Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 56(5); 2022 > Article
-
Original Article
Evaluation of the characteristics of multiple human papillomavirus (HPV) infections identified using the BD Onclarity HPV assay and comparison with those of single HPV infection -
Jinhee Kim
 , Moonsik Kim
, Moonsik Kim , Ji Young Park
, Ji Young Park
-
Journal of Pathology and Translational Medicine 2022;56(5):289-293.
DOI: https://doi.org/10.4132/jptm.2022.08.02
Published online: September 13, 2022
Department of Pathology, School of Medicine, Kyungpook National University, Kyungpook National University Chilgok Hospital, Daegu, Korea
- Corresponding Author: Ji Young Park, MD, PhD, Department of Pathology, School of Medicine, Kyungpook National University, Kyungpook National University Chilgok Hospital, 807 Hoguk-ro, Buk-gu, Daegu 41404, Korea, Tel: +82-53-200-3391, Fax: +82-53-200-3399, E-mail: jyparkmd@knu.ac.kr
© 2022 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Human papillomavirus (HPV) infection is a major cause of cervical cancer and associated precursor lesions. Multiple HPV genotype infections have been reported. However, their clinicopathological characteristics still remain elusive.
-
Methods
- For this study, 814 consecutive patients who had undergone colposcopy and HPV genotyping test using BD Onclarity HPV assay were retrospectively selected. Clinicopathological parameters of multiple HPV infections were compared with those of single HPV infection.
-
Results
- Multiple HPV infections were found in 110 out of 814 cases (13.5%). Multiple HPV infections were associated with a significantly higher incidence of high-grade intraepithelial lesions (HSILs) compared with single HPV infection. Other high-risk HPV genotypes, in addition to HPV 16, were found more frequently in the multiple HPV infections group; these included HPV 51, 52, 33/58, 56/59/66, and 35/39/68. No specific coinfection pattern was not identified. Additionally, the number of HPV genotypes in multiple HPV infections was not associated with the progression to HSIL or squamous cell carcinoma.
-
Conclusions
- Multiple HPV infections have distinct clinicopathological characteristics (compared with single HPV infection). As their biological behavior is uncertain, close and frequent follow-up is warranted.
- Study population
- We retrospectively selected 814 consecutive patients who had undergone colonoscopy as well as HPV genotyping tests in the department of pathology, Kyungpook National University Chilgok Hospital in 2019. These patients underwent HPV genotyping assays owing to abnormal cytology or biopsy results, or during routine health check-ups. The clinicopathological data, including age and biopsy results, of the patients were retrieved from the electronic medical records of the hospital.
- Histological evaluation
- Specimens obtained during colposcopy were fixed in 10% neutral-buffered formalin and embedded in paraffin blocks. Paraffin blocks were cut into 4 μm sections and stained with hematoxylin and eosin. Two independent pathologists with specialization in gynecological pathology (M.K. and J.Y.P.) reviewed all the samples. All patients were diagnosed and classified in accordance to the recommendations of the LAST project working group [19].
- HPV genotyping test
- Onclarity testing uses the automated BD Viper Lt platform (BD Diagnostics, Sparks, MD, USA), the full workflow for which has been described in detail in a previous paper [20]. Briefly, 0.5 mL of original resuspended SurePath material was aliquoted into a BD tube containing 1.7 mL of sample medium. The samples were prewarmed for 30 minutes at 120°C. They were then transferred to the fully automated BD Viper Lt platform and tested using Onclarity according to the manufacturer’s instructions.
- Statistical analysis
- All statistical analyses were performed using IBM SPSS for Windows ver. 23.0 (IBM Corp., Armonk, NY, USA). The association between clinicopathological parameters was analyzed using the chi-square test or Fisher exact test. p-values < .05 were considered significant. Adjusted p-values using Bonferroni correction were used for multiple comparisons.
