Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(1); 2012 > Article
-
Original Article
The Ratio of Atypical Ductal Hyperplasia Foci to Core Numbers in Needle Biopsy: A Practical Index Predicting Breast Cancer in Subsequent Excision - Jeong-Ju Lee, Hee Jin Lee, Jun Kang, Jeong-Hyeon Jo, Gyungyub Gong
-
Korean Journal of Pathology 2012;46(1):15-21.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.1.15
Published online: February 23, 2012
Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- Corresponding Author: Gyungyub Gong, M.D. Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, 388-1 Pungnap 2-dong, Songpa-gu, Seoul 138-736, Korea. Tel: +82-2-3010-4554, Fax: +82-2-472-7898, gygong@amc.seoul.kr
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Although core needle biopsy (CNB) is considered to be the standard technique for histological diagnosis of breast lesions, it is less reliable for diagnosing atypical ductal hyperplasia (ADH). We therefore assessed the characteristics of CNB-diagnosed ADH that are more likely to be associated with more advanced lesions on subsequent surgical excision.
-
Methods
- We retrospectively examined 239 consecutive CNBs, 127 of which were diagnosed as ADH following surgical excision, performed at Asan Medical Center between 1995 and 2010. Archival slides were analyzed for the number of cores per specimen, the number of ADH foci, and the ratio of ADH foci to number of cores (FC ratio).
-
Results
