Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(5); 2014 > Article
-
Original Article
Preparation of Compact Agarose Cell Blocks from the Residues of Liquid-Based Cytology Samples - Suk Jin Choi, Yeon Il Choi, Lucia Kim, In Suh Park, Jee Young Han, Joon Mee Kim, Young Chae Chu
-
Korean Journal of Pathology 2014;48(5):351-360.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.5.351
Published online: October 27, 2014
Department of Pathology, Inha University Hospital, Inha University School of Medicine, Incheon, Korea
- Corresponding Author: Suk Jin Choi, M.D. Department of Pathology, Inha University Hospital, Inha University School of Medicine, 27 Inhang-ro, Jung-gu, Incheon 400-711, Korea Tel: +82-32-890-3980 Fax: +82-32-890-3464 E-mail: 204058@inha.ac.kr
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Inevitable loss of diagnostic material should be minimized during cell block preparation. We introduce a modified agarose cell block technique that enables the synthesis of compact cell blocks by using the entirety of a cell pellet without the loss of diagnostic material during cell block preparations. The feasibility of this technique is illustrated by high-throughput immunocytochemistry using high-density cell block microarray (CMA).
-
Methods
- The cell pellets of Sure- Path residues were pre-embedded in ultra-low gelling temperature agarose gel and re-embedded in standard agarose gel. They were fixed, processed, and embedded in paraffin using the same method as tissue sample processing. The resulting agarose cell blocks were trimmed and represented on a CMA for high-throughput analysis using immunocytochemical staining.
-
Results
- The SurePath residues were effectively and entirely incorporated into compact agarose cell buttons and embedded in paraffin. Sections of the agarose cell blocks revealed cellularities that correlated well with corresponding SurePath smears and had immunocytochemical features that were sufficient for diagnosis of difficult cases.
-
Conclusions
- This agarose-based compact cell block technique enables preparation of high-quality cell blocks by using up the residual SurePath samples without loss of diagnostic material during cell block preparation.
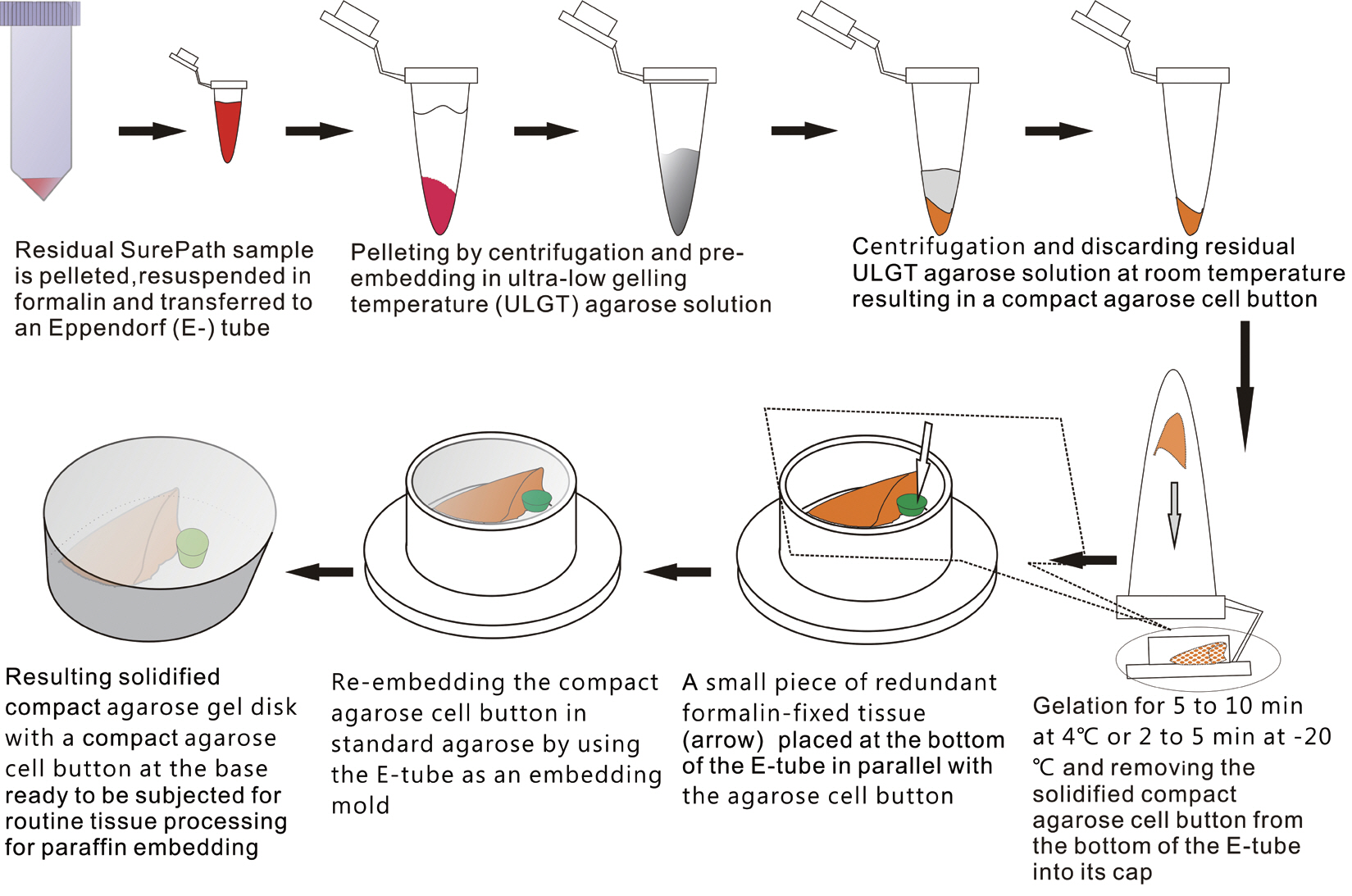
- The schematic flow chart of the protocol is represented in Fig. 1.
- Preparation of resuspending medium and re-embedding medium
- An ultra-low gelling temperature (ULGT) agarose (Agarose Type IX-A, Sigma-Aldrich, St. Louis, MO, USA) that gels at temperature <17°C was used as a resuspending medium. A standard agarose (Agarose Type I-A, Sigma-Aldrich) that gels at <36°C was used as a re-embedding medium. Each agarose material was melted in boiling water at 3% (w/v). In order to preserve gelation quality of the agarose solutions, they were kept at 4°C with the cap fully tightened. When ready to be used, they were re-melted using a microwave oven. The re-melted ULGT agarose solution was then kept at room temperature while the re-melted standard agarose solution was kept in the oven set at 60°C to prevent premature solidification prior to use.