MATERIALS AND METHODS
- HPV genotype prevalence in multiple and single HPV infections
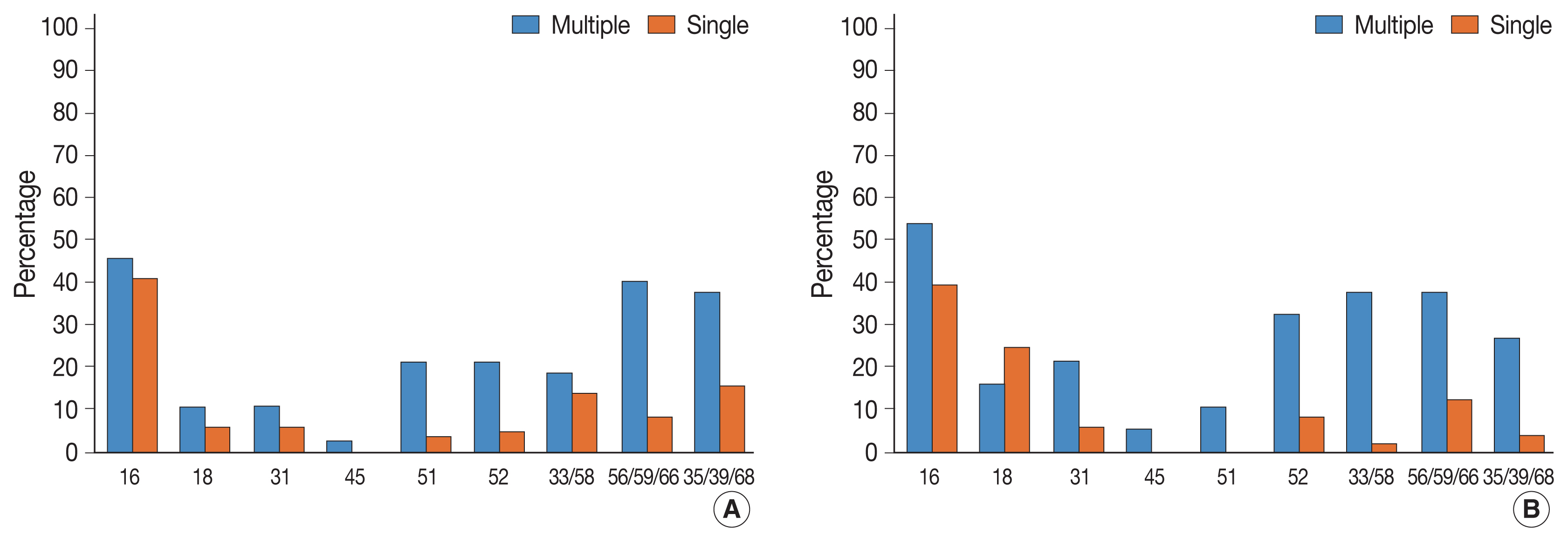
- Table 1 presents HPV genotype prevalence in multiple and single HPV infections, respectively. Detailed clinicopathological characteristics of the patient cohorts are listed in Supplementary Table S1. Among 814 cases wherein HPV genotyping tests were performed, multiple HPV infection was found in 110 cases (13.5%, 110/814) and single HPV infections were found in 361 cases (44.3%, 361/814). The mean age of the patients was 58.2 years in the multiple HPV infections group and 55.5 years in the single HPV infection group. In the multiple HPV infections group, HPV 35/39/68 was most frequently detected, followed by 56/59/66, 16, and 52. In the single HPV infection group, HPV 16 was most frequently detected, followed by 56/59/66, 35/39/68, and 52. HPV 31 (p = .005), 51 (p < .001), 52 (p < .001), 33/58 (p < .001), 56/59/66 (p < .001), and 35/39/68 (p < .001) were significantly more frequently detected in the multiple HPV infections group. Multiple infections among grouped HPV strains, for example, HPV 33 and 58, can possibly happen. However, such possible cases were counted as single HPV infection, based on the previous study [17]. The result of full HPV genotyping assay in a different cohort using Seegene Anyplex II also suggested that multiple infections within three bulk groups (33/35, 56/59/66, and 35/39/68) were not frequent (Supplementary Table S2).
