Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 51(2); 2017 > Article
-
Case Study
Mucinous Carcinoma with Extensive Signet Ring Cell Differentiation: A Case Report - Hye Min Kim, Eun Kyung Kim, Ja Seung Koo
-
Journal of Pathology and Translational Medicine 2017;51(2):176-179.
DOI: https://doi.org/10.4132/jptm.2016.08.17
Published online: December 5, 2016
Department of Pathology, Yonsei University College of Medicine, Seoul, Korea
- Corresponding Author Ja Seung Koo, MD Department of Pathology, Severance Hospital, Yonsei University College of Medicine, 50 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea Tel: +82-2-2228-1772 Fax: +82-2-362-0860 E-mail: kjs1976@yuhs.ac
• Received: June 29, 2016 • Revised: August 11, 2016 • Accepted: August 17, 2016
© 2017 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Breast cancers that present with mucin include mucinous carcinoma and carcinoma with signet ring cell differentiation. The former shows extracellular mucin and the latter shows abundant intracellular mucin. Here, we report a case of breast cancer showing both extracellular mucin and extensive signet ring cell differentiation due to abundant intracellular mucin. Unlike mucinous carcinoma, this case had the features of high-grade nuclear pleomorphism, high mitotic index, estrogen receptor negativity, progesterone receptor negativity, human epidermal growth factor receptor-2 positivity, and ductal type with positivity for E-cadherin. In a case with signet ring cell differentiation, differential diagnosis with metastatic signet ring cell carcinoma of the stomach and colon is essential. In this case, the presence of accompanied ductal carcinoma in situ component and mammaglobin and gross cystic disease fluid protein-15 positivity were findings that suggested the breast as the origin.
- A 64-year-old woman presented with nipple discharge from right breast for 3 months. On physical examination, a palpable mass was noted in the right breast without other remarkable findings. She had no remarkable medical history or familial history. Diagnostic mammogram revealed a 5.4-cm-sized mass with microcalcification in the palpable area in the right upper medial portion of the breast. In magnification view, the parenchymal distortion measured about 6.3 cm in maximal diameter