- Preparation of compact cell buttons
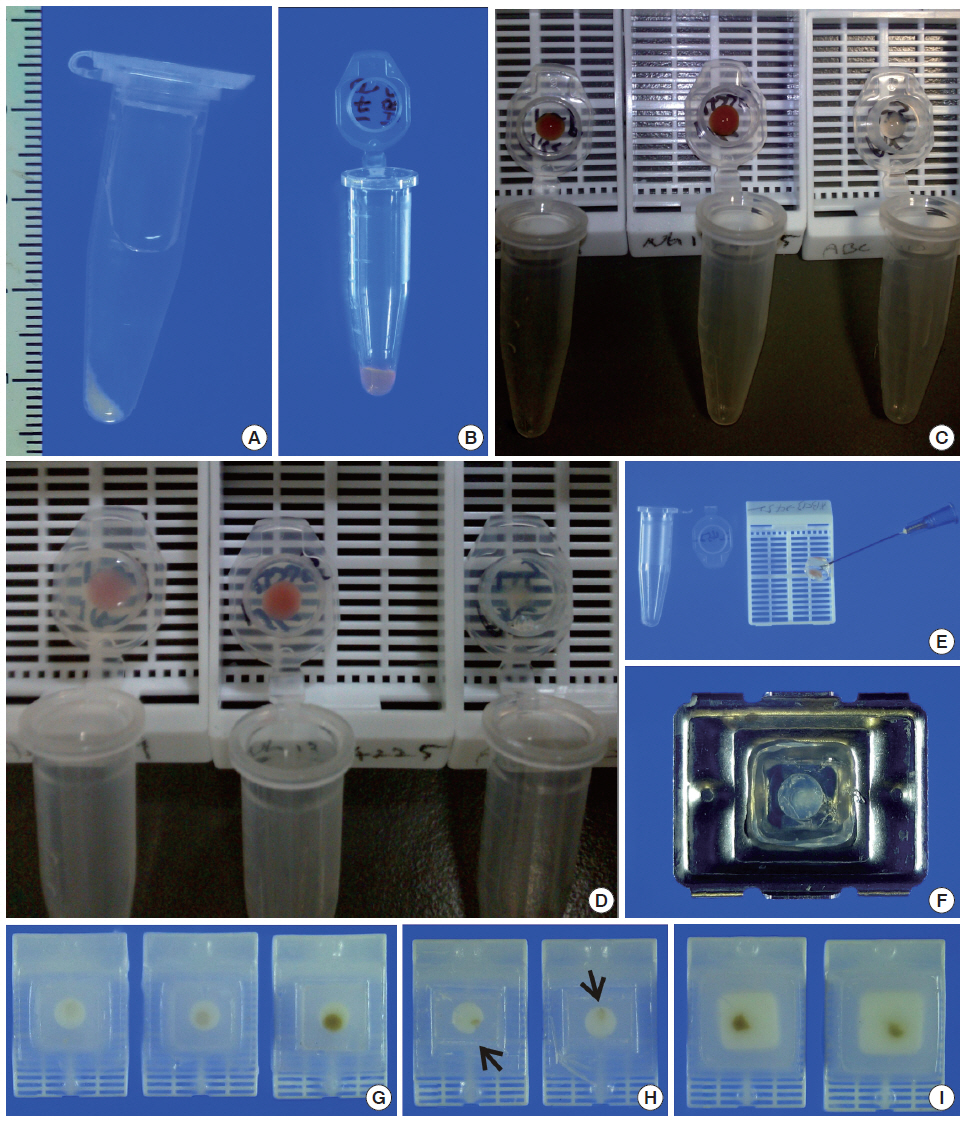
- We recruited consecutive 37 cytology case samples to be included in this study. There were cases that were from residual LBC samples after rendering the cytodiagnosis based on SurePath smears (SurePath, TriPath Care Technologies, Burlington, NC, USA), including 30 ultrasound-guided thyroid fine needle aspirates (FNAs), two lymph node FNAs and five serous effusions. Each SurePath residue was subjected to centrifugation and the cell pellet was resuspended in 200–300 µL buffered formalin. After incubation for 1 hour at room temperature, the suspension was entirely transferred to a 1.5 mL Eppendorf reaction tube and was centrifuged on a table-top centrifuge for 30 seconds at 15,000 rpm (Fig. 2A). Then, the supernatant was carefully discarded by pipetting, leaving a formalin-fixed cell pellet.
- Depending on the size of the cell pellet or cellularity of the corresponding SurePath smear, the cell pellet was resuspended with 50 to 100 µL ULGT agarose solution (Fig. 2B). Following re-centrifugation of the suspension on a table-top centrifuge at 15,000 rpm for 30 seconds, the supernatant ULGT agarose solution was carefully discarded by pipetting. Finally, the resulting compact agarose cell button was allowed to solidify for 5 to 10 minutes at 4°C or 2 to 5 minutes at –20°C.
- Preparation of compact agarose cell blocks
- The solidified compact agarose cell button was removed into the cap of an Eppendorf reaction tube by tapping upside down on a hard surface or manually with a metal ear-pick (Fig. 2C).Then, the cap of the Eppendorf reaction tube was filled with standard agarose solution so that the agarose cell button was embedded at the base of the standard agarose gel (Fig. 2D). When the initial agarose cell button was nearly transparent, it was marked in advance with a tissue marking dye that could be used to indicate the optimal cutting level of the cell block. Alternatively, a small piece of redundant formalin-fixed tissue was embedded in parallel with the agarose cell button. Following solidification of the standard agarose gel at room temperature for 1 to 2 minutes, the resulting agarose gel disk with an agarose cell button at the base was carefully removed from the cap of the tube using a 22-gauge needle (Fig. 2E). When the agarose gel disk was fractured during this step, it was carefully reconstructed and re-embedded in standard agarose gel (Fig. 2F). The resulting agarose gel disk was placed in a tissue cassette without additional wrapping and then was subjected to routine tissue processing under standard conditions using an automated tissue processor machine and embedded in paraffin. Finally, the cell block was trimmed to expose the agarose cell button (Fig. 2G–I), from which serial 3 to 5 sections were cut at 4-µm thickness and mounted on a slide for hematoxylin and eosin (H&E) staining.
- Construction of cell block microarray for high throughput immunocytochemistry
- To save time and resources in examining the applicability of the agarose cell blocks to adjuvant immunocytochemistry, the cores of paraffin-embedded agarose cell buttons in the agarose cell blocks were represented in a CMA[16]. In order to incorporate the entire cases into a single CMA, we used a self-made manual microarray kit and a homemade recipient block as described previously (Fig. 3)[17,18]. Tissue cores from redundant FFPET were also arrayed in the same CMA to be used as macroscopic orientation markers of the CMA sections. Serial sections were cut from the CMA and routinely processed for H&E and immunostaining. An automated slide stainer (Ventana BenchMark XT, Ventana Medical Systems Inc., Tucson, AZ, USA) and the Ventana OptiView DAB detection kit were used to analyze expression of markers (Table 1) using the same antibodies and protocols standardized for immunostaining of FFPET sections. A digital slide scanner (VM600, Motic, Xiamen, China) was used to acquire the entire image of the H&E and immunostained CMA sections.
