Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 53(4); 2019 > Article
-
Original Article
Serous Adenocarcinoma of Fallopian Tubes: Histological and Immunohistochemical Aspects -
Natalia Hyriavenko
 , Mykola Lyndin
, Mykola Lyndin , Kateryna Sikora1
, Kateryna Sikora1 , Artem Piddubnyi
, Artem Piddubnyi , Ludmila Karpenko
, Ludmila Karpenko , Olha Kravtsova1
, Olha Kravtsova1 , Dmytrii Hyriavenko2
, Dmytrii Hyriavenko2 , Olena Diachenko2
, Olena Diachenko2 , Vladyslav Sikora
, Vladyslav Sikora , Anatolii Romaniuk
, Anatolii Romaniuk
-
Journal of Pathology and Translational Medicine 2019;53(4):236-243.
DOI: https://doi.org/10.4132/jptm.2019.03.21
Published online: April 11, 2019
Department of Pathology, Sumy State University, Sumy, Ukraine
1Sumy Regional Clinical Perinatal Center, Sumy, Ukraine
2Sumy State University, Sumy, Ukraine
- Corresponding Author Natalia Hyriavenko, MD, PhD Department of Pathology, Sumy State University, st. Privokzalnaya, 31, m. Sumy 40022, Ukraine Tel: +380-997466578 Fax: +380-542334058 E-mail: n.gyryavenko@med.sumdu.edu.ua
© 2019 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Although primary cancer of the fallopian tubes is a relatively rare type of tumor in female reproductive organs, its mortality is quite high. It is important to identify molecular and biological markers of this malignancy that determine its specific phenotype.
-
Methods
- The study was carried out on samples received from 71 female patients with primary cancer of the fallopian tubes. The main molecular and biological properties, including hormone status (estrogen receptor [ER], progesterone receptor [PR]), human epidermal growth factor receptor (HER2)/neu expression, proliferative potential (Ki-67), apoptosis (p53, Bcl-2), and pro-angiogenic (vascular endothelial growth factor) quality of serous tumors were studied in comparison with clinical and morphological characteristics.
-
Results
- ER and PR expression is accompanied by low grade neoplasia, early clinical disease stage, and absence of lymphogenic metastasis (p < .001). HER2/neu expression is not typical for primary cancer of the fallopian tubes. Ki-67 expression is characterized by an inverse correlation with ER and PR (p < .05) and is associated with lymphogenic metastasis (p < .01). p53+ status correlates with high grade malignancy, tumor progression, metastasis, negative ER/PR (p < .001), and negative Bcl-2 status (p < .05). Positive Bcl-2 status is positively correlated with ER and PR expression and low grade malignancy.
-
Conclusions
- Complex morphologic (histological and immunohistochemical) study of postoperative material allows estimation of the degree of malignancy and tumor spread to enable appropriate treatment for each case.
- The material was collected from Sumy Regional Pathology Clinical Center and Sumy Regional Clinical Oncology Center. Written informed consent to investigate tissue was obtained from patients. The Bioethics Commission of the Medical Institute of Sumy State University approved the experimental protocol (No. 8 from 29.04.2016).
- The research was performed on surgical material obtained from 71 female patients with PCFT. Pathologic findings were identified using SEE-FIM protocols of FT tissue examination. Determination of the primacy of FT lesions was based on the following criteria: main tumor focus localized in the FT with spread from the endosalpinx for ovary and/or uterus involvement; FT contained more neoplastic mass tissue than other sites; border between normal and affected (neoplastic) epithelium of the tube was visualized; no malignant neoplasms of other localizations.
- Hematoxylin-eosin stained tissue sections of 5-μm thickness allowed determination of the histogenesis, degree of tumor malignancy, depth of invasion, spread into pelvic organs, and presence of metastases in regional lymph nodes.
- For immunohistochemical study, 4-μm sections were made from paraffin blocks, subjected to standard deparaffinization, dehydrated in xylene and ethanol of graded concentrations, and mounted on 3-aminopropyltriethoxysilane-coated slides. Antigen retrieval was carried out in a water-bath at 97°C–98°C. Antigen-antibody reaction was visualized using the Ultra Vision Quanto Detection System HRP DAB Chromogen (Thermo Fisher Scientific, Waltham, MA, USA), which included blocking endogenous peroxidase activity, blocking nonspecific background staining using the Ultra V block, and enhancing the reaction by Primary Antibody Amplifier Quant. The final visualization was carried out with diaminobenzidine with additional nuclei counterstaining with Modified Mayer’s Hematoxylin (Thermo Fisher Scientific).
- To exclude influences of tumor histogenesis on the immunophenotype of cancer cells, the presence of serous adenocarcinoma of the FT (SAcFT) was a criterion for inclusion in the studied group to investigate its molecular-biological markers. All other types (mucinous, squamous, etc.) of PCFT were excluded. While there were few excluded cases, they may have influenced the obtained results. The antibody panel specified in Table 1 was used for this research (Thermo Fisher Scientific).
- Obtained specimens were examined using a microscope with digital camera Zeiss AxioCam ERs 5s and ZEN 2 (blue edition) software (Zeiss, Jena, Germany).
- Data processing was carried out using Microsoft Excel 2010 with the application Attestat 12.0.5. Estimation of probable differences between comparable indicators was carried out using the Student’s t-test. Detection and evaluation of the links between indicators was carried out using the nonparametric Spearman correlation coefficient (r). A p-value of 0.05 and 95% confidence interval was considered statistically significant.
