Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(1); 2012 > Article
-
Original Article
Clinicopathologic Features of Q Fever Patients with Acute Hepatitis - Miji Lee, Jae Jeong Jang, Yang Soo Kim1, Sang-Oh Lee1, Sang-Ho Choi1, Sung-Han Kim1, Eunsil Yu
-
Korean Journal of Pathology 2012;46(1):10-14.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.1.10
Published online: February 23, 2012
Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
1Department of Infectious Disease, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- Corresponding Author: Eunsil Yu, M.D. Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, 388-1 Pungnap 2-dong, Songpa-gu, Seoul 138-736, Korea. Tel: +82-2-3010-4560, Fax: +82-2-472-7898, esyu@amc.seoul.kr
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Q fever caused by Coxiella burnetii presents with diverse clinical and pathological features including subclinical or cholestatic hepatitis. However, the pathological features of liver biopsies from patients with Q fever have not been well described.
-
Methods
- Clinical features and pathological findings of liver biopsies were reviewed in seven cases of Q fever that were confirmed by serological, microbiological, or molecular tests.
-
Results
- All cases presented with fever. Liver enzymes were mildly elevated except one case with marked hyperbilirubinemia. Characteristic fibrin ring granulomas were present in three cases, epithelioid granulomas with eosinophilic infiltration in two cases, extensive extravasated fibrins without ring configuration mimicking necrotizing granuloma in one case, and acute cholangitis without granuloma in one case. All cases were treated with antibiotics for 20 days. Six cases were completely cured, but one suffered from multiorgan failure.
-
Conclusions
- C. burnetii infection is uncommon, but should always be considered in patients with acute hepatitis and fever. Because variable-sized circumferential or radiating fibrin deposition was a consistent feature of the present cases, Q fever can be strongly suggested by pathological features and confirmed by serological and/or molecular tests.
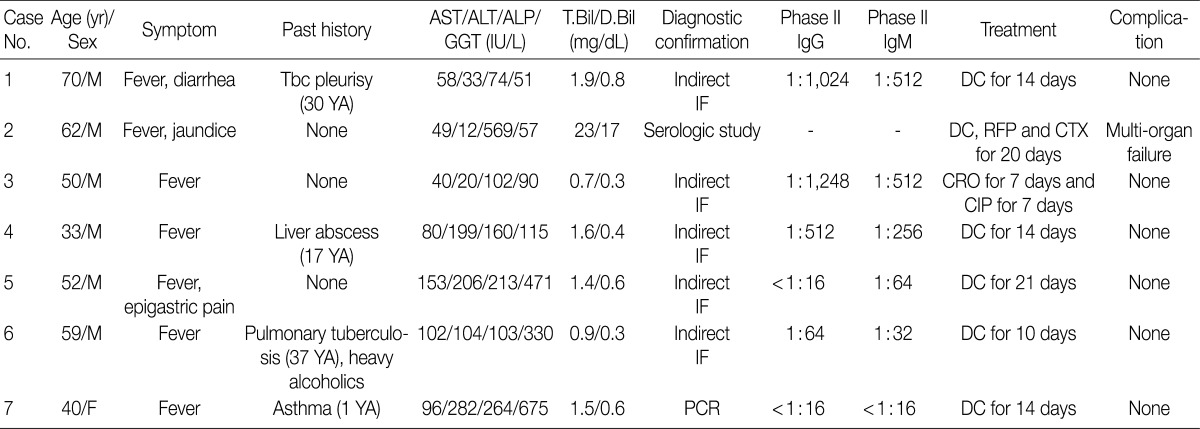
- Seven liver biopsies from patients with acute Q fever were retrieved from the archives of the Department of Pathology, Asan Medical Center from January 1, 1989 to June 31, 2010. The confirmative diagnosis was obtained by serological or molecular tests. Among seven cases, five were diagnosed by indirect microimmuno-fluorescence assay. At least a four-fold increase in phase II IgG titers or phase II IgG titers of >1/200 and phase II IgM titers of >1/25 were regarded as diagnostic for acute Q fever. The other two cases were diagnosed by detecting C. burnetii DNA using nested polymerase chain reaction (PCR) and identifying intracellular C. burnetii by a serological test. Clinical findings and laboratory data were reviewed from electronic medical records.