including grouped coarse heterogeneous calcification. In diagnostic ultrasound, a 3-cm-sized heterogeneous area including calcification in the inner part was observed 3 cm from the nipple in the right upper medial 2 o’clock direction and five core needle biopsies were performed. The pathologic diagnosis of biopsy was ductal carcinoma in situ (DCIS) with a suspicious area of invasion showing mucinous differentiation. Breast magnetic resonance imaging showed right nipple retraction without pathologic lymph node, and skeletal metastasis was not observed in whole body bone scan. The patient underwent total mastectomy and sentinel lymph node dissection of the right breast.
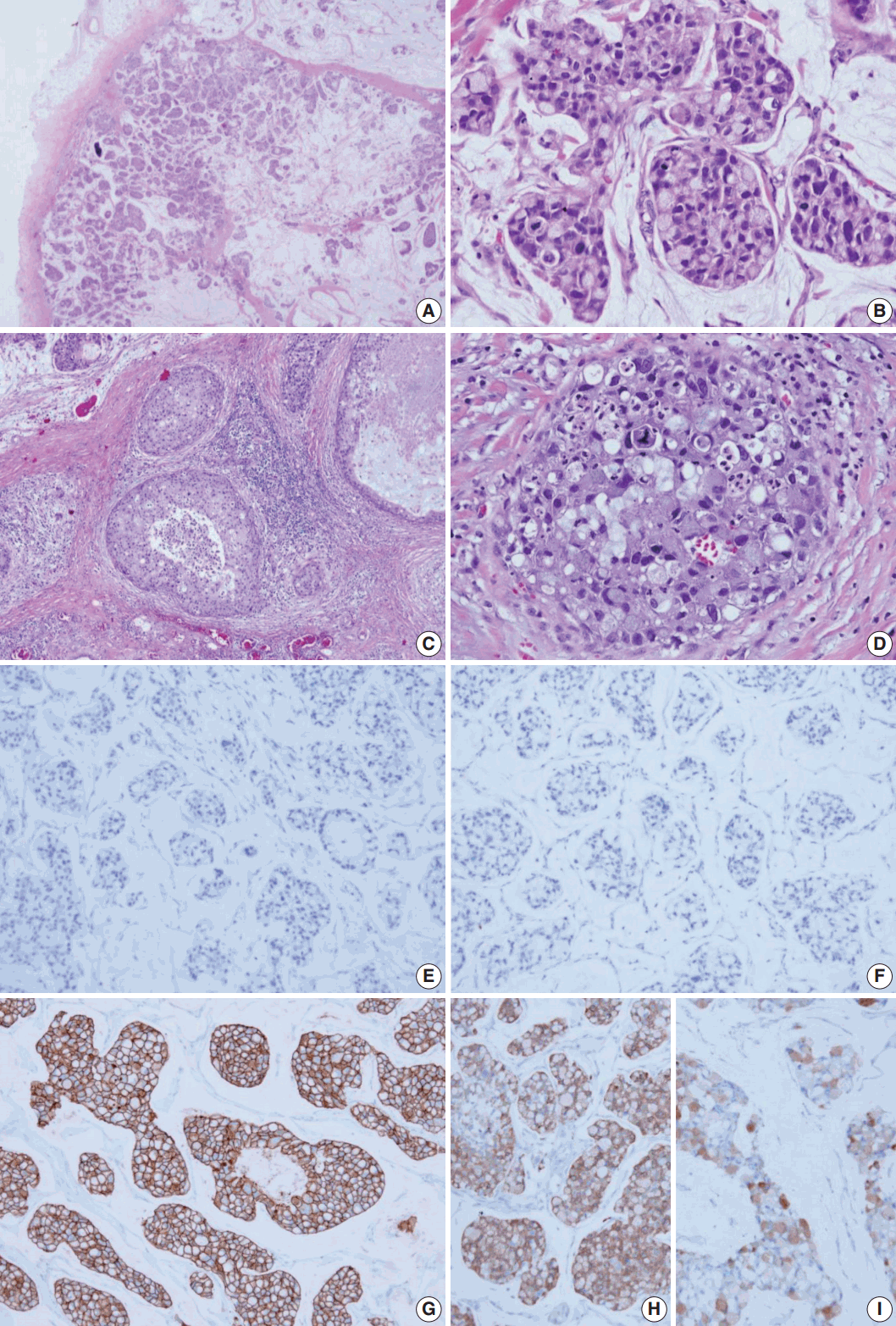
- The surgical specimen was sent to the Department of Pathology. On gross examination, the cut surface revealed a gelatinous gray white mass (2.2×2.0 cm). On histologic examination, the tumor with expanding margin was observed in the low-power view (Fig. 1A). The tumor cell clusters were floating in the mucin pool and the cell density was higher in the periphery than in the center (Fig. 1A). In the high-power view, the tumor cell cluster floating in the mucin pool showed nuclear atypia suitable for nuclear grade 3 and the mitotic count was 14 in 10 high power fields. Many tumor cells were seen as signet ring cells with the tumor nucleus pushed into a corner by abundant intracellular mucin (Fig. 1B). DCIS was observed in the periphery of the expanding invasive nodule, comprising 60% of invasive tumor area (Fig. 1C). The DCIS component showed a significantly high nuclear grade and signet ring cell differentiation, but extracellular mucin was not observed (Fig. 1D). Serial immunohistochemical staining results showed that tumor cells were negative for ER (Fig. 1E) and PR (Fig. 1F) and positive for HER-2 (3+) (Fig. 1G), with a Ki-67 labeling index of about 30%. In addition, tumor cells were positive for mammaglobin (Fig. 1H), gross cystic disease fluid protein-15 (GCDFP-15) (Fig. 1I), E-cadherin, and MUC-1, and tumor mucin was positive for Alcian blue and mucicarmine. A total of 12 axillary lymph nodes were evaluated, but no metastasis was noted. The patient has been followed on an outpatient basis after surgery and to date, there is no evidence of recurrence or metastasis.
