Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(3); 2014 > Article
-
Brief Case Report
Primary Gastric Histiocytic Sarcoma Reminiscent of Inflammatory Pseudotumor: A Case Report with Review of the Literature - Dakeun Lee, Young-Bae Kim, Sook Hee Chung1, Sang-Ryung Lee, Cheul Su Byun2, Sang-Uk Han2, Jae Ho Han
-
Korean Journal of Pathology 2014;48(3):258-262.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.3.258
Published online: June 26, 2014
Department of Pathology, Ajou University School of Medicine, Suwon, Korea.
1Department of Gastroenterology, Ajou University School of Medicine, Suwon, Korea.
2Department of Surgery, Ajou University School of Medicine, Suwon, Korea.
- Corresponding Author: Young-Bae Kim, M.D. Department of Pathology, Ajou University School of Medicine, 164 Worldcup-ro, Yeongtong-gu, Suwon 443-380, Korea. Tel: +82-31-219-5936, Fax: +82-31-219-5934, ybkim@ajou.ac.kr
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
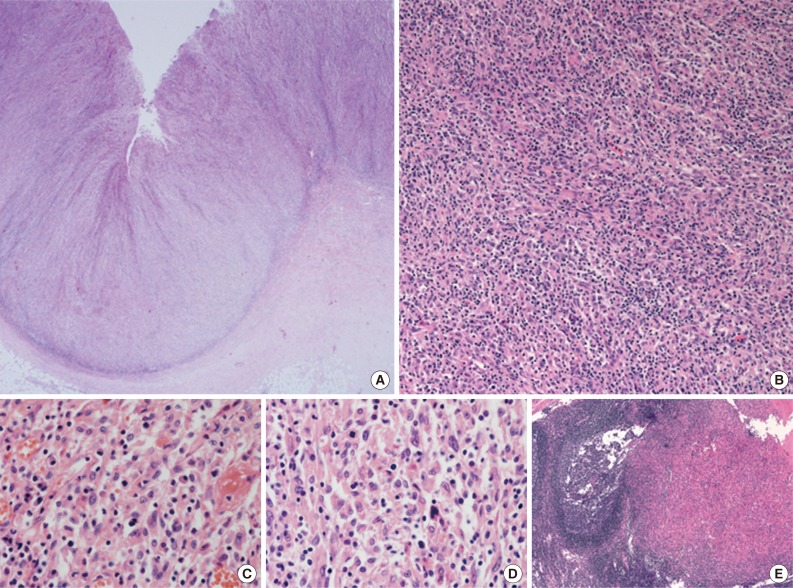
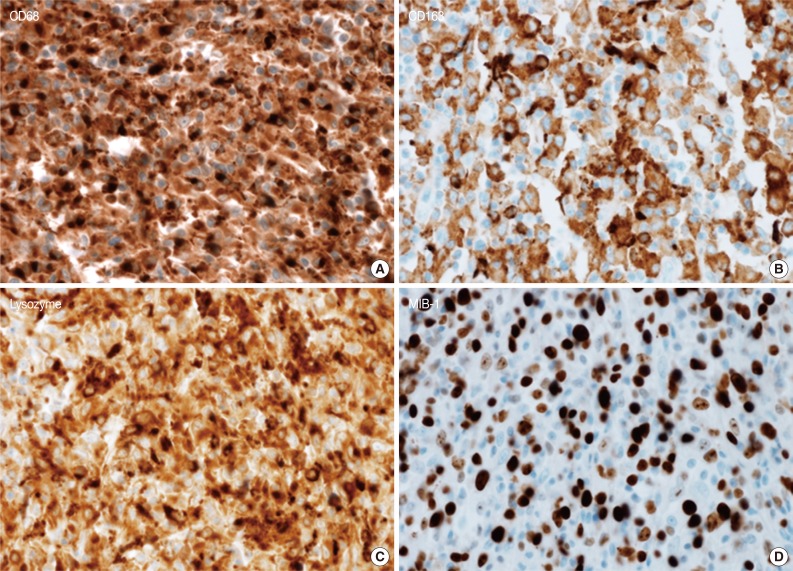
- A 45-year-old previously healthy woman presented with 40 days history of epigastric pain. Laboratory findings were unremarkable with the exception of mild anemia. Esophagogastroduodenoscopy revealed an irregular ulcerofungating lesion in the gastric antrum. A biopsy report suggested the presence of an inflammatory pseudotumor rather than a malignant lesion. However, a positron emission tomography scan showed high fludeoxyglucose uptake in the stomach, perigastric lymph nodes, and right hilar space. The patient underwent palliative distal gastrectomy due to distal gastric obstruction causing difficulties in eating. The resected specimen showed a huge, ulcerofungating mass in the antrum and pylorus of the stomach (Fig. 1A). The duodenal bulb and resection margins were also involved by the tumor. On cut section, the tumor was white-tan, solid, and somewhat rubbery in consistency (Fig. 1B). Microscopically, tumor cells showed band-like infiltration mainly in the mucosa and submucosa, but tumor cells also penetrated into the serosa in some areas (Fig. 2A). Inflammatory cells were intimately admixed with tumor cells (Fig. 2B). Tumor cells mostly had intermediate-sized nuclei with plump, pink cytoplasm (Fig. 2C). Nuclei were ovoid to elongated and frequently showed convoluted or overlapping shapes. The chromatin was fairly vesicular, and one or two micronucleoli were discernible, but they were not prominent. Pleomorphic or multinucleated tumor giant cells were scarcely observed (Fig. 2D). Mitotic figures were found up to 2-3/10 high power fields. The majority of admixed inflammatory cells were mature lymphocytes followed by plasma cells. Some neutrophils and occasional eosinophils were also noted. Metastatic tumor deposits were found in two perigastric lymph nodes (Fig. 2E). Immunohistochemical staining was performed, and the tumor cells were strongly reactive for CD68 (Fig. 3A), CD163 (Fig. 