poletika, V. V. Novitskii, O. I. Urazova
Bulletin of Experimental Biology and Medicine.2018; 165(2): 256. CrossRef - The expression of CCL11/eotaxin, CCR3 receptor and eosinophil peroxidase in tumor tissue in gastric and colon cancers
Yu. V. Kolobovnikova, K. I. Yankovich, E. V. Romanova, A. I. Dmitrieva, V. V. Novitskiy, O. I. Urazova
Bulletin of Siberian Medicine.2018; 17(3): 80. CrossRef - Friend or foe?
Tommaso Colangelo, Giovanna Polcaro, Livio Muccillo, Giovanna D'Agostino, Valeria Rosato, Pamela Ziccardi, Angelo Lupo, Gianluigi Mazzoccoli, Lina Sabatino, Vittorio Colantuoni
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer.2017; 1867(1): 1. CrossRef - CCL24 contributes to HCC malignancy via RhoB- VEGFA-VEGFR2 angiogenesis pathway and indicates poor prognosis
Lei Jin, Wei-Ren Liu, Meng-Xin Tian, Xi-Fei Jiang, Han Wang, Pei-Yun Zhou, Zhen-Bin Ding, Yuan-Fei Peng, Zhi Dai, Shuang-Jian Qiu, Jian Zhou, Jia Fan, Ying-Hong Shi
Oncotarget.2017; 8(3): 5135. CrossRef - Inflammatory cytokines are associated with response and prognosis in patients with esophageal cancer
Susanne Blank, Henrik Nienhüser, Lena Dreikhausen, Leila Sisic, Ulrike Heger, Katja Ott, Thomas Schmidt
Oncotarget.2017; 8(29): 47518. CrossRef - The formation mechanisms of tumor-associated tissue eosinophilia in gastric cancer and colon cancer
В.В. Новицкий, К.И. Янкович, Ю.В. Колобовникова, А.И. Дмитриева, О.И. Уразова, И.Л. Пурлик, Л.А. Кудяков, С.П. Чумакова
ZHurnal «Patologicheskaia fiziologiia i eksperimental`naia terapiia».2017; (4(61)): 74. CrossRef - LncRNAs expression in adjuvant-induced arthritis rats reveals the potential role of LncRNAs contributing to rheumatoid arthritis pathogenesis
Hui Jiang, Xiu-Juan Qin, Wei-Ping Li, Rong Ma, Ting Wang, Zhu-Qing Li
Gene.2016; 593(1): 131. CrossRef - Emerging Roles for Eosinophils in the Tumor Microenvironment
Hadar Reichman, Danielle Karo-Atar, Ariel Munitz
Trends in Cancer.2016; 2(11): 664. CrossRef
- Fibroblast Activation Protein-α Expression in Cancer-Associated Fibroblasts Shows the Poor Survival of Colorectal Cancer via Immune-Mediated Pathways
- Pathologic Factors Associated with Prognosis after Adjuvant Chemotherapy in Stage II/III Microsatellite-Unstable Colorectal Cancers
- Jung Ho Kim, Jeong Mo Bae, Hyeon Jeong Oh, Hye Seung Lee, Gyeong Hoon Kang
- J Pathol Transl Med. 2015;49(2):118-128. Published online March 12, 2015
- DOI: https://doi.org/10.4132/jptm.2015.02.05
- 13,007 View
- 120 Download
- 20 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Although there are controversies regarding the benefit of fluoropyrimidine-based adjuvant chemotherapy in patients with microsatellite instability–high (MSI-H) colorectal cancer (CRC), the pathologic features affecting postchemotherapeutic prognosis in these patients have not been fully identified yet. Methods: A total of 26 histopathologic and immunohistochemical factors were comprehensively evaluated in 125 stage II or III MSI-H CRC patients who underwent curative resection followed by fluoropyrimidine-based adjuvant chemotherapy. We statistically analyzed the associations of these factors with disease-free survival (DFS). Results: Using a Kaplan- Meier analysis with log-rank test, we determined that ulceroinfiltrative gross type (p=.003), pT4 (p<.001), pN2 (p=.002), perineural invasion (p=.001), absence of peritumoral lymphoid reaction (p=.041), signet ring cell component (p=.006), and cribriform comedo component (p=.004) were significantly associated with worse DFS in patients receiving oxaliplatin-based adjuvant chemotherapy (n=45). By contrast, pT4 (p<.001) and tumor budding-positivity (p=.032) were significant predictors of poor survival in patients receiving non-oxaliplatin–based adjuvant chemotherapy (n=80). In Cox proportional hazards regression model-based univariate and multivariate analyses, pT category (pT1-3 vs pT4) was the only significant prognostic factor in patients receiving non-oxaliplatin–based adjuvant chemotherapy, whereas pT category, signet ring cell histology and cribriform comedo histology remained independent prognostic factors in patients receiving oxaliplatin-based adjuvant chemotherapy. Conclusions: pT4 status is the most significant pathologic determinant of poor outcome after fluoropyrimidine-based adjuvant chemotherapy in patients with stage II/III MSI-H CRC. -
Citations
Citations to this article as recorded by- Evaluation of D-Mannoheptulose and Doxorubicin as Potential Therapeutic Agents for Breast Cancer by Targeting Glycolysis and Inducing Apoptosis
Ahmed Ghdhban Al-Ziaydi
Indian Journal of Clinical Biochemistry.2025; 40(3): 412. CrossRef - Clinicopathological features and evaluation of microsatellite stability of colorectal carcinoma with cribriform comedo pattern
Tuğba Günler, Pinar Karabağli, Hicret Tiyek, Özge Keskin, Muslu K. Körez
Indian Journal of Pathology and Microbiology.2024; 67(2): 275. CrossRef - Cribriform colon cancer: a morphological growth pattern associated with extramural venous invasion, nodal metastases and microsatellite stability
Alexander S Taylor, Natalia Liu, Jiayun M Fang, Nicole Panarelli, Lili Zhao, Jerome Cheng, Purva Gopal, Suntrea Hammer, Jing Sun, Henry Appelman, Maria Westerhoff
Journal of Clinical Pathology.2022; 75(7): 483. CrossRef - HSP110 as a Diagnostic but Not a Prognostic Biomarker in Colorectal Cancer With Microsatellite Instability
Gaelle Tachon, Arnaud Chong-Si-Tsaon, Thierry Lecomte, Audelaure Junca, Éric Frouin, Elodie Miquelestorena-Standley, Julie Godet, Camille Evrard, Violaine Randrian, Romain Chautard, Marie-Luce Auriault, Valérie Moulin, Serge Guyetant, Gaelle Fromont, Luci
Frontiers in Genetics.2022;[Epub] CrossRef - Comparative expression of immunohistochemical biomarkers in cribriform and pattern 4 non-cribriform prostatic adenocarcinoma
Guang-Qian Xiao, Elise Nguyen, Pamela D. Unger, Andy E. Sherrod
Experimental and Molecular Pathology.2020; 114: 104400. CrossRef - Prognostic Predictability of American Joint Committee on Cancer 8th Staging System for Perihilar Cholangiocarcinoma: Limited Improvement Compared with the 7th Staging System
Jong Woo Lee, Jae Hoon Lee, Yejong Park, Woohyung Lee, Jaewoo Kwon, Ki Byung Song, Dae Wook Hwang, Song Cheol Kim
Cancer Research and Treatment.2020; 52(3): 886. CrossRef - Prognostic predictability of the new American Joint Committee on Cancer 8th staging system for distal bile duct cancer: limited usefulness compared with the 7th staging system
Jae Seung Kang, Seungyeoun Lee, Donghee Son, Youngmin Han, Kyung Bun Lee, Jae Ri Kim, Wooil Kwon, Sun‐Whe Kim, Jin‐Young Jang
Journal of Hepato-Biliary-Pancreatic Sciences.2018; 25(2): 124. CrossRef - Invasion Depth Measured in Millimeters is a Predictor of Survival in Patients with Distal Bile Duct Cancer: Decision Tree Approach
Kyueng‐Whan Min, Dong‐Hoon Kim, Byoung Kwan Son, Eun‐Kyung Kim, Sang Bong Ahn, Seong Hwan Kim, Yun Ju Jo, Young Sook Park, Jinwon Seo, Young Ha Oh, Sukjoong Oh, Ho Young Kim, Mi Jung Kwon, Soo Kee Min, Hye‐Rim Park, Ji‐Young Choe, Jang Yong Jeon, Hong Il
World Journal of Surgery.2017; 41(1): 232. CrossRef - BRAF-Mutated Colorectal Cancer Exhibits Distinct Clinicopathological Features from Wild-TypeBRAF-Expressing Cancer Independent of the Microsatellite Instability Status
Min Hye Jang, Sehun Kim, Dae Yong Hwang, Wook Youn Kim, So Dug Lim, Wan Seop Kim, Tea Sook Hwang, Hye Seung Han
Journal of Korean Medical Science.2017; 32(1): 38. CrossRef - Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma
Hye Eun Park, Jung Ho Kim, Nam-Yun Cho, Hye Seung Lee, Gyeong Hoon Kang
Virchows Archiv.2017; 471(3): 329. CrossRef - Dominant high expression of wild‐type HSP110 defines a poor prognostic subgroup of colorectal carcinomas with microsatellite instability: a whole‐section immunohistochemical analysis
Hyeon Jeong Oh, Jung Ho Kim, Tae Hun Lee, Hye Eun Park, Jeong Mo Bae, Hye Seung Lee, Gyeong Hoon Kang
APMIS.2017; 125(12): 1076. CrossRef - TNM Staging of Colorectal Cancer Should be Reconsidered According to Weighting of the T Stage
Jun Li, Cheng-Hao Yi, Ye-Ting Hu, Jin-Song Li, Ying Yuan, Su-Zhan Zhang, Shu Zheng, Ke-Feng Ding
Medicine.2016; 95(6): e2711. CrossRef - Molecular genetics of colorectal cancer
James Church
Seminars in Colon and Rectal Surgery.2016; 27(4): 172. CrossRef - Characterisation of PD-L1-positive subsets of microsatellite-unstable colorectal cancers
Jung Ho Kim, Hye Eun Park, Nam-Yun Cho, Hye Seung Lee, Gyeong Hoon Kang
British Journal of Cancer.2016; 115(4): 490. CrossRef - Distinct features betweenMLH1-methylated and unmethylated colorectal carcinomas with the CpG island methylator phenotype: implications in the serrated neoplasia pathway
Jung Ho Kim, Jeong Mo Bae, Nam-Yun Cho, Gyeong Hoon Kang
Oncotarget.2016; 7(12): 14095. CrossRef - Tumor deposits: markers of poor prognosis in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy
Lu-Ning Zhang, Wei-Wei Xiao, Shao-Yan Xi, Pu-Yun OuYang, Kai-Yun You, Zhi-Fan Zeng, Pei-Rong Ding, Hui-Zhong Zhang, Zhi-Zhong Pan, Rui-Hua Xu, Yuan-Hong Gao
Oncotarget.2016; 7(5): 6335. CrossRef
- Evaluation of D-Mannoheptulose and Doxorubicin as Potential Therapeutic Agents for Breast Cancer by Targeting Glycolysis and Inducing Apoptosis
- Differential Features of Microsatellite-Unstable Colorectal Carcinomas Depending on EPCAM Expression Status
- Jung Ho Kim, Jeong Mo Bae, Kyung-Ju Kim, Ye-Young Rhee, Younghoon Kim, Nam-Yun Cho, Hye Seung Lee, Mee Soo Chang, Gyeong Hoon Kang
- Korean J Pathol. 2014;48(4):276-282. Published online August 26, 2014
- DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.4.276
- 13,145 View
- 63 Download
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF Background Recent studies have revealed that a small subset of Lynch syndrome-associated colorectal carcinomas (CRCs) is caused by a germline
EPCAM deletion-inducedMSH2 epimutation. Based on the finding of this genetic alteration, we investigated the implications of EPCAM expression changes in microsatellite instability-high (MSI-H) CRCs.Methods Expression of EPCAM and DNA mismatch repair proteins was assessed by immunohistochemistry in 168 MSI-H CRCs. Using DNA samples of these tumors,
MLH1 promoter methylation status was also determined by methylation-specific real-time polymerase chain reaction method (MethyLight).Results Among 168 MSI-H CRCs, complete loss (CL) and focal loss (FL) of EPCAM expression was observed in two (1.2%) and 22 (13.1%) cases, respectively. Both of the EPCAM-CL cases were found in MSH2-negative tumors without
MLH1 promoter methylation. However, only nine of the 22 EPCAM-FL tumors had MSH2 deficiency. Of the 22 EPCAM-FL tumors, 13 showed MLH1 loss, and among them, nine cases were determined to haveMLH1 methylation. EPCAM-FL was significantly associated with advanced stage (p=.043), distant metastasis (p=.003), poor differentiation (p=.001), and signet ring cell component (p=.004).Conclusions Loss of EPCAM expression is differentially associated with clinicopathological and molecular features, depending on the completeness of the loss, in MSI-H CRCs.
-
Citations
Citations to this article as recorded by- Clinical Significance of EPCAM Pathogenic Germline Variant
Jun-Ha Jang, Kyoung-Bo Kim, Jong Eun Park, Mi-Ae Jang, Dongju Won, Boyoung Park, Jung-Sook Ha, Sun-Young Kong
Laboratory Medicine Online.2025; 15(4): 263. CrossRef - Prognostic significance of microsatellite instability in colon cancer: Insights from a Propensity Score-Matched Study
Hilmi Yazici, Ayse Eren Kayaci, Melike Zeynep Can Sahin, Cisil Bayir, Aysenur Yildiz, Esin Zeynep Cinal, Muhammer Ergenc, Tevfik Kivilcim Uprak
Current Problems in Surgery.2024; 61(12): 101633. CrossRef - Unraveling the multifaceted role of EpCAM in colorectal cancer: an integrated review of its function and interplay with non-coding RNAs
Xingyu Jiang, Sumeng Wang, Qi Liang, Yiqian Liu, Lingxiang Liu
Medical Oncology.2023;[Epub] CrossRef - Outcomes of Definitive Treatment of Signet Ring Cell Carcinoma of the Rectum: Is Minimal Invasive Surgery Detrimental in Signet Ring Rectal Cancers?
