Search
- Page Path
- HOME > Search
- Diagnostic yield of fine needle aspiration with simultaneous core needle biopsy for thyroid nodules
- Mohammad Ali Hasannia, Ramin Pourghorban, Hoda Asefi, Amir Aria, Elham Nazar, Hojat Ebrahiminik, Alireza Mohamadian
- J Pathol Transl Med. 2025;59(3):180-187. Published online April 16, 2025
- DOI: https://doi.org/10.4132/jptm.2025.03.04
- 1,916 View
- 139 Download
-
 Abstract
Abstract
 PDF
PDF - Background
Fine needle aspiration (FNA) is a widely utilized technique for assessing thyroid nodules; however, its inherent non-diagnostic rate poses diagnostic challenges. The present study aimed to evaluate and compare the diagnostic efficacy of FNA, core needle biopsy (CNB), and their combined application in the assessment of thyroid nodules.
Methods
A total of 56 nodules from 50 patients was analyzed using both FNA and simultaneous CNB. The ultrasound characteristics were categorized according to the American College of Radiology Thyroid Imaging Reporting and Data Systems classification system. The study compared the sensitivity, specificity, and accuracy of FNA, CNB, and the combination of the two techniques.
Results
The concordance between FNA and CNB was notably high, with a kappa coefficient of 0.837. The sensitivity for detecting thyroid malignancy was found to be 25.0% for FNA, 66.7% for CNB, and 83.3% for the combined FNA/CNB approach, with corresponding specificities of 84.6%, 97.4%, and 97.4%. The accuracy of the FNA/CNB combination was the highest at 94.1%.
Conclusions
The findings of this study indicate that both CNB and the FNA/CNB combination offer greater diagnostic accuracy for thyroid malignancy compared to FNA alone, with no significant complications reported. Integrating CNB with FNA findings may enhance management strategies and treatment outcomes for patients with thyroid nodules.
- Fine needle aspiration cytology diagnoses of follicular thyroid carcinoma: results from a multicenter study in Asia
- Hee Young Na, Miyoko Higuchi, Shinya Satoh, Kaori Kameyama, Chan Kwon Jung, Su-Jin Shin, Shipra Agarwal, Jen-Fan Hang, Yun Zhu, Zhiyan Liu, Andrey Bychkov, Kennichi Kakudo, So Yeon Park
- J Pathol Transl Med. 2024;58(6):331-340. Published online November 7, 2024
- DOI: https://doi.org/10.4132/jptm.2024.10.12
- 2,491 View
- 235 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
This study was designed to compare diagnostic categories of thyroid fine needle aspiration cytology (FNAC) and incidence of thyroid tumors in the multi-institutional Asian series with a special focus on diagnostic category IV (suspicious for a follicular neoplasm) and follicular thyroid carcinomas (FTCs). Methods: Distribution of FNAC categories, incidence of thyroid tumors in resection specimens and cytologic diagnoses of surgically confirmed follicular adenomas (FAs) and FTCs were collected from 10 institutes from five Asian countries and were compared among countries and between FAs and FTCs. Results: The frequency of category IV diagnoses (3.0%) in preoperative FNAC were significantly lower compared to those in Western countries (10.1%). When comparing diagnostic categories among Asian countries, category IV was more frequent in Japan (4.6%) and India (7.9%) than in Taiwan (1.4%), Korea (1.4%), and China (3.6%). Similarly, incidence of FAs and FTCs in surgical resection specimens was significantly higher in Japan (10.9%) and India (10.1%) than in Taiwan (5.5%), Korea (3.0%), and China (2.5%). FTCs were more commonly diagnosed as category IV in Japan (77.5%) than in Korea (33.3%) and China (35.0%). Nuclear pleomorphism, nuclear crowding, microfollicular pattern, and dyshesive cell pattern were more common in FTCs compared with FAs. Conclusions: Our study highlighted the difference in FNAC diagnostic categories of FTCs among Asian countries, which is likely related to different reporting systems and thyroid cancer incidence. Cytologic features such as nuclear pleomorphism, nuclear crowding, microfollicular pattern, and dyshesive cell pattern were found to be useful in diagnosing FTCs more effectively.
- Cytologic hallmarks and differential diagnosis of papillary thyroid carcinoma subtypes
- Agnes Stephanie Harahap, Chan Kwon Jung
- J Pathol Transl Med. 2024;58(6):265-282. Published online November 7, 2024
- DOI: https://doi.org/10.4132/jptm.2024.10.11
- 4,523 View
- 407 Download
- 2 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - Papillary thyroid carcinoma (PTC) is the most common thyroid malignancy, characterized by a range of subtypes that differ in their cytologic features, clinical behavior, and prognosis. Accurate cytologic evaluation of PTC using fine-needle aspiration is essential but can be challenging due to the morphologic diversity among subtypes. This review focuses on the distinct cytologic characteristics of various PTC subtypes, including the classic type, follicular variant, tall cell, columnar cell, hobnail, diffuse sclerosing, Warthin-like, solid/trabecular, and oncocytic PTCs. Each subtype demonstrates unique nuclear features, architectural patterns, and background elements essential for diagnosis and differentiation from other thyroid lesions. Recognizing these distinct cytologic patterns is essential for identifying aggressive subtypes like tall cell, hobnail, and columnar cell PTCs, which have a higher risk of recurrence, metastasis, and poorer clinical outcomes. Additionally, rare subtypes such as diffuse sclerosing and Warthin-like PTCs present unique cytologic profiles that must be carefully interpreted to avoid diagnostic errors. The review also highlights the cytologic indicators of lymph node metastasis and high-grade features, such as differentiated high-grade thyroid carcinoma. The integration of molecular testing can further refine subtype diagnosis by identifying specific genetic mutations. A thorough understanding of these subtype-specific cytologic features and molecular profiles is vital for accurate diagnosis, risk stratification, and personalized management of PTC patients. Future improvements in diagnostic techniques and standardization are needed to enhance cytologic evaluation and clinical decision-making in thyroid cancer.
