Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 59(5); 2025 > Article
-
Original Article
National quality assurance program using digital cytopathology: a 5-year digital transformation experience by the Korean Society for Cytopathology -
Yosep Chong1
 , Hyeong Ju Kwon2
, Hyeong Ju Kwon2 , Soon Auck Hong3
, Soon Auck Hong3 , Sung Soon Kim4
, Sung Soon Kim4 , Bo-Sung Kim5
, Bo-Sung Kim5 , Younghee Choi6
, Younghee Choi6 , Yoon Jung Choi7
, Yoon Jung Choi7 , Jung-Soo Pyo8
, Jung-Soo Pyo8 , Ji Yun Jeong9
, Ji Yun Jeong9 , Soo Jin Jung10
, Soo Jin Jung10 , Hoon Kyu Oh11
, Hoon Kyu Oh11 , Seung-Sook Lee12
, Seung-Sook Lee12 , The Committee of Quality Improvement of the Korean Society for Cytopathology
, The Committee of Quality Improvement of the Korean Society for Cytopathology -
Journal of Pathology and Translational Medicine 2025;59(5):320-333.
DOI: https://doi.org/10.4132/jptm.2025.06.27
Published online: September 15, 2025
1Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
2Department of Pathology, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea
3Department of Pathology, Chung-Ang University College of Medicine, Seoul, Korea
4Department of Pathology, Chonnam National University Medical School, Gwangju, Korea
5Department of Pathology, Green Cross Laboratories, Yongin, Korea
6Department of Pathology, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Korea
7Department of Pathology, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea
8Department of Pathology, Eulji University Hospital, Eulji University School of Medicine, Seoul, Korea
9Department of Pathology, Seegene Medical Foundation, Daegu Gyeongbuk Laboratory, Daegu, Korea
10Department of Pathology, Inje University Busan Paik Hospital, Busan, Korea
11Department of Pathology, Daegu Catholic University School of Medicine, Daegu, Korea
12Department of Pathology, Korea Institute of Radiological and Medical Sciences, Seoul, Korea
- Corresponding Author: Yosep Chong, MD, PhD, Department of Hospital Pathology, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 271 Cheonbo-ro, Uijeongbu 11765, Korea Tel: +82-31-820-3160, Fax: +82-31-820-3877, E-mail: ychong@catholic.ac.kr
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,564 Views
- 96 Download
- 1 Web of Science
Abstract
-
Background
- Digital cytopathology (DC) is emerging as a transformative approach in quality assurance programs (QAP), though its comprehensive evaluation remains limited. Since 2020, the Korean Society for Cytopathology has progressively incorporated DC into its national QAP, including digital proficiency testing (PT), sample adequacy testing (SAT), a customizable PT module, and a self-assessment module (SAM), aiming for full digital implementation by 2026.
-
Methods
- This 5-year study assessed diagnostic concordance between conventional and digital PT formats and analyzed participant feedback on service quality and digital image usability across PT, SAT, and SAM. Parallel testing was conducted during the transitional phase, and satisfaction was measured through structured surveys.
-
Results
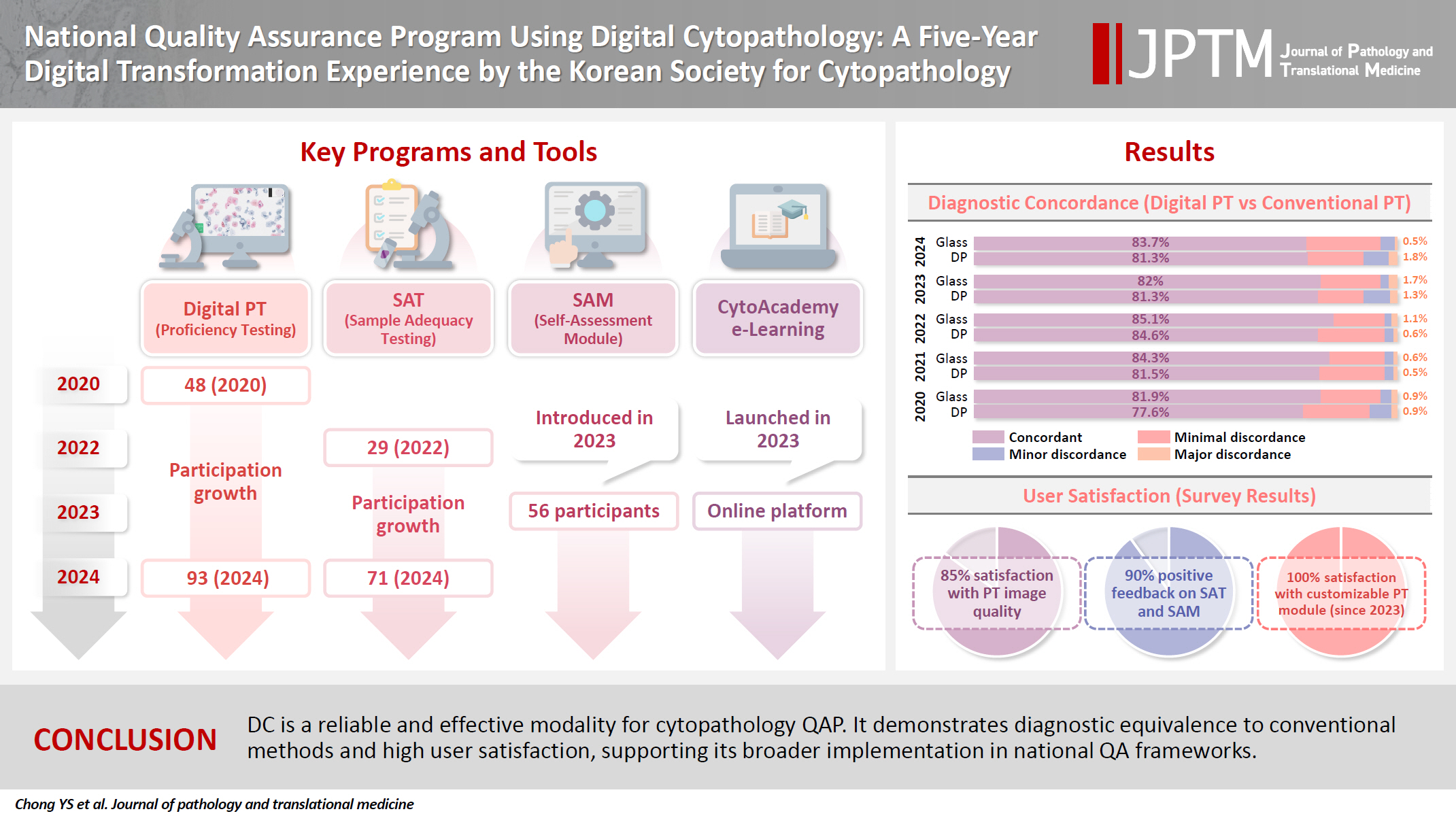
- Participation in digital PT increased from 48 institutions in 2020 to 93 in 2024, while digital SAT participation rose from 29 to 71 between 2022 and 2024. In 2023, 56 institutions joined SAM. Diagnostic concordance rates were comparable between digital and conventional PTs (78.6%–84.6% vs. 82.0%–85.1%), including similar category C (major discordance) rates. Satisfaction with digital PT services and image quality exceeded 85%, and over 90% of institutions reported positive feedback on SAT and SAM. Over 80% were satisfied with the customizable PT module.