S. Raghavan, Deepak Kumar Singh, J. Rohila, A. DeSouza, R. Engineer, A. Ramaswamy, V. Ostwal, A. Saklani
Indian Journal of Surgical Oncology.2020; 11(4): 597. CrossRef - Evaluation of the Role of Circulating Tumor Cells and Microsatellite Instability Status in Predicting Outcome of Advanced CRC Patients
Ippokratis Messaritakis, Maria Sfakianaki, Konstantinos Vogiatzoglou, Asimina Koulouridi, Chara Koutoulaki, Dimitrios Mavroudis, Maria Tzardi, Nikolaos Gouvas, John Tsiaoussis, John Souglakos
Journal of Personalized Medicine.2020; 10(4): 235. CrossRef - Is Ep-CAM Expression a Diagnostic and Prognostic Biomarker for Colorectal Cancer? A Systematic Meta-Analysis
Susu Han, Shaoqi Zong, Qi Shi, Hongjia Li, Shanshan Liu, Wei Yang, Wen Li, Fenggang Hou
EBioMedicine.2017; 20: 61. CrossRef - Prognostic factors in sporadic colon cancer with high-level microsatellite instability
Bo Young Oh, Jung Wook Huh, Yoon Ah Park, Yong Beom Cho, Seong Hyeon Yun, Hee Cheol Kim, Woo Yong Lee, Ho-Kyung Chun
Surgery.2016; 159(5): 1372. CrossRef - Clinical significance of fibroblast growth factor receptor 2 expression in patients with residual rectal cancer after preoperative chemoradiotherapy: relationship with KRAS or BRAF mutations and MSI status
Ghilsuk Yoon, Hwayoung Lee, Jae-Hoon Kim, Keun Hur, An Na Seo
Tumor Biology.2016; 37(8): 10209. CrossRef - Subcellular differential expression of Ep-ICD in oral dysplasia and cancer is associated with disease progression and prognosis
Raj Thani Somasundaram, Jatinder Kaur, Iona Leong, Christina MacMillan, Ian J. Witterick, Paul G. Walfish, Ranju Ralhan
BMC Cancer.2016;[Epub] CrossRef - BRAF, PIK3CA, and HER2 Oncogenic Alterations According to KRAS Mutation Status in Advanced Colorectal Cancers with Distant Metastasis
Soo Kyung Nam, Sumi Yun, Jiwon Koh, Yoonjin Kwak, An Na Seo, Kyoung Un Park, Duck-Woo Kim, Sung-Bum Kang, Woo Ho Kim, Hye Seung Lee, Wayne A Phillips
PLOS ONE.2016; 11(3): e0151865. CrossRef - Twist1-induced epithelial-mesenchymal transition according to microsatellite instability status in colon cancer cells
Bo Young Oh, So-Young Kim, Yeo Song Lee, Hye Kyung Hong, Tae Won Kim, Seok Hyung Kim, Woo Yong Lee, Yong Beom Cho
Oncotarget.2016; 7(35): 57066. CrossRef - Clinicopathologic, molecular, and prognostic implications of the loss of EPCAM expression in colorectal carcinoma
Jung Ho Kim, Jeong Mo Bae, Young Seok Song, Nam-Yun Cho, Hye Seung Lee, Gyeong Hoon Kang
Oncotarget.2016; 7(12): 13372. CrossRef - Pathologic Factors Associated with Prognosis after Adjuvant Chemotherapy in Stage II/III Microsatellite-Unstable Colorectal Cancers
Jung Ho Kim, Jeong Mo Bae, Hyeon Jeong Oh, Hye Seung Lee, Gyeong Hoon Kang
Journal of Pathology and Translational Medicine.2015; 49(2): 118. CrossRef - HER3 protein expression in relation to HER2 positivity in patients with primary colorectal cancer: clinical relevance and prognostic value
An Na Seo, Yoonjin Kwak, Woo Ho Kim, Duck-Woo Kim, Sung-Bum Kang, Gheeyoung Choe, Hye Seung Lee
Virchows Archiv.2015; 466(6): 645. CrossRef - Clinical and prognostic value of MET gene copy number gain and chromosome 7 polysomy in primary colorectal cancer patients
An Na Seo, Kyoung Un Park, Gheeyoung Choe, Woo Ho Kim, Duck-Woo Kim, Sung-Bum Kang, Hye Seung Lee
Tumor Biology.2015; 36(12): 9813. CrossRef - c-MYC Copy-Number Gain Is an Independent Prognostic Factor in Patients with Colorectal Cancer
Kyu Sang Lee, Yoonjin Kwak, Kyung Han Nam, Duck-Woo Kim, Sung-Bum Kang, Gheeyoung Choe, Woo Ho Kim, Hye Seung Lee, Andreas Krieg
PLOS ONE.2015; 10(10): e0139727. CrossRef - Nuclear Ep-ICD accumulation predicts aggressive clinical course in early stage breast cancer patients
Gunjan Srivastava, Jasmeet Assi, Lawrence Kashat, Ajay Matta, Martin Chang, Paul G Walfish, Ranju Ralhan
BMC Cancer.2014;[Epub] CrossRef
- Clinical Significance of EPCAM Pathogenic Germline Variant
- Progressive Increase of Regulatory T Cells and Decrease of CD8+ T Cells and CD8+ T Cells/Regulatory T Cells Ratio during Colorectal Cancer Development
- Tae Jung Jang
- Korean J Pathol. 2013;47(5):443-451. Published online October 25, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.5.443
- 10,110 View
- 67 Download
- 26 Crossref
-
 Abstract
Abstract
 PDF
PDF Background We examined the distribution of CD8+ T cells and regulatory T cells (Tregs), measured the CD8+ T cell/Tregs ratio, investigated the relationship between Tregs and cyclooxygenase-2 (COX-2) expression during colorectal cancer (CRC) development.
Methods We performed immunohistochemical staining for CD8, forkhead box P3, E-cadherin, and COX-2 in 32 cases of invasive CRC, 10 cases of intramucosal CRC, 27 cases of high-grade tubular adenoma, 22 cases of low-grade tubular adenoma, and 32 cases of non-neoplastic conditions.
Results We observed a progressive increase in Tregs, and a decrease in CD8+ T cells and the CD8+ T cells/Tregs ratio during CRC development. The alterations were most severe in high-grade tubular adenoma and CRC. COX-2 expression was positively associated with Tregs infiltration. The degree of T cell infiltration differed among tumor compartment and the ratio in the tumor center was the lowest of all areas. The ratio and number of CD8+ T cells in the tumor center and the invasive front of invasive CRC were associated with gender, differentiation, node metastasis and tumor budding.
Conclusions Alteration in the distribution of both CD8+T cells and Tregs may contribute to the generation of an immune environment suitable for the development and progression of CRC.
-
Citations
Citations to this article as recorded by- The Roles of Vitamin D Receptor (VDR) and CD8+ T‐Lymphocytes in Acral and Mucosal Melanoma Invasion Depth
Hermin Aminah Usman, Fitria Sholihah, Birgitta M. Dewayani, Octavianus Giovani
Journal of Cutaneous Pathology.2025; 52(3): 227. CrossRef - The Immune Environment in Colorectal Adenoma: A Systematic Review
Ugne Silinskaite, Jurate Valciukiene, Matas Jakubauskas, Tomas Poskus
Biomedicines.2025; 13(3): 699. CrossRef - Tumor Microenvironment and Immune Response in Lip Cancer
Anastasia G. Gkegka, Michael I. Koukourakis, Maria Lambropoulou, Alexandra Giatromanolaki
Cancers.2023; 15(5): 1478. CrossRef - Regenerating gene 4 promotes chemoresistance of colorectal cancer by affecting lipid droplet synthesis and assembly
Cong-Yu Zhang, Rui Zhang, Li Zhang, Zi-Mo Wang, Hong-Zhi Sun, Zheng-Guo Cui, Hua-Chuan Zheng
World Journal of Gastroenterology.2023; 29(35): 5104. CrossRef - Molecular mechanisms of tumour budding and its association with microenvironment in colorectal cancer
Phimmada Hatthakarnkul, Jean A. Quinn, Aula Ammar, Gerard Lynch, Hester Van Wyk, Donald C. McMillan, Chanitra Thuwajit, Joanne Edwards
Clinical Science.2022; 136(8): 521. CrossRef - Attackers and defenders: tumor buds and lymphocytes as morphological biomarkers in colorectal cancer
Sonay Kus Öztürk, Tariq S. Haddad, Inti Zlobec, Alessandro Lugli, Iris D. Nagtegaal
Diagnostic Histopathology.2022; 28(11): 480. CrossRef - The prognostic impact of the immune microenvironment in small-cell neuroendocrine carcinoma of the uterine cervix: PD-L1 and immune cell subtypes
Xiaoying Sun, Lili Liu, Ting Wan, Qidan Huang, Jieping Chen, Rongzhen Luo, Jihong Liu
Cancer Cell International.2022;[Epub] CrossRef - Preinvasive Colorectal Lesions of African Americans Display an Immunosuppressive Signature Compared to Caucasian Americans
Kristin Wallace, Georges J. Nahhas, Christine Bookhout, David N. Lewin, Chrystal M. Paulos, Nana Nikolaishvili-Feinberg, Stephanie M. Cohen, Silvia Guglietta, Ali Bakhtiari, E. Ramsay Camp, Elizabeth G. Hill, John A. Baron, Jennifer D. Wu, Alexander V. Al
Frontiers in Oncology.2021;[Epub] CrossRef - Cancer Immunoprevention: Current Status and Future Directions
Mahsa Keshavarz-Fathi, Nima Rezaei
Archivum Immunologiae et Therapiae Experimentalis.2021;[Epub] CrossRef - Transcriptomic Analyses of the Adenoma-Carcinoma Sequence Identify Hallmarks Associated With the Onset of Colorectal Cancer
Qin Hong, Bing Li, Xiumei Cai, Zhengtao Lv, Shilun Cai, Yunshi Zhong, Bo Wen
Frontiers in Oncology.2021;[Epub] CrossRef - Immune Responses Vary in Preinvasive Colorectal Lesions by Tumor Location and Histology
Kristin Wallace, Georges J. El Nahas, Christine Bookhout, Jessica E. Thaxton, David N. Lewin, Nana Nikolaishvili-Feinberg, Stephanie M. Cohen, J. Grant Brazeal, Elizabeth G. Hill, Jennifer D. Wu, John A. Baron, Alexander V. Alekseyenko
Cancer Prevention Research.2021; 14(9): 885. CrossRef - CD103+ T Lymphocyte Count Linked to the Thickness of Invasion on Acral Melanoma without E-Cadherin Involvement
Fauzan Ali Zainal Abidin, Hermin Aminah Usman, Sri Suryanti, Bethy S Hernowo
Clinical, Cosmetic and Investigational Dermatology.2021; Volume 14: 1783. CrossRef - FOXP3 and CD25 double staining antibody cocktails identify regulatory T cells in different types of tumor tissues using tissue microarrays
Xinmin Liu, Xiaohong Wang, Jianmin Ding, Yan Gao, Yiming Zhao, Ruihua Zhao, Qigang Sun, Songlin Zhang
Annals of Diagnostic Pathology.2019; 38: 67. CrossRef - Epithelial CD80 promotes immune surveillance of colonic preneoplastic lesions and its expression is increased by oxidative stress through STAT3 in colon cancer cells
Chiara Marchiori, Melania Scarpa, Andromachi Kotsafti, Susan Morgan, Matteo Fassan, Vincenza Guzzardo, Andrea Porzionato, Imerio Angriman, Cesare Ruffolo, Stefania Sut, Stefano Dall’Acqua, Romeo Bardini, Raffaele De Caro, Carlo Castoro, Marco Scarpa, Igna
Journal of Experimental & Clinical Cancer Research.2019;[Epub] CrossRef - Automated Analysis of Lymphocytic Infiltration, Tumor Budding, and Their Spatial Relationship Improves Prognostic Accuracy in Colorectal Cancer
Ines P. Nearchou, Kate Lillard, Christos G. Gavriel, Hideki Ueno, David J. Harrison, Peter D. Caie
Cancer Immunology Research.2019; 7(4): 609. CrossRef - Colorectal cancer prevention: Immune modulation taking the stage
Rochelle Fletcher, Yi-Jun Wang, Robert E. Schoen, Olivera J. Finn, Jian Yu, Lin Zhang
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer.2018; 1869(2): 138. CrossRef - The Major Role of NF-κB in the Depth of Invasion on Acral Melanoma by Decreasing CD8+ T Cells
Hermin Aminah Usman, Bethy S. Hernowo, Maringan Diapari Lumban Tobing, Reti Hindritiani
Journal of Pathology and Translational Medicine.2018; 52(3): 164. CrossRef - Increased number of arginase 1-positive cells in the stroma of carcinomas compared to precursor lesions and nonneoplastic tissues
Tae Jung Jang, Sun A. Kim, Min Kyung Kim
Pathology - Research and Practice.2018; 214(8): 1179. CrossRef - Hematopoietic stem cell specific V-ATPase controls breast cancer progression and metastasis via cytotoxic T cells
Manoranjan Sahoo, Gajendra K. Katara, Mahmood Y. Bilal, Safaa A. Ibrahim, Arpita Kulshrestha, Sara Fleetwood, Kimiko Suzue, Kenneth D. Beaman
Oncotarget.2018; 9(69): 33215. CrossRef - Morphoproteomic-Guided Host-Directed Therapy for Tuberculosis
Robert E. Brown, Robert L. Hunter, Shen-An Hwang
Frontiers in Immunology.2017;[Epub] CrossRef - O-GlcNAcylation is associated with the development and progression of gastric carcinoma
Tae Jung Jang, Ui Jung Kim
Pathology - Research and Practice.2016; 212(7): 622. CrossRef - The Immune Landscapes of Polypoid and Nonpolypoid Precancerous Colorectal Lesions
Antonella Maglietta, Rosalia Maglietta, Teresa Staiano, Ramona Bertoni, Nicola Ancona, Giancarlo Marra, Leonardo Resta, Hiroshi Shiku
PLOS ONE.2016; 11(7): e0159373. CrossRef - Analysis of CD8+ Treg cells in patients with ovarian cancer: a possible mechanism for immune impairment
Shuping Zhang, Xing Ke, Suyun Zeng, Meng Wu, Jianfang Lou, Lei Wu, Peijun Huang, Lei Huang, Fang Wang, Shiyang Pan
Cellular & Molecular Immunology.2015; 12(5): 580. CrossRef - The Distribution of CD8- and Foxp3-positive T Cells in Skin Squamous Cell Tumors and Basal Cell Carcinomas
Tae Jung Jang
Journal of Life Science.2015; 25(6): 686. CrossRef - Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancer
Jingying Hou, Zhong Yu, Rengyun Xiang, Chuqiang Li, Lin Wang, Shufen Chen, Qingyun Li, Mei Chen, Linyun Wang
Experimental and Molecular Pathology.2014; 96(3): 284. CrossRef - Methionine enkephalin (MENK) improves lymphocyte subpopulations in human peripheral blood of 50 cancer patients by inhibiting regulatory T cells (Tregs)
Qiushi Wang, Xinghua Gao, Zhe Yuan, Zhe Wang, Yiming Meng, Yan Cao, Nicolas P Plotnikoff, Noreen Griffin, Fengping Shan
Human Vaccines & Immunotherapeutics.2014; 10(7): 1836. CrossRef
- The Roles of Vitamin D Receptor (VDR) and CD8+ T‐Lymphocytes in Acral and Mucosal Melanoma Invasion Depth
- The Expression of CD10 and CD15 Is Progressively Increased during Colorectal Cancer Development
- Tae Jung Jang, Jeong Bae Park, Jong Im Lee
- Korean J Pathol. 2013;47(4):340-347. Published online August 26, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.4.340
- 12,399 View
- 99 Download
- 39 Crossref
-
 Abstract
Abstract
 PDF
PDF Background The aim of this study was to examine the expression of CD10 and CD15 in tumor cells, stromal cells and infiltrating inflammatory cells during colorectal carcinoma (CRC) development and to investigate their expression levels between the tumor center and invasive front and compare them to clinicopathological parameters in invasive CRC.
