Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 54(2); 2020 > Article
-
Brief Case Report
Pseudomesotheliomatous carcinoma of the lung in the parietal pleura -
Ae Ri An
 , Kyoung Min Kim
, Kyoung Min Kim , Jong Hun Kim
, Jong Hun Kim , Gong Yong Jin
, Gong Yong Jin , Young Hoon Choe
, Young Hoon Choe , Myoung Ja Chung,
, Myoung Ja Chung,
-
Journal of Pathology and Translational Medicine 2020;54(2):192-195.
DOI: https://doi.org/10.4132/jptm.2019.11.14
Published online: January 29, 2020
Department of Pathology, Jeonbuk National University Medical School, Jeonju, Korea
- Corresponding Author: Myoung Ja Chung, MD, PhD Department of Pathology, Jeonbuk National University Medical School, 567 Baekje-daero, Deokjin-gu, Jeonju 54896, Korea Tel: +82-63-270-3072, Fax: +82-63-270-3135, E-mail: mjchung@jbnu.ac.kr
• Received: September 27, 2019 • Revised: November 11, 2019 • Accepted: November 14, 2019
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
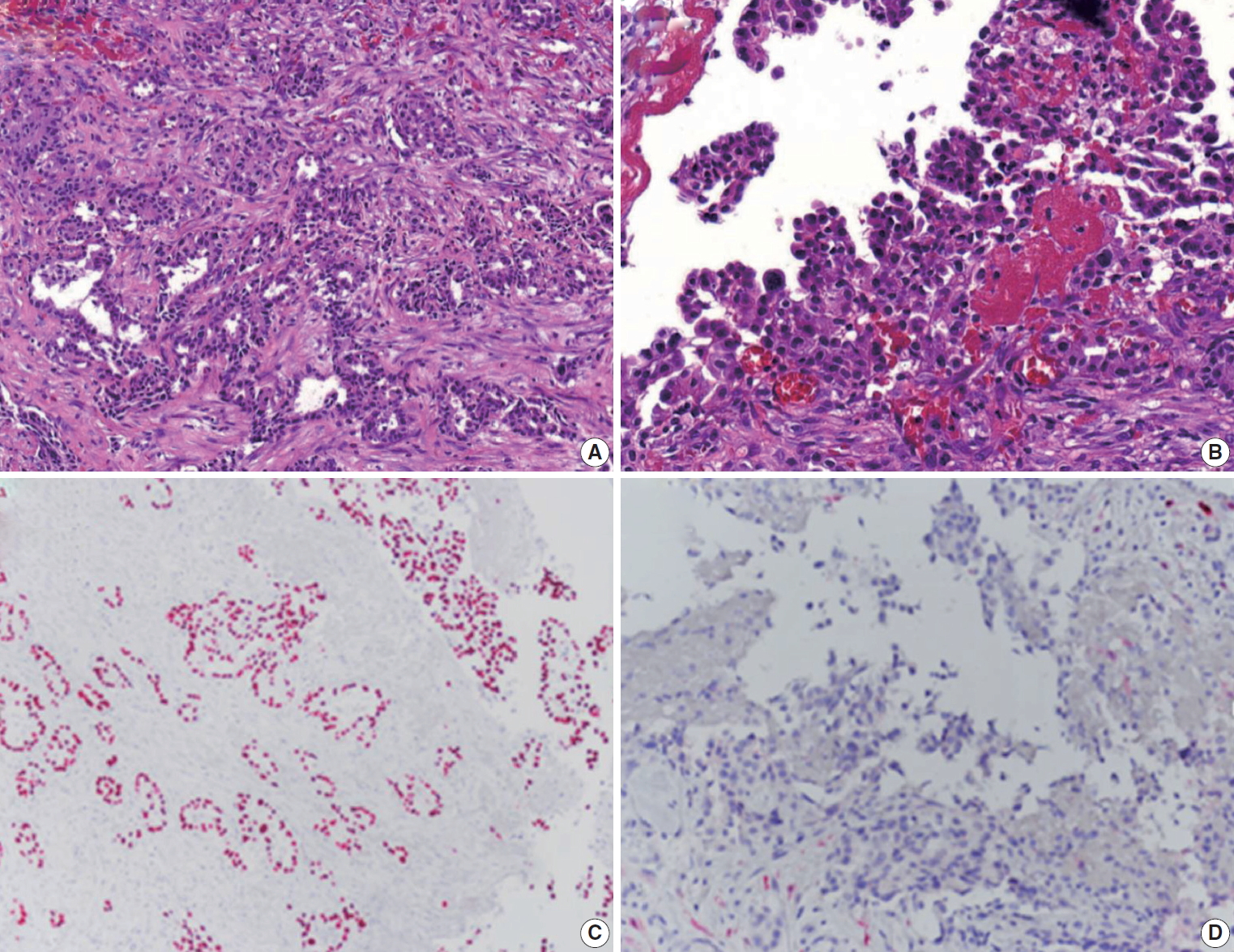
- A 69-year-old male patient presented with dyspnea and recurrent pleural effusion. He was a current smoker with a 30-pack year. He had no history of chronic pulmonary disease or asbestos exposure. The patient received thoracentesis and antibiotic treatment, but his condition did not improve. A computed tomography (CT) of the chest revealed left pleural effusion and pleural thickening with multiple nodules, suggestive of metastatic carcinoma. No parenchymal mass was observed in the lung (Fig. 1A). Positron emission tomography (PET)-CT showed fluorodeoxyglucose uptake in the areas of pleural thickening (Fig. 1B). Video-assisted thoracoscopic surgery (VATS) showed diffuse multiple nodules in the parietal pleura, and a biopsy was performed for histologic diagnosis. The histopathological examination presented glandular and cord-like structures of tumor cells within desmoplastic stroma (Fig. 2A). Under high-power field, a hobnail-like appearance of tumor cells with hyperchromatic nuclei and inconspicuous nucleoli was identified (Fig. 2B). Immunohistochemistry (IHC) was performed using an automatic immunostainer (BenchMark XT, Ventana Medical Systems, Tucson, AZ, USA) according to the manufacturer’s instructions. Immunohistochemically, the tumor cells were positive for thyroid transcription factor-1 (ready to use [RTU], Ventana Medical Systems) (Fig. 2C), carcinoembryonic antigen (RTU, Ventana Medical Systems), Napsin A (RTU, Ventana Medical Systems), and epithelial membrane antigen (1:50, Dako, Glostrup, Denmark). Calretinin (RTU, Ventana Medical Systems) (Fig. 2D), cytokeratin 5/6 (RTU, Ventana Medical Systems), Wilms’ tumor product-1 (1:100, Dako), and D2-40 (RTU, Ventana Medical Systems) were negative. With these immunohistochemical results and clinical presentation, the tumor was diagnosed as PCL. The patient refused chemotherapy and is only receiving treatment for pleural effusion.