- We found that ADH foci in 3 or more cores (p=0.003) and the presence of ADH in 3 or more foci (p=0.002) were correlated with malignancy following excision lesion. Moreover, an FC>1.1 was significantly associated with malignancy in the subsequent excision (p=0.000).
-
Conclusions
- Including the number of ADH foci, the number of cores involved according to ADH, FC ratio, and histologic type in a pathology report of CNB may help in making clinical decisions about surgical excision.
- We examined the surgical pathology files of Asan Medical Center, Seoul, Korea, for all patients with CNB-diagnosed ADH between January 1995 and August 2010. Of the 239 CNB-diagnosed patients, 62 were diagnosed with DCIS or invasive carcinoma in the same breast and were therefore excluded. Of the 177 patients diagnosed by CNB with ADH as the most significant lesion, 127 underwent follow-up surgical excision, and 50 did not. Of the latter, some refused further surgery, and others underwent excision at other institutions, with their slides unavailable for review; these patients were therefore excluded. Thus, our study population consisted of 127 patients. All underwent biopsies using a vacuum assisted device with an 11-gauge automated needle or nonvacuum assisted device with a 14-gauge automated needle. All core biopsies and subsequently excised tissue sections were fixed in 10% buffered formaldehyde, embedded in paraffin, and stained with hematoxylin and eosin (H&E).