-
Conclusions
- DC is a reliable and effective modality for cytopathology QAP. It demonstrates diagnostic equivalence to conventional methods and high user satisfaction, supporting its broader implementation in national quality assurance frameworks.
- Quality assurance programs (QAP) are fundamental in ensuring the accuracy, reliability, and consistency of diagnostic cytopathology. In recent years, digital cytopathology (DC) has emerged as a transformative innovation in enhancing QAP frameworks, leveraging advancements in whole-slide imaging (WSI) technology and artificial intelligence (AI) [1-6]. While DC is increasingly adopted for applications like proficiency testing (PT), its broader efficacy in structured and multifaceted QAP implementations remains underexplored [4]. Current limitations include insufficient experience and unfamiliarity with digital tools, suboptimal image quality, and a lack of comprehensive validation studies, which hinder its full-scale adoption in conventional QAP programs and individual lab’s routine cytopathology quality control activities.
- The Korean Society for Cytopathology (KSC) has a long-standing history of conducting national QAPs, beginning with the Continuous Quality Improvement (CQI) program in 1995 [7-9]. This program includes five main components: an annual survey of cytopathological statistics, an on-site laboratory evaluation, a diagnostic PT, a sample adequacy test (SAT), submission of candidate slides for diagnostic PT. The diagnostic PT was performed using five glass slides per laboratory, including two gynecologic (GYN) samples, two body fluid samples/urine, and one fine-needle aspiration (FNA) cytology sample. These components provided a structured approach to evaluate laboratory performance and improve diagnostic accuracy and have shown a significant contribution to the improved cytopathology practice performance quality by enhancing diagnostic accuracy that was presented in a gradual decrease of major discordant cases in diagnostic PT over time. However, challenges in conventional QAP using glass slides such as donation glass slide shortages, labor-intensive workflows for QAP committee members, and logistical risks, including slide damage and patient information leaks, necessitated an adoption of DC. Since 2020, the KSC has gradually incorporated DC into its QAP, including digital PT and SAT, conducting parallel evaluations by allowing institutions to participate in digital PT and SAT on an optional basis [10]. During this digital transformation, more than 3000 slides from the archives of QAP were digitized and could also be used for launching CytoAcademy, a mobile/web-based online education platform, in 2023 [11]. With plans for full digital implementation by 2026, participation in DC QAP activities has steadily grown, with 93 out of 213 institutions now engaged in digital PT and 71 in SAT by 2024.
- This study is to report the 5-year digital transformation journey of the KSC QAP, focusing on its design, implementation, and outcomes. Through a comparative analysis of diagnostic concordance rates between digital and conventional PTs, as well as feedback on service and image quality, the study aims to assess the effectiveness and user satisfaction of DC in QAP. Additionally, it seeks to provide practical insights and key takeaways gained from real-world QAP experiences.
