Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(3); 2013 > Article
-
Original Article
Cytologic Features ofALK -Positive Pulmonary Adenocarcinoma - Seung Yeon Ha, Jungsuk Ahn, Mee Sook Roh1, Joungho Han2, Jae Jun Lee2, Boin Lee2, Jun Yim3
-
Korean Journal of Pathology 2013;47(3):252-257.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.3.252
Published online: June 25, 2013
Department of Pathology, Gachon University Gil Medical Center, Incheon, Korea.
1Department of Pathology, Dong-A University College of Medicine, Busan, Korea.
2Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
3Department of Preventive Medicine, Graduate School of Medicine, Gachon University, Incheon, Korea.
- Corresponding Author: Joungho Han, M.D. Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 135-710, Korea. Tel: +82-2-3410-2765, Fax: +82-2-3410-6396, hanjho@skku.edu
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- The aim of this study was to determine the cytologic features of anaplastic lymphoma kinase (ALK) expressing pulmonary adenocarcinoma.
-
Methods
- We analyzed the cytopathological findings of 15 cases of endobronchial ultrasound guided aspiration and a case of bronchial washing. These cases were selected based on the histomorphology of ALK-rearranged lung adenocarcinoma.
-
Results
- Cytology showed mucinous (81.3%) and hemorrhagic (50%) backgrounds. The cells were arranged in tubulopapillary or tubulocribriform patterns (93.8%), and clusters (56.3%) admixed with signet ring cell features (87.5%). The tumor cells were monotonous and uniform with vesicular nuclei and a small nucleolus.
-
Conclusions
- The characteristic findings were sheets showing a tubulopapillary or tubulocribriform appearance, with vesicular nuclei and a bland chromatin pattern (p<0.001). Scattered signet ring cells were helpful in suggesting ALK-positive adenocarcinoma (p<0.001).
- Patient selection
- We selected 107 cases of pulmonary adenocarcinoma diagnosed at Samsung Medical Center and Gachon University Gil Medical Center between March 2010 and July 2012. All tumors, except one, were collected using endobronchial-ultrasound guided biopsy (EBUS). In 15 cases, the patients had undergone EBUS aspiration, and had undergone bronchial washing. The 15 cases were diagnosed by biopsy, and examined by immunohistochemistry using ALK protein (1:40, clone 5A4, Novocastra, Newcastle upon Tyne, UK). EML4-ALK rearrangements were confirmed in all ALK-positive immunoreactive cases by FISH analysis using an ALK break-apart probe (Vysis LSI ALK Dual Color, break-apart rearrangement probe, Abbott Molecular, Abbott Park, IL, USA). We identified 16 cases of immunohistochemically and FISH proven ALK-positive adenocarcinoma. Fine needle aspiration cytologies from the 16 cases of ALK-negative pulmonary adenocarcinoma were also evaluated. All of these ALK-negative patients underwent lobectomy. The histologic finding was conventional acinar-type adenocarcinoma.
- Cytologic and histologic analysis
- Hematoxylin and eosin stained slides of histologic sections from 32 cases were retrospectively reviewed. All smeared cytology slides were reviewed by two pathologists. Scoring was as follows: 0, no evidence of cytologic features; 1, <30% of cytologic features in smeared slides; 2, ≥30% of cytologic features in smeared slides.
- Each cytologic smear was evaluated for the following features: 1) background pattern, mucin, hemorrhage, and necrosis; 2) cellular arrangement, such as tubulopapillary, tubulocribriform, acini, sheet, cluster, and single; 3) individual cell morphology such as vesicular nuclei, presence of nucleoli, dense chromatin, and signet ring cell features. 'Acinus' refers to a small saclike dilatation, particularly one of glandular appearance. A two dimensional arrangement was 'sheet.' 'Cluster' was used to indicate a three dimensional group of cells.
- Histologic sections were assessed for histologic patterns (solid, micropapillary, acinar, lepidic, mucinous, and signet ring cells scored as two parts: <10%, ≥10%) and nuclear grade (I, II, III).
