Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 59(2); 2025 > Article
-
Case Study
Mucocele of the rectal stump: mucinous cystic neoplasm with low-grade dysplasia simulating low-grade appendiceal mucinous neoplasm -
Hasan Basri Aydin1
 , Maria Faraz1
, Maria Faraz1 , A. David Chismark2
, A. David Chismark2 , Haiyan Qiu1
, Haiyan Qiu1 , Hwajeong Lee1
, Hwajeong Lee1
-
Journal of Pathology and Translational Medicine 2025;59(2):139-146.
DOI: https://doi.org/10.4132/jptm.2024.12.27
Published online: February 26, 2025
1Department of Pathology and Laboratory Medicine, Albany Medical Center, Albany, NY, USA
2Department of Surgery, Albany Medical Center, Albany, NY, USA
- Corresponding author Hwajeong Lee, MD, Department of Pathology and Laboratory Medicine, Albany Medical Center, 47 New Scotland Ave., MC81, Albany, NY 12208, USA Tel: +1-518-262-6254, Fax: +1-518-262-3663, E-mail: LeeH5@amc.edu
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,771 Views
- 170 Download
Abstract
- Mucoceles, commonly observed in the appendix, are mucin-filled, dilated structures arising from a range of etiologies. Cases associated with dysplastic or neoplastic epithelium can rupture and disseminate within the abdominopelvic cavity. Similar lesions in other parts of the colon are exceedingly rare, with only 16 colonic mucoceles having been reported. The first case of a colonic mucinous neoplasm with dysplasia resembling a low-grade appendiceal mucinous neoplasm involving rectal stump was described in 2016. Here, we present the second such case arising in the rectal stump, identified in a 44-year-old male with extensive surgical history. Microscopic examination revealed low-grade dysplastic epithelium lining the cyst and mucin dissecting into the stroma, without evidence of rupture or extramural mucin. The patient was followed for 16 months without recurrence or peritoneal disease. The exact etiology and outcome of these rare lesions remain unknown, requiring close follow-up.
- Despite the relative prevalence of appendiceal mucocele, colonic mucoceles are extremely rare, with only sixteen cases reported in the literature [1-14]. The term 'mucocele' in pathology literature is traditionally a clinical or gross descriptive term used to describe a dilated, mucin-filled bowel segment or tissue cavity, most commonly the appendix [15]. It does not correspond to any specific diagnosis but rather denotes the macroscopic appearance and presentation of a lesion. Pathologically, mucoceles can result from various underlying processes, and the type of epithelial lining or lack thereof plays a critical role in determining their nature. The term ‘mucinous neoplasm’ however, refers to a pathologic entity characterized by the presence of dysplastic or neoplastic mucin-producing epithelium [16]. For clarity, in this study, 'mucocele' is not used as a synonym for low-grade appendiceal mucinous neoplasm (LAMN) but rather in its general descriptive sense, consistent with its gross presentation.
- LAMN is a distinct diagnostic entity with well-defined histopathologic criteria and significant clinical implications. The presence of low-grade dysplastic mucinous epithelium in the appendix in an appropriate clinical and histological context supports the diagnosis of LAMN [17]. Even in cases where acellular mucin pools are present in the appendiceal stroma without identifiable residual epithelial lining, the lesion may be classified as LAMN, as dysplastic epithelium may no longer be visible in sampled sections following mucin extravasation and rupture [16,18].
- To date, only one case, reported by Tanaka et al. in 2016 [10], described a distal rectal stump mucocele with low-grade epithelial dysplasia resembling LAMN. More recently, in 2024, Chen et al. [14] reported five cases of mucinous neoplasms originating in extra-appendiceal segments of the colon, reminiscent of appendiceal mucinous neoplasms. In their discussion, they proposed the term “extra-appendiceal mucinous neoplasm” for such lesions. Three of the five cases grossly presented as mucoceles, similar in clinical context and pathologic findings to previously reported colorectal mucoceles, and are therefore included in our analysis. To the best of our knowledge, our case of a mucocele with dysplasia arising in a rectal stump represents the second reported instance. These cases collectively suggest a potential link between longstanding mucin stasis, mucocele formation, and neoplastic progression.
