Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 54(1); 2020 > Article
-
Review
HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation -
Soomin Ahn1
 , Ji Won Woo1,2
, Ji Won Woo1,2 , Kyoungyul Lee3
, Kyoungyul Lee3 , So Yeon Park,1,2
, So Yeon Park,1,2
-
Journal of Pathology and Translational Medicine 2020;54(1):34-44.
DOI: https://doi.org/10.4132/jptm.2019.11.03
Published online: November 6, 2019
1Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea
2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea
3Department of Pathology, Kangwon National University Hospital, Chuncheon, Korea
- Corresponding Author: So Yeon Park, MD, PhD, Department of Pathology, Seoul National University Bundang Hospital, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea Tel: +82-31-787-7712, Fax: +82-31-787-4012, E-mail: sypmd@snu.ac.kr
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Human epidermal growth factor receptor 2 (HER2) protein overexpression and/or HER2 gene amplification is found in about 20% of invasive breast cancers. It is a sole predictive marker for treatment benefits from HER2 targeted therapy and thus, HER2 testing is a routine practice for newly diagnosed breast cancer in pathology. Currently, HER2 immunohistochemistry (IHC) is used for a screening test, and in situ hybridization is used as a confirmation test for HER2 IHC equivocal cases. Since the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines on HER2 testing was first released in 2007, it has been updated to provide clear instructions for HER2 testing and accurate determination of HER2 status in breast cancer. During HER2 interpretation, some pitfalls such as intratumoral HER2 heterogeneity and increase in chromosome enumeration probe 17 signals may lead to inaccurate assessment of HER2 status. Moreover, HER2 status can be altered after neoadjuvant chemotherapy or during metastatic progression, due to biologic or methodologic issues. This review addresses recent updates of ASCO/CAP guidelines and factors complicating in the interpretation of HER2 status in breast cancers.
- Currently, immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), and chromogenic in situ hybridization (CISH) including silver in situ hybridization (SISH) are regarded as standard methods for determination of HER2 status in breast cancer, and some of them have been approved by the U.S. Food and Drug Administration (FDA) for HER2 testing in breast cancer since 1998.
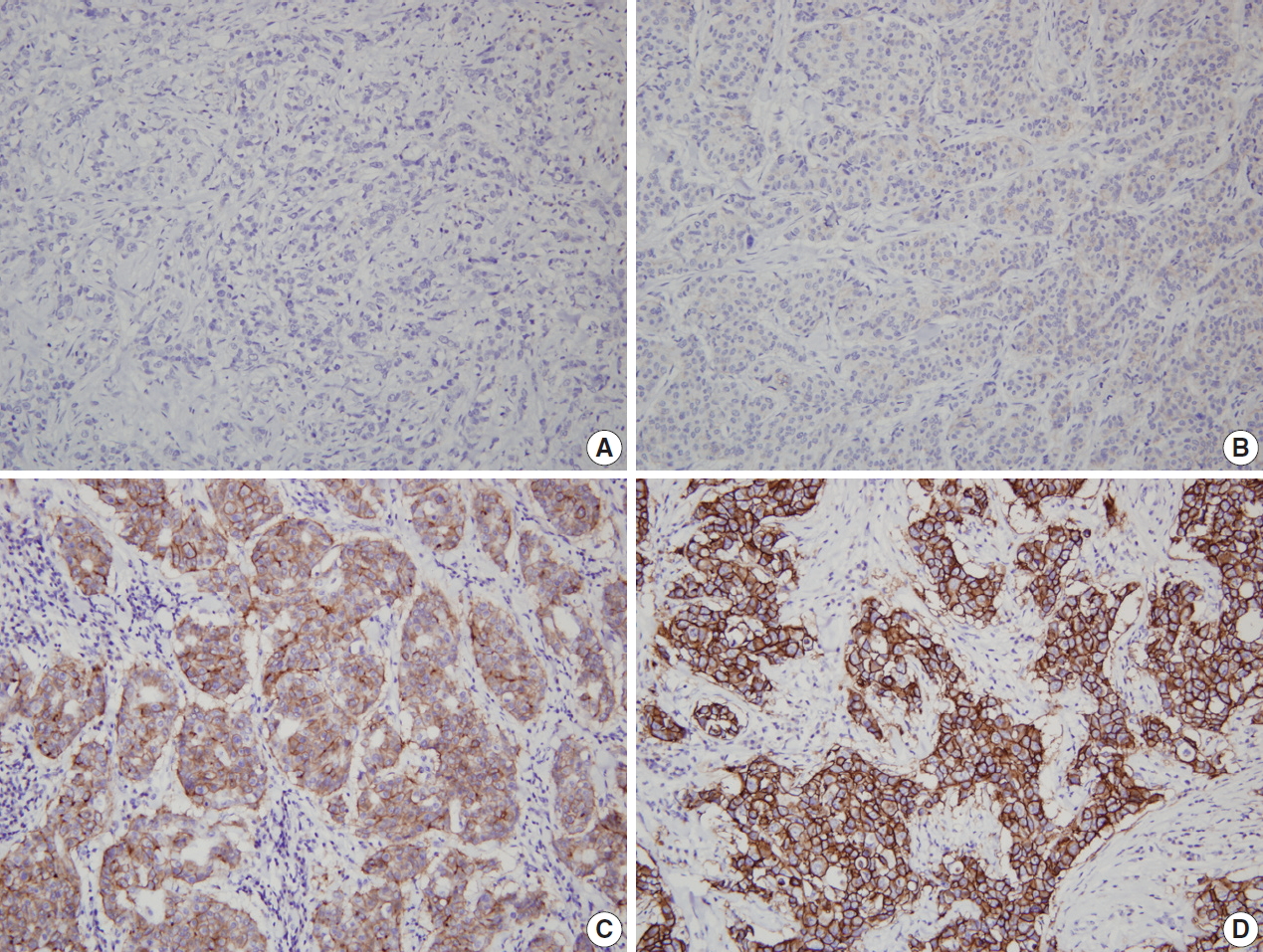
- Although HER2 status can be directly tested by in situ hybridization (ISH), many laboratories have adopted IHC as a screening test, and FISH as a confirmation test for HER2 IHC equivocal cases, considering higher failure rate, longer procedure time and higher reagent cost of FISH, compared to that of IHC. Moreover, high concordance has been found between HER2 protein overexpression by IHC and gene amplification by FISH [3-5]. Finally, the 2007 ASCO/CAP guidelines stated that HER2 status should be initially assessed by IHC using a semi-quantitative scoring system (Fig. 1), and confirmed by FISH in all IHC score 2+ equivocal cases [6].
- Bright-field in situ hybridization such as CISH and SISH has advantage in that it allows histologic evaluation of tumors, utilizes ordinary microscope, leaves permanent signals for archival storage, and can be fully automated [7]. Moreover, it shows more than 95% concordance rate with FISH [8-11]. Thus, CISH and SISH are now admitted as an alternative to FISH.
METHODS OF HER2 TESTING
- For uniformity in accuracy and reproducibility of HER2 testing in breast cancer, ASCO/CAP jointly released guidelines and recommendations for HER2 testing first in 2007, addressing a wide range of pre-analytic, analytic and post-analytic variables [6]. This guideline focused on limiting the false-positive results, adopting a higher cutoff of 30% for HER2 IHC positivity (3+), instead of 10% cutoff previously recommended by FDA (Table 1). For FISH analysis, HER2 gene was regarded as amplified if HER2/chromosome enumeration probe 17 (CEP17) ratio >2.2 for dual-probe assay (instead of the previously recommended ratio of 2.0) or HER2 gene copy >6 signals per cell for single-probe assay.
- After then, the revised 2013 ASCO/CAP guideline focused on maximizing identification of patients who can benefit from HER2-targeted therapy and minimizing false-negative results [12]. The range of HER2 IHC equivocal cases (2+) was widened, and HER2 IHC 3+ was defined using 10% as cutoff value, not using 30% (Table 1). When using validated dual-probe ISH assay, HER2/CEP17 ratio ≥2.0 or HER2/CEP17 ratio <2.0 and average HER2 copy number ≥6.0 was regarded as ISH positive (Table 2). The 2007 and 2013 ASCO/CAP guidelines included ISH equivocal results, which had been a problem for clinical decision making.
- Since the publication of the 2013 guideline, several laboratories and clinical investigators have reported on the practical implications of the 2013 guidelines and increased frequencies of equivocal results [13]. The HER2 testing Expert Panel wished to clarify controversial issues in the 2013 guideline, and in that context the 2018 updated ASCO/CAP guidelines on HER2 testing was reported [13]. The updated guideline addressed five clinical questions. The first question was about the definition of HER2 IHC 2+, and it was revised as weak to moderate complete membrane staining observed in >10% of tumor cells. The second question was about repeated HER2 test of initially negative case, and it was recommended that if the initial HER2 testing result in a core needle biopsy specimen is negative, a new HER2 test may (not ‘‘must’’) be ordered on the excision specimen based on specific clinical criteria. These two clinical questions were addressed in a previous correspondence by the Expert Panel [14]. The remaining three questions were about less common ISH patterns, and the updated HER2 testing algorithm addressed the workup for these three clinical scenarios, occasionally found when using a dual-probe ISH assay. These scenarios were described as ISH group 2 (HER2/CEP17 ratio ≥2.0; average HER2 copy number < 4.0 signals per cell), ISH group 3 (HER2/CEP17 ratio <2.0; average HER2 copy number ≥6.0 signals per cell), and ISH group 4 (HER2/CEP17 ratio <2.0; average HER2 copy number ≥ 4.0 and <6.0 signals per cell) [13]. The diagnostic approach includes more rigorous interpretation criteria for ISH and requires concomitant IHC review for dual-probe ISH groups 2 to 4 to achieve the most accurate determination of HER2 status based on combined interpretation of the ISH and IHC assay [13]. It was recommended that laboratories using single-probe ISH assays include concomitant IHC review as part of the interpretation of all ISH assay results [13]. By this approach, the HER2 status was designated to positive or negative with no equivocal category.
- Although complicated, HER2 status is basically determined by IHC results in the dual-probe ISH groups 2 to 4. While cases with HER2 IHC 3+ are regarded as HER2 positive, those with HER2 IHC 0 or 1+ are regarded as HER2 negative. In cases with HER2 IHC 2+, an additional observer blinded to previous result recounts ISH. As for ISH groups 2 and 4, if the repeated ISH result is designated to the same ISH group, it is finally regarded as HER2 negative. On the contrary, as for ISH group 3, if the repeated ISH result is categorized to the same group, it is finally regarded as HER2 positive. If the repeated ISH shows other ISH result, the results should be adjudicated per internal procedures to determine final category. The changes on HER2 interpretation in the updated 2018 ASCO/CAP guidelines in comparison with previous 2007 and 2013 guidelines are summarized in Tables 1 and 2.
- Recent studies on implementation of the updated 2018 ASCO/CAP guidelines have shown significant increases of HER2 negative rates through reclassification of ISH equivocal cases by the 2013 guidelines [15-17]. The updated guidelines seem to provide much clearer instructions for HER2 status designation by using concomitant IHC review in ISH groups 2, 3 and 4, and eliminating ISH equivocal category [15].
CHANGES IN GUIDELINES ON INTERPRETATION OF HER2 STATUS
- Intratumoral HER2 heterogeneity
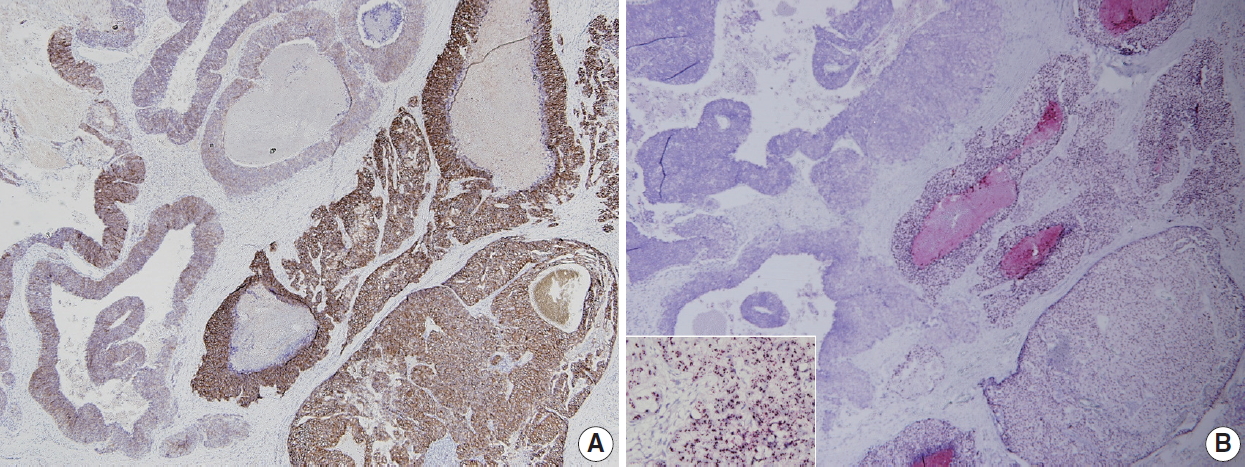
- HER2 status has been thought to be fairly homogeneous within a tumor. However, intratumoral heterogeneity of HER2 gene amplification, that is, intratumoral HER2 heterogeneity, is found in a subset of breast cancers. It has important clinical implications, in that it can contribute to inaccurate assessment of HER2 status and affect treatment responses to HER2-targeted therapy [18]. In previous studies, our group has shown that intratumoral HER2 heterogeneity is more common in breast cancer with equivocal HER2 protein expression and low-grade HER2 gene amplification [19,20]. Intratumoral HER2 heterogeneity was associated with poor clinical outcome in patients with HER2-positive primary breast cancer [19]. Moreover, it was related to poor response to trastuzumab and decreased survival in patients with HER2-positive metastatic breast cancer [20]. In a subsequent study, Lee et al. [21] reported that cases with HER2 regional heterogeneity showed a decreased disease-free survival rate compared to those without heterogeneity in the hormone receptor-positive subgroup of breast cancer patients treated with adjuvant trastuzumab. In a neoadjuvant setting, intratumoral HER2 heterogeneity was also found to be associated with incomplete response to anti-HER2 chemotherapy [22].
- The CAP addressed this issue and published a separate recommendation in 2009 and defined HER2 genetic heterogeneity as the presence of tumor cells with HER2/CEP17 signal ratios greater than 2.2 in 5% to 50% of the tumor cells tested [23]. However, this recommendation has some problems, in that it was based on expert opinion rather than evidence, and artificial heterogeneity caused by technical issues could be regarded as HER2 genetic heterogeneity. Finally, the 2013 ASCO/CAP guidelines added a recommendation about HER2 heterogeneity for HER2 ISH interpretation, stating that if there is a second population of cells with increased HER2 signals/cell and this cell population is more than 10% of tumor cells on the slide, a separate counting of at least 20 non-overlapping cells must also be performed within this cell population and reported [12].
- HER2 heterogeneity can be found as distinct clusters of amplified cells among non-amplified cells or appear as intermixed amplified and non-amplified cells (Fig. 2). Basically, it is important to scan all fields when observing ISH slide and to match it with HER2 IHC slide to detect areas with HER2 heterogeneity. From this point of view, CISH or SISH has an advantage in evaluating HER2 heterogeneity, because it can be easily matched with HER2 IHC slide under light microscope. The HER2/CEP17 ratios or HER2 copy number should be calculated separately for amplified and non-amplified areas. Suggestions for how to assess intratumoral HER2 heterogeneity are summarized in Table 3 [24,25].
- CEP17 copy number gain
- CEP17 copy number gain is a genetic change commonly observed during dual-probe HER2 ISH for breast cancer, with reported frequency of 3% to 46% in breast cancers [26]. In our study using 945 cases of invasive breast cancer, CEP17 copy number gain was observed in 29.9% when using the definition of CEP17 copy number gain as mean CEP17 ≥2.6, and was found in 19.7% with the definition of CEP17 copy number gain as CEP17 ≥3.0 [27]. This had been thought to result from increasing numbers of whole chromosome 17, which is referred to as polysomy 17. However, subsequent studies have revealed that true polysomy 17 is a rare phenomenon in breast cancers, and CEP17 copy number gain results from amplification or copy number gain in the centromeric or pericentromeric region [28-32].
- Although it is still controversial, CEP17 copy number gain has been found to be associated with increased HER2 protein expression [27,33-35]. However, CEP17 copy number gain without HER2 gene amplification was not associated with benefit from HER2-targeted therapy in breast cancers [36,37]. Moreover, CEP17 copy number gain in breast cancers has been reported to be associated with adverse clinicopathological features [27,38-40] and response to anthracycline-based therapy in breast cancer [41,42]. However, as for its prognostic significance, there have been conflicting results [43-46]. Recently, our group has shown that CEP17 copy number gain is an independent poor prognostic factor in patients with luminal/HER2-negative breast cancers, suggesting that CEP17 copy number gain may reflect chromosomal instability in breast cancer [27].