Methods We performed immunohistochemical staining for CD10, CD15, and E-cadherin in 42 cases of CRC, 49 of tubular adenoma, 15 of hyperplastic polyp, and 17 of non-neoplastic colon.
Results CD10 was expressed in tumor cells (tCD10), stromal cells (sCD10) and infiltrating inflammatory cells (iCD10), and CD15 was expressed in tumor cells (tCD15) and infiltrating inflammatory cells (iCD15). Their expressions were progressively increased during CRC development and the iCD10 expression level was significantly correlated with the iCD15 expression level in invasive CRC. Invasive front revealed a higher expression level of iCD10 and iCD15 than the tumor center. Moreover, the iCD15 expression level of invasive front was significantly correlated with the degree of tumor budding and tCD15 in whole tissue sections was closely associated with tumor depth.
Conclusions The present study suggests that the expression of CD10 and CD15 is associated with the development and progression of CRC.
-
Citations
Citations to this article as recorded by- From Ulcerative Colitis to Metastatic Colorectal Cancer
Iliana I. León-Vega, Reyna Oregon, Michael Schnoor, Eduardo Vadillo
The American Journal of Pathology.2025; 195(5): 814. CrossRef - CD10 Expression Correlates with Earlier Tumour Stages and Left-Sided Tumour Location in Colorectal Cancer but Has No Prognostic Impact in a European Cohort
Julia-Kristin Grass, Katharina Grupp, Martina Kluth, Claudia Hube-Magg, Ronald Simon, Marius Kemper, Jakob R. Izbicki, Guido Sauter, Nathaniel Melling
Cancers.2024; 16(8): 1473. CrossRef - Exploring cell-derived extracellular vesicles in peripheral blood and bone marrow of B-cell acute lymphoblastic leukemia pediatric patients: proof-of-concept study
Fábio Magalhães-Gama, Marina Malheiros Araújo Silvestrini, Juliana Costa Ferreira Neves, Nilberto Dias Araújo, Fabíola Silva Alves-Hanna, Marlon Wendell Athaydes Kerr, Maria Perpétuo Socorro Sampaio Carvalho, Andréa Monteiro Tarragô, Gemilson Soares Ponte
Frontiers in Immunology.2024;[Epub] CrossRef - Pathophysiological roles and applications of glycosphingolipids in the diagnosis and treatment of cancer diseases
Xuefeng Jin, Guang-Yu Yang
Progress in Lipid Research.2023; 91: 101241. CrossRef - Expression of CD10 and CD15 in colorectal mucinous and signet ring adenocarcinomas and its relation to clinicopathological features and prognosis
Abd AlRahman Mohammad Foda, Haitham Abdulkarem Alamer, Nadeem Ikram, Hadi Abdulhadi Helali, Fayza Sami Fayad, Sara Waleed Hussian, Khaled Abdelwahab, Tamer Akl, Ziad Emarah, Ahmed M. Ramez
Cancer Biomarkers.2022; 33(1): 143. CrossRef -
A Defucosylated Mouse Anti-CD10 Monoclonal Antibody (31-mG
2a
-f) Exerts Antitumor Activity in a Mouse Xenograft Model of Renal Cell Cancers
Hiroki Kawabata, Tomokazu Ohishi, Hiroyuki Suzuki, Teizo Asano, Manabu Kawada, Hiroyoshi Suzuki, Mika K. Kaneko, Yukinari Kato
Monoclonal Antibodies in Immunodiagnosis & Immunotherapy.2022; 41(6): 320. CrossRef - Single-cell proteomics defines the cellular heterogeneity of localized prostate cancer
Laura De Vargas Roditi, Andrea Jacobs, Jan H. Rueschoff, Pete Bankhead, Stéphane Chevrier, Hartland W. Jackson, Thomas Hermanns, Christian D. Fankhauser, Cedric Poyet, Felix Chun, Niels J. Rupp, Alexandra Tschaebunin, Bernd Bodenmiller, Peter J. Wild
Cell Reports Medicine.2022; 3(4): 100604. CrossRef - Targeting CD10 on B-Cell Leukemia Using the Universal CAR T-Cell Platform (UniCAR)
Nicola Mitwasi, Claudia Arndt, Liliana R. Loureiro, Alexandra Kegler, Frederick Fasslrinner, Nicole Berndt, Ralf Bergmann, Vaclav Hořejší, Claudia Rössig, Michael Bachmann, Anja Feldmann
International Journal of Molecular Sciences.2022; 23(9): 4920. CrossRef - Prognostic and Therapeutic Role of CD15 and CD15s in Cancer
Wojciech Szlasa, Karol Wilk, Klaudia Knecht-Gurwin, Adam Gurwin, Anita Froń, Natalia Sauer, Wojciech Krajewski, Jolanta Saczko, Tomasz Szydełko, Julita Kulbacka, Bartosz Małkiewicz
Cancers.2022; 14(9): 2203. CrossRef - Expression Profile of CD10, BCL-2, p63, and EMA in the Normal Skin and Basal Cell Carcinomas: An Immunohistochemical Reappraisal
M.R. Hussein, A.M. Ahmed
Actas Dermo-Sifiliográficas.2022; 113(9): 848. CrossRef - Targeting Aberrantly Elevated Sialyl Lewis A as a Potential Therapy for Impaired Endometrial Selection Ability in Unexplained Recurrent Miscarriage
Zhi Ma, Huixia Yang, Mirjana Kessler, Markus Sperandio, Sven Mahner, Udo Jeschke, Viktoria von Schönfeldt
Frontiers in Immunology.2022;[Epub] CrossRef - [Artículo traducido] Perfil de expresión de CD10, BCL-2, p63 y EMA en los carcinomas normales de piel y de células basales: Revaloración inmunohistoquímica
M.R. Hussein, A.M. Ahmed
Actas Dermo-Sifiliográficas.2022; 113(9): T848. CrossRef - Multiomics surface receptor profiling of the NCI-60 tumor cell panel uncovers novel theranostics for cancer immunotherapy
Simon Heumos, Sandra Dehn, Konstantin Bräutigam, Marius C. Codrea, Christian M. Schürch, Ulrich M. Lauer, Sven Nahnsen, Michael Schindler
Cancer Cell International.2022;[Epub] CrossRef - High level of CD10 expression is associated with poor overall survival in patients with head and neck cancer
Q. Li, Y. Wang, L. Xu, L. Wang, Y. Guo, C. Guo
International Journal of Oral and Maxillofacial Surgery.2021; 50(7): 857. CrossRef - Expression of the Carbohydrate Lewis Antigen, Sialyl Lewis A, Sialyl Lewis X, Lewis X, and Lewis Y in the Placental Villi of Patients With Unexplained Miscarriages
Zhi Ma, Huixia Yang, Lin Peng, Christina Kuhn, Anca Chelariu-Raicu, Sven Mahner, Udo Jeschke, Viktoria von Schönfeldt
Frontiers in Immunology.2021;[Epub] CrossRef - CD15+ tumor infiltrating granulocytic cells can predict recurrence and their depletion is accompanied by good responses to S‐1 with oral cancer
Mai Seki‐Soda, Takaaki Sano, Masaru Ogawa, Satoshi Yokoo, Tetsunari Oyama
Head & Neck.2021; 43(8): 2457. CrossRef - Relationship between Immunophenotype and Clinicopathological Findings for Superficial Nonampullary Duodenal Epithelial Tumor
Shigeki Fukusada, Takaya Shimura, Hiroyasu Iwasaki, Yusuke Okuda, Takahito Katano, Ruriko Nishigaki, Takanori Ozeki, Mika Kitagawa, Hirotada Nishie, Mamoru Tanaka, Keiji Ozeki, Eiji Kubota, Satoshi Tanida, Hiromi Kataoka
Digestion.2021; 102(6): 870. CrossRef - Relationship between inflammation and the severity of Recurrent Respiratory Papillomatosis
Vivian Narana Ribeiro El Achkar, Andressa Duarte, Román Carlos, Jorge Esquiche León, Alfredo Ribeiro-Silva, Shirley Shizue Nagata Pignatari, Estela Kaminagakura
American Journal of Otolaryngology.2020; 41(2): 102321. CrossRef - Broad and thematic remodeling of the surfaceome and glycoproteome on isogenic cells transformed with driving proliferative oncogenes
Kevin K. Leung, Gary M. Wilson, Lisa L. Kirkemo, Nicholas M. Riley, Joshua J. Coon, James A. Wells
Proceedings of the National Academy of Sciences.2020; 117(14): 7764. CrossRef - Prognostic Implications of CD10 and CD15 Expression in Papillary Thyroid Carcinoma
Eun Ji Oh, Andrey Bychkov, Haejin Cho, Tae-Min Kim, Ja Seong Bae, Dong-Jun Lim, Chan Kwon Jung
Cancers.2020; 12(6): 1413. CrossRef - Is There Such a Thing as a Genuine Cancer Stem Cell Marker? Perspectives from the Gut, the Brain and the Dental Pulp
Crende Olatz, García-Gallastegui Patricia, Luzuriaga Jon, Badiola Iker, de la Hoz Carmen, Unda Fernando, Ibarretxe Gaskon, Pineda Jose Ramon
Biology.2020; 9(12): 426. CrossRef - Neprilysin expression and functions in development, ageing and disease
NN Nalivaeva, IA Zhuravin, AJ Turner
Mechanisms of Ageing and Development.2020; 192: 111363. CrossRef - A Risk Signature With Inflammatory and T Immune Cells Infiltration in Colorectal Cancer Predicting Distant Metastases and Efficiency of Chemotherapy
Xiang Hu, Ya-Qi Li, Xiao-ji Ma, Long Zhang, San-Jun Cai, Jun-Jie Peng
Frontiers in Oncology.2019;[Epub] CrossRef - Tumor-infiltrating Neutrophils is Prognostic and Predictive for Postoperative Adjuvant Chemotherapy Benefit in Patients With Gastric Cancer
Heng Zhang, Hao Liu, Zhenbin Shen, Chao Lin, Xuefei Wang, Jing Qin, Xinyu Qin, Jiejie Xu, Yihong Sun
Annals of Surgery.2018; 267(2): 311. CrossRef - Fucosylated Antigens in Cancer: An Alliance toward Tumor Progression, Metastasis, and Resistance to Chemotherapy
Athanasios Blanas, Neha M. Sahasrabudhe, Ernesto Rodríguez, Yvette van Kooyk, Sandra J. van Vliet
Frontiers in Oncology.2018;[Epub] CrossRef - Biomarkers in Urachal Cancer and Adenocarcinomas in the Bladder: A Comprehensive Review Supplemented by Own Data
Henning Reis, Ulrich Krafft, Christian Niedworok, Orsolya Módos, Thomas Herold, Mark Behrendt, Hikmat Al-Ahmadie, Boris Hadaschik, Peter Nyirady, Tibor Szarvas
Disease Markers.2018; 2018: 1. CrossRef - CD10 inhibits cell motility but expression is associated with advanced stage disease in colorectal cancer
Teresa P. Raposo, Mireia Sueca Comes, Adeyemi Idowu, Bora Agit, James Hassall, Wakkas Fadhil, Robert Nica, Rupert Ecker, Takashi Yao, Mohammad Ilyas
Experimental and Molecular Pathology.2018; 104(3): 190. CrossRef - High levels of tumor-associated neutrophils are associated with improved overall survival in patients with stage II colorectal cancer
Ryan S. Berry, Meng-Jun Xiong, Alissa Greenbaum, Parisa Mortaji, Robert A. Nofchissey, Fred Schultz, Cathleen Martinez, Li Luo, Katherine T. Morris, Joshua A. Hanson, Masaru Katoh
PLOS ONE.2017; 12(12): e0188799. CrossRef - Tissue expression of CD10 and CD15 proteins in gastric lesions
Omneya Y. Bassyoni, Sarah N. Nasif
Egyptian Journal of Pathology.2017; 37(2): 321. CrossRef - Menin and Daxx Interact to Suppress Neuroendocrine Tumors through Epigenetic Control of the Membrane Metallo-Endopeptidase
Zijie Feng, Lei Wang, Yanmei Sun, Zongzhe Jiang, John Domsic, Chiying An, Bowen Xing, Jingjing Tian, Xiuheng Liu, David C. Metz, Xiaolu Yang, Ronen Marmorstein, Xiaosong Ma, Xianxin Hua
Cancer Research.2017; 77(2): 401. CrossRef - Does CD10 Expression Predict Lymph Node Metastasis in Colorectal Cancer?