INTRODUCTION
- Overview of CQI program of the KSC
- Fig. 1 provides an overview of the CQI program conducted by the KSC. The program operates through a structured annual cycle, beginning with the Cytology Statistics Survey in January and February, where participating institutions report data on their cytopathological activities in the previous year. This is followed by On-site Assessments in conjunction with Korean Society of Pathologists (KSP) between April and June, during which two or more KSC and KSP members evaluates the facilities, workflows, and practices of participating laboratories to ensure compliance with quality standards using QAP cytopathology checklists. In May and June, institutions take part in PT, also known as diagnostic accuracy testing, which assesses diagnostic accuracy through either conventional (glass slides) or DC methods. Subsequently, in September and October, the SAT evaluates the quality of sample preparation and the authenticity of the report of each laboratory, providing an additional layer of quality assurance. Finally, the program concludes with the donation of slides for PT from November to December, where laboratories contribute samples that will be used for the next cycle of PT. Since 2020, from PT to SAT, KSC introduced a parallel testing using either glass slides or digital WSIs on an optional basis. This systematic approach ensures a continuous feedback loop for enhancing diagnostic quality and supports the gradual integration of digital tools into routine cytopathology practices.
- Annual survey on the cytopathology statistics using online CQI system
- In 2018, KSC renovated a web-based online CQI system for the annual survey (Supplementary Fig. S1). This system collects questionnaires and written informed consent from all registered cytopathology laboratories performing cytopathologic examinations in Korea. The survey gathers statistical data on overall cytologic examinations, including the number of GYN cases by diagnostic category, GYN sample adequacy, cytology-histology correlation review (CHCR) results, the number of discordant cases based on concordance assessment criteria, and the case number screened by cytotechnicians.
- The diagnostic concordance between cytologic findings and corresponding histology is categorized as concordant (category O) or one of three discordant categories: category A (minimal clinical impact), category B (minor clinical impact), and category C (major clinical impact). Each institution develops its CHCR criteria based on internal laboratory quality assurance (QA) guidelines, while the CQI KSC provides discordance assessment criteria for PT as a reference for CHCR evaluations. Participating institutions are classified into three groups: university hospitals, general hospitals, and commercial laboratories. The survey results analyze overall cytologic examination statistics by category, including GYN, FNA, and non-GYN/non-FNA samples. The non-GYN/non-FNA category encompasses urine, body fluids, respiratory tract samples (such as sputum, bronchial washing, brushing, and bronchoalveolar lavage), cerebrospinal fluid, and more. Endoscopic bronchial ultrasonography-assisted aspiration cytology samples are classified as FNA rather than body fluids. Additionally, cystic fluids from anatomical body cavities, including the pleural, peritoneal, and pericardial cavities, are categorized as body fluids, even when obtained via needle aspiration.
- On-site laboratory assessment by CQI KSC/KSP committee members using QAP checklists (Redbook)
- Fig. 3 illustrates the on-site laboratory evaluation process conducted by the KSC as part of its CQI program, occurring between April and June each year in conjunction with KSP. During this phase, evaluators (volunteer members of KSC and KSP) visit laboratories in person, including university hospital labs and commercial labs, to assess their diagnostic processes and overall compliance with quality standards. The evaluation involves reviewing laboratory workflows, documentation, and diagnostic records to identify areas for improvement according to KSP/KSC QAP checklist. The checklist is organized into multiple chapters covering key areas of pathology QAP. It includes sections on various diagnostic and research domains such as autopsy (A), biobank management (B), cytopathology (C), basic information of the institute and personnel (D), electron microscopy (E), morphometric analysis (F), general considerations for lab management (G), immunohistochemistry (I), molecular pathology including next-generation sequencing (M), diagnostic quality improvement (Q), surgical pathology (S), and digital pathology (W). Each institution is evaluated only for the pathology examinations it performs. After thorough assessment, evaluators provide detailed feedback through official reports, which include QAP scores, observations, and recommendations tailored to each institution. These reports are then distributed back to the laboratories, serving as actionable guides for addressing deficiencies and enhancing quality practices. This process ensures that both academic and commercial labs maintain high standards in cytopathology, fostering a culture of continuous improvement and accountability.
- For university hospitals and general hospitals, two evaluators conduct an on-site inspection every 2 years. If the laboratory receives grade A, the following year’s inspection is replaced with a document review. If it receives grade B, another on-site inspection is conducted the next year. A grade C results in conditional accreditation, and if a laboratory receives conditional accreditation for two consecutive years, its accreditation is revoked. For commercial laboratories, a team of four expert evaluators conducts an on-site inspection annually, with the same grade-based measures applied.
- Diagnostic PT
- Fig. 3 illustrates the diagnostic PT process conducted by the KSC, which occurs annually from May to June. Among the histology-confirmed cytology cases collected from participating laboratories 2 years in advance, five randomly selected cases (2 GYN, 1 respiratory tract, 1 body fluid or urine, 1 FNA) are dispatched to each participating laboratory. The laboratories are then asked to analyze the test slides and submit their diagnostic interpretations to the KSC via an online platform. For those wishing to apply for digital PT, six digital WSIs (2 GYN, 1 respiratory tract, 1 body fluid, 1 urine, 1 FNA) are randomly assigned to each laboratory online (Fig. 4). During this process, approximately 30 WSIs are selected from a pool of 60 or more WSIs by CQI Committee members in advance, and a different set of randomly selected 6 WSIs is assigned to each laboratory to prevent potential misconduct due to communication between participants. These interpretations are then assessed by the KSC CQI Committee members to determine diagnostic accuracy, with results and feedback provided to the laboratories in detailed reports. The diagnostic concordance between original and submitted cytologic diagnoses is categorized as concordant (category O) or one of three discordant categories: category A (minimal clinical impact), category B (minor clinical impact), and category C (major clinical impact) according to the discordance assessment criteria developed and provided by CQI KSC. To assess the efficiency and differences between these two methods, the diagnostic performance of participating institutions is collected and compared.