- Targeted NGS was performed using formalin-fixed paraffin-embedded (FFPE) tumor tissue. Total nucleic acid was isolated from FFPE tumor tissue using RecoverAll Total Nucleic Acid Isolation Kit for FFPE according to the manufacturer’s specifications (Thermo Fisher Scientific, Waltham, MA, USA). The samples were sequenced using the Oncomine Comprehensive Assay Cancer Panel (Ion torrent S5 XL, Thermo Fisher Scientific), which covers 2737 amplicons (2530 DNA+207 RNA) within 143 cancer-related genes. The number of mapped DNA reads was 8,755,214 (≥Q20) and the mean coverage per target amplicon was ×3,616. The percentage of amplicons that were covered by greater than 20% of the mean amplicon coverage was 95%. Reads were aligned to the hg19 reference genome and allele frequencies <5% were excluded. We identified one pathogenic somatic mutations in splicing factor 3B subunit 1 (SF3B1) gene, NM_012433.3(SF3B1):c.2098A>G (p.Lys700Glu).
- Ethics statement
- This study was approved by the Institutional Review Board of Chonbuk National University Hospital with a waiver of informed consent (IRB No. 2019-04-040).
CASE REPORT
- PCLs were first reported by Harwood et al. in 1976 [1]. The authors defined this tumor entity as a malignant epithelial neoplasm, which presents with radiological, macroscopic, and microscopic features similar to those of diffuse malignant pleural mesothelioma [1]. PCL is mostly found in men, and the age of onset is between the sixth and seventh decades of life. The majority of patients are cigarette smokers [3]. In association with asbestos exposure, some researchers reported on the presence of asbestos bodies in cancer tissue, and Koss et al. [3] reported the possibility of occupational exposure in 21% of the patients. However, the relationship between cancer development and asbestos exposure is unclear. The tumor growth pattern makes it difficult to differentiate PCL from MM based on radiologic findings alone, and histologic confirmation is needed. IHC provides an adequate sensitivity and specificity for distinguishing PCL from MM. The tumor cells are positive for thyroid transcription factor-1, Napsin A, epithelial membrane antigen, and carcinoembryonic antigen and negative for mesothelial markers such as calretinin and D2-40.