Irina Bernescu, Ari C. Reichstein, Martin Luchtefeld, James W. Ogilvie
Diseases of the Colon & Rectum.2016; 59(1): 22. CrossRef - Epigenetic suppression of neprilysin regulates breast cancer invasion
H M Stephen, R J Khoury, P R Majmudar, T Blaylock, K Hawkins, M S Salama, M D Scott, B Cosminsky, N K Utreja, J Britt, R E Conway
Oncogenesis.2016; 5(3): e207. CrossRef - Substance P and thiorphan synergically enhance angiogenesis in wound healing
Jihyun Um, Jinyeong Yu, Maria Jose Dubon, Ki-Sook Park
Tissue Engineering and Regenerative Medicine.2016; 13(2): 149. CrossRef - Neutral endopeptidase (NEP) is differentially involved in biological activities and cell signaling of colon cancer cell lines derived from various stages of tumor development
Magdalena Mizerska-Kowalska, Agnieszka Bojarska-Junak, Joanna Jakubowicz-Gil, Martyna Kandefer-Szerszeń
Tumor Biology.2016; 37(10): 13355. CrossRef - Stage-Specific Embryonic Antigen-1 (SSEA-1) Expression in Thyroid Tissues
Jin Xu, Heather Hardin, Ranran Zhang, Kaitlin Sundling, Darya Buehler, Ricardo V. Lloyd
Endocrine Pathology.2016; 27(4): 271. CrossRef - Role of B Cell Development Marker CD10 in Cancer Progression and Prognosis
Deepshikha Mishra, Sunita Singh, Gopeshwar Narayan
Molecular Biology International.2016; 2016: 1. CrossRef - Increase in Both CD14-Positive and CD15-Positive Myeloid-Derived Suppressor Cell Subpopulations in the Blood of Patients With Glioma But Predominance of CD15-Positive Myeloid-Derived Suppressor Cells in Glioma Tissue
Paul R. Gielen, Barbara M. Schulte, Esther D. Kers-Rebel, Kiek Verrijp, Harriëtte M.J.M. Petersen-Baltussen, Mark ter Laan, Pieter Wesseling, Gosse J. Adema
Journal of Neuropathology & Experimental Neurology.2015; 74(5): 390. CrossRef - Immunohistochemical Characterization of Large Intestinal Adenocarcinoma in the Rhesus Macaque (Macaca mulatta)
C. E. Harbison, F. Taheri, H. Knight, A. D. Miller
Veterinary Pathology.2015; 52(4): 732. CrossRef - Tumor-Associated Neutrophils as a New Prognostic Factor in Cancer: A Systematic Review and Meta-Analysis
Meixiao Shen, Pingping Hu, Frede Donskov, Guanghui Wang, Qi Liu, Jiajun Du, William B. Coleman
PLoS ONE.2014; 9(6): e98259. CrossRef
- From Ulcerative Colitis to Metastatic Colorectal Cancer
- Early Colorectal Epithelial Neoplasm in Korea: A Multicenter Survey of Pathologic Diagnosis
- Yun Kyung Kang, So-Young Jin, Mee Soo Chang, Jung Yeon Kim, Gyeong Hoon Kang, Hye Seung Lee, Jin Hee Sohn, Ho Sung Park, Kye Won Kwon, Mi Jin Gu, Young Hee Maeng, Jong Eun Joo, Haeng Ji Kang, Hee Kyung Kim, Kee-Taek Jang, Mi Ja Lee, Hee Kyung Chang, Joon Mee Kim, Hye Seung Han, Won Ae Lee, Yoon Jung Choi, Dong Wook Kang, Sunhoo Park, Jae Hyuk Lee, Mee-Yon Cho
- Korean J Pathol. 2013;47(3):245-251. Published online June 25, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.3.245
- 11,524 View
- 56 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Background The incidence of early colorectal epithelial neoplasm (ECEN) is increasing, and its pathologic diagnosis is important for patient care. We investigated the incidence of ECEN and the current status of its pathologic diagnosis.
Methods We collected datasheets from 25 institutes in Korea for the incidence of colorectal adenoma with high grade dysplasia (HGD) and low grade dysplasia in years 2005, 2007, and 2009; and early colorectal carcinoma in the year 2009. We also surveyed the diagnostic terminology of ECEN currently used by the participating pathologists.
Results The average percentage of diagnoses of adenoma HGD was 7.0%, 5.0%, and 3.4% in years 2005, 2007, and 2009, respectively. The range of incidence rates of adenoma HGD across the participating institutes has gradually narrowed over the years 2005 to 2009. The incidence rate of early colorectal carcinoma in the year 2009 was 21.2%. The participants did not share a single criterion or terminology for the diagnosis of adenoma HGD. The majority accepted the diagnostic terms that distinguished noninvasive, mucosal confined, and submucosal invasive carcinoma.
Conclusions Further research requirements suggested are a diagnostic consensus for the histopathologic diagnosis of ECEN; and standardization of diagnostic terminology critical for determining the disease code.
-
Citations
Citations to this article as recorded by- Diminutive and Small Colorectal Polyps: The Pathologist's Perspective
Yun Kyung Kang
Clinical Endoscopy.2014; 47(5): 404. CrossRef
- Diminutive and Small Colorectal Polyps: The Pathologist's Perspective
- Radiotherapy Response in Microsatellite Instability Related Rectal Cancer
- Joo-Shik Shin, Thein Ga Tut, Tao Yang, C. Soon Lee
- Korean J Pathol. 2013;47(1):1-8. Published online February 25, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.1.1
- 12,102 View
- 99 Download
- 31 Crossref
-
 Abstract
Abstract
 PDF
PDF Preoperative radiotherapy may improve the resectability and subsequent local control of rectal cancers. However, the extent of radiation induced regression in these tumours varies widely between individuals. To date no reliable predictive marker of radiation sensitivity in rectal cancer has been identified. At the cellular level, radiation injury initiates a complex molecular network of DNA damage response (DDR) pathways that leads to cell cycle arrest, attempts at re-constituting the damaged DNA and should this fail, then apoptosis. This review presents the details which suggest the roles of DNA mismatch repair proteins, the lack of which define a distinct subset of colorectal cancers with microsatellite instability (MSI), in the DDR pathways. Hence routine assessment of the MSI status in rectal cancers may potentially serve as a predictor of radiotherapy response, thereby improving patient stratification in the administration of this otherwise toxic treatment.
-
Citations
Citations to this article as recorded by- Neoadjuvant chemoradiotherapy up-regulates PD-L1 in radioresistant colorectal cancer
Sung Uk Bae, Hye Won Lee, Jee Young Park, Incheol Seo, Jae-Min Cho, Jin Young Kim, Ju Yup Lee, Yoo Jin Lee, Seong Kyu Baek, Nam Kyu Kim, Sang Jun Byun, Shin Kim
Clinical and Translational Radiation Oncology.2025; 51: 100906. CrossRef - Incidence and Outcomes of Patients With Mismatch Repair Deficient Rectal Cancer Operated in 2016: A Nationwide Cohort From The Netherlands
Eline G.M. van Geffen, Cornelis R.C. Hogewoning, Sanne-Marije J.A. Hazen, Tania C. Sluckin, Marilyne M. Lange, Petur Snaebjornsson, Regina G.H. Beets-Tan, Corrie A.M. Marijnen, Cornelis Verhoef, Myriam Chalabi, Pieter J. Tanis, Miranda Kusters, Tjeerd S.
Clinical Colorectal Cancer.2025; 24(2): 188. CrossRef - Clinicopathological features and mucin expression of early papillary gastric adenocarcinoma
Jiaqi Chen, Xiujie Cui, Chengjun Zhou, Mulan Jin
Scientific Reports.2025;[Epub] CrossRef - Colorectal cancers associated with mismatch repair deficiency
Mingzhu Sun, Kevin Monahan, Jayne Moquet, Stephen Barnard
Frontiers in Medicine.2025;[Epub] CrossRef - Potential risks associated with the use of ionizing radiation for imaging and treatment of colorectal cancer in Lynch syndrome patients
Mingzhu Sun, Jayne Moquet, Michele Ellender, Simon Bouffler, Christophe Badie, Rachel Baldwin-Cleland, Kevin Monahan, Andrew Latchford, David Lloyd, Susan Clark, Nicola A. Anyamene, Elizabeth Ainsbury, David Burling
Familial Cancer.2023; 22(1): 61. CrossRef - Not All Patients With Locally Advanced Rectal Cancer Benefit From Neoadjuvant Therapy
Carolyn Chang, Jonathan T. Bliggenstorfer, Jessie Liu, Jennifer Shearer, Paul Dreher, Katherine Bingmer, Sharon L. Stein, Emily Steinhagen
The American Surgeon™.2023; 89(11): 4327. CrossRef - Survival outcomes in locally advanced dMMR rectal cancer: surgery plus adjunctive treatment vs. surgery alone
Kemin Ni, Yixiang Zhan, Zhaoce Liu, Zhen Yuan, Shuyuan Wang, Xuan-zhu Zhao, Hangyu Ping, Yaohong Liu, Wanting Wang, Suying Yan, Ran Xin, Qiurong Han, Qinghuai Zhang, Guoxun Li, Xipeng Zhang, Guihua Wang, Zili Zhang, Hong Ma, Chunze Zhang
BMC Cancer.2023;[Epub] CrossRef - Rectal Cancer in Patients with Hereditary Nonpolyposis Colorectal Cancer Compared with Sporadic Cases: Response to Neoadjuvant Chemoradiation and Local Recurrence
Khaled M Madbouly, Sameh Hany Emile, Yasmine Amr Issa
Journal of the American College of Surgeons.2022; 234(5): 793. CrossRef - Molecular Recognition and Quantification of MLH1, MSH2, MSH6, PMS2, and KRAS in Biological Samples
Raluca-Ioana Stefan-van Staden, Ruxandra-Maria Ilie-Mihai, Maria Coros, Stela Pruneanu
ECS Sensors Plus.2022; 1(3): 031606. CrossRef - Magnetic Resonance Imaging Downstaging, Pathological Response, and Microsatellite Instability Status in Patients with Signet-Ring Cell Carcinoma Rectum Undergoing Preoperative Long-course Chemoradiation
B. Rajkrishna, Saikat Das, Dipti Masih, Tharani Putta, Rajat Raghunath, Thomas Samuel Ram
Current Medical Issues.2022; 20(3): 154. CrossRef - Biomarkers and cell-based models to predict the outcome of neoadjuvant therapy for rectal cancer patients
Aylin Alkan, Tobias Hofving, Eva Angenete, Ulf Yrlid
Biomarker Research.2021;[Epub] CrossRef - Microsatellite Instability (MSI) as an Independent Predictor of Pathologic Complete Response (PCR) in Locally Advanced Rectal Cancer
Shaakir Hasan, Paul Renz, Rodney E. Wegner, Gene Finley, Moses Raj, Dulabh Monga, James McCormick, Alexander Kirichenko
Annals of Surgery.2020; 271(4): 716. CrossRef - Delivery of Personalized Care for Locally Advanced Rectal Cancer: Incorporating Pathological, Molecular Genetic, and Immunological Biomarkers Into the Multimodal Paradigm
Éanna J. Ryan, Ben Creavin, Kieran Sheahan
Frontiers in Oncology.2020;[Epub] CrossRef - Association of mismatch repair status with survival and response to neoadjuvant chemo(radio)therapy in rectal cancer
Shu-Biao Ye, Yi-Kan Cheng, Lin Zhang, Yi-Feng Zou, Ping Chen, Yan-Hong Deng, Yan Huang, Jian-Hong Peng, Xiao-Jian Wu, Ping Lan
npj Precision Oncology.2020;[Epub] CrossRef - Implication of expression of MMR proteins and clinicopathological characteristics in gastric cancer
Renu Verma, Puja Sakhuja, Ritu Srivastava, Prakash Chand Sharma
Asia-Pacific Journal of Oncology.2020; : 1. CrossRef - Mismatch Repair–Deficient Rectal Cancer and Resistance to Neoadjuvant Chemotherapy
Andrea Cercek, Gustavo Dos Santos Fernandes, Campbell S. Roxburgh, Karuna Ganesh, Shu Ng, Francisco Sanchez-Vega, Rona Yaeger, Neil H. Segal, Diane L. Reidy-Lagunes, Anna M. Varghese, Arnold Markowitz, Chao Wu, Bryan Szeglin, Charles-Etienne Gabriel Sauvé
Clinical Cancer Research.2020; 26(13): 3271. CrossRef - The Mismatch Repair System (MMR) in Head and Neck Carcinogenesis and Its Role in Modulating the Response to Immunotherapy: A Critical Review
Maria Cilona, Luca Giovanni Locatello, Luca Novelli, Oreste Gallo
Cancers.2020; 12(10): 3006. CrossRef - Genetics of rectal cancer and novel therapies: primer for radiologists
Sebastian Mondaca, Rona Yaeger
Abdominal Radiology.2019; 44(11): 3743. CrossRef - Mismatch repair deficiency as a predictive marker for response to adjuvant radiotherapy in endometrial cancer
Casper Reijnen, Heidi V.N. Küsters-Vandevelde, Clemens F. Prinsen, Leon F.A.G. Massuger, Marc P.M.L. Snijders, Stefan Kommoss, Sara Y. Brucker, Janice S. Kwon, Jessica N. McAlpine, Johanna M.A. Pijnenborg
Gynecologic Oncology.2019; 154(1): 124. CrossRef - Mismatch Repair System Deficiency Is Associated With Response to Neoadjuvant Chemoradiation in Locally Advanced Rectal Cancer
Nicolas Meillan, Dewi Vernerey, Jérémie H. Lefèvre, Gilles Manceau, Magali Svrcek, Jeremy Augustin, Jean-François Fléjou, Olivier Lascols, Jean-Marc Simon, Romain Cohen, Philippe Maingon, Jean-Baptiste Bachet, Florence Huguet
International Journal of Radiation Oncology*Biology*Physics.2019; 105(4): 824. CrossRef - Promoter methylation and expression of DNA repair genes MGMT and ERCC1 in tissue and blood of rectal cancer patients
Sally M. Shalaby, Amal S. El-Shal, Lobna A. Abdelaziz, Eman Abd-Elbary, Mostafa M. Khairy
