Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(5); 2014 > Article
-
Original Article
Mdm2 and p53 Expression in Radiation-Induced Sarcomas of the Head and Neck: Comparison with De Novo Sarcomas - Min Jeong Song, Joon Seon Song, Jong-Lyel Roh1, Seung-Ho Choi1, Soon Yuhl Nam1, Sang Yoon Kim1, Sung Bae Kim2, Sang-wook Lee3, Kyung-Ja Cho
-
Korean Journal of Pathology 2014;48(5):346-350.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.5.346
Published online: October 27, 2014
Departments of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
1Departments of Head and Neck Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
2Departments of Medical Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
3Departments of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- Corresponding Author: Kyung-Ja Cho, M.D. Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 138-736, Korea Tel: +82-2-3010-4545 Fax: +82-2-472-7898 E-mail: kjc@amc.seoul.kr
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- The pathogenesis of radiation-induced sarcomas (RISs) is not well known. In RIS, TP53 mutations are frequent, but little is known about Mdm2-p53 interaction, which is a recent therapeutic target of sarcomas.
-
Methods
- We studied the immunohistochemical expression of Mdm2 and p53 of 8 RISs. The intervals between radiation therapy and diagnosis of secondary sarcomas ranged from 3 to 17 years.
-
Results
- Mdm2 expression was more common in de novo sarcomas than RISs (75% vs 37.5%), and p53 expression was more common in RISs than in de novo cases (75% vs 37.5%). While half of the RISs were Mdm2(–)/p53(+), none of de novo cases showed such combination; while half of de novo sarcomas were Mdm2(+)/p53(–), which are a candidate group of Mdm2 inhibitors, only 1 RIS showed such a combination. Variable immunoprofiles observed in both groups did not correlate with tumor types, except that all of 2 myxofibrosarcomas were Mdm2(+)/p53(+).
-
Conclusions
- In conclusion, we speculated that both radiation-induced and de novo sarcomagenesis are not due to a unique genetic mechanism. Mdm2-expression without p53 overexpression in 1 case of RIS decreases the future possibility of applying Mdm2 inhibitors on a subset of these difficult tumors.
- Eight cases of RIS of the head and neck were found from data of Asan Medical Center, Seoul, Korea from 2006 through 2012. The selection was based on the slightly modified criteria applied by Bjerkehagen et al.[12]: the sarcoma’s location in the field of previous radiotherapy; a latency time of at least 2 years; and histomorphology different from that of the primary tumors. The patients’ primary tumors were nasopharyngeal carcinoma in 3, olfactory neuroblastoma in 1, malignant lymphoma in 1, liposarcoma in 1, malignant melanoma in 1, and astrocytoma in 1, and the secondary sarcomas comprised 3 undifferentiated pleomorphic sarcomas (UPS), 3 osteosarcomas, 1 fibrosarcoma, and 1 myxofibrosarcoma. The latency period ranged from 4 to 17 years (Table 1). Eight cases of de novo sarcomas of the head and neck were retrieved for comparison, and they included 3 osteosarcomas of the maxillary sinus or nasal cavity, 2 UPS of the maxillary sinus or scalp, 2 fibrosarcomas of the nasal cavity, and 1 myxofibrosarcoma of the maxillary sinus.
- Tumor tissues from 16 cases were subjected to immunohistochemical staining for Mdm2 (1:100, Zeta, Arcadia, CA, USA) and p53 (1:1,500, Dako, Glostrup, Denmark). The staining was processed on 4-μm sections using a Ventana autostainer and ultraView DAB detection kit (Ventana, Tucson, AZ, USA) according to the manufacturer’s instructions. Nuclear staining in more than 10% of tumor cells was rendered positive for both Mdm2 and p53. Statistical analysis of immunohistochemical results between the two groups was performed by chi-square and Fisher’s exact test (ver. 18.0, SPSS Inc., Chicago, IL, USA).
MATERIALS AND METHODS
- Mdm2 expression was less common in RIS (3/8, 37.5%) than in de novo cases (6/8, 75%) (p<.05), while p53 expression was more common in RIS cases (75% vs 37.5%) (p<.05). Variable combination types of expression were observed in both groups (Table 2, Fig. 1); however, while half of RISs were Mdm2(–)/p53(+), none of de novo cases showed such combination, and while half of de novo sarcomas were Mdm2(+)/p53(–), which can be the candidate group of Mdm2 inhibitors, only 1 RIS showed such a combination. The expression profiles of Mdm2 and p53 did not correlate with tumor types, except that both of the myxofibrosarcomas, 1 RIS case and 1 de novo case, were positive for both Mdm2 and p53 (Table 2, Fig. 1).
