Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 56(1); 2022 > Article
-
Case Study
TTF1-positive SMARCA4/BRG1 deficient lung adenocarcinoma -
Anurag Mehta1
 , Himanshi Diwan1
, Himanshi Diwan1 , Divya Bansal1
, Divya Bansal1 , Manoj Gupta2
, Manoj Gupta2
-
Journal of Pathology and Translational Medicine 2022;56(1):53-56.
DOI: https://doi.org/10.4132/jptm.2021.09.16
Published online: November 16, 2021
1Department of Laboratory, Molecular and Transfusion Services, Rajiv Gandhi Cancer Institute and Research Centre (RGCIRC), New Delhi, India
2Department of Nuclear Medicine, Rajiv Gandhi Cancer Institute and Research Centre (RGCIRC), New Delhi, India
- Corresponding Author: Himanshi Diwan, MD, Department of Laboratory, Molecular and Transfusion Services, Rajiv Gandhi Cancer Institute and Research Centre, New Delhi 110085, India, Tel: +91-9413542252, Fax: +91-11-27051037, E-mail: himanshidiwan89@gmail.com
© 2022 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- SMARCA4/BRG1-deficient lung adenocarcinoma (SD-LUAD) is being recognized as a distinct subtype based on subtle differences in its clinical, morphological, and immunophenotypic attributes compared to other non–small cell lung carcinomas. We present here a case of SD-LUAD with curious thyroid transcription factor 1 (TTF1) expression in a morphologically heterogenous lung adenocarcinoma. The better differentiated area showed preservation of TTF1 expression, and a poorly differentiated tumor had loss of TTF1 expression with universal BRG1 loss.
