Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 59(6); 2025 > Article
-
Newsletter
What’s new in hematopathology 2025: myeloid neoplasms in the WHO 5th edition and ICC -
Barina Aqil

-
Journal of Pathology and Translational Medicine 2025;59(6):472-475.
DOI: https://doi.org/10.4132/jptm.2025.09.24
Published online: October 22, 2025
Department of Pathology, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA
- Corresponding Author: Barina Aqil Department of Pathology, Feinberg School of Medicine, Northwestern University, Chicago, IL, 60611, USA Email: barina.aqil@northwestern.edu
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 7,418 Views
- 258 Download
- Abstract
- GENERAL CHANGES IN TERMINOLOGY & TAXONOMY
- CLONAL HEMATOPOIESIS (CH)
- MYELOPROLIFERATIVE NEOPLASMS
- MASTOCYTOSIS
- MYELODYSPLASTIC SYNDROME/NEOPLASM
- MYELODYSPLASTIC/MYELOPROLIFERATIVE NEOPLASMS
- MYELOID/LYMPHOID NEOPLASMS WITH EOSINOPHILIA WITH TYROSINE KINASE GENE FUSIONS
- ACUTE MYELOID LEUKEMIA
- ACUTE LEUKEMIAS OF MIXED OR AMBIGUOUS LINEAGE
- SECONDARY MYELOID NEOPLASMS
- Meet the Author
Abstract
- The previous edition of the World Health Organization (WHO) classification of hematolymphoid neoplasms was published in 2008 and later revised in 2017. A new 5th edition of the WHO classification of hematolymphoid neoplasms was released in 2022. Additionally, the Clinical Advisory Committee developed the International Consensus Classification (ICC) of hematolymphoid tumors, which differs from the WHO classification in several key defining features as outlined below.
- ● Most of the updates in this newsletter are based on the WHO 5th edition, with significant differences noted between the ICC and WHO classifications.
- ● Classification structure is lineage defined (based on flow cytometry and/or immunohistochemistry) with further designation under the following parameters:
- ○ Category, such as precursor lesions, acute and chronic neoplasms.
- ○ Family: myeloproliferative neoplasms (MPN), myelodysplastic neoplasm/syndrome (MDS), mastocytosis, myelodysplastic/myeloproliferative neoplasms (MDS/MPN), acute myeloid leukemia (AML), secondary myeloid neoplasms (post cytotoxic therapy [pCT], germline predisposition), myeloid/lymphoid neoplasms with eosinophilia and tyrosine kinase gene fusions (M/LN-E-TK) and acute leukemias of mixed or ambiguous lineage, including mixed phenotype acute leukemia (MPAL) and acute leukemias of ambiguous lineage (ALAL).
- ○ Subtypes: gene fusions, rearrangements, and mutations.
- ●Disease entity-defining genetic abnormalities are prioritized where necessary.
- ●Emerging entities that are rare/new are now under the category of other defined genetic alterations, replacing the terminology of provisional entities.
- ●The Human Genome Organization (HUGO) Gene Nomenclature Classification System is used for gene symbols and names, including the new designation of gene fusions using double colon marks (::).
GENERAL CHANGES IN TERMINOLOGY & TAXONOMY
- ●Clonal hematopoiesis of indeterminate potential (CHIP):
- ○ Presence of somatic mutations (Table 1) with variant allele frequency (VAF) of ≥2% (≥4% for X-linked gene mutations in male patients) in peripheral blood (PB) or bone marrow (BM).
- ○ No unexplained cytopenias.
- ○ No morphological features of defined myeloid neoplasms.
- ●Clonal cytopenia of undetermined significance (CCUS):
- ○ Clonal hematopoiesis in presence of unexplained persistent cytopenias (4 months or longer in duration).
- ○ The cytopenia thresholds used for CCUS are similar to those for MDS and MDS/MPN: hemoglobin (female <12 g/dL; male <13 g/dL), absolute neutrophil count (<1.8 × 109/L) and platelet count (<150 × 109/L).
- ○ Lacks morphological features of defined myeloid neoplasms.