-
Citations
Citations to this article as recorded by- Nuclear pseudoinclusion is associated with BRAFV600E mutation: Analysis of nuclear features in papillary thyroid carcinoma
Agnes Stephanie Harahap, Dina Khoirunnisa, Salinah, Maria Francisca Ham
Annals of Diagnostic Pathology.2025; 75: 152434. CrossRef - 2025 Korean Thyroid Association Clinical Management Guideline on Active Surveillance for Low-Risk Papillary Thyroid Carcinoma
Eun Kyung Lee, Min Joo Kim, Seung Heon Kang, Bon Seok Koo, Kyungsik Kim, Mijin Kim, Bo Hyun Kim, Ji-hoon Kim, Shin Je Moon, Kyorim Back, Young Shin Song, Jong-hyuk Ahn, Hwa Young Ahn, Ho-Ryun Won, Won Sang Yoo, Min Kyoung Lee, Jeongmin Lee, Ji Ye Lee, Kyo
International Journal of Thyroidology.2025; 18(1): 30. CrossRef - Structure-based molecular screening and dynamic simulation of phytocompounds targeting VEGFR-2: a novel therapeutic approach for papillary thyroid carcinoma
Shuai Wang, Lingqian Zhang, Wenjun Zhang, Xiong Zeng, Jie Mei, Weidong Xiao, Lijie Yang
Frontiers in Pharmacology.2025;[Epub] CrossRef - 2025 Korean Thyroid Association Clinical Management Guideline on Active Surveillance for Low-Risk Papillary Thyroid Carcinoma
Eun Kyung Lee, Min Joo Kim, Seung Heon Kang, Bon Seok Koo, Kyungsik Kim, Mijin Kim, Bo Hyun Kim, Ji-hoon Kim, Shinje Moon, Kyorim Back, Young Shin Song, Jong-hyuk Ahn, Hwa Young Ahn, Ho-Ryun Won, Won Sang Yoo, Min Kyoung Lee, Jeongmin Lee, Ji Ye Lee, Kyon
Endocrinology and Metabolism.2025; 40(3): 307. CrossRef
- Nuclear pseudoinclusion is associated with BRAFV600E mutation: Analysis of nuclear features in papillary thyroid carcinoma
- Contribution of cytologic examination to diagnosis of poorly differentiated thyroid carcinoma
- Na Rae Kim, Jae Yeon Seok, Yoo Seung Chung, Joon Hyop Lee, Dong Hae Chung
- J Pathol Transl Med. 2020;54(2):171-178. Published online February 5, 2020
- DOI: https://doi.org/10.4132/jptm.2019.12.03
- 7,222 View
- 201 Download
- 3 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
The cytologic diagnosis of poorly differentiated thyroid carcinoma (PDTC) is difficult because it lacks salient cytologic findings and shares cytologic features with more commonly encountered neoplasms. Due to diverse cytologic findings and paucicellularity of PDTC, standardization of cytologic diagnostic criteria is limited. The purpose of this study is to investigate and recognize diverse thyroid findings of fine needle aspiration (FNA) cytology and frozen smear cytology in diagnosis of this rare but aggressive carcinoma.
Methods
The present study included six cases of FNA cytology and frozen smears of histologically diagnosed PDTCs.
Results
PDTC showed cytologic overlap with well-differentiated thyroid carcinomas (WDTCs). Five of six cases showed dedifferentiation arising from well differentiated thyroid carcinomas. Only one de novo PDTC showed highly cellular smears composed of discohesive small cells, high nuclear/cytoplasmic (N/C) ratio, prominent micronucleoli, and irregular nuclei. Retrospectively reviewed, these findings are highly suspicious for PDTC. Cytologic findings of nuclear atypia, pleomorphism, and irregularity were frequently found, whereas scattered small cells were seen only in the de novo case.
Conclusions
Heterogeneous cytologic findings of PDTCs are shared with those of WDTCs and contribute to difficult preoperative cytologic diagnoses. Most PDTCs show dedifferentiation from WDTCs. Albeit rare, de novo PDTC should be considered with cytology showing discohesive small cells with high N/C ratio. This will enable precise diagnosis and prompt treatment of this aggressive malignancy -
Citations
Citations to this article as recorded by- Non-papillary thyroid carcinoma diagnoses in The Bethesda System for Reporting Thyroid Cytopathology categories V and VI: An institutional experience
Myunghee Kang, Na Rae Kim, Jae Yeon Seok
Annals of Diagnostic Pathology.2024; 71: 152263. CrossRef - Cytologic features of differentiated high‐grade thyroid carcinoma: A multi‐institutional study of 40 cases
Vanda F. Torous, Tikamporn Jitpasutham, Zubair Baloch, Richard L. Cantley, Darcy A. Kerr, Xiaoying Liu, Zahra Maleki, Ross Merkin, Vania Nosé, Liron Pantanowitz, Isabella Tondi Resta, Esther D. Rossi, William C. Faquin
Cancer Cytopathology.2024; 132(8): 525. CrossRef - An Unexpected Finding of Poorly Differentiated Thyroid Carcinoma in a Toxic Thyroid Nodule
Kimberly Yuang, Huda Al-Bahadili, Alan Chang
JCEM Case Reports.2023;[Epub] CrossRef - Revisiting the cytomorphological features of poorly differentiated thyroid carcinoma: a comparative analysis with indeterminate thyroid fine-needle aspiration samples
Yazeed Alwelaie, Ali Howaidi, Mohammed Tashkandi, Ahmad Almotairi, Hisham Saied, Moammar Muzzaffar, Doaa Alghamdi
Journal of the American Society of Cytopathology.2023; 12(5): 331. CrossRef - Characterization of the genomic alterations in poorly differentiated thyroid cancer
Yeeun Lee, SeongRyeol Moon, Jae Yeon Seok, Joon-Hyop Lee, Seungyoon Nam, Yoo Seung Chung
Scientific Reports.2023;[Epub] CrossRef
- Non-papillary thyroid carcinoma diagnoses in The Bethesda System for Reporting Thyroid Cytopathology categories V and VI: An institutional experience
- 2019 Practice guidelines for thyroid core needle biopsy: a report of the Clinical Practice Guidelines Development Committee of the Korean Thyroid Association
- Chan Kwon Jung, Jung Hwan Baek, Dong Gyu Na, Young Lyun Oh, Ka Hee Yi, Ho-Cheol Kang
- J Pathol Transl Med. 2020;54(1):64-86. Published online January 15, 2020
- DOI: https://doi.org/10.4132/jptm.2019.12.04
- 22,357 View
- 978 Download
- 39 Web of Science
- 47 Crossref
-
 Abstract
Abstract
 PDF
PDF - Ultrasound-guided core needle biopsy (CNB) has been increasingly used for the pre-operative diagnosis of thyroid nodules. Since the Korean Society of the Thyroid Radiology published the ‘Consensus Statement and Recommendations for Thyroid CNB’ in 2017 and the Korean Endocrine Pathology Thyroid CNB Study Group published ‘Pathology Reporting of Thyroid Core Needle Biopsy’ in 2015, advances have occurred rapidly not only in the management guidelines for thyroid nodules but also in the diagnostic terminology and classification schemes. The Clinical Practice Guidelines Development Committee of the Korean Thyroid Association (KTA) reviewed publications on thyroid CNB from 1995 to September 2019 and updated the recommendations and statements for the diagnosis and management of thyroid nodules using CNB. Recommendations for the resolution of clinical controversies regarding the use of CNB were based on expert opinion. These practical guidelines include recommendations and statements regarding indications for CNB, patient preparation, CNB technique, biopsy-related complications, biopsy specimen preparation and processing, and pathology interpretation and reporting of thyroid CNB.