CASE REPORT
- Representative breast cancers that secrete mucin are mucinous carcinoma and carcinoma with signet ring cell differentiation according to WHO classification [1]. In mucinous carcinoma, mucin appears as extracellular mucin [2], whereas in carcinoma with signet ring cell differentiation, mucin is shown as abundant intracellular mucin [3]. However, the case reported here had the typical findings of both extracellular mucin and extensive signet ring cell differentiation due to abundant intracellular mucin. In addition, this case was different from typical mucinous carcinoma due to the existence of the following features: (1) high-grade nuclear pleomorphism, (2) high mitotic index, and (3) ER negativity, PR negativity, and HER-2 positivity. Carcinoma with signet ring cell differentiation has been reported to appear mainly in lobular carcinoma [9]. However, we suggest that this case is ductal type due to the positivity of E-cadherin. In the case of carcinoma with signet ring cell differentiation, hormone receptors are reported to be positive [7]; however, in this case, there was a difference in that the ER and PR immunohistochemical staining results were negative. In previous studies of carcinoma with signet ring cell differentiation, some reported that mucinous carcinoma is accompanied by signet ring cell component, which is a similar finding with this case. However, the proportion of signet ring cell component in mucinous carcinoma was reported to be 8%–17%, which shows significant difference with this case in the amount of signet ring cell component [4]. Previously published case reports that are most similar to this case include the report by Leung et al. [10], which showed very similar histologic feature with this case. Furthermore, Kuroda et al. [11] reported a case of invasive ductal carcinoma of breast with signet ring cell and mucinous carcinoma components, which is a similar finding to that in this report. To the best of our knowledge, the case reported here is the first case reported in Korea.
- In such a case with signet ring cell differentiation, the most important differential diagnosis is metastatic signet ring cell carcinoma. In signet ring cell carcinoma of the stomach and colon extracellular mucin and signet ring cell differentiation due to abundant intracellular mucin are frequently observed. The presence of accompanied DCIS component and mammaglobin and GCDFP-15 positivity in this case suggest breast as the origin. The immunohistochemical markers widely used to help differentiate between signet ring cell carcinoma of the stomach and colon and signet ring cell carcinoma of the breast are CDX2, cytokeratin 20, MUC-1, and ER [12].
- The case reported here shows both extracellular mucin and extensive signet ring cell differentiation due to abundant intracellular mucin. Notably, this finding does not appear in separate regions in the tumor, but as a pattern of floating signet ring cell in a mucin pool. Therefore, we speculate that this finding is not a result of a mixed form of mucinous carcinoma and carcinoma with signet ring cell differentiation, but rather is signet ring cell differentiation in mucinous carcinoma cells. We report this case because it has the features of high-grade nuclear pleomorphism, high mitotic index, ER negativity, PR negativity, and HER-2 positivity, which differ from typical mucinous carcinoma.
DISCUSSION
Fig. 1.Histologic features and biomarker status in mucinous carcinoma with extensive signet ring cell differentiation. (A) In the low-power view, a tumor with an expanding margin is observed. Tumor cell clusters floating in the mucin pool are shown and the cell density is higher in the periphery than in the center. (B) In the high-power view, tumor cell cluster floating in the mucin pool shows dysplasia suitable for the nuclear grade 3. Many tumor cells are seen as signet ring cell with the nucleus pushed into the corner by abundant intracellular mucin. (C) Ductal carcinoma in situ (DCIS) is observed in the periphery of the expanding invasive nodule. (D) The DCIS component shows significantly high nuclear grade and signet ring cell differentiation. Mucinous carcinoma cells are negative for estrogen receptor (E) and progesterone receptor (F) and positive for human epidermal growth factor receptor-2 (3+) (G). Mucinous carcinoma cells are positive for mammaglobin (H) and gross cystic disease fluid protein-15 (I).

- 1. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, Vijver MJ. WHO classification of tumours of the breast. 4th ed. Lyon: International Agency for Research on Cancer, 2012.