- Transjugular liver biopsy specimens of all cases were formalin-fixed and paraffin-embedded. Four-micrometer thick sections were stained with hematoxylin and eosin. All specimens were reviewed to assess the characteristic features of granulomas, the grades of portal and lobular activities, the types of cellular infiltrates, and the degree of fatty changes.
MATERIALS AND METHODS
- The cases included six males and one female with acute hepatitis. Patient age ranged from 33 to 70 years (mean age, 52 years). All patients had a history of acute onset fever. One patient (case 2) presented with jaundice, and another patient (case 1) suffered from diarrhea. The duration of symptoms ranged from 5-20 days, and the mean duration from the onset of symptoms to liver biopsy was 17 days (range, 12 to 30 days). Two cases (cases 1 and 6) had a history of tuberculous pleurisy and pulmonary tuberculosis, respectively. They were treated with antituberculous drugs and have recovered without complications. No patient had a history of chronic liver disease.
- Abnormalities on liver function tests were variable as shown in Table 1. One patient (case 2) with severe jaundice showed marked elevation of total and direct bilirubin levels up to 23-fold higher than reference levels, whereas other liver enzymes were only slightly increased. No bacterial growth was noted on blood cultures of any patient. Antibodies for leptospirosis, brucellosis, hepatitis A, B, and C, as well as autoantibodies including anti-nuclear antibody, anti-double strand DNA antibody, and anti-neutrophilic cytoplasmic antibody were all negative.
- In case 1, the PCR for Epstein Barr virus (EBV) using bone marrow aspirates was positive (1,560 copies/mL), and phase II IgG (1:512) and IgM (1:512) titers for EBV were elevated, whereas in situ hybridization for EBV early RNA using liver biopsy specimens, which was performed as an ancillary study, was negative. Although EBV-viral capsid antigen (VCA) IgG and EBV-Epstein Barr nuclear antigen IgG were detected on serological tests, the results of EBV-VCA IgM and EBV-early antigen IgM were negative. No additional viral copies were identified on two follow-up serum EBV PCR tests.
- The sole female patient (case 7), who had taken a dose of prednisolone 6 months ago due to an asthma attack, had a positive serological result for herpes simplex virus-1 (HSV-1) by PCR at an outside hospital. She had been treated with an antiviral agent (acyclovir) for 3 days, but her symptoms did not subside, so she was transferred to our hospital for a second opinion. Unlike the previous result from the outside hospital, PCR tests in serum, urine, and bone marrow were negative for HSV-1 DNA, but a PCR test for C. burnetii was positive, so she was diagnosed with Q fever hepatitis.
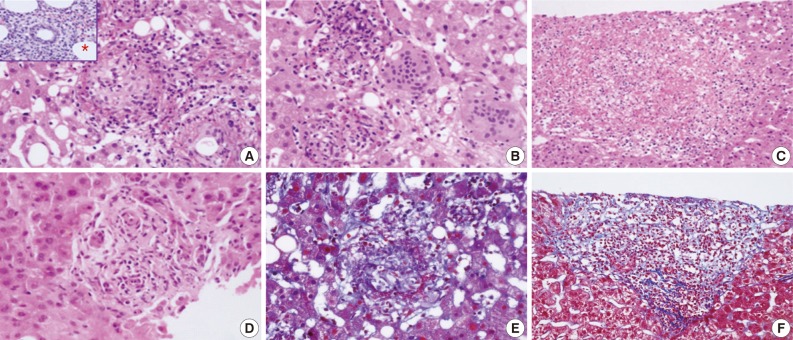
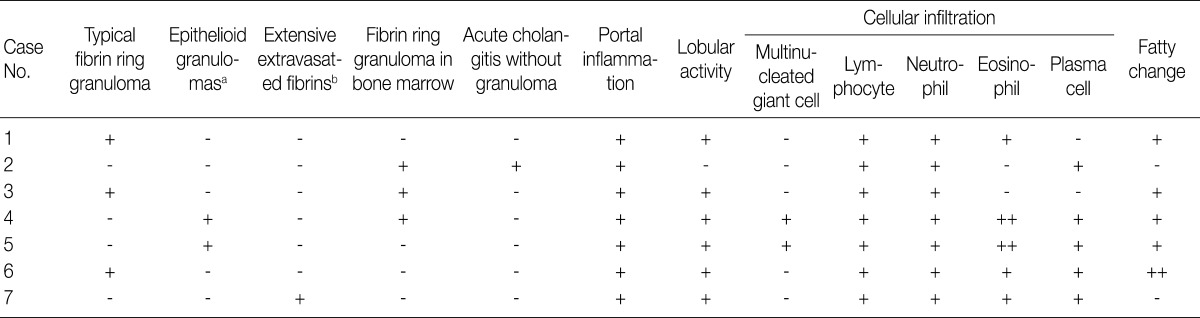
