Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 50(2); 2016 > Article
-
Original Article
Prognostic Significance of Aquaporin 5 Expression in Non-small Cell Lung Cancer - Young Min Jo, Tae In Park,, Hwa Young Lee1, Ji Yun Jeong, Won Kee Lee2
-
Journal of Pathology and Translational Medicine 2016;50(2):122-128.
DOI: https://doi.org/10.4132/jptm.2015.10.31
Published online: February 8, 2016
Department of Pathology, Kyungpook National University Hospital, Kyungpook National University School of Medicine, Daegu, Korea
1Department of Pathology, Kyungpook National University School of Medicine, Daegu, Korea
2Biostatistics, Biomedical Research Institute, Kyungpook National University School of Medicine, Daegu, Korea
- Corresponding Author: Tae In Park, MD, PhD Department of Pathology, Kyungpook National University Hospital, Kyungpook National University School of Medicine, 680 Gukchaebosang-ro, Jung-gu, Daegu 41944, Korea Tel: +82-53-200-4854 Fax: +82-53-426-1525 E-mail: tipark@knu.ac.kr
© 2016 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background:
- Aquaporins are water channel proteins that play a major role in the movement of water in various human tissues. Recently, it has been found that aquaporins have influence in the carcinogenesis of human malignancies. We analyzed the prognostic impact of aquaporin 5 (AQP5) in non-small lung cancer (NSCLC).
-
Methods:
- Seventy-six cases of NSCLC were studied, including 44 cases of adenocarcinoma (ADC) and 32 cases of squamous cell carcinoma (SQCC). Tissue microarray was constructed and immunohistochemical staining for AQP5 was performed.
-
Results:
- AQP5 was positive in 59.2% of the total enrolled NSCLCs (63.7% in ADC and 53.1% in SQCC). The difference in expression of AQP5 according to the histologic grade of the tumor was significant (p<.047), but not in a serial order. When ADC and SQCC were separately evaluated, no significant difference was observed according to the histologic grade of the tumor (p=.076 in ADC and p=.631 in SQCC). No difference was observed between AQP5 expression and other demographic data and tumor characteristics. Disease-free survival (DFS) was higher in AQP5 negative cases than positive cases in ADC (p=.047), but no significance was found in SQCC (p=.068). We were unable to find a significance between AQP5 overexpression and overall survival in either ADC (p=.210) or SQCC (p=.533).
-
Conclusions:
- AQP5 expression is associated with DFS in ADC of the lung and tumor grade of NSCLC. The present study suggests that AQP5 can be a prognostic factor of NSCLC.
- Patients and tissue selection
- All tissues used in this study were formalin-fixed and paraffinembedded NSCLC tissues obtained from the Kyungpook National University Hospital between 1998 and 2008. Clinicopathologic information was obtained from electronic medical records. Two pathologists examined the slides of selected cases and reconfirmed the diagnoses of ADC and SQCC according to the World Health Organization classifications. The pathologic TNM stages were evaluated based on the seventh edition of the American Joint Committee on Cancer (AJCC) cancer staging system. A total of 385 cases of NSCLC were identified, of which 338 cases were ADC and SQCC. Other types of NSCLC such as large cell neuroendocrine carcinoma were excluded due to their rare incidence. However, only 76 cases were used in this study; cases with loss of tissue due to other experiments, cases lost to follow-up, and cases that lacked certain clinicopathologic data were all excluded. Consequently, 44 cases of ADC and 32 cases of SQCC were enrolled in the study. This study has been approved by the Institutional Review Board of Kyungpook National University.
- Tissue microarray and immunohistochemical staining
- Tissue microarrays were constructed from the representative tumor areas, each core measuring 3 mm in diameter. Normal human lung tissues were used as controls. Immunohistochemical staining was performed using the BenchMark automated staining instrument (Ventana Medical System, Tucson, AZ, USA). The AQP5 antibody was an affinity-purified goat antibody, used at a dilution of 1:50. Tissue sections were cut in 4 µm thickness and deparaffinized in xylene, rehydrated in three graded alcohol chambers, and treated with 3% hydrogen peroxide in methanol. The avidin-biotin-peroxidase technique was used for visualization (DAKO LSAB Kit, DAKO Cytomation, Carpinteria, CA, USA). All the stained slides were examined by two pathologists who were blinded from all clinicopathologic data.