- Association of multiple HPV infection with histology
- We then investigated the association between HPV infection status and histological diagnosis of cervical lesions. Of note, multiple HPV infections showed more significant association with high-grade squamous intraepithelial lesions (HSILs) compared with single HPV infection (p = .033) (Table 2). Low-grade squamous intraepithelial lesions (LSILs), squamous cell carcinoma (SQCC), and adenocarcinoma (ADC) were also more frequent in the multiple HPV infections group, albeit this was not significant. In the multiple HPV infections group, HPV 16 was the most frequently detected HPV genotype in LSILs, HSILs, SQCC, and ADC (Table 3). In the single HPV infection group, HPV 16 was the most frequently detected HPV genotype in HSILs and SQCC. In addition to HPV 16, other high-risk HPV genotypes were also detected more frequently in the patients with ≥ HSIL lesions (HSIL and SQCC), which suggests clinical significance. Among these, HPV 51, 52, 33/58, 56/59/66, and 35/39/68 were more frequently found in the multiple HPV infections group than in the single HPV infection group (Table 4, Fig. 1). In the multiple HPV group, there was no correlation between the number of infected HPV strains and the progression to cervical cancer and their precursor lesions (Supplementary Table S3). Although HPV 16 was the most frequently detected HPV genotype in multiple HPV infections, the presence of HPV 16 genotype in multiple HPV infections was not associated with the progression to cervical neoplastic lesions (compared with non-HPV 16 type of multiple HPV infections) (Supplementary Table S4). We further divided the study cases into two groups according to the HPV 16 or/and HPV 18 infection status (group 1: HPV 16 or/and HPV18 infection vs. group 2: non–HPV 16 and non–HPV 18 high-risk HPV). In both groups, there were no statistically significant correlation between multiple HPV infection and cervical lesions. However, HSIL tended to occur more frequently in group 2 (Supplementary Tables S5, S6).
RESULTS
- In this study, we demonstrated the clinicopathological implications of multiple HPV infections in Korean patients, using the BD Onclarity HPV assay. Multiple HPV infections were more closely related to HSILs than single HPV infection. In addition, we report that high-risk HPV genotypes other than HPV 16 were detected more frequently in the multiple HPV infections group. In our previous study, using Seegene Anyplex II HPV28 detection kit, we showed that multiple HPV infections were related to HSIL and persistent HPV infection [21]. However, Seegene Anyplex II and BD Onclarity assays have different ranges and use different probes for the detection of HPV [22]. The two HPV genotyping assays, thus, might have different performance capacity for the detection of multiple HPV infections. Therefore, we re-investigated the clinicopathological aspects of multiple HPV infections using the BD Onclarity HPV assay.
- The clinicopathological significance of multiple HPV infections still remains debatable. Overall, HPV 31, 51, 52, 33/58, 56/59/66, and 35/39/68 genotypes were more frequently detected in multiple HPV infections than in single HPV infection. The incidence of HPV genotypes in multiple HPV infections greatly differs across studies, depending on regional variation, ethnicity, and participant characteristics [12,14,23].
- In this study, multiple HPV infection was significantly associated with HSIL, which was consistent with the results from our previous study. Oncogenic risk of multiple HPV infections is still unclear. While some previous studies have shown that multiple HPV infections contribute to cervical carcinogenesis, other studies have shown that multiple HPV infections do not confer additional carcinogenic effect (compared to single HPV infection) [10,16, 24,25]. Thus, a larger cohort study may be instrumental in validating the oncogenic potential of multiple HPV infection.
- In multiple HPV infection, HPV 16 was the most prevalent HPV genotype in SIL and SQCC. However, other high-risk HPV genotypes were also more frequently detected in multiple HPV infections than in single HPV infection. In particular, HPV 51, 52, 33/58, 56/59/66, and 35/39/68 showed significant association with ≥HSILs in multiple HPV infections. There was no difference between the HPV 16–positive multiple HPV infections group and the HPV 16–negative multiple HPV infections group. We also divided study cases into HPV 16 or/and HPV18 infection group and non–HPV 16 and non–HPV 18 high-risk group, and further investigated the association between the multiple infection and the histology of cervical lesions in both groups; however, significant associations were not found. In non–HPV 16 and non–HPV 18 high-risk HPV group, HSIL tended to occur more frequently among multiple HPV infections. Thus, coinfection with non–HPV 16 and non–HPV 18 high-risk HPV genotypes might have synergistic effect on cervical carcinogenesis. Subsequent larger cohort study or expanded full genotyping assay should be followed to validate this hypothesis.
