Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(3); 2013 > Article
-
Review
Current Concepts and Occurrence of Epithelial Odontogenic Tumors: I. Ameloblastoma and Adenomatoid Odontogenic Tumor - Suk Keun Lee, Yeon Sook Kim1
-
Korean Journal of Pathology 2013;47(3):191-202.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.3.191
Published online: June 25, 2013
Department of Oral Pathology, College of Dentistry, Gangneung-Wonju National University, Gangneung, Korea.
1Department of Dental Hygiene, College of Health Sciences, Cheongju University, Cheongju, Korea.
- Corresponding Author: Suk Keun Lee, D.D.S., Ph.D. Department of Oral Pathology, College of Dentistry, Gangneung-Wonju National University, 123 7 Jukheongil, Gangneung 210-702, Korea. Tel: +82-33-640-2228, Fax: +82-33-642-6410, sukkeunlee@hanmail.net
• Received: March 19, 2013 • Accepted: April 25, 2013
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Ameloblastomas and adenomatoid odontogenic tumors (AOTs) are common epithelial tumors of odontogenic origin. Ameloblastomas are clinico-pathologically classified into solid/multicystic, unicystic, desmoplastic, and peripheral types, and also divided into follicular, plexiform, acanthomatous, granular types, etc., based on their histological features. Craniopharyngiomas, derived from the remnants of Rathke's pouch or a misplaced enamel organ, are also comparable to the odontogenic tumors. The malignant transformation of ameloblastomas results in the formation of ameloblastic carcinomas and malignant ameloblastomas depending on cytological dysplasia and metastasis, respectively. AOTs are classified into follicular, extrafollicular, and peripheral types. Ameloblastomas are common, have an aggressive behavior and recurrent course, and are rarely metastatic, while AOTs are hamartomatous benign lesions derived from the complex system of the dental lamina or its remnants. With advances in the elucidation of molecular signaling mechanisms in cells, the cytodifferentiation of epithelial tumor cells in ameloblastomas and AOTs can be identified using different biomarkers. Therefore, it is suggested that comprehensive pathological observation including molecular genetic information can provide a more reliable differential diagnosis for the propagation and prognosis of ameloblastomas and AOTs. This study aimed to review the current concepts of ameloblastomas and AOTs and to discuss their clinico-pathological features relevant to tumorigenesis and prognosis.
- The pathogenetic mechanism of odontogenic tumors is closely related to the developmental processes of teeth. As a result, the molecular signaling mechanisms for normal enamel organs and odontogenic tumors have been closely compared. For the organogenesis of tooth germs, the reciprocal induction between odontogenic mesenchyme and enamel epithelium sequentially progresses to differentiate odontoblasts into odontogenic mesenchyme and ameloblasts in the enamel epithelium.1,2 During these processes of tooth formation, the tumorigenesis of odontogenic epithelium occurs in the state of immature odontogenic tissues, resulting in different histological features and variable potentials of tumor propagation. However, different epithelial odontogenic tumors can be classified into ameloblastomas, adenomatoid odontogenic tumors (AOTs), calcifying epithelial odontogenic tumors, odontogenic ghost cell tumors, squamous odontogenic tumors, ameloblastic fibromas, ameloblastic fibroodontomas, or odontomas, etc. This study was conducted to review the current concepts and occurrences of ameloblastomas and AOTs.
EPITHELIAL ODONTOGENIC TUMORS
- Once designated as an adamantinoma in 1885 (Malassez) but renamed to ameloblastoma in 1930 (Ivey and Churchil), ameloblastoma is a representative benign tumor of odontogenic epithelium. Some authors still misuse the term adamantinoma to describe ameloblastomas, even though an adamantinoma, meaning "very hard," is a rare bone tumor that differs in histology and frequency of malignancy from ameloblastomas.3 Ameloblastomas are common, have an aggressive behavior and recurrent course, and are rarely metastatic. However, adamantinomatous craniopharyngioma and adamantinoid basal cell carcinoma are terms that are still used to describe their histological characteristics.4
- Characteristics of ameloblastoma
- Ameloblastoma is a slow growing odontogenic epithelial tumor of the jaw and accounts for about 1% of all oral tumors and about 18% of odontogenic tumors. It is primarily seen in adults in the third to fifth decades of life, with almost equal sex predilection.5 Radiographically, it appears as an expansile radiolucency with thinned and perforated cortices, frequently causing root resorption. These features are common in giant cell tumors, aneurysmal bone cysts, and renal cell carcinoma metastasis, thus a definitive diagnosis can be made through histopathology.
- A total of 5,213 cases of ameloblastomas are found in literature, including 3,677 cases of ameloblastomas available in literature from 1960 to 1993,5 340 cases of ameloblastomas in the Malaysian population (1993 through 2008),6 and 1,196 cases of ameloblastomas diagnosed during the years of 1993 to 2009 in Chulalongkorn University, Bangkok.7 These studies revealed the mean age of patients as 36.1 years, with 2,709 (51.9%) patients being male, and mandibular tumors (81.7%) outnumbering maxillary tumors. In the variants of ameloblastomas, approximately 10.7% were unicystic ameloblastomas and 1.8% were peripheral ameloblastomas.
- In Korea, about 500 cases of ameloblastomas were sporadically reported, and among them, 452 cases were available to be statistically analyzed.8-10 The ratio of males to females was about 57:43, and the average age was approximately 33.8 years. The most frequent site of involvement was around the mandibular molar area (57.7%). The tumors were composed of conventional ameloblastomas (48.9%), unicystic ameloblastomas (25.3%), and peripheral ameloblastomas (3.1%). The recurrence rate for conventional ameloblastomas (17.1%) was significantly higher than for the unicystic type (9.1%). According to the histology, the acanthomatous, plexiform, and follicular patterns of the conventional ameloblastomas had similar recurrence rates of 16.2%, 15.9%, and 12.7%, respectively.
- Molecular mechanisms of ameloblastoma
- Every cellular changes, including proliferation, differentiation, senescence, tumorigenesis, etc., occur through the activation or inactivation of related molecular signaling pathways. The overexpression or underexpression of important signaling molecules may play an important role in the tumorigenesis of ameloblastomas. First of all, several proteins expressed in the enamel epithelium during the early stage of tooth formation, including amelogenin,11 tuftelin,12 and ameloblastin,13,14 are specifically expressed in tumor cells of ameloblastomas, but enamelin and amelotin expressed in the mineralizing stage of tooth formation are rarely positive in tumor cells of ameloblastomas.15,16
- Different proteins functioning in the signaling of the proliferation and differentiation of enamel epithelium and odontogenic tumors have been identified by many authors. The cellular and stromal proteins of syndecan-1,17 perlecan, α-dystroglycan, integrin β1,18 CD10, and osteopontin19 are overexpressed in ameloblastomas that exhibit locally invasive behavior and a high risk of recurrence. Solid ameloblastomas show an intense expression of fibronectin at the epithelial-mesenchymal interface, whereas desmoplastic ameloblastomas reveal no immunoexpression of fibronectin at this site. Ameloblastomas present a stronger immunoreaction of tenascin than AOTs, especially at the epithelial-mesenchymal interface, while AOTs and desmoplastic ameloblastomas exhibit an intense labeling for type I collagen. These expression patterns of matrix proteins agree with the more locally invasive behavior of ameloblastomas in comparison to AOTs.20
- The local invasiveness of ameloblastoma is also related to the expressions of metalloproteinase (MMP)-2 and MMP-9. The overexpression of MMP-9 in ameloblastomas is possibly modulated by the unmethylation of the gene and may influence the aggressive behavior and high recurrence rates of ameloblastoma.21
- The facts that receptor activator of nuclear factor κB ligand (RANKL) and MMP-9 are expressed in ameloblastomas may suggest that ameloblastoma cells have the potential to induce osteoclastogenesis, resulting in the rapid destruction of bone marrow.22,23 The higher expression rates of interleukin (IL)-1α and IL-6 are associated with tumor size in ameloblastomas. Therefore, it is suggested that the IL-1α and IL-6 cytokines play a role in the aggressive behavior of ameloblastomas by increasing bone resorption.24
- The overexpression of cyclin D1, a member of G1 cyclins controlling the cell-cycle transit from the G1 to S phase, is found in some plexiform ameloblastomas but is relatively mild in AOTs. The peripheral columnar and central stellate reticulum-like cells of ameloblastomas exhibit a dominant immunoreaction of cyclin D1, which is almost non-existent in the squamous and granular cells of ameloblastomas.25
- Midkine is a heparin-binding growth factor overexpressed in various human cancers and is expressed in ameloblastomas and ameloblastic carcinomas. This midkine expression in the majority of ameloblastomas may suggest a role of the protein in tumor development, progression, and behavior.26
- Epithelial growth factor receptor expression had no statistical significance for local recurrence, patient age, or tumor size, whereas a significant relation existed between CD10 expression and the Ki-67 labeling index, which is significantly related to the recurrence of ameloblastomas.27
- The overexpression of p53 and murine double minute 2 (MDM2) are associated with the pathogenesis and oncogenesis of ameloblastomas.28 The overexpression of p-mTOR, p-4E-BP1, and p-p70S6K in the nucleus also are related to the invasiveness of ameloblastomas.29
- The transforming growth factor-β (TGF-β)/SMAD signaling pathway is commonly activated in ameloblastomas, AOTs, and calcifying cystic odontogenic tumors. Meanwhile, the TGF-β/SMAD immunoreaction is significantly reduced in ameloblastomas in comparison to AOTs and calcifying cystic odontogenic tumors. These changes may lead to the more aggressive biological behavior of ameloblastomas through increased cell proliferation and reduced apoptosis and differentiation.30
- WNT proteins (except WNT-10b) are heterogeneously expressed in different types of ameloblastomas, indicating that WNT expression is closely related to tumor cell differentiation and invasion.31 However, the WNT-related bone-forming genes of WDR5 and runt-related transcription factor 2 (RUNX2) are down-regulated in ameloblastomas.32
- Alterations in the Sonic Hedgehog signaling pathway, including protein patched homolog (PTCH) gene mutations, have been associated with the pathogenesis of some odontogenic tumors including ameloblastomas and are closely related to nevoid basal cell carcinoma syndrome through the genetic alteration of PTCH. Therefore, ameloblastoma diagnosis possibly warrants a search for associated cutaneous basal cell carcinomas and other benign and malignant tumors related to nevoid basal cell carcinoma syndrome.33-35
- There appears to be no difference in the expression of E-cadherin or β-catenin between tooth germs and solid and unicystic ameloblastomas. The expression of these molecules seems to be mainly related to the process of cell differentiation.36
- Rho GTPases that regulate the cell cycle, shape, polarization, invasion, migration, and apoptosis are overexpressed in ameloblastomas. Therefore, it is suggested that these GTPases play a role in the neoplastic epithelial cell phenotype determination of ameloblastomas (polarized or non-polarized), as well as in variant (solid or unicystic) and subtype (follicular or plexiform) determination.37
- On the other hand, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and TRAIL receptor are diffusely expressed in ameloblastomas and are possibly involved in the neoplastic transformation of odontogenic epithelium. FAS (CD95), FAS ligand, and caspase-3 are also expressed in ameloblastomas.38 These expressions may suggest some intrinsic regulation of neoplastic cell proliferation and death in ameloblastomas, thus explaining their slow growth and inability to metastasize.39
- Histological types of ameloblastoma
- Histologically, ameloblastomas are classified into follicular, plexiform, acanthomatous, and granular cell types. Less common histologic variants are clear cell, desmoplastic, basal cell, papilliferous, and keratoameloblastoma types. Generally, one-third of ameloblastomas are plexiform, one-third are follicular, and other variants such as acanthomatous types usually occur in older patients.