- Statistical analysis
- We used the chi-square test to evaluate the differences in cytologic features between ALK-positive adenocarcinomas and ALK-negative adenocarcinomas. Fisher exact test was used instead of the chi-square test in cases in which 50% of the cells had expected counts of less than 5.
MATERIALS AND METHODS
- The patients ranged in age from 25 to 67 years (mean age, 52 years). There were nine female and seven male patients with ALK-positive adenocarcinoma. Twelve patients with ALK-negative adenocarcinoma were females.
- Most EBUSs of ALK-positive adenocarcinomas showed mucinous (13/16, 81.3%), hemorrhagic (8/16, 50.0%), and necrotic (3/16, 18.8%) backgrounds. An extensive mucinous background was observed in two cases, and 11 cases were focally mucinous admixed with tumor cells. These contained cellular aspirates, even though they were less cellular than fine needle aspiration cytology. The tumor cells were arranged in sheets (15/16, 93.8%), clusters (9/16, 56.3%), and single cells (11/16, 68.8%). The clusters were relatively loose and the sheets were somewhat tubulopapillary or tubulocribriform in appearance (p<0.001) (Fig. 1G, H), and contained cells showing intracytoplasmic mucin. The scattered single cells showed vesicular nuclei (14/16, 87.5%; p<0.001) and signet ring cell features (14/16, 87.5%; p<0.001) (Fig. 1J, N). The nuclei had an open chromatin appearance-similar to the nuclei of papillary carcinoma of the thyroid (ground glass appearance). The nuclear membrane is distinct and thickened. The single cells had the appearance of small histiocyte-like cells and sometimes also contained intracytoplasmic mucin vacuoles. The nucleoli were mostly small in size (10/16, 62.5%). Large prominent nucleoli were observed in two cases (2/16, 12.5%) (Fig. 1K, L). A summary of the cytological features of 16 cases is shown in Table 1. When the cytologic features were scored, a high score (more than 2) was found to be associated with a single cell pattern (four cases), a signet ring cell pattern (six cases), and significant mucinous background (two cases).
- The cellularity and background of ALK-negative pulmonary adenocarcinomas showed: cells were in loosely cohesive groups, syncytial tissue fragments, acini (13/16, 81.3%), and tubule. Individual cells were variable in size: from small to large. Cytoplasms were pale to dense, foamy, or lacy containing small several intracytoplasmic vacuoles, not single vacuoles. The nuclei displayed relatively uniform, smooth to irregular nuclear membranes with small to large prominent nucleoli, that were larger compared with those of ALK-positive adenocarcinomas. These features are summarized in Table 1. The specificity of the tubulopapillary or tubulocribriform patterns and the vesicular chromatin in cytologic smears to predict for ALK-positive adenocarcinoma was 81.3%. Those of signet ring cell morphology and single cell distribution were 93.8% and 81.3%, respectively. The sensitivity of tubulopapillary-tubulocribriform pattern and vesicular chromatin was 93.8%. Those of signet ring cell morphology and single cell distribution were 87.5% and 68.8%, respectively.
- In histologic findings, backgrounds were hemorrhagic (8/16, 50.0%), mucinous (4/16, 25.0%), and necrotic (0/16, 0%). Eight (50.0%) cases showed a cribriform formation with a rigid or flaccid appearance. A solid pattern was observed in four (25.0%) cases. Significant extracellular mucus was observed in four (25.0%) cases. Fifteen cases showed intraglandular or intracribriform mucinous lumen and intracytoplasmic mucin. One case showed a psammoma body. Individual signet ring cells were identified in 14 (87.5%) cases, accounting for 0% of tumor cellularity (2/16, 12.5%), <10% (7/16, 43.8%), and ≥10% (7/16, 43.8%) (Fig. 1).