- In 2023, a customizable PT module was introduced to digital PT to address the growing demand for flexibility. Most commercial laboratories primarily handle GYN cytology cases, whereas university hospital laboratories more commonly deal with non-GYN cytology. To accommodate these differences, participating institutions were given the option to select an additional PT module tailored to their case characteristics. From 2023 onward, each laboratory could choose either four additional GYN or non-GYN cases, in addition to the original PT module.
- Sample adequacy test
- Fig. 5 illustrates the annual process of conventional and digital SAT from September to October. For conventional SAT, participating institutions collect and submit their physical GYN cytology slides with five consecutive numbers on the designated date of the previous year, along with corresponding reports on sample adequacy. The collected slides are assessed under a microscope by KSC CQI Committee members to determine whether the sample adequacy of each slide correlates with the corresponding report. Alternatively, for digital SAT, a pool of GYN cytology slides with satisfactory and unsatisfactory sample adequacy is prepared and digitized by KSC CQI Committee members in advance and distributed electronically, allowing participants to evaluate the adequacy of samples via digital platforms, similar to digital PT.
- Donation of samples (slides/digital images) for the next rounds of PT
- Fig. 6 illustrates the process of slide/digital image donation and preparation for the next rounds of diagnostic PT. Institutions are invited to contribute cytologic slides between November and December, which serve as the foundation for subsequent PT rounds. These donated slides undergo a rigorous selection and review process managed by the KSC CQI Committee members to ensure they meet the required quality and represent a wide spectrum of diagnostic scenarios. Once curated, the slides are digitized using WSI systems, facilitating their use in both traditional microscopy and DC formats. Since 2023, for institutions that are unable to participate in sample donation due to a limited number of eligible cases—such as those that only handle cytology and do not have matching histology slides, the formal program was replaced by self-assessment module (SAM) utilizing the CytoAcademy online education platform.
- Feedback Survey for CQI programs using DC
- After the completion of the digital diagnostic PT, SAT, and SAM, a feedback survey is conducted to gather participants’ insights on the quality and effectiveness of these programs. The survey questionnaire for feedback collection is provided in Supplementary Table S1. The survey questionnaire for digital diagnostic PT includes overall satisfaction with the digital method, image quality, case eligibility for assigned cytological specimens, and overall satisfaction with the customizable PT modules. The survey questionnaire for SAT includes overall satisfaction with the SAT, and digital slide image quality. The survey questionnaire for SAM includes the overall satisfaction with the SAM and the cases.
MATERIALS AND METHODS
- Participating institutions for digital PT, SAT, and SAM
- Fig. 7A shows the number of institutions participating in conventional glass slide-based versus DC-based diagnostic PT from 2020 to 2024. In 2020, 48 institutions participated in the trial digital PT, and 107 institutions participated first official digital PT while 108 institutions participated conventional in 2021. Although the number of institutions participating digital PT transiently decreased to 81 institutions in 2022, it has been increasing gradually as 86 and 93 in the following years. Fig. 7B shows the number of institutions participating SAT. In 2022, 29 institutions joined the digital SAT program, growing to 57 in 2023 and reaching 71 in 2024. Concurrently, participation in the conventional SAT program declined, indicating a gradual shift towards digital method. Fig. 7C shows a breakdown of institutions engaged in the process of slide/digital image donation or SAM initiated in 2023. Out of 150 participating institutions, 10 submitted digital images, 56 engaged in the SAM, and 84 remained to submit conventional glass slides with matching histology slides.
- Digital diagnostic PT
- Fig. 8 shows the diagnostic concordance rates between digital and conventional PT tests, categorized into four levels: Category O (concordant), A (minimal discordance), B (minor discordance), and C (major discordance). For conventional PT using glass slides, the concordant rates ranged from 81.9% to 85.1% between 2020 and 2024, while for digital PT, the concordant rates varied from 78.6% to 84.6%. These ranges were not significantly different from each other. The gap between the concordance rates of digital and conventional PT gradually decreased from 4.3 percentage points in 2020 to 0.7 percentage points in 2023, but increased to 5 percentage points in 2024. This reversal in 2024 may be attributable to the inclusion of more challenging cases that year and the influx of new participants still adapting to the digital format, as well as subtle variations in image quality despite overall high image quality satisfaction.
- In category A, conventional PT results ranged from 13.4% to 17.0% and digital PT showed a similar range of 13.3% to 17.5%. For category B, both modalities consistently remained under 2.0%, with conventional PT ranging between 0.6% and 1.7% and digital PT between 0.5% and 1.9%. Finally, category C, reflecting major discordance, showed the lowest rates for both methods. Glass slide results varied from 0.5% to 0.6%, while digital PT displayed a slightly higher range from 0.5% to 1.8%.
- It is impossible to directly compare the diagnostic concordance between conventional and digital PT because the cases used in each method were different. However, the overall performance of participating institutions in PT was not significantly different between different methods.
