HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation
Article information
Abstract
Human epidermal growth factor receptor 2 (HER2) protein overexpression and/or HER2 gene amplification is found in about 20% of invasive breast cancers. It is a sole predictive marker for treatment benefits from HER2 targeted therapy and thus, HER2 testing is a routine practice for newly diagnosed breast cancer in pathology. Currently, HER2 immunohistochemistry (IHC) is used for a screening test, and in situ hybridization is used as a confirmation test for HER2 IHC equivocal cases. Since the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines on HER2 testing was first released in 2007, it has been updated to provide clear instructions for HER2 testing and accurate determination of HER2 status in breast cancer. During HER2 interpretation, some pitfalls such as intratumoral HER2 heterogeneity and increase in chromosome enumeration probe 17 signals may lead to inaccurate assessment of HER2 status. Moreover, HER2 status can be altered after neoadjuvant chemotherapy or during metastatic progression, due to biologic or methodologic issues. This review addresses recent updates of ASCO/CAP guidelines and factors complicating in the interpretation of HER2 status in breast cancers.
Human epidermal growth factor receptor 2 (HER2) is a protooncogene that encodes epidermal growth factor receptor with tyrosine kinase activity, located on chromosome 17 at q21. In breast cancers, HER2 gene is amplified in 15%–20% of invasive breast cancers and its amplification is closely linked to HER2 protein overexpression [1,2]. HER2 amplification is a poor prognostic factor associated with a high rate of recurrence and mortality, and is a predictive factor for response to anthracycline-based chemotherapies in patients with breast cancer [1,2]. Most importantly, it is a sole predictive marker for treatment benefits from HER2-targeting agents such as trastuzumab, lapatinib, and pertuzumab. As HER2-targeted therapy is exclusively effective in HER2-overexpressed and/or HER2-amplified breast cancers, precise assessment of HER2 status is an essential step for treatment of breast cancer. In this review, we focused on changes in the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines on HER2 interpretation and some pitfalls in the interpretation of HER2 status in breast cancers.
METHODS OF HER2 TESTING
Currently, immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), and chromogenic in situ hybridization (CISH) including silver in situ hybridization (SISH) are regarded as standard methods for determination of HER2 status in breast cancer, and some of them have been approved by the U.S. Food and Drug Administration (FDA) for HER2 testing in breast cancer since 1998.
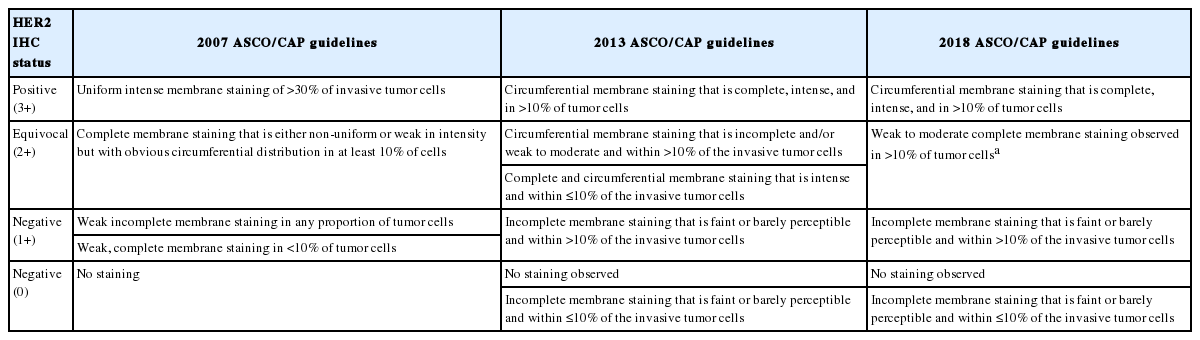
Although HER2 status can be directly tested by in situ hybridization (ISH), many laboratories have adopted IHC as a screening test, and FISH as a confirmation test for HER2 IHC equivocal cases, considering higher failure rate, longer procedure time and higher reagent cost of FISH, compared to that of IHC. Moreover, high concordance has been found between HER2 protein overexpression by IHC and gene amplification by FISH [3-5]. Finally, the 2007 ASCO/CAP guidelines stated that HER2 status should be initially assessed by IHC using a semi-quantitative scoring system (Fig. 1), and confirmed by FISH in all IHC score 2+ equivocal cases [6].
