Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 56(5); 2022 > Article
-
Original Article
Special AT-rich sequence-binding protein 2 (SATB2) in the differential diagnosis of osteogenic and non-osteogenic bone and soft tissue tumors -
Sharon Milton1
 , Anne Jennifer Prabhu1
, Anne Jennifer Prabhu1 , V. T. K. Titus2
, V. T. K. Titus2 , Rikki John3
, Rikki John3 , Selvamani Backianathan4
, Selvamani Backianathan4 , Vrisha Madhuri5
, Vrisha Madhuri5
-
Journal of Pathology and Translational Medicine 2022;56(5):270-280.
DOI: https://doi.org/10.4132/jptm.2022.07.11
Published online: September 13, 2022
1Department of Pathology, Christian Medical College Vellore, Tamil Nadu, India
2Department of Orthopaedics, Christian Medical College Vellore, Tamil Nadu, India
3Department of Paediatric Oncology and Haematology, Christian Medical College Vellore, Tamil Nadu, India
4Department of Radiotherapy, Christian Medical College Vellore, Tamil Nadu, India
5Department of Paediatric Orthopaedics, Christian Medical College Vellore, Tamil Nadu, India
- Corresponding Author: Anne Jennifer Prabhu, MD, Department of Pathology, Christian Medical College Vellore, 632004, Tamil Nadu, India, Tel: +91-9488780525, Fax: +91-04162282005, E-mail: annejennifer91@gmail.com
© 2022 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- The diagnosis of osteosarcoma (OSA) depends on clinicopathological and radiological correlation. A biopsy is considered the gold standard for OSA diagnosis. However, since OSA is a great histological mimicker, diagnostic challenges exist. Immunohistochemistry (IHC) can serve as an adjunct for the histological diagnosis of OSA. Special AT-rich sequence-binding protein 2 (SATB2) was recently described as a reliable adjunct immunohistochemical marker for the diagnosis of OSA.
-
Methods
- We investigated the IHC expression of SATB2 in 95 OSA and 100 non-osteogenic bone and soft tissue tumors using a monoclonal antibody (clone EPNCIR30A). The diagnostic utility of SATB2 and correlation with clinicopathological parameters were analyzed.
-
Results
- SATB2 IHC was positive in 88 out of 95 cases (92.6%) of OSA and 50 out of 100 cases (50.0%) of primary non-osteogenic bone and soft tissue tumors. Of the 59 bone tumors, 37 cases (62.7%) were positive for SATB2, and of the 41 soft tissue tumors, 13 cases (31.7%) were positive for SATB2. The sensitivity of SATB2 as a diagnostic test was 92.6%, specificity 50%, positive predictive value 63.8%, and negative predictive value 87.7%.
-
Conclusions
- Although SATB2 is a useful diagnostic marker for OSA, other clinical, histological and immunohistochemical features should be considered for the interpretation of SATB2.
- This study is retrospective and was carried out in the Department of Pathology, Christian Medical College Vellore, Tamil Nadu, India. The study used archived stained and mounted hematoxylin and eosin (H&E) slides and formalin-fixed paraffin-embedded (FFPE) blocks of all small biopsies diagnosed as OSA, before chemotherapy, and selected cases diagnosed as non-osteogenic soft tissue and bone tumors on small biopsies and resection specimens, over 2 years.
- Ultimately, 95 cases of OSA and 100 cases of non-osteogenic soft tissue and bone tumors were included. Of the 95 cases of OSA, eight cases (8.4%) had undergone decalcification, 38 cases (40.0%) had undergone mild decalcification and 49 (52%) did not undergo decalcification at the time of processing. Of the 100 non-osteogenic tumors, 18 cases (18.0%) had undergone decalcification, 17 cases (17.0%) had undergone mild decalcification and 65 (65.0%) did not undergo decalcification at the time of processing.
- Of the 100 cases in the non-osteogenic tumors group (Table 1), 59 were bone tumors, and 41 were soft tissue tumors.
- Relevant clinical and radiological data were retrieved from electronic medical records and pathology reports and comprised age, sex, radiological location, radiological tumor size, staging (Enneking staging), metastasis, history of radiation exposure, or Paget’s disease. The elevated serum levels of alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) were noted when available.
- The slides were reviewed for histology and grading by two pathologists (A.J.P. and S.M.). The 8th edition of the American Joint Committee on Cancer and International Union Against Cancer staging system for bone tumors includes a 3-grade system but effectively collapses into high grade and low grade. Conventional OSA is considered grade 3 (high-grade) sarcoma. Since our assessment of the grade of the tumor was on small biopsy specimens, those cases in which the degree of atypia was insufficient for the diagnosis of grade 3 (high grade) were classified as grade 2 (intermediate grade). However, for treatment purposes, the tumors included in the intermediate histological grade were considered as a high grade for biological grading.
- A representative FFPE block from each specimen was chosen for analysis by IHC after H&E examination. The primary SATB2 antibody (rabbit monoclonal antibody, clone EPNCIR30A, Abcam, Cambridge, UK) was used in all cases with a dilution of 1:100. Colonic epithelium served as the positive control. IHC assays were performed on a VENTANA BenchMark XT autostainer (Tucson, AZ, USA) according to the manufacturer’s instructions. We analyzed SATB2 expression on the entire 4 μm tissue sections of the representative FFPE block.
- SATB2 expression was assessed in neoplastic cells. Neoplastic cells of OSA are spindle to polygonal with moderate to markedly pleomorphic nuclei and conspicuous nucleoli. Nuclear staining in the tumor cells was considered positive staining. Nuclear staining of osteoblasts and osteocytes was not considered positive. Faint non-specific staining of stromal cells was also considered negative.
- In the non-osteogenic tumors, immunoreactivity was interpreted as positive or negative based on the nuclear staining in the neoplastic cells of that tumor.
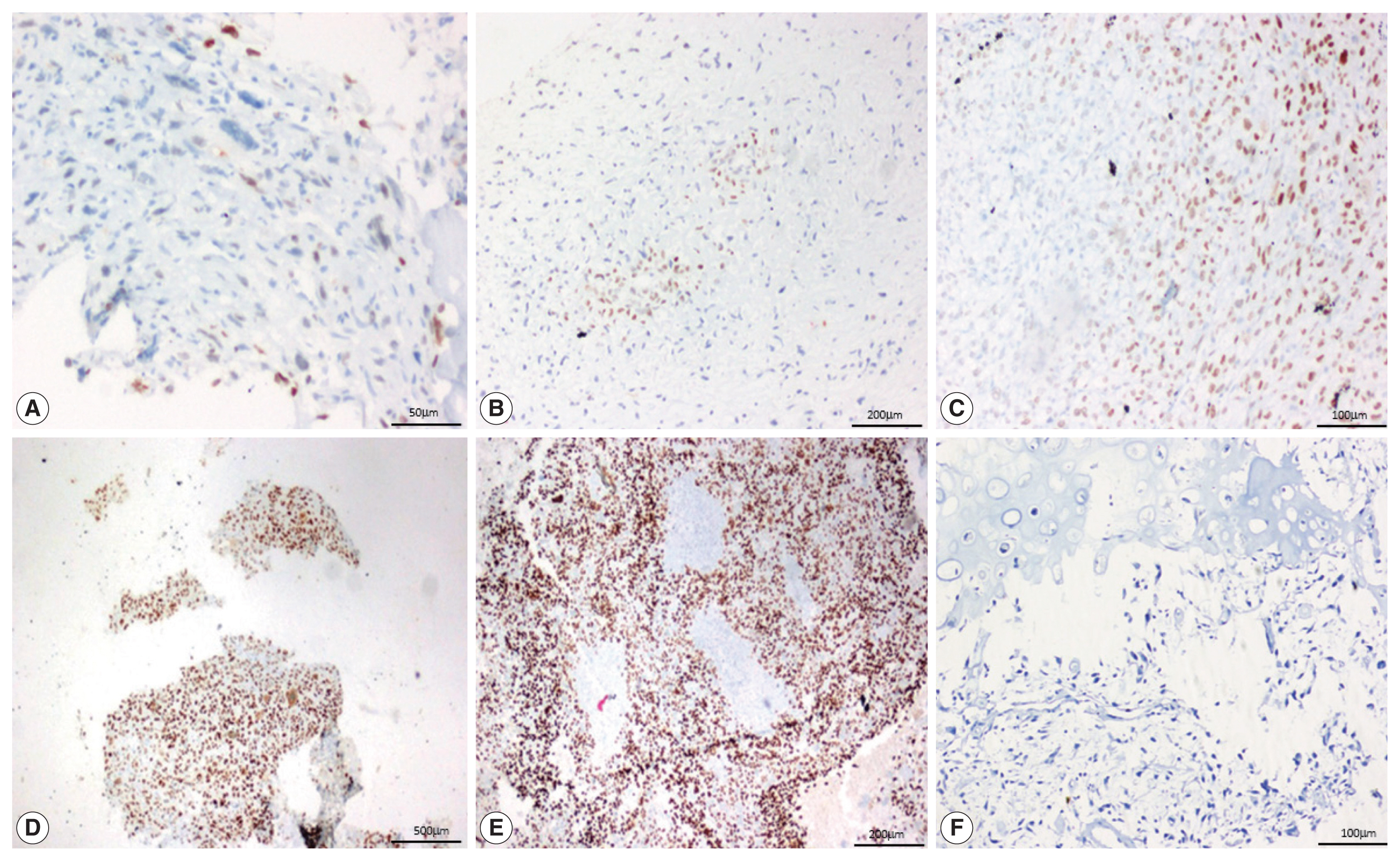
- SATB2 expression proportion score was assessed as the percentage of positive nuclear expression on tumor cells. SATB2 proportion score was graded as 0, no staining; 1+, < 5%; 2+, 5%–25%; 3+, 26%–50%, 4+, 51%–75%; and 5+, 76%–100% [8] and staining intensity was recorded as weak, moderate and strong (Fig 1). For the purpose of analysis, cases with no SATB2 staining (proportion score 0) were considered SATB2 negative and the cases with any proportion of SATB2 staining (proportion scores 1+ to 5+) were considered SATB2 positive. The staining intensities were also observed and recorded in all the cases, with no definite intensity cutoff [10].