INTRODUCTION
- A 44-year-old male who was born with imperforate anus with subsequent pull-through procedure underwent proctectomy with end-colostomy at age 12 due to poor anorectal function. Throughout his childhood, he suffered from draining sinuses from the perineum, which were ablated each time. His symptoms eventually dissipated in his 20s and he remained asymptomatic for the next 20 years. Recently he presented with a complaint of intractable lower back pain radiating down to his left leg. On initial presentation, he also had acute kidney injury (AKI) with a creatinine level of 13.0 g/dL (reference, <1.0 g/dL). An abdominal computed tomography scan revealed a large cystic mass in the pelvis causing obstructive uropathy with bilateral hydroureteronephrosis as well as compressive neuropathy, leading to lower back pain (Fig. 1). Bilateral percutaneous nephrostomy tubes were placed to address obstructive uropathy. This led to improvement of AKI and creatinine level trended down to 4.78 g/dL. Eventually, the pelvic mass was resected and sent to pathology. Other than severe intraabdominal adhesions, no implants, nodules, mucin, or other lesion were noted in the abdominopelvic cavity by the surgery team. The operative note did not specify the exact origin of the mass or any direct anatomical connection to a bowel segment. The resected mass appeared intact without any surface disruptions, excrescences or mucin, and measured 17.5 × 10.7 × 4.5 cm. Sectioning of the mass revealed a multiloculated cyst containing amorphous, tan and gelatinous material.
- Sections revealed a cystic structure that was largely denuded but partially lined by attenuated anorectal-type mucosa, consisting of both colorectal glandular and anorectal transitional-type epithelium (Fig. 2). Notably, focal areas of low-grade dysplasia were identified. Pools of acellular mucin were observed dissecting into the stromal tissues, accompanied by degenerative changes such as calcifications and fibrosis (Fig. 3). Importantly, there was no evidence of high-grade dysplasia, invasive carcinoma, or metastasis.
- The findings were reminiscent of a LAMN as defined by the Peritoneal Surface Oncology Group International (PSOGI), by fulfilling five of the six criteria, namely low-grade cytologic atypia (dysplastic mucin-producing epithelium), loss of the lamina propria and muscularis mucosae consistent with pressure-related atrophy and fibrosis, fibrosis of the submucosa, non-infiltrative pushing growth pattern without destructive stromal invasion and mucin dissection into the wall without evidence of extra-cystic mucin or peritoneal spread [17].
- Given the absence of high-grade features, invasive carcinoma, or extra-cystic mucin/neoplastic cells, a descriptive diagnosis, mucinous cystic neoplasm of uncertain malignant potential, was rendered. The patient has been followed for 16 months without evidence of recurrence or peritoneal disease.
CASE REPORT
- Mucoceles are most commonly encountered in the appendix within the luminal gastrointestinal tract. They are rarely symptomatic and rather incidentally found during clinical care for other conditions. They constitute less than 1% of appendectomies [19]. However, their malignant potential is well recognized. Therefore, incidental identification of any dysplastic epithelium in an appendectomy usually prompts additional examination of the specimen. Cases of LAMN typically lack infiltrative growth pattern, destructive invasion, stromal desmoplastic reaction, or distant metastasis [20]. Nevertheless, if left untreated or incompletely excised, they can grow further and eventually rupture at weaker points of the appendiceal wall and result in mucin and dysplastic epithelium spreading within the abdominopelvic cavity, termed pseudomyxoma peritonei [21]. Despite the relative prevalence of appendiceal mucocele, colonic mucocele including colonic mucinous neoplasm similar to LAMN is exceedingly rare, with only seventeen cases including the present case [1-14] summarized in Table 1.
- In brief, the mean age of the patients was 58 (range 12 to 92) years with female predominance (12 out of 17). Most patients had a complex surgical history including colostomy, endorectal pull-through, Hartmann’s procedure, total colectomy, total hysterectomy, and hemorrhoidectomy. A number of patients had colonic diverticulosis and ended up developing mucoceles in a diverticulum [9,14]. The presentation commonly involved large mucoceles, often distending the rectum or pelvic area, some causing compressive symptoms similar to our case. Typically, the unused bowel segment such as the pouch or diverticulum where the fecal stream is not present was the predilection site for mucin accumulation, similar to the present case. Histologic findings varied, with most cases showing nondysplastic benign epithelium, while five cases exhibited dysplastic changes similar to LAMN [9,10,14]. Another case (2014) displayed conventional colonic villous adenoma within the mucocele [8]. None of the cases showed invasive malignancy. Follow-up durations ranged from 13 days to 3 years, and no recurrence or malignant transformation were reported.
