Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(5); 2012 > Article
-
Case Report
Cytologic Features of Giant Cell Ependymoma: A Case Report and Review of the Literature - Myoung Ju Koh, Sun Och Yoon,, Hyae Min Jeon, Hyeon Joo Jeong, Soon Won Hong, Se Hoon Kim,
-
Korean Journal of Pathology 2012;46(5):507-513.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.5.507
Published online: October 25, 2012
Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- Corresponding Author: Se Hoon Kim, M.D. Department of Pathology, Yonsei University College of Medicine, 250 Seongsan-ro, Seodaemun-gu, Seoul 120-752, Korea. Tel: +82-2-2228-1769, Fax: +82-2-362-0860, paxco@yuhs.ac, Sun Och Yoon, M.D. Department of Pathology, Yonsei University College of Medicine, 211 Eonju-ro, Gangnam-gu, Seoul 135-720, Korea. Tel: +82-2-2019-2791, Fax: +82-2-362-0860, soyoon@yuhs.ac
- *Sun Och Yoon and Se Hoon Kim contributed equally to this work.
*Dr. Yoon consulted this case to Dr. Kim.
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Here, we present a case of anaplastic giant cell ependymoma (GCE) occurring in a 15-year-old woman. Squash smear slides for intraoperative frozen section diagnosis revealed oval to round cell clusters with a papillary structure in a fibrillary background. This was occasionally accompanied by the presence of bizarre pleomorphic giant cells with hyperchromatic nuclei and prominent intranuclear inclusions. These intranuclear inclusions were a key clue to diagnosis of ependymoma. Histologic analysis revealed features of a high-grade tumor with perivascular pseudorosettes and bizarre pleomorphic giant cells, which established the diagnosis of GCE. We performed a review of literatures about the cytologic features of GCE, including our case, thus proposing that intraoperative frozen diagnosis of GCE would be established by squash smear preparations featuring the mitosis and necrosis, as well as the high cellularity, and the presence of giant cells showing hyperchromatic nuclei with eosinophilic cytoplasm and intranuclear inclusions/pseudoinclusions.
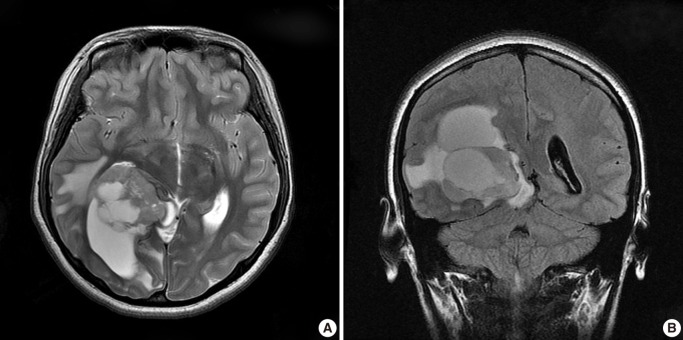
- A healthy 15-year-old woman was admitted to the department of neurosurgery of our institution with a chief complaint of a 1-month history of headache and dizziness. Magnetic resonance imaging (MRI) (Fig. 1) demonstrated a 6.8×5 cm-sized, solid and cystic intra-axial mass in the right temporooccipital area, compressing the posterior horn of the right lateral ventricle. The solid portion of the mass included a calcification, thus presumably partly infiltrating into the brain parenchyma. This was accompaned by the presence of edema of the adjacent brain parenchyma. Differential diagnoses based on radiology include astroblastoma, ependymoma, pleomorphic xanthoastrocytoma and supratentorial primitive neuroectodermal tumor.
- The patient underwent a craniotomy. Thus, grossly, the supratentorial tumor was completely resected. The surgeon noted that the tumor was highly vascularized. Postoperatively, the patient received a focal fractionated radiotherapy with a total dose of 5,040 cGy. A follow-up MRI was taken on postoperative month 5, which revealed no recurrence or progression of the tumor.