- The follicular type of ameloblastoma frequently forms various sizes of dental follicle structures and possesses an outer arrangement of columnar or palisaded ameloblast-like cells and inner zone of triangular-shaped cells resembling the stellate reticulum in the bell stage of the tooth germ (Fig. 1A, E). Histopathology shows cells that have a tendency for nuclei to move away from the basement membrane. This process is referred to as "reverse polarization." The central cells sometimes degenerate to form central microcysts.
- The follicular type of ameloblastomas grow and usually form multicystic nodules, resulting in multilocular tumor masses with frequent recurrence compared to the other types of ameloblastomas.40
- The plexiform type contains epithelium that proliferates in a "fish net pattern," or in a "cord-like fashion anastomosing each other," hence the name "plexiform." There are layers of cells between the proliferating epithelium with well-formed desmosomal junctions, simulating spindle cell layers (Fig. 1B, F). Plexiform ameloblastomas with multiple cyst formation are rare and benign odontogenic tumors which may reach grotesque proportions, affecting a large region of the jaw.41
- Acanthomatous ameloblastoma is the extremely rare variant exhibiting solid epithelial cell nests with peripheral palisading ameloblastic cells and central squamous cell differentiation (Fig. 1C, G).42 The acanthomatous ameloblastoma masquerades as a squamous cell carcinoma and also appears as a "hybrid" ameloblastoma admixed with a pronounced desmoplastic pattern.43
- Granular cell ameloblastoma is a variant of ameloblastoma, where the tumor cells located in the central portion of the follicles have granular eosinophilic cytoplasm and the peripheral tumor cells resemble ameloblasts. The granular cells are a transitional or matured phase in the life cycle of ameloblastomas, starting with normal stellate reticulum-like cells, leading to a production of granules and finally resulting in degeneration and the formation of cystic areas, differing from the aggressive tumor nature reported previously.44 However, the granular cell type is rare and accounts for only 4% of ameloblastomas.45
- Keratoameloblastoma is a very rare ameloblastoma variant defined by extensive squamous metaplasia and keratinization. Hemangiomatous ameloblastoma is also described as a rare ameloblastic variant.46
- Basal cell ameloblastoma is also a very rare variant of ameloblastoma resembling basaloid squamous cell carcinoma but possessing the pathological features of conventional ameloblastoma.47
- Adenoid ameloblastoma is a rare variant in this category and can cause problems in diagnosis due to the presence of areas resembling AOT and the occurrence of varying degrees of dentinoid formation.48
- Clinico-pathological classification of ameloblastoma
- According to the World Health Organization (WHO), ameloblastomas are classified into the following types depending on the origin of tumorigenesis: solid/multicystic, extraosseous/peripheral, desmoplastic, and unicystic. The common solid/multicystic ameloblastomas arise from enamel epithelial rests in jaw bone, while unicystic ameloblastomas arise from the epithelium of odontogenic cysts. Desmoplastic ameloblastomas exhibit active stromal proliferation, while peripheral ameloblastomas arise from dental lamina rests and oral mucosa epithelium. These tumor types differ in biological behavior and rate of recurrence. Therefore, each type of ameloblastoma requires different forms of treatment.49 Additionally craniopharyngiomas arising from the rests of Rathke's pouch epithelium are comparable to ameloblastomas.
- Conventional ameloblastomas are the most common and prevalent among odontogenic tumors. Clinico-radiographically, they present as intraosseous lesions which exhibit slow, painless swelling or expansion of the jaw, with multilocular expansile radiolucency most frequently found in the mandibular molar/ramus area. Depending on the histological features, the conventional ameloblastoma is also called a solid/multicystic ameloblastoma, in contrast to the unicystic ameloblastoma.50
- Unicystic ameloblastomas refer to the cystic lesions that exhibit clinical, radiographic, or gross features of a mandibular cyst but upon histologic examination show typical ameloblastoma epithelium lining part of the cyst cavity, with or without luminal and/or mural tumor growth (Fig. 2A, B).51 The unicystic ameloblastoma is believed to be less aggressive than the conventional ameloblastoma. Since this tumor shows considerable clinical and radiographical similarities with a dentigerous cyst, a differential diagnosis is necessary. Moreover, the recurrence of unicystic ameloblastomas may be long delayed, and long-term postoperative follow-up is essential for the proper management of these patients.52
- Unicystic ameloblastomas also demonstrate the variable propensity for recurrence. As a result, there is a difference in the biological behavior between mural unicystic ameloblastomas and those which are simply cystic or exhibit intraluminal proliferation.53 However, unicystic ameloblastomas have a distinct trait of less aggressive behavior compared to conventional ameloblastomas.54
- Desmoplastic ameloblastoma is a benign but locally invasive variant of the solid/multicystic ameloblastoma (Fig. 1D, H). The desmoplastic ameloblastoma accounts for only 4% to 13% of all ameloblastomas and can induce abundant stromal osteoplasia.55 Simple desmoplastic ameloblastomas (88.0%) and desmoplastic ameloblastomas with osteoplasia (12.0%) were the histologic variants reported, however, desmoplastic ameloblastomas showed slower growth rate than conventional ameloblastomas.56 In the immunoreactions of proliferating cell nuclear antigen (PCNA) and Ki-67, the desmoplastic ameloblastoma demonstrated a significantly lower proliferation rate compared to solid/multicystic ameloblastomas, unicystic ameloblastomas, and ameloblastic carcinomas.57
- Peripheral ameloblastoma is a rare extraosseous odontogenic tumor with histological characteristics similar to those found in conventional intraosseous ameloblastomas. Peripheral ameloblastomas clearly originated from odontogenic epithelial remnants located at the outside of tooth germs rather than from common oral epithelium.58,59 This tumor accounts for approximately 2-10% of all ameloblastomas, similar to classical ameloblastomas.60
- Craniopharyngiomas are low-grade epithelial neoplasms occurring almost exclusively in the sellar/suprasellar region. Craniopharyngiomas are generally considered to arise from the remnants of Rathke's pouch or a misplaced enamel organ. The overall incidence rate of craniopharyngiomas is approximately 1.3 per million. During adulthood, there is a peak incidence between 40 and 44 years. Based on the striking histological similarity of craniopharyngiomas and odontogenic tumors, craniopharyngiomas can be divided into adamantinomatous and papillary variants according to the WHO classification of odontogenic tumors. Approximately 50% of craniopharyngiomas corresponded histologically to calcifying odontogenic cysts, 24% to ameloblastomas, and 15% exhibited features of both calcifying odontogenic cysts and ameloblastomas either within the same specimen or in specimens derived from different resections. Only a rare 5% of craniopharyngiomas resembled calcifying epithelial odontogenic tumors, with approximately 2% of craniopharyngiomas resembling AOTs. However, no odontogenic counterparts could be established for papillary craniopharyngiomas.61
- The adamantinomatous craniopharyngioma is a locally aggressive neoplasm with a significant rate of recurrence.62 All adamantinomatous craniopharyngiomas show a variable degree of enamel protein expression, mainly in ghost cells that includes amelogenin, enamelin, and enamelysin. Lymphoid enhancer binding factor 1 (LEF1) is also heterogeneously expressed in adamantinomatous craniopharyngiomas. Remarkably, the expression pattern of LEF1 is identical to that of nuclear β-catenin accumulation. In contrast, none of the papillary craniopharyngiomas expresses enamel proteins or LEF1. These findings suggest that adamantinomatous craniopharyngiomas consistently exhibit odontogenic epithelial differentiation. Since adamantinomatous craniopharyngiomas are possibly caused by a β-catenin mutation, the inappropriate activation of β-catenin/LEF1 complex-dependent transcription may play a critical role in their tumorigenesis.63
- Only shadow cells present in adamantinomatous craniopharyngiomas are positive for human hair keratin, which may indicate their follicular differentiation. Rathke's cleft cyst ciliated cuboidal cells are cytokeratin (CK)-7+/CK-8+/CK-14-, and metaplastic squamous cells are CK-7+/CK-8-/CK-14+. These findings suggest that adamantinomatous craniopharyngiomas may be related to heterotopic ectodermal tissue, which can differentiate into hair follicles, while papillary craniopharyngiomas may arise from Rathke's cleft cyst.49
- In the immunological expressions of KL1 (high molecular weight cytokeratins), 5D3 (low molecular weight cytokeratins) and involucrin (characteristic of terminally differentiated keratinocytes), adamantinomatous craniopharyngiomas are similar to those reported in ameloblastomas for squamous differentiation, implying that adamantinomatous craniopharyngiomas and ameloblastomas are homologous lesions.64
- The papillary type of craniopharyngiomas occurs almost exclusively in adult patients, shows frequent radiologic solidity and absence of calcification, demonstrates a macroscopic papillary nature, and, microscopically, exhibits a well-differentiated papillary squamous epithelium without calcification, palisaded cells, or keratoid nodules.65
- Malignant transformation in craniopharyngiomas, although rare, does occur. It assumes varied histologic appearances, usually after multiple recurrences and radiation therapy, and has a nearly uniform fatal outcome. De novo malignancy in odontogenic tumors of the sella is even more unusual but also has an ominous prognosis.66
- Malignant transformation of ameloblastomas
- The malignant transformation of ameloblastomas can exhibit an aggressive clinical course, including multiple recurrences, a short disease-free interval, pulmonary metastasis, and extensive skull-base infiltration. They constitute less than 1% of all ameloblastomas. Malignancies in ameloblastomas may involve local dysplastic change or metastasis in the tissue. The former are classified as ameloblastic carcinomas, the latter as malignant ameloblastomas.