- Taken together, chronic luminal stasis and resultant increased intraluminal pressure and impaired anal drainage associated with complex surgical history appear to contribute to mucocele development in the unused segment of colon [8]. The prolonged stasis within the surgically altered bowel segment creates an environment conducive to mucin accumulation. This mechanism is analogous to that observed in appendiceal mucoceles, where luminal obstruction and stasis are key factors [22]. Appendix is more prone to luminal obstruction, due to blind-ended anatomy and narrower lumen, explaining the relatively high incidence of appendiceal mucinous neoplasms [22]. Likewise, postoperative intraabdominal adhesions can be another contributing factor as they can restrict luminal diameter, potentiate intraluminal pressure buildup, and impair drainage [13]. Extensive intraabdominal adhesions were noted in our case as well.
- Histopathologically, our case exhibited features akin to LAMN, including epithelium with low-grade dysplasia and acellular mucin pools dissecting stromal tissues with degenerative features. Our and previous observations underscore the potential for neoplastic transformation in colonic mucoceles. However, dysplastic epithelium was observed in seven out of 17 (to include our current case) cases. Therefore, it is likely that mucoceles arise as passive processes in patients with complex surgical history, with neoplasia developing as a secondary event in a subset, possibly in those with genetic predisposition.
- Given its rarity, there is currently no consensus or published guidelines on the management of colonic mucocele. Since the malignant potential of the appendiceal counterpart is well-documented, complete surgical resection might be appropriate. However, as the malignant potential of colonic mucoceles in general and those with dysplasia in particular, is unknown, vigilant long-term follow-up and monitoring for recurrence or progression to malignancy may be also justified. Further case documentation is needed to better understand the behavior and long-term outcomes of colonic mucoceles, particularly those with dysplastic changes.
- Our study has several limitations that warrant acknowledgment. First, a gross photograph of the cystic mass from our case is unavailable. Second, in our effort to curate and summarize previously reported cases of colonic mucoceles, we encountered incomplete reporting in some of the cases, limiting our ability to perform a uniform comparison. This also highlights the need for more standardized reporting of such rare cases in the literature. Thirdly, we acknowledge that certain reported cases reflect dilated, mucin-filled segments of bowel secondary to outlet obstruction rather than true neoplastic or cystic lesions. These cases would appropriately fall under the category of “retention cysts” in a broader sense.
DISCUSSION
Ethics Statement
Institutional Review Board approval was waived due to the use of retrospective, de-identified data.
Availability of Data and Material
All data generated or analyzed during the study are included in this published article (and its supplementary information files).
Code Availability
Not applicable.
Author Contributions
Conceptualization: HL. Investigation: HBA, MF, ADC, HQ. Supervision: HL. Writing—original draft: HBA. Writing—review & editing: MF, ADC, HQ. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
Acknowledgments
The authors thank Dale Veasey for his contribution in grossing.