RESULTS
- RISs develop in a field of prior radiation after a latent period as high-grade sarcoma, most frequently osteosarcoma or undifferentiated pleomorphic sarcoma (former malignant fibrous histiocytoma)[12,13]. The pathogenesis of RIS is still unknown even though the initiating events have been identified. Gonin-Laurent et al.[1] detected a high incidence (58%) of inactivating mutation of TP53 gene in 36 RIS, and the inactivation was subsequently shown to be unrelated to MDM2 amplification/expression[2]. The same study group recently described a transcriptome signature distinguishing sporadic sarcomas from RISs, and the signature suggested that RISs are characterized by chronic endogenous oxidative stress[14]. On the contrary, Rumenapp et al.[15] showed that 6 of 7 radiation-induced osteosarcomas harbored a high degree of genomic instability similar to that identified earlier in primary osteosarcomas with poor prognosis. They speculated that the poor prognosis is caused by genetic instability, and not by an initiating event.
- Apart from the genetic instability, prognosis of RIS is expected to be poor, considering the high histologic grade, limitations in further radiotherapy, and the presence of pre-existing malignancy. Bjerkehagen et al.[5] compared 98 patients with RIS and 239 sporadic high-grade sarcomas and concluded that the poorer prognosis of RIS is related to the central tumor site, incomplete surgical remission, microscopic tumor necrosis, and the presence of metastases. McHugh et al.[6] studied primary versus radiation-associated craniofacial osteosarcomas and showed radiation-associated cases were more aggressive, because of their unresectability, high grade, and expression of adverse markers including p53, Ki-67, and ezrin.
- The Mdm2-p53 interaction in sarcomas has recently received attention on account of a new therapeutic approach targeting this interaction[10,11,16]. MDM2 amplification/overexpression is characteristic of well-differentiated/dedifferentiated liposarcomas (DDLS), but is also identified in a small percentage of other sarcomas[17,18]. Studies on Mdm2 in RIS are very rare. Gonin-Laurent et al.[2] identified MDM2 mRNA expression in only 5 of 36 RIS cases (13.9%). In contrast, Roch-Lefevre et al.[3] detected recurrent gains/amplifications at many chromosomal regions including the loci of MDM2 in a series of 16 rat osteosarcomas induced by plutonium-238. In a chemical sarcomagenesis experiment, a high percentage of Mdm2 immunoreactivity was observed in mouse RMS, and the authors suggested that Mdm2 expression is an important pathogenetic event in this sarcomagenesis[4].
- Our study is the first immunohistochemical study on Mdm2 expression of RIS. Mdm2 expression was more common in de novo than RISs (p<.05), and p53 expression was more common in RIS than de novo cases (p<.05). Frequent Mdm2 expression of de novo nonlipogenic sarcomas in this series might be due to the high percentage of included osteosarcoma cases. Frequent p53 overexpression in RISs suggests mutations of TP53 are pathogenetic events in radiation-induced sarcomagenesis, as proposed earlier[1]. Mutations in TP53 are basically not affected by Mdm2, but concurrent overexpression of Mdm2 was observed in 2 cases. While half of the RISs were Mdm2(–)/p53(+), none of de novo cases showed such combination, and while half of de novo sarcomas were Mdm2(+)/p53(–), which can be the candidate group for Mdm2 inhibitors, only 1 RIS showed such combination. Mdm2-p53 interaction, that is p53 inactivation by Mdm2 activation, appears to not be a major pathogenetic step in radiation-induced sarcomagenesis.
- RMSs are aggressive pediatric tumors, and there have been recent preclinical trials of Mdm2 inhibitors in RMS[10,11]. Radiation-induced RMS has rarely been described, mostly among Chinese patients treated for nasopharyngeal cancer[8,9,19,20]. Since our series included no RMS cases, Mdm2-p53 status in radiation-induced RMS is not known. Among osteosarcomas, UPS and fibrosarcomas, Mdm2-p53 status showed no correlation with tumor type, but 2 myxofibrosarcomas (1 radiation-induced and the 1 de novo) were both positive for both Mdm2 and p53, but the meaning of this is uncertain for now.
