Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 57(4); 2023 > Article
-
Newsletter
What’s new in hematopathology 2023: updates on mature T-cell neoplasms in the 5th edition of the WHO classification -
Mario L. Marques-Piubelli, MD1
 , Roberto N. Miranda, MD2
, Roberto N. Miranda, MD2
-
Journal of Pathology and Translational Medicine 2023;57(4):246-249.
DOI: https://doi.org/10.4132/jptm.2023.06.15
Published online: June 27, 2023
1Department of Translational Molecular Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
2Department of Hematopathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
- Corresponding Author: Roberto N. Miranda, MD Department of Hematopathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA E-mail: roberto.miranda@mdanderson.org
- This article has been published jointly, with consent, in both Journal of Pathology and Translational Medicine and PathologyOutlines.com.
• Received: February 23, 2023 • Accepted: June 15, 2023
© 2023 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Abstract
- MATURE T-CELL AND NK-CELL LEUKEMIAS
- • Sezary syndrome
- PRIMARY CUTANEOUS T-CELL LYMPHOID PROLIFERATIONS AND LYMPHOMAS (CTCL)
- INTESTINAL T-CELL AND NK-CELL LYMPHOID PROLIFERATION AND LYMPHOMAS
- HEPATOSPLENIC T-CELL LYMPHOMA (HSTCL)
- ANAPLASTIC LARGE CELL LYMPHOMAS (ALCL)
- NODAL T-FOLLICULAR HELPER CELL LYMPHOMAS (NTFHL)
- OTHER PERIPHERAL T-CELL AND NK-CELL LYMPHOMAS
- EBV-POSITIVE NK/T-CELL LYMPHOMAS
- EBV-POSITIVE T- AND NK-CELL LYMPHOID PROLIFERATIONS AND LYMPHOMAS OF CHILDHOOD
- Meet the Authors
- REFERENCES
Abstract
- The overview of the upcoming Blue Book of the 5th edition of the World Health Organization Classification of Hematolymphoid Tumors was published in Leukemia in June 2022. The updates on mature T-/NK-cell lymphomas and leukemias are organized in nine groups based on cell of origin, morphology, clinical scenario, and localization, and are highlighted in this newsletter.
- • T-prolymphocytic leukemia (T-PLL)
- • T-large granular lymphocytic leukemia (T-LGLL)
- • Adult T-cell leukemia/lymphoma (ATLL)
- • Aggressive NK-cell leukemia (ANKL)
MATURE T-CELL AND NK-CELL LEUKEMIAS
- This group includes entities that mostly present in a leukemic phase. T-PLL requires monoclonal T-cell lymphocytosis (> 5 × 109/L), T-cell monoclonality and aberrations in TCL1A or MTCP1. T-LGLL with STAT3 mutations is associated with cytopenias, splenomegaly and autoimmune diseases. ATLL has shown new immune evasion mechanisms: CTLA4::CD28, ICOS::CD28, REL truncations, variations of CD274, and alterations in HLA-A and HLA-B. ANKL is associated with mutations in the JAK/STAT pathway, epigenetic modifiers and immune checkpoints. Although Sezary syndrome is much closer to mycosis fungoides, it is discussed here because of its leukemic presentation, mimicking the other leukemic processes.
• Sezary syndrome
- • Primary cutaneous CD4+ small or medium T-cell lymphoproliferative disorder
- • Primary cutaneous acral CD8+ lymphoproliferative disorder
- • Mycosis fungoides
- • Primary cutaneous CD30-positive T-cell lymphoproliferative disorder: lymphomatoid papulosis
- • Primary cutaneous CD30-positive T-cell lymphoproliferative disorder: primary cutaneous anaplastic large cell lymphoma
- • Subcutaneous panniculitis-like T-cell lymphoma
- • Primary cutaneous gamma/delta T-cell lymphoma
- • Primary cutaneous CD8-positive aggressive epidermotropic cytotoxic T-cell lymphoma
- • Primary cutaneous peripheral T-cell lymphoma (PTCL), NOS
- These are well-known disorders as noted in previous WHO editions, 9 in total.