| Case No. | Report year | Age (yr)/Sex | Presentation | Surgical history | Gross findings | Histologic findings | Follow-up duration | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 1974 [1] | 38/F | Pelvic mucocele | Colostomy and mucous fistula for traumatic colonic injury, stenosis of mucus fistula and anal canal | Mucocele of the distal colonic segment, 21 cm in size. The bowel was chronically inflamed, thickened (1 cm), and distended with mucus. | Marked mucosal atrophy with fibrosis of the muscularis mucosae and submucosa. Multiple histiocytes containing brown pigment and chronic inflammatory cells present in the terminal rectum | N/A | No known recurrence or malignant transformation |
| 2 | 1987 [2] | 12/M | Perirectal mucocele | Endorectal pull-through for imperforate anus | 1,500 mL mucosal lined pelvic mucocele, obstructing the rectum and ureter | N/A | 1 year | No known recurrence or malignant transformation |
| 3 | 1991 [3] | 59/F | Rectal mucocele | Hartmann's procedure for perforated diverticulitis | Retrouterine fluid collection (pelvic abscess) within the pelvis, 9 cm in diameter. | N/A | N/A | No known recurrence or malignant transformation |
| 4 | 2011 [4] | 39/F | Rectal mucocele | Hemorrhoidectomy | Two small lesions located in 6 and 10 o’clock direction and anal canal scarring | Mucocele with benign colorectal glands floating in mucin pool | 9 mo | No known recurrence or malignant transformation |
| 5 | 2011 [5] | 73/F | Colonic mucocele | Ileo-sigmoid bypass surgery for adenocarcinoma of splenic flexure | Dilated ascending and transverse colon with features of mucocele (12 cm) | Closed loop obstruction of a colonic segment with subsequent mucin accumulation, no cystic lesion is present. | 6 wk | No known recurrence or malignant transformation |
| 6 | 2011 [6] | 36/M | Colonic mucocele | N/A | 1.0 × 0.9 cm polyp at hepatic flexure, which was removed with hot snare | Mucocele without dysplasia, hyperplasia of the crypt epithelium, mucinous cystadenoma or mucinous cystadenocarcinoma | 12 mo | No known recurrence or malignant transformation |
| 7 | 2013 [7] | 40/F | Rectal mucocele | Total colectomy and end ileostomy for Crohn’s disease | Grossly distended rectal stump, 15 × 9 cm in size | Mucin was transrectally drained, no sections are submitted for histologic examination. | 1 mo | No known recurrence or malignant transformation |
| 8 | 2014 [8] | 92/M | Rectal mucocele | Subtotal colectomy and end ileostomy for ulcerative colitis | Grossly distended rectal stump filled with three liters of mucin | Benign, mucus secreting, rectal villous adenoma within the rectal stump, size unspecified | N/A | No known recurrence or malignant transformation |
| 9 | 2015 [9] | 66/F | Colonic mucocele in a cecal diverticulum | Total hysterectomy | 3.5 × 3.0 cm cystic mass with mucin collection found within a cecal diverticulum | Mucin collection with dysplastic epithelial lining in muscularis propria with colonic lamina propria curving into muscularis propria, thus forming a diverticulum | 12 mo | No known recurrence or malignant transformation |