- PCL is characterized mainly by visceral pleural thickening; however, our case showed a unique presentation of only parietal pleural thickening without visceral pleural thickening. These clinical features created a diagnostic difficulty, and in this case, it was important to recognize PCL for an accurate diagnosis.
- PCL shows a distinctly different biological behavior from the usual type of adenocarcinoma of the lung. Because of its extensive invasion of the pleura and lack of effective treatment, patients have a poor prognosis [4]. Recently, identification of the genetic alteration of cancer-related genes through sequencing in cancer has been widely used for application of targeted cancer treatment. Because PCL does not yet have an established guideline on effective treatment and the prognosis is poor, it would be meaningful to know the genetic alterations of PLC in terms of therapeutic development. No studies about genetic alterations in PCL have been reported [5]. We investigated the mutational status of cancer-related genes using targeted NGS and identified genetic alteration in SF3B1 gene, which is involved in transcription and mRNA processing. The mutation of SF3B1 gene has been recurrently observed in hematologic malignancies, breast cancer, pancreas cancer, prostate cancer, and others. SF3B1 mutations have been reported to be associated with poor outcome and drug resistance in chronic lymphocytic leukemia [6]. Although neither the mutation of known genes that have target drugs nor driver mutation were observed in this study, an accumulation of information on genetic alteration is expected to be useful for understanding the pathogenesis or developing an effective treatment in the future.
- The histogenesis of PCL is not clear. Harwood et al. [1] reported a small subpleural adenocarcinoma associated with some PCL and suggested a possible origin of PCL, as this small subpleural tumor became widely disseminated via subpleural lymphatics. A second suggestion is that the fibrous thickening of the pleura may be an early event. Furthermore, the cancer developed in subpleural lung parenchyma and subsequently spread rapidly through the thickened pleura. In the present case, VATS showed diffuse pleural thickening with multiple nodules in the parietal pleura instead of the visceral pleura. Neither chest CT nor PET-CT revealed lung parenchymal lesions. In our case, with these presentation characteristics, the possibility of different histogenesis can be considered. The possibility of pulmonary heterotopia of the pleura can be considered based on reports that lung tissue is found in an ectopic location [7]. However, there is no report of ectopic lung tissue in the parietal pleura, and there is no evidence to support this hypothesis in our case.
- We report a case of PCL presented with parietal pleural thickening without lung parenchymal lesions. The present report is the first case of PCL with gene mutation results approached through NGS.
DISCUSSION
Author contributions
Conceptualization: MJC.
Data curation: ARA, KMK, YHC, JHK, GYJ.
Investigation: ARA, KMK.
Writing: ARA, MJC.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding
No funding to declare.
Fig. 1.(A) Computed tomography (CT) of the chest shows the extremely thickened pleura along the left lobe. (B) Positron emission tomography–CT shows fluorodeoxyglucose uptake in the left pleural thickening areas.