- The most significant change is the removal of the umbrella term of PTCL from which 4 different entities have been derived, including PTCL, NOS to emphasize that the CTCL are still difficult to classify [1]. It is expected that the publication of the 5th edition will abound with underlying molecular mechanisms for each of the disorders in this group. The diagnostic criteria for each entity are well defined from previous editions. It is acknowledged that mycosis fungoides has a range of clinical and histologic subtypes, and it is of interest that its folliculotropic subtype has divergent outcomes when comparing early with advanced stage cases. Because of overlapping clinical, pathologic and immunophenotypic features of these 9 entities, clinical correlation is more valid than ever for achieving the most accurate diagnosis.
PRIMARY CUTANEOUS T-CELL LYMPHOID PROLIFERATIONS AND LYMPHOMAS (CTCL)
- • Indolent T-cell lymphoma of the gastrointestinal tract
- • Indolent NK-cell lymphoproliferative disorder (LPD) of the gastrointestinal tract
- • Enteropathy-associated T-cell lymphoma
- • Monomorphic epitheliotropic intestinal T-cell lymphoma
- • Intestinal T-cell lymphoma, NOS
- There may be an increased frequency of reported intestinal lymphoma, likely due to its recognition as a distinct group. The category of intestinal LPD and lymphomas includes 2 indolent disorders. The indolent T-cell lymphoma (Fig. 1) has been observed to have significant morbidity over time, including dissemination. Alterations in the JAK/STAT pathway and mutations of epigenetic modifiers are more common in CD4+, CD4+/CD8+, and CD4-/CD8- subsets. The indolent NK-cell LPD seems to behave more as an enteropathy, have no aggressive behavior and carry JAK3 mutations. Lesions are well circumscribed and small, but cells show moderate atypia, therefore NK-cell LPD can be confused with extranodal NK/T-cell lymphoma which is associated with EBV. The other entities in the group of intestinal lymphomas remain unchanged.
INTESTINAL T-CELL AND NK-CELL LYMPHOID PROLIFERATION AND LYMPHOMAS
- This rare lymphoma is difficult to diagnose due to its well-known protean presentation, histopathology (Fig. 2, 3), phenotype and clinical aggressiveness; it is also a diagnosis that may portend allogenic stem cell transplant as the only means of achieving cure. Not only young, but also adult patients can be affected. HSTCL usually presents at stage IV. The diagnosis can be missed in a bone marrow that mimics a myelodysplastic syndrome, and the spectrum of tumor cells may resemble blasts of acute leukemia. TCRγδ is expressed in ~75% of cases, TCRαβ is expressed in ~20% and TCR is silent in 5% of cases.
HEPATOSPLENIC T-CELL LYMPHOMA (HSTCL)
- • ALK+ ALCL
- • ALK- ALCL
- • Primary cutaneous CD30-positive T-cell lymphoproliferative disorder: primary cutaneous ALCL (see also CTCL)
- • Breast implant-associated ALCL These lymphomas have in common a pleomorphic cytomorphology, uniform and strong expression of CD30 and conspicuous absence of T-cell lineage markers. Four entities are recognized, 2 systemic and 2 site-specific forms. The systemic entities are divided by the presence of ALK gene rearrangement, as ALK+ and ALK- ALCL. Patients with ALK+ ALCL are usually young, while ALK- ALCL (Fig. 4) patients are adults and elders. Patients with ALK+ have better outcomes than patients with ALKALCL. ALK- ALCL can have DUSP22 or TP63 rearrangements, with the latter conveying the worst outcomes. Of interest, cases with DUSP22 rearrangements show a uniform cytomorphology, with doughnut-like cells and LEF1 expression.
- The site-specific forms of ALCL include the primary cutaneous ALCL that is grouped together with primary cutaneous T-cell LPD and lymphomas, and breast implant-associated ALCL (BIAALCL). BIA-ALCL (Fig. 5–7) is associated with textured implants and has an excellent outcome if surgically excised with negative margins. Patients with non-resectable disease require chemo- or immunotherapy.
