Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 54(1); 2020 > Article
-
Review
Tumor immune response and immunotherapy in gastric cancer -
Yoonjin Kwak1,2
 , An Na Seo3
, An Na Seo3 , Hee Eun Lee4
, Hee Eun Lee4 , Hye Seung Lee2,5
, Hye Seung Lee2,5
-
Journal of Pathology and Translational Medicine 2020;54(1):20-33.
DOI: https://doi.org/10.4132/jptm.2019.10.08
Published online: November 1, 2019
1Department of Pathology, Seoul National University Hospital, Seoul, Korea
2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea
3Department of Pathology, School of Medicine, Kyungpook National University, Kyungpook National University Chilgok Hospital, Daegu, Korea
4Division of Anatomic Pathology, Mayo Clinic, Rochester, MN, USA
5Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea
- Corresponding Author: Hye Seung Lee, MD, PhD, Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea Tel: +82-31-787-7714, Fax: +82-31-787-4012, E-mail: hye2@snu.ac.kr
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Using glucocorticoid receptor-related genes to create and validate a survival model predicting gastric cancer
Ke Guo, Ping Huang, Jiasheng Zhang, Bo Zhang, Linyang Li, Jiaxin Li
Computational Biology and Chemistry.2026; 120: 108726. CrossRef - Mechanisms of Metabolic Reprogramming Regulating Immunosuppression in the Gastric Cancer Tumor Microenvironment
Wenting Dong, Xuepeng Qian, Honglin Liu, Jinhai Huo, Weiming Wang
Biomolecules.2026; 16(1): 160. CrossRef - Perineural invasion in digestive tract tumors: Immune system interactions and therapeutic strategies
Jia-Yu Pang, Rong-Yao Jin, Han-Xiao Zhang, Ying-Hua Zhang, Xin-Yue Wei, Wen-Bo Cao, Yi-Xiao Chen, Jun-Ling Wang, Sai-Jun Mo
World Journal of Clinical Oncology.2026;[Epub] CrossRef - FOXP3+/CD8+ ratio associated with aggressive behavior in RUNX3‐methylated diffuse esophagogastric junction tumor
Suguru Maruyama, Yu Imamura, Tasuku Toihata, Ikumi Haraguchi, Manabu Takamatsu, Makiko Yamashita, Yuichiro Nakashima, Eiji Oki, Kenichi Taguchi, Manabu Yamamoto, Shinji Mine, Akihiko Okamura, Jun Kanamori, Souya Nunobe, Takeshi Sano, Shigehisa Kitano, Tet
Cancer Science.2025; 116(1): 178. CrossRef - Immune biomarkers and predictive signatures in gastric cancer: Optimizing immunotherapy responses
Sundaram Vickram, Shofia Saghya Infant, S. Manikandan, D. Jenila Rani, C.M. Mathan Muthu, Hitesh Chopra
Pathology - Research and Practice.2025; 265: 155743. CrossRef - Korean Practice Guidelines for Gastric Cancer 2024: An Evidence-based, Multidisciplinary Approach (Update of 2022 Guideline)
In-Ho Kim, Seung Joo Kang, Wonyoung Choi, An Na Seo, Bang Wool Eom, Beodeul Kang, Bum Jun Kim, Byung-Hoon Min, Chung Hyun Tae, Chang In Choi, Choong-kun Lee, Ho Jung An, Hwa Kyung Byun, Hyeon-Su Im, Hyung-Don Kim, Jang Ho Cho, Kyoungjune Pak, Jae-Joon Kim
Journal of Gastric Cancer.2025; 25(1): 5. CrossRef - Targeted therapy and immunotherapy for gastric cancer: rational strategies, novel advancements, challenges, and future perspectives
Dong Luo, Yunmei Liu, Zhengmao Lu, Lei Huang
Molecular Medicine.2025;[Epub] CrossRef - Prognostic value of the triglyceride-glucose index in gastric cancer
Tugce Eskazan, Suat Saribas, Bekir Kocazeybek
World Journal of Gastroenterology.2025;[Epub] CrossRef - Deciphering the dual role of autophagy in gastric cancer and gastroesophageal junction cancer: from tumor suppression to cancer progression
Lili Lei, Junling Zhang, Ran Wei, Bingqi Dong, Xin Wang, Ying Zhou
Discover Oncology.2025;[Epub] CrossRef - Comparison of clinicopathological parameters with the presence of Epstein–Barr virus and the absence of DNA mismatch repair proteins in gastric adenocarcinomas
Özge Eyeoğlu, Serra Kayaçetin
Asian Biomedicine.2025; 19(2): 86. CrossRef - The impact of combined immunotherapy on the cellular composition of the tumor microenvironment in patients with gastric carcinoma
