Clinicopathologic Features of Q Fever Patients with Acute Hepatitis
Article information
Abstract
Background
Q fever caused by Coxiella burnetii presents with diverse clinical and pathological features including subclinical or cholestatic hepatitis. However, the pathological features of liver biopsies from patients with Q fever have not been well described.
Methods
Clinical features and pathological findings of liver biopsies were reviewed in seven cases of Q fever that were confirmed by serological, microbiological, or molecular tests.
Results
All cases presented with fever. Liver enzymes were mildly elevated except one case with marked hyperbilirubinemia. Characteristic fibrin ring granulomas were present in three cases, epithelioid granulomas with eosinophilic infiltration in two cases, extensive extravasated fibrins without ring configuration mimicking necrotizing granuloma in one case, and acute cholangitis without granuloma in one case. All cases were treated with antibiotics for 20 days. Six cases were completely cured, but one suffered from multiorgan failure.
Conclusions
C. burnetii infection is uncommon, but should always be considered in patients with acute hepatitis and fever. Because variable-sized circumferential or radiating fibrin deposition was a consistent feature of the present cases, Q fever can be strongly suggested by pathological features and confirmed by serological and/or molecular tests.
Q fever is a worldwide zoonotic disease caused by Coxiella burnetii, an obligate intracellular Gram negative bacterium.1 The main reservoirs are farm animals including cattle, sheep, goats, as well as pets.2 Transmission to humans occurs following inhalation of contaminated aerosols and dusts derived from infected animal products or the ingestion of raw milk or fresh goat cheese.2-4 The incubation period is 2-3 weeks after exposure.5
Clinical manifestations of C. burnetii infection are diverse, so the diagnosis can be made systematically. Q fever can be categorized into acute and chronic forms.5 Acute Q fever is more common than the chronic form. The acute form commonly presents as a self-limited febrile illness with flu-like symptoms, pneumonia, and subclinical hepatitis.6 Frank hepatitis with jaundice is rare,3 but hepatomegaly and mildly elevated liver enzyme levels are common. Chronic Q fever can develop months7 or years after acute illness,8 and there may be no history of acute illness. Sixty to seventy percent of the chronic form presents as endocarditis in patients with risk factors such as valvular heart disease, immunocompromised status, and pregnancy.9
Q fever is a rarely developed disease entity in Korea. According to a report by the National Institute of Health of Korea, there were 24 cases of Q fever diagnosed by the national reference laboratory from 2006 to March 2008.10 The most frequent presenting symptom of Q fever was fever of unknown origin (FUO). Many studies have described that most common cause of FUO is infectious disease, and useful diagnostic methods are biopies and radiological tests.11 A liver biopsy can be helpful for diagnosis, particularly in patients with acute Q fever presenting as hepatitis. Although fibrin ring granulomas are characteristic in acute Q fever hepatitis,12 details of the pathological features have not been described. Herein, we report the clinical and pathological features of liver biopsies from seven cases of acute Q fever.
MATERIALS AND METHODS
Seven liver biopsies from patients with acute Q fever were retrieved from the archives of the Department of Pathology, Asan Medical Center from January 1, 1989 to June 31, 2010. The confirmative diagnosis was obtained by serological or molecular tests. Among seven cases, five were diagnosed by indirect microimmuno-fluorescence assay. At least a four-fold increase in phase II IgG titers or phase II IgG titers of >1/200 and phase II IgM titers of >1/25 were regarded as diagnostic for acute Q fever. The other two cases were diagnosed by detecting C. burnetii DNA using nested polymerase chain reaction (PCR) and identifying intracellular C. burnetii by a serological test. Clinical findings and laboratory data were reviewed from electronic medical records.
Transjugular liver biopsy specimens of all cases were formalin-fixed and paraffin-embedded. Four-micrometer thick sections were stained with hematoxylin and eosin. All specimens were reviewed to assess the characteristic features of granulomas, the grades of portal and lobular activities, the types of cellular infiltrates, and the degree of fatty changes.
RESULTS
The cases included six males and one female with acute hepatitis. Patient age ranged from 33 to 70 years (mean age, 52 years). All patients had a history of acute onset fever. One patient (case 2) presented with jaundice, and another patient (case 1) suffered from diarrhea. The duration of symptoms ranged from 5-20 days, and the mean duration from the onset of symptoms to liver biopsy was 17 days (range, 12 to 30 days). Two cases (cases 1 and 6) had a history of tuberculous pleurisy and pulmonary tuberculosis, respectively. They were treated with antituberculous drugs and have recovered without complications. No patient had a history of chronic liver disease.