ANAPLASTIC LARGE CELL LYMPHOMAS (ALCL)
- • Nodal T-follicular helper cell lymphoma angioimmunoblastic-type (nTFHLAI): formerly angioimmunoblastic T-cell lymphoma
- • Nodal T-follicular helper cell lymphoma follicular-type (nTFHL-F): formerly follicular T-cell lymphoma
- • Nodal T-follicular helper cell lymphoma, NOS (nTFHL-NOS); formerly PTCL with TFH phenotype that does not meet criteria for nTFHL-AI or nTFHL-F
- This group includes 3 nodal TCL with T-follicular helper phenotype (≥ 2 markers are required for diagnosis: PD1, ICOS, CXCL13, CD10, BCL6, CXCR5, SAP, c-MAF and CD200), derived from gene expression signature of CD4+ lymphocytes. There is morphologic overlap between these entities, and the prototype is nTFHL-AI (Fig. 8, 9). While the 3 entities have mutations of RHOA and IDH2, nTFHL-AI has also recurrent mutations of TET2 and DNMT3A in hematopoietic precursors. The diagnosis of nTFHL is recommended for small samples and to avoid misclassification.
NODAL T-FOLLICULAR HELPER CELL LYMPHOMAS (NTFHL)
- • PTCL, NOS
- This entity remains as a heterogeneous group of neoplasms and the diagnosis is performed based on the exclusion of other described entities. Two distinct biological groups can be identified using T-cell markers by immunohistochemistry: PTCL-TBX21 (PTCL-TH1) and PTCL-GATA3 (PTCL-TH2). The PTCL-TH1 group is usually associated with a cytotoxic phenotype, while the PTCL-TH2 group is a more heterogeneous group and is associated with poorer outcomes. Although these important biological mechanisms have been described, the experts concluded that there is still insufficient data to perform systematic classification in subtypes [1].
OTHER PERIPHERAL T-CELL AND NK-CELL LYMPHOMAS
- • EBV-positive nodal T- and NK-cell lymphoma (EBV+ NTNKL)
- • Extranodal NK-T-cell lymphoma (ENKTL)
- This is a group of mature lymphomas with NK/T phenotype that are associated with EBV infection. The EBV+ NTNKL is a distinct entity that was under the PTCL, NOS umbrella in the previous WHO classification. The disease is more common in East Asians and presents with extensive lymphadenopathy and sometimes extranodal involvement. Angioinvasion and coagulative necrosis are usually absent and there is no clear cut-off for EBV positivity. The ENKTL is an updated denomination where the qualifier “nasal-type” is dropped, following primary tumors found in diverse extranodal sites. Importantly, the use of L-asparaginase-based regimens has resulted in improved outcomes in affected patients.
EBV-POSITIVE NK/T-CELL LYMPHOMAS
- • Chronic active EBV disease (CAEBVD)
- - Severe mosquito bite allergy (SMBA)
- - Hydroa vacciniforme lymphoproliferative disorder (HVLPD) classic form
- - Hydroa vacciniforme lymphoproliferative disorder (HVLPD) systemic form
- - Systemic CAEBVD
- • Systemic EBV-positive T-cell lymphoma of childhood
- EBV+ NK/T-cell LPD and lymphomas of childhood comprise an uncommon group of disorders that mostly affect children of Asian and native American ethnic ancestry. CAEBVD has a broad clinical spectrum, which ranges from localized, mostly cutaneous forms to systemic disease with fever, hepatosplenomegaly and lymphadenopathy. The systemic form of HVLPD should be distinguished from systemic CAEBVD, which has a more aggressive behavior.
EBV-POSITIVE T- AND NK-CELL LYMPHOID PROLIFERATIONS AND LYMPHOMAS OF CHILDHOOD
- Dr. Marques-Piubelli has been part of the PathologyOutlines.com hematopathology editorial board since 2021. He is an Assistant Professor in the Department of Translational Molecular Pathology, at The University of Texas MD Anderson Cancer Center.
- Dr. Miranda has been part of the PathologyOutlines.com hematopathology editorial board since 2021. He is a Professor in the Department of Hematopathology, The University of Texas MD Anderson Cancer Center.
Meet the Authors
Fig. 1.Expansion of the lamina propria by small-sized and mature-appearing lymphocytes in indolent T-cell lymphoma of the gastrointestinal tract.