RESULTS
- Molecular targeted therapy has played an increasingly important role in cancer treatment, particularly in genetically defined subsets of patients. Identification of genetic alterations is extremely important for treatments using specific molecular targeted agents. ALK gene rearrangements in NSCLC carcinogenesis were first reported in 2007.14 The EML4-ALK fusion gene has been identified in 1-5% of cases of NSCLCs.2,8-11 ALK inhibitors have been developed and have shown effective activity in ALK-rearranged NSCLCs. Confirmation of EML4-ALK gene rearrangement is necessary for NCSLC patients.
- The histologic findings of ALK-rearranged NSCLCs included an acinar growth pattern, extracellular mucus production, and signet-ring cell morphologies in associated with solid growth.9-12 Some researchers have reported findings of solid or acinar growth patterns, cribriform structure, presence of mucous cells (signet ring cells or goblet cells), abundant extracellular mucus, lack of lepidic growth, and lack of a significant nuclear pleomorphism. In particular, a combination of two findings, such as a solid signet ring cell pattern and a mucinous cribriform pattern, were observed in the majority of ALK-positive tumors.15
- In this study, most cytologic smears of ALK-positive adenocarcinomas showed mucinous background (81.3%), even though it was focal, not extensive, except in two cases. Other backgrounds, such as hemorrhage (50.0%) and necrosis (18.8%), might be nonspecific for malignant tumors. The background of ALK-negative adenocarcinoma was variable, rather than specific as with malignant tumors. The majority of cells of ALK-positive tumors were arranged in sheets with tubulopapillary or tubulocribriform patterns (93.8%). These findings were significant on statistical analysis (p<0.001). However acinus pattern was also found to be significant on statistical analysis, and was relatively frequently observed for glandular forming adenocarcinoma and therefore might be an unspecific feature. Cluster formation showed a relatively loose arrangement (56.3%), however clusters contained cells having mucous vacuoles (goblet-cell-like feature). Scattered signet ring cells, goblet-like cells, or mucous cells, were found in 87.5% of cases (p<0.001). Some of these showed a histiocyte-like appearance with uniform nuclei. Song et al.16 suggested that the histologic findings of adenocarcinomas expressing ALK show intra- and extra-cytoplasmic mucin and show a signet ring cell appearance, or a cribriform pattern with extracytoplasmic mucin.16 These histologic findings are compatible with cytologic features. Most individual cells had vesicular monotonous nuclei without or with a small nucleolus. Prominent nucleoli and dense chromatin were found in only two cases. The chromatin pattern was homogenous, showing a ground-glass appearance. These findings were reminiscent of ALK-rearranged anaplastic large cell lymphoma, which tends to show less nuclear pleomorphism than the ALK-wild type variety.17 The specificity and sensitivity of the tubulopapillary or tubulocribriform patterns and vesicular chromatin in cytologic smears to predict ALK-positive adenocarcinoma were higher than those of other cytologic findings. However there were only 16 cases of ALK-positive adenocarcinoma, it will be necessary to study a larger group.
- The morphologic criteria of fine needle aspiration cytology for classic adenocarcinoma include small aggregates with glandular, acinar, and papillary architecture. The cell membrane is poorly defined, and cells show scanty and vacuolated cytoplasm with prominent nucleoli.18 The differences in features between classic adenocarcinoma (ALK-negative adenocarcinoma) and ALK-positive adenocarcinoma are cell architecture (tubulopapillary, tubulocribriform, and scattered single cells) and in chromatin pattern (vesicular and bland, and small tiny nucleoli). Based on the specificity and sensitivity findings, the specific cytological findings to predict ALK-positive adenocarcinoma might be tubulopapillary or tubulocribriform architectures, signet ring cells, and vesicular chromatin. One of the cytologic features of mucinous adenocarcinoma is the presence of abundant extracellular mucin admixed with a variable number of neoplastic cells, histiocytes, multinucleated giant cells, fibroblasts, and stromal fragments.19 The tumor cells showed sharply delineated cell borders and unevenly distributed nuclei, forming a characteristic "drunken honeycomb" pattern with tall columnar cells.20 On the other hand, ALK-positive adenocarcinoma frequently showed a relatively small amount of mucin, and did not have tall columnar cells. In addition, no multinucleated giant cells or fibroblasts were observed. The nuclear membrane was smooth and thickened. However, that of mucinous adenocarcinoma was slightly irregular. A lepidic pattern in pulmonary adenocarcinoma tends to exfoliate as single cells, papillary fronds, and sheets. The nuclei are characteristically round to oval and uniform in size, with finely granular or powdery chromatin and inconspicuous nucleoli. ALK-positive adenocarcinoma had similar nuclear features of a lepidic pattern.20 The tumor cells were arranged in a tubulopapillary or tubulocribriform pattern and had small nucleoli. Scattered single cells were more common in the fields of cytologic smears. Making a differential diagnosis may be difficult, however there are several morphological features that can help provide a diagnosis.
