Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 52(2); 2018 > Article
-
Case Study
Fine-Needle Aspiration Cytology of Carcinosarcoma in the Salivary Gland: An Extremely Rare Case Report - Hyo Jung An1, Hye Jin Baek2,3,4, Jin Pyeong Kim2,3,5, Min Hye Kim6, Dae Hyun Song,1,2,3
-
Journal of Pathology and Translational Medicine 2018;52(2):136-139.
DOI: https://doi.org/10.4132/jptm.2017.07.27
Published online: December 28, 2017
1Deparment of pathology, Gyeongsang National University Changwon Hospital, Changwon, Korea
2Gyeongsang National University School of Medicine, Jinju, Korea
3Gyeongsang Institute of Health Science, Jinju, Korea
4Department of Radiology, Gyeongsang National University Changwon Hospital, Changwon, Korea
5Department of Otorhinolaryngology, Gyeongsang National University Changwon Hospital, Changwon, Korea
6Department of pathology, Gyeongsang National University Hospital, Jinju, Korea
- Corresponding Author Dae Hyun Song, MD Department of Pathology, Gyeongsang National University School of Medicine, 15 Jinju-daero 816beon-gil, Jinju 52727, Korea Tel: +82-55-214-3150 Fax: +82-55-214-3174 E-mail: golgy@hanmail.net
© 2018 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Carcinosarcoma of the salivary gland is an extremely rare tumor that is composed of both malignant epithelial and mesenchymal components. Diagnosing carcinosarcoma with fine-needle aspiration cytology is challenging because of its overlapping cytomorphologic characteristics with other high-grade malignant salivary gland tumors. Among the many features, including pleomorphic oncocytoid epithelial components, necrotic background, and mitoses, recognizing the singly scattered atypical spindle cells is most essential in carcinosarcoma. We present a case of a 66-year-old male patient with characteristic features of carcinosarcoma, who was successfully treated by wide local excision and subsequent radiation therapy.
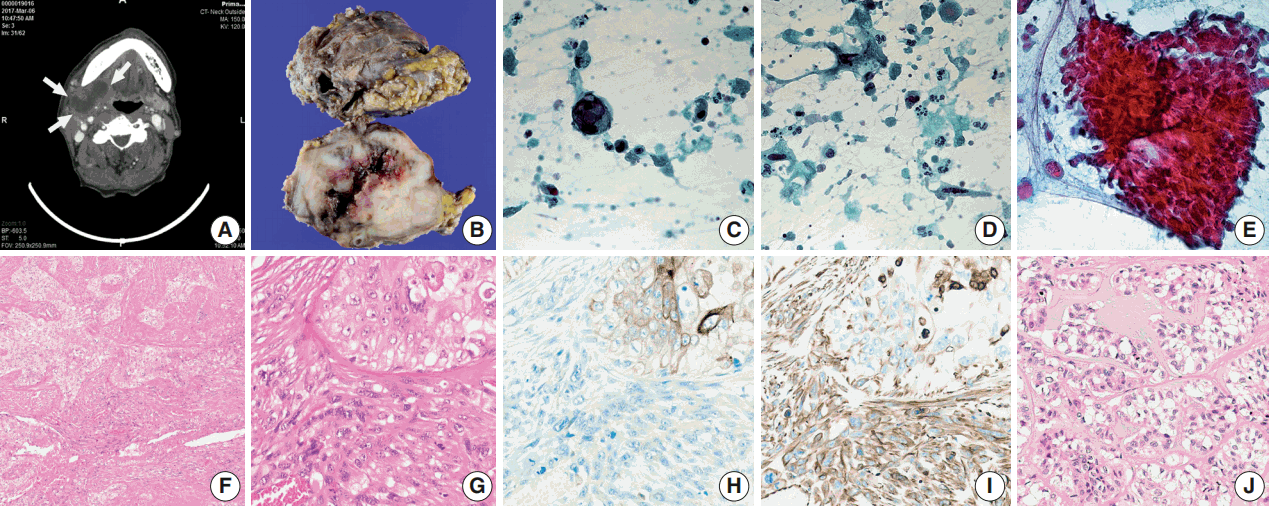
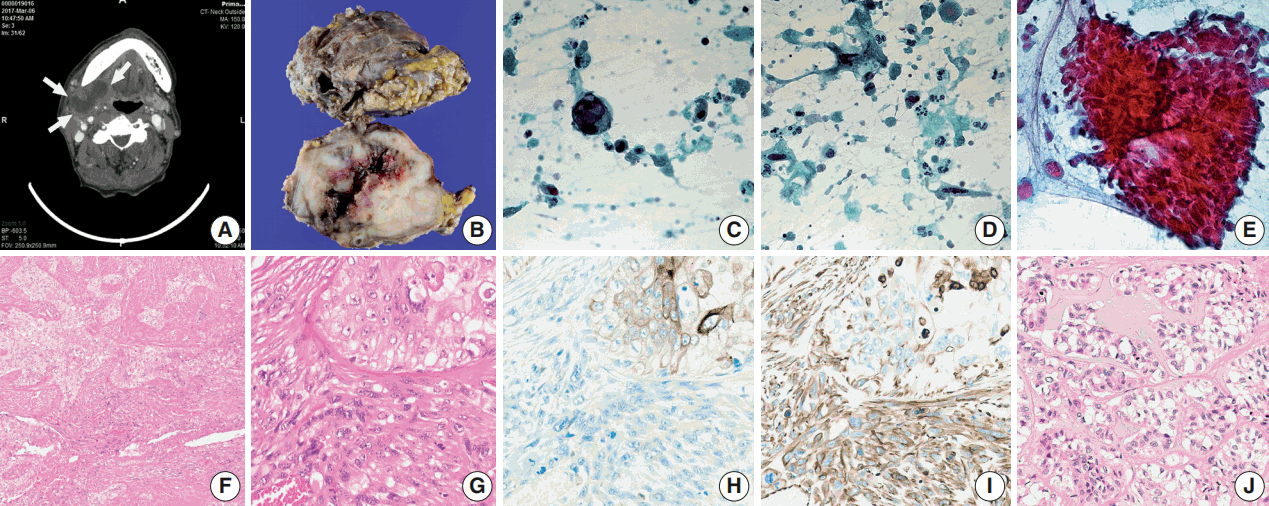