- Scoring of AQP5 immunohistochemistry
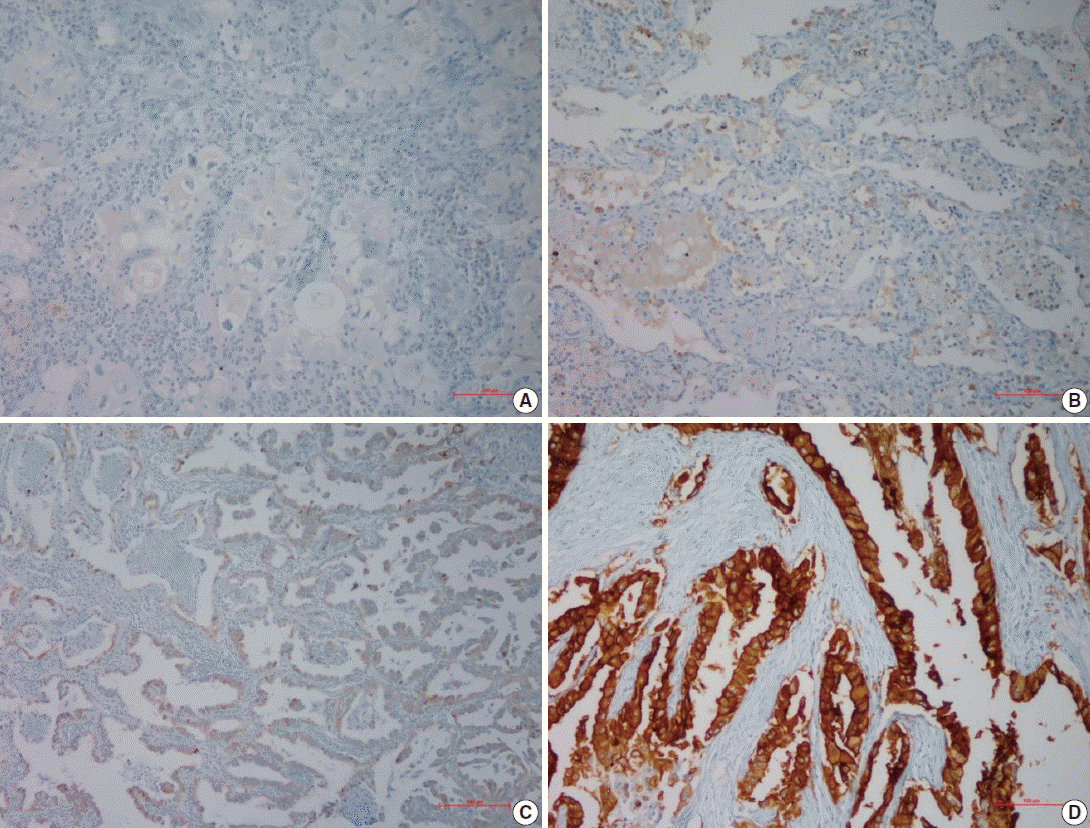
- For semi-quantitative analysis of the AQP5 immunoreactivity, we used H-score method [15]. All cells had cytoplasmic staining pattern. We counted 100 tumor cells in the hot spot, and the H-score was calculated by adding the percentages of strongly stained (3×), moderately stained (2×), and weakly stained (1×) nuclei, resulting in a possible range of 0–300. Two pathologists evaluated and obtained this score. We divided the H-score interval into 0–15 (less than 5% of the maximum score, AQP5 negative), 15–75 (5%–25%, AQP5 weak positivity, 1+), 75–150 (25%–50%, AQP5 moderate positivity, 2+), and over 151 (50%–100%, AQP5 strong positivity, 3+). The median values of H-score for each interval were 4 for negative cases, 53 for weak, 108 for moderate, and 267 for strong staining. We considered over 1+ immunohistochemical staining as positive.
- Statistical analysis
- Statistical analysis was performed using SAS ver. 9.4 (SAS Institute Inc., Cary, NC, USA) and R ver. 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria), and the t test and log-rank test were used. Patient’s mean age, histologic grade, and tumor stage were evaluated by t test. Survival curves were produced by log-rank test to evaluate the disease-free survival (DFS) status and overall survival (OS) status. Age, sex, histological grade, and tumor stage were accounted. All p-values were two-sided, and differences at p<.05 were considered statistically significant.
MATERIALS AND METHODS
- Patient characteristics
- Patient characteristics are shown in Table 1. Of the 76 cases, 52 were men and 24 were women. The mean age was 64 years (range, 41 to 82 years). All cases were diagnosed as either ADC (n=44) or SQCC (n=32). The most prevalent histologic grade was moderately differentiated, with 43 cases (56.6%). The pathologic stages were as follows: stage I (n=36, 47.3%), stage II (25, 32.9%), stage III (15, 19.8%), and stage IV (0, 0%). AQP5 showed significantly different expressions depending on the histologic grade (p<.047), but not in a serial order. When ADC and SQCC were separately evaluated, there was no significance regarding the histologic grade (in ADC, p=.076 and in SQCC, p= .631). No difference was identified between AQP5 expression and other clinical and tumor characteristics. We included N3 patients who underwent surgery due to tumor burden. In all cases, complete resection was performed, and resection margins were free from tumor in all cases.
- AQP5 immunoexpression
- Following immunohistochemical staining, AQP5 expression was evaluated by H-score with staining intensity in the hot spot and categorized into the following: no staining (–), weak or mild staining (1+), moderate staining (2+), and strong staining (3+). The representative images of each score are shown in Fig. 1 and the results are shown in Table 2. The positive cases totaled 28 (57.9%) in ADC and 17 (42.1%) in SQCC. Specifically, 1+, 2+, and 3+ were scored in six ADC and three SQCC cases (total nine), seven ADC and six SQCC cases (total 13), and 15 ADC and eight SQCC cases (total 23), respectively.