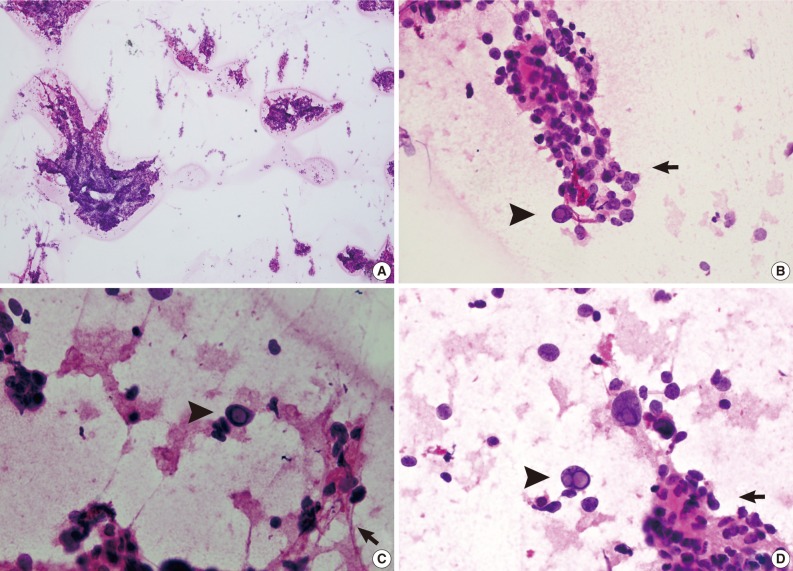
- For intraoperative frozen diagnosis, we used the square-smear technique (Fig. 2). This revealed a hypercellular smear in a fibrillary background. Most of the small- to medium-sized cells with papillary structures had hyperchromatic nuclei and coarse chromatin. This was occasionally accompanied by the presence of bizarre pleomorphic giant cells. They had a round-to-oval shape and contained hyperchromatic nuclei, eosinophilic cytoplasm and prominent eosinophilic intranuclear inclusions. These intranuclear inclusions were a key clue to differential diagnosis of ependymoma and meningioma. Considering the cytologic features along with the clinical and radiological data, we made an intraoperative frozen diagnosis of ependymoma. With a retrospective review of the slides, we identified a perivasculasr pseudorosettes-like lesion. Thus, we supported a frozen diagnosis of ependymoma.
- The surgical specimens consisted of multiple pieces of soft red-to-grey tissue. They were fixed with a 10% buffered formalin and then paraffin-embedded. Then, the specimens were sectioned at a thickness of 2 µm and then stained with a hematoxylin and eosin dye. For immunohistochemistry, we used antibodies against glial fibrillary acidic protein (GFAP), epithelial membrane antigen (EMA), cytokeratin, Ki-67, synaptophysin, and CD99 (MIC2).
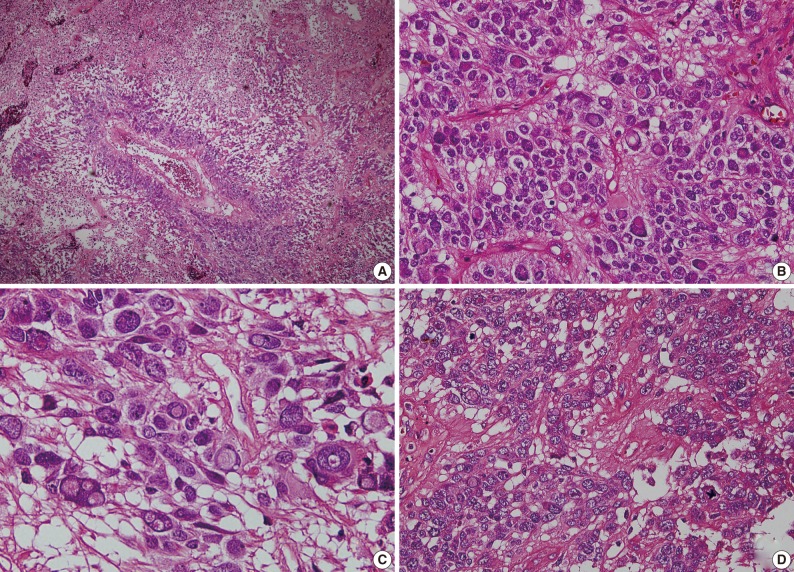
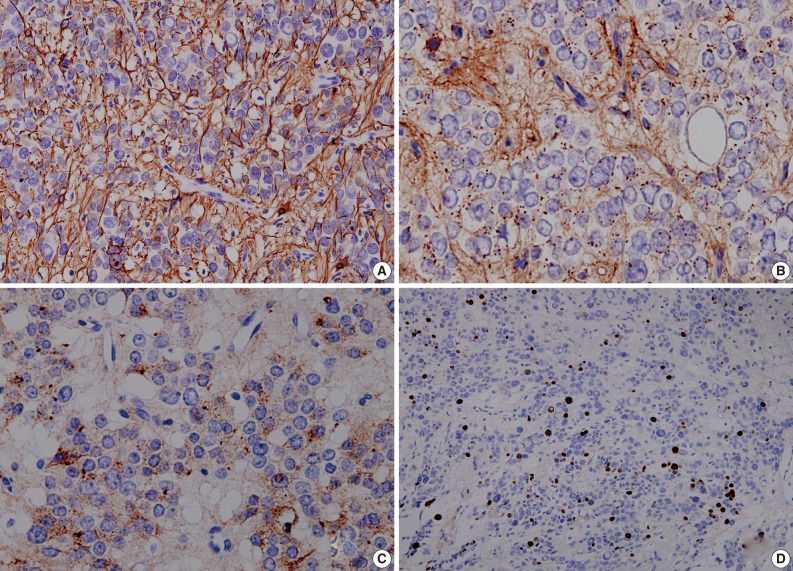
- As shown in Fig. 3, a histopathologic examination showed that the tumor had perivascular pseudorosettes; this is one of the characteristic features of ependymoma. The tumor cells had histopathological findings that are consistent with squash smear ones described above. This was also accompanied by the frequent presence of bizarre pleomorphic giant cells with prominent intranuclear eosinophilic inclusions. According to the WHO criteria, it had features of