Gene.2018; 644: 66. CrossRef - RBBP6 increases radioresistance and serves as a therapeutic target for preoperative radiotherapy in colorectal cancer
Chao Xiao, Yupeng Wang, Miao Zheng, Jian Chen, Guohe Song, Zhijie Zhou, Chongzhi Zhou, Xing Sun, Lin Zhong, Erxun Ding, Yi Zhang, Liu Yang, Gang Wu, Shifeng Xu, Hong Zhang, Xiaoliang Wang
Cancer Science.2018; 109(4): 1075. CrossRef - Baseline MAPK signaling activity confers intrinsic radioresistance to KRAS-mutant colorectal carcinoma cells by rapid upregulation of heterogeneous nuclear ribonucleoprotein K (hnRNP K)
Stefan Eder, Annette Arndt, Andreas Lamkowski, Wassiliki Daskalaki, Alexis Rump, Markus Priller, Felicitas Genze, Eva Wardelmann, Matthias Port, Konrad Steinestel
Cancer Letters.2017; 385: 160. CrossRef - The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing
E. Ryan, K. Sheahan, B. Creavin, H.M. Mohan, D.C. Winter
Critical Reviews in Oncology/Hematology.2017; 116: 38. CrossRef - Exploration of Mutation and DNA Methylation of Polo-Like Kinase 1 (PLK1) in Colorectal Cancer
Wayne Ng, Joo-Shik Shin, Bin Wang, Cheok Soon Lee
Open Journal of Pathology.2017; 07(03): 45. CrossRef - Expression of Mismatch Repair Proteins in Early and Advanced Gastric Cancer in Poland
Katarzyna Karpińska-Kaczmarczyk, Magdalena Lewandowska, Małgorzata Ławniczak, Andrzej Białek, Elżbieta Urasińska
Medical Science Monitor.2016; 22: 2886. CrossRef - DNA Mismatch Repair Deficiency in Rectal Cancer: Benchmarking Its Impact on Prognosis, Neoadjuvant Response Prediction, and Clinical Cancer Genetics
Nicole de Rosa, Miguel A. Rodriguez-Bigas, George J. Chang, Jula Veerapong, Ester Borras, Sunil Krishnan, Brian Bednarski, Craig A. Messick, John M. Skibber, Barry W. Feig, Patrick M. Lynch, Eduardo Vilar, Y. Nancy You
Journal of Clinical Oncology.2016; 34(25): 3039. CrossRef -
miRNA-148b regulates radioresistance in non-small lung cancer cells via regulation of MutL homologue 1
Guangsheng Zhai, Gaozhong Li, Bo Xu, Tongfu Jia, Yinping Sun, Jianbo Zheng, Jianbin Li
Bioscience Reports.2016;[Epub] CrossRef - Predictive and prognostic biomarkers for neoadjuvant chemoradiotherapy in locally advanced rectal cancer
S.H. Lim, W. Chua, C. Henderson, W. Ng, J.-S. Shin, L. Chantrill, R. Asghari, C.S. Lee, K.J. Spring, P. de Souza
Critical Reviews in Oncology/Hematology.2015; 96(1): 67. CrossRef - Potential of DNA methylation in rectal cancer as diagnostic and prognostic biomarkers
Ruth Exner, Walter Pulverer, Martina Diem, Lisa Spaller, Laura Woltering, Martin Schreiber, Brigitte Wolf, Markus Sonntagbauer, Fabian Schröder, Judith Stift, Fritz Wrba, Michael Bergmann, Andreas Weinhäusel, Gerda Egger
British Journal of Cancer.2015; 113(7): 1035. CrossRef - Study of Liver Transplant Rejection in Alcoholism-Induced Cirrhosis
J. Tinoco González, C. Bernal Bellido, G. Jimenez Riera, V. Camacho Marente, L.M. Marín Gómez, G. Suárez Artacho, J.M. Álamo Martínez, J. Serrano Díez-Canedo, R.J. Padillo Ruíz, M.A. Gómez Bravo
Transplantation Proceedings.2013; 45(10): 3650. CrossRef
- Neoadjuvant chemoradiotherapy up-regulates PD-L1 in radioresistant colorectal cancer
- Expression of Cortactin and Focal Adhesion Kinase in Colorectal Adenocarcinoma: Correlation with Clinicopathologic Parameters and Their Prognostic Implication
- Yo Na Kim, Ji Eun Choi, Jun Sang Bae, Kyu Yun Jang, Myoung Ja Chung, Woo Sung Moon, Myoung Jae Kang, Dong Geun Lee, Ho Sung Park
- Korean J Pathol. 2012;46(5):454-462. Published online October 25, 2012
- DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.5.454
- 9,371 View
- 47 Download
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Background Cortactin and focal adhesion kinase (FAK) are two important components among actin cross-linking proteins that play a central role in cell migration.
Methods The aims of this study were to evaluate the expression of cortactin and FAK in normal colorectal mucosa and colorectal adenocarcinoma (CRC) using tissue microarray of 2 mm cores to correlate their expression with other clinicopathological factors and, investigate their prognostic significance.
Results Twenty (9%) and 24 cases (11%) of normal colorectal mucosa were immunoreactive for cortactin and FAK. In addition, 184 (84%) and 133 cases (61%) of CRCs were immunoreactive for cortactin and FAK, respectively. Cortactin expression was associated with histologic differentiation and FAK expression. Cortactin, but not FAK expression was also correlated with poor overall and relapse-free survival and served well as an independent prognostic factor for poor survival.
Conclusions Cortactin expression, in association with FAK expression, may plays an important role in tumor progression. Furthermore, it may also be a satisfactory biomarker to predict tumor progression and survival in CRC patients.
-
Citations
Citations to this article as recorded by- Identification of a Subset of Stage I Colorectal Cancer Patients With High Recurrence Risk
Lik Hang Lee, Lindy Davis, Lourdes Ylagan, Angela R Omilian, Kristopher Attwood, Canan Firat, Jinru Shia, Philip B Paty, William G Cance
JNCI: Journal of the National Cancer Institute.2022; 114(5): 732. CrossRef - Profiling the expression of pro-metastatic genes in association with the clinicopathological features of primary breast cancer
Seyed-Mohammad Mazloomi, Mitra Foroutan-Ghaznavi, Vahid Montazeri, Gholamreza Tavoosidana, Ashraf Fakhrjou, Hojjatollah Nozad-Charoudeh, Saeed Pirouzpanah
Cancer Cell International.2021;[Epub] CrossRef - PZR promotes metastasis of colorectal cancer through increasing FAK and Src phosphorylation
Dan Tan, Wenpeng Zhang, Yu Tao, Yesseyeva Galiya, Mingliang Wang
Acta Biochimica et Biophysica Sinica.2019; 51(4): 356. CrossRef - Overexpression and Tyr421-phosphorylation of cortactin is induced by three-dimensional spheroid culturing and contributes to migration and invasion of pancreatic ductal adenocarcinoma (PDAC) cells
Katharina Stock, Rebekka Borrink, Jan-Henrik Mikesch, Anna Hansmeier, Jan Rehkämper, Marcel Trautmann, Eva Wardelmann, Wolfgang Hartmann, Jan Sperveslage, Konrad Steinestel
Cancer Cell International.2019;[Epub] CrossRef - Cortactin promotes colorectal cancer cell proliferation by activating the EGFR-MAPK pathway
Xiaojian Zhang, Kun Liu, Tao Zhang, Zhenlei Wang, Xuan Qin, Xiaoqian Jing, Haoxuan Wu, Xiaopin Ji, Yonggang He, Ren Zhao
Oncotarget.2017; 8(1): 1541. CrossRef - Prognostic Value of Focal Adhesion Kinase (FAK) in Human Solid Carcinomas: A Meta-Analysis
Xiao-Qing Zeng, Na Li, Li-Li Ma, Yu-Jen Tseng, Nai-Qing Zhao, Shi-Yao Chen, Han-Chung Wu
PLOS ONE.2016; 11(9): e0162666. CrossRef - Regulators of Actin Dynamics in Gastrointestinal Tract Tumors
Konrad Steinestel, Eva Wardelmann, Wolfgang Hartmann, Inga Grünewald
Gastroenterology Research and Practice.2015; 2015: 1. CrossRef
- Identification of a Subset of Stage I Colorectal Cancer Patients With High Recurrence Risk
- Tumor Budding and Recurrence in Submucosal Invasive Colorectal Cancers of Favorable Histology: Case Reports of Two Early Colorectal Cancers with Advanced Recurrences
- Heae Surng Park, Hee Jin Chang, Ji Won Park, Byung Chang Kim, Dae Kyung Sohn, Chang Won Hong, Ji-Yeon Baek, Sun Young Kim, Hyo Seong Choi, Jae Hwan Oh
- Korean J Pathol. 2012;46(3):272-277. Published online June 22, 2012
- DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.3.272
- 9,897 View
- 68 Download
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Complete resection of submucosal invasive colorectal cancer (SICC) showing favorable histology is regarded as curative. We report on two cases of SICC showing recurrence within 5 years despite complete resection. The first patient was a 68-year-old woman with well differentiated rectal adenocarcinoma invading the superficial submucosa, which recurred after 4.7 years. The second patient was a 53-year-old man with pT1N0 moderately differentiated colonic adenocarcinoma. He developed widespread tumor recurrence after 3.9 years. Retrospective pathologic review of the original tumors showed multiple foci of tumor budding at the invasive front. Immunohistochemical staining for D2-40 of deeper levels of the paraffin blocks showed rare foci of small lymphatic invasion. Tumor budding at the invasive front may be an important indicator for SICC aggressiveness or may reflect early lymphatic invasion. More aggressive pathologic examination and follow-up is required for patients with SICC showing tumor budding, even in the absence of unfavorable histologic findings.
-
Citations
Citations to this article as recorded by- Estudio de factores histológicos predictivos de metástasis ganglionar locorregional en adenocarcinoma colorrectal mínimamente invasivo pT1
Isidro Machado, Miriam Valera-Alberni, Fernando Martínez de Juan, José A. López-Guerrero, Alfonso García Fadrique, Julia Cruz, Carmen Martínez Lapiedra, Fernanda Maia de Alcantara, Ricardo Yaya, Jorge Campos, Carlos Fernández-Martos, Rafael Estevan
Gastroenterología y Hepatología.2016; 39(1): 1. CrossRef - Histological factors predicting loco-regional lymph node metastasis in early invasive colorectal adenocarcinoma pT1
Isidro Machado, Miriam Valera-Alberni, Fernando Martínez de Juan, José A. López-Guerrero, Alfonso García Fadrique, Julia Cruz, Carmen Martínez Lapiedra, Fernanda Maia de Alcantara, Ricardo Yaya, Jorge Campos, Carlos Fernández-Martos, Rafael Estevan
Gastroenterología y Hepatología (English Edition).2016; 39(1): 1. CrossRef - Tumor budding in the clinical management of colon and rectal cancer
Viktor H Koelzer, Inti Zlobec, Alessandro Lugli
Colorectal Cancer.2014; 3(4): 387. CrossRef
- Estudio de factores histológicos predictivos de metástasis ganglionar locorregional en adenocarcinoma colorrectal mínimamente invasivo pT1
- Inhibitors of Apoptosis Proteins Expression and Their Prognostic Significance in Colorectal Carcinoma.
- Kyung Hwa Lee, Soong Lee, Hyeon Min Lee, Seung Chul Back, Sung Bum Cho, Jae Hyuk Lee
- Korean J Pathol. 2011;45(4):397-405.
- DOI: https://doi.org/10.4132/KoreanJPathol.2011.45.4.397
- 4,541 View
- 22 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The expression of the inhibitor of apoptosis proteins (IAPs) family has not been fully investigated in colorectal carcinomas. This study investigated IAP expression in colorectal carcinomas and assessed their prognostic significance.
METHODS
Livin, XIAP, and SMAC/DIABLO expression was assessed by immunohistochemistry in 159 colorectal carcinomas. Correlations between protein expression and clinicopathological features were evaluated. The survival data analysis was estimated according to the Kaplan-Meier method.
RESULTS
Increased expression of IAPs in cancer tissues compared to surrounding nonneoplastic counterparts was observed in 67 cases (42.1%) for Livin, 50 cases (31.4%) for XIAP, and 68 cases (42.8%) for SMAC. A significant correlation was found between Livin expression and tumor differentiation, and SMAC expression and tumor location. The recurrence-free and overall survival of patients with low Livin expression were inferior to those of patients with high Livin expression (p=0.054 and 0.095, respectively). High XIAP expression was significantly associated with shorter progression-free survival (p= 0.041).
CONCLUSIONS
Our study demonstrated that altered expression of IAP family members, including Livin, XIAP, and SMAC, is frequent in colorectal carcinoma. This result suggests that high Livin expression and low XIAP expression may be a favorable prognostic implication related to patient survival. -
Citations
Citations to this article as recorded by- A Review of the Current Impact of Inhibitors of Apoptosis Proteins and Their Repression in Cancer
Pierina Cetraro, Julio Plaza-Diaz, Alex MacKenzie, Francisco Abadía-Molina
Cancers.2022; 14(7): 1671. CrossRef
- A Review of the Current Impact of Inhibitors of Apoptosis Proteins and Their Repression in Cancer
- Rapid and Sensitive Detection of KRAS Mutation by Peptide Nucleic Acid-based Real-time PCR Clamping: A Comparison with Direct Sequencing between Fresh Tissue and Formalin-fixed and Paraffin Embedded Tissue of Colorectal Cancer.
