Search
- Page Path
- HOME > Search
Review
- Professional biobanking education in Korea based on ISO 20387
- Jong Ok Kim, Chungyeul Kim, Sangyong Song, Eunah Shin, Ji-Sun Song, Mee Sook Roh, Dong-chul Kim, Han-Kyeom Kim, Joon Mee Kim, Yeong Jin Choi
- J Pathol Transl Med. 2025;59(1):11-25. Published online January 15, 2025
- DOI: https://doi.org/10.4132/jptm.2024.11.04
- 5,527 View
- 181 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
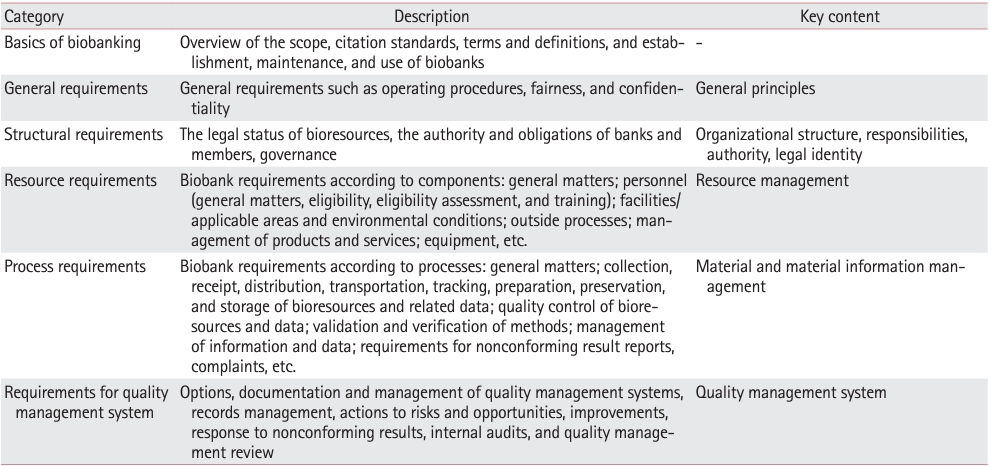
PDF - To ensure high-quality bioresources and standardize biobanks, there is an urgent need to develop and disseminate educational training programs in accordance with ISO 20387, which was developed in 2018. The standardization of biobank education programs is also required to train biobank experts. The subdivision of categories and levels of education is necessary for jobs such as operations manager (bank president), quality manager, practitioner, and administrator. Essential training includes programs tailored for beginner, intermediate, and advanced practitioners, along with customized training for operations managers. We reviewed and studied ways to develop an appropriate range of education and training opportunities for standard biobanking education and the training of experts based on KS J ISO 20387. We propose more systematic and professional biobanking training programs in accordance with ISO 20387, in addition to the certification programs of the National Biobank and the Korean Laboratory Accreditation System. We suggest various training programs appropriate to a student’s affiliation or work, such as university biobanking specialized education, short-term job training at unit biobanks, biobank research institute symposiums by the Korean Society of Pathologists, and education programs for biobankers and researchers. Through these various education programs, we expect that Korean biobanks will satisfy global standards, meet the needs of users and researchers, and contribute to the advancement of science.
-
Citations
Citations to this article as recorded by- Development of a big data platform for collecting and utilizing clinical information from the Korea Biobank Network
Yun Seon Im, Seol Whan Oh, Ki Hoon Kim, Wona Choi, In Young Choi
BMC Medical Informatics and Decision Making.2025;[Epub] CrossRef - Frozen section histopathology and preanalytical factors affecting nucleic acid integrity in biobanked fresh-frozen human cancer tissues
Soungeun Kim, Jaewon Kang, Boyeon Kim, Yoonjin Kwak, Hye Seung Lee
Journal of Pathology and Translational Medicine.2025; 59(6): 398. CrossRef
- Development of a big data platform for collecting and utilizing clinical information from the Korea Biobank Network

 E-submission
E-submission

 First
First Prev
Prev