- Various types of granulomas were observed in six cases on liver biopsy (Table 2, Fig. 1). Typical fibrin ring granulomas consisted of a central fat vacuole, a fibrin ring, activated macrophages, and lymphocytes (Fig. 1A and E) and were found in three cases including a patient with a positive EBV PCR result in bone marrow (case 1). Epithelioid granulomas with numerous eosinophils and multinucleated giant cells were present in two cases (Fig. 1B). Distinctively extensive extravasated fibrin deposits resembling necrotizing granulomas were noted in one case (case 7) (Fig. 1C and F). A case with acute cholangitis showed no granulomas (case 2) (Fig. 1D). The grades of portal and lobular activity were mild in all cases. Lymphocytic and neutrophilic infiltrations were predominant, whereas eosinophils and plasma cells were variably noted. Fatty changes were observed to varying degrees; none in two cases, mild in four cases, and moderate in one case. No viral inclusion, acid-fast bacilli or periodic acid Schiff-positive fungi were observed.
- A bone marrow biopsy was performed in all patients. Characteristic fibrin ring granulomas were identified in three of seven bone marrow biopsies (Fig. 1A), and the other bone marrow biopsies showed normocellular marrow.
- Six patients were treated with doxycyclin for 10-21 days, but one patient (case 3), who was clinically suspected of contracting typhoid fever, was treated with ceftriaxone and ciprofloxacine. One patient (case 2) died of multiorgan failure despite antibiotic therapy (doxycyclin, rifampin, and cefotaxim) for 20 days. The other six patients were cured without any sequelae after acute symptoms subsided gradually with antibiotic therapy.
RESULTS
- The clinical presentations of Q fever are variable.6 The most frequent clinical feature of acute Q fever is a self-limiting febrile illness with a varying degree of pneumonia and hepatitis. Acute Q fever with hepatitis has been reported at rates of 9.0-67% in endemic areas, such as France, southern Spain, and Taiwan.13-15 In Korea, two cases of acute Q fever associated with hepatitis have been reported.16,17 Only one of the two cases underwent a liver biopsy, and typical fibrin ring granulomas were observed.17
- Fever was the first symptom in all seven patients in the present study. Because FUO is a liver biopsy indication, a percutaneous or transjugular liver biopsy was performed before confirmative serological or molecular tests in all cases. Acute hepatitis, the next frequent clinical feature of patients with Q fever, generally presents with mild elevation of liver enzymes, hepatomegaly, and/or splenomegaly. However, one case (case 2) in the present study showed severe jaundice, which is rare in Q fever,3 and died of multiorgan failure. Chang et al.15 reported two of eight cases of acute Q fever hepatitis in south Taiwan who had >10 mg/dL total bilirubin. Although a correlation between hyperbilirubinemia and severity of acute Q fever hepatitis has not been demonstrated, the severity of hyperbilirubinemia is thought to be associated with prolongation of hospitalization, longer fever duration, and delayed recovery time.15
- Acute Q fever with rapidly progressive hepatic failure has been reported in a patient with alcoholism,18 as in case 6 of the present study. The previously reported case revealed severe jaundice, hepatomegaly, elevated hepatic enzyme levels, and showed fibrous bands around regenerative liver parenchyma on the liver biopsy, indicating progression to cirrhotic change. In contrast, the present case presented only fever with a normal bilirubin level, a typical fibrin ring granuloma, and moderate fatty changes without significant fibrosis on the liver biopsy. The relationship between alcoholism and the severity of Q fever hepatitis needs to be investigated.