- All H&E-stained slides of the CNB specimens were reviewed by two pathologists, blind to the results of subsequent excisional biopsy, to confirm the diagnosis of ADH using established criteria.10,11
- Following the verification of the presence of ADH in CNB specimens, the extent of involvement was determined by evaluation of the number of large ducts and/or terminal duct lobular units affected by ADH, as described previously.5 When multiple foci of ADH were present, the size of the largest focus was determined.
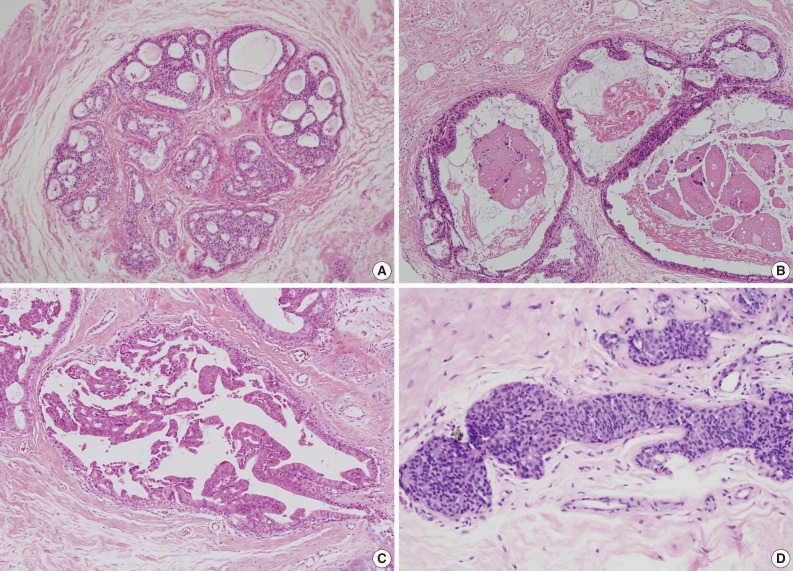
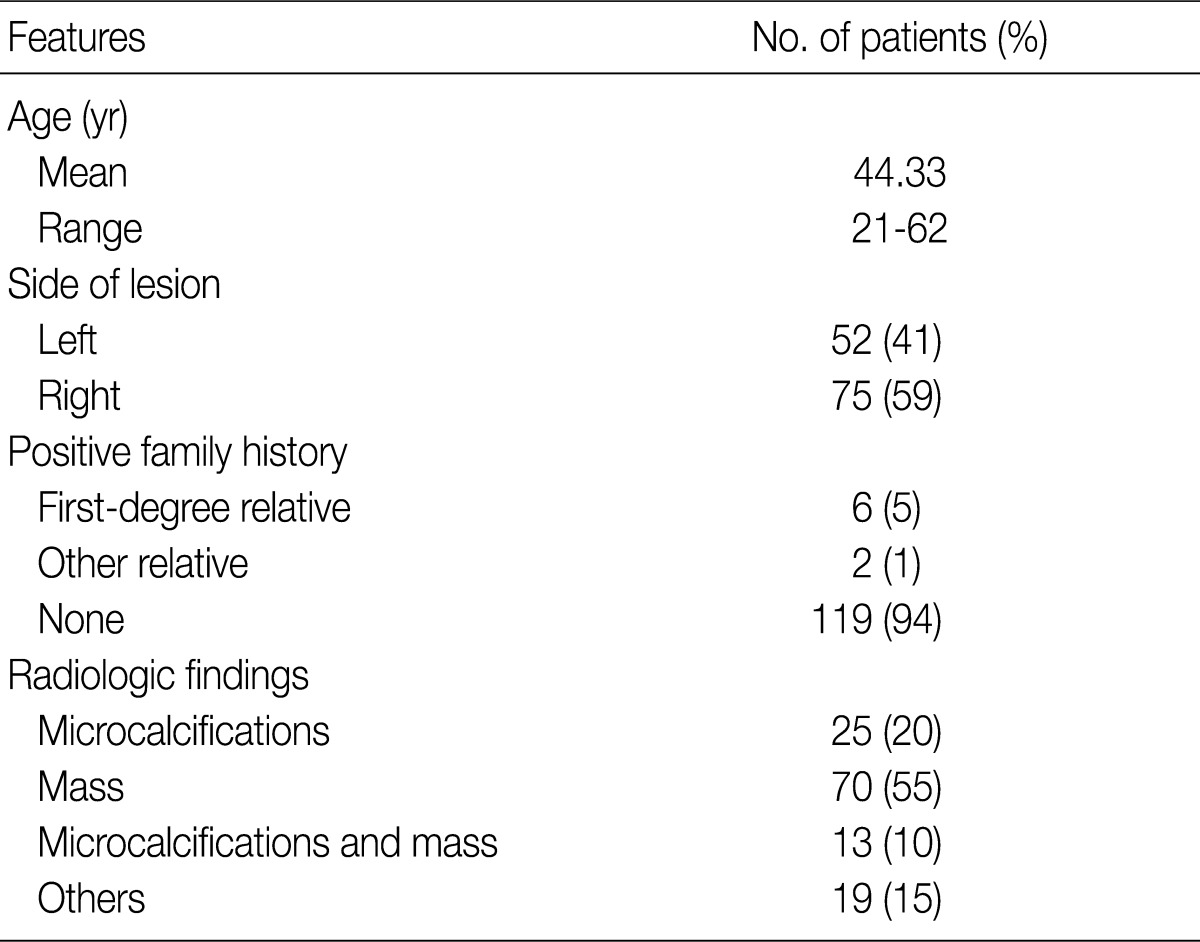
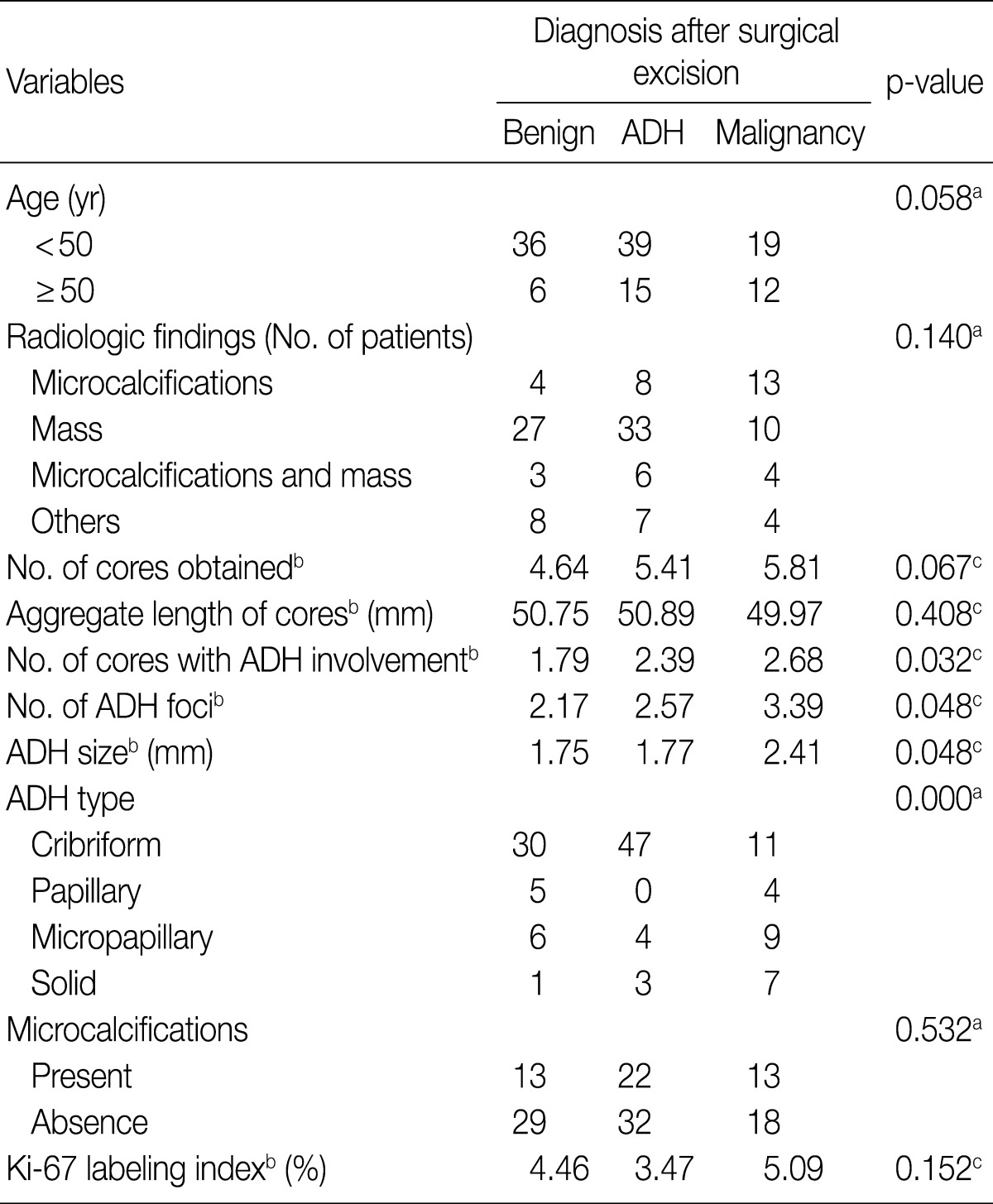
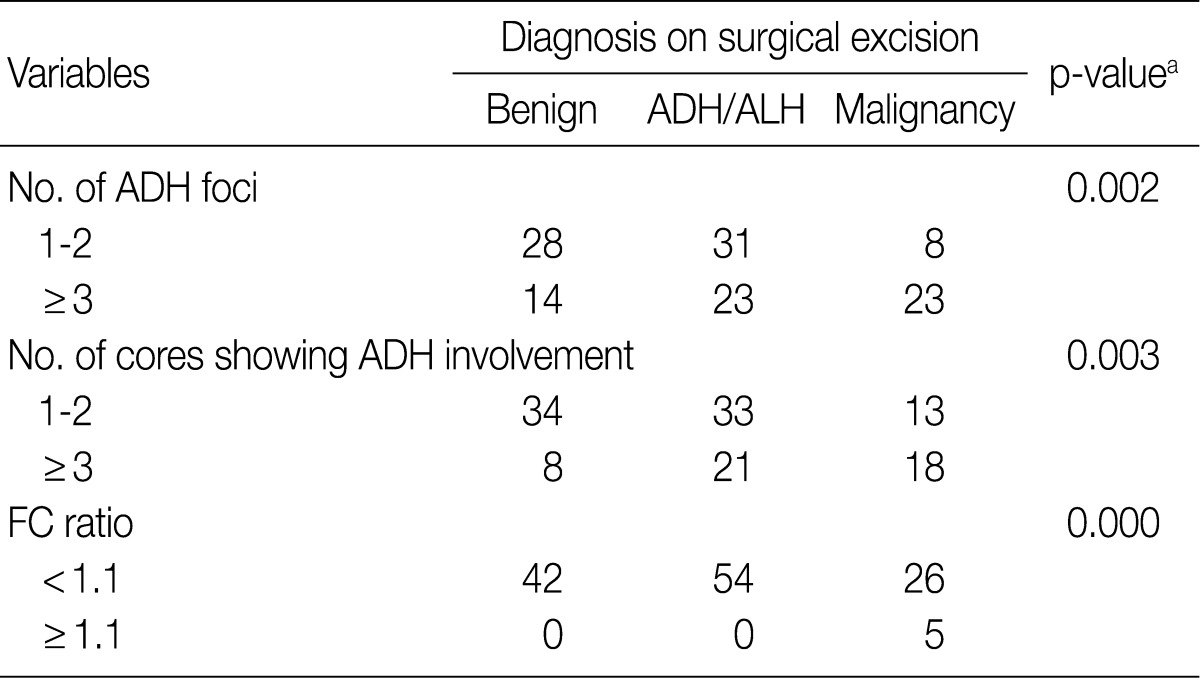
- We also analyzed the predominant histologic pattern of ADH (cribriform, micropapillary, papillary or solid) (Fig. 1), the presence of calcifications, number of tissue cores obtained, and number of tissue cores showing ADH involvement. Clinical parameters, including patient age, affected side, and mammographic and ultrasonographic findings, were obtained from patients' electronic medical records.
- All H&E-stained slides of the subsequent surgical excisions were reviewed by two additional pathologists, blind to the CNB results, to confirm the final diagnosis. Surgically excised specimens were evaluated for the presence of invasive carcinoma, DCIS, lobular carcinoma in situ (LCIS), and residual ADH, using established criteria.10 The presence of a prior biopsy site was confirmed in all cases.