- CEP17 copy number gain may affect the interpretation of HER2 ISH, and lead to an underestimation of HER2 status. Therefore, the 2013 ASCO/CAP guideline recommended to repeat HER2 testing using an alternative probe for CEP17 or for another gene in chromosome 17 not expected to co-amplify with HER2 for the ISH equivocal cases (now ISH group 4 in the updated 2018 ASCO/CAP guideline) [12,26]. However, following studies have shown that with alternative probes, HER2 ISH equivocal cases were upgraded to HER2 ISH positive status in a significant proportion, and clinicopathologic features of those upgraded cases were not compatible with those of HER2-amplified breast cancers, suggesting that use of alternative probe is not reliable in clinical practice [32,47]. The updated 2018 ASCO/CAP guidelines do not recommend the use of alternative probe as standard practice due to limited evidence on its analytical and clinical validity [13].
- Changes in HER2 status after neoadjuvant chemotherapy
- Neoadjuvant chemotherapy (NAC) is currently considered as standard treatment for locally advanced breast cancer [48]. Alteration of biomarker status after NAC is occasionally found in breast cancer [49,50]. Hormone receptor status changed more often than HER2 status, and as for hormone receptors, positive to negative conversion was more common than negative to positive conversion [51,52]. The frequency of HER2 change after NAC is reported in up to 15%, and both positive to negative conversion and negative to positive conversion were found with no preponderance [52-66]. Previous studies on HER2 change after NAC are summarized in Table 4. In our study, HER2 status was altered after NAC in 3.4% with positive to negative conversion in 0.9% and negative to positive conversion in 2.5% [52]. Most cases with negative to positive conversion of HER2 status after NAC showed low level of HER2 amplification, and the HER2/CEP17 ratio ranged from 2.2 to 4.4 (data not shown in a previous study) [52]. Cockburn et al. [61] also reported the mean HER2/CEP17 ratio in resection specimens with HER2 positive conversion was 3.7. Although there are no guidelines about whether treatment should be modified based on altered biomarker status after NAC, the change of HER2 status may have an impact on the therapeutic management in certain patients. Accordingly, re-evaluation of biomarkers including HER2 after NAC is recommended for proper management.
- The mechanism of HER2 conversion after NAC is not well understood. This can be partly explained by the selection of HER2-positive or HER2-negative clones after NAC, tumor heterogeneity, and pre-analytical and analytical pitfalls, Guarneri et al. [67] evaluated HER2 status change on 107 HER2-positive patients treated with NAC with or without anti-HER2 agents. They reported that patients with tumors undergoing HER2 negative conversion following treatment had significantly reduced disease-free survival compared to patients with maintained HER2 positivity [67]. However, the prognostic significance of HER2 status change is still unclear.
- Finally, caution is needed in interpretation of HER2 ISH after NAC in distinguishing between a true HER2 amplification and increase of HER2 copy number by chromosome 17 polysomy [68]. Increase in HER2 copy number could not be attributed to true HER2 amplifications, but instead could reflect polyploidization after chemotherapy, which presumably affects all chromosomes [68]. Careful evaluation using dual-probe ISH with concomitant IHC review is recommended.
- Change in HER2 status with metastatic progression
- Change in receptor status during metastatic progression, a phenomenon called “receptor conversion” occurs not only in hormonal receptors, but also in HER2. The reported frequency of HER2 conversion varies a lot between studies, but it is usually observed less often than hormone receptor conversion. Schrijver et al. [69] reported in their meta-analysis that pooled frequency of HER2 positive to negative conversion was 21.3%, and that of negative to positive conversion was 9.5%. Previous studies on HER2 change during metastatic progression are summarized in Table 5 [70-79]. In our study, HER2 receptor status changed in 12 out of 152 cases (7.9%) during metastatic progression: nine cases (5.9%) were negative conversion, and three cases (2.0%) were positive conversion [79].
- Similar to HER2 status alteration after NAC, little is known about the true mechanism of HER2 status conversion during metastatic progression. It would be reasonable to postulate intratumoral heterogeneity and selection pressure from treatment play a role in HER2 status conversion. In our study, cases with positive to negative conversion showed significantly lower level of HER2 protein expression and heterogeneous HER2 gene amplification, compared to consistently positive cases [79]. From this observation, it can be inferred that tumors with HER2 heterogeneity have a propensity to show different status in metastatic sites, because HER2-targeted therapy drives susceptible clones to fade away.
- HER2 conversion is not a common event, but it is important to discover it because of its relation with treatment. There are some studies which reported response to trastuzumab treatment in patients who gained HER2 positivity in metastatic lesions [77,80]. For this reason, it is now widely recommended to reevaluate receptor status including HER2 in metastatic lesions, if possible [13,81].
COMPLICATING FACTORS FOR HER2 INTERPRETATION
- The ASCO/CAP guidelines on HER2 interpretation in breast cancer, which were released first in 2007 and subsequently updated in 2013 and 2018, have been changing to provide much clearer instructions for HER2 status designation. The updated 2018 ASCO/CAP guideline focused on three dual-probe ISH groups (groups 2, 3, and 4) with less common ISH patterns, and recommended concomitant IHC review for these ISH groups to achieve the most accurate determination of HER2 status. Finally, in the updated 2018 ASCO/CAP guideline, HER2 status, as determined by ISH, is categorized to positive or negative with no equivocal results.
- There are some complicating factors for HER2 interpretation including HER2 heterogeneity, CEP17 copy number gain, and HER2 status alteration after NAC or during metastatic progression. It is important to scan the entire ISH slide and to match it with HER2 IHC to detect HER2 heterogeneity. Separate counting should be performed in both amplified and non-amplified areas. CEP17 copy number gain may lead to an underestimation of HER2 status, but the use of alternative probe is not recommended in the updated 2018 ASCO/CAP guidelines due to the limited evidence on its analytical and clinical validity. HER2 conversion is occasionally found after NAC or during metastasis progression. The change of HER2 status may have an impact on the therapeutic decision and response to treatment. Accordingly, re-evaluation of HER2 status should be performed in postNAC specimens and metastatic lesions.
CONCLUSION
Author contributions
Project administration: SA.
Supervision: SYP.
Writing—original draft: SA, JWW, KL, SYP.
Writing—review & editing: SYP.
Conflicts of Interest
S.Y.P. is the editor-in-chief of the Journal of Pathology and Translational Medicine and was not involved in the editorial evaluation or decision to publish this article. All remaining authors declare that they have no potential conflicts of interest.
Funding
No funding to declare.
| HER2 IHC status | 2007 ASCO/CAP guidelines | 2013 ASCO/CAP guidelines | 2018 ASCO/CAP guidelines |
|---|---|---|---|
| Positive (3+) | Uniform intense membrane staining of >30% of invasive tumor cells | Circumferential membrane staining that is complete, intense, and in >10% of tumor cells | Circumferential membrane staining that is complete, intense, and in >10% of tumor cells |
| Equivocal (2+) | Complete membrane staining that is either non-uniform or weak in intensity but with obvious circumferential distribution in at least 10% of cells | Circumferential membrane staining that is incomplete and/or weak to moderate and within >10% of the invasive tumor cells | Weak to moderate complete membrane staining observed in >10% of tumor cellsa |
| Complete and circumferential membrane staining that is intense and within ≤10% of the invasive tumor cells | |||
| Negative (1+) | Weak incomplete membrane staining in any proportion of tumor cells | Incomplete membrane staining that is faint or barely perceptible and within >10% of the invasive tumor cells | Incomplete membrane staining that is faint or barely perceptible and within >10% of the invasive tumor cells |
| Weak, complete membrane staining in <10% of tumor cells | |||
| Negative (0) | No staining | No staining observed | No staining observed |
| Incomplete membrane staining that is faint or barely perceptible and within ≤10% of the invasive tumor cells | Incomplete membrane staining that is faint or barely perceptible and within ≤10% of the invasive tumor cells |
ASCO, American Society of Clinical Oncology; CAP, College of American Pathologists; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry.
aUnusual staining patterns of HER2 by IHC can be encountered that are not covered by these definitions. As one example, some specific subtypes of breast cancers can show IHC staining that is moderate to intense but incomplete (basolateral or lateral) and can be found to be HER2 amplified. Another example is circumferential membrane staining that is intense but in ≤ 10% tumor cells. Such cases can be considered equivocal (2+).
| HER2 ISH status | 2007 ASCO/CAP guidelines | 2013 ASCO/CAP guidelines | 2018 ASCO/CAP guidelines |
|---|---|---|---|
| ISH positive | HER2/CEP17 ratio > 2.2 | HER2/CEP17 ratio ≥ 2.0 | HER2/CEP17 ratio ≥ 2.0 and average HER2 copy number ≥ 4.0 (group 1) |
| HER2/CEP17 ratio < 2.0 and average HER2 copy number ≥ 6.0 | HER2/CEP17 ratio ≥ 2.0 and average HER2 copy number < 4.0 (group 2) with concurrent IHC 3+ | ||
| HER2/CEP17 ratio < 2.0 and average HER2 copy number ≥ 6.0 (group 3) with concurrent IHC 2+a | |||
| HER2/CEP17 ratio < 2.0 and average HER2 copy number ≥ 6.0 (group 3) with concurrent IHC 3+ | |||
| HER2/CEP17 ratio < 2.0 with average HER2 copy number ≥ 4.0 and < 6.0 (group 4) with concurrent IHC 3+ | |||
| ISH equivocal | HER2/CEP17 ratio of 1.8-2.2 | HER2/CEP17 ratio < 2.0 with average HER2 copy number ≥ 4.0 and < 6.0 | (no equivocal category) |
| ISH negative | HER2/CEP17 ratio of < 1.8 | HER2/CEP17 ratio < 2.0 with average HER2 copy number < 4.0 | HER2/CEP17 ratio < 2.0 with average HER2 copy number < 4.0 (group 5) |
| HER2/CEP17 ratio ≥ 2.0 and average HER2 copy number < 4.0 (group 2) with concurrent IHC 2+b | |||
| HER2/CEP17 ratio < 2.0 with average HER2 copy number ≥ 4.0 and < 6.0 (group 4) with concurrent IHC 2+b | |||
| Groups 2, 3, and 4 with concurrent IHC 0 or 1 + |
ASCO, American Society of Clinical Oncology; CAP, College of American Pathologists; HER2, human epidermal growth factor receptor 2; ISH, in situ hybridization; CEP17, chromosome enumeration probe 17; IHC, immunohistochemistry.
aAn additional observer blinded to previous result recounts ISH. If the repeated ISH result is categorized to the same group, it is finally regarded as HER2 positive;
bAn additional observer blinded to previous result recounts ISH. If the repeated ISH result is designated to same ISH group, it is finally regarded as HER2 negative.
| Year | Author | Total No. of cases | Method |
Frequency of HER2 alteration, n (%) |
||
|---|---|---|---|---|---|---|
| Total | + to – | – to + | ||||
| 2018 | De La Cruz et al. [53] | 54 | IHC/FISH | 2/54 (3.7) | 1/54 (1.9) | 1/54 (1.9) |
| 2018 | Ahn et al. [52] | 442 | IHC/SISH | 15/442 (3.4) | 4/442 (0.9) | 11/442 (2.5) |
| 2017 | Xian et al. [54] | 77 | IHC/FISH | 6/77 (7.8) | 5/77 (6.5) | 1/77 (1.3) |
| 2017 | Reddy et al. [55] | 140 | IHC/FISH | 8/97 (8.2) | 5/97 (5.2) | 3/97 (3.1) |
| 2016 | Gahlaut et al. [56] | 133 | IHC/FISH | 8/133 (6.0) | 5/133 (3.8) | 3/133 (2.3) |
| 2016 | Lim et al. [57] | 290 | IHC/FISH | 17/290 (5.9) | 17/290 (5.9) | 0/290 (0) |
| 2016 | Zhou et al. [58] | 107 | IHC/FISH | 5/107 (4.7) | 3/107 (2.8) | 2/107 (1.9) |
| 2015 | Jin et al. [59] | 423 | IHC/FISH | 40/423 (9.5) | 27/423 (6.4) | 13/423 (3.1) |
| 2013 | Yang et al. [60] | 113 | IHC | 17/113 (15.0) | 9/113 (8.0) | 8/113 (7.1) |
| 2013 | Cockburn et al. [61] | 133 | IHC/FISH | 16/133 (12.0) | 9/133 (6.8) | 7/133 (5.3) |
| 2013 | Lee et al. [62] | 120 | IHC/FISH | 11/107 (10.3) | 6/107 (5.6) | 5/107 (4.7) |
| 2009 | Hirata et al. [63] | 368 | IHC/FISH | 35/368 (9.5) | 22/368 (6.0) | 13/368 (3.5) |
| 2008 | Kasami et al. [64] | 173 | IHC/FISH | 0/173 (0) | 0/173 (0) | 0/173 (0) |
| 2006 | Neubauer et al. [65] | 87 | IHC | 13/87 (14.9) | 11/87 (12.6) | 2/87 (2.3) |
| 2003 | Faneyte et al. [66] | 50 | IHC | 3/50 (6.0) | 2/50 (4.0) | 1/50 (2.0) |