- Feedback survey for digital PT, digital SAT, and SAM
- Fig. 9 shows the satisfaction levels of participating institutions with the digital PT, digital SAT, and SAM. For the digital PT, satisfaction with general services and image quality showed relatively good response that only less than 15% of response was negative throughout the years (Fig. 9A, B). For the customizable PT module (introduced in 2023), there was no negative response demonstrating widespread acceptance of the flexibility and relevance of this module (Fig. 9C). Similarly, in the digital SAT and SAM conducted in 2023, there was no negative response (Fig. 9D, E). Across all tests, “Bad” and “Little bad” ratings were minimal, indicating the overall successful digital transformation and its alignment with participant expectations.
RESULTS
- The five-year digital transformation of the CQI program of KSC has been substantial, progressing at a moderate yet steady pace, marking a significant milestone in modernizing QAP in cytopathology in Korea. Participation in digital PT and SAT has steadily increased, with over 43.6% and 33.3% of total institutions engaged by 2024, respectively. Concordance rates between digital and conventional PT have shown comparable results, validating the feasibility of DC for PT. Moreover, participating institutions reported high levels of satisfaction with the integration of DC into PT and SAT, particularly appreciating the customizable PT modules tailored to meet the specimen characteristics of each individual laboratory. Through the online education platform, CytoAcademy—developed using digitized QAP WSIs—cytopathologists from participating laboratories have become more familiar with interpreting digital WSIs via SAM, supported by comprehensive feedback.
- Advantage of DC in QAP
- DC provides significant advantages in the QAP by addressing the logistical and operational limitations of traditional glass slide-based systems. The use of WSI eliminates risks associated with slide damage, misplacement, and transportation. Digital systems also streamline workflows by enabling remote evaluation and facilitating interlaboratory comparisons through cloud-based platforms. The customizable testing modules introduced in 2023 further enhance the program's flexibility, allowing institutions to tailor their testing approaches to meet specific needs. Conventional glass slide-based QAP faces several challenges, including the considerable time and effort required to collect and distribute QA slides from participating institutions. There are persistent difficulties in securing an adequate number of high-quality cases, concerns regarding the diagnostic reliability of certain slides, and biases due to the overrepresentation of specific diagnostic categories. Additionally, the variability in slide assignments across institutions can result in inconsistent levels of difficulty for diagnostic PT.
- DC effectively overcomes these limitations. By utilizing digital slides, it ensures standardized case distribution, providing equal levels of difficulty and consistent diagnostic challenges for all participants. This approach eliminates biases related to case selection and improves the reliability of diagnostic assessments. Moreover, the ease of sharing and replicating digital cases facilitates the creation of a diverse and balanced case pool, enhancing the overall quality and fairness of QAP evaluations. Overall, DC contributes to increased efficiency, better resource management, and improved accessibility for participating laboratories.
- Overall feedback for user experience
- Feedback from participants over the 5-year period highlights generally high satisfaction rates with the digital transformation. By 2024, over 85% of participants rated positively for general service and image quality. Similarly, 71% of participants expressed high satisfaction with the customizable module introduced in 2023, and 92% rated the SAM positively. However, the lack of familiarity with DC among many cytopathologists still remains one of the biggest barriers to its widespread adoption, emphasizing the need for continuous education and training.
- Current status of QAP/PT using DC in major countries
- Globally, QAP have begun incorporating DC, particularly in the domain of PT. The UK National External Quality Assessment Service (UK NEQAS) and the Royal College of Pathologists of Australasia Quality Assurance Programs (RCPAQAP) have implemented digital diagnostic PT, primarily for non-gynecologic samples, utilizing WSI [12]. These initiatives remain at variable stages of maturity, with standardization of assessment criteria and accreditation integration still evolving. The KSC provides a valuable benchmark, having successfully established a fully digital, nationwide cytology PT program, demonstrating both scalability and practical feasibility.
- United States, Canada, and Australia
- In the United States, the Clinical Laboratory Improvement Amendments (CLIA) mandate participation in Centers for Medicare & Medicaid Services (CMS)–approved PT for individuals interpreting gynecologic cytology [12]. As of 2025, the College of American Pathologists (CAP) and the American Society for Clinical Pathology (ASCP) continue to offer such programs, primarily using glass slides, although CAP has introduced digital modules for non-gynecologic cytology [4,12-14]. Digital PT adoption is expanding gradually, currently only for educational modules, with ongoing validation of diagnostic concordance between digital and conventional formats.
- Canada has adopted a hybrid model for cytology PT. The Institute for Quality Management in Healthcare (IQMH) incorporates digital WSI cases alongside conventional glass slides in both gynecologic and non-gynecologic surveys [1,15]. Despite this progress, digital PT remains supplementary, with broader implementation under evaluation.
- Australia has made significant strides, with RCPAQAP offering biannual digital PT for non-gynecologic cytology using secure web-based WSI platforms [12,16]. Gynecologic cytology PT continues to rely on glass slides. RCPAQAP’s digital programs are ISO 17043-accredited and exemplify the region’s advanced integration of digital formats into cytology QA.