- Samples from 79 patients were available for paraffin blocks. Sections were cut at 3 µm thicknesses, deparaffinized in xylene, and rehydrated with graded ethanol. Samples were assayed for Ki-67 expression using a monoclonal mouse anti-human Ki-67 antibody (Zymed, San Francisco, CA, USA) using standard immunohistochemical methods and a Ventana BenchMark XT immunostainer (Ventana Medical Systems, Tucson, AZ, USA). These slides were evaluated by a pathologist blind to the clinical data, who calculated the Ki-67 percentage (representing the proportion of the nuclear positivity for Ki-67) in each ADH lesion.
- The number of cores obtained, ADH foci, and cores showing ADH involvement, the size of the largest ADH focus, and the Ki-67 labeling index were compared using the Kruskal-Wallis 1-way analysis of variance by ranks. Other clinical and pathological characteristics were cross-tabulated with diagnostic yield in a C×R contingency table. Pearson chi-squared test statistics were estimated for every contingency table to test the null hypothesis that the diagnostic yields and clinical characteristics were independent against the alternative that there was an association between them. A 2-sided p-value<0.05 was considered statistically significant. All statistical analyses were performed using SPSS ver.15 (SPSS Inc., Chicago, IL, USA).
MATERIALS AND METHODS
- The 127 women assessed ranged in age from 21 to 62 years (mean, 44.33 years); their clinical and demographic data are summarized in Table 1. Mammographic and/or ultrasonographic evaluation showed that 25 lesions were microcalcifications, 70 were masses, 13 were combined masses with microcalcifications, and 19 showed other findings.
- The number of cores obtained from each lesion ranged from 1 to 14 (median, 5), the aggregate length of cores obtained from each lesion ranged from 7 to 240 mm (mean, 51 mm) and the median number of cores showing ADH involvement was 2. The median number of ADH foci was 2, and the mean size of ADH foci was 1.0 mm. The predominant histologic pattern of ADH in CNB specimens was cribriform, observed in 88 patients (69.3%), followed by micropapillary in 19 (15.0%), papillary in 9 (7.1%), and solid in 11 (8.7%) patients. The presence of microcalcifications was confirmed histologically in 48 CNBs (37.8%). The Ki-67 labeling index ranged from 0% to 30% (mean, 4.3%).
- Histologic evaluation of the surgically excised specimens showed that 42 (33.1%) were benign breast tissue without evidence of atypia, 54 (42.5%) were residual ADH, and 31 (24.4%) were malignant, including DCIS in 25 patients, LCIS in 3, DCIS with LCIS in 1, and invasive carcinoma in 2.
- Utilizing the Nottingham modification of Scarff-Bloom-Richardson histologic grading system, 13 cases (52%) of DCIS were nuclear grade 2, 11 cases (44%) of DCIS were nuclear grade 1, and one case (4%) of DCIS was nuclear grade 3. Two cases of LCIS were accompanied with ADH, but one case of LCIS was purely LCIS. Invasive carcinomas included invasive ductal carcinoma and papillary carcinoma which were nuclear grade 2 and histologic grade 2.
- The correlations between the extent and types of ADH lesions and other features of the CNB specimens with the results of the surgical excisional specimens are summarized in Table 2. There were no statistically significant differences related to the side of the lesion, family history of breast cancer and radiologically observed microcalcifications. The number of ADH foci, the number of cores showing ADH involvement and ADH size differed significantly among the groups according to their diagnosis based on surgically excised specimens. In contrast, patient age, number of cores obtained, aggregate length of cores, presence of microcalcification in CNB specimens and Ki-67 labeling index were not related to the diagnosis on excised specimens.
