Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(4); 2013 > Article
-
Original Article
ERG Immunohistochemistry as an Endothelial Marker for Assessing Lymphovascular Invasion - Sehun Kim, Hyung Kyu Park, Ho Young Jung, So-Young Lee, Kyueng-Whan Min, Wook Youn Kim, Hye Seung Han, Wan Seop Kim, Tae Sook Hwang, So Dug Lim
-
Korean Journal of Pathology 2013;47(4):355-364.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.4.355
Published online: August 26, 2013
Department of Pathology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
- Corresponding Author: So Dug Lim, M.D. Department of Pathology, Konkuk University Medical Center, 120-1 Neungdong-ro, Gwangjin-gu, Seoul 143-729, Korea. Tel: +82-2-2030-5643, Fax: +82-2-2030-5629, sdlim@kuh.ac.kr
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- ERG, a member of the ETS family of transcription factors, is a highly specific endothelial marker. We investigated whether the use of ERG immunostaining can help pathologists detect lymphovascular invasion (LVI) and decrease interobserver variability in LVI diagnosis.
-
Methods
- Fifteen cases of surgically resected colorectal cancers with hepatic metastasis were selected and the most representative sections for LVI detection were immunostained with ERG, CD31, and D2-40. Eight pathologists independently evaluated LVI status on hematoxylin and eosin (H&E) and the corresponding immunostained sections and then convened for a consensus meeting. The results were analyzed by kappa (κ) statistics.
-
Results
- The average rate of LVI positivity was observed in 43% with H&E only, 10% with CD31, 29% with D2-40, and 16% with ERG. Agreement among pathologists was fair for H&E only (κ=0.27), D2-40 (κ=0.21), ERG (κ=0.23), and was moderate for CD31 (κ=0.55). Consensus revealed that ERG nuclear immunoreactivity showed better visual contrast of LVI detection than the other staining, with improved agreement and LVI detection rate (κ=0.65, LVI positivity rate 80%).
-
Conclusions
- The present study demonstrated a superiority with ERG immunostaining and indicated that ERG is a promising panendothelial marker that might help pathologists increase LVI detection and decrease interobserver variability in LVI diagnosis.
- All cases of resected colorectal cancers from the pathologic archives of Konkuk University Medical Center, Seoul, Korea between 2005 and 2011 were reviewed through the use of medical records. The present study included only 15 cases of moderately differentiated colorectal adenocarcinomas that revealed concurrent or subsequent hepatic metastasis because it was intended that all the cases would have LVI. Other histologic types such as mucinous adenocarcinoma were excluded to achieve homogeneity in the study. This study was approved by the Institutional Review Board of Konkuk University Medical Center.
- Histologic sections of the 15 cases were reviewed and the most representative sections for assessing LVI were selected by two pathologists (S.D.L. and S.H.K.). ERG, CD31, and D2-40 immunostaining was performed on the corresponding paraffin sections and each stained slide, including H&E sections that were de-identified with no clinical or pathological information. Each set of 15 stained slides was separately circulated to the other eight pathologists and they independently reviewed all H&E slides and each corresponding immunostained slide using their own criteria of LVI assessment. They were asked to record presence or absence of LVI for each stained slide.
- After the circulation of the slides to the pathologists, a consensus meeting was held to discuss the results and the current issues of LVI detection, particularly using ERG immunostaining. Cases were reviewed again using a 10 headed-conference microscope and the pathologists had the chance to modify their previous decision of LVI detection with ERG immunostaining. The consensus decision of the final LVI status was given as presence or absence of LVI when more than 75% of the pathologists agreed based on ERG immunostaining. As for the remaining cases, they remained equivocal.
- On post-consensus meeting, a total of 60 digital images of suspicious or debatable LVI immunostained by ERG were presented to the pathologists for a quiz in a projected PowerPoint slide show. To evaluate interobserver variability, they were independently asked to record as presence, absence or equivocal of LVI.
- ERG immunostaining was also performed on the most representative sections of LVI detection for 100 other tumors including breast, stomach and thyroid cancers in order to discuss further interpretation issues of LVI detection.
- Immunohistochemistry (IHC) was performed using the Automatic Ventana ES IHC staining machine (Ventana Medical Systems, Tucson, AZ, USA). Representative sections from paraffin blocks were cut to 4 µm thickness and were deparaffinized by treatment with xylene followed by rehydration. Primary antibodies were CD31 (1:25, Thermo Fisher Scientific Inc., Kalamazoo, MI, USA), D2-40 (predilution, Dako, Glostrup, Denmark), and ERG (predilution, Biocare Medical, Concord, CA, USA). The appropriate internal controls of the vessels were used during the immunostaining procedures to ensure the quality of the staining. For negative control, the primary antibody was replaced by normal goat serum.
- The statistical analyses focusing on the estimation of the agreement rate were executed using the kappa (κ) statistic, which is a statistical measure of interobserver agreement occurring by chance. Interpretations for the κ-values were previously described.14 It is suggested that κ values less than 0.00 indicate poor agreement, values of 0.00-0.20 slight agreement, values of 0.21-0.40 fair agreement, values of 0.41-0.60 moderate agreement, values of 0.61-0.80 substantial agreement and values of 0.81-1.00 almost perfect agreement. The statistics was performed using SPSS ver. 17 (IBM, Endicott, NY, USA) and dbSTAT ver. 5 (dbSTAT, Seoul, Korea).
MATERIALS AND METHODS
- Clinicopathologic characteristics of all 15 cases are shown in Table 1. There were 12 concurrent and three subsequent hepatic metastases of the colorectal cancers. Seven patients (47%) had metastatic lymph nodes and 8 patients (53%) had no metastatic lymph nodes at the time of surgery. Eight cases (53.3%) were recorded to have presence of LVI at the initial pathology reports. The mean follow-up period was 22 months (range, 13 to 68 months). Two patients died of disease and 13 patients were alive without recurrence or disease during follow-up.
- The average rate of LVI positivity for 15 colorectal cancers as assessed by each pathologist was 43% with H&E only, 10% with CD31, 29% with D2-40, and 16% with ERG. Agreements for LVI positivity among pathologists were moderate for CD31 (κ=0.55; 95% confidence interval [CI], 0.28 to 0.82) and fair for H&E only (κ=0.27; 95% CI, 0.16 to 0.38), D2-40 (κ=0.21; 95% CI, 0.05 to 0.37), and ERG (κ=0.23; 95% CI, 0.02 to 0.48), respectively (Fig. 1).
- During the consensus meeting, the pathologists indicated that they knew the published criteria of LVI based on the 1999 CAP Consensus Statement6,7 and applied the criteria strictly in their individual evaluation. The pathologists indicated that retraction artifact was the most complicated difficulty in detecting LVI, which might result in high detection rate with H&E only (Figs. 1, 2). They suggested that CD31 immunostaining, with the lowest LVI detection rate (10%), could result in a higher level of agreement for CD31 immunostaining (Fig. 1).