- Europe
- The UK NEQAS has developed a web-based Digital Interpretative Diagnostic Cytopathology Program, currently conducted twice yearly with 12 distributed cases per round [12]. This voluntary scheme, operated by the UK NEQAS for Cellular Pathology Technique (CPT), is not yet tied to formal laboratory accreditation but serves as a recognized educational and QA tool [12]. The program enables participants to evaluate whole slide images of non-gynecologic cytology cases using an online viewer, promoting digital competency and interlaboratory consistency.
- In France, external quality assessment (EQA) in cytopathology has traditionally been managed by Association Française d’Assurance Qualité en Anatomie et Cytologie Pathologiques (AFAQAP), which primarily relies on glass slide circulation and static image sets [12,17]. While digital pathology is gaining ground in histopathology EQA schemes, full-scale implementation of digital PT in cytology is still limited. Recent discussions within the French Society of Clinical Cytology (SFCC) have emphasized the need to explore WSI-based digital testing, particularly in response to technical limitations and logistical challenges in distributing physical slides. However, as of 2025, DC PT remains exploratory and lacks integration into formal accreditation requirements.
- In Germany, the QuIP GmbH consortium (Quality Assurance Initiative Pathology) oversees EQA programs in collaboration with the German Society of Pathology [12,18]. Cytology PT primarily uses conventional formats, though digital pathology is expanding through educational initiatives and preliminary pilot studies. Technical challenges related to z-stack scanning and cytologic detail have slowed adoption of digital PT. Nonetheless, QuIP and the German cytology community are actively evaluating WSI applications, particularly in non-gynecologic cytology, as part of ongoing QA modernization.
- In Italy, regional initiatives have demonstrated early implementation of digital PT [12]. Notably, the Emilia-Romagna region conducted DC PT programs using scanned slides and centralized review platforms. These efforts, supported by local pathology societies and regional health authorities, showed promising results in diagnostic concordance and participant satisfaction. Although Italy lacks a nationwide digital PT framework, the Italian Society of Pathology and Cytology (SIAPeC-IAC) is advocating for broader adoption, leveraging successful regional pilots as models for national integration.
- In the Scandinavian countries, particularly Sweden and Denmark, digital PT has progressed through collaborative programs organized by national cytology societies [12]. Sweden’s KVAlitets- och STandardiseringskommittén (KVAST) group has incorporated WSI into national QA rounds, particularly for non-gynecologic cytology such as effusions and FNAs. Equalis, a major EQA provider, offers digital slide-based assessments accessible through secure platforms. These programs are voluntary but widely accepted, reflecting a high degree of digital readiness. Denmark and Finland have shown growing interest and often share digital infrastructure and QA protocols within the Nordic collaborative network.
- In Spain, the Spanish Society of Cytology (SEC) has implemented a web-based digital QAP for liquid-based cervical cytology since 2020 (https://www.secitologia.org) [19]. The program, consisting of two rounds per year with eight cases each, is voluntary but has seen growing participation. It has been ISO 17043-accredited and aims to become a mandatory component of cytology laboratory accreditation. Other sample types have yet to be included, largely due to technical barriers in scanning and standardizing certain cytologic specimens.
- Japan and Taiwan
- In Japan, PT in cytology continues to rely on traditional methodologies. The Japanese Society of Clinical Cytology (JSCC) oversees certification and QA, with digital PT not yet nationally implemented [20]. While digital pathology has seen increased use for education and teleconsultation, formal digital PT programs in cytology remain limited to small-scale pilots without national standardization.
- Taiwan has not yet established a formal DC PT program [21]. PT by The Taiwan Society of Clinical Cytology remains glass-based, though leading institutions have adopted WSI for diagnostics and education. Participation in international digital PT programs, such as those by CAP, has provided indirect exposure. The Taiwan Society of Clinical Cytology is evaluating the feasibility of digital QA integration in the near future, reflecting a growing readiness to transition toward digital PT frameworks.
- Research projects, education platform development using digitized QAP archive slides
- Through the digitization of QAP slides. KSC has undertaken significant initiatives to enhance cytopathology education and research [11,22-25]. By digitizing over 7,000 QAP slides, the KSC developed “CytoAcademy,” a web- and mobile-based e-learning platform designed to provide comprehensive educational resources for cytopathologists and cytotechnologists [11]. Launched in March 2023, CytoAcademy offers basic sessions for each organ system, on-demand lectures, a WSI archive, and SAM, facilitating continuous medical education in cytopathology.
- In parallel, National Cancer Center and three other major institutions in Korea conducted a national AI dataset project in collaboration with KSC CQI Committee, “The OPEN AI Dataset Project,” funded by National Information Society Agency (NIA) in Korea, aiming to construct a comprehensive dataset for AI applications in non-gynecological cytopathology [22-25]. This project amassed 5,500 WSIs encompassing 11 cancer types across five specimen categories, serving as a foundation for developing over ten AI models focused on cancer detection. These efforts show the significant potential for QAP utilizing DC to be leveraged in various national-level AI research and cytopathology education endeavors [26,27].