- 2. Coady AT, Shousha S, Dawson PM, Moss M, James KR, Bull TB. Mucinous carcinoma of the breast: further characterization of its three subtypes. Histopathology 1989; 15: 617-26. ArticlePubMed
- 3. Hull MT, Seo IS, Battersby JS, Csicsko JF. Signet-ring cell carcinoma of the breast: a clinicopathologic study of 24 cases. Am J Clin Pathol 1980; 73: 31-5. ArticlePubMed
- 4. Bartosch C, Mendes N, Rios E, et al. Morphological features and mucin expression profile of breast carcinomas with signet-ring cell differentiation. Pathol Res Pract 2015; 211: 588-95. ArticlePubMed
- 5. Barkley CR, Ligibel JA, Wong JS, Lipsitz S, Smith BL, Golshan M. Mucinous breast carcinoma: a large contemporary series. Am J Surg 2008; 196: 549-51. ArticlePubMed
- 6. Lacroix-Triki M, Suarez PH, MacKay A, et al. Mucinous carcinoma of the breast is genomically distinct from invasive ductal carcinomas of no special type. J Pathol 2010; 222: 282-98. ArticlePubMedPDF
- 7. O’Connell FP, Wang HH, Odze RD. Utility of immunohistochemistry in distinguishing primary adenocarcinomas from metastatic breast carcinomas in the gastrointestinal tract. Arch Pathol Lab Med 2005; 129: 338-47. ArticlePubMedPDF
- 8. Frost AR, Terahata S, Yeh IT, Siegel RS, Overmoyer B, Silverberg SG. The significance of signet ring cells in infiltrating lobular carcinoma of the breast. Arch Pathol Lab Med 1995; 119: 64-8. PubMed
- 9. Raju U, Ma CK, Shaw A. Signet ring variant of lobular carcinoma of the breast: a clinicopathologic and immunohistochemical study. Mod Pathol 1993; 6: 516-20. PubMed
- 10. Leung KM, Yeoh GP, Chan JK, Cheung PS, Chan KW. Ductal type signet ring cell carcinoma of breast with growth pattern of pure mucinous carcinoma. Pathology 2011; 43: 282-4. ArticlePubMed
- 11. Kuroda N, Fujishima N, Ohara M, Hirouchi T, Mizuno K, Lee GH. Invasive ductal carcinoma of the breast with signet-ring cell and mucinous carcinoma components: diagnostic utility of immunocytochemistry of signet-ring cells in aspiration cytology materials. Diagn Cytopathol 2007; 35: 171-3. ArticlePubMed
- 12. Chu PG, Weiss LM. Immunohistochemical characterization of signet-ring cell carcinomas of the stomach, breast, and colon. Am J Clin Pathol 2004; 121: 884-92. ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Signet ring cell carcinoma of the urachus: survival and pathologic outcomes from the national cancer database