- Fibrin ring granulomas (doughnut granulomas), which were the most frequent finding in this study, are not diagnostic but characteristic for Q fever, although they can be seen in various diseases such as tuberculosis, cytomegaloviral hepatitis, EBV hepatitis, toxoplasmosis, leishmaniasis, Hodgkin's disease, Crohn's disease, sarcoidosis, and drug-induced granulomatous hepatitis.19,20
- Because EBV hepatitis may exhibits diverse histopathological features, including epithelioid noncaseating granulomas and extensive fibrin deposits resembling necrotizing granulomas in addition to characteristic findings of EBV hepatitis such as sinusoidal lymphocytic infiltration and atypical lymphocytes, the possibility of combined EBV hepatitis with acute Q fever hepatitis cannot be ruled out in case 1. A combined EBV and C. burnetii infection has not been reported in the English literature. The positive PCR result for EBV was thought to be an asymptomatic latent infection rather than an acute EBV infection such as infectious mononucleosis.
- Herpes viral hepatitis could be excluded in case 7, because characteristic features of herpes viral infection, including confluent hemorrhagic necrosis, scattered acidophilic bodies, and intranuclear "ground glass" inclusions were absent on the liver biopsy, and C. burnetii DNA was detected by PCR. However, herpes simplex hepatitis should be considered in cases undergoing steroid administration21 as in this case.
- Although acute Q fever is very rare in Korea, it should always be considered in patients with acute FUO and features of acute hepatitis, and may be strongly suggested by liver biopsy findings such as granulomas with either circumferential or radiating fibrin deposition. Furthermore, pathologists should recommend serological or molecular tests for differential or confirmative diagnosis of various infections including C. burnetii infection.
DISCUSSION
- 1. Angelakis E, Raoult D. Q fever. Vet Microbiol 2010; 140: 297-309. ArticlePubMed
- 2. Fishbein DB, Raoult D. A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am J Trop Med Hyg 1992; 47: 35-40. ArticlePubMed
- 3. Maurin M, Raoult D. Q fever. Clin Microbiol Rev 1999; 12: 518-553. ArticlePubMedPMCPDF
- 4. Marmion BP, Stoker MG, Walker CB, Carpenter RG. Q fever in Great Britain: epidemiological information from a serological survey of healthy adults in Kent and East Anglia. J Hyg (Lond) 1956; 54: 118-140. ArticlePubMedPMC
- 5. Bolaños M, Santana OE, Pérez-Arellano JL, et al. Q fever in Gran Canaria: 40 new cases. Enferm Infecc Microbiol Clin 2003; 21: 20-23. ArticlePubMed
- 6. Raoult D, Marrie T. Q fever. Clin Infect Dis 1995; 20: 489-495. ArticlePubMed
- 7. Fenollar F, Raoult D. Molecular genetic methods for the diagnosis of fastidious microorganisms. APMIS 2004; 112: 785-807. ArticlePubMed
- 8. Wilson HG, Neilson GH, Galea EG, Stafford G, O'Brien MF. Q fever endocarditis in Queensland. Circulation 1976; 53: 680-684. ArticlePubMed
- 9. Vogel JP. Turning a tiger into a house cat: using Legionella pneumophila to study Coxiella burnetii. Trends Microbiol 2004; 12: 103-105. ArticlePubMed
- 10. Korea Centers for Disease Control and Prevention. Q fever in Korea. Public Health Wkly Rep 2008; 1: 149-153.
- 11. Kim YK, Kim MS, Lee KS, et al. A comparison of causes of fever of unknown origin between the 1980s and the 1990s. Korean J Med 2001; 61: 546-552.