- Diagnostic concordance assessment criteria for diagnostic proficiency test
- In diagnostic PT, various systems have been proposed to assess diagnostic concordance between cytotechnologists and pathologists, as well as between cytological and histological diagnoses. For instance, the CAP utilizes a PT program that evaluates laboratories based on their concordance with intended responses, categorizing results as acceptable or unacceptable. An unsuccessful event indicates that the laboratory did not achieve overall acceptable concordance with the intended responses, necessitating a review and assessment of all unacceptable responses to identify areas requiring improvement. In the recent study by Caputo et al. [28], the authors assessed interobserver variability in lymph node cytology using the Sydney system. They introduced a “delta” metric to quantify diagnostic discordance, where a delta of 0 indicates perfect agreement, and a delta of 1 signifies a one-step disagreement, such as classifying a benign case as atypical. This approach allowed for a nuanced analysis of diagnostic discrepancies among cytopathologists.
- Despite these efforts, there is currently no universally established system or consensus for such assessments. In this context, the KSC has implemented a diagnostic concordance assessment system that classifies results into four categories according to clinical impact of the discordancy level: O, concordant; A, minimal discordance; B, minor discordance; and C, major discordance. This stratification allows for a nuanced evaluation of diagnostic accuracy and helps identify specific areas needing improvement.
- Looking ahead, as AI models become increasingly integrated into cytopathology, it is crucial to develop discordancy assessment criteria that go beyond simple diagnostic accuracy [26,27]. Comparative evaluations between AI-based cytological diagnoses and human cytological diagnoses, as well as between AI-based cytological diagnoses and histological diagnoses, will be essential. Such comprehensive assessment systems will not only facilitate the validation of AI models but also ensure their reliability and effectiveness in clinical practice. Therefore, further research and development in this area are very important.
- Future strategy to increase digital intimacy
- To further enhance digital intimacy, the KSC plans to expand training programs and develop more intuitive user interfaces to improve the accessibility of digital tools. Introducing AI-driven analytics for rapid feedback and error detection can also support laboratories in adapting to digital workflows. Additionally, fostering international collaboration and knowledge sharing through workshops and conferences will help establish global standards for DC, promoting its adoption in QAP worldwide.
DISCUSSION
Supplementary Information
Ethics Statement
This study was reviewed and approved by the Institutional Review Board of the Catholic University of Korea College of Medicine (UC21ZCSI0133). The informed consent was waived by the Institutional Review Board of the Catholic University of Korea College of Medicine.
Availability of Data and Material
Data and materials for this work are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: YC (Yosep Chong), HJK, SAH, HKO, SJJ, BSK, JYJ. Data curation: YC (Yosep Chong), HJK, YC (Younghee Choi), SSK, JSP, SAH. Formal analysis: YC (Yosep Chong). Funding acquisition: YC (Yosep Chong), YJC. Investigation: YC (Yosep Chong), HJK, SAH, HKO, SJJ, YC (Younghee Choi), SSK, JSP, BSK, JYJ. Methodology: YC (Yosep Chong), YC (Younghee Choi), SSK, JSP, SAH, HKO, SJJ, BSK, JYJ. Project administration: YC (Yosep Chong), YJC, SSL. Resources: HJK, SAH, YC (Younghee Choi), SSK, JSP, HKO, SJJ, BSK, JYJ. Software: YC (Yosep Chong). Supervision: YJC, SSL. Validation: HJK, SAH, YC (Younghee Choi), SSK, JSP. Visualization: YC (Yosep Chong), HJK. Writing—original draft: YC (Yosep Chong). Writing—review & editing: YC (Yosep Chong), HJK. Approval of final manuscript: all authors.
Conflicts of Interest
Y.C., a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
This study was partially supported by The Korean Society for Cytopathology Grant (No.24-01) and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2021R1A2C2013630/RS-2021-NR059550).
Acknowledgments
We would like to thank Songyeon Kim for data curation. We appreciate Dr. Zaibo Li, Ma Jesús Fdez-Aceñero, and Julia Pagliuso for reviewing the manuscript.








- 1. Demichelis F, Della Mea V, Forti S, Dalla Palma P, Beltrami CA. Digital storage of glass slides for quality assurance in histopathology and cytopathology. J Telemed Telecare 2002; 8: 138-42. ArticlePubMedPDF
- 2. Marchevsky AM, Wan Y, Thomas P, Krishnan L, Evans-Simon H, Haber H. Virtual microscopy as a tool for proficiency testing in cytopathology: a model using multiple digital images of Papanicolaou tests. Arch Pathol Lab Med 2003; 127: 1320-4. ArticlePubMedPDF
- 3. Stewart J 3rd, Miyazaki K, Bevans-Wilkins K, Ye C, Kurtycz DF, Selvaggi SM. Virtual microscopy for cytology proficiency testing: are we there yet? Cancer 2007; 111: 203-9. ArticlePubMed
- 4. Wilbur DC. Proficiency testing in the digital pathology age [Internet]. Concord: Corista, 2025 [cited 2025 Feb 4]. Available from: https://blog.corista.com/corista-digital-pathology-blog/proficiency-testing-in-the-digital-pathology-age.