L.A. Tashireva, A.Yu. Kalinchuk, D.M. Loos, E.A. Tsarenkova, A.V. Avgustinovich, S.G. Afanas’ev, S.V. Vtorushin
Russian Journal of Archive of Pathology.2025; 87(4): 24. CrossRef - Immunotherapy in gastric adenocarcinoma – a rapidly evolving treatment landscape
Yang Wang, Geoffrey Chong
Critical Reviews in Oncology/Hematology.2025; 216: 104941. CrossRef - Ubiquilin-4 induces immune escape in gastric cancer by activating the notch signaling pathway
Quan Jiang, Hao Chen, Shixin Zhou, Tao Zhu, Wenshuai Liu, Hao Wu, Yong Zhang, Fenglin Liu, Yihong Sun
Cellular Oncology.2024; 47(1): 303. CrossRef - Expression and prognostic value of APOBEC2 in gastric adenocarcinoma and its association with tumor-infiltrating immune cells
Lipan Wei, Xiuqian Wu, Lan Wang, Ling Chen, Xuejun Wu, Tiantian Song, Yuanyuan Wang, Wenjun Chang, Aizhen Guo, Yongdong Niu, Haihua Huang
BMC Cancer.2024;[Epub] CrossRef - Identification and characterization of CLEC11A and its derived immune signature in gastric cancer
Qing Zheng, Zhenqi Gong, Baizhi Li, Runzi Cheng, Weican Luo, Cong Huang, Huaiming Wang
Frontiers in Immunology.2024;[Epub] CrossRef - Cervical cancer subtype identification and model building based on lipid metabolism and post-infection microenvironment immune landscape
Yongzhi Chen, Rongjie Cui, Dun Xiong, Yuan Zhao, Jianyu Pang, Samina Gul, Qi Qi, Yuheng Tang, Xuhong Zhou, Wenru Tang
Heliyon.2024; 10(9): e30746. CrossRef - Systematic Analysis of Tumor Stem Cell-related Gene Characteristics
to Predict the PD-L1 Immunotherapy and Prognosis of Gastric
Cancer
Chenchen Wang, Ying Chen, Ru Zhou, Ya’nan Yang, Yantian Fang
Current Medicinal Chemistry.2024; 31(17): 2467. CrossRef - Comprehensive landscape of m6A regulator-related gene patterns and tumor microenvironment infiltration characterization in gastric cancer
Bin Peng, Yinglin Lin, Gao Yi, Mingzhen Lin, Yao Xiao, Yezhenghong Qiu, Wenxia Yao, Xinke Zhou, Zhaoyu Liu
Scientific Reports.2024;[Epub] CrossRef - Distinctive Phenotypic and Microenvironmental Characteristics of Neuroendocrine Carcinoma and Adenocarcinoma Components in Gastric Mixed Adenoneuroendocrine Carcinoma
Yoonjin Kwak, Soo Kyung Nam, Yujun Park, Yun-Suhk Suh, Sang-Hoon Ahn, Seong-Ho Kong, Do Joong Park, Hyuk-Joon Lee, Hyung-Ho Kim, Han-Kwang Yang, Hye Seung Lee
Modern Pathology.2024; 37(10): 100568. CrossRef - Computed tomography-detected extramural venous invasion-related gene signature: a potential negative biomarker of immune checkpoint inhibitor treatment in patients with gastric cancer
Hao Yang, Xinyi Gou, Caizhen Feng, Yinli Zhang, Fan Chai, Nan Hong, Yingjiang Ye, Yi Wang, Bo Gao, Jin Cheng
Journal of Translational Medicine.2023;[Epub] CrossRef - A standardized pathology report for gastric cancer: 2nd edition
Young Soo Park, Myeong-Cherl Kook, Baek-hui Kim, Hye Seung Lee, Dong-Wook Kang, Mi-Jin Gu, Ok Ran Shin, Younghee Choi, Wonae Lee, Hyunki Kim, In Hye Song, Kyoung-Mee Kim, Hee Sung Kim, Guhyun Kang, Do Youn Park, So-Young Jin, Joon Mee Kim, Yoon Jung Choi,
Journal of Pathology and Translational Medicine.2023; 57(1): 1. CrossRef - A Standardized Pathology Report for Gastric Cancer: 2nd Edition
Young Soo Park, Myeong-Cherl Kook, Baek-hui Kim, Hye Seung Lee, Dong-Wook Kang, Mi-Jin Gu, Ok Ran Shin, Younghee Choi, Wonae Lee, Hyunki Kim, In Hye Song, Kyoung-Mee Kim, Hee Sung Kim, Guhyun Kang, Do Youn Park, So-Young Jin, Joon Mee Kim, Yoon Jung Choi,
Journal of Gastric Cancer.2023; 23(1): 107. CrossRef - Korean Practice Guidelines for Gastric Cancer 2022: An Evidence-based, Multidisciplinary Approach
Tae-Han Kim, In-Ho Kim, Seung Joo Kang, Miyoung Choi, Baek-Hui Kim, Bang Wool Eom, Bum Jun Kim, Byung-Hoon Min, Chang In Choi, Cheol Min Shin, Chung Hyun Tae, Chung sik Gong, Dong Jin Kim, Arthur Eung-Hyuck Cho, Eun Jeong Gong, Geum Jong Song, Hyeon-Su Im
Journal of Gastric Cancer.2023; 23(1): 3. CrossRef - Could Toll-like Receptor 2 Serve as Biomarker to Detect Advanced Gastric Cancer?