- Prognostic impact of AQP5 in NSCLC
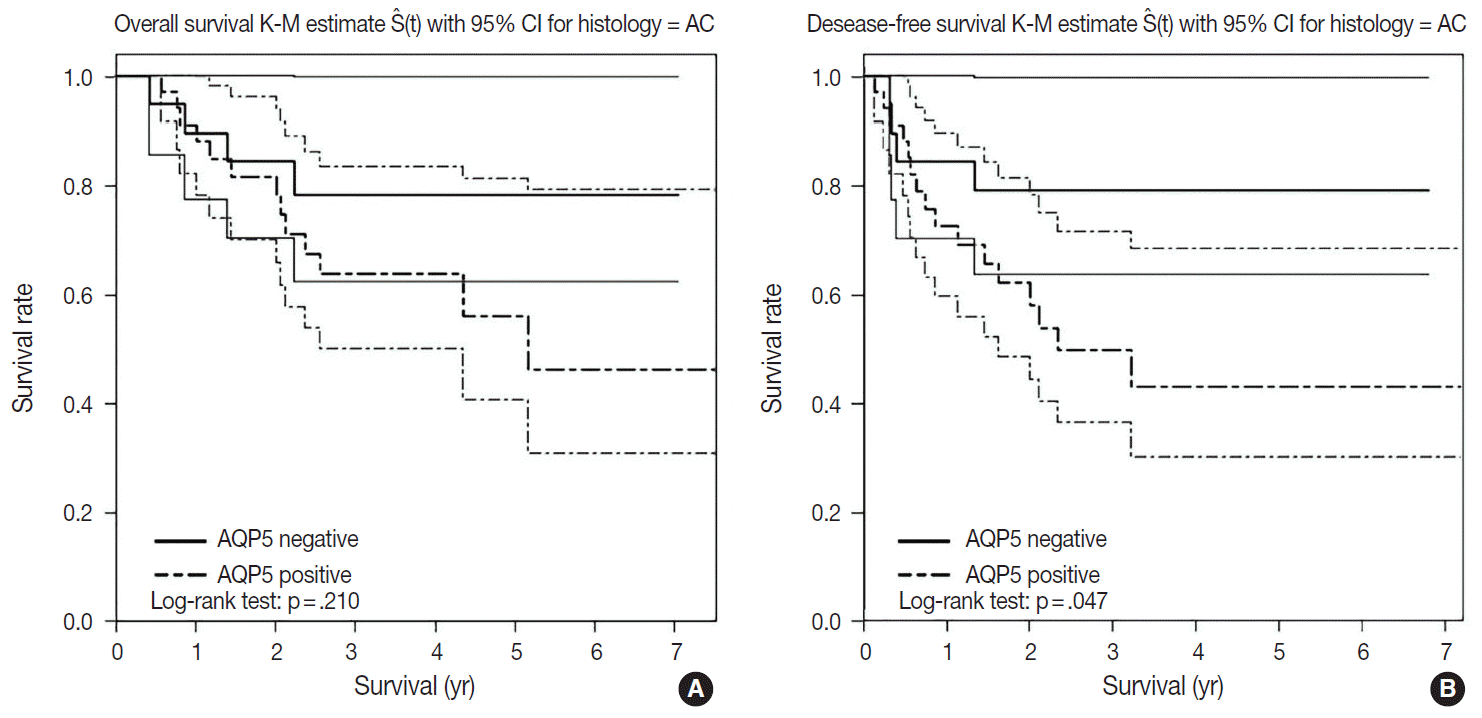
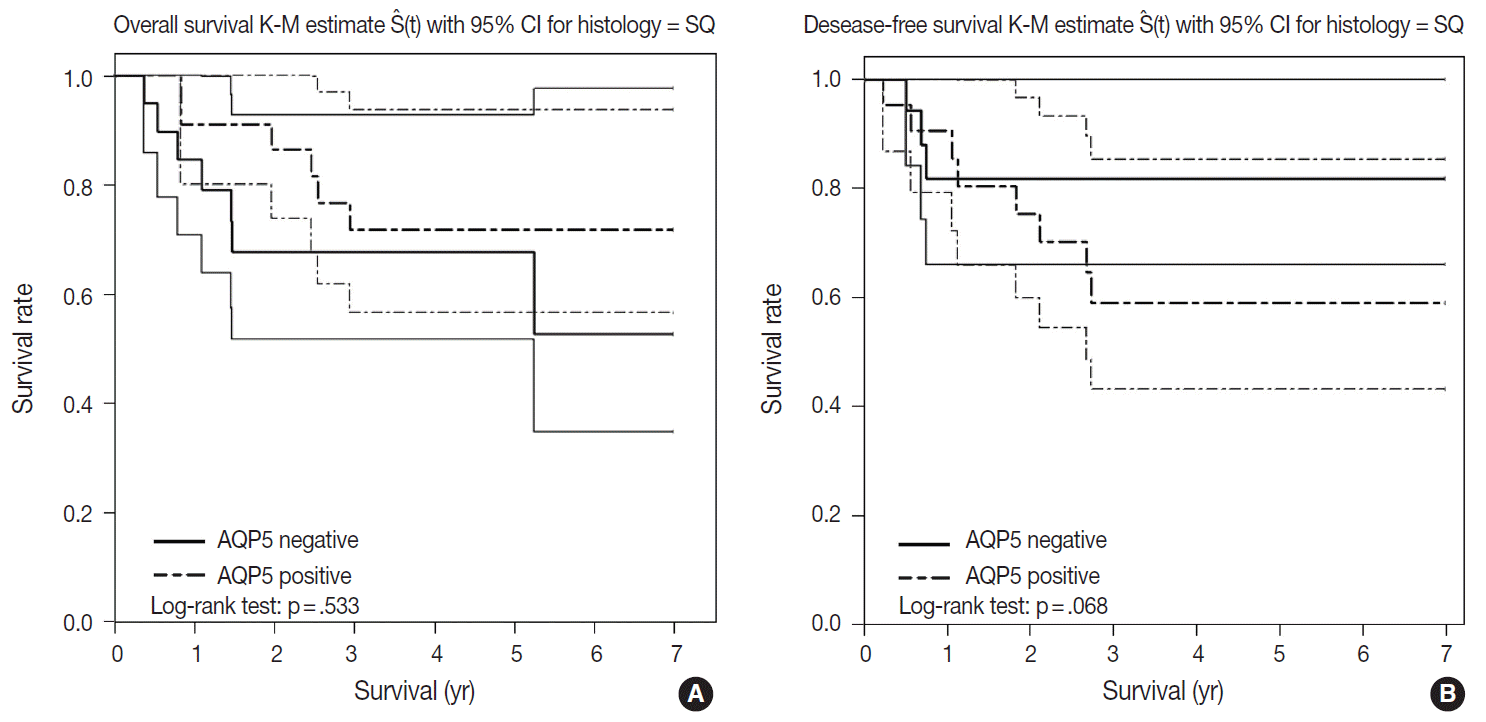
- We had long-term follow-up data for all patients included in this study, ranging from 1 year to 9 years. Within that period, 31 patients died of primary lung cancer or related complications. Eighteen of the 44 patients with ADC and 13 of the 32 patients with SQCC died. Seven cases of death in ADC were due to the primary disease and others deaths were due to complications including pneumonia, cachexia and treatment related death. In SQCC, five cases were died due to the primary disease and others were due to complications. The number of patients with recurrent lung cancer was 21 in ADC and 12 in SQCC. DFS and OS along with AQP5 positive and negative cases are shown in Fig. 2 for ADC and in Fig. 3 for SQCC. In the log-rank test graph, AQP5 positive cases are represented with a dotted line and AQP5 negative cases are represented with a solid line in both ADC and SQCC with 95% confidence interval.
- In the DFS graph, the p-value of AQP5 positive patients is .047 in ADC, which shows the significance in terms of prognostic impact. However, the other parameters were not significant, as shown in Figs. 2 and 3. We did find more correlation with AQP5 positive status in the earlier stage (stage I) than in the higher stages in the DFS, but statistical correlation was not significant (p=.152 vs. p=.929). Meanwhile, recurrence rate, stage at the time of diagnosis and the cause of patient death (primary disease vs. other complications) did not correlate with the AQP5 status.
RESULTS
- Although the role of AQP5 in both normal control and tumor tissue has not been clarified, few studies have reported the effect of AQPs in carcinogenesis and tumor progression. Some studies reported that expression of AQP1 was frequently involved in the development of colon cancer, pancreatic cancer, brain tumors, and renal cell carcinoma with paralleling angiogenesis [5,6]. Additionally, AQP5 expression in various human cancers has been studied. Burghardt et al. [7] reported the expression of AQP5 in pancreatic cancer and ovarian cancer. In another study conducted by Kang et al. [16], AQP5 showed a good correlation with lymph node metastasis in patients with colon cancer, but the OS or DFS was not found to be significant. Lee et