- 5. Kim D, Sundling KE, Virk R, et al. Digital cytology part 1: digital cytology implementation for practice: a concept paper with review and recommendations from the American Society of Cytopathology Digital Cytology Task Force. J Am Soc Cytopathol 2024; 13: 86-96. ArticlePubMed
- 6. Pantanowitz L, Sinard JH, Henricks WH, et al. Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med 2013; 137: 1710-22. ArticlePubMedPMC
- 7. Choi YD, Oh HK, Kim SJ, et al. Continuous quality improvement program and its results of Korean Society for Cytopathology. J Pathol Transl Med 2020; 54: 246-52. ArticlePDF
- 8. Chong Y, Jung H, Pyo JS, Hong SW, Oh HK. Current status of cytopathology practices in Korea: annual report on the Continuous Quality Improvement program of the Korean Society for Cytopathology for 2018. J Pathol Transl Med 2020; 54: 318-31. ArticlePDF
- 9. Hong SA, Jung H, Kim SS, et al. Current status of cytopathology practice in Korea: impact of the coronavirus pandemic on cytopathology practice. J Pathol Transl Med 2022; 56: 361-9. ArticlePubMedPMCPDF
- 10. Chong Y, Hong SA, Oh HK, et al. Diagnostic proficiency test using digital cytopathology and comparative assessment of whole slide images of cytologic samples for quality assurance program in Korea. J Pathol Transl Med 2023; 57: 251-64. ArticlePubMedPMCPDF
- 11. Hong R, Chong Y, Chae SW, Lee SS, Gong G. Development of CytoAcademy: a new web- and mobile-based E-learning platform for cytopathologists and cytotechnologists by the Korean Society for Cytopathology in the post-pandemic era. J Pathol Transl Med 2024; 58: 261-4. ArticlePubMedPMCPDF
- 12. Srebotnik Kirbis I, Strojan Flezar M. External quality assessment in diagnostic cytopathology: current programs, challenges, and perspectives. Acta Cytol 2025 May 28 [Epub]. https://doi.org/10.1159/000546537. ArticlePubMed
- 13. Centers for Medicare and Medicaid Services [Internet]. Baltimore: U.S. Centers for Medicare and Medicaid Services, 2025 [cited 2025 Feb 3]. Available from: https://www.cms.gov/medicare/quality/clinical-laboratory-improvement-amendments/proficiency-testing.
- 14. CAP non-gyn digital cytology program overview [Internet]. Northfield: College of American Pathologists, 2025 [cited 2025 Apr 5]. Available from: https://www.cap.org.
- 15. Digital cytology integration in Institute for Quality Management in Healthcare proficiency test [Internet]. North York: Institute for Quality Management in Healthcare, 2025 [cited 2025 Apr 5]. Available from: https://iqmh.org.
- 16. Shield PW, Frost F, Finnimore JL, Wright RG, Cummings MC. External quality assurance in nongynecologic cytology: the Australasian experience. Cancer Cytopathol 2017; 125: 349-61. ArticlePDF
- 17. Cochand-Priollet B, Vincent S, Vielh P. Cytopathology in France. Cytopathology 2004; 15: 163-6. ArticlePubMed
- 18. QuIP GmbH [Internet]. Berlin: Quality in Pathology (QuIP GmbH), 2025 [cited 2025 Apr 5]. Available from: https://www.qualityinpathology.com/en_GB/.
- 19. Fernández-Aceñero M, Lorente AM, Comba J. Implementation of a national control of quality program in Spain. Proceedings of the 45th European Congress of Cytopathology; 2024 Jun 23-26; Leipzig, Germany.
- 20. The Japanese Society of Clinical Cytology [Internet]. Tokyo: The Japanese Society of Clinical Cytology, 2025 [cited 2025 Apr 5]. Available from: https://jscc.or.jp/cs/.
- 21. The Taiwan Society of Clinical Cytology. Participant manual for the 1st cytology proficiency test in 2025. Ver. 3.2. Taipei: The Taiwan Society of Clinical Cytology, 2025.
- 22. Kim HK, Han E, Lee J, et al. Artificial-intelligence-assisted detection of metastatic colorectal cancer cells in ascitic fluid. Cancers (Basel) 2024; 16: 1064.Article
- 23. Kim T, Chang H, Kim B, et al. Deep learning-based diagnosis of lung cancer using a nationwide respiratory cytology image set: improving accuracy and inter-observer variability. Am J Cancer Res 2023; 13: 5493-503. PubMedPMC
- 24. Lee Y, Alam MR, Park H, et al. Improved diagnostic accuracy of thyroid fine-needle aspiration cytology with artificial intelligence technology. Thyroid 2024; 34: 723-34. ArticlePubMed
- 25. Park HS, Chong Y, Lee Y, et al. Deep learning-based computational cytopathologic diagnosis of metastatic breast carcinoma in pleural fluid. Cells 2023; 12: 1847.ArticlePubMedPMC
- 26. Kim D, Sundling KE, Virk R, et al. Digital cytology part 2: artificial intelligence in cytology: a concept paper with review and recommendations from the American Society of Cytopathology Digital Cytology Task Force. J Am Soc Cytopathol 2024; 13: 97-110. Article
- 27. Thakur N, Alam MR, Abdul-Ghafar J, Chong Y. Recent application of artificial intelligence in non-gynecological cancer cytopathology: a systematic review. Cancers (Basel) 2022; 14: 3529.ArticlePubMedPMC
- 28. Caputo A, Fraggetta F, Cretella P, et al. Digital Examination of LYmph node CYtopathology Using the Sydney system (DELYCYUS): an international, multi-institutional study. Cancer Cytopathol 2023; 131: 679-92. Article
REFERENCES
Figure & Data
References
Citations











 E-submission
E-submission


 Cite this Article
Cite this Article