- Ameloblastic carcinoma is a rare, odontogenic, malignant tumor that has the features of ameloblastoma in addition to cytologic atypia with or without metastasis. It is classified as primary type; secondary type, intraosseous; secondary type, and peripheral type according to the WHO classification of 2005.67
- The majority of cases reported are secondary type ameloblastic carcinoma. The mandible is the most common site of occurrence for both ameloblastic carcinoma types. The tumor cells resemble the cells seen in ameloblastomas but exhibit cytologic atypia (Fig. 2C), including plexiform invasion, hyperchromatism, mitosis, and necrosis that are associated with history of recurrence and death by disease, as well as tumors with clear cells, especially in the secondary type of ameloblastic carcinoma. Secondary type ameloblastic carcinoma appears to correlate with recurrence and mortality.68 Direct extension of the tumor with lymph node involvement and metastasis to various sites (frequently the lungs) have been reported. Wide local excision is the treatment of choice. Regional lymph node dissection should be considered and performed selectively.69
- In the analysis of six ameloblastic carcinomas from the literature up to 2009, the mean age was 49.2 years with a wide age range (7-91 years). The rate of occurrence was higher in males, and the most common site of occurrence was the mandible. Most cases (70%) involved the posterior portion of the jaw. Metastatic lesions were detected in 22% of patients during follow-up, and the lung was the most common area of distant metastasis.70
- Ameloblastic carcinomas may present de novo, ex ameloblastomas, or ex odontogenic cysts. Most ameloblastic carcinomas are presumed to present de novo. The clinical course of ameloblastic carcinomas is typically aggressive, with extensive local destruction.71
- Peripheral ameloblastic carcinoma is an extremely rare odontogenic tumor derived from the remnants of dental lamina and/or mucosal epithelium of the oral mucosa. The histology of the biopsy tissue and surgically-removed specimens reveal characteristic features resembling squamous cell carcinoma, basal cell carcinoma, and benign follicles of ameloblastoma. These neoplastic structures, as well as the proliferation and elongation of the mucosal epithelium, comprise an extensive network. The varied cytopathologic findings may be related to the proliferation and transformation of basal cells of the mucosal epithelium toward ameloblastic carcinoma and variable squamous differentiation.72
- A rare variant of spindle-cell ameloblastic carcinoma resulting in extensive metastasis and unfavorable outcomes have been reported in about seven cases in the literature.73 Ultrastructural and immunohistochemical examinations also show the spindle-cell component of the tumor to be epithelial in character.73
- In genome analysis, the CpG methylation of p16 (cyclin-dependent kinase inhibitor 2A) is observed in all ameloblastic carcinoma samples, but only one ameloblastoma specimen exhibits the mutation. Therefore, it is presumed that p16 alteration may play a role in the malignant progression of ameloblastic carcinoma.74
- The WHO defines malignant ameloblastoma as a lesion exhibiting features of an ameloblastoma in primary and metastatic growths. The WHO classification emphasizes metastasis as a diagnostic criterion but is rather vague in defining its histopathologic aspects. It is advocated that the term malignant ameloblastoma be reserved for those lesions that, in spite of a seemingly innocuous histology, have produced metastatic growth. The WHO classification should be modified to include ameloblastic carcinoma as a diagnostic term for lesions that combine features of an ameloblastoma with a less-differentiated histomorphology.75
- It is not possible to distinguish conventional intraosseous ameloblastomas from malignant ameloblastomas according to histopathologic features. It is necessary to pay special attention, especially in elderly patients, and to carry out further clinical, radiological, and pathohistological diagnostic procedures, such as immunohistochemical analysis.76 When metastases occur, although uncommon, lungs (71%) constitute the most frequent site involved, followed by cervical lymph nodes (28%). The female-to-male ratio is about 1:1.1. Primary tumor is diagnosed in about 28% of cases at ages ≤20 years, with a maxilla-to-mandible ratio of approximately 1:5.2. The mean disease-free interval and survival for pulmonary metastasis is about 14.4 years and 3 years, respectively, and about 13 years and 6 years for cervical metastasis, respectively.76 So far, less than 50 cases of ameloblastoma with metastases have been reported.77
AMELOBLASTOMA
Follicular type
Plexiform type
Acanthomatous type
Granular cell type
Others
Solid/multicystic ameloblastoma (intraosseous ameloblastoma, central ameloblastoma, conventional ameloblastoma)
Unicystic ameloblastoma (mural ameloblastoma)
Desmoplastic ameloblastoma
Peripheral ameloblastoma
Craniopharyngioma compared with odontogenic tumors
Ameloblastic carcinoma
Malignant ameloblastoma
- Characteristics of adenomatoid odontogenic tumor
- AOT is an uncommon, hamartomatous, benign, epithelial lesion of odontogenic origin that was first described by Driebaldt in 1907 as a pseudo-adenoameloblastoma. The current WHO classification of odontogenic tumors defines AOT as being composed of odontogenic epithelium in a variety of histoarchitectural patterns, embedded in mature connective tissue stroma, and characterized by slow but progressive growth.78
- The introduction of the name "AOT" has resulted in simpler and fruitful surgical management like enucleation and curettage with no reports of recurrence. In the past, a similar lesion with the diagnosis of adeno-ameloblastoma resulted in unnecessary mutilating surgery.79
- Compared to ameloblastomas, AOT is a benign, nonaggressive tumor with limited growth and no tendency of recurrence. This is often misdiagnosed as an odontogenic cyst and accounts for about 1% to 9% of all odontogenic tumors. It is predominantly found in young and female patients, is more often located in the maxilla and is typically associated with an unerupted permanent tooth. There are three variants of AOT-follicular, extrafollicular, and peripheral.80
- Among 15 cases of AOT, the anterior maxilla was the most common site (66.6%), and radiographically, most cases showed a unilocular radiolucency with well-defined borders (57.1%). Histologically, most cases exhibited a predominantly solid growth pattern (46.7%) or a similar proportion of solid and cribriform patterns (46.7%) (Fig. 3A, B, E, F). Eosinophilic amorphous material ("tumor droplets") was found in all cases (100%) (Fig. 3C, D, G, H). Most tumors contained duct-like spaces (93.3%) (Fig. 3B, C, F, G) and convoluted structures (60.0%), whereas a minor proportion of the cases presented with calcifying epithelial odontogenic tumor-like areas (26.7%). Variable amounts of calcified materials were found in most AOTs (80.0%) (Fig. 3A, D, E, H), whereas osteodentin and perivascular hyalinization were seen only rarely (6.7% for each). About five (33.3%) cases had areas mimicking a dentigerous cyst and were mostly diagnosed in females (80.0%).81
- AOT has numerous dispersed or clustered radiopaque foci compared to the presence of calcification in calcifying cystic odontogenic tumors showing a thin radiopaque line and discrete radiopaque foci. Therefore, radiolucency with numerous radiopaque foci (particularly when the radiolucency surrounds a portion of the root or entire tooth) is suggestive of an AOT rather than a calcifying cystic odontogenic tumor.82
- In a review of 272 AOTs with special emphasis on radiological features, the patient age at time of diagnosis ranged from 3 years to 82 years (mean, 18.4 years). The maxilla-to-mandible ratio was 1.7:1. Small opacities were present in 77% of the lesions and were associated with expansion of the cortex. The significant radiological features in patients aged 30 years and above were root resorption and lesions crossing the midline.83