| Year | Author | Total No. of cases | Method | Site |
Frequency of HER2 alteration, n (%) |
||
|---|---|---|---|---|---|---|---|
| Total | + to – | – to + | |||||
| 2019 | Woo et al. [79] | 152 | IHC/SISH | All | 12/152 (7.9) | 9/152 (5.9) | 3/152 (2.0) |
| 2014 | de Duenas et al. [70] | 165 | IHC/FISH | All | 5/165 (3.0) | 0/165 (0.0) | 5/165 (3.0) |
| 2013 | Curtit et al. [71] | 219 | IHC/FISH | All | 8/219 (3.7) | 6/219 (2.7) | 2/219 (0.9) |
| 2013 | Nakamura et al. [72] | 156 | IHC/FISH | All | 13/156 (8.3) | 5/156 (3.2) | 8/156 (5.1) |
| 2013 | Aurilio et al. [73] | 86 | IHC/FISH | Bone | 6/86 (7.0) | 2/86 (2.3) | 4/86 (4.7) |
| 2012 | Duchnowska et al. [74] | 119 | IHC/FISH | Brain | 17/119 (14.3) | 7/119 (5.9) | 10/119 (8.4) |
| 2012 | Jensen et al. [75] | 114 | IHC/FISH | All | 10/114 (8.8) | 2/114 (1.8) | 8/114 (7.0) |
| 2011 | Bogina et al. [76] | 136 | IHC/SISH | All | 1/136 (0.7) | 0/136 (0.0) | 1/136 (0.7) |
| 2011 | Chang et al. [77] | 56 | IHC/FISH | All | 7/56 (12.5) | 2/56 (3.6) | 5/56 (8.9) |
| 2010 | Hoefnagel et al. [78] | 233 | IHC/SISH | All | 12/233 (5.2) | 6/233 (2.6) | 6/233 (2.6) |
- 1. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235: 177-82. ArticlePubMed
- 2. Tandon AK, Clark GM, Chamness GC, Ullrich A, McGuire WL. HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol 1989; 7: 1120-8. ArticlePubMed
- 3. Couturier J, Vincent-Salomon A, Nicolas A, et al. Strong correlation between results of fluorescent in situ hybridization and immunohistochemistry for the assessment of the ERBB2 (HER-2/neu) gene status in breast carcinoma. Mod Pathol 2000; 13: 1238-43. ArticlePubMedPDF
- 4. Lebeau A, Deimling D, Kaltz C, et al. Her-2/neu analysis in archival tissue samples of human breast cancer: comparison of immunohistochemistry and fluorescence in situ hybridization. J Clin Oncol 2001; 19: 354-63. ArticlePubMed
- 5. Yaziji H, Goldstein LC, Barry TS, et al. HER-2 testing in breast cancer using parallel tissue-based methods. JAMA 2004; 291: 1972-7. ArticlePubMed
- 6. Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007; 25: 118-45. PubMed
- 7. Tanner M, Gancberg D, Di Leo A, et al. Chromogenic in situ hybridization: a practical alternative for fluorescence in situ hybridization to detect HER-2/neu oncogene amplification in archival breast cancer samples. Am J Pathol 2000; 157: 1467-72. PubMedPMC
- 8. Dandachi N, Dietze O, Hauser-Kronberger C. Chromogenic in situ hybridization: a novel approach to a practical and sensitive method for the detection of HER2 oncogene in archival human breast carcinoma. Lab Invest 2002; 82: 1007-14. ArticlePubMed
- 9. Gong Y, Gilcrease M, Sneige N. Reliability of chromogenic in situ hybridization for detecting HER-2 gene status in breast cancer: comparison with fluorescence in situ hybridization and assessment of interobserver reproducibility. Mod Pathol 2005; 18: 1015-21. ArticlePubMedPDF
- 10. Dietel M, Ellis IO, Hofler H, et al. Comparison of automated silver enhanced in situ hybridisation (SISH) and fluorescence ISH (FISH) for the validation of HER2 gene status in breast carcinoma according to the guidelines of the American Society of Clinical Oncology and the College of American Pathologists. Virchows Arch 2007; 451: 19-25. ArticlePubMedPDF
- 11. Koh YW, Lee HJ, Lee JW, Kang J, Gong G. Dual-color silver-enhanced in situ hybridization for assessing HER2 gene amplification in breast cancer. Mod Pathol 2011; 24: 794-800. ArticlePubMedPDF
- 12. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013; 31: 3997-4013. PubMed
- 13. Wolff AC, Hammond ME, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol 2018; 36: 2105-22. PubMed
- 14. Wolff AC, Hammond ME, Hicks DG, et al. Reply to E.A. Rakha et al. J Clin Oncol 2015; 33: 1302-4. ArticlePubMed
- 15. Liu ZH, Wang K, Lin DY, et al. Impact of the updated 2018 ASCO/CAP guidelines on HER2 FISH testing in invasive breast cancer: a retrospective study of HER2 FISH results of 2233 cases. Breast Cancer Res Treat 2019; 175: 51-7. ArticlePubMedPDF
- 16. Gordian-Arroyo AM, Zynger DL, Tozbikian GH. Impact of the 2018 ASCO/CAP HER2 Guideline Focused Update. Am J Clin Pathol 2019; 152: 17-26. ArticlePubMedPDF
- 17. Xu B, Shen J, Guo W, Zhao W, Zhuang Y, Wang L. Impact of the 2018 ASCO/CAP HER2 guidelines update for HER2 testing by FISH in breast cancer. Pathol Res Pract 2019; 215: 251-5. ArticlePubMed
- 18. Moeder CB, Giltnane JM, Harigopal M, et al. Quantitative justification of the change from 10% to 30% for human epidermal growth factor receptor 2 scoring in the American Society of Clinical Oncology/College of American Pathologists guidelines: tumor heterogeneity in breast cancer and its implications for tissue microarray based assessment of outcome. J Clin Oncol 2007; 25: 5418-25. ArticlePubMed
- 19. Seol H, Lee HJ, Choi Y, et al. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: its clinicopathological significance. Mod Pathol 2012; 25: 938-48. ArticlePubMedPDF
- 20. Lee HJ, Seo AN, Kim EJ, et al. HER2 heterogeneity affects trastuzumab responses and survival in patients with HER2-positive metastatic breast cancer. Am J Clin Pathol 2014; 142: 755-66. ArticlePubMedPDF
- 21. Lee HJ, Kim JY, Park SY, et al. Clinicopathologic significance of the intratumoral heterogeneity of HER2 gene amplification in HER2- positive breast cancer patients treated with adjuvant trastuzumab. Am J Clin Pathol 2015; 144: 570-8. ArticlePubMedPDF
- 22. Hou Y, Nitta H, Wei L, et al. HER2 intratumoral heterogeneity is independently associated with incomplete response to anti-HER2 neoadjuvant chemotherapy in HER2-positive breast carcinoma. Breast Cancer Res Treat 2017; 166: 447-57. ArticlePubMedPDF
- 23. Vance GH, Barry TS, Bloom KJ, et al. Genetic heterogeneity in HER2 testing in breast cancer: panel summary and guidelines. Arch Pathol Lab Med 2009; 133: 611-2. ArticlePubMedPDF
- 24. Walker RA, Bartlett JM, Dowsett M, et al. HER2 testing in the UK: further update to recommendations. J Clin Pathol 2008; 61: 818-24. ArticlePubMed
- 25. Starczynski J, Atkey N, Connelly Y, et al. HER2 gene amplification in breast cancer: a rogues' gallery of challenging diagnostic cases: UKNEQAS interpretation guidelines and research recommendations. Am J Clin Pathol 2012; 137: 595-605. ArticlePubMedPDF
- 26. Hanna WM, Ruschoff J, Bilous M, et al. HER2 in situ hybridization in breast cancer: clinical implications of polysomy 17 and genetic heterogeneity. Mod Pathol 2014; 27: 4-18. ArticlePMCPDF
- 27. Lee K, Jang MH, Chung YR, et al. Prognostic significance of centromere 17 copy number gain in breast cancer depends on breast cancer subtype. Hum Pathol 2017; 61: 111-20. ArticlePubMed
- 28. Gunn S, Yeh IT, Lytvak I, et al. Clinical array-based karyotyping of breast cancer with equivocal HER2 status resolves gene copy number and reveals chromosome 17 complexity. BMC Cancer 2010; 10: 396.ArticlePubMedPMCPDF
- 29. Yeh IT, Martin MA, Robetorye RS, et al. Clinical validation of an array CGH test for HER2 status in breast cancer reveals that polysomy 17 is a rare event. Mod Pathol 2009; 22: 1169-75. ArticlePubMedPDF
- 30. Moelans CB, de Weger RA, van Diest PJ. Absence of chromosome 17 polysomy in breast cancer: analysis by CEP17 chromogenic in situ hybridization and multiplex ligation-dependent probe amplification. Breast Cancer Res Treat 2010; 120: 1-7. ArticlePubMedPDF
- 31. Marchio C, Lambros MB, Gugliotta P, et al. Does chromosome 17 centromere copy number predict polysomy in breast cancer? A fluorescence in situ hybridization and microarray-based CGH analysis. J Pathol 2009; 219: 16-24. ArticlePubMedPDF
- 32. Jang MH, Kim EJ, Kim HJ, Chung YR, Park SY. Assessment of HER2 status in invasive breast cancers with increased centromere 17 copy number. Breast Cancer Res Treat 2015; 153: 67-77. ArticlePubMedPDF
- 33. Ma Y, Lespagnard L, Durbecq V, et al. Polysomy 17 in HER-2/neu status elaboration in breast cancer: effect on daily practice. Clin Cancer Res 2005; 11: 4393-9. ArticlePubMedPDF
- 34. Merola R, Mottolese M, Orlandi G, et al. Analysis of aneusomy level and HER-2 gene copy number and their effect on amplification rate in breast cancer specimens read as 2+ in immunohistochemical analysis. Eur J Cancer 2006; 42: 1501-6. ArticlePubMed
- 35. Hyun CL, Lee HE, Kim KS, et al. The effect of chromosome 17 polysomy on HER-2/neu status in breast cancer. J Clin Pathol 2008; 61: 317-21. ArticlePubMed
- 36. Downey L, Livingston RB, Koehler M, et al. Chromosome 17 polysomy without human epidermal growth factor receptor 2 amplification does not predict response to lapatinib plus paclitaxel compared with paclitaxel in metastatic breast cancer. Clin Cancer Res 2010; 16: 1281-8. ArticlePubMedPDF
- 37. Perez EA, Reinholz MM, Hillman DW, et al. HER2 and chromosome 17 effect on patient outcome in the N9831 adjuvant trastuzumab trial. J Clin Oncol 2010; 28: 4307-15. ArticlePubMedPMC
- 38. Orsaria M, Khelifa S, Buza N, Kamath A, Hui P. Chromosome 17 polysomy: correlation with histological parameters and HER2NEU gene amplification. J Clin Pathol 2013; 66: 1070-5. ArticlePubMed
- 39. Krishnamurti U, Hammers JL, Atem FD, Storto PD, Silverman JF. Poor prognostic significance of unamplified chromosome 17 polysomy in invasive breast carcinoma. Mod Pathol 2009; 22: 1044-8. ArticlePubMedPDF
- 40. Vanden Bempt I, Van Loo P, Drijkoningen M, et al. Polysomy 17 in breast cancer: clinicopathologic significance and impact on HER-2 testing. J Clin Oncol 2008; 26: 4869-74. ArticlePubMed
- 41. Bartlett JM, Munro AF, Dunn JA, et al. Predictive markers of anthracycline benefit: a prospectively planned analysis of the UK National Epirubicin Adjuvant Trial (NEAT/BR9601). Lancet Oncol 2010; 11: 266-74. ArticlePubMed
- 42. Tibau A, Lopez-Vilaro L, Perez-Olabarria M, et al. Chromosome 17 centromere duplication and responsiveness to anthracycline-based neoadjuvant chemotherapy in breast cancer. Neoplasia 2014; 16: 861-7. ArticlePubMedPMC
- 43. Kim A, Shin HC, Bae YK, et al. Multiplication of chromosome 17 centromere is associated with prognosis in patients with invasive breast cancers exhibiting normal HER2 and TOP2A status. J Breast Cancer 2012; 15: 24-33. ArticlePubMedPMC
- 44. Nielsen KV, Ejlertsen B, Moller S, et al. Lack of independent prognostic and predictive value of centromere 17 copy number changes in breast cancer patients with known HER2 and TOP2A status. Mol Oncol 2012; 6: 88-97. ArticlePubMedPDF
- 45. Zaczek A, Markiewicz A, Supernat A, et al. Prognostic value of TOP2A gene amplification and chromosome 17 polysomy in early breast cancer. Pathol Oncol Res 2012; 18: 885-94. ArticlePubMedPDF
- 46. Fountzilas G, Dafni U, Bobos M, et al. Evaluation of the prognostic role of centromere 17 gain and HER2/topoisomerase II alpha gene status and protein expression in patients with breast cancer treated with anthracycline-containing adjuvant chemotherapy: pooled analysis of two Hellenic Cooperative Oncology Group (HeCOG) phase III trials. BMC Cancer 2013; 13: 163.ArticlePubMedPMCPDF
- 47. Sneige N, Hess KR, Multani AS, Gong Y, Ibrahim NK. Prognostic significance of equivocal human epidermal growth factor receptor 2 results and clinical utility of alternative chromosome 17 genes in patients with invasive breast cancer: a cohort study. Cancer 2017; 123: 1115-23. ArticlePubMedPDF
- 48. Thompson AM, Moulder-Thompson SL. Neoadjuvant treatment of breast cancer. Ann Oncol 2012; 23 Suppl 10: x231-6. ArticlePubMedPDF
- 49. Lee SH, Chung MA, Quddus MR, Steinhoff MM, Cady B. The effect of neoadjuvant chemotherapy on estrogen and progesterone receptor expression and hormone receptor status in breast cancer. Am J Surg 2003; 186: 348-50. ArticlePubMed
- 50. Makris A, Powles TJ, Allred DC, et al. Quantitative changes in cytological molecular markers during primary medical treatment of breast cancer: a pilot study. Breast Cancer Res Treat 1999; 53: 51-9. ArticlePubMed
- 51. Zhang N, Moran MS, Huo Q, Haffty BG, Yang Q. The hormonal receptor status in breast cancer can be altered by neoadjuvant chemotherapy: a meta-analysis. Cancer Invest 2011; 29: 594-8. ArticlePubMed
- 52. Ahn S, Kim HJ, Kim M, et al. Negative conversion of progesterone receptor status after primary systemic therapy is associated with poor clinical outcome in patients with breast cancer. Cancer Res Treat 2018; 50: 1418-32. ArticlePubMedPMCPDF
- 53. De La Cruz LM, Harhay MO, Zhang P, Ugras S. Impact of neoadjuvant chemotherapy on breast cancer subtype: does subtype change and, if so, how?: IHC profile and neoadjuvant chemotherapy. Ann Surg Oncol 2018; 25: 3535-40. ArticlePubMedPDF
- 54. Xian Z, Quinones AK, Tozbikian G, Zynger DL. Breast cancer biomarkers before and after neoadjuvant chemotherapy: does repeat testing impact therapeutic management? Hum Pathol 2017; 62: 215-21. ArticlePubMed
- 55. Reddy OL, Apple SK. Breast cancer biomarker changes after neoadjuvant chemotherapy: a single institution experience and literature review. Clin Oncol 2017; 2: 1245.