- All pathologists agreed that ERG nuclear immunoreactivity demonstrated vessels better than the other methods showing cytoplasmic immunoreactivity. ERG immunostaining showed distinct nuclear immunoreactivity of endothelial cells in the artery, vein, and lymphatic vessels without cross immunoreactivity compared to CD31 stainability in inflammatory cells and D2-40 stainability in fibroblasts (Fig. 3).
- Pertaining to the use of ERG immunostaining on consensus, improved detection of LVI (80%) and interobserver agreement (κ=0.65; 95% CI, 0.57 to 0.74) were obvious among pathologists (Fig. 1).
- Through the quiz, higher rates of concordance among the pathologists were obtained with concordance rates of 100% for 18 photos (30%), 87.5% for 18 photos (30%), and 75% for 12 photos (20%) (Table 2). Twelve photos (20%) with concordance rates of 62.5 % and 50% remained debatable (Table 2). Some interesting digital images of LVI are shown in Figs. 3-6.
- The tumor cells surrounded by a vascular space were considered LVI positive with a concordance rate of 100% (Fig. 4A, B). A single-cell metastasis even in a single vascular space or even in inflammatory background reached agreement for LVI positivity with high concordance (Table 2, Fig. 4C, D).
- The cases that raised the issue of retraction artifact are shown in Fig. 4. Endothelial cells protruding into the vascular space were highlighted by ERG immunostaining and considered as LVI positivity with a concordance rate of 100%. The presence of a thin fibrocollagenous layer between the tumor and ERG-positive endothelial cells reached LVI negativity with a concordance rate of 100% (Fig. 5A). A "kissing pattern" showing tumor cells surrounded by endothelial nuclei was noticeable as LVI positivity with the concordance rate of 87.5% (Fig. 5B).
- All pathologists recognized that vessels embraced by tumor cells, referred to as the "hugging pattern," was an interesting finding particularly highlighted by ERG nuclear immunoreactivity, and should be further discussed in the future (Fig. 5C, D).
- ERG immunostaining for breast, stomach and thyroid cancers also revealed better visual contrast of LVI detection than CD31, particularly in the tumors showing severe retraction artifact as well as in obvious LVI such as in tumor cells protruding into the lymphovascular space in gastric carcinoma and LVI that is well distinguished from retraction artifact in thyroid cancer (Fig. 6A-C).
- Examples of unequivocal or debatable LVIs are shown in Fig. 7. Some inflammatory cells in the lymphovascular space of the noninflammatory background reached LVI negativity with a 75% concordance rate, but 25% of the pathologists suggested that the significance was unknown and further investigation was necessary (Fig. 7A). There was a different threshold among the pathologists in calling LVI positivity with respect to retraction artifacts and distribution of vessels, even though the endothelial nuclei were well shown by ERG staining (Fig. 7B, C).
RESULTS
- Many studies have reported LVI is not only a simple prognostic factor, but also a practical and powerful parameter for determination of treatment in patients with various cancers15-18 and the modality of treatment and patient care could be quite different according to LVI status in cancers.15,16,19,20 However, lack of standardized criteria and interobserver variability contribute to difficulties in LVI diagnosis, hence, LVI has been adopted in the staging systems of only a few tumor types, such as testicular cancer, produced by the American Joint Committee on Cancer/International Union Against Cancer (AJCC/UICC).21
- Recently, the issues of LVI diagnosis have been studied intensively from this perspective, but only a few studies of interobserver variability with respect to LVI detection have been performed.7,9 Kryvenko and Epstein8 described many issues associated with LVI detection in prostate cancer, including unique histologic features of high frequency of retraction artifact and LVI mimickers. They discussed how to distinguish LVI on H&E sections and recommended that H&E section alone, without immunostaining, is sufficient for detecting LVI. Kirsch et al.9 discussed venous invasion in colorectal cancers and suggested new features of venous invasion such as "orphan arteriaoles" and "protruding tongue" signs as diagnostic clues. They highlighted the role of elastin staining in improving the detection of venous invasion and interobserver agreement. Harris et al.7 reported that the use of endothelial markers such as CD31 and D2-40 did not improve LVI detection in colorectal cancer mainly due to the poor degree of interobserver agreement, lack of criteria and different threshold for LVI diagnosis. These studies have disclosed the limitations of the current methods and have shown little improvement pertaining to LVI detection. Therefore, the development of improved methods including identifying vessels is critical.
- The present study was intended to determine which debatable features of LVI should be considered as a true LVI because LVI develops in all the cases of hepatic metastasis, although it does not mean that a few foci on a single slide are true indicators of LVI. In this study, ERG immunostaining helped pathologists make a confirmatory decision about a LVI status that was suspicious or debatable on H&E examination. Retraction artifact is known to be the most common mimicker of LVI. As the peritumoral stroma surrounding tumor glands may be interpreted incorrectly interpreted as endothelial cells, identification of at least 2 endothelial nuclei has been advocated for LVI by another study.8,22 ERG immunostaining shows more reliable and sufficient visual contrast of the endothelial nuclei protruding to the vascular lumen, which is clearly distinguished from the retraction artifact, in comparison to H&E section alone or the staining with other endothelial markers.
- There were new features of LVI that were not noticeable by other methods. The "kissing pattern" of obvious tumor cells surrounded by the nuclei of endothelial cells showed a concordance rate of 87.5% for LVI positivity. It is interesting to see this pattern even with the retraction artifact. The "hugging" pattern was not interpreted as LVI positivity, but pathologists agreed that its significance should be further investigated in future studies with respect to the vessels in tumor cells. These findings are attributable to the nuclear immunoreactivity of ERG, which is not demonstrable by other markers with cytoplasmic immunoreactivity.
- The discrepancy of LVI diagnosis among pathologists could be caused by differences in criteria used for diagnosing LVI. The pathologists indicated they have a tendency to strictly apply their own criteria for LVI, which resulted in relatively low LVI detection rate. During the consensus meeting, pathologists discussed the patterns of ERG immunostaining for LVI detection and tried to reach an agreement on diagnosing LVI status. This kind of consensus and education appeared to increase the detection rate and decrease the interobserver variability for LVI detection in this study. Although there is a different threshold with respect to whether only one LVI can be considered as LVI positivity, ERG immunostaining appeared to decrease this threshold difference for LVI positivity with a concordance rate of 100%, even in a single cell metastasis in one vascular space because of the distinct visual contrast of the nuclear immunoreactivity.
- There have been reports of dual or triple immunohistochemical staining combining cytokeratin and vascular markers including CD31, CD34, and D2-40 being sensitive in detecting LVI than standardized H&E alone.10,11 It might be necessary to investigate dual or triple immunostaining including ERG to improve LVI detection. However, the issue of cost-effectiveness would also need to be addressed if such an approach were to be used in routine diagnostic work.