Marek Majewski, Kamil Torres, Paulina Mertowska, Sebastian Mertowski, Izabela Korona-Głowniak, Jan Korulczyk, Witold Zgodziński, Ewelina Grywalska
International Journal of Molecular Sciences.2023; 24(6): 5824. CrossRef - Research Progress of Immunotherapy for Gastric Cancer
Zhipeng Zhang, Ningning Liu, Mingyu Sun
Technology in Cancer Research & Treatment.2023;[Epub] CrossRef - Case Report: A rare synchronous multiple gastric carcinoma achieved progression-free disease through NGS-guided serial treatment
Xinyi Shao, Jin Yin, Di Wang, Erjiong Huang, Yini Zhang, Jiani C. Yin, Chen Huang, Hao Wu, Xiaoli Wu
Frontiers in Oncology.2023;[Epub] CrossRef - Artificial Intelligence-Enabled Gastric Cancer Interpretations
Mustafa Yousif, Liron Pantanowitz
Surgical Pathology Clinics.2023; 16(4): 673. CrossRef - The Optimal Tumor Mutational Burden Cutoff Value as a Novel Marker for Predicting the Efficacy of Programmed Cell Death-1 Checkpoint Inhibitors in Advanced Gastric Cancer
Jae Yeon Jang, Youngkyung Jeon, Sun Young Jeong, Sung Hee Lim, Won Ki Kang, Jeeyun Lee, Seung Tae Kim
Journal of Gastric Cancer.2023; 23(3): 476. CrossRef - Biomarkers for Predicting Response to Personalized Immunotherapy in Gastric Cancer
Moonsik Kim, Ji Yun Jeong, An Na Seo
Diagnostics.2023; 13(17): 2782. CrossRef - The Prognostic Value of the Prognostic Nutritional Index in Patients with Advanced or Metastatic Gastric Cancer Treated with Immunotherapy
Yuting Pan, Yue Ma, Guanghai Dai
Nutrients.2023; 15(19): 4290. CrossRef - Molecular classification of gastric cancer predicts survival in patients undergoing radical gastrectomy based on project HOPE
Kenichiro Furukawa, Keiichi Hatakeyama, Masanori Terashima, Takeshi Nagashima, Kenichi Urakami, Keiichi Ohshima, Akifumi Notsu, Takashi Sugino, Taisuke Yagi, Keiichi Fujiya, Satoshi Kamiya, Makoto Hikage, Yutaka Tanizawa, Etsuro Bando, Yae Kanai, Yasuto A
Gastric Cancer.2022; 25(1): 138. CrossRef - Immunotherapy for Gastric Cancer: A 2021 Update
Christo Kole, Nikolaos Charalampakis, Sergios Tsakatikas, Nikolaos-Iasonas Kouris, George Papaxoinis, Michalis V Karamouzis, Anna Koumarianou, Dimitrios Schizas
Immunotherapy.2022; 14(1): 41. CrossRef - The immune microenvironment in gastric adenocarcinoma
Yana Zavros, Juanita L. Merchant
Nature Reviews Gastroenterology & Hepatology.2022; 19(7): 451. CrossRef - Immunomodulation by Gut Microbiome on Gastrointestinal Cancers: Focusing on Colorectal Cancer
Raghad Khalid AL-Ishaq, Lenka Koklesova, Peter Kubatka, Dietrich Büsselberg
Cancers.2022; 14(9): 2140. CrossRef - An Immunity-Associated lncRNA Signature for Predicting Prognosis in Gastric Adenocarcinoma
Xiaowen Zhao, Pingfan Wu, Dongling Liu, Changtian Li, Ling Xue, Zhe Liu, Meng Zhu, Jie Yang, Ziyi Chen, Yaling Li, Yali She, Kathiravan Srinivasan