Abnormalities on liver function tests were variable as shown in Table 1. One patient (case 2) with severe jaundice showed marked elevation of total and direct bilirubin levels up to 23-fold higher than reference levels, whereas other liver enzymes were only slightly increased. No bacterial growth was noted on blood cultures of any patient. Antibodies for leptospirosis, brucellosis, hepatitis A, B, and C, as well as autoantibodies including anti-nuclear antibody, anti-double strand DNA antibody, and anti-neutrophilic cytoplasmic antibody were all negative.
In case 1, the PCR for Epstein Barr virus (EBV) using bone marrow aspirates was positive (1,560 copies/mL), and phase II IgG (1:512) and IgM (1:512) titers for EBV were elevated, whereas in situ hybridization for EBV early RNA using liver biopsy specimens, which was performed as an ancillary study, was negative. Although EBV-viral capsid antigen (VCA) IgG and EBV-Epstein Barr nuclear antigen IgG were detected on serological tests, the results of EBV-VCA IgM and EBV-early antigen IgM were negative. No additional viral copies were identified on two follow-up serum EBV PCR tests.
The sole female patient (case 7), who had taken a dose of prednisolone 6 months ago due to an asthma attack, had a positive serological result for herpes simplex virus-1 (HSV-1) by PCR at an outside hospital. She had been treated with an antiviral agent (acyclovir) for 3 days, but her symptoms did not subside, so she was transferred to our hospital for a second opinion. Unlike the previous result from the outside hospital, PCR tests in serum, urine, and bone marrow were negative for HSV-1 DNA, but a PCR test for C. burnetii was positive, so she was diagnosed with Q fever hepatitis.
Various types of granulomas were observed in six cases on liver biopsy (Table 2, Fig. 1). Typical fibrin ring granulomas consisted of a central fat vacuole, a fibrin ring, activated macrophages, and lymphocytes (Fig. 1A and E) and were found in three cases including a patient with a positive EBV PCR result in bone marrow (case 1). Epithelioid granulomas with numerous eosinophils and multinucleated giant cells were present in two cases (Fig. 1B). Distinctively extensive extravasated fibrin deposits resembling necrotizing granulomas were noted in one case (case 7) (Fig. 1C and F). A case with acute cholangitis showed no granulomas (case 2) (Fig. 1D). The grades of portal and lobular activity were mild in all cases. Lymphocytic and neutrophilic infiltrations were predominant, whereas eosinophils and plasma cells were variably noted. Fatty changes were observed to varying degrees; none in two cases, mild in four cases, and moderate in one case. No viral inclusion, acid-fast bacilli or periodic acid Schiff-positive fungi were observed.
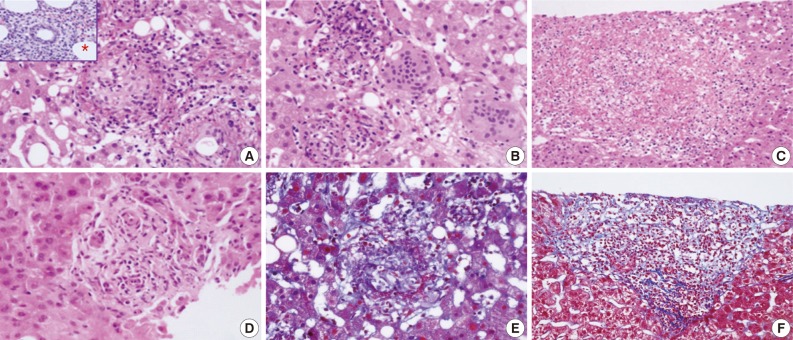
Granuloma types in liver biopsies from patients with acute Q fever hepatitis. (A) Typical fibrin ring granuloma consists of central lipid cores surrounded by a fibrin ring with mixed cellular infiltration. (B) Epithelioid granuloma with numerous eosinophils and multiple giant cells. (C) Extensive extravasated fibrin without ring configuration mimicking necrotizing granuloma. (D) Acute cholangitis without granulomas. (E) Typical fibrin ring granuloma (Masson's-trichrome stain). (F) Extravasated fibrin without ring configuration (Masson's-trichrome stain). *Inset of (A), fibrin ring granuloma from a bone marrow biopsy.
