Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 58(1); 2024 > Article
-
Original Article
Identification of invasive subpopulations using spatial transcriptome analysis in thyroid follicular tumors -
Ayana Suzuki1,2
 , Satoshi Nojima1
, Satoshi Nojima1 , Shinichiro Tahara1
, Shinichiro Tahara1 , Daisuke Motooka3
, Daisuke Motooka3 , Masaharu Kohara1
, Masaharu Kohara1 , Daisuke Okuzaki3,4
, Daisuke Okuzaki3,4 , Mitsuyoshi Hirokawa2
, Mitsuyoshi Hirokawa2 , Eiichi Morii1,4
, Eiichi Morii1,4
-
Journal of Pathology and Translational Medicine 2024;58(1):22-28.
DOI: https://doi.org/10.4132/jptm.2023.11.21
Published online: January 10, 2024
1Department of Pathology, Graduate School of Medicine, Osaka University, Suita, Osaka, Japan
2Department of Diagnostic Pathology and Cytology, Kuma Hospital, Kobe, Hyogo, Japan
3Genome Information Research Center, Research Institute for Microbial Diseases, Osaka University, Suita, Osaka, Japan
4Institute for Open and Transdisciplinary Research Initiatives, Osaka University, Suita, Osaka, Japan
- Corresponding Author: Ayana Suzuki, MHSc, Department of Diagnostic Pathology and Cytology, Kuma Hospital, Kobe, 8-2-35, Shimoyamate-dori, Chuo-ku, Kobe, Hyogo, 650-0011, Japan Tel: +81-78-371-3721, Fax: +81-78-371-3645, E-mail: suzuki01@kuma-h.or.jp
© 2024 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Follicular tumors include follicular thyroid adenomas and carcinomas; however, it is difficult to distinguish between the two when the cytology or biopsy material is obtained from a portion of the tumor. The presence or absence of invasion in the resected material is used to differentiate between adenomas and carcinomas, which often results in the unnecessary removal of the adenomas. If nodules that may be follicular thyroid carcinomas are identified preoperatively, active surveillance of other nodules as adenomas is possible, which reduces the risk of surgical complications and the expenses incurred during medical treatment. Therefore, we aimed to identify biomarkers in the invasive subpopulation of follicular tumor cells.
-
Methods
- We performed a spatial transcriptome analysis of a case of follicular thyroid carcinoma and examined the dynamics of CD74 expression in 36 cases.
-
Results
- We identified a subpopulation in a region close to the invasive area, and this subpopulation expressed high levels of CD74. Immunohistochemically, CD74 was highly expressed in the invasive and peripheral areas of the tumor.
-
Conclusions
- Although high CD74 expression has been reported in papillary and anaplastic thyroid carcinomas, it has not been analyzed in follicular thyroid carcinomas. Furthermore, the heterogeneity of CD74 expression in thyroid tumors has not yet been reported. The CD74-positive subpopulation identified in this study may be useful in predicting invasion of follicular thyroid carcinomas.
- Patients
- Between May 2016 and December 2022, 283 follicular thyroid carcinoma nodules were resected at the Kuma Hospital in Japan. Of these, 36 nodules (minimally invasive [n = 18], encapsulated angioinvasive [n = 8], widely invasive [n = 10]) were extracted after excluding those with papillary-like nuclear features, poorly differentiated carcinoma components, and very small invasive areas. A nodule with an invasive area within a 5-mm-diameter circle was subjected to spatial transcriptome analysis, and all cases were subjected to immunohistochemical staining. The clinicopathological features of the enrolled 36 cases are shown in Table 1.
- Spatial transcriptome analysis
- We performed spatial transcriptome analysis using Visium CytAssist Spatial Gene Expression for FFPE (10x Genomics, Pleasanton, CA, USA). In this analysis, the whole RNA transcriptome of cells in each spot of the specialized slides was obtained from FFPE tissue sections. Each spot contained approximately 10–20 cells, and the RNA transcriptome of these cells revealed the features of RNA expression in each spot. The tissues were sectioned as described in the Visium CytAssist Spatial Gene Expression for FFPE Tissue Preparation Guide (CG000518). Sections were stained with hematoxylin and eosin (H&E), imaged, and de-cover-slipped, followed by H&E de-staining and de-crosslinking. Glass slides with tissue sections were processed using a Visium CytAssist instrument to transfer analytes to a Visium CytAssist Spatial Gene Expression slide with a 0.42 cm2 capture area. Probe extension and library construction steps followed the standard Visium for the FFPE workflow. Libraries were sequenced using a DNBSEQ-G400 sequencer (BGI) (read 1: 28 bp, read 2: 100 bp). Spatial data were pre-processed and aligned using 10x Genomics Space Ranger v1.3.0 with the reference human genome GRCh38 (refdata-gex-GRCh38-2020-A) to generate raw unique molecular identifier count spot matrices. Dimensionality reduction was performed using classical principal component analysis integrated uniform manifold approximation and projection (UMAP) [15]. Unsupervised clustering of the data spots was performed using Louvain clustering [16] integrated into the BioTuring Lens with a resolution of 0.1. Further downstream analysis and gene expression visualization were conducted using the BBrowser (BioTuring, San Digo, CA, USA).
- CD74 immunohistochemical analysis
- Immunohistochemical staining was performed using 3-μmthick FFPE specimens. Anti-CD74 (1:100, LN2, ab9514, Abcam, Cambridge, UK) and anti–thyroid transcription factor-1 (TTF-1; 1:100, SP141, ab227652, Abcam) were used as primary antibodies. Staining was performed using a Dako Autostainer Link 48+ (Dako, Carpinteria, CA, USA), according to the manufacturer’s recommendations. The expression of CD74 was assessed using a visual grading system based on staining intensity under light microscopy. High intensity (++), low intensity (+), and no signal (–) were defined as strong, weak, and no staining, respectively. The histological index was calculated using the following formula:
- Histological index = 2 × (% cells of high intensity) + 1 × (% cells of low intensity).