an anaplastic tumor, including a marked cellularity, abundant mitoses, vascular proliferation and necrosis. Immunohistochemically, the tumor showed a diffuse expression of both GFAP and synaptophysin. That is, it had a high intensity, a dot-like expression of CD99 and that of EMA. In addition, the tumor cells had a Ki-67 labeling index of about 10% (Fig. 4). Based on all of these findings, a diagnosis of anaplastic GCE was established.
CASE REPORT
- A rare variant of ependymoma, GCE poses a diagnostic challenge for the pathologists on the intraoperative frozen section as well as the permanent section. The presence of perivascular pseudorosettes is a key histologic clue to diagnosis of ependymoma. But pseudorosettes are not present in all the case of GCE. According to Zec et al.,2 who first described two cases of GCE in 1996, the absence of perivascular pseudorosettes in GCE might reflect the failure of the neoplastic cells to elaborate perivascular process. Moreover, perivascular pseudorosettes cannot be easily found on the intraoperative frozen section. This often leads to the misdiagnosis of GCE as glioblastoma multiforme,11 anaplastic astrocytoma,6 subependymomal giant cell astrocytoma or tanycytic ependymoma5 on intraoperative frozen section. In addition, GCE should also be differentially diagnosed from anaplastic oligodendroglioma, clear cell ependymoma, pleomorphic xanthoastrocytoma and giant cell glioblastoma.10
- Despite these diagnostic challenges, there has been an increase in the demand for rapid intraoperative diagnosis. This is particularly case with the neurosurgical practice. A simple, reliable, and rapid method, the squash smear technique is useful to present detailed cytologic features of lesions. It is useful in making an intraoperative diagnosis of central nervous system lesions.14 To our knowledge, however, there are no reports about the cytologic features of GCE.