Journal of Healthcare Engineering.2022; 2022: 1. CrossRef - RNA modification writers influence tumor microenvironment in gastric cancer and prospects of targeted drug therapy
Peng Song, Sheng Zhou, Xiaoyang Qi, Yuwen Jiao, Yu Gong, Jie Zhao, Haojun Yang, Zhifen Qian, Jun Qian, Liming Tang
Journal of Bioinformatics and Computational Biology.2022;[Epub] CrossRef - Identification of the three subtypes and the prognostic characteristics of stomach adenocarcinoma: analysis of the hypoxia-related long non-coding RNAs
Zehua Fan, Yanqun Wang, Rong Niu
Functional & Integrative Genomics.2022; 22(5): 919. CrossRef - Complete Response of High Microsatellite Instability Gastric Cancer and Synchronous Microsatellite Stability Rectal Cancer
Zachary E Hunzeker, Pooja Bhakta, Sindusha R Gudipally, Sri Bharathi Kavuri, Rohit Venkatesan, Chukwuyejulumafor Nwanze
Cureus.2022;[Epub] CrossRef - Immune Profiling in Gastric Cancer Reveals the Dynamic Landscape of Immune Signature Underlying Tumor Progression
Yuhan Wei, Jianwei Zhang, Xueke Fan, Zhi Zheng, Xiaoyue Jiang, Dexi Chen, Yuting Lu, Yingrui Li, Miao Wang, Min Hu, Qi Du, Liuting Yang, Hongzhong Li, Yi Xiao, Yongfu Li, Jiangtao Jin, Deying Wang, Xiangliang Yuan, Qin Li
Frontiers in Immunology.2022;[Epub] CrossRef - Tumor vessel normalization and immunotherapy in gastric cancer
Xianzhe Yu, Shan He, Jian Shen, Qiushi Huang, Peng Yang, Lin Huang, Dan Pu, Li Wang, Lu Li, Jinghua Liu, Zelong Liu, Lingling Zhu
Therapeutic Advances in Medical Oncology.2022;[Epub] CrossRef - FN1 is a prognostic biomarker and correlated with immune infiltrates in gastric cancers
Han Wang, Junchang Zhang, Huan Li, Hong Yu, Songyao Chen, Shuhao Liu, Changhua Zhang, Yulong He
Frontiers in Oncology.2022;[Epub] CrossRef - Molecular Pathology of Gastric Cancer
Moonsik Kim, An Na Seo
Journal of Gastric Cancer.2022; 22(4): 264. CrossRef - Bioinformatics Analysis and Structure of Gastric Cancer Prognosis Model Based on Lipid Metabolism and Immune Microenvironment
Yongzhi Chen, Hongjun Yuan, Qian Yu, Jianyu Pang, Miaomiao Sheng, Wenru Tang
Genes.2022; 13(9): 1581. CrossRef - Clinical implications of interleukins-31, 32, and 33 in gastric cancer
Qing-Hua Liu, Ji-Wei Zhang, Lei Xia, Steven G Wise, Brett David Hambly, Kun Tao, Shi-San Bao
World Journal of Gastrointestinal Oncology.2022; 14(9): 1808. CrossRef - Microbiota and the Immune System—Actors in the Gastric Cancer Story
Marek Majewski, Paulina Mertowska, Sebastian Mertowski, Konrad Smolak, Ewelina Grywalska, Kamil Torres
Cancers.2022; 14(15): 3832. CrossRef - Bioinformatics and Experimental Analyses Reveal MAP4K4 as a Potential Marker for Gastric Cancer
Junping Zhang, Xiaoping Cai, Weifeng Cui, Zheng Wei
Genes.2022; 13(10): 1786. CrossRef - Common strategies for effective immunotherapy of gastroesophageal cancers using immune checkpoint inhibitors
Shuang Ma, Fei Chen