- When the predominant histologic pattern of ADH seen in the CNB specimens was correlated with the corresponding findings on subsequent surgical excision, there was an association between solid ADH and subsequent malignancy, as well as an association between cribriform ADH and subsequent benign lesions.
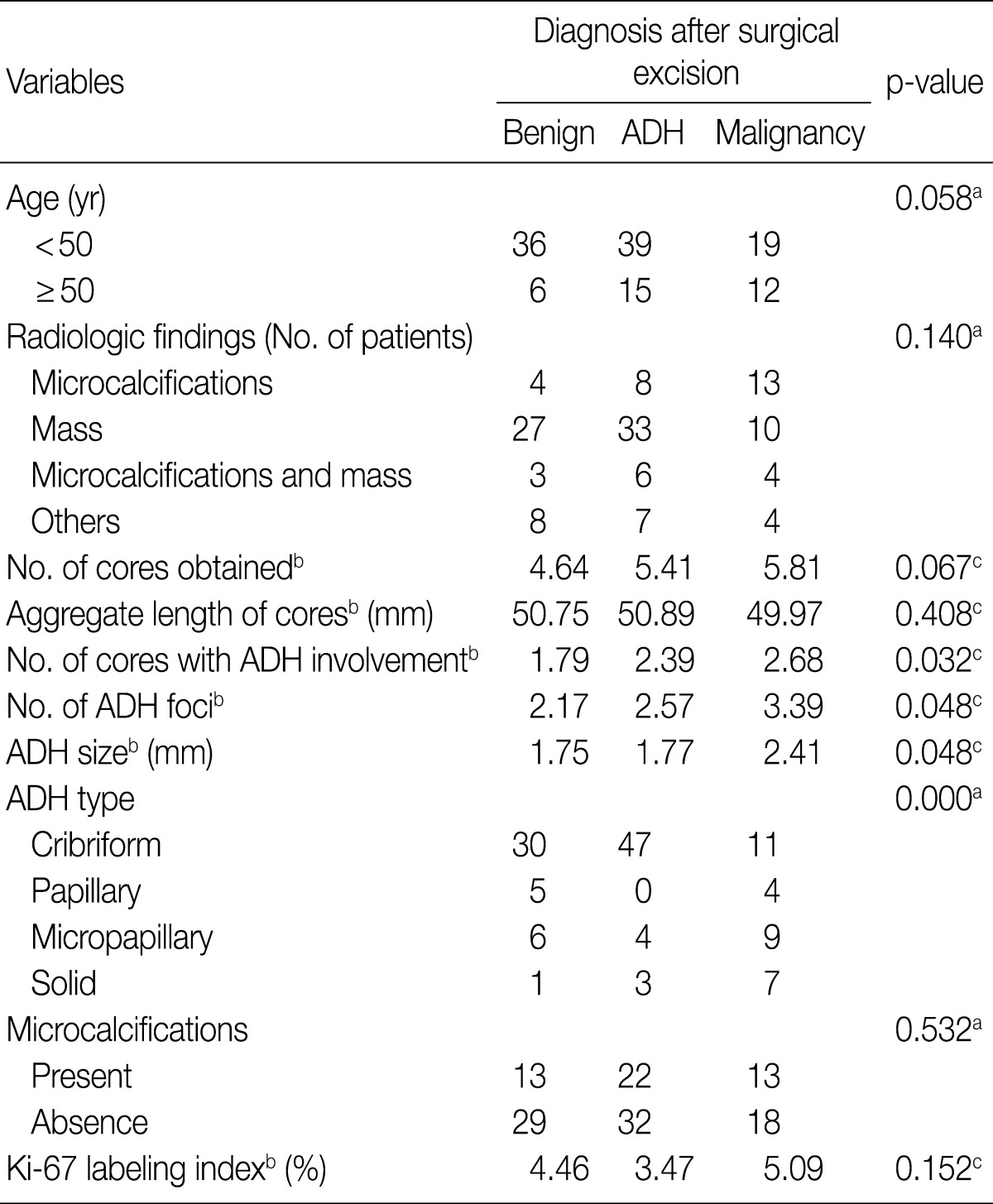
- Among the 67 patients with ADH limited to 1 or 2 foci in CNB specimens, 8 (12%) were diagnosed with malignancy, 31 (46%) with residual ADH, and 28 (42%) with lesions having benign histologic features without atypia (Table 3). Among the 60 patients with ADH affecting 3 or more foci in CNB specimens, 23 (38%) were diagnosed with malignancy, 23 (38%) with ADH, and 14 (24%) with benign lesions without atypia. The sensitivity and specificity of ADH involving 3 or more foci in predicting the presence of a malignancy were 74.2% and 61.5%, respectively.
- Similar results were observed when we considered the number of cores showing ADH involvement. The sensitivity and specificity of ADH involving 3 or more cores in predicting worse lesions (DCIS/LCIS or invasive carcinoma) were 58.1% and 69.8%, respectively.
- We divided the CNB specimens into two groups. The cut off value was an FC ratio of 1.1 based on the maximum area under the receiver operating characteristic curve. Of the 122 patients with CNB specimens having FC ratios<1.1, 42 (35%) were diagnosed with malignancy, 54 (44%) with residual ADH, and 26 (21%) with benign lesions without atypia (Table 3). In contrast, of the 5 patients with CNB specimens having FC ratios≥1.1, all 5 (100%) were diagnosed with malignancy after surgical excision. The sensitivity and specificity of an FC ratio≥1.1 in predicting the presence of a malignancy were 16.1% and 100%, respectively.
RESULTS
- We have assessed the clinicopathological and radiological findings of ADH diagnosed by CNB to determine the factors that can distinguish between ADH and cancer, including DCIS, LCIS, and invasive carcinoma. ADH is a type of histologically borderline lesion that is difficult to distinguish from low grade DICS on small tissue samples provided by CNB. Because of this difficulty, clinicopathologic and radiologic findings that can distinguish between ADH and DCIS are valuable in planning patient management.
- Several studies have examined various mammographic, clinical, and pathologic factors that may predict the presence of a more significant lesion following surgical excision after a CNB diagnosis of ADH.7,15,16 For example, the combination of a mass with microcalcifications on ultrasonography has been associated with an underestimation of cancer,17 although we found no association between microcalcifications, on ultrasonography or mammography, and subsequent cancer on excision.
- Although no universal agreement has been reached about the number of cores necessary to achieve an accurate histologic diagnosis, previous studies have demonstrated that underestimation of malignancy decreases as the average number of cores obtained per lesion increases.2,18 For example, underestimation at CNB diminished as the mean number of cores per lesion increased from 6.3 to 9.5, with an even further reduction in the underestimation rate when more than 10 cores were acquired per lesion.19,20 While acknowledging that a certain number of tissue cores are needed for categorization of the lesion, other studies found that the underestimation rate was not related to the number of cores obtained, suggesting that numbers alone may not accurately reflect how thoroughly the lesion is sampled by CNB.21-24 In contrast to previous findings,19,20 however, we did not observe a statistically significant correlation between the number of tissue cores removed and the results on final surgical excision.