- ●VEXAS syndrome, classified as a CH-related disorder, is included in the CH section and is commonly associated with cytopenias.
- ○ VEXAS syndrome consists of vacuoles, E1 enzyme, X-linked, autoinflammatory symptoms and somatic UBA1 mutations.
- ○ Cytoplasmic vacuoles can be seen in myeloid and erythroid precursors.
- ○ Subset with progression is associated with MDS, typically low risk features, and limited number of mutations aside from UBA1.
CLONAL HEMATOPOIESIS (CH)
- ●Chronic myeloid leukemia (CML) (Fig. 1): the accelerated phase (AP) is generally considered less responsive to tyrosine kinase inhibitors (TKIs), in contrast to the chronic phase (CP) and blast phase (BP).
- ○ Introduction of a concept of high-risk features associated with CP progression and resistance to TKIs:
- ▪ 10-19% blasts in BM or PB
- ▪ ≥20% basophils in PB
- ▪ additional chromosomal abnormalities: isochromosome 17q, trisomy 19, trisomy 21, additional Ph chromosome, monosomy 7, trisomy 8, 3q26.2 (MECOM) rearrangements, 11q23 rearrangements
- ○ Per ICC, all three phases (CP, AP and BP) in CML are retained and >5% lymphoid blasts in PB or BM represent impending lymphoid BP.
- ○ CML, BP according to WHO includes ≥20% myeloid blasts in PB or BM or presence of an extramedullary blast proliferation or demonstration of lymphoblasts in PB or BM (even if <10%).
- ●The diagnostic criteria for polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF) remain the same with only minor refinements.
- ○ For PV, increased red cell mass with 51Cr-labeled red cells has been removed.
- ○ EZH2, IDH1, IDH2, SRSF2, U2AF1 and ASXL1 mutations in PMF are known to carry poor prognosis.
- ○ TP53 mutations are associated with leukemic transformation.
- ●Chronic neutrophilic leukemia criteria have not changed in the new WHO.
- ○ ICC suggests decreasing the WBC threshold from ≥25 × 109/L to ≥13 × 109/L in cases with CSF3R T618I or other activating CSF3R mutations.
- ●Chronic eosinophilic leukemia has been revised with several changes.
- ○ “Not otherwise specified” qualifier has been removed per WHO, but ICC still retains the NOS qualifier.
- ○ Duration of hypereosinophilia is reduced from 6 months to 4 weeks.
- ○ Abnormal BM morphology (such as dysplasia) and the evidence of clonality (by cytogenetic or molecular studies) are included.
- ○ Exclusion of increased blasts (≥2% in PB or 5-19% in BM).
- ●Juvenile myelomonocytic leukemia (JMML) is now considered a childhood myeloproliferative neoplasm. Updates to diagnostic criteria include:
- ○ No KMT2A rearrangements.
- ○ Elimination of monosomy 7 (-7) as a cytogenetic criterion.
- ○ Including genetic criteria that demonstrate mutations of RAS pathway (such as somatic mutations of NRAS, KRAS or PTPN11 as well as somatic or germline NF1 or CBL mutations).
MYELOPROLIFERATIVE NEOPLASMS
- ● Modification of systemic mastocytosis (SM) diagnostic criteria:
- ○ CD30 has been added as an aberrant expression in mast cells, like CD2 and CD25.
- ○ Presence of any type of KIT mutation is accepted as a minor criterion.
- ○ ICC added tryptase and KIT (CD117) immunostains among the major criteria for identification of mast cell aggregates.
- ●BM mastocytosis is a new SM subtype without skin lesions, no B-findings and basal serum tryptase <125 ng/ml.
- ●The nomenclature of SM with an associated hematologic neoplasm (SM-AHN) is still retained in WHO but renamed in ICC as SM with associated myeloid neoplasm (SM-AMN).
- ●Well-differentiated SM (WDSM) is characterized by round and well-granulated mast cells that express CD30 and are negative for CD2 and CD25. KIT codon 816 mutation is not present.
MASTOCYTOSIS
- ● Introduction of new terminology of “myelodysplastic neoplasm” instead of “myelodysplastic syndrome” per WHO.