- 56. Gahlaut R, Bennett A, Fatayer H, et al. Effect of neoadjuvant chemotherapy on breast cancer phenotype, ER/PR and HER2 expression: implications for the practising oncologist. Eur J Cancer 2016; 60: 40-8. ArticlePubMed
- 57. Lim SK, Lee MH, Park IH, et al. Impact of molecular subtype conversion of breast cancers after neoadjuvant chemotherapy on clinical outcome. Cancer Res Treat 2016; 48: 133-41. ArticlePubMedPDF
- 58. Zhou X, Zhang J, Yun H, et al. Alterations of biomarker profiles after neoadjuvant chemotherapy in breast cancer: tumor heterogeneity should be taken into consideration. Oncotarget 2015; 6: 36894-902. ArticlePubMedPMC
- 59. Jin X, Jiang YZ, Chen S, Yu KD, Shao ZM, Di GH. Prognostic value of receptor conversion after neoadjuvant chemotherapy in breast cancer patients: a prospective observational study. Oncotarget 2015; 6: 9600-11. ArticlePubMedPMC
- 60. Yang YF, Liao YY, Li LQ, Xie SR, Xie YF, Peng NF. Changes in ER, PR and HER2 receptors status after neoadjuvant chemotherapy in breast cancer. Pathol Res Pract 2013; 209: 797-802. ArticlePubMed
- 61. Cockburn A, Yan J, Rahardja D, et al. Modulatory effect of neoadjuvant chemotherapy on biomarkers expression; assessment by digital image analysis and relationship to residual cancer burden in patients with invasive breast cancer. Hum Pathol 2014; 45: 249-58. ArticlePubMed
- 62. Lee HC, Ko H, Seol H, et al. Expression of immunohistochemical markers before and after neoadjuvant chemotherapy in breast carcinoma, and their use as predictors of response. J Breast Cancer 2013; 16: 395-403. ArticlePubMedPMC
- 63. Hirata T, Shimizu C, Yonemori K, et al. Change in the hormone receptor status following administration of neoadjuvant chemotherapy and its impact on the long-term outcome in patients with primary breast cancer. Br J Cancer 2009; 101: 1529-36. ArticlePubMedPMCPDF
- 64. Kasami M, Uematsu T, Honda M, et al. Comparison of estrogen receptor, progesterone receptor and Her-2 status in breast cancer pre- and post-neoadjuvant chemotherapy. Breast 2008; 17: 523-7. ArticlePubMed
- 65. Neubauer H, Gall C, Vogel U, et al. Changes in tumour biological markers during primary systemic chemotherapy (PST). Anticancer Res 2008; 28: 1797-804. PubMed
- 66. Faneyte IF, Schrama JG, Peterse JL, Remijnse PL, Rodenhuis S, van de Vijver MJ. Breast cancer response to neoadjuvant chemotherapy: predictive markers and relation with outcome. Br J Cancer 2003; 88: 406-12. ArticlePubMedPMCPDF
- 67. Guarneri V, Dieci MV, Barbieri E, et al. Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann Oncol 2013; 24: 2990-4. ArticlePubMedPDF
- 68. Valent A, Penault-Llorca F, Cayre A, Kroemer G. Change in HER2 (ERBB2) gene status after taxane-based chemotherapy for breast cancer: polyploidization can lead to diagnostic pitfalls with potential impact for clinical management. Cancer Genet 2013; 206: 37-41. ArticlePubMed
- 69. Schrijver W, Suijkerbuijk KP, van Gils CH, van der Wall E, Moelans CB, van Diest PJ. Receptor conversion in distant breast cancer metastases: a systematic review and meta-analysis. J Natl Cancer Inst 2018; 110: 568-80. ArticlePubMedPDF
- 70. de Duenas EM, Hernandez AL, Zotano AG, et al. Prospective evaluation of the conversion rate in the receptor status between primary breast cancer and metastasis: results from the GEICAM 2009-03 ConvertHER study. Breast Cancer Res Treat 2014; 143: 507-15. ArticlePubMedPMCPDF
- 71. Curtit E, Nerich V, Mansi L, et al. Discordances in estrogen receptor status, progesterone receptor status, and HER2 status between primary breast cancer and metastasis. Oncologist 2013; 18: 667-74. ArticlePubMedPMCPDF
- 72. Nakamura R, Yamamoto N, Onai Y, Watanabe Y, Kawana H, Miyazaki M. Importance of confirming HER2 overexpression of recurrence lesion in breast cancer patients. Breast Cancer 2013; 20: 336-41. ArticlePubMedPDF
- 73. Aurilio G, Monfardini L, Rizzo S, et al. Discordant hormone receptor and human epidermal growth factor receptor 2 status in bone metastases compared to primary breast cancer. Acta Oncol 2013; 52: 1649-56. ArticlePubMed
- 74. Duchnowska R, Dziadziuszko R, Trojanowski T, et al. Conversion of epidermal growth factor receptor 2 and hormone receptor expression in breast cancer metastases to the brain. Breast Cancer Res 2012; 14: R119.ArticlePubMedPMCPDF
- 75. Jensen JD, Knoop A, Ewertz M, Laenkholm AV. ER, HER2, and TOP2A expression in primary tumor, synchronous axillary nodes, and asynchronous metastases in breast cancer. Breast Cancer Res Treat 2012; 132: 511-21. ArticlePubMedPDF
- 76. Bogina G, Bortesi L, Marconi M, et al. Comparison of hormonal receptor and HER-2 status between breast primary tumours and relapsing tumours: clinical implications of progesterone receptor loss. Virchows Arch 2011; 459: 1-10. ArticlePubMedPMC
- 77. Chang HJ, Han SW, Oh DY, et al. Discordant human epidermal growth factor receptor 2 and hormone receptor status in primary and metastatic breast cancer and response to trastuzumab. Jpn J Clin Oncol 2011; 41: 593-9. ArticlePubMedPDF
- 78. Hoefnagel LD, van de Vijver MJ, van Slooten HJ, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res 2010; 12: R75.ArticlePubMedPMCPDF
- 79. Woo JW, Chung YR, Ahn S, et al. Changes in biomarker status in metastatic breast cancer and their prognostic value. J Breast Cancer 2019; 22: 439-52. ArticlePubMedPMCPDF
- 80. Fabi A, Di Benedetto A, Metro G, et al. HER2 protein and gene variation between primary and metastatic breast cancer: significance and impact on patient care. Clin Cancer Res 2011; 17: 2055-64. ArticlePubMedPDF
- 81. Van Poznak C, Harris LN, Somerfield MR. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American Society of Clinical Oncology clinical practice guideline. J Oncol Pract 2015; 11: 514-6. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- A Comprehensive Model Outperformed the Single Radiomics Model in Noninvasively Predicting the HER2 Status in Patients with Breast Cancer
Weimin Liu, Yiqing Yang, Xiaohong Wang, Chao Li, Chen Liu, Xiaolei Li, Junzhe Wen, Xue Lin, Jie Qin
Academic Radiology.2025; 32(1): 24. CrossRef - Multiparametric MRI Radiomics With Machine Learning for Differentiating HER2-Zero, -Low, and -Positive Breast Cancer: Model Development, Testing, and Interpretability Analysis
Yongxin Chen, Siyi Chen, Wenjie Tang, Qingcong Kong, Zhidan Zhong, Xiaomeng Yu, Yi Sui, Wenke Hu, Xinqing Jiang, Yuan Guo
American Journal of Roentgenology.2025;[Epub] CrossRef - Cervical Lymph Node Metastasis of Unknown Origin and Remote Primary at a Tertiary Cancer Centre in North India: Case Series with Review of Literature
Kriti Grover, Siddharth Arora, Mansi Dey, Deepti Awasthi, Harshad Sharma, Bibhu Prasad Mishra, Nitesh Mohan, Cheena Garg, Arjun Agarwal
Indian Journal of Otolaryngology and Head & Neck Surgery.2025; 77(1): 424. CrossRef - Pooled clinical trial analyses evaluating outcomes of HER2-low vs HER2-0 expression in patients with metastatic breast cancer following chemotherapy
Elizabeth B. Lamont, Emily Stein, Paolo Tarantino, Sara M. Tolaney, Corinne Ahlberg, Krishna Chinnathambu, Jiezhi Qi, Jackie Bilan, Ruthie Davi, Lisa Ensign
Breast Cancer Research and Treatment.2025; 210(1): 11. CrossRef - Insights into AI advances in immunohistochemistry for effective breast cancer treatment: a literature review of ER, PR, and HER2 scoring
Genevieve Chyrmang, Kangkana Bora, Anup Kr. Das, Gazi N. Ahmed, Lopamudra Kakoti
Current Medical Research and Opinion.2025; 41(1): 115. CrossRef - Prediction of Lung Adenocarcinoma Driver Genes Through Protein–Protein Interaction Networks Utilizing GenePlexus
Fei Yuan, Yu‐Hang Zhang, FeiMing Huang, Xiaoyu Cao, Lei Chen, JiaBo Li, WenFeng Shen, KaiYan Feng, YuSheng Bao, Tao Huang, Yu‐Dong Cai
PROTEOMICS.2025;[Epub] CrossRef - Quantitative expression of estrogen, progesterone and human epidermal growth factor receptor-2 and their correlation with immunohistochemistry in breast cancer at Uganda Cancer Institute
Henry Wannume, Nixon Niyonzima, Sam Kalungi, Julius Boniface Okuni, Tonny Okecha, Edward Kakungulu, Steven Mpungu Kiwuwa, Geoffrey Waiswa, Sylvester Kadhumbula, Monica Namayanja, Martin Nabwana, Jackson Orem, Kenji Fujiwara
PLOS ONE.2025; 20(1): e0311185. CrossRef - Automated Quantification of HER2 Amplification Levels Using Deep Learning
Ching-Wei Wang, Kai-Lin Chu, Ting-Sheng Su, Keng-Wei Liu, Yi-Jia Lin, Tai-Kuang Chao
IEEE Journal of Biomedical and Health Informatics.2025; 29(1): 333. CrossRef - CAR-macrophage therapy for HER2-overexpressing advanced solid tumors: a phase 1 trial
Kim A. Reiss, Mathew G. Angelos, E. Claire Dees, Yuan Yuan, Naoto T. Ueno, Paula R. Pohlmann, Melissa L. Johnson, Joseph Chao, Olga Shestova, Jonathan S. Serody, Maggie Schmierer, Madison Kremp, Michael Ball, Rehman Qureshi, Benjamin H. Schott, Poonam Son
Nature Medicine.2025; 31(4): 1171. CrossRef - Human Cytomegalovirus Infection and Breast Cancer: A Literature Review of Clinical and Experimental Data
Rancés Blanco, Juan P. Muñoz
Biology.2025; 14(2): 174. CrossRef - An antibody-photosensitiser bioconjugate overcomes trastuzumab resistance in HER2-positive breast cancer
Mireia Jordà-Redondo, Ana Piqueras, Ana Castillo, Pedro Luis Fernández, Roger Bresolí-Obach, Lidia Blay, Joan Francesc Julián Ibáñez, Santi Nonell
European Journal of Medicinal Chemistry.2025; 290: 117511. CrossRef - Dynamic HER2-low status among patients with triple negative breast cancer (TNBC) and the impact of repeat biopsies
Yael Bar, Geoffrey Fell, Aylin Dedeoglu, Natalie Moffett, Neelima Vidula, Laura Spring, Seth A. Wander, Aditya Bardia, Naomi Ko, Beverly Moy, Leif W. Ellisen, Steven J. Isakoff
npj Breast Cancer.2025;[Epub] CrossRef - Clinical significances of RPL15 gene expression in circulating tumor cells of patients with breast cancer
Ying Zhuang, Keli Su, Shushu Liu, Wei Fan, Huijuan Lv, Wei Zhong
Biomedical Reports.2025; 22(5): 1. CrossRef - Advancing Breast Cancer Treatment: The Role of Immunotherapy and Cancer Vaccines in Overcoming Therapeutic Challenges
Marco Palma
Vaccines.2025; 13(4): 344. CrossRef - Complete preclinical evaluation of the novel antibody mimetic Nanofitin-IRDye800CW for diverse non-invasive diagnostic applications in the management of HER-2 positive tumors
Margherita Iaboni, Federico Crivellin, Francesca Arena, Francesca La Cava, Alessia Cordaro, Francesco Stummo, Daniele Faletto, Simon Huet, Leo Candela, Jessy Pedrault, Eugenia R. Zanella, Andrea Bertotti, Francesco Blasi, Alessandro Maiocchi, Luisa Poggi,
Scientific Reports.2025;[Epub] CrossRef - New insights on Galectin-9 expression in cancer prognosis: An updated systemic review and meta-analysis
Chun Yan So, Yusong Li, Kwan Ting Chow, Xiaosheng Tan
PLOS ONE.2025; 20(3): e0320441. CrossRef - Role of artificial intelligence –based machine learning model in predicting HER2/neu gene status in breast cancer
Ghada Mohamed, Omar Hamdy, Anwar Alkallas, Youssef Tahoun, Mohammed Mohammed Gomaa, Inas Moaz, Ahmed Orabi, Yasmine Hany elzohery, AL-Shimaa Zakaria, Mahitab Ibrahim Eltohamy
Pathology - Research and Practice.2025; 270: 155927. CrossRef - HER2 mRNA Score From Quantitative ERBB2 mRNA Expression of Oncotype Dx
Hyunwoo Lee, Jai Min Ryu, Se Kyung Lee, Byung Joo Chae, Jonghan Yu, Jeong Eon Lee, Seok Won Kim, Seok Jin Nam, Yoon Ah Cho, Eun Yoon Cho
American Journal of Surgical Pathology.2025; 49(8): 807. CrossRef - Neratinib and metformin: A novel therapeutic approach against HER2-Positive Breast Cancer
Hadeel Kheraldine, Arij Fouzat Hassan, Sumayyah Saeed, Maysaloun Merhi, Jericha Miles Mateo, Monika Ulamec, Melita Peric-Balja, Semir Vranic, Hamda Al-Thawadi, Ala-Eddin Al Moustafa
Biomedicine & Pharmacotherapy.2025; 187: 118034. CrossRef - Machine learning prediction of HER2-low expression in breast cancers based on hematoxylin–eosin-stained slides
Jun Du, Jun Shi, Dongdong Sun, Yifei Wang, Guanfeng Liu, Jingru Chen, Wei Wang, Wenchao Zhou, Yushan Zheng, Haibo Wu
Breast Cancer Research.2025;[Epub] CrossRef - Predicting sentinel lymph node metastatic burden with intravoxel incoherent motion diffusion-weighted imaging and dynamic contrast-enhanced magnetic resonance imaging in clinical early-stage breast cancer patients
Mingli Jin, Fang Xiao, Qi Zhao, Ying Jiang, Zhihua Pan, Zhicai Duan, Juxi Jiang, Miaoqi Zhang, Jian Shu
Magnetic Resonance Imaging.2025; 121: 110397. CrossRef - HbA1c levels and breast cancer prognosis in women without diabetes
Jonas Busk Holm, Jens Meldgaard Bruun, Peer Christiansen, Reimar Wernich Thomsen, Jan Frystyk, Deirdre Cronin-Fenton, Signe Borgquist
BMC Cancer.2025;[Epub] CrossRef - Clinical Outcomes in Early-Stage HER2-Low and HER2-Zero Breast Cancer: Single-Center Experience
Jamshid Hamdard, Mehmet Haluk Yücel, Harun Muğlu, Özgür Açıkgöz, Aslı Çakır, Ahmet Bilici, Ömer Fatih Ölmez
Journal of Clinical Medicine.2025; 14(9): 2937. CrossRef - Molecular Mechanism of Breast Cancer and Predisposition of Mouse Mammary Tumor Virus Propagation Cycle
Arya Ghosh, Subash C.B. Gopinath
Current Medicinal Chemistry.2025; 32(12): 2330. CrossRef - ASSESSMENT OF RECEPTOR CONVERSION ROLE FOR ADVANCED BREAST CANCER ON THE CHEMORESISTANCE OCCURRENCE
Oleksii V. Movchan, Ivan I. Smolanka, Andriy O. Lyashenko, Anton D. Loboda, Iryna V. Dosenko, Oksana M. Ivankova
Clinical and Preventive Medicine.2025; (3): 56. CrossRef - Combining conventional magnetic resonance imaging (MRI) parameters with clinicopathologic data for differentiation of the three-tiered human epidermal growth factor receptor 2 (HER2) status in breast cancer
W. Liu, C. Liu, Y. Yang, Y. Chen, A. Muhetaier, Z. Lin, Z. Weng, X. Wang, P. Zhang, J. Qin
Clinical Radiology.2025; 86: 106955. CrossRef - IN SILICO STUDY: THE POTENTIAL OF KILEMO (Litsea cubeba) ENDEMIC PLANT FROM KALIMANTAN AS ANTI-BREAST CANCER THROUGH HER2 INHIBITION
Dwi Utami, Tarisha Elmaningtyas Zahro, Khairun Nisa, Muhammad Farid
JIIS (Jurnal Ilmiah Ibnu Sina): Ilmu Farmasi dan Kesehatan.2025; 10(1): 166. CrossRef - Trastuzumab Deruxtecan in Previously Treated HER2-Low Metastatic Breast Cancer: Real-World Multicentric Study in the Portuguese Population
Luísa Soares Miranda, Maria João Sousa, Miguel Martins Braga, Marisa Couto, Isabel Vieira Fernandes, Francisca Abreu, Inês Eiriz, Catarina Lopes Fernandes, Alice Fonseca Marques, Maria Teresa Marques, Raquel Romão, Fernando Gonçalves, Joana Simões, Antóni
Cancers.2025; 17(12): 1911. CrossRef - Factors influencing pathological complete response to neoadjuvant chemotherapy in resectable breast cancer: A retrospective study
