Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 57(5); 2023 > Article
-
Original Article
Diagnostic proficiency test using digital cytopathology and comparative assessment of whole slide images of cytologic samples for quality assurance program in Korea -
Yosep Chong1
 , Soon Auck Hong2
, Soon Auck Hong2 , Hoon Kyu Oh3
, Hoon Kyu Oh3 , Soo Jin Jung4
, Soo Jin Jung4 , Bo-Sung Kim5
, Bo-Sung Kim5 , Ji Yun Jeong6
, Ji Yun Jeong6 , Ho-Chang Lee7
, Ho-Chang Lee7 , Gyungyub Gong8
, Gyungyub Gong8 , The Committee of Quality Improvement of Korean Society for Cytopathology
, The Committee of Quality Improvement of Korean Society for Cytopathology -
Journal of Pathology and Translational Medicine 2023;57(5):251-264.
DOI: https://doi.org/10.4132/jptm.2023.07.17
Published online: August 24, 2023
1Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
2Department of Pathology, Chung-Ang University College of Medicine, Seoul, Korea
3Department of Pathology, Daegu Catholic University School of Medicine, Daegu, Korea
4Department of Pathology, Inje University Busan Paik Hospital, Busan, Korea
5Department of Pathology, Green Cross Laboratories, Yongin, Korea
6Department of Pathology, Kyungpook National University Chilgok Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
7Department of Pathology, Chungbuk National University College of Medicine, Cheongju, Korea
8Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- Corresponding Author: Yosep Chong, MD, PhD, Department of Hospital Pathology, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 271 Cheonbo-ro, Uijeongbu 11765, Korea Tel: +82-31-820-3160, Fax: +82-31-820-3877, E-mail: ychong@catholic.ac.kr
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- The Korean Society for Cytopathology introduced a digital proficiency test (PT) in 2021. However, many doubtful opinions remain on whether digitally scanned images can satisfactorily present subtle differences in the nuclear features and chromatin patterns of cytological samples.
-
Methods
- We prepared 30 whole-slide images (WSIs) from the conventional PT archive by a selection process for digital PT. Digital and conventional PT were performed in parallel for volunteer institutes, and the results were compared using feedback. To assess the quality of cytological assessment WSIs, 12 slides were collected and scanned using five different scanners, with four cytopathologists evaluating image quality through a questionnaire.
-
Results
- Among the 215 institutes, 108 and 107 participated in glass and digital PT, respectively. No significant difference was noted in category C (major discordance), although the number of discordant cases was slightly higher in the digital PT group. Leica, 3DHistech Pannoramic 250 Flash, and Hamamatsu NanoZoomer 360 systems showed comparable results in terms of image quality, feature presentation, and error rates for most cytological samples. Overall satisfaction was observed with the general convenience and image quality of digital PT.
-
Conclusions
- As three-dimensional clusters are common and nuclear/chromatin features are critical for cytological interpretation, careful selection of scanners and optimal conditions are mandatory for the successful establishment of digital quality assurance programs in cytology.
- Parallel digital pathology and glass PTs and post-test feedback survey
- Annually, more than 1,000 glass slides were collected by the KSC from the donation of participating institutes/hospital in South Korea. Out of 7,000 slides of the PT archive, we initially selected 258 slides and 85 slides were chosen and scanned after the first round of review by the KSC CQI members. The 85 scanned images were carefully reviewed by five KSC board members and only 30 WSIs were finally selected for the digital PT. Digital and conventional PT were performed in parallel for volunteer institutes among the 215 registered cytopathology laboratories in South Korea, and the results were compared with feedback. Conventional PT was performed using five glass slides, including two gynecology (Pap smear), one body fluid, one urine, and one FNAC sample. Digital PT was performed using six whole slide cytologic images, including two gynecology, two body fluids, one urine, and one FNAC sample. The diagnostic concordance between the cytological diagnosis submitted by the institutes and the original diagnosis of the histologically confirmed case was categorized as either concordant (category O) or one of the three discordant categories: category A (minimal clinical impact), category B (minor clinical impact), or category C (major clinical impact). The criteria for discordance assessment according to sample type were developed by the CQI KSC and provided to each institute (Supplementary Table S1–S3).
- Comparative assessment of whole-slide images of cytologic samples based on scanners
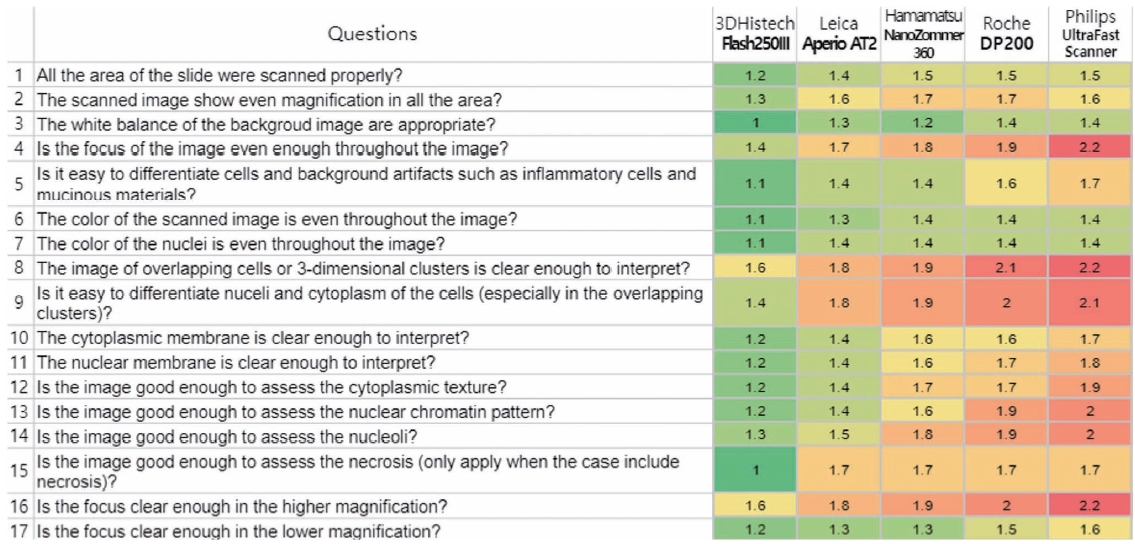
- For the comparative assessment of cytological assessment WSIs, 12 cytopathology slides were selected after careful review out of the PT archive independently to the PT. The scanning of these 12 cytological slide was performed using five different scanners from major scanner vendors. Each system utilizes its own specific image viewer software, it applies to the scanning of glass slides and the accompanying image viewer software provided by each scanner used under various scanning conditions (Table 1, Supplementary Fig. S1). For instance, AT2 (Leica Biosystems, Nussloch, Germany) utilizes ImageScope, Flash 250 III (3DHistech, Budapest, Hungary) employs SlideViewer, NanoZoomer S360 (Hamamatsu, Japan) utilizes NDP.view2, and Ventana DP200 (Roche, Basel, Switzerland) operates with uPath software. Since the IMS viewer of the Ultra-Fast Scanner (Philips, Amsterdam, Netherlands) was unavailable, we utilized the Pathomation image viewer instead. Scanner specifications, such as capacity, z-stacking, file format, size, scan time, and error rate, were assessed. Four cytopathologists assessed image quality using a questionnaire on focus, color balance, nuclear/cytoplasmic/chromatin features, etc., using different monitors and workstations of their own (Table 2). The questionnaire consisted of 17 questions that evaluated the quality of the scanned image of a tissue sample. The questions assessed the evenness of the magnification, white balance, and color of the image, as well as the clarity of the focus and ability to differentiate cells and artifacts. Additionally, questions focused on the clarity of the cytoplasmic and nuclear membranes, ability to assess the texture and chromatin pattern, and presence of necrosis in the image. Finally, questions addressed the clarity of the image at higher and lower magnifications. For each question, the quality was rated as 1 (yes/all), 2 (partially no, <10%), or 3 (no, >10%).