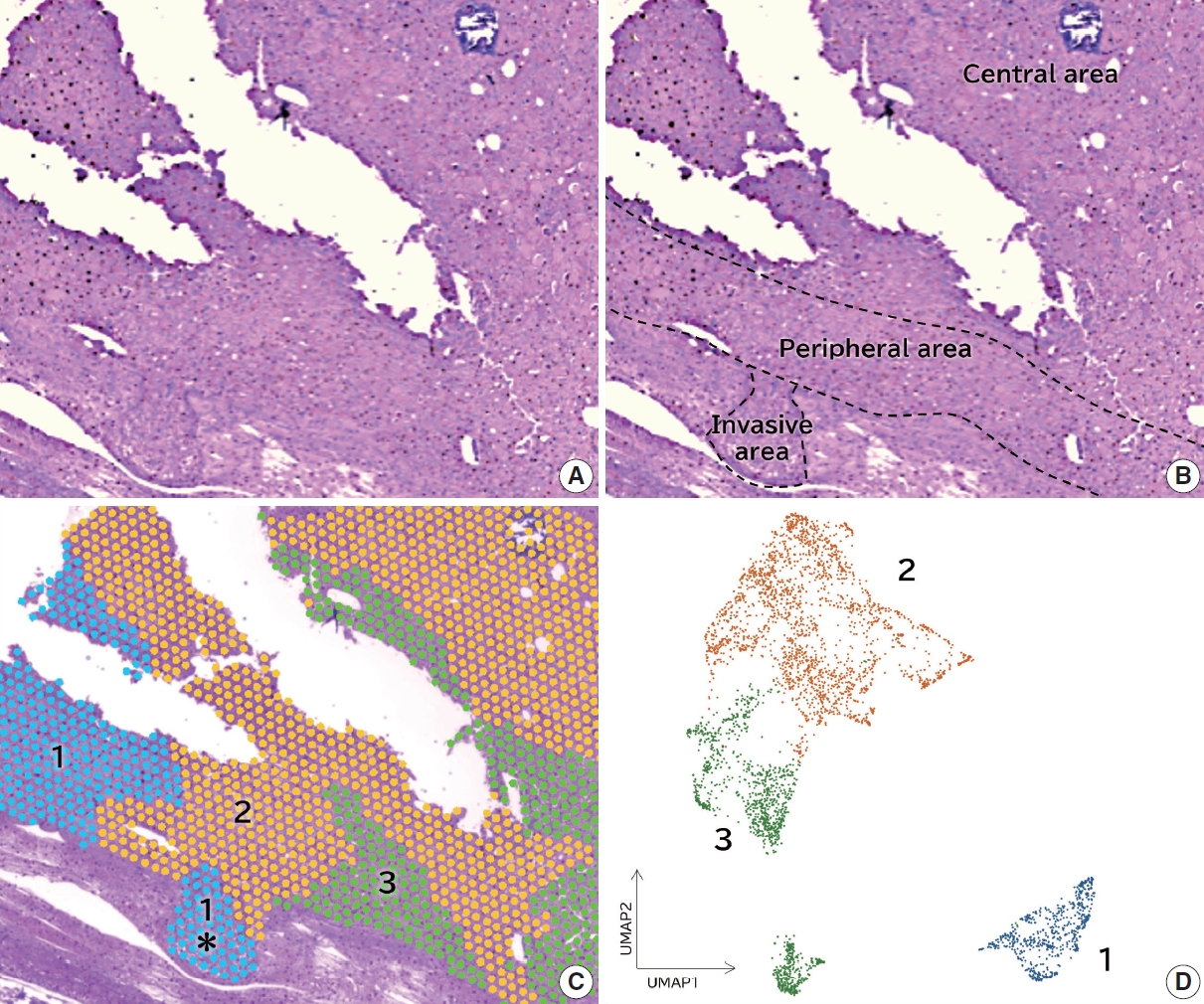
- The invasive area was defined as a tumor lesion invading or over the capsule or an angioinvasive lesion. The peripheral area of the tumor was defined as the tumor lesion within 1 mm of the tumor border. The tumor central area was defined as the tumor lesion > 1 mm from the border. One pathology assistant (A.S) and one pathologist (E.M) scored the data independently.
MATERIALS AND METHODS
- Spatial transcriptome analysis
- Among the 36 cases of follicular thyroid carcinoma, a nodule with an invasive area within a circle of 5 mm diameter was subjected to spatial transcriptome analysis (Fig. 1A). As tissue samples in the circle could be examined in the spatial transcriptome analysis, we selected a case in which the invasive and non-invasive areas were involved in the circle. A minimally invasive case was selected for spatial transcriptome analysis. The invasive, peripheral, and central areas were shown in Fig. 1B. Louvain clustering with a resolution of 0.1 showed three clusters, and the H&E staining image merged with the three clusters (Fig. 1C). Dimensionality reduction using classical principal component analysis integrated with UMAP showed that the features of cluster 1 were distinct from those of clusters 2 and 3 (Fig. 1D). Cluster 1 was detected in the invasive area, where clusters 2 or 3 were not found (Fig. 1C, asterisk portion). The BioTuring Lens showed 10 highly expressed genes in each cluster (Table 2). CD74 was highly expressed in cluster 1 but not in clusters 2 or 3. We examined the expression of CD74 with immunohistochemistry in all 36 cases of follicular thyroid carcinoma.