| 10 | 2016 [10] | 37/F | Distal colonic stump mucocele | Transverse loop colostomy for colonic obstruction due to a yolk sac tumor, anterior of the sacrum | Yellowish mucin-filled cyst; no lesions suggestive of malignant change was found at the mucosal surface of the cyst and stricture segment | Dysplastic changes similar to that of low-grade appendiceal mucinous neoplasms, including pseudo-stratified nuclei, papillary-proliferating cells with mucin content, and loss of the lamina muscularis mucosae and the stroma in the lamina propria, mucosae replaced with fibrous tissue; no invasive carcinoma or severe atypia | 1 mo | No known recurrence or malignant transformation |
| 11 | 2018 [11] | 84/M | Rectal mucocele | Hemorrhoidectomy | 5 × 7 cm in size, mucin-filled unilocular cyst, with a relatively strong film and a mucosal interior | Unilocular cystic lesion with the majority of the wall formed of mucous columnar epithelium, with a component of laminated stratified squamous epithelium. | 3 yr | No known recurrence or malignant transformation |
| 12 | 2018 [12] | 74/F | Rectal mucocele | Subtotal colectomy with end ileostomy and a mucous fistula at the descending colon due to Crohn’s disease | Dilated rectum and sigmoid with large amounts of partly calcified mucus | No evidence of dysplasia, malignancy, or Crohn’s manifestation in the completely obliterated proximal colon and the anus | 13 days | No known recurrence or malignant transformation |
| 13 | 2021 [13] | 85/F | Rectal stump mucocele causing mechanical ileus | Hartmann’s procedure | Digital rectal examination led to immediate drainage of a citrine viscous fluid, consistent with mucus. Consequently, a rectal catheter was placed in the stump, which drained approximately 2 L of fluid. | Cyst not excised surgically due to age and comorbidities of the patient, instead drained via catheter | 6 mo | No known recurrence or malignant transformation |
| 14 | 2024 [14] | 77/F | Colonic mucocele in a diverticulum | Diverticulosis | 1.7 cm cyst in sigmoid colon, arising in a diverticulum | Focal high-grade mucinous neoplasm of the colon arising in association with SSL extending into subserosal fat | N/A | No known recurrence or malignant transformation |
| 15 | 2024 [14] | 58/F | Rectosigmoid mucocele | Diverticulosis, possible duplication cyst | 6.1 cm cyst in rectosigmoid colon without connection to the lumen | Focal high-grade mucinous neoplasm of the colon arising in a duplication cyst or obstructed diverticulum, extending into muscularis propria | N/A | No known recurrence or malignant transformation |
| 16 | 2024 [14] | 67/F | Colonic mucocele | Appendix with fibrous obliteration | 4.6 cm submucosal mucin-filled cystic mass in cecum | Low-grade mucinous neoplasm, limited to submucosa | N/A | No known recurrence or malignant transformation |
| 17 | 2024 (current study) | 44/M | Distal colonic stump mucocele | Congenital imperforate anus with subsequent pull-through procedure, proctectomy with end-colostomy | 17.5 × 10.7 × 4.5 cm cystic mass in distal rectal stump | Cyst partially lined by low-grade dysplastic epithelium with pools of acellular mucin dissecting stroma, associated calcifications and fibrosis | 16 mo | No known recurrence or malignant transformation |
- 1. Nicosia JF, Abcarian H. Mucocele of the distal colonic segment, a late sequela of trauma to the perineum: report of a case. Dis Colon Rectum 1974; 17: 536-9. ArticlePubMed
- 2. Hitch DC, Patil UB, Panicek DM. Mucocele after endorectal pull-through for imperforate anus. J Pediatr Surg 1987; 22: 1023-4. ArticlePubMed
- 3. Creagh MF, Chan TY. Case report: rectal mucocele following Hartmann's procedure. Clin Radiol 1991; 43: 358-9. ArticlePubMed