-
Citations
Citations to this article as recorded by- Comparison of core-needle biopsy and repeat fine-needle aspiration biopsy for thyroid nodules with initially inconclusive findings: a systematic review, diagnostic accuracy meta-analysis, and meta-regression
Hendra Zufry, Timotius Ivan Hariyanto
Journal of the American Society of Cytopathology.2025; 14(3): 159. CrossRef - Ultrasound-guided core-needle biopsy for diagnosis of thyroid cancer
D.D. Dolidze, S.D. Kovantsev, Z.A. Bagatelia, A.V. Bumbu, Yu.V. Barinov, G.M. Chechenin, N.V. Pichugina, D.G. Gogolashvili
Pirogov Russian Journal of Surgery.2025; (3): 87. CrossRef - Superior Diagnostic Yield of Core Needle Biopsy Over Fine Needle Aspiration in Diagnosing Follicular-Patterned Neoplasms: A Multicenter Study Focusing on Bethesda IV Results
Leehi Joo, Jung Hwan Baek, Jungbok Lee, Dong Eun Song, Sae Rom Chung, Young Jun Choi, Jeong Hyun Lee
Korean Journal of Radiology.2025; 26(6): 604. CrossRef - Diagnostic yield of fine needle aspiration with simultaneous core needle biopsy for thyroid nodules
Mohammad Ali Hasannia, Ramin Pourghorban, Hoda Asefi, Amir Aria, Elham Nazar, Hojat Ebrahiminik, Alireza Mohamadian
Journal of Pathology and Translational Medicine.2025; 59(3): 180. CrossRef - Lessons learned from the first 2 years of experience with thyroid core needle biopsy at an Indonesian national referral hospital
Agnes Stephanie Harahap, Maria Francisca Ham, Retno Asti Werdhani, Erwin Danil Julian, Rafi Ilmansyah, Chloe Indira Arfelita Mangunkusumso, Tri Juli Edi Tarigan
Journal of Pathology and Translational Medicine.2025; 59(3): 149. CrossRef - Preoperative Fine-Needle Aspiration in Goiter With Compressive Symptoms: A Systematic Review and Meta-analysis
Moeen Sbeit, Rania Faris, Ohad Ronen
Endocrine Practice.2025;[Epub] CrossRef - A comparative analysis of core needle biopsy and repeat fine needle aspiration in patients with inconclusive initial cytology of thyroid nodules
Xuejiao Su, Can Yue, Wanting Yang, Buyun Ma
Frontiers in Endocrinology.2024;[Epub] CrossRef - A Narrative Review of the 2023 Korean Thyroid Association Management Guideline for Patients with Thyroid Nodules
Eun Kyung Lee, Young Joo Park, Chan Kwon Jung, Dong Gyu Na
Endocrinology and Metabolism.2024; 39(1): 61. CrossRef - Doing more with less: integrating small biopsies in cytology practice
Anjali Saqi, Michiya Nishino, Mauro Saieg, Amy Ly, Abberly Lott Limbach
Journal of the American Society of Cytopathology.2024; 13(4): 233. CrossRef - 2023 Update of the Korean Thyroid Association Guidelines for the Management of Thyroid Nodules
Eun Kyung Lee, Young Joo Park
Clinical Thyroidology®.2024; 36(4): 153. CrossRef - Korean Thyroid Association Guidelines on the Management of Differentiated Thyroid Cancers; Part I. Initial Management of Differentiated Thyroid Cancers - Chapter 2. Surgical Management of Thyroid Cancer 2024
Yoon Young Cho, Cho Rok Lee, Ho-Cheol Kang, Bon Seok Koo, Hyungju Kwon, Sun Wook Kim, Won Woong Kim, Jung-Han Kim, Dong Gyu Na, Young Joo Park, Kyorim Back, Young Shin Song, Seung Hoon Woo, Ho-Ryun Won, Chang Hwan Ryu, Jee Hee Yoon, Min Kyoung Lee, Eun Ky
International Journal of Thyroidology.2024; 17(1): 30. CrossRef - Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules 2024
Young Joo Park, Eun Kyung Lee, Young Shin Song, Su Hwan Kang, Bon Seok Koo, Sun Wook Kim, Dong Gyu Na, Seung-Kuk Baek, So Won Oh, Min Kyoung Lee, Sang-Woo Lee, Young Ah Lee, Yong Sang Lee, Ji Ye Lee, Dong-Jun Lim, Leehi Joo, Yuh-Seog Jung, Chan Kwon Jung,
International Journal of Thyroidology.2024; 17(1): 208. CrossRef - Korean Thyroid Association Guidelines on the Management of Differentiated Thyroid Cancers; Overview and Summary 2024
Young Joo Park, Eun Kyung Lee, Young Shin Song, Bon Seok Koo, Hyungju Kwon, Keunyoung Kim, Mijin Kim, Bo Hyun Kim, Won Gu Kim, Won Bae Kim, Won Woong Kim, Jung-Han Kim, Hee Kyung Kim, Hee Young Na, Shin Je Moon, Jung-Eun Moon, Sohyun Park, Jun-Ook Park, J
International Journal of Thyroidology.2024; 17(1): 1. CrossRef - Educational exchange in thyroid core needle biopsy diagnosis: enhancing pathological interpretation through guideline integration and peer learning
Agnes Stephanie Harahap, Chan Kwon Jung
Journal of Pathology and Translational Medicine.2024; 58(5): 205. CrossRef - Current role of interventional radiology in thyroid nodules
Onur Taydas, Erbil Arik, Omer Faruk Sevinc, Ahmet Burak Kara, Mustafa Ozdemir, Hasret Cengiz, Zulfu Bayhan, Mehmet Halil Ozturk
Frontiers in Endocrinology.2024;[Epub] CrossRef - Neck Schwannoma Masking as Thyroid Tumour: Into the Deep of Diagnostics and Anatomy
Serghei Covantsev, Anna Bumbu, Anna Sukhotko, Evghenii Zakurdaev, Ivan Kuts, Andrey Evsikov
Diagnostics.2024; 14(20): 2332. CrossRef - Thermal ablation for Bethesda III and IV thyroid nodules: current diagnosis and management
Wen-Hui Chan, Pi-Ling Chiang, An-Ni Lin, Yen-Hsiang Chang, Wei-Che Lin
Ultrasonography.2024; 43(6): 395. CrossRef - A new LNC89/LNC60-Col11a2 axis revealed by whole-transcriptome analysis may be associated with goiters related to excess iodine nutrition
Guanying Nie, Shuang Li, Wei Zhang, Fangang Meng, Zixuan Ru, Jiahui Li, Dianjun Sun, Ming Li
Frontiers in Endocrinology.2024;[Epub] CrossRef - A simplified four-tier classification for thyroid core needle biopsy
M. Paja, J. L. Del Cura, R. Zabala, I. Korta, Mª T. Gutiérrez, A. Expósito, A. Ugalde
Journal of Endocrinological Investigation.2024; 48(4): 895. CrossRef - Risk of thyroid cancer in a lung cancer screening population of the National Lung Screening Trial according to the presence of incidental thyroid nodules detected on low-dose chest CT
Hyobin Seo, Kwang Nam Jin, Ji Sang Park, Koung Mi Kang, Eun Kyung Lee, Ji Ye Lee, Roh-Eul Yoo, Young Joo Park, Ji-hoon Kim
Ultrasonography.2023; 42(2): 275. CrossRef - Preoperative Risk Stratification of Follicular-patterned Thyroid Lesions on Core Needle Biopsy by Histologic Subtyping and RAS Variant-specific Immunohistochemistry
Meejeong Kim, Sora Jeon, Chan Kwon Jung
Endocrine Pathology.2023; 34(2): 247. CrossRef - Differential regional importance mapping for thyroid nodule malignancy prediction with potential to improve needle aspiration biopsy sampling reliability
Liping Wang, Yuan Wang, Wenliang Lu, Dong Xu, Jincao Yao, Lijing Wang, Lei Xu
Frontiers in Oncology.2023;[Epub] CrossRef - Preoperative evaluation of thyroid nodules – Diagnosis and management strategies
Tapoi Dana Antonia, Lambrescu Ioana Maria, Gheorghisan-Galateanu Ancuta-Augustina
Pathology - Research and Practice.2023; 246: 154516. CrossRef - 2023 Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules
Young Joo Park, Eun Kyung Lee, Young Shin Song, Soo Hwan Kang, Bon Seok Koo, Sun Wook Kim, Dong Gyu Na, Seung-Kuk Baek, So Won Oh, Min Kyoung Lee, Sang-Woo Lee, Young Ah Lee, Yong Sang Lee, Ji Ye Lee, Dong-Jun Lim, Leehi Joo, Yuh-Seog Jung, Chan Kwon Jung