MATERIALS AND METHODS
- Parallel digital pathology and glass PTs and post-test feedback survey
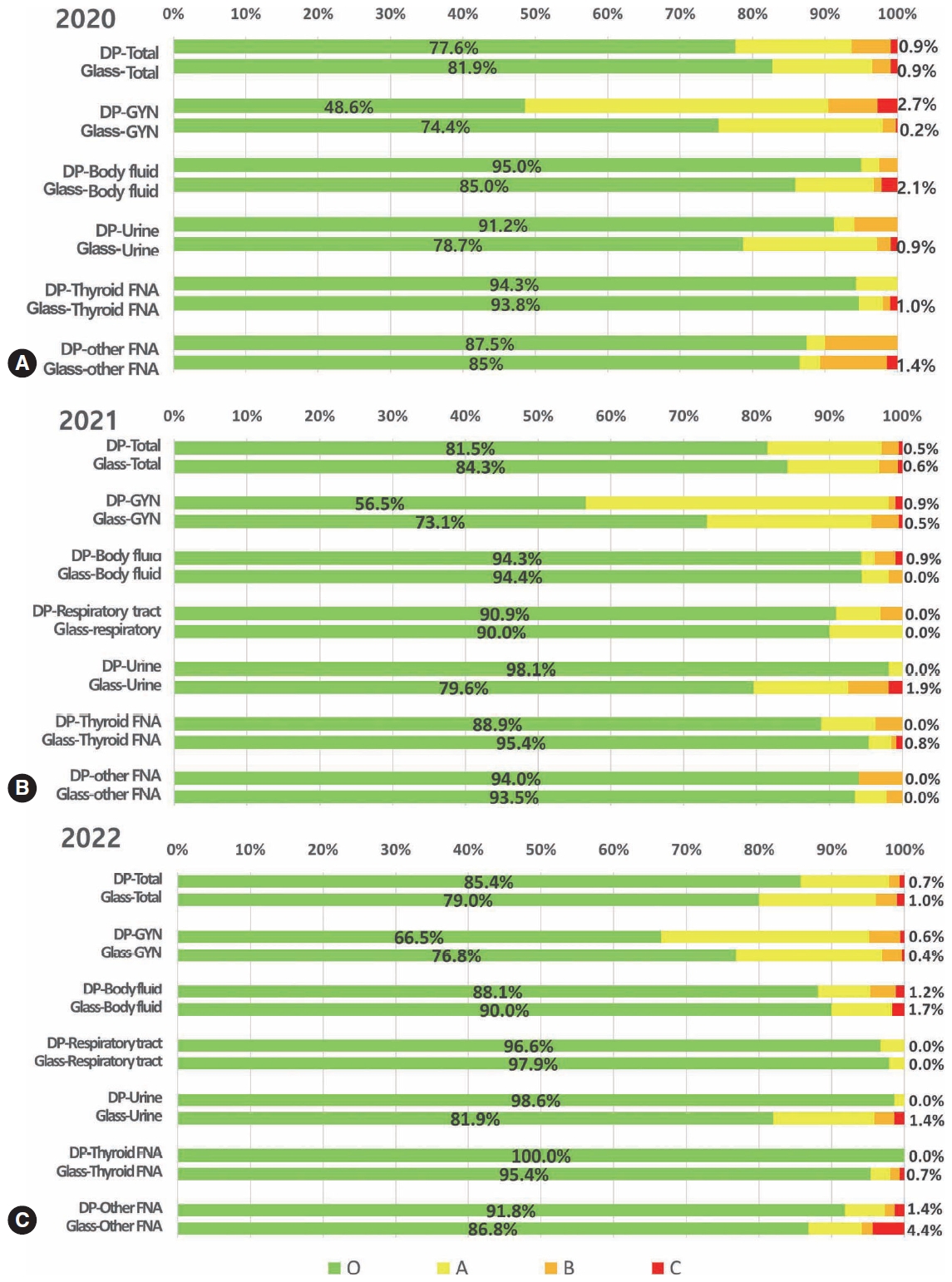
- In 2020, 216 institutes participated in conventional PT, and 48 institutes participated in trial digital PT as a supplementary test for the third and fourth programs. In 2021, 215 institutes, including 85 university hospitals, 80 general hospitals, and 45 commercial laboratories, participated in PT, 108 institutes participated in digital PT, and 107 institutes participated in conventional glass-slide PT. The glass slides for conventional PT were collected 2 years prior to PT as a fourth program. In 2022, 211 institutes, including 85 university hospitals, 80 general hospitals, and 45 commercial laboratories, participated in PT, 81 institutes participated in digital PT, and 130 institutes participated in conventional glass-slide PT. The concordance rates of digital and conventional PT based on various sample types are summarized in Fig. 1.
- In 2020, the overall concordance rates were 77.6% for the digital PT and 81.9% for the conventional PT using glass slides (Fig. 1A). The concordance rates were not significantly different in thyroid fine-needle aspiration (FNA) (digital vs. conventional PT, 94.3% vs. 93.8%) and other FNA samples (87.5% vs. 85.0%), whereas they were significantly lower in the digital PT of gynecologic samples (48.6% vs. 74.4%) and significantly higher in the digital PT of body fluid (95.0% vs. 85.0%) and urine samples (91.2% vs. 78.7%) (Fig. 1A). More cases of minor and minimal discordance exist in digital PT than in conventional PT. The number of cases with major discordance affecting clinical practice was similar, less than 1% for both digital and glass PT (0.9%).
- In 2021, the overall concordance rates were 81.5% for digital PT and 84.3% for conventional PT using glass slides (Fig. 1B). The concordance rates were not significantly different in body fluid samples (digital vs. conventional, 94.3% vs. 94.4%), respiratory tract samples (90.9% vs. 90.0%), and other FNA samples (94.0% vs. 93.5%), while they were moderately lower in digital PT of gynecologic samples (56.5% vs. 73.1%) and thyroid FNA (88.9% vs. 95.4%) and significantly higher in digital PT of urine samples (98.1% vs. 79.6%) (Fig. 1B). Cases with minor discordance were more common in digital PT, whereas cases with minimal discordance were similar in both digital and conventional PT. The cases with major discordance were similar, with <1% in both digital and glass PT (0.9%).
- In 2022, the overall concordance rates were 85.4% for digital PT and 79.0% for conventional PT using glass slides (Fig. 1C). The concordance rates were not significantly different in body fluid samples (digital vs. conventional, 88.1 vs. 90.0%) and respiratory tract samples (96.6% vs. 97.9%), while they were moderately lower in digital PT of gynecologic samples (66.5% vs. 76.8%), and significantly higher in digital PT of urine (98.6% vs. 81.9%), thyroid FNA (100.0% vs. 95.4%), and other FNA samples (91.8% vs. 86.8%) (Fig. 1C). Cases with minor and minimal discordance were more in conventional PT, and cases with major discordance were similar, with <1% in both digital and glass PT (0.7% vs. 1.0%).
- Significant changes were noted in the results over time in that the concordant cases of digital PT significantly increased every year, showing better concordance than conventional PT in 2022. The cases with minor and minimal discordance in digital PT significantly reduced than conventional PT over time, although the cases with major discordance were relatively similar every year. Regarding the sample types, only cases with minimal discordance were more frequent in gynecologic samples in digital PT than in conventional PT in 2022. These results indicate that participants gradually became familiar with digital platforms in most sample types, yet room for progress remains in gynecologic samples, where diagnostic categories are complex and highly segmented.