MATERIALS AND METHODS
- Compact agarose cell blocks
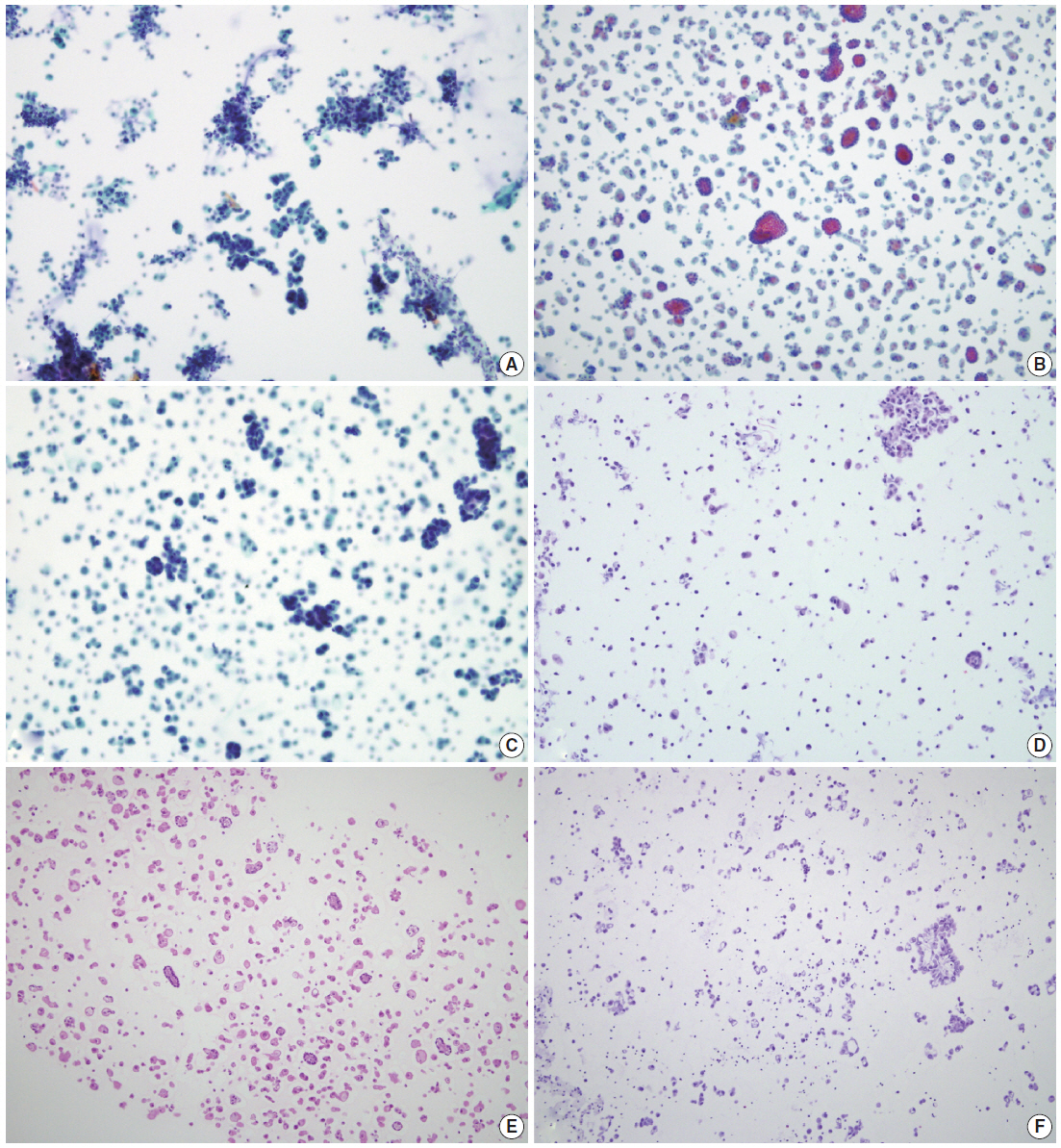
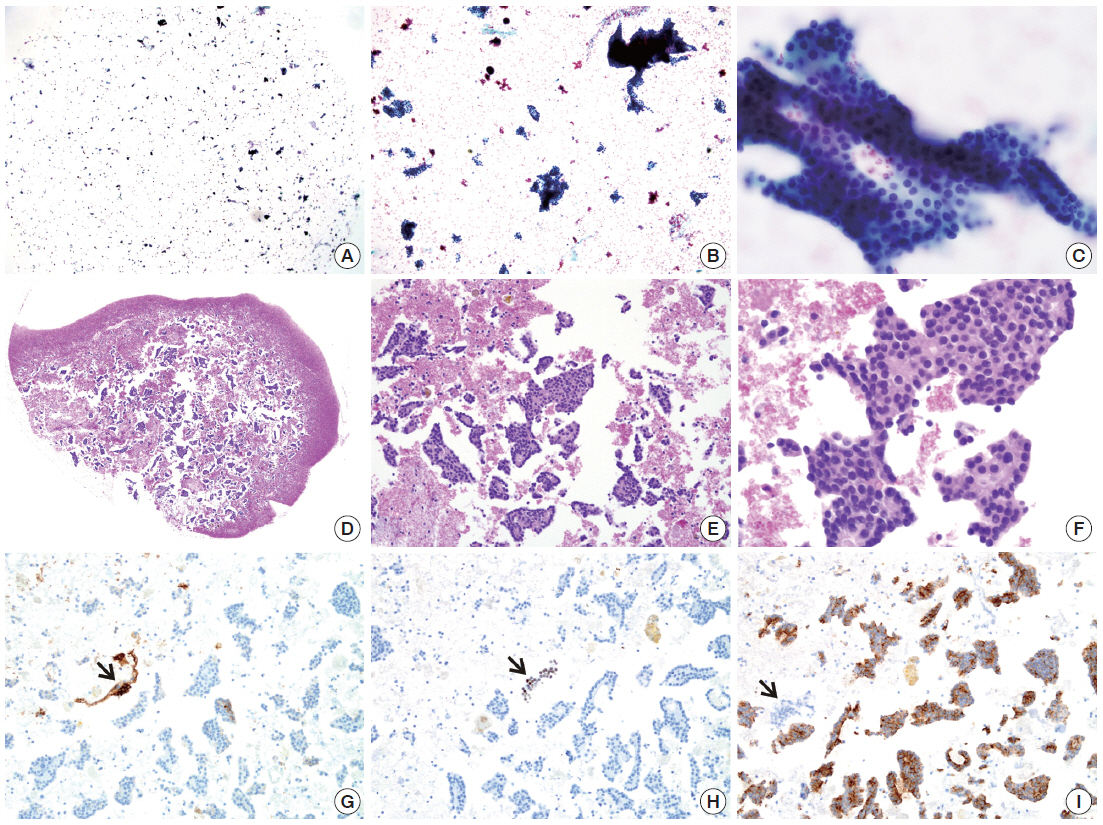
- By using ULGT agarose solution as a resuspending medium, the residual diagnostic materials in the residual SurePath sample was easily and entirely incorporated into a compact agarose cell button which measured 3 to 5 mm in diameter. By using the cap of an Eppendorf reaction tube as an embedding mold and the standard agarose as a pre-embedding medium, the compact agarose cell button was easily integrated at the base of an agarose gel disk, which was easy to manipulate for further procedures for tissue processing and paraffin embedding. Following the routine 12- to 13-hour tissue processing, all of the agarose gel disks were well impregnated with paraffin and retained their original size and shape. With the aid of FFPET embedded in parallel or a marking dye applied in advance to the agarose cell buttons, it was not difficult for the histotechnologists to determine the optimal cutting level of the cell blocks. Although a thin layer of nearly transparent agarose gel was present in the H&E-stained sections, it did not obscure the cytologic and architectural features because it was retained outside the cells or tissue fragments. The cytomorphologic features of the cell block sections were supportive for the original diagnosis of the corresponding SurePath smears (Figs. 4, 5).