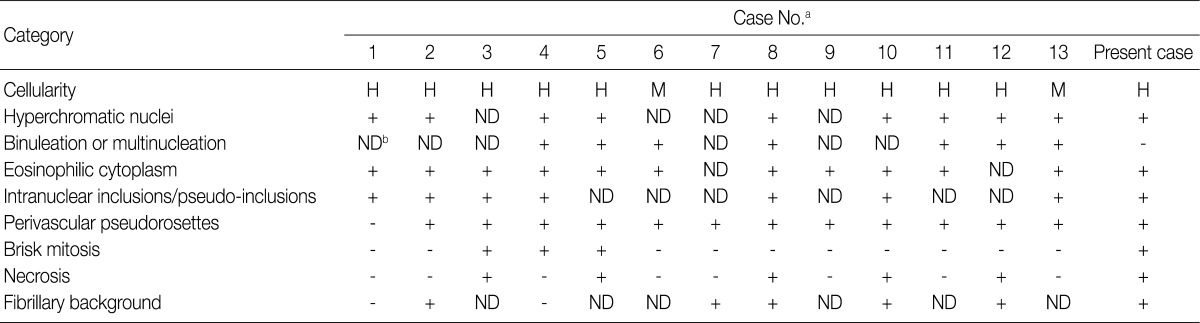
- We performed a review of literatures about GCE, focusing on the cytologic features seen on the tissue sections, whose results including our case are summarized in Table 2. The cytologic features are classified based on the cellularity, hyperchromatic nuclei, binucleation or multinucleation, eosinophilic cytoplasm, intranuclear inclusion/pseudoinclusions, perivascular pseudorosettes, brisk mitosis, necrosis and fibrillary background. Basically, all the 14 cases showed hypercellularity, mitosis and necrosis. Of the total cases, 93% (13/14) had eosinophilic cytoplasm and perivascular pseudorosettes; 71% (10/14) did hyperchromatic nuclei; 57% (8/14) did intranuclear inclusions/pseudo-inclusions; and 50% (7/14) did binucleation or multinucleation.
- The cytologic features of GEC are described in Table 2. It is noteworthy, however, that these features are based on tissue sections of GCE rather than cytology specimens such as the squash smear preparations. It is, therefore, a matter of course that there is no consistency in the cytologic features between the tissue sections and the cytology specimens. In our case, there were perivascular pseudorosettes on the tissue sections, but not found on the squash smear preparations. But both diagnostic modalities showed such findings as mitosis and necrosis, giant cells and intranuclear inclusions/pseudoinclusions. Further comparative descriptions are warranted to define the cytologic features of GCE between tissue sections and cytology specimens.
- In making an intraoperative frozen diagnosis based on squash smear preparations featuring the mitosis and necrosis, as well as the high cellularity, and the presence of giant cells showing hyperchromatic nuclei with eosinophilic cytoplasm and intranuclear inclusions/pseudoinclusions would be key histologic features that are helpful for establishing a diagnosis of GCE. This is particularly true to our case; the presence of giant cells with intranuclear inclusions and papillary structures was a critical clue to intraoperative frozen diagnosis. In addition to the cytologic features, the clinical and radiologic findings are helpful for improving the diagnostic accuracy.
- Due to a relatively smaller number of reported cases, we failed to establish the relationship between the histological pattern of GCE and its prognosis. In patients with anaplastic GCE, however, a poor prognosis is expected with a relatively higher rate of recurrence (Table 1). In our patient, there was no disease progression or recurrence. Due to a shorter length of follow-up, however, further long-term follow-up studies are warranted to predict clinical outcomes of anaplastic GCE.
DISCUSSION
Acknowledgments
Acknowledgments
- 1. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO classification of tumours of the central nervous system. 2007; 4th ed. Lyon: IARC Press, 74-78.