- Dongjun Jeong, Yujun Jeong, Jonghyun Lee, Moo Jun Baek, Yongbae Kim, Ji Hye Lee, Hyun Deuk Cho, Mee Hye Oh, Chang Jin Kim
- Korean J Pathol. 2011;45(2):151-159.
- DOI: https://doi.org/10.4132/KoreanJPathol.2011.45.2.151
- 6,027 View
- 84 Download
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Rapid and sensitive detection of KRAS mutation is needed to maximize the benefits for patients who are being treated with monoclonal antibodies to target the epidermal growth factor receptor in colorectal cancer. The aim of this study is to evaluate the efficacy of the peptide nucleic acid clamp real-time PCR (PCqPCR) as compared to that of direct sequencing (DS) between using fresh colorectal cancer tissue and the matched formalin-fixed and paraffin-embedded (FFPE) colorectal cancer tissue.
METHODS
The efficacy of PCqPCR was evaluated and compared with that of DS using fresh tissue and matched FFPE tissue from 30 cases of colorectal cancer.
RESULTS
PCqPCR is more sensitive than DS for detecting KRAS mutation. PCqPCR detected 1% of mutants in 1 ng DNA. PCqPCR detected mutation in 1% of mutant cells, while DS barely detected, by manual reading, that in 20-50% of mutant cells. In the clinical samples, PCqPCR detected KRAS mutation in 60.0% while DS detected KRAS mutation in 53.3% of the colorectal cancers. The two methods showed a 100% concordance rate for detecting KRAS mutation between the fresh tissue and FFPE tissue.
CONCLUSIONS
The PCqPCR method is efficiently applicable for the detection of KRAS mutation in a clinical setting. -
Citations
Citations to this article as recorded by- NTRK oncogenic fusions are exclusively associated with the serrated neoplasia pathway in the colorectum and begin to occur in sessile serrated lesions
Jung Ho Kim, Jeong Hoon Hong, Yoon‐La Choi, Ji Ae Lee, Mi‐kyoung Seo, Mi‐Sook Lee, Sung Bin An, Min Jung Sung, Nam‐Yun Cho, Sung‐Su Kim, Young Kee Shin, Sangwoo Kim, Gyeong Hoon Kang
The Journal of Pathology.2021; 255(4): 399. CrossRef - Discrimination of the V600E Mutation in BRAF by Rolling Circle Amplification and Förster Resonance Energy Transfer
Mariia Dekaliuk, Xue Qiu, Frédéric Troalen, Pierre Busson, Niko Hildebrandt
ACS Sensors.2019; 4(10): 2786. CrossRef - Sensitive Genotyping of Somatic Mutations in the EGFR, KRAS, PIK3CA, BRAF Genes from NSCLC Patients Using Hydrogel Biochips
Marina Emelyanova, Ksenia Arkhipova, Natalia Mazurenko, Alexander Chudinov, Irina Demidova, Irina Zborovskaya, Lyudmila Lyubchenko, Alexander Zasedatelev, Tatiana Nasedkina
Applied Immunohistochemistry & Molecular Morphology.2015; 23(4): 255. CrossRef - Low Frequency of KRAS Mutation in Pancreatic Ductal Adenocarcinomas in Korean Patients and Its Prognostic Value
Mi Jung Kwon, Jang Yong Jeon, Hye-Rim Park, Eun Sook Nam, Seong Jin Cho, Hyung Sik Shin, Ji Hyun Kwon, Joo Seop Kim, Boram Han, Dong Hoon Kim, Yoon-La Choi
Pancreas.2015; 44(3): 484. CrossRef - Comparison of PNA clamping and direct sequencing for detecting KRAS mutations in matched tumour tissue, cell block, pleural effusion and serum from patients with malignant pleural effusion
Ji Young Kang, Chan Kwon Park, Chang Dong Yeo, Hea Yeon Lee, Chin Kook Rhee, Seung Joon Kim, Seok Chan Kim, Young Kyoon Kim, Mi Sun Park, Hyeon Woo Yim
Respirology.2015; 20(1): 138. CrossRef - BRAF V600E Mutation Analysis in Papillary Thyroid Carcinomas by Peptide Nucleic Acid Clamp Real-time PCR
Dongjun Jeong, Yujun Jeong, Ji Hye Park, Sun Wook Han, Sung Yong Kim, Yeo Joo Kim, Sang Jin Kim, Young Hwangbo, Soyoung Park, Hyun Deuk Cho, Mee Hye Oh, Seung Ha Yang, Chang Jin Kim
Annals of Surgical Oncology.2013; 20(3): 759. CrossRef - Detection and comparison of EGFR mutations in matched tumor tissues, cell blocks, pleural effusions, and sera from patients with NSCLC with malignant pleural effusion, by PNA clamping and direct sequencing
Chang Dong Yeo, Jin Woo Kim, Kwan Hyoung Kim, Jick Hwan Ha, Chin Kook Rhee, Seung Joon Kim, Young Kyoon Kim, Chan Kwon Park, Sang Haak Lee, Mi Sun Park, Hyeon Woo Yim
Lung Cancer.2013; 81(2): 207. CrossRef - Detection ofBRAFV600EMutations in Papillary Thyroid Carcinomas by Peptide Nucleic Acid Clamp Real-Time PCR: A Comparison with Direct Sequencing
Dongjun Jeong, Yujun Jeong, Sungche Lee, Hyeran Lee, Wanju Lee, Hyungjoo Kim, Doosan Park, Soyoung Park, Wenxia Mu, Hyun-Deuk Cho, Mee-Hye Oh, Sung Soo Lee, Seung-Ha Yang, Chang-Jin Kim
Korean Journal of Pathology.2012; 46(1): 61. CrossRef
- NTRK oncogenic fusions are exclusively associated with the serrated neoplasia pathway in the colorectum and begin to occur in sessile serrated lesions
- Validation of Gene Expression Changes of Osteopontin and MMP-1 in Primary and Metastatic Colorectal Carcinomas.
- Junjeong Choi, Sangkyum Kim, Jeon Han Park, Nam Kyu Kim, Hoguen Kim
- Korean J Pathol. 2010;44(3):225-233.
- DOI: https://doi.org/10.4132/KoreanJPathol.2010.44.3.225
- 4,608 View
- 28 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Metastasis is one of the most important characteristics of cancer in terms of its impact on patient survival. Unfortunately, identification of altered genes during tumor metastasis is limited.
METHODS
Using high-throughput microarrays containing 19K spotted human oligonucleotides, gene expression of primary and matched metastatic colon cancer were compared in previous study. Although DNA microarray analysis did not demonstrate complete classification of primary and metastatic carcinoma, 80 differentially expressed genes were identified. Among these, expression of osteopontin, matrix metalloproteinase-1 (MMP-1) and serpin A1 was assessed using immunohistochemistry in a validation set containing 43 pairs from tissue microarrays.
RESULTS
The expression of osteopontin was significantly higher in metastatic carcinoma than in primary carcinoma, as indicated by mRNA expression. The expression of MMP-1 was significantly lower in metastatic carcinoma. Expression of serpin A1 was not correlated with the microarray results.
CONCLUSIONS
Osteopontin and MMP-1 expression successfully classified primary and metastatic colorectal carcinomas and further studies on their clinical application is encouraged.
- Aberrant Promoter Methylation of the Vimentin Gene in Colorectal Cancer Associated with the Adenoma-Carcinoma Sequence.
- Mi Hee Cho, Yu Mi Lee, Jin Sook Kim, Hyun Soo Kim, Kyung Hwa Lee, Sang Woo Juhng, Jae Hyuk Lee
- Korean J Pathol. 2010;44(2):179-186.
- DOI: https://doi.org/10.4132/KoreanJPathol.2010.44.2.179
- 4,660 View
- 37 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
DNA hypermethylation is a common epigenetic finding in human cancers and is closely associated with transcriptional silencing. In the present study, we investigated the proportion of colorectal neoplasms that showed the adenoma-carcinoma progression and vimentin gene methylation.
METHODS
Methylation status of the vimentin gene was examined in nontumoral mucosa, adenomas, and adenocarcinomas from 45 colorectal cancer patients who had adenoma and adenocarcinoma together. Methylation status was determined by bisulfite modification and the methylation-specific polymerase chain reaction. The expression of the vimentin gene product was also examined by immunohistochemistry.
RESULTS
Promoter methylation of vimentin was detected in 80% (36 out of 45 cases) of adenocarcinomas, 82.2% (37 of 45) of adenomas, and 28.9% (13 of 45) of normal epithelia, and the difference between neoplastic and normal specimens was statistically significant (p < 0.001). However, no significant correlations were observed between methylation frequency and clinicopathologic variables. Immunohistochemically, vimentin expression was not observed in either normal epithelial cells or tumor cells. Protein expression and vimentin promoter methylation were not associated.
CONCLUSIONS
The frequency of aberrant methylation of the vimentin gene was high in colonic adenomas and adenocarcinomas. This result suggests that the methylation status of vimentin may be clinically beneficial in screening for colorectal cancer patients and may be helpful in clarifying colorectal cancer biology. -
Citations
Citations to this article as recorded by- Epigenetic Modifications as Biomarkers of Tumor Development, Therapy Response, and Recurrence across the Cancer Care Continuum
Margaret Thomas, Paola Marcato
Cancers.2018; 10(4): 101. CrossRef - Aberrant promoter methylation of beta‐1,4 galactosyltransferase 1 as potential cancer‐specific biomarker of colorectal tumors
Maria Luana Poeta, Emanuela Massi, Paola Parrella, Pasquale Pellegrini, Mariangela De Robertis, Massimiliano Copetti, Carla Rabitti, Giuseppe Perrone, Andrea Onetti Muda, Francesca Molinari, Elena Zanellato, Stefano Crippa, Damiano Caputo, Marco Caricato,
Genes, Chromosomes and Cancer.2012; 51(12): 1133. CrossRef
- Epigenetic Modifications as Biomarkers of Tumor Development, Therapy Response, and Recurrence across the Cancer Care Continuum
- Clinicopathologic Significances of EGFR Expression at Invasive Front of Colorectal Cancer.
- Yeo Ju Kang, Chan Kwon Jung, Yeong Jin Choi, Kyo Young Lee, Hyung Jin Kim, Won Kyung Kang, Seong Taek Oh
- Korean J Pathol. 2010;44(1):16-21.
- DOI: https://doi.org/10.4132/KoreanJPathol.2010.44.1.16
- 4,222 View
- 41 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Epidermal growth factor receptor (EGFR) is frequently expressed in the invasive front of colorectal cancer (CRC), but its clinicopathologic significance remains unclear. We investigated the clinical value of the EGFR expression at the invasive front of CRC.
METHODS
We performed an immunohistochemical analysis in order to examine the expression and distribution of EGFR in 214 cases of CRC. The EGFR status was considered positive when > or =1% of the tumor cells had membranous staining.
RESULTS
Overall, an EGFR expression was observed in 144 (67%) cases and it had no significant relationship with the clinicopathologic parameters. However, an EGFR expression at the invasive front was correlated with lymphatic invasion, lymph node metastasis and a high level of serum carcinoembryonic antigen (p = 0.028, p = 0.043, and p = 0.045, respectively). For the budding-positive CRCs liver metastases were found in the cases with an EGFR expression at the budding, but no liver metastasis occurred in the EGFR negative cases at the budding (p = 0.030).
CONCLUSIONS
An EGFR expression at the invasive front has clinicopathologic significances in patients with CRC. An EGFR expression at tumor cell budding is a pathologic marker that suggests the high potential for liver metastasis in CRC.
- Clinicopathologic Characteristics of Left-Sided Colon Cancers with High Microsatellite Instability.
- Sang Kyum Kim, Junjeong Choi, Hyun Ki Kim, Young Nyun Park, Si Young Song, Hoguen Kim
- Korean J Pathol. 2009;43(5):428-434.
- DOI: https://doi.org/10.4132/KoreanJPathol.2009.43.5.428
- 4,346 View
- 42 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
High microsatellite instability (MSI-H) colorectal carcinomas (CRCs) with numerous mutations in the microsatellite sequence are characterized by a right-sided preponderance, frequent peritumoral and intratumoral lymphocytic infiltration, and frequent mucin production. However, no study has correlated anatomic site and type of genetic changes with clinicopathologic changes.
METHODS
We analyzed the histopathologic features of 135 MSI-H CRCs and compared them to 140 microsatellite stable (MSS) CRCs. Histopathologic changes in MSI-H were further analyzed according to anatomic sites and genetic changes.
RESULTS
MSI-H CRCs showed previously reported clinicopathologic findings; a right-sided preponderance, an increased number of mucinous carcinomas, and peritumoral lymphoid reactions (p<0.001 for each variable). Increased serum CEA levels showed an MSS CRC preponderance (p=0.013). We further analyzed the histologic differences between right- and left-sided MSI-H tumors. We found that MSI-H CRCs on both sides had similar clinicopathologic findings, except for higher tumor stage (p=0.048) and less frequent abnormal CEA levels in left-sided MSI-H tumors (p=0.027). We found that not all clinicopathologic features were different between hereditary nonpolyposis colorectal cancers (HNPCCs) and sporadic MSI-H CRCs.
CONCLUSIONS
These findings indicate that MSI-H CRCs of the left colon have similar clinicopathologic characteristics as right-sided MSI-H CRCs. We did not find any significant clinicopathological difference between HNPCCs and sporadic MSI-H CRCs. -
Citations
Citations to this article as recorded by- Fibroblast Growth Factor Receptor 1 Gene Copy Number and mRNA Expression in Primary Colorectal Cancer and Its Clinicopathologic Correlation
Yoonjin Kwak, Soo Kyung Nam, An Na Seo, Duck-Woo Kim, Sung-Bum Kang, Woo Ho Kim, Hye Seung Lee
Pathobiology.2015; 82(2): 76. CrossRef
- Fibroblast Growth Factor Receptor 1 Gene Copy Number and mRNA Expression in Primary Colorectal Cancer and Its Clinicopathologic Correlation
- Microsatellite Instability in Colorectal Carcinomas.
- Hee Jeong Cha, Dong Kyun Woo, Sun Hee Kim, Yong ll Kim, Jae Gahb Park, Woo Ho Kim
- Korean J Pathol. 2001;35(2):111-114.