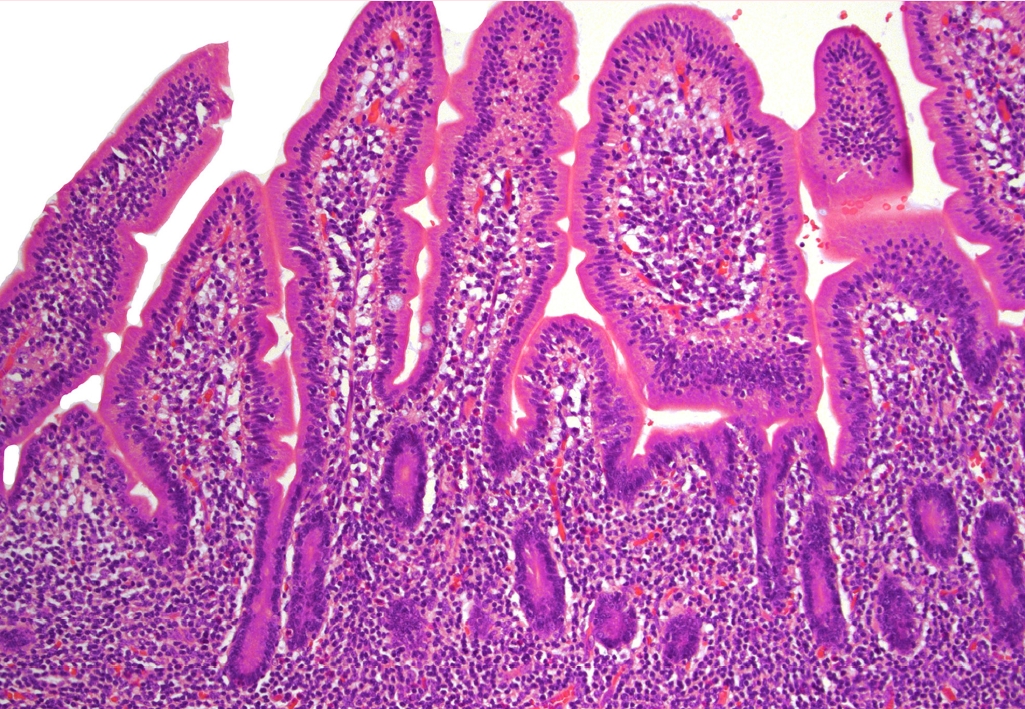

Fig. 2.Bone marrow core biopsy involved with HSTCL shows hypercellularity displaying small and hypolobated megakaryocytes, morphologically consistent with dysmegakaryopoiesis, raising the possibility of underlying myelodysplastic syndrome.
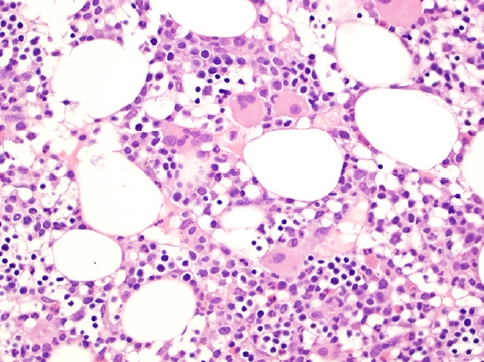

Fig. 3.CD3 immunostaining shows that the lymphocytes have a sinusoidal pattern characterized by clusters of lymphocytes in a cord-like pattern in HSTCL.
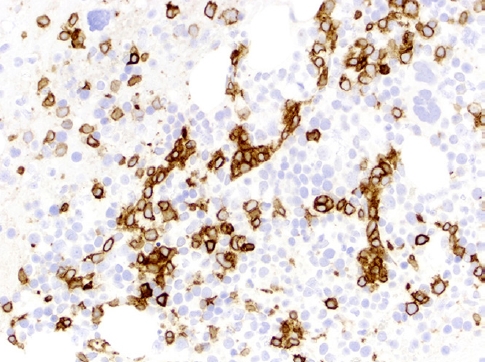

Fig. 4.Lymph node involved by ALK- ALCL shows diffuse replacement of normal architecture by large and anaplastic lymphocytes with no distinct nucleoli; some of them have a doughnut-like shape.