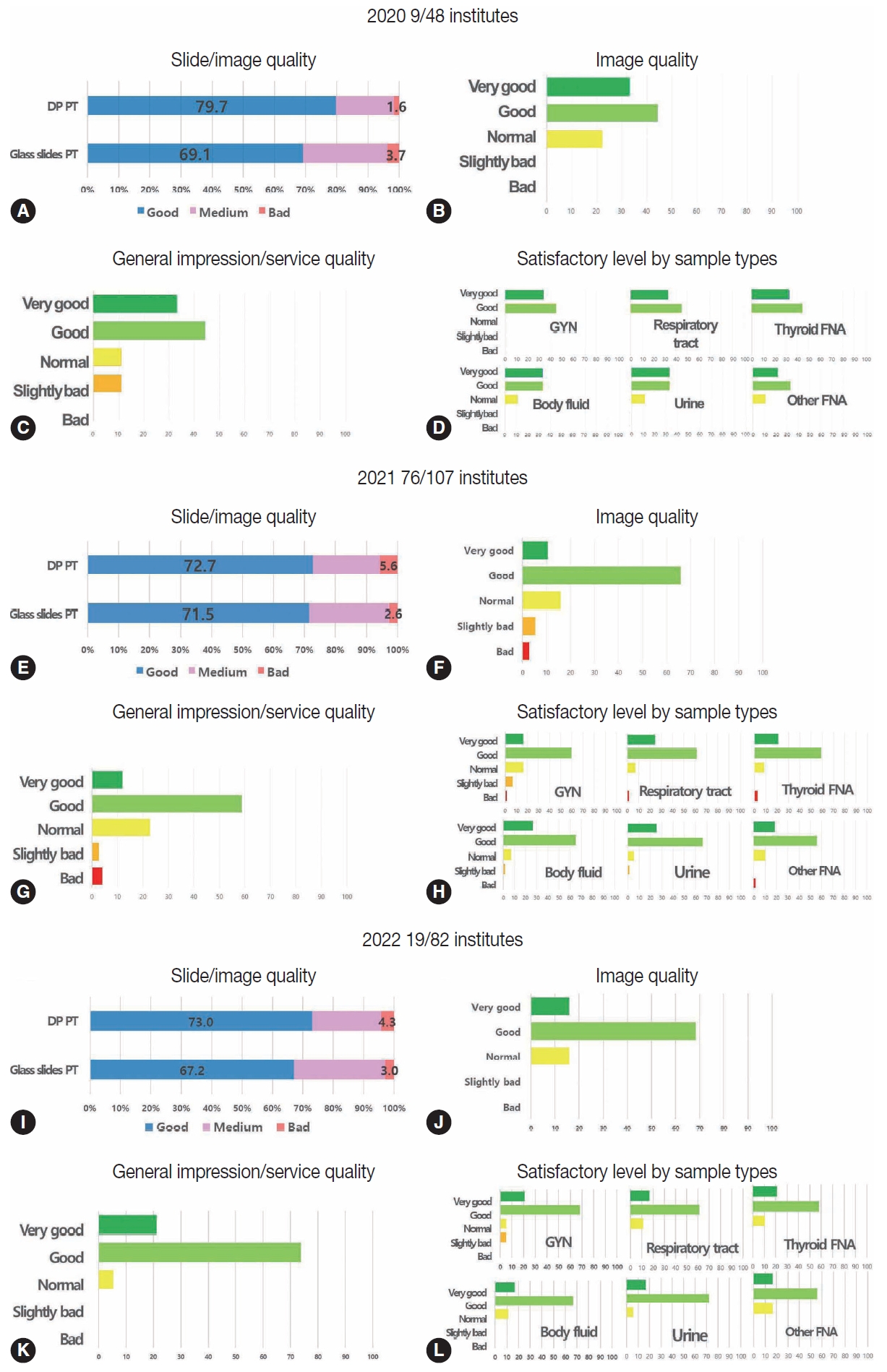
- Fig. 2 summarizes the post-test feedback survey after digital PT in 2020, 2021, and 2022. All participants (48 institutes for digital PT and 168 institutes for conventional PT in 2020, 107 for digital PT and 108 for conventional PT in 2021, and 82 for digital PT and 133 for conventional PT in 2022) submitted ratings for the given slides/image quality (Fig. 2A, E, I). In 2020, 79.7% of the respondents said that the quality of the digital images was good, whereas only 69.1% said that the conventional slides were of good quality (Fig. 2A). In 2021, 72.7% and 71.5% of the respondents reported good-quality digital images and conventional slides, respectively (Fig. 2E). In 2022, 73.7% and 71.5% of the respondents reported good-quality digital images and conventional slides, respectively (Fig. 2I). In 2020, the percentage of respondents who reported bad quality of slides/images was slightly higher in the conventional PT group than in the digital group by 3.7% vs. 1.6 %, although these numbers were slightly higher in the digital group than in the conventional group in 2021 (5.6% vs. 2.6%) and 2022 (4.3% vs. 3.0%). The respondents who reported good slide/image quality were similar or slightly higher in the digital group than in the conventional group for all 3 years. In 2020, nine out of 48 institutes that participated in digital PT as a supplementary test responded to the survey, while 76 out of 107 institutes responded to the survey after participation in digital PT in 2021, and 19 out of 82 institutes responded to the survey after participation in digital PT in 2022 (Fig. 2B–D, 2F–H, 2J–L). The majority of the respondents reported generally good or very good image, service quality, or satisfactory levels by sample type in 2020, and similar results were found in 2021 and 2022 except for a very limited number of respondents reporting bad or slightly bad image, service quality, or satisfactory levels by sample type in 2021 as the number of gross participants increased (Fig. 2B–D, 2F–H, 2J–L).
- Comparative assessment of whole slide images of cytologic samples according to scanners
- The product specifications of the five digital scanners are listed in Table 3. The Pannoramic 250 Flash of 3DHistech offers a high-speed slide scanning with a maximum resolution of 0.23 μm/pixel, making it an ideal choice for high-throughput laboratories. The Pannoramic 250 flash scanner had a slide capacity of 250 and a scan speed of 3 minutes for a 5×5-mm-sized slide at 40× magnification with five layers of z-stacking. It has an excellent graphical user interface and produces an excellent image quality at 40× magnification. The file size of a 15×15-mm-sized slide at 40× magnification with five layers of z-stacking is 10 GB. The scanner has a weekly capacity of 200 slides and can operate in the bright-field and fluorescent imaging modes. It supports MRXS, JPG, and JPG2000 digital slide formats and has a multilayer support system with either a Z-stack or an extended focus. The error rate per run is 2 and has a special feature of continuous loading.
- Conversely, the NanoZoomer 360 of Hamamatsu has a fast-scanning speed with a maximum resolution of 0.23 μm/pixel and allows multiple users to access the system simultaneously. It has a slide capacity of 360 and a scan speed of 1.5 min for a 5×5-mm-sized slide at 40× magnification with five layers of z-stacking. It has a good GUI and produces good image quality at 40× magnification. The file size of a 15×15-mm-sized slide at 40× magnification with five layers of z-stacking is 10 GB. The scanner has a weekly capacity of 300 slides and can operate in the bright-field imaging mode. It supports the JPG and NDPI digital slide formats and has a multilayer support system with either a Z-stack or an extended focus. The error rate per run was 3, and it has special features of quality scoring and intelligent rescans.
- The Leica Aperio AT2 scanner had a slide capacity of 400 and a scan speed of 2.5 min for a 5×5-mm-sized slide at 40× magnification with five layers of z-stacking. It has a satisfactory GUI and produces an excellent image quality at 40× magnification. The file size of a 15×15-mm-sized slide at 40× magnification with five layers of z-stacking is 8 GB. The scanner has a weekly capacity of 150 slides and can operate in the bright-field and fluorescent imaging modes. It supports the TIFF (SVS) digital slide format and has a multilayer support system with a Z-stack. The error rate is 4 per run and has a special feature of automated scanning.
- The Roche Ventana DP200 is a fully automated slide scanner that can scan up to 200 slides simultaneously with a maximum resolution of 0.25 μm/pixel. It has a slide capacity of 6 and a scan speed of 1.5 min for a 5×5-mm-sized slide at 40× magnification with five layers of z-stacking. It has a satisfactory GUI and produces good image quality at 40× magnification. The file size of a 15×15 mm-sized slide at 40× magnification with five layers of z-stacking is 12 GB. The scanner has a weekly capacity of less than 100 slides and can operate in the bright-field imaging mode. It supports the BIF digital slide format and has a multilayer support system with an extended focus. The error rate for each run was 3.
- Philips’ Ultra-Fast Scanner has a unique dual-camera system that allows the scanning of both bright-field and fluorescent slides with a maximum resolution of 0.25 μm/pixel. The slide capacity is 300, and the scan speed is extraordinarily fast for general HE-stained tissue slides but does not provide z-stacking because it was originally not targeting cytologic samples. As a result, it generally produces suboptimal image quality for cytological samples at 40× magnification. It supports only the bright-field imaging mode, syntax and fic digital slide formats. No information was provided on the weekly capacity or error rate.