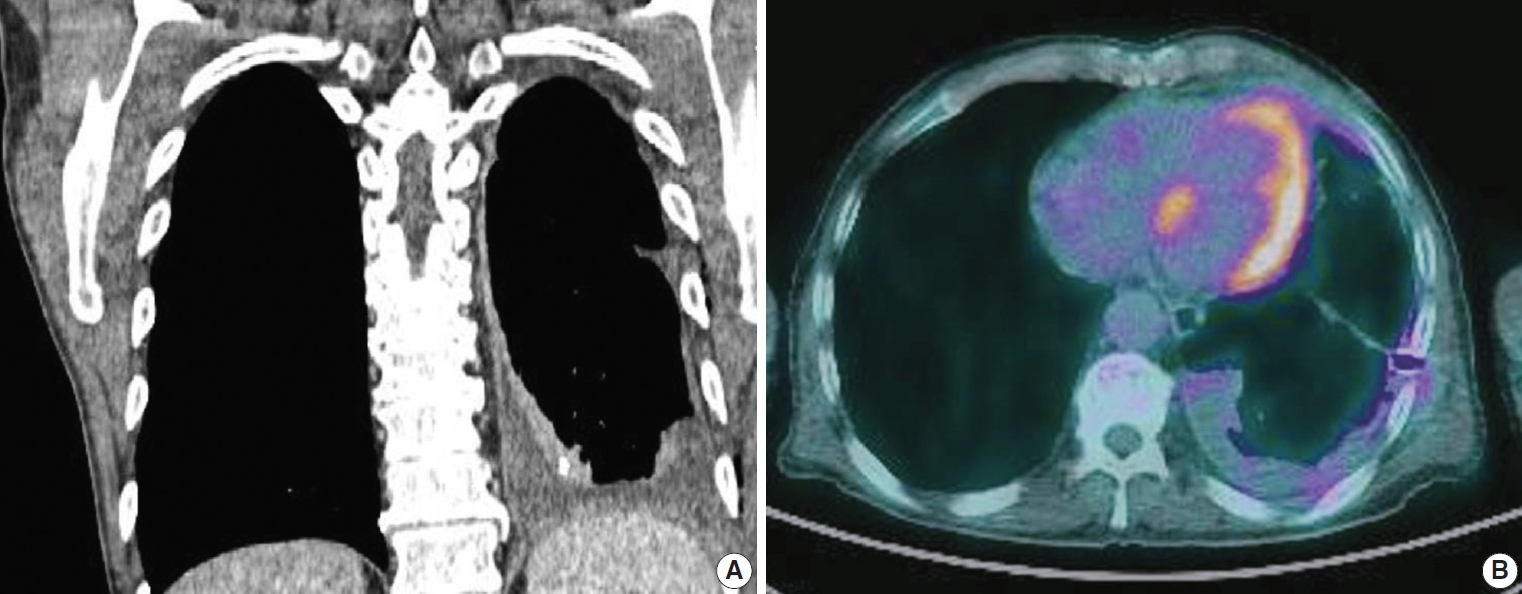

Fig. 2.Histopathological sections showing tumor cells with glandular and papillary, cord-like structures (A) and a hobnail-like appearance of tumor cells with irregular and hyperchromatic nuclei resembling malignant mesothelioma (B). Immunohistochemical evaluation demonstrates the tumor cells to be positive for thyroid transcription factor-1 (C) and negative for calretinin (D).

- 1. Harwood TR, Gracey DR, Yokoo H. Pseudomesotheliomatous carcinoma of the lung: a variant of peripheral lung cancer. Am J Clin Pathol 1976; 65: 159-67. ArticlePubMedPDF
- 2. Attanoos RL, Gibbs AR. ‘Pseudomesotheliomatous’ carcinomas of the pleura: a 10-year analysis of cases from the Environmental Lung Disease Research Group, Cardiff. Histopathology 2003; 43: 444-52. ArticlePubMedPDF
- 3. Koss MN, Fleming M, Przygodzki RM, Sherrod A, Travis W, Hochholzer L. Adenocarcinoma simulating mesothelioma: a clinicopathologic and immunohistochemical study of 29 cases. Ann Diagn Pathol 1998; 2: 93-102. ArticlePubMed
- 4. Vukovic´ J, Plavec G, Ac´imovic´ S, et al. Pseudomesotheliomatous carcinoma of the lung. Vojnosanit Pregl 2016; 73: 1168-72. ArticlePubMed