- 12. Cone LA, Curry N, Shaver P, Brooks D, DeForge J, Potts BE. Q fever in the Southern California desert: epidemiology, clinical presentation and treatment. Am J Trop Med Hyg 2006; 75: 29-32. ArticlePubMed
- 13. Raoult D, Tissot-Dupont H, Foucault C, et al. Q fever 1985-1998: clinical and epidemiologic features of 1,383 infections. Medicine (Baltimore) 2000; 79: 109-123. ArticlePubMed
- 14. Domingo P, Muñoz C, Franquet T, Gurguí M, Sancho F, Vazquez G. Acute Q fever in adult patients: report on 63 sporadic cases in an urban area. Clin Infect Dis 1999; 29: 874-879. ArticlePubMed
- 15. Chang K, Yan JJ, Lee HC, Liu KH, Lee NY, Ko WC. Acute hepatitis with or without jaundice: a predominant presentation of acute Q fever in southern Taiwan. J Microbiol Immunol Infect 2004; 37: 103-108. PubMed
- 16. Park HS, Lee EG, Lee SY, et al. A case of Q fever: associated with pancytopenia, hepatitis, and myocarditis. Korean J Infect Dis 1992; 24: 45-54.
- 17. Choi HC, Lee SH, Kim J, et al. A case of acute Q fever with severe acute cholestatic hepatitis. Gut Liver 2009; 3: 141-144. ArticlePubMedPMC
- 18. Lin PH, Lo YC, Chiang FT, et al. Acute Q fever presenting as fever of unknown origin with rapidly progressive hepatic failure in a patient with alcoholism. J Formos Med Assoc 2008; 107: 896-901. ArticlePubMed
- 19. Riechman N, Raz R, Keysary A, Goldwasser R, Flatau E. Chronic Q fever and severe thrombocytopenia in a pregnant woman. Am J Med 1988; 85: 253-254. ArticlePubMed
- 20. Rodriguez JM, Yañez RJ, Pan R, Rodriguez JF, Salas ML, Viñuela E. Multigene families in African swine fever virus: family 505. J Virol 1994; 68: 2746-2751. ArticlePubMedPMCPDF
- 21. Kaufman B, Gandhi SA, Louie E, Rizzi R, Illei P. Herpes simplex virus hepatitis: case report and review. Clin Infect Dis 1997; 24: 334-338. ArticlePubMed
REFERENCES
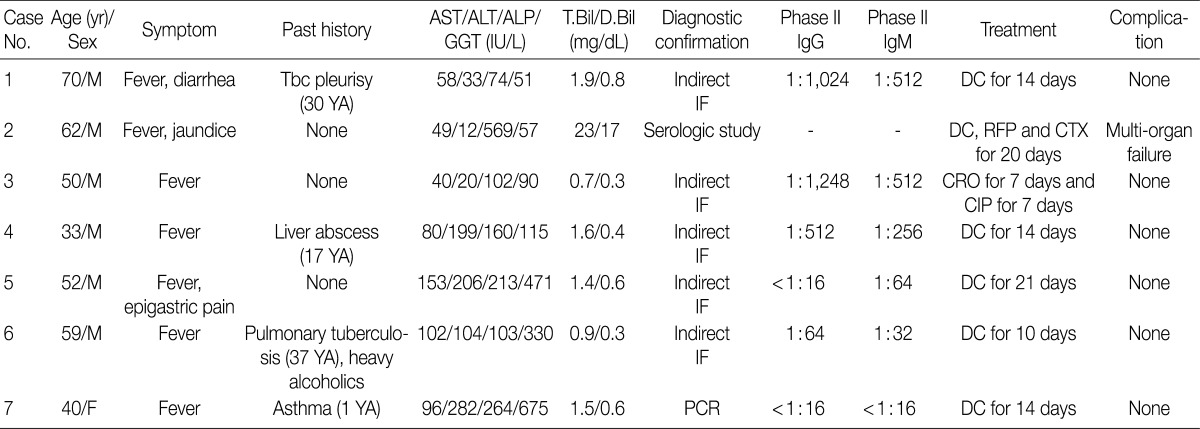
AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkaline phosphatase; GGT, gamma glutamyl transferase; T.Bil, total bilirubin; D.Bil, direct bilirubin; M, male; Tbc, tuberculosis; IF, immunofluorescence; DC, doxycyclin; RFP, rifampin; CTX, cefotaxime; CRO, ceftriaxone; CIP, ciprofloxacin; F, female; PCR, polymerase chain reaction.