- A difference in the scanning area coverage was noted between the whole slide scanners (WSSs), and a representative case example is shown in Fig. 3. The Panoramic Flash 250 III of 3DHistech showed the largest coverage in general, AT2, NanoZoomer S360, and Ventana DP200 showed similar coverage, while the UltraFast Scanner by Philips showed the smallest scanning area coverage. Scanning coverage is directly related to scanning time and file size. The larger the scanning coverage, the longer the scanning time, and the larger the file size. In other words, as the Pannoramic Flash 250 III covers the largest scanning area, it takes the longest scanning time and generates the largest file size, whereas the Ultra-Fast Scanner takes the shortest scanning time and generates the smallest file size because it covers the smallest scanning area. The difference in scanning coverage did not appear to be very effective for proper or impaired diagnosis in the included cases, although concluding that the coverage represents the eligibility for proper diagnosis and the integrity of digital slide images is not possible.
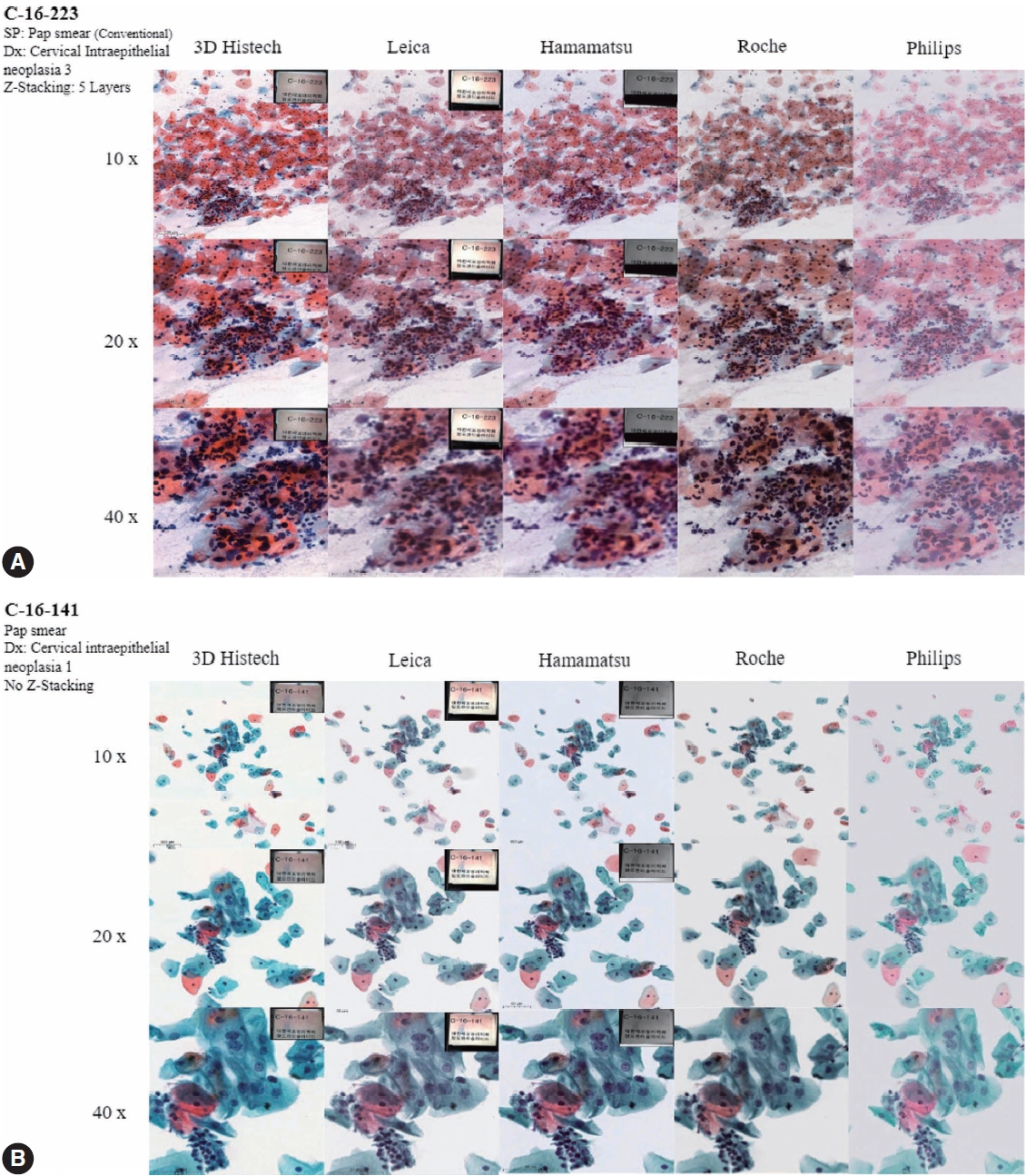
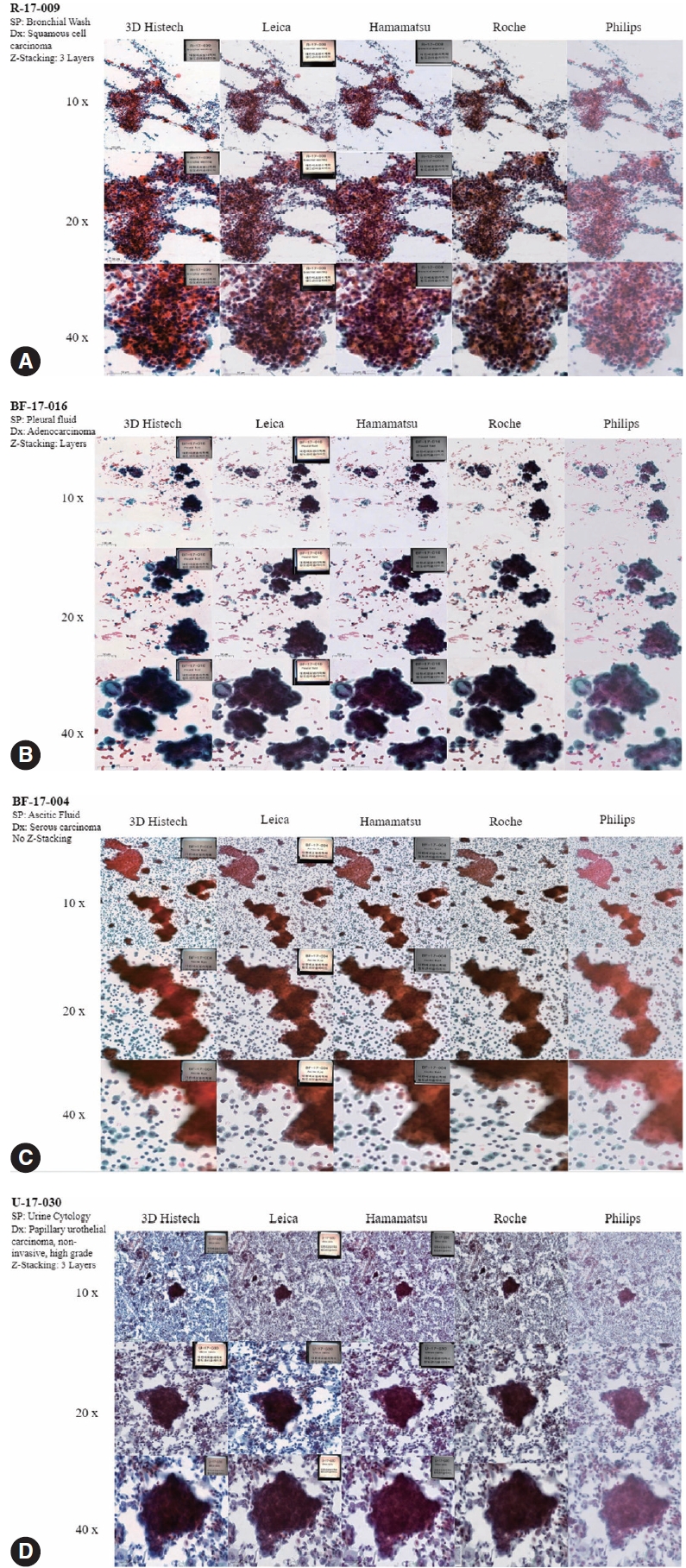
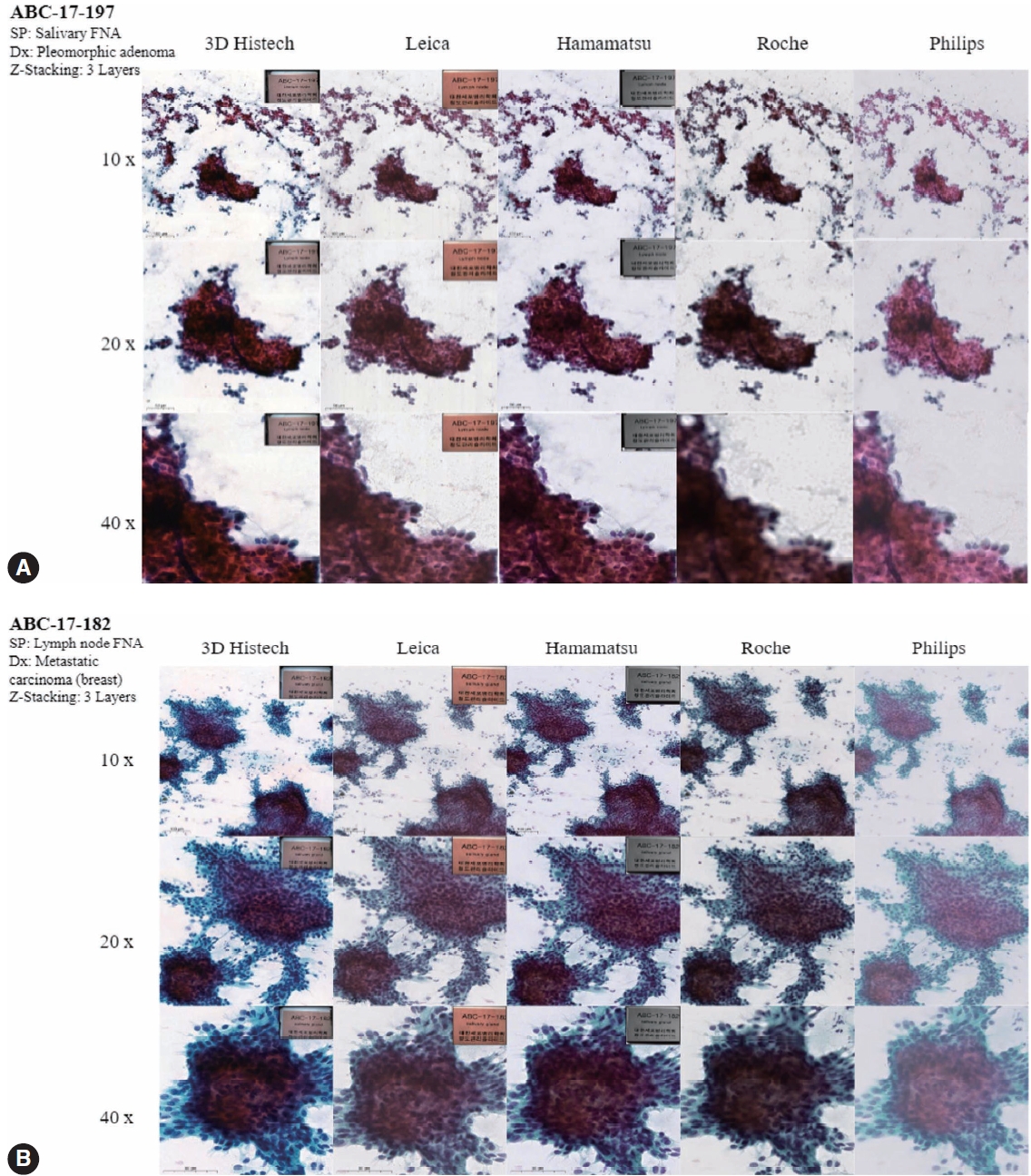
- Figs. 4 to 6 show the differences between the scanned images of representative samples. In general, color differences exist in terms of contrast, temperature, hue, saturation, sharpness, brightness, exposure, clarity, and background based on the WSSs. This may be a subjective matter that varies among individuals. It should also be noted that the color can be adjusted or optimized using different color subsets or profiles within the viewer program and monitor settings. The color of the WSIs scanned by 3DHistech tends to show more vivid images with a slightly exaggerated contrast. We can see orangophilic squamous cells in the pap smear of squamous intraepithelial lesion more eminently in the WSIs scanned by 3DHistech (Fig. 4A, B). The color of the WSIs scanned using the Roche WSS was slightly higher than that of the green tint. The WSIs scanned using Philips WSS presented realistic colors, although the images were fuzzy and less clear than the others. The background of the WSIs was the brightest among the 3DHistech WSIs. Fig. 5 shows the differences between the scanned images of representative body fluid samples, squamous cell carcinoma of the lungs on a conventional bronchial washing smear (Fig. 5A), metastatic adenocarcinoma on a conventional pleural fluid smear (Fig. 5B), serous carcinoma on a conventional ascitic fluid smear (Fig. 5C), and high-grade noninvasive papillary urothelial carcinoma on a urine cytology sample (Fig. 5D) scanned using three Z-stacking layers. Fig. 6 shows the difference in scanned images of FNA samples including pleomorphic adenoma of salivary gland on conventional smear with three layers of z-stacking (Fig. 6A), and metastatic ductal carcinoma of lymph node on a conventional smear scanned with three layers of z-stacking (Fig. 6B). The images present different image qualities and characteristics according to the scanners in terms of nuclei and nucleoli features, three-dimensional clusters, and singly dispersed cells, as the scanners provide different technical specifications (see also Supplementary Fig. S2 for the rest of the samples).