- According to all reports regarding AOTs and cited in PubMed since 1990, AOTs account for approximately 1% to 9% of all odontogenic tumors. These tumors are predominantly found in young female patients, are more often located in the maxilla, and are typically associated with an unerupted permanent tooth. AOTs frequently resemble other odontogenic lesions such as dentigerous cysts or ameloblastomas.84
- Molecular mechanisms of adenomatoid odontogenic tumor
- AOT is a noninvasive tumor that never infiltrates surrounding normal tissues. However, the mean values of the labeling index for Ki-67 in solid ameloblastomas and AOTs are 4% and 1%, respectively. These values for B-cell lymphoma 2 (BCL-2) in solid ameloblastomas and AOTs are 63% and 26%, respectively. These findings suggest the harmatomatous behavior of AOT compared to ameloblastomas.85 AOT demonstrates a similar level of PCNA immunoreaction to ameloblastomas but exhibits weaker expressions of p53 and MDM2. This implies that AOT has less aggressive behavior than ameloblastomas.86,87 In AOTs, some epithelial tumor cells show a strong cytoplasmic reaction to β-catenin,88 possess whirling epithelial cells expressing cyclin D1, and have spindle-shaped tumor cells that are positive for podoplanin.89 These findings are suggestive for the active proliferation of AOTs.25
- Since the consistent and strong immunolocalization of hepatocyte growth factor (HGF) and c-met in squamous cells are present in AOTs, the HGF/c-met interaction may have an influence on squamous differentiation in the odontogenic epithelium of AOT.90 Enamel proteins including amelogenin, ameloblastin, and amelotin, as well as TGF-β/SMADs, are more intensely expressed in AOTs than in ameloblastomas. Therefore, AOTs exhibit less aggressive biological behavior and increased cytodifferentiation and apoptosis than ameloblastomas.16,30
- Types of adenomatoid odontogenic tumor
- AOT is an uncommon, progressively growing, and asymptomatic benign non-invasive lesion that occurs twice as often in females and usually in the second decade of life. The three variants of AOT are characteristic-a follicular variant (73%) associated with an impacted and displaced tooth, an extrafollicular variant (24%) mimicking a radicular cyst around the apex of a tooth, and a peripheral (epulis-like) variant (3%) exhibiting a periodontal bone defect or ectopic growth. On reappraisal of the origin and pathogenesis of AOT, it would seem that this tumor or hamartomatous lesion is derived from the odontogenic epithelium of the dental lamina complex or its remnants.91
- The follicular variant of the AOT is thought to originate from the reduced enamel epithelium of the dental follicle. All the variants of AOT exhibit identical histologic features. The follicular variant (male:female ratio, 1:1.9) accounts for 73.0% to 97.2% of all AOTs and is three times as frequent as the extrafollicular variant. The follicular variant is diagnosed earlier in life (mean age, 17 years) compared to the extrafollicular variant (mean age, 24 years), whereas 53.1% of all the variants occur during the teenage years (13-19 years). Follicular AOT is associated with one embedded tooth in 93.2% of cases. Maxillary permanent canines account for 41.7% of the cases, and all four canines are involved in 60.1% of AOT-associated cases.92
- Compared to the follicular variant of AOT, the origin of the extrafollicular variant remains unclear. However, the available reviews in the literature suggest that some extrafollicular AOTs may arise as a secondary phenomenon within pre-existing odontogenic cysts or cystic tumors. For example, the tumor may originate from the epithelial lining of an odontogenic cyst or unicystic ameloblastoma.93,94 A rare subvariant of the extrafollicular type of AOT may radiographically mimic periapical diseases, which is initially suspicious of small periapical pathology. This subvariant is very rare, with only 12 cases reported in the literature.95
- Peripheral AOT occurring far distant from tooth germ structures is rarely encountered. So far, there have been only 14 reported cases of peripheral AOTs. A marked female predominance was apparent in peripheral AOTs, and approximately 90% of the peripheral AOTs occurred in the maxilla,96 showing a striking tendency to occur in the anterior maxilla. The tumor primarily manifested in the incisor and can involve the maxillary antrum.97
ADENOMATOID ODONTOGENIC TUMOR
Follicular type
Extrafollicular type
Peripheral type
- Ameloblastomas and AOTs are benign epithelial tumors of odontogenic origin. The former demonstrates an aggressive behavior and frequent recurrence, while the latter exhibits limited growth and no tendency of recurrence. The differential diagnosis between ameloblastomas and AOTs is essential. Due to the local invasive growth and the possible potential for the malignant transformation of ameloblastomas, it is recommended by WHO that ameloblastomas be classified into solid/multicystic, unicystic, desmoplastic, and peripheral ameloblastomas according to their clinicopathological features rather than the previous histological types including follicular, plexiform, acanthomatous, granular types, etc. AOTs are also classified into follicular, extrafollicular, and peripheral types, even though all types exhibit similar histological features. With advance in the elucidation of molecular signaling mechanisms in cells, the cytodifferentiation of epithelial tumor cells in ameloblastomas and AOTs can be identified using different biomarkers. Therefore, it is suggested that comprehensive pathological observation including molecular genetic information can provide a more reliable differential diagnosis for the propagation and prognosis of ameloblastomas and AOTs.
SUMMARY
- 1. Sarkar L, Cobourne M, Naylor S, Smalley M, Dale T, Sharpe PT. Wnt/Shh interactions regulate ectodermal boundary formation during mammalian tooth development. Proc Natl Acad Sci U S A 2000; 97: 4520-4524. ArticlePubMedPMC
- 2. Thesleff I, Vaahtokari A, Kettunen P, Aberg T. Epithelial-mesenchymal signaling during tooth development. Connect Tissue Res 1995; 32: 9-15. PubMed
- 3. Kim JY, Kang GH, Chi JG. Adamantinoma of tibia with predominant features of fibrous dysplasia: a case report. J Korean Med Sci 1996; 11: 444-448. ArticlePubMedPMC
- 4. Kato N, Endo Y, Tamura G, Motoyama T. Ameloblastoma with basal cell carcinoma-like feature emerging as a nasal polyp. Pathol Int 1999; 49: 747-751. ArticlePubMed
- 5. Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol 1995; 31B: 86-99. ArticlePubMed
- 6. Siar CH, Lau SH, Ng KH. Ameloblastoma of the jaws: a retrospective analysis of 340 cases in a Malaysian population. J Oral Maxillofac Surg 2012; 70: 608-615. ArticlePubMed
- 7. Dhanuthai K, Chantarangsu S, Rojanawatsirivej S, et al. Ameloblastoma: a multicentric study. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113: 782-788. ArticlePubMed
- 8. Hong J, Yun PY, Chung IH, et al. Long-term follow up on recurrence of 305 ameloblastoma cases. Int J Oral Maxillofac Surg 2007; 36: 283-288. ArticlePubMed
- 9. Doscher JC, Kramer JM, Fantasia JE. A large calcifying lesion of the maxilla in a child. J Am Dent Assoc 2011; 142: 1026-1030. ArticlePubMed
- 10. Lim J, Ahn H, Min S, Hong SD, Lee JI, Hong SP. Oligonucleotide microarray analysis of ameloblastoma compared with dentigerous cyst. J Oral Pathol Med 2006; 35: 278-285. ArticlePubMed
- 11. Snead ML, Luo W, Hsu DD, Melrose RJ, Lau EC, Stenman G. Human ameloblastoma tumors express the amelogenin gene. Oral Surg Oral Med Oral Pathol 1992; 74: 64-72. ArticlePubMed
- 12. Deutsch D, Fermon E, Lustmann J, et al. Tuftelin mRNA is expressed in a human ameloblastoma tumor. Connect Tissue Res 1998; 39: 177-184. ArticlePubMed
