Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 59(3); 2025 > Article
-
Original Article
Primary Merkel cell carcinoma of the salivary gland: a clinicopathologic study of four cases with a review of literature -
Gyuheon Choi1
 , Joon Seon Song1
, Joon Seon Song1 , Hee Jin Lee1
, Hee Jin Lee1 , Gi Hwan Kim1
, Gi Hwan Kim1 , Young Ho Jung2
, Young Ho Jung2 , Yoon Se Lee2
, Yoon Se Lee2 , Kyung-Ja Cho,1
, Kyung-Ja Cho,1
-
Journal of Pathology and Translational Medicine 2025;59(3):171-179.
DOI: https://doi.org/10.4132/jptm.2025.03.25
Published online: April 30, 2025
1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
2Department of Otorhinolaryngology – Head and Neck Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- Corresponding Author Kyung-Ja Cho, MD, PhD Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea Tel: +82-2-3010-1640, Fax: +82-2-472-7898 E-mail: kjc@amc.seoul.kr
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,612 Views
- 146 Download
Abstract
-
Background
- Primary Merkel cell carcinoma of the salivary gland is currently not listed in the World Health Organization classification. However, cases of Merkel cell type neuroendocrine carcinomas of the salivary gland with perinuclear cytokeratin 20 positivity have been intermittently reported. We here investigated the clinicopathologic features of additional cases.
-
Methods
- Data of four cases of Merkel cell type small cell neuroendocrine carcinoma of the salivary gland were retrieved. To confirm the tumors’ primary nature, clinical records and pathologic materials were reviewed. Optimal immunohistochemical staining was performed to support the diagnosis.
-
Results
- All tumors were located in the parotid gland. Possibilities of metastasis were excluded in all cases through a meticulous clinicopathological review. Tumor histology was consistent with the diagnosis of small cell neuroendocrine carcinoma. Tumors’ immunohistochemical phenotypes were consistent with Merkel cell carcinoma, including Merkel cell polyomavirus large T antigen positivity in two of the four cases.
-
Conclusions
- Merkel cell carcinomas can originate in salivary glands and are partly associated with Merkel cell polyomavirus infection as in cutaneous Merkel cell carcinomas.
- Small cell neuroendocrine carcinoma (SCNEC) is a high-grade neuroendocrine carcinoma (NEC) and can arise in several organs. It rarely originates in the salivary gland, accounting for <1% of salivary tumors [1]. SCNEC in the salivary gland deserves special attention as some of them have distinctive features compared to SCNEC of other sites, including diffuse perinuclear cytokeratin 20 (CK20) positivity [2]. A study showed that CK20-positive salivary SCNEC had significantly better prognosis than CK20-negative tumors [1]. Due to its histopathological resemblance to Merkel cell carcinoma (MCC), this group of CK20-positive SCNEC has been referred to by various names, including Merkel cell-like, Merkel cell variant, Merkel cell type SCNEC, or MCC [1-6].
- MCC is a type of NEC mostly arising in the skin with characteristic features different from typical neuroendocrine tumors. The association with the oncogenic virus, Merkel cell polyomavirus (MCPyV), is the most remarkable finding of MCC. MCPyV is a type of polyomavirus identified from MCC tissue [7]. Subsequently, MCPyV infection has been observed in the healthy population with high prevalence, usually being asymptomatic [8-10]. Its oncogenic potential proved highly specific for cutaneous MCC, being detected in approximately 80% of all MCC [11,12]. The presence of MCPyV can be detected using immunohistochemical staining targeting MCPyV large or small T antigen. In contrast, ultraviolet (UV)-related mutations are believed to be a significant pathogenetic factor in the remaining 20% of all MCC, with UV mutations and MCPyV infections being mutually exclusive [13]. Additionally, MCC are distinguished from typical neuroendocrine tumors by the expression of several proteins, including CK20, CD5, terminal deoxynucleotidyl transferase (TdT), paired box 5 (PAX5), and SRY-box transcription factor 2 (SOX2) [6,14-16].
- Although most MCC originate in the skin, they could arise in extracutaneous mucosal sites in the genitourinary tract, anorectal area, and head and neck areas [17-19]. MCC primarily located in the lymph node is regarded as metastasis from regressed occult cutaneous MCC [20]. Some researchers regarded salivary SCNEC of Merkel cell type as being a metastasis from occult cutaneous MCC on the basis of the presence of a UV signature mutation [21]. In this study, we investigated four cases of salivary gland Merkel cell type SCNEC and discussed the controversy associated with this tumor with a review of the literature.
INTRODUCTION
- Case selection and review
- Six cases of NEC of the salivary gland were retrieved from the database of the Department of Pathology of Asan Medical Center, Seoul, Korea, from 2000 to 2023. Two of them were excluded because one case was a large cell NEC, and the other showed positive immunostaining for thyroid transcription factor 1 (TTF-1) and enlarged subaortic lymph nodes, not completely excluding a metastasis of a regressed pulmonary small cell carcinoma. To confirm the absence of tumors from the outside of the salivary gland, a meticulous review of the remaining four cases for available clinical and radiological resources was performed. Two pathologists (G.H.K. and K.J.C.) performed a pathologic review to analyze tumor histology and immunohistochemistry (IHC) results.
- Immunohistochemistry
- One representative formalin-fixed and paraffin-embedded tissue block was retrieved from each case, and 3-µm-thick sections were acquired. IHC staining was performed using Benchmark XT autostainer (Ventana Medical Systems, Tucson, AZ, USA) and OptiView DAB IHC Detection Kit (Ventana Medical Systems) following the manufacturer’s instructions. Detailed information of antibodies is provided in Table 1. All markers except p53 were assessed as positive or negative on the basis of the nuclear (TTF-1, MCPyV large T antigen, PAX5, retinoblastoma (Rb), p53, TdT, SOX2, and PTEN) or cytoplasmic (CK20, synaptophysin, chromogranin, CD56, and BRAF) staining, regardless of intensity or extent. The p53 expression was evaluated in four categories on the basis of the proportion and intensity of expression–negative, 1+ (weak), 2+ (moderate), and 3+ (strong).