Pathology - Research and Practice.2022; 238: 154110. CrossRef - High-level of intratumoral GITR+ CD4 T cells associate with poor prognosis in gastric cancer
Shouyu Ke, Feng Xie, Yixian Guo, Jieqiong Chen, Zeyu Wang, Yimeng Yu, Haigang Geng, Danhua Xu, Xu Liu, Xiang Xia, Fengrong Yu, Chunchao Zhu, Zizhen Zhang, Gang Zhao, Bin Li, Wenyi Zhao
iScience.2022; 25(12): 105529. CrossRef - Characteristics of Adenosine-to-Inosine RNA editing-based subtypes and novel risk score for the prognosis and drug sensitivity in stomach adenocarcinoma
Jingjing Pan, Xinyuan Gu, Jing Luo, Xinye Qian, Qiang Gao, Tianjie Li, Longying Ye, Chenlu Li
Frontiers in Cell and Developmental Biology.2022;[Epub] CrossRef - Inhibition of NF‐κB is required for oleanolic acid to downregulate PD‐L1 by promoting DNA demethylation in gastric cancer cells
Xirong Lu, Yuyi Li, Wei Yang, Minghao Tao, Yanmiao Dai, Jinkang Xu, Qianfei Xu
Journal of Biochemical and Molecular Toxicology.2021;[Epub] CrossRef - Prognostic Value of C-Reactive Protein to Albumin Ratio in Gastric Cancer: A Meta-Analysis
Liang Yue, Yi Lu, Yulin Li, Yilin Wang
Nutrition and Cancer.2021; 73(10): 1864. CrossRef - Immunogenic characteristics of microsatellite instability‐low esophagogastric junction adenocarcinoma based on clinicopathological, molecular, immunological and survival analyses
Yu Imamura, Tasuku Toihata, Ikumi Haraguchi, Yoko Ogata, Manabu Takamatsu, Aya Kuchiba, Norio Tanaka, Osamu Gotoh, Seiichi Mori, Yuichiro Nakashima, Eiji Oki, Masaki Mori, Yoshinao Oda, Kenichi Taguchi, Manabu Yamamoto, Masaru Morita, Naoya Yoshida, Hideo
International Journal of Cancer.2021; 148(5): 1260. CrossRef - Two Similar Signatures for Predicting the Prognosis and Immunotherapy Efficacy of Stomach Adenocarcinoma Patients
Taohua Yue, Shuai Zuo, Jing Zhu, Shihao Guo, Zhihao Huang, Jichang Li, Xin Wang, Yucun Liu, Shanwen Chen, Pengyuan Wang
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - Tumor microenvironment characterization in stage IV gastric cancer
Feng Yang, Zhenbao Wang, Xianxue Zhang
Bioscience Reports.2021;[Epub] CrossRef - E2F2 inhibition induces autophagy via the PI3K/Akt/mTOR pathway in gastric cancer
Hui Li, Shufen Zhao, Liwei Shen, Peige Wang, Shihai Liu, Yingji Ma, Zhiwei Liang, Gongjun Wang, Jing Lv, Wensheng Qiu
Aging.2021; 13(10): 13626. CrossRef - Chemoradiation induces upregulation of immunogenic cell death-related molecules together with increased expression of PD-L1 and galectin-9 in gastric cancer
S. H. Petersen, L. F. Kua, S. Nakajima, W. P. Yong, K. Kono
Scientific Reports.2021;[Epub] CrossRef - Establishment of an Immune Cell Infiltration Score to Help Predict the Prognosis and Chemotherapy Responsiveness of Gastric Cancer Patients
Quan Jiang, Jie Sun, Hao Chen, Chen Ding, Zhaoqing Tang, Yuanyuan Ruan, Fenglin Liu, Yihong Sun
Frontiers in Oncology.2021;[Epub] CrossRef - Microsatellite instability in Gastric Cancer: Between lights and shadows