- A 66-year-old man presented with a mass in the right submandibular gland that had been rapidly enlarging for several months. His medical history included hypertension, cardiovascular attack, and unstable angina. The computed tomography scan showed a 5-cm-sized movable mass with sialolithiasis in the right submandibular area (Fig. 1A). The FNAC specimen contained numerous single malignant epithelial cells that had marked nuclear pleomorphism, increased nuclear-cytoplasmic ratio, and coarse chromatin pattern with prominent nucleoli (Fig. 1C). Additionally, a few atypical mucin-containing cells, reminiscent of mucoepidermoid carcinoma, were found. Abundant necrotic debris and a mixture of inflammatory cells were scattered in the background of dispersed atypical spindle cells (Fig. 1D). There were sheet-like fragments, which showed squamous differentiation with a few mitosis in only one out of ten FNAC slides (Fig. 1E). The patient underwent surgery to remove the mass and stones at Gyeongsang National University Changwon Hospital.
- Overall, the cut surface showed a relatively well-circumscribed, ivory, heterogeneous mass that measured 5.5 × 4 cm and extended to the extra-parenchymal area (Fig. 1B). Microscopically, the tumor was mainly composed of two components—undifferentiated carcinoma (UC) and undifferentiated pleomorphic sarcoma (UPS) with a central necrosis (Fig. 1F). Under higher magnification, the carcinomatous component was haphazardly arranged with numerous mitoses. The sarcomatous components were permeating to the UC (Fig. 1G). The immunohistochemical findings were in a sharp contrast in these two components. Carcinoma cells were positive for cytokeratin (Fig. 1H), whereas sarcoma cells were negative for cytokeratin and positive for vimentin (Fig. 1I). Focal areas mimicking epithelial-myoepithelial carcinoma were observed (Fig. 1J).
- All dissected 11 lymph nodes had no metastatic focus. The patient was successfully managed by wide local excision and subsequent radiation therapy. This study was approved by the Institutional Review Board of Gyeongsang National University Changwon Hospital with a waiver of informed consent (GNUCH 2017-09-009).