MATERIALS AND METHODS
- Clinical features
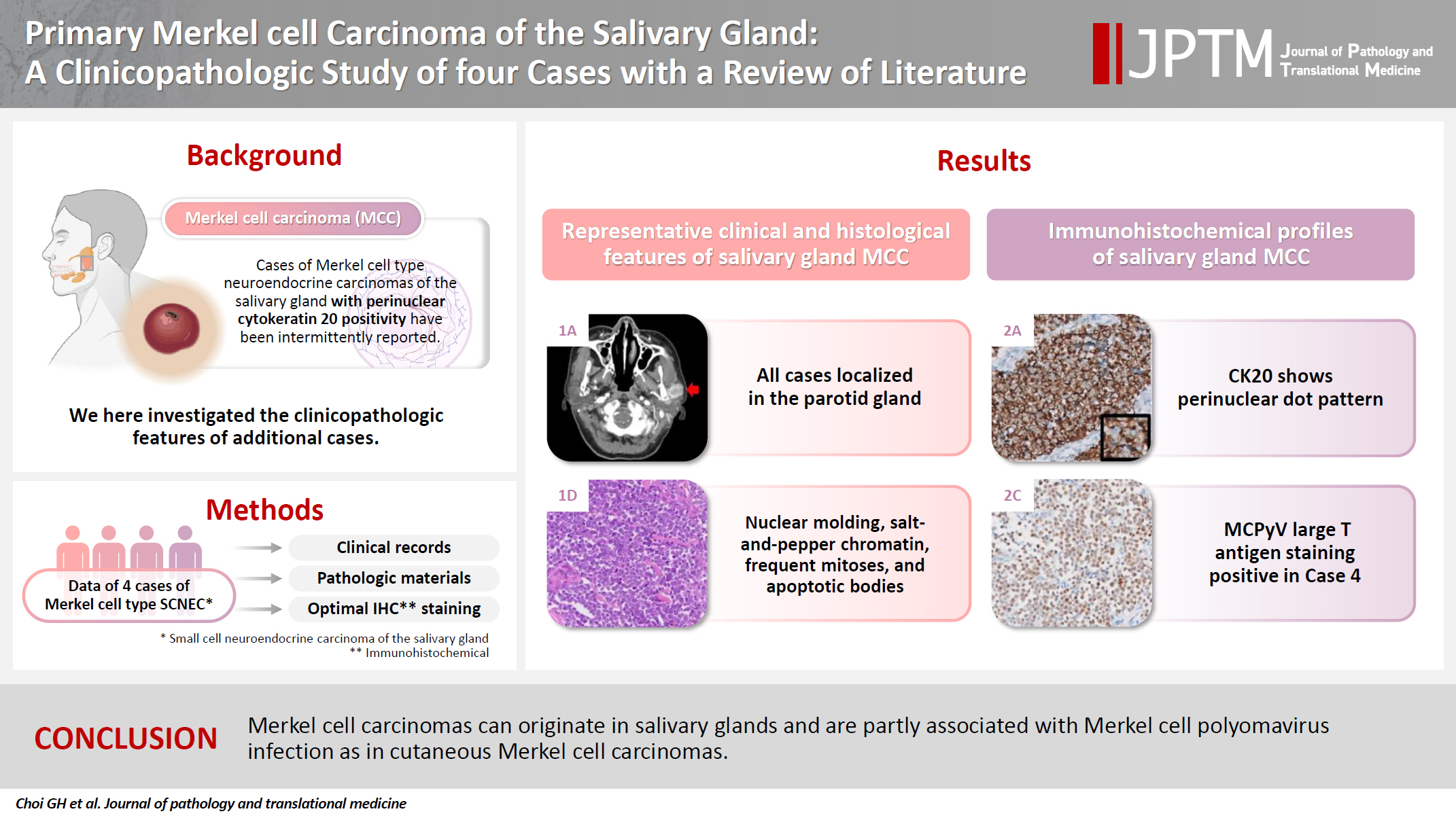
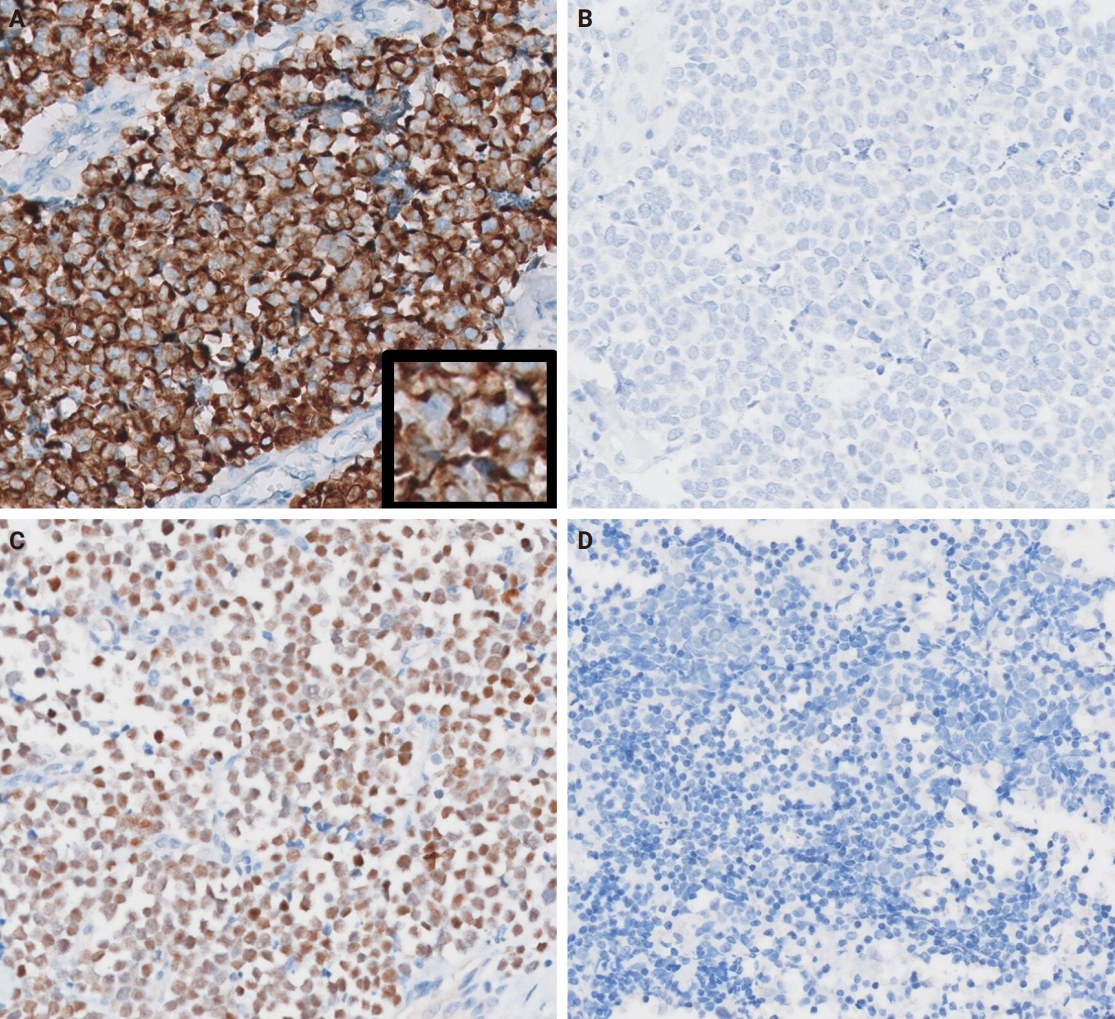
- The patients were three females and one male whose ages ranged from 59 to 78 years (mean, 69.7 years). All patients presented with a rapidly increasing palpable mass in the preauricular area, with accompanying pain in two of them. Computed tomography revealed enhancing mass in the parotid gland with a lobulating or partially infiltrating border (Fig. 1A). The following is the medical history of each patient: Case 1 has diabetes mellitus (DM) and rheumatoid arthritis treated with medication, case 2 has DM treated with medication, case 3 has DM treated with medication and experienced a cerebral stroke 5 months ago, and case 4 underwent a liver transplant due to hepatitis B-associated liver cirrhosis 1 year and 3 months ago. Each patient underwent total (cases 1, 2, and 4) or partial (case 3) parotidectomy. Additionally, cases 2, 3, and 4 underwent neck dissection. Notably, widespread lymph node metastases were identified in the unilateral cervical lymph nodes of case 3 (24 of 62 lymph nodes with the largest tumor dimension of 15 mm and extranodal extension) and case 4 (22 of 44 lymph nodes with the largest tumor dimension of 22 mm and extranodal extension). Adjuvant therapy was administered to two patients. Case 1 received radiotherapy as 66 Gy/25 fraction, whereas case 3 received concurrent chemoradiotherapy as 60 Gy/30 fraction with etoposide and cisplatin. In case 3, tumor recurrence involving the left neck and bilateral external iliac lymph nodes was observed, leading to additional systemic chemotherapy with etoposide and cisplatin. The average follow-up period was 19.4 months. Three patients were referred to local clinics within 1 year. No cases of patient mortality were observed until the last follow-up. Clinical data are presented in Table 2.