- The study data were summarized using descriptive statistics. Data was entered using EpiData ver. 3.1 and statistical analysis was carried out using SPSS software ver. 17 (SPSS Inc., Chicago, IL, USA). In all the statistical analyses a p-value of <.05 was considered to be statistically significant. The sensitivity, specificity, positive predictive value, and negative predictive value were calculated.
MATERIALS AND METHODS
- The study group included 95 OSA cases and 100 non-osteogenic primary bone and soft tissue tumors. In the OSA cases, there was a male predominance (75.8%), the mean was 18.0 years (range, 6 to 65 years), and 58% were under the age of 20 years. The most common site was the femur (49.5%) followed by the tibia (28.4%). Radiologically, the most common location was meta-diaphyseal (62.1%) with a mean size of 12 cm, and the majority were Enneking staging IIB (61%). Of the 95 cases, 29 had metastasis at presentation. Two cases had a history of Paget’s disease, 42% of cases had elevated ALP levels, and 20% had elevated LDH levels.
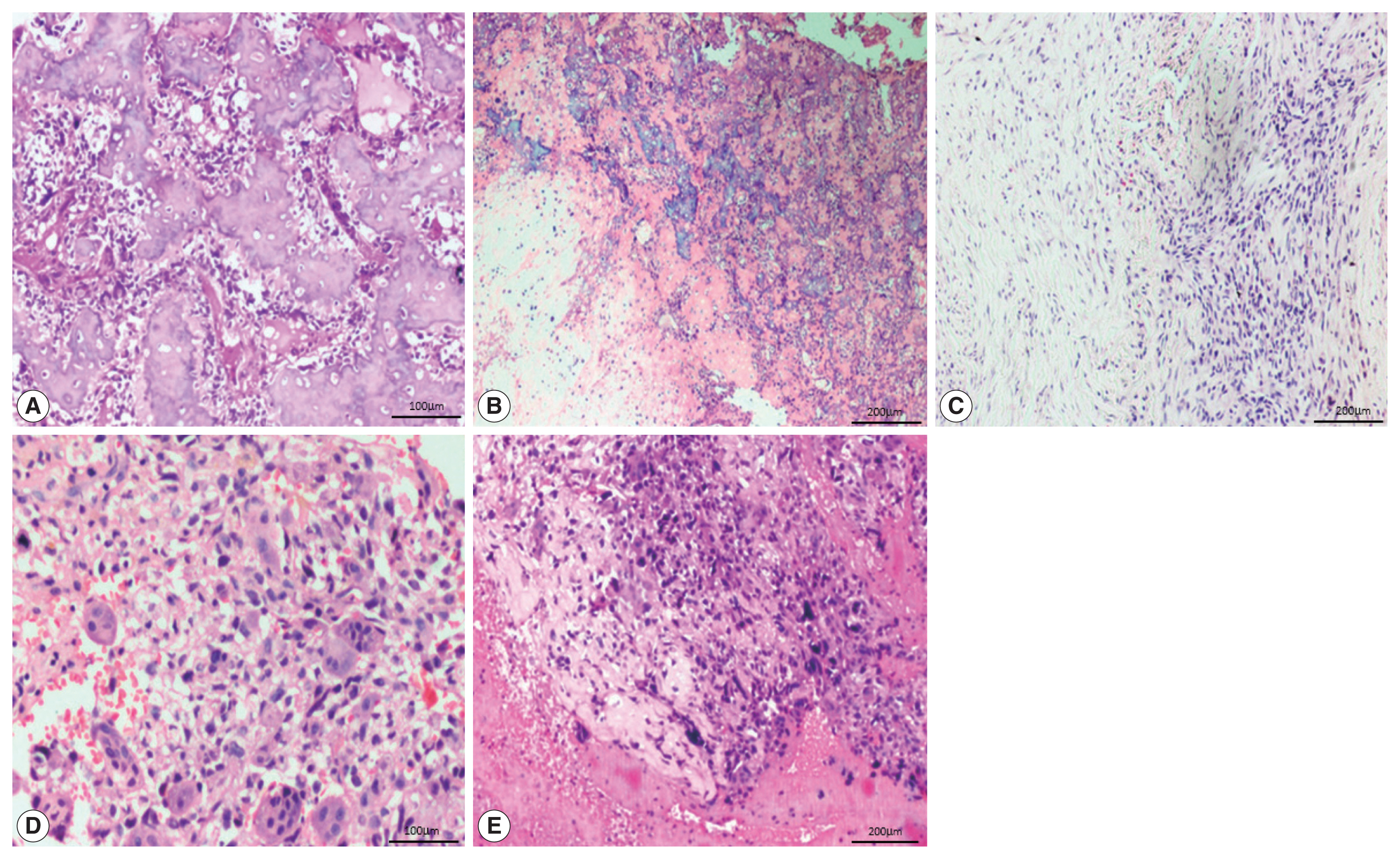
- The most common histological subtype (Fig. 2) observed in our study was osteoblastic (64.2%) followed by chondroblastic (30.5%). Other subtypes were giant cell-rich (2.1%), fibroblastic (2.1%), and telangiectatic (1.1%). The most common cell morphology observed was spindle cells, along with polygonal and bizarre cells (30.5%). The presence of osteoid was found in 88.4% of the OSA cases, and 78.9% of cases had high-grade morphology.
- SATB2 expression in OSA cases
- Of the 95 cases of OSA, 88 (92.6%) showed nuclear positivity for SATB2 in the neoplastic cells with a median staining proportion score of 4+ (Table 2). These 88 cases included 57 osteoblastic cases, 26 chondroblastic cases, both of the fibroblastic and giant cell-rich cases, and one telangiectatic case.
- In 27/95 of the OSA cases, the staining was 4+. On further analysis, 20/61 cases of the osteoblastic subtype and 7/29 cases of the chondroblastic subtype showed SATB2 positivity in osteoblastic/fibroblastic differentiation and showed limited staining in chondroblastic differentiation. Two fibroblastic OSA, one case of telangiectatic OSA and two cases of giant cell-rich OSA showed SATB2 positive staining in the tumor cells but were negative in the osteoclast-type giant cells.
- On analyzing the intensity of the staining, a strong intensity of SATB2 staining was seen in 45 (51.1%) OSA cases (Table 3), comprising 29 osteoblastic cases, 12 chondroblastic cases, one fibroblastic case, both giant cell-rich cases, and the one telangiectatic case. However, interpretation of intensity is too subjective to be used in routine practice with a definite cutoff.
- The clinical, biochemical, radiological, and histological parameters did not correlate with the SATB2 expression indicated by IHC, as depicted in Table 4.