- 2. Zec N, De Girolami U, Schofield DE, Scott RM, Anthony DC. Giant cell ependymoma of the filum terminale: a report of two cases. Am J Surg Pathol 1996; 20: 1091-1101. PubMed
- 3. Brown DF, Chason DP, Schwartz LF, Coimbra CP, Rushing EJ. Supratentorial giant cell ependymoma: a case report. Mod Pathol 1998; 11: 398-403. PubMed
- 4. Pimentel J, Kepes JJ, Moura Nunes JF, Bentes C, Miguéns J, Antunes JL. Supratentorial giant cell ependymoma. Clin Neuropathol 2001; 20: 31-37. PubMed
- 5. Jeon YK, Jung HW, Park SH. Infratentorial giant cell ependymoma: a rare variant of ependymoma. Pathol Res Pract 2004; 200: 717-725. ArticlePubMed
- 6. Fourney DR, Siadati A, Bruner JM, Gokaslan ZL, Rhines LD. Giant cell ependymoma of the spinal cord: case report and review of the literature. J Neurosurg 2004; 100(1 Suppl Spine):75-79. PubMed
- 7. Cooper PB, Katus M, Moores L, et al. Rare giant cell ependymoma in an octogenarian: case report and review of the literature. J Neurosurg 2006; 105: 908-911. PubMed
- 8. Szpak GM, Lewandowska E, Schmidt-Sidor B, et al. Giant cell ependymoma of the spinal cord and fourth ventricle coexisting with syringomyelia. Folia Neuropathol 2008; 46: 220-231. PubMed
- 9. Sangoi AR, Lim M, Dulai M, Vogel H, Chang S. Suprasellar giant cell ependymoma: a rare neoplasm in a unique location. Hum Pathol 2008; 39: 1396-1401. ArticlePubMed
- 10. Adamek D, Dec M, Sobol G, Urbanowicz B, Jaworski M. Giant cell ependymoma: a case report. Clin Neurol Neurosurg 2008; 110: 176-181. ArticlePubMed
- 11. Shamji MF, Benoit BG, Perry A, Jansen GH. Giant cell ependymoma of the thoracic spine: pathology case report. Neurosurgery 2009; 64: E566-E567. PubMed
- 12. Shintaku M, Sakamoto T. Tanycytic ependymoma of the filum terminale with pleomorphic giant cells. Brain Tumor Pathol 2009; 26: 79-82. ArticlePubMedPDF
- 13. Barbagallo GM, Caltabiano R, Parisi G, Albanese V, Lanzafame S. Giant cell ependymoma of the cervical spinal cord: case report and review of the literature. Eur Spine J 2009; 18(Suppl 2):186-190. ArticlePubMedPDF