Jian Kang, Huifen Xiong, Xiaoliu Jiang, Zhaohui Huang, Yonghong Guo, Yali Cao, Jingxian Ding
Oncology Letters.2025; 30(1): 1. CrossRef - Association of HER2-low with clinicopathological features in patients with early invasive lobular breast cancer: an international multicentric study
Karen Van Baelen, Ha-Linh Nguyen, François Richard, Gitte Zels, Maria Margarete Karsten, Guilherme Nader-Marta, Peter Vermeulen, Luc Dirix, Adam David Dordevic, Evandro de Azambuja, Denis Larsimont, Marion Maetens, Elia Biganzoli, Hans Wildiers, Ann Smeet
Breast Cancer Research.2025;[Epub] CrossRef - Collagenase type IV improves the quality of HER2 fluorescence in situ hybridization for breast cancers
Shang-En Lee, Sheng-Chi Hsu, Tsai-Hsien Hung, Kwai-Fong Ng, Tse-Ching Chen
American Journal of Clinical Pathology.2025; 164(3): 342. CrossRef - Resonant multi-focal scanning super-resolution microscopy with extended depth and field-of-view
Kidan Tadesse, Keyi Han, Wenhao Liu, Oliver S. Lee, Shu Jia
Cell Reports Physical Science.2025; 6(7): 102680. CrossRef - Scalable Nuclei Detection in HER2-SISH Whole Slide Images via Fine-Tuned Stardist with Expert-Annotated Regions of Interest
Zaka Ur Rehman, Mohammad Faizal Ahmad Fauzi, Wan Siti Halimatul Munirah Wan Ahmad, Fazly Salleh Abas, Phaik-Leng Cheah, Seow-Fan Chiew, Lai-Meng Looi
Diagnostics.2025; 15(13): 1584. CrossRef - Molecular landscape of HER2-mutated non-small cell lung cancer in Northeastern Brazil: Clinical, histopathological, and genomic insights
Cleto Dantas Nogueira, Samuel Frota, Huylmer Lucena Chaves, Juliana Cordeiro de Sousa, Guilherme de Sousa Veloso, Francisco Jonathan dos Santos Araujo, Gabriel Barbosa Silva, Samuel Silva Ferreira, Marclesson Santos Alves, Fabio Nasser, Ezequiel Rangel, F
Oncotarget.2025; 16(1): 467. CrossRef - Correlation between CTMP expression levels and resistance to trastuzumab in HER2 + metastatic breast cancer
Mania Makhoul, Maher Saifo, Fariz Ahmad, Jumana Saleh
Discover Oncology.2025;[Epub] CrossRef - The association between body mass index and neoadjuvant chemotherapy response in patients with breast cancer
Jonas Busk Holm, Stine Blaabjerg Skovbjerg, Hanne Melgaard Nielsen, Peer Christiansen, Jens Meldgaard Bruun, Jan Alsner, Deirdre Cronin-Fenton, Signe Borgquist
Breast Cancer Research.2025;[Epub] CrossRef - An immunohistochemical characterization of basal cell carcinoma in patients below 40 years of age
Vincent Waller, Myriam Boeschen, Thomas Lingscheidt, Mathias Stiller, Lena Eickenscheidt, Thomas Wilhelm, Sonja Grunewald, Mirjana Ziemer, Jan‐Christoph Simon, Hendrik Bläker, Maximilian von Laffert
JDDG: Journal der Deutschen Dermatologischen Gesellschaft.2025;[Epub] CrossRef - Clinical Relationship Between Serum ApoB, HER2, and Myocardial Ischemia Risk in Breast Cancer Patients
Yeyan Lei, Dongmei Li, Shuang Bai, Xing Zeng, Rongyuan Yang, Qing Liu
Cancer Reports.2025;[Epub] CrossRef - Dual-targeting and steric hindrance resolution in HER2 IHC: a novel approach to improve diagnostic sensitivity
Li Luo, Xi Zhang, Linqiong Chen, Zhuohan Chen, Yuchen Wang, Kaihao Huang, Xiaoyun Lin, Hongxiang Zhu, Wangqi Du
BMC Cancer.2025;[Epub] CrossRef - Time‐Dependent Diffusion MRI‐Based Microstructural Mapping for Characterizing HER2‐Zero, ‐Low, ‐Ultra‐Low, and ‐Positive Breast Cancer
Xiaoxia Wang, Yao Huang, Ying Cao, Huifang Chen, Xueqin Gong, Xiaosong Lan, Jiuquan Zhang, Zhaoxiang Ye
Journal of Magnetic Resonance Imaging.2025;[Epub] CrossRef - Digital pathology enabling lean management of HER2/neu testing in breast Cancer
Aishwarya Sharma, Prarthna Shah, Manali Ranade, Trupti Pai, Ayushi Sahay, Asawari Patil, Tanuja Shet, Heena Gupta, Devika Chauhan, Puneet Somal, Sankalp Sancheti, Sangeeta Desai
Journal of Pathology Informatics.2025; : 100515. CrossRef - Enhancing HER2-low breast cancer detection with quantitative transcriptomics
Maria-Anna Misiakou, Maj-Britt Jensen, Maj-Lis Talman, Bent Ejlertsen, Maria Rossing
npj Breast Cancer.2025;[Epub] CrossRef - Caracterización inmunohistoquímica del cáncer de mama correlacionado con histopatología,estudio realizado en un hospital de Ecuador
María Fernanda Calderón León, Diego Mauricio Cabrera Moyano, Jorge Daniel Cárdenas Rodríguez, Paula Andrea Vásquez Jaramillo, Andrea Alexandra Saltos Román, Maryoli González Sánchez
Journal of the Selva Andina Research Society.2025; 16(2): 116. CrossRef - Relationship of vitamin D receptor expression with hormone receptors and other clinicopathological features in primary breast carcinomas: A retrospective cross-sectional study
Yaşar Ünlü, Ethem Ömeroğlu, Abdülhalim Serden Ay, Meryem İlkay Eren Karanis, Kilinç Ayşe Nur Uğur, Esra Yilmaz
Medicine.2025; 104(35): e44222. CrossRef - Improving HER2 Diagnostics with Digital Real‐Time PCR for Ultrafast, Precise Prediction of Anti‐HER2 Therapy Response in Patients with Breast Cancer
Hee‐Joo Choi, Soo Young Park, Minsik Song, Jinhyuk Chang, YoonSik Kim, Hosub Park, Chihwan David Cha, Sohyeon Yang, Nam Hun Heo, Min Ji Song, Da Sol Kim, Hayeon Kim, Minuk Kim, Jae Eun Park, Yesung Lee, EunChae Ji, Heekyoung Chung, Ilecheon Jeong, Mineui
Small Methods.2025;[Epub] CrossRef - Identification of novel chemical scaffolds against kinase domain of cancer causing human epidermal growth factor receptor 2: a systemic chemoinformatic approach
Faris Alrumaihi
Journal of Biomolecular Structure and Dynamics.2024; 42(12): 6269. CrossRef - Standardized pathology report for HER2 testing in compliance with 2023 ASCO/CAP updates and 2023 ESMO consensus statements on HER2-low breast cancer
Mariia Ivanova, Francesca Maria Porta, Marianna D’Ercole, Carlo Pescia, Elham Sajjadi, Giulia Cursano, Elisa De Camilli, Oriana Pala, Giovanni Mazzarol, Konstantinos Venetis, Elena Guerini-Rocco, Giuseppe Curigliano, Giuseppe Viale, Nicola Fusco
Virchows Archiv.2024; 484(1): 3. CrossRef - Analytical Performance Evaluation of a 523-Gene Circulating Tumor DNA Assay for Next-Generation Sequencing–Based Comprehensive Tumor Profiling in Liquid Biopsy Samples
Johannes Harter, Eleonora Buth, Janina Johaenning, Florian Battke, Maria Kopp, Henning Zelba, Martin Schulze, Jiri Koedding, Saskia Biskup
The Journal of Molecular Diagnostics.2024; 26(1): 61. CrossRef - Are There More HER2 FISH in the Sea? An Institution’s Experience in Identifying HER2 Positivity Using Fluorescent In Situ Hybridization in Patients with HER2 Negative Immunohistochemistry
Camille Suydam, Fairouz Chibane, Nicole Brown, Madeleine Schlafly, Alicia H. Arnold, Intisar Ghleilib, Melissa Easley, Joseph White
Annals of Surgical Oncology.2024; 31(1): 376. CrossRef - HER2 copy number determination in breast cancer using the highly sensitive droplet digital PCR method
Beate Alinger-Scharinger, Cornelia Kronberger, Georg Hutarew, Wolfgang Hitzl, Roland Reitsamer, Klaassen-Federspiel Frederike, Martina Hager, Thorsten Fischer, Karl Sotlar, Heidi Jaksch-Bogensperger
Virchows Archiv.2024; 485(1): 53. CrossRef - Impact of HER2-low status for patients with early-stage breast cancer and non-pCR after neoadjuvant chemotherapy: a National Cancer Database Analysis
Huiyue Li, Jennifer K. Plichta, Kan Li, Yizi Jin, Samantha M. Thomas, Fei Ma, Li Tang, Qingyi Wei, You-Wen He, Qichen Chen, Yuanyuan Guo, Yueping Liu, Jian Zhang, Sheng Luo
Breast Cancer Research and Treatment.2024; 204(1): 89. CrossRef - Qualification of a multiplexed tissue imaging assay and detection of novel patterns of HER2 heterogeneity in breast cancer
Jennifer L. Guerriero, Jia-Ren Lin, Ricardo G. Pastorello, Ziming Du, Yu-An Chen, Madeline G. Townsend, Kenichi Shimada, Melissa E. Hughes, Siyang Ren, Nabihah Tayob, Kelly Zheng, Shaolin Mei, Alyssa Patterson, Krishan L. Taneja, Otto Metzger, Sara M. Tol
npj Breast Cancer.2024;[Epub] CrossRef - Clinical Utility and Benefits of Comprehensive Genomic Profiling in Cancer
Melissa Yuwono Tjota, Jeremy P Segal, Peng Wang
The Journal of Applied Laboratory Medicine.2024; 9(1): 76. CrossRef - High contrast breast cancer biomarker semi-quantification and immunohistochemistry imaging using upconverting nanoparticles
Sanathana Konugolu Venkata Sekar, Hui Ma, Katarzyna Komolibus, Gokhan Dumlupinar, Matthias J. Mickert, Krzysztof Krawczyk, Stefan Andersson-Engels
Biomedical Optics Express.2024; 15(2): 900. CrossRef - HER2-low breast cancer and response to neoadjuvant chemotherapy: a population-based cohort study
Ximena Baez-Navarro, Mieke R. van Bockstal, Agnes Jager, Carolien H.M. van Deurzen
Pathology.2024; 56(3): 334. CrossRef - Fast-tracking drug development with biomarkers and companion diagnostics
Noreen McBrearty, Devika Bahal, Suso Platero
Journal of Cancer Metastasis and Treatment.2024;[Epub] CrossRef - Emerging Landscape of Targeted Therapy of Breast Cancers With Low Human Epidermal Growth Factor Receptor 2 Protein Expression
Gary Tozbikian, Savitri Krishnamurthy, Marilyn M. Bui, Michael Feldman, David G. Hicks, Shabnam Jaffer, Thaer Khoury, Shi Wei, Hannah Wen, Paula Pohlmann
Archives of Pathology & Laboratory Medicine.2024; 148(2): 242. CrossRef - Treatment Patterns and Health Outcomes among Patients with HER2 IHC0/-Low Metastatic or Recurrent Breast Cancer
Eliya Farah, Chantelle Carbonell, Devon J. Boyne, Darren R. Brenner, Jan-Willem Henning, Daniel Moldaver, Simran Shokar, Winson Y. Cheung
Cancers.2024; 16(3): 518. CrossRef - Clinical Internal Dosimetry and Biodistribution of 177Lu-DOTA-Trastuzumab in HER2-Positive Metastatic and Locally Advanced Breast Carcinoma
Yoga S. Narwadkar, Rahul V. Parghane, Sudeep Sahu, Sangita Lad, Kamal Deep, Gaurav Wanage, Tejal Suralkar, Sharmila Banerjee, Sudeep Gupta, Sandip Basu, Rajendra A. Badwe
Clinical Nuclear Medicine.2024; 49(4): e149. CrossRef - Molecular Classifications in Gastric Cancer: A Call for Interdisciplinary Collaboration
Cristina Díaz del Arco, María Jesús Fernández Aceñero, Luis Ortega Medina
International Journal of Molecular Sciences.2024; 25(5): 2649. CrossRef - The combined immunohistochemical expression of AMBRA1 and SQSTM1 identifies patients with poorly differentiated cutaneous squamous cell carcinoma at risk of metastasis: A proof of concept study
Michael H. Alexander, William J. Cousins, Tom Ewen, Andrew P. South, Penny Lovat, Niki Stefanos
Journal of Cutaneous Pathology.2024; 51(6): 450. CrossRef - Concordance between pathologists and between specimen types in detection of HER2-low breast carcinoma by immunohistochemistry
Jing Wang, Esther Yoon, Savitri Krishnamurthy
Annals of Diagnostic Pathology.2024; 70: 152288. CrossRef - Detection of HER2 expression using 99mTc-NM-02 nanobody in patients with breast cancer: a non-randomized, non-blinded clinical trial
Lingzhou Zhao, Yan Xing, Changcun Liu, Shaofei Ma, Wenhua Huang, Zhen Cheng, Jinhua Zhao
Breast Cancer Research.2024;[Epub] CrossRef - Pyrotinib and Trastuzumab Plus Chemotherapy Serve as an Acceptable Neoadjuvant Regimen Exhibiting Good Efficacy and Tolerance in HER2-Positive Breast Cancer Patients
Yibo Chen, Tianyi Zhang, Rui Zhang, Xuchen Cao
Cancer Biotherapy and Radiopharmaceuticals.2024; 39(6): 435. CrossRef - Clinicopathological and prognostic significance of VEGF, PDGF-B, and HER2/neu expression in gallbladder cancer
Pooja Shukla, Kumudesh Mishra, Ratnakar Shukla, Ruchira Vishwakarma, Niraj Kumari, Narendra Krishnani, Anu Behari, Vinay K. Kapoor
Journal of Cancer Research and Therapeutics.2024; 20(1): 349. CrossRef - Open questions, current challenges, and future perspectives in targeting human epidermal growth factor receptor 2-low breast cancer
G. Curigliano, R. Dent, H. Earle, S. Modi, P. Tarantino, G. Viale, S.M. Tolaney
ESMO Open.2024; 9(4): 102989. CrossRef - Concordance of HER2 status between core needle biopsy and surgical resection specimens of breast cancer: an analysis focusing on the HER2-low status
Sei Na, Milim Kim, Yujun Park, Hyun Jung Kwon, Hee-Chul Shin, Eun-Kyu Kim, Mijung Jang, Sun Mi Kim, So Yeon Park
Breast Cancer.2024; 31(4): 705. CrossRef - Impact of Neoadjuvant Chemotherapy (NAC) on Biomarker Expression in Breast Cancer
Suji Lee, Jee Yeon Kim, So Jeong Lee, Chung Su Hwang, Hyun Jung Lee, Kyung Bin Kim, Jung Hee Lee, Dong Hoon Shin, Kyung Un Choi, Chang Hun Lee, Gi Yeong Huh, Ahrong Kim
Medicina.2024; 60(5): 737. CrossRef - Clinical Use of Molecular Biomarkers in Canine and Feline Oncology: Current and Future
Heike Aupperle-Lellbach, Alexandra Kehl, Simone de Brot, Louise van der Weyden
Veterinary Sciences.2024; 11(5): 199. CrossRef - Clinical Validation of Artificial Intelligence–Powered PD-L1 Tumor Proportion Score Interpretation for Immune Checkpoint Inhibitor Response Prediction in Non–Small Cell Lung Cancer
Hyojin Kim, Seokhwi Kim, Sangjoon Choi, Changhee Park, Seonwook Park, Sergio Pereira, Minuk Ma, Donggeun Yoo, Kyunghyun Paeng, Wonkyung Jung, Sehhoon Park, Chan-Young Ock, Se-Hoon Lee, Yoon-La Choi, Jin-Haeng Chung
JCO Precision Oncology.2024;[Epub] CrossRef - Mesenchymal-epithelial transition factor amplification correlates with adverse pathological features and poor clinical outcome in colorectal cancer
Qiu-Xiao Yu, Ping-Ying Fu, Chi Zhang, Li Li, Wen-Ting Huang
World Journal of Gastrointestinal Surgery.2024; 16(5): 1395. CrossRef - Lipid nanoparticles-based RNA therapies for breast cancer treatment
Luigia Serpico, Yuewen Zhu, Renata Faria Maia, Sumedha Sumedha, Mohammad-Ali Shahbazi, Hélder A. Santos
Drug Delivery and Translational Research.2024; 14(10): 2823. CrossRef - Human epidermal growth factor receptor 2 (HER2) status in breast cancer: practice points and challenges
Natthawadee Laokulrath, Mihir Gudi, Syed Ahmed Salahuddin, Angela Phek Yoon Chong, Cristine Ding, Jabed Iqbal, Wei Qiang Leow, Benjamin Yongcheng Tan, Gary Tse, Emad Rakha, Puay Hoon Tan
Histopathology.2024; 85(3): 371. CrossRef - Comprehensive Immunohistochemical Analysis of Epithelial–Mesenchymal Transition Biomarkers in the Invasive Micropapillary Cancer of the Breast
Ozden Oz, Funda Alkan Tasli, Resmiye Irmak Yuzuguldu, Baha Zengel, Demet Kocatepe Cavdar, Merih Guray Durak, Raika Durusoy, Pranshu Sahgal
International Journal of Breast Cancer.2024;[Epub] CrossRef - Advancing HER2-low breast cancer management: enhancing diagnosis and treatment strategies
Simona Borstnar, Ivana Bozovic-Spasojevic, Ana Cvetanovic, Natalija Dedic Plavetic, Assia Konsoulova, Erika Matos, Lazar Popovic, Savelina Popovska, Snjezana Tomic, Eduard Vrdoljak
Radiology and Oncology.2024; 58(2): 258. CrossRef - Peer‐to‐peer validation of Ki‐67 scoring in a pathology quality circle as a tool to assess interobserver variability: are we better than we thought?