al. [17] showed that the AQP5 upregulation led to unfavorable prognoses in patients with estrogen receptor positive breast cancer, and it led to significantly worse prognosis especially in the case of early breast cancer. In lung cancer, Guo and Jin [14] showed that NFAT5 promotes proliferation and migration of ADC partly via regulation of AQP5 expression. However, very few studies have been conducted in this field until recently. Based on these observations, we hypothesized that the NSCLCs with AQP5 positive staining would have more frequent tumor recurrence, shorter DFS, and poorer OS.
- In our study, APQ5 was expressed in the portion of the lung cancer patients in both ADC and SQCC. Among various clinicopathologic features, tumor grade was significantly correlated with p-value of .047. This is suggestive of a prognostic implication of AQP5 in NSCLCs. Although the percentage of AQP5 positive cases is not serially ordered as the degree of differentiation in our results, more extensive study should be conducted to verify the exact significance. In addition, when we separately evaluated the tumor grade with AQP5 in both ADC and SQCC, no significant correlation was identified. For the other variables, we could not find any significant correlation with AQP5 expression.
- AQP5 positive cases showed a poorer DFS rate than the negative ones (p=.047) in ADC. However, the AQP5 positive status was not significantly correlated with OS in ADC cases (p=.210). The crude hazard ratio (HR) and 95% confidence interval of ADC in DFS were 2.291 (0.97–8.75) and the HR and 95% confidence interval after adjustment for smoking status, age, tumor stage, etc. were 2.95 (0.98–8.92). Median survival rate in AQP5 positive cases was 1.94 year. While median survival rate in AQP5 negative cases was not evaluated. Its level was not fall below the 0.75. In the DFS graph for SQCC, the p-value of AQP5 positive patients is .068, and the prognostic impact was not significant for OS between AQP5 positive and negative cases (p=.533). Additionally, neither recurrence rate nor tumor stage correlated with the AQP5 status. That is, no parameter correlated with AQP5 positivity in SQCC cases. This may be explained by the small number of cases involved in the study, and perhaps a study with a large number of cases can demonstrate its significance as a prognostic marker.