- SATB2 expression in non-osteogenic tumors
- Of the 59 bone tumors, 37 cases (62.7%) were positive and 22 cases (37.3%) were negative for SATB2, and of the 41 soft tissue tumors, 13 cases (31.7%) were positive and 28 cases (68.3%) were negative for SATB2. The proportion and intensity of SATB2 in non-osteogenic tumors are detailed in Tables 5 and 6.
- Non-osteogenic bone tumors
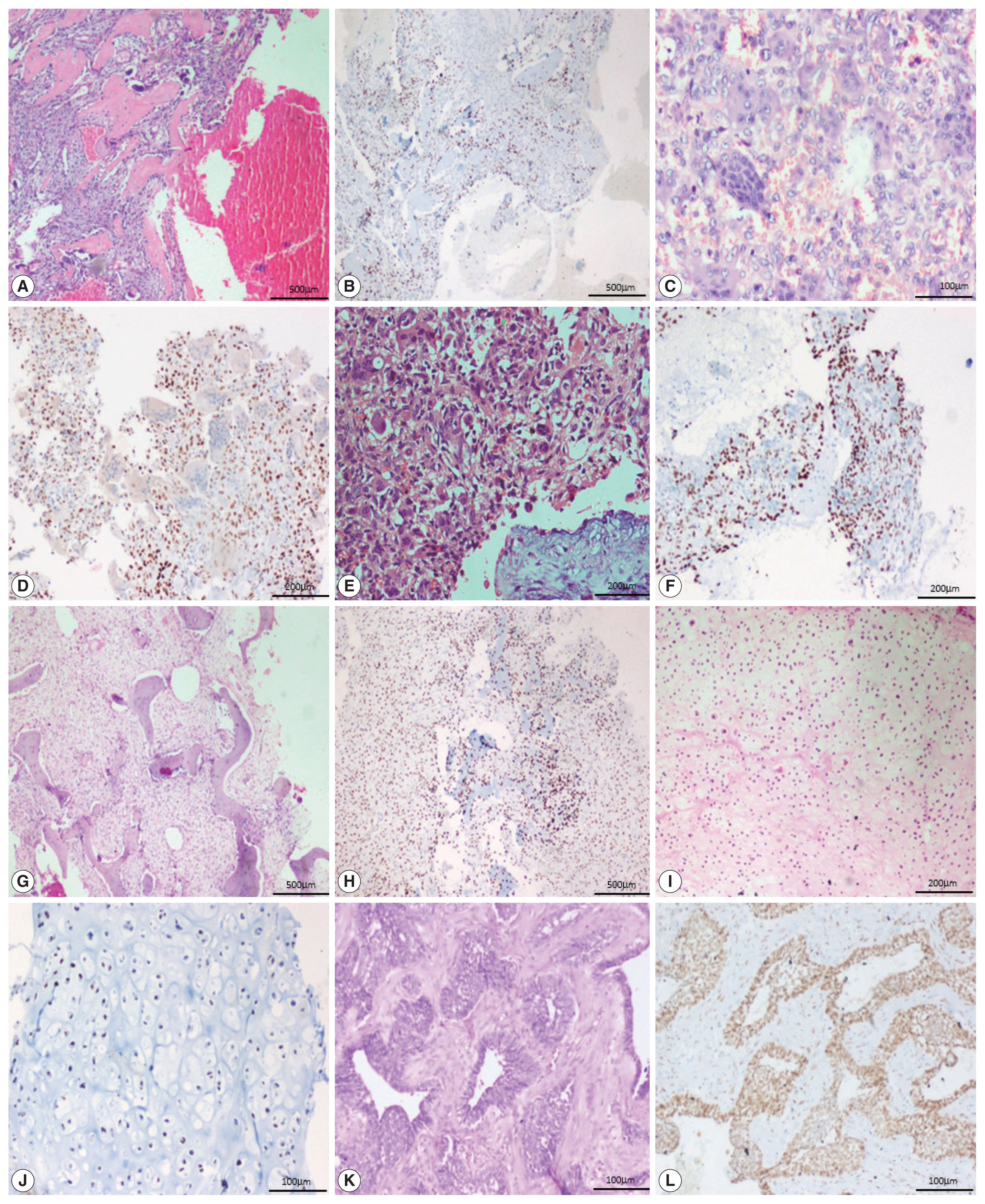
- All 13 cases of giant cell tumor (GCT) were positive for SATB2 in the neoplastic mononuclear cells and all four cases of fibrous dysplasia were positive for SATB2 in the bland fibroblastic spindle cells. Thirteen out of 18 cases of aneurysmal bone cyst (ABC) were positive for SATB2 in the bland spindle-shaped cells. Four out of five cases of chondroblastoma were positive for SATB2 in the chondroblasts. Two out of eight cases of chondrosarcoma showed positive staining in the neoplastic chondrocytes. One case of adamantinoma was negative and one case showed positive staining in both mesenchymal and epithelial components. All six cases of Ewing sarcoma, two cases of mesenchymal chondrosarcoma, and one case of poorly differentiated round cell tumor were negative for SATB2.
- In 12/13 cases of GCT, all four cases of fibrous dysplasia, two cases of chondroblastoma and one case of adamantinoma showed a strong intensity of staining. Seven out of 18 cases of ABC showed moderate staining. Two cases of chondroblastoma showed weak staining (Fig. 3).
- Non-osteogenic soft tissue tumors
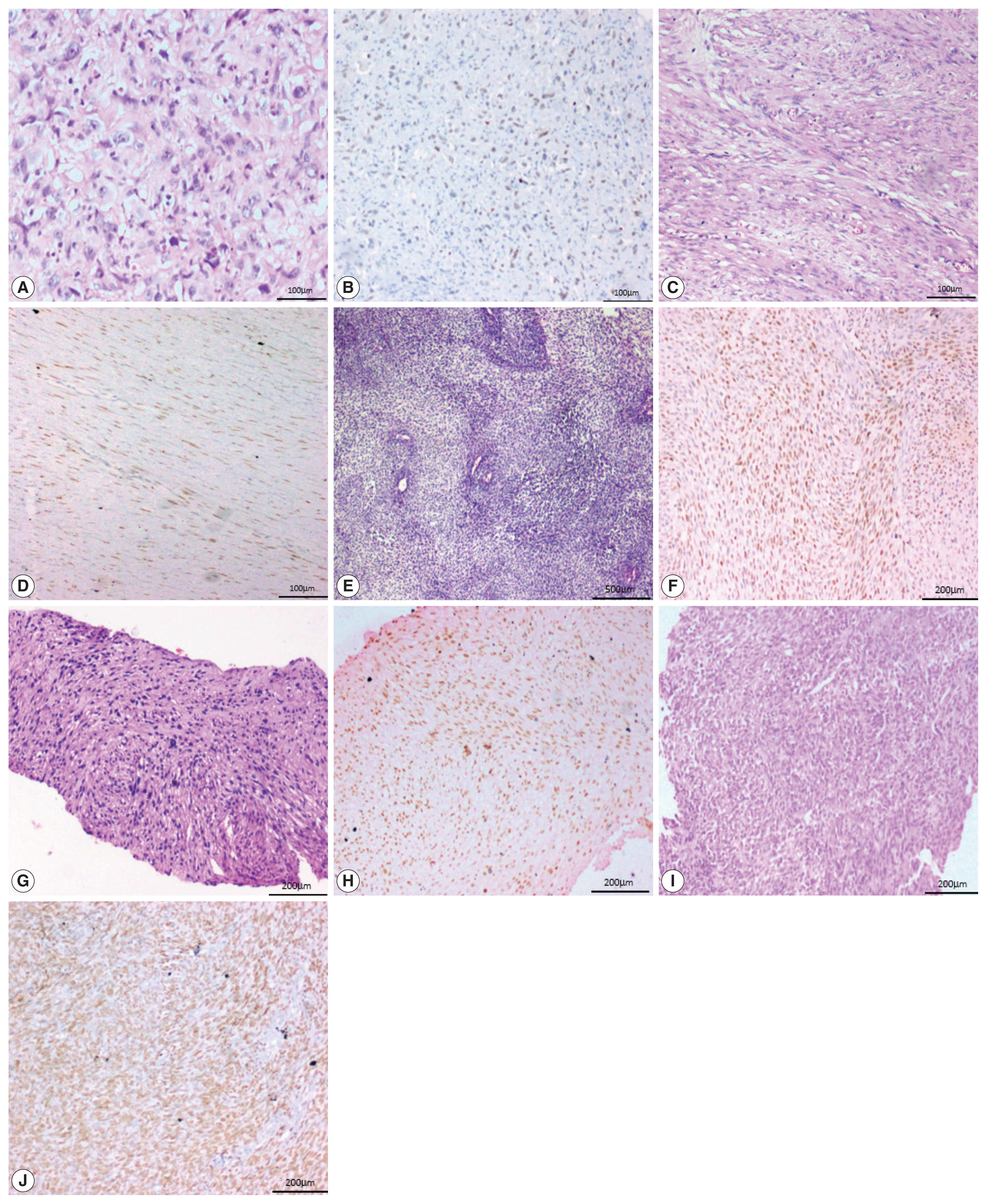
- Two of the five cases of desmoid fibromatosis, two of nine cases of leiomyosarcoma, one case of epithelioid sarcoma (proven by loss of INI-1 immunohistochemistry staining), one out of six cases of undifferentiated pleomorphic sarcoma, one out of three cases of malignant peripheral nerve sheath tumors (MPNST), one embryonal subtype out of three rhabdomyosarcomas (RMS) and one (positive for SYT-SSX1 translocation by polymerase chain reaction) of the six synovial sarcoma cases showed positive staining in neoplastic cells. All the three cases of well-differentiated liposarcoma, the one case of extraskeletal myxoid chondrosarcoma, and the two cases of clear cell sarcoma of soft tissue were negative for SATB2.