Marit Bernhardt, Leonie Weinhold, Christine Sanders, Oliver Hommerding, Jan‐Frederic Lau, Marieta Toma, Verena Tischler, Matthias Schmid, Tomasz Zienkiewicz, Ralf Hildenbrand, Peter Gerlach, Hui Zhou, Martin Braun, Gunnar Müller, Erich Sieber, Christian M
APMIS.2024; 132(10): 718. CrossRef - Immunohistochemical Expression of Cyclin D1 and p16 in Invasive Breast Carcinoma and Its Association with Clinicopathological Parameters
Shaivy Malik, Shakthivel V., Sana Ahuja, Charanjeet Ahluwalia
Indian Journal of Surgical Oncology.2024; 15(4): 864. CrossRef - Novel engineered HER2 specific recombinant protein nanocages for targeted drug delivery
Javad Kheshti, Mohammad Ahmadyousefi, Meysam Soleimani
Molecular Biology Reports.2024;[Epub] CrossRef - Circulating C-reactive protein levels as a prognostic biomarker in breast cancer across body mass index groups
J. B. Holm, E. Baggesen, D. Cronin-Fenton, J. Frystyk, J. M. Bruun, P. Christiansen, S. Borgquist
Scientific Reports.2024;[Epub] CrossRef - Standardized molecular pathology workflow for ctDNA-based ESR1 testing in HR+/HER2- metastatic breast cancer
Elena Guerini-Rocco, Konstantinos Venetis, Giulia Cursano, Eltjona Mane, Chiara Frascarelli, Francesco Pepe, Mariachiara Negrelli, Edoardo Olmeda, Davide Vacirca, Alberto Ranghiero, Dario Trapani, Carmen Criscitiello, Giuseppe Curigliano, Christian Rolfo,
Critical Reviews in Oncology/Hematology.2024; 201: 104427. CrossRef - The differences between pure and mixed invasive micropapillary breast cancer: the epithelial–mesenchymal transition molecules and prognosis
Ozden Oz, Resmiye Irmak Yuzuguldu, Ayse Yazici, Demet Kocatepe Cavdar, Cengiz Yilmaz, Mucteba Ozturk, Hilal Duzel, Duygu Gurel
Breast Cancer Research and Treatment.2024; 208(1): 41. CrossRef - Best practices for achieving consensus in HER2‐low expression in breast cancer: current perspectives from practising pathologists
Gary Tozbikian, Marilyn M. Bui, David G Hicks, Shabnam Jaffer, Thaer Khoury, Hannah Y Wen, Savitri Krishnamurthy, Shi Wei
Histopathology.2024; 85(3): 489. CrossRef - Consensus Guidelines on Human Epidermal Growth Factor Receptor 2 (HER2)-Low Testing in Breast Cancer in Malaysia
Pathmanathan Rajadurai, Sarala Ravindran, Bang Rom Lee, Suria Hayati Md Pauzi, Seow Fan Chiew, Kean Hooi Teoh, Navarasi S. Raja Gopal, Mastura Md Yusof, Cheng Har Yip
Cancers.2024; 16(13): 2325. CrossRef - Revolutionizing Pathology with Artificial Intelligence: Innovations in Immunohistochemistry
Diana Gina Poalelungi, Anca Iulia Neagu, Ana Fulga, Marius Neagu, Dana Tutunaru, Aurel Nechita, Iuliu Fulga
Journal of Personalized Medicine.2024; 14(7): 693. CrossRef - Interobserver Variability in HER‐2 Immunostaining Interpretation of Metastatic HER2 Low Breast Cancers in Cytology Specimens
Niyati Desai, Courtney F. Connelly, Simon Sung, Adela Cimic, Swikrity U. Baskota
Diagnostic Cytopathology.2024; 52(12): 722. CrossRef - Weakly-supervised deep learning models enable HER2-low prediction from H &E stained slides
Renan Valieris, Luan Martins, Alexandre Defelicibus, Adriana Passos Bueno, Cynthia Aparecida Bueno de Toledo Osorio, Dirce Carraro, Emmanuel Dias-Neto, Rafael A. Rosales, Jose Marcio Barros de Figueiredo, Israel Tojal da Silva
Breast Cancer Research.2024;[Epub] CrossRef - Understanding the spectrum of HER2 status in breast cancer: From HER2-positive to ultra-low HER2
Sana Ahuja, Adil Aziz Khan, Sufian Zaheer
Pathology - Research and Practice.2024; 262: 155550. CrossRef - Systematic review of added immunotherapy in traditional treatment for HER2 positive breast cancer patients
Rohan Choudhari
Innovative Practice in Breast Health.2024; 3-4: 100013. CrossRef - Detecting early-stage breast cancer with GATA3-positive circulating tumor cells
Chun-Hsin Hsieh, Ya-Herng Chang, Pei-Ying Ling, Ying-Tai Jin, Pei-Hsuan Lo, Hei-Jen Jou
Taiwanese Journal of Obstetrics and Gynecology.2024; 63(5): 745. CrossRef - First-in-human study of DP303c, a HER2-targeted antibody-drug conjugate in patients with HER2 positive solid tumors
Jian Zhang, Yiqun Du, Yanchun Meng, Xiaojun Liu, Yuxin Mu, Yunpeng Liu, Yehui Shi, Jufeng Wang, Aimin Zang, Shanzhi Gu, Tianshu Liu, Huan Zhou, Hongqian Guo, Silong Xiang, Xialu Zhang, Suqiong Wu, Huanhuan Qi, Mengke Li, Xichun Hu
npj Precision Oncology.2024;[Epub] CrossRef - Targeting CD276 for T cell-based immunotherapy of breast cancer
Ilona Hagelstein, Laura Wessling, Alexander Rochwarger, Latifa Zekri, Boris Klimovich, Christian M. Tegeler, Gundram Jung, Christian M. Schürch, Helmut R. Salih, Martina S. Lutz
Journal of Translational Medicine.2024;[Epub] CrossRef - Correlation of Androgen Receptor Expression With Ki67 Proliferative Index and Other Clinicopathological Characteristics in Invasive Mammary Carcinomas
D Keerthana Devi, V Pavithra, Leena D Joseph, Chithra Bhanu Challa
Cureus.2024;[Epub] CrossRef - CD81-guided heterologous EVs present heterogeneous interactions with breast cancer cells
Elena Gurrieri, Giulia Carradori, Michela Roccuzzo, Michael Pancher, Daniele Peroni, Romina Belli, Caterina Trevisan, Michela Notarangelo, Wen-Qiu Huang, Agata S. A. Carreira, Alessandro Quattrone, Guido Jenster, Timo L. M. Ten Hagen, Vito Giuseppe D’Agos
Journal of Biomedical Science.2024;[Epub] CrossRef - Deciphering HER2-low breast cancer (BC): insights from real-world data in early stage breast cancer
Anna Pous, Adrià Bernat-Peguera, Assumpció López-Paradís, Beatriz Cirauqui, Vanesa Quiroga, Iris Teruel, Eudald Felip, Angelica Ferrando-Díez, Milana Bergamino, Laia Boronat, Margarita Romeo, Gemma Soler, Christian Mariño, Paula Rodríguez-Martínez, Laura
Therapeutic Advances in Medical Oncology.2024;[Epub] CrossRef - Liquid biopsy-based technologies: a promising tool for biomarker identification in her2-low breast cancer patients for improved therapeutic outcomes
Aldo D’Alessandro, Anca Florentina Deaconu, Sandro Mandolesi, Federico Pio Fabrizio, Massimo Lombardi, Giovanna Liguori, Giovanni Pepe, Nicola Marino, Aureliano Stingi, Alessandro D’Alessandro, Antonio Giordano
Journal of Cancer Metastasis and Treatment.2024;[Epub] CrossRef - SPP1 mRNA Expression Is Associated with M2 Macrophage Infiltration and Poor Prognosis in Triple-Negative Breast Cancer
Yu-Chia Chen, Chia-Ching Chen, Rong-Fu Chen, Hsin-Hung Chen, Po-Ming Chen
Current Issues in Molecular Biology.2024; 46(12): 13499. CrossRef - MicroRNAs and their role in breast cancer metabolism (Review)
Wen Lee, Bann Yeo, Rozi Mahmud, Geok Tan, Mohamed Wahid, Yoke Cheah
International Journal of Oncology.2024;[Epub] CrossRef - CDH1 methylation and expression of E-cadherin and other markers in breast cancer
Luiz Fernando de Queiroz, Marcelo Soares da Mota e Silva, Fernando Colonna Rosman, Siane Lopes Bittencourt Rosas, Heitor Siffert Pereira de Souza, Maria da Glória da Costa Carvalho
Mastology.2024;[Epub] CrossRef - Surgical Management of the Axilla in HR+/HER2– Breast Cancer in the Z1071 Era: A Propensity Score-Matched Analysis of the National Cancer Database
Vayda R. Barker, Samer A. Naffouje, Melissa A. Mallory, Susan A. Hoover, Christine Laronga
Annals of Surgical Oncology.2023; 30(13): 8371. CrossRef - Noninvasive identification of HER2-low-positive status by MRI-based deep learning radiomics predicts the disease-free survival of patients with breast cancer
Yuan Guo, Xiaotong Xie, Wenjie Tang, Siyi Chen, Mingyu Wang, Yaheng Fan, Chuxuan Lin, Wenke Hu, Jing Yang, Jialin Xiang, Kuiming Jiang, Xinhua Wei, Bingsheng Huang, Xinqing Jiang
European Radiology.2023; 34(2): 899. CrossRef - Systemic investigation of inetetamab in combination with small molecules to treat HER2-overexpressing breast and gastric cancers
Lan Deng, Le Zhao, Lifen Liu, Haomin Huang
Open Life Sciences.2023;[Epub] CrossRef - HER2-low expression in patients with advanced or metastatic solid tumors
B. Uzunparmak, C. Haymaker, G. Raso, S. Masciari, L. Wang, H. Lin, A. Gorur, B. Kirby, A.-M. Cimo, A. Kennon, Q. Ding, G. Urschel, Y. Yuan, G. Feng, Y. Rizvi, A. Hussain, C. Zhu, P. Kim, G. Abbadessa, V. Subbiah, T.A. Yap, J. Rodon, S.A. Piha-Paul, F. Mer
Annals of Oncology.2023; 34(11): 1035. CrossRef - Data on 2D culture characterisation of potential markers in human HER2-positive breast cancer cell lines
Son H. Pham, Lyn R. Griffiths, Rachel K. Okolicsanyi, Larisa M. Haupt
Data in Brief.2023; 46: 108880. CrossRef - Determining HER2 Status by Artificial Intelligence: An Investigation of Primary, Metastatic, and HER2 Low Breast Tumors
Christiane Palm, Catherine E. Connolly, Regina Masser, Barbara Padberg Sgier, Eva Karamitopoulou, Quentin Simon, Beata Bode, Marianne Tinguely
Diagnostics.2023; 13(1): 168. CrossRef - Gene amplification mutations originate prior to selective stress in Acinetobacter baylyi
Jennifer A Herrmann, Agata Koprowska, Tesa J Winters, Nancy Villanueva, Victoria D Nikityuk, Feini Pek, Elizabeth M Reis, Constancia Z Dominguez, Daniel Davis, Eric McPherson, Staci R Rocco, Cynthia Recendez, Shyla M Difuntorum, Kelly Faeth, Mario D Lopez
G3.2023;[Epub] CrossRef - Flow Rate-Independent Multiscale Liquid Biopsy for Precision Oncology
Jie Wang, Robert Dallmann, Renquan Lu, Jing Yan, Jérôme Charmet
ACS Sensors.2023; 8(3): 1200. CrossRef - Single-cell HER2 quantification via instant signal amplification in microdroplets
Xiaoxian Liu, Yifan Zhu, Caoxin Li, Yanyun Fang, Jinna Chen, Fei Xu, Yanqing Lu, Perry Ping Shum, Ying Liu, Guanghui Wang
Analytica Chimica Acta.2023; 1251: 340976. CrossRef - Molecular imaging of HER2 receptor: Targeting HER2 for imaging and therapy in nuclear medicine
Daniela Miladinova
Frontiers in Molecular Biosciences.2023;[Epub] CrossRef - CORRELATION BETWEEN DIFFERENT CLINICOPATHOLOGICAL PARAMETERS AND MOLECULAR SUBTYPES OF FEMALE BREAST CARCINOMA IN SOUTH REGION OF IRAQ
Yassir Alaa Muhammed Hassan Shubbar
Wiadomości Lekarskie.2023; 76(1): 97. CrossRef - Pathological identification of HER2-low breast cancer: Tips, tricks, and troubleshooting for the optimal test
Elham Sajjadi, Elena Guerini-Rocco, Elisa De Camilli, Oriana Pala, Giovanni Mazzarol, Konstantinos Venetis, Mariia Ivanova, Nicola Fusco
Frontiers in Molecular Biosciences.2023;[Epub] CrossRef - Protective Effect of HER2 Gene Polymorphism rs24537331 in the Outcome of Canine Mammary Tumors
Ana Canadas-Sousa, Marta Santos, Patrícia Dias-Pereira
Animals.2023; 13(8): 1384. CrossRef - Can Patients with HER2-Low Breast Cancer Benefit from Anti-HER2 Therapies? A Review
Jin Wang, Dongying Liao, Xuemin Zhang, Changhong Miao, Kuang Chen
Breast Cancer: Targets and Therapy.2023; Volume 15: 281. CrossRef - HER2 Low Breast Cancer: A New Subtype or a Trojan for Cytotoxic Drug Delivery?
Marina Popović, Tajana Silovski, Marija Križić, Natalija Dedić Plavetić
International Journal of Molecular Sciences.2023; 24(9): 8206. CrossRef - HER2-Low Breast Cancer—Diagnostic Challenges and Opportunities for Insights from Ongoing Studies: A Podcast
Aditya Bardia, Giuseppe Viale
Targeted Oncology.2023; 18(3): 313. CrossRef - Histopathological and immunohistochemical analysis of predictive and prognostic markers in spontaneous canine mammary cancer
Vladimír Tancoš, Marcel Kovalik, Martin Levkut, Martina Bobrovská, Petra Kolenčíková, Ľubomír Straka, Zuzana Ševčíková, Ondřej Škor, Martina Antošová, Lukáš Plank, Keith L. Thoday
Acta Veterinaria Brno.2023; 92(2): 143. CrossRef - Dissecting sources of variability in patient response to targeted therapy: anti-HER2 therapies as a case study
Timothy Qi, Yanguang Cao
European Journal of Pharmaceutical Sciences.2023; 186: 106467. CrossRef - The Effect of HER2-Low Status on Pathological Complete Response and Survival in Triple-Negative Breast Cancer: A Systemic Review and Meta-Analysis
Yakup Ergun, Baran Akagunduz, Cengiz Karacin, Sema Turker, Gokhan Ucar
Clinical Breast Cancer.2023; 23(6): 567. CrossRef - Efficacy, toxicity and prognostic factors of pyrotinib‑involved neoadjuvant therapy in HER2‑positive breast cancer: A retrospective study
Hao Wang, Hailing Cao, Zhiyun Guo
Oncology Letters.2023;[Epub] CrossRef - Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer
Feng Ye, Saikat Dewanjee, Yuehua Li, Niraj Kumar Jha, Zhe-Sheng Chen, Ankush Kumar, Vishakha, Tapan Behl, Saurabh Kumar Jha, Hailin Tang
Molecular Cancer.2023;[Epub] CrossRef - Weakly supervised bilayer convolutional network in segmentation of HER2 related cells to guide HER2 targeted therapies
Ching-Wei Wang, Kun-Lin Lin, Hikam Muzakky, Yi-Jia Lin, Tai-Kuang Chao
Computerized Medical Imaging and Graphics.2023; 108: 102270. CrossRef - Immune Biomarkers in Triple-Negative Breast Cancer: Improving the Predictivity of Current Testing Methods
Francesca Maria Porta, Elham Sajjadi, Konstantinos Venetis, Chiara Frascarelli, Giulia Cursano, Elena Guerini-Rocco, Nicola Fusco, Mariia Ivanova
Journal of Personalized Medicine.2023; 13(7): 1176. CrossRef - HER2 Equivocal (Score = 2+) Breast Carcinoma Cases Identified by Immunohistochemistry at a South African Hospital. What is the Impact of Fluorescent In Situ Hybridization Testing?