- In addition to the relatively small sample size, our study had several limitations. We originally sought to determine the clinical usefulness of immunohistochemical staining for AQP5 protein for determining prognosis in NSCLC. We examined earlier studies for the significance of AQP5 in detecting and predicting prognosis in patients with ADC or SQCC. In addition, we focused on correlating DFS rate, OS rate, recurrence rate, and histological stage with AQP5. However, the correlation of immunoexpression of AQP5 in malignant tissue with poor prognosis has already been reported. However, to date, only a few studies on this topic have been published, and our study will provide an additional evidence to support the correlation of AQP5 with survival rates in ADC.
- We examined the usefulness of immunohistochemical staining of AQP5 for clinical significance. However, we did not evaluate the molecular pathogenesis, molecular pathway, or carcinogenetic mechanisms. Many such pathways and molecules were unknown until recently. More molecules and carcinogenetic processes related to invasion, adhesion, migration, and motility should be identified and studied.
- Lung cancer subtypes may have distinct carcinogenetic pathways. Although ADC and SQCC are the predominant subtypes, others such as large cell neuroendocrine carcinoma and small cell carcinoma do exist. Even though they are rare, they should be included when studying carcinogenetic molecules and pathways in NSCLC. Lastly, many studies referenced in our study had been performed using a single cell line. More studies using other cell lines and subtypes of NSCLC can be the basis for future research.
- In conclusion, the results of the current study show a correlation between AQP5 expression and poorer DFS in the ADC subtype of NSCLC. We believe that AQP5 can be a potential novel prognostic biomarker in ADC. Further research is needed to determine its utility in other subtypes of lung cancer.
DISCUSSION
Acknowledgments
- 1. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart. 4th ed. Lyon: IARC Press, 2015; 26-52.
- 2. King LS, Agre P. Pathophysiology of the aquaporin water channels. Annu Rev Physiol 1996; 58: 619-48. ArticlePubMed
- 3. Verkman AS, van Hoek AN, Ma T, et al. Water transport across mammalian cell membranes. Am J Physiol 1996; 270(1 Pt 1):C12-30. ArticlePubMed