3B), leukocyte common antigen, lysozyme (Fig. 3C), and vimentin. CD4 was weakly stained in the cytoplasm of the tumor cells, and some individual tumor cells were also reactive for CD31. S-100 protein showed patch immunoreactivity for the tumor cytoplasm. Notably, MIB-1 labeling index was about 60% to 70% (Fig. 3D). Tumor cells were negative for epithelial cell markers (cytokeratin [AE1/AE3], cytokeratin 8&18, epithelial membrane antigen), follicular dendritic cell markers (CD21, CD35), Langerhans cell marker (CD1a), specific myeloid markers (CD34, myeloperoxidase), and other markers including c-kit, smooth muscle actin, and CD30. CD3 and CD20 were not stained in the tumor cells. The infiltrating lymphocytes were predominantly T cells. In-situ hybridization for Epstein-Barr virus-encoded RNA was performed, and no apparent positive signal was noted. The deeply infiltrative growth of the tumor and the presence of lymph node metastases indicated the malignant nature of this tumor. Ultimately, a primary gastric histiocytic sarcoma was diagnosed. The postoperative course was uneventful, but the patient is still vulnerable. Bone marrow biopsy was done, and there was no evidence of tumor infiltration. Postoperative adjuvant chemotherapy with cyclophophomide, doxorubicin, vincristine, and prednisolone (CHOP) is planned.
CASE REPORT
- Histiocytic sarcomas usually consist of diffuse, noncohesive proliferation of large cells. These cells are round to oval and are commonly pleomorphic. Large multinucleated nuclei are frequently observed, and single prominent nucleoli are found in most of the tumor cells. Based on morphological similarities, diffuse large B-cell lymphoma or anaplastic large cell lymphoma has long been misdiagnosed as a malignant histiocytic lesion.7 Thus, the malignant nature of this lesion is easily detected in general, even though the specific diagnosis is laborious. On the other hand, the tumor cells in the current case were not highly atypical or pleomorphic, and, instead, most of the tumor cells had appearances similar to reactive histiocytes. The diagnosis on a biopsy specimen was even more difficult, and the findings were more suggestive of an inflammatory lesion such as an inflammatory pseudotumor than of a malignant process. However, the resected specimen clearly demonstrated the malignant nature of this tumor. The cellular atypia of histiocytic sarcoma has been reported to be variable, but its diagnostic confusion with a benign reactive lesion is exceptional.8 In line with this, sometimes the neoplastic cells in histiocytic sarcomas involving the central nervous system are obscured by a heavy inflammatory infiltrate including neutrophils and lymphocytes, thereby mimicking an inflammatory lesion.9 Therefore, histiocytic sarcoma also should be included in the differential diagnoses of lesions with even bland-looking histiocytic proliferation, and clinicopathologic correlation is mandatory for the diagnosis.