- CD74 immunohistochemical analyses
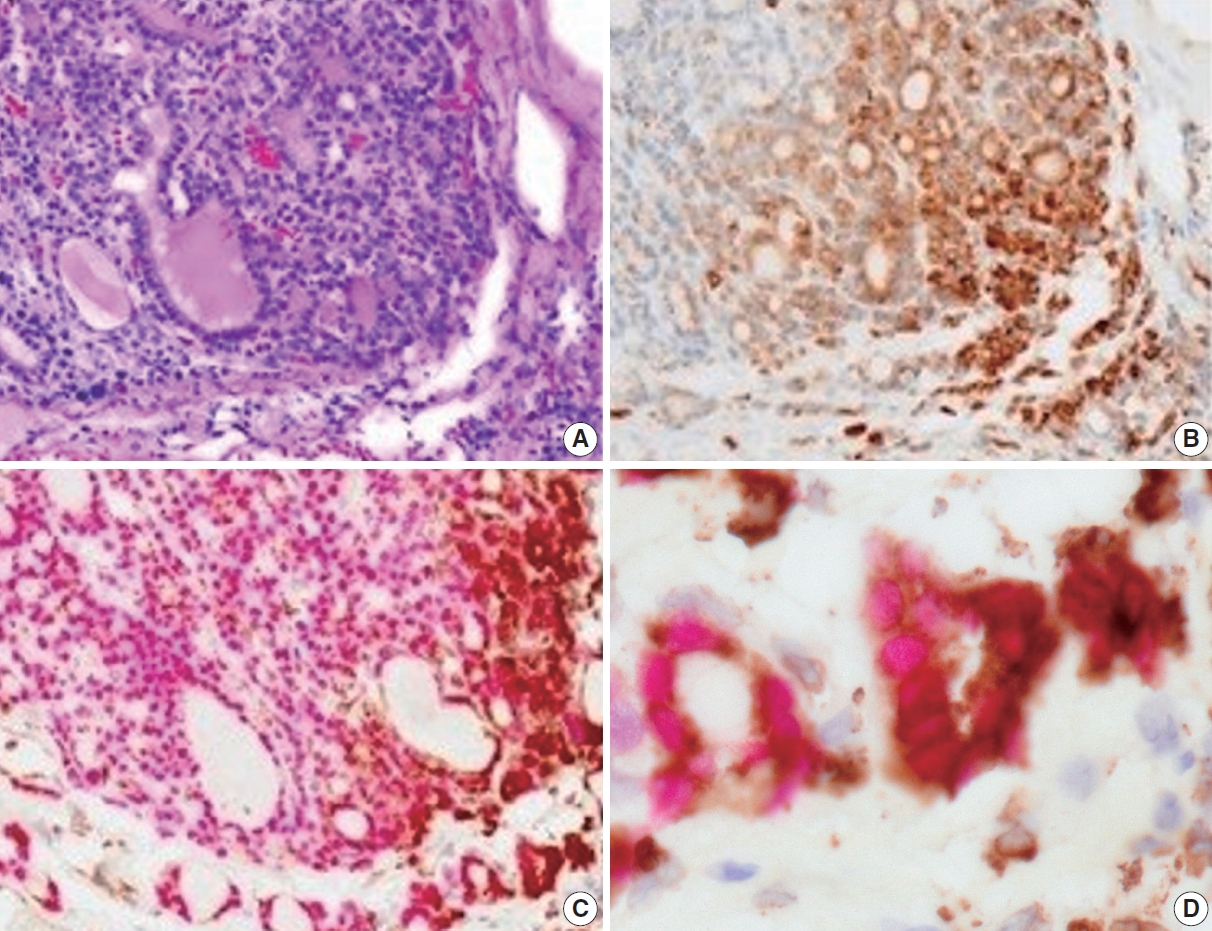
- Immunohistochemically, the CD74 staining intensity was heterogeneous. The intensity increased in the invasive and peripheral areas of the tumors (Fig. 2A, B). Since CD74 is expressed in macrophages, double staining for CD74 and TTF-1 was performed to confirm that CD74-positive cells were follicular thyroid carcinoma cells. CD74-positive cells in the invasive and peripheral areas were TTF-1 positive, indicating that CD74 positivity was detected in tumor cells located in the invasive and peripheral areas (Fig. 2C, D).