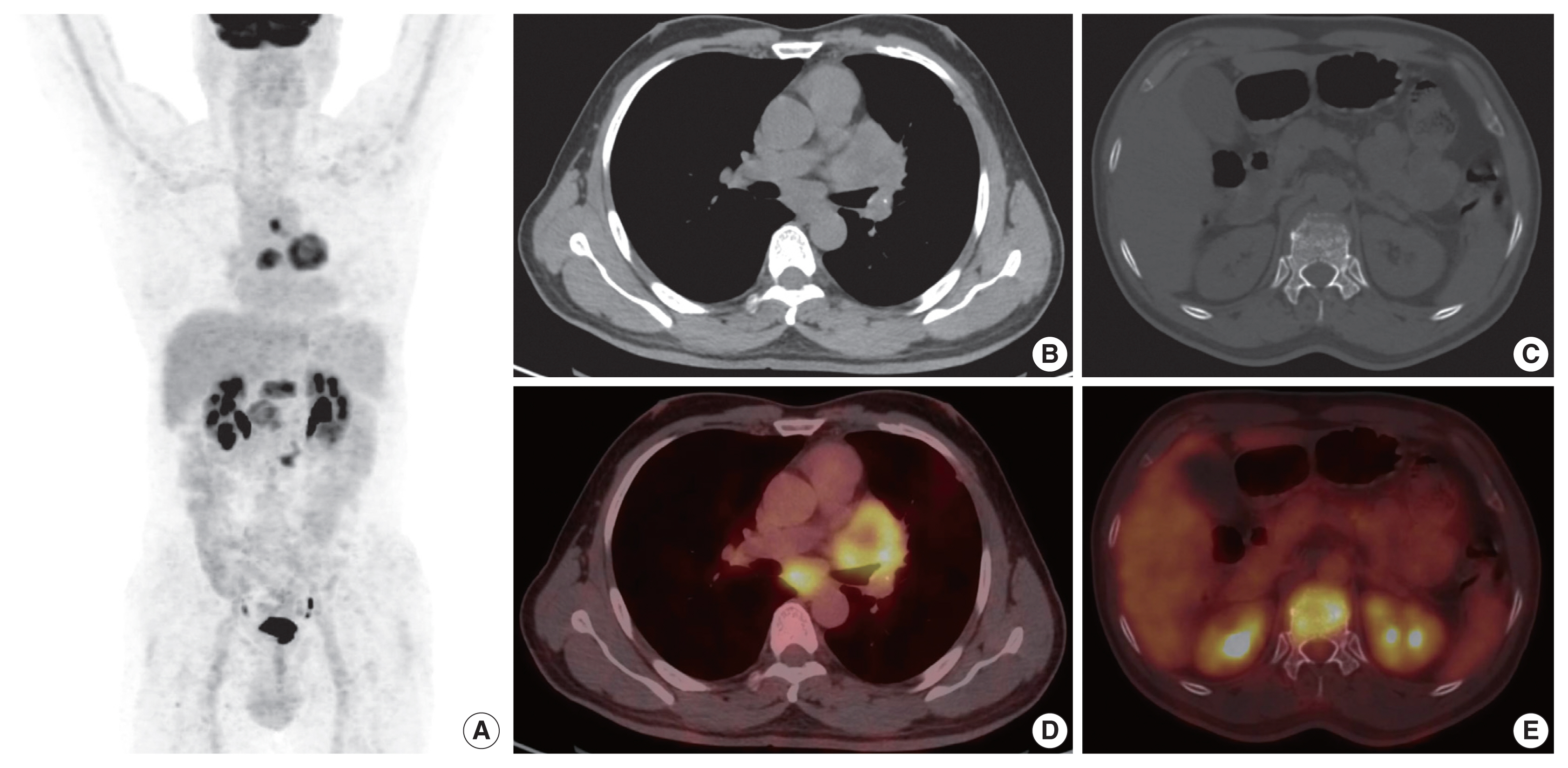
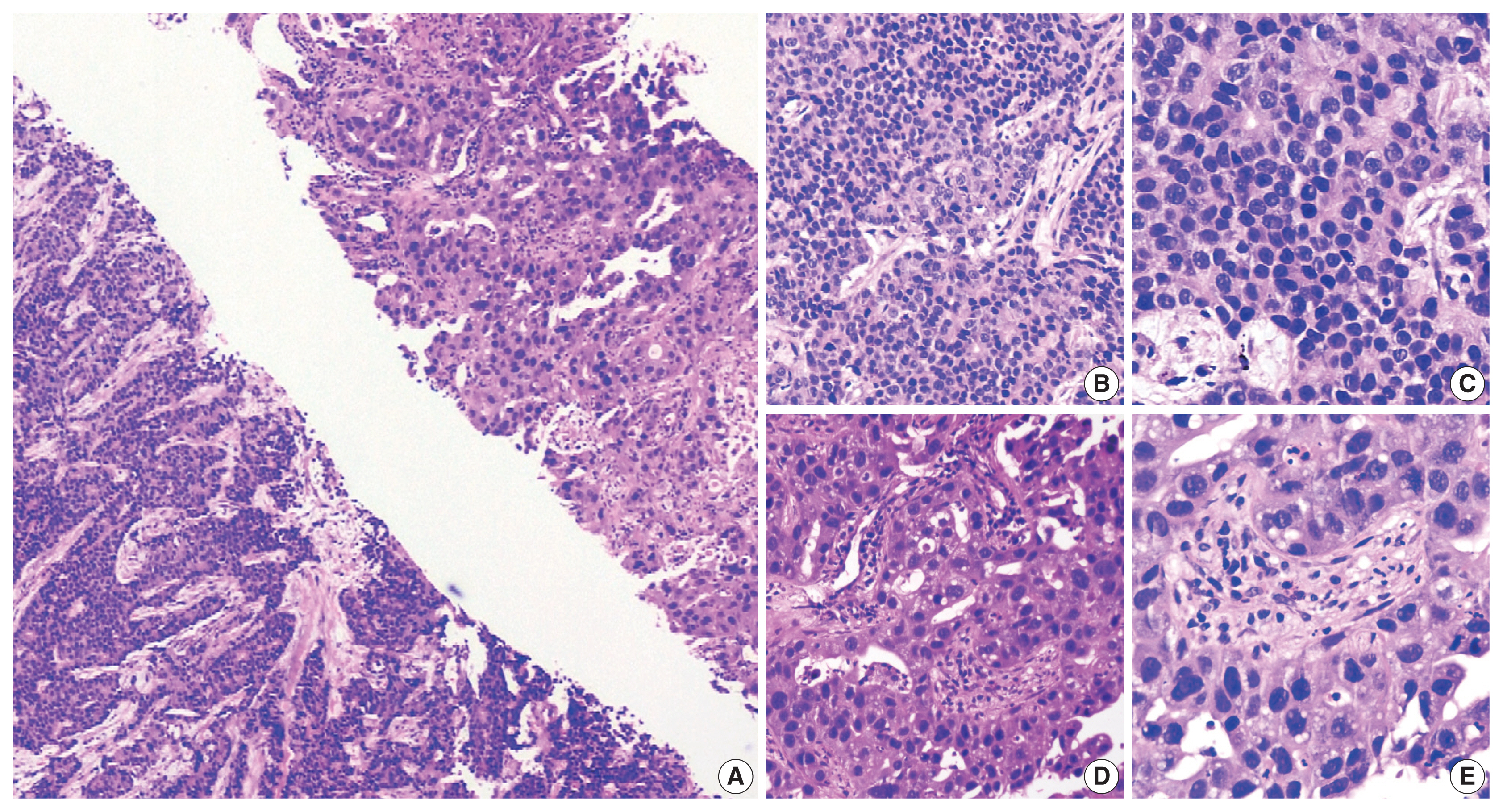
- A 54-year-old male, chronic smoker with a 40 pack-year history, presented with complaints of breathlessness and hemoptysis. Routine laboratory investigations were within normal limits. Chest X-ray revealed a large rounded mass in the parahilar region of the left lung with mediastinal widening. Whole-body positron emission tomography and non-diagnostic computed tomography (PET-CT) revealed a parahilar mass in the left lung measuring 6.2 × 4.8 cm with bulky mediastinal lymphadenopathy and bony metastases (Fig. 1). Computed tomography (CT)–guided trucut biopsy from the left lung mass revealed a heterogeneous tumor with solid pattern (Fig. 2A). The tumor cells in the better differentiated area had bland nuclei, high nuclear to cytoplasmic ratio, and a compact morphology (Fig. 2B, C). The tumor cells in other areas had abundant pink bubbly cytoplasm, pleomorphic vesicular nuclei with prominent nucleoli, and brisk mitoses (Fig. 2D, E). Neutrophilic emperipolesis and inflamed stroma were also seen in these areas (Fig. 2D, E).