- Histiocytic sarcoma rarely occurs in the stomach; we found only 5 such cases through our Pubmed search. The clinical characteristics of primary gastric histiocytic sarcomas including ours are summarized in Table 1. Initially, we found two more single case reports in the literature, but these were not included in our analysis because they were written in Chinese, and even the abstracts were not available online. Among the 6 patients including ours, there were 2 men and 4 women. There is a wide age range, from 28 to 89 years old (average, 57.8 years), but most cases occurred in the middle-aged or elderly. Two cases showed multiple tumor involvement (colon and jejunum), while the rest presented with a solitary gastric mass. Tumor size ranged from 2 to 20 cm, and most cases presented with a large, ulcerofungating mass, which, preoperatively, had been suspected as an advanced gastric cancer. All patients underwent a gastrectomy, and 4 of 6 (66.7%) patients had regional lymph node metastases at the time of surgery. Two patients succumbed to the disease during the follow-up period. The remaining four patients were alive at the last follow-up, but the follow-up periods were all short. Histiocytic sarcoma is usually aggressive, and the clinical course is grim due to the poor response to conventional chemotherapy.2 With regard to gastric histiocytic sarcomas, one previous case showed a relatively long survival (3 years) after surgery and adjuvant chemotherapy.5 On the other hand, a different case with small tumor size (2 cm) and no initial regional lymph node involvement developed multiple distant metastases before long, and this patient had not received chemotherapy.6 These examples suggest that adjuvant chemotherapy may be effective and prolong the survival of patients with gastric histiocytic sarcoma.
- In summary, histiocytic sarcoma rarely occurs in the stomach, and it usually presents with a large, ulcerofungating mass with an advanced clinical stage. Histologically, since the cellular atypia of this tumor is variable and may be minimal, clinical correlation is warranted before a definite diagnosis is made. Like other extranodal histiocytic sarcomas, primary gastric histiocytic sarcoma appears to be an aggressive neoplasm, but adjuvant chemotherapy may be effective in some cases.
DISCUSSION
- 1. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press, 2008.
- 2. Hornick JL, Jaffe ES, Fletcher CD. Extranodal histiocytic sarcoma: clinicopathologic analysis of 14 cases of a rare epithelioid malignancy. Am J Surg Pathol 2004; 28: 1133-1144. PubMed
