Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 57(2); 2023 > Article
-
Original Article
Clinicopathologic significance of the delta-like ligand 4, vascular endothelial growth factor, and hypoxia-inducible factor-2α in gallbladder cancer -
Sujin Park1
 , Junsik Kim2
, Junsik Kim2 , Woncheol Jang2, Kyoung-Mee Kim1
, Woncheol Jang2, Kyoung-Mee Kim1 , Kee-Taek Jang,1
, Kee-Taek Jang,1
-
Journal of Pathology and Translational Medicine 2023;57(2):113-122.
DOI: https://doi.org/10.4132/jptm.2023.02.01
Published online: March 14, 2023
1Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
2Department of Statistics, Duksung Women’s University, Seoul, Korea
- Corresponding Author: Kee-Taek Jang, MD, PhD, Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea Tel: +82-2-3410-2763, Fax: +82-2-3410-0025, E-mail: kt12.jang@samsung.com
© 2023The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Gallbladder cancer (GBC) is usually detected in advanced stages with a low 5-year survival rate. Delta-like ligand 4 (DLL4), vascular endothelial growth factor (VEGF), and hypoxia-inducible factor-2alpha (HIF2α) have been studied for their role in tumorigenesis and potential for therapeutic target, and multiple clinical trials of the agents targeting them are ongoing. We investigated the expression of these markers in surgically resected GBC and tried to reveal their association with the clinicopathologic features, mutual correlation of their expression, and prognosis of the GBC patients by their expression.
-
Methods
- We constructed the tissue microarray blocks of 99 surgically resected GBC specimens and performed immunohistochemistry of DLL4, VEGF, and HIF2α. We used the quantitative digital image analysis to evaluate DLL4 and VEGF expression, while the expression of HIF2α was scored manually.
-
Results
- The expression of VEGF and HIF2α showed a significant trend with tumor differentiation (p = .028 and p = .006, respectively). We found that the high DLL4 and VEGF expression were significantly correlated with lymph node metastasis (p = .047, both). The expression of VEGF and HIF2α were significantly correlated (p < .001). The GBC patients with low HIF2α expression showed shorter recurrence-free survival than those with high HIF2α expression.
-
Conclusions
- This study suggested the possibility of the usage of DLL4 and VEGF to predict the lymph node metastasis and the possibility of VEGF and HIF2α to predict the expression level mutually. Further studies may be needed to validate our study results and eventually accelerate the introduction of the targeted therapy in GBC.
- Patient selection and tissue samples
- We collected the tissues of the GBC patients that underwent surgical resection between January 2010 and December 2017 from the surgical pathology database of Samsung Medical Center (Seoul, Korea). Initially, 101 cases were found, but one was excluded because the tumor was a metastatic tumor from the liver, and another one was excluded due to pre-operative chemotherapy. Finally, we enrolled a total study population of 99 GBC cases. Clinical data, including age, sex, date of surgery, history of post-operative chemotherapy, recurrence-free survival (RFS), overall survival, and duration of follow-up, were extracted from electronic medical records. As all hematoxylin and eosin (H&E)-stained slides were reviewed by two pathologists (K.T.J. and S.P.), the histologic type and differentiation were reviewed for all tumor tissues. We checked the tumor staging according to the American Joint Committee on Cancer staging system (8th edition) [22].
- Tissue microarray construction and immunohistochemistry
- Representative tumor areas confirmed for the absence of hemorrhage or necrosis were marked on the formalin-fixed paraffin-embedded blocks. Two tissue cores with a diameter of 2.0 mm were acquired from each donor block and were arranged in the recipient paraffin blocks. Each tissue microarray (TMA) block contained up to 40 tumor tissue cores and two control tissue cores. One normal pancreas tissue core and one normal tonsil tissue core were used as control cores.
- Immunohistochemistry (IHC) was performed on 4-μm-thick tissue sections obtained from TMA blocks. For detection of DLL4 and HIF2α, automated Ventana BenchMark Ultra instrument (Ventana Medical Systems, Tucson, AZ, USA) was used for antigen retrieval and primary antibody reaction. After the antigen retrieval for 92 minutes with CC1 in Ventana BenchMark Ultra, the sections were incubated with anti-DLL4 antibody (HPA023392, 1:50, Sigma-Aldrich, St. Louis, MO, USA) for 60 minutes in 37°C and with EPAS-1 (sc-46691, 1:50, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for HIF2α detection for 120 minutes in 37°C, respectively. For EPAS-1, the chromogenic reactions were carried out for 12 minutes with OptiView Amplification Kit (860-099, Ventana Medical Systems) and OptiView DAB IHC Detection kit (760-700, Ventana Medical Systems), but for anti-DLL4 antibody, with OptiView DAB IHC Detection kit (760-700, Ventana Medical Systems) only. For VEGF protein detection, the sections were incubated with a mouse monoclonal anti-VEGF antibody (sc-7269, 1:500, Santa Cruz Biotechnology) for 20 minutes in a Bond-max autoimmunostainer (Leica Biosystems, Melbourne, Australia) after antigen retrieval with ER1 buffer (pH 6.0, Leica Biosystems) in 100°C. Antigen-antibody chromogenic reactions were developed for 10 minutes using the Bond Polymer refine detection kit, DS9800 (Vision Biosystems, Melbourne, Australia).
- Quantitative digital image analysis and manual scoring
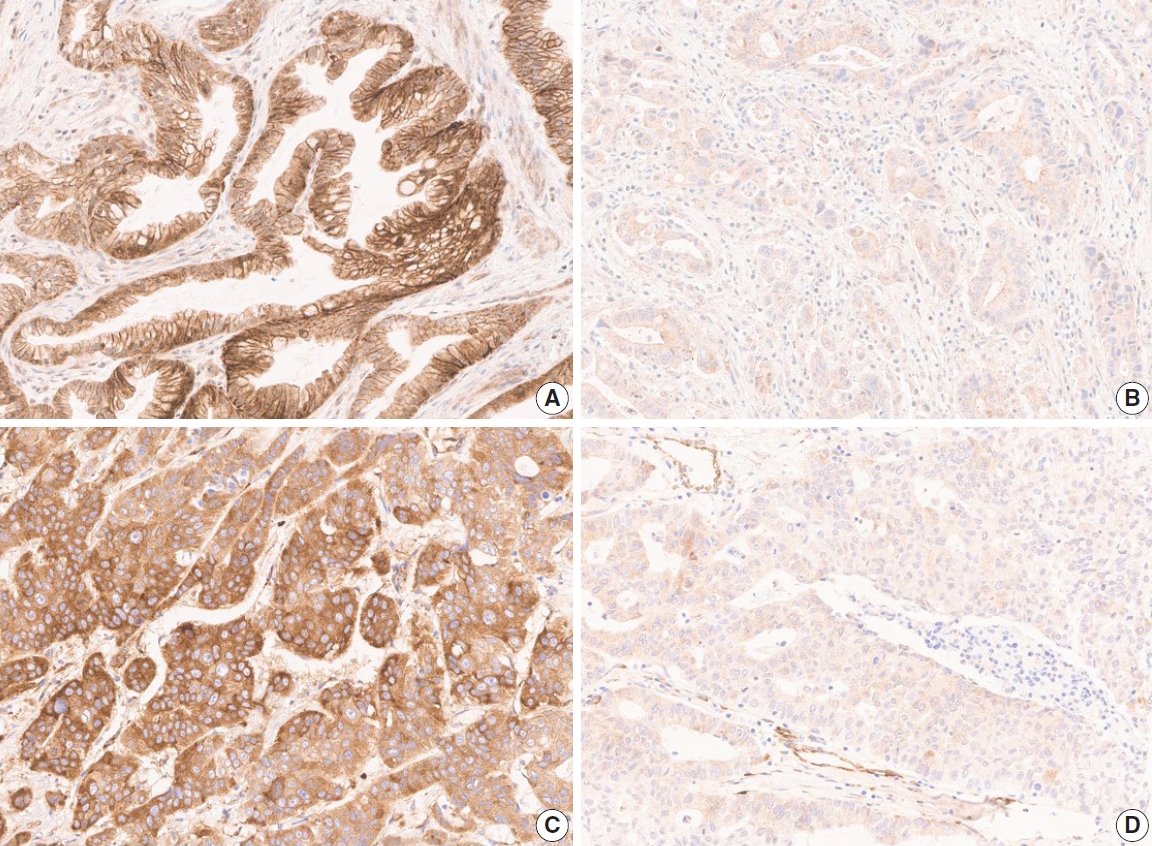
- The TMA slide stained with H&E and IHC was digitized by Aperio AT2 scanner (Leica Biosystems, Buffalo Grove, IL, USA) at 20× magnification. For DLL4 and VEGF expression analysis, Aperio ImageScope software (ver. 12.4.2, Leica Biosystems, Buffalo Grove, IL, USA) was used. All tumor cells except those in lymphocyte-rich areas were exclusively annotated in each TMA core. According to the expression patterns of the antibodies, DLL4 expression and VEGF expression were analyzed with membrane v9 algorithm and cytoplasm v2 algorithm (Fig. 1), respectively. The algorithms were available as a component in the commercial version of Aperio ImageScope software. Both algorithms automatically counted the VEGF- or DLL4-positive cells based on their staining intensity (0, 1+, 2+, and 3+). The annotation was performed by one pathologist (S.P.), and reviewed by an additional pathologist (K.T.J.) before the automatic analysis. Two pathologists (S.P. and K.T.J.) jointly reviewed the digitally scanned slides and results of the automatic analysis to confirm its performance. The H-scores could be automatically derived from the results (cytoplasm v2), or be calculated from the values of the results (membrane v9).