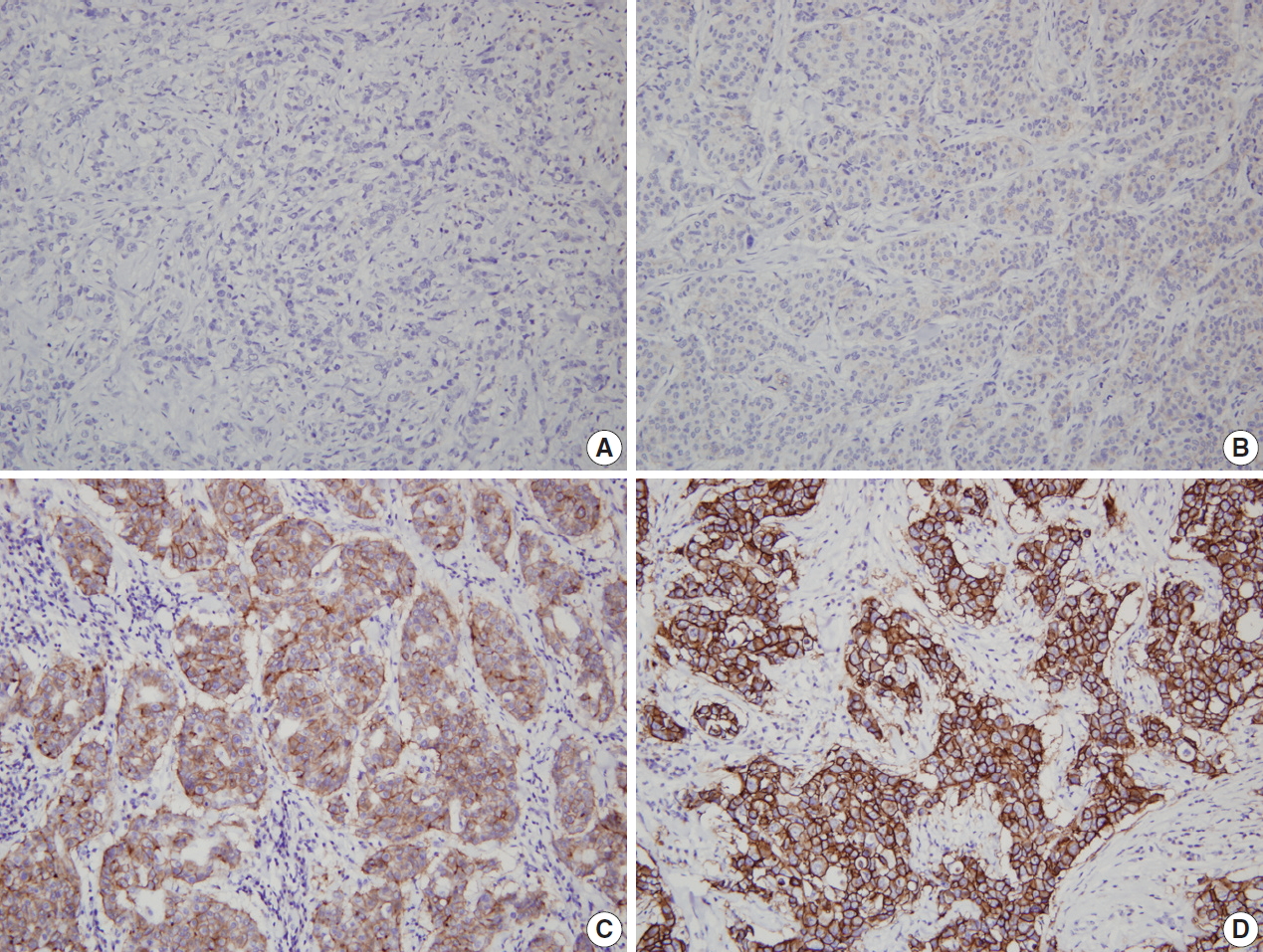
Representative examples of human epidermal growth factor receptor 2 (HER2) immunohistochemistry (IHC) in breast cancer. (A) HER2 IHC negative (0). (B) HER2 IHC negative (1+). (C) HER2 IHC equivocal (2+). (D) HER2 IHC positive (3+).