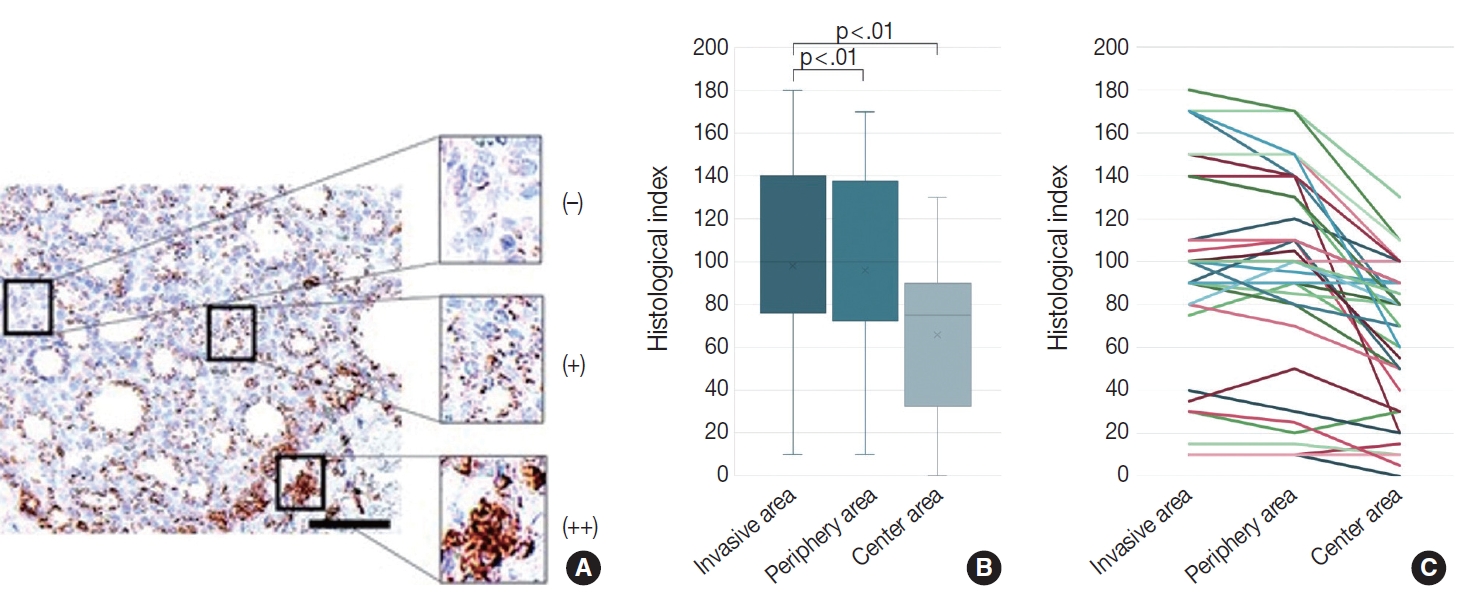
- We evaluated the CD74 staining intensity in follicular thyroid carcinoma cells using a histological index (Fig. 3A). The histological index was 98.1 ± 50.6 (mean ± standard deviation) in invasive areas, 96.0 ± 48.5 in peripheral areas, and 65.8 ± 36.2 in central areas (Fig. 3B, C). Significant differences were detected between the invasive and central areas and between the peripheral and central areas (p < .01).
RESULTS
- Follicular thyroid adenomas and carcinomas are collectively treated as follicular tumors. They are difficult to differentiate using fine-needle aspiration cytology [2], and the clinical management is not differentiated [3]. However, because follicular thyroid adenomas, which comprise the majority of follicular tumors, are benign, unnecessary resection is often performed in many cases. Several studies have proposed the use of various markers for distinguishing between follicular thyroid adenomas and carcinomas. Sato et al. [5] reported the utility of p53-binding protein 1 (53BP1) expression in nuclear foci, a marker reflecting the DNA damage response, to distinguish follicular thyroid carcinomas from adenomas. 53BP1 showed high sensitivity (89.3%) and specificity (83.3%), although the interpretation of results required advanced techniques. Suzuki et al. [6] attempted to differentiate between the two tumor types by determining the cell proliferation index reflecting increased nuclear DNA levels using a special flow cytometry device, LC-1000 (Sysmex Corporation, Tokyo, Japan). This method automatically calculates the index; however, a huge initial investment is required. An easier method to incorporate is immunohistochemistry, and antibodies such as ki-67, bax, secreted protein acidic and rich in cysteine, HBME1, Rac1, Galectin-3, and CD61 have been investigated, but none of them is currently in clinical use [7-12].
- RNA sequences of microdissected samples are useful for identifying molecular markers; however, they only provide information on the bulk expression of tumor cells in the microdissected area. The most precise investigation of RNA expression is a single-cell RNA sequence; however, this method loses the spatial information of each individual cell. Spatial transcriptome analysis is a new technique that enables whole-transcriptome analysis without microdissection of FFPE specimens while maintaining morphological information; it has been investigated in a variety of tissues [13,14]. In this study, we performed spatial transcriptome analysis using follicular thyroid carcinoma and identified a subpopulation in the invasive area that was found to express high levels of CD74.
- CD74 is known to play an important role in antigen presentation by mediating the construction of major histocompatibility complex class II complexes and intracellular trafficking [17]. CD74 has also been reported to be upregulated in malignant tumors and involved in increased growth and metastatic potential [18-28]. In a study on urothelial bladder carcinomas by Choi et al. [18], urothelial bladder carcinomas with high CD74 expression were characterized by older age, high World Health Organization grade, and advanced stages of TNM classification. In a study on gastrointestinal carcinomas, Gold et al. [26] found that the expression of CD74 in gastrointestinal carcinomas was significantly greater than that in their normal tissue counterparts (p < .001 or lower). With regard to thyroid carcinomas, Varinelli et al. [27] and Cheng et al. [28] reported that the CD74 axis plays an important role in the biology of aggressive cases in papillary and anaplastic thyroid carcinomas. In these two studies [27,28], CD74 staining was detected in all tumor cells of aggressive cases, and no heterogeneous staining was reported. To our knowledge, the present study is the first report of CD74 staining in follicular thyroid carcinomas and the heterogeneous staining intensity of CD74 was detected for the first time, suggesting the presence of an invasive subpopulation of follicular tumor cells. In a study by Cheng et al. [28], treatment with anti-CD74 antibody in papillary thyroid carcinoma cell lines inhibited cell growth, colony formation, cell migration and invasion, and vascular endothelial growth factor secretion. Our results suggested that CD74 might play a role in cell invasion and might be a novel therapeutic target for follicular thyroid carcinomas, such as papillary thyroid carcinoma.