- UPSs with MDM2 amplification/expression, especially of the retroperitoneum, were considered DDLS by one research group[21], and the same group recently published a similar result on peripheral UPS[22]. In addition, it has been documented that immunostaining results for Mdm2 are well correlated with MDM2 gene amplification[23]. Our study resulted in 2 cases of Mdm2-positive UPS, 1 radiation-induced and 1 de novo. Nevertheless, it appears to be hasty to regard these nonlipogenic sarcomas as DDLS, since no example of radiation-induced DDLS has been reported.
- In summary, in the comparison of Mdm2 and p53 expression of RIS with that of de novo sarcomas of the head and neck, we speculated that radiation-induced and de novo sarcomagenesis are not due to a unique genetic mechanism. Mdm2 expression without p53 overexpression in 1 case of RIS decreases the future possibility of applying Mdm2 inhibitors on a subset of these difficult tumors.
DISCUSSION
| Mdm2(+)/p53(+) | Mdm2(+)/p53(–) | Mdm2(–)/p53(+) | Mdm2(–)/p53(–) | |
|---|---|---|---|---|
| Radiation-induced | ||||
| Osteosarcoma | 0 | 1 | 2 | 0 |
| UPS | 1 | 0 | 2 | 0 |
| Fibrosarcoma | 0 | 0 | 0 | 1 |
| Myxofibrosarcoma | 1 | 0 | 0 | 0 |
| De novo | ||||
| Osteosarcoma | 0 | 2 | 0 | 1 |
| UPS | 0 | 1 | 1 | 0 |
- 1. Gonin-Laurent N, Gibaud A, Huygue M, et al. Specific TP53 mutation pattern in radiation-induced sarcomas. Carcinogenesis 2006; 27: 1266-72. ArticlePubMed
- 2. Gonin-Laurent N, Hadj-Hamou NS, Vogt N, et al. RB1 and TP53 pathways in radiation-induced sarcomas. Oncogene 2007; 26: 6106-12. ArticlePubMedPDF
- 3. Roch-Lefevre S, Daino K, Altmeyer-Morel S, Guilly MN, Chevillard S. Cytogenetic and molecular characterization of plutonium-induced rat osteosarcomas. J Radiat Res 2010; 51: 243-50. ArticlePubMed
- 4. Wu H, Inoue M. Immunohistochemical analysis for Mdm2 and p53 proteins in methylcholanthrene-induced mouse rhabdomyosarcomas. J Vet Med Sci 2006; 68: 427-31. ArticlePubMed
- 5. Bjerkehagen B, Småstuen MC, Hall KS, Skjeldal S, Smeland S, Fossa SD. Why do patients with radiation-induced sarcomas have a poor sarcoma-related survival? Br J Cancer 2012; 106: 297-306. ArticlePubMedPMCPDF
- 6. McHugh JB, Thomas DG, Herman JM, et al. Primary versus radiation-associated craniofacial osteosarcoma: biologic and clinicopathologic comparisons. Cancer 2006; 107: 554-62. ArticlePubMed
- 7. Thijssens KM, van Ginkel RJ, Suurmeijer AJ, et al. Radiation-induced sarcoma: a challenge for the surgeon. Ann Surg Oncol 2005; 12: 237-45. ArticlePubMedPDF
- 8. Xi M, Liu MZ, Wang HX, et al. Radiation-induced sarcoma in patients with nasopharyngeal carcinoma: a single-institution study. Cancer 2010; 116: 5479-86. ArticlePubMed
- 9. Chan JY, Wong ST, Lau GI, Wei WI. Postradiation sarcoma after radiotherapy for nasopharyngeal carcinoma. Laryngoscope 2012; 122: 2695-9. ArticlePubMed