- Fig. 7 shows the overall average results of the image quality assessment using a questionnaire administered by four experienced cytopathologists. The number in each cell represents the average rating and is marked as a color spectrum from green for 1 (yes/all) to red for 3 (no/>10%). As we can see in the figure, the cells are more likely to be red in DP200 (Roche) and Ultra-Fast Scanner (Philips), which are originally not designed for cytology and do not support z-stacking. The differences between the Flash 250 III by 3DHistech, Aperio AT2 by Leica, and NanoZoomer 360 by Hamamatsu were generally not significant, although the Flash 250 III by 3DHistech showed the best satisfactory results in most of the questionnaires.
RESULTS
General product specification according to scanners
Scanning area coverage according to scanners
Qualitative analysis of the image quality of the scanned images
Image quality assessment using a questionnaire by experienced cytopathologists
- The results of the digital and glass PT were found to be comparable in 2021 and 2022. Feedback from participating institutes was generally positive for the DP PT. The 3DHistech Pannoramic 250 Flash III showed generally satisfactory image quality with high capacity, featuring large coverage, even focus, good z-stacking features, low error rates, and continuous loading [11]. The device had the highest contrast and strongest saturation but resulted in a longer scanning time and larger file size. Conversely, the Leica AT2 showed generally satisfactory image quality with high capacity, offering middle coverage with sharp and well-focused images, good z-stacking features, and high compatibility. However, it had a slightly dark hue, slightly outdated user interface and usability, longer scanning time, and slightly higher error rate.
- The Hamamatsu NanoZoomer S360 generally showed satisfactory image quality with high capacity, featuring acceptable coverage, good scanning speed, even focus, good z-stacking features, and appropriate color and saturation [12]. Despite having the smallest file size, it remains in the process of registration with the KFDA. The Roche Ventana DP200 also demonstrated generally satisfactory image quality, but with limited capacity, offering good coverage, good focus with extended z-stacking, good color, and specialization for companion diagnosis and image analysis [13]. However, its capacity is limited, although with a good scanning speed. The Philips Intellisite Ultra-Fast Scanner has good image quality, but poor focus for 3D-cluster-rich samples [14]. The device had good speed and capacity, and the most realistic hue, color, saturation, and contrast, but poor coverage and poor focus owing to the lack of z-stacking (good image quality in GYN LBP), a slightly high error rate, and limited compatibility.