- Our results demonstrated a significantly higher staining score in the invasive area than in the central area (p < .01), suggesting that the CD74-positive subpopulation may be used to predict the invasion of follicular tumors. Although there was no significant difference between the invasive and peripheral areas just below the capsule (p = .429), Gold et al. [26] stated that precursor lesions might express the same or higher CD74 levels as the respective cancers, as the activation of survival pathways was particularly important in the early stages of tumorigenesis. It was possible that the positive cells observed immediately below the capsule in our study were near invasion. Although this study was performed on resected material, we expect that immunocytochemical staining for CD74 will be performed in the future on nodules suspected of having follicular tumors on cytological examination or biopsy tissue specimens to determine the possibility of invasion before surgery, which will contribute to reducing unnecessary resection of nodules with a low possibility of invasion.
- In this study, we only included follicular thyroid carcinomas with a clear invasion. Future studies are required to examine CD74 immunostaining in patients with follicular tumors of uncertain malignant potential [1] with unclear histological status to identify whether they are follicular thyroid adenomas or carcinomas and prospectively observe their prognosis. The correlation between the staining results and prognosis provided a better confirmation of the relationship between CD74 immunostaining and invasion.
DISCUSSION
Ethics Statement
The study protocol was reviewed and approved by the Institutional Review Board of Kuma Hospital (no. 20230112-2, 12/01/2023) and Osaka University Clinical Research Review Committee (no. 22425, 17/02/2023). This study complied with the 1964 Declaration of Helsinki and its later amendments. All study participants provided informed consent.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: SN, EM, AS. Data curation: SN, EM. Formal analysis: SN, AS. Investigation: SN, AS, MK. Methodology: SN, ST, DM, DO, MK. Project administration: SN, EM. Resources: EM. Supervision: SN, EM. Writing—original draft: AS, EM. Writing—review & editing: SN, ST, DM, DO, MH, EM. Approval of the final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
This work was supported by JSPS KAKENHI under Grant Numbers A19H034520, T22K194330 and A23H027000, and by AMED under Grant Number JP21ae0121049.
Acknowledgments
| Minimally invasive | Encapsulated angioinvasive | Widely invasive | Total | |
|---|---|---|---|---|
| No. | 18 | 8 | 10 | 36 |
| Sex (male:female) | 3:15 | 4:4 | 2:8 | 9:27 |
| Age (yr), mean (range) | 49.1 (27–83) | 43.5 (11–68) | 47.3 (12–69) | 47.4 (11–83) |
- 1. World Health Organization. WHO classification of tumours online. Endocrine and neuroendocrine tumours. 5th ed. Thyroid tumours [Internet]. Geneva: World Health Organization, 2022 [cited 2023 Oct 25]. Available from: https://tumourclassification.iarc.who.int/chapters/53.
- 2. Auger M, Callegari F, Fadda G, Hirokawa M, Rooper L. Follicular neoplasm. In: Ali SZ, VanderLaan PA, eds. The Bethesda system for reporting thyroid cytopathology: definitions, criteria, and explanatory notes. In: Ali SZ, VanderLaan PA, eds. The Bethesda system for reporting thyroid cytopathology: definitions, criteria, and explanatory notes. 3rd ed. Cham: Springer, 2023; 81-95.
- 3. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016; 26: 1-133. PubMedPMC
- 4. Hirokawa M, Suzuki A, Kawakami M, Kudo T, Miyauchi A. Criteria for follow-up of thyroid nodules diagnosed as follicular neoplasm without molecular testing: the experience of a high-volume thyroid centre in Japan. Diagn Cytopathol 2022; 50: 223-9. ArticlePubMedPMCPDF
- 5. Sato A, Matsuda K, Motoyama T, et al. 53BP1 expression as a biomarker to differentiate thyroid follicular tumors. Endocr Connect 2021; 10: 309-15. ArticlePubMedPMC
- 6. Suzuki A, Hirokawa M, Furutate M, Hirai Y, Miyauchi A. LC-1000 Flow cytometry system improves risk stratification of thyroid nodules with suspected follicular neoplasm. JMA J 2022; 5: 124-6. ArticlePubMed
- 7. Kim HK, Lee DW, Jin SY, Kim DW. Ki-67 labelling index and bax expression according to the capsular invasion in the follicular neoplasms of the thyroid. Korean J Pathol 2001; 35: 531-5.
- 8. Kim CY, Cho SJ, Kim MK, Chae YS. SPARC expression in thyroid follicular adenomas and carcinomas. Korean J Pathol 2000; 34: 1016-21.