- In conclusion, a tubulopapillary or tubulocribriform arrangement and scattered single monotonous cells (signet ring cell) with uniform vesicular nuclei are characteristic features of ALK-expressing pulmonary adenocarcinoma (p<0.001). Areas of mucinous background may be helpful. These finding might not be definitive; however, they are helpful in suggesting a diagnosis.
DISCUSSION
- 1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007; 57: 43-66. ArticlePubMed
- 2. Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000: the global picture. Eur J Cancer 2001; 37(Suppl 8):S4-S66. ArticlePubMed
- 3. Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6: 244-285. PubMedPMC
- 4. Gold KA, Wistuba II, Kim ES. New strategies in squamous cell carcinoma of the lung: identification of tumor drivers to personalize therapy. Clin Cancer Res 2012; 18: 3002-3007. ArticlePubMedPMCPDF
- 5. Perez-Moreno P, Brambilla E, Thomas R, Soria JC. Squamous cell carcinoma of the lung: molecular subtypes and therapeutic opportunities. Clin Cancer Res 2012; 18: 2443-2451. ArticlePubMedPDF
- 6. Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 2008; 455: 1069-1075. PubMedPMC
- 7. Chirieac LR, Dacic S. Targeted therapies in lung cancer. Surg Pathol Clin 2010; 3: 71-82. ArticlePubMedPMC
- 8. Paik JH, Choi CM, Kim H, et al. Clinicopathologic implication of ALK rearrangement in surgically resected lung cancer: a proposal of diagnostic algorithm for ALK-rearranged adenocarcinoma. Lung Cancer 2012; 76: 403-409. ArticlePubMed
- 9. Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol 2009; 22: 508-515. ArticlePubMedPDF
- 10. Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol 2008; 3: 13-17. ArticlePubMed
- 11. Jokoji R, Yamasaki T, Minami S, et al. Combination of morphological feature analysis and immunohistochemistry is useful for screening of EML4-ALK-positive lung adenocarcinoma. J Clin Pathol 2010; 63: 1066-1070. ArticlePubMed
- 12. Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res 2009; 15: 5216-5223. ArticlePubMedPMCPDF