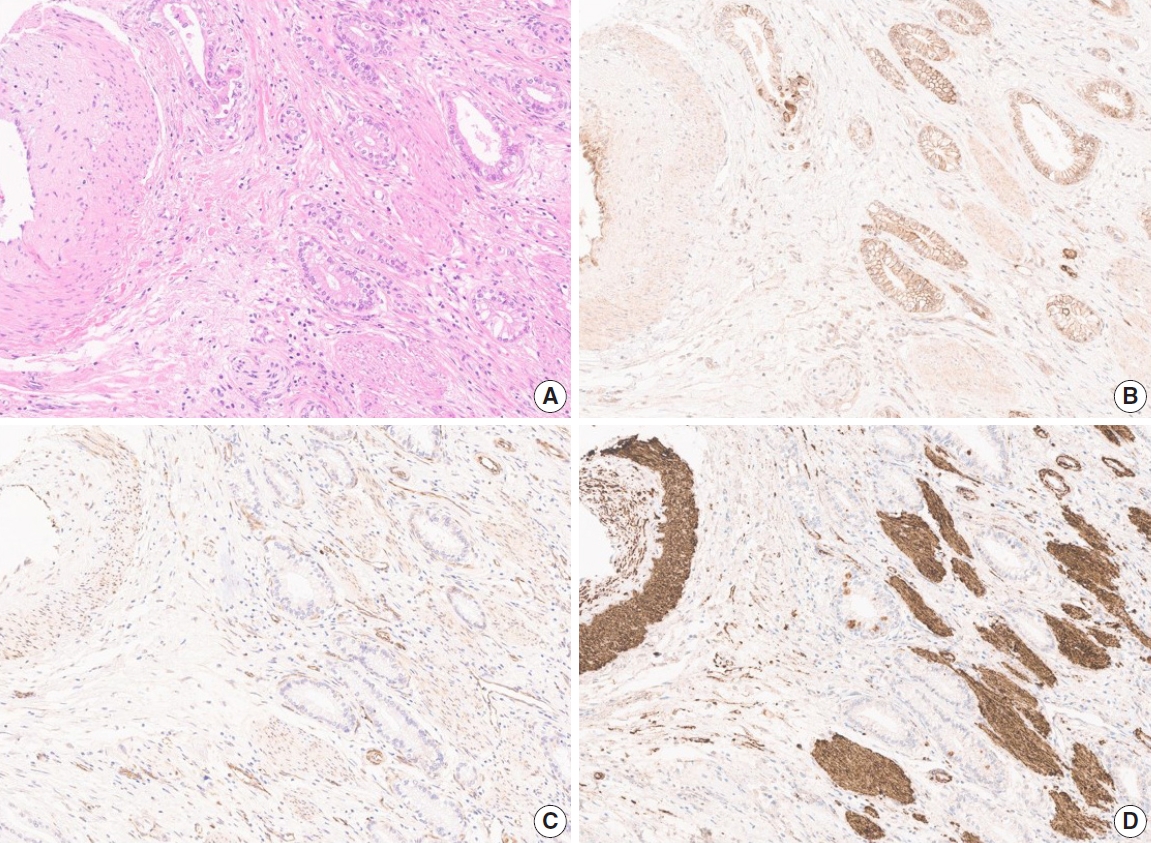
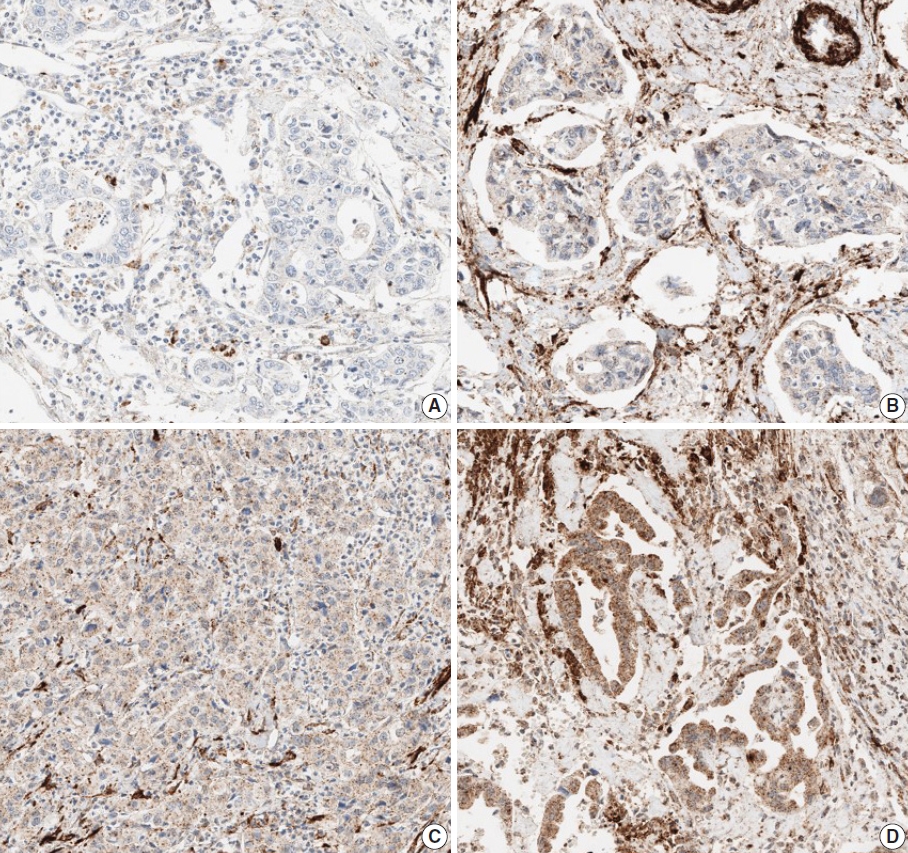
- Due to the extensive background staining of HIF2α compared to DLL4 and VEGF (Fig. 2), the expression of HIF2α was manually scored according to the staining intensity (0, 1+, 2+, and 3+) (Fig. 3) to calculate the H-scores, by one pathologist (S.P.). As an additional pathologist (K.T.J.) reviewed, consensus was achieved between two pathologists for any discrepancy.
- Statistical analysis
- As appropriate, Pearson’s chi-square test or Cochran-Armitage test was used to analyze the correlation between DLL4, VEGF, and HIF2α expression and clinicopathologic parameters. Pearson’s chi-square test and Spearman’s ρ rank correlation test were used to analyze the correlations between the expression levels of three markers. The Kaplan-Meier survival method was used to analyze survival rates. The Cox proportional hazard regression was used to describe the effects of one or more predictors on survival time or time-to-event outcomes. In all statistical analyses, IBM SPSS ver. 27.0 for Windows (IBM Corp., Armonk, NY, USA) and R software (R Foundation for Statistical Computing, Vienna, Austria) were used.
MATERIALS AND METHODS
- Clinicopathologic features of the GBC patients
- The clinicopathologic features of the GBC patients and the association of these features with DLL4, VEGF, and HIF2α expression are summarized in Table 1. For the chi-square test, the expression levels of the markers were divided into two groups: high when the H-score ≥120, low when <120 (Fig. 1). Among the 99 patients, 40 patients (40.4%) were male, and 59 patients (59.6%) were female, and their age at the time of diagnosis ranged widely from 31 to 85 years (median age, 63 years). All tumors were primary GBCs, consist of conventional adenocarcinoma (81 cases, 81.8%), adenosquamous carcinoma (8 cases, 8.1%), neuroendocrine carcinoma (5 cases, 5.1%), mixed neuroendocrine carcinoma and adenocarcinoma, hepatoid adenocarcinoma (1 case, 1.0%), and undifferentiated carcinoma (3 cases, 3.0%, respectively). About half of the cases were well, moderately, or well to moderately differentiated tumors (56 cases, 56.6%). Forty cases (40.4%) had any proportion of poor differentiation, and the remaining three cases (3.0%) were classified as undifferentiated tumors. As a result of surgical resection, most cases were T3 (88 cases, 88.9%). Lymph node metastasis was present in 68 cases (68.7%), and distant metastasis was present in 11 cases (11.1%). Recurrence occurred in 53 cases (53.5%) during the follow-up period (median, 11.6 months; range, 0.7 to 126.9 months) and 36 patients (36.4%) died during the follow-up period (median, 20.0 months; range, 0.7 to 126.9 months).