- 5. Sakata S, Sakamoto Y, Takaki A, Ishizuka S, Saeki S, Fujii K. Reversible restrictive lung disease in pseudomesotheliomatous carcinoma in a lung harboring a HER2-mutation. Intern Med 2018; 57: 2223-6. ArticlePubMedPMC
- 6. Rossi D, Bruscaggin A, Spina V, et al. Mutations of the SF3B1 splicing factor in chronic lymphocytic leukemia: association with progression and fludarabine-refractoriness. Blood 2011; 118: 6904-8. ArticlePubMedPMCPDF
- 7. Jeon GW, Han SW, Jung JM, Kang MS, Sin JB. The first Korean case of cutaneous lung tissue heterotopia. J Korean Med Sci 2010; 25: 1387-9. ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Pseudomesotheliomatous lung cancer mimicking malignant pleural mesothelioma: A case report
Supakorn Chansaengpetch, Ruchira Ruangchira-urai, Nisa Muangman, Rathachai Kaewlai, Trongtum Tongdee, Teerapat Singwicha, Narongpon Dumavibhat
The ASEAN Journal of Radiology.2025; 26(1): 24. CrossRef - Pseudomesotheliomatous Carcinoma of the Lung with Morphological Characteristics of Signet Ring Cell Carcinoma: An Autopsy Case Report
Tetsu Hirakawa, Takuya Tanimoto, Yui Hattori, Ryo Katsura, Shinya Miyake, Suguru Fujita, Sayaka Ueno, Ken Masuda, Takashi Nishisaka, Nobuhisa Ishikawa
Internal Medicine.2024; 63(7): 979. CrossRef - Intrapulmonary Biphasic Mesothelioma Misdiagnosed as Adenocarcinoma: Case Report and a Potential Diagnostic Pitfall