- Histology and IHC
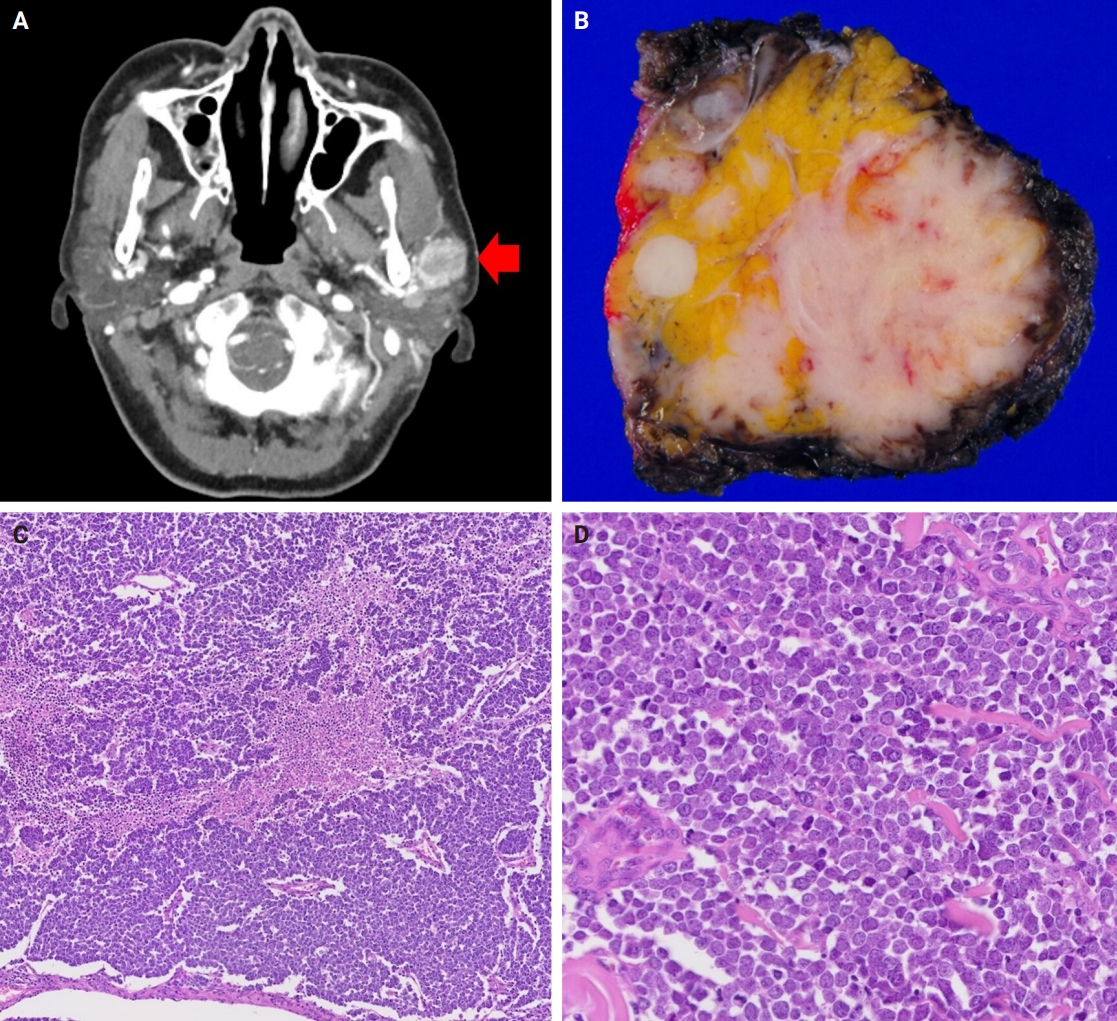
- Grossly, all tumors appeared as ill-demarcated solid masses involving the parotid gland with fleshy cut surface (Fig. 1B). The tumor size ranged from 1.3 to 4.6 cm (mean, 2.85 cm). Microscopic examination revealed infiltrating tumors composed of small monotonous tumor cells demonstrating diverse histological patterns within the tumor, including diffuse, nested, and trabecular patterns. Tumor cells exhibited hyperchromatic finely granular nuclei with nuclear molding and crush artifact. Significant mitotic activity was observed, with counts exceeding 20 per high-power field in some regions. Spotty necrosis and apoptotic debris were frequently observed (Fig. 1C, D). Results of IHC staining for MCC-associated markers are shown in Table 3. In all cases, neuroendocrine markers (synaptophysin, chromogranin, and CD56) were positive. CK20 showed diffuse expression with a perinuclear dot pattern in all cases. TTF-1 was negative in all cases (Fig. 2A, B). MCPyV large T antigen was identified in two cases (cases 3 and 4) (Fig. 2C, D). In both cases, more than 90% of cells showed nuclear staining with variable intensity. PAX5 was focally positive in one case (case 2). PTEN loss was observed in two cases (cases 1 and 3). Rb and p53 were positive in all cases with heterogeneous expression. TdT and BRAF were negative in all cases. SOX2 showed diffuse positive staining in all cases with strong intensity.