- One case of angiosarcoma and one case of epithelioid sarcoma showed strong staining. Two cases of leiomyosarcoma and one case each of MPNST, RMS, and synovial sarcoma showed moderate staining. All the three cases of desmoid fibromatosis, two cases of leiomyosarcoma and one case of undifferentiated pleomorphic sarcoma showed weak staining (Fig. 4).
- SATB2 as a diagnostic test
- The diagnostic capability of SATB2 IHC for OSA compared to non-osteogenic bone and soft tissue tumors was calculated.
- The sensitivity of SATB2 as a diagnostic test was 92.6% (95% confidence interval, 85.4% to 97.0%). The specificity was 50.0% (95% confidence interval, 39.8% to 60.2%). The positive predictive was 63.8% (95% confidence interval, 55.2% to 71.8%). The negative predictive value was 87.7% (95% confidence interval, 76.3% to 94.9%). Hence, in our study, we found that SATB2 as a diagnostic marker is highly sensitive with a good negative predictive value.
RESULTS
- Differentiating OSA from non-osteogenic bone and soft tissue tumors is crucial as neo-adjuvant chemotherapy and surgical management are significantly different between OSA and other malignant tumors [11]. OSA is a great histological mimicker and causes diagnostic challenges, especially in small tissue biopsies [9]. The diagnostic difficulties are especially pertinent in lesions in very young children where bone lymphomas form a major differential diagnosis and in unusual lesions such as a post-radiation tumor. The diagnosis is relatively straightforward in the presence of neoplastic osteoid, but in the following circumstances can pose challenges: (1) primary bone tumors with scant or absent osteoid formation; (2) differentiating osteoid from hyalinised collagen; (3) sclerosing stromal tissue or reactive bone; (4) small cell OSA mimicking other small round cell malignancies of the bone; (5) low-grade OSA that can mimic fibrous dysplasia; (6) differentiating extraskeletal OSA from other primary soft tissue tumors; (7) the identification of giant cell-rich OSA vs. other giant cell-rich tumors; (8) bizarre tumor cell morphology and heterologous differentiation; (9) metastatic spindle cell sarcoma in the lung with no osteoid; and (10) sampling error in terms of the biopsy under-representing the diagnostically important components of the tumor. In such cases, IHC can be used as an adjunct to histological diagnosis, especially to rule out OSA in cases where osteoid is not present.
- In our study, 92.6% of OSA cases were positive for SATB2. The median score of OSA staining was 4+.
- Of the 7/95 (7.4%) OSA cases that were negative for SATB2, six cases showed osteoid, and three cases showed the presence of cartilage. However, three of these cases had undergone decalcification while processing, which may have affected the SATB2 immunoreactivity. The radiology of two of these cases was suggestive of OSA while the radiology of four cases was only reported as suggestive of primary bone tumor. Only one case was followed by resection, which proved the diagnosis of OSA. In a study by Davis and Horvai [10], three out of 48 cases (6.2% cases) were negative for SATB2. In another study by Machado et al. [12], four out of 42 cases (9.6% cases) were negative for SATB2.
- Of the 59 non-osteogenic bone tumors, 62.7% were positive for SATB2 and of the 41 soft tissue tumors, 31.7% were positive for SATB2.
- The majority of GCT cases were strongly positive for SATB2 in our study. This finding is consistent with those of previous studies, where the authors explain that stromal cells may be either immature proliferating osteogenic elements or specialized osteoblast-like cells that fail to show neoplastic features but can induce the differentiation of osteoclast precursors [13–15]. In our study, all four cases of fibrous dysplasia were strongly positive for SATB2 and had a staining score of 4+ and 5+ in two cases, each in the spindle cells. This is in keeping with the study by Conner and Hornick [8]. In a study by Grad-Akrish et al. [16], all cases of fibro-osseous lesions of the jaw (n=42) including nine fibrous dysplasia showed diffuse staining patterns in the spindle cells and the staining intensity was moderate or strong in 96% of the intra-osseous fibro-osseous lesions included in the study. However, they observed that SATB2 showed only weak and focal expression in the spindle cells adjacent to reactive bone tissue (periosteal bone reaction). In fibrous dysplastic bone, the increased expression of cAMP by the mutated lesional cells is associated with abnormal osteoblast differentiation and the formation of defective bone, characterized by changes in the bone matrix organization and in mineralization [17]. Most of the cases of aneurysmal bone cysts in our study were positive for SATB2. This is in contrast to the study by Conner et al. where all five cases of ABC were negative for SATB2. We hypothesize that it is because of the osteoblast lineage associated with cadherin-11 rearrangement [18]. SATB2 was positive in ABC in our study. However, further studies are needed to validate this hypothesis and to determine the exact cause of SATB2 expression in ABC.
- In our study, most cases of chondroblastoma were weak to strongly positive for SATB2, which is in keeping with the study by Conner and Hornick [8]. Some authors have proposed that these tumors can produce osteoid, lack collagen type II expression, and isocitrate dehydrogenase mutations [19]. In the present study, two cases of grade 2 chondrosarcoma showed a weak positivity for SATB2, while five cases of grade 1 and one case of grade 2 chondrosarcoma were negative for SATB2. In comparison, 7/29 cases of chondroblastic OSA showed a staining proportion of 4+ in the areas with osteoblastic differentiation and showed limited staining in areas with chondroblastic differentiation. In a study by Conner and Hornick [8], all 10 cases of conventional chondrosarcoma were negative for SATB2 while 8/11 dedifferentiated chondrosarcoma were positive for SATB2 because of heterologous osteoblastic differentiation. In another study [12], of the 14/31 cases of conventional chondrosarcoma, the staining was more frequent and intense in grade 3 chondrosarcoma and uncommon in chondrosarcoma grade 1. The occasional weak positivity observed in undifferentiated pleomorphic sarcoma in our study is similar to that of Conner and Hornick [8], which could possibly represent foci of early osteoblastic differentiation.
- In our study, the sensitivity of SATB2 was 92.6% with a negative predictive value of 87.7% and a specificity of 50.0%. These observations are similar to those of Davis and Horvai [10] who while evaluating the expression of SATB2 in 48 pre-treated OSA biopsies and 36 non-osteogenic bone sarcomas, showed a sensitivity of 94% and a specificity of 55%. The stronger intensity staining they observed in OSA was very similar to our results. However, due to the high subjectivity in the interpretation of intensity [10], statistical analysis of intensity was not attempted.
- To the best of our knowledge, this is the first southeast Asian study that aimed to assess the immunohistochemical expression of SATB2 in OSA while also comparing it with non-osteogenic primary bone and soft tissue tumors.
- However, there are a few limitations to our study. Of the 7/95 cases of OSA that were negative for SATB2, only one had a subsequent resection and was reported as OSA. Also, 3/7 SATB2 negative OSA cases had undergone decalcification while processing which may have affected SATB2 immunoreactivity. In addition, though there were 100 cases in the comparison arm, the number of cases of each histological subtype was small.