- Construction of cell block microarray for high-throughput immunocytochemistry
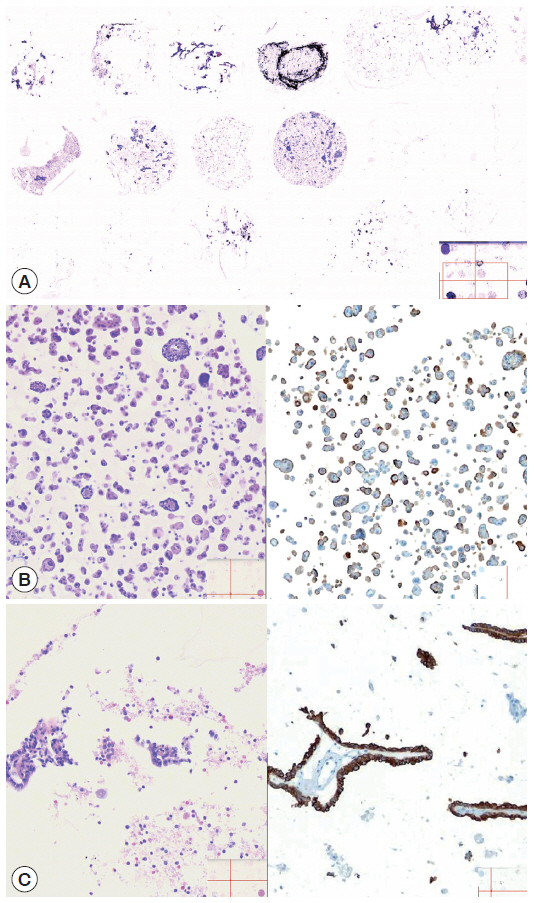
- The cellularity yield of each cytology spot on the CMA section correlated well with that of the corresponding section of the donor agarose cell blocks (Fig 6A). With the entirety of the diagnostic material concentrated on a viewing cytology spot of each cell block, it was possible to minimize differences in celluarity yield through serial sections of the CMA cut for a series of immunocytochemistry using panels of diagnostic markers (Table 2). Immunocytochemical analyses of the cytology spots on the CMA provided additional information supportive for the diagnosis of cytologically difficult cases (Figs. 6B, 6C, 7).
RESULTS
- Although smears of alcohol-fixed liquid-based or cytocentrifuged preparations of cytology samples can be used for complementary immunocytochemistry, the quality of the immunostains from smear preparations cannot be always identical to that of formalin-fixed paraffin embedded cell block sections[4,5]. This is because the cell block provides the most reliable form of sample preparation that produces results identical to that of FFPET. Furthermore, multiple sections can be cut from the cell blocks that can be effectively used when definitive differential diagnosis should be rendered only based on appropriate histologic, immunocytochemical or cytochemical findings[19].
- In order to be used in routine ancillary tests such as immunocytochemistry, a cell block should retain a yield of cellularity sufficient enough for ancillary tests. More often than not, however, H&E-stained and/or immunostained sections of conventionally prepared cell blocks fail to provide additional clues for differential diagnosis even though the corresponding smear preparations of the cytology sample retain sufficient cellularities. This is because the informative residual materials remained after routine smear preparations cannot always be incorporated entirely into the cell blocks due to technical difficulity[3,7]. This is because considerable loss or dilution of diagnostically important materials can occur during the preparation of cell blocks using the conventional simple sedimentation method. This problem mainly occurs when cell pellets are manually removed from the tubes, wrapped in lens paper and transferred into tissue cassettes before subjected to routine tissue processing for paraffinization[20]. Bloody samples should be another major cause of scarce cellularity in cell block sections because the peripheral blood further dilutes the cells or tissue fragments of interest.
- To overcome the loss of material during cell block preparation, various supporting media such as bacterial agar, egg albumin, and plasma-thrombin have been used[21]. Among these, preparation of agarose cell blocks by using small volumes of agarose solution as pre-embedding media can be a simple and cost-effective method because cell buttons pre-embedded in standard agarose gel are easy to handle during the procedures for paraffin embedding[8-10]. The advantages of the agarose cell block technique has been already confirmed[22-24]. However, the major drawback of this technique is that the agarose solution should be kept in a temperature not lower than the gelling temperature of agarose solution to prevent premature solidification of the agarose solution while cell pellets are resuspended. If this inadvertently occurs, the cells or tissue fragments within the cell pellets will not be evenly distributed throughout the agarose cell button. Although the agarose solution can be kept in a preheated heat block in an oven set at 60°C during the resuspending step, it could be technically difficult for routine cell block preparation in daily practice.
- Mansy[11] took the advantage of the agarose cell block technique using low melting temperature (LGT) agarose to make agarose cell blocks from urine cytology samples. In several preceding trials with this method, however, we have found that the usual LGT agarose solution should also be kept at a temperature above room temperature (usually >30°C) to prevent premature solidification. Otherwise, it was not possible to have diagnostic cells or tissue fragments evenly distributed throughout different levels. This was especially the case when we tried to resuspend cell pellets with small volumes of agarose solution to make small agarose cell button as compact as possible. To overcome this problem, we modified the method for agarose cell block preparation using two types of agarose that become gels at different temperatures: ULGT agarose is for preparation of agarose cell buttons and standard agarose is for re-embedding in agarose gel disks. Because the ULGT agarose solution does not solidify at room temperature, it can be effectively used to resuspend the formalin-fixed cell pellet from the LBC sample that remains after smear preparation. In addition, the suspension can be centrifuged again at room temperature in order to get more concentrated agarose cell buttons. We presume that this modified technique enables the diagnostic material in the residual LBC samples to be transferred as entirely as possible into small agarose cell buttons that are as compact as possible.
- In the preliminary experiment to modify the agarose cell block technique, we found that the gelation property of the agarose solution significantly deteriorates with time when the agarose solution was kept in the oven at 60°C for more than 1 month. Furthermore, the agarose solution kept in the oven more than 2 months changed to brownish yellow color and the gelation property was completely lost. To prevent this problem, we aliquoted the agarose solutions into one time use aliquots and stored them at 4°C until they were re-melted using a microwave. Because only a small amount of agarose is needed for agarose cell block preparations, there was no significant increase in cost for agarose cell block preparation. Furthermore, because solidified agarose buttons and agarose gel disks are easier to manipulate compared to wrapping the cell pellet with lens paper, the time and work load do not substantially increase compared to conventional simple sedimentation method, although we did not analyze this observation statistically.
- In conclusion, we illustrated a modified agarose cell block technique to obtain compact agarose cell blocks that prevents significant loss of diagnostic cells or tissue during cell block preparation. This modified technique can be widely applied in daily cytopathology practice, especially when the cellularity of the cytologic sample is expected to be low.
DISCUSSION
Acknowledgments


- 1. Khan S, Omar T, Michelow P. Effectiveness of the cell block technique in diagnostic cytopathology. J Cytol 2012; 29: 177-82. ArticlePubMedPMC
- 2. Ikeda K, Tate G, Suzuki T, Mitsuya T. Comparison of immunocytochemical sensitivity between formalin-fixed and alcohol-fixed specimens reveals the diagnostic value of alcohol-fixed cytocentrifuged preparations in malignant effusion cytology. Am J Clin Pathol 2011; 136: 934-42. ArticlePubMed
- 3. Fetsch PA, Simsir A, Brosky K, Abati A. Comparison of three commonly used cytologic preparations in effusion immunocytochemistry. Diagn Cytopathol 2002; 26: 61-6. ArticlePubMed
- 4. Udasimath S, Arakeril SU, Karigowdar MH, Yelikar BR. The role of the cell block method in the diagnosis of malignant ascitic fluid effusions. J Clin Diagn Res 2012; 6: 1280-3.