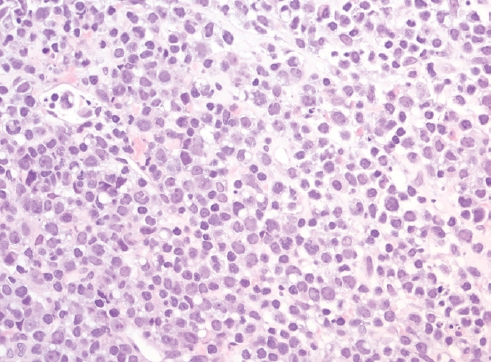

Fig. 5.Cytologic preparation of an effusion around a breast implant in a case of BIA-ALCL shows large and atypical cells with irregular nuclear contours, inconspicuous nuclei and abundant cytoplasm with small inclusions.
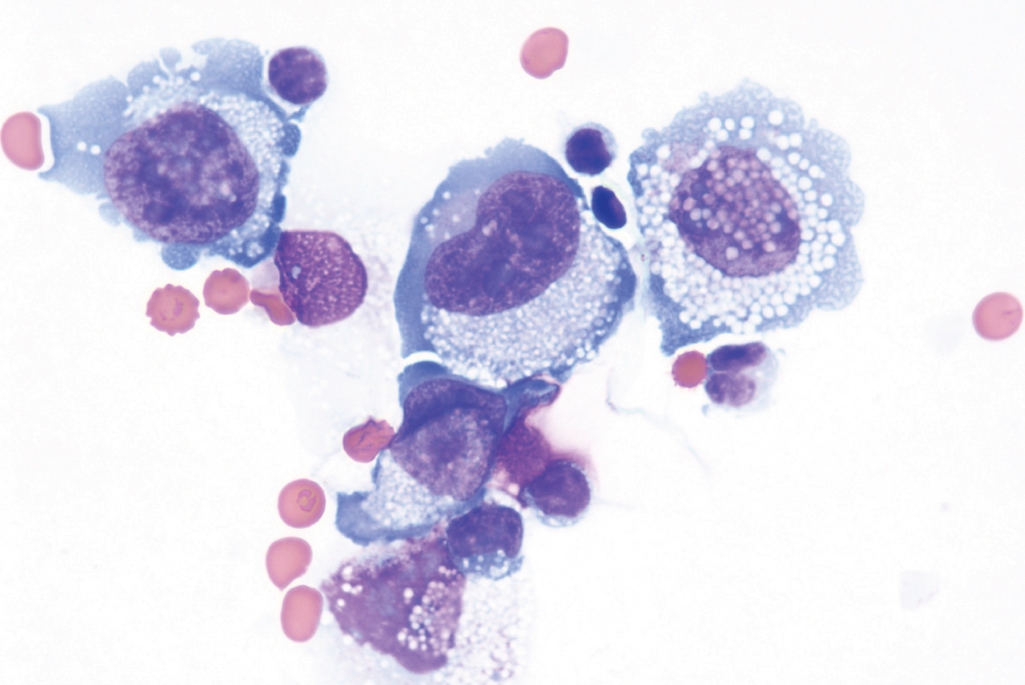

Fig. 6.Breast capsule with BIA-ALCL shows large and neoplastic cells confined to the luminal space in a necrotic background.
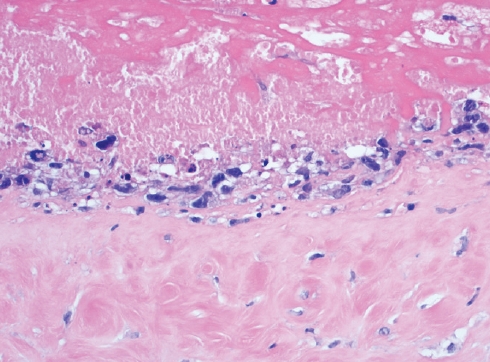

Fig. 7.CD30 immunostaining shows diffuse and strong cytoplasmic and nuclear positivity in the BIAALCL cells.