- 13. Lee SK, Kim SM, Lee YJ, Yamada KM, Yamada Y, Chi JG. The structure of the rat ameloblastin gene and its expression in amelogenesis. Mol Cells 2003; 15: 216-225. ArticlePubMed
- 14. Lee SK, Krebsbach PH, Matsuki Y, Nanci A, Yamada KM, Yamada Y. Ameloblastin expression in rat incisors and human tooth germs. Int J Dev Biol 1996; 40: 1141-1150. ArticlePubMedPDF
- 15. Saku T, Okabe H, Shimokawa H. Immunohistochemical demonstration of enamel proteins in odontogenic tumors. J Oral Pathol Med 1992; 21: 113-119. ArticlePubMed
- 16. Crivelini MM, Felipini RC, Miyahara GI, de Sousa SC. Expression of odontogenic ameloblast-associated protein, amelotin, ameloblastin, and amelogenin in odontogenic tumors: immunohistochemical analysis and pathogenetic considerations. J Oral Pathol Med 2012; 41: 272-280. ArticlePubMed
- 17. Al-Otaibi O, Khounganian R, Anil S, Rajendran R. Syndecan-1 (CD 138) surface expression marks cell type and differentiation in ameloblastoma, keratocystic odontogenic tumor, and dentigerous cyst. J Oral Pathol Med 2013; 42: 186-193. ArticlePubMed
- 18. Mishra M, Naik VV, Kale AD, Ankola AV, Pilli GS. Perlecan (basement membrane heparan sulfate proteoglycan) and its role in oral malignancies: an overview. Indian J Dent Res 2011; 22: 823-826. ArticlePubMed
- 19. Masloub SM, Abdel-Azim AM, Elhamid ES. CD10 and osteopontin expression in dentigerous cyst and ameloblastoma. Diagn Pathol 2011; 6: 44.ArticlePubMedPMCPDF
- 20. de Medeiros AM, Nonaka CF, Galvão HC, de Souza LB, Freitas Rde A. Expression of extracellular matrix proteins in ameloblastomas and adenomatoid odontogenic tumors. Eur Arch Otorhinolaryngol 2010; 267: 303-310. ArticlePubMedPDF
- 21. Farias LC, Gomes CC, Rodrigues MC, et al. Epigenetic regulation of matrix metalloproteinase expression in ameloblastoma. BMC Clin Pathol 2012; 12: 11.ArticlePubMedPMCPDF
- 22. Qian Y, Huang HZ. The role of RANKL and MMP-9 in the bone resorption caused by ameloblastoma. J Oral Pathol Med 2010; 39: 592-598. ArticlePubMed
- 23. Tekkesin MS, Mutlu S, Olgac V. The role of RANK/RANKL/OPG signalling pathways in osteoclastogenesis in odontogenic keratocysts, radicular cysts, and ameloblastomas. Head Neck Pathol 2011; 5: 248-253. ArticlePubMedPMCPDF
- 24. Sengüven B, Oygür T. Investigation of interleukin-1 alpha and interleukin-6 expression and interleukin-1 alpha gene polymorphism in keratocystic odontogenic tumors and ameloblastomas. Med Oral Patol Oral Cir Bucal 2011; 16: e467-e472. PubMed
- 25. Kumar H, Vandana R, Kumar G. Immunohistochemical expression of cyclin D1 in ameloblastomas and adenomatoid odontogenic tumors. J Oral Maxillofac Pathol 2011; 15: 283-287. ArticlePubMedPMC
- 26. Scheper MA, Duarte EC, Intapa C, et al. Expression of midkine in ameloblastomas and its correlation with clinicopathologic parameters. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 114: 497-502. ArticlePubMed
- 27. Abdel-Aziz A, Amin MM. EGFR, CD10 and proliferation marker Ki67 expression in ameloblastoma: possible role in local recurrence. Diagn Pathol 2012; 7: 14.ArticlePubMedPMCPDF
- 28. Sharifi-Sistani N, Zartab H, Babakoohi S, et al. Immunohistochemical comparison of the expression of p53 and MDM2 proteins in ameloblastomas and keratocystic odontogenic tumors. J Craniofac Surg 2011; 22: 1652-1656. ArticlePubMed
- 29. Li N, Zhong M, Song M. Expression of phosphorylated mTOR and its regulatory protein is related to biological behaviors of ameloblastoma. Int J Clin Exp Pathol 2012; 5: 660-667. PubMedPMC
- 30. Karathanasi V, Tosios KI, Nikitakis NG, et al. TGF-beta1, Smad-2/-3, Smad-1/-5/-8, and Smad-4 signaling factors are expressed in ameloblastomas, adenomatoid odontogenic tumors, and calcifying cystic odontogenic tumors: an immunohistochemical study. J Oral Pathol Med 2013; 42: 415-423. PubMed
- 31. Sukarawan W, Simmons D, Suggs C, Long K, Wright JT. WNT5A expression in ameloblastoma and its roles in regulating enamel epithelium tumorigenic behaviors. Am J Pathol 2010; 176: 461-471. ArticlePubMedPMC
- 32. Sathi GA, Tsujigiwa H, Ito S, et al. Osteogenic genes related to the canonic WNT pathway are down-regulated in ameloblastoma. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 114: 771-777. ArticlePubMed
- 33. Ponti G, Pastorino L, Pollio A, et al. Ameloblastoma: a neglected criterion for nevoid basal cell carcinoma (Gorlin) syndrome. Fam Cancer 2012; 11: 411-418. ArticlePubMedPDF
- 34. Park HR, Han YI, Sol MY, Lee SK. Nevoid basal cell carcinoma syndrome: report of a case. Korean J Pathol 1995; 29: 263-267.
- 35. Song KY, Choi YH, Kim MK, Lee KK, Ham EK. Pathological analysis of the basal cell carcinoma. Korean J Pathol 1994; 28: 160-167.
- 36. Alves Pereira KM, do Amaral BA, dos Santos BR, Galvão HC, Freitas Rde A, de Souza LB. Immunohistochemical expression of E-cadherin and beta-catenin in ameloblastomas and tooth germs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109: 425-431. PubMed
- 37. Modolo F, Biz MT, de Sousa SM, Fachinelli Rde L, Crema VO. Immunohistochemical expression of Rho GTPases in ameloblastomas. J Oral Pathol Med 2012; 41: 400-407. ArticlePubMed
- 38. Kumamoto H, Kimi K, Ooya K. Immunohistochemical analysis of apoptosis-related factors (Fas, Fas ligand, caspase-3 and single-stranded DNA) in ameloblastomas. J Oral Pathol Med 2001; 30: 596-602. ArticlePubMedPDF
- 39. Rizzardi C, Leocata P, Ventura L, et al. Apoptosis-related factors (TRAIL, DR4, DR5, DcR1, DcR2, apoptotic cells) and proliferative activity in ameloblastomas. Anticancer Res 2009; 29: 1137-1142. PubMed
- 40. Shahidi S, Bronoosh P, Daneshbod Y. Follicular ameloblastoma presenting as a sinonasal tumor. Iran Red Crescent Med J 2012; 14: 113-116. PubMedPMC
- 41. Castro-Silva II, Israel MS, Lima GS, de Queiroz Chaves Lourenço S. Difficulties in the diagnosis of plexiform ameloblastoma. Oral Maxillofac Surg 2012; 16: 115-118. ArticlePubMedPDF
- 42. Bansal M, Chaturvedi TP, Bansal R, Kumar M. Acanthomatous ameloblastoma of anterior maxilla. J Indian Soc Pedod Prev Dent 2010; 28: 209-211. ArticlePubMed
- 43. Ahuja A, Mathur S, Iyer VK. Acanthomatous ameloblastoma masquerading as a squamous cell carcinoma. Cytopathology 2012 4 25 [Epub]. http://dx.doi.org/10.1111/j.1365-2303.2012.00978.x. ArticlePMC
- 44. Gupta S, Grewal H, Sah K. Granular cell ameloblastoma showing desmoplasia. Ann Saudi Med 2012; 32: 537-540. ArticlePubMedPMC
- 45. Bansal A, Bhatnagar A, Saxena S. Metastasizing granular cell ameloblastoma. J Oral Maxillofac Pathol 2012; 16: 122-124. ArticlePubMedPMC
- 46. Sharma VK, Verma SK, Goyal L, Chaudhary PK. Hemangiomatous ameloblastoma in maxilla: a report of a very rare case. Dent Res J (Isfahan) 2012; 9: 345-349. PubMedPMC
- 47. Ide F, Shimoyama T, Horie N. Basaloid squamous cell carcinoma versus basal cell ameloblastoma. Oral Oncol 1998; 34: 154-155. ArticlePubMed
- 48. Saxena K, Jose M, Chatra LK, Sequiera J. Adenoid ameloblastoma with dentinoid. J Oral Maxillofac Pathol 2012; 16: 272-276. ArticlePubMedPMC
- 49. Tateyama H, Tada T, Okabe M, Takahashi E, Eimoto T. Different keratin profiles in craniopharyngioma subtypes and ameloblastomas. Pathol Res Pract 2001; 197: 735-742. ArticlePubMed
- 50. Kim TJ, Lee YS, Kim BK, Lee KY. Ameloblastoma associated with dentinogenic ghost cell tumor: a case report. Korean J Pathol 2006; 40: 297-302.
- 51. Chaudhary Z, Sangwan V, Pal US, Sharma P. Unicystic ameloblastoma: a diagnostic dilemma. Natl J Maxillofac Surg 2011; 2: 89-92. ArticlePubMedPMC
- 52. Gupta N, Saxena S, Rathod VC, Aggarwal P. Unicystic ameloblastoma of the mandible. J Oral Maxillofac Pathol 2011; 15: 228-231. ArticlePubMedPMC
- 53. De Melo WM, Pereira-Santos D, Sonoda CK, Pereira-Freitas SA, de Moura WL, de Paulo Cravinhos JC. Large unicystic ameloblastoma of the mandible: management guided by biological behavior. J Craniofac Surg 2012; 23: e499-e502. PubMed
- 54. Kim J, Choi IJ. Ameloblastoma arising in odontogenic cysts: report of 5 cases and its histologic characteristics. Korean J Pathol 1986; 20: 435-441.