Bright-field in situ hybridization such as CISH and SISH has advantage in that it allows histologic evaluation of tumors, utilizes ordinary microscope, leaves permanent signals for archival storage, and can be fully automated [7]. Moreover, it shows more than 95% concordance rate with FISH [8-11]. Thus, CISH and SISH are now admitted as an alternative to FISH.
CHANGES IN GUIDELINES ON INTERPRETATION OF HER2 STATUS
For uniformity in accuracy and reproducibility of HER2 testing in breast cancer, ASCO/CAP jointly released guidelines and recommendations for HER2 testing first in 2007, addressing a wide range of pre-analytic, analytic and post-analytic variables [6]. This guideline focused on limiting the false-positive results, adopting a higher cutoff of 30% for HER2 IHC positivity (3+), instead of 10% cutoff previously recommended by FDA (Table 1). For FISH analysis, HER2 gene was regarded as amplified if HER2/chromosome enumeration probe 17 (CEP17) ratio >2.2 for dual-probe assay (instead of the previously recommended ratio of 2.0) or HER2 gene copy >6 signals per cell for single-probe assay.
After then, the revised 2013 ASCO/CAP guideline focused on maximizing identification of patients who can benefit from HER2-targeted therapy and minimizing false-negative results [12]. The range of HER2 IHC equivocal cases (2+) was widened, and HER2 IHC 3+ was defined using 10% as cutoff value, not using 30% (Table 1). When using validated dual-probe ISH assay, HER2/CEP17 ratio ≥2.0 or HER2/CEP17 ratio <2.0 and average HER2 copy number ≥6.0 was regarded as ISH positive (Table 2). The 2007 and 2013 ASCO/CAP guidelines included ISH equivocal results, which had been a problem for clinical decision making.
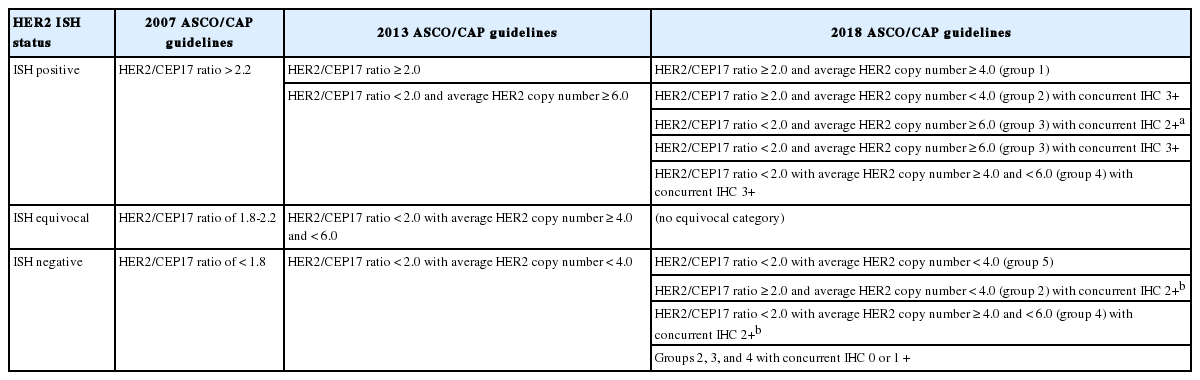
Changes in the ASCO/CAP guidelines: interpretation of HER2 status using dual-probe in situ hybridization assay
Since the publication of the 2013 guideline, several laboratories and clinical investigators have reported on the practical implications of the 2013 guidelines and increased frequencies of equivocal results [13]. The HER2 testing Expert Panel wished to clarify controversial issues in the 2013 guideline, and in that context the 2018 updated ASCO/CAP guidelines on HER2 testing was reported [13]. The updated guideline addressed five clinical questions. The first question was about the definition of HER2 IHC 2+, and it was revised as weak to moderate complete membrane staining observed in >10% of tumor cells. The second question was about repeated HER2 test of initially negative case, and it was recommended that if the initial HER2 testing result in a core needle biopsy specimen is negative, a new HER2 test may (not ‘‘must’’) be ordered on the excision specimen based on specific clinical criteria. These two clinical questions were addressed in a previous correspondence by the Expert Panel [14]. The remaining three questions were about less common ISH patterns, and the updated HER2 testing algorithm addressed the workup for these three clinical scenarios, occasionally found when using a dual-probe ISH assay. These scenarios were described as ISH group 2 (HER2/CEP17 ratio ≥2.0; average HER2 copy number < 4.0 signals per cell), ISH group 3 (HER2/CEP17 ratio <2.0; average HER2 copy number ≥6.0 signals per cell), and ISH group 4 (HER2/CEP17 ratio <2.0; average HER2 copy number ≥ 4.0 and <6.0 signals per cell) [13]. The diagnostic approach includes more rigorous interpretation criteria for ISH and requires concomitant IHC review for dual-probe ISH groups 2 to 4 to achieve the most accurate determination of HER2 status based on combined interpretation of the ISH and IHC assay [13]. It was recommended that laboratories using single-probe ISH assays include concomitant IHC review as part of the interpretation of all ISH assay results [13]. By this approach, the HER2 status was designated to positive or negative with no equivocal category.