Figure & Data
References
Citations

- Actualités sur la fièvre Q
Maxime Colson, Matthieu Million, Pierre-Edouard Fournier, Sophie Edouard
Revue Francophone des Laboratoires.2025; 2025(573): 39. CrossRef - Coxiella burnetii: Emerging threats, molecular insights, and advances in diagnosis and control measures
Mohammad Reza Mohammadi, Safoura Moradkasani, Mina Latifian, Saber Esmaeili
Journal of Microbiological Methods.2025; 237: 107213. CrossRef - Q fever as a cause of fever of unknown origin in a patient on hemodialysis
Emilio Guirao-Arrabal, Ana Delgado-Ureña, Elena Borrego-García, Rosa Ríos-Pelegrina
Nefrología.2024; 44(6): 906. CrossRef - Q fever as a cause of fever of unknown origin in a patient on hemodialysis
Emilio Guirao-Arrabal, Ana Delgado-Ureña, Elena Borrego-García, Rosa Ríos-Pelegrina
Nefrología (English Edition).2024; 44(6): 906. CrossRef - (Seltene) infektiöse Hepatitiden als wichtige Differenzialdiagnose der unklaren Hepatopathie
Michael Wührl, Marc Ringelhan, Ursula Ehmer, Jochen Schneider, Juliane Kager, Tobias Lahmer, Anna Schneider, Wilko Weichert, Carolin Mogler
Die Pathologie.2023; 44(1): 53. CrossRef - Sero-epidemiological study of zoonotic bacterial abortifacient agents in small ruminants
Muhammad Abid Zeeshan, Sarmad Ali, Ishtiaq Ahmed, Aziz ur Rehman, Muhammad Kamran Rafique, Amar Nasir, Aman Ullah Khan, Muhammad Kashif, Katja Mertens-Scholz, Muhammad Imran Arshad, Syed Ehtisham-ul-Haque, Heinrich Neubauer
Frontiers in Veterinary Science.2023;[Epub] CrossRef - The First Case of Coxiella Burnetti Infection Detected Through Bone Marrow Biopsy in Vietnam
Do Thi Vinh An, Bui Thi Viet Ha, Dao Xuan Co, Vu Minh Tam, Le Thi Diem Tuyet, Vu Van Truong
Clinical Pathology.2022;[Epub] CrossRef - Pathological study and molecular detection of zoonotic diseases in small ruminants at slaughter houses in Mymensingh, Bangladesh
Nazneen Sultana, Munmun Pervin, Sajeda Sultana, Mahmuda Islam, Moutuza Mostaree, Mohammad Abu Hadi Noor Ali Khan
Veterinary World.2022; : 2119. CrossRef - The First Report of Coxiella burnetii as a Potential Neglected Pathogen of Acute Hepatitis of Unknown Causes in Egypt
Mohamed A. El-Mokhtar, Ibrahim M. Sayed, Ayat M. Kamel, Ahmed Atef Mesalam, Elsayed A. Elgohary, Khaled Abo bakr Khalaf, Sara Adel, Azza Abo Elfadl, Walaa A. Khalifa, Haidi Karam-Allah Ramadan
Microorganisms.2022; 10(11): 2168. CrossRef - A case of coexistent acute severe alcoholic and Q fever hepatitis: The useful contribution of repeated liver biopsies