- 3. Congyang L, Xinggui W, Hao L, Weihua H. Synchronous histiocytic sarcoma and diffuse large B cell lymphoma involving the stomach: a case report and review of the literature. Int J Hematol 2011; 93: 247-252. ArticlePubMedPDF
- 4. Shen XZ, Liu F, Ni RJ, Wang BY. Primary histiocytic sarcoma of the stomach: a case report with imaging findings. World J Gastroenterol 2013; 19: 422-425. ArticlePubMedPMC
- 5. Sundersingh S, Majhi U, Seshadhri RA, Tenali GS. Multifocal histiocytic sarcoma of the gastrointestinal tract. Indian J Pathol Microbiol 2012; 55: 233-235. ArticlePubMed
- 6. Yoo KD, Han DS, Chung SM, et al. A case of extranodal histiocytic sarcoma of stomach mimicking gastric adenocarcinoma. Korean J Gastroenterol 2010; 55: 127-132. ArticlePubMed
- 7. Wilson MS, Weiss LM, Gatter KC, Mason DY, Dorfman RF, Warnke RA. Malignant histiocytosis. A reassessment of cases previously reported in 1975 based on paraffin section immunophenotyping studies. Cancer 1990; 66: 530-536. ArticlePubMed
- 8. Boisseau-Garsaud AM, Vergier B, Beylot-Barry M, et al. Histiocytic sarcoma that mimics benign histiocytosis. J Cutan Pathol 1996; 23: 275-282. ArticlePubMed
- 9. Sun W, Nordberg ML, Fowler MR. Histiocytic sarcoma involving the central nervous system: clinical, immunohistochemical, and molecular genetic studies of a case with review of the literature. Am J Surg Pathol 2003; 27: 258-265. PubMed
REFERENCES

| Reference | Age (yr) | Sex | Tumor location | Tumor size (cm) | Accompanying lesion | Regional LN involvement | Surgical treatment | Adjuvant chemotherapy | Distant metastasis | Survival |
|---|---|---|---|---|---|---|---|---|---|---|
| Hornick et al. [2] (2004) | 89 | Male | Stomach+colon | 12 | No | Yes | Gastrectomya | No | Sternum | Died (5 mo) |
| Yoo et al. [6] (2010) | 71 | Male | Stomach (F & B) | 3, 1 (2 distinct masses) | No | No | Total gastrectomy | No | Spleen and intra-abdominal LNs | Alive with disease (9 mo) |
| Congyang et al. [3] (2011) | 62 | Female | Stomach (B & A) | 20 | DLBCL | Yes | Total gastrectomy | No | No | Died (7 mo) |
| Sundersingh et al. [5](2012) | 28 | Female | Stomach (A & P)+jejunum | 9 | No | Yes | Distal gastrectomy+jejunal resection | Yes (CHOP, 4 cycles) | No | Alive without disease (3 yr) |
| Shen et al. [4] (2013) | 52 | Female | Stomach (F) | 7 | No | No | Total gastrectomy | Yes | No | Alive without disease (4 mo) |
| Present case | 45 | Female | Stomach (A & P) | 7.5 | No | Yes | Distal gastrectomy | Not yet | No | Alive with residual disease (1 mo) |
Figure & Data
References
Citations