- This study revealed relatively low lymph node metastasis (47%) (Table 1) compared to the published articles, which show more than 60% lymph node metastasis in stage IV colorectal cancers.23-25 This study also revealed many small capillaries that were more vividly identified in the lamina propria in coloinic mucosa, which is known to have no lymphatic vessels.26-28 The single focus of LVI in the lamina propria was easily detected by ERG staining in this study, which might explain why hematogenous metastasis is more frequent in our cohort. A better understanding of early vascular invasion by carcinoma in the lamina propria may offer new insight into the understanding of metastasis in colon cancer. Many researchers have investigated tumor angiogenesis assessed by the quantization of vessels, and thus, improved information for angiogenesis demonstrated by ERG immunostaining may be beneficial to the researchers and patients.
- A better visual detection of LVI using ERG immunostaining was also observed in 100 other carcinomas including stomach, breast, and thyroid. Therefore, ERG immunostaining could be a better endothelial marker for evaluating LVI on a routine diagnostic basis.
- In this study, LVI positivity on the H&E sections was higher than that on immunostained slides and the interobserver variability did not decrease with the immunostained slides. This result may be attributable to the unfamiliar immunostaining pattern, especially for ERG, and to the short time period spent by each pathologist searching each slide. The pathologists concurred that they needed more time with eager attentiveness to detect LVI and need to be educated with a standardized guideline in interpreting LVI and the immunostaining.
- In conclusion, this study demonstrated that a promising new panendothelial marker, ERG, might help increase LVI detection and decrease interobserver variability. However, this study also identified the problems and limitations of LVI detection that were noticed in the previous studies. Further studies on a larger study group in many clinical contexts of all organ cancers are necessary to confirm true predictive or prognostic values of LVI using ERG immunostaining. In addition, more emphasis on LVI detection as well as developing more reliable methods is needed to improve cancer research and patient care.
DISCUSSION
Acknowledgments
Acknowledgments
- 1. Knudsen JB, Nilsson T, Sprechler M, Johansen A, Christensen N. Venous and nerve invasion as prognostic factors in postoperative survival of patients with resectable cancer of the rectum. Dis Colon Rectum 1983; 26: 613-617. ArticlePubMed
- 2. Cheng L, Jones TD, Lin H, et al. Lymphovascular invasion is an independent prognostic factor in prostatic adenocarcinoma. J Urol 2005; 174: 2181-2185. ArticlePubMed
- 3. Manoharan M, Katkoori D, Kishore TA, Jorda M, Luongo T, Soloway MS. Lymphovascular invasion in radical cystectomy specimen: is it an independent prognostic factor in patients without lymph node metastases? World J Urol 2010; 28: 233-237. ArticlePubMedPDF
- 4. Guntupalli SR, Zighelboim I, Kizer NT, et al. Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol Oncol 2012; 124: 31-35. ArticlePubMed
- 5. Hassell L, Aldinger W, Moody C, et al. Electronic capture and communication of synoptic cancer data elements from pathology reports: results of the Reporting Pathology Protocols 2 (RPP2) project. J Registry Manag 2009; 36: 117-124. PubMed
- 6. Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000; 124: 979-994. PubMed
- 7. Harris EI, Lewin DN, Wang HL, et al. Lymphovascular invasion in colorectal cancer: an interobserver variability study. Am J Surg Pathol 2008; 32: 1816-1821. PubMedPMC
- 8. Kryvenko ON, Epstein JI. Histologic criteria and pitfalls in the diagnosis of lymphovascular invasion in radical prostatectomy specimens. Am J Surg Pathol 2012; 36: 1865-1873. ArticlePubMed
- 9. Kirsch R, Messenger DE, Riddell RH, et al. Venous invasion in colorectal cancer: impact of an elastin stain on detection and interobserver agreement among gastrointestinal and nongastrointestinal pathologists. Am J Surg Pathol 2013; 37: 200-210. PubMed
- 10. Lim CS, Alexander-Sefre F, Allam M, et al. Clinical value of immunohistochemically detected lymphovascular space invasion in early stage cervical carcinoma. Ann Surg Oncol 2008; 15: 2581-2588. ArticlePubMedPDF
- 11. O'Donnell RK, Feldman M, Mick R, Muschel RJ. Immunohistochemical method identifies lymphovascular invasion in a majority of oral squamous cell carcinomas and discriminates between blood and lymphatic vessel invasion. J Histochem Cytochem 2008; 56: 803-810. ArticlePubMedPMCPDF
- 12. Miettinen M, Wang ZF, Paetau A, et al. ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol 2011; 35: 432-441. ArticlePubMedPMC
- 13. Birdsey GM, Dryden NH, Amsellem V, et al. Transcription factor Erg regulates angiogenesis and endothelial apoptosis through VE-cadherin. Blood 2008; 111: 3498-3506. ArticlePubMedPMCPDF
- 14. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159-174. ArticlePubMed
- 15. Branchereau J, Larue S, Vayleux B, Karam G, Bouchot O, Rigaud J. Prognostic value of the lymphovascular invasion in high-grade stage pT1 bladder cancer. Clin Genitourin Cancer 2013; 11: 182-188. ArticlePubMed
- 16. Weber SK, Sauerwald A, Pölcher M, et al. Detection of lymphovascular invasion by D2-40 (podoplanin) immunoexpression in endometrial cancer. Int J Gynecol Cancer 2012; 22: 1442-1448. ArticlePubMed
- 17. Belsante M, Darwish O, Youssef R, et al. Lymphovascular invasion in clear cell renal cell carcinoma: association with disease-free and cancer-specific survival. Urol Oncol 2013 2 18 [Epub]. http://dx.doi.org/10.1016/j.urolonc.2012.11.002. Article
- 18. Matsuo K, Sheridan TB, Yoshino K, et al. Significance of lymphovascular space invasion in epithelial ovarian cancer. Cancer Med 2012; 1: 156-164. ArticlePubMedPMCPDF
- 19. Massabeau C, Filleron T, Wakil G, et al. The prognostic significance of lymphovascular invasion on biopsy specimens in lung cancer treated with definitive chemoradiotherapy. Clin Lung Cancer 2012; 13: 59-67. ArticlePubMed
- 20. Rami-Porta R. Adjuvant therapy for resected non-small-cell lung cancer with lymphovascular invasion. J Thorac Oncol 2013; 8: e9-e10. ArticlePubMed
- 21. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer, 2010.