- 2,180 View
- 13 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Microsatellite instability (MSI), which is caused by a deficient mismatch repair system, is seen in most of the hereditary non-polyposis colon cancers and a portion of sporadic colorectal cancers.
METHODS
Two hundreds forty-six consecutive sporadic colorectal cancer patients were analyzed for MSI using an ABI 377 automatic sequencer and fluorescent dye-labelled primers (BAT-25 and BAT-26).
RESULTS
The overall incidence of MSI in studied cases was 9.8% (24/246). This incidence is lower than most of the reported incidences in western countries. The incidence of MSI tumors in the proximal colon was 29.6%, while that of the distal colon was only 4.2% (p<0.001). MSI in sporadic colorectal cancers was more prevalent in poorly differentiated adenocarcinoma. In contrast to western countries, mucinous carcinoma did not show higher incidence of MSI.
CONCLUSION
The results suggest that MSI frequently occurs in cancers of the proximal colon and in tumors with poorly differentiated histology.
- The Expression of Transforming Growth Factor-1 and Its Signaling Receptors in Human Colorectal Carcinoma.
- Gyeong Seon Kim, Joo Heon Kim, Woo Sung Moon, Myoung Ja Chung, Dong Geun Lee, Myoung Jae Kang
- Korean J Pathol. 2001;35(2):115-122.
- 2,061 View
- 14 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Resistance to the potent growth inhibitory effects of TGF- (transforming growth factor-) is a characteristic of many malignancies. TGF- insensitivity has been attributed to alterations in the number and function of the TGF- receptors as well as disturbances of downstream signal transduction. The aim of this study was to examine the expression of TGF-1 and its receptors in human colorectal cancer tissue and determine its relationship with cancer growth and with prognostic factors.
METHODS
Immunohistochemical staining of TGF-1, TGF-RI, and TGF-RII was performed on 20 human colorectal adenomas, 30 carcinomas and 10 normal mucosas as a control.
RESULTS
The staining indices of TGF-1, TGF-RI, and TGF-RII increased in adenomas and carcinomas compared with normal mucosas and adenomas, respectively. In adenomas the staining index of TGF-1 significantly increased with the severity of atypism. The staining index of TGF-RII increased in the carcinomas in the right colon and rectum, compared with those in the left colon.
CONCLUSION
The enhanced expression of TGF-1, TGF-RI and II in the colorectal carcinoma suggests an important role of colorectal carcinogenesis and tumor progression.
- Epidermal Growth Factor Receptor Overexpression and the Tumor Response to Preoperative Radiochemotherapy for Patients with Advanced Rectal Cancer.
- Jinyoung Yoo, Ju Won Chyung, Ji Han Jung, Hyun Joo Choi, Seok Jin Kang, Kyo Young Lee
- Korean J Pathol. 2007;41(5):316-323.
- 2,108 View
- 25 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
An association between the epidermal growth factor receptor (EGFR) signaling pathway and the response of cancer cells to ionizing radiation has been previously described. Preoperative radiochemotherapy (PRCT) has been administered for treating locally advanced rectal cancer to improve the outcomes, and to preserve the sphincter from lowlying tumor. However, the responses of tumors to PRCT are variable and there are currently no reliable markers that predict the therapeutic benefits. We studied the association between EGFR overexpression and the tumor response to PRCT in rectal cancer.
METHODS
The EGFR protein expression, as determined by immunohistochemistry, was analyzed in the pretreatment biopsy specimens from 120 patients with advanced rectal cancer. The tumor response was graded in the surgically resected specimens by using a three-scale grading system: no response (NR), partial remission (PR) and complete remission (CR).
RESULTS
NR was identified in 70 cases (58.3%). Fifty patients (41.7%) responded to PRCT; 27 (22.5%) achieved a PR and 23 (19.2%) achieved a CR. EGFR overexpression was detected in 78 (65%) cases. Seventy-eight percent (39/50) of the tumors with a CR/PR revealed EGFR reactivity, whereas 55.7% (39/70) of the tumors with NR showed an EGFR expression (p=0.048).
CONCLUSIONS
The EGFR protein expression might be a valuable marker for identifying those patients who are most likely to benefit from PRCT.
- K-ras Gene Mutations and Expression of K-ras, p16, Cyclin D1 and p53 in Synchronous Lesions of The Colon Adenoma-Carcinoma Sequences.
- Hwa Eun Oh, Seong Jin Cho, Nam Hee Won, Dale Lee, Insun Kim, Bom Woo Yeom
- Korean J Pathol. 2001;35(4):291-298.
- 2,200 View
- 26 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The colorectal adenoma-carcinoma sequence represents a well-known para-digm for the sequential development of cancer driven by the accumulation of genomic defects. Although the colorectal adenoma-carcinoma sequence has been well investigated, the studies about tumors of different dignity co-existent in the same patient are rare. K-ras mutation is an early genetic change in colon cancer. The genes involved in the cell cycle such as cyclin D1, p16, and p53 are important in the tumorigenesis of the colon. The aims of this study were to determine K-ras gene mutation and expression of K-ras, p16, cyclin D1 and p53 in synchronous lesions of the colon adenoma-carcinoma sequences and their possible relationship with K-ras mutation.
METHODS
The materials included 45 colonic adenocarcinomas which were accompanied by adenoma (22 low grade and 26 high grade). By using polymerase chain reaction-single strand conformational polymorphism (PCR-SSCP), we detected K-ras mutation of codon 12. An aberrant K-ras, p16, cyclin D1 and p53 expressions were stained using an immunohistochemical method. RESULTS: K-ras mutation was 52.4% (11/21) of high grade adenomas. K-ras expression was 65.4% (17/26) of high grade adenomas. p16 and cyclin D1 expressions were 50% (11/22) and 90.9% (20/22) of low grade adenomas, respectively. p53 expression was 75.6% (34/45) of adenocarcinomas. There were statistical correlations among K-ras, p16 and cyclin D1.
CONCLUSIONS
These results indicate that the ras gene mutation is an early event and the overexpressions of p16, cyclin D1 and p53 are associated with K-ras mutation and expression in adenoma-carcinoma sequences.
- Adenocarcinoma of the Sigmoid Colon with Prominent Rhabdoid Features: A Case Report.
- Hoon Kyu Oh, Chang Ho Cho, Yoon Seup Kum
- Korean J Pathol. 2008;42(1):63-65.
- 2,094 View
- 29 Download
-
 Abstract
Abstract
 PDF
PDF - Colorectal adenocarcinoma with rhabdoid features is extremely rare and only two cases have been previously reported. We report here on a case of colorectal adenocarcinoma with prominent rhabdoid features in a 69-year-old female. The specimen was an ulcerative mass from the sigmoid colon, and it measured 3.5x3 cm. Microscopic examination of the tumor showed mostly rhabdoid cells that had eccentrically located large nuclei and foci of glandular formation. A transitional area from the poorly differentiated adenocarcinoma to the rhabdoid tumor was also noted. Immunohistochemical studies showed strong reactivity of the glandular forming cells for pan-cytokeratin, and the cells were occasionally positive for vimentin. The cells with rhabdoid features were diffusely positive for vimentin and focally positive for pan-cytokeratin. These results suggested that the cells with rhabdoid features originated from dedifferentiated primary adenocarcinoma. Since colorectal adenocarcinoma with rhabdoid features is highly aggressive and unresponsive to conventional therapy, making the preoperative diagnosis is important to facilitate the treatment.
- An Analysis of p53, bcl-2 and Ki-67 Expressions, and Apoptosis in Rectal Cancer: Their Correlation with the Tumor Response after Preoperative Radiochemotherapy.
- Jinyoung Yoo, Su Zy Kim, Hyeon Min Cho, Sung Whan Kim, Hyung Min Chin, Jung Yong Lee, Jun Ki Kim, Seok Jin Kang, Chung Soo Chun, Chang Suk Kang
- Korean J Pathol. 2005;39(4):222-228.
- 2,112 View
- 22 Download
-
 Abstract
Abstract
 PDF
PDF - Background
: Preoperative radiochemotherapy (RCT) has been administered for locally advanced rectal cancer to increase the therapeutic benefits, and to preserve the sphincter in low-lying tumors, however, tumor responses after RCT are variable. Methods : Apoptotic index (AI), and expressions of Ki-67, p53 and bcl-2 were analyzed in pretreatment biopsies from 69 patients with rectal cancer by immunohistochemistry. Tumor response was graded in surgically resected specimens by using a three-scale grading system: no response (NR), partial remission (PR) and complete remission (CR). Results : CR was identified in 19 cases (28%), PR in 24 cases (35%), and NR in 26 cases (38%) of 69 cases. p53 protein was expressed in 49 cases (71%), whereas bcl-2 was in 42 cases (61%). The pretreatment Ki-67 labeling index was 65.4+/-3.4%. The tumor response was not associated with any of these markers. Tumors with CR/PR showed a higher AI (0.84+/-.84%/0.66+/-.52%) than that of tumors with NR (0.58+/-0.54%). There was a significant correlation between tumor response and the histologic differentiation (p=0.008) or recurrence (p=0.039). Conclusions : The AI revealed a tendency to increase in tumors with CR/PR, while expressions of p53 and bcl-2, and Ki-67 labeling index had little direct association with tumor response.
- Expression of Cytokeratin 7 and 20 According to The Anatomical Location of Colon Cancer and The Differential Diagnosis with Cholangiocarcinoma.
- Yoon Kyung Jeon, Sun Lee, Byoung Kwon Kim, Woo Ho Kim, Gyeong Hoon Kang
- Korean J Pathol. 2002;36(3):146-153.
- 3,137 View
- 59 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Colonic adenocarcinoma usually shows CK7 negativity and CK20 positivity, which helps to differentiate it from cholangiocarcinoma usually showing a reverse immunohistochemical profile. We immunohistochemically investigated the pattern of CK7 and 20 expressions according to the anatomical location of colon cancer to refine the usefulness of CK expression in differential diagnosis.
METHODS
Immunohistochemical staining was done on 90 cases of surgically resected colon cancers and 84 cases of cholangiocarcinomas.
RESULTS
When the cases of colon cancer were divided into CATD (from the cecum to the descending colon) (32), sigmoid (26), and rectum (32), the positivity of CK7 was 41%, 15% and 28%, respectively, and the negativity of CK20 was 25%, 0 and 9% (p=0.013), respectively. In sigmoid colon cancers, 22 cases (85%) exhibited CK7-/CK20+ immunophenotype. However, the percentage decreased to 63% in the rectum and 47% in CATD. The CK7+/CK20- immunophenotype was found only in cancers in the cecum and ascending colon. The expression of CK7 was related to histologic differentiation (p=0.017).
CONCLUSIONS
The aberrant expressions of CKs were frequent in cancers of the rectum and ascending colon which are located in the transition site from the anus and small bowel, respectively. If adenocarcinoma in the liver were CK7+/CK20+ or CK7-/CK20-, the possibility of metastatic adenocarcinoma from CATD and rectum should be considered.
- Expression of p21, p53 and Ki-67, and Apoptosis in Colorectal Adenocarcinoma.
- Young Euy Park, Kyung Chan Choi, Jin Hee Sohn, Young Hee Choi, Hyung Sik Shin
- Korean J Pathol. 2002;36(5):296-304.
- 2,285 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The purpose of this study is to assess the roles of p21 protein, p53 protein, and Ki-67 expressions and apoptosis in colorectal tumorigenesis.
METHODS
Fifty-seven colorectal cancers and 15 villotubular adenomas were investigated by immunohistochemical staining for p21 protein, p53 protein, Ki-67, and in situ labeling of apoptotic cells. Clinicopathologic values (tumor size, histologic grade, Dukes stage, and lymph node metastasis) were compared with the incidence of expressions of p21 protein and p53 protein, index of Ki-67 expression, and apoptosis.
RESULTS
The incidence of p21 protein expression was decreased with lymph node metastasis (p<0.005), and that of p53 expression was increased with lymph node metastasis (p<0.005). There were no statistically significant correlations among the p21 protein or p53 protein expressions, tumor size, histologic grade and stage. The correlation between the Ki-67 labeling index and the clinicopathologic values was not statistically significant. The labeling index of apoptosis was increased with the Astler-Coller stage (p<0.05). Statistical analysis revealed a significant inverse correlation between the p21 protein and p53 protein expressions (p<0.05).
CONCLUSIONS
It is suggested that p21 protein, p53 protein and the apoptotic labeling index are useful variables for the prognostic assessment of colorectal adenocarcinoma. Down-regulation of p21 protein expression may be associated with poor prognosis. Also, the expressions of p21 protein and p53 protein may play an important role in the tumorigenesis and progression of the colorectal adenoma-carcinoma sequence.
- Lipoma of Rectum: A Case Report.
- Sung Chul Lim, Hye Keun Oh, Young Don Min
- Korean J Pathol. 2002;36(5):341-343.
- 2,536 View
- 41 Download
-
 Abstract
Abstract
 PDF
PDF - Gastrointestinal lipomas are rare and are most common in the right colon. They are in opposite distribution of predilection site in comparison to adenocarcinomas and adenomatous polyps. The peak incidence for lipoma of the large bowel is in the sixth decade when there is a high incidence of colorectal carcinoma. Because of their location and the age of the patients at presentation, large bowel lipomas are usually treated on the basis of a presumptive malignant diagnosis. A 79-year-old male is presented with a 1-year history of rectal bleeding. Colonoscopy demonstrated a pedunculated mass nearly obstructing the rectum. Anterior resection was performed. The mass consisted of submucosal lobulated mature fatty tissue with ulcerated mucosa. The authors describe a case of a submucosal lipoma of the rectum with review of literatures.