- 55. Cervelli D, Marianetti TM, Boniello R, et al. Giant neglected desmoplastic ameloblastoma: reconstruction with free fibula flap. J Craniofac Surg 2012; 23: e171-e174. PubMed
- 56. Effiom OA, Odukoya O. Desmoplastic ameloblastoma: analysis of 17 Nigerian cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011; 111: e27-e31. Article
- 57. Bologna-Molina R, Mosqueda-Taylor A, Molina-Frechero N, Mori-Estevez AD, Sánchez-Acuña G. Comparison of the value of PCNA and Ki-67 as markers of cell proliferation in ameloblastic tumors. Med Oral Patol Oral Cir Bucal 2013; 18: e174-e179. ArticlePubMed
- 58. Kato H, Ota Y, Sasaki M, et al. Peripheral ameloblastoma of the lower molar gingiva: a case report and immunohistochemical study. Tokai J Exp Clin Med 2012; 37: 30-34. PubMed
- 59. Ide F, Mishima K, Miyazaki Y, Saito I, Kusama K. Peripheral ameloblastoma in-situ: an evidential fact of surface epithelium origin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 108: 763-767. ArticlePubMed
- 60. Beena VT, Choudhary K, Heera R, Rajeev R, Sivakumar R, Vidhyadharan K. Peripheral ameloblastoma: a case report and review of literature. Case Rep Dent 2012; 2012: 571509.ArticlePubMedPMCPDF
- 61. Paulus W, Stöckel C, Krauss J, Sörensen N, Roggendorf W. Odontogenic classification of craniopharyngiomas: a clinicopathological study of 54 cases. Histopathology 1997; 30: 172-176. ArticlePubMedPDF
- 62. Giangaspero F, Burger PC, Osborne DR, Stein RB. Suprasellar papillary squamous epithelioma ("papillary craniopharyngioma"). Am J Surg Pathol 1984; 8: 57-64. ArticlePubMed
- 63. Sekine S, Takata T, Shibata T, et al. Expression of enamel proteins and LEF1 in adamantinomatous craniopharyngioma: evidence for its odontogenic epithelial differentiation. Histopathology 2004; 45: 573-579. ArticlePubMed
- 64. Badger KV, Gardner DG. The relationship of adamantinomatous craniopharyngioma to ghost cell ameloblastoma of the jaws: a histopathologic and immunohistochemical study. J Oral Pathol Med 1997; 26: 349-355. ArticlePubMed
- 65. Zoicas F, Schöfl C. Craniopharyngioma in adults. Front Endocrinol (Lausanne) 2012; 3: 46.ArticlePubMedPMC
- 66. Rodriguez FJ, Scheithauer BW, Tsunoda S, Kovacs K, Vidal S, Piepgras DG. The spectrum of malignancy in craniopharyngioma. Am J Surg Pathol 2007; 31: 1020-1028. ArticlePubMed
- 67. Horváth A, Horváth E, Popşor S. Mandibular ameloblastic carcinoma in a young patient. Rom J Morphol Embryol 2012; 53: 179-183. PubMed
- 68. Casaroto AR, Toledo GL, Filho JL, Soares CT, Capelari MM, Lara VS. Ameloblastic carcinoma, primary type: case report, immunohistochemical analysis and literature review. Anticancer Res 2012; 32: 1515-1525. PubMed
- 69. Bedi RS, Chugh A, Pasricha N. Ameloblastic carcinoma of maxilla. Natl J Maxillofac Surg 2012; 3: 70-74. ArticlePubMedPMC
- 70. Yoon HJ, Hong SP, Lee JI, Lee SS, Hong SD. Ameloblastic carcinoma: an analysis of 6 cases with review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 108: 904-913. ArticlePubMed
- 71. Pundir S, Saxena S, Rathod V, Aggrawal P. Ameloblastic carcinoma: secondary dedifferentiated carcinoma of the mandible: report of a rare entity with a brief review. J Oral Maxillofac Pathol 2011; 15: 201-204. ArticlePubMedPMC
- 72. Fujita S, Anami M, Satoh N, et al. Cytopathologic features of secondary peripheral ameloblastic carcinoma: a case report. Diagn Cytopathol 2011; 39: 354-358. ArticlePubMed
- 73. Kawauchi S, Hayatsu Y, Takahashi M, et al. Spindle-cell ameloblastic carcinoma: a case report with immunohistochemical, ultrastructural, and comparative genomic hybridization analyses. Oncol Rep 2003; 10: 31-34. ArticlePubMed
- 74. Khojasteh A, Khodayari A, Rahimi F, et al. Hypermethylation of p16 tumor-suppressor gene in ameloblastic carcinoma, ameloblastoma, and dental follicles. J Oral Maxillofac Surg 2013; 71: 62-65. ArticlePubMed
- 75. Slootweg PJ, Müller H. Malignant ameloblastoma or ameloblastic carcinoma. Oral Surg Oral Med Oral Pathol 1984; 57: 168-176. ArticlePubMed
- 76. Dissanayake RK, Jayasooriya PR, Siriwardena DJ, Tilakaratne WM. Review of metastasizing (malignant) ameloblastoma (METAM): pattern of metastasis and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011; 111: 734-741. ArticlePubMed
- 77. Lai H, Wang J. Benign metastasizing ameloblastoma or malignant ameloblastoma? J Craniofac Surg 2011; 22: 995-997. ArticlePubMed
- 78. Baskaran P, Misra S, Kumar MS, Mithra R. Adenomatoid odontogenic tumor: a report of two cases with histopathology correlation. J Clin Imaging Sci 2011; 1: 64.ArticlePubMedPMC
- 79. Vasudevan K, Kumar S, Vijayasamundeeswari , Vigneswari S. Adenomatoid odontogenic tumor, an uncommon tumor. Contemp Clin Dent 2012; 3: 245-247. ArticlePubMedPMC
- 80. Sharma N, Passi S, Kumar VV. Adenomatoid odontogenic tumor: as an unusual mandibular manifestation. Contemp Clin Dent 2012; 3(Suppl 1): S29-S32. PubMedPMC
- 81. de Matos FR, Nonaka CF, Pinto LP, de Souza LB, de Almeida Freitas R. Adenomatoid odontogenic tumor: retrospective study of 15 cases with emphasis on histopathologic features. Head Neck Pathol 2012; 6: 430-437. ArticlePubMedPMCPDF
- 82. Chindasombatjaroen J, Poomsawat S, Kakimoto N, Shimamoto H. Calcifying cystic odontogenic tumor and adenomatoid odontogenic tumor: radiographic evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 114: 796-803. ArticlePubMed
- 83. Becker T, Buchner A, Kaffe I. Critical evaluation of the radiological and clinical features of adenomatoid odontogenic tumour. Dentomaxillofac Radiol 2012; 41: 533-540. ArticlePubMedPMC
- 84. Handschel JG, Depprich RA, Zimmermann AC, Braunstein S, Kübler NR. Adenomatoid odontogenic tumor of the mandible: review of the literature and report of a rare case. Head Face Med 2005; 1: 3.ArticlePubMedPMCPDF
- 85. Razavi SM, Tabatabaie SH, Hoseini AT, Hoseini ET, Khabazian A. A comparative immunohistochemical study of Ki-67 and Bcl-2 expression in solid ameloblastoma and adenomatoid odontogenic tumor. Dent Res J (Isfahan) 2012; 9: 192-197. ArticlePubMedPMC
- 86. Salehinejad J, Zare-Mahmoodabadi R, Saghafi S, et al. Immunohistochemical detection of p53 and PCNA in ameloblastoma and adenomatoid odontogenic tumor. J Oral Sci 2011; 53: 213-217. ArticlePubMed
- 87. Krishna A, Kaveri H, Naveen Kumar RK, Kumaraswamy KL, Shylaja S, Murthy S. Overexpression of MDM2 protein in ameloblastomas as compared to adenomatoid odontogenic tumor. J Cancer Res Ther 2012; 8: 232-237. ArticlePubMed
- 88. Harnet JC, Pedeutour F, Raybaud H, Ambrosetti D, Fabas T, Lombardi T. Immunohistological features in adenomatoid odontogenic tumor: review of the literature and first expression and mutational analysis of beta-catenin in this unusual lesion of the jaws. J Oral Maxillofac Surg 2013; 71: 706-713. PubMed
- 89. Tsuneki M, Maruyama S, Yamazaki M, Cheng J, Saku T. Podoplanin expression profiles characteristic of odontogenic tumor-specific tissue architectures. Pathol Res Pract 2012; 208: 140-146. ArticlePubMed
- 90. Poomsawat S, Punyasingh J, Vejchapipat P, Larbcharoensub N. Co-expression of hepatocyte growth factor and c-met in epithelial odontogenic tumors. Acta Histochem 2012; 114: 400-405. ArticlePubMed
- 91. Philipsen HP, Samman N, Ormiston IW, Wu PC, Reichart PA. Variants of the adenomatoid odontogenic tumor with a note on tumor origin. J Oral Pathol Med 1992; 21: 348-352. ArticlePubMed
- 92. Philipsen HP, Reichart PA, Zhang KH, Nikai H, Yu QX. Adenomatoid odontogenic tumor: biologic profile based on 499 cases. J Oral Pathol Med 1991; 20: 149-158. ArticlePubMed
- 93. Jivan V, Altini M, Meer S, Mahomed F. Adenomatoid odontogenic tumor (AOT) originating in a unicystic ameloblastoma: a case report. Head Neck Pathol 2007; 1: 146-149. ArticlePubMedPMCPDF
- 94. Prasad G, Nair P, Thomas S, Gharote H, Singh N, Bhambal A. Extrafollicular adenomatoid odontogenic tumour. BMJ Case Rep 2011; 2011: http://dx.doi.org/10.1136/bcr.03.2011.3963. Article
- 95. Avinash Tejasvi ML, Prashanth Shenai K, Chatra L. Atypical case of periapical adenomatoid odontogenic tumour. J Maxillofac Oral Surg 2010; 9: 99-101. ArticlePubMedPMCPDF
- 96. Ide F, Mishima K, Saito I, Kusama K. Rare peripheral odontogenic tumors: report of 5 cases and comprehensive review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 106: e22-e28. Article
- 97. Sandhu SV, Narang RS, Jawanda M, Rai S. Adenomatoid odontogenic tumor associated with dentigerous cyst of the maxillary antrum: a rare entity. J Oral Maxillofac Pathol 2010; 14: 24-28. ArticlePubMedPMC
REFERENCES
Fig. 1Photomicrographs of the different types of ameloblastoma. (A, E) Follicular type. (B, F) Plexiform type. (C, G) Acanthomatous type. (D, H) Desmoplastic type. Panels (E), (F), (G), and (H) are the magnifications of panels (A), (B), (C), and (D), respectively.