- Upon immunohistochemistry (IHC) analysis, tumor cells in the better differentiated area (Fig. 3A) expressed cytokeratin (CK) (Fig. 3B), but lacked BRG1 expression (Fig. 3C). These tumor cells were positive for TTF1 (Fig. 3D) while tumor cells in lesser differentiated area (Fig. 3E) expressed weaker CK (Fig. 3F), and were devoid of BRG1 (Fig. 3G) and TTF1 (Fig. 3H). In summary, tumor cells in both the areas were devoid of BRG1 and expressed CK, CK7, epithelial membrane antigen, and BerEp4, while TTF1 expression was noted only in better differentiated component. On additional IHC, the tumor cells in the better differentiated area revealed aberrant expression of SRY-box transcription factor (SOX) 2 and SOX11, and were negative for p40, Hep Par 1, spalt like transcription factor 4 (SALL4), and CD34 immunoexpression. Other areas with poor differentiation exhibited immunopositivity for SALL4 and were negative for p40, Hep Par 1, SOX2, SOX11, and CD34 expression. This contrasts with the well-defined expression of Hep Par 1 in SD-LUAD and SALL4, SOX2, and CD34 in SMARCA4/BRG1-deficient thoracic sarcoma (SD-TS) [4]. A final diagnosis of SD-LUAD was established on the basis of epithelial marker expression and BRG1 loss. Programmed death-ligand 1 immunoexpression showed a tumor proportion score of < 1%. No targetable driver mutation was identified for epidermal growth factor receptor (EGFR), reactive oxygen species 1 (ROS1), and anaplastic lymphoma kinase 1 (ALK-1).