- 4. Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med 2004; 10: 789-99. ArticlePubMedPDF
- 5. Saadoun S, Papadopoulos MC, Davies DC, Bell BA, Krishna S. Increased aquaporin 1 water channel expression in human brain tumours. Br J Cancer 2002; 87: 621-3. ArticlePubMedPMCPDF
- 6. Moon C, Soria JC, Jang SJ, et al. Involvement of aquaporins in colorectal carcinogenesis. Oncogene 2003; 22: 6699-703. ArticlePubMedPDF
- 7. Burghardt B, Elkaer ML, Kwon TH, et al. Distribution of aquaporin water channels AQP1 and AQP5 in the ductal system of the human pancreas. Gut 2003; 52: 1008-16. ArticlePubMedPMC
- 8. Woo J, Lee J, Chae YK, et al. Overexpression of AQP5, a putative oncogene, promotes cell growth and transformation. Cancer Lett 2008; 264: 54-62. ArticlePubMedPMC
- 9. Vacca A, Frigeri A, Ribatti D, et al. Microvessel overexpression of aquaporin 1 parallels bone marrow angiogenesis in patients with active multiple myeloma. Br J Haematol 2001; 113: 415-21. ArticlePubMedPDF
- 10. Moon C, Williams JB, Preston GM, et al. The mouse aquaporin-1 gene. Genomics 1995; 30: 354-7. PubMed
- 11. Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 2005; 434: 786-92. ArticlePubMedPDF
- 12. Moon C, Rousseau R, Soria JC, et al. Aquaporin expression in human lymphocytes and dendritic cells. Am J Hematol 2004; 75: 128-33. ArticlePubMed
- 13. Chae YK, Woo J, Kim MJ, et al. Expression of aquaporin 5 (AQP5) promotes tumor invasion in human non small cell lung cancer. PLoS One 2008; 3: e2162. Article
- 14. Guo K, Jin F. NFAT5 promotes proliferation and migration of lung adenocarcinoma cells in part through regulating AQP5 expression. Biochem Biophys Res Commun 2015; 465: 644-9. ArticlePubMed
- 15. Ishibashi H, Suzuki T, Suzuki S, et al. Sex steroid hormone receptors in human thymoma. J Clin Endocrinol Metab 2003; 88: 2309-17. ArticlePubMed
- 16. Kang BW, Kim JG, Lee SJ, et al. Expression of aquaporin-1, aquaporin-3, and aquaporin-5 correlates with nodal metastasis in colon cancer. Oncology 2015; 88: 369-76. ArticlePubMedPDF
- 17. Lee SJ, Chae YS, Kim JG, et al. AQP5 expression predicts survival in patients with early breast cancer. Ann Surg Oncol 2014; 21: 375-83. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

- AQP5 promotes epithelial-mesenchymal transition and tumor growth through activating the Wnt/β-catenin pathway in triple-negative breast cancer
Zhengcai Zhu, Tao Li, Honggang Wang, Lianghe Jiao