- ● MDS is now subtyped based on defining genetic abnormalities (Table 2) and morphology (Table 3).
- ● Detection of ≥15% ring sideroblasts (RS) in WHO substitutes for SF3B1 mutation and defines the entity as MDS with low blasts and ring sideroblasts. This is unlike ICC, where MDS-RS with wild type SF3B1 is classified as MDS, NOS, irrespective of the percentage of RS.
- ● Due to inclusion of CCUS in the classification schemata, the verbiage of “NOS” or “unclassifiable” is not required in WHO, so MDS, unclassifiable is omitted.
- ● Single lineage and multilineage dysplasia in MDS have been retained according to ICC in the subclassification of MDS, NOS.
- ● ICC has a slight variation in the MDS subtype names: MDS with del(5q) and MDS with mutated TP53.
- ● With the introduction of the MDS/AML category in ICC, there is now only one – MDS with increased blasts (MDS-IB) – subtype. MDS/AML is characterized by cytopenia with dysplasia, 10-19% PB or BM blasts with exception of AML-defining cytogenetic abnormalities, NPM1, bZIP domain in CEBPA and TP3 mutations.
MYELODYSPLASTIC SYNDROME/NEOPLASM
- ●ICC included cytopenia along with cytosis as the defining feature of MDS/MPN neoplasms.
- ●ICC introduced two new CMML precursor entities, which are not mentioned in WHO:
- ○ Clonal monocytosis of undetermined significance (CMUS), based on persistent monocytosis (relative monocytes ≥10% and absolute monocytes ≥0.5 × 109/L), along with the presence of myeloid neoplasm-associated mutation(s) and without BM morphologic findings of CMML.
- ○ If cytopenia is present in addition to above findings, the nomenclature of clonal cytopenia and monocytosis of undetermined significance (CCMUS) is suggested.
- ● Chronic myelomonocytic leukemia (CMML) diagnostic criteria per WHO are updated with refinement of subgrouping based on blast percentages and clinical features.
- ○ Absolute monocytosis is lowered from 1.0 × 109/L to 0.5 × 109/L in cases with presence of clonality (cytogenetic or molecular mutations).
- ○ PB monocyte subtypes are included as a supporting criterion. Monocytes are classified into three subsets based on CD14 and CD16 expression: classic monocytes (CD14+/CD16-), intermediate monocytes (CD14+/CD16+), and non-classic monocytes (CD14-low/CD16+).
- ○ CMML demonstrates an increase in the fraction of classic monocytes (>94%).
- ○ Further subtyping of CMML is based on white blood cell (WBC) count and blast percentage:
- ▪ Myelodysplastic (CMML-MD) with WBC <13 × 109/L and myeloproliferative (CMML-MP) with WBC >13 × 109/L.
- ▪ CMML-1 with <5% blasts in peripheral blood (PB) and <10% blasts in BM vs. CMML-2 with 5–19% blasts in PB and 10–19% blasts in BM. The previous CMML-0 category has been removed in the new edition.
- ● Atypical chronic myeloid leukemia (aCML) is renamed in WHO as MDS/MPN with neutrophilia, but ICC retained aCML terminology without BCR::ABL1.
- ● MDS/MPN with ring sideroblasts and thrombocytosis (MDS/MPN-RS-T) is redefined with incorporation of SF3B1 mutation and rephrased as MDS/MPN with SF3B1 mutation and thrombocytosis (MDS-SF3B1-T).
- ○ In cases with wild type SF3B1 and ≥15% RS, the terminology of MDS/MPN-RS-T is acceptable.
- ● JMML is moved into MPN according to WHO and into pediatric and/or germline mutation associated disorders per ICC.
- ● MDS/MPN, unclassifiable is changed to MDS/MPN, NOS (not otherwise specified) per ICC.
- ● ICC brought forth a new provisional sub-entity in MDS/MPN, NOS category: MDS/MPN with isolated isochromosome 17q.