- This study has a few limitations. First, this study has a relatively small sample size. Subsequent studies with larger cohort would be valuable to further strengthen the major findings of this study. In addition, we were unable to evaluate the effect of multiple HPV genotypes on the duration of HPV infection, as we could not perform the follow-up HPV genotyping test using BD Onclarity HPV assay.
- In conclusion, multiple HPV infections have distinct clinicopathological characteristics. As their clinicopathological characteristics are still uncertain, close follow-up is warranted for the patients with multiple HPV infections.
DISCUSSION
Supplementary Information
Ethics Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional review board of Kyungpook National University Chilgok Hospital (KNUCH 2019-04-002-002). The requirement for written informed consent from the patients was waived due to the retrospective nature of the study.
Availability of Data and Material
All data generated or analyzed during the study are included in this published article (and its supplementary information files).
Code Availability
Not applicable.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Author Contributions
Conceptualization: JYP. Data curation: JK, MK. Formal analysis: JK, MK. Funding acquisition: JYP. Investigation: JK. Methodology: JK, MK. Supervision: JYP. Writing—original draft: JK. Writing—review & editing: MK, JYP. Approval of final manuscript: all authors.
Funding Statement
This research was supported (in part) by The Korean Society for Cytopathology Grant No. 2019-01
Acknowledgments
| Variable | No. (%) | HPV infection status (n = 471) | p-value | |
|---|---|---|---|---|
|
|
||||
| Multiple (n = 110) | Single (n = 361) | |||
| Age (yr) | 58.2 ± 12.6 | 55.5 ± 11.9 | .043 | |
| HPV type | ||||
| 16 | 125 (26.5) | 39 (35.5) | 86 (23.8) | .019 |
| 18 | 36 (7.6) | 11 (10.0) | 25 (6.9) | .107 |
| 31 | 34 (7.2) | 15 (13.6) | 19 (5.3) | .005a |
| 45 | 7 (1.5) | 5 (4.5) | 2 (5.5) | .091 |
| 51 | 33 (7.0) | 19 (17.3) | 14 (3.9) | < .001a |
| 52 | 85 (18.0) | 36 (32.7) | 49 (13.6) | < .001a |
| 33/58 | 64 (13.6) | 29 (26.4) | 35 (9.7) | < .001a |
| 56/59/66 | 113 (24.0) | 46 (41.8) | 67 (18.6) | < .001a |
| 35/39/68 | 111 (23.6) | 47 (42.7) | 64 (17.7) | < .001a |
| HPV type | No. (%) | HPV infection status (n = 199) | p-value | |
|---|---|---|---|---|
|
|
||||
| Multiple ( n = 60) | Single (n = 139) | |||
| 16 | 86 (43.2) | 30 (50.0) | 56 (40.3) | .216 |
| 18 | 30 (15.1) | 8 (13.1) | 22 (15.8) | .676 |
| 31 | 16 (8.0) | 8 (13.1) | 8 (5.8) | .089 |
| 45 | 2 (1.0) | 2 (3.3) | 0 | .091 |
| 51 | 13 (6.5) | 10 (16.7) | 3 (2.2) | < .001a |
| 52 | 25 (12.6) | 17 (28.3) | 8 (5.8) | < .001a |
| 33/58 | 28 (14.1) | 15 (25.0) | 13 (9.4) | .005a |
| 56/59/66 | 36 (18.1) | 23 (38.3) | 13 (9.4) | < .001a |
| 35/39/68 | 37 (18.6) | 21 (35.0) | 16 (11.5) | < .001a |
Values are presented as number (%).