- 22. Epstein JI, Srigley J, Grignon D, Humphrey P. Association of Directors of Anatomic and Surgical Pathology. Recommendations for the reporting of prostate carcinoma: Association of Directors of Anatomic and Surgical Pathology. Am J Clin Pathol 2008; 129: 24-30. ArticlePubMed
- 23. Suzuki H, Masuda N, Shimura T, et al. Nuclear β-catenin expression at the invasive front and in the vessels predicts liver metastasis in colorectal carcinoma. Anticancer Res 2008; 28: 1821-1830. PubMed
- 24. Ishibashi K, Ishida H, Ohsawa T, Okada N, Kumamoto K, Haga N. Impact of hepatic lymph node metastasis on survival of patients with synchronous resectable or unresectable liver metastases of colorectal cancer. Tech Coloproctol 2013; 17: 51-57. ArticlePubMedPDF
- 25. Seo SI, Lim SB, Yoon YS, et al. Comparison of recurrence patterns between ≤5 years and >5 years after curative operations in colorectal cancer patients. J Surg Oncol 2013; 108: 9-13. ArticlePubMedPDF
- 26. Dobbins WO 3rd. The intestinal mucosal lymphatic in man. A light and electron microscopic study. Gastroenterology 1966; 51: 994-1003. ArticlePubMed
- 27. Fenoglio CM, Kaye GI, Lane N. Distribution of human colonic lymphatics in normal, hyperplastic, and adenomatous tissue. Its relationship to metastasis from small carcinomas in pedunculated adenomas, with two case reports. Gastroenterology 1973; 64: 51-66. ArticlePubMed
- 28. Kenney BC, Jain D. Identification of lymphatics within the colonic lamina propria in inflammation and neoplasia using the monoclonal antibody D2-40. Yale J Biol Med 2008; 81: 103-113. PubMedPMC
REFERENCES
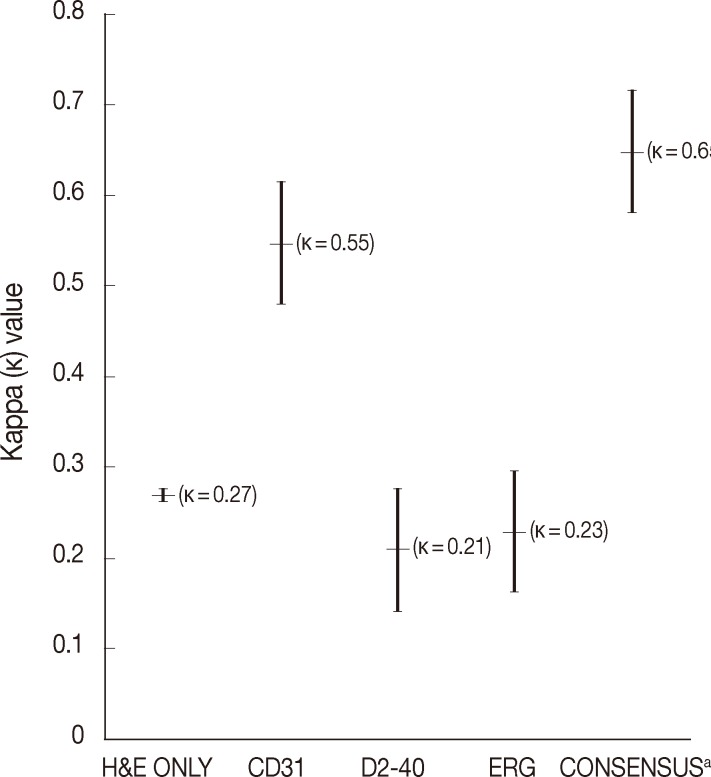
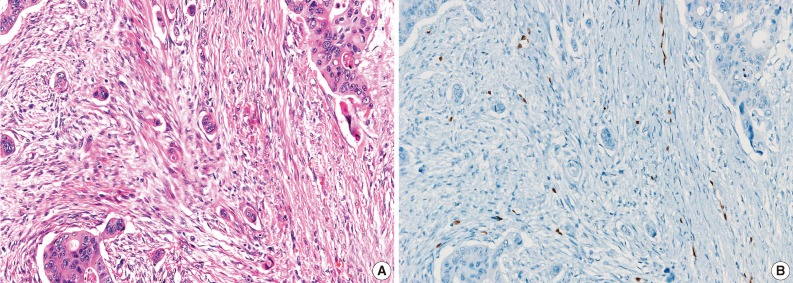
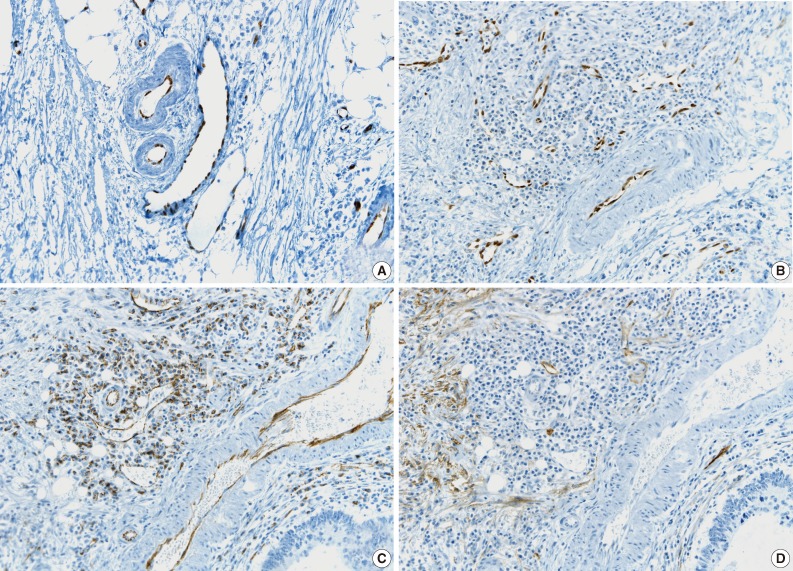
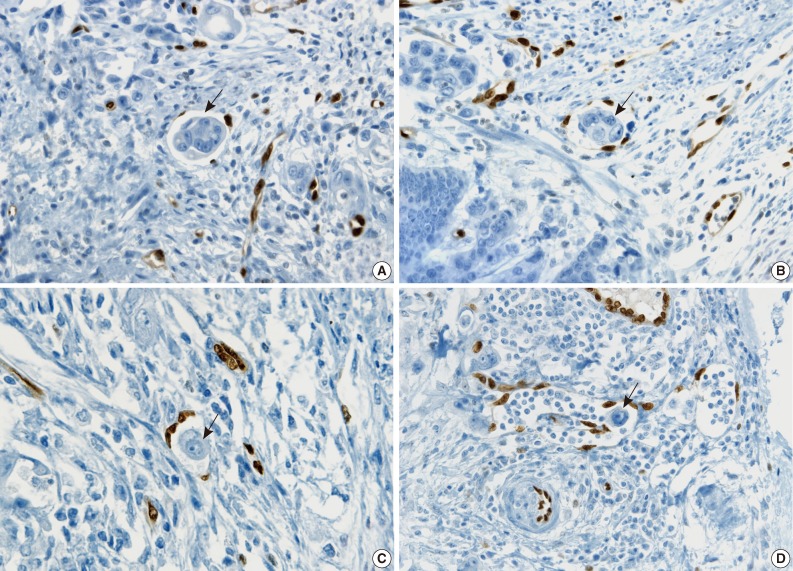
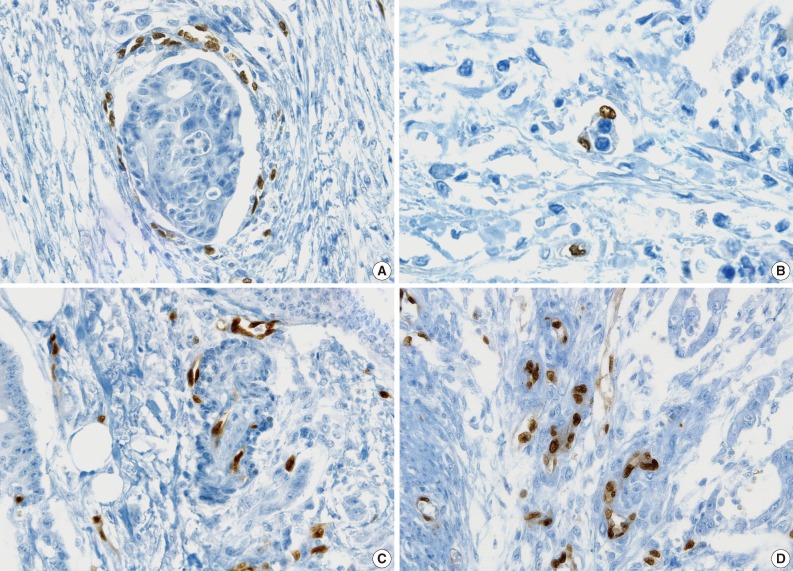
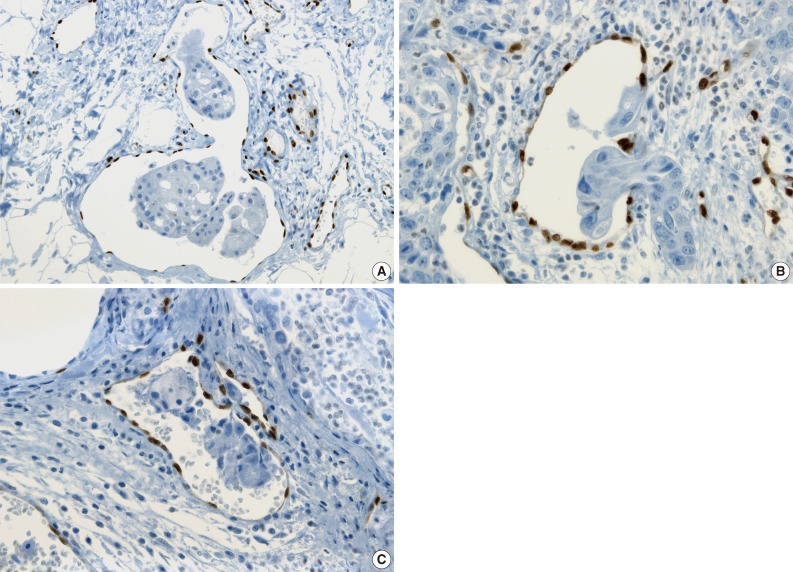
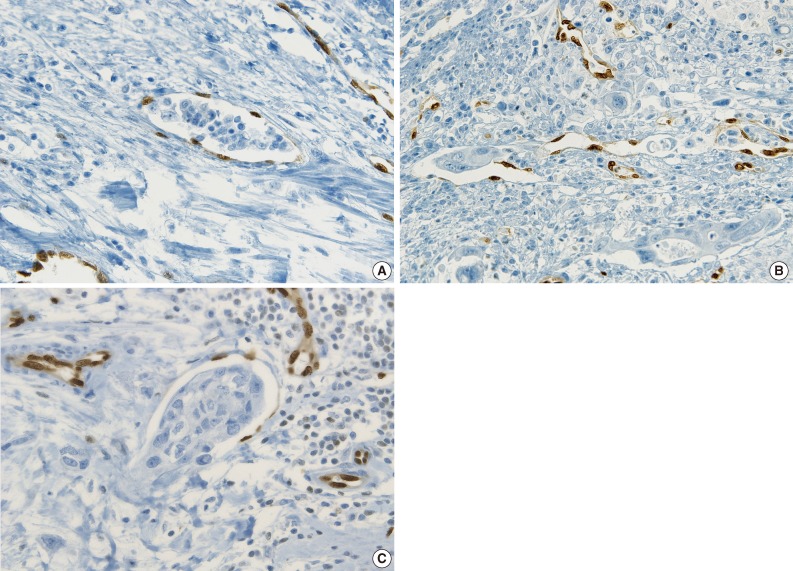
Figure & Data
References
Citations

- Exercise mitigates flow recirculation and activates metabolic transducer SCD1 to catalyze vascular protective metabolites
Susana Cavallero, Mehrdad Roustaei, Sandro Satta, Jae Min Cho, Henry Phan, Kyung In Baek, Ana M. Blázquez-Medela, Sheila Gonzalez-Ramos, Khoa Vu, Seul-Ki Park, Tomohiro Yokota, Jennifer Sumner, Julia J. Mack, Curt D. Sigmund, Srinivasa T. Reddy, Rongsong