A bone marrow biopsy was performed in all patients. Characteristic fibrin ring granulomas were identified in three of seven bone marrow biopsies (Fig. 1A), and the other bone marrow biopsies showed normocellular marrow.
Six patients were treated with doxycyclin for 10-21 days, but one patient (case 3), who was clinically suspected of contracting typhoid fever, was treated with ceftriaxone and ciprofloxacine. One patient (case 2) died of multiorgan failure despite antibiotic therapy (doxycyclin, rifampin, and cefotaxim) for 20 days. The other six patients were cured without any sequelae after acute symptoms subsided gradually with antibiotic therapy.
DISCUSSION
The clinical presentations of Q fever are variable.6 The most frequent clinical feature of acute Q fever is a self-limiting febrile illness with a varying degree of pneumonia and hepatitis. Acute Q fever with hepatitis has been reported at rates of 9.0-67% in endemic areas, such as France, southern Spain, and Taiwan.13-15 In Korea, two cases of acute Q fever associated with hepatitis have been reported.16,17 Only one of the two cases underwent a liver biopsy, and typical fibrin ring granulomas were observed.17
Fever was the first symptom in all seven patients in the present study. Because FUO is a liver biopsy indication, a percutaneous or transjugular liver biopsy was performed before confirmative serological or molecular tests in all cases. Acute hepatitis, the next frequent clinical feature of patients with Q fever, generally presents with mild elevation of liver enzymes, hepatomegaly, and/or splenomegaly. However, one case (case 2) in the present study showed severe jaundice, which is rare in Q fever,3 and died of multiorgan failure. Chang et al.15 reported two of eight cases of acute Q fever hepatitis in south Taiwan who had >10 mg/dL total bilirubin. Although a correlation between hyperbilirubinemia and severity of acute Q fever hepatitis has not been demonstrated, the severity of hyperbilirubinemia is thought to be associated with prolongation of hospitalization, longer fever duration, and delayed recovery time.15
Acute Q fever with rapidly progressive hepatic failure has been reported in a patient with alcoholism,18 as in case 6 of the present study. The previously reported case revealed severe jaundice, hepatomegaly, elevated hepatic enzyme levels, and showed fibrous bands around regenerative liver parenchyma on the liver biopsy, indicating progression to cirrhotic change. In contrast, the present case presented only fever with a normal bilirubin level, a typical fibrin ring granuloma, and moderate fatty changes without significant fibrosis on the liver biopsy. The relationship between alcoholism and the severity of Q fever hepatitis needs to be investigated.
Fibrin ring granulomas (doughnut granulomas), which were the most frequent finding in this study, are not diagnostic but characteristic for Q fever, although they can be seen in various diseases such as tuberculosis, cytomegaloviral hepatitis, EBV hepatitis, toxoplasmosis, leishmaniasis, Hodgkin's disease, Crohn's disease, sarcoidosis, and drug-induced granulomatous hepatitis.19,20
Because EBV hepatitis may exhibits diverse histopathological features, including epithelioid noncaseating granulomas and extensive fibrin deposits resembling necrotizing granulomas in addition to characteristic findings of EBV hepatitis such as sinusoidal lymphocytic infiltration and atypical lymphocytes, the possibility of combined EBV hepatitis with acute Q fever hepatitis cannot be ruled out in case 1. A combined EBV and C. burnetii infection has not been reported in the English literature. The positive PCR result for EBV was thought to be an asymptomatic latent infection rather than an acute EBV infection such as infectious mononucleosis.
Herpes viral hepatitis could be excluded in case 7, because characteristic features of herpes viral infection, including confluent hemorrhagic necrosis, scattered acidophilic bodies, and intranuclear "ground glass" inclusions were absent on the liver biopsy, and C. burnetii DNA was detected by PCR. However, herpes simplex hepatitis should be considered in cases undergoing steroid administration21 as in this case.
Although acute Q fever is very rare in Korea, it should always be considered in patients with acute FUO and features of acute hepatitis, and may be strongly suggested by liver biopsy findings such as granulomas with either circumferential or radiating fibrin deposition. Furthermore, pathologists should recommend serological or molecular tests for differential or confirmative diagnosis of various infections including C. burnetii infection.
Notes
No potential conflict of interest relevant to this article was reported.