International Journal of Thyroidology.2023; 16(1): 1. CrossRef - Fast Track Management of Primary Thyroid Lymphoma in the Very Elderly Patient
Pierre Yves Marcy, Frederic Bauduer, Juliette Thariat, Olivier Gisserot, Edouard Ghanassia, Bruno Chetaille, Laurys Boudin, Jean Baptiste Morvan
Current Oncology.2023; 30(6): 5816. CrossRef - Reevaluating diagnostic categories and associated malignancy risks in thyroid core needle biopsy
Chan Kwon Jung
Journal of Pathology and Translational Medicine.2023; 57(4): 208. CrossRef - Diagnostic performance of shear wave elastography in thyroid nodules with indeterminate cytology: A systematic review and meta-analysis
Yuxuan Qiu, Zhichao Xing, Qianru Yang, Yan Luo, Buyun Ma
Heliyon.2023; 9(10): e20654. CrossRef - Comparison of the diagnostic value of fine needle aspiration and ultrasound in thyroid pathology
P. S. Glushkov, R. Kh. Azimov, N. L. Aleshenko, E. A. Maruchak, Y. P. Sych, G. N. Minkova, K. A. Shemyatovsky, V. A. Gorsky
Endocrine Surgery.2023; 17(3): 43. CrossRef - Comparison of Core Needle Biopsy and Repeat Fine-Needle Aspiration in Avoiding Diagnostic Surgery for Thyroid Nodules Initially Diagnosed as Atypia/Follicular Lesion of Undetermined Significance
Leehi Joo, Dong Gyu Na, Ji-hoon Kim, Hyobin Seo
Korean Journal of Radiology.2022; 23(2): 280. CrossRef - Diagnostic efficacy, performance and safety of side-cut core needle biopsy for thyroid nodules: comparison of automated and semi-automated biopsy needles
Ji Yeon Park, Seong Yoon Yi, Soo Heui Baek, Yu Hyun Lee, Heon-Ju Kwon, Hee Jin Park
Endocrine.2022; 76(2): 341. CrossRef - Thyroid Cancer Diagnostics Related to Occupational and Environmental Risk Factors: An Integrated Risk Assessment Approach
Gabriela Maria Berinde, Andreea Iulia Socaciu, Mihai Adrian Socaciu, Andreea Cozma, Armand Gabriel Rajnoveanu, Gabriel Emil Petre, Doina Piciu
Diagnostics.2022; 12(2): 318. CrossRef - Approach to Bethesda system category III thyroid nodules according to US-risk stratification
Jieun Kim, Jung Hee Shin, Young Lyun Oh, Soo Yeon Hahn, Ko Woon Park
Endocrine Journal.2022; 69(1): 67. CrossRef - Clinicopathological and Molecular Features of Secondary Cancer (Metastasis) to the Thyroid and Advances in Management
Marie Nguyen, George He, Alfred King-Yin Lam
International Journal of Molecular Sciences.2022; 23(6): 3242. CrossRef - Diagnostic Performance of Thyroid Core Needle Biopsy Using the Revised Reporting System: Comparison with Fine Needle Aspiration Cytology
Kwangsoon Kim, Ja Seong Bae, Jeong Soo Kim, So Lyung Jung, Chan Kwon Jung
Endocrinology and Metabolism.2022; 37(1): 159. CrossRef - Core Needle Biopsy Can Early and Precisely Identify Large Thyroid Masses
Antonio Matrone, Luigi De Napoli, Liborio Torregrossa, Aleksandr Aghababyan, Piermarco Papini, Carlo Enrico Ambrosini, Rosa Cervelli, Clara Ugolini, Fulvio Basolo, Eleonora Molinaro, Rossella Elisei, Gabriele Materazzi
Frontiers in Oncology.2022;[Epub] CrossRef - Primary thyroid leiomyosarcoma with transvenous extension to the right atrium: a case report
Juraj Dubrava, Peter Martanovic, Marina Pavlovicova, Pavel Babal, Akhil Narang, Maria Mattioli, Nidhish Tiwari, Zhiyu Liu, Mariame Chakir
European Heart Journal - Case Reports.2022;[Epub] CrossRef - Radiofrequency ablation for management of thyroid nodules in quarantine zone of COVID-19 pandemic setting in Indonesia
Kristanto Yuli Yarso, Sumadi Lukman Anwar
Annals of Medicine and Surgery.2022; 81: 104132. CrossRef - A Matched-Pair Analysis of Nuclear Morphologic Features Between Core Needle Biopsy and Surgical Specimen in Thyroid Tumors Using a Deep Learning Model
Faridul Haq, Andrey Bychkov, Chan Kwon Jung
Endocrine Pathology.2022; 33(4): 472. CrossRef - Diagnostic performance of core needle biopsy as a first‐line diagnostic tool for thyroid nodules according to ultrasound patterns: Comparison with fine needle aspiration using propensity score matching analysis
Hye Shin Ahn, Inyoung Youn, Dong Gyu Na, Soo Jin Kim, Mi Yeon Lee
Clinical Endocrinology.2021; 94(3): 494. CrossRef - Hydrodissection: A Novel Approach for Safe Core Needle Biopsy of Small High-Risk Subcapsular Thyroid Nodules
Hojat Ebrahiminik, Hossein Chegeni, Javad Jalili, Rambod Salouti, Hadi Rokni, Afshin Mohammadi, Ali Mosaddegh Khah, Seyed Mohammad Tavangar, Zahra Ebrahiminik
CardioVascular and Interventional Radiology.2021; 44(10): 1651. CrossRef - Application of biomarkers in the diagnosis of uncertain samples of core needle biopsy of thyroid nodules
Yan Xiong, Xin Li, Li Liang, Dong Li, Limin Yan, Xueying Li, Jiting Di, Ting Li
Virchows Archiv.2021; 479(5): 961. CrossRef - VE1 immunohistochemistry is an adjunct tool for detection of BRAFV600E mutation: Validation in thyroid cancer patients
Faiza A. Rashid, Sobia Tabassum, Mosin S. Khan, Hifzur R. Ansari, Muhammad Asif, Ahmareen K. Sheikh, Syed Sameer Aga
Journal of Clinical Laboratory Analysis.2021;[Epub] CrossRef - The Diagnostic Value of the American College of Radiology Thyroid Imaging Reporting and Data System Classification and Shear-Wave Elastography for the Differentiation of Thyroid Nodules
Gül Bora Makal, Aydın Aslan
Ultrasound in Medicine & Biology.2021; 47(5): 1227. CrossRef - Comparison of the diagnostic performance of the modified Korean Thyroid Imaging Reporting and Data System for thyroid malignancy with three international guidelines
Eun Ju Ha, Jung Hee Shin, Dong Gyu Na, So Lyung Jung, Young Hen Lee, Wooyul Paik, Min Ji Hong, Yeo Koon Kim, Chang Yoon Lee
Ultrasonography.2021; 40(4): 594. CrossRef - VE1 Immunohistochemistry Improves the Limit of Genotyping for Detecting BRAFV600E Mutation in Papillary Thyroid Cancer
Sonam Choden, Somboon Keelawat, Chan Kwon Jung, Andrey Bychkov
Cancers.2020; 12(3): 596. CrossRef - The 2019 core-needle biopsy practice guidelines
So Yeong Jeong, Jung Hwan Baek
Ultrasonography.2020; 39(3): 311. CrossRef - Re: The 2019 core-needle biopsy practice guidelines
Ji-hoon Kim
Ultrasonography.2020; 39(3): 313. CrossRef
- Comparison of core-needle biopsy and repeat fine-needle aspiration biopsy for thyroid nodules with initially inconclusive findings: a systematic review, diagnostic accuracy meta-analysis, and meta-regression
- The Usefulness of Immunocytochemistry of CD56 in Determining Malignancy from Indeterminate Thyroid Fine-Needle Aspiration Cytology
- Hyunseo Cha, Ju Yeon Pyo, Soon Won Hong
- J Pathol Transl Med. 2018;52(6):404-410. Published online October 15, 2018
- DOI: https://doi.org/10.4132/jptm.2018.09.20
- 8,379 View
- 171 Download
- 5 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Fine-needle aspiration cytology serves as a safe, economical tool in evaluating thyroid nodules. However, about 30% of the samples are categorized as indeterminate. Hence, many immunocytochemistry markers have been studied, but there has not been a single outstanding marker. We studied the efficacy of CD56 with human bone marrow endothelial cell marker-1 (HBME-1) in diagnosis in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) category III.