Deerush Kannan Sakthivel, Pushan Prabhakar, Mohamed Javid Raja Iyub, Rohan Garje, Murugesan Manoharan
International Urology and Nephrology.2025;[Epub] CrossRef - Research on the Histological Features and Pathological Types of Gastric Adenocarcinoma With Mucinous Differentiation
Nian-Long Meng, Yang-kun Wang, Hai-Li Wang, Jun-Ling Zhou, Su-nan Wang
Frontiers in Medicine.2022;[Epub] CrossRef - Clinicopathologic characteristics of HER2-positive pure mucinous carcinoma of the breast
Yunjeong Jang, Hera Jung, Han-Na Kim, Youjeong Seo, Emad Alsharif, Seok Jin Nam, Seok Won Kim, Jeong Eon Lee, Yeon Hee Park, Eun Yoon Cho, Soo Youn Cho
Journal of Pathology and Translational Medicine.2020; 54(1): 95. CrossRef - Human Epidermal Growth Factor Receptor 2-positive Mucinous Carcinoma with Signet Ring Cell Differentiation, Which Showed Complete Response after Neoadjuvant Chemotherapy
Yunjeong Jang, Eun Yoon Cho, Soo Youn Cho
Journal of Breast Cancer.2019; 22(2): 336. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Mucinous Carcinoma with Extensive Signet Ring Cell Differentiation: A Case Report
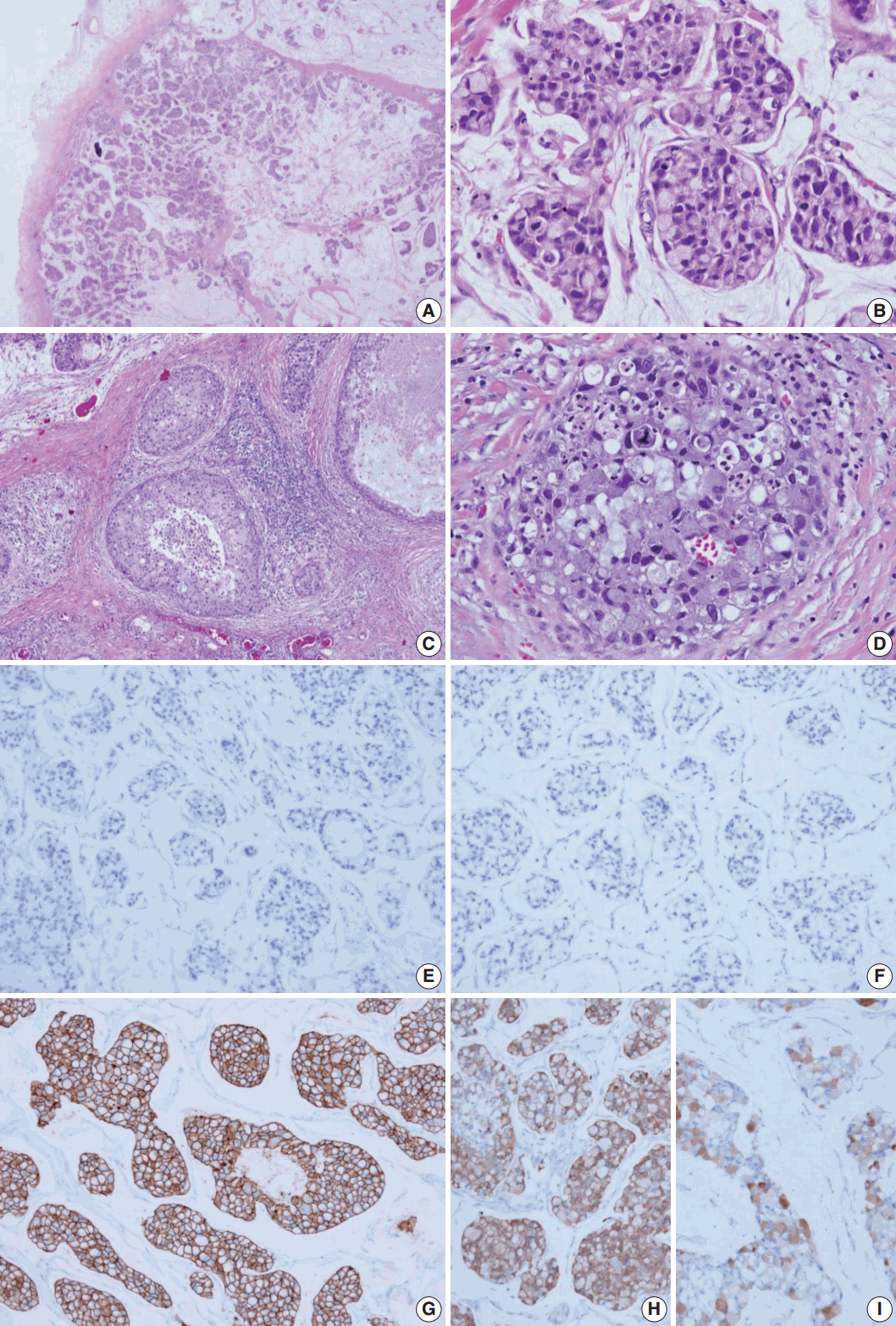
Fig. 1. Histologic features and biomarker status in mucinous carcinoma with extensive signet ring cell differentiation. (A) In the low-power view, a tumor with an expanding margin is observed. Tumor cell clusters floating in the mucin pool are shown and the cell density is higher in the periphery than in the center. (B) In the high-power view, tumor cell cluster floating in the mucin pool shows dysplasia suitable for the nuclear grade 3. Many tumor cells are seen as signet ring cell with the nucleus pushed into the corner by abundant intracellular mucin. (C) Ductal carcinoma in situ (DCIS) is observed in the periphery of the expanding invasive nodule. (D) The DCIS component shows significantly high nuclear grade and signet ring cell differentiation. Mucinous carcinoma cells are negative for estrogen receptor (E) and progesterone receptor (F) and positive for human epidermal growth factor receptor-2 (3+) (G). Mucinous carcinoma cells are positive for mammaglobin (H) and gross cystic disease fluid protein-15 (I).
Fig. 1.
Mucinous Carcinoma with Extensive Signet Ring Cell Differentiation: A Case Report

 E-submission
E-submission