- The patient received six cycles of 5 Gy radiotherapy, but unfortunately succumbed to his illness.
CASE REPORT
- The literature describes a varied morphology of SD-LUAD in the form of solid adenocarcinoma, large cell carcinoma, hepatoid, rhabdoid, spindling, and signet ring cell along with inflamed stroma, emperipolesis, necrosis, and brisk mitosis [4–9]. There is only a handful of cases with acinar/papillary morphology [3,6]. SD-LUAD are typically negative for TTF1 as described in the literature [5,6]. Contrary observations were described by Herpel et al. [3] and Agaimy et al. [4] with intact TTF1 expression in 20% and 10% of their cases, respectively. The present case is unique with heterogeneous tumor morphology and heterogenous TTF1 expression, but with diffuse loss of BRG1. Despite having a typical morphology of adenocarcinoma in one core, BRG1 immunoexpression was studied in view of the morphological telltale signs of SD-LUAD in other areas.
- SD-LUAD can variably express Hep Par 1 and stem cell markers are more often expressed in SD-TS [4]. Our case unfolds the dynamic and evolving genetic events of lung adenocarcinoma. TTF1 was expressed only by the better differentiated component and BRG1 loss was exhibited by both areas in the present case. The loss of BRG1 in the better differentiated component is germane to our hypothesis that BRG1 loss was the primary event, which eventually had downregulated the transcription of TTF1 and was reflected as loss of TTF1 in poorly differentiated area and suggested that SMARCA4 mutation was the driver mutation. The observation of SALL4 expression in lesser differentiated area with aberrant SOX2 and SOX11 expression in better differentiated area is difficult to explain in the present case. However, it clearly unravels the evolutionary pathway of oncogenesis with SMARCA4 protein loss being the primary oncogenic event. There is continuous remodeling of chromatin responsible for gain and loss of many genes and protein expression, which is reflected as aberrant and heterogenous expression of SALL4, SOX2, and SOX11 in the present case. Rekhtman et al. also proposed that SMARCA4-deficient thoracic sarcomas are indeed undifferentiated or dedifferentiated lung carcinoma associated with smoking [10]. The IHC in the present case imitates the ongoing continuous remodeling, thereby leaving a probability of possible dedifferentiation of adenocarcinoma into sarcoma over time.
- Given an almost indistinctive morphology, a need to minimize IHC, the unique biology propelling a rapid progression, and lack of actionable driver mutation, this entity needs separation from other lung adenocarcinoma, so that it can be studied more exhaustively to develop a genome-directed therapy. This task however, is rendered more difficult by the fact that some of the SD-LUAD may express TTF1, and this finding opens up the question whether all non–small cell lung carcinomas should be assessed for SMARCA4/BRG1 protein expression.
DISCUSSION
Ethics Statement
All procedures performed in the study were approved by the Institutional Review Board (IRB) (vide letter no: Nos. RGCIRC/IRB-BHR/61/2021 dated 3rd July 2021) in accordance with the 1964 Helsinki declaration and its latest amendments. Informed consent was obtained from each of the individuals included in the study.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: AM. Data curation: HD. Formal analysis: AM, HD. Investigation: AM, HD, DB, MG. Methodology: AM. Project administration: AM, DB. Resources: MG. Supervision: AM. Visualization: HD. Writing—original draft: HD. Writing—review & editing: AM, DB. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.