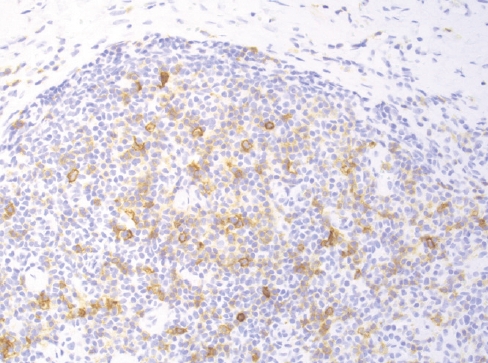
Fig. 8.nTFHL-AI with effacement of the nodal architecture and infiltration of the capsule and subcapsular sinus.
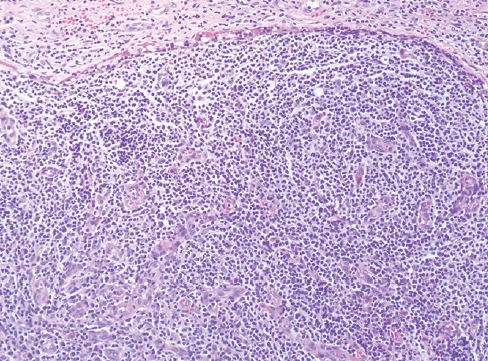

Figure & Data
References
Citations
Citations to this article as recorded by 

- Primary intestinal T-cell lymphoma discovered upon surgical resection: report of a monocentric Moroccan case series with diagnostic challenges
Sabrine Derqaoui, Siham Mesmoudi, Taha Yassine Aaboudech, Soukaina Haidouri, Yahya Khedid, Hamza Sekkat, Ahmad Jahid, Kaoutar Znati, Zoubida Tazi Mezalek, Zakia Bernoussi, Gilbert Sterling Octavius
Research Connections.2025;[Epub] CrossRef - The Significance of the Microenvironment in T/Nk-Cell Neoplasms
Ivan Petković, Michele Ritucci, Ana Stojković, Slavica Stojnev, Aleksandar Popović, Irena Conić, Milica Radić, Miljana Džunić, Miljan Krstić
International Journal of Molecular Sciences.2025; 26(22): 11225. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
- Related articles
-
- What’s new in hematopathology 2025: myeloid neoplasms in the WHO 5th edition and ICC
- What’s new in medical renal pathology 2025: Updates on podocytopathy and immunofluorescence staining in medical kidney