- 4. Hsu KF, Hsieh CB, Yu JC, Chan DC, Wu CC, Jin JS, et al. Rare rectal mucocele mimic tumor following hemorrhoidectomy in an adult patient. Rev Esp Enferm Dig 2011; 103: 276-7. PubMed
- 5. Ali A, Krishnan A, Rehman S, Rao V, Pearson HJ. Giant colonic mucocele following palliative surgery for metastatic adenocarcinoma. J Surg Case Rep 2011; 2011: 1-4. Article
- 6. Ai XB, Feng JC, Gong FY, Wang A, Ren DH, Sui K. Endoscopic therapy of colonic liver flexure mucocele. Case Rep Gastroenterol 2011; 5: 433-7. ArticlePubMedPMC
- 7. Teoh AY, Lee JF, Chong CC, Tang RS. Endoscopic ultrasonography-guided drainage of a rectal mucocele after total colectomy for Crohn's disease. Endoscopy 2013; 45 Suppl 2 UCTN: E252-3. ArticlePubMed
- 8. Appleton N, Day N, Walsh C. Rectal mucocoele following subtotal colectomy for colitis. Ann R Coll Surg Engl 2014; 96: e13-4. Article
- 9. Nakatani K, Tokuhara K, Sakaguchi T, Ryota H, Yoshioka K, Kon M. Low-grade mucinous neoplasia in a cecal diverticulum: A case report. Int J Surg Case Rep 2015; 15: 66-9. ArticlePubMedPMC
- 10. Tanaka T, Kawai K, Abe H, Murono K, Otani K, Nishikawa T, et al. Giant mucocele of the colon at the distal stump due to low-grade mucinous neoplasia. Surg Case Rep 2016; 2: 117.ArticlePubMedPMCPDF
- 11. Ishii D, Aoki T, Inaba S, Yabuki H. Rectal mucocele in the anterior wall of the rectum. BMJ Case Rep 2018; 2018: bcr2018225097.ArticlePubMedPMC
- 12. Schneider R, Kraljevic M, von Flue M, Fuglistaler I. Giant symptomatic rectal mucocele following subtotal colectomy. Case Rep Gastroenterol 2018; 12: 143-6. ArticlePubMedPMCPDF
- 13. Longchamp G, Colucci N, Ris F, Buchs NC. Rectal stump mucocele after a Hartmann's procedure causing mechanical ileus. BMJ Case Rep 2021; 14: e237543. ArticlePubMedPMC
- 14. Chen F, Harvey SE, Young ED, Liang TZ, Larman T, Voltaggio L. Extra-appendiceal mucinous neoplasms: a tumour with clinicopathologic similarities to low- and high-grade appendiceal counterpart. Hum Pathol 2024; 148: 23-31. ArticlePubMed
- 15. Faure M, Salgado R, Op de Beeck B, Bellinck P, Termote JL, Parizel PM. Mucocele of the appendix: case report and review of literature. JBR-BTR 2014; 97: 217-21. ArticlePubMed
- 16. Carr NJ, Cecil TD, Mohamed F, Sobin LH, Sugarbaker PH, Gonzalez-Moreno S, et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: the results of the Peritoneal Surface Oncology Group International (PSOGI) modified Delphi process. Am J Surg Pathol 2016; 40: 14-26. ArticlePubMed
- 17. Govaerts K, Lurvink RJ, De Hingh I, Van der Speeten K, Villeneuve L, Kusamura S, et al. Appendiceal tumours and pseudomyxoma peritonei: literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur J Surg Oncol 2021; 47: 11-35. ArticlePubMed
- 18. Guner M, Aydin C. Low-grade appendiceal mucinous neoplasm: what is the best treatment? Cureus 2023; 15: e46591. ArticlePubMedPMC
- 19. Cristian DA, Grama FA, Becheanu G, Pop A, Popa I, Surlin V, et al. Low-grade appendiceal mucinous neoplasm mimicking an adnexal mass. Rom J Morphol Embryol 2015; 56: 837-42. PubMed
- 20. Pai RK, Longacre TA. Appendiceal mucinous tumors and pseudomyxoma peritonei: histologic features, diagnostic problems, and proposed classification. Adv Anat Pathol 2005; 12: 291-311. ArticlePubMed
- 21. Misdraji J. Mucinous epithelial neoplasms of the appendix and pseudomyxoma peritonei. Mod Pathol 2015; 28 Suppl 1: S67-79. ArticlePubMedPDF