- We found a significant correlation between the number of cores showing ADH involvement and the likelihood of malignant lesions after subsequent excision. The number of cores showing ADH involvement has been shown to be significantly higher in patients with a follow-up excisional diagnosis of DCIS compared with patients with a benign lesion or an ADH by excisional biopsy.25
- Although all patients with ADH limited to 1 or 2 foci did not have more malignant lesions on open biopsy, patients with ADH involving 4 or more foci had a higher probability of an underlying, more advanced lesion on excision, with 86.6% of the latter having DCIS or invasive carcinoma.5 Moreover, we found that ADH involving 3 or more foci in CNB is an indicator of a more significant lesion after excision.
- Although a previous study reported no association between largest ADH size and subsequent malignancy,25 we found that largest ADH size was correlated with subsequent malignancy after surgical excision of the lesion. Moreover, a strong association was observed between micropapillary ADH and subsequent DCIS,5 whereas another study found no association between increased risk of carcinoma on excision and a micropapillary pattern of ADH.26 We also observed an association between solid ADH and subsequent cancer on excision, whereby 7 of these 11 patients (64%) were diagnosed with malignancy.
- Histologic examination has shown calcifications in mammary ducts within areas of ADH, but without cell necrosis.27 In DCIS, the calcifications develop in secretions and are dystrophic calcifications secondary to necrotic tumor cells.28,29 Our findings were not in agreement with these earlier results.
- One study found an association between a high Ki-67 labeling index (>2%) in atypical hyperplasia and increased risk of breast cancer within 10 years after atypia.30 The cumulative incidence of breast cancer at 10 years was also high in patients with a high Ki-67 labeling index.24,30 In contrast, we found that the Ki-67 labeling index was not significantly correlated with the short-term risk of breast cancer.
- We also found that FC ratio, representing the number of ADH foci divided by the number of cores, is an indicator for surgical excision in patients with core biopsy samples of breast lesion with ADH. An FC ratio≥1.1 is associated with a significantly higher probability of malignancy on subsequent excisions (p=0.000). The FC ratio compensates for the variability in the number of biopsy cores, is highly specific and is useful for predicting malignancy on follow-up excision.
- In summary, our study in a series of 127 cases of ADH diagnosed by CNB indicates that microcalcifications on ultrasonography or mammography, determination of the extent (ADH foci, cores involved with ADH), FC ratio, and solid histologic type of ADH can be applied to predict the presence of malignancy found by surgical excision. Including the number of ADH foci, the number of cores involved according to ADH, FC ratio, and histologic type in a pathology report may help in making clinical decisions about surgical excision.
DISCUSSION
- 1. Dahlstrom JE, Sutton S, Jain S. Histological precision of stereotactic core biopsy in diagnosis of malignant and premalignant breast lesions. Histopathology 1996; 28: 537-541. ArticlePubMed