MATERIALS AND METHODS
- Archive data from the Sumy Regional Pathology Clinical Center and Sumy Regional Clinical Oncology Center indicated that PCFT accounted for 1.13% of malignant neoplasms of the female reproductive system and 4.36% of malignant neoplasms of the uterine appendages (peak incidence was between 50 and 69 years). FT carcinomas were predominantly localized on one side, and bilateral involvement of tubes was detected in only 16.9% of cases. The tumor process involved the ampulla segment more often (87.3%) than the isthmus (7.1%) or infundibulum (5.6%). In most cases, FT neoplasias (60.6%) were identified at stages I–II (Table 2).
- Neoplastic spread outside the FT was found in 66.2% of women during diagnosis. Metastatic tumor dissemination into the omentum, peritoneum, ovaries, and regional lymph nodes did not depend on the size of the primary focus in the FT. Nevertheless, metastases in iliac and lumbar lymph nodes were detected in 26.8% of cases.
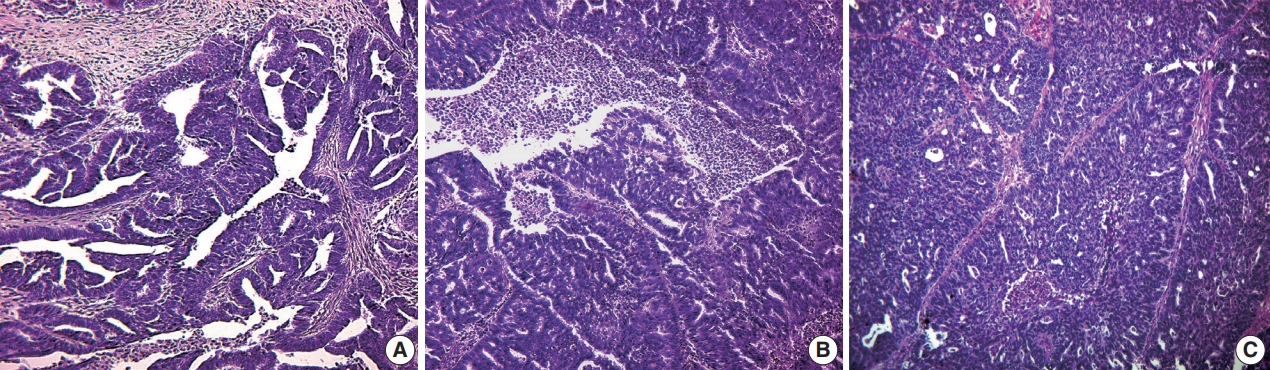
- Our histologic study showed that the overwhelming majority of malignant neoplasms of the FT were SAcFT (93.0% of cases) (Fig. 1). Mucinous (2.8%), clear cell (2.8%), and squamous cell carcinomas (1.4%) were quite rare.
- A total of 88.4% of SAcFT cases showed a high degree of malignancy (low [G3] and moderately differentiated [G2]). Their metastases were more often present in regional lymph nodes (28.2%), omentum, peritoneum (11.3%), and ovaries (5.6%).
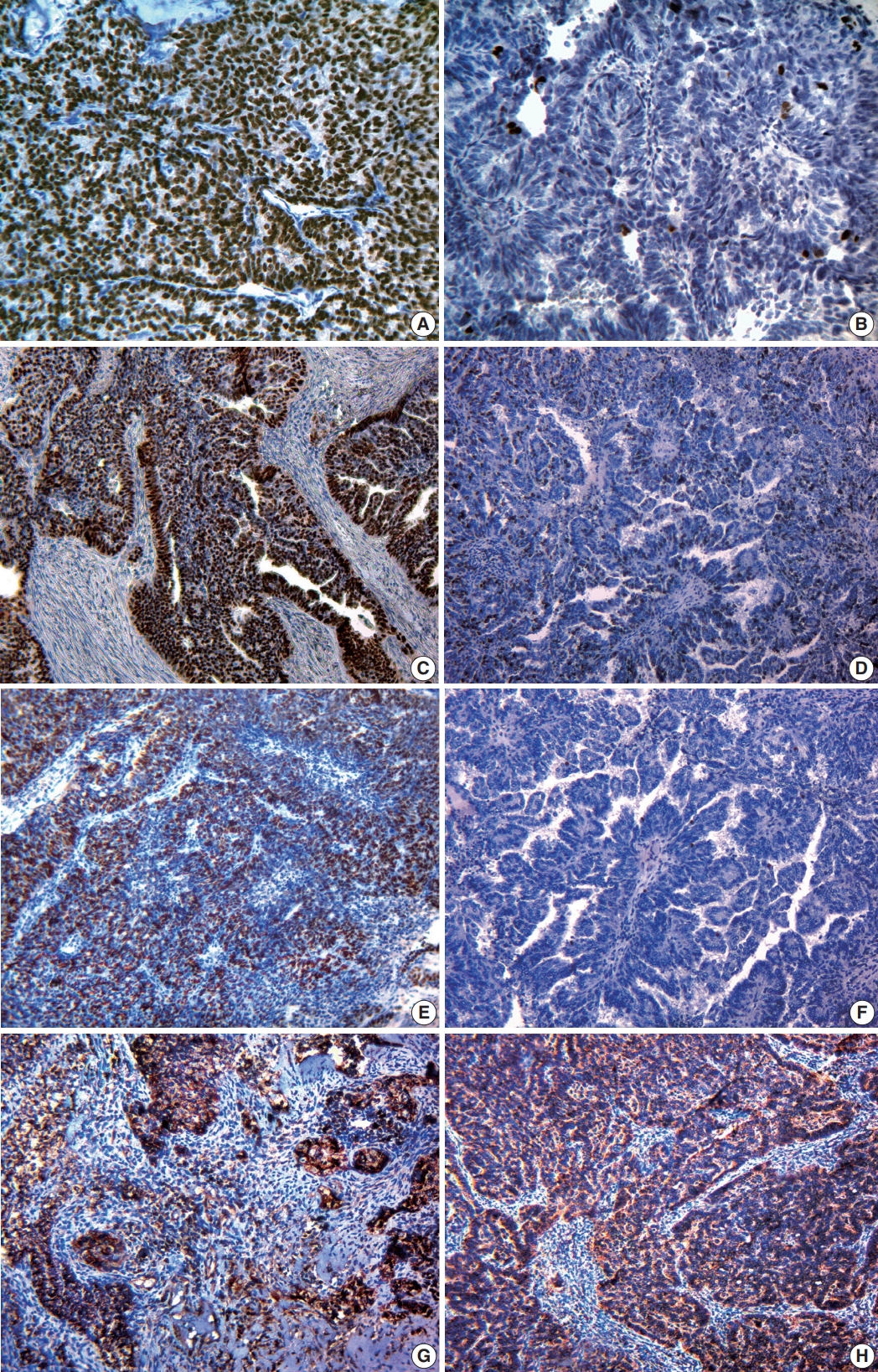
- Immunohistochemical study showed variable expression of ER and PR (Fig. 2A, B): ER+PR+, 62.1%; ER+PR–, 21.2%. Strong expression of ER and PR in SAcFT correlated with well-differentiated tumors (low grade cancer) and initial clinical stages of disease (p < .001). The presence of metastases in lymph nodes was accompanied by significantly lower expression of ER (p < .001) and PR (p < .001) in cancer cells compared to cases without metastases (p < .05).