- NF1 mutation and TUBB3 amplification in gastric histiocytic sarcoma: a case report and literature review
Yi Yang, Wei Fan, Xiaoping Liu, Qiongrong Chen
Medical Molecular Morphology.2024; 57(3): 244. CrossRef - Small bowel and lung histiocytic sarcoma revealed by acute peritonitis: A case report with review of literature
Ahlem Bellalah, Ibtissem korbi, Seifeddine Ben Hammouda, Asma Achour, Nouha Ben Abdeljelil, Manel Njima, Amira Daldoul, Rim Hadhri, Leila Njim, Abdelfatteh Zakhama
Annals of Medicine and Surgery.2021; 68: 102638. CrossRef - An unexpected diagnosis of histiocytic sarcoma
Joshua T. Byers, Samuel W. French
Experimental and Molecular Pathology.2019; 106: 60. CrossRef - NLRX1 suppresses tumorigenesis and attenuates histiocytic sarcoma through the negative regulation of NF-λB signaling
Sheryl Coutermarsh-Ott, Alysha Simmons, Vittoria Capria, Tanya LeRoith, Justin E. Wilson, Bettina Heid, Casandra W. Philipson, Qizhi Qin, Raquel Hontecillas-Magarzo, Josep Bassaganya-Riera, Jenny P-Y Ting, Nikolaos Dervisis, Irving C. Allen
Oncotarget.2016; 7(22): 33096. CrossRef
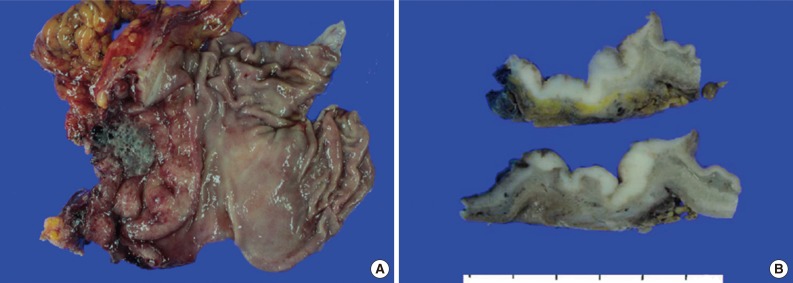
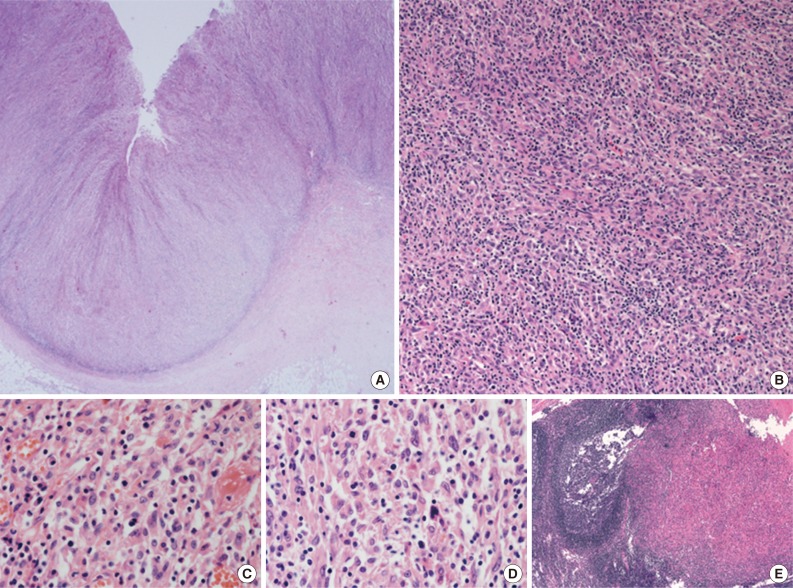
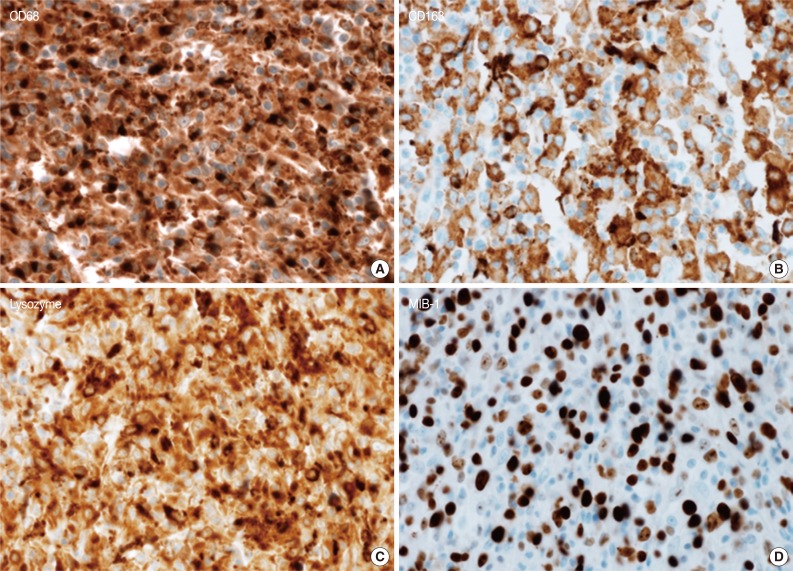
Fig. 1
Fig. 2
Fig. 3
| Reference | Age (yr) | Sex | Tumor location | Tumor size (cm) | Accompanying lesion | Regional LN involvement | Surgical treatment | Adjuvant chemotherapy | Distant metastasis | Survival |
|---|---|---|---|---|---|---|---|---|---|---|
| Hornick et al. [2] (2004) | 89 | Male | Stomach+colon | 12 | No | Yes | Gastrectomy |
No | Sternum | Died (5 mo) |
| Yoo et al. [6] (2010) | 71 | Male | Stomach (F & B) | 3, 1 (2 distinct masses) | No | No | Total gastrectomy | No | Spleen and intra-abdominal LNs | Alive with disease (9 mo) |
| Congyang et al. [3] (2011) | 62 | Female | Stomach (B & A) | 20 | DLBCL | Yes | Total gastrectomy | No | No | Died (7 mo) |
| Sundersingh et al. [5](2012) | 28 | Female | Stomach (A & P)+jejunum | 9 | No | Yes | Distal gastrectomy+jejunal resection | Yes (CHOP, 4 cycles) | No | Alive without disease (3 yr) |
| Shen et al. [4] (2013) | 52 | Female | Stomach (F) | 7 | No | No | Total gastrectomy | Yes | No | Alive without disease (4 mo) |
| Present case | 45 | Female | Stomach (A & P) | 7.5 | No | Yes | Distal gastrectomy | Not yet | No | Alive with residual disease (1 mo) |
LN, lymph node; F, fundus; B, body; A, antrum; DLBCL, diffuse large B cell lymphoma; P, pylorus; CHOP, cyclophophomide, doxorubicin, vincristine, and prednisolone. The surgical treatment for the colonic mass is not described in the paper.

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article