Science Advances.2024;[Epub] CrossRef - Cardiac delivery of modified mRNA using lipid nanoparticles: Cellular targets and biodistribution after intramyocardial administration
M.C.I. Labonia, M. Estapé Senti, P.H. van der Kraak, M.A.D. Brans, I. Dokter, T.J. Streef, A.M. Smits, A.K. Deshantri, S.C.A. de Jager, R.M. Schiffelers, J.P.G. Sluijter, P. Vader
Journal of Controlled Release.2024; 369: 734. CrossRef - Heterogeneous Patterns of Endothelial NF-κB p65 and MAPK c-Jun Activation, Adhesion Molecule Expression, and Leukocyte Recruitment in Lung Microvasculature of Mice with Sepsis
Zhendong Wang, Erna-Zulaikha Dayang, Peter J. Zwiers, Martha L. Hernandez Garcia, Matthijs Luxen, Matijs van Meurs, Jill Moser, Jan A. A. M. Kamps, Grietje Molema
Biomedicines.2024; 12(8): 1672. CrossRef - Recruitment of neutrophils in glomeruli in early mouse sepsis is associated with E-selectin expression and activation of endothelial nuclear factor kappa-light-chain-enhancer of activated B cells and mitogen-activated protein kinase pathways
Zhendong Wang, Erna-Zulaikha Dayang, Peter J Zwiers, Martha L Hernandez Garcia, Matthijs Luxen, Matijs van Meurs, Jan A A M Kamps, Jill Moser, Grietje Molema
Journal of Leukocyte Biology.2024; 116(6): 1479. CrossRef - Endometrial cancer: predictors and oncological safety of tumor tissue manipulation
Raquel Quintana-Bertó, Pablo Padilla-Iserte, Víctor Lago, Carmen Tauste, Berta Díaz-Feijoo, Silvia Cabrera, Reyes Oliver-Pérez, Pluvio J. Coronado, María Belén Martín-Salamanca, Manuel Pantoja-Garrido, Josefa Marcos-Sanmartin, Eduardo Cazorla, Cristina Lo
Clinical and Translational Oncology.2023; 26(5): 1098. CrossRef - Universal method for the isolation of microvessels from frozen brain tissue: A proof-of-concept multiomic investigation of the neurovasculature
Marina Wakid, Daniel Almeida, Zahia Aouabed, Reza Rahimian, Maria Antonietta Davoli, Volodymyr Yerko, Elena Leonova-Erko, Vincent Richard, René Zahedi, Christoph Borchers, Gustavo Turecki, Naguib Mechawar
Brain, Behavior, & Immunity - Health.2023; 34: 100684. CrossRef - The Usefulness of Elastin Staining to Detect Vascular Invasion in Cancer
Jeffrey Gonzalez, Hisham F. Bahmad, Stephanie Ocejo, Alvaro Abreu, Meagan Popp, Samantha Gogola, Vielka Fernandez, Monica Recine, Robert Poppiti