MYELODYSPLASTIC/MYELOPROLIFERATIVE NEOPLASMS
- ●This family is renamed from prior myeloid/lymphoid neoplasm with eosinophilia (M/LN-eo) and gene rearrangement to myeloid/lymphoid neoplasms with eosinophilia and tyrosine kinase gene fusions (M/LN-E-TK).
- ●Identification of three new entities under this category:
- ○ Myeloid/lymphoid neoplasms with JAK2 rearrangement
- ○ Myeloid/lymphoid neoplasms with FLT3 rearrangement
- ○ Myeloid/lymphoid neoplasms with ETV6::ABL1 fusion
MYELOID/LYMPHOID NEOPLASMS WITH EOSINOPHILIA WITH TYROSINE KINASE GENE FUSIONS
- ●This entity is described by ICC but not mentioned by WHO.
- ○ TP53 mutation VAF has a defined lower threshold (VAF >10%).
- ○ This category includes separate diagnoses of MDS (0–9%), MDS/AML (10–19%), and AML with mutated TP53 (>20%) (including AEL), based on blast percentage.
- ○ Irrespective of blast count, this group shows aggressive behavior and poor prognosis.
MYELOID NEOPLASMS WITH MUTATED TP53
- ● AML with defining genetic abnormalities is separated from AML defined by differentiation, so the term AML, NOS is omitted.
- ● Blast requirement of ≥20% is eliminated in both WHO and ICC for AML with defining genetic abnormalities, with some differences (Fig. 2). WHO has no lower threshold for blasts in cases of recurrent genetic abnormalities, while ICC requires ≥10% blasts.
- ● AML with KMT2A, MECOM and NUP98 are recognized. AML with KMT2A rearrangement replaces the prior terminology of AML with t(9;11)(p22;q23); KMT2A::MLLT3.
- ● AML with CEBPA mutation is changed to include biallelic (biCEBPA) as well as single mutations located in the basic leucine zipper (bZIP) region of the gene (smbZIP-CEBPA).
- ● AML with other defined genetic alterations is added.
- ● AML with myelodysplasia-related changes (AML-MRC) is changed to AML, myelodysplasia-related (AML-MR) in WHO. While the category of AML with myelodysplasia-related cytogenetic abnormalities is retained, new categories of AML with mutated TP53 and AML with myelodysplasia-related gene mutations are added in ICC.
- ○ Morphology alone is taken out from diagnostic criteria of AML-MR.
- ○ Cytogenetic abnormalities are updated, and somatic mutations are included (Table 4).
- ○ AML with mutated TP53 according to ICC requires >20% blasts and VAF of >10%.
- ○ MDS and MDS/MPN progression to AML is still considered under AML-MR in WHO, but ICC introduced the addition of qualifier to the diagnosis in AML progressing from MDS and MDS/MPN.
- ● AML with somatic RUNX1 mutation is no longer recognized as a separate entity.
- ● In WHO, the diagnosis of acute erythroid leukemia (AEL) supersedes AML-MR. Because AEL is typically associated with TP53 mutations, it is included within the category of AML with TP53 mutations in the ICC.
ACUTE MYELOID LEUKEMIA
- ● Two new subtypes in the defining genetic alterations are added, which are MPAL with ZNF384 rearrangement and ALAL with BCL11B rearrangement.
- ● Lineage assignment criteria for MPAL have been refined with greater emphasis on the intensity and pattern of antigen expression on blasts as well as their association with normal hematopoietic counterparts.
ACUTE LEUKEMIAS OF MIXED OR AMBIGUOUS LINEAGE
- ● Myeloid neoplasms (MN) that arise after exposure to cytotoxic therapy (CT) or germline predisposition (GP) are included in this WHO category.
- ● The terminology of therapy-related is replaced with MN-pCT per WHO but retained in ICC.
- ● Exposure to PARP1 inhibitors is added as a qualifying criterion for MN-pCT, and methotrexate therapy has been excluded.