- The chi-square test showed that lower DLL4 expression and VEGF expression was associated with lymph node metastasis (p= .047, both). The Cochran-Amitage test revealed that there is a statistically significant linear-trend between the degree of differentiation and VEGF or HIF2α expression (p=.028 and p= .006, respectively). The test suggests strong evidence of a linearity between the expression of these markers and the tumor differentiation.
- Expression of DLL4, VEGF, and HIF2α
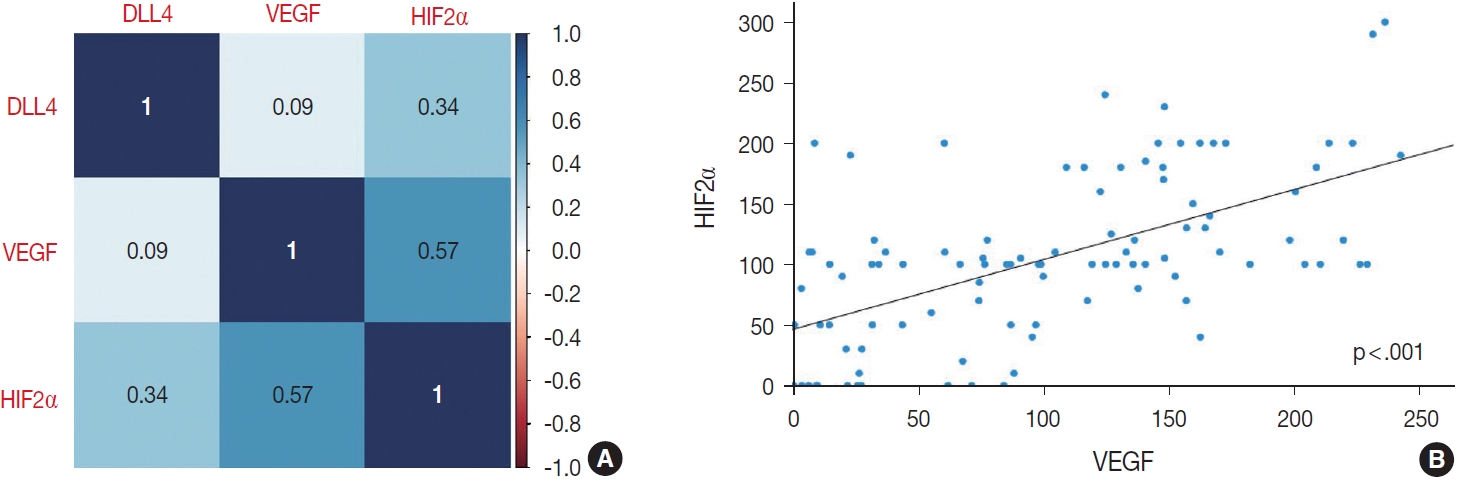
- When compared by the chi-square test as described above, DLL4 expression did not correlate with the expression of other markers. VEGF and HIF2α expression, however, was significantly correlated (p<.001), tumors with high VEGF expression would display higher expression of HIF2α, vice versa.
- To further confirm the statistical significance of the correlation between expressions of the three markers, we performed an additional statistical analysis. By correlation matrix, the correlations were visualized, showing weak correlations between DLL4 vs. VEGF, and DLL4 vs. HIF2α, but a relatively strong correlation between VEGF vs. HIF2α (Fig. 4).
- Considering that the H-score values of three makers are not normally distributed, we used Spearman’s ρ rank correlation coefficient to test the significances of correlations between the H-score values of three makers. Between DLL4 and VEGF, and between DLL4 and HIF2α, the Spearman’s rank correlation co-efficients are 0.09 (p = .356) and 0.34 (p < .001), respectively, which is consistent with the insignificant results obtained with previous chi-square test. Between H-score values of VEGF and HIF2α, there was a statistically significant positive correlation, with correlation coefficient of 0.57 (p < .001). The Spearman’s rank correlation coefficient values between each marker are reflected in Fig. 4.
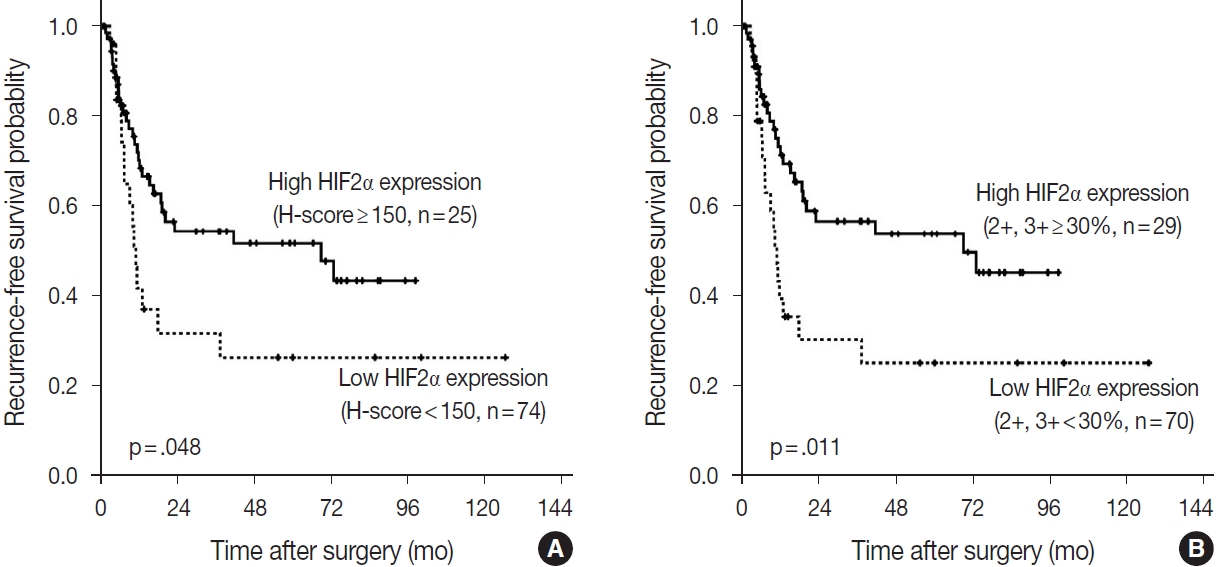
- Impact of DLL4, VEGF, and HIF2α expression on the prognosis
- According to the Kaplan-Meier survival analysis result, expression of DLL4 and VEGF did not affect recurrence or death. According to HIF2α expression, however, recurrence rates showed a statistically significant difference. When the cutoff for high or low expression was set as H-score=150, patients with low HIF2α expression (n= 74) showed shorter RFS than the patients with higher HIF2α expression (n=25) (p=.048). When the high expression group was defined as the tumor with more than 30% of 2+ and 3+ cells, patients with low HIF2α expression (n=70), again, showed shorter RFS than those with higher HIF2α expression (n=29) (p=.011) (Fig. 5). By performing Cox proportional hazard regression, HIF2α expression was confirmed to be a significant predictive factor of the time to recurrence (p=.020), along with the presence of nodal metastasis of the patients (p< .001). The result regarding HIF2α expression shows consistency with the result of Kaplan-Meier survival analysis. Overall survival rate did not differ among patients according to HIF2α expression by Kaplan-Meier survival analysis, and all the negative results were consistent with the result of Cox proportional hazard regression model.
RESULTS
- Although several previous studies investigated the expression of DLL4, VEGF, or HIF2α in GBC separately [23-27], this is the first study to perform the IHC of these three markers coincidently, attempting to integrate and confirm the results of the previous studies. As the study material, we used the TMA constructed with 99 surgically resected gallbladder cases from a single institution. These cases were followed up for a relatively long period of time – at least 0.7 months to a maximum of 11 years. For analysis of IHC, we digitized the slides and utilized a digital image analysis platform. Complete annotation of tumor area of 99 cases was laborious and time-consuming, but with this tool, we could assure the objectivity of the analysis. Most of the previous studies scored the IHC stain manually with a light microscope, which is inevitably subjective, and this might affect the study results. With digital image analysis, we attempted to overcome such limitations on the aspect of DLL4 and VEGF, but still not for the analysis related to HIF2α, due to the background staining (Fig. 2D). Quantitative digital image analysis has been used in multiple previous studies in various platforms such as Aperio ImageScope, QuPath, ASAP, etc. Since subjectivity of interpretation has long been a hurdle to be overcome and a task for pathologists to conquer, we believe this can be achieved in part by digital image analysis.