- To date, evidence for the validity of DP application in cytological samples is insufficient. This is mainly because of the requirement of a higher resolution such as 100× for cytologic samples that can demonstrate good visibility for nuclear-level features, such as chromatin patterns and nucleolar features, the so-called image quality. Another main reason that pathologists use DPS for cytology is the longer scanning time, larger file size of digital cytological images, and higher error rates, which can be a burden for managing and storing systems as well as image analysis processes.
- At the 2023 Annual Meeting of the United States of America and Canadian Association of Pathologists, Akbar et al. presented a poster at Ohio State University Wexner Medical Center [15]. In this study, the authors compared the technical performance of four WSI scanners, including the Ultra-Fast Scanner by Philips, AT2 and GT450 by Leica, and the Genius Digital Diagnostics System by Hologic, on 250 cytology slides with different preparations [15]. The overall successful scan rate ranges from 38% to 96%, with the Hologic scanner showing the best performance and ThinPrep slides showing the highest success rate [15]. The fail-to-scan rates remained significant, indicating that a digital cytology workflow for primary diagnosis is currently infeasible. Further experience and evidence should be provided for the safe implementation of digital cytology.
- Recently, Hologic launched a new scanner system for cytologic specimens, along with an artificial intelligence (AI) algorithm for gynecologic samples, called Genius Digital Diagnostics [16], which is a pioneering digital cytology platform that has obtained CE-mark certification through integrating advanced volumetric imaging technology with a novel AI algorithm to assist cytotechnologists and pathologists in detecting precancerous lesions and cancer cells in women. The platform can swiftly examine every cell on a ThinPrep Pap digital image, reducing tens of thousands of cells to an AI-curated gallery showing the most diagnostically significant images [16,17]. This system provides an AI algorithm for gynecologic samples and its API is planned to open to third-party applications to expand its coverage to AI algorithms for non-gynecologic samples, such as urine, body fluids, and FNAs.
- Currently, one of the major challenges in applying AI to cytology is the significantly larger size of cytologic whole-slide images compared with histology [18,19]. This not only leads to a more time-consuming scanning process but also demands increased computational resources for image analysis. This was largely due to the Z-stacking process, which is essential for cytological samples [18,20]. In addition, the image quality can vary depending on the scanner used, which can affect the effectiveness of AI algorithms. Another challenge in cytology is the difficulty of annotating images, particularly when dealing with image patches or cell clusters. Furthermore, the limited availability of well-annotated large datasets, publicly accessible datasets, and significant challenges can hinder the development and testing of AI models [21-24]. Volumetric scanning technology, which was recently introduced by the Genius Digital Diagnostics system, can be a good solution to these issues by scanning slides tangentially at once and combining the acquired images into a single layer of images by post-processing computation. This allows fast scanning with an optimal focus resulting in a much smaller file size.
- Unfortunately, the latest systems, including Genius Digital Diagnostics by Hologic and GT450 by Leica, were not included in this study because they were not publicly released at the time of the study design. Several new scanners, such as Optrascan, Morphle, and Olympus, have been developed and introduced by traditional and new companies. Further studies comparing scanners from various vendors are required. In addition, it should also be clearly understood that the DP200 by Roche and the Ultra-Fast Scanner by Philips were not originally intended to be applied for cytological samples, but for histological samples.
- Based on the analysis of different scanner models, having at least three layers of z-stacking is recommended for LBP and five layers of z-stacking for conventional smears to achieve optimal image quality. The selection of a scanner model should be based on careful consideration of the institutional characteristics of the cytopathology practices. To ensure the best fit in practice, a thorough test run of the candidate scanner models is recommended.
- Digital scanners are essential tools in modern pathology laboratories. They provide high-resolution digital images of the tissue samples that can be easily viewed, stored, and shared electronically. The aim of this study was to compare the product specifications and image quality of cytologic slides scanned using five digital scanners: Pannoramic 250 Flash of 3DHistech, NanoZoomer 360 of Hamamatsu, Aperio AT2 of Leica, Ventana DP200 of Roche, and Ultra-Fast Scanner of Philips. In conclusion, each digital scanner has strengths and limitations. The Pannoramic 250 Flash of 3DHistech and NanoZoomer 360 (Hamamatsu) are best suited for high-throughput laboratories, whereas the Aperio AT2 (Leica) and Ventana DP200 (Roche) are best suited for the high-resolution scanning of a large number of slides. Philips’ Ultra-Fast Scanner is an excellent choice for laboratories that require both bright-field and fluorescence imaging. Therefore, the selection of an appropriate digital scanner depends on specific laboratory requirements.
DISCUSSION
Supplementary Information
Supplementary Table S1.
Supplementary Table S2.
Supplementary Table S3.
Supplementary Fig. S1.
Supplementary Fig. S2.
Ethics Statement
This study was reviewed and approved by the Institutional Review Board of the Catholic University of Korea College of Medicine (UC21ZCSI0133). The informed consent was waived by the Institutional Review Board of the Catholic University of Korea College of Medicine.
Availability of Data and Material
Data and materials for this work are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
Author contributions
Conceptualization: YC, SAH, HKO, SJJ, BSK, JYJ, GG. Data curation: YC, SAH, HKO, SJJ, BSK. Formal analysis: YC. Funding acquisition: YC, GG. Investigation: YC, SAH, HKO, SJJ, BSK, JYJ. Methodology: YC, SAH, HKO, SJJ, BSK, JYJ. Project administration: YC, SAH. Resources: SAH, HKO, SJJ, BSK, JYJ. Software: YC. Supervision: GG, HCL.
Conflicts of Interest
Y.C., a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
This study was partially supported by a Korean Society for Cytopathology grant (No. 2019-01) and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2021R1A2C2013630).
Acknowledgments
| Manufacturer | 3DHistech | Hamamatsu | Leica | Roche | Philips |
|---|---|---|---|---|---|
| Model | Pannoramic 250 Flash | NanoZoomer 360 | Aperio AT2 | Ventana DP 200 | Ultra-Fast Scanner |
| Slide capacity | 250 | 360 | 400 | 6 | 300 |
| Scan speed 5 × 5 mm (40×) – 5 layers | 3 min | 1.5 min | 2.5 min | 1.5 min | - |
| GUI (user friendliness) | Excellent | Good | Satisfactory | Satisfactory | - |
| Image quality (40×) | Excellent | Good | Excellent | Good | - |
| File size 15 × 15 mm (40×) 5 layers | 10 GB | 10 GB | 8 GB | 12 GB | - |
| Magnification | 20×, 40× | 20×, 40× | 20×, 40× | 20×, 40× | 20×, 40× |
| Weekly capacity (slides) | 200 | 300 | 150 | < 100 | - |
| Imaging mode(s) | Bright field, fluorescent | Bright field | Bright field, fluorescent | Bright field | Bright field |
| Digital slide format | MRXS, JPG, and JPG2000 | JPG, ndpi | TIFF (SVS) | BIF | Insyntax, fic |
| Multilayer support | Z-stack or extended focus | Z-stack or extended focus | Z-stack | Extended focus | Not support |
| Error ratea | 2 | 3 | 4 | 3 | - |
| Special features | Continuous loading | Quality scoring and intelligent rescans | Automated scanning | - | LCD touchscreen |
- 1. Lee HK, Kim SN, Khang SK, Kang CS, Yoon HK. Quality control program and its results of Korean Society for Cytopathologists. Korean J Cytopathol 2008; 19: 65-71. Article
- 2. The Korean Society for Cytopathology [Internet]. Seoul: The Korean Society for Cytopathology, 2023 [cited 2023 Apr 25]. Availabe from: http://eng.cytopathol.or.kr/index.asp.