Mutation Research - Fundamental and Molecular Mechanisms of Mutagenesis.2024; 829: 111868. CrossRef - Aquaporin-mediated dysregulation of cell migration in disease states
Ian M. Smith, Shohini Banerjee, Allison K. Moses, Kimberly M. Stroka
Cellular and Molecular Life Sciences.2023;[Epub] CrossRef - The Role of Aquaporin 5 (AQP5) in Lung Adenocarcinoma: A Review Article
Lukasz Jaskiewicz, Anna Romaszko-Wojtowicz, Anna Doboszynska, Agnieszka Skowronska
Cells.2023; 12(3): 468. CrossRef - Aquaporins 1, 3 and 5 in Different Tumors, their Expression, Prognosis Value and Role as New Therapeutic Targets
Mahdieh-Sadat Moosavi, Yalda Elham
Pathology & Oncology Research.2020; 26(2): 615. CrossRef - Aquaporins in lung health and disease: Emerging roles, regulation, and clinical implications
Ekta Yadav, Niket Yadav, Ariel Hus, Jagjit S. Yadav
Respiratory Medicine.2020; 174: 106193. CrossRef - Combined Systematic Review and Transcriptomic Analyses of Mammalian Aquaporin Classes 1 to 10 as Biomarkers and Prognostic Indicators in Diverse Cancers
Pak Hin Chow, Joanne Bowen, Andrea J Yool
Cancers.2020; 12(7): 1911. CrossRef - Prognostic Role of S100A8 and S100A9 Protein Expressions in Non-small Cell Carcinoma of the Lung
Hyun Min Koh, Hyo Jung An, Gyung Hyuck Ko, Jeong Hee Lee, Jong Sil Lee, Dong Chul Kim, Jung Wook Yang, Min Hye Kim, Sung Hwan Kim, Kyung Nyeo Jeon, Gyeong-Won Lee, Se Min Jang, Dae Hyun Song
Journal of Pathology and Translational Medicine.2019; 53(1): 13. CrossRef - Effect of FGF/FGFR pathway blocking on lung adenocarcinoma and its cancer‐associated fibroblasts
Ahmed E Hegab, Mari Ozaki, Naofumi Kameyama, Jingtao Gao, Shizuko Kagawa, Hiroyuki Yasuda, Kenzo Soejima, Yongjun Yin, Robert D Guzy, Yoshikazu Nakamura, David M Ornitz, Tomoko Betsuyaku
The Journal of Pathology.2019; 249(2): 193. CrossRef - Anti-cancer effect of Aquaporin 5 silencing in colorectal cancer cells in association with inhibition of Wnt/β-catenin pathway
Wei Wang, Qing Li, Tao Yang, Dongsheng Li, Feng Ding, Hongzhi Sun, Guang Bai
Cytotechnology.2018; 70(2): 615. CrossRef - Knockdown of aquaporin-5 sensitizes colorectal cancer cells to 5-fluorouracil via inhibition of the Wnt–β-catenin signaling pathway
Qing Li, Tao Yang, Dongsheng Li, Feng Ding, Guang Bai, Wei Wang, Hongzhi Sun
Biochemistry and Cell Biology.2018; 96(5): 572. CrossRef - Implications of KRAS mutations in acquired resistance to treatment in NSCLC