HSIL, high-grade intraepithelial lesion; SQCC, squamous cell carcinoma; HPV, human papillomavirus.
aSignificant p-value obtained after Bonferroni correction (corrected p-value: .05/9 = .006; all 9 HPV classes were included) was found between multiple and single HPV infection groups.
- 1. Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer 2007; 7: 11-22. ArticlePubMedPDF
- 2. Abreu AL, Souza RP, Gimenes F, Consolaro ME. A review of methods for detect human papillomavirus infection. Virol J 2012; 9: 262.ArticlePubMedPMCPDF
- 3. Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348: 518-27. ArticlePubMed
- 4. Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189: 12-9. ArticlePubMed
- 5. de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology 2004; 324: 17-27. ArticlePubMed
- 6. Van Doorslaer K, Li Z, Xirasagar S, et al. The papillomavirus episteme: a major update to the papillomavirus sequence database. Nucleic Acids Res 2017; 45: D499-506. ArticlePubMedPMC
- 7. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum 2007; 90: 1-636. PubMedPMC
- 8. de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11: 1048-56. PubMed
- 9. Munagala R, Dona MG, Rai SN, et al. Significance of multiple HPV infection in cervical cancer patients and its impact on treatment response. Int J Oncol 2009; 34: 263-71. PubMed
- 10. Dickson EL, Vogel RI, Geller MA, Downs LS Jr. Cervical cytology and multiple type HPV infection: a study of 8182 women ages 31–65. Gynecol Oncol 2014; 133: 405-8. ArticlePubMedPMC
- 11. Soto-De Leon S, Camargo M, Sanchez R, et al. Distribution patterns of infection with multiple types of human papillomaviruses and their association with risk factors. PLoS One 2011; 6: e14705.ArticlePubMedPMC
- 12. Cuschieri KS, Cubie HA, Whitley MW, et al. Multiple high risk HPV infections are common in cervical neoplasia and young women in a cervical screening population. J Clin Pathol 2004; 57: 68-72. ArticlePubMedPMC
- 13. Lee SA, Kang D, Seo SS, et al. Multiple HPV infection in cervical cancer screened by HPVDNAChip. Cancer Lett 2003; 198: 187-92. ArticlePubMed
- 14. Oyervides-Munoz MA, Perez-Maya AA, Sanchez-Dominguez CN, et al. Multiple HPV infections and viral load association in persistent cervical lesions in Mexican women. Viruses 2020; 12: 380.ArticlePubMedPMC
- 15. De Brot L, Pellegrini B, Moretti ST, et al. Infections with multiple high-risk HPV types are associated with high-grade and persistent low-grade intraepithelial lesions of the cervix. Cancer Cytopathol 2017; 125: 138-43. ArticlePubMedPDF
- 16. Salazar KL, Zhou HS, Xu J, et al. Multiple human papilloma virus infections and their impact on the development of high-risk cervical lesions. Acta Cytol 2015; 59: 391-8. ArticlePubMedPDF