- 3. Oh EJ, Jung CK, Kim DH, et al. Current cytology practices in Korea: a nationwide survey by the Korean Society for Cytopathology. J Pathol Transl Med 2017; 51: 579-87. ArticlePubMedPMCPDF
- 4. Chong Y, Bae JM, Kang DW, Kim G, Han HS. Development of quality assurance program for digital pathology by the Korean Society of Pathologists. J Pathol Transl Med 2022; 56: 370-82. ArticlePubMedPMCPDF
- 5. Hong SA, Jung H, Kim SS, et al. Current status of cytopathology practice in Korea: impact of the coronavirus pandemic on cytopathology practice. J Pathol Transl Med 2022; 56: 361-9. ArticlePubMedPMCPDF
- 6. Chong Y, Jung H, Pyo JS, Hong SW, Oh HK. Current status of cytopathology practices in Korea: annual report on the Continuous Quality Improvement program of the Korean Society for Cytopathology for 2018. J Pathol Transl Med 2020; 54: 318-31. ArticlePubMedPMCPDF
- 7. Choi YD, Oh HK, Kim SJ, et al. Continuous quality improvement program and its results of Korean Society for Cytopathology. J Pathol Transl Med 2020; 54: 246-52. ArticlePubMedPMCPDF
- 8. Chong Y, Kim DC, Jung CK, et al. Recommendations for pathologic practice using digital pathology: consensus report of the Korean Society of Pathologists. J Pathol Transl Med 2020; 54: 437-52. ArticlePubMedPMCPDF
- 9. The Royal College of Pathologist of Australasia Quality Assurance Programs (RCPAQAP) [Internet]. St. Leonards: The Royal College of Pathologist of Australasia, 2023 [cited 2023 Apr 15]. Available from: https://rcpaqap.com.au/about-us/.
- 10. UK National External Quality Assessment Service (NEQAS) [Internet]. Sheffield: UK NEQAS, 2023 [cited 2023 Apr 25]. Available from: https://ukneqas.org.uk/about-us/.
- 11. Pannoramic 250 Flash Series Digital Scnners [Internet]. Budapest: 3DHISTECH, 2023 [cited 2023 Apr 26]. Avaliable from: https://www.3dhistech.com/research/pannoramic-digital-slide-scanners/pannoramic-250-flash-iii/.
- 12. Nano Zoomer S360 Digital slide scanner [Internet]. Hanamatsu: HAMAMATSU Photonics K.K., 2023 [cited 2023 Apr 26]. Available from: https://www.hamamatsu.com/us/en/product/life-science-and-medical-systems/digital-slide-scanner/C13220-01.html/.
- 13. VENTANA DP 200 slide scanner [Internet]. Rotkreuz: F. HoffmannLa Roche Ltd., 2023 [cited 2023 Apr 26]. Available from: https://diagnostics.roche.com/global/en/products/instruments/ventana-dp200-ins-6320.html.
- 14. Ultra Fast Scanner [Internet]. Amsterdam: Philips Intellisite, 2023 [cited 2023 Apr 26]. Available from: https://www.usa.philips.com/healthcare/product/HCNOCTN442/ultra-fast-scanner-digital-pathology-slide-scanner/.
- 15. Akbar S, Sincair D, Shen R, Parwani AV, Li Z. Comparative assessment of digital sccnners for scanning cytology slides. In: USCAP Annual Meeting; 2023 Mar 11-16; New Orleans, LA.
- 16. Genius Digital Diagnostics System [Internet]. Marlborough: HOLOGIC, 2023 [cited 2023 Apr 25]. Available from: https://www.hologic.com/hologic-products/cytology/genius-digital-diagnosticssystem/.
- 17. Yao K, Sadimin E, Chang S, Schmolze D, Li Z. Current applications and challenges of digital pathology in cytopathology. Hum Pathol Rep 2022; 28: 300634.Article
- 18. Capitanio A, Dina RE, Treanor D. Digital cytology: a short review of technical and methodological approaches and applications. Cytopathology 2018; 29: 317-25. ArticlePubMedPDF
- 19. Niazi MK, Parwani AV, Gurcan MN. Digital pathology and artificial intelligence. Lancet Oncol 2019; 20: e253-61. ArticlePubMedPMC
- 20. Hanna MG, Monaco SE, Cuda J, Xing J, Ahmed I, Pantanowitz L. Comparison of glass slides and various digital-slide modalities for cytopathology screening and interpretation. Cancer Cytopathol 2017; 125: 701-9. ArticlePubMedPDF
- 21. Xu C, Li M, Li G, Zhang Y, Sun C, Bai N. Cervical cell/clumps detection in cytology images using transfer learning. Diagnostics (Basel) 2022; 12: 2477.ArticlePubMedPMC
- 22. Hipp JD, Sica J, McKenna B, et al. The need for the pathology community to sponsor a whole slide imaging repository with technical guidance from the pathology informatics community. J Pathol Inform 2011; 2: 31.ArticlePubMedPMC
- 23. Hartman DJ, Van Der Laak J, Gurcan MN, Pantanowitz L. Value of public challenges for the development of pathology deep learning algorithms. J Pathol Inform 2020; 11: 7.ArticlePubMedPMC
- 24. Thakur N, Alam MR, Abdul-Ghafar J, Chong Y. Recent application of artificial intelligence in non-gynecological cancer cytopathology: a systematic review. Cancers (Basel) 2022; 14: 3529.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Sensitivity, Specificity, and Cost–Benefit Effect Between Primary Human Papillomavirus Testing, Primary Liquid‐Based Cytology, and Co‐Testing Algorithms for Cervical Lesions
Chang Gok Woo, Seung‐Myoung Son, Hye‐Kyung Hwang, Jung‐Sil Bae, Ok‐Jun Lee, Ho‐Chang Lee
Diagnostic Cytopathology.2025; 53(1): 35. CrossRef - Integration of AI‐Assisted in Digital Cervical Cytology Training: A Comparative Study
Yihui Yang, Dongyi Xian, Lihua Yu, Yanqing Kong, Huaisheng Lv, Liujing Huang, Kai Liu, Hao Zhang, Weiwei Wei, Hongping Tang
Cytopathology.2025; 36(2): 156. CrossRef - National quality assurance program using digital cytopathology: a 5-year digital transformation experience by the Korean Society for Cytopathology
Yosep Chong, Hyeong Ju Kwon, Soon Auck Hong, Sung Soon Kim, Bo-Sung Kim, Younghee Choi, Yoon Jung Choi, Jung-Soo Pyo, Ji Yun Jeong, Soo Jin Jung, Hoon Kyu Oh, Seung-Sook Lee