International Journal of Molecular Sciences.2023; 24(20): 15264. CrossRef - Single‐nucleus transcriptome analysis reveals disease‐ and regeneration‐associated endothelial cells in white matter vascular dementia
Daniel N. Mitroi, Min Tian, Riki Kawaguchi, William E. Lowry, S. Thomas Carmichael
Journal of Cellular and Molecular Medicine.2022; 26(11): 3183. CrossRef - Gene of the month: ERG
Dorinda Mullen, Klaudia Nowak, Runjan Chetty
Journal of Clinical Pathology.2022; 75(9): 577. CrossRef - Oncological safety of hysteroscopy in endometrial cancer
Raquel Quintana-Bertó, Pablo Padilla-Iserte, Antonio Gil-Moreno, Reyes Oliver-Pérez, Pluvio J. Coronado, María Belén Martín-Salamanca, Manuel Pantoja-Garrido, Cristina Lorenzo, Eduardo Cazorla, Juan Gilabert-Estellés, Lourdes Sánchez, Fernando Roldán-Riva
International Journal of Gynecological Cancer.2022; 32(11): 1395. CrossRef - Upregulated PD-1 signaling antagonizes glomerular health in aged kidneys and disease
Jeffrey W. Pippin, Natalya Kaverina, Yuliang Wang, Diana G. Eng, Yuting Zeng, Uyen Tran, Carol J. Loretz, Anthony Chang, Shreeram Akilesh, Chetan Poudel, Hannah S. Perry, Christopher O’Connor, Joshua C. Vaughan, Markus Bitzer, Oliver Wessely, Stuart J. Sh
Journal of Clinical Investigation.2022;[Epub] CrossRef - Primary Soft Tissue Sarcoma of the Heart: An Emerging Chapter in Cardio-Oncology
Pietro Scicchitano, Maria Chiara Sergi, Matteo Cameli, Marcelo H. Miglioranza, Marco Matteo Ciccone, Marica Gentile, Camillo Porta, Marco Tucci
Biomedicines.2021; 9(7): 774. CrossRef - Analysis on the healing of gastrointestinal ulceration by using Hemospray
Christoph R. Werner, Lena Brücklmeier, Thomas Kratt, Nisar P. Malek, Bence Sipos, Dörte Wichmann, Martin Götz
Scientific Reports.2021;[Epub] CrossRef - Immunohistochemistry for diagnosis and prognosis of breast cancer: a review
T. Bonacho, F. Rodrigues, J. Liberal
Biotechnic & Histochemistry.2020; 95(2): 71. CrossRef - TMEM100 is a key factor for specification of lymphatic endothelial progenitors
Eun-Hye Moon, Yong Hwan Kim, Phuong-Nhung Vu, Hyunjin Yoo, Kwonho Hong, Young Jae Lee, S. Paul Oh
Angiogenesis.2020; 23(3): 339. CrossRef - Spectral photon-counting CT imaging of colorectal peritoneal metastases: initial experience in rats