Although complicated, HER2 status is basically determined by IHC results in the dual-probe ISH groups 2 to 4. While cases with HER2 IHC 3+ are regarded as HER2 positive, those with HER2 IHC 0 or 1+ are regarded as HER2 negative. In cases with HER2 IHC 2+, an additional observer blinded to previous result recounts ISH. As for ISH groups 2 and 4, if the repeated ISH result is designated to the same ISH group, it is finally regarded as HER2 negative. On the contrary, as for ISH group 3, if the repeated ISH result is categorized to the same group, it is finally regarded as HER2 positive. If the repeated ISH shows other ISH result, the results should be adjudicated per internal procedures to determine final category. The changes on HER2 interpretation in the updated 2018 ASCO/CAP guidelines in comparison with previous 2007 and 2013 guidelines are summarized in Tables 1 and 2.
Recent studies on implementation of the updated 2018 ASCO/CAP guidelines have shown significant increases of HER2 negative rates through reclassification of ISH equivocal cases by the 2013 guidelines [15-17]. The updated guidelines seem to provide much clearer instructions for HER2 status designation by using concomitant IHC review in ISH groups 2, 3 and 4, and eliminating ISH equivocal category [15].
COMPLICATING FACTORS FOR HER2 INTERPRETATION
Intratumoral HER2 heterogeneity
HER2 status has been thought to be fairly homogeneous within a tumor. However, intratumoral heterogeneity of HER2 gene amplification, that is, intratumoral HER2 heterogeneity, is found in a subset of breast cancers. It has important clinical implications, in that it can contribute to inaccurate assessment of HER2 status and affect treatment responses to HER2-targeted therapy [18]. In previous studies, our group has shown that intratumoral HER2 heterogeneity is more common in breast cancer with equivocal HER2 protein expression and low-grade HER2 gene amplification [19,20]. Intratumoral HER2 heterogeneity was associated with poor clinical outcome in patients with HER2-positive primary breast cancer [19]. Moreover, it was related to poor response to trastuzumab and decreased survival in patients with HER2-positive metastatic breast cancer [20]. In a subsequent study, Lee et al. [21] reported that cases with HER2 regional heterogeneity showed a decreased disease-free survival rate compared to those without heterogeneity in the hormone receptor-positive subgroup of breast cancer patients treated with adjuvant trastuzumab. In a neoadjuvant setting, intratumoral HER2 heterogeneity was also found to be associated with incomplete response to anti-HER2 chemotherapy [22].
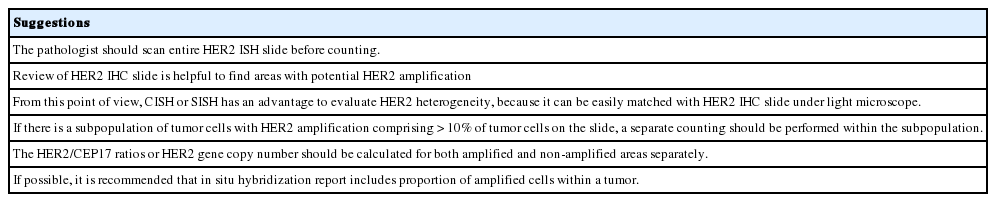
The CAP addressed this issue and published a separate recommendation in 2009 and defined HER2 genetic heterogeneity as the presence of tumor cells with HER2/CEP17 signal ratios greater than 2.2 in 5% to 50% of the tumor cells tested [23]. However, this recommendation has some problems, in that it was based on expert opinion rather than evidence, and artificial heterogeneity caused by technical issues could be regarded as HER2 genetic heterogeneity. Finally, the 2013 ASCO/CAP guidelines added a recommendation about HER2 heterogeneity for HER2 ISH interpretation, stating that if there is a second population of cells with increased HER2 signals/cell and this cell population is more than 10% of tumor cells on the slide, a separate counting of at least 20 non-overlapping cells must also be performed within this cell population and reported [12].