- 13. Kawahara A, Akiba J, Abe H, et al. EML4-ALK-positive lung adenocarcinoma with signet-ring cells. Diagn Cytopathol 2012 11 16 [Epub]. http://dx.doi.org/10.1002/dc.22936. ArticlePMC
- 14. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007; 448: 561-566. ArticlePubMedPDF
- 15. Yoshida A, Tsuta K, Nakamura H, et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol 2011; 35: 1226-1234. ArticlePubMed
- 16. Song HJ, Jeong JY, Choi Y, Han J. Histologic features of ALK-expressing adenocarciomas of the lung. J Lung Cancer 2011; 10: 32-36. Article
- 17. Shiota M, Nakamura S, Ichinohasama R, et al. Anaplastic large cell lymphomas expressing the novel chimeric protein p80NPM/ALK: a distinct clinicopathologic entity. Blood 1995; 86: 1954-1960. ArticlePubMedPDF
- 18. Nizzoli R, Tiseo M, Gelsomino F, et al. Accuracy of fine needle aspiration cytology in the pathological typing of non-small cell lung cancer. J Thorac Oncol 2011; 6: 489-493. ArticlePubMed
- 19. Chhieng DC. Fine-needle aspiration cytology of pulmonary mucinous cystadenocarcinoma. Diagn Cytopathol 2008; 36: 581-585. ArticlePubMed
- 20. Morency E, Rodriguez Urrego PA, Szporn AH, Beth Beasley M, Chen H. The "drunken honeycomb" feature of pulmonary mucinous adenocarcinoma: a diagnostic pitfall of bronchial brushing cytology. Diagn Cytopathol 2013; 41: 63-66. ArticlePubMed
REFERENCES
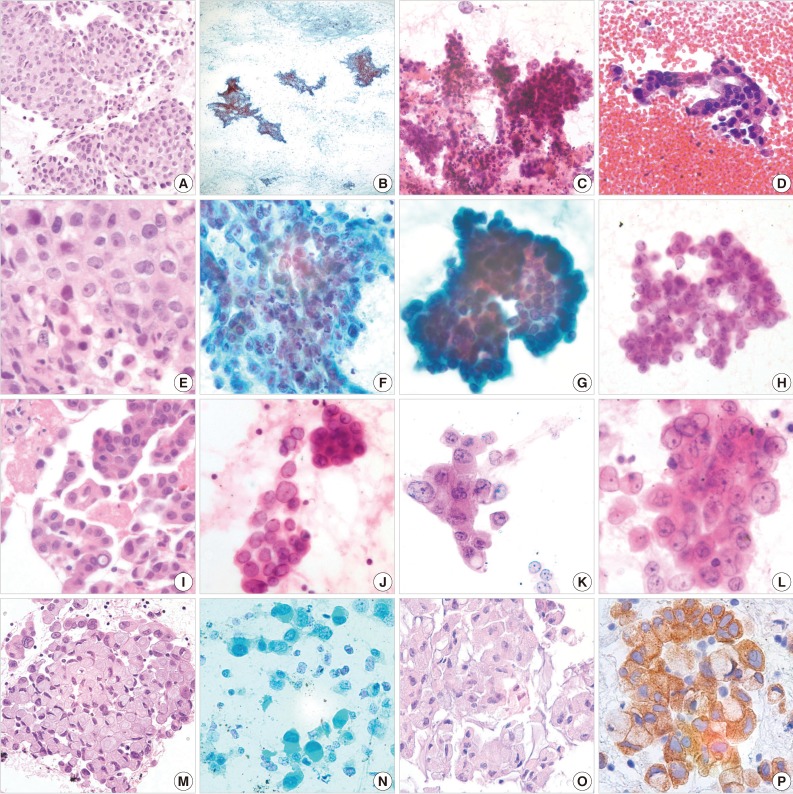
| Cytologic feature | ALK-positive adenocarcinoma (%) | ALK-negative adenocarcinoma (%) | p-valuea | |
|---|---|---|---|---|
| Background | Mucin | 13/16 (81.3) | 6/16 (37.5) | 0.012 |
| Hemorrhagic | 8/16 (50.0) | 5/16 (31.3) | 0.280 | |
| Necrotic | 3/16 (18.8) | 8/16 (50.0) | 0.063 | |
| Architecture | Tubulopapillary | 15/16 (93.8) | 3/16 (18.8) | < 0.001 |
| Tubulocribriform | 15/16 (93.8) | 3/16 (18.8) | < 0.001 | |
| Acini | 6/16 (37.5) | 13/16 (81.3) | < 0.001 | |
| Sheet | 15/16 (93.8) | 14/16 (87.5) | 0.387b | |
| Cluster | 9/16 (56.3) | 11/16 (68.8) | 0.465 | |
| Single | 11/16 (68.8) | 3/16 (18.8) | 0.004 | |