- While SATB2 is a highly sensitive marker due to the high negative predictive value, it lacks specificity owing to the immunopositivity observed in various non-osteogenic bone and soft tissue tumors such as giant cell tumors, fibrous dysplasia, aneurysmal bone cysts, chondroblastoma, chondrosarcoma, adamantinoma, desmoid fibromatosis, leiomyosarcoma, epithelioid sarcoma, undifferentiated pleomorphic sarcoma, malignant peripheral nerve sheath tumors, rhabdomyosarcoma, and synovial sarcoma.
- The high negative predictive value of 87.7% makes SATB2 a useful negative marker for a rule-out test. In small biopsies, SATB2 serves as a valuable adjunct in the appropriate clinical setting. However, in view of the low specificity, its expression needs to be correlated clinicoradiologically, especially in the absence of detectable osteoid matrix in a small biopsy.
DISCUSSION
Ethics Statement
This study was approved by the institutional review board (IRB No. 10470). Formal written informed consent was not required after a waiver by the IRB.
Availability of Data and Material
The datasets generated or analyzed during the study are not publicly available due [to maintain patient confidentiality] but are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: AJP, SM. Data curation: AJP, SM, VTKT, VM, SB, RJ. Formal analysis: SM, AJP. Investigation: SM, AJP. Methodology: SM, AJP. Project administration: AJP, SM. Resources: AJP, SM, VTKT, SB, RJ, VM. Supervision: AJP. Validation: SM, AJP. Visualization: SM, AJP. Writing—original draft: SM. Writing—review & editing: SM, AJP, VTKT, SB, RJ, VM. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
- 1. Ramaswamy A, Rekhi B, Bakhshi S, Hingmire S, Agarwal M. Indian data on bone and soft tissue sarcomas: a summary of published study results. South Asian J Cancer 2016; 5: 138-45. ArticlePubMedPMC
- 2. Komaranchath A, Appaji L, Lakshmaiah KC, Kamath M, Kumar RV. Demographic profile of pediatric osteosarcoma in south India: a single institution experience. Int J Med Res Health Sci 2015; 4: 551-4. Article
- 3. Hasegawa T, Hirose T, Kudo E, Hizawa K, Usui M, Ishii S. Immunophenotypic heterogeneity in osteosarcomas. Hum Pathol 1991; 22: 583-90. ArticlePubMed
- 4. Chua K, Virshup DM, Odono EG, et al. YJ5 as an immunohistochemical marker of osteogenic lineage. Pathology 2021; 53: 229-38. ArticlePubMed
- 5. Docker D, Schubach M, Menzel M, et al. Further delineation of the SATB2 phenotype. Eur J Hum Genet 2014; 22: 1034-9. ArticlePubMedPMCPDF
- 6. SATB2 Gene - GeneCards | SATB2 Protein | SATB2 Antibody [Internet] Rehovot: Weizmann Institute of Science, 2016 [cited 2016 Nov 20]. Available from: http://www.genecards.org/cgi-bin/carddisp.pl?gene=SATB2 .
- 7. Zhang J, Tu Q, Grosschedl R, et al. Roles of SATB2 in osteogenic differentiation and bone regeneration. Tissue Eng Part A 2011; 17: 1767-76. ArticlePubMedPMC
- 8. Conner JR, Hornick JL. SATB2 is a novel marker of osteoblastic differentiation in bone and soft tissue tumors. Histopathology 2013; 63: 36-49. ArticlePubMed
- 9. Wadhwa N. Osteosarcoma: Diagnostic dilemmas in histopathology and prognostic factors. Indian J Orthop 2014; 48: 247-54. ArticlePubMedPMCPDF
- 10. Davis JL, Horvai AE. Special AT-rich sequence-binding protein 2 (SATB2) expression is sensitive but may not be specific for osteosarcoma as compared with other high-grade primary bone sarcomas. Histopathology 2016; 69: 84-90. ArticlePubMed