Journal of Pathology and Translational Medicine.2025; 59(5): 320. CrossRef - Integration of Digital Cytology in Quality Assurance Programs for Cytopathology
Yosep Chong, Maria Jesús Fernández Aceñero, Zaibo Li, Andrey Bychkov
Acta Cytologica.2025; : 1. CrossRef - Practice of Cytopathology in Korea: A 40‐Year Evolution Through Standardization, Digital Transformation, and Global Partnership
Yosep Chong, Ran Hong, Hyeong Ju Kwon, Haeryoung Kim, Lucia Kim, Soon Jae Kim, Yoon Jung Choi
Diagnostic Cytopathology.2025;[Epub] CrossRef - Quantitative Assessment of Focus Quality in Whole-Slide Imaging of Thyroid Liquid-Based Cytology Using Laplacian Variance
Chan Kwon Jung, Chankyung Kim, Sora Jeon, Andrey Bychkov
Endocrine Pathology.2025;[Epub] CrossRef - Validation of digital image slides for diagnosis in cervico-vaginal cytology
Francisco Tresserra, Gemma Fabra, Olga Luque, Miriam Castélla, Carla Gómez, Carmen Fernández-Cid, Ignacio Rodríguez
Revista Española de Patología.2024; 57(3): 182. CrossRef - Improved Diagnostic Accuracy of Thyroid Fine-Needle Aspiration Cytology with Artificial Intelligence Technology
Yujin Lee, Mohammad Rizwan Alam, Hongsik Park, Kwangil Yim, Kyung Jin Seo, Gisu Hwang, Dahyeon Kim, Yeonsoo Chung, Gyungyub Gong, Nam Hoon Cho, Chong Woo Yoo, Yosep Chong, Hyun Joo Choi
Thyroid®.2024; 34(6): 723. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
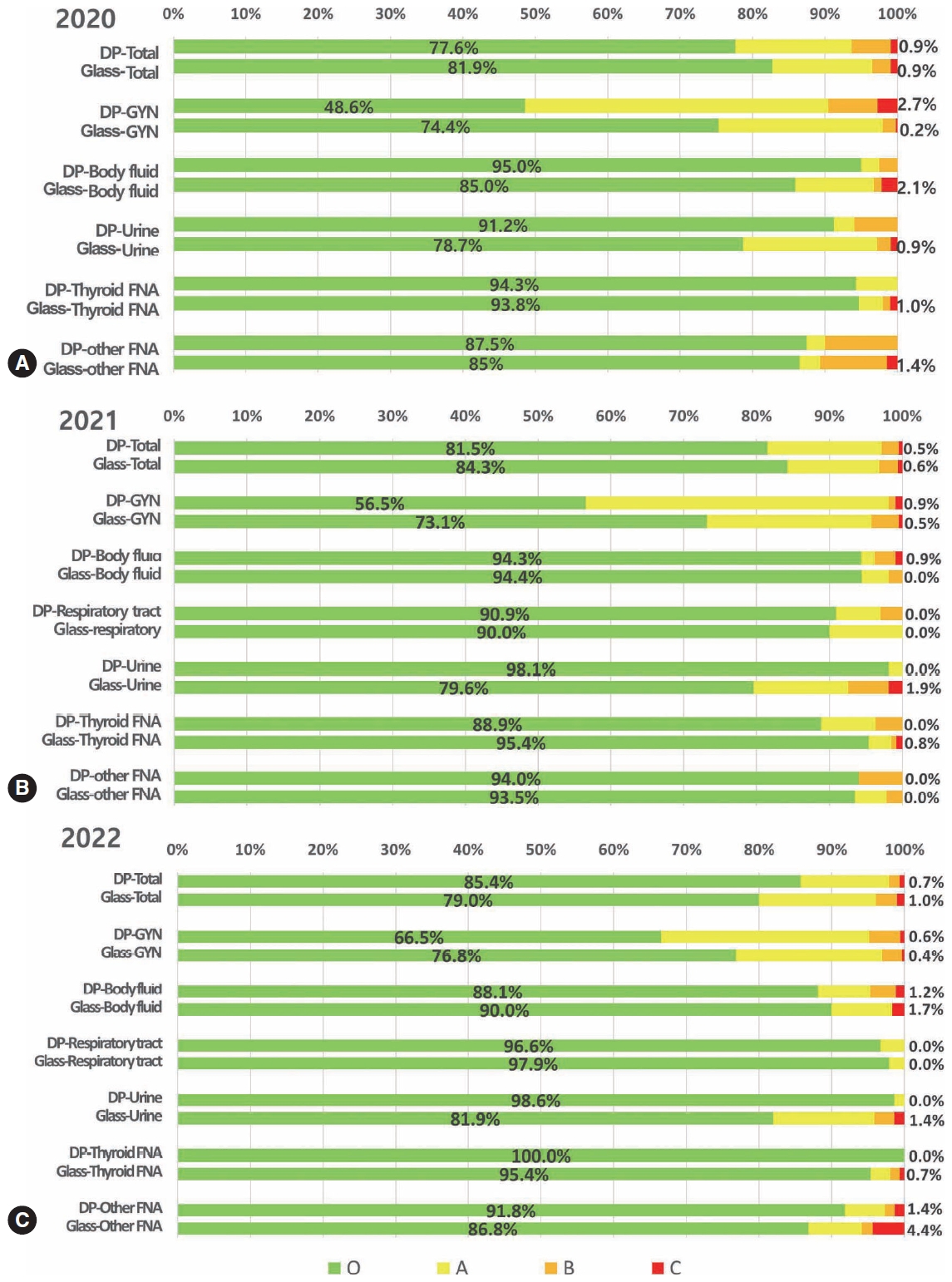






Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
| Label | Specimen type | Diagnosis | Z-stacking | Layers |
|---|---|---|---|---|
| C16-092 | Pap smear (conventional) | Squamous cell carcinoma | Yes | 5 |
| C16-223 | Pap smear (conventional) | High grade squamous intraepithelial lesion | Yes | 5 |
| C-16-141 | Pap smear (LBP) | Low grade squamous intraepithelial lesion | No | 1 |
| C-16-005 | Pap smear (LBP) | Adenocarcinoma | No | 1 |
| R-17-009 | Bronchial washing | Squamous cell carcinoma | Yes | 3 |
| R-18-037 | Sputum | Squamous cell carcinoma | Yes | 3 |
| BF-17-016 | Pleural fluid | Adenocarcinoma | Yes | 3 |
| BF-17-004 | Ascitic fluid | Serous carcinoma, metastatic | No | 1 |
| U-17-030 | Urine cytology | Papillary urothelial carcinoma, non-invasive, high grade | Yes | 3 |
| ABC-18-006 | Thyroid FNA | Papillary carcinoma | Yes | 3 |
| ABC-17-182 | Salivary FNA | Pleomorphic adenoma | Yes | 3 |
| ABC-17-197 | Lymph node FNA | Metastatic carcinoma (breast) | Yes | 3 |
| No. | Questions | 1 | 2 | 3 |
|---|---|---|---|---|
| 1 | All the area of the slide were scanned properly? | All | Partly no (<10%) | No (>10%) |
| 2 | The scanned image show even magnification in all the area? | Yes | Partly no | No |
| 3 | The white balance of the background image is appropriate? | Yes | Partly no | No |
| 4 | Is the focus of the image even enough throughout the image? | Yes | Partly no | No |
| 5 | Is it easy to differentiate cells and background artifacts such as inflammatory cells and mucinous materials? | Yes | Partly no | No |
| 6 | The color of the scanned image is even throughout the image? | Yes | Partly no | No |
| 7 | The color of the nuclei is even throughout the image? | Yes | Partly no | No |
| 8 | The image of overlapping cells or 3-dimensional clusters is clear enough to interpret? | Yes | Partly no | No |
| 9 | Is it easy to differentiate nuclei and cytoplasm of the cells (especially in the overlapping clusters)? | Yes | Partly no | No |
| 10 | The cytoplasmic membrane is clear enough to interpret? | Yes | Partly no | No |
| 11 | The nuclear membrane is clear enough to interpret? | Yes | Partly no | No |
| 12 | Is the image good enough to assess the cytoplasmic texture? | Yes | Partly no | No |
| 13 | Is the image good enough to assess the nuclear chromatin pattern? | Yes | Partly no | No |
| 14 | Is the image good enough to assess the nucleoli? | Yes | Partly no | No |
| 15 | Is the image good enough to assess the necrosis (only apply when the case includes necrosis)? | Yes | Partly no | No |
| 16 | Is the focus clear enough in the higher magnification? | Yes | Partly no | No |
| 17 | Is the focus clear enough in the lower magnification? | Yes | Partly no | No |
| Manufacturer | 3DHistech | Hamamatsu | Leica | Roche | Philips |
|---|---|---|---|---|---|
| Model | Pannoramic 250 Flash | NanoZoomer 360 | Aperio AT2 | Ventana DP 200 | Ultra-Fast Scanner |
| Slide capacity | 250 | 360 | 400 | 6 | 300 |
| Scan speed 5 × 5 mm (40×) – 5 layers | 3 min | 1.5 min | 2.5 min | 1.5 min | - |
| GUI (user friendliness) | Excellent | Good | Satisfactory | Satisfactory | - |
| Image quality (40×) | Excellent | Good | Excellent | Good | - |
| File size 15 × 15 mm (40×) 5 layers | 10 GB | 10 GB | 8 GB | 12 GB | - |
| Magnification | 20×, 40× | 20×, 40× | 20×, 40× | 20×, 40× | 20×, 40× |
| Weekly capacity (slides) | 200 | 300 | 150 | < 100 | - |
| Imaging mode(s) | Bright field, fluorescent | Bright field | Bright field, fluorescent | Bright field | Bright field |
| Digital slide format | MRXS, JPG, and JPG2000 | JPG, ndpi | TIFF (SVS) | BIF | Insyntax, fic |
| Multilayer support | Z-stack or extended focus | Z-stack or extended focus | Z-stack | Extended focus | Not support |
| Error rate |
2 | 3 | 4 | 3 | - |
| Special features | Continuous loading | Quality scoring and intelligent rescans | Automated scanning | - | LCD touchscreen |
LBP, liquid-based preparation; FNA, fine-needle aspiration.
GUI, graphical user interface. Error rate per run.

 E-submission
E-submission