Reena Dhansukh Mohanlal, Nikki Bouwer, Pascale Willem
Applied Immunohistochemistry & Molecular Morphology.2023; 31(8): 555. CrossRef - Discordance of HER2 Expression and/or Amplification on Repeat Testing
Timothy P. DiPeri, Kathleen Kong, Kaushik Varadarajan, Daniel D. Karp, Jaffer A. Ajani, Shubham Pant, Michael F. Press, Sarina A. Piha-Paul, Ecaterina E. Dumbrava, Funda Meric-Bernstam
Molecular Cancer Therapeutics.2023; 22(8): 976. CrossRef - Low and Ultra-Low HER2 in Human Breast Cancer: An Effort to Define New Neoplastic Subtypes
Mariausilia Franchina, Cristina Pizzimenti, Vincenzo Fiorentino, Maurizio Martini, Giuseppina Rosaria Rita Ricciardi, Nicola Silvestris, Antonio Ieni, Giovanni Tuccari
International Journal of Molecular Sciences.2023; 24(16): 12795. CrossRef - Genetic analysis of oligo-recurrence breast cancer: correlation with clinical outcomes
Kuikui Jiang, Danyang Zhou, Fei Xu, Wen Xia, Qiufan Zheng, Qianyi Lu, Rongzhen Luo, Ruoxi Hong, Shusen Wang
BMC Cancer.2023;[Epub] CrossRef - Single domain antibodies specific for HER2 dimerization domain effectively disrupts HER2 dimerization
Ahmad Najafi, Reza Valadan, Hossein Asgarian-Omran, Alireza Rafiei, Mohsen Tehrani
International Immunopharmacology.2023; 124: 110999. CrossRef - Récepteur du facteur de croissance épidermique HER2, tests utilisés pour rechercher son amplification dans le cancer du sein : principes et limites
Imane Eliahiai, Mohammed Eljiar, Sanae Chaib, Jinane KHarmoum, Mariame Chraïbi
Bulletin du Cancer.2023; 110(12): 1301. CrossRef - Integrated Molecular Characterization of HER2-Low Breast Cancer Using Next Generation Sequencing (NGS)
Jean-Louis Merlin, Marie Husson, Nassim Sahki, Pauline Gilson, Vincent Massard, Alexandre Harlé, Agnès Leroux
Biomedicines.2023; 11(12): 3164. CrossRef - GLUCOSE LEVELS OF PLEURAL EFFUSION FLUID AND HER2 STATUS IN PLEURAL-METASTATIC BREAST CANCER
Muhammad Dhanny Irawan, Desak Gede Agung Suprabawati, Heru Purwanto
Majalah Biomorfologi.2023; 33(2): 75. CrossRef - Design of a Ratiometric Plasmonic Biosensor for Herceptin Detection in HER2-Positive Breast Cancer
Neda Shahbazi, Rouholah Zare-Dorabei, Seyed Morteza Naghib
ACS Biomaterials Science & Engineering.2022; 8(2): 871. CrossRef - A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
Mahdi Sadeghi, Soheila Kashanian, Seyed Morteza Naghib, Esfandyar Askari, Fateme Haghiralsadat, Davood Tofighi
Nanotechnology Reviews.2022; 11(1): 793. CrossRef - Anti-HER2 therapy in metastatic breast cancer: many choices and future directions
Carrie S. Wynn, Shou-Ching Tang
Cancer and Metastasis Reviews.2022; 41(1): 193. CrossRef - Electroanalytical overview: screen-printed electrochemical sensing platforms for the detection of vital cardiac, cancer and inflammatory biomarkers
Robert D. Crapnell, Alejandro Garcia-Miranda Ferrari, Nina C. Dempsey, Craig E. Banks
Sensors & Diagnostics.2022; 1(3): 405. CrossRef - FTO genotype was associated with breast cancer in HER2 negative patients
Fateme Montazeri, Hossein Hatami, Soroor Fathi, Naeemeh Hasanpour Ardekanizadeh, Fatemeh Bourbour, Samira Rastgoo, Fatemeh Shafiee, Mohammad Esmail Akbari, Maryam Gholamalizadeh, Seyed Alireza Mosavi Jarrahi, Saeid Doaei
Clinical Nutrition ESPEN.2022; 49: 495. CrossRef - Breast Cancer Human Epidermal Growth Factor Receptor 2 mRNA Molecular Testing Compared to Immunohistochemistry with Correlation to Neoadjuvant Therapy Response
Mahmoud Behairy, Samia Mohamed Gabal, Mohamed Sherif Negm
Open Access Macedonian Journal of Medical Sciences.2022; 10(A): 352. CrossRef - Validity and utility of HER2/ERBB2 copy number variation assessed in liquid biopsies from breast cancer patients: A systematic review
Noortje Verschoor, Teoman Deger, Agnes Jager, Stefan Sleijfer, Saskia M. Wilting, John W.M. Martens
Cancer Treatment Reviews.2022; 106: 102384. CrossRef - RETRACTED: Longitude Variation of the microRNA-497/FGF-23 Axis during Treatment and Its Linkage with Neoadjuvant/Adjuvant Trastuzumab-Induced Cardiotoxicity in HER2-Positive Breast Cancer Patients
Hui Liu, Xiaoyan Hu, Lingyun Wang, Tao Du, Jing Feng, Ming Li, Lei Liu, Xiaofang Liu
Frontiers in Surgery.2022;[Epub] CrossRef - Use of Radionuclide-Based Imaging Methods in Breast Cancer
Betül Altunay, Agnieszka Morgenroth, Felix M. Mottaghy
Seminars in Nuclear Medicine.2022; 52(5): 561. CrossRef - Functional regulations between genetic alteration-driven genes and drug target genes acting as prognostic biomarkers in breast cancer
Li Wang, Lei Yu, Jian Shi, Feng Li, Caiyu Zhang, Haotian Xu, Xiangzhe Yin, Lixia Wang, Shihua Lin, Anastasiia Litvinova, Yanyan Ping, Shangwei Ning, Hongying Zhao
Scientific Reports.2022;[Epub] CrossRef - Human epidermal growth factor receptor-2 and endocrine resistance in hormone-dependent breast cancer
Anastasia Alataki, Mitch Dowsett
Endocrine-Related Cancer.2022; 29(8): R105. CrossRef - The Evolution of Targeted Radionuclide Diagnosis of HER2-Positive Breast Cancer
Olga D. Bragina, Sergei M. Deyev, Vladimir I. Chernov, Vladimir M. Tolmachev
Acta Naturae.2022; 14(2): 4. CrossRef - Deriving tumor purity from cancer next generation sequencing data: applications for quantitative ERBB2 (HER2) copy number analysis and germline inference of BRCA1 and BRCA2 mutations
Stephanie E. Siegmund, Danielle K. Manning, Phani K. Davineni, Fei Dong
Modern Pathology.2022; 35(10): 1458. CrossRef - Current challenges and unmet needs in treating patients with human epidermal growth factor receptor 2-positive advanced breast cancer
Matti Aapro, Fatima Cardoso, Giuseppe Curigliano, Alexandru Eniu, Joseph Gligorov, Nadia Harbeck, Andreas Mueller, Olivia Pagani, Shani Paluch-Shimon, Elzbieta Senkus, Beat Thürlimann, Khalil Zaman
The Breast.2022; 66: 145. CrossRef - [Retracted] Application of Fluorescence In Situ Hybridization Assisted by Fluorescence Microscope in Detection of Her2 Gene in Breast Cancer Patients
Fang Lu, Tingting Zhou, Yan Liu, Liying Song, Bin Zhang, Yuyan Li, Sorayouth Chumnanvej
Contrast Media & Molecular Imaging.2022;[Epub] CrossRef - High sensitivity label-free detection of HER2 using an Al–GaN/GaN high electron mobility transistor-based biosensor
Shivanshu Mishra, Pharyanshu Kachhawa, Amber Kumar Jain, Rajiv Ranjan Thakur, Nidhi Chaturvedi
Lab on a Chip.2022; 22(21): 4129. CrossRef - Indian Data on HER2 Fluorescence In Situ Hybridization in Invasive Breast Cancer with Immunohistochemically Equivocal Results As Per 2018 ASCO/CAP Guidelines
B. R. Nagarjun, Biren Parikh, Manaswi Nareshkumar Patel, Pina J. Trivedi, Dharmesh M. Patel
South Asian Journal of Cancer.2022; 11(04): 281. CrossRef - S‑phase fraction, lymph node status and disease staging as the main prognostic factors to differentiate between young and older patients with invasive breast carcinoma
António Pinto, João Matos, Teresa Pereira, Giovani Silva, Saudade André
Oncology Letters.2022;[Epub] CrossRef - Inferring tumor-specific cancer dependencies through integrating ex vivo drug response assays and drug-protein profiling
Alina Batzilla, Junyan Lu, Jarno Kivioja, Kerstin Putzker, Joe Lewis, Thorsten Zenz, Wolfgang Huber, James Gallo
PLOS Computational Biology.2022; 18(8): e1010438. CrossRef - Clinical possibilities of HER2-positive breast cancer diagnosis using alternative scaffold proteins
O. D. Bragina, V. I. Chernov, S. M. Deyev, V. M. Tolmachev
Bulletin of Siberian Medicine.2022; 21(3): 132. CrossRef - Molecular Pathology of Gastric Cancer
Moonsik Kim, An Na Seo
Journal of Gastric Cancer.2022; 22(4): 264. CrossRef - Loss of NECTIN1 triggers melanoma dissemination upon local IGF1 depletion
Julien Ablain, Amira Al Mahi, Harriet Rothschild, Meera Prasad, Sophie Aires, Song Yang, Maxim E. Dokukin, Shuyun Xu, Michelle Dang, Igor Sokolov, Christine G. Lian, Leonard I. Zon
Nature Genetics.2022; 54(12): 1839. CrossRef - Advanced diagnosis technologies for HER2 breast cancer markers
Mengxue Zhang
Highlights in Science, Engineering and Technology.2022; 14: 44. CrossRef - An Overview of Clinical Development of Agents for Metastatic or Advanced Breast Cancer Without ERBB2 Amplification (HER2-Low)
Aleix Prat, Aditya Bardia, Giuseppe Curigliano, M. Elizabeth H. Hammond, Sibylle Loibl, Sara M. Tolaney, Giuseppe Viale
JAMA Oncology.2022; 8(11): 1676. CrossRef - Development of T-cell engagers selective for cells co-expressing two antigens
Danielle M. Dicara, Sunil Bhakta, Mary Ann Go, James Ziai, Ron Firestein, Bill Forrest, Chen Gu, Steven R. Leong, Genee Lee, Shang-Fan Yu, Andrew G. Polson, Nicholas J. Agard
mAbs.2022;[Epub] CrossRef - The clinical significance of HER2 expression in DCIS
Ioanna Akrida, Francesk Mulita
Medical Oncology.2022;[Epub] CrossRef - Antibody-Drug Conjugates in Breast Cancer: Spotlight on HER2
Rachel Occhiogrosso Abelman, Arielle Medford, Laura Spring, Aditya Bardia
The Cancer Journal.2022; 28(6): 423. CrossRef - The Clinical Utility of Droplet Digital PCR for Profiling Circulating Tumor DNA in Breast Cancer Patients
Ugur Gezer, Abel J. Bronkhorst, Stefan Holdenrieder
Diagnostics.2022; 12(12): 3042. CrossRef - Lapatinib and lapatinib plus trastuzumab therapy versus trastuzumab therapy for HER2 positive breast cancer patients: an updated systematic review and meta-analysis
Ye Yuan, Xumei Liu, Yi Cai, Wenyuan Li
Systematic Reviews.2022;[Epub] CrossRef - Interactions dietary components with expression level of breast cancer-related genes
Fatemeh Bourbour, Azam Pourtaheri, Khadijeh Abbasi, Naeemeh Hasanpour Ardekanizadeh, Maryam Gholamalizadeh, Azadeh Hajipour, Sepideh Abdollahi, Seyedeh Elaheh Bagheri, Mina Ahmadzadeh, Saeid Doaei, Arezoo Haghighian
Egyptian Journal of Medical Human Genetics.2022;[Epub] CrossRef - HER2-directed antibodies, affibodies and nanobodies as drug-delivery vehicles in breast cancer with a specific focus on radioimmunotherapy and radioimmunoimaging
Betül Altunay, Agnieszka Morgenroth, Mohsen Beheshti, Andreas Vogg, Nicholas C. L. Wong, Hong Hoi Ting, Hans-Jürgen Biersack, Elmar Stickeler, Felix M. Mottaghy
European Journal of Nuclear Medicine and Molecular Imaging.2021; 48(5): 1371. CrossRef - Risk-based decision-making in the treatment of HER2-positive early breast cancer: Recommendations based on the current state of knowledge
Christian Jackisch, Patricia Cortazar, Charles E. Geyer, Luca Gianni, Joseph Gligorov, Zuzana Machackova, Edith A. Perez, Andreas Schneeweiss, Sara M. Tolaney, Michael Untch, Andrew Wardley, Martine Piccart
Cancer Treatment Reviews.2021; 99: 102229. CrossRef - Histologic Patterns of Cutaneous Metastases of Breast Carcinoma: A Clinicopathologic Study of 232 Cases
Shira Ronen, David Suster, Wei-Shen Chen, Natali Ronen, Sri Krishna C. Arudra, Celestine Trinidad, Doina Ivan, Victor G. Prieto, Saul Suster
The American Journal of Dermatopathology.2021; 43(6): 401. CrossRef - Standardized pathology report for breast cancer
Soo Youn Cho, So Yeon Park, Young Kyung Bae, Jee Yeon Kim, Eun Kyung Kim, Woo Gyeong Kim, Youngmee Kwon, Ahwon Lee, Hee Jin Lee, Ji Shin Lee, Jee Young Park, Gyungyub Gong, Hye Kyoung Yoon
Journal of Pathology and Translational Medicine.2021; 55(1): 1. CrossRef - The impact of oral contraceptive use on breast cancer risk: State of the art and future perspectives in the era of 4P medicine
R. Bonfiglio, M.L. Di Pietro
Seminars in Cancer Biology.2021; 72: 11. CrossRef - The Co-Expression of Melanoma-Antigen Family a Proteins and New York Esophageal Squamous Cell Carcinoma-1 in Breast Cancer: A Pilot Study
Yu-Xin Wang, Feng-Lian Li, Li-Xin Du, Jun-Fang Liu, Li-Gang Huo, Shu-Qing Li, Bin Tian
Cancer Management and Research.2021; Volume 13: 6123. CrossRef - Targeting HER2 protein in individual cells using ICP-MS detection and its potential as prognostic and predictive breast cancer biomarker
A. Fernández Asensio, M. Corte-Rodríguez, J. Bettmer, L.M. Sierra, M. Montes-Bayón, E. Blanco- González
Talanta.2021; 235: 122773. CrossRef - Development of a 99mTc-Labeled Single-Domain Antibody for SPECT/CT Assessment of HER2 Expression in Breast Cancer
Lingzhou Zhao, Changcun Liu, Yan Xing, Jin He, Jim O’Doherty, Wenhua Huang, Jinhua Zhao
Molecular Pharmaceutics.2021; 18(9): 3616. CrossRef - WITHDRAWN: Nouvelles stratégies thérapeutiques dans les cancers du sein HER2-surexprimé
Benoîte Mery, Philippe Toussaint, Pierre-Etienne Heudel, Armelle Dufresne, Mélodie Carbonnaux, Hélène Vanacker, Thomas Bachelot, Olivier Trédan
Bulletin du Cancer.2021;[Epub] CrossRef - Retrospective observational study of HER2 immunohistochemistry in borderline breast cancer patients undergoing neoadjuvant therapy, with an emphasis on Group 2 (HER2/CEP17 ratio ≥2.0, HER2 copy number <4.0 signals/cell) cases
Emad A. Rakha, Islam M. Miligy, Cecily M. Quinn, Elena Provenzano, Abeer M. Shaaban, Caterina Marchiò, Michael S. Toss, Grace Gallagy, Ciara Murray, Janice Walshe, Ayaka Katayama, Karim Eldib, Nahla Badr, Bruce Tanchel, Rebecca Millican-Slater, Colin Purd
British Journal of Cancer.2021; 124(11): 1836. CrossRef - Loss of HER2‐positivity following neoadjuvant targeted therapy for breast cancer is not associated with inferior oncologic outcomes
Catherine L. Wetzel, Thomas L. Sutton, Stuart Gardiner, Maryam Farinola, Nathalie Johnson, Jennifer R. Garreau
Journal of Surgical Oncology.2021; 124(8): 1224. CrossRef - Clinical and Genetic Predictive Models for the Prediction of Pathological Complete Response to Optimize the Effectiveness for Trastuzumab Based Chemotherapy
Lun Li, Min Chen, Shuyue Zheng, Hanlu Li, Weiru Chi, Bingqiu Xiu, Qi Zhang, Jianjing Hou, Jia Wang, Jiong Wu
Frontiers in Oncology.2021;[Epub] CrossRef - tRNA‐derived fragments: tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 as novel diagnostic biomarkers for breast cancer
Yue Zhang, Zhao Bi, Xiaohan Dong, Miao Yu, Kangyu Wang, Xingguo Song, Li Xie, Xianrang Song
Thoracic Cancer.2021; 12(17): 2314. CrossRef - Detection of secondary metastatic breast cancer by measurement of plasma CA 15.3
L. De Cock, J. Heylen, A. Wildiers, K. Punie, A. Smeets, C. Weltens, P. Neven, J. Billen, A. Laenen, H. Wildiers
ESMO Open.2021; 6(4): 100203. CrossRef - Standardized Pathology Report for Breast Cancer
Soo Youn Cho, So Yeon Park, Young Kyung Bae, Jee Yeon Kim, Eun Kyung Kim, Woo Gyeong Kim, Youngmee Kwon, Ahwon Lee, Hee Jin Lee, Ji Shin Lee, Jee Young Park, Gyungyub Gong, Hye Kyoung Yoon
Journal of Breast Cancer.2021; 24(1): 1. CrossRef - Circular RNA circ-ERBB2 promotes HER2-positive breast cancer progression and metastasis via sponging miR-136-5p and miR-198
Jin-xiu Zhong, Yun-yuan Kong, Rong-guang Luo, Guo-jin Xia, Wen-xing He, Xue-zhong Chen, Wei-wei Tan, Qing-jie Chen, Yu-yin Huang, Yan-xing Guan
Journal of Translational Medicine.2021;[Epub] CrossRef - Nouvelles stratégies thérapeutiques dans les cancers du sein HER2-surexprimé
Benoîte Mery, Philippe Toussaint, Pierre-Etienne Heudel, Armelle Dufresne, Mélodie Carbonnaux, Hélène Vanacker, Thomas Bachelot, Olivier Trédan
Bulletin du Cancer.2021; 108(11): 11S8. CrossRef - Association of Estrogen and Progesterone Receptors with Clinicopathological Prognostic Factors in Breast Cancer
Ali Abdul Hadi Abdul-Kareem, Qahtan A. Mahdi
Medical Journal of Babylon.2021; 18(2): 111. CrossRef - HER2 alterations in non-small-cell lung cancer – Druggable or undruggable?