- The tumors were divided into three groups according to the degree of differentiation. The tumors that consisted of only well-differentiated and/or moderately differentiated portions were classified as the first group, and the tumors with any poorly differentiated portion were the second group. Only three undifferentiated tumors were classified as the third group. As a result, there was a positive trend between the differentiation and the expression of VEGF or HIF2α; the worse the differentiation tumors showed, the lower expression they exhibited. This trend was not consistent with the results of previous studies: some studies showed that VEGF expression was higher in poorly differentiated tumors compared to more differentiated tumors [25,27], and in other studies, no association was revealed [23,24]. To our knowledge, there have been no previous studies to report the significant association of degree of tumor differentiation and HIF2α expression. Although this study demonstrated a different result than the previous ones because the expression of VEGF and HIF2α are positively correlated, which is consistent with what is stated in other studies. Therefore, the result regarding the trend with differentiation might not be discarded. Instead, such conflicting results should be explained by investigating underlying mechanisms in future studies.
- As stated above, VEGF and HIF2α expression were significantly correlated – the tumors with higher VEGF expression tend to show higher HIF2α expression, vice versa. In a study by Giatromanolaki et al. [23], IHC was performed in 60 GBC samples and showed a similar result compared to ours. Since the data showing the association between VEGF and HIF2α expression are being accumulated, the application of these data onto the therapeutic aspects of these markers could be considered. VEGF has long been regarded as a well-established anti-neoplastic therapy. Several anti-VEGF inhibitors have been developed and are currently used, including bevacizumab [28]. Anti-VEGF inhibitor inhibits angiogenesis by reducing endothelial cell proliferation and thus tumor growth. Recently, anti-DLL4/anti-VEGF bispecific monoclonal antibody has been developed to enhance the anti-neoplastic activity and avoid the cardiac toxicity observed in patients when treated with anti-DLL4 inhibitors [16]. HIF2α, on the other hand, playing a pivotal role in tumor progression and metastasis [19], and being the main driver in the development of clear cell RCC [29], is an attractive therapeutic target. Multiple agents have been designed, and some have shown promising results in preclinical level and clinical trials [30,31]. Since the role of both VEGF and HIF2α and drugs that inhibit their action are being vigorously investigated, if VEGF and HIF2α could work as surrogate markers for each other or helps predict the expression level of each other, it might be considerably useful and convenient, in possible future occasions that expression level of these markers may work as a treatment indication.
- By chi-square test, we found that DLL4 and VEGF are correlated with lymph node metastasis status. DLL4 and VEGF expression tended to be lower in cases with lymph node metastasis (p=.047). Although our result did not reveal any prognostic significance of DLL4 and VEGF, because lymph node metastasis is determining factor for TNM stage that reflects patient survival, this correlation may point to the potential prognostic implication. In the cases that show low expression when stained with DLL4 and VEGF and if it is detected in biopsy sample prior to surgical resections, surgeons should perform meticulous lymph node dissection considering the higher possibility of lymph node metastasis. The pathologists should also spend more time evaluating the presence of tumor cells in dissected lymph nodes. If such a patient is subject to concurrent chemotherapy and radiotherapy, it would provide information for oncologists or radiologists’ decision. Low expression groups of DLL4 and VEGF, however, account for more than half of the patients (65.7% and 56.6%, respectively), there is the possibility that they might not work as the effective screening tool. A study by Liu et al. [32] showed a relevant result in non-small cell lung cancer cases, stating that low DLL4 expression was significantly correlated with lymph node metastasis. A study with a conflicting result compared to ours [33] revealed that high DLL4 expression predicted pelvic lymph node metastasis in early cervical cancer patients. Moreover, high DLL4 expression was an independent predictor of poor survival in these cervical cancer patients. Such conflict is possibly due to the bi-functional cellular responses that Notch signaling pathway may induce during tumorigenesis in different tumors [17]. DLL4, working as a ligand in the Notch signal pathway, may either promote or inhibit tumor cell proliferation or survival, and this might be different according to tumor cell origin of tumor cell types. Further studies are necessary to clarify the underlying mechanism of DLL4 activity in GBC to confirm our results on lymph node metastasis.
- Except for HIF2α, two other markers did not show any correlation with prognosis. The correlation of HIF2α with recurrence was not clear when the patients were divided into two groups: high as H-score ≥ 120 or low as H-score < 120. When the high group was set with more conservative criteria (higher H-score) or the proportion of 2+ and 3+ cells, patients with lower HIF2α expression showed shorter RFS than those with higher HIF2α expression. By Cox proportional hazard regression model, HIF2α expression was confirmed as a significant predictive factor for recurrence. High HIF2α expression, therefore, may help to expect a better prognosis regarding recurrence, but the threshold for “high” expression should be relatively high to gain reliable results. In our study cohort, some of the patients were transferred to different hospitals right after surgery for subsequent treatment and follow-up. The data regarding recurrence, death, and additional treatment could be incomplete, which might have affected our results.
- In this study, multiple cutoffs for statistical analysis was used. For example, H-score = 120, H-score = 150 or the tumor with more than 30% of 2+ and 3+ cells, in each analysis. In studies using quantitative measuring of expression level, especially regarding the studies using immunohistochemical stain, a certain cutoff is needed for grouping the patients. The gold standard for setting the cutoff value, however, is not established or even recommended for pathologists. H-score=120 was helpful in dividing the patients into two groups with adequate population in each group, for all three markers (DLL4: n= 65 in low group, n=34 in high group; VEGF: n=56 in low group, n=43 in high group; HIF2α: n= 65 in low group, n= 34 in high group). For survival analysis, however, with the same cutoff no statistically significant result was yielded, so that other cutoff values were adopted and utilized in this study. Considering that all researchers should report any meaningful data they obtained during the analysis, it was unavoidable to report the statistically significant results with multiple cutoffs.
- In a previous study that demonstrated DLL4 expression was a prognostic marker and predicted gemcitabine effect in pancreatic cancer [34], two cohorts of patients, total 154, were enrolled and their clinicopathologic and treatment data for at least 6 years were collect. When a larger number of patients with complete data for survival and post-operative treatment is available in our study, DLL4 expression is worth being re-evaluated for its prognostic significance in association with treatment effect like the study by Drouillard et al. [34]. Our study has other limitations. The study cohort only includes surgically resected cases. Although 25 cases were advanced diseases as to be staged surgically as IVB, including 11 cases with distant metastasis and 14 cases without distant metastasis but with N2 lymph node metastasis, the majority of this cohort was relatively early GBC cases. Because GBC is one of the lately detected cancers, most of the patients are subject to systemic treatment rather than surgical resection at the time of diagnosis. Our cohort, therefore, may not represent the whole GBC patients. Another limitation is that we could not evaluate the HIF2α expression digitally. Objectivity gained by quantitative digital image analysis for DLL4 and VEGF is one of the strengths of our study. However, due to the intensive background stain of HIF2α (Fig. 2), there was no available tool to annotate and evaluate the intensity of staining exactly. Although the consensus was made between two pathologists, manual evaluation might be relatively crude and subjective compared to digitized analysis.
- In conclusion, this study studied the expression patterns and levels of DLL4, VEGF, and HIF2α in surgically resected GBC. We demonstrated that VEGF and HIF2α expression intensity is positively correlated, suggesting the possibility for these markers to work as mutually substitutable markers. Low DLL4 and VEGF expression levels were significantly associated with the status of lymph node metastasis, presumably with prognosis, although such a result was not yielded in this study. Lastly, patients with lower HIF2α expression showed shorter RFS in our cohort. The cellular mechanisms of DLL4, VEGF, and HIF2α in GBC are worth further investigating to explain these results, to accelerate the application the target therapy for these molecules to treat GBC patients.