- 14. Goel D, Sundaram C, Paul TR, et al. Intraoperative cytology (squash smear) in neurosurgical practice: pitfalls in diagnosis experience based on 3057 samples from a single institution. Cytopathology 2007; 18: 300-308. ArticlePubMed
REFERENCES
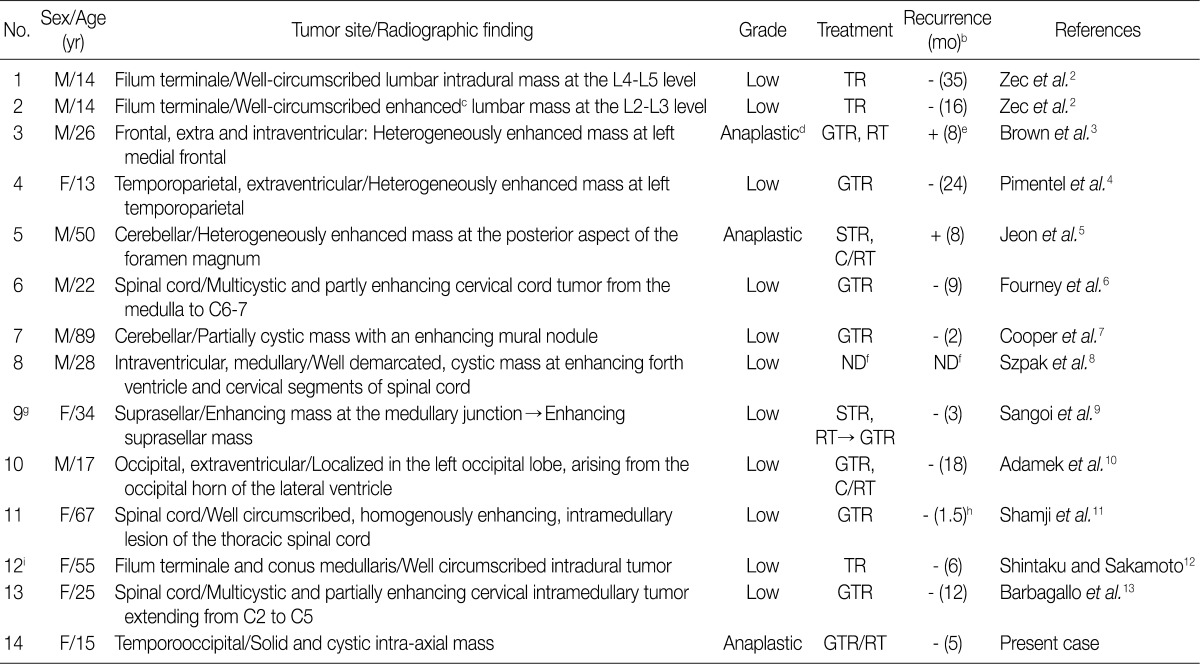
M, male; F, female;TR, total resection; GTR, grossly total resection; STR, subtotal resection; C/RT, chemotherapy/radiotherapy; ND, not described.
aThe table is a modified version of Table 1 from the work of Adamek et al.10; bMonths from the date of operation to last follow-up visit or recurrence; cAfter gadolinium injection; dOn the basis of criteria set forth by the World Health Organization (WHO),1 the neoplasm we described with marked cellularity, abundant mitoses, perivascular pseudorosettes, vascular proliferation, and necrosis would be classified as an anaplastic ependymoma (WHO Grade III); eThe patient was alive 8 months after surgery with recent evidence of enhancement at the tumor resection site. It was not clear at that time whether the enhancement represented tumor recurrence or postoperative/radiation sequelae; fThe patient died suddenly with signs of respiratory insufficiency; gThey report the suprasellar giant cell ependymoma occurring in a 34-year-old female 7 years after an initial diagnosis of a medullary ependymoma with rare atypical giant cells. Incomplete resection and, possibly, incomplete radiotherapy may have contributed to tumoral seeding; hThe patient had a recurrence of severe Clostridium difficile colitis, complicated by renal failure requiring dialysis. This was further complicated by congestive heart failure and pneumonia, and the patient died 6 weeks after the surgery; iThey report tanycytic ependymoma with many pleomorphic giant cells and suggest that it could be termed "giant cell tanycytic ependymoma."
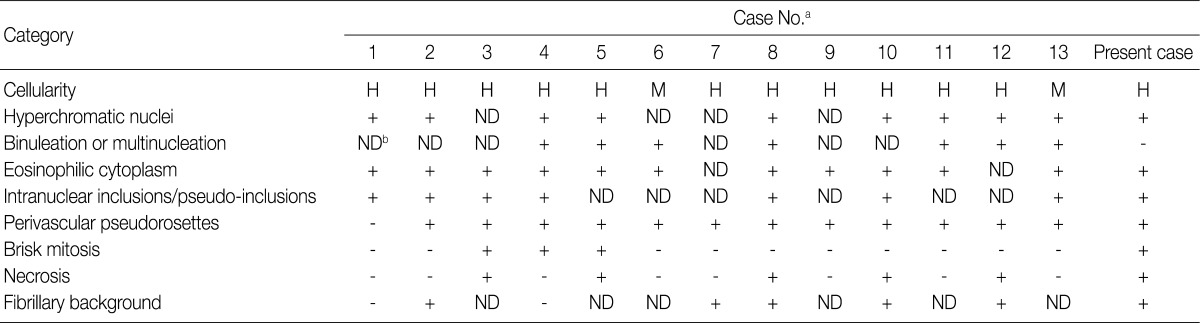
H, high; M, moderate; ND, not described.
aCase No., same as the Table 1; bThe cytologic feature is not described at the literature.