HER2 heterogeneity can be found as distinct clusters of amplified cells among non-amplified cells or appear as intermixed amplified and non-amplified cells (Fig. 2). Basically, it is important to scan all fields when observing ISH slide and to match it with HER2 IHC slide to detect areas with HER2 heterogeneity. From this point of view, CISH or SISH has an advantage in evaluating HER2 heterogeneity, because it can be easily matched with HER2 IHC slide under light microscope. The HER2/CEP17 ratios or HER2 copy number should be calculated separately for amplified and non-amplified areas. Suggestions for how to assess intratumoral HER2 heterogeneity are summarized in Table 3 [24,25].
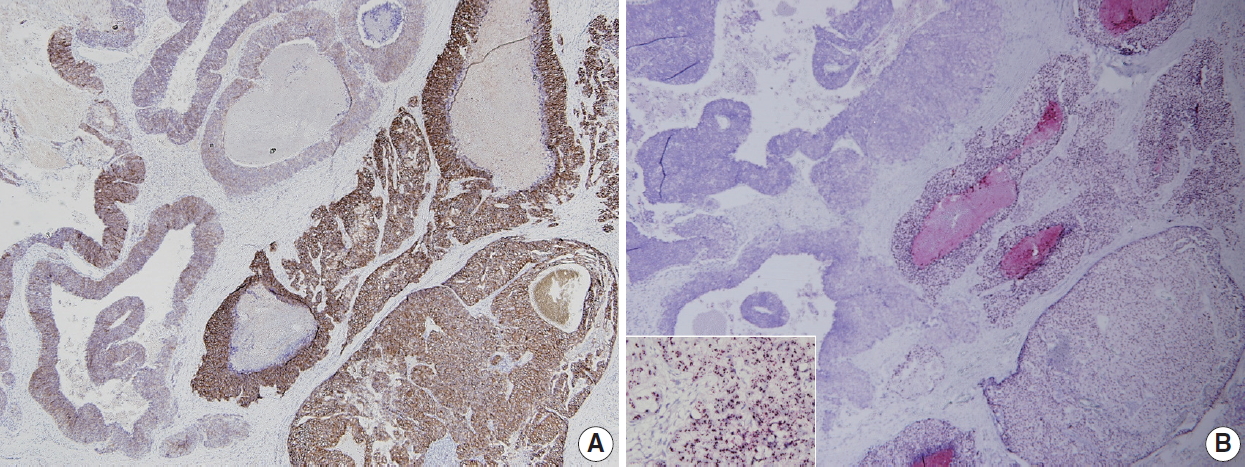
A representative breast cancer with intratumoral human epidermal growth factor receptor 2 (HER2) heterogeneity. (A) HER2 immunohistochemistry shows heterogeneous expression with strong, complete membranous expression on the right, and weak to moderate, incomplete membranous expression on the left. (B) HER2 silver in situ hybridization reveals high-level amplification on the right and no amplification on the left (inset, area of high-level amplification).
CEP17 copy number gain
CEP17 copy number gain is a genetic change commonly observed during dual-probe HER2 ISH for breast cancer, with reported frequency of 3% to 46% in breast cancers [26]. In our study using 945 cases of invasive breast cancer, CEP17 copy number gain was observed in 29.9% when using the definition of CEP17 copy number gain as mean CEP17 ≥2.6, and was found in 19.7% with the definition of CEP17 copy number gain as CEP17 ≥3.0 [27]. This had been thought to result from increasing numbers of whole chromosome 17, which is referred to as polysomy 17. However, subsequent studies have revealed that true polysomy 17 is a rare phenomenon in breast cancers, and CEP17 copy number gain results from amplification or copy number gain in the centromeric or pericentromeric region [28-32].