Methods
We reviewed ThinPrep liquid-based cytology (LBC) samples with Papanicolaou stain from July 1 to December 31, 2016 (2,195 cases) and selected TBSRTC category III cases (n = 363). Twenty-six cases were histologically confirmed as benign (six cases, 23%) or malignant (20 cases, 77%); we stained 26 LBC slides with HBME-1 and CD56 through the cell transfer method. For evaluation of reactivity of immunocytochemistry, we chose atypical follicular cell clusters.
Results
CD56 was not reactive in 18 of 20 cases (90%) of malignant nodules and showed cytoplasmic positivity in five of six cases (83%) of benign nodules. CD56 showed high sensitivity (90.0%) and relatively low specificity (83.3%) in detecting malignancy (p = .004). HBME-1 was reactive in 17 of 20 cases (85%) of malignant nodules and was not reactive in five of six cases (83%) of benign nodules. HBME-1 showed slightly lower sensitivity (85.0%) than CD56. The specificity in detecting malignancy by HBME-1 was similar to that of CD56 (83.3%, p = .008). CD56 and HBME-1 tests combined showed lower sensitivity (75.0% vs 90%) and higher specificity (93.8% vs 83.3%) in detecting malignancy compared to using CD56 alone.
Conclusions
Using CD56 alone showed relatively low specificity despite high sensitivity for detecting malignancy. Combining CD56 with HBME-1 could increase the specificity. Thus, we suggest that CD56 could be a useful preoperative marker for differential diagnosis of TBSRTC category III samples. -
Citations
Citations to this article as recorded by- Preoperative evaluation of thyroid nodules – Diagnosis and management strategies
Tapoi Dana Antonia, Lambrescu Ioana Maria, Gheorghisan-Galateanu Ancuta-Augustina
Pathology - Research and Practice.2023; 246: 154516. CrossRef - Immunocytochemistry in thyroid cytology and its multiple roles: a systematic review
Federica Policardo, Pietro Tralongo, Angela Feraco, Federica Vegni, Angela Carlino, Alfredo Pontecorvi, Celestino Pio Lombardi, Marco Raffaelli, Francesco Pierconti, Luigi Maria Larocca, Esther Diana Rossi
Diagnostic Histopathology.2023; 29(8): 386. CrossRef - Paving the path toward multi-omics approaches in the diagnostic challenges faced in thyroid pathology
Isabella Piga, Vincenzo L’Imperio, Giulia Capitoli, Vanna Denti, Andrew Smith, Fulvio Magni, Fabio Pagni
Expert Review of Proteomics.2023; 20(12): 419. CrossRef - CD56 Expression in Papillary Thyroid Carcinoma Is Highly Dependent on the Histologic Subtype: A Potential Diagnostic Pitfall
Uiju Cho, Yourha Kim, Sora Jeon, Chan Kwon Jung
Applied Immunohistochemistry & Molecular Morphology.2022; 30(5): 389. CrossRef
- Preoperative evaluation of thyroid nodules – Diagnosis and management strategies
- Preoperative Cytologic Diagnosis of Warthin-like Variant of Papillary Thyroid Carcinoma
- Jisup Kim, Beom Jin Lim, Soon Won Hong, Ju Yeon Pyo
- J Pathol Transl Med. 2018;52(2):105-109. Published online February 12, 2018
- DOI: https://doi.org/10.4132/jptm.2017.12.26
- 8,179 View
- 152 Download
- 9 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Warthin-like variant of papillary thyroid carcinoma (WLV-PTC) is a relatively rare variant of papillary thyroid carcinoma with favorable prognosis. However, preoperative diagnosis using fine-needle aspiration (FNA) specimens is challenging especially with lymphocytic thyroiditis characterized by Hürthle cells and lymphocytic background. To determine a helpful cytological differential point, we compared WLV-PTC FNA findings with conventional papillary thyroid carcinoma with lymphocytic thyroiditis (PTC-LT) and conventional papillary thyroid carcinoma without lymphocytic thyroiditis (PTC) regarding infiltrating inflammatory cells and their distribution. Preoperative diagnosis or potential for WLV-PTC will be helpful for surgeons to decide the scope of operation.
Methods
Of the 8,179 patients treated for papillary thyroid carcinoma between January 2007 and December 2012, 16 patients (0.2%) were pathologically confirmed as WLV-PTC and four cases were available for cytologic review. For comparison, we randomly selected six PTC-LT cases and five PTC cases during the same period. The number of intratumoral and background lymphocytes, histiocytes, neutrophils, and the presence of giant cells were evaluated and compared using conventional smear and ThinPrep preparations.
Results
WLV-PTC showed extensive lymphocytic smear with incorporation of thyroid follicular tumor cell clusters and frequent histiocytes. WLV-PTC was associated with higher intratumoral and background lymphocytes and histiocytes compared with PTC-LT or PTC. The difference was more distinct in liquid-based cytology.
Conclusions
The lymphocytic smear pattern and the number of inflammatory cells of WLV-PTC are different from those of PTC-LT or PTC and will be helpful for the differential diagnosis of WLV-PTC in preoperative FNA. -
Citations
Citations to this article as recorded by- An Algorithmic Approach to Defining Variants of Papillary Thyroid Carcinoma: Accuracy of Fine Needle Aspiration Cytology
Neha Nigam, Neha Kumari, Rishabh Sahai, Nandita Chaudhary, Sabaretnam Mayilvaganan, Pallavi Prasad, Prabhakar Mishra
Journal of Cytology.2025; 42(1): 27. CrossRef - Warthin-like variant of papillary thyroid carcinoma with lymph node metastases: a case report and review of the literature
Andrii Hryshchyshyn, Andrii Bahrii, Pavlina Botsun, Volodymyr Chuba
Journal of Medical Case Reports.2024;[Epub] CrossRef - Cytologic hallmarks and differential diagnosis of papillary thyroid carcinoma subtypes
Agnes Stephanie Harahap, Chan Kwon Jung
Journal of Pathology and Translational Medicine.2024; 58(6): 265. CrossRef - Warthin-like papillary thyroid carcinoma: a case report and comprehensive review of the literature
Abdel Mouhaymen Missaoui, Fatma Hamza, Wafa Belabed, Manel Mellouli, Mohamed Maaloul, Slim Charfi, Issam Jardak, Tahya Sellami-Boudawara, Nabila Rekik, Mohamed Abid
Frontiers in Endocrinology.2023;[Epub] CrossRef - The Warthin-like variant of papillary thyroid carcinomas: a clinicopathologic analysis report of two cases
Xing Zhao, Yijia Zhang, Pengyu Hao, Mingzhen Zhao, Xingbin Shen
Oncologie.2023; 25(5): 581. CrossRef - Challenges in Cytology Specimens With Hürthle Cells
Eleni Thodou, Sule Canberk, Fernando Schmitt
Frontiers in Endocrinology.2021;[Epub] CrossRef - Warthin-like variant of Papillary thyroid carcinoma—Case report of an uncommon tumour with review of literature
Pradyumna Kumar Sahoo, Rashmi Patnayak, Perwez Alam Khan, Amitabh Jena
International Journal of Surgery Case Reports.2020; 77: 9. CrossRef
- An Algorithmic Approach to Defining Variants of Papillary Thyroid Carcinoma: Accuracy of Fine Needle Aspiration Cytology
- Fine-Needle Aspiration Cytology of Carcinosarcoma in the Salivary Gland: An Extremely Rare Case Report
- Hyo Jung An, Hye Jin Baek, Jin Pyeong Kim, Min Hye Kim, Dae Hyun Song
- J Pathol Transl Med. 2018;52(2):136-139. Published online December 28, 2017
- DOI: https://doi.org/10.4132/jptm.2017.07.27
- 6,674 View
- 133 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - Carcinosarcoma of the salivary gland is an extremely rare tumor that is composed of both malignant epithelial and mesenchymal components. Diagnosing carcinosarcoma with fine-needle aspiration cytology is challenging because of its overlapping cytomorphologic characteristics with other high-grade malignant salivary gland tumors. Among the many features, including pleomorphic oncocytoid epithelial components, necrotic background, and mitoses, recognizing the singly scattered atypical spindle cells is most essential in carcinosarcoma. We present a case of a 66-year-old male patient with characteristic features of carcinosarcoma, who was successfully treated by wide local excision and subsequent radiation therapy.