DISCUSSION
Ethics Statement
The institutional review board of Samsung Medical Center approved this study (2021-10-053) and waived informed consent.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author contributions
Conceptualization: KMK, KTJ. Data curation: SP. Formal analysis: SP, JK. Investigation: SP. Methodology: SP, KMK. Resources: KMK, KTJ. Software: SP, JK. Supervision: KTJ. Visualization: SP, JK. Writing—original draft: SP. Writing—review & editing: KTJ.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
| Total (n=99) |
DLL4 expression |
VEGF expression |
HIF2α expression |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (n=65, 65.7%) | High (n=34, 34.3%) | p-value | Low (n=56, 56.6%) | High (n=43, 43.4%) | p-value | Low (n=65, 65.7%) | High (n=34, 34.3%) | p-value | |||
| Age (yr) | .654 | .410 | .648 | ||||||||
| ≥ 60 | 67 (67.7) | 43 (64.2) | 24 (35.8) | 36 (53.7) | 31 (46.3) | 45 (67.2) | 22 (32.8) | ||||
| < 60 | 32 (32.3) | 22 (68.8) | 10 (31.3) | 20 (62.5) | 12 (37.5) | 20 (62.5) | 12 (37.5) | ||||
| Sex | .159 | .796 | .023 | ||||||||
| Female | 59 (59.6) | 42 (71.2) | 17 (28.8) | 34 (57.6) | 25 (42.4) | 44 (74.6) | 15 (25.4) | ||||
| Male | 40 (40.4) | 23 (57.5) | 17 (42.5) | 22 (55.0) | 18 (45.0) | 21 (52.5) | 19 (47.5) | ||||
| Diagnosis | NA | NA | NA | ||||||||
| Adenocarcinoma | 81 (81.8) | 48 (59.3) | 33 (40.7) | 51 (63.0) | 30 (37.0) | 52 (64.2) | 29 (35.8) | ||||
| Adenosquamous carcinoma | 8 (8.1) | 7 (87.5) | 1 (12.5) | 2 (25.0) | 6 (75.0) | 6 (75.0) | 2 (25.0) | ||||
| NEC | 5 (5.1) | 5 (100) | 0 | 1 (20.0) | 4 (80.0) | 4 (80.0) | 1 (20.0) | ||||
| Mixed NEC and adenocarcinoma | 1 (1.0) | 1 (100) | 0 | 0 | 1 (100) | 1 (100) | 0 | ||||
| Hepatoid adenocarcinoma | 1 (1.0) | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 | ||||
| Undifferentiated carcinoma | 3 (3.0) | 3 (100) | 0 | 1 (33.3) | 2 (66.7) | 1 (33.3) | 2 (66.7) | ||||
| Differentiationa | .068 | .028 | .006 | ||||||||
| WD, MD | 56 (56.6) | 33 (58.9) | 23 (41.1) | 37 (66.1) | 19 (33.9) | 43 (76.8) | 13 (23.2) | ||||
| PD | 40 (40.4) | 29 (72.5) | 11 (27.5) | 18 (45.0) | 22 (55.0) | 21 (52.5) | 19 (47.5) | ||||
| UD | 3 (3.0) | 3 (100) | 0 | 1 (33.3) | 2 (66.7) | 1 (33.3) | 2 (66.7) | ||||
| T categorya | .827 | .792 | .827 | ||||||||
| 1b, 2a, 2b | 5 (5.1) | 1 (20.0) | 4 (80.0) | 3 (60.0) | 2 (40.0) | 4 (80.0) | 1 (20.0) | ||||
| 3 | 88 (88.9) | 62 (70.5) | 26 (29.5) | 49 (55.7) | 39 (44.3) | 56 (63.6) | 32 (36.4) | ||||
| 4 | 6 (6.1) | 2 (33.3) | 4 (66.7) | 4 (66.7) | 2 (33.3) | 5 (83.3) | 1 (16.7) | ||||
| N category | .047 | .047 | .872 | ||||||||
| N0 | 31 (31.3) | 16 (51.6) | 15 (48.4) | 13 (41.9) | 18 (58.1) | 20 (64.5) | 11 (35.5) | ||||
| N1, N2 | 68 (68.7) | 49 (72.1) | 19 (27.9) | 43 (63.2) | 25 (36.8) | 45 (66.2) | 23 (33.8) | ||||
| M category | .410 | .616 | .410 | ||||||||
| M0 | 88 (88.9) | 59 (67.0) | 29 (33.0) | 49 (55.7) | 39 (44.3) | 59 (67.0) | 29 (33.0) | ||||
| M1 | 11 (11.1) | 6 (54.5) | 5 (45.5) | 7 (63.6) | 4 (36.4) | 6 (54.5) | 5 (45.5) | ||||
| AJCC stage | .112 | .155 | .772 | ||||||||
| I–III | 71 (71.7) | 50 (70.4) | 21 (29.6) | 37 (52.1) | 34 (47.9) | 46 (64.8) | 25 (35.2) | ||||
| IV | 28 (28.3) | 15(53.6) | 13 (46.4) | 19 (67.9) | 9 (32.1) | 19 (67.9) | 9 (32.1) | ||||
| Recurrence | .610 | .690 | .174 | ||||||||
| Yes | 46 (46.5) | 29 (63.0) | 17 (37.0) | 27 (58.7) | 19 (41.3) | 27 (58.7) | 19 (41.3) | ||||
| No | 53 (53.5) | 36 (67.9) | 17 (32.1) | 29 (54.7) | 24 (45.3) | 38 (71.7) | 15 (28.3) | ||||
| Death | .873 | .789 | .549 | ||||||||
| Yes | 63 (63.6) | 41 (65.1) | 22 (34.9) | 35 (55.6) | 28 (44.4) | 40 (63.5) | 23 (36.5) | ||||
| No | 36 (36.4) | 24 (66.7) | 12 (33.3) | 21 (58.3) | 15 (41.7) | 25 (69.4) | 11 (30.6) | ||||
| DLL4 expression | .340 | .054 | |||||||||
| Low | 65 (65.7) | 39 (60.0) | 26 (40.0) | 47 (72.3) | 18 (27.7) | ||||||
| High | 34 (34.3) | 17 (50.0) | 17 (50.0) | 18 (52.9) | 16 (47.1) | ||||||
| VEGF expression | .340 | <.001 | |||||||||
| Low | 56 (56.6) | 39 (69.6) | 17 (30.4) | 49 (87.5) | 7 (12.5) | ||||||
| High | 43 (43.4) | 26 (60.5) | 17 (39.5) | 16 (37.2) | 27 (62.8) | ||||||
| HIF2α expression | .054 | < .001 | |||||||||
| Low | 34 (34.3) | 47 (72.3) | 18 (27.7) | 49 (75.4) | 16 (24.6) | ||||||
| High | 65 (65.7) | 18 (52.9) | 16 (47.1) | 7 (20.6) | 27 (79.4) | ||||||
Values are presented as number (%).
DLL4, delta-like ligand 4; VEGF, vascular endothelial growth factor; HIF2α, hypoxia-inducible factor-2α; NEC, neuroendocrine carcinoma; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; UD, undifferentiated; AJCC, American Joint Committee on Cancer.
aBy Cochran-Armitage trend test, otherwise by chi-square test.
- 1. Jung KW, Won YJ, Hong S, Kong HJ, Im JS, Seo HG. Prediction of cancer incidence and mortality in Korea, 2021. Cancer Res Treat 2021; 53: 316-22. ArticlePubMedPMCPDF
- 2. Wi Y, Woo H, Won YJ, Jang JY, Shin A. Trends in gallbladder cancer incidence and survival in Korea. Cancer Res Treat 2018; 50: 1444-51. ArticlePubMedPMCPDF
- 3. Ministry of Health and Welfare; Korea Central Cancer Registry; National Cancer Center. Annual report of cancer statistics in Korea in 2018. Sejong: Ministry of Health and Welfare, 2020.