Although it is still controversial, CEP17 copy number gain has been found to be associated with increased HER2 protein expression [27,33-35]. However, CEP17 copy number gain without HER2 gene amplification was not associated with benefit from HER2-targeted therapy in breast cancers [36,37]. Moreover, CEP17 copy number gain in breast cancers has been reported to be associated with adverse clinicopathological features [27,38-40] and response to anthracycline-based therapy in breast cancer [41,42]. However, as for its prognostic significance, there have been conflicting results [43-46]. Recently, our group has shown that CEP17 copy number gain is an independent poor prognostic factor in patients with luminal/HER2-negative breast cancers, suggesting that CEP17 copy number gain may reflect chromosomal instability in breast cancer [27].
CEP17 copy number gain may affect the interpretation of HER2 ISH, and lead to an underestimation of HER2 status. Therefore, the 2013 ASCO/CAP guideline recommended to repeat HER2 testing using an alternative probe for CEP17 or for another gene in chromosome 17 not expected to co-amplify with HER2 for the ISH equivocal cases (now ISH group 4 in the updated 2018 ASCO/CAP guideline) [12,26]. However, following studies have shown that with alternative probes, HER2 ISH equivocal cases were upgraded to HER2 ISH positive status in a significant proportion, and clinicopathologic features of those upgraded cases were not compatible with those of HER2-amplified breast cancers, suggesting that use of alternative probe is not reliable in clinical practice [32,47]. The updated 2018 ASCO/CAP guidelines do not recommend the use of alternative probe as standard practice due to limited evidence on its analytical and clinical validity [13].
Changes in HER2 status after neoadjuvant chemotherapy
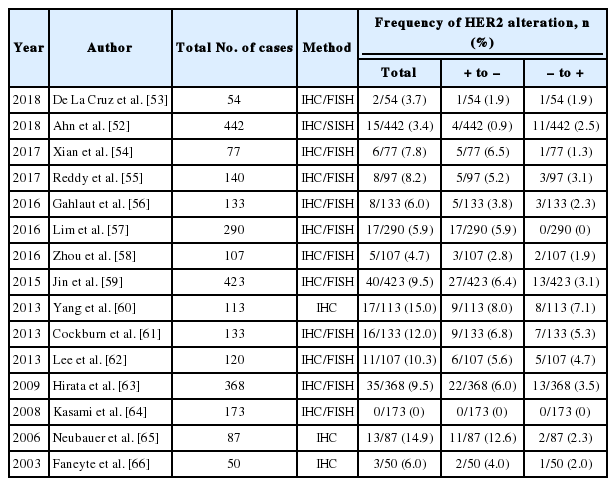
Neoadjuvant chemotherapy (NAC) is currently considered as standard treatment for locally advanced breast cancer [48]. Alteration of biomarker status after NAC is occasionally found in breast cancer [49,50]. Hormone receptor status changed more often than HER2 status, and as for hormone receptors, positive to negative conversion was more common than negative to positive conversion [51,52]. The frequency of HER2 change after NAC is reported in up to 15%, and both positive to negative conversion and negative to positive conversion were found with no preponderance [52-66]. Previous studies on HER2 change after NAC are summarized in Table 4. In our study, HER2 status was altered after NAC in 3.4% with positive to negative conversion in 0.9% and negative to positive conversion in 2.5% [52]. Most cases with negative to positive conversion of HER2 status after NAC showed low level of HER2 amplification, and the HER2/CEP17 ratio ranged from 2.2 to 4.4 (data not shown in a previous study) [52]. Cockburn et al. [61] also reported the mean HER2/CEP17 ratio in resection specimens with HER2 positive conversion was 3.7. Although there are no guidelines about whether treatment should be modified based on altered biomarker status after NAC, the change of HER2 status may have an impact on the therapeutic management in certain patients. Accordingly, re-evaluation of biomarkers including HER2 after NAC is recommended for proper management.
The mechanism of HER2 conversion after NAC is not well understood. This can be partly explained by the selection of HER2-positive or HER2-negative clones after NAC, tumor heterogeneity, and pre-analytical and analytical pitfalls, Guarneri et al. [67] evaluated HER2 status change on 107 HER2-positive patients treated with NAC with or without anti-HER2 agents. They reported that patients with tumors undergoing HER2 negative conversion following treatment had significantly reduced disease-free survival compared to patients with maintained HER2 positivity [67]. However, the prognostic significance of HER2 status change is still unclear.