-
Citations
Citations to this article as recorded by- Carcinosarcoma of the parotid gland: a case report and review of the literature
Swachi Jain, Mohammed Abdelwahed, Daniel Hector Chavarria, Lucio Pereira, Gary Stone, Alan Johnson, Jian Yi Li
Journal of Medical Case Reports.2024;[Epub] CrossRef - Is Primary Poorly Differentiated Sarcomatoid Malignancy of the Parotid Gland Sarcomatoid Undifferentiated/Dedifferentiated Melanoma? Report of Three Unusual Cases Diagnosed by Fine-Needle Aspiration Combined with Histological, Immunohistochemical, and Mol
Jerzy Klijanienko, Julien Masliah-Planchon, Olivier Choussy, Guillaume Rougier, Antoine Dubray Vautrin, Maria Lesnik, Nathalie Badois, Wahib Ghanem, Jan Klos, Christophe Le Tourneau, Gregoire Marret, Raymond Barnhill, Adel K. El-Naggar
Acta Cytologica.2024; 68(2): 107. CrossRef - Carcinosarcoma of the deep lobe of the parotid gland in the parapharyngeal region: A case report
Yue-Yang Tang, Gui-Quan Zhu, Zhi-Jian Zheng, Li-Hong Yao, Zi-Xin Wan, Xin-Hua Liang, Ya-Ling Tang
World Journal of Clinical Cases.2023; 11(31): 7663. CrossRef - Carcinosarcoma of Submandibular Salivary Gland with a Rare Sarcomatous Variant
Shalini Bhalla, Naseem Akhtar, Puneet Prakash, Malti Kumari, Madhu Mati Goel
Indian Journal of Surgical Oncology.2019; 10(1): 61. CrossRef
- Carcinosarcoma of the parotid gland: a case report and review of the literature
- Thyroid Fine-Needle Aspiration in Taiwan: The History and Current Practice
- Jen-Fan Hang, Chih-Yi Hsu, Chiung-Ru Lai
- J Pathol Transl Med. 2017;51(6):560-564. Published online October 18, 2017
- DOI: https://doi.org/10.4132/jptm.2017.09.20
- 8,916 View
- 108 Download
- 10 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF - In Taiwan, thyroid cancer is the most common endocrine gland malignancy and the incidence of thyroid cancer has increased four-fold in the past two decades. Fine-needle aspiration is an accurate and cost-effective method of evaluating thyroid nodules and has been the gold-standard diagnostic tool for thyroid tumors in Taiwan since the 1980s. This article reviews the history, current practice, reporting systems, training, and quality assurance for thyroid fine-needle aspiration cytology in Taiwan.
-
Citations
Citations to this article as recorded by- Association between Thyroid Profile Levels and Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Retrospective Study
Yu-Shan Hsieh, Ting-Teng Yang, Chung-Huei Hsu, Yan-Yu Lin
Reports.2024; 7(3): 78. CrossRef - An ultrasonography of thyroid nodules dataset with pathological diagnosis annotation for deep learning
Xiaowen Hou, Menglei Hua, Wei Zhang, Jianxin Ji, Xuan Zhang, Huiru Jiang, Mengyun Li, Xiaoxiao Wu, Wenwen Zhao, Shuxin Sun, Lei Cao, Liuying Wang
Scientific Data.2024;[Epub] CrossRef - The Asian Thyroid Working Group, from 2017 to 2023
Kennichi Kakudo, Chan Kwon Jung, Zhiyan Liu, Mitsuyoshi Hirokawa, Andrey Bychkov, Huy Gia Vuong, Somboon Keelawat, Radhika Srinivasan, Jen-Fan Hang, Chiung-Ru Lai
Journal of Pathology and Translational Medicine.2023; 57(6): 289. CrossRef - Machine Learning–Assisted Diagnostic System for Indeterminate Thyroid Nodules
Lei Chen, Minda Chen, Qian Li, Viksit Kumar, Yu Duan, Kevin A. Wu, Theodore T. Pierce, Anthony E. Samir
Ultrasound in Medicine & Biology.2022; 48(8): 1547. CrossRef - Radiomics Nomogram for Identifying Sub-1 cm Benign and Malignant Thyroid Lesions
Xinxin Wu, Jingjing Li, Yakui Mou, Yao Yao, Jingjing Cui, Ning Mao, Xicheng Song
Frontiers in Oncology.2021;[Epub] CrossRef - Biokinetic model of radioiodine I-131 in nine thyroid cancer patients subjected to in-vivo gamma camera scanning: A simplified five-compartmental model
Chao-Chun Huang, Ya-Hui Lin, Samrit Kittipayak, Yi-Shi Hwua, Shan-Ying Wang, Lung-Kwang Pan, Juan Pardo-Montero
PLOS ONE.2020; 15(5): e0232480. CrossRef - Cancer Incidence Characteristic Evolution Based on the National Cancer Registry in Taiwan
Yu-Ching Huang, Yu-Hung Chen
Journal of Oncology.2020; 2020: 1. CrossRef - Thyroid fine-needle aspiration cytology in Taiwan: a nationwide survey and literature update
Chien-Chin Chen, Jen-Fan Hang, Chih-Yi Liu, Yeh-Han Wang, Chiung-Ru Lai
Journal of Pathology and Translational Medicine.2020; 54(5): 361. CrossRef - Machine Learning–Assisted System for Thyroid Nodule Diagnosis
Bin Zhang, Jie Tian, Shufang Pei, Yubing Chen, Xin He, Yuhao Dong, Lu Zhang, Xiaokai Mo, Wenhui Huang, Shuzhen Cong, Shuixing Zhang
Thyroid.2019; 29(6): 858. CrossRef - Thyroid FNA cytology in Asian practice—Active surveillance for indeterminate thyroid nodules reduces overtreatment of thyroid carcinomas
K. Kakudo, M. Higuchi, M. Hirokawa, S. Satoh, C. K. Jung, A. Bychkov
Cytopathology.2017; 28(6): 455. CrossRef - The Use of Fine-Needle Aspiration (FNA) Cytology in Patients with Thyroid Nodules in Asia: A Brief Overview of Studies from the Working Group of Asian Thyroid FNA Cytology
Chan Kwon Jung, SoonWon Hong, Andrey Bychkov, Kennichi Kakudo
Journal of Pathology and Translational Medicine.2017; 51(6): 571. CrossRef
- Association between Thyroid Profile Levels and Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Retrospective Study
- The Use of the Bethesda System for Reporting Thyroid Cytopathology in Korea: A Nationwide Multicenter Survey by the Korean Society of Endocrine Pathologists
- Mimi Kim, Hyo Jin Park, Hye Sook Min, Hyeong Ju Kwon, Chan Kwon Jung, Seoung Wan Chae, Hyun Ju Yoo, Yoo Duk Choi, Mi Ja Lee, Jeong Ja Kwak, Dong Eun Song, Dong Hoon Kim, Hye Kyung Lee, Ji Yeon Kim, Sook Hee Hong, Jang Sihn Sohn, Hyun Seung Lee, So Yeon Park, Soon Won Hong, Mi Kyung Shin
- J Pathol Transl Med. 2017;51(4):410-417. Published online June 14, 2017
- DOI: https://doi.org/10.4132/jptm.2017.04.05
- 10,275 View
- 224 Download
- 25 Web of Science
- 23 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) has standardized the reporting of thyroid cytology specimens. The objective of the current study was to evaluate the nationwide usage of TBSRTC and assess the malignancy rates in each category of TBSRTC in Korea.