- 11. Wesolowski R, Budd GT. Use of chemotherapy for patients with bone and soft-tissue sarcomas. Cleve Clin J Med 2010; 77 (Suppl 1): S23-6. ArticlePubMed
- 12. Machado I, Navarro S, Picci P, Llombart-Bosch A. The utility of SATB2 immunohistochemical expression in distinguishing between osteosarcomas and their malignant bone tumor mimickers, such as Ewing sarcomas and chondrosarcomas. Pathol Res Pract 2016; 212: 811-6. ArticlePubMed
- 13. Horvai AE, Roy R, Borys D, O’Donnell RJ. Regulators of skeletal development: a cluster analysis of 206 bone tumors reveals diagnostically useful markers. Mod Pathol 2012; 25: 1452-61. ArticlePubMedPDF
- 14. Salerno M, Avnet S, Alberghini M, Giunti A, Baldini N. Histogenetic characterization of giant cell tumor of bone. Clin Orthop Relat Res 2008; 466: 2081-91. ArticlePubMedPMC
- 15. Goldring SR, Roelke MS, Petrison KK, Bhan AK. Human giant cell tumors of bone identification and characterization of cell types. J Clin Invest 1987; 79: 483-91. ArticlePubMedPMC
- 16. Grad-Akrish S, Rachmiel A, Ben-Izhak O. SATB2 is not a reliable diagnostic marker for distinguishing between oral osteosarcoma and fibro-osseous lesions of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol 2021; 131: 572-81. ArticlePubMed
- 17. Feller L, Wood NH, Khammissa RA, Lemmer J, Raubenheimer EJ. The nature of fibrous dysplasia. Head Face Med 2009; 5: 22.ArticlePubMedPMCPDF
- 18. Oliveira AM, Hsi BL, Weremowicz S, et al. USP6 (Tre2) fusion oncogenes in aneurysmal bone cyst. Cancer Res 2004; 64: 1920-3. PubMed
- 19. Konishi E, Nakashima Y, Iwasa Y, Nakao R, Yanagisawa A. Immunohistochemical analysis for Sox9 reveals the cartilaginous character of chondroblastoma and chondromyxoid fibroma of the bone. Hum Pathol 2010; 41: 208-13. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- The diagnostic utility of SATB2 immunohistochemistry as an adjunct for differentiating osteogenic from non-osteogenic bone tumors: A systematic review and Meta-analysis
Yuchen Lou, Xuan Liu, Chenxiao Ma, Xin Liu
Bone.2026; 203: 117721. CrossRef - Immunohistochemical Characterization of Feline Giant Cell Tumor of Bone (GCTb): What We Know and What We Can Learn from the Human Counterpart
Ilaria Porcellato, Giuseppe Giglia, Leonardo Leonardi
Animals.2025; 15(5): 699. CrossRef - Epigallocatechin gallate impels osteogenic differentiation of human BMSCs by targeting the METTL3/SATB2/Wnt/β-catenin axis
Qiao Ren, Kang Chen, Lin Wang
Letters in Drug Design & Discovery.2025; 22(3): 100027. CrossRef - A case of cardiac undifferentiated pleomorphic sarcoma treated with post-operative radiotherapy followed by heart transplantation
Sungyeon Jung, Eun Na Kim, Hye In Lee, Hak Jae Kim, Jiwon Koh
Cardiovascular Pathology.2025; 79: 107760. CrossRef - Osteoblastic Osteosarcoma With Diverse Histomorphology: Diagnostic Insights From SATB2 and CD56 Immunoexpression
Padmaraj Hegde, Reshma Amin, Vijith Vittal Shetty, Pushparaja Shetty
Journal of Health and Allied Sciences NU.2025; 0: 1. CrossRef - High-throughput 3D engineered paediatric tumour models for precision medicine
MoonSun Jung, Valentina Poltavets, Joanna N Skhinas, Gabor Tax, Alvin Kamili, Jinhan Xie, Sarah Ghamrawi, Philipp Graber, Jie Mao, Marie Wong-Erasmus, Louise Cui, Kathleen Kimpton, Pooja Venkat, Chelsea Mayoh, Angela Lin, Emmy D G Fleuren, Ashleigh M Ford
Molecular Systems Biology.2025; 21(12): 1748. CrossRef - SATB2 immunohistochemistry in osteosarcoma: Utility in diagnosis and differentiation from histologic mimics