- 10. Barone G, Tweddle DA, Shohet JM, et al. MDM2-p53 interaction in paediatric solid tumours: preclinical rationale, biomarkers and resistance. Curr Drug Targets 2014; 15: 114-23. ArticlePubMed
- 11. Ohnstad HO, Castro R, Sun J, et al. Correlation of TP53 and MDM2 genotypes with response to therapy in sarcoma. Cancer 2013; 119: 1013-22. ArticlePubMed
- 12. Bjerkehagen B, Smeland S, Walberg L, et al. Radiation-induced sarcoma: 25-year experience from the Norwegian Radium Hospital. Acta Oncol 2008; 47: 1475-82. ArticlePubMed
- 13. Murray EM, Werner D, Greeff EA, Taylor DA. Postradiation sarcomas: 20 cases and a literature review. Int J Radiat Oncol Biol Phys 1999; 45: 951-61. ArticlePubMed
- 14. Hadj-Hamou NS, Ugolin N, Ory C, et al. A transcriptome signature distinguished sporadic from postradiotherapy radiation-induced sarcomas. Carcinogenesis 2011; 32: 929-34. ArticlePubMedPMC
- 15. Rumenapp C, Smida J, Gonzalez-Vasconcellos I, et al. Secondary radiation-induced bone tumours demonstrate a high degree of genomic instability predictive of a poor prognosis. Curr Genomics 2012; 13: 433-7. ArticlePubMedPMC
- 16. Canner JA, Sobo M, Ball S, et al. MI-63: a novel small-molecule inhibitor targets MDM2 and induces apoptosis in embryonal and alveolar rhabdomyosarcoma cells with wild-type p53. Br J Cancer 2009; 101: 774-81. ArticlePubMedPMCPDF
- 17. Hameed M. Pathology and genetics of adipocytic tumors. Cytogenet Genome Res 2007; 118: 138-47. ArticlePubMedPDF
- 18. Sabah M, Cummins R, Leader M, Kay E. Immunoreactivity of p53, Mdm2, p21(WAF1/CIP1) Bcl-2, and Bax in soft tissue sarcomas: correlation with histologic grade. Appl Immunohistochem Mol Morphol 2007; 15: 64-9. PubMed
- 19. Wei Z, Xu J, Zeng X, et al. Radiation-induced sarcoma in the head and neck region: a clinicopathologic and immunohistochemical study of 13 cases. Asian Pac J Cancer Prev 2011; 12: 2995-9. PubMed
- 20. Wei Z, Xie Y, Xu J, et al. Radiation-induced sarcoma of head and neck: 50 years of experience at a single institution in an endemic area of nasopharyngeal carcinoma in China. Med Oncol 2012; 29: 670-6. ArticlePubMedPDF
- 21. Coindre JM, Mariani O, Chibon F, et al. Most malignant fibrous histiocytomas developed in the retroperitoneum are dedifferentiated liposarcomas: a review of 25 cases initially diagnosed as malignant fibrous histiocytoma. Mod Pathol 2003; 16: 256-62. ArticlePubMed
- 22. Le Guellec S, Chibon F, Ouali M, et al. Are peripheral purely undifferentiated pleomorphic sarcomas with MDM2 amplification dedifferentiated liposarcomas? Am J Surg Pathol 2014; 38: 293-304. ArticlePubMed