Elisabetta Puliga, Simona Corso, Filippo Pietrantonio, Silvia Giordano
Cancer Treatment Reviews.2021; 95: 102175. CrossRef - Survival-associated alternative splicing events interact with the immune microenvironment in stomach adenocarcinoma
Zai-Sheng Ye, Miao Zheng, Qin-Ying Liu, Yi Zeng, Sheng-Hong Wei, Yi Wang, Zhi-Tao Lin, Chen Shu, Qiu-Hong Zheng, Lu-Chuan Chen
World Journal of Gastroenterology.2021; 27(21): 2871. CrossRef - Immunotherapy of gastric cancer: Past, future perspective and challenges
Jun Xie, Liping Fu, Li Jin
Pathology - Research and Practice.2021; 218: 153322. CrossRef - Clinicopathologic and Prognostic Association of GRP94 Expression in Colorectal Cancer with Synchronous and Metachronous Metastases
Sumi Yun, Sukmook Lee, Ho-Young Lee, Hyeon Jeong Oh, Yoonjin Kwak, Hye Seung Lee
International Journal of Molecular Sciences.2021; 22(13): 7042. CrossRef - Injectable shear-thinning polylysine hydrogels for localized immunotherapy of gastric cancer through repolarization of tumor-associated macrophages
Yan Yang, Yang Yang, Meili Chen, Jianquan Chen, Jinyan Wang, Yajun Ma, Hanqing Qian
Biomaterials Science.2021; 9(19): 6597. CrossRef - Correlation between LRP1B Mutations and Tumor Mutation Burden in Gastric Cancer
Sizhe Hu, Xiaokang Zhao, Feng Qian, Cancan Jin, Kaishun Hou, Tao Huang
Computational and Mathematical Methods in Medicine.2021; 2021: 1. CrossRef - Comprehensive Analysis to Identify MAGEA3 Expression Correlated With Immune Infiltrates and Lymph Node Metastasis in Gastric Cancer
Jinji Jin, Jianxin Tu, Jiahuan Ren, Yiqi Cai, Wenjing Chen, Lifang Zhang, Qiyu Zhang, Guanbao Zhu
Frontiers in Oncology.2021;[Epub] CrossRef - Effect of P2X7 receptor on tumorigenesis and its pharmacological properties
Wen-jun Zhang, Ce-gui Hu, Zheng-ming Zhu, Hong-liang Luo
Biomedicine & Pharmacotherapy.2020; 125: 109844. CrossRef - Current status and future potential of predictive biomarkers for immune checkpoint inhibitors in gastric cancer
Byung Woog Kang, Ian Chau
ESMO Open.2020; 5(4): e000791. CrossRef - Is Ramucirumab Still the Only Second-Line Treatment in Metastatic Gastric Cancer?
Khalil El Gharib, Hampig Raphael Kourie
Pharmacogenomics.2020; 21(17): 1203. CrossRef - Deep Learning Predicts Underlying Features on Pathology Images with Therapeutic Relevance for Breast and Gastric Cancer
Renan Valieris, Lucas Amaro, Cynthia Aparecida Bueno de Toledo Osório, Adriana Passos Bueno, Rafael Andres Rosales Mitrowsky, Dirce Maria Carraro, Diana Noronha Nunes, Emmanuel Dias-Neto, Israel Tojal da Silva
Cancers.2020; 12(12): 3687. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
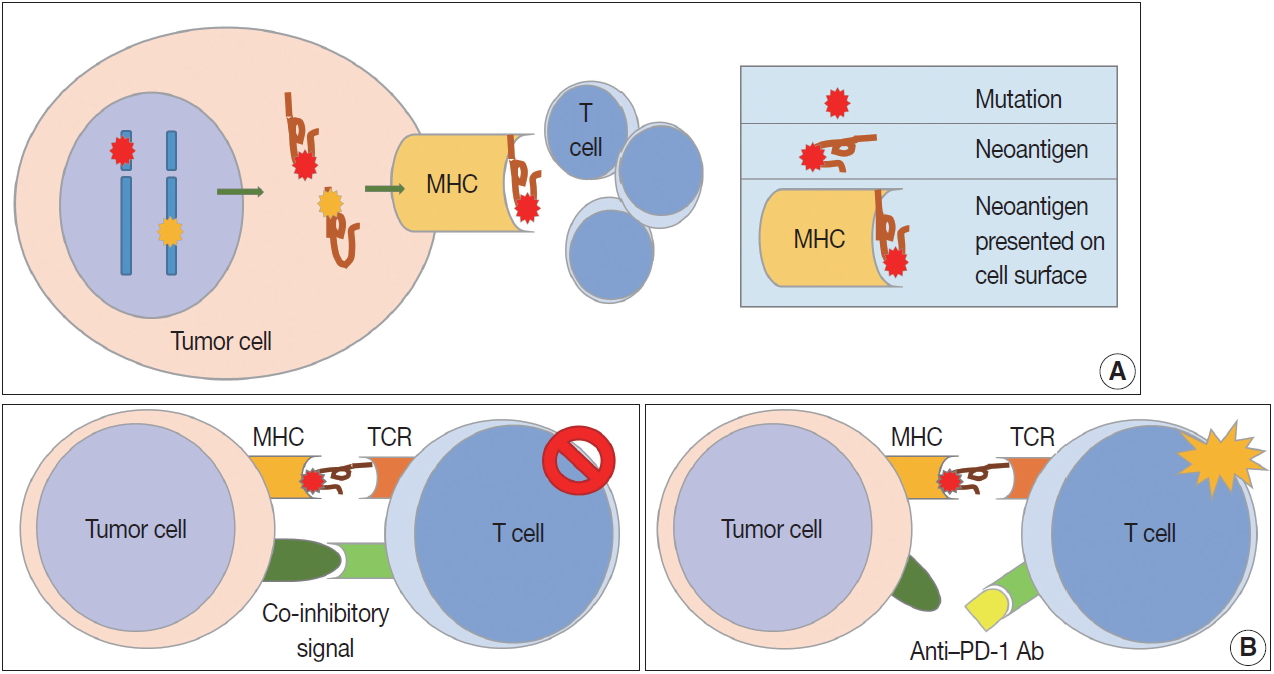
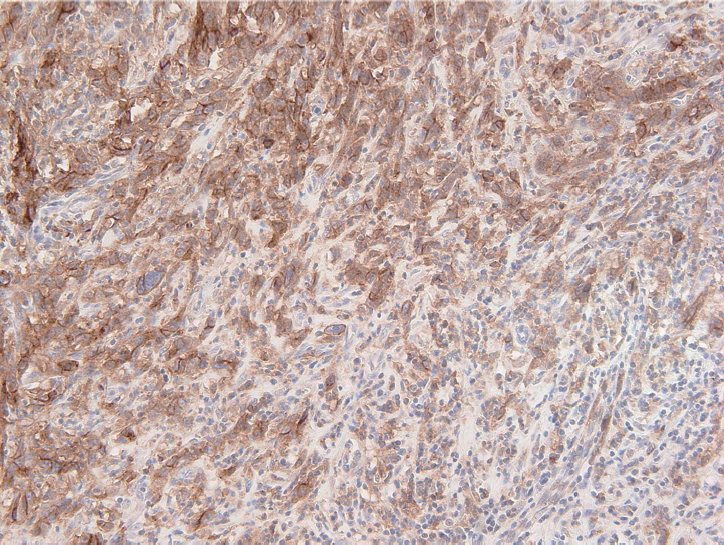
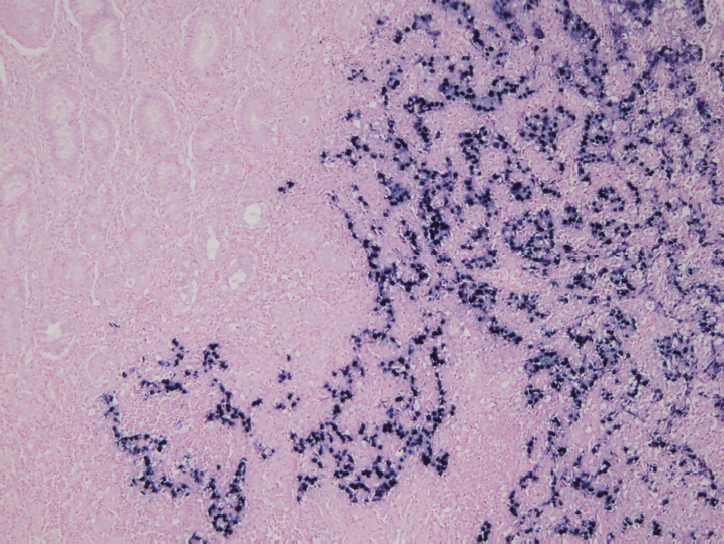
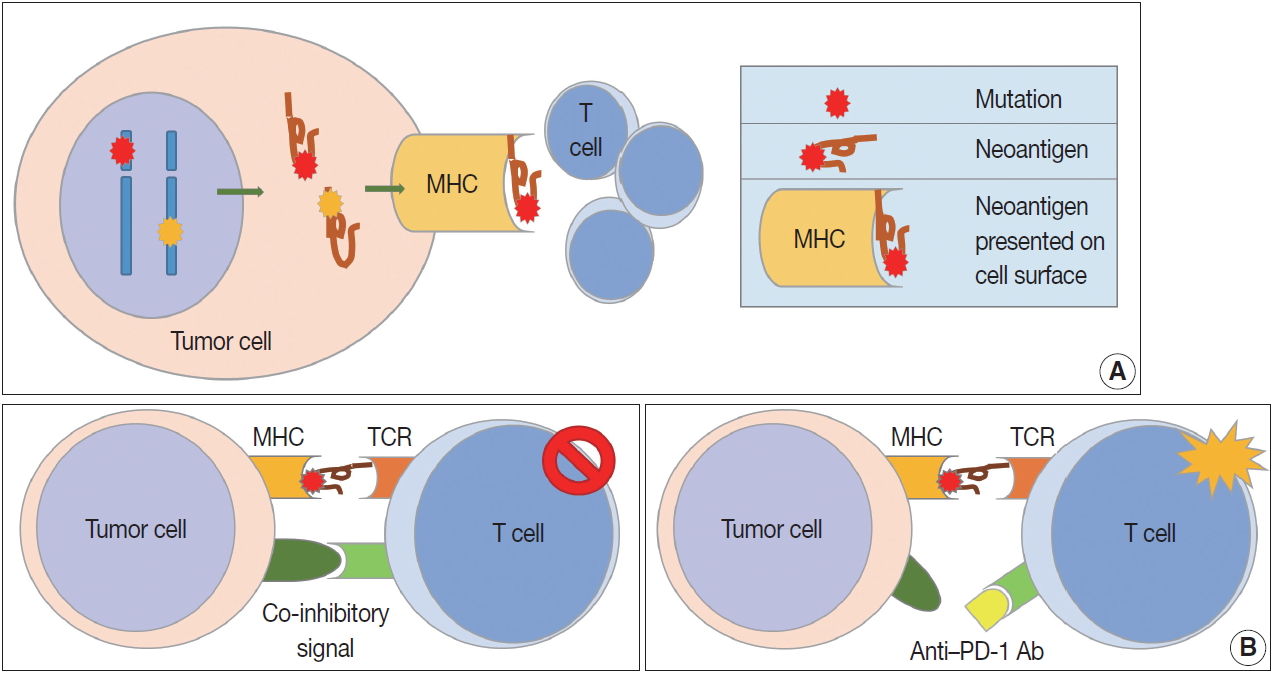
Fig. 1.