Lucia Zampaglione, Aurélie Bornand, Nicolas Goossens, Lucas Ramer, Giulia Magini, Marie Ongaro, Andreas Cerny, Laura Rubbia-Brandt, Jean-Louis Frossard, Laurent Spahr
Annals of Clinical Gastroenterology and Hepatology.2022; 6(1): 034. CrossRef - Q-fever associated granulomatous hepatitis
Nicolas Dauby, Maria Gomez Galdon, Isabel Montesinos, Marjan Van Esbroeck, Thomas Sersté
International Journal of Infectious Diseases.2020; 95: 113. CrossRef - Pathologic changes and immune responses against Coxiella burnetii in mice following infection via non-invasive intratracheal inoculation
Xueyuan Hu, Yonghui Yu, Junxia Feng, Mengjiao Fu, Lupeng Dai, Zhiyu Lu, Wenbo Luo, Jinglin Wang, Dongsheng Zhou, Xiaolu Xiong, Bohai Wen, Baohua Zhao, Jun Jiao, Daniel E. Voth
PLOS ONE.2019; 14(12): e0225671. CrossRef - Fibrin Ring Granulomas in Checkpoint Inhibitor-induced Hepatitis
Jamie Everett, Amitabh Srivastava, Joseph Misdraji
American Journal of Surgical Pathology.2017; 41(1): 134. CrossRef - Clinical and Genetic Features ofCoxiella burnetiiin a Patient with an Acute Febrile Illness in Korea
Seung Hun Lee, Jung Yeon Heo, Hae Kyung Lee, Yeong Seon Lee, Hye Won Jeong, Seon Do Hwang
Journal of Korean Medical Science.2017; 32(6): 1038. CrossRef - Q Fever Presented as a Large Retroperitoneal Pseudotumoral Mass
Behdokht Nowroozizadeh, Negar Haghighi Mehmandari, Nicolas Gallegos, Mari Perez-Rosendahl, Di Lu
Case Reports in Pathology.2017; 2017: 1. CrossRef - From Q Fever to Coxiella burnetii Infection: a Paradigm Change
Carole Eldin, Cléa Mélenotte, Oleg Mediannikov, Eric Ghigo, Matthieu Million, Sophie Edouard, Jean-Louis Mege, Max Maurin, Didier Raoult
Clinical Microbiology Reviews.2017; 30(1): 115. CrossRef - Prolonged Pyrexia and Hepatitis: Q fever
Caitlin Dugdale, Brian Chow, Evgeny Yakirevich, Erna Kojic, Bettina Knoll
The American Journal of Medicine.2014; 127(10): 928. CrossRef
 PubReader
PubReader-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
- Related articles
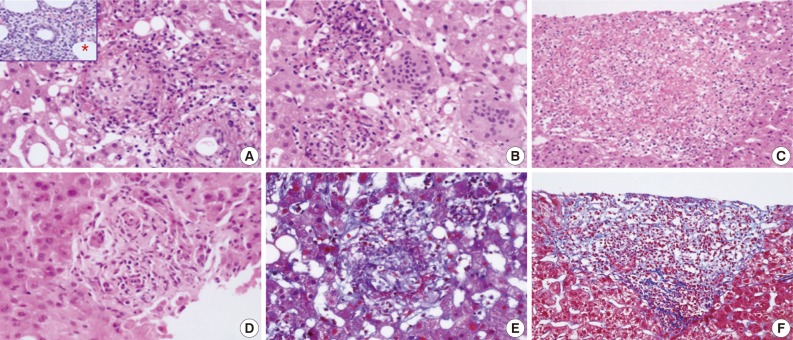
Fig. 1
AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkaline phosphatase; GGT, gamma glutamyl transferase; T.Bil, total bilirubin; D.Bil, direct bilirubin; M, male; Tbc, tuberculosis; IF, immunofluorescence; DC, doxycyclin; RFP, rifampin; CTX, cefotaxime; CRO, ceftriaxone; CIP, ciprofloxacin; F, female; PCR, polymerase chain reaction.
aEpithelioid granulomas with numerous eosinophils and multi-nucleated giant cells; bExtensive extravasated fibrins without ring configuration mimicking necrotizing granuloma.

 E-submission
E-submission