Figure & Data
References
Citations

- A case of myxopapillary ependymoma with predominant giant cell morphology: A rare entity with comprehensive genomic profiling and review of literature
Bryan Morales‐Vargas, Hassan Saad, Daniel Refai, Matthew Schniederjan, Zied Abdullaev, Kenneth Aldape, Malak Abedalthagafi
Neuropathology.2025; 45(1): 13. CrossRef - Report of a case of giant cell ependymoma with unusual clinical and pathological presentation
Mónica B. Mezmezian, Victor Del Caño, Liliana G. Olvi
Neuropathology.2019; 39(4): 313. CrossRef - Giant Cell Ependymoma of Cervicomedullary Junction: A Case Report of a Long-Term Survivor and Literature Review
Martina Cappelletti, Andrea G. Ruggeri, Giorgia Iacopino, Roberto Delfini
World Neurosurgery.2018; 116: 121. CrossRef - Immunohistochemical features of giant cell ependymoma of the filum terminale with unusual clinical and radiological presentation
Fernando Candanedo-Gonzalez, Cindy Sharon Ortiz-Arce, Samuel Rosales-Perez, Ana Lilia Remirez-Castellanos, Candelaria Cordova-Uscanga, Armando Gamboa-Dominguez
Diagnostic Pathology.2017;[Epub] CrossRef - Giant Cell Ependymoma of Lateral Ventricle: Case Report, Literature Review, and Analysis of Prognostic Factors and Genetic Profile