- 5. Fowler LJ, Lachar WA. Application of immunohistochemistry to cytology. Arch Pathol Lab Med 2008; 132: 373-83. ArticlePubMedPDF
- 6. Zito FA, Gadaleta CD, Salvatore C, et al. A modified cell block technique for fine needle aspiration cytology. Acta Cytol 1995; 39: 93-9. PubMed
- 7. Hecht SA, McCormack M. Comparison of three cell block techniques for detection of low frequency abnormal cells. Pathol Lab Med Int 2013; 5: 1-7. Article
- 8. Olson NJ, Gogel HK, Williams WL, Mettler FA Jr. Processing of aspiration cytology samples. An alternative method. Acta Cytol 1986; 30: 409-12. PubMed
- 9. Kerstens HM, Robben JC, Poddighe PJ, et al. AgarCyto: a novel cell-processing method for multiple molecular diagnostic analyses of the uterine cervix. J Histochem Cytochem 2000; 48: 709-18. ArticlePubMedPDF
- 10. Mayall F, Chang B, Darlington A. A review of 50 consecutive cytology cell block preparations in a large general hospital. J Clin Pathol 1997; 50: 985-90. ArticlePubMedPMC
- 11. Mansy SS. Agarose cell block: innovated technique for the processing of urine cytology for electron microscopy examination. Ultrastruct Pathol 2004; 28: 15-21. ArticlePubMed
- 12. Schieven LW, Smedts F, Hopman AH, van der Wijk J, Nijman RJ, de Jong IJ. Fine needle aspiration using improved agar microbiopsy is highly concordant with renal mass final diagnosis and subclassification. J Urol 2009; 182: 2590-3. ArticlePubMed
- 13. Wagner DG, Russell DK, Benson JM, Schneider AE, Hoda RS, Bonfiglio TA. Cellient automated cell block versus traditional cell block preparation: a comparison of morphologic features and immunohistochemical staining. Diagn Cytopathol 2011; 39: 730-6. ArticlePubMed
- 14. Zhao J, Lin DL, Zhai LH, Wang JG. Evaluation of ultrasound-processed rapid cell blocks in the cytopathologic diagnosis of cavity fluids. Acta Cytol 2014; 58: 182-91. ArticlePubMedPDF
- 15. Crapanzano JP, Heymann JJ, Monaco S, Nassar A, Saqi A. The state of cell block variation and satisfaction in the era of molecular diagnostics and personalized medicine. Cytojournal 2014; 11: 7.ArticlePubMedPMC
- 16. Pu RT, Giordano TJ, Michael CW. Utility of cytology microarray constructed from effusion cell blocks for immunomarker validation. Cancer 2008; 114: 300-6. ArticlePubMed
- 17. Choi CH, Kim KH, Song JY, et al. Construction of high-density tissue microarrays at low cost by using self-made manual microarray kits and recipient paraffin blocks. Korean J Pathol 2012; 46: 562-8. ArticlePubMedPMC
- 18. Kim KH, Choi SJ, Choi YI, et al. In-house manual construction of high-density and high-quality tissue microarrays by using homemade recipient agarose-paraffin blocks. Korean J Pathol 2013; 47: 238-44. ArticlePubMedPMC
- 19. Liu K, Dodge R, Glasgow BJ, Layfield LJ. Fine-needle aspiration: comparison of smear, cytospin, and cell block preparations in diagnostic and cost effectiveness. Diagn Cytopathol 1998; 19: 70-4. ArticlePubMed
- 20. Hecht SA, McCormack M. Comparison of three cell block techniques for detection of low frequency abnormal cells. Pathol Lab Med Int 2013; 5: 1-7. Article
- 21. Bales CE, Durfee GR. Cytological technique, part 1. In: Koss LG, Melamed MR, eds. Koss’ diagnostic cytology and its histopathologic bases. 4th ed. Philadelphia: Lippincott JB, 1992; 1451-74.