Suresh Kumar Bondili, Ravindra Nandhana, Vanita Noronha, Swayamprabha Pawar, Nandini Menon, Omshree Shetty, Anuradha Chougule, Abhishek Mahajan, Rajiv Kumar, Vijay M. Patil, Amit Joshi, Kumar Prabhash
Cancer Research, Statistics, and Treatment.2021; 4(2): 374. CrossRef - UCNP-based Photoluminescent Nanomedicines for Targeted Imaging and Theranostics of Cancer
Evgenii L. Guryev, Anita S. Smyshlyaeva, Natalia Y. Shilyagina, Evgeniya A. Sokolova, Samah Shanwar, Alexey B. Kostyuk, Alexander V. Lyubeshkin, Alexey A. Schulga, Elena V. Konovalova, Quan Lin, Indrajit Roy, Irina V. Balalaeva, Sergey M. Deyev, Andrei V.
Molecules.2020; 25(18): 4302. CrossRef - Impact of the Updated Guidelines on Human Epidermal Growth Factor Receptor 2 (HER2) Testing in Breast Cancer
Min Chong Kim, Su Hwan Kang, Jung Eun Choi, Young Kyung Bae
Journal of Breast Cancer.2020; 23(5): 484. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
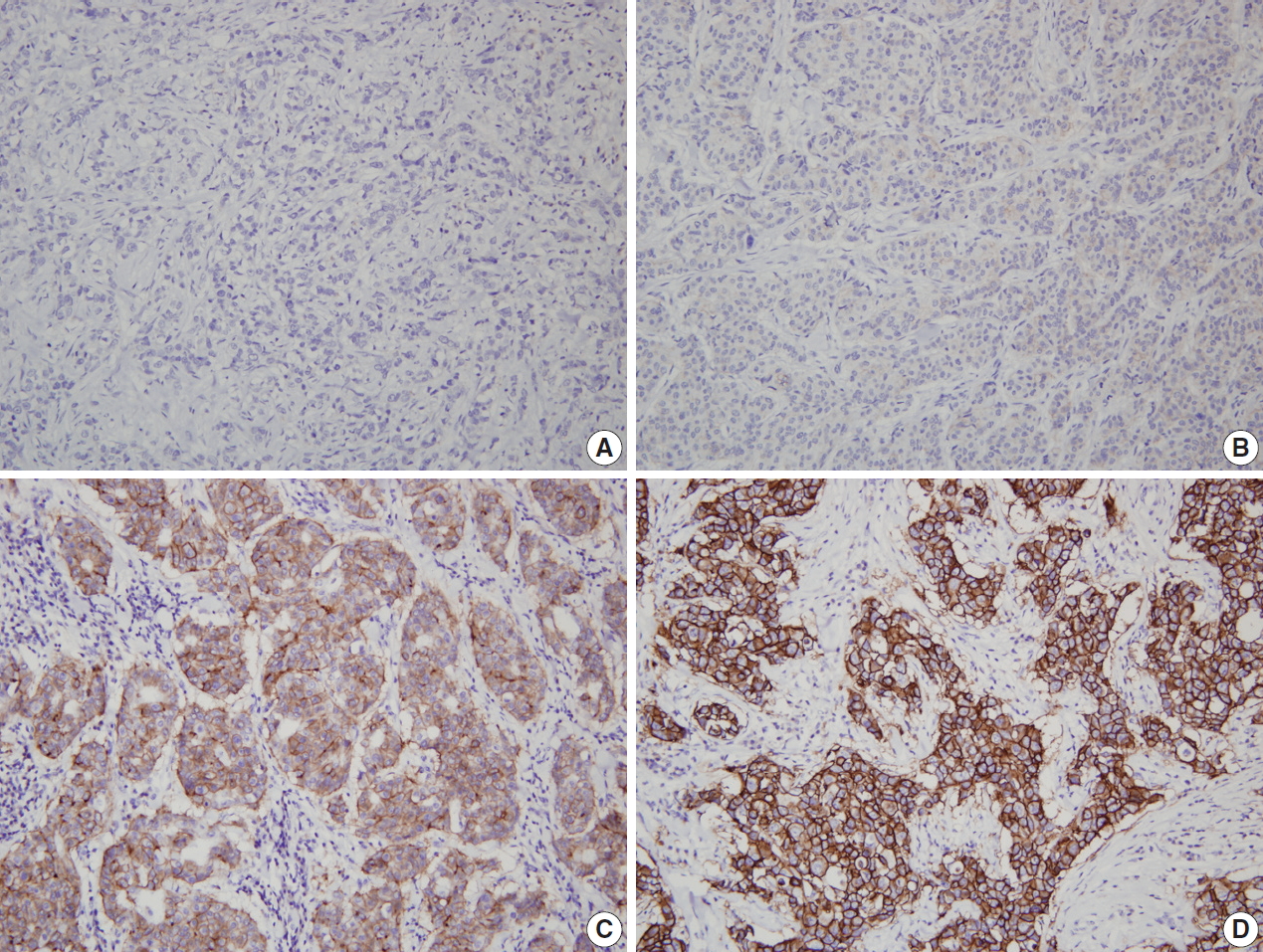
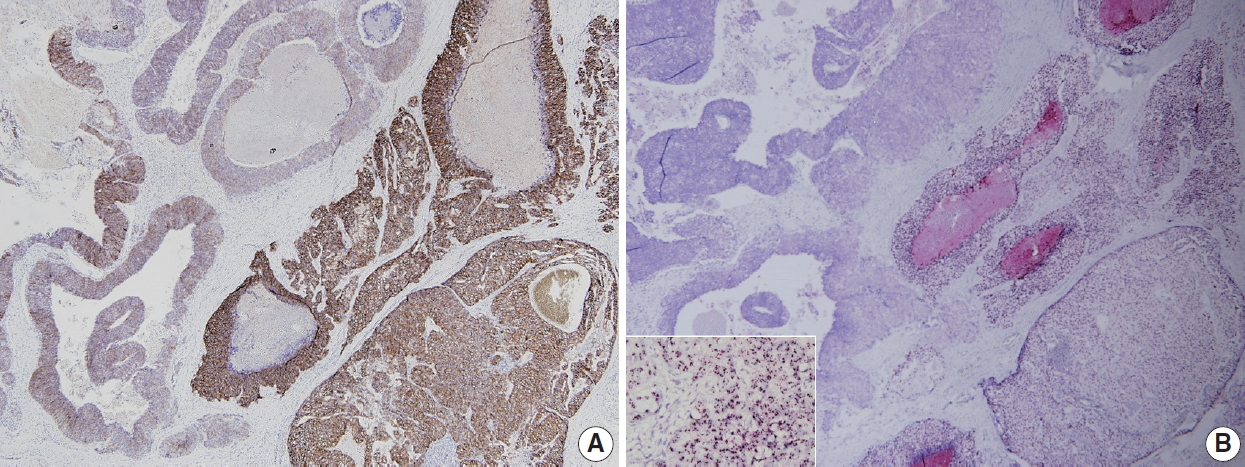
Fig. 1.
Fig. 2.
| HER2 IHC status | 2007 ASCO/CAP guidelines | 2013 ASCO/CAP guidelines | 2018 ASCO/CAP guidelines |
|---|---|---|---|
| Positive (3+) | Uniform intense membrane staining of >30% of invasive tumor cells | Circumferential membrane staining that is complete, intense, and in >10% of tumor cells | Circumferential membrane staining that is complete, intense, and in >10% of tumor cells |
| Equivocal (2+) | Complete membrane staining that is either non-uniform or weak in intensity but with obvious circumferential distribution in at least 10% of cells | Circumferential membrane staining that is incomplete and/or weak to moderate and within >10% of the invasive tumor cells | Weak to moderate complete membrane staining observed in >10% of tumor cells |
| Complete and circumferential membrane staining that is intense and within ≤10% of the invasive tumor cells | |||
| Negative (1+) | Weak incomplete membrane staining in any proportion of tumor cells | Incomplete membrane staining that is faint or barely perceptible and within >10% of the invasive tumor cells | Incomplete membrane staining that is faint or barely perceptible and within >10% of the invasive tumor cells |
| Weak, complete membrane staining in <10% of tumor cells | |||
| Negative (0) | No staining | No staining observed | No staining observed |
| Incomplete membrane staining that is faint or barely perceptible and within ≤10% of the invasive tumor cells | Incomplete membrane staining that is faint or barely perceptible and within ≤10% of the invasive tumor cells |
| HER2 ISH status | 2007 ASCO/CAP guidelines | 2013 ASCO/CAP guidelines | 2018 ASCO/CAP guidelines |
|---|---|---|---|
| ISH positive | HER2/CEP17 ratio > 2.2 | HER2/CEP17 ratio ≥ 2.0 | HER2/CEP17 ratio ≥ 2.0 and average HER2 copy number ≥ 4.0 (group 1) |
| HER2/CEP17 ratio < 2.0 and average HER2 copy number ≥ 6.0 | HER2/CEP17 ratio ≥ 2.0 and average HER2 copy number < 4.0 (group 2) with concurrent IHC 3+ | ||
| HER2/CEP17 ratio < 2.0 and average HER2 copy number ≥ 6.0 (group 3) with concurrent IHC 2+ |
|||
| HER2/CEP17 ratio < 2.0 and average HER2 copy number ≥ 6.0 (group 3) with concurrent IHC 3+ | |||
| HER2/CEP17 ratio < 2.0 with average HER2 copy number ≥ 4.0 and < 6.0 (group 4) with concurrent IHC 3+ | |||
| ISH equivocal | HER2/CEP17 ratio of 1.8-2.2 | HER2/CEP17 ratio < 2.0 with average HER2 copy number ≥ 4.0 and < 6.0 | (no equivocal category) |
| ISH negative | HER2/CEP17 ratio of < 1.8 | HER2/CEP17 ratio < 2.0 with average HER2 copy number < 4.0 | HER2/CEP17 ratio < 2.0 with average HER2 copy number < 4.0 (group 5) |
| HER2/CEP17 ratio ≥ 2.0 and average HER2 copy number < 4.0 (group 2) with concurrent IHC 2+ |
|||
| HER2/CEP17 ratio < 2.0 with average HER2 copy number ≥ 4.0 and < 6.0 (group 4) with concurrent IHC 2+ |
|||
| Groups 2, 3, and 4 with concurrent IHC 0 or 1 + |
| Suggestions |
|---|
| The pathologist should scan entire HER2 ISH slide before counting. |
| Review of HER2 IHC slide is helpful to find areas with potential HER2 amplification |
| From this point of view, CISH or SISH has an advantage to evaluate HER2 heterogeneity, because it can be easily matched with HER2 IHC slide under light microscope. |
| If there is a subpopulation of tumor cells with HER2 amplification comprising > 10% of tumor cells on the slide, a separate counting should be performed within the subpopulation. |
| The HER2/CEP17 ratios or HER2 gene copy number should be calculated for both amplified and non-amplified areas separately. |
| If possible, it is recommended that in situ hybridization report includes proportion of amplified cells within a tumor. |
| Year | Author | Total No. of cases | Method | Frequency of HER2 alteration, n (%) |
||
|---|---|---|---|---|---|---|
| Total | + to – | – to + | ||||
| 2018 | De La Cruz et al. [53] | 54 | IHC/FISH | 2/54 (3.7) | 1/54 (1.9) | 1/54 (1.9) |
| 2018 | Ahn et al. [52] | 442 | IHC/SISH | 15/442 (3.4) | 4/442 (0.9) | 11/442 (2.5) |
| 2017 | Xian et al. [54] | 77 | IHC/FISH | 6/77 (7.8) | 5/77 (6.5) | 1/77 (1.3) |
| 2017 | Reddy et al. [55] | 140 | IHC/FISH | 8/97 (8.2) | 5/97 (5.2) | 3/97 (3.1) |
| 2016 | Gahlaut et al. [56] | 133 | IHC/FISH | 8/133 (6.0) | 5/133 (3.8) | 3/133 (2.3) |
| 2016 | Lim et al. [57] | 290 | IHC/FISH | 17/290 (5.9) | 17/290 (5.9) | 0/290 (0) |
| 2016 | Zhou et al. [58] | 107 | IHC/FISH | 5/107 (4.7) | 3/107 (2.8) | 2/107 (1.9) |
| 2015 | Jin et al. [59] | 423 | IHC/FISH | 40/423 (9.5) | 27/423 (6.4) | 13/423 (3.1) |
| 2013 | Yang et al. [60] | 113 | IHC | 17/113 (15.0) | 9/113 (8.0) | 8/113 (7.1) |
| 2013 | Cockburn et al. [61] | 133 | IHC/FISH | 16/133 (12.0) | 9/133 (6.8) | 7/133 (5.3) |
| 2013 | Lee et al. [62] | 120 | IHC/FISH | 11/107 (10.3) | 6/107 (5.6) | 5/107 (4.7) |
| 2009 | Hirata et al. [63] | 368 | IHC/FISH | 35/368 (9.5) | 22/368 (6.0) | 13/368 (3.5) |
| 2008 | Kasami et al. [64] | 173 | IHC/FISH | 0/173 (0) | 0/173 (0) | 0/173 (0) |
| 2006 | Neubauer et al. [65] | 87 | IHC | 13/87 (14.9) | 11/87 (12.6) | 2/87 (2.3) |
| 2003 | Faneyte et al. [66] | 50 | IHC | 3/50 (6.0) | 2/50 (4.0) | 1/50 (2.0) |
| Year | Author | Total No. of cases | Method | Site | Frequency of HER2 alteration, n (%) |
||
|---|---|---|---|---|---|---|---|
| Total | + to – | – to + | |||||
| 2019 | Woo et al. [79] | 152 | IHC/SISH | All | 12/152 (7.9) | 9/152 (5.9) | 3/152 (2.0) |
| 2014 | de Duenas et al. [70] | 165 | IHC/FISH | All | 5/165 (3.0) | 0/165 (0.0) | 5/165 (3.0) |
| 2013 | Curtit et al. [71] | 219 | IHC/FISH | All | 8/219 (3.7) | 6/219 (2.7) | 2/219 (0.9) |
| 2013 | Nakamura et al. [72] | 156 | IHC/FISH | All | 13/156 (8.3) | 5/156 (3.2) | 8/156 (5.1) |
| 2013 | Aurilio et al. [73] | 86 | IHC/FISH | Bone | 6/86 (7.0) | 2/86 (2.3) | 4/86 (4.7) |
| 2012 | Duchnowska et al. [74] | 119 | IHC/FISH | Brain | 17/119 (14.3) | 7/119 (5.9) | 10/119 (8.4) |
| 2012 | Jensen et al. [75] | 114 | IHC/FISH | All | 10/114 (8.8) | 2/114 (1.8) | 8/114 (7.0) |
| 2011 | Bogina et al. [76] | 136 | IHC/SISH | All | 1/136 (0.7) | 0/136 (0.0) | 1/136 (0.7) |
| 2011 | Chang et al. [77] | 56 | IHC/FISH | All | 7/56 (12.5) | 2/56 (3.6) | 5/56 (8.9) |
| 2010 | Hoefnagel et al. [78] | 233 | IHC/SISH | All | 12/233 (5.2) | 6/233 (2.6) | 6/233 (2.6) |
ASCO, American Society of Clinical Oncology; CAP, College of American Pathologists; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry. Unusual staining patterns of HER2 by IHC can be encountered that are not covered by these definitions. As one example, some specific subtypes of breast cancers can show IHC staining that is moderate to intense but incomplete (basolateral or lateral) and can be found to be
ASCO, American Society of Clinical Oncology; CAP, College of American Pathologists; HER2, human epidermal growth factor receptor 2; ISH, in situ hybridization; CEP17, chromosome enumeration probe 17; IHC, immunohistochemistry. An additional observer blinded to previous result recounts ISH. If the repeated ISH result is categorized to the same group, it is finally regarded as HER2 positive; An additional observer blinded to previous result recounts ISH. If the repeated ISH result is designated to same ISH group, it is finally regarded as HER2 negative.
HER2, human epidermal growth factor receptor 2; ISH, in situ hybridization; IHC, immunohistochemistry; CISH, chromogenic in situ hybridization; SISH, silver in situ hybridization; CEP17, chromosome enumeration probe 17.
HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; SISH, silver in situ hybridization.
HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; SISH, silver in situ hybridization; FISH, fluorescence in situ hybridization.

 E-submission
E-submission