- 4. Tajima H, Ohta T, Shinbashi H, et al. Successful treatment of unresectable gallbladder cancer with low-dose paclitaxel as palliative chemotherapy after failure of gemcitabine and oral S-1: a case report. Oncol Lett 2012; 4: 1281-4. ArticlePubMedPMC
- 5. Mishra SK, Kumari N, Krishnani N. Molecular pathogenesis of gallbladder cancer: an update. Mutat Res 2019; 816-818: 111674.ArticlePubMed
- 6. Neyaz A, Husain N, Gupta S, et al. Investigation of targetable predictive and prognostic markers in gallbladder carcinoma. J Gastrointest Oncol 2018; 9: 111-25. ArticlePubMedPMC
- 7. D’Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene 2008; 27: 5148-67. ArticlePubMedPMCPDF
- 8. Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer 2011; 11: 338-51. ArticlePubMedPDF
- 9. Lobov IB, Renard RA, Papadopoulos N, et al. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A 2007; 104: 3219-24. ArticlePubMedPMC
- 10. Li JL, Sainson RC, Shi W, et al. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res 2007; 67: 11244-53. ArticlePubMedPDF
- 11. Kim Y, Byeon SJ, Hur J, et al. High delta-like ligand 4 expression correlates with a poor clinical outcome in gastric cancer. J Cancer 2019; 10: 3172-8. ArticlePubMedPMC
- 12. Chen HT, Cai QC, Zheng JM, et al. High expression of delta-like ligand 4 predicts poor prognosis after curative resection for pancreatic cancer. Ann Surg Oncol 2012; 19 Suppl 3: S464-74. ArticlePubMedPDF
- 13. Kontomanolis E, Panteliadou M, Giatromanolaki A, et al. Delta-like ligand 4 (DLL4) in the plasma and neoplastic tissues from breast cancer patients: correlation with metastasis. Med Oncol 2014; 31: 945.ArticlePubMedPDF
- 14. Xiao M, Yang S, Ning X, Huang Y. Aberrant expression of delta-like ligand 4 contributes significantly to axillary lymph node metastasis and predicts postoperative outcome in breast cancer. Hum Pathol 2014; 45: 2302-10. PubMed
- 15. Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL. High DLL4 expression in tumour-associated vessels predicts for favorable radiotherapy outcome in locally advanced squamous cell head-neck cancer (HNSCC). Angiogenesis 2013; 16: 343-51. ArticlePubMedPDF
- 16. Jimeno A, Moore KN, Gordon M, et al. A first-in-human phase 1a study of the bispecific anti-DLL4/anti-VEGF antibody navicixizumab (OMP-305B83) in patients with previously treated solid tumors. Invest New Drugs 2019; 37: 461-72. ArticlePubMedPDF
- 17. Katoh M, Katoh M. Precision medicine for human cancers with Notch signaling dysregulation (Review). Int J Mol Med 2020; 45: 279-97. ArticlePubMed
- 18. Mutvei AP, Landor SK, Fox R, et al. Notch signaling promotes a HIF2alpha-driven hypoxic response in multiple tumor cell types. Oncogene 2018; 37: 6083-95. ArticlePubMedPMCPDF
- 19. Moreno Roig E, Yaromina A, Houben R, Groot AJ, Dubois L, Vooijs M. Prognostic role of hypoxia-inducible factor-2alpha tumor cell expression in cancer patients: a meta-analysis. Front Oncol 2018; 8: 224.PubMedPMC
- 20. Wierzbicki PM, Klacz J, Kotulak-Chrzaszcz A, et al. Prognostic significance of VHL, HIF1A, HIF2A, VEGFA and p53 expression in patients with clear‑cell renal cell carcinoma treated with sunitinib as first‑line treatment. Int J Oncol 2019; 55: 371-90. ArticlePubMedPMC
- 21. Choueiri TK, Kaelin WG Jr. Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat Med 2020; 26: 1519-30. ArticlePubMedPDF
- 22. Amin MB, Edge SB, Greene FL, et al. AJCC cancer staging manual. 8th. New York: Springer, 2017.
- 23. Giatromanolaki A, Sivridis E, Simopoulos C, et al. Hypoxia inducible factors 1alpha and 2alpha are associated with VEGF expression and angiogenesis in gallbladder carcinomas. J Surg Oncol 2006; 94: 242-7. ArticlePubMed