RESULTS
- MCC is primarily of a cutaneous origin; however, extracutaneous cases, accounting for approximately 2% of all MCC, have been steadily reported with the salivary glands being the most common site [22]. In the literature, we could identify 45 cases of Merkel cell type (CK20-positive) NEC of the salivary gland (Table 4) [1,3-6,23-34]. Including our four cases, the mean age of the total 49 cases was 70.9 years, with a male predilection (65.3%). The majority of cases (46 cases, 93.9%) originated in the parotid gland, with three originating in the submandibular gland. MCPyV was detected in six of 15 cases tested. Immunosuppression and chronic inflammatory conditions including DM and rheumatic disorders contribute as risk factors for cutaneous MCCs [35,36]. Among our cases, three patients had DM (including one with concurrent rheumatoid arthritis), and one had a liver transplantation history. The underlying mechanisms generating a correlation between MCC and immunosuppression or chronic inflammatory disorders are not well-established; however, immunosuppression is linked with poor prognosis [37] and MCPyV negativity [38]. Although our patients demonstrated a relatively favorable prognosis, the small sample size and the relatively short follow-up duration were limitations. Out of a total 49 cases, 38 had documented survival status and follow-up duration. The survival duration ranged from 2 to 156 months, with a median of 36 months. This finding is better than the previously reported median survival of salivary SCNEC (25–28.5 months) [39,40], which is consistent with the previous finding that CK20-positive salivary SCNEC show better prognosis [1].
- The Fifth World Health Organization classification of head and neck tumors presents that most salivary SCNEC carrying MCPyV or UV signature mutations are best classified as metastatic MCC [41]. However, no robust tools have been developed yet, which could be used for differentiating primary or metastatic MCC, if there have been regressed or occult skin tumors. Meticulous clinical and radiological investigations for exploring primary site appear to be the most critical for proper diagnosis. Nevertheless, as the skin is the most superficial part of the body, the absence of any skin tumors in patients’ histories is considered relatively reliable. MCPyV does not exclusively infect the skin cells but can be detected in systemic tissues, such as the saliva, aerodigestive tract, or liver [42]. It has been detected in extracutaneous MCC, including cases involving the maxillary sinus [18], stomach [43], and salivary glands [3-5]. UV mutation signatures are often cited as supporting evidence for a skin origin in tumors; however, they have also been observed in a range of extracutaneous tumors [44] including salivary squamous cell carcinoma cases [45,46]. Given that whole exome sequencing is the standard method for detecting UV signature mutations, its application was not feasible in our study due to the age of our specimens. To explore UV-related mutations, we instead employed IHC for PTEN and BRAF as surrogate markers, as mutations in these genes have been commonly associated with UV-induced damage [47,48]. Unfortunately, the IHC results did not reveal significant findings; specifically, we found no evidence of UV mutations in the MCPyV-negative cases. While previous studies have used UV mutation signatures to argue that cases of lung melanoma [49] and salivary squamous cell carcinoma [45] may represent occult cutaneous origin metastases, relying on UV mutation signatures as definitive evidence of a cutaneous origin remains challenging due to numerous variables [50]. That is, although certain DNA damage patterns are relatively strongly associated with UV exposure, it is difficult to completely rule out other causes of similar damage, and the lack of a standardized analytical approach can lead to variation in results depending on the specific genes analyzed and the threshold values applied. Therefore, after excluding other possible primary sites through clinical evaluation, it seems reasonable to consider salivary gland MCC as a primary salivary tumor.
- Interestingly, MCC tumor cells show various lymphocytic markers, including TdT, PAX5, and CD5 [14,15], and it could pose a diagnostic pitfall when distinguishing MCCs from lymphocytic neoplasms. However, our cases were nearly all negative for TdT and PAX5, except for one case with trivial positivity for PAX5. Conversely, all cases showed diffuse positivity for SOX2, which is a Merkel cell differentiation regulator and an essential oncogene of MCC [51,52]. It can be used for distinguishing MCC from other skin tumors but not from extracutaneous NEC [16]. An essential pathogenetic mechanism of MCC is Rb protein inactivation [53]. However, loss of Rb expression is frequently observed in a minor portion of MCPyV-negative cases [16,54,55]. Although our cases consistently exhibited Rb and SOX2 positivity, it is less likely that this finding is specific for salivary gland Merkel cell type NEC. Overall, salivary gland Merkel type NEC has similar clinicopathological characteristics with cutaneous MCC.
- In conclusion, we propose that MCC can arise in the salivary glands, in addition to the skin. After thoroughly ruling out the possibility of metastasis, these cases should be treated in a manner appropriate for a primary salivary gland malignancy. Salivary gland MCC exhibits a phenotype identical with cutaneous MCC, including MCPyV and SOX2 positivity. We expect that MCC would be listed as a primary salivary gland malignancy in the classification.
DISCUSSION
Ethics Statement
All procedures performed in the current study were approved by the Institutional Review Board of Asan Medical Center (approval No. 2023-1328) in accordance with the 1964 Helsinki Declaration and its later amendments. Formal written informed consent was not required with a waiver by the Institutional Review Board of Asan Medical Center.
Availability of Data and Material
1. The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
2. All data generated or analyzed during the study are included in this published article (and its supplementary information files).
3. The datasets generated or analyzed during the study are not publicly available due [REASON(S) WHY DATA ARE NOT PUBLIC] but are available from the corresponding author on reasonable request.
4. The datasets generated or analyzed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS]
5. Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Code Availability
Not applicable.
Author Contributions
Conceptualization: KJC. Data curation: GC, GHK. Formal analysis: GC, GHK. Investigation: GC, GHK. Methodology: KJC. Project administration: GC, KJC. Resources: JSS, HJL, YHJ, YSL, KJC. Supervision: KJC. Validation: JSS, HJL, KJC. Visualization: GC. Writing—original draft: GC. Writing—review & editing: JSS, HJL, KJC. Approval of final manuscript: all authors.
Conflicts of Interest
J.S.S., a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
No funding to declare.
R, right; TP, total parotidectomy; RT, radiotherapy; DM, diabetes mellitus; RA, rheumatoid arthritis; NED, no evidence of the disease; SND, selective neck dissection; RP, radical parotidectomy; MRND, modified radical neck dissection; CCRT, concurrent chemoradiotherapy; CVA, cerebrovascular accident; AWD, alive with the disease; L, left; LT, liver transplantation.