- 17. Bonde JH, Pedersen H, Quint W, Xu L, Arbyn M, Ejegod DM. Clinical and analytical performance of the BD onclarity HPV assay with SurePath screening samples from the Danish Cervical Screening Program using the VALGENT framework. J Clin Microbiol 2020; 58: e01518-19. ArticlePubMedPMCPDF
- 18. Kim MS, Lee EH, Park MI, et al. Utility of human papillomavirus testing for cervical cancer screening in Korea. Int J Environ Res Public Health 2020; 17: 1726.ArticlePubMedPMC
- 19. Darragh TM, Colgan TJ, Cox JT, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med 2012; 136: 1266-97. PubMed
- 20. Ejegod D, Bottari F, Pedersen H, Sandri MT, Bonde J. The BD onclarity HPV assay on samples collected in SurePath medium meets the international guidelines for human papillomavirus test requirements for cervical screening. J Clin Microbiol 2016; 54: 2267-72. ArticlePubMedPMCPDF
- 21. Kim M, Park NJ, Jeong JY, Park JY. Multiple human papilloma virus (HPV) infections are associated with HSIL and persistent HPV infection status in Korean patients. Viruses 2021; 13: 1342.ArticlePubMedPMC
- 22. Bonde J, Ejegod DM, Cuschieri K, et al. The Valgent4 protocol: Robust analytical and clinical validation of 11 HPV assays with genotyping on cervical samples collected in SurePath medium. J Clin Virol 2018; 108: 64-71. ArticlePubMed
- 23. Li M, Du X, Lu M, et al. Prevalence characteristics of single and multiple HPV infections in women with cervical cancer and precancerous lesions in Beijing, China. J Med Virol 2019; 91: 473-81. ArticlePubMedPDF
- 24. Schmitt M, Depuydt C, Benoy I, et al. Multiple human papillomavirus infections with high viral loads are associated with cervical lesions but do not differentiate grades of cervical abnormalities. J Clin Microbiol 2013; 51: 1458-64. ArticlePubMedPMCPDF
- 25. Trottier H, Mahmud S, Costa MC, et al. Human papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2006; 15: 1274-80. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

- The Prevalence of Multi-Type Infections Among Human Papillomavirus Types in Korean Women
Jang Mook Kim, Hee Seung Song, Jieun Hwang, Jae Kyung Kim
Pathogens.2025; 14(4): 369. CrossRef - Multiple high-risk human papillomavirus infections exacerbate cervical lesion risk: epidemiological evidence from suining, Sichuan
Yaling Jing, Jianhui Chen, Fang Lin, Xiaonan Huang, Yulin Liu, Mingcai Zhao, Chuan Ye, Lianfang Zhao, Xiaofang Liu, Jiayan Yang
Virology Journal.2025;[Epub] CrossRef - The cervical cancer related distribution, coinfection and risk of 15 HPV types in Baoan, Shenzhen, in 2017–2023