- 9. Pujani M, Arora B, Pujani M, Singh SK, Tejwani N. Role of Ki-67 as a proliferative marker in lesions of thyroid. Indian J Cancer 2010; 47: 304-7. ArticlePubMed
- 10. Mase T, Funahashi H, Koshikawa T, et al. HBME-1 immunostaining in thyroid tumors especially in follicular neoplasm. Endocr J 2003; 50: 173-7. ArticlePubMed
- 11. Agustina H, Ahyati R, Suryanti S, Hernowo BS. The potential diagnostic value of Rac1 immunohistochemistry in follicular thyroid carcinoma. Malays J Pathol 2022; 44: 225-33. PubMed
- 12. Cracolici V, Parilla M, Henriksen KJ, Cipriani NA. An evaluation of CD61 immunohistochemistry in identification of vascular invasion in follicular thyroid neoplasms. Head Neck Pathol 2020; 14: 399-405. ArticlePubMedPDF
- 13. Gracia Villacampa E, Larsson L, Mirzazadeh R, et al. Genome-wide spatial expression profiling in formalin-fixed tissues. Cell Genom 2021; 1: 100065.ArticlePubMedPMC
- 14. Watanabe R, Miura N, Kurata M, Kitazawa R, Kikugawa T, Saika T. Spatial gene expression analysis reveals characteristic gene expression patterns of de novo neuroendocrine prostate cancer coexisting with androgen receptor pathway prostate cancer. Int J Mol Sci 2023; 24: 8955.ArticlePubMedPMC
- 15. McInnes L, Healy J, Saul N, Grossberger L. UMAP: Uniform Manifold Approximation and Projection. J Open Source Softw 2018; 3: 861.ArticlePDF
- 16. Blondel VD, Guillaume JL, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J Stat Mech 2008; 10: P10008.Article
- 17. Schroder B. The multifaceted roles of the invariant chain CD74: more than just a chaperone. Biochim Biophys Acta 2016; 1863: 1269-81. ArticlePubMed
- 18. Choi JW, Kim Y, Lee JH, Kim YS. CD74 expression is increased in high-grade, invasive urothelial carcinoma of the bladder. Int J Urol 2013; 20: 251-5. ArticlePubMed
- 19. Hong WC, Lee DE, Kang HW, et al. CD74 promotes a pro-inflammatory tumor microenvironment by inducing S100A8 and S100A9 secretion in pancreatic cancer. Int J Mol Sci 2023; 24: 12993.ArticlePubMedPMC