CASE REPORT
- FNAC is a simple, safe, cost-effective, well-tolerated, and in particular, minimally invasive method [3]. On average, the salivary gland FNAC has high specificity (97%), but the sensitivity is relatively low (80%) [4]. This means that the diagnosis on FNAC is very reliable, but the false-negative rate associated with FNAC (20%) may not be acceptable [4]. FNAC determines the extent of surgery needed after malignant tumor is diagnosed. It helps in deciding whether the facial nerve can be spared during the surgery and therefore, it is still important [3]. Diagnosing a high-grade salivary gland tumor, especially carcinosarcoma, on FNAC is challenging; thus, we should approach more systematically. Griffith et al. [5] proposed a risk stratification of FNAC in salivary gland tumors, which are classified as non-neoplastic and neoplastic. Among the neoplastic lesions, they proposed using the term oncocytoid and basaloid neoplasm rather than pleomorphic adenoma and Warthin tumor, the two most common tumors of the salivary gland. They also subdivided the oncocytoid and basaloid groups based on their nuclear grade (as monomorphic and pleomorphic groups), background characteristics, and stromal features. The pleomorphic oncocytoid neoplasm group was universally high-grade malignancies (21/21) and most of these 21 cases were of salivary duct carcinomas and several other high-grade carcinoma types including three high-grade mucoepidermoid carcinoma, one poorly differentiated carcinoma, and one UC [5].
- Based on the criteria of abundant cytoplasm, high nuclear grade with pleomorphism and hyperchromasia, and increased mitotic activity, the “pleomorphic oncocytoid neoplasm” was very identical to the carcinomatous components in our case. In addition, some other findings were observed, including isolated giant cells with vesicular nuclei and macronucleoli and isolated atypical spindle cells with hyperchromatic nuclei. These two different cells were classified as atypical because they had variations in size and shape more than three times the normal [6]. When FNAC findings were correlated with histological findings, the giant epithelial cells seemed to have come from the UC component, and the atypical spindle cells, either isolated or clustered with epithelial cells, seemed to have been exfoliated from the UPS component. In histologic section, UPS and UC were intermingled, showing different patterns of immunohistochemical staining for cytokeratin and vimentin, which is the key finding in confirming carcinosarcoma.
- Because there were squamoid clusters without keratinization in the FNA specimen, mucoepidermoid carcinoma (MEC) with squamous elements and moderate to poorly differentiated squamous cell carcinoma (SCC) were included in differential diagnosis. If it were MEC, there must be numerous tumor cell clusters due to its hard and solid consistency in high-grade types [6]. Also, non-keratinizing, moderate to poorly differentiated SCCs usually exfoliate in clusters or sheets. Rapidly growing tumors like our case frequently produce central necrosis and often show cellular debris with necrotic background in their aspirates [6]. In our case, there were plenty of individual pleomorphic cells in abundant necrotic background. Only up to 23 cell clusters were contained in 10 aspirated slides. We suggest that more aggressive tumors including malignant mixed tumor and carcinosarcoma must be considered, if there are fewer carcinomatous clusters and abundant individual pleomorphic cells in necrotic background.
- The sarcomatous components in our case occupied nearly 40% of the total tumor volume. However, these components were hardly seen in the FNAC. Both isolated spindle cells and sheet-like tissue fragments were found in one out of 10 FNAC slides. We hypothesized that matrix-forming characteristics of carcinosarcoma might have affected the hypocellularity of sarcomatous cells in the FNAC specimen. The malignant mesenchymal elements of carcinosarcomas are most commonly in the form of chondrosarcoma [2,7,8]. In the present case, however, features of chondrosarcoma were not observed in both cytologic and surgical specimens. In addition, tumor cells in sheets were not as pleomorphic as the isolated atypical spindle cells in the FNAC specimen. We should be concentrating on the scattered atypical cells or matrix-forming cells when we approach high-grade pleomorphic salivary gland tumors in FNAC. Frequently, isolated cells, which accurately reveal their characteristic morphologic features, are more important than three-dimensional clusters in cytologic specimens.
- The histogenesis and pathogenesis of the carcinosarcoma are still under discussion. Some authors insist that pleomorphic adenoma and carcinosarcomas may share a common precursor cell in which the myoepithelial cell is a major component in their development [8,9]. In the present case, approximately 20% of the total volume of epithelial myoepithelial carcinoma-mimicking area was observed in the histologic specimen. However, these components did not show any immunoactivities for smooth muscle actin and S100, a marker of myoepithelial cells. Furthermore, histologic evidence of a preexisting or coexisting pleomorphic adenoma was not observed. These findings may indirectly prove that myoepithelial cells may not be the sole origin of carcinosarcoma. We agree with Kwon and Gu [10] that the primitive mesenchymal cells, which can be differentiated in diverse directions, may contribute to the development of different types of sarcomas in carcinosarcomas; thus, they have heterogeneous combinations of both epithelial and mesenchymal components [7].