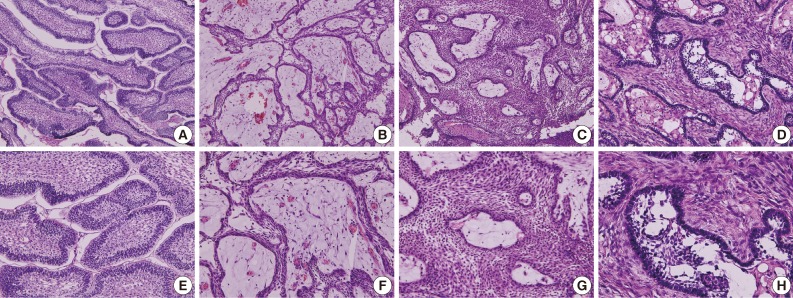

Fig. 2Photomicrographs of unicystic ameloblastoma (A, B) and ameloblastic carcinoma (C). (A, B) Ameloblastoma occurs from the epithelium of odontogenic keratocyst. (C) Ameloblastic carcinoma shows severe atypia of tumor cells.
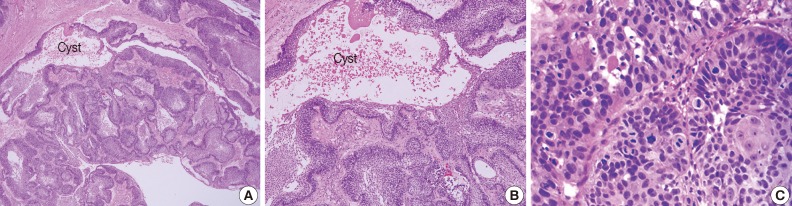

Fig. 3Variable histological features of adenomatoid odontogenic tumor. (A, E) Multiple epithelial follicles with calcifications (arrows). (B, F) Whirling epithelial cells with adenomatoid structures. (C, G) Multifocal calcification (arrows) with eosinophilic coagulum (arrowheads). (D, H) Abortive tooth materials (asterisk) produced by epithelial tumor cells.
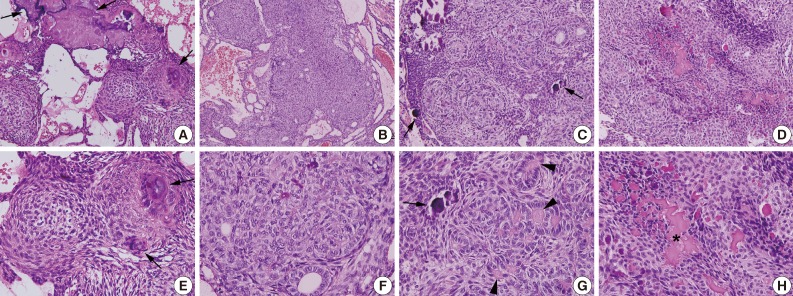

Figure & Data
References
Citations
Citations to this article as recorded by 

- Metabolic Analysis of Tumor Cells Within Ameloblastoma at the Single‐Cell Level
Rui‐Fang Li, Yi Zhao, Qi‐Wen Man
Oral Diseases.2025; 31(7): 2206. CrossRef - Granular cell ameloblastoma: A rare case report with public health implications
Shyamkumar Sriram, Shamimul Hasan, Mambakkam Jayakanth, Syed Ansar Ahmad, Anoop Kumar Narayanan
Medicine.2025; 104(19): e41992. CrossRef - Exploratory Advanced Radiotherapies for Ameloblastoma and Ameloblastic Carcinoma—A Concise Review
Heba Turkstani, Afrah Alfaifi, Sunday O. Akintoye
Oral Diseases.2025; 31(10): 2848. CrossRef - An incidentally discovered interradicular radiolucency of the anterior mandible
Joshua S. Goldfaden, Joanne L. Prasad, Husniye Demirturk, Steven C. Licht, Richard J. Vargo
The Journal of the American Dental Association.2025;[Epub] CrossRef - Adenomatoid odontogenic tumor: clinicopathological analysis of 34 cases from Karachi, Pakistan
Summaya Zafar, Sehar Sulaiman, Madeeha Nisar, Poonum Khan, Nasir Ud Din
Journal of Pathology and Translational Medicine.2025; 59(6): 390. CrossRef - Differential Profile of Primary and Recurrent Ameloblastomas Among Afro-descendants and Non-Afro-descendants—a Systematic Review
Parth Patel, Olajumoke A. Effiom, Abdul-Warith O. Akinshipo, Sunday O. Akintoye
Journal of Racial and Ethnic Health Disparities.2024; 11(1): 92. CrossRef - Adenomatoid Odontogenic Tumor: A 33-Year Retrospective Study with SEM Insight
Sandhya Tamgadge, Avinash Tamgadge, Treville Pereira, Hritika Mehta, Divya More
Journal of Microscopy and Ultrastructure.2024;[Epub] CrossRef - Hybrid Ameloblastoma of Jaw-A Case Report
Sudipa Ghosh, Shivaprasad S., Ashok L., Shambulingappa P.