Finally, caution is needed in interpretation of HER2 ISH after NAC in distinguishing between a true HER2 amplification and increase of HER2 copy number by chromosome 17 polysomy [68]. Increase in HER2 copy number could not be attributed to true HER2 amplifications, but instead could reflect polyploidization after chemotherapy, which presumably affects all chromosomes [68]. Careful evaluation using dual-probe ISH with concomitant IHC review is recommended.
Change in HER2 status with metastatic progression
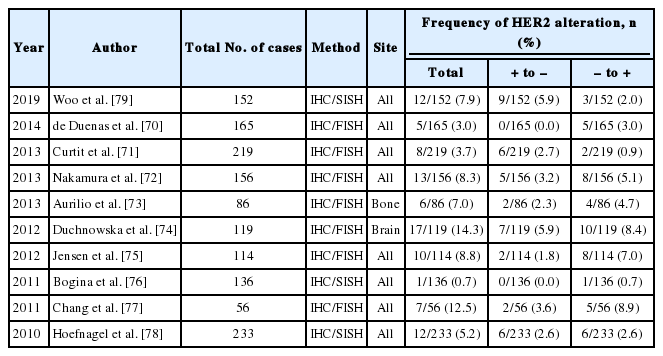
Change in receptor status during metastatic progression, a phenomenon called “receptor conversion” occurs not only in hormonal receptors, but also in HER2. The reported frequency of HER2 conversion varies a lot between studies, but it is usually observed less often than hormone receptor conversion. Schrijver et al. [69] reported in their meta-analysis that pooled frequency of HER2 positive to negative conversion was 21.3%, and that of negative to positive conversion was 9.5%. Previous studies on HER2 change during metastatic progression are summarized in Table 5 [70-79]. In our study, HER2 receptor status changed in 12 out of 152 cases (7.9%) during metastatic progression: nine cases (5.9%) were negative conversion, and three cases (2.0%) were positive conversion [79].
Similar to HER2 status alteration after NAC, little is known about the true mechanism of HER2 status conversion during metastatic progression. It would be reasonable to postulate intratumoral heterogeneity and selection pressure from treatment play a role in HER2 status conversion. In our study, cases with positive to negative conversion showed significantly lower level of HER2 protein expression and heterogeneous HER2 gene amplification, compared to consistently positive cases [79]. From this observation, it can be inferred that tumors with HER2 heterogeneity have a propensity to show different status in metastatic sites, because HER2-targeted therapy drives susceptible clones to fade away.
HER2 conversion is not a common event, but it is important to discover it because of its relation with treatment. There are some studies which reported response to trastuzumab treatment in patients who gained HER2 positivity in metastatic lesions [77,80]. For this reason, it is now widely recommended to reevaluate receptor status including HER2 in metastatic lesions, if possible [13,81].
CONCLUSION
The ASCO/CAP guidelines on HER2 interpretation in breast cancer, which were released first in 2007 and subsequently updated in 2013 and 2018, have been changing to provide much clearer instructions for HER2 status designation. The updated 2018 ASCO/CAP guideline focused on three dual-probe ISH groups (groups 2, 3, and 4) with less common ISH patterns, and recommended concomitant IHC review for these ISH groups to achieve the most accurate determination of HER2 status. Finally, in the updated 2018 ASCO/CAP guideline, HER2 status, as determined by ISH, is categorized to positive or negative with no equivocal results.
There are some complicating factors for HER2 interpretation including HER2 heterogeneity, CEP17 copy number gain, and HER2 status alteration after NAC or during metastatic progression. It is important to scan the entire ISH slide and to match it with HER2 IHC to detect HER2 heterogeneity. Separate counting should be performed in both amplified and non-amplified areas. CEP17 copy number gain may lead to an underestimation of HER2 status, but the use of alternative probe is not recommended in the updated 2018 ASCO/CAP guidelines due to the limited evidence on its analytical and clinical validity. HER2 conversion is occasionally found after NAC or during metastasis progression. The change of HER2 status may have an impact on the therapeutic decision and response to treatment. Accordingly, re-evaluation of HER2 status should be performed in postNAC specimens and metastatic lesions.
Notes
Author contributions
Project administration: SA.
Supervision: SYP.
Writing—original draft: SA, JWW, KL, SYP.
Writing—review & editing: SYP.
Conflicts of Interest
S.Y.P. is the editor-in-chief of the Journal of Pathology and Translational Medicine and was not involved in the editorial evaluation or decision to publish this article. All remaining authors declare that they have no potential conflicts of interest.
Funding
No funding to declare.