- 22. Gupta P, Mishra AK, Deo A, Yadav R, K CM, Bhattarai A. Complete small intestinal obstruction due to band formation in low-grade appendiceal mucinous neoplasm: a rare case report and literature review. Int J Surg Case Rep 2023; 108: 108422.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
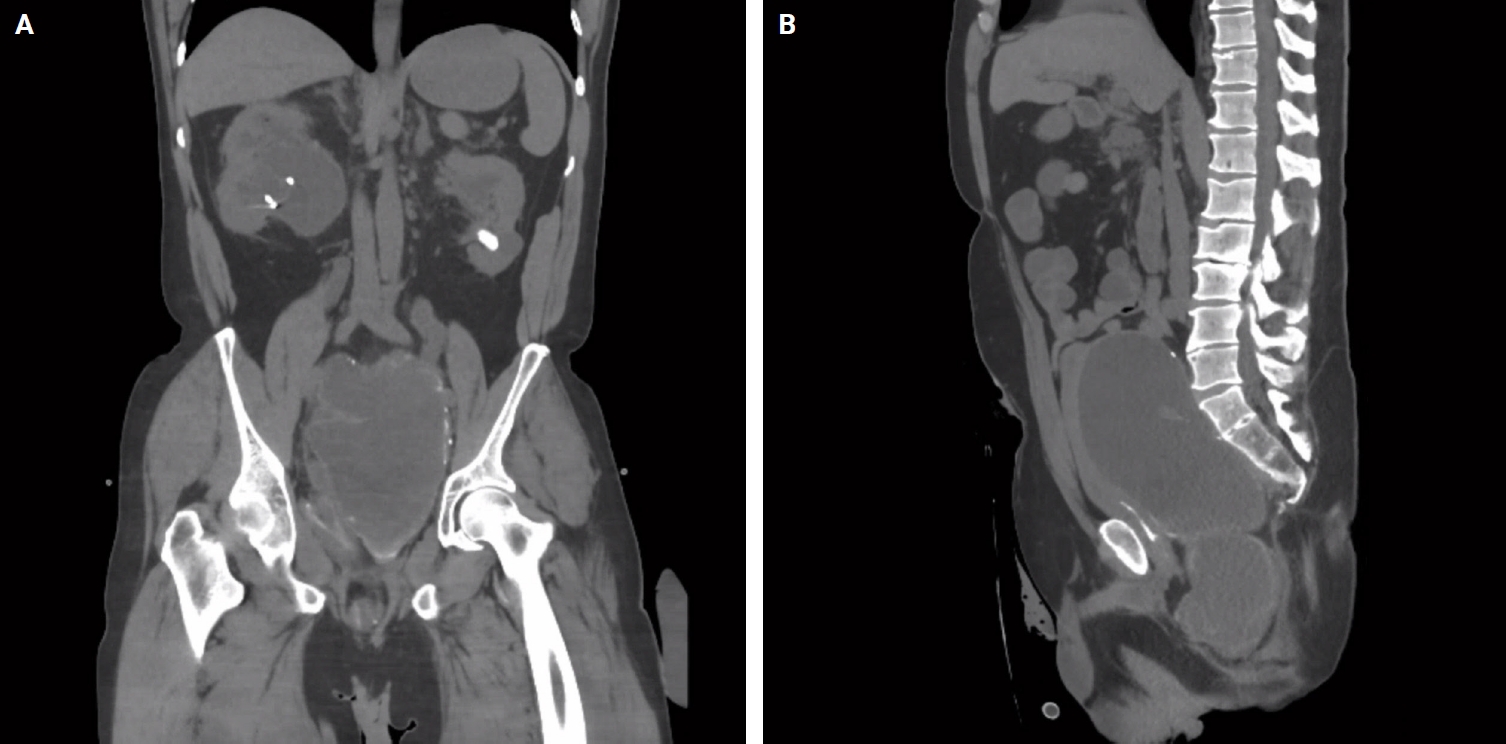


Fig. 1.
Fig. 2.
Fig. 3.
| Case No. | Report year | Age (yr)/Sex | Presentation | Surgical history | Gross findings | Histologic findings | Follow-up duration | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 1974 [1] | 38/F | Pelvic mucocele | Colostomy and mucous fistula for traumatic colonic injury, stenosis of mucus fistula and anal canal | Mucocele of the distal colonic segment, 21 cm in size. The bowel was chronically inflamed, thickened (1 cm), and distended with mucus. | Marked mucosal atrophy with fibrosis of the muscularis mucosae and submucosa. Multiple histiocytes containing brown pigment and chronic inflammatory cells present in the terminal rectum | N/A | No known recurrence or malignant transformation |
| 2 | 1987 [2] | 12/M | Perirectal mucocele | Endorectal pull-through for imperforate anus | 1,500 mL mucosal lined pelvic mucocele, obstructing the rectum and ureter | N/A | 1 year | No known recurrence or malignant transformation |
| 3 | 1991 [3] | 59/F | Rectal mucocele | Hartmann's procedure for perforated diverticulitis | Retrouterine fluid collection (pelvic abscess) within the pelvis, 9 cm in diameter. | N/A | N/A | No known recurrence or malignant transformation |
| 4 | 2011 [4] | 39/F | Rectal mucocele | Hemorrhoidectomy | Two small lesions located in 6 and 10 o’clock direction and anal canal scarring | Mucocele with benign colorectal glands floating in mucin pool | 9 mo | No known recurrence or malignant transformation |
| 5 | 2011 [5] | 73/F | Colonic mucocele | Ileo-sigmoid bypass surgery for adenocarcinoma of splenic flexure | Dilated ascending and transverse colon with features of mucocele (12 cm) | Closed loop obstruction of a colonic segment with subsequent mucin accumulation, no cystic lesion is present. | 6 wk | No known recurrence or malignant transformation |
| 6 | 2011 [6] | 36/M | Colonic mucocele | N/A | 1.0 × 0.9 cm polyp at hepatic flexure, which was removed with hot snare | Mucocele without dysplasia, hyperplasia of the crypt epithelium, mucinous cystadenoma or mucinous cystadenocarcinoma | 12 mo | No known recurrence or malignant transformation |
| 7 | 2013 [7] | 40/F | Rectal mucocele | Total colectomy and end ileostomy for Crohn’s disease | Grossly distended rectal stump, 15 × 9 cm in size | Mucin was transrectally drained, no sections are submitted for histologic examination. | 1 mo | No known recurrence or malignant transformation |
| 8 | 2014 [8] | 92/M | Rectal mucocele | Subtotal colectomy and end ileostomy for ulcerative colitis | Grossly distended rectal stump filled with three liters of mucin | Benign, mucus secreting, rectal villous adenoma within the rectal stump, size unspecified | N/A | No known recurrence or malignant transformation |
| 9 | 2015 [9] | 66/F | Colonic mucocele in a cecal diverticulum | Total hysterectomy | 3.5 × 3.0 cm cystic mass with mucin collection found within a cecal diverticulum | Mucin collection with dysplastic epithelial lining in muscularis propria with colonic lamina propria curving into muscularis propria, thus forming a diverticulum | 12 mo | No known recurrence or malignant transformation |