Arnaud Thivolet, Salim Si-Mohamed, Pierre-Emmanuel Bonnot, Christophe Blanchet, Vahan Képénékian, Loïc Boussel, Philippe Douek, Pascal Rousset
Scientific Reports.2020;[Epub] CrossRef - Diagnostic and reporting issues of preneoplastic polyps of the large intestine with early carcinoma
Dale C. Snover
Annals of Diagnostic Pathology.2019; 39: 1. CrossRef - Feasibility of Endoscopic Resection in Early Gastric Cancer with Lymphovascular Invasion
Jeung Hui Pyo, Hyuk Lee, Yang Won Min, Byung-Hoon Min, Jun Haeng Lee, Kyoung-Mee Kim, Hyeseung Kim, Kyunga Kim, Jae J. Kim
Annals of Surgical Oncology.2019; 26(2): 449. CrossRef - Lymphovascular Invasion and the Decision for Postmastectomy Radiation Therapy: A Cautionary Case Report
J. Arthur Jensen, Rania Bakkar, Michele Burnison, Armando E. Giuliano
Plastic and Reconstructive Surgery - Global Open.2019; 7(2): e2115. CrossRef - Utility of ERG Immunohistochemistry for Evaluation of Lymphovascular Invasion in Testicular Germ Cell Tumors: A Retrospective Pilot Study
Aaron M. Udager, Jonathan B. McHugh, Todd M. Morgan, Daniel E. Spratt, Arul M. Chinnaiyan, Rohit Mehra
Applied Immunohistochemistry & Molecular Morphology.2019; 27(5): 392. CrossRef - An interesting observation from our experiences with circulating tumour cells
Kok H. Lim, Yong W. Chua, Tony K.H. Lim, Angela M. Takano
Lung Cancer.2017; 108: 247. CrossRef - Differences in Prevalence of Lymphovascular Invasion among Early Gastric Cancers between Korea and Japan
Sun-Young Lee, Naohisa Yoshida, Osamu Dohi, Sang Pyo Lee, Daisuke Ichikawa, Jeong Hwan Kim, In-Kyung Sung, Hyung Seok Park, Eigo Otsuji, Yoshito Itoh, Chan Sup Shim, Hye Seung Han, Mitsuo Kishimoto, Yuji Naito
Gut and Liver.2017; 11(3): 383. CrossRef - Tumor Budding Is an Independent Adverse Prognostic Factor in Pancreatic Ductal Adenocarcinoma
Kate O’Connor, Hector H. Li-Chang, Steven E. Kalloger, Renata D. Peixoto, Douglas L. Webber, David A. Owen, David K. Driman, Richard Kirsch, Stefano Serra, Charles H. Scudamore, Daniel J. Renouf, David F. Schaeffer
American Journal of Surgical Pathology.2015; 39(4): 472. CrossRef - P2X7Receptor Expression in Coexistence of Papillary Thyroid Carcinoma with Hashimoto's Thyroiditis
Ji Hyun Kwon, Eun Sook Nam, Hyung Sik Shin, Seong Jin Cho, Hye Rim Park, Mi Jung Kwon