| Study | No. | Age (yr) | Sex | Site | Size (cm) | MCPyV | LN metastasis | Recurrence | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Fornelli et al. [23] | 2 | 65 | M | Parotid | 4 | NA | - | + | DOD (28 mo) |
| 70 | M | Parotid | 2.5 | NA | - | + | AWD (2 yr) | ||
| Nagao et al. [1] | 11 | 77 | F | Parotid | 1.8 | NA | - | - | NED (28 mo) |
| 78 | M | Parotid | 1.5 | NA | + | - | DOD (45 mo) | ||
| 81 | F | Parotid | 3 | NA | + | - | DOD (17 mo) | ||
| 85 | M | Submandibular | 3.8 | NA | - | - | DOD (9 mo) | ||
| 50 | M | Parotid | 0.7 | NA | + | - | NED (155 mo) | ||
| 66 | M | Parotid | 5 | NA | - | - | DOD (20 mo) | ||
| 76 | M | Parotid | 11 | NA | + | - | DOD (2 mo) | ||
| 72 | M | Parotid | 2.9 | NA | - | - | NED (4 mo) | ||
| 72 | M | Parotid | 2 | NA | + | - | DOD (34 mo) | ||
| 67 | M | Parotid | 8 | NA | + | - | AWD (18 mo) | ||
| 52 | F | Parotid | 1.5 | NA | - | - | NED (4 mo) | ||
| Jorcano et al. [24] | 1 | 91 | M | Parotid | 4 | NA | + | - | DOC (3 yr) |
| Mulder et al. [25] | 1 | 78 | M | Parotid | NA | NA | + | + | DOD (3 yr) |
| Ghaderi et al. [26] | 1 | 35 | F | Parotid | 2 | NA | - | - | NA |
| Baca et al. [27] | 1 | 77 | M | Parotid | 8.5 | NA | + | NA | NA |
| Chernock et al. [6] | 5 | 68 | M | Parotid | NA | - | + | - | DOD (<6 mo) |
| 66 | M | Parotid | NA | - | + | + | NED (112 mo) | ||
| 74 | M | Parotid | NA | - | + | - | NED (2 yr) | ||
| 22 | M | Parotid | NA | - | + | - | DOD (<6 mo) | ||
| 60 | M | Parotid | NA | - | - | - | NED (13 yr) | ||
| De Biase et al. [5] | 1 | 64 | M | Parotid | 4 | + | + | NA | DOD (1 yr) |
| Kanazawa et al. [28] | 1 | 87 | F | Parotid | 5.5 | NA | + | - | NED (108 mo) |
| Fisher et al. [4] | 3 | 64 | F | Parotid | 1.4 | + | - | - | NED (41 mo) |
| 82 | M | Parotid | 6.5 | - | - | - | DOC (8 mo) | ||
| 82 | M | Parotid | 2.8 | + | + | - | NED (31 mo) | ||
| Lombardi et al. [3] | 1 | 67 | F | Submandibular | 2.2 | + | + | - | NED (12 mo) |
| Bizzaro et al. [29] | 1 | 65 | M | Parotid | 1.7 | NA | - | - | NED (24 mo) |
| Knopf et al. [30] | 8 | Mean 75 | 3M, 5F | Parotid | NA | NA | 3 (37.5%) | 5-yr RFI, 71% | 5-yr OS, 29% |
| Alotaibi et al. [31] | 1 | 71 | M | Parotid | 3.5 | NA | - | - | NA |
| Astreidis et al. [32] | 1 | 76 | M | Parotid | 2.7 | NA | - | - | NED (60 mo) |
| Young et al. [33] | 1 | 69 | M | Parotid | 4.9 | - | - | + | AWD (20 mo) |
| De Luca et al. [34] | 5 | 57 | M | Parotid | 1.5 | NA | + | + | DOD (45 mo) |
| 79 | F | Parotid | NA | NA | NA | + | DOC (24 mo) | ||
| 92 | F | Submandibular | 2.8 | NA | - | - | DOC (16 mo) | ||
| 70 | M | Parotid | 3.4 | NA | NA | + | DOD (14 mo) | ||
| 88 | M | Parotid | 2.5 | NA | NA | - | DOC (46 mo) |
- 1. Nagao T, Gaffey TA, Olsen KD, Serizawa H, Lewis JE. Small cell carcinoma of the major salivary glands: clinicopathologic study with emphasis on cytokeratin 20 immunoreactivity and clinical outcome. Am J Surg Pathol 2004; 28: 762-70. ArticlePubMed
- 2. Chan JK, Suster S, Wenig BM, Tsang WY, Chan JB, Lau AL. Cytokeratin 20 immunoreactivity distinguishes Merkel cell (primary cutaneous neuroendocrine) carcinomas and salivary gland small cell carcinomas from small cell carcinomas of various sites. Am J Surg Pathol 1997; 21: 226-34. ArticlePubMed
- 3. Lombardi D, Accorona R, Ungari M, Melocchi L, Bell D, Nicolai P. Primary Merkel cell carcinoma of the submandibular gland: when CK20 status complicates the diagnosis. Head Neck Pathol 2015; 9: 309-14. ArticlePubMedPMCPDF
- 4. Fisher CA, Harms PW, McHugh JB, et al. Small cell carcinoma in the parotid harboring Merkel cell polyomavirus. Oral Surg Oral Med Oral Pathol Oral Radiol 2014; 118: 703-12. ArticlePubMed
- 5. de Biase D, Ragazzi M, Asioli S, Eusebi V. Extracutaneous Merkel cell carcinomas harbor polyomavirus DNA. Hum Pathol 2012; 43: 980-5. ArticlePubMed
- 6. Chernock RD, Duncavage EJ, Gnepp DR, El-Mofty SK, Lewis JS Jr. Absence of Merkel cell polyomavirus in primary parotid high-grade neuroendocrine carcinomas regardless of cytokeratin 20 immunophenotype. Am J Surg Pathol 2011; 35: 1806-11. ArticlePubMed
- 7. Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008; 319: 1096-100. ArticlePubMedPMC
- 8. Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog 2009; 5: e1000363.ArticlePubMedPMC
- 9. Carter JJ, Paulson KG, Wipf GC, et al. Association of Merkel cell polyomavirus-specific antibodies with Merkel cell carcinoma. J Natl Cancer Inst 2009; 101: 1510-22. ArticlePubMedPMC
- 10. Tolstov YL, Knauer A, Chen JG, et al. Asymptomatic primary Merkel cell polyomavirus infection among adults. Emerg Infect Dis 2011; 17: 1371-80. ArticlePubMedPMC
- 11. Duncavage EJ, Le BM, Wang D, Pfeifer JD. Merkel cell polyomavirus: a specific marker for Merkel cell carcinoma in histologically similar tumors. Am J Surg Pathol 2009; 33: 1771-7. ArticlePubMed
- 12. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology 2013; 435: 118-30. ArticlePubMedPMC
- 13. Harms PW, Vats P, Verhaegen ME, et al. The distinctive mutational spectra of polyomavirus-negative Merkel cell carcinoma. Cancer Res 2015; 75: 3720-7. ArticlePubMedPMCPDF
- 14. Kolhe R, Reid MD, Lee JR, Cohen C, Ramalingam P. Immunohistochemical expression of PAX5 and TdT by Merkel cell carcinoma and pulmonary small cell carcinoma: a potential diagnostic pitfall but useful discriminatory marker. Int J Clin Exp Pathol 2013; 6: 142-7. PubMedPMC