- 2. Jackman RJ, Nowels KW, Shepard MJ, Finkelstein SI, Marzoni FA Jr. Stereotaxic large-core needle biopsy of 450 nonpalpable breast lesions with surgical correlation in lesions with cancer or atypical hyperplasia. Radiology 1994; 193: 91-95. ArticlePubMed
- 3. Parker SH, Lovin JD, Jobe WE, Burke BJ, Hopper KD, Yakes WF. Nonpalpable breast lesions: stereotactic automated large-core biopsies. Radiology 1991; 180: 403-407. ArticlePubMed
- 4. Bonnett M, Wallis T, Rossmann M, et al. Histopathologic analysis of atypical lesions in image-guided core breast biopsies. Mod Pathol 2003; 16: 154-160. ArticlePubMed
- 5. Ely KA, Carter BA, Jensen RA, Simpson JF, Page DL. Core biopsy of the breast with atypical ductal hyperplasia: a probabilistic approach to reporting. Am J Surg Pathol 2001; 25: 1017-1021. PubMed
- 6. Harvey JM, Sterrett GF, Frost FA. Atypical ductal hyperplasia and atypia of uncertain significance in core biopsies from mammographically detected lesions: correlation with excision diagnosis. Pathology 2002; 34: 410-416. ArticlePubMed
- 7. Jackman RJ, Birdwell RL, Ikeda DM. Atypical ductal hyperplasia: can some lesions be defined as probably benign after stereotactic 11-gauge vacuum-assisted biopsy, eliminating the recommendation for surgical excision? Radiology 2002; 224: 548-554. ArticlePubMed
- 8. Renshaw AA, Cartagena N, Schenkman RH, Derhagopian RP, Gould EW. Atypical ductal hyperplasia in breast core needle biopsies: correlation of size of the lesion, complete removal of the lesion, and the incidence of carcinoma in follow-up biopsies. Am J Clin Pathol 2001; 116: 92-96. ArticlePubMed
- 9. Sneige N, Lim SC, Whitman GJ, et al. Atypical ductal hyperplasia diagnosis by directional vacuum-assisted stereotactic biopsy of breast microcalcifications: considerations for surgical excision. Am J Clin Pathol 2003; 119: 248-253. ArticlePubMed
- 10. Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast: a long-term follow-up study. Cancer 1985; 55: 2698-2708. ArticlePubMed
- 11. Tavassoli FA, Norris HJ. A comparison of the results of long-term follow-up for atypical intraductal hyperplasia and intraductal hyperplasia of the breast. Cancer 1990; 65: 518-529. ArticlePubMed
- 12. Bassett L, Winchester DP, Caplan RB, et al. Stereotactic core-needle biopsy of the breast: a report of the Joint Task Force of the American College of Radiology, American College of Surgeons, and College of American Pathologists. CA Cancer J Clin 1997; 47: 171-190. ArticlePubMed
- 13. Jacobs TW, Connolly JL, Schnitt SJ. Nonmalignant lesions in breast core needle biopsies: to excise or not to excise? Am J Surg Pathol 2002; 26: 1095-1110. PubMed
- 14. Liu X, Elojeimy S, Turner LS, et al. Acid ceramidase inhibition: a novel target for cancer therapy. Front Biosci 2008; 13: 2293-2298. ArticlePubMed
- 15. Brown TA, Wall JW, Christensen ED, et al. Atypical hyperplasia in the era of stereotactic core needle biopsy. J Surg Oncol 1998; 67: 168-173. ArticlePubMed
- 16. Hoang JK, Hill P, Cawson JN. Can mammographic findings help discriminate between atypical ductal hyperplasia and ductal carcinoma in situ after needle core biopsy? Breast 2008; 17: 282-288. ArticlePubMed
- 17. Chae BJ, Lee A, Song BJ, Jung SS. Predictive factors for breast cancer in patients diagnosed atypical ductal hyperplasia at core needle biopsy. World J Surg Oncol 2009; 7: 77.ArticlePubMedPMCPDF