- 22. Zanoni DS, Grandi F, Cagnini DQ, Bosco SM, Rocha NS. Agarose cell block technique as a complementary method in the diagnosis of fungal osteomyelitis in a dog. Open Vet J 2012; 2: 19-22. ArticlePubMedPMC
- 23. Zanoni DS, Grandi F, Rocha NS. Use of the agarose cell block technique in veterinary diagnostic cytopathology: an “old and forgotten” method. Vet Clin Pathol 2012; 41: 307-8. ArticlePubMed
- 24. Mansy SS, Abbas MA, Yehia HA, Abdelrazik SM, Ghanem LY, Amin TM. Value of the innovated technique agarose cell block in improving the sensitivity of urine cytology in cases of bladder carcinoma. Ultrastruct Pathol 2006; 30: 379-85. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Establishing 3D organoid models from patient-derived conditionally reprogrammed cells to bridge preclinical and clinical insights in pancreatic cancer
Jin Su Kim, Chan Hee Park, Eunyoung Kim, Hee Seung Lee, Jinyoung Lee, Jeehoon Kim, Eun Hee Kam, Sanghee Nam, Moon Jae Chung, Jeong Youp Park, Seung Woo Park, Sangwoo Kim, Galam Leem, Seungmin Bang
Molecular Cancer.2025;[Epub] CrossRef - Assessing Determinants of Response to PARP Inhibition in Germline ATM Mutant Melanoma
Eleonora Allavena, Michela Croce, Bruna Dalmasso, Cecilia Profumo, Valentina Rigo, Virginia Andreotti, Irene Vanni, Benedetta Pellegrino, Antonino Musolino, Nicoletta Campanini, William Bruno, Luca Mastracci, Gabriele Zoppoli, Enrica Teresa Tanda, Frances
International Journal of Molecular Sciences.2025; 26(15): 7420. CrossRef - Immunocytochemistry on frozen-embedded cell block for the diagnosis of hematolymphoid cytology specimen: a straightforward alternative to the conventional cell block
Youjeong Seo, Sanzida Alam Prome, Lucia Kim, Jee Young Han, Joon Mee Kim, Suk Jin Choi
Journal of Hematopathology.2024; 17(1): 1. CrossRef - Comparison of liquid-based cytology and cell blocks prepared from cell remnants for diagnosis of cervical pathology
Elif Kuzucular, Ferhat Ozden, Bahar Muezzinoglu
Annals of Diagnostic Pathology.2024; 69: 152265. CrossRef - Advances in diagnostic liquid‐based cytology
Hideyuki Abe, Akihiko Kawahara, Jun Akiba, Rin Yamaguchi
Cytopathology.2024; 35(6): 682. CrossRef - Enhancing diagnostic precision in thyroid nodule assessment: evaluating the efficacy of a novel cell preservation technique in fine-needle aspiration cytology
Diana-Raluca Streinu, Octavian Constantin Neagoe, Andreea Borlea, Ion Icma, Mihnea Derban, Dana Stoian
Frontiers in Endocrinology.2024;[Epub] CrossRef - Cell blocks in cytology: review of preparation methods, advantages, and limitations
Vanda F. Torous, Jacqueline M. Cuda, Varsha Manucha, Melissa L. Randolph, Qiuying Shi, Christopher J. VandenBussche
Journal of the American Society of Cytopathology.2023; 12(2): 77. CrossRef - Cerebral Organoid Arrays for Batch Phenotypic Analysis in Sections and Three Dimensions
Juan Chen, Haihua Ma, Zhiyu Deng, Qingming Luo, Hui Gong, Ben Long, Xiangning Li
International Journal of Molecular Sciences.2023; 24(18): 13903. CrossRef - Diagnosis of pancreatic solid pseudopapillary neoplasms using cell‐blocks and immunohistochemical evaluation of endoscopic ultrasound‐guided fine needle aspiration biopsy specimens
José Celso Ardengh, César Vivian Lopes, Filadélfio Euclides Venco, Marcel Autran Machado