- 20. Xu S, Li X, Tang L, Liu Z, Yang K, Cheng Q. CD74 correlated with malignancies and immune microenvironment in gliomas. Front Mol Biosci 2021; 8: 706949.ArticlePubMedPMC
- 21. Zeiner PS, Zinke J, Kowalewski DJ, et al. CD74 regulates complexity of tumor cell HLA class II peptidome in brain metastasis and is a positive prognostic marker for patient survival. Acta Neuropathol Commun 2018; 6: 18.ArticlePubMedPMCPDF
- 22. Gai JW, Wahafu W, Song L, et al. Expression of CD74 in bladder cancer and its suppression in association with cancer proliferation, invasion and angiogenesis in HT-1376 cells. Oncol Lett 2018; 15: 7631-8. ArticlePubMedPMC
- 23. Wang P, Shi Q, Zuo T, He X, Yu J, Wang W. Expression of cluster of differentiation 74 in gallbladder carcinoma and the correlation with epithelial growth factor receptor levels. Oncol Lett 2016; 11: 2061-6. ArticlePubMedPMC
- 24. Liu Z, Chu S, Yao S, et al. CD74 interacts with CD44 and enhances tumorigenesis and metastasis via RHOA-mediated cofilin phosphorylation in human breast cancer cells. Oncotarget 2016; 7: 68303-13. ArticlePubMedPMC
- 25. Zhang JT, Zhang J, Wang SR, et al. Spatial downregulation of CD74 signatures may drive invasive component development in part-solid lung adenocarcinoma. iScience 2023; 26: 107699.ArticlePubMedPMC
- 26. Gold DV, Stein R, Burton J, Goldenberg DM. Enhanced expression of CD74 in gastrointestinal cancers and benign tissues. Int J Clin Exp Pathol 2010; 4: 1-12. PubMedPMC
- 27. Varinelli L, Caccia D, Volpi CC, et al. 4-IPP, a selective MIF inhibitor, causes mitotic catastrophe in thyroid carcinomas. Endocr Relat Cancer 2015; 22: 759-75. ArticlePubMed
- 28. Cheng SP, Liu CL, Chen MJ, et al. CD74 expression and its therapeutic potential in thyroid carcinoma. Endocr Relat Cancer 2015; 22: 179-90. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Diagnosis of invasive encapsulated follicular variant papillary thyroid carcinoma by protein-based machine learning