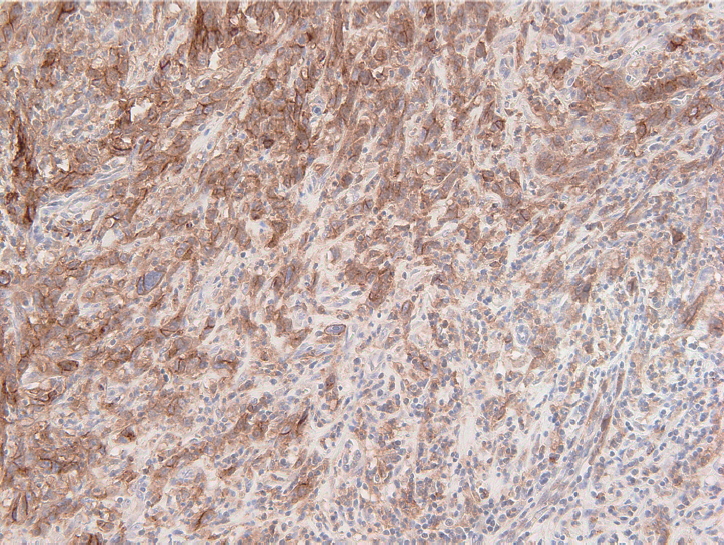
Fig. 2.
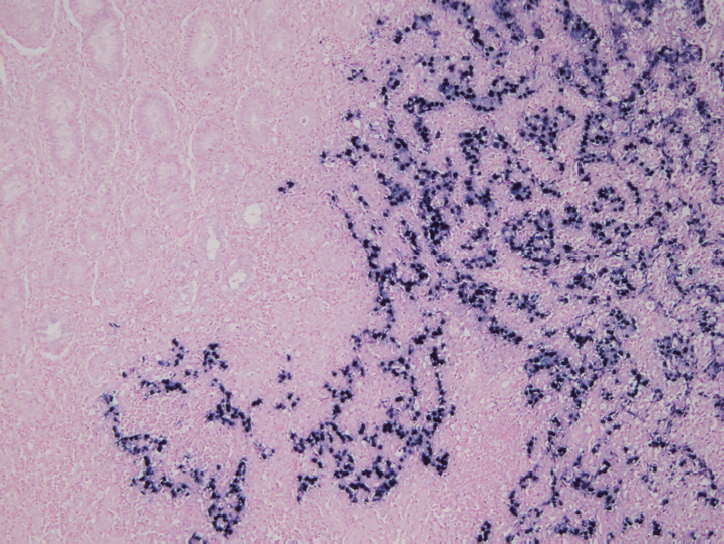
Fig. 3.
| Study | Region | No. | Subsets | Outcomes | TMA | Study | Selected area | CD8 cutoff point | CD8 cutoff number (/mm2) |
|---|---|---|---|---|---|---|---|---|---|
| Lee et al. (2008) [29] | Korea | 220 | CD3/CD8/CD45RO | OS | Yes | Consecutive GC | Representative one area | Mean | 435.73 |
| Haas et al. (2009) [30] | Germany | 52 | CD3/CD8/CD20/Foxp3/Granzyme B/M | OS | Yes | Gastric cardia cancer | Six representative areas | Median | 21.6 (epithelial) |
| 212.7 (stromal) | |||||||||
| Shen et al. (2010) [31] | China | 133 | CD4/CD8/Foxp3 | OS | Yes | GC with R0 resection | Average of two centers and two invasive border | Median | 946.22 (intratumoral) |
| 744.40 (peritumoral) | |||||||||
| Kim et al. (2011) [32] | Korea | 180 | CD3/CD4/CD8/Foxp3/Granzyme B | OS/RFS | No | Gastric cardia cancer | Mean of 5 HPFs (center areas) | Median | 60.8/HPF (253.33) |
| Kim et al. (2014) [33] | Korea | 99 | CD8/Foxp3 | OS | Yes | MSI-H advanced GC | Average of 3 representative areas | 60th percentile | 601.5 (median, 542.6) |
| Li e al. (2015) [34] | China | 192 | CD4/CD8 | OS | No | Advanced GC | Representative one slide | 26%-100% staining | Not available |
| Liu et al. (2015) [35] | China | 166 | CD3/CD4/CD8/Foxp3/CD57/M | OS | No | Surgical resection cases | Average of 5 noncontiguous and the densest random areas, in intratumoral and stromal area | Median | 839.69 (intratumoral) |
| 523.90 (stromal) | |||||||||
| Hennequin et al. (2016) [36] | France | 82 | CD8/CD20/Foxp3/Tbet | RFS | No | Consecutive GC (including preop-chemotherapy) | Mean of 3 HPF in core and invasive margin | Median | Not available |
| Kim et al. (2016) [37] | Korea | 243 | CD3/CD4/CD8 | DFS | Yes | Consecutive GC | Representative one core | Median | 375.48 |
| Giampieri et al. (2017) [38] | Italy | 73 | CD3 | OS | No | Metastatic GC with 1st-line chemotherapy | Biopsy or resected specimens | More than 50%-60% stromal area covered by TILs | Not available |
| Kawazoe et al. (2017) [39] | Japan | 383 | CD3/CD4/CD8/Foxp3 | OS | Yes | Advanced GC | Invasive area (two cores) | Median | 384 |
| Koh et al. (2017) [40] | Korea | 392 | CD3/CD4/CD8/Foxp3 | OS | Yes | Stage II and III GC | Tumor center and invasive border | 25th percentile | 130.07 (center) |
| 101.76 (border) | |||||||||
| Pernot et al. (2019) [41] | France | 67 | CD8/Foxp3/CD57 | OS | No | Locally advanced or metastatic GC | Mean of 3 representative HPFs | Median | 31/HPF |
| Kim et al. (2019) [42] | Korea | 297 | CD3/CD8/Foxp3 | OS | Yes | Early GC with submucosal invasion and advanced GC | Tumor center and invasive border | Median | Not available |
| Trials | Target | Phase | Treatment arms | Setting (line) | No. of patients | Results/primary endpoints | |
|---|---|---|---|---|---|---|---|
| Pembralizumab | |||||||
| KEYNOTE-012 [49] | PD-1 | 1b | Pembrolizumab | Any | 39 with positive PD-L1 | ORR (%): 22 (95% CI, 10-39; all PR) | |
| KEYNOTE-059 (cohort 1) [50] | PD-1 | 2 | Pembrolizumab | ≥ 3rd | 259 | ORR (%): 11.6 (95% CI, 8.0–16.1; CR in 2.3) | |
| Median response duration (mo): 8.4 (1.6 + |
|||||||
| OS (mo): 5.6; PFS (mo): 2.0 | |||||||