Cytopathology.2021; 32(1): 50. CrossRef - Somatostatin receptor 2 expression in nasopharyngeal cancer is induced by Epstein Barr virus infection: impact on prognosis, imaging and therapy
Matt Lechner, Volker H. Schartinger, Christopher D. Steele, Wen Long Nei, Marc Lucas Ooft, Liesa-Marie Schreiber, Christodoulos P. Pipinikas, Grace Tin-Yun Chung, Yuk Yu Chan, Feng Wu, Ka-Fai To, Chi Man Tsang, Wayne Pearce, Daniele Morelli, Martin Philpo
Nature Communications.2021;[Epub] CrossRef - Utility of PD‐L1 immunocytochemistry using body‐fluid cell blocks in patients with non‐small‐cell lung cancer
Seung Geun Song, Jonghoon Lee, Jaemoon Koh, Sehui Kim, Doo Hyun Chung, Yoon Kyung Jeon
Diagnostic Cytopathology.2020; 48(4): 291. CrossRef - Agarose cell block and ancillary molecular tests enhance diagnostic efficacy of liquid-based cytology samples
EmanS Abusinna, MervatM El-Deftar, YasmineF El-Esawy
Egyptian Journal of Pathology.2020; 40(2): 169. CrossRef - Brain-Derived Neurotrophin and TrkB in Head and Neck Squamous Cell Carcinoma
József Dudás, Anna Riml, Raphaela Tuertscher, Christian Pritz, Teresa Bernadette Steinbichler, Volker Hans Schartinger, Susanne Sprung, Rudolf Glueckert, Anneliese Schrott-Fischer, Lejo Johnson Chacko, Herbert Riechelmann
International Journal of Molecular Sciences.2019; 20(2): 272. CrossRef - Pleiotropic Effects of Epithelial Mesenchymal Crosstalk on Head and Neck Cancer: EMT and beyond
T. B. Steinbichler, D. Savic, D. Dejaco, A. Romani, B. Kofler, I. I. Skvortsova, H. Riechelmann, J. Dudas
Cancer Microenvironment.2019; 12(2-3): 67. CrossRef - Microarray Embedding/Sectioning for Parallel Analysis of 3D Cell Spheroids
Jonathan Gabriel, David Brennan, Jennifer H. Elisseeff, Vince Beachley
Scientific Reports.2019;[Epub] CrossRef - Photodynamic Effect of Methylene Blue and Low Level Laser Radiation in Head and Neck Squamous Cell Carcinoma Cell Lines
Barbara Kofler, Angela Romani, Christian Pritz, Teresa Steinbichler, Volker Schartinger, Herbert Riechelmann, Jozsef Dudas
International Journal of Molecular Sciences.2018; 19(4): 1107. CrossRef - Predictors of Response to Autologous Dendritic Cell Therapy in Glioblastoma Multiforme
Chia-Ing Jan, Wan-Chen Tsai, Horng-Jyh Harn, Woei-Cherng Shyu, Ming-Chao Liu, Hsin-Man Lu, Shao-Chih Chiu, Der-Yang Cho
Frontiers in Immunology.2018;[Epub] CrossRef - Nerve Growth Factor (NGF)—Receptor Survival Axis in Head and Neck Squamous Cell Carcinoma
József Dudás, Wolfgang Dietl, Angela Romani, Susanne Reinold, Rudolf Glueckert, Anneliese Schrott-Fischer, Daniel Dejaco, Lejo Johnson Chacko, Raphaela Tuertscher, Volker Hans Schartinger, Herbert Riechelmann
International Journal of Molecular Sciences.2018; 19(6): 1771. CrossRef - Cell blocks in cytopathology: An update
Aruna Nambirajan, Deepali Jain
Cytopathology.2018; 29(6): 505. CrossRef - Cell transfer technique for constructing cytological microarrays for immunocytochemical analysis
C.‐H. Wen, C.‐H. Lin, P.‐L. Ko, Y.‐F. Kuo, Y.‐J. Chen, C.‐Y. Chai
Cytopathology.2017; 28(2): 157. CrossRef - Cell and Tissue Display
Nicholas Theodosakis, Goran Micevic, Marcus W. Bosenberg, Nemanja Rodić