Supriya Gangula, Monalisa Hui, Shantveer G. Uppin, B Arvind Kumar, K Nageshwara Rao, B Rajeev Reddy, G Sadashivudu
Indian Journal of Pathology and Microbiology.2025; 68(3): 518. CrossRef - Early-onset metastatic fibroblastic osteosarcoma of the metatarsus in a young cat: a case report
Mojtaba Kiakojoori, Hossein Kazemi Mehrjerdi, Ali Mirshahi, Mahdieh Zaeemi, Mohsen Maleki
BMC Veterinary Research.2025;[Epub] CrossRef - Favorable treatment response to high‐grade sarcoma in neurofibromatosis 1
Michelle H. Talukder, Mauli M. Patel, Tala Al‐Saghir, Ghadir K. Katato, Janet Poulik, William J. Powell, Alysia K. Kemp, Steven Miller, Danielle Bell, Jeffrey W. Taub
Pediatric Blood & Cancer.2023;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
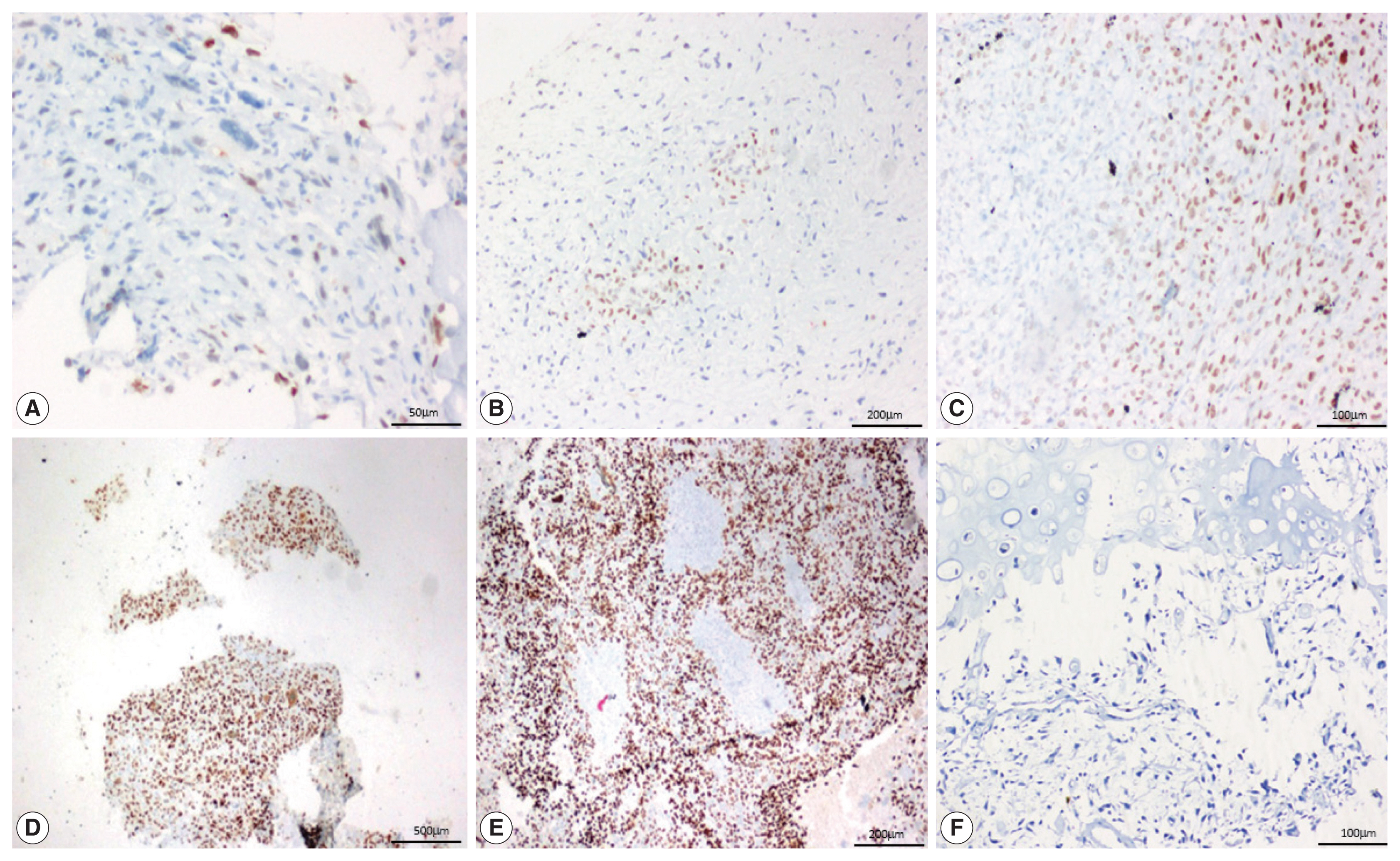
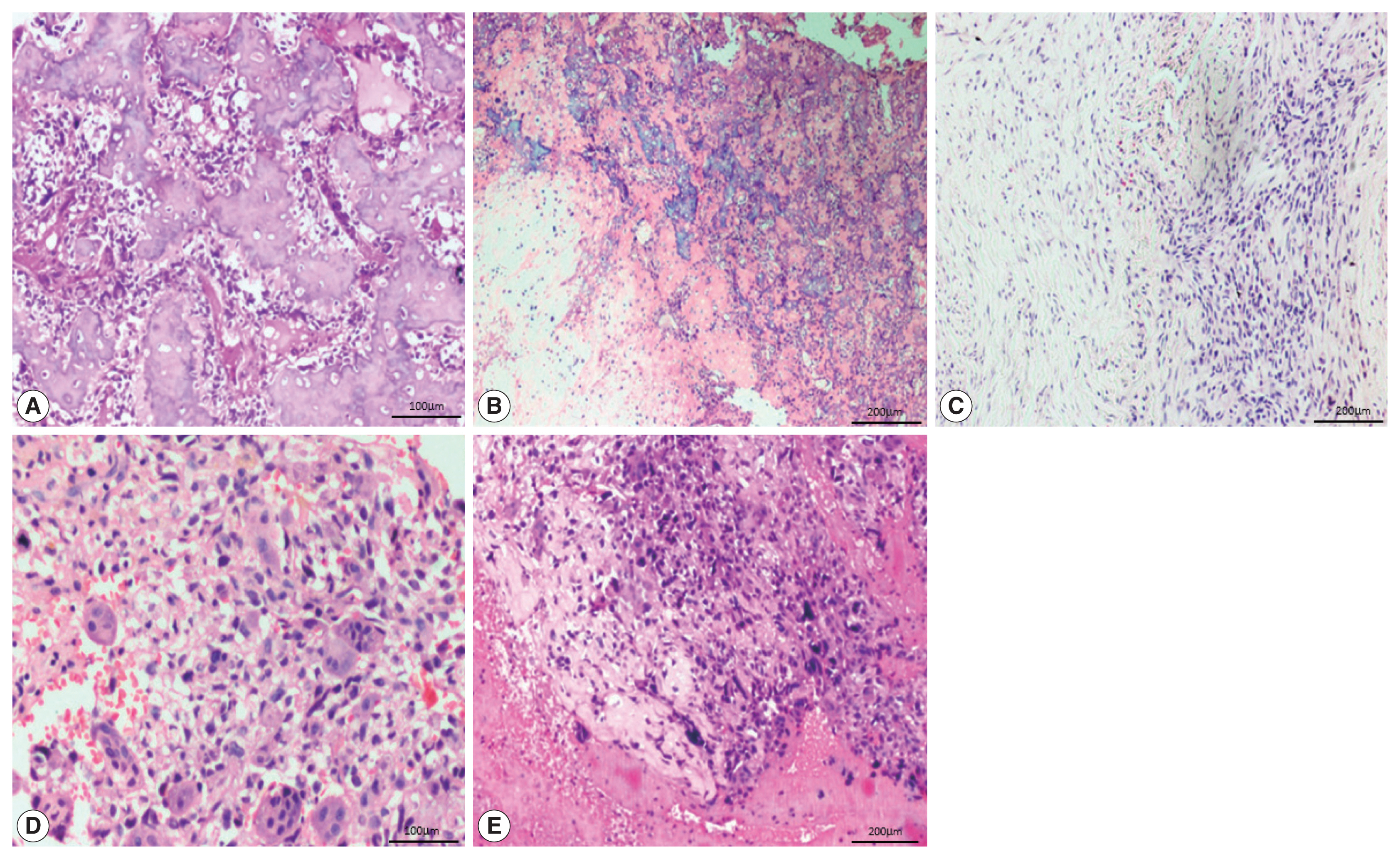
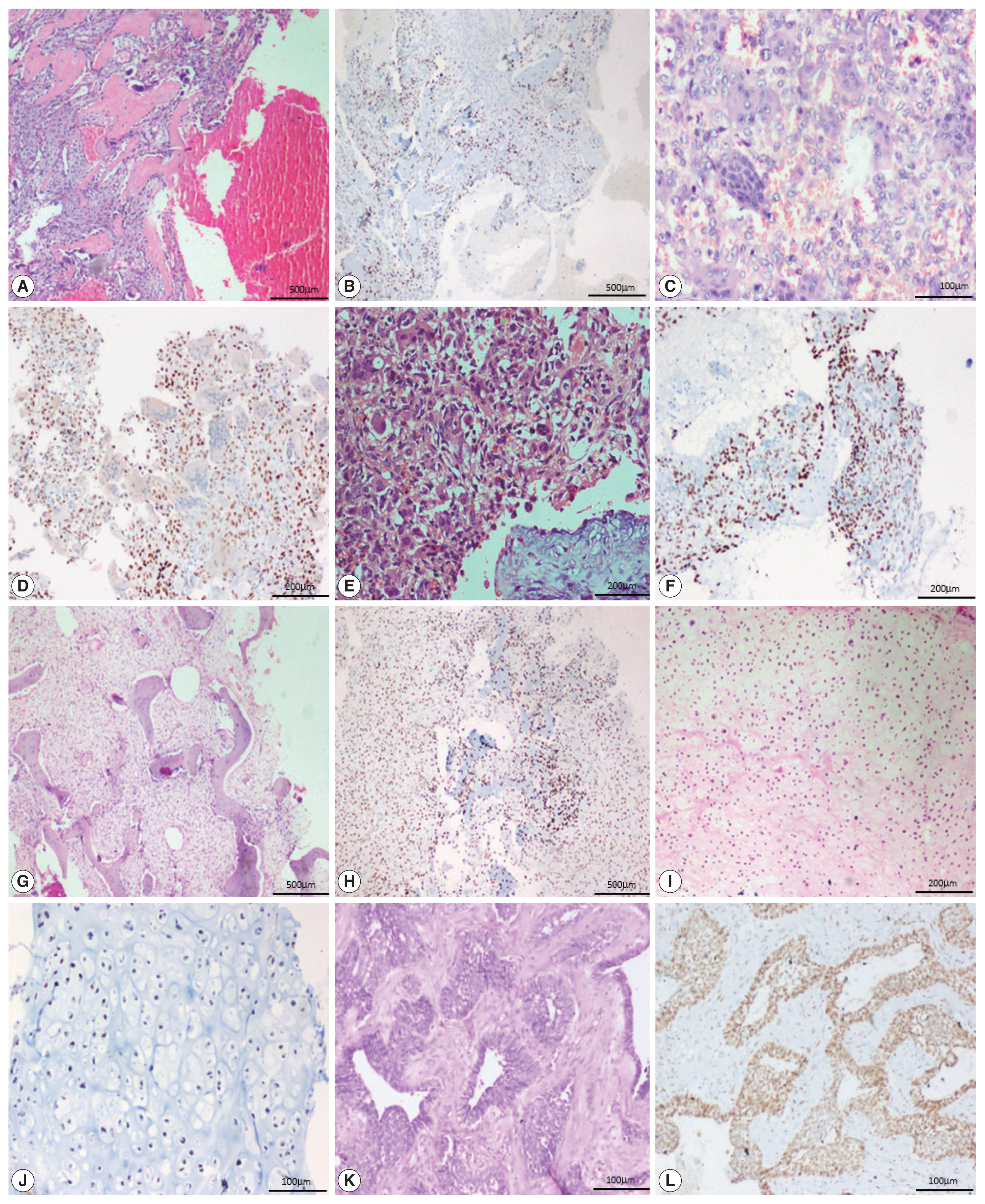
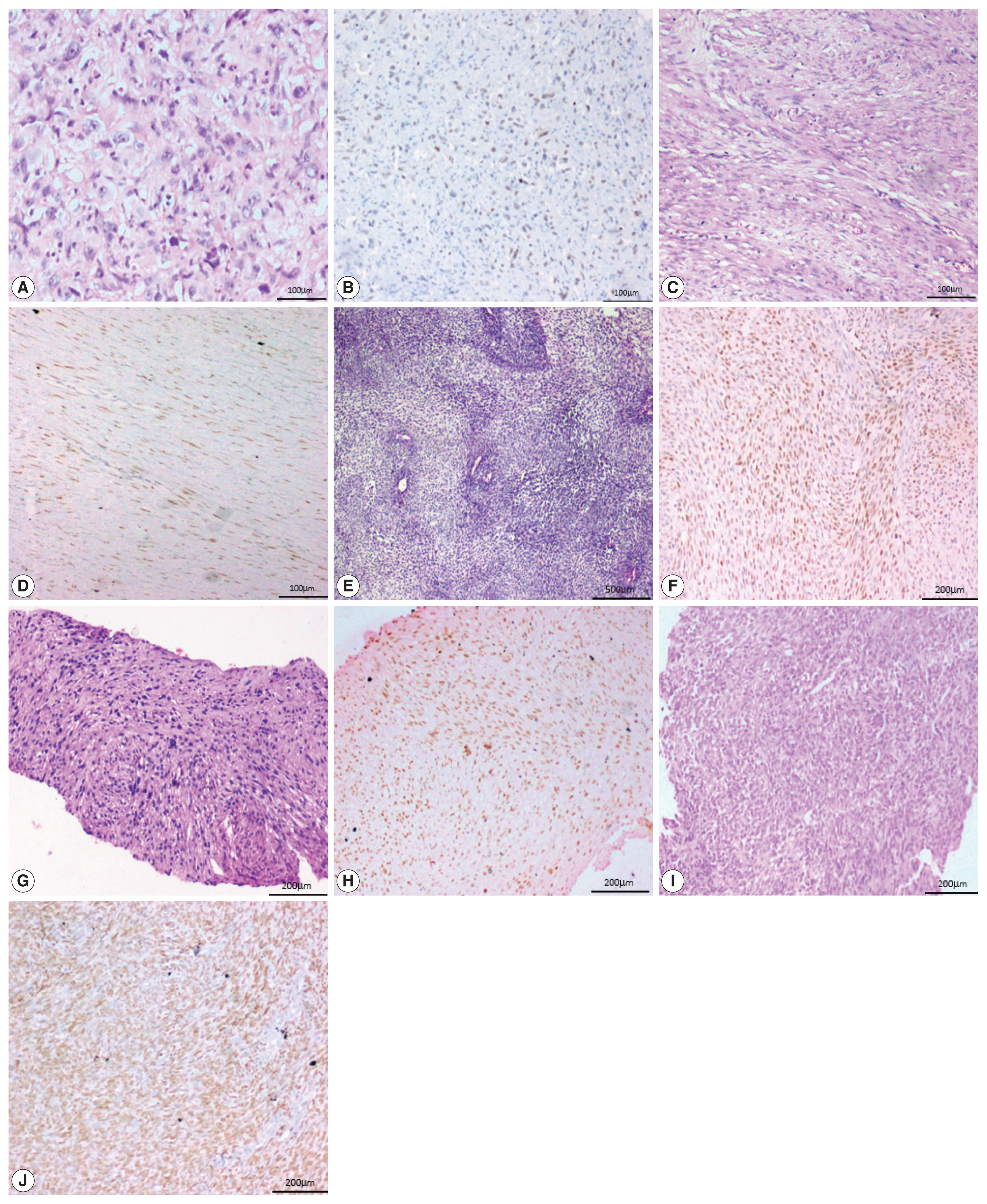
Fig. 1
Fig. 2
Fig. 3
Fig. 4
| Bone tumors (n = 59) | Soft tissue tumors (n = 41) |
|---|---|
| Aneurysmal bone cyst (18) | Leiomyosarcoma (9) |
| Giant cell tumor (13) | Synovial sarcoma (7) |
| Chondrosarcoma (8) | Undifferentiated pleomorphic sarcoma (6) |
| Ewing sarcoma (6) | Fibromatosis (5) |
| Chondroblastoma (5) | Liposarcoma (3) |
| Fibrous dysplasia (4) | Malignant peripheral nerve sheath tumor (3) |
| Adamantinoma (2) | Rhabdomyosarcoma (3) |
| Mesenchymal chondrosarcoma (2) | Clear cell sarcoma of soft tissue (2) |
| Poorly differentiated round cell sarcoma (1) | Epithelioid sarcoma (1) |
| Extraskeletal myxoid chondrosarcoma (1) | |
| Angiosarcoma (1) |
| Histological subtype | SATB2 negative | SATB2 1+ | SATB2 2+ | SATB2 3+ | SATB2 4+ | SATB2 5+ |
|---|---|---|---|---|---|---|
| Osteoblastic (n = 61) | 4 (6.5) | 4 (6.5) | 8 (13.1) | 7 (11.5) | 18 (29.5) | 20 (32.8) |
| Chondroblastic (n = 29) | 3 (10.4) | 5 (17.2) | 2 (6.9) | 6 (20.7) | 7 (24.1) | 6 (20.7) |
| Fibroblastic (n = 2) | 0 | 1 (50.0) | 0 | 0 | 1 (50.0) | 0 |
| Telangiectatic (n = 1) | 0 | 0 | 0 | 0 | 1 (100) | 0 |
| Giant cell rich (n = 2) | 0 | 0 | 0 | 0 | 1 (50.0) | 1 (50.0) |
| Histological subtype | SATB2 strong | SATB2 moderate | SATB2 weak |
|---|---|---|---|
| Osteoblastic (n = 57) | 29 (50.9) | 14 (24.5) | 14 (24.5) |
| Chondroblastic (n = 26) | 12 (46.2) | 11 (42.3) | 3 (10.5) |
| Fibroblastic (n = 2) | 1 (50.0) | 0 | 1 (50.0) |
| Telangiectatic (n = 1) | 1 (100) | 0 | 0 |
| Giant cell rich (n = 2) | 2 (100) | 0 | 0 |
| Parameter | SATB2 positive | SATB2 negative | p-value |
|---|---|---|---|
| Age (yr) | >.99 | ||
| < 20 | 51 (58.0) | 4 (57.1) | |
| ≥ 20 | 37 (42.0) | 3 (42.9) | |
| Sex | >.99 | ||
| Male | 66 (75.0) | 6 (85.7) | |
| Female | 22 (25.0) | 1 (14.3) | |
| Site | .203 | ||
| Femur and tibia | 67 (76.1) | 7 (100) | |
| Fibula, humerus, pelvis and others | 21 (23.9) | 0 | |
| Radiological location | >.99 | ||
| Metaphyseal and diaphyseal | 55 (63.2) | 4 (57.1) | |
| Others | 33 (37.5) | 3 (42.9) | |
| Radiological size (cm) | .425 | ||
| < 20 | 82 (93.2) | 6 (87.5) | |
| ≥ 20 | 6 (6.8) | 1 (14.3) | |
| Enneking staging | .433 | ||
| Stage III | 26 (29.9) | 3 (42.9) | |
| Stage IB and IIB | 62 (70.5) | 4 (57.1) | |
| Histological subtype | .684 | ||
| Osteoblastic and chondroblastic | 83 (94.3) | 7 (100) | |
| Fibroblastic, telangiectatic and giant cell rich | 5 (5.7) | 0 | |
| Cell morphology | .698 | ||
| Spindle and polygonal and spindle, polygonal and bizarre | 46 (52.9) | 4 (57.1) | |
| Others | 42 (47.1) | 3 (42.9) | |
| Cartilage | .684 | ||