Rukai Li, Weiwei Meng, Yunhai Zuo, Yanli Xu, Shaonan Wu
Virology Journal.2024;[Epub] CrossRef - Molecular findings and virological assessment of bladder papillomavirus infection in cattle
Francesca De Falco, Anna Cutarelli, Francesca Luisa Fedele, Cornel Catoi, Sante Roperto
Veterinary Quarterly.2024; 44(1): 1. CrossRef - Patterns of single and multiple HPV infections in female: A systematic review and meta-analysis
Dan Zhou, Jing Xue, Yaqiong Sun, Liling Zhu, Ming Zhao, Meimei Cui, Min Zhang, Jingjing Jia, Limei Luo
Heliyon.2024; 10(17): e35736. CrossRef - Age distribution of patients with multiple High-Risk Human Papilloma Virus (HR-HPV) genotypes and HPV vaccine recommendations by age
Gülçin Çetin Uysal, Nil Tekin
Family Practice and Palliative Care.2024; 9(3): 80. CrossRef - Relative distribution of HPV genotypes in histological cervical samples and associated grade lesion in a women population over the last 16 years in Burgundy, France
Christelle Auvray, Serge Douvier, Odile Caritey, Jean-Baptiste Bour, Catherine Manoha
Frontiers in Medicine.2023;[Epub] CrossRef - Epidemiologic characteristics of high-risk HPV and the correlation between multiple infections and cervical lesions
Qinli Luo, Xianghua Zeng, Hanyi Luo, Ling Pan, Ying Huang, Haiyan Zhang, Na Han
BMC Infectious Diseases.2023;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
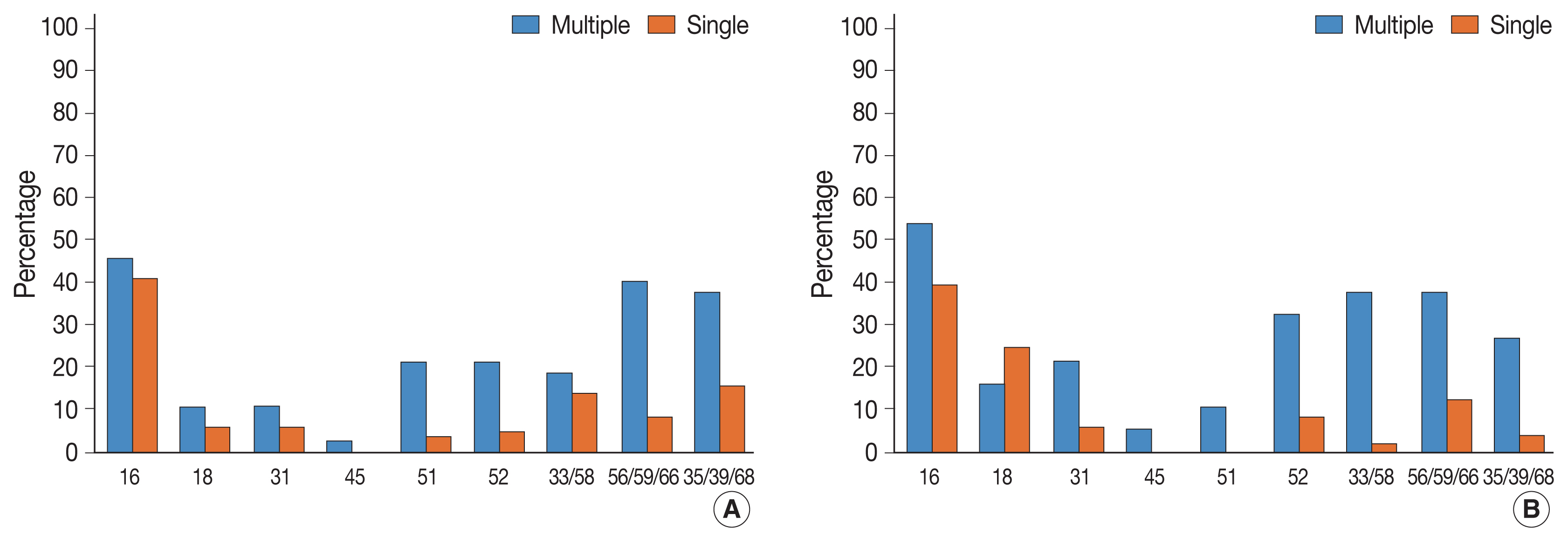
Fig. 1
| Variable | No. (%) | HPV infection status (n = 471) | p-value | |
|---|---|---|---|---|
|
| ||||
| Multiple (n = 110) | Single (n = 361) | |||
| Age (yr) | 58.2 ± 12.6 | 55.5 ± 11.9 | .043 | |
| HPV type | ||||
| 16 | 125 (26.5) | 39 (35.5) | 86 (23.8) | .019 |
| 18 | 36 (7.6) | 11 (10.0) | 25 (6.9) | .107 |
| 31 | 34 (7.2) | 15 (13.6) | 19 (5.3) | .005 |
| 45 | 7 (1.5) | 5 (4.5) | 2 (5.5) | .091 |
| 51 | 33 (7.0) | 19 (17.3) | 14 (3.9) | < .001 |
| 52 | 85 (18.0) | 36 (32.7) | 49 (13.6) | < .001 |
| 33/58 | 64 (13.6) | 29 (26.4) | 35 (9.7) | < .001 |
| 56/59/66 | 113 (24.0) | 46 (41.8) | 67 (18.6) | < .001 |
| 35/39/68 | 111 (23.6) | 47 (42.7) | 64 (17.7) | < .001 |
| Variable | No. (%) | HPV infection status (n = 471) | p-value | |
|---|---|---|---|---|
|
| ||||
| Multiple (n = 110) | Single (n = 361) | |||
| Histology | ||||
| Normal | 210 (44.5) | 34 (30.9) | 176 (48.8) | .001 |
| LSIL | 62 (13.2) | 16 (14.5) | 46 (12.7) | .632 |
| HSIL | 120 (25.5) | 37 (33.6) | 83 (23.0) | .033 |
| Squamous cell carcinoma | 65 (13.8) | 18 (16.4) | 47 (13.0) | .654 |
| Adenocarcinoma | 14 (3.0) | 5 (4.6) | 9 (2.5) | .332 |
| HPV type | Multiple HPV infection | Single HPV infection | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