- Human epidermal growth factor receptor (HER2)/neu overexpression was not typical for SAcFT (80.9% of tumors were receptor-negative). Nevertheless, most neoplastic FT cells (55.0 ± 12.2%) expressed Ki-67 receptors (Fig. 2C, D). Table 3 shows the division of patients by Ki-67 expression level.
- Tumor cell proliferation was characterized by an inverse relationship with steroid hormone expression (p < .05). In groups with positive receptor status (ER+PR+), Ki-67 overexpression (more than 60%) was detected in only 46.3% of cases. Conversely, tumors with completely negative ER and PR or with only ER positivity had significantly higher proliferative activity (63.4% and 71.4%, respectively). There were no differences in Ki-67 expression, clinical tumor stage, or degree of differentiation (p > .05).
- In 13 cases (19.7%), a negative reaction was detected for the pro-apoptotic protein p53 (Fig. 2F). Fifty-three cases of SAcFT (80.3%) were p53-positive. Among them, nine cases (13.6%) were weakly positive, 13 cases (19.7%) were moderately positive, and 31 cases (47%) were strongly positive (Fig. 2E). The presence of these receptors in tumor tissue was found in patients of all clinical stages. Strong correlations were identified between the presence of p53 and clinical stage (r = 0.77, p < .001), as well as between p53 and degree of differentiation (r = 0.58, p < .001) (Table 4). Significantly higher p53 expression was identified in the group of patients with metastases (80.6% ± 2.7%) compared to those without metastases (29.7% ± 3.6%).
- A total of 81.8% of SAcFT cases were Bcl-2–positive. Most of them (96.3%) also had ER and PR (71.4%) expression. Positive correlations were identified between Bcl-2 and expression of ER (r = 0.84, p < .001) and PR (r = 0.69, p < .001). In contrast to p53, a moderate negative correlation was identified between Bcl-2 and clinical disease stage (r = –0.44, p < .001), as well as between Bcl-2 and degree of anaplasia (r = –0.52, p < .001). SAcFT metastases were associated with decreased anti-apoptotic protein expression (r = –0.39, p < .01) (Table 5).
- The correlation between Ki-67 and p53 expression (p < .05) has been established. Patients with high p53 expression (58.3% of cases) were predominant in the high Ki-67 expression group. There was a negative correlation between expression of Ki-67 and Bcl-2 (p < .05).
- Cytoplasmic expression of VEGF was detected in both tumor cells and vascular endothelium. Most patients had moderate or strong cytoplasmic signals in endothelial cells (87.9%) and in more than 70% of tumor cells located diffusely in all fields of view (Fig. 2G, H). Only six cases (9.1%) had weak and moderate focal expression of 10%–50%. Focal weak cytoplasmic reaction, which was present in less than 10% of tumor cells and in the endothelium of single vessels, was observed in only two cases (3%).
- The angiogenic potential of PCFT depends on the degree of differentiation (p < .05) and clinical disease stage (p < .05). Moreover, all cases of regional lymph node metastases were accompanied by strong VEGF expression. This result may confirm that metastasis of PCFT depends on angiogenesis. In general, steroid-negative FT tumors were characterized by a higher level of VEGF expression. There was weak correlation between expression of VEGF and p53 (r = 0.25, p < .05). There was no significant correlation between VEGF expression and the presence of Ki-67 and Bcl-2 receptors (p > .05).
RESULTS
- Numerous scientific publications on PCFT include single observations of this disease [15,16]. Patients with malignant FT neoplasms are typically older, which may be due to increased amount of secretory cells in older women [17,18]. Morphologically, malignant epithelial tumors of the FT can be comprised of carcinomas of all cell types. In our research, the overwhelming majority of malignant neoplasms were SAcFT, which demonstrate a high degree of malignancy and very aggressive behavior.
- The presence of steroid hormone receptors in FT tumors is a substantial indicator for the use of hormonal therapy [19]. Conversely, steroid-negative tumors are contraindicated for hormone therapy. The medical literature includes sporadic reports on the use of hormonal therapy combined with cytostatics for treatment of FT neoplasia [20-22]. Increased anaplasia and significant tumor spread are associated with decreased steroid-positive cells and an increased proportion of steroid-negative FT neoplasms. This can be explained by functional and phenotypic simplification of tumor cells with decreased ER and PR expression associated with tumor progression (increased tumor autonomy degree). Moreover, neoplasias with lymph node metastases expressed significantly less ER and PR than cases without lymph node metastases (p < .001). Our results demonstrate that the presence of steroid hormone receptors in SAcFT is an important prognostic biologic characteristic of these neoplasias. Reduced or lost expression of both ER and PR in FT tumors is associated with invasion, metastasis, and an adverse disease course.