- 24. Giatromanolaki A, Koukourakis MI, Simopoulos C, Polychronidis A, Sivridis E. Vascular endothelial growth factor (VEGF) expression in operable gallbladder carcinomas. Eur J Surg Oncol 2003; 29: 879-83. ArticlePubMed
- 25. Letelier P, Garcia P, Leal P, et al. Immunohistochemical expression of vascular endothelial growth factor A in advanced gallbladder carcinoma. Appl Immunohistochem Mol Morphol 2014; 22: 530-6. ArticlePubMed
- 26. Luo Y, Yang ZL, Wang C, et al. The clinicopathological significance of Jagged1 and DLL4 in gallbladder cancer. Tumori 2017; 103: 557-65. ArticlePubMedPDF
- 27. Xu D, Li J, Jiang F, Cai K, Ren G. The effect and mechanism of vascular endothelial growth factor (VEGF) on tumor angiogenesis in gallbladder carcinoma. Iran J Public Health 2019; 48: 713-21. ArticlePubMedPMCPDF
- 28. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 2004; 3: 391-400. ArticlePubMedPDF
- 29. Hoefflin R, Harlander S, Schafer S, et al. HIF-1alpha and HIF-2alpha differently regulate tumour development and inflammation of clear cell renal cell carcinoma in mice. Nat Commun 2020; 11: 4111.PubMedPMC
- 30. Choueiri TK, Bauer TM, Papadopoulos KP, et al. Inhibition of hypoxia-inducible factor-2alpha in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis. Nat Med 2021; 27: 802-5. ArticlePubMedPMCPDF
- 31. Wallace EM, Rizzi JP, Han G, et al. A small-molecule antagonist of HIF2alpha is efficacious in preclinical models of renal cell carcinoma. Cancer Res 2016; 76: 5491-500. PubMed
- 32. Liu H, Peng J, Zhao M, et al. Downregulation of DLL4 predicts poor survival in non‑small cell lung cancer patients due to promotion of lymph node metastasis. Oncol Rep 2018; 40: 2988-96. ArticlePubMed
- 33. Yang S, Liu Y, Xia B, et al. DLL4 as a predictor of pelvic lymph node metastasis and a novel prognostic biomarker in patients with early-stage cervical cancer. Tumour Biol 2016; 37: 5063-74. ArticlePubMedPDF
- 34. Drouillard A, Puleo F, Bachet JB, et al. DLL4 expression is a prognostic marker and may predict gemcitabine benefit in resected pancreatic cancer. Br J Cancer 2016; 115: 1245-52. ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations

- Dedifferentiated Leiomyosarcoma of the Uterine Corpus with Heterologous Component: Clinicopathological Analysis of Five Consecutive Cases from a Single Institution and Comprehensive Literature Review
Suyeon Kim, Hyunsik Bae, Hyun-Soo Kim
Diagnostics.2024; 14(2): 160. CrossRef - Identification of Key Immune Infiltration Related Genes Involved in Aortic Dissection Using Bioinformatic Analyses and Experimental Verification
Lin Zheng, Yusi Yang, Jie Liu, Tianliang Zhao, Xin Zhang, Lihua Chen
Journal of Inflammation Research.2024; Volume 17: 2119. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
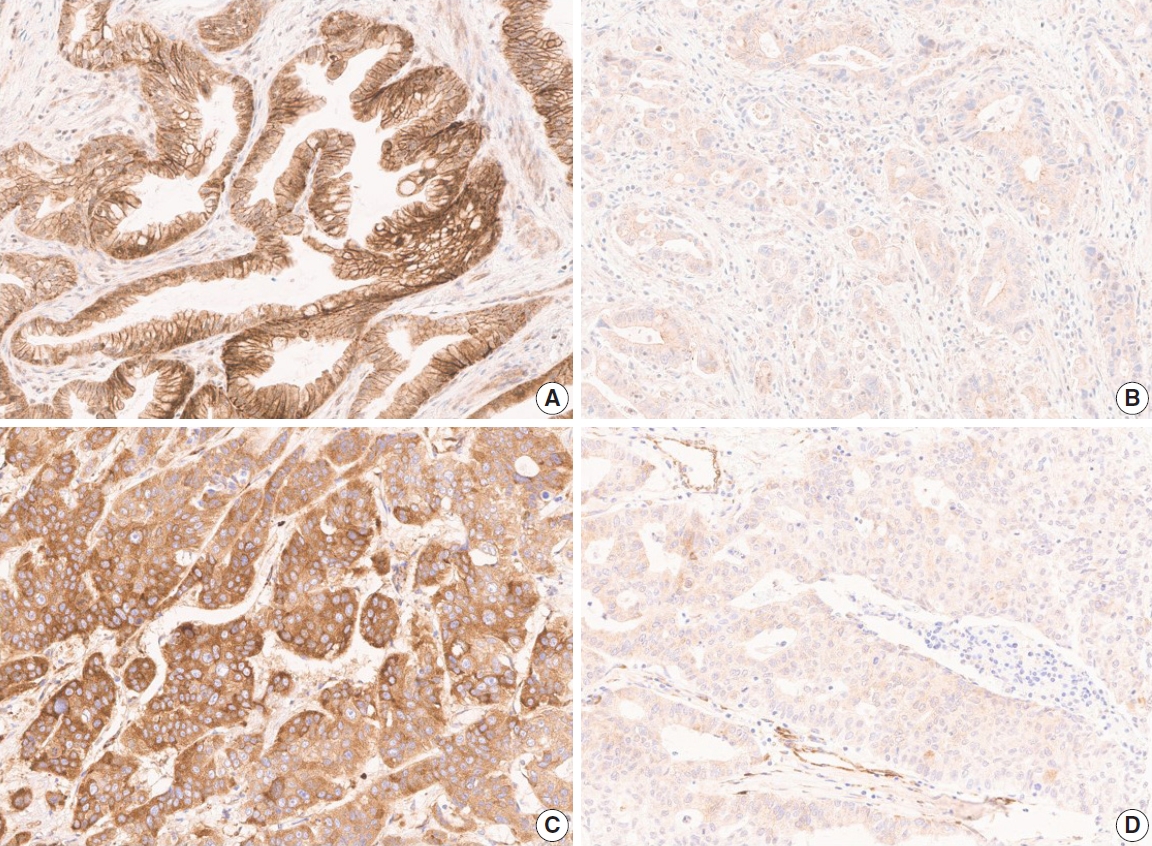
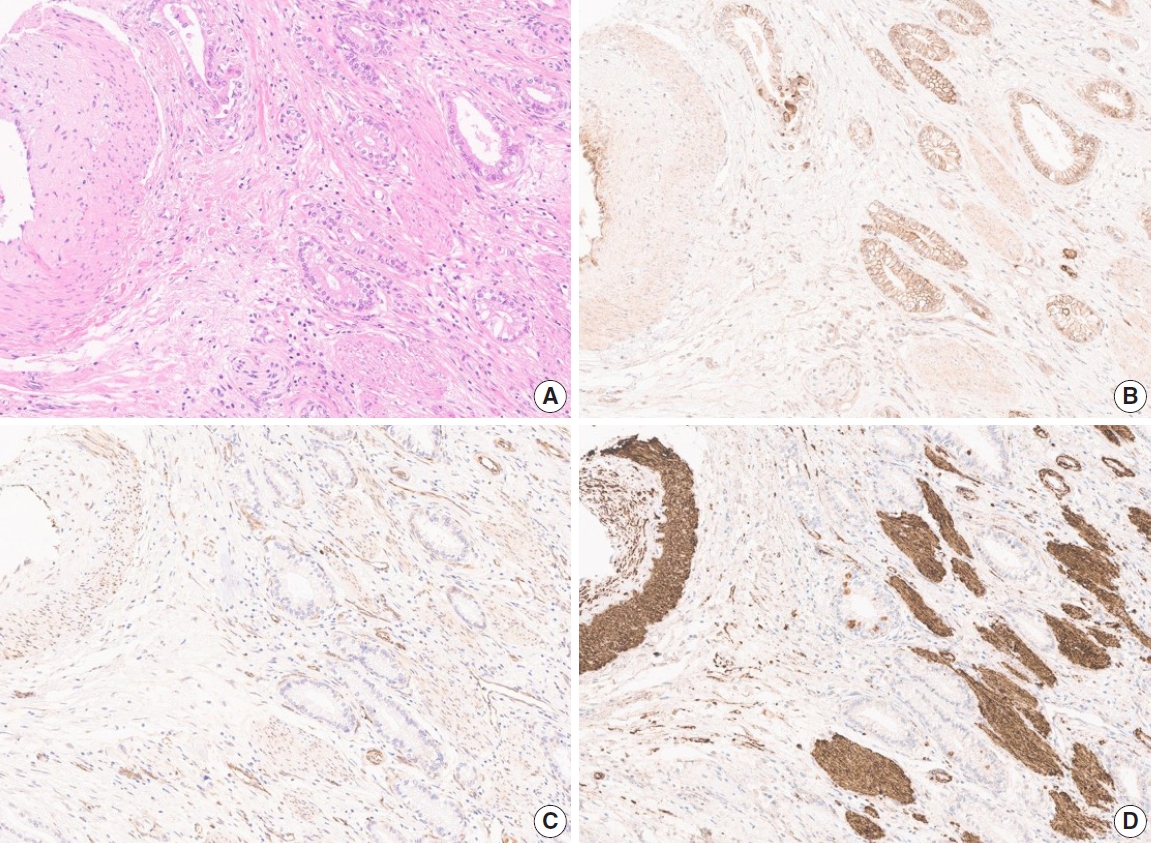
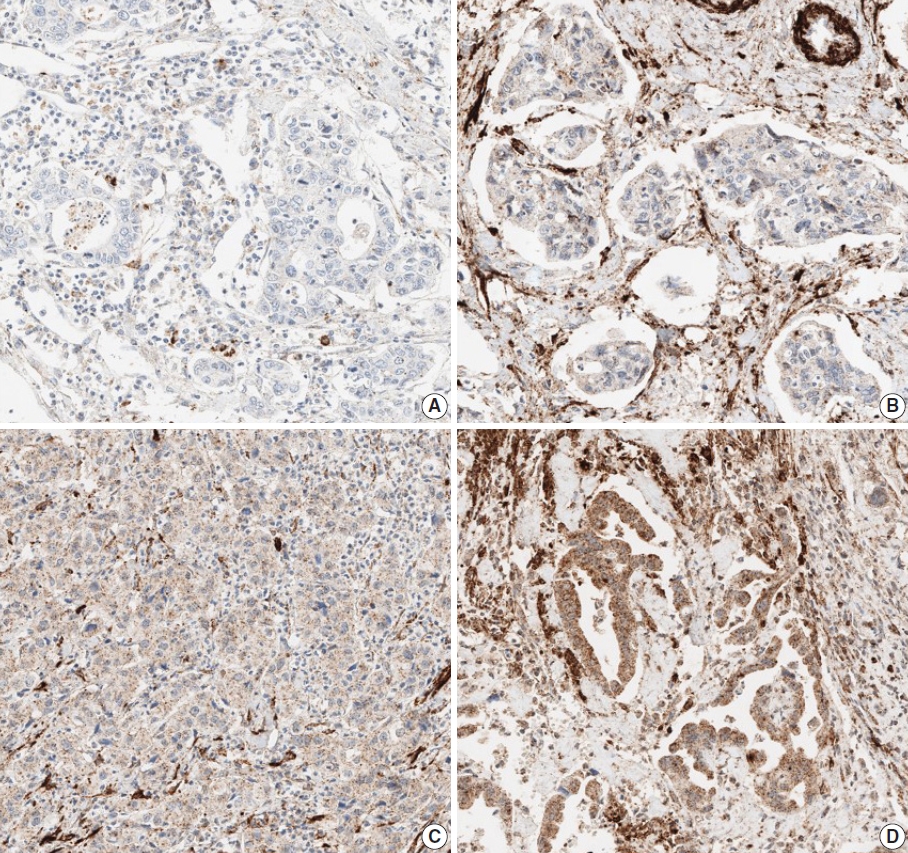
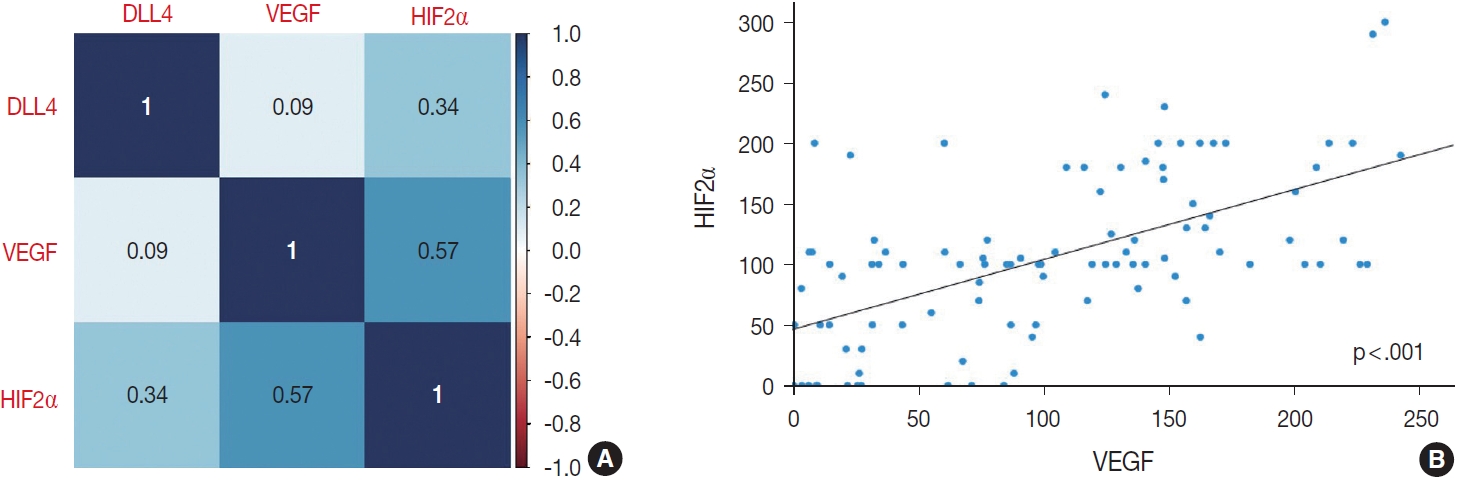
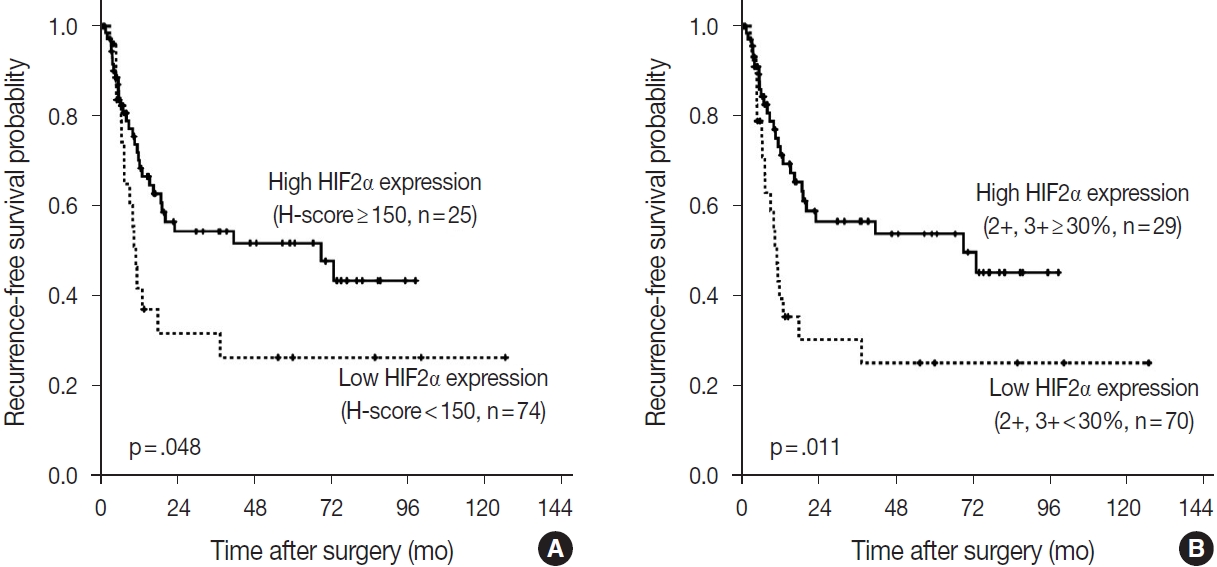
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
| Total (n=99) | DLL4 expression |
VEGF expression |
HIF2α expression |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (n=65, 65.7%) | High (n=34, 34.3%) | p-value | Low (n=56, 56.6%) | High (n=43, 43.4%) | p-value | Low (n=65, 65.7%) | High (n=34, 34.3%) | p-value | |||
| Age (yr) | .654 | .410 | .648 | ||||||||
| ≥ 60 | 67 (67.7) | 43 (64.2) | 24 (35.8) | 36 (53.7) | 31 (46.3) | 45 (67.2) | 22 (32.8) | ||||
| < 60 | 32 (32.3) | 22 (68.8) | 10 (31.3) | 20 (62.5) | 12 (37.5) | 20 (62.5) | 12 (37.5) | ||||
| Sex | .159 | .796 | .023 | ||||||||