- Although carcinosarcoma is an extremely rare tumor, pathologists should be aware of this entity because the diagnosis of carcinosarcoma warrants concurrent radiation therapy extended to regional lymph nodes even if those lymph nodes are not metastatic.
- We described the cytologic features of carcinosarcoma arising in the submandibular salivary gland. In FNAC of the salivary gland tumor, carcinosarcoma should be considered if atypical spindle cells and highly pleomorphic epithelial cells are identified in abundant necrotic background.
DISCUSSION
- 1. King OH Jr. Carcinosarcoma of accessory salivary gland: first report of a case. Oral Surg Oral Med Oral Pathol 1967; 23: 651-9. PubMed
- 2. Keh SM, Tait A, Ahsan F. Primary carcinosarcoma of the parotid gland. Clin Pract 2011; 1: e117.ArticlePubMedPMC
- 3. Schmidt RL, Hall BJ, Wilson AR, Layfield LJ. A systematic review and meta-analysis of the diagnostic accuracy of fine-needle aspiration cytology for parotid gland lesions. Am J Clin Pathol 2011; 136: 45-59. ArticlePubMedPDF
- 4. Schmidt RL, Hunt JP, Hall BJ, Wilson AR, Layfield LJ. A systematic review and meta-analysis of the diagnostic accuracy of frozen section for parotid gland lesions. Am J Clin Pathol 2011; 136: 729-38. ArticlePubMedPDF
- 5. Griffith CC, Pai RK, Schneider F, et al. Salivary gland tumor fine-needle aspiration cytology: a proposal for a risk stratification classification. Am J Clin Pathol 2015; 143: 839-53. PubMedPMCPDF
- 6. Naib ZM. Cytopathology. 4th ed. New York: Little Brown & Company, 1996.
- 7. Marcotullio D, de Vincentiis M, Iannella G, Cerbelli B, Magliulo G. Mucoepidermoid carcinoma associated with osteosarcoma in a true malignant mixed tumor of the submandibular region. Case Rep Otolaryngol 2015; 2015: 694684.ArticlePubMedPMCPDF
- 8. Stephen J, Batsakis JG, Luna MA, von der Heyden U, Byers RM. True malignant mixed tumors (carcinosarcoma) of salivary glands. Oral Surg Oral Med Oral Pathol 1986; 61: 597-602. ArticlePubMed
- 9. Bleiweiss IJ, Huvos AG, Lara J, Strong EW. Carcinosarcoma of the submandibular salivary gland. Immunohistochemical findings. Cancer 1992; 69: 2031-5. ArticlePubMed
- 10. Kwon MY, Gu M. True malignant mixed tumor (carcinosarcoma) of parotid gland with unusual mesenchymal component: a case report and review of the literature. Arch Pathol Lab Med 2001; 125: 812-5. PubMed
REFERENCES
Figure & Data
References
Citations

- Carcinosarcoma of the parotid gland: a case report and review of the literature
Swachi Jain, Mohammed Abdelwahed, Daniel Hector Chavarria, Lucio Pereira, Gary Stone, Alan Johnson, Jian Yi Li
Journal of Medical Case Reports.2024;[Epub] CrossRef - Is Primary Poorly Differentiated Sarcomatoid Malignancy of the Parotid Gland Sarcomatoid Undifferentiated/Dedifferentiated Melanoma? Report of Three Unusual Cases Diagnosed by Fine-Needle Aspiration Combined with Histological, Immunohistochemical, and Mol
Jerzy Klijanienko, Julien Masliah-Planchon, Olivier Choussy, Guillaume Rougier, Antoine Dubray Vautrin, Maria Lesnik, Nathalie Badois, Wahib Ghanem, Jan Klos, Christophe Le Tourneau, Gregoire Marret, Raymond Barnhill, Adel K. El-Naggar
Acta Cytologica.2024; 68(2): 107. CrossRef - Carcinosarcoma of the deep lobe of the parotid gland in the parapharyngeal region: A case report
Yue-Yang Tang, Gui-Quan Zhu, Zhi-Jian Zheng, Li-Hong Yao, Zi-Xin Wan, Xin-Hua Liang, Ya-Ling Tang
World Journal of Clinical Cases.2023; 11(31): 7663. CrossRef - Carcinosarcoma of Submandibular Salivary Gland with a Rare Sarcomatous Variant
Shalini Bhalla, Naseem Akhtar, Puneet Prakash, Malti Kumari, Madhu Mati Goel
Indian Journal of Surgical Oncology.2019; 10(1): 61. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

 E-submission
E-submission