- What’s new in neuropathology 2024: CNS WHO 5th edition updates
- What’s new in thyroid pathology 2024: updates from the new WHO classification and Bethesda system
- What’s new in dermatopathology 2023: WHO 5th edition updates
What’s new in hematopathology 2023: updates on mature T-cell neoplasms in the 5th edition of the WHO classification
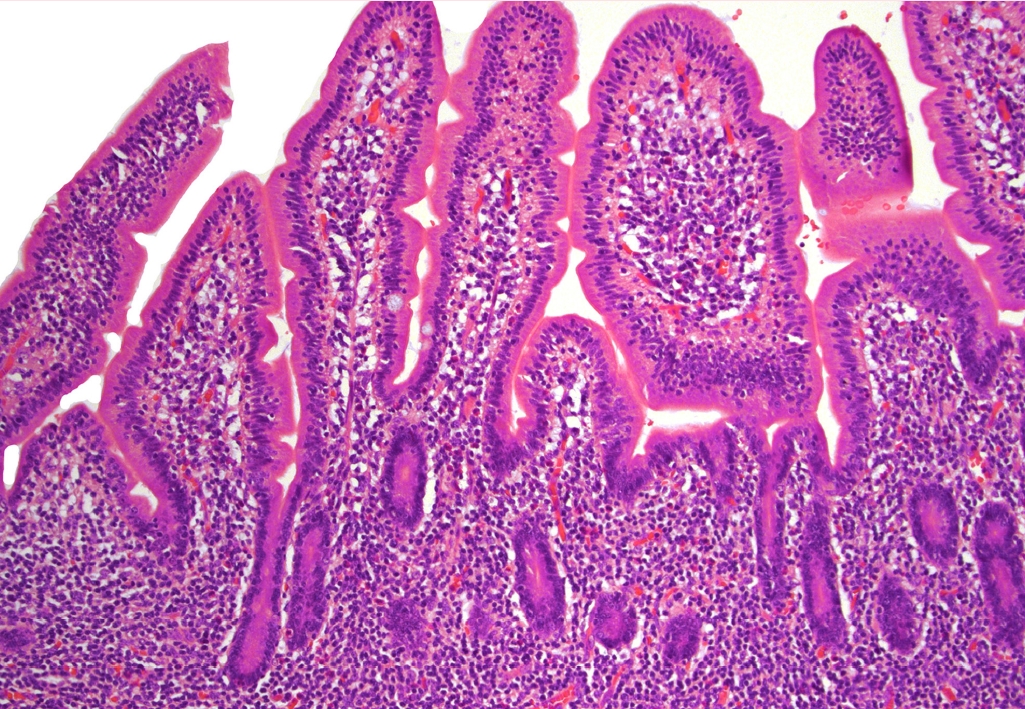
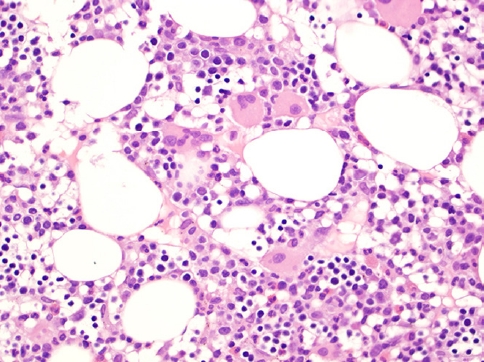
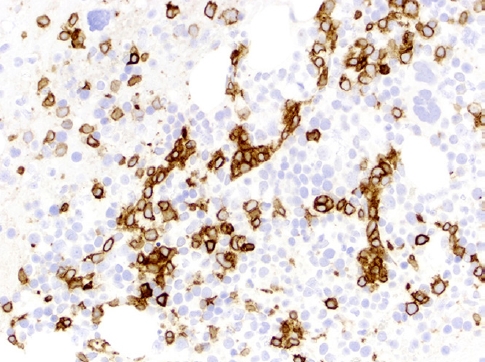
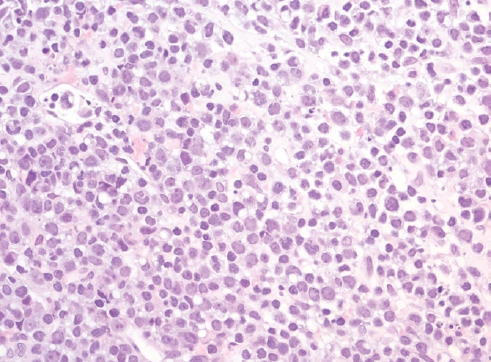
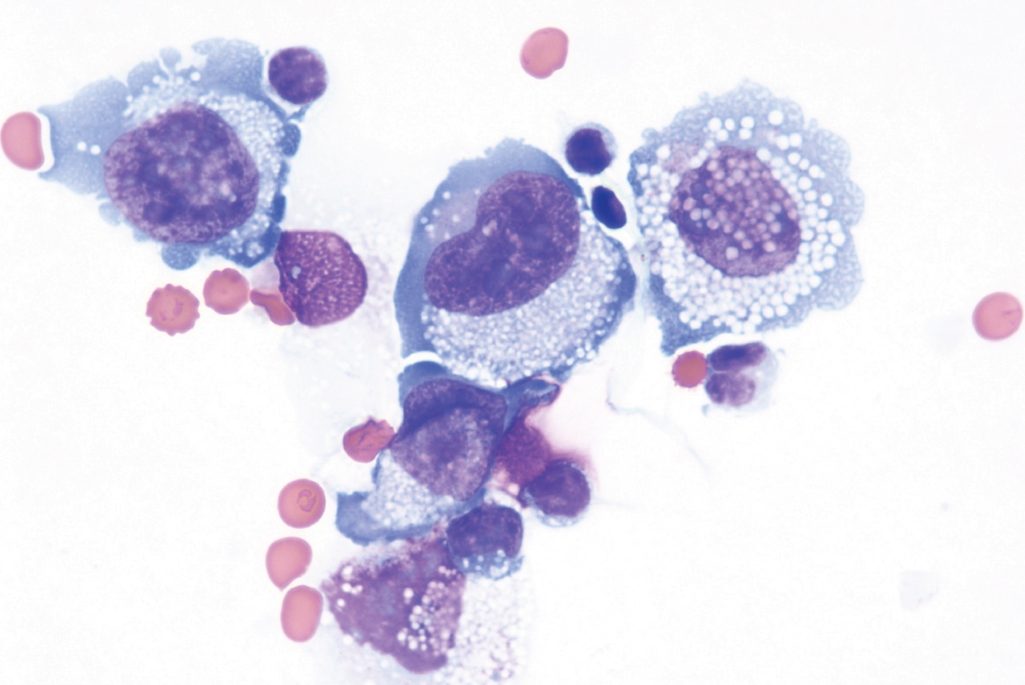
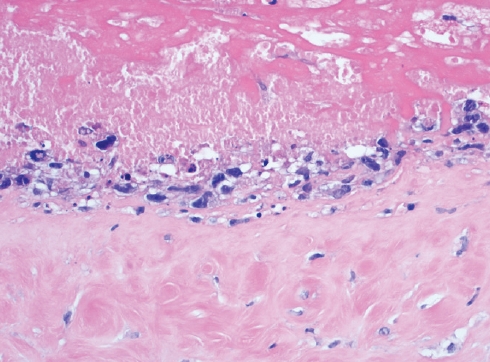

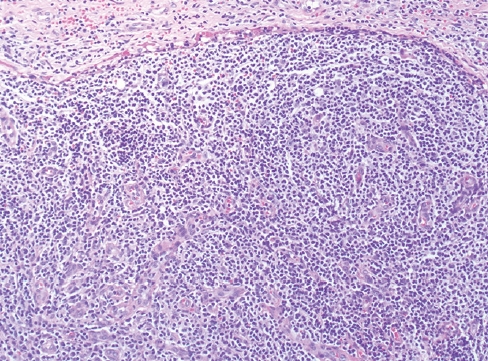
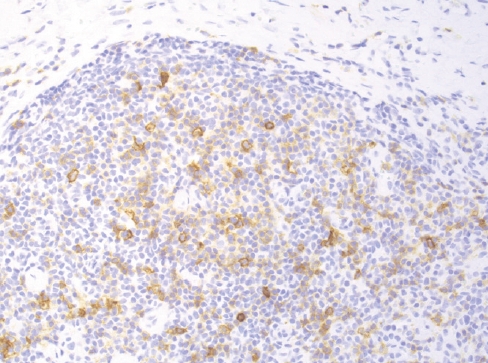
Fig. 1. Expansion of the lamina propria by small-sized and mature-appearing lymphocytes in indolent T-cell lymphoma of the gastrointestinal tract.
Fig. 2. Bone marrow core biopsy involved with HSTCL shows hypercellularity displaying small and hypolobated megakaryocytes, morphologically consistent with dysmegakaryopoiesis, raising the possibility of underlying myelodysplastic syndrome.
Fig. 3. CD3 immunostaining shows that the lymphocytes have a sinusoidal pattern characterized by clusters of lymphocytes in a cord-like pattern in HSTCL.
Fig. 4. Lymph node involved by ALK- ALCL shows diffuse replacement of normal architecture by large and anaplastic lymphocytes with no distinct nucleoli; some of them have a doughnut-like shape.
Fig. 5. Cytologic preparation of an effusion around a breast implant in a case of BIA-ALCL shows large and atypical cells with irregular nuclear contours, inconspicuous nuclei and abundant cytoplasm with small inclusions.
Fig. 6. Breast capsule with BIA-ALCL shows large and neoplastic cells confined to the luminal space in a necrotic background.
Fig. 7. CD30 immunostaining shows diffuse and strong cytoplasmic and nuclear positivity in the BIAALCL cells.
Fig. 8. nTFHL-AI with effacement of the nodal architecture and infiltration of the capsule and subcapsular sinus.
Fig. 9. Positivity for the checkpoint molecule PD-1 in scattered lymphoma cells supports a T follicular helper phenotype in a case of nTFHL-AI.
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
Fig. 8.
Fig. 9.
What’s new in hematopathology 2023: updates on mature T-cell neoplasms in the 5th edition of the WHO classification

 E-submission
E-submission