 E-submission
E-submission

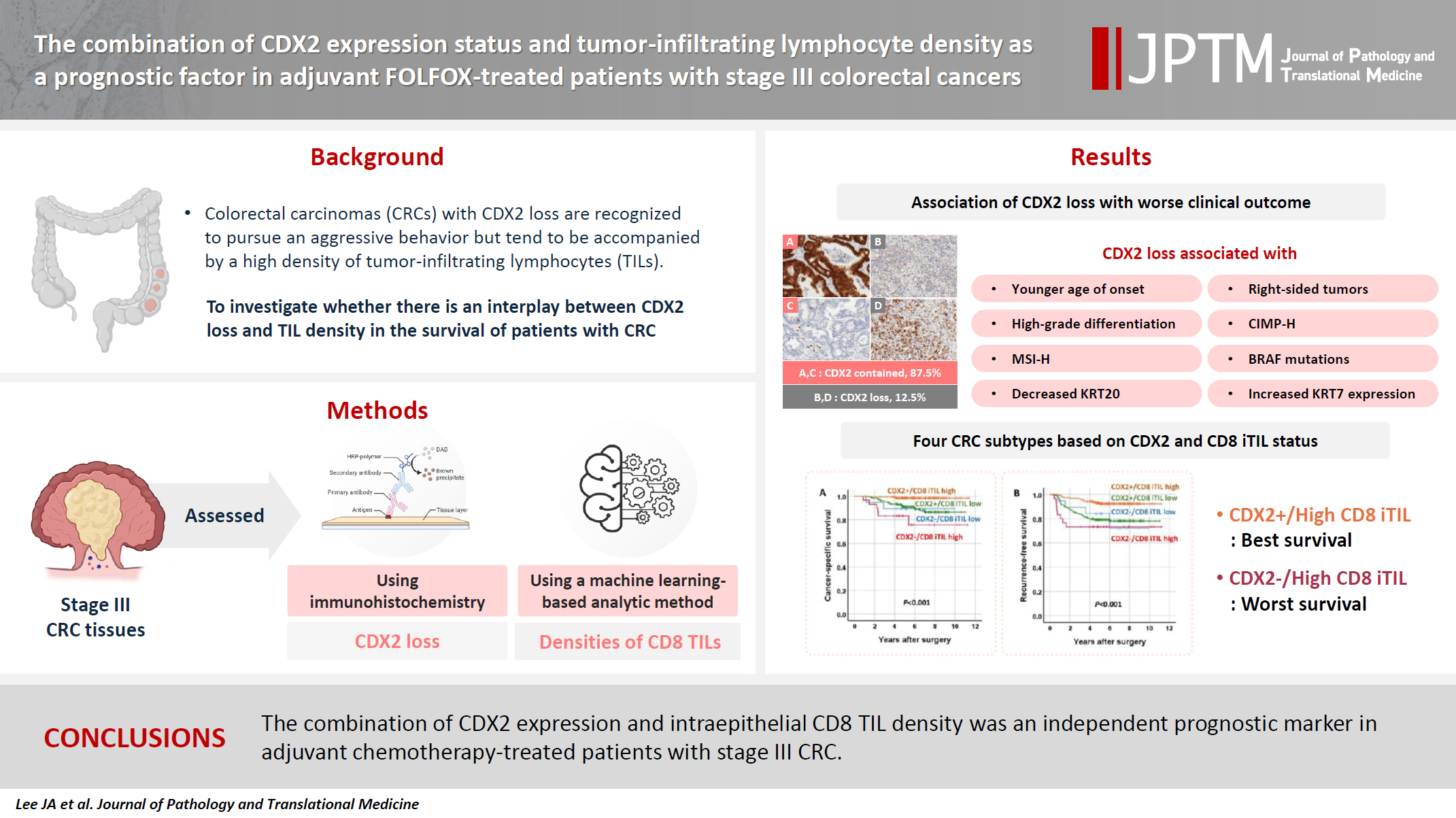



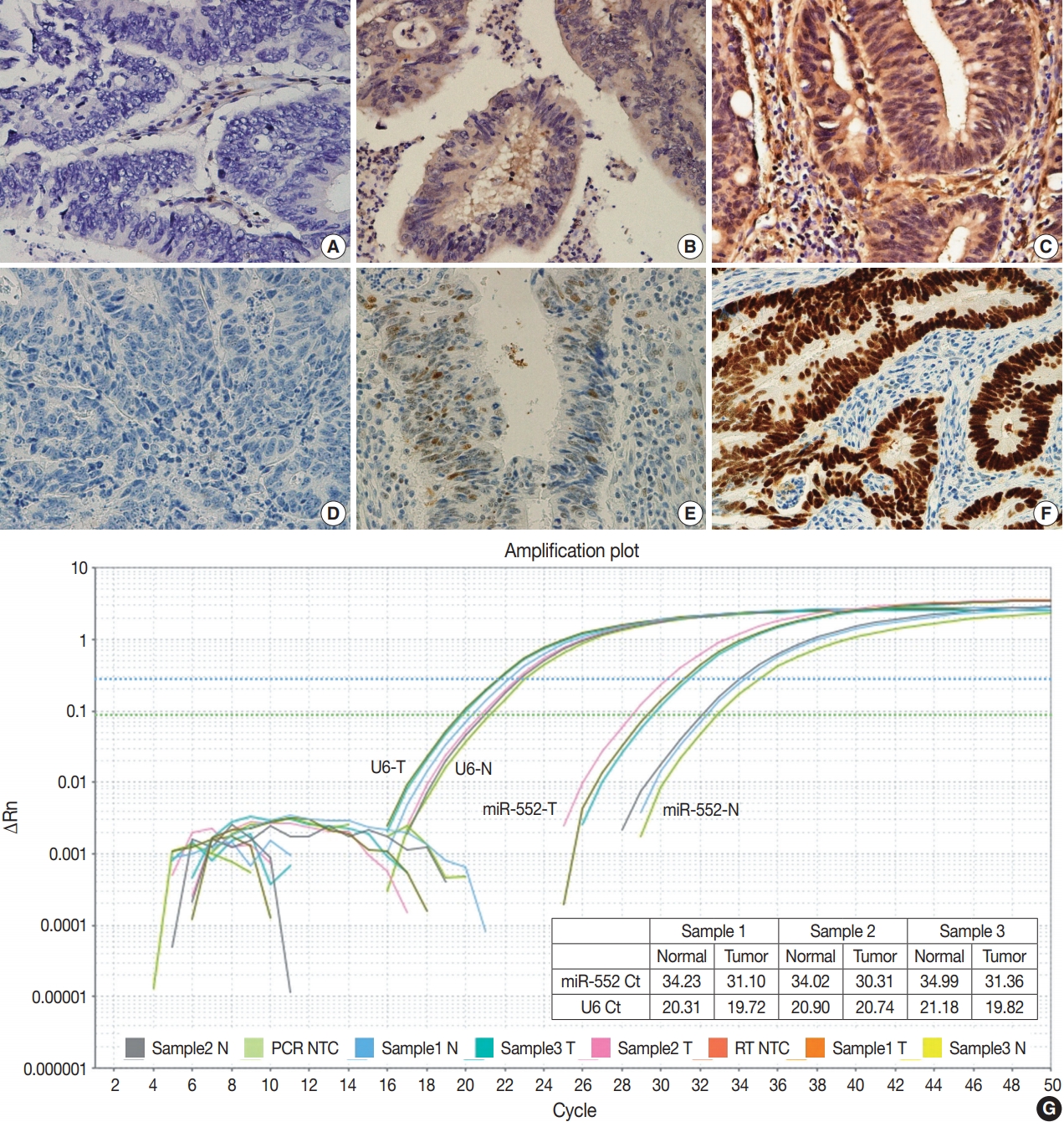
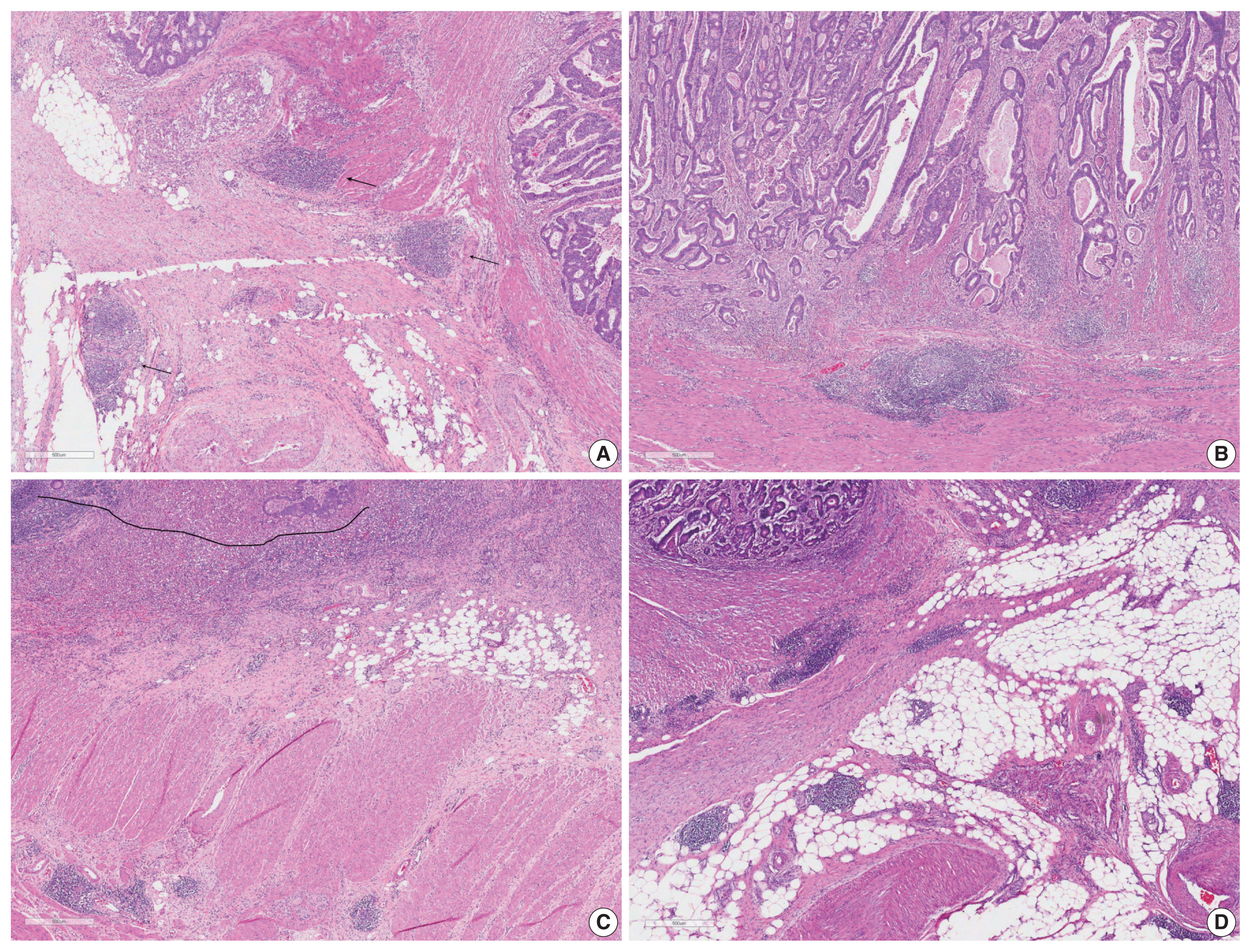
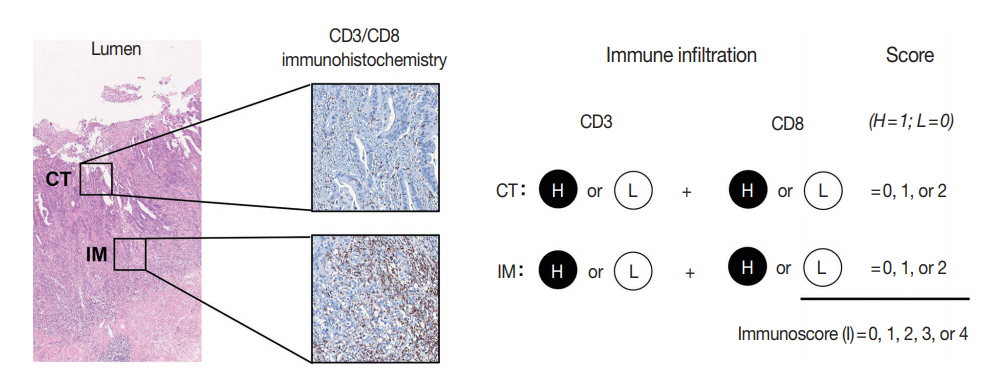
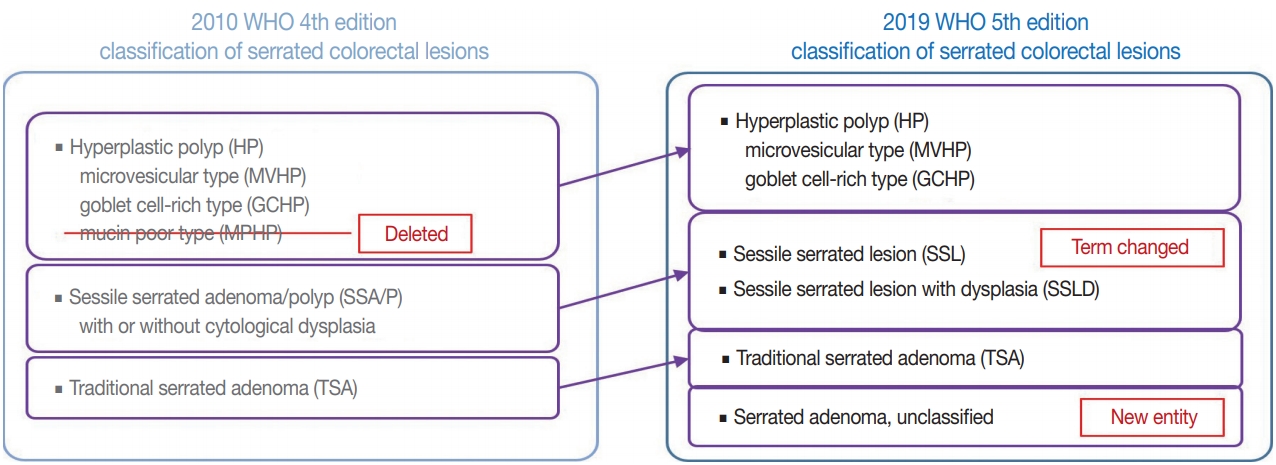

 First
First Prev
Prev