- The results of our research indicate that overexpression of HER2/neu is not typical for PCFT. In contrast to our data, overexpression of HER2/neu in neoplastic FT tissue has been demonstrated by some authors in 25.6% [23], 31% [24], and even 89% [25] of SAcFT. According to our results, we cannot recommend testing for this oncoprotein as a prognostic biomarker.
- Immunohistochemistry demonstrated a mean Ki-67 of 55.0% ± 12.2%. Most FT tumors have a proliferation index of more than 60% (54.5%). PCFT has high proliferative activity, indicating an extremely aggressive disease course. In the presence of metastases, the level of Ki-67 expression can be considered an independent prognostic marker for N-status in PCFT. This contributes to the allocation of certain women to the high-risk patient group.
- The received data suggests that there is no direct dependency between a cell’s steroid status and its ability to divide. This indicates that complex hormonal mechanisms contribute to proliferation. The significant increase in proliferative activity of PR cells may demonstrate the significant effect of progesterone on the ability of cells to divide.
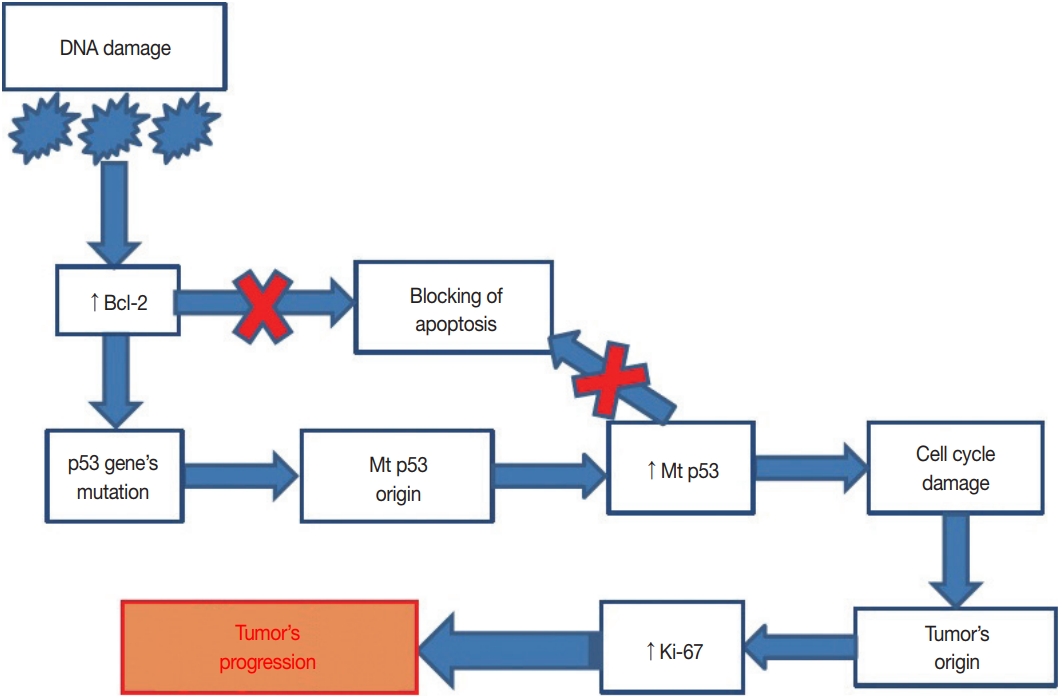
- In our study, 80.3% of SAcFT cases were p53-positive. p53 mutations were associated with tumor spread. In contrast to p53, 72.4% of cases expressed the anti-apoptotic Bcl-2 protein in the first stages of the neoplastic process. Blockage of apoptosis likely occurs in one of two tumor growth stages via different mechanisms; in the early stages, Bcl-2 is activated, while in the late stages, mutations in the TP53 gene occur. This results in the loss of functional qualities of the protein that it encodes. Exuberant amounts of mutant p53 and decreased wild-type p53 lead to suppression of the apoptosis blockade, which consequently increases cell proliferation. Cell division becomes uncontrolled and the malignant process actively progresses (Fig. 3).
- The p53+ status of SAcFT correlates with a high degree of malignancy, significant tumor spread, metastases, and high proliferation. In other words, p53+ status can act as an independent prognostic factor for PCFT. In addition, our studies show that the Bcl-2+ status of FT tumors correlates with positivity of steroid hormone receptors, low degree of anaplasia, and low proliferation. Bcl-2 overexpression indicates more intense inhibition of apoptosis and should be accompanied by more aggressive tumor behavior. Nonetheless, according to our obtained data, Bcl-2 overexpression is a positive predictor for PCFT. This is likely due to a combination of Bcl-2 and steroid hormone receptor expression since the Bcl-2 gene is estrogen-dependent [26,27].
- FT tumors are characterized by robust angiogenesis. Sometimes, tumor cells can stimulate angiogenesis (angiogenetic activation) by forming and releasing angiogenetic factors (e.g., VEGFs) that stimulate endothelial cell proliferation and capillary growth [28,29]. In our research, the majority of patients (87.9%) had moderate or strong VEGF cytoplasmic expression in endothelial cells and in more than 70% of tumor cells were located diffusely in all fields of view. Accordingly, constant and intense angiogenesis in PCFT determines their fast growth and has adverse prognostic implications. We have also detected that the angiogenic potential of malignant FT tumors correlates with the degree of differentiation, clinical disease stage, negative ER/PR status, and p53-positive status (p < .05). All cases of metastasis in regional lymph nodes were accompanied by strong VEGF expression.