Journal of Indian Dental Association.2024;[Epub] CrossRef - Role of HIF-1α in Ameloblastoma: A Systematic Review
Ayushi Jain, Pooja Sharma, N Sivakumar, Priya Devi, Shalini Gupta, Shaleen Chandra
Indian Journal of Otolaryngology and Head & Neck Surgery.2023; 75(4): 3136. CrossRef - Machine learning-based radiomics for predicting BRAF-V600E mutations in ameloblastoma
Wen Li, Yang Li, Xiaoling Liu, Li Wang, Wenqian Chen, Xueshen Qian, Xianglong Zheng, Jiang Chen, Yiming Liu, Lisong Lin
Frontiers in Immunology.2023;[Epub] CrossRef -
Clinicopathological relevance of
BRAF
and
SMO
mutations in Chinese patients with ameloblastoma
Chen Ruixue, Li Hexiang, Hou Yali, Li Xiangjun, Sun Xu, Wang Jie, Zhang Xudong
All Life.2023;[Epub] CrossRef - Immunohistochemical expression of Ki-67 and Glypican-3 to distinguish aggressive from nonaggressive benign odontogenic tumors
T. P. Chaturvedi, Kanupriya Gupta, Rahul Agrawal, P. G. Naveen Kumar, Jatin Gupta
Journal of Cancer Research and Therapeutics.2022; 18(Suppl 2): S205. CrossRef - Hypoxia enhances basal autophagy of epithelial‐derived ameloblastoma cells
Anwar A. A. Y. AlMuzaini, Kathleen Boesze‐Battaglia, Faizan Alawi, Sunday O. Akintoye
Oral Diseases.2022; 28(8): 2175. CrossRef - Giant ameloblastoma
Muthuvel Ramesh, A. N. Gurumoorthy, Jeevan G. Sanjive
Formosan Journal of Surgery.2022; 55(1): 27. CrossRef - Hemangiomatous Ameloblastoma with Spindle Cell Proliferation: A Rare Case Report and Review of Literature
Pavan D Puri, Abhinandh Krishna, Suchitra Gosavi, Vivek Nayyar
Journal of Oral and Maxillofacial Pathology.2022; 26(1): 132. CrossRef - Clinical, Radiographic and Histopathological Analysis of Craniopharyngiomas and Ameloblastomas: A Systematic Review
Luana Amorim Morais da Silva, Solimar Ribeiro Carlete Filho, Marcelo Jales Diniz Saraiva, Caio Rodrigues Maia, Camila Dannyelle Fernandes Dutra Pe Santos, Pedro Paulo de Andrade Santos
Head and Neck Pathology.2022; 16(4): 1195. CrossRef - CDC7 Expression in Selected Odontogenic Tumors
Zohreh Jaafari-Ashkavandi, Nahid Alizadeh, Luca Testarelli
International Journal of Dentistry.2022;[Epub] CrossRef - Immunohistochemical differential expression of p16 proteins in follicular type and plexiform type ameloblastoma
Haris Budi Widodo, Anung Saptiwulan, Helmi Hirawan, Christiana Cahyani Prihastuti, Tirta Wardana
Dental Journal (Majalah Kedokteran Gigi).2022; 55(3): 137. CrossRef - Fibroblastic Growth Factor as a Diagnostic and Prognostic Marker in Odontogenic Cysts and Tumors: A Systematic Review
Gururaj Narayana Rao, Adlin Saroja Rosaian, Gowthami Jawahar, P. Hari Nivas Raj, J. Beryl Rachel, P. Blessing Emmanuel
Journal of Pharmacy and Bioallied Sciences.2021; 13(Suppl 1): S6. CrossRef - A View of Adenomatoid Odontogenic Tumor in Ameloblastoma: A Hybrid Variant
Priya Thomas, Sapna Chandran Lathakumari
Journal of Health Sciences & Research.2021; 12(1): 21. CrossRef - Development and Validation of a Prognostic Nomogram for Postoperative Recurrence-Free Survival of Ameloblastoma
Yao-Cheng Yang, Jun-Jie Wang, Yun Huang, Wei-Xin Cai, Qian Tao
Cancer Management and Research.2021; Volume 13: 4403. CrossRef Peripheral Adenomatoid Odontogenic Tumor — A Rare Cause of Gingival Enlargement: A Case Report with CBCT Findings
Arun Sadasivan, Roshni Ramesh, Nikhil M Kurien
Clinical, Cosmetic and Investigational Dentistry.2020; Volume 12: 297. CrossRef- Adenomatoid odontogenic tumour: A rare threat to orthodontic treatment planning
Laura Han, Alison Downing, David Farr, Kaushik Dasgupta, Duncan Stewart
Journal of Orthodontics.2019; 46(3): 259. CrossRef - Recurrence of plexiform ameloblastoma as acanthomatous ameloblastoma: A rare case report
SanatKumar Bhuyan, Ruchi Bhuyan, TapanKumar Sahoo, Pinali Das
Contemporary Clinical Dentistry.2019; 10(1): 178. CrossRef - Immunoexperssion of cancer stem cell marker (CD44) in ameloblastoma
Manjushri Madhukar Vanje, Shahela Tanveer, Syed Afroz Ahmed, Shravan Kumar, Tejashree Vanje
Journal of Oral and Maxillofacial Pathology.2019; 23(3): 400. CrossRef - Unklare Schwellung im Bereich eines Oberkiefereckzahns
S. H. Baum, C. Loef, D. Baumhoer, C. Mohr
Der MKG-Chirurg.2018; 11(2): 111. CrossRef - Ameloblastoma Secondary to Third Molar Extraction and Sagittal Split Ramus Osteotomy : A Case Report
Sung-Tak Lee, Santhiya Iswarya Vinothini Udayakumar, Tae-Geon Kwon, Hong-In Shin, So-Young Choi
The Korean Journal of Oral and Maxillofacial Pathology.2018; 42(2): 39. CrossRef - Glypican‐3 distinguishes aggressive from non‐aggressive odontogenic tumors: a preliminary study
Ramon Barreto Mendes, Rosane Borges Dias, Andreia Leal Figueiredo, Clarissa Araújo Gurgel, Manoel Santana Filho, Leonardo Araújo Melo, Marília Trierveiler, Patrícia Ramos Cury, Rosalia Leonardi, Jean Nunes Dos Santos
Journal of Oral Pathology & Medicine.2017; 46(4): 297. CrossRef - Immunoexpression of BMP-2 and BMP-4 and their receptors, BMPR-IA and BMPR-II, in ameloblastomas and adenomatoid odontogenic tumors
Marcelo Anderson Barbosa Nascimento, Cassiano Francisco Weege Nonaka, Carlos Augusto Galvão Barboza, Roseana de Almeida Freitas, Leão Pereira Pinto, Lélia Batista de Souza
Archives of Oral Biology.2017; 73: 223. CrossRef - Rare case of ameloblastoma with pulmonary metastases
Ivan Valkadinov, Nikolay Conev, Dian Dzhenkov, Ivan Donev
Intractable & Rare Diseases Research.2017; 6(3): 211. CrossRef - High strength oil palm shell concrete beams reinforced with steel fibres
S. Poh-Yap, U. Johnson-Alengaram, K. Hung-Mo, M. Zamin-Jumaat
Materiales de Construcción.2017; 67(328): e142. CrossRef - A novel marker of ameloblastoma and systematic review of immunohistochemical findings
Bacem A.E.O. Khalele, Rami A. Al-Shiaty
Annals of Diagnostic Pathology.2016; 22: 18. CrossRef - Adenoid variant of peripheral ameloblastoma with cellular atypia in the retromolar pad area: A case report
Bacem A.E.O. Khalele
Future Dental Journal.2016; 2(2): 91. CrossRef - Ameloblastoma during pregnancy: a case report
Helbert Eustáquio Cardoso da Silva, Erika do Socorro Ramos Costa, Antônio Carlos Quintão Medeiros, Paulo Sérgio dos Santos Pereira
Journal of Medical Case Reports.2016;[Epub] CrossRef - A case report and short review on changing trends in the site of occurrence of adenomatoid odontogenic tumor: Unravelling the past 15 years
Sneha Sethi, Manish Kumar, Pratul Aggarwal, HS Indra Kumar, ChetanD Sugandhi, Silvie Singh
Dental Research Journal.2016; 13(5): 462. CrossRef - De novo adamantinomatous craniopharyngioma presenting anew in an elderly patient with previous normal CT and MRI studies: A case report and implications on pathogenesis
Amy Walker, Radmehr Torabi, Michael Punsoni, Edward Stopa, Curtis Doberstein
Interdisciplinary Neurosurgery.2015; 2(3): 149. CrossRef - Understanding ameloblastomas through tooth development
Amer Sehic
Journal of Dentistry and Oral Care.2015;[Epub] CrossRef - New Features in Mucous-Ameloblastoma. A Case Report of rare Entity
IS Gataa
International Journal of Oral and Craniofacial Science.2015; : 001. CrossRef - Aggressive granular cell ameloblastoma: Report of a rare case
N. Aravindha Babu, S. Leena Sankari, N. Anitha, Gouse Mohideen
Journal of Pharmacy and Bioallied Sciences.2015; 7(Suppl 1): S276. CrossRef - Adenomatoid odontogenic tumor associated with a dentigerous cyst: A case report
Ludmila de Faro Valverde, Tássia Amaral Gomes, Maria Lúcia Neves, Rosane Borges Dias, Manuela Torres Andion Vidal, Caroline Brandi Schlaepfer Sales, Clarissa Araújo Gurgel Rocha, Jean Nunes dos Santos
Indian Journal of Dentistry.2014; 5: 82. CrossRef - Adenomatoid odontogen tumor: «To tredje-delstumoren»
Bjarte Grung, Anne Christine Johannessen
Den norske tannlegeforenings Tidende.2014; 124(9): 740. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Current Concepts and Occurrence of Epithelial Odontogenic Tumors: I. Ameloblastoma and Adenomatoid Odontogenic Tumor
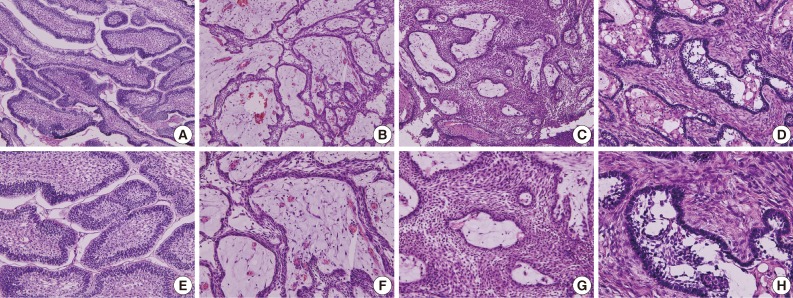


Fig. 1 Photomicrographs of the different types of ameloblastoma. (A, E) Follicular type. (B, F) Plexiform type. (C, G) Acanthomatous type. (D, H) Desmoplastic type. Panels (E), (F), (G), and (H) are the magnifications of panels (A), (B), (C), and (D), respectively.
Fig. 2 Photomicrographs of unicystic ameloblastoma (A, B) and ameloblastic carcinoma (C). (A, B) Ameloblastoma occurs from the epithelium of odontogenic keratocyst. (C) Ameloblastic carcinoma shows severe atypia of tumor cells.
Fig. 3 Variable histological features of adenomatoid odontogenic tumor. (A, E) Multiple epithelial follicles with calcifications (arrows). (B, F) Whirling epithelial cells with adenomatoid structures. (C, G) Multifocal calcification (arrows) with eosinophilic coagulum (arrowheads). (D, H) Abortive tooth materials (asterisk) produced by epithelial tumor cells.
Fig. 1
Fig. 2
Fig. 3
Current Concepts and Occurrence of Epithelial Odontogenic Tumors: I. Ameloblastoma and Adenomatoid Odontogenic Tumor

 E-submission
E-submission