| KEYNOTE-061 [51] | PD-1 | 3 | Pembrolizumab | 2nd | 592 (395 with CPS ≥ 1) | OS (mo): 9.1 vs. 8.3 (HR, 0.82; 95% CI, 0.66-1.03) | |
| PaCIitaxel | PFS (mo): 1.5 vs. 4.1 (HR, 1.27; 95% CI, 1.03-1.57) (both analyses in the subgroup of positive PD-L1) | ||||||
| KEYNOTE-062 [52] | PD-1 | 3 | Pembrolizumab vs | 1st | 763 with CPS ≥ 1 (281 with CPS ≥ 10) | OS (mo): 10.6 vs. 12.5 vs. 11.1 (CPS ≥ 1) | |
| Pembrolizumab + cisplatin + 5-FU or capecitabine | OS (mo): 17.4 vs. 12.3 vs. 10.8 (CPS ≥ 10) | ||||||
| PFS (mo): 2.0 vs. 6.9 vs. 6.4 (CPS ≥ 1) | |||||||
| Placebo + cisplatin + 5-FU or capecitabine | PFS (mo): 2.9 vs. 5.7 vs. 6.1 (CPS ≥ 10) | ||||||
| Nivolumab (± ipilimumab) | |||||||
| CheckMate-032 [53] | PD-1 | 1/2 | Nivolumab 3 mg/kg nivolumab 1 mg/kg + ipilimumab 3 mg/kg | ≥ 2nd | 160 | ORR (%): 12 vs. 24 vs. 8 (independent of PD-L1 status) | |
| CTLA-4 | |||||||
| Nivolumab 3 mg/kg+ipilimumab 1 mg/kg | 12-mo PFS rates (%): 8 vs. 17 vs. 10 | ||||||
| 12-mo OS rates (%): 39 vs. 35 vs. 24 | |||||||
| ONO-4538-12, ATTRACTION-2 [46] | PD-1 | 3 | Nivolumab alone placebo | ≥ 3rd | 493 | OS (mo): 5.26 vs. 4.14 (HR, 0.63; 95% CI, 0.51-0.78) | |
| 12-mo OS rates (%): 26.2 vs. 10.9 | |||||||
| ATTRACTION-4, part 1 [54] | PD-1 | 2 | Nivolumab + oxaliplatin+capecitabine | 1st | 40 | ORR (%): 76.5 vs. 57.1 | |
| Nivdumab+oxaliplatin+S-1 | PFS (mo): 10.6 vs. 9.7 | ||||||
| ATTRACTION-4, part 2 | PD-1 | 3 | Nivolumab + oxaliplatin+S-1 or capecitabine Placebo + oxaliplatin+S-1 or capecitabine | 1st | Approx. 650 | Ongoing | |
| CheckMate-649 [55] | PD-1 | 3 | Nivolumab + ipilimumab | 1st | 870 | Ongoing | |
| CTLA-4 | Nivolumab + oxaliplatin + 5-FU or capecitabine Oxaliplatin + 5-FU or capecitabine | ||||||
| Others | |||||||
| JAVELIN Gastric 100 [58] | PD-L1 | 3 | Avelumab | Maintenance after 1st-line | 499 | Ongoing | |
| BSC after response or stability to oxaliplatinb + fluoropyrimidine | |||||||
| JAVELIN Gastric 300 [57] | PD-L1 | 3 | Avelumab | 3rd | 371 | OS (mo): 4.6 vs. 5.0 (HR, 1.1; 95% CI, 0.9-1.4) | |
| PaCIitaxel or irinotecan | PFS (mo): 1.4 vs. 2.7 (HR, 1.73; 95% CI, 1.4-2.2) | ||||||
| ORR (%): 2.2 vs. 4.3 | |||||||
| NCT02340975 [59] | PD-L1 | 1b/2 | Durvalumab (anti-PD-L1) | ≥ 2nd | 94 (phase 2; as of Sep 13, 2017) | Ongoing | |
| CTLA-4 | Tremelimumab (anti-CTLA-4) | ||||||
| Durvalumab+tremelimumab | |||||||
| NCT01968109 [60] | LAG-3 | 1/2a | Relatlimab (anti-LAG3) vs. Relatlimab + nivolumab | Last | Advanced solid tumors | Ongoing | |
| PD-1 | |||||||
TMA, tissue microarray; OS, overall survival; GC, gastric cancer; RFS, relapse-free survival; HPF, high power field; MSI-H, microsatellite instability-high; DFS, disease-free survival; TIL, tumor-infiltrating lymphocyte.
PD-1, programmed death-1; PD-L1, programmed death-ligand 1; ORR, objective response rate; CI, confidence interval; PR, partial response; CR, complete response; OS, overall survival; PFS, progression-free survival; CPS, Combined Positive Score for PD-L1; HR, hazard ratio; CI, confidence interval; 5-FU, 5-fluorouracil; CTLA-4, cytotoxic T lymphocyte–associated protein 4; LAG-3, lymphocyte-activation gene 3. + indicates that patients had no progressive disease at their last assessment.

 E-submission
E-submission