- The results of our complex morphological study of PCFT demonstrate factors that should be considered when determining the strategy for treating patients with malignant FT tumors, including use of antihormonal therapy in cases of steroid positive tumors, adjuvant chemotherapy in cases with proapoptotic protein values, p53 higher than 10%, proliferative activity more than 60%, and use of anti-VEGF therapy in cases with high angiogenic potential. Our study was limited by a lack of data on patient lifestyle, which may be important, as it is in neoplastic processes in other female reproductive organs [30,31].
- In most cases, PCFT is serous adenocarcinoma. Neoplastic cells have high rates of expression for estrogen receptors (83.3%), progesterone receptors (62.1%), Ki-67 (55.0% ± 12.2%), p53 (80.3%), Bcl-2 (81.8%), and VEGF (87.9%), which influence tumor aggressiveness. The use of complex morphologic (histological and immunohistochemical) study of postoperative material allows assessment of the degree of malignancy and tumor spread. This allows selection of appropriate therapeutic tactics for each individual case.
DISCUSSION
Author Contributions
Conceptualization: NH, ML, VS, AR.
Data curation: NH, ML, KS, AP, LK, VS, OK.
Formal analysis: NH, DH, OD.
Investigation: NH, ML, KS, AP, LK, OK, VS, DH, OD.
Methodology: NH, ML, KS, AR, VS.
Project administration: NH, ML, VS, AR.
Resources: OK, KS, AR.
Software: AP, LK, DH.
Supervision: AR.
Validation: NH, ML, KS, AP, LK, OK, VS.
Visualization: NH, KS, AP, LK, OK .
Writing—original draft: NH, ML, KS, AP, LK, OK, OD, VS.
Writing—review & editing: NH, ML, AR.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Acknowledgments
| Average index of proliferation (%) | No. of female patients with SAcFT (%) |
|---|---|
| 0 | 0 |
| 0–30 | 12 (18.2) |
| 30–60 | 18 (27.3) |
| > 60 | 36 (54.5) |
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69-90. ArticlePubMed
- 2. Azodi M, Langer A, Jenison EL. Primary fallopian tube carcinoma with isolated torsion of involved tube. Eur J Gynaecol Oncol 2000; 21: 364-7. PubMed
- 3. Singhal P, Odunsi K, Rodabaugh K, Driscoll D, Lele S. Primary fallopian tube carcinoma: a retrospective clinicopathologic study. Eur J Gynaecol Oncol 2006; 27: 16-8. PubMed
- 4. Kalampokas E, Sofoudis C, Boutas I, Kalampokas T, Tourountous I. Primary fallopian tube carcinoma: a case report and mini-review of the literature. Eur J Gynaecol Oncol 2014; 35: 595-6. PubMed
- 5. Riska A, Leminen A. Updating on primary fallopian tube carcinoma. Acta Obstet Gynecol Scand 2007; 86: 1419-26. ArticlePubMed
- 6. Callahan MJ, Crum CP, Medeiros F, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol 2007; 25: 3985-90. ArticlePubMed
- 7. Kalampokas E, Kalampokas T, Tourountous I. Primary fallopian tube carcinoma. Eur J Obstet Gynecol Reprod Biol 2013; 169: 155-61. ArticlePubMed
- 8. Kessler M, Fotopoulou C, Meyer T. The molecular fingerprint of high grade serous ovarian cancer reflects its fallopian tube origin. Int J Mol Sci 2013; 14: 6571-96. ArticlePubMedPMC
- 9. Reade CJ, McVey RM, Tone AA, et al. The fallopian tube as the origin of high grade serous ovarian cancer: review of a paradigm shift. J Obstet Gynaecol Can 2014; 36: 133-40. ArticlePubMed
- 10. Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci U S A 2012; 109: 3921-6. ArticlePubMedPMC
- 11. Przybycin CG, Kurman RJ, Ronnett BM, Shih IM, Vang R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol 2010; 34: 1407-16. ArticlePubMed
- 12. Riska A, Leminen A, Pukkala E. Sociodemographic determinants of incidence of primary fallopian tube carcinoma, Finland 1953-97. Int J Cancer 2003; 104: 643-5. ArticlePubMed
- 13. Potapov S, Sidorenko R, Galata D, Stratiy N, Gargin V. Peculiarities of catenin activity in the embryonal testicular carcinoma. Georgian Med News 2016; (261):68-73.
- 14. Romaniuk A, Lyndin M, Smiyanov V, Sikora V, Rieznik A, Kuzenko Y, et al. Primary multiple tumor with affection of the thyroid gland, uterus, urinary bladder, mammary gland and other organs. Pathol Res Pract 2017; 213: 574-9. ArticlePubMed
- 15. Oliveira C, Duarte H, Bartosch C, Fernandes D. Small fallopian tube carcinoma with extensive upper abdominal dissemination: a case report. J Med Case Rep 2013; 7: 252.ArticlePubMedPMCPDF
- 16. Pectasides D, Pectasides E, Papaxoinis G, et al. Primary fallopian tube carcinoma: results of a retrospective analysis of 64 patients. Gynecol Oncol 2009; 115: 97-101. ArticlePubMed
- 17. Wang Y, Li L, Wang Y, Tang SN, Zheng W. Fallopian tube secretory cell expansion: a sensitive biomarker for ovarian serous carcinogenesis. Am J Transl Res 2015; 7: 2082-90. PubMedPMC
- 18. Wang Y, Wang Y, Li D, et al. IMP3 signatures of fallopian tube: a risk for pelvic serous cancers. J Hematol Oncol 2014; 7: 49.ArticlePubMedPMCPDF
- 19. Farooq S, Tasleem R, Nazir N, Reshi R, Hassan Z. Histopathological pattern of ovarian neoplasms and estrogen and progesterone receptor expression in primary epithelial tumours and their histopathological correlation. Int J Curr Res Rev 2013; 5: 70-7.