- ● The clinical manifestations of the diseases within germline predisposition are grouped into three subtypes:
- ○ MN with GP without a pre-existing platelet disorder or organ dysfunction
- ○ MN with GP and pre-existing platelet disorder
- ○ MN with GP and potential organ dysfunction
SECONDARY MYELOID NEOPLASMS
- Dr. Barina Aqil joined the Department of Pathology at Northwestern University Feinberg School of Medicine in 2019 as an Assistant Professor, where she specializes in Hematopathology. She has been writing for PathologyOutlines.com since 2023 and joined as a Board of Reviewers for Hematopathology in 2024. Dr. Aqil’s research interests include understanding genetic markers in myeloid and lymphoid neoplasms. She is also passionate about the use of newer methodologies for interactive teaching.
Meet the Author
Fig. 1.Chronic myeloid leukemia. (A) Peripheral blood smear with absolute neutrophilia, basophilia and eosinophilia (Wright-Giemsa, 200×). (B) Bone core biopsy in chronic myeloid leukemia showing hypercellular marrow with increased left shifted myeloid maturation, increased eosinophils and small hypolobated megakaryocytes.


Fig. 2.Differences in blast requirement (red) for AML with defining genetic abnormalities between WHO and ICC.


Table 1.Driver gene mutations associated with CHIP
Table 2.MDS subtypes based on defining genetic abnormalities
Table 3.MDS subtypes based on the morphology
Table 4.WHO and ICC based cytogenetic and somatic mutations in AML-MR
Figure & Data
References
Citations
Citations to this article as recorded by 

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
- Related articles
-
- What’s new in neuropathology 2024: CNS WHO 5th edition updates
- What’s new in adrenal gland pathology: WHO 5th edition for adrenal cortex
- What’s new in thyroid pathology 2024: updates from the new WHO classification and Bethesda system
- What’s new in genitourinary pathology 2023: WHO 5th edition updates for urinary tract, prostate, testis, and penis
- What’s new in dermatopathology 2023: WHO 5th edition updates
What’s new in hematopathology 2025: myeloid neoplasms in the WHO 5th edition and ICC
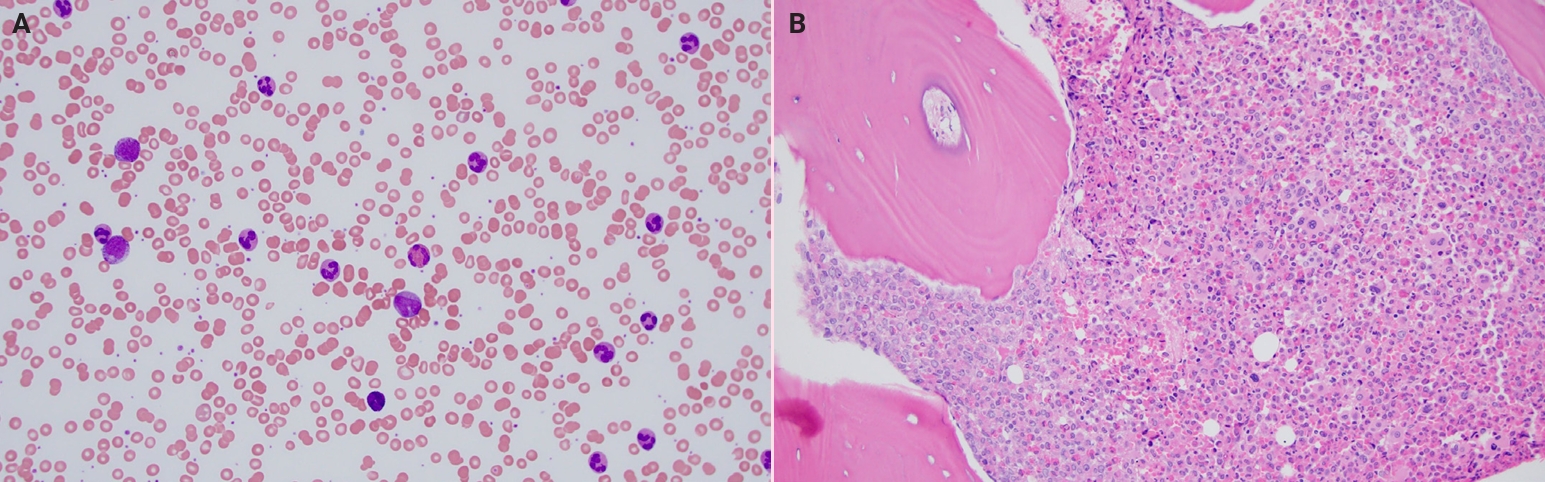

Fig. 1. Chronic myeloid leukemia. (A) Peripheral blood smear with absolute neutrophilia, basophilia and eosinophilia (Wright-Giemsa, 200×). (B) Bone core biopsy in chronic myeloid leukemia showing hypercellular marrow with increased left shifted myeloid maturation, increased eosinophils and small hypolobated megakaryocytes.