- 1. Kadoch C, Hargreaves DC, Hodges C, et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet 2013; 45: 592-601. ArticlePubMedPMCPDF
- 2. Errichiello E, Mustafa N, Vetro A, et al. SMARCA4 inactivating mutations cause concomitant Coffin-Siris syndrome, microphthalmia and small-cell carcinoma of the ovary hypercalcaemic type. J Pathol 2017; 243: 9-15. ArticlePubMedPMCPDF
- 3. Herpel E, Rieker RJ, Dienemann H, et al. SMARCA4 and SMARCA2 deficiency in non-small cell lung cancer: immunohistochemical survey of 316 consecutive specimens. Ann Diagn Pathol 2017; 26: 47-51. ArticlePubMed
- 4. Agaimy A, Fuchs F, Moskalev EA, Sirbu H, Hartmann A, Haller F. SMARCA4-deficient pulmonary adenocarcinoma: clinicopathological, immunohistochemical, and molecular characteristics of a novel aggressive neoplasm with a consistent TTF1(neg)/CK7(pos)/HepPar-1(pos) immunophenotype. Virchows Arch 2017; 471: 599-609. ArticlePubMedPDF
- 5. Mehta A, Bansal D, Tripathi R, Jajodia A. SMARCA4/BRG1 protein-deficient thoracic tumors dictate re-examination of small biopsy reporting in non-small cell lung cancer. J Pathol Transl Med 2021; 55: 307-16. ArticlePubMedPMCPDF
- 6. Nambirajan A, Singh V, Bhardwaj N, Mittal S, Kumar S, Jain D. SMARCA4/BRG1-deficient non-small cell lung carcinomas: a case series and review of the literature. Arch Pathol Lab Med 2021; 145: 90-8. ArticlePubMedPDF
- 7. Naito T, Udagawa H, Umemura S, et al. Non-small cell lung cancer with loss of expression of the SWI/SNF complex is associated with aggressive clinicopathological features, PD-L1-positive status, and high tumor mutation burden. Lung Cancer 2019; 138: 35-42. ArticlePubMed
- 8. Oike T, Ogiwara H, Tominaga Y, et al. A synthetic lethality-based strategy to treat cancers harboring a genetic deficiency in the chromatin remodeling factor BRG1. Cancer Res 2013; 73: 5508-18. ArticlePubMedPDF
- 9. Yoshida A, Kobayashi E, Kubo T, et al. Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities. Mod Pathol 2017; 30: 797-809. ArticlePubMedPDF
- 10. Rekhtman N, Montecalvo J, Chang JC, et al. SMARCA4-deficient thoracic sarcomatoid tumors represent primarily smoking-related undifferentiated carcinomas rather than primary thoracic sarcomas. J Thorac Oncol 2020; 15: 231-47. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- SMARCA4-deficient Non–small Cell Lung Cancer on 18F-FDG PET/CT
Tao Liu, Hengshan Ji, Siyuan Jiang, Rongxin Qi, Xiaodie Zhou, Jingjing Sun, Jiang Wu
Clinical Nuclear Medicine Open.2025;[Epub] CrossRef - Case Report: SMARCA4-deficient NSCLC with brain metastasis harboring co-mutations in chromatin remodeling and DNA damage repair pathways
Jiaqin Song, Shikun Yang, Lei Xia
Frontiers in Oncology.2025;[Epub] CrossRef - One Case of Non-Small Cell Lung Cancer with SMARCA4 Deletion Was Reported
允龙 宋
Medical Diagnosis.2024; 14(01): 137. CrossRef - Delineation of a SMARCA4-specific competing endogenous RNA network and its function in hepatocellular carcinoma
Lei Zhang, Ting Sun, Xiao-Ye Wu, Fa-Ming Fei, Zhen-Zhen Gao
World Journal of Clinical Cases.2022; 10(29): 10501. CrossRef - Novel germline SMARCA4 mutation in Small Cell Carcinoma of the Ovary, Hypercalcemic Type
Anurag Mehta, Himanshi Diwan, Diksha Karki, Divya Bansal, Meenakshi Kamboj, Anila Sharma, Shrinidhi Nathany, Sakshi Mattoo, Dushyant Kumar
Current Problems in Cancer: Case Reports.2022; 8: 100205. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



 E-submission
E-submission