- 20. Rose PG, Piver MS, Tsukada Y. Fallopian tube cancer. The Roswell Park experience. Cancer 1990; 66: 2661-7. ArticlePubMed
- 21. Rosen AC, Reiner A, Klein M, et al. Prognostic factors in primary fallopian tube carcinoma. Austrian Cooperative Study Group for Fallopian Tube Carcinoma. Gynecol Oncol 1994; 53: 307-13. PubMed
- 22. Thomas C, Gustafsson JÅ. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer 2011; 11: 597-608. ArticlePubMedPDF
- 23. Lacy MQ, Hartmann LC, Keeney GL, et al. c-erbB-2 and p53 expression in fallopian tube carcinoma. Cancer 1995; 75: 2891-6. ArticlePubMed
- 24. Alvarado-Cabrero I, Kiyokawa T, Piña P, Valencia-Cedillo R, Santiago-Payán H, Stolnicu S. HER2/neu, p53, MIB1 and PAX8 immunoexpression in primary serous fallopian tube carcinomas. Rev Esp Patol 2016; 49: 219-25. Article
- 25. Chung TK, Cheung TH, To KF, Wong YF. Overexpression of p53 and HER-2/neu and c-myc in primary fallopian tube carcinoma. Gynecol Obstet Invest 2000; 49: 47-51. ArticlePubMedPDF
- 26. Dawson SJ, Makretsov N, Blows FM, et al. BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer 2010; 103: 668-75. ArticlePubMedPMCPDF
- 27. Romaniuk A, Lyndin M. Immune microenvironment as a factor of breast cancer progression. Diagn Pathol 2015; 10: 79.ArticlePubMedPMCPDF
- 28. Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005; 69 Suppl 3: 4-10. ArticlePubMedPDF
- 29. Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell 2010; 140: 268-79. ArticlePubMed
- 30. Lytvynenko M, Bocharova T, Zhelezniakova N, Narbutova T, Gargin V. Cervical transformation in alcohol abuse patients. Georgian Med News 2017; (271):12-7.
- 31. Lytvynenko M, Shkolnikov V, Bocharova T, Sychova L, Gargin V. Peculiarities of proliferative activity of cervical squamous cancer in HIV infection. Georgian Med News 2017; (270):10-5.