Korean Journal of Pathology.2014; 48(1): 30. CrossRef - Evaluation of colon cancer histomorphology: a comparison between formalin and PAXgene tissue fixation by an international ring trial
Sibylle Gündisch, Julia Slotta-Huspenina, Paolo Verderio, Chiara Maura Ciniselli, Sara Pizzamiglio, Christina Schott, Enken Drecoll, Christian Viertler, Kurt Zatloukal, Marcel Kap, Peter Riegman, Irene Esposito, Katja Specht, Gregor Babaryka, Martin Assla
Virchows Archiv.2014; 465(5): 509. CrossRef - Tumor Sprouting in Papillary Thyroid Carcinoma Is Correlated with Lymph Node Metastasis and Recurrence
Eunjung Lee, Wonkyung Jung, Jeong-Soo Woo, Jae Bok Lee, Bong Kyung Shin, Han Kyeom Kim, Aeree Kim, Baek-hui Kim
Korean Journal of Pathology.2014; 48(2): 117. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
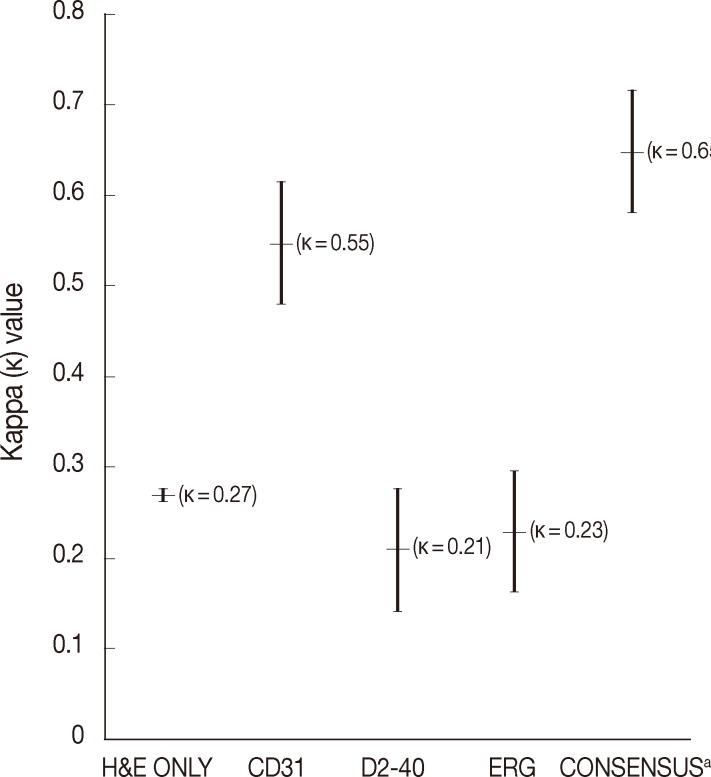
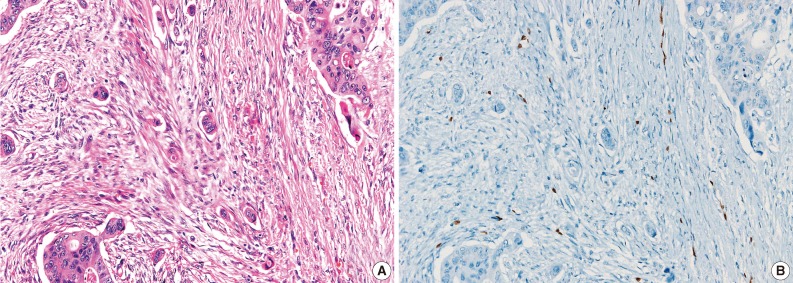
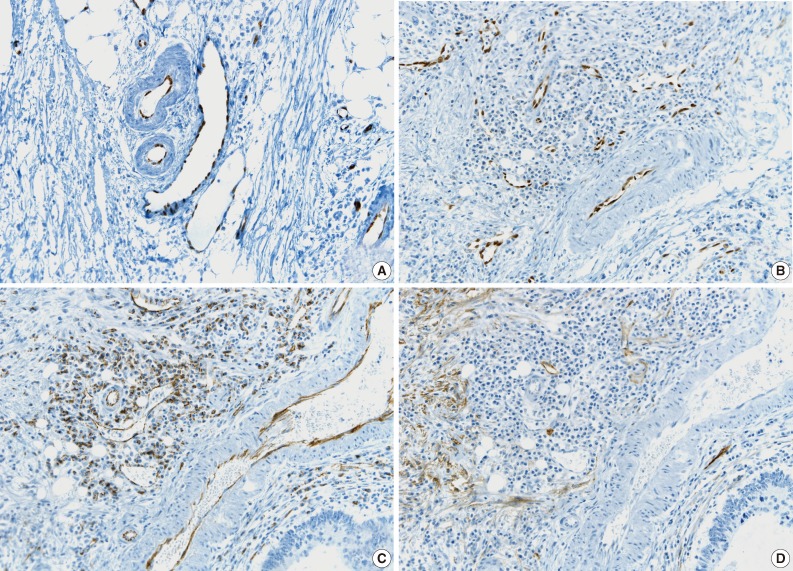
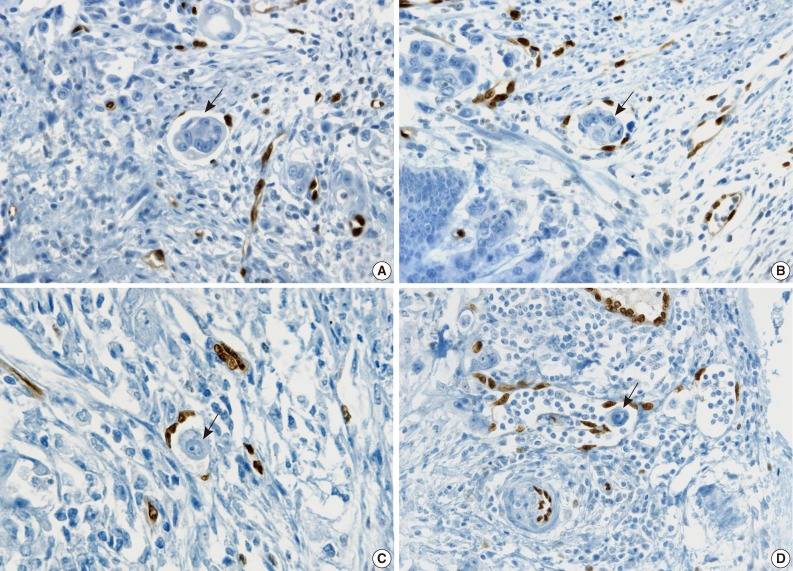
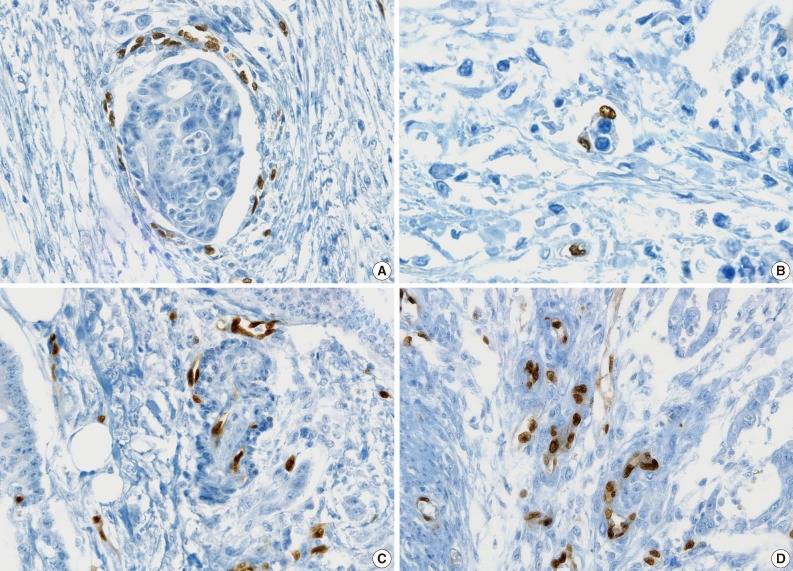
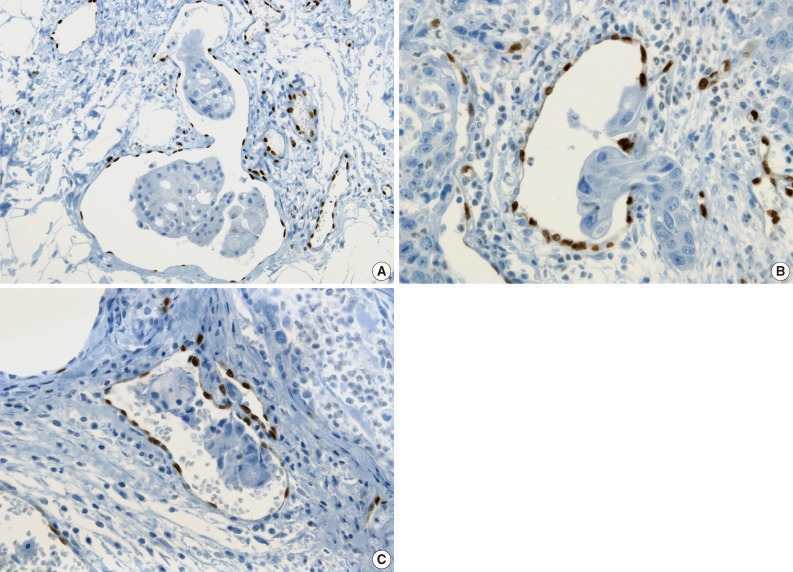
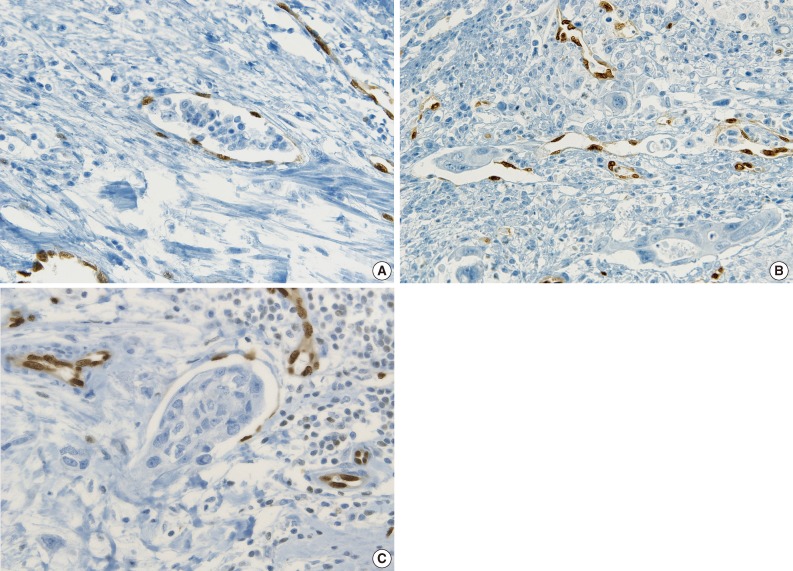
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Fig. 6
Fig. 7
| Characteristic | No. (%) (Total n=15) |
|---|---|
| Mean age (range, yr) | 62.4 (44-89) |
| Pathologic T stage | 11 (73) |
| pT3 | 11 (73) |
| pT4 | 4 (37) |
| LN metastasis | |
| Absent | 8 (53) |
| Present | 7 (47) |
| LVI | |
| Absent | 7 (47) |
| Present | 8 (53) |
| Hepatic metastasis | |
| Concurrent | 12 (80) |
| Subsequent | 3 (20) |
| Mean follow-up (range, mo) | 22 (13-68) |
| DOD | 2 (13) |
| AWD | 13 (87) |
| LVI status | Concordance rate |
Total No. | ||||
|---|---|---|---|---|---|---|
| 100% | 87.50% | 75% | 62.50% | 50% | ||
| LVI-P | 5 | 4 | 3 | 4 | 0 | 16 |
| LVI-N | 13 | 14 | 11 | 4 | 0 | 40 |
| Equivocal |
0 | 0 | 0 | 0 | 4 | 4 |
| Total No. | 18 | 18 | 12 | 8 | 4 | 60 |
Values are presented as number (% or range). LN, lymph node; LVI, lymphovascular invasion at the initial diagnosis; DOD, died of disease; AWD, alive without disease.
LVI-P, lymphovascular invasion (LVI) positivity; LVI-N, LVI negativity. Concordance rate between participants for LVI detection by ERG immunostaining; Equivocal: unknown significance of certain pattern or suspicious LVI.

 E-submission
E-submission