| 10 | 2016 [10] | 37/F | Distal colonic stump mucocele | Transverse loop colostomy for colonic obstruction due to a yolk sac tumor, anterior of the sacrum | Yellowish mucin-filled cyst; no lesions suggestive of malignant change was found at the mucosal surface of the cyst and stricture segment | Dysplastic changes similar to that of low-grade appendiceal mucinous neoplasms, including pseudo-stratified nuclei, papillary-proliferating cells with mucin content, and loss of the lamina muscularis mucosae and the stroma in the lamina propria, mucosae replaced with fibrous tissue; no invasive carcinoma or severe atypia | 1 mo | No known recurrence or malignant transformation |
| 11 | 2018 [11] | 84/M | Rectal mucocele | Hemorrhoidectomy | 5 × 7 cm in size, mucin-filled unilocular cyst, with a relatively strong film and a mucosal interior | Unilocular cystic lesion with the majority of the wall formed of mucous columnar epithelium, with a component of laminated stratified squamous epithelium. | 3 yr | No known recurrence or malignant transformation |
| 12 | 2018 [12] | 74/F | Rectal mucocele | Subtotal colectomy with end ileostomy and a mucous fistula at the descending colon due to Crohn’s disease | Dilated rectum and sigmoid with large amounts of partly calcified mucus | No evidence of dysplasia, malignancy, or Crohn’s manifestation in the completely obliterated proximal colon and the anus | 13 days | No known recurrence or malignant transformation |
| 13 | 2021 [13] | 85/F | Rectal stump mucocele causing mechanical ileus | Hartmann’s procedure | Digital rectal examination led to immediate drainage of a citrine viscous fluid, consistent with mucus. Consequently, a rectal catheter was placed in the stump, which drained approximately 2 L of fluid. | Cyst not excised surgically due to age and comorbidities of the patient, instead drained via catheter | 6 mo | No known recurrence or malignant transformation |
| 14 | 2024 [14] | 77/F | Colonic mucocele in a diverticulum | Diverticulosis | 1.7 cm cyst in sigmoid colon, arising in a diverticulum | Focal high-grade mucinous neoplasm of the colon arising in association with SSL extending into subserosal fat | N/A | No known recurrence or malignant transformation |
| 15 | 2024 [14] | 58/F | Rectosigmoid mucocele | Diverticulosis, possible duplication cyst | 6.1 cm cyst in rectosigmoid colon without connection to the lumen | Focal high-grade mucinous neoplasm of the colon arising in a duplication cyst or obstructed diverticulum, extending into muscularis propria | N/A | No known recurrence or malignant transformation |
| 16 | 2024 [14] | 67/F | Colonic mucocele | Appendix with fibrous obliteration | 4.6 cm submucosal mucin-filled cystic mass in cecum | Low-grade mucinous neoplasm, limited to submucosa | N/A | No known recurrence or malignant transformation |
| 17 | 2024 (current study) | 44/M | Distal colonic stump mucocele | Congenital imperforate anus with subsequent pull-through procedure, proctectomy with end-colostomy | 17.5 × 10.7 × 4.5 cm cystic mass in distal rectal stump | Cyst partially lined by low-grade dysplastic epithelium with pools of acellular mucin dissecting stroma, associated calcifications and fibrosis | 16 mo | No known recurrence or malignant transformation |
F, female; M, male; N/A, not applicable; SSL, sessile serrated lesion.

 E-submission
E-submission