- 15. Legrand M, Tallet A, Guyetant S, Samimi M, Ortonne N, Kervarrec T. CD5 expression in Merkel cell carcinoma and extracutaneous neuroendocrine carcinomas. Pathology 2023; 55: 141-3. ArticlePubMed
- 16. Thanguturi S, Tallet A, Miquelestorena-Standley E, et al. Investigation of the RB1-SOX2 axis constitutes a tool for viral status determination and diagnosis in Merkel cell carcinoma. Virchows Arch 2022; 480: 1239-54. ArticlePubMedPDF
- 17. Aron M, Zhou M. Merkel cell carcinoma of the genitourinary tract. Arch Pathol Lab Med 2011; 135: 1067-71. ArticlePubMedPDF
- 18. Sheldon JD, Lott Limbach AA. Merkel cell carcinoma of the maxillary sinus: an unusual presentation of a common tumor. Head Neck Pathol 2021; 15: 691-7. ArticlePubMedPMCPDF
- 19. van Wyk AC, Moolla Z, Motala AI, et al. Merkel cell carcinoma of the anorectum: a case report and review of the literature. Clin J Gastroenterol 2022; 15: 740-5. ArticlePubMedPDF
- 20. Lawrence LEB, Saleem A, Sahoo MK, et al. Is Merkel cell carcinoma of lymph node actually metastatic cutaneous Merkel cell carcinoma? Am J Clin Pathol 2020; 154: 369-80. ArticlePubMedPDF
- 21. Sun L, Cliften PF, Duncavage EJ, Lewis JS Jr, Chernock RD. UV signature mutations reclassify salivary high-grade neuroendocrine carcinomas as occult metastatic cutaneous Merkel cell carcinomas. Am J Surg Pathol 2019; 43: 682-7. ArticlePubMed
- 22. Albores-Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, Henson DE. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol 2010; 37: 20-7. ArticlePubMed
- 23. Fornelli A, Eusebi V, Pasquinelli G, Quattrone P, Rosai J. Merkel cell carcinoma of the parotid gland associated with Warthin tumour: report of two cases. Histopathology 2001; 39: 342-6. ArticlePubMedPDF
- 24. Jorcano S, Casado A, Berenguer J, Arenas M, Rovirosa A, Colomo L. Primary neuroendocrine small cell undifferentiated carcinoma of the parotid gland. Clin Transl Oncol 2008; 10: 303-6. ArticlePubMedPDF
- 25. Mulder DC, Rosenberg AJ, Storm-Bogaard PW, Koole R. Spontaneous regression of advanced merkel-cell-like small cell carcinoma of the parotid gland. Br J Oral Maxillofac Surg 2010; 48: 199-200. ArticlePubMed
- 26. Ghaderi M, Coury J, Oxenberg J, Spector H. Primary Merkel cell carcinoma of the parotid gland. Ear Nose Throat J 2010; 89: E24-7. ArticlePubMedPDF
- 27. Baca JM, Chiara JA, Strenge KS, Keylock JB, Jones CL, Harsha WJ. Small-cell carcinoma of the parotid gland. J Clin Oncol 2011; 29: e34-6. ArticlePubMed
- 28. Kanazawa T, Fukushima N, Tanaka H, et al. Parotid small cell carcinoma presenting with long-term survival after surgery alone: a case report. J Med Case Rep 2012; 6: 431.ArticlePubMedPMCPDF
- 29. Bizzarro T, Buda R, Ricci M, Bernardi L. Cytological diagnosis of a rare case of primary Merkel cell carcinoma of the parotid gland. Cytopathology 2017; 28: 552-4. ArticlePubMedPDF
- 30. Knopf A, Bas M, Hofauer B, Mansour N, Stark T. Clinicopathological characteristics of head and neck Merkel cell carcinomas. Head Neck 2017; 39: 92-7. ArticlePubMedPDF
- 31. Alotaibi FH, Lugo R, Patel SY, Abdulsattar J, Ghali GE. Primary Merkel cell carcinoma of the parotid gland: unusual location and clinical presentation. Oral Maxillofac Surg Cases 2020; 6: 100197.Article
- 32. Astreidis I, Kalaitsidou I, Papaemmanouil S, Vachtsevanos K, Antoniadis K. Small cell neuroendocrine carcinoma of the parotid gland: a chronicle of publications based on the largest case series and a report of the first Greek case. Res Rep Oral Maxillofac Surg 2020; 4: 044.Article
- 33. Young S, Oh J, Bukhari H, Ng T, Chau N, Tran E. Primary parotid Merkel type small cell neuroendocrine carcinoma with oligometastasis to the brain and adrenal gland: case report and review of literature. Head Neck Pathol 2021; 15: 311-8. ArticlePubMedPMCPDF
- 34. De Luca P, Simone M, De Seta D, et al. Small cell neuroendocrine carcinoma "Merkel-like" of major salivary glands: presentation of a multicenter case series of this exceptional histological entity. Oral Oncol 2023; 138: 106329.ArticlePubMed
- 35. Cook M, Baker K, Redman M, et al. Differential outcomes among immunosuppressed patients with Merkel cell carcinoma: impact of immunosuppression type on cancer-specific and overall survival. Am J Clin Oncol 2019; 42: 82-8. ArticlePubMedPMC
- 36. Sahi H, Sihto H, Artama M, Koljonen V, Bohling T, Pukkala E. History of chronic inflammatory disorders increases the risk of Merkel cell carcinoma, but does not correlate with Merkel cell polyomavirus infection. Br J Cancer 2017; 116: 260-4. ArticlePubMedPMCPDF
- 37. Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol 2013; 133: 642-6. ArticlePubMedPMC
- 38. Starrett GJ, Thakuria M, Chen T, et al. Clinical and molecular characterization of virus-positive and virus-negative Merkel cell carcinoma. Genome Med 2020; 12: 30.ArticlePubMedPMCPDF
- 39. Bai J, Zhao F, Pan S. Clinicopathological characteristics and survival of small cell carcinoma of the salivary gland: a population-based study. Cancer Manag Res 2019; 11: 10749-57. ArticlePubMedPMC
- 40. Zhan KY, Din HA, Muus JS, Nguyen SA, Lentsch EJ. Predictors of survival in parotid small cell carcinoma: a study of 344 cases. Laryngoscope 2016; 126: 2036-40. ArticlePubMedPDF
- 41. Lisa M, Vania N, Peter S, Byardo P, Marion C. Small cell neuroendocrine carcinoma. In: WHO Classification of Tumours Editorial Board, ed. WHO classification of tumours. 5th ed. Vol. 9. Head and neck tumours. Lyon: International Agency for Research on Cancer, 2022; 646-8.