| Signet ring cell | 14/16 (87.5) | 1/16 (6.3) | < 0.001 | |
| Nucleus | Vesicular chromatin | 14/16 (87.5) | 2/16 (12.5) | < 0.001 |
| Small nucleoli | 10/16 (62.5) | 8/16 (50.0) | 0.476 |
Figure & Data
References
Citations

- Cytomorphological and histomorphological features of lung adenocarcinoma with epidermal growth factor receptor mutation and anaplastic lymphoma kinase gene rearrangement
Nikola Gardić, Aleksandra Lovrenski, Vanesa Sekeruš, Svetlana Lečić, Milorad Bijelović, Tanja Lakić, Aleksandra Ilić, Bojan Zarić, Sofija Glumac
Oncology Letters.2024;[Epub] CrossRef - Machine learning‐based gene alteration prediction model for primary lung cancer using cytologic images
Shuhei Ishii, Manabu Takamatsu, Hironori Ninomiya, Kentaro Inamura, Takeshi Horai, Akira Iyoda, Naoko Honma, Rira Hoshi, Yuko Sugiyama, Noriko Yanagitani, Mingyon Mun, Hitoshi Abe, Tetuo Mikami, Kengo Takeuchi
Cancer Cytopathology.2022; 130(10): 812. CrossRef - Fine‐needle aspiration cytology of non‐small cell lung carcinoma: A paradigm shift
Pranab Dey, Ratan Kumar Ghosh
Diagnostic Cytopathology.2019; 47(4): 351. CrossRef - Qualitative and quantitative cytomorphological features of primary anaplastic lymphoma kinase‐positive lung cancer
Ryuko Tsukamoto, Hiroyuki Ohsaki, Sho Hosokawa, Yasunori Tokuhara, Shingo Kamoshida, Toshiko Sakuma, Tomoo Itoh, Chiho Ohbayashi
Cytopathology.2019; 30(3): 295. CrossRef - Primary signet-ring adenocarcinoma of the lung: A rare lung tumor
Varun Rajpal, Rahul Kumar Sharma, Charul Dabral, Deepak Talwar
South Asian Journal of Cancer.2019; 08(04): 257. CrossRef - Cytological features in eight patients with ALK‐rearranged lung cancer
Naoto Kuroda, Masahiko Ohara, Yukari Wada, Kaori Yasuoka, Keiko Mizuno, Kenji Yorita, Chiho Obayashi, Kengo Takeuchi
Diagnostic Cytopathology.2018; 46(6): 516. CrossRef - Cytological markers for predicting ALK‐positive pulmonary adenocarcinoma
K. Miyata, S. Morita, H. Dejima, N. Seki, N. Matsutani, M. Mieno, F. Kondo, Y. Soejima, F. Tanaka, M. Sawabe