- 18. Zografos GC, Zagouri F, Sergentanis TN, et al. Minimizing underestimation rate of microcalcifications excised via vacuum-assisted breast biopsy: a blind study. Breast Cancer Res Treat 2008; 109: 397-402. ArticlePubMedPDF
- 19. Jackman RJ, Burbank F, Parker SH, et al. Stereotactic breast biopsy of nonpalpable lesions: determinants of ductal carcinoma in situ underestimation rates. Radiology 2001; 218: 497-502. ArticlePubMed
- 20. Jackman RJ, Burbank F, Parker SH, et al. Atypical ductal hyperplasia diagnosed at stereotactic breast biopsy: improved reliability with 14-gauge, directional, vacuum-assisted biopsy. Radiology 1997; 204: 485-488. ArticlePubMed
- 21. Darling ML, Smith DN, Lester SC, et al. Atypical ductal hyperplasia and ductal carcinoma in situ as revealed by large-core needle breast biopsy: results of surgical excision. AJR Am J Roentgenol 2000; 175: 1341-1346. PubMed
- 22. Liberman L, Cohen MA, Dershaw DD, Abramson AF, Hann LE, Rosen PP. Atypical ductal hyperplasia diagnosed at stereotaxic core biopsy of breast lesions: an indication for surgical biopsy. AJR Am J Roentgenol 1995; 164: 1111-1113. ArticlePubMed
- 23. Philpotts LE, Lee CH, Horvath LJ, Lange RC, Carter D, Tocino I. Underestimation of breast cancer with II-gauge vacuum suction biopsy. AJR Am J Roentgenol 2000; 175: 1047-1050. ArticlePubMed
- 24. Winchester DJ, Bernstein JR, Jeske JM, et al. Upstaging of atypical ductal hyperplasia after vacuum-assisted 11-gauge stereotactic core needle biopsy. Arch Surg 2003; 138: 619-622. ArticlePubMed
- 25. Wagoner MJ, Laronga C, Acs G. Extent and histologic pattern of atypical ductal hyperplasia present on core needle biopsy specimens of the breast can predict ductal carcinoma in situ in subsequent excision. Am J Clin Pathol 2009; 131: 112-121. ArticlePubMedPDF
- 26. Doren E, Hulvat M, Norton J, et al. Predicting cancer on excision of atypical ductal hyperplasia. Am J Surg 2008; 195: 358-361. ArticlePubMed
- 27. Helvie MA, Hessler C, Frank TS, Ikeda DM. Atypical hyperplasia of the breast: mammographic appearance and histologic correlation. Radiology 1991; 179: 759-764. ArticlePubMed
- 28. Holland R, Hendriks JH. Microcalcifications associated with ductal carcinoma in situ: mammographic-pathologic correlation. Semin Diagn Pathol 1994; 11: 181-192. PubMed
- 29. Slanetz PJ, Giardino AA, Oyama T, et al. Mammographic appearance of ductal carcinoma in situ does not reliably predict histologic subtype. Breast J 2001; 7: 417-421. ArticlePubMed
- 30. Santisteban M, Reynolds C, Barr Fritcher EG, et al. Ki67: a time-varying biomarker of risk of breast cancer in atypical hyperplasia. Breast Cancer Res Treat 2010; 121: 431-437. ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations

- Active Surveillance for Atypical Ductal Hyperplasia and Ductal Carcinoma In Situ
Rachel Miceli, Cecilia L Mercado, Osvaldo Hernandez, Chloe Chhor
Journal of Breast Imaging.2023; 5(4): 396. CrossRef
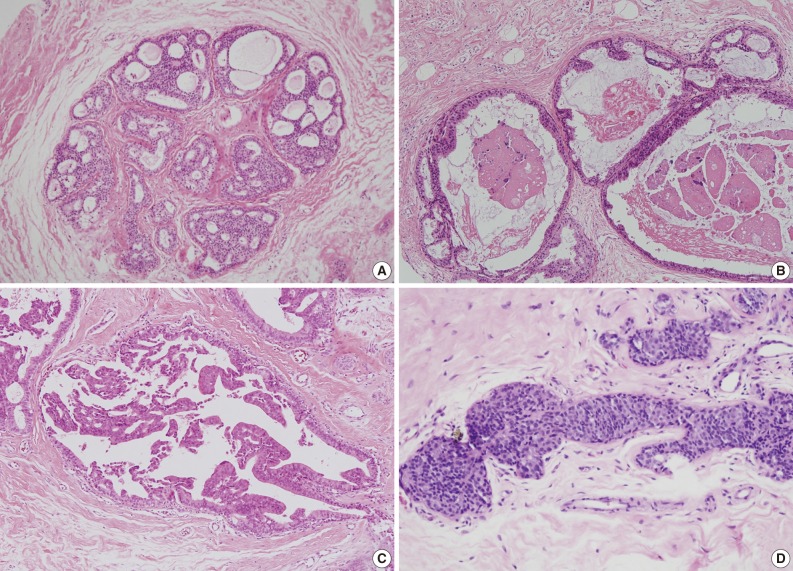
Fig. 1
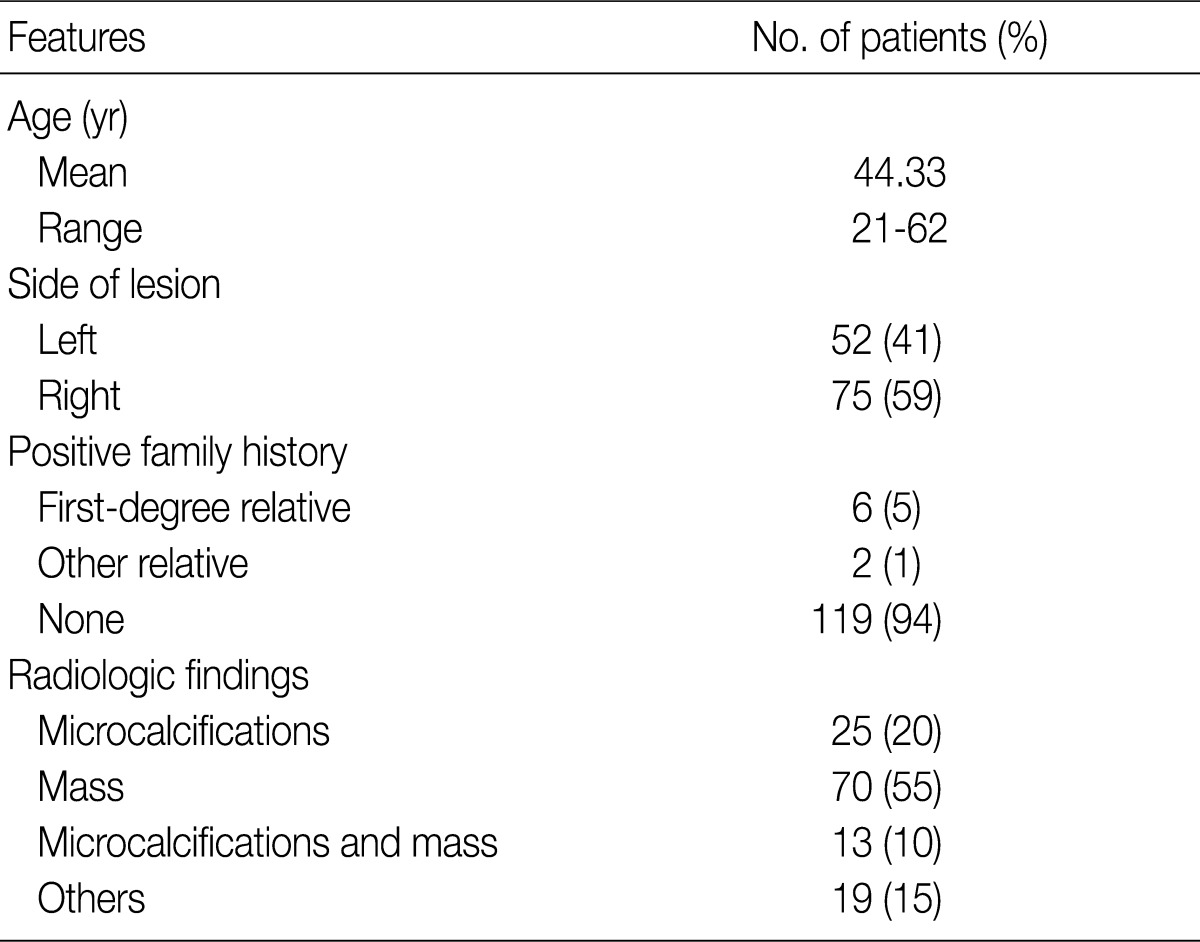
ADH, atypical ductal hyperplasia; CNB, core needle biopsy. aChi-squared test; bMean value; cKruskal-Wallis test.
ADH, atypical ductal hyperplasia; CNB, core needle biopsy; ALH, atypical lobular hyperplasia; FC ratio, ratio of ADH foci to number of cores. aChi-squared test.

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article