Methods
Questionnaire surveys were used for data collection on the fine needle aspiration (FNA) of thyroid nodules at 74 institutes in 2012. The incidences and follow-up malignancy rates of each category diagnosed from January to December, 2011, in each institute were also collected and analyzed.
Results
Sixty out of 74 institutes answering the surveys reported the results of thyroid FNA in accordance with TBSRTC. The average malignancy rates for resected cases in 15 institutes were as follows: nondiagnostic, 45.6%; benign, 16.5%; atypical of undetermined significance, 68.8%; suspicious for follicular neoplasm (SFN), 30.2%; suspicious for malignancy, 97.5%; malignancy, 99.7%.
Conclusions
More than 80% of Korean institutes were using TBSRTC as of 2012. All malignancy rates other than the SFN and malignancy categories were higher than those reported by other countries. Therefore, the guidelines for treating patients with thyroid nodules in Korea should be revisited based on the malignancy rates reported in this study. -
Citations
Citations to this article as recorded by- High Rates of Unnecessary Surgery for Indeterminate Thyroid Nodules in the Absence of Molecular Test and the Cost-Effectiveness of Utilizing Molecular Test in an Asian Population: A Decision Analysis
Man Him Matrix Fung, Ching Tang, Gin Wai Kwok, Tin Ho Chan, Yan Luk, David Tak Wai Lui, Carlos King Ho Wong, Brian Hung Hin Lang
Thyroid®.2025; 35(2): 166. CrossRef - Inconclusive cytology results of fine-needle aspiration for thyroid nodules: the importance of strict guideline implementation
Sangwoo Cho, Kyunghwa Han, Jung Hyun Yoon, Vivian Youngjean Park, Miribi Rho, Jiyoung Yoon, Jin Young Kwak
Ultrasonography.2025; 44(4): 285. CrossRef - Improved Diagnostic Accuracy of Thyroid Fine-Needle Aspiration Cytology with Artificial Intelligence Technology
Yujin Lee, Mohammad Rizwan Alam, Hongsik Park, Kwangil Yim, Kyung Jin Seo, Gisu Hwang, Dahyeon Kim, Yeonsoo Chung, Gyungyub Gong, Nam Hoon Cho, Chong Woo Yoo, Yosep Chong, Hyun Joo Choi
Thyroid®.2024; 34(6): 723. CrossRef - Welcoming the new, revisiting the old: a brief glance at cytopathology reporting systems for lung, pancreas, and thyroid
Rita Luis, Balamurugan Thirunavukkarasu, Deepali Jain, Sule Canberk
Journal of Pathology and Translational Medicine.2024; 58(4): 165. CrossRef - Fine needle aspiration cytology diagnoses of follicular thyroid carcinoma: results from a multicenter study in Asia
Hee Young Na, Miyoko Higuchi, Shinya Satoh, Kaori Kameyama, Chan Kwon Jung, Su-Jin Shin, Shipra Agarwal, Jen-Fan Hang, Yun Zhu, Zhiyan Liu, Andrey Bychkov, Kennichi Kakudo, So Yeon Park
Journal of Pathology and Translational Medicine.2024; 58(6): 331. CrossRef - Predictors of Malignancy in Thyroid Nodules Classified as Bethesda Category III
Xiaoli Liu, Jingjing Wang, Wei Du, Liyuan Dai, Qigen Fang
Frontiers in Endocrinology.2022;[Epub] CrossRef - Risk stratification of indeterminate thyroid nodules by novel multigene testing: a study of Asians with a high risk of malignancy
Chunfang Hu, Weiwei Jing, Qing Chang, Zhihui Zhang, Zhenrong Liu, Jian Cao, Linlin Zhao, Yue Sun, Cong Wang, Huan Zhao, Ting Xiao, Huiqin Guo
Molecular Oncology.2022; 16(8): 1680. CrossRef - CD56 Expression in Papillary Thyroid Carcinoma Is Highly Dependent on the Histologic Subtype: A Potential Diagnostic Pitfall
Uiju Cho, Yourha Kim, Sora Jeon, Chan Kwon Jung
Applied Immunohistochemistry & Molecular Morphology.2022; 30(5): 389. CrossRef - Malignancy rates in thyroid nodules: a long-term cohort study of 17,592 patients
M Grussendorf, I Ruschenburg, G Brabant
European Thyroid Journal.2022;[Epub] CrossRef - Subclassification of the Bethesda Category III (AUS/FLUS): A study of thyroid FNA cytology based on ThinPrep slides from the National Cancer Center in China
Huan Zhao, HuiQin Guo, LinLin Zhao, Jian Cao, Yue Sun, Cong Wang, ZhiHui Zhang
Cancer Cytopathology.2021; 129(8): 642. CrossRef - Effect of the Noninvasive Follicular Thyroid Neoplasm With Papillary-Like Nuclear Features (NIFTP) Nomenclature Revision on Indian Thyroid Fine-Needle Aspiration Practice
Chanchal Rana, Pooja Ramakant, Divya Goel, Akanksha Singh, KulRanjan Singh, Suresh Babu, Anand Mishra
American Journal of Clinical Pathology.2021; 156(2): 320. CrossRef - Comprehensive DNA Methylation Profiling Identifies Novel Diagnostic Biomarkers for Thyroid Cancer
Jong-Lyul Park, Sora Jeon, Eun-Hye Seo, Dong Hyuck Bae, Young Mun Jeong, Yourha Kim, Ja Seong Bae, Seon-Kyu Kim, Chan Kwon Jung, Yong Sung Kim
Thyroid.2020; 30(2): 192. CrossRef - Differences in surgical resection rate and risk of malignancy in thyroid cytopathology practice between Western and Asian countries: A systematic review and meta‐analysis
Huy Gia Vuong, Hanh Thi Tuyet Ngo, Andrey Bychkov, Chan Kwon Jung, Trang Huyen Vu, Kim Bach Lu, Kennichi Kakudo, Tetsuo Kondo
Cancer Cytopathology.2020; 128(4): 238. CrossRef - Thyroid cancer among patients with thyroid nodules in Yemen: a three-year retrospective study in a tertiary center and a specialty clinic
Butheinah A. Al-Sharafi, Jamila A. AlSanabani, Ibraheem M. Alboany, Amani M. Shamsher
Thyroid Research.2020;[Epub] CrossRef - Is Bethesda classification sufficient to predict thyroid cancer in endemic regions?