Fig. 2. Differences in blast requirement (red) for AML with defining genetic abnormalities between WHO and ICC.
Fig. 1.
Fig. 2.
What’s new in hematopathology 2025: myeloid neoplasms in the WHO 5th edition and ICC
| DNMT3A | TET2 | ASXL1 | JAK2 | TP53 |
| SF3B1 | PPM1D | SRSF2 | IDH1 | IDH2 |
| U2AF1 | KRAS | NRAS | CTCF | CBL |
| GNB1 | BRCC3 | PTPN11 | GNAS | BCOR |
| BCORL1 | BRAF | CALR | CEBPA | CREBBP |
| CSF3R | CUX1 | ETV6 | EZH2 | GATA2 |
| JAK3 | KDM6A | KIT | KMT2A | MPL |
| MYD88 | NOTCH1 | PHF6 | PIGA | PRPF40B |
| SF3A1 | SMC1A | SMC3 | STAG2 | STAT3 |
| PTEN | RAD21 | RUNX1 | SETBP1 | SF1 |
| U2AF2 | WT1 | ZRSR2 | ||
| MDS subtypes | Blasts | Cytogenetic findings | Somatic mutations |
|---|---|---|---|
| MDS with low blasts and isolated 5q deletion (MDS-5q) | <5% BM and <2% PB | 5q deletion or with 1 other abnormality (exception of -7 or del7q) | |
| MDS with low blasts and SF3B1 mutation (MDS-SF3B1) | Absence of del5q, -7 or complex karyotype | SF3B1 (VAF >5%) | |
| MDS with biallelic TP53 inactivation (MDS-biTP53) | <20% BM/PB | Complex karyotype | ≥2 TP53 mutations or 1 mutation with evidence of TP53 copy number loss or copy-neutral loss of heterozygosity (cnLOH) |
| MDS subtypes | Blasts |
|---|---|
| MDS, morphologically defined | |
| MDS with low blasts (MDS-LB) | <5% BM and <2% PB |
| MDS, hypoplastic (MDS-h) (<25% marrow cellularity) | |
| MDS with increased blasts (MDS-IB) | |
| MDS-IB1 | 5-9% BM or 2-4% PB |
| MDS-IB2 | 10-19% BM or 5-19% PB or Auer rods |
| MDS with fibrosis (MDS-f) | 5-19% BM |
| 2-19% PB | |
| WHO | ICC | WHO | ICC |
|---|---|---|---|
| Cytogenetic abnormalities | Somatic gene mutations (includes one additional gene in ICC) | ||
| Complex karyotype | +8* | SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, STAG2 | RUNX1* in addition to 8 genes in WHO |
| 5q del/ loss | 20q del* | ||
| -7, 7q del/loss | 5q del/ loss | ||
| 11q del* | -7, 7q del/loss | ||
| 12p del/loss | 12p del/loss | ||
| -13 or 13q del* | 17p del/loss | ||
| 17p del/loss | Isochromosome 17q | ||
| Isochromosome 17q | idic(X)(q13) | ||
| idic(X)(q13) | Complex karyotype | ||
Table 1. Driver gene mutations associated with CHIP
Table 2. MDS subtypes based on defining genetic abnormalities
Table 3. MDS subtypes based on the morphology
Table 4. WHO and ICC based cytogenetic and somatic mutations in AML-MR
*difference between WHO and ICC.

 E-submission
E-submission