Journal of Histochemistry & Cytochemistry.2016; 64(7): 403. CrossRef - Diagnostic Usefulness of Claudin-3 and Claudin-4 for Immunocytochemical Differentiation between Metastatic Adenocarcinoma Cells and Reactive Mesothelial Cells in Effusion Cell Blocks
Nah Ihm Kim, Ga-Eon Kim, Ji Shin Lee
Acta Cytologica.2016; 60(3): 232. CrossRef - Do We Know What Is in Our Samples?
Louise Gilroy, Kathy Walsh, Anca Oniscu
Journal of Thoracic Oncology.2015; 10(12): e122. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
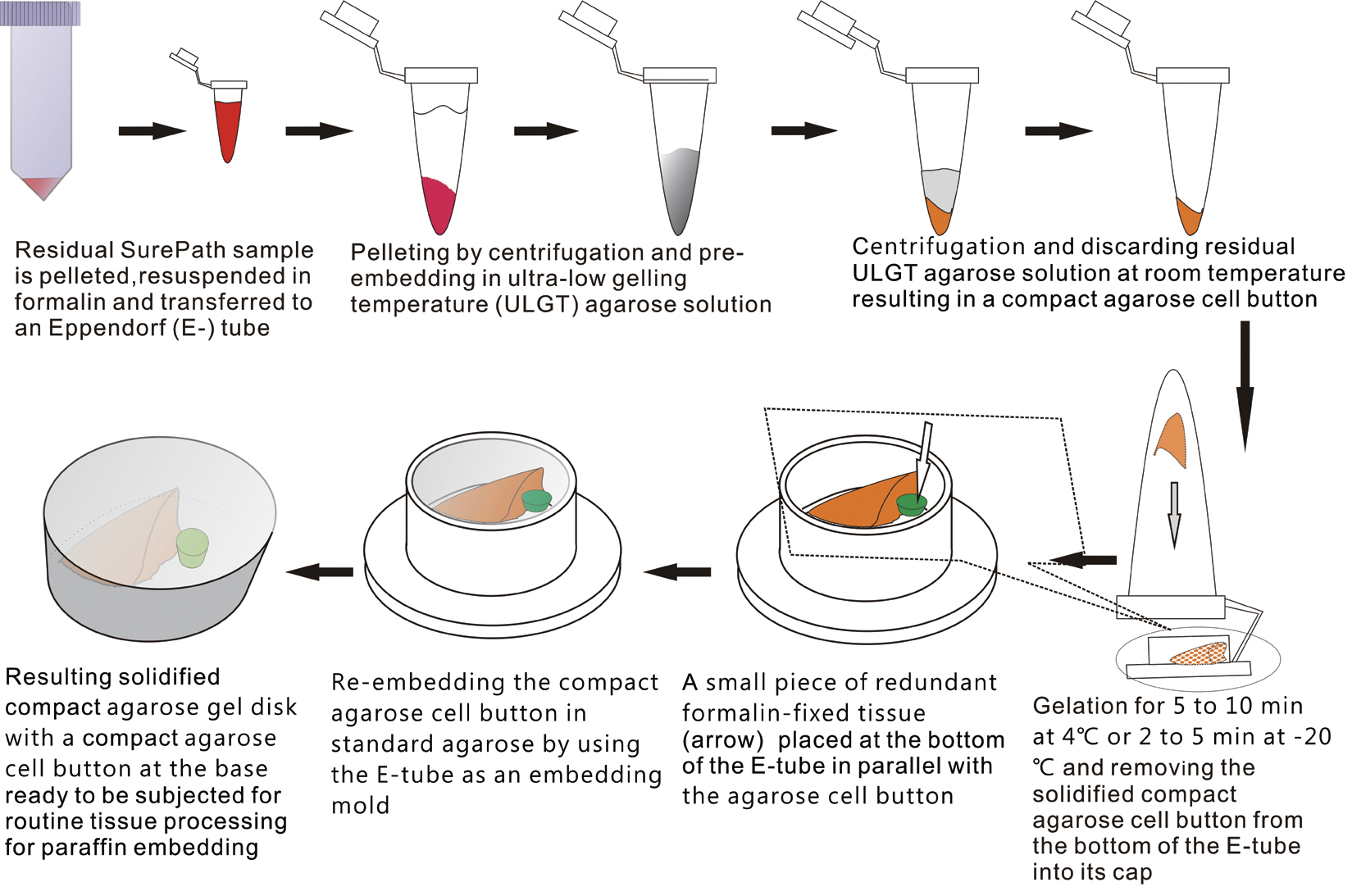
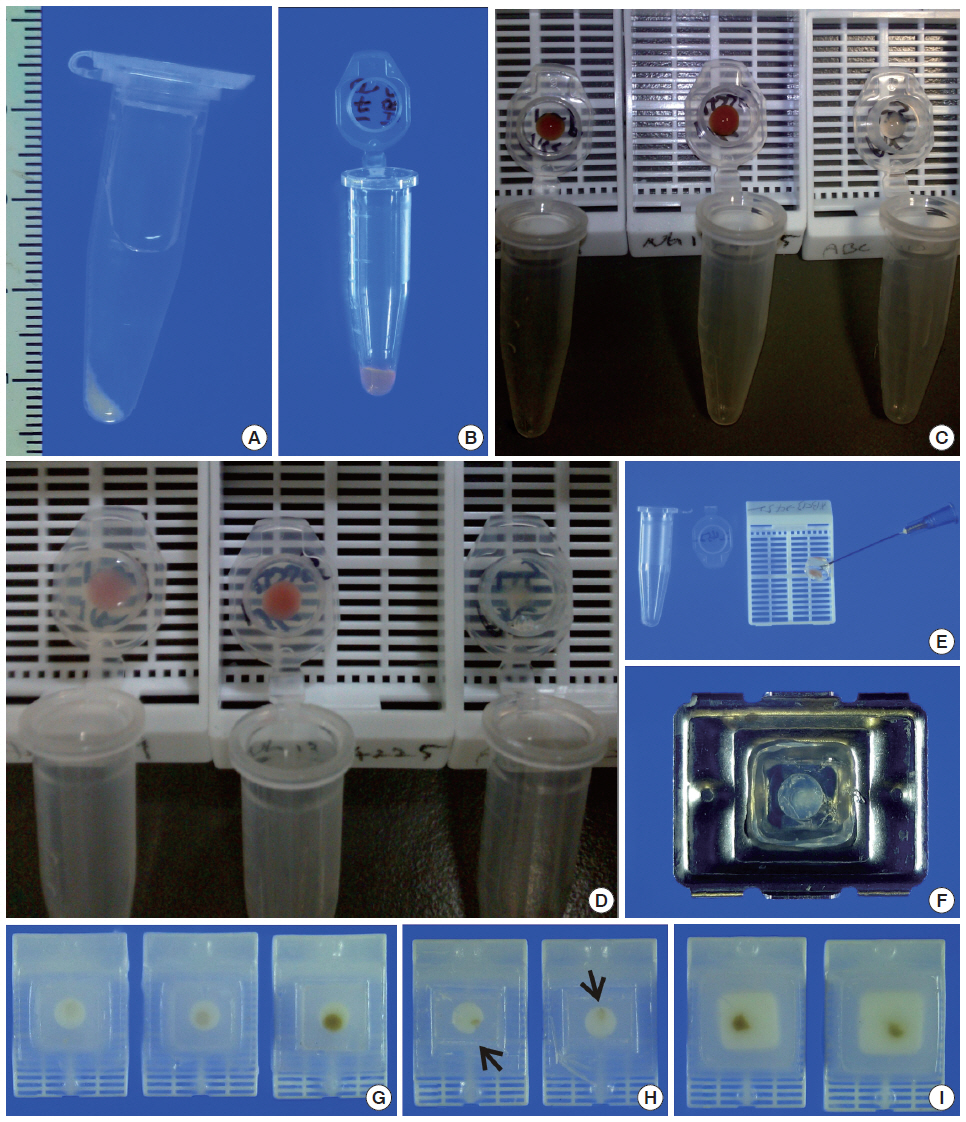

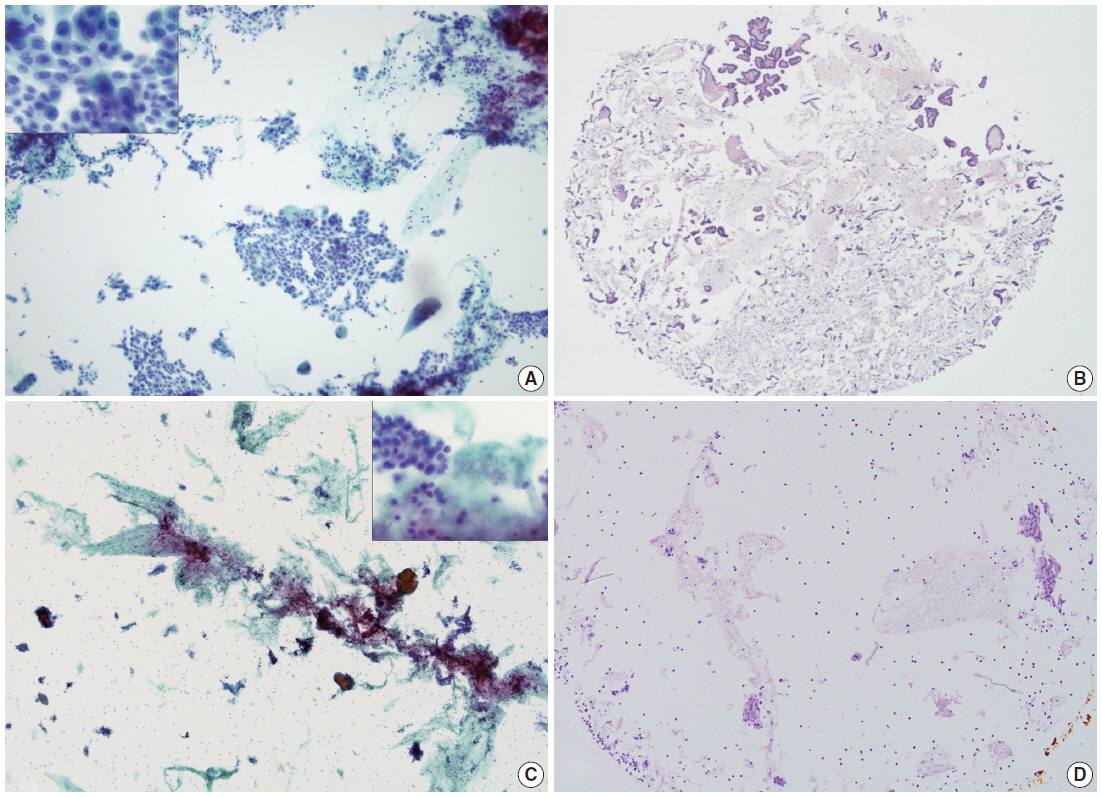

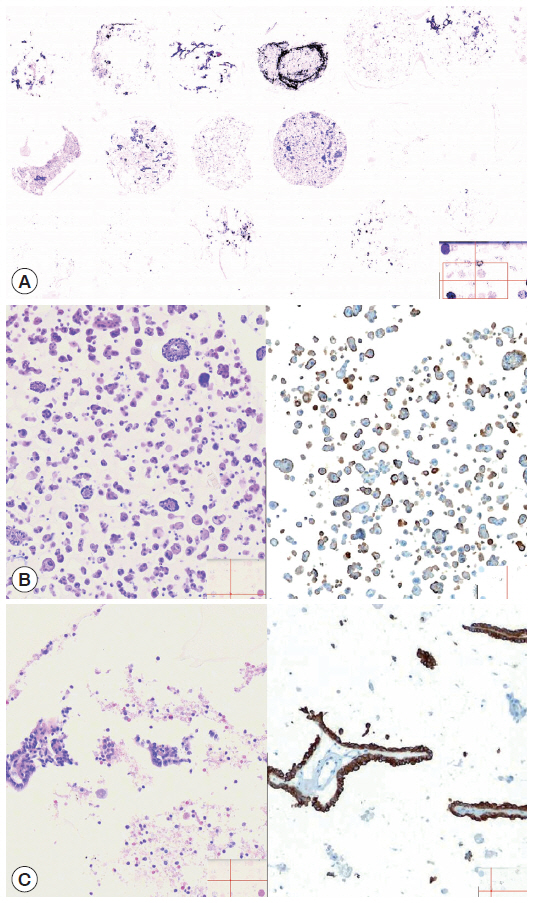
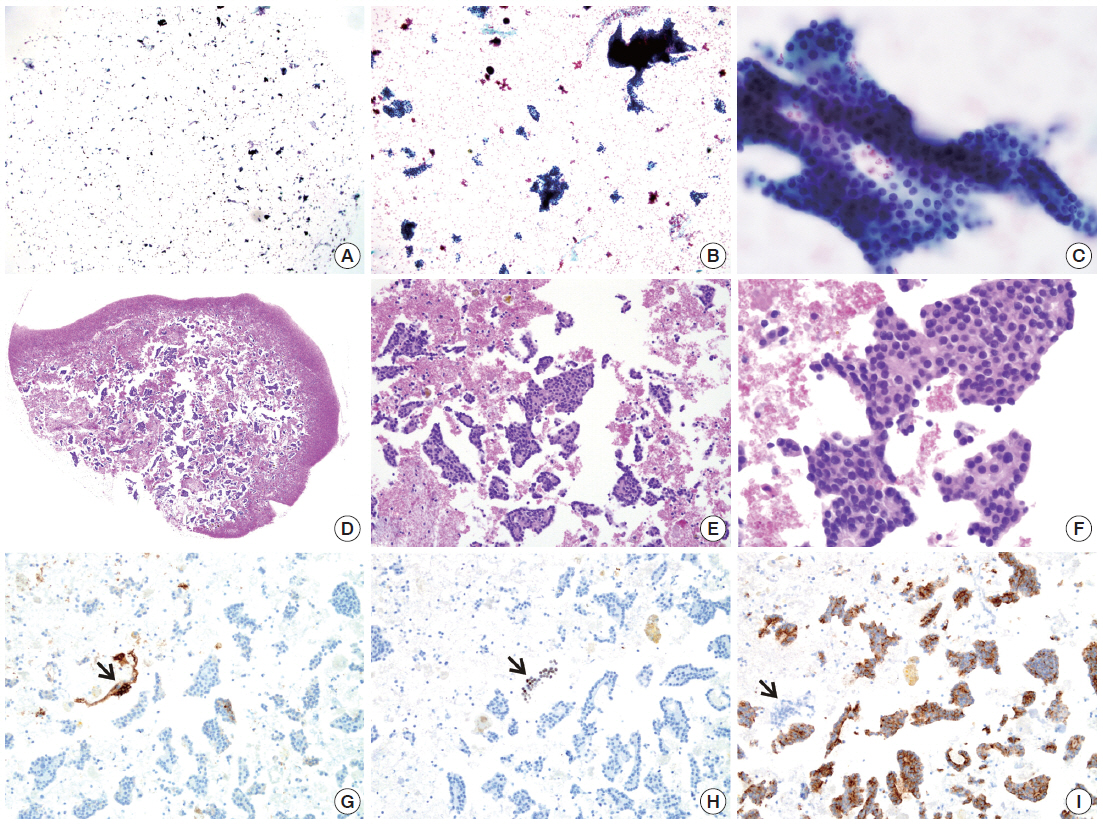
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
| Target | Source | Dilution |
|---|---|---|
| Cytokeratin 19 | Dako | 1:200 |
| Galectin-3 | Novocastra | 1:500 |
| Thyroid transcription factor-1 | NeoMarkers | 1:1,500 |
| Thyroglobulin | NeoMarkers | 1:1,500 |
| Parathyroid hormone | NeoMarkers | 1:400 |
| CD56 | Novocastra | 1:400 |
| HBME1 | Cell Marque | 1:400 |
| D2-40 | Dako | 1:300 |
| Wilms’ tumor-1 | Cell Marque | 1:500 |
| Calretinin | Chemicon | 1:1,800 |
| Leukocyte common antigen (CD45) | Dako | 1:2,000 |
| CD20 | Pharmingen | 1:100 |
| CD3 | Dako | 1:100 |
| Terminal deoxynucleotidyl transferase | Cell Marque | 1:400 |
| PAX5 | Cell Marque | 1:400 |
| Samples | Site | Diagnosis of smears | Supportive immunocytochemistry of cell block* | Diagnosis based on ICC |
|---|---|---|---|---|
| LBC_FNAs (SurePath) | Thyroid | Papillary carcinoma (n=8) | TG+, TTF1+, CK19+, galectin 3+, CD56–, HBME1+, PTH– | Papillary thyroid carcinoma |
| Suspicious for papillary carcinoma (n=2) | TG+, TTF1+, CK19+, galectin 3+, CD56–, HBME1+, PTH– | Papillary thyroid carcinoma | ||
| Nodular hyperplasia (n=12) | TG+, TTF1+, CK19–, galectin 3–, CD56+, HBME1–, PTH– | Nodular hyperplasia | ||
| Atypia (n=4) | TG+, TTF1+, CK19–, galectin 3–, CD56+, HBME1–, PTH– | Nodular hyperplasia | ||
| Atypia (n=1) | TG–, TTF1–, CK19+, galectin 3–, CD56–, HBME1–, PTH+ | Parathyroid lesion | ||
| Benign Hurthle cell proliferation (n=2) | TG+, TTF1+, CK19–, galectin 3–, CD56+, HBME1–, PTH– | Benign Hurthle cell lesion | ||
| Chronic lymphocytic thyroiditis (n=2) | N/A due to scanty cellularity | N/A | ||
| Lymph node | Lymphoid malignancy (n=2) | CD45–, CD20–, CD3–, TdT+, PAX5+, CD56– | B-lymphoblastic lymphoma | |
| CD45+, CD20+, CD3–, TdT–, PAX5+, CD56– | Large B-cell lymphoma | |||
| LBC_serous effusions (SurePath) | Ascitic fluid | Lymphoid malignancy (n=1) | CD45+, CD20–, CD3–, TdT+, PAX5+, CD56– | B-lymphoblastic lymphoma |
| Adenocarcinoma (n=1) | CK7+, CK19+, calretinin–, D2-40, WT1+, CD56–, TTF1– | Ovarian serous carcinoma | ||
| Pleural effusion | Adenocarcinoma (n=1) | CK7+, CK19+, calretinin–, D2-40–, HBME1–, WT1–, TTF1+ | Adenocarcinoma of lung primary | |
| Favor malignant mesothelioma (n=1) | CK7+, CK19+, calretinin+, D2-40+, HBME1+, WT1+, TTF1– | Malignant mesothelioma | ||
| Negative for malignancy (n=1) | N/A | N/A |
ICC, immunocytochemistry; LBC, liquid-based cytology; FNA, fine needle aspiration; cytokeratin; TG, thyroglobulin; TTF1, thyroid transcription factor-1; CK, cytokeratin; PTH, parathyroid hormone; N/A, not applicable; TdT, terminal deoxynucleotidyl transferase; WT1, Wilms’ tumor-1.

 E-submission
E-submission