Diagnostic Cytopathology.2017; 45(11): 963. CrossRef - ALK-rearranged adenocarcinoma with extensive mucin production can mimic mucinous adenocarcinoma: clinicopathological analysis and comprehensive histological comparison with KRAS-mutated mucinous adenocarcinoma
Yoon Jin Cha, Joungho Han, Soo Hyun Hwang, Tae Bum Lee, Hojoong Kim, Jea Ill Zo
Pathology.2016; 48(4): 325. CrossRef - Cytomorphological identification of advanced pulmonary adenocarcinoma harboring KRAS mutation in lymph node fine‐needle aspiration specimens: Comparative investigation of adenocarcinoma with KRAS and EGFR mutations
Dae Hyun Song, Boram Lee, Yooju Shin, In Ho Choi, Sang Yun Ha, Jae Jun Lee, Min Eui Hong, Yoon‐La Choi, Joungho Han, Sang‐Won Um
Diagnostic Cytopathology.2015; 43(7): 539. CrossRef - Comprehensive analysis of RET and ROS1 rearrangement in lung adenocarcinoma
Seung Eun Lee, Boram Lee, Mineui Hong, Ji-Young Song, Kyungsoo Jung, Maruja E Lira, Mao Mao, Joungho Han, Jhingook Kim, Yoon-La Choi
Modern Pathology.2015; 28(4): 468. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
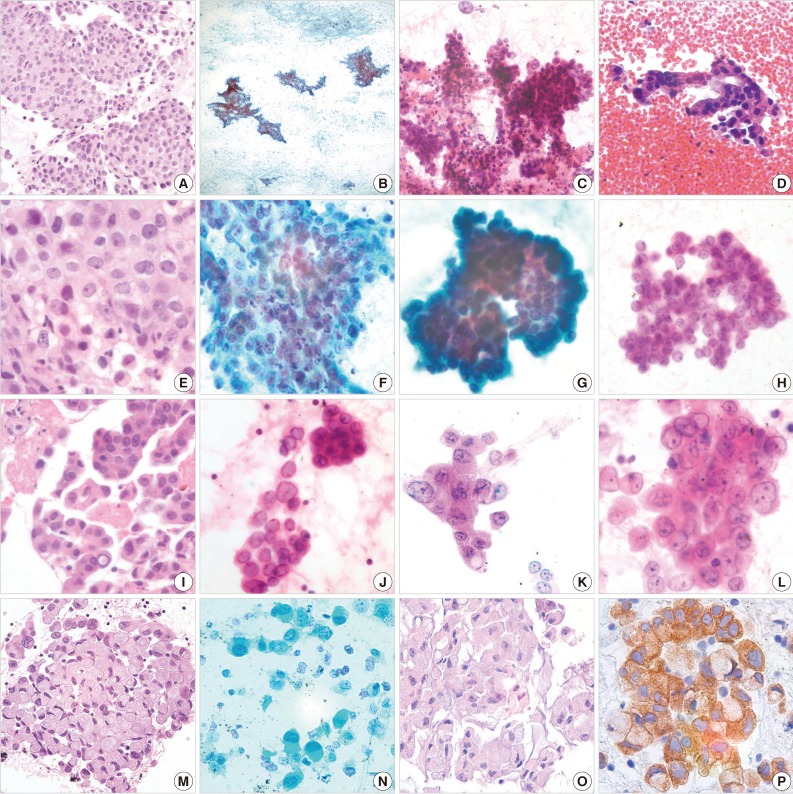
Fig. 1
| Cytologic feature | ALK-positive adenocarcinoma (%) | ALK-negative adenocarcinoma (%) | p-value |
|
|---|---|---|---|---|
| Background | Mucin | 13/16 (81.3) | 6/16 (37.5) | 0.012 |
| Hemorrhagic | 8/16 (50.0) | 5/16 (31.3) | 0.280 | |
| Necrotic | 3/16 (18.8) | 8/16 (50.0) | 0.063 | |
| Architecture | Tubulopapillary | 15/16 (93.8) | 3/16 (18.8) | < 0.001 |
| Tubulocribriform | 15/16 (93.8) | 3/16 (18.8) | < 0.001 | |
| Acini | 6/16 (37.5) | 13/16 (81.3) | < 0.001 | |
| Sheet | 15/16 (93.8) | 14/16 (87.5) | 0.387 |
|
| Cluster | 9/16 (56.3) | 11/16 (68.8) | 0.465 | |
| Single | 11/16 (68.8) | 3/16 (18.8) | 0.004 | |
| Signet ring cell | 14/16 (87.5) | 1/16 (6.3) | < 0.001 | |
| Nucleus | Vesicular chromatin | 14/16 (87.5) | 2/16 (12.5) | < 0.001 |
| Small nucleoli | 10/16 (62.5) | 8/16 (50.0) | 0.476 |
p-value for chi-square test; p-value for Fisher exact test.

 E-submission
E-submission