- 42. Loyo M, Guerrero-Preston R, Brait M, et al. Quantitative detection of Merkel cell virus in human tissues and possible mode of transmission. Int J Cancer 2010; 126: 2991-6. ArticlePubMedPMC
- 43. Capella C, Marando A, Longhi E, et al. Primary gastric Merkel cell carcinoma harboring DNA polyomavirus: first description of an unusual high-grade neuroendocrine carcinoma. Hum Pathol 2014; 45: 1310-4. ArticlePubMed
- 44. Mata DA, Williams EA, Sokol E, et al. Prevalence of UV mutational signatures among cutaneous primary tumors. JAMA Netw Open 2022; 5: e223833.ArticlePubMedPMC
- 45. Fishbach S, Steinhardt G, Zhen CJ, Puranik R, Segal JP, Cipriani NA. High rates of ultraviolet-signature mutations in squamous cell carcinomas of the parotid gland and prognostic implications. Head Neck Pathol 2022; 16: 236-47. ArticlePubMedPMCPDF
- 46. Mai ZM, Sargen MR, Curtis RE, Pfeiffer RM, Tucker MA, Cahoon EK. Ambient ultraviolet radiation and major salivary gland cancer in the United States. J Am Acad Dermatol 2020; 83: 1775-7. ArticlePubMedPMC
- 47. Besaratinia A, Pfeifer GP. Sunlight ultraviolet irradiation and BRAF V600 mutagenesis in human melanoma. Hum Mutat 2008; 29: 983-91. ArticlePubMed
- 48. Ming M, Han W, Maddox J, et al. UVB-induced ERK/AKT-dependent PTEN suppression promotes survival of epidermal keratinocytes. Oncogene 2010; 29: 492-502. ArticlePubMedPMCPDF
- 49. Yang C, Sanchez-Vega F, Chang JC, et al. Lung-only melanoma: UV mutational signature supports origin from occult cutaneous primaries and argues against the concept of primary pulmonary melanoma. Mod Pathol 2020; 33: 2244-55. ArticlePubMedPMCPDF
- 50. Brash DE. UV signature mutations. Photochem Photobiol 2015; 91: 15-26. ArticlePubMedPMC
- 51. Harold A, Amako Y, Hachisuka J, et al. Conversion of Sox2-dependent Merkel cell carcinoma to a differentiated neuron-like phenotype by T antigen inhibition. Proc Natl Acad Sci U S A 2019; 116: 20104-14. ArticlePubMedPMC
- 52. Laga AC, Lai CY, Zhan Q, et al. Expression of the embryonic stem cell transcription factor SOX2 in human skin: relevance to melanocyte and merkel cell biology. Am J Pathol 2010; 176: 903-13. ArticlePubMedPMC
- 53. DeCaprio JA. Molecular pathogenesis of Merkel cell carcinoma. Annu Rev Pathol 2021; 16: 69-91. ArticlePubMed
- 54. Sihto H, Kukko H, Koljonen V, Sankila R, Bohling T, Joensuu H. Merkel cell polyomavirus infection, large T antigen, retinoblastoma protein and outcome in Merkel cell carcinoma. Clin Cancer Res 2011; 17: 4806-13. ArticlePubMedPDF
- 55. Houben R, Schrama D, Alb M, et al. Comparable expression and phosphorylation of the retinoblastoma protein in Merkel cell polyoma virus-positive and negative Merkel cell carcinoma. Int J Cancer 2010; 126: 796-8. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
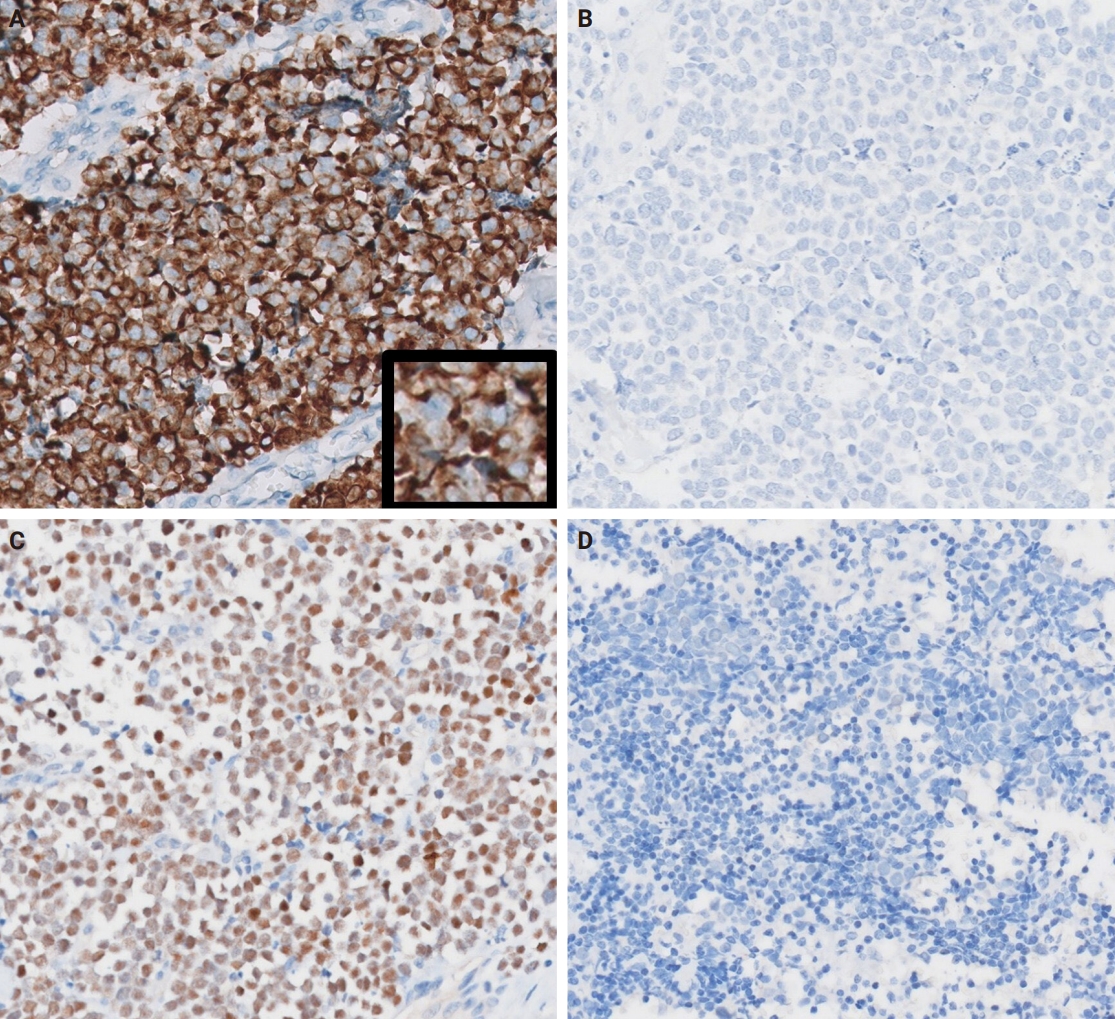
Fig. 1.
Fig. 2.