REFERENCES
Figure & Data
References
Citations

- Clinical and Morphological Analysis of Odontogenic Tumors and Tooth Developmental Anomalies
Yе.V. Kuzenko, S.M. Hermanchuk, O.O. Mykhno, D.H. Tsepochko, O.V. Kuzenko, A.Yu. Olishkevych
Kharkiv Dental Journal.2025; : 275. CrossRef - Rare non-serous fallopian tube cancers: institutional experience and literature review
Dmitrii Sumtsov, Georgyi Sumtsov, Nataliia Hyriavenko, Mykola Lyndin, Kateryna Sikora, Nataliia Kalashnik, Svitlana Smiian, Igor Gladchuk
Wiener Medizinische Wochenschrift.2024; 174(9-10): 199. CrossRef - UŞAQLIQ BORULARININ BİRİNCİLİ XƏRÇƏNGİ: DİAQNOSTİKASI VƏ MÜALİCƏSİNİN NƏTİCƏLƏRİ
D.G. Sumtsov, G.O. Sumtsov, N.I. Hyriavenko, S.A. Smiian, N.V. Kalashnyk, K.O. Sikora, N.M. Rozhkovska, I.Z. Gladchuk
Azerbaijan Medical Journal.2023; (4): 75. CrossRef - FEATURES OF ENDOMETRIUM STRUCTURE IN ALCOHOL-ABUSING HIV-INFECTED INDIVIDUALS
M. Lytvynenko
Inter Collegas.2021; 8(1): 52. CrossRef - Concurrent Clostridial Enteritis and Oviductal Adenocarcinoma with Carcinomatosis in an Adult Alpaca (Vicugna pacos)
Mandy Womble, Megan E. Schreeg, Allison Hoch, Enoch B. de Souza Meira, Derek Foster, Christopher Premanandan, Tatiane T. Negrão Watanabe
Journal of Comparative Pathology.2021; 189: 52. CrossRef - Problems of primary fallopian tube cancer diagnostics during and after surgery
D.G. Sumtsov, I.Z. Gladchuk, G.O. Sumtsov, N.I. Hyriavenko, M.S. Lyndin, V.V. Sikora, V.M. Zaporozhan
REPRODUCTIVE ENDOCRINOLOGY.2021; (59): 66. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
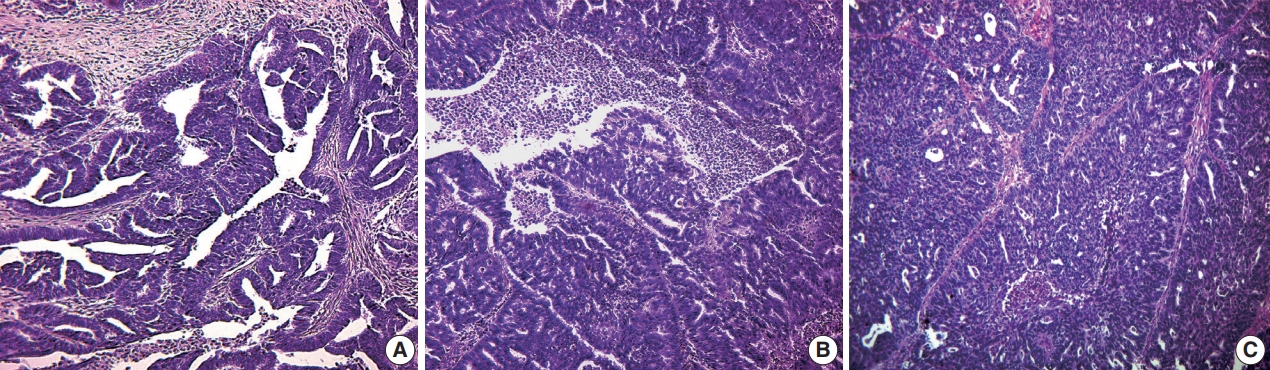
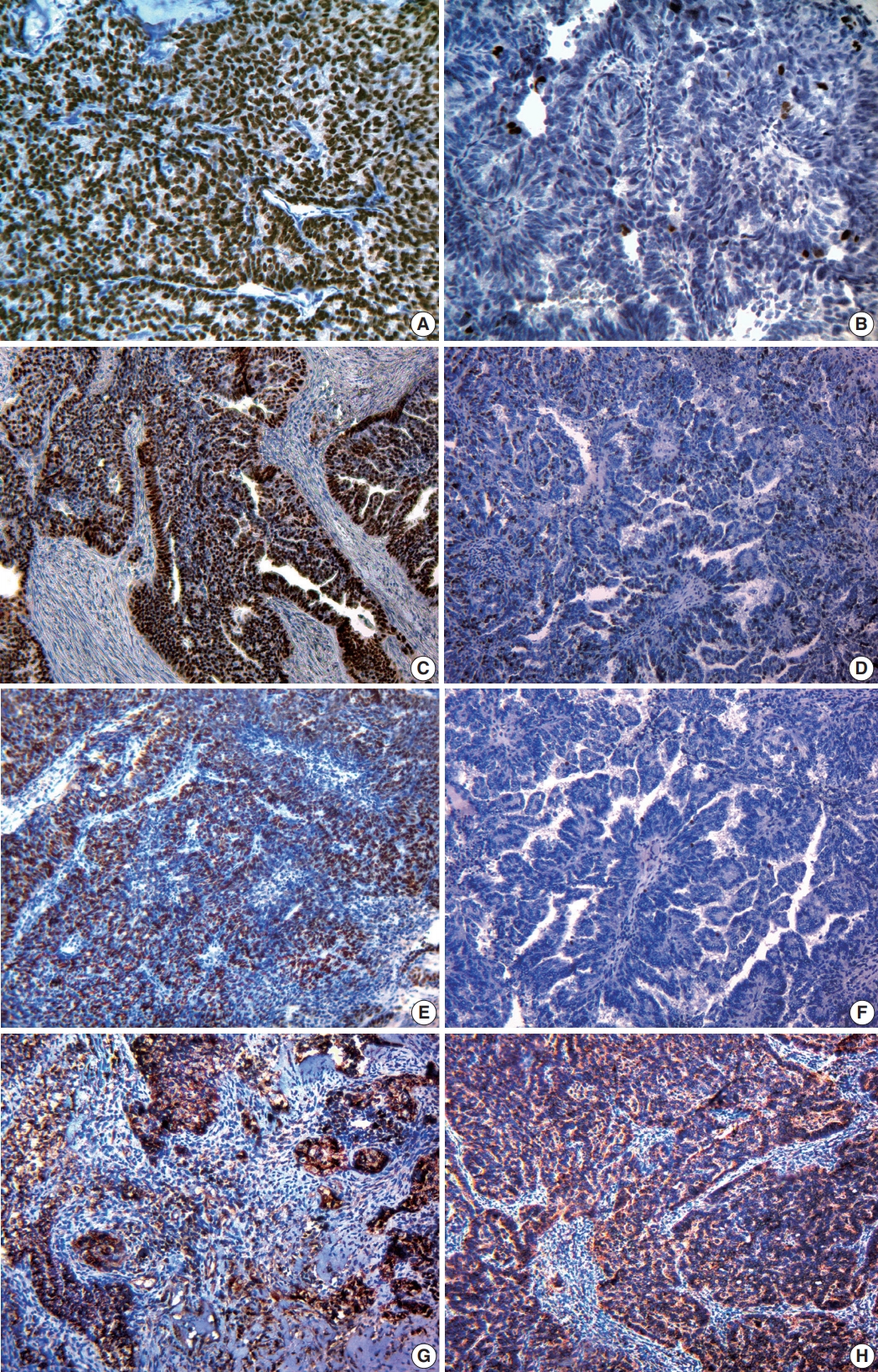
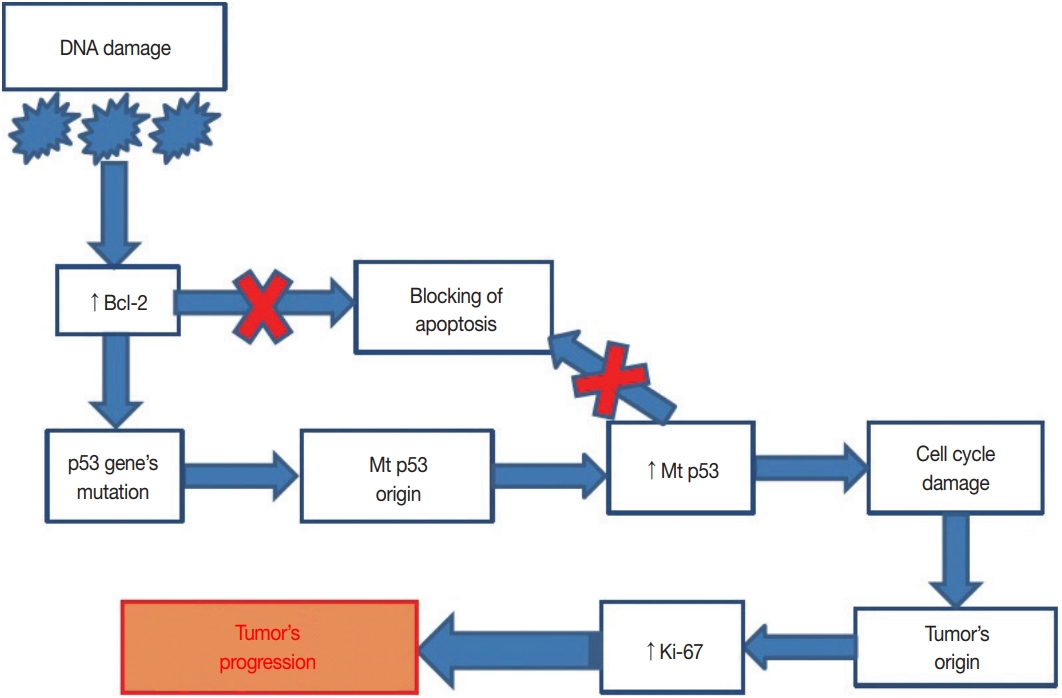
Fig. 1.