Marzia Del Re, Eleonora Rofi, Giuliana Restante, Stefania Crucitta, Elena Arrigoni, Stefano Fogli, Massimo Di Maio, Iacopo Petrini, Romano Danesi
Oncotarget.2018; 9(5): 6630. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
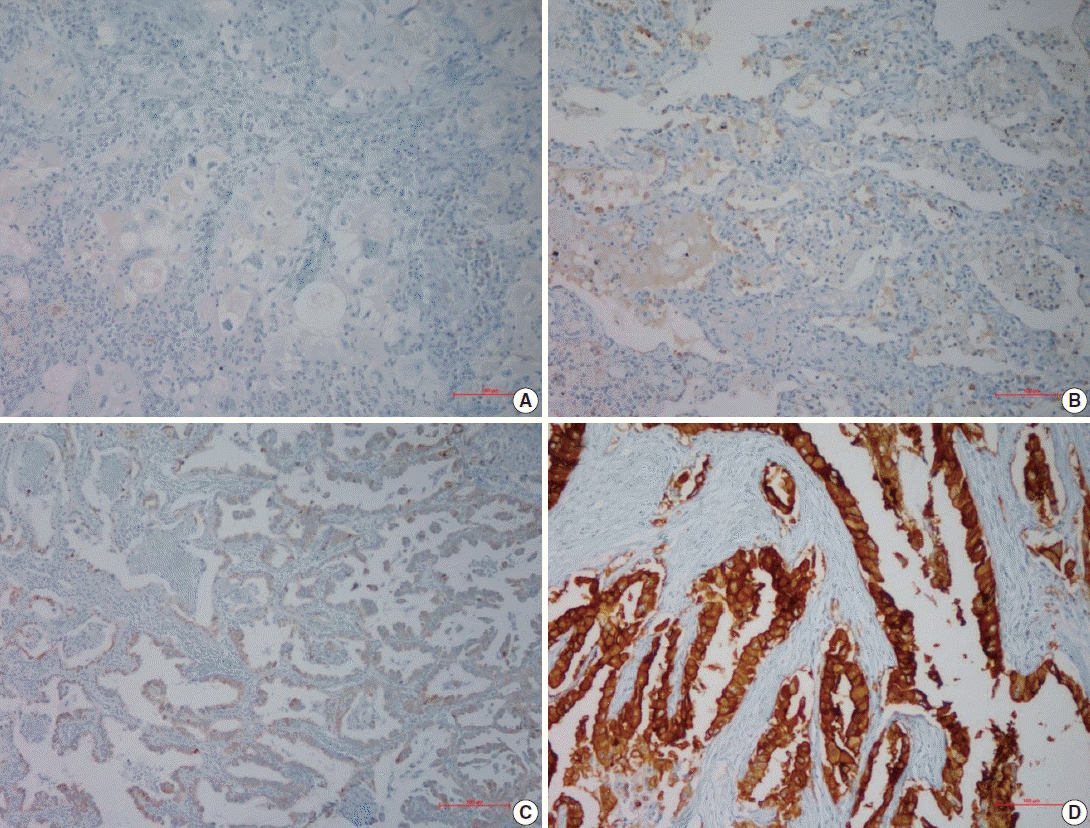
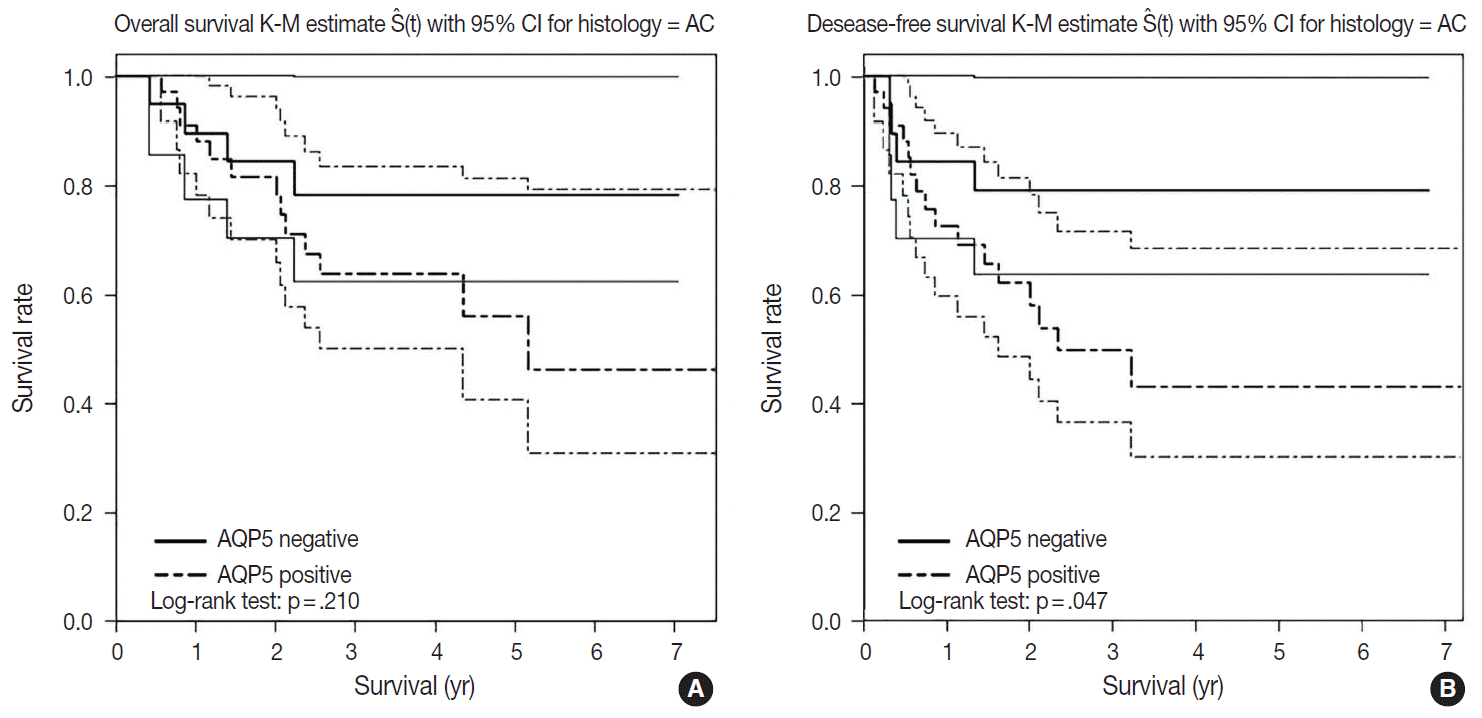
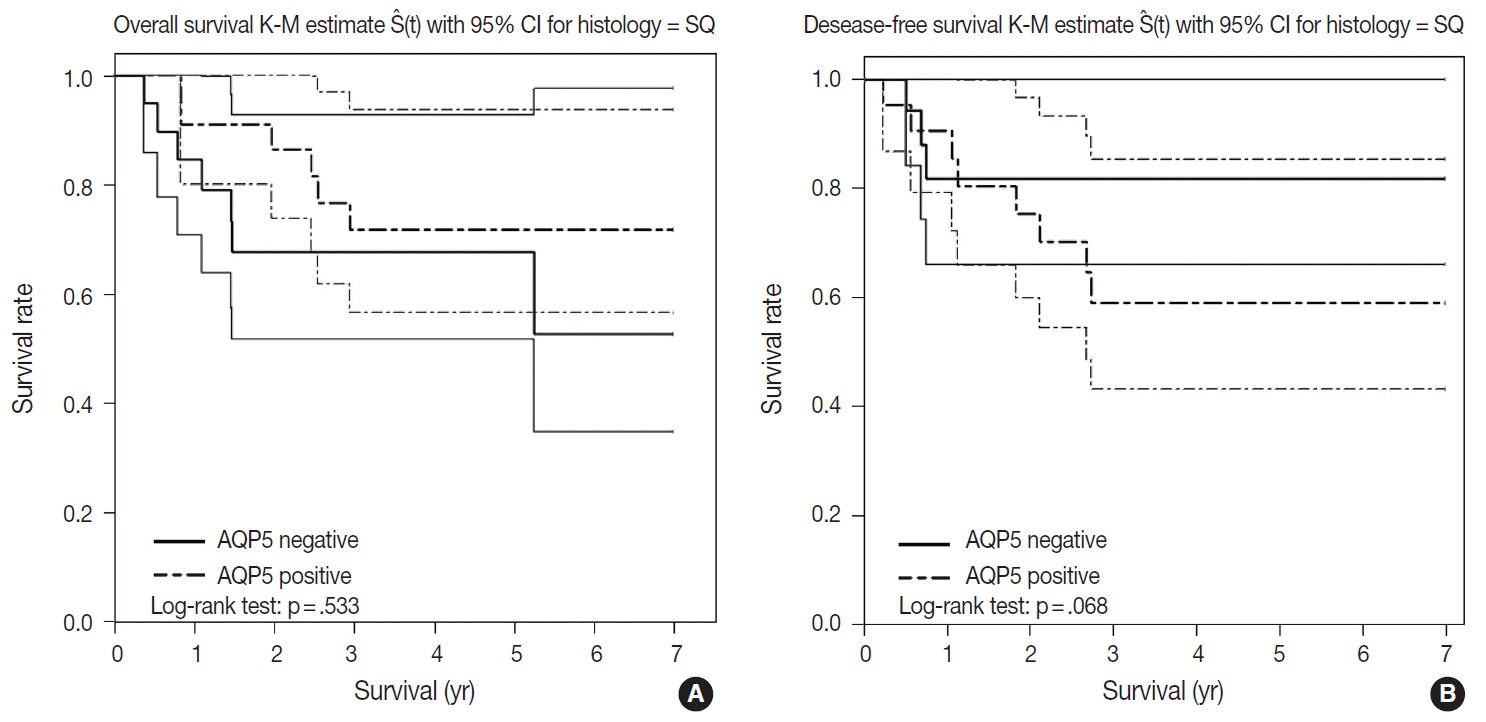
Fig. 1.
Fig. 2.
Fig. 3.
| Clinical and tumor characteristic | AQP5 status in NSCLC |
p-value | |
|---|---|---|---|
| AQP5 (–) (n = 31, 40.8%) | AQP5 (+) (n = 45, 59.2%) | ||
| Age, mean ± SD (range, yr) | 64 ± 8.3 (41–78) | 64 ± 8.1 (49–82) | .985 |
| Sex | |||
| Male | 22 (42.3) | 30 (57.7) | .692 |
| Female | 9 (37.5) | 15 (62.5) | |
| Histologic subtype | |||
| Adenocarcinoma | 16 (36.4) | 28 (63.6) | .357 |
| Squamous cell carcinoma | 15 (47) | 17 (53) | |
| Grade | |||
| Well differentiated | 7 (36.9) | 12 (63.1) | .047 |
| Moderately differentiated | 22 (51.2) | 21 (48.8) | |
| Poorly differentiated | 2 (14.3) | 12 (85.7) | |
| Grade among ADCs | |||
| Well differentiated | 5 (38.5) | 8 (61.5) | .076 |
| Moderately differentiated | 10 (50) | 10 (50) | |
| Poorly differentiated | 1 (9.1) | 10 (90.9) | |
| Grade among SQCCs | |||
| Well differentiated | 2 (33.3) | 4 (66.7) | .631 |
| Moderately differentiated | 12 (52.2) | 11 (47.8) | |
| Poorly differentiated | 1 (33.3) | 2 (66.7) | |
| T stage | |||
| T1 | 7 (43.7) | 9 (56.3) | .305 |
| T2 | 16 (38.1) | 26 (61.9) | |
| T3 | 4 (36.4) | 7 (63.6) | |
| T4 | 4 (57.2) | 3 (42.8) | |
| N stage | |||
| N0 | 20 (40.8) | 29 (59.2) | .479 |
| N1 | 4 (30.8) | 9 (69.2) | |
| N2 | 4 (40) | 6 (60) | |
| N3 | 3 (75) | 1 (25) | |
| TNM stage | |||
| I | 18 (50) | 18 (50) | .094 |
| II | 7 (28) | 18 (72) | |
| III | 4 (30.8) | 9 (69.2) | |
| IV | 0 | 0 | |
| Aquaporin 5 immunohistochemical staining status | 0 | 1 + | 2 + | 3 + | Total |
|---|---|---|---|---|---|
| Adenocarcinoma | 16 (36.3) | 6 (13.6) | 7 (15.9) | 15 (34.2) | 44 (57.9) |
| Squamous cell carcinoma | 15 (46.9) | 3 (9.3) | 6 (18.8) | 8 (25) | 32 (42.1) |
| Total | 31 (40.8) | 9 (11.8) | 13 (17.1) | 23 (30.3) | 76 (100) |
Values are presented as number (%) unless otherwise indicated. AQP5, aquaporin 5; NSCLC, non-small lung cancer; SD, standard deviation; ADC, adenocarcinoma; SQCC, squamous cell carcinoma.
Values are presented as number (%).

 E-submission
E-submission