| |||||||||
| Normal (n = 34) | LSIL (n = 16) | HSIL (n = 37) | SQCC (n = 18) | ADC (n = 5) | Normal (n = 176) | LSIL (n = 46) | HSIL (n = 83) | SQCC (n = 47) | ADC (n = 9) | |
| 16 | 2 (5.9) | 7 (43.8) | 17 (45.9) | 10 (55.6) | 3 (60.0) | 24 (13.6) | 6 (13.0) | 34 (41.0) | 19 (40.4) | 3 (33.3) |
| 18 | 2 (5.9) | 1 (6.3) | 4 (10.8) | 3 (16.7) | 1 (20.0) | 1 (0.6) | 2 (4.3) | 5 (6.0) | 12 (25.5) | 5 (55.6) |
| 31 | 6 (17.6) | 1 (6.3) | 4 (10.8) | 4 (22.2) | 0 | 8 (4.5) | 3 (6.5) | 5 (6.0) | 3 (6.4) | 0 |
| 45 | 1 (2.9) | 2 (12.5) | 1 (2.7) | 1 (5.6) | 0 | 2 (1.1) | 0 | 0 | 0 | 0 |
| 51 | 9 (26.5) | 0 | 8 (21.6) | 2 (11.1) | 0 | 9 (5.1) | 2 (4.3) | 3 (3.6) | 0 | 0 |
| 52 | 12 (35.3) | 7 (43.8) | 8 (21.6) | 6 (33.3) | 3 (60.0) | 35 (19.9) | 7 (15.2) | 4 (4.8) | 4 (8.5) | 0 |
| 33/58 | 11 (32.3) | 3 (18.8) | 7 (18.9) | 7 (38.9) | 1 (20.0) | 19 (10.8) | 3 (6.5) | 12 (14.5) | 1 (2.1) | 0 |
| 56/59/66 | 15 (44.1) | 8 (50.0) | 15 (40.5) | 7 (38.9) | 1 (20.0) | 42 (23.9) | 12 (26.1) | 7 (8.4) | 6 (12.8) | 0 |
| 35/39/68 | 18 (52.9) | 8 (50.0) | 14 (37.8) | 5 (27.8) | 2 (40.0) | 37 (21.0) | 11 (23.9) | 13 (15.7) | 2 (4.3) | 1 (11.1) |
| HPV type | No. (%) | HPV infection status (n = 199) | p-value | |
|---|---|---|---|---|
|
| ||||
| Multiple ( n = 60) | Single (n = 139) | |||
| 16 | 86 (43.2) | 30 (50.0) | 56 (40.3) | .216 |
| 18 | 30 (15.1) | 8 (13.1) | 22 (15.8) | .676 |
| 31 | 16 (8.0) | 8 (13.1) | 8 (5.8) | .089 |
| 45 | 2 (1.0) | 2 (3.3) | 0 | .091 |
| 51 | 13 (6.5) | 10 (16.7) | 3 (2.2) | < .001 |
| 52 | 25 (12.6) | 17 (28.3) | 8 (5.8) | < .001 |
| 33/58 | 28 (14.1) | 15 (25.0) | 13 (9.4) | .005 |
| 56/59/66 | 36 (18.1) | 23 (38.3) | 13 (9.4) | < .001 |
| 35/39/68 | 37 (18.6) | 21 (35.0) | 16 (11.5) | < .001 |
Values are presented as mean ± standard deviation or number (%). Significant p-value obtained after Bonferroni correction (corrected p-value: .05/9 = .006; all 9 human papillomavirus [HPV] classes were included) was found between multiple and single HPV infection groups.
Values are presented as number (%). HPV, human papillomavirus; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion.
Values are presented as number (%). HPV, human papillomavirus; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; SQCC, squamous cell carcinoma; ADC, adenocarcinoma.
Values are presented as number (%). HSIL, high-grade intraepithelial lesion; SQCC, squamous cell carcinoma; HPV, human papillomavirus. Significant p-value obtained after Bonferroni correction (corrected p-value: .05/9 = .006; all 9 HPV classes were included) was found between multiple and single HPV infection groups.

 E-submission
E-submission