Fig. 2.
Fig. 3.
| Antibody | Immunized animal | Clone | Dilution | Pattern | Evaluation features |
|---|---|---|---|---|---|
| ER | Rabbit | SP1 | 1:200 | Nucleus | According to the recommendations of D.C. Allred and taking into account the proportion of colored nuclei and the intensity of their staining |
| PR | Rabbit | YR85 | 1:150 | Nucleus | |
| HER2/neu | Rabbit | SP3 | 1:100 | Membrane | In points (from 0 to 3), according to the manufacturer’s recommendations and taking into account the completeness and intensity of membrane staining |
| Ki-67 | Rabbit | SP6 | 1:100 | Nucleus | 0 point: negative reaction |
| 1 point: weakly positive reaction (n = 0%–30%) | |||||
| 2 points: moderately positive reaction (30% < n < 60%) | |||||
| 3 points: strong positive reaction (n > 60%) | |||||
| p53 | Mouse | SP5 | 1:100 | Nucleus | Weak reaction (10–25% of positively stained tumor cells), moderate positive |
| Bcl-2 | Mouse | 100/D5 | 1:100 | Cytoplasm | Weak expression (1+): weak cytoplasmic staining of more than 10% of tumor cells |
| Moderate (2+): staining of average intensity more than 10% | |||||
| Strong (3+): intense staining of more than 10% of tumor cells | |||||
| VEGF | Rabbit | Polyclon | 1:200 | Cytoplasm and membrane | 0 points: absence of cytoplasmic expression |
| 1 point: weak cytoplasmic staining less than 10% of cells | |||||
| 2 points: weak or moderate expression in 10%–50% of cells | |||||
| 3 points: strong or moderate expression in more than 50% of cells |
| Stage | No. of patients with PCFT (%) |
|---|---|
| 0 | - |
| I | 24 (33.8) |
| IA–T1aN0M0 | 5 (7.0) |
| IB–T1bN0M0 | 8 (11.3) |
| IC–11cN0M0 | 11 (15.5) |
| II | 19 (26.8) |
| IIA–T2aN0M0 | 10 (14.1) |
| IIB–T2bN0M0 | 3 (4.2) |
| IIC–T2cN0M0 | 6 (8.5) |
| III | 28 (39.4) |
| IIIA–T3aN0 M0 | 2 (2.8) |
| IIIB–T3bN0 M0 | 5 (7.0) |
| IIIC–T3c N0M0 | 2 (2.8) |
| IIIC–T1-3N1M0 | 19 (26.8) |
| IV–T1-3N0-1M1 | - |
| Total | 71 (100) |
| Average index of proliferation (%) | No. of female patients with SAcFT (%) |
|---|---|
| 0 | 0 |
| 0–30 | 12 (18.2) |
| 30–60 | 18 (27.3) |
| > 60 | 36 (54.5) |
| Evaluation’s criteria | Average No. of p53 (%) |
|---|---|
| Disease stage according to FIGO classification | |
| I | 10.5 ± 2.2 |
| II | 46.8 ± 5.2 |
| III | 71.7 ± 3.9 |
| Presence of lesion in regional lymph nodes | |
| N0 | 29.7 ± 3.6 |
| N1 | 80.6 ± 2.7 |
| Degree of tumor differentiation | |
| G1 | 5.6 ± 2.4 |
| G2 | 35.6 ± 6.2 |
| G3 | 62.2 ± 4.2 |
| Evaluation’s criteria | No. of Bcl-2–positive tumor cases |
|||
|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | |
| Disease stage according to FIGO classification | ||||
| I (n = 23) | - | 1 | 4 | 18 |
| II (n = 16) | 3 | 2 | 5 | 6 |
| III (n = 27) | 8 | 4 | 9 | 6 |
| The presence of lesion in regional lymph nodes | ||||
| N0 (n = 47) | 2 | 5 | 12 | 28 |
| N1 (n = 19) | 9 | 2 | 6 | 2 |
| Degree of tumor differentiation | ||||
| G1 (n = 10) | - | - | - | 100 |
| G2 (n = 23) | 3 | - | 5 | 15 |
| G3 (n = 33) | 8 | 7 | 13 | 5 |
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor; VEGF, vascular endothelial growth factor.
PCFT, primary cancer of the fallopian tubes; FIGO, International Federation of Gynecology and Obstetrics.
SAcFT, serous adenocarcinoma of the fallopian tubes.
SAcFT, serous adenocarcinoma of the fallopian tubes; FIGO, International Federation of Gynecology and Obstetrics.
SAcFT, serous adenocarcinoma of the fallopian tubes; FIGO, International Federation of Gynecology and Obstetrics.

 E-submission
E-submission