| Absent | 59 (67) | 4 (57.1) | |
| Present | 29 (33) | 3 (42.9) | |
| Osteoid | .590 | ||
| Present | 78 (88.6) | 6 (85.7) | |
| Absent | 10 (11.4) | 1 (14.3) | |
| Cellularity | .763 | ||
| Mild | 12 (13.6) | 1 (14.3) | |
| Moderate | 66 (75.0) | 6 (85.7) | |
| Marked | 10 (11.4) | 0 | |
| Pleomorphism | .770 | ||
| Mild | 3 (3.4) | 0 | |
| Moderate | 53 (60.2) | 5 (71.4) | |
| Severe | 32 (36.4) | 2 (28.6) | |
| Mitosis | .413 | ||
| 0–5/10 hpf | 53 (60.2) | 4 (57.1) | |
| 6–9/10 hpf | 22 (25) | 3 (42.9) | |
| ≥ 10/10 hpf | 13 (14.8) | 0 | |
| Necrosis | >.99 | ||
| Yes | 45 (51.1) | 4 (57.1) | |
| No | 43 (48.9) | 3 (42.9) | |
| Grade | .673 | ||
| Intermediate (conventional OSA) | 19 (21.6) | 1 (14.3) | |
| High (conventional OSA) | 69 (78.4) | 6 (85.7) | |
| Elevated ALP | .782 | ||
| Yes | 36 (40.9) | 4 (57.1) | |
| No | 33 (37.5) | 2 ( 28.6) | |
| Not available | 19 (21.6) | 1 (14.3) | |
| Tumor | SATB2 negative | 1+ | 2+ | 3+ | 4+ | 5+ |
|---|---|---|---|---|---|---|
| Bone tumor | ||||||
| Aneurysmal bone cyst (n = 18) | 5 | 2 | 2 | 8 | 1 | - |
| Giant cell tumor (n = 13) | - | 1 | 2 | 2 | 8 | |
| Chondrosarcoma (n = 8) | 6 | - | 2 | - | - | - |
| Ewing sarcoma (n = 6) | 6 | - | - | - | - | - |
| Chondroblastoma (n = 5) | 1 | 2 | - | 1 | 1 | - |
| Fibrous dysplasia (n = 4) | - | - | - | - | 2 | 2 |
| Adamantinoma (n = 2) | 1 | - | - | - | - | 1 |
| Mesenchymal chondrosarcoma (n = 2) | 2 | - | - | - | - | - |
| Poorly differentiated round cell sarcoma (n = 1) | 1 | - | - | - | - | - |
| Total (n = 59) | 22 | 5 | 4 | 11 | 6 | 11 |
| Soft tissue tumor | ||||||
| Leiomyosarcoma (n = 9) | 5 | 1 | 2 | 1 | - | - |
| Synovial sarcoma (n = 7) | 6 | - | - | - | 1 | - |
| Undifferentiated pleomorphic sarcoma (n = 6) | 5 | - | - | 1 | - | - |
| Fibromatosis (n = 5) | 2 | - | 1 | 2 | - | - |
| Liposarcoma (n = 3) | 3 | - | - | - | - | - |
| Malignant peripheral nerve sheath tumor (n = 3) | 2 | - | - | 1 | - | - |
| Rhabdomyosarcoma (n = 3) | 2 | - | 1 | - | - | - |
| Clear cell sarcoma of soft tissue (n = 2) | 2 | - | - | - | - | - |
| Epithelioid sarcoma (n = 1) | - | - | - | - | 1 | - |
| Extraskeletal myxoid chondrosarcoma (n = 1) | 1 | - | - | - | - | - |
| Angiosarcoma (n = 1) | - | 1 | ||||
| Total (n = 41) | 28 | 2 | 4 | 5 | 2 | - |
| Tumor | Strong | Moderate | Weak |
|---|---|---|---|
| Bone tumor | |||
| Giant cell tumor (n = 13) | 12 | 1 | - |
| Aneurysmal bone cyst (n = 13) | 5 | 7 | 1 |
| Chondroblastoma (n = 4) | 2 | - | 2 |
| Fibrous dysplasia (n = 4) | 4 | - | - |
| Chondrosarcoma (n = 2) | - | - | 2 |
| Adamantinoma (n = 1) | 1 | - | - |
| Total | 24 | 8 | 5 |
| Soft tissue tumor | |||
| Leiomyosarcoma (n = 4) | - | 2 | 2 |
| Fibromatosis (n = 3) | - | - | 3 |
| Angiosarcoma (n = 1) | 1 | - | - |
| Epithelioid sarcoma (n = 1) | 1 | - | - |
| Undifferentiated pleomorphic sarcoma (n = 1) | - | - | 1 |
| Malignant peripheral nerve sheath tumor (n = 1) | 1 | - | |
| Rhabdomyosarcoma (n = 1) | - | 1 | - |
| Synovial sarcoma (n = 1) | - | 1 | - |
| Total | 2 | 5 | 6 |
Values are presented as number (%). SATB2, special AT-rich sequence-binding protein 2; OSA, osteosarcoma.
Values are presented as number (%). SATB2, special AT-rich sequence-binding protein 2; OSA, osteosarcoma.
SATB2, special AT-rich sequence-binding protein 2; OSA, osteosarcoma; ALP, alkaline phosphatase.
SATB2, special AT-rich sequence-binding protein 2.
SATB2, special AT-rich sequence-binding protein 2.

 E-submission
E-submission