| Female | 59 (59.6) | 42 (71.2) | 17 (28.8) | 34 (57.6) | 25 (42.4) | 44 (74.6) | 15 (25.4) | ||||
| Male | 40 (40.4) | 23 (57.5) | 17 (42.5) | 22 (55.0) | 18 (45.0) | 21 (52.5) | 19 (47.5) | ||||
| Diagnosis | NA | NA | NA | ||||||||
| Adenocarcinoma | 81 (81.8) | 48 (59.3) | 33 (40.7) | 51 (63.0) | 30 (37.0) | 52 (64.2) | 29 (35.8) | ||||
| Adenosquamous carcinoma | 8 (8.1) | 7 (87.5) | 1 (12.5) | 2 (25.0) | 6 (75.0) | 6 (75.0) | 2 (25.0) | ||||
| NEC | 5 (5.1) | 5 (100) | 0 | 1 (20.0) | 4 (80.0) | 4 (80.0) | 1 (20.0) | ||||
| Mixed NEC and adenocarcinoma | 1 (1.0) | 1 (100) | 0 | 0 | 1 (100) | 1 (100) | 0 | ||||
| Hepatoid adenocarcinoma | 1 (1.0) | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 | ||||
| Undifferentiated carcinoma | 3 (3.0) | 3 (100) | 0 | 1 (33.3) | 2 (66.7) | 1 (33.3) | 2 (66.7) | ||||
| Differentiation |
.068 | .028 | .006 | ||||||||
| WD, MD | 56 (56.6) | 33 (58.9) | 23 (41.1) | 37 (66.1) | 19 (33.9) | 43 (76.8) | 13 (23.2) | ||||
| PD | 40 (40.4) | 29 (72.5) | 11 (27.5) | 18 (45.0) | 22 (55.0) | 21 (52.5) | 19 (47.5) | ||||
| UD | 3 (3.0) | 3 (100) | 0 | 1 (33.3) | 2 (66.7) | 1 (33.3) | 2 (66.7) | ||||
| T category |
.827 | .792 | .827 | ||||||||
| 1b, 2a, 2b | 5 (5.1) | 1 (20.0) | 4 (80.0) | 3 (60.0) | 2 (40.0) | 4 (80.0) | 1 (20.0) | ||||
| 3 | 88 (88.9) | 62 (70.5) | 26 (29.5) | 49 (55.7) | 39 (44.3) | 56 (63.6) | 32 (36.4) | ||||
| 4 | 6 (6.1) | 2 (33.3) | 4 (66.7) | 4 (66.7) | 2 (33.3) | 5 (83.3) | 1 (16.7) | ||||
| N category | .047 | .047 | .872 | ||||||||
| N0 | 31 (31.3) | 16 (51.6) | 15 (48.4) | 13 (41.9) | 18 (58.1) | 20 (64.5) | 11 (35.5) | ||||
| N1, N2 | 68 (68.7) | 49 (72.1) | 19 (27.9) | 43 (63.2) | 25 (36.8) | 45 (66.2) | 23 (33.8) | ||||
| M category | .410 | .616 | .410 | ||||||||
| M0 | 88 (88.9) | 59 (67.0) | 29 (33.0) | 49 (55.7) | 39 (44.3) | 59 (67.0) | 29 (33.0) | ||||
| M1 | 11 (11.1) | 6 (54.5) | 5 (45.5) | 7 (63.6) | 4 (36.4) | 6 (54.5) | 5 (45.5) | ||||
| AJCC stage | .112 | .155 | .772 | ||||||||
| I–III | 71 (71.7) | 50 (70.4) | 21 (29.6) | 37 (52.1) | 34 (47.9) | 46 (64.8) | 25 (35.2) | ||||
| IV | 28 (28.3) | 15(53.6) | 13 (46.4) | 19 (67.9) | 9 (32.1) | 19 (67.9) | 9 (32.1) | ||||
| Recurrence | .610 | .690 | .174 | ||||||||
| Yes | 46 (46.5) | 29 (63.0) | 17 (37.0) | 27 (58.7) | 19 (41.3) | 27 (58.7) | 19 (41.3) | ||||
| No | 53 (53.5) | 36 (67.9) | 17 (32.1) | 29 (54.7) | 24 (45.3) | 38 (71.7) | 15 (28.3) | ||||
| Death | .873 | .789 | .549 | ||||||||
| Yes | 63 (63.6) | 41 (65.1) | 22 (34.9) | 35 (55.6) | 28 (44.4) | 40 (63.5) | 23 (36.5) | ||||
| No | 36 (36.4) | 24 (66.7) | 12 (33.3) | 21 (58.3) | 15 (41.7) | 25 (69.4) | 11 (30.6) | ||||
| DLL4 expression | .340 | .054 | |||||||||
| Low | 65 (65.7) | 39 (60.0) | 26 (40.0) | 47 (72.3) | 18 (27.7) | ||||||
| High | 34 (34.3) | 17 (50.0) | 17 (50.0) | 18 (52.9) | 16 (47.1) | ||||||
| VEGF expression | .340 | <.001 | |||||||||
| Low | 56 (56.6) | 39 (69.6) | 17 (30.4) | 49 (87.5) | 7 (12.5) | ||||||
| High | 43 (43.4) | 26 (60.5) | 17 (39.5) | 16 (37.2) | 27 (62.8) | ||||||
| HIF2α expression | .054 | < .001 | |||||||||
| Low | 34 (34.3) | 47 (72.3) | 18 (27.7) | 49 (75.4) | 16 (24.6) | ||||||
| High | 65 (65.7) | 18 (52.9) | 16 (47.1) | 7 (20.6) | 27 (79.4) | ||||||
Values are presented as number (%). DLL4, delta-like ligand 4; VEGF, vascular endothelial growth factor; HIF2α, hypoxia-inducible factor-2α; NEC, neuroendocrine carcinoma; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; UD, undifferentiated; AJCC, American Joint Committee on Cancer. By Cochran-Armitage trend test, otherwise by chi-square test.

 E-submission
E-submission