Hirokazu Takami, Christopher S. Graffeo, Avital Perry, Aditya Raghunathan, Robert B. Jenkins, Caterina Giannini, Terry C. Burns
World Neurosurgery.2017; 108: 997.e9. CrossRef
 PubReader
PubReader-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
- Related articles
-
- Cytological characteristics of Müllerian adenosarcoma of the uterine corpus: a case report and literature review
- Cytological features of atypical adenomatous hyperplasia and adenocarcinoma in situ of the lung: a case report
- Metastatic choroidal melanoma in the breast: a case report and review of the literature
- Hepatic carcinoma expressing inhibin: case report of a proposed novel entity and review of the literature
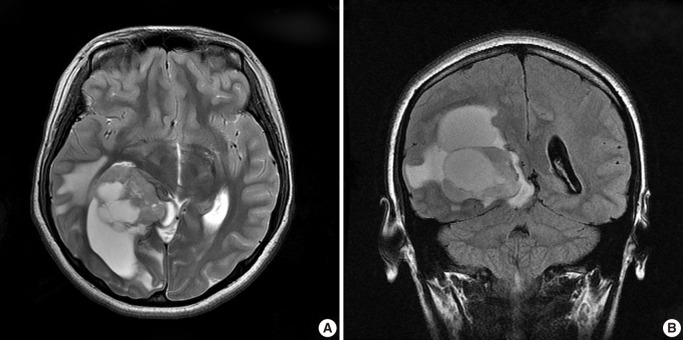
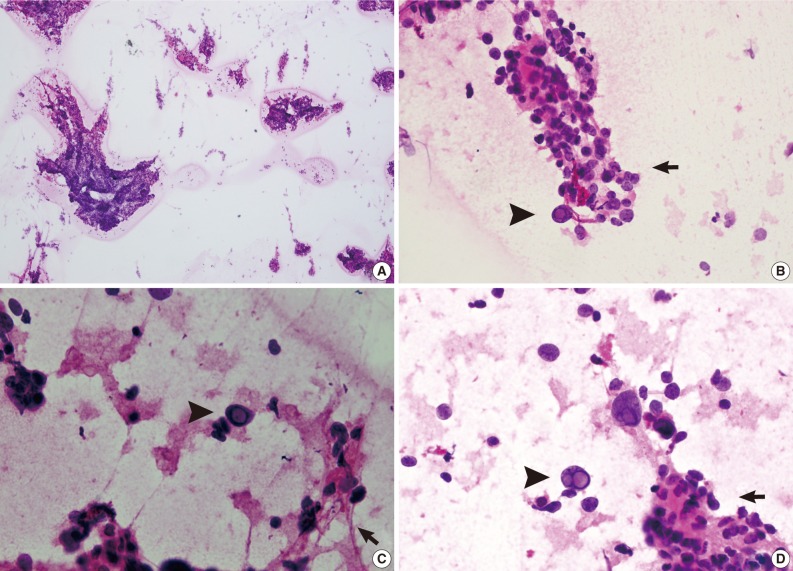
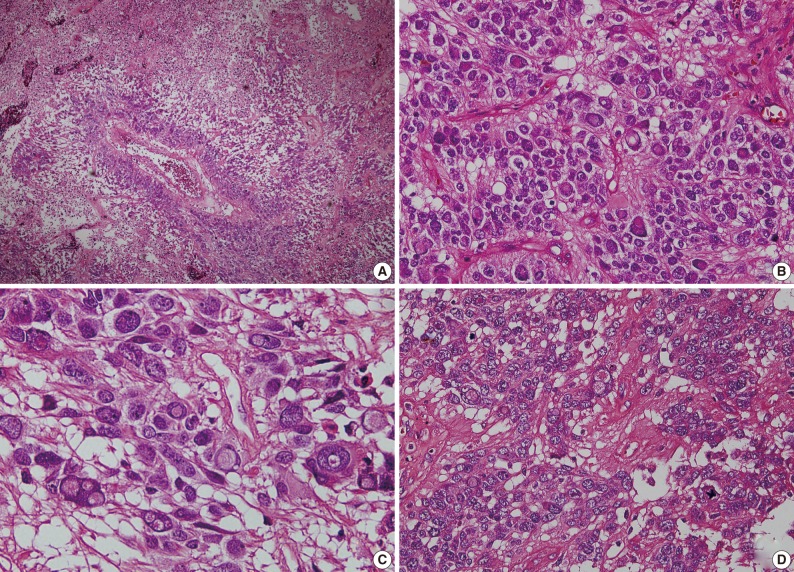
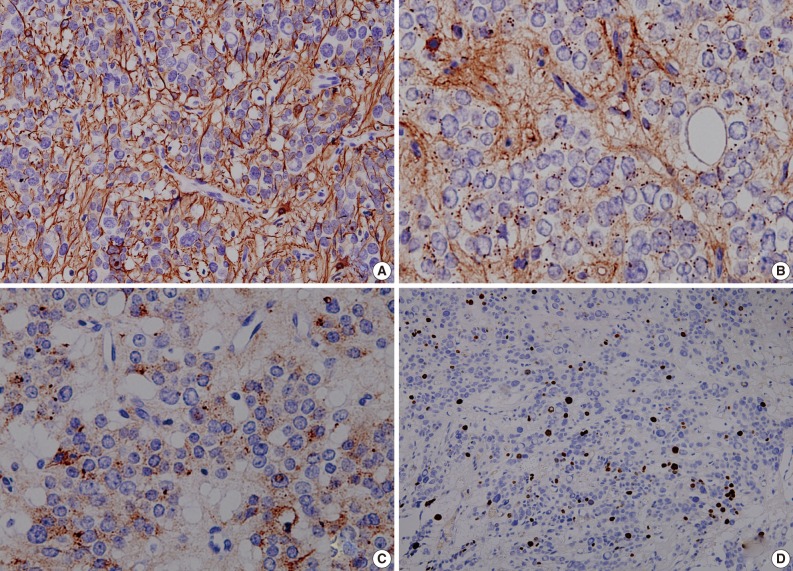
Fig. 1
Fig. 2
Fig. 3
Fig. 4

M, male; F, female;TR, total resection; GTR, grossly total resection; STR, subtotal resection; C/RT, chemotherapy/radiotherapy; ND, not described. aThe table is a modified version of Table 1 from the work of Adamek et al.
H, high; M, moderate; ND, not described. aCase No., same as the

 E-submission
E-submission