Truong Phan-Xuan Nguyen, Minh-Khang Le, Sittiruk Roytrakul, Shanop Shuangshoti, Nakarin Kitkumthorn, Somboon Keelawat
Journal of Pathology and Translational Medicine.2025; 59(1): 39. CrossRef - Spatial Transcriptomics in Thyroid Cancer: Applications, Limitations, and Future Perspectives
Chaerim Song, Hye-Ji Park, Man S. Kim
Cells.2025; 14(12): 936. CrossRef - A New Tool to Decrease Interobserver Variability in Biomarker Annotation in Solid Tumor Tissue for Spatial Transcriptomic Analysis
Sravya Palavalasa, Emily Baker, Jack Freeman, Aditri Gokul, Weihua Zhou, Dafydd Thomas, Wajd N. Al-Holou, Meredith A. Morgan, Theodore S. Lawrence, Daniel R. Wahl
Current Issues in Molecular Biology.2025; 47(7): 531. CrossRef - Carbonic Anhydrase 12 as a Novel Prognostic Biomarker and Therapeutic Target for High‐Risk Follicular Thyroid Carcinoma
Masashi Tanida, Tsuyoshi Takashima, Shinichiro Tahara, Masaharu Kohara, Haruka Kanai, Masami Suzuki, Motoyuki Suzuki, Mitsuyoshi Hirokawa, Ayana Suzuki, Shinya Sato, Daisuke Okuzaki, Satoshi Nojima, Takahiro Matsui, Hidenori Inohara, Eiichi Morii
Cancer Science.2025;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
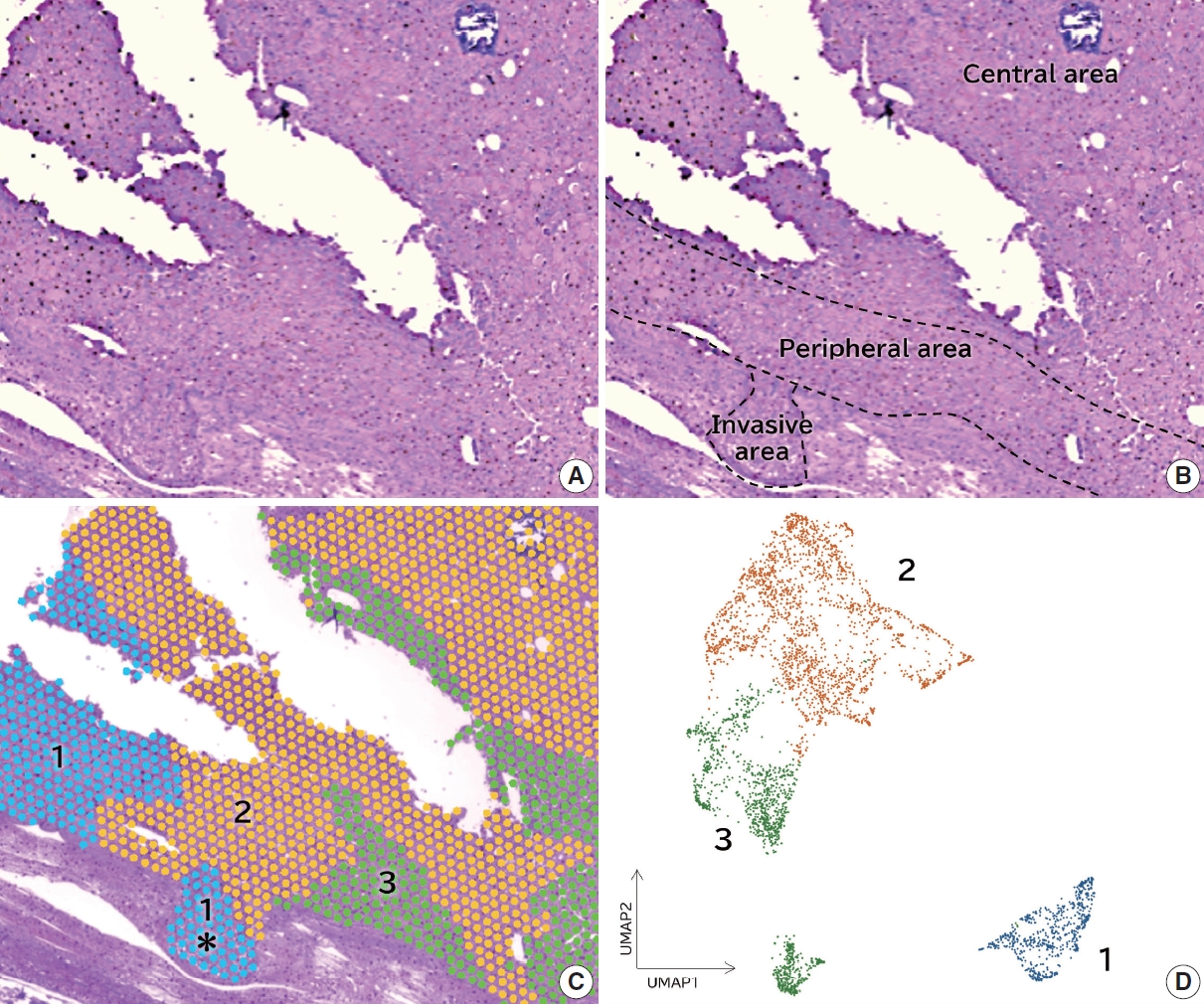
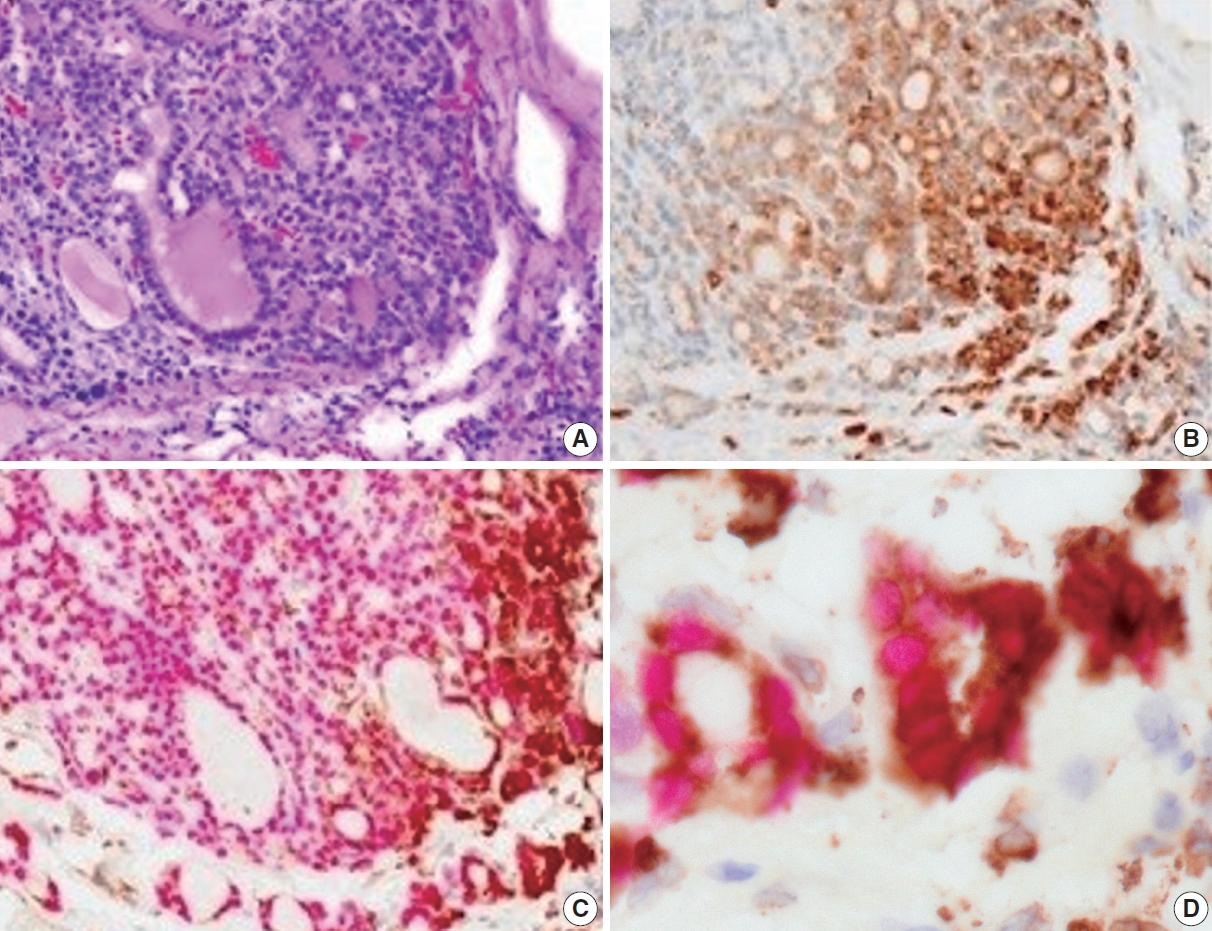
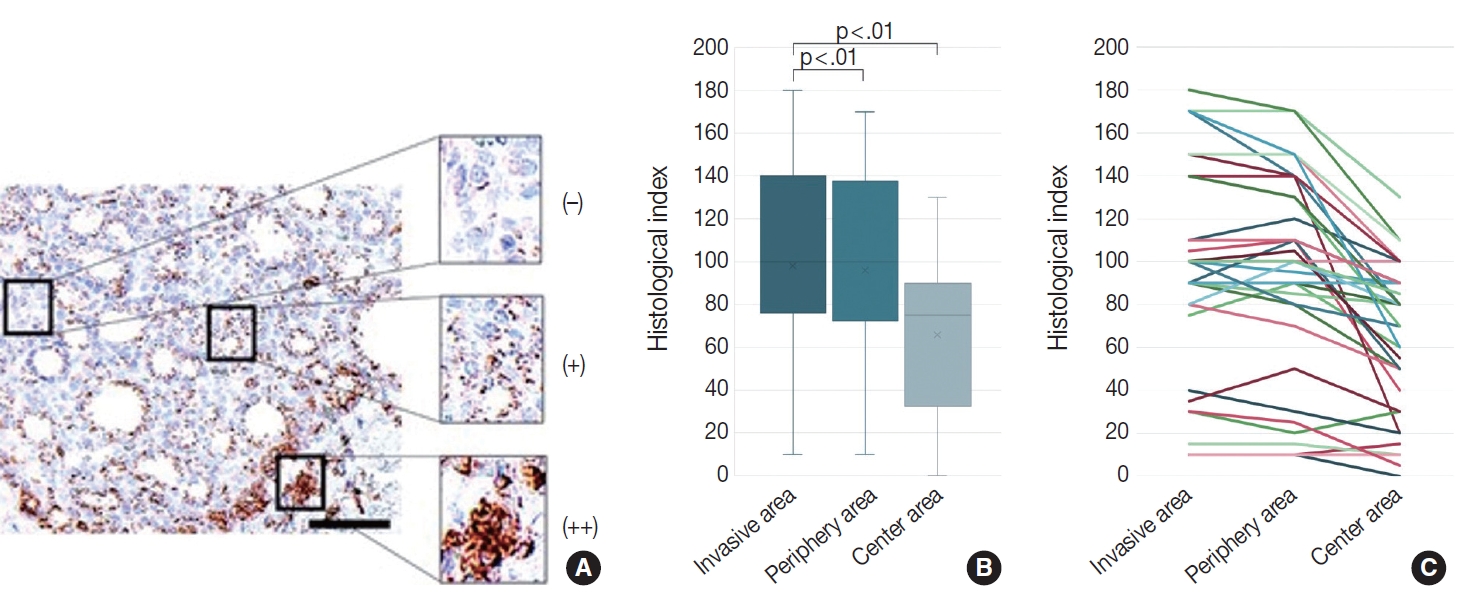
Fig. 1.
Fig. 2.
Fig. 3.
| Minimally invasive | Encapsulated angioinvasive | Widely invasive | Total | |
|---|---|---|---|---|
| No. | 18 | 8 | 10 | 36 |
| Sex (male:female) | 3:15 | 4:4 | 2:8 | 9:27 |
| Age (yr), mean (range) | 49.1 (27–83) | 43.5 (11–68) | 47.3 (12–69) | 47.4 (11–83) |
| Cluster 1 | Cluster 2 | Cluster 3 |
|---|---|---|
| CD74 | COL9A3 | COL9A3 |
| IGHG1 | SFRP1 | APLP2 |
| IGHG3 | APLP2 | MT1G |
| IGHM | CD24 | CD24 |
| B2M | GPX3 | SFRP1 |
| IGHA1 | MT1G | SLC26A7 |
| COL1A1 | PDCD4 | PDCD4 |
| COL3A1 | SLC26A7 | GPX3 |
| ACTB | IGFG1 | FCGBP |
| FOS | B2M | APP |

 E-submission
E-submission