Wenfeng Xu, XingYan Zhu, Hao Tang, Qijian Ying, Yujuan Xu, Deyu Guo
OncoTargets and Therapy.2024; Volume 17: 925. CrossRef -
A RARE CASE OF UNCLASSIFIED CARCINOMA OF THE LUNG: DIAGNOSTIC CHALLENGES
B.M. Fylenko, N.V. Royko, I.I. Starchenko, O.V. Starchenko, O.Y. Horodynska, S.A. Proskurnia
Azerbaijan Medical Journal.2024; (4): 182. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Pseudomesotheliomatous carcinoma of the lung in the parietal pleura
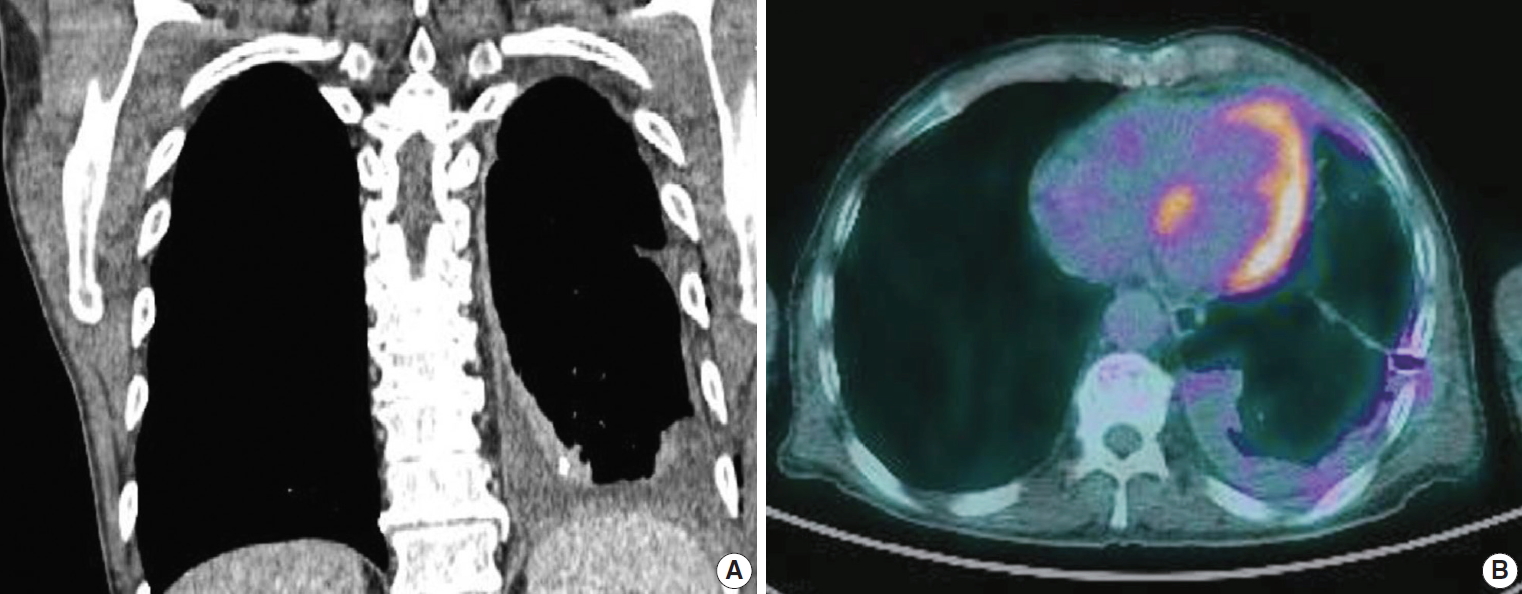
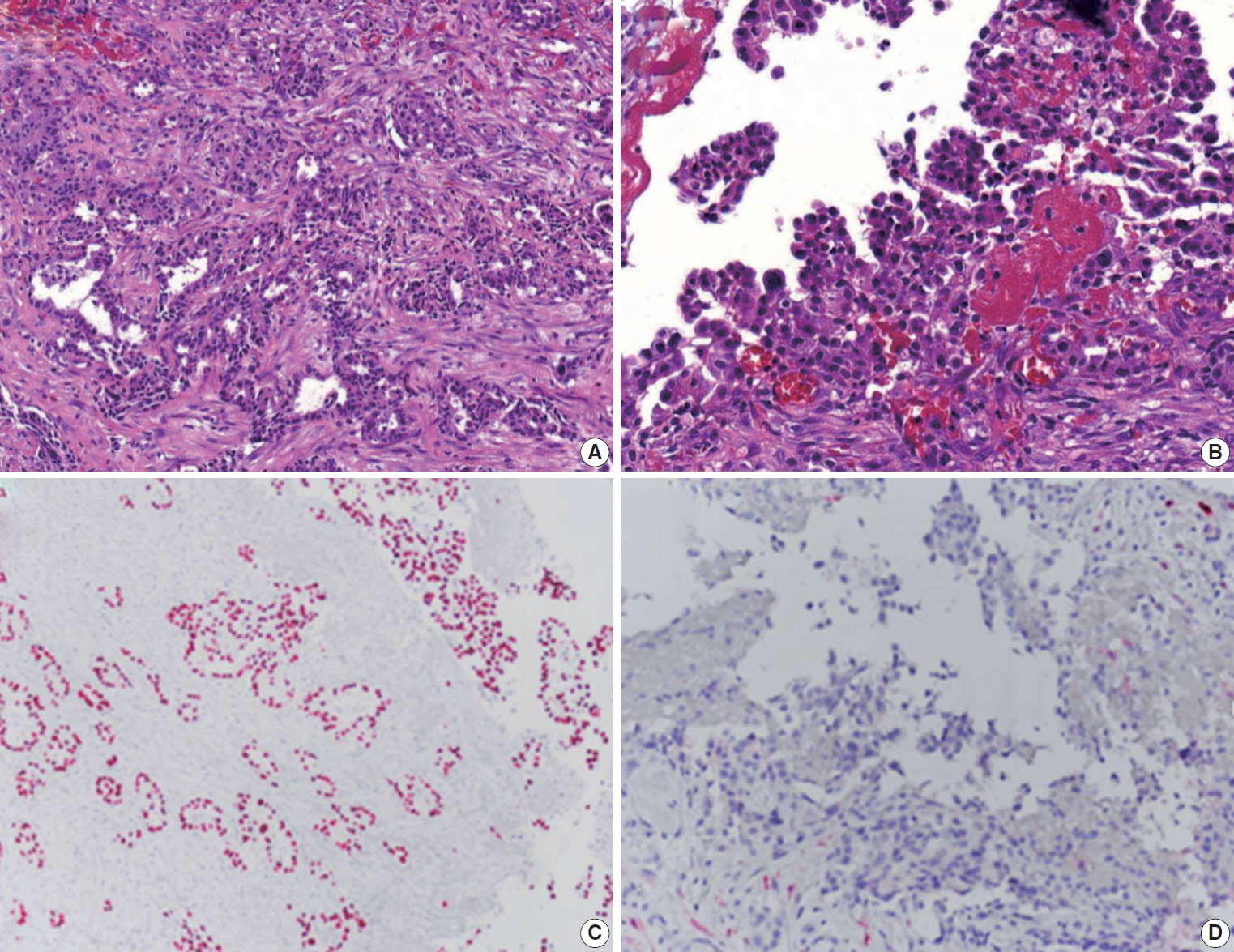
Fig. 1. (A) Computed tomography (CT) of the chest shows the extremely thickened pleura along the left lobe. (B) Positron emission tomography–CT shows fluorodeoxyglucose uptake in the left pleural thickening areas.
Fig. 2. Histopathological sections showing tumor cells with glandular and papillary, cord-like structures (A) and a hobnail-like appearance of tumor cells with irregular and hyperchromatic nuclei resembling malignant mesothelioma (B). Immunohistochemical evaluation demonstrates the tumor cells to be positive for thyroid transcription factor-1 (C) and negative for calretinin (D).
Fig. 1.
Fig. 2.
Pseudomesotheliomatous carcinoma of the lung in the parietal pleura

 E-submission
E-submission