Graphical abstract
| Antibody | Source | Clone | Dilution |
|---|---|---|---|
| CK20 | DAKO, Glostrup, Denmark | Ks 20.8 | 1:200 |
| Synaptophysin | Cell Marque, Rocklin, CA, USA | MRQ-40 | 1:100 |
| Chromogranin | DAKO, Carpinteria, CA, USA | DAK-A3 | 1:1,600 |
| CD56 (NCAM) | Cell Marque, Rocklin, CA, USA | 156R-96 | 1:250 |
| TTF-1 | NOVO, Gatwick, UK | SPT24 | 1:200 |
| MCPyV LTA | Santa Cruz Biotechnology, Santa Cruz, CA, USA | CM2B4 | 1:50 |
| PAX5 | Cell Marque, Rocklin, CA, USA | SP34 | 1:100 |
| Rb | QED Bioscience, San Diego, CA, USA | 3C8 | 1:10,000 |
| p53 | DAKO, Carpinteria, CA, USA | DO-7 | 1:1,000 |
| TdT | Cell Marque, Rocklin, CA, USA | Polyclonal | 1:100 |
| SOX2 | Abcam, Cambridge, UK | Polyclonal | 1:250 |
| PTEN | Cell Signaling, Danvers, MA, USA | 138G6 | 1:100 |
| BRAF | Ventana Medical Systems, Tucson, AZ, USA | VE1 | Prediluent |
| Case No. | Age (yr) | Sex | Site | Size (cm) | Treatment | History | LN metastasis | Recurrence (mo) | Follow-up (mo) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 74 | F | Parotid (R) | 3.5 | TP + RT | DM, RA | - | - | NED (9.1) |
| 2 | 78 | F | Parotid (R) | 2 | TP + SND | DM | - | - | NED (73.0) |
| 3 | 66 | M | Parotid (R) | 1.3 | RP + MRND + CCRT | DM, CVA | + | 5.6 | AWD (8.4) |
| 4 | 59 | F | Parotid (L) | 4.6 | TP + MRND | LT | + | - | NED (6.5) |
| Case No. | Synaptophysin | Chromogranin | CD56 | CK20 | TTF-1 | MCPyV LTA | PAX5 | Rb | p53 | TdT | SOX2 | BRAF (VE1) | PTEN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | NA | + | – | – | – | + | 1+ | – | + | – | – |
| 2 | + | + | + | + | – | – | + | + | 1+ | – | + | – | + |
| 3 | + | + | + | + | – | + | – | + | 2+ | – | + | – | – |
| 4 | + | – | + | + | – | + | – | + | 2+ | – | + | – | + |
| Study | No. | Age (yr) | Sex | Site | Size (cm) | MCPyV | LN metastasis | Recurrence | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Fornelli et al. [23] | 2 | 65 | M | Parotid | 4 | NA | - | + | DOD (28 mo) |
| 70 | M | Parotid | 2.5 | NA | - | + | AWD (2 yr) | ||
| Nagao et al. [1] | 11 | 77 | F | Parotid | 1.8 | NA | - | - | NED (28 mo) |
| 78 | M | Parotid | 1.5 | NA | + | - | DOD (45 mo) | ||
| 81 | F | Parotid | 3 | NA | + | - | DOD (17 mo) | ||
| 85 | M | Submandibular | 3.8 | NA | - | - | DOD (9 mo) | ||
| 50 | M | Parotid | 0.7 | NA | + | - | NED (155 mo) | ||
| 66 | M | Parotid | 5 | NA | - | - | DOD (20 mo) | ||
| 76 | M | Parotid | 11 | NA | + | - | DOD (2 mo) | ||
| 72 | M | Parotid | 2.9 | NA | - | - | NED (4 mo) | ||
| 72 | M | Parotid | 2 | NA | + | - | DOD (34 mo) | ||
| 67 | M | Parotid | 8 | NA | + | - | AWD (18 mo) | ||
| 52 | F | Parotid | 1.5 | NA | - | - | NED (4 mo) | ||
| Jorcano et al. [24] | 1 | 91 | M | Parotid | 4 | NA | + | - | DOC (3 yr) |
| Mulder et al. [25] | 1 | 78 | M | Parotid | NA | NA | + | + | DOD (3 yr) |
| Ghaderi et al. [26] | 1 | 35 | F | Parotid | 2 | NA | - | - | NA |
| Baca et al. [27] | 1 | 77 | M | Parotid | 8.5 | NA | + | NA | NA |
| Chernock et al. [6] | 5 | 68 | M | Parotid | NA | - | + | - | DOD (<6 mo) |
| 66 | M | Parotid | NA | - | + | + | NED (112 mo) | ||
| 74 | M | Parotid | NA | - | + | - | NED (2 yr) | ||
| 22 | M | Parotid | NA | - | + | - | DOD (<6 mo) | ||
| 60 | M | Parotid | NA | - | - | - | NED (13 yr) | ||
| De Biase et al. [5] | 1 | 64 | M | Parotid | 4 | + | + | NA | DOD (1 yr) |
| Kanazawa et al. [28] | 1 | 87 | F | Parotid | 5.5 | NA | + | - | NED (108 mo) |
| Fisher et al. [4] | 3 | 64 | F | Parotid | 1.4 | + | - | - | NED (41 mo) |
| 82 | M | Parotid | 6.5 | - | - | - | DOC (8 mo) | ||
| 82 | M | Parotid | 2.8 | + | + | - | NED (31 mo) | ||
| Lombardi et al. [3] | 1 | 67 | F | Submandibular | 2.2 | + | + | - | NED (12 mo) |
| Bizzaro et al. [29] | 1 | 65 | M | Parotid | 1.7 | NA | - | - | NED (24 mo) |
| Knopf et al. [30] | 8 | Mean 75 | 3M, 5F | Parotid | NA | NA | 3 (37.5%) | 5-yr RFI, 71% | 5-yr OS, 29% |
| Alotaibi et al. [31] | 1 | 71 | M | Parotid | 3.5 | NA | - | - | NA |
| Astreidis et al. [32] | 1 | 76 | M | Parotid | 2.7 | NA | - | - | NED (60 mo) |
| Young et al. [33] | 1 | 69 | M | Parotid | 4.9 | - | - | + | AWD (20 mo) |
| De Luca et al. [34] | 5 | 57 | M | Parotid | 1.5 | NA | + | + | DOD (45 mo) |
| 79 | F | Parotid | NA | NA | NA | + | DOC (24 mo) | ||
| 92 | F | Submandibular | 2.8 | NA | - | - | DOC (16 mo) | ||
| 70 | M | Parotid | 3.4 | NA | NA | + | DOD (14 mo) | ||
| 88 | M | Parotid | 2.5 | NA | NA | - | DOC (46 mo) |
CK, cytokeratin; TTF-1, thyroid transcription factor 1; MCPyV LTA, Merkel cell polyomavirus large T antigen; PAX5, paired box 5; Rb, retinoblastoma; TdT, terminal deoxynucleotidyl transferase; SOX2, SRY-box transcription factor 2.
R, right; TP, total parotidectomy; RT, radiotherapy; DM, diabetes mellitus; RA, rheumatoid arthritis; NED, no evidence of the disease; SND, selective neck dissection; RP, radical parotidectomy; MRND, modified radical neck dissection; CCRT, concurrent chemoradiotherapy; CVA, cerebrovascular accident; AWD, alive with the disease; L, left; LT, liver transplantation.
CK, cytokeratin; TTF-1, thyroid transcription factor 1; MCPyV LTA, Merkel cell polyomavirus large T antigen; PAX5, paired box 5; Rb, retinoblastoma; TdT, terminal deoxynucleotidyl transferase; SOX2, SRY-box transcription factor 2; NA, not available.
MCPyV, Merkel cell polyomavirus; LN, lymph node; NA, not available; DOD, died of the disease; AWD, alive with the disease; NED, no evidence of the disease; DOC, died of other causes; RFI, recurrence-free interval; OS, overall survival.

 E-submission
E-submission