- 23. Binh MB, Sastre-Garau X, Guillou L, et al. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: a comparative analysis of 559 soft tissue neoplasms with genetic data. Am J Surg Pathol 2005; 29: 1340-7. PubMed
REFERENCES
Figure & Data
References
Citations

- Radiation-induced osteosarcoma in the head and neck region: Case report and literature review
Iara Vieira Ferreira, Marcelo Elias Schempf Cattan, Carlos Takahiro Chone, Arthur Antolini, Erika Said Abu Egal, Albina Altemani, Fernanda Viviane Mariano
Oral Oncology.2025; 162: 107216. CrossRef - Radiation-Induced Sarcomas of the Head and Neck: A Systematic Review
Andrés Coca-Pelaz, Antti A. Mäkitie, Primož Strojan, June Corry, Avraham Eisbruch, Jonathan J. Beitler, Sandra Nuyts, Robert Smee, Johannes A. Langendijk, William M. Mendenhall, Cesare Piazza, Alessandra Rinaldo, Alfio Ferlito
Advances in Therapy.2021; 38(1): 90. CrossRef - Genomic Characterization of Radiation-Induced Intracranial Undifferentiated Pleomorphic Sarcoma
Christopher S. Hong, Edwin Partovi, James Clune, Anita Huttner, Henry S. Park, Sacit Bulent Omay, Balraj Mittal
Case Reports in Genetics.2021; 2021: 1. CrossRef - Radiation-Induced Sarcoma of the Head and Neck: A Review of the Literature
Lorenzo Giannini, Fabiola Incandela, Marco Fiore, Alessandro Gronchi, Silvia Stacchiotti, Claudia Sangalli, Cesare Piazza
Frontiers in Oncology.2018;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
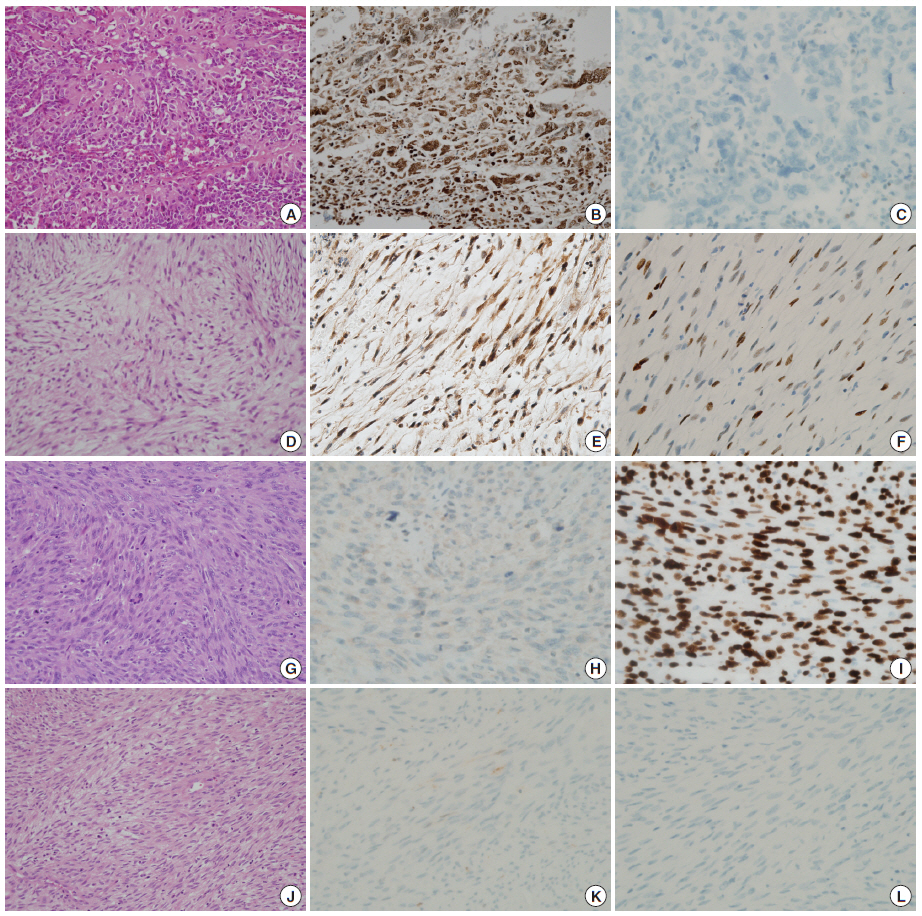
Fig. 1.
| Tumor type | Site | Age (yr)/Sex | Previous tumor | Treatment | Interval between RT & sarcoma |
|---|---|---|---|---|---|
| Osteosarcoma | Mandible | 61/F | Malignant lymphoma of palatine tonsil | CT and RT | 9 yr 3 mo |
| Osteosarcoma | Mandible | 17/M | Pleomorphic liposarcoma of parotid gland | Wide excision and RT | 4 yr |
| Osteosarcoma | Skull | 33/F | Astrocytoma | Tumor resection and RT | 3 yr 6 mo |
| UPS | Nasal cavity | 22/M | Olfactory neuroblastoma | Medial maxillectomy, CT and RT | 3 yr 3 mo |
| UPS | Nasopharynx | 42/F | Nasopharyngeal carcinoma | RT | 6 yr |
| UPS | Posterior neck | 60/M | Nasopharyngeal carcinoma | RT | 5 yr 5 mo |
| Fibrosarcoma | Hard palate | 50/F | Malignant melanoma of maxillary sinus | Partial maxillectomy, CT and RT | 17 yr |
| MFS | Submandibular area | 51/F | Nasopharyngeal carcinoma | RT | 12 yr |
| Mdm2(+)/p53(+) | Mdm2(+)/p53(–) | Mdm2(–)/p53(+) | Mdm2(–)/p53(–) | |
|---|---|---|---|---|
| Radiation-induced | ||||
| Osteosarcoma | 0 | 1 | 2 | 0 |
| UPS | 1 | 0 | 2 | 0 |
| Fibrosarcoma | 0 | 0 | 0 | 1 |
| Myxofibrosarcoma | 1 | 0 | 0 | 0 |
| De novo | ||||
| Osteosarcoma | 0 | 2 | 0 | 1 |
| UPS | 0 | 1 | 1 | 0 |
F, female; CT, chemotherapy; RT, radiotherapy; M, male; UPS, undifferentiated pleomorphic sarcoma; MFS, myxofibrosarcoma.
RIS, radiation-induced sarcoma; UPS, undifferentiated pleomorphic sarcoma.

 E-submission
E-submission