Gamze ÇITLAK, Bahar CANBAY TORUN
Journal of Surgery and Medicine.2020; 4(9): 794. CrossRef - Preoperative diagnostic categories of fine needle aspiration cytology for histologically proven thyroid follicular adenoma and carcinoma, and Hurthle cell adenoma and carcinoma: Analysis of cause of under- or misdiagnoses
Hee Young Na, Jae Hoon Moon, June Young Choi, Hyeong Won Yu, Woo-Jin Jeong, Yeo Koon Kim, Ji-Young Choe, So Yeon Park, Paula Soares
PLOS ONE.2020; 15(11): e0241597. CrossRef - Nuclear features of papillary thyroid carcinoma: Comparison of Core needle biopsy and thyroidectomy specimens
Jae Yeon Seok, Jungsuk An, Hyun Yee Cho, Younghye Kim, Seung Yeon Ha
Annals of Diagnostic Pathology.2018; 32: 35. CrossRef - Clinical utility of EZH1 mutations in the diagnosis of follicular-patterned thyroid tumors
Chan Kwon Jung, Yourha Kim, Sora Jeon, Kwanhoon Jo, Sohee Lee, Ja Seong Bae
Human Pathology.2018; 81: 9. CrossRef - The History of Korean Thyroid Pathology
Soon Won Hong, Chan Kwon Jung
International Journal of Thyroidology.2018; 11(1): 15. CrossRef - Thyroid FNA cytology in Asian practice—Active surveillance for indeterminate thyroid nodules reduces overtreatment of thyroid carcinomas
K. Kakudo, M. Higuchi, M. Hirokawa, S. Satoh, C. K. Jung, A. Bychkov
Cytopathology.2017; 28(6): 455. CrossRef - Thyroid Fine-Needle Aspiration Cytology Practice in Korea
Yoon Jin Cha, Ju Yeon Pyo, SoonWon Hong, Jae Yeon Seok, Kyung-Ju Kim, Jee-Young Han, Jeong Mo Bae, Hyeong Ju Kwon, Yeejeong Kim, Kyueng-Whan Min, Soonae Oak, Sunhee Chang
Journal of Pathology and Translational Medicine.2017; 51(6): 521. CrossRef - Current Practices of Thyroid Fine-Needle Aspiration in Asia: A Missing Voice
Andrey Bychkov, Kennichi Kakudo, SoonWon Hong
Journal of Pathology and Translational Medicine.2017; 51(6): 517. CrossRef - Current Status of Thyroid Fine-Needle Aspiration Practice in Thailand
Somboon Keelawat, Samreung Rangdaeng, Supinda Koonmee, Tikamporn Jitpasutham, Andrey Bychkov
Journal of Pathology and Translational Medicine.2017; 51(6): 565. CrossRef
- High Rates of Unnecessary Surgery for Indeterminate Thyroid Nodules in the Absence of Molecular Test and the Cost-Effectiveness of Utilizing Molecular Test in an Asian Population: A Decision Analysis
- Evaluation of the VE1 Antibody in Thyroid Cytology Using Ex Vivo Papillary Thyroid Carcinoma Specimens
- Yon Hee Kim, Hyunee Yim, Yong-Hee Lee, Jae Ho Han, Kyi Beom Lee, Jeonghun Lee, Euy Young Soh, Seon-Yong Jeong, Jang-Hee Kim
- J Pathol Transl Med. 2016;50(1):58-66. Published online December 14, 2015
- DOI: https://doi.org/10.4132/jptm.2015.10.10
- 10,489 View
- 76 Download
- 9 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Recently, VE1, a monoclonal antibody against the BRAFV600E mutant protein, has been investigated in terms of its detection of the BRAFV600E mutation. Although VE1 immunostaining and molecular methods used to assess papillary thyroid carcinoma in surgical specimens are in good agreement, evaluation of VE1 in thyroid cytology samples is rarely performed, and its diagnostic value in cytology has not been well established. In present study, we explored VE1 immunoexpression in cytology samples from ex vivo papillary thyroid carcinoma specimens in order to minimize limitations of low cellularity and sampling/targeting errors originated from thyroid fineneedle aspiration and compared our results with those obtained using the corresponding papillary thyroid carcinoma tissues. Methods: The VE1 antibody was evaluated in 21 cases of thyroid cytology obtained directly from ex vivo thyroid specimens. VE1 immunostaining was performed using liquid-based cytology, and the results were compared with those obtained using the corresponding tissues. Results: Of 21 cases, 19 classic papillary thyroid carcinomas had BRAFV600E mutations, whereas two follicular variants expressed wild-type BRAF. VE1 immunoexpression varied according to specimen type. In detection of the BRAFV600E mutation, VE1 immunostaining of the surgical specimen exhibited 100% sensitivity and 100% specificity, whereas VE1 immunostaining of the cytology specimen exhibited only 94.7% sensitivity and 0% specificity. Conclusions: Our data suggest that VE1 immunostaining of a cytology specimen is less specific than that of a surgical specimen for detection of the BRAFV600E mutation, and that VE1 immunostaining of a cytology specimen should be further evaluated and optimized for clinical use. -
Citations
Citations to this article as recorded by- Principles of Analytic Validation of Immunohistochemical Assays: Guideline Update
Jeffrey D. Goldsmith, Megan L. Troxell, Sinchita Roy-Chowdhuri, Carol F. Colasacco, Mary Elizabeth Edgerton, Patrick L. Fitzgibbons, Regan Fulton, Thomas Haas, Patricia L. Kandalaft, Tanja Kalicanin, Christina Lacchetti, Patti Loykasek, Nicole E. Thomas,
Archives of Pathology & Laboratory Medicine.2024; 148(6): e111. CrossRef - Inter- and Intra-observer Reproducibility of Thyroid Fine Needle Aspiration Cytology: An investigation of Bethesda 2023 Using Immunohistochemical BRAFV600E Antibody
Erdogan Bahattin, Dündar Emine, Çetin Kısmet Çivi, Yılmaz Fatih
Journal of Cytology.2024; 41(4): 221. CrossRef - VE1 immunohistochemistry is an adjunct tool for detection of BRAFV600E mutation: Validation in thyroid cancer patients
Faiza A. Rashid, Sobia Tabassum, Mosin S. Khan, Hifzur R. Ansari, Muhammad Asif, Ahmareen K. Sheikh, Syed Sameer Aga
Journal of Clinical Laboratory Analysis.2021;[Epub] CrossRef - Effective utilization of liquid-based cytology for thyroid lesions
Yukie YAMAYA
The Journal of the Japanese Society of Clinical Cytology.2021; 60(3): 164. CrossRef - Diagnostic Efficacy of BRAFV600E Immunocytochemistry in Thyroid Aspirates in Bethesda Category IV and Papillary Thyroid Carcinoma
Nidhi Anand, Tushar Agrawal, Anurag Gupta, Saumya Shukla, Roma Pradhan, Nuzhat Husain
Journal of Cytology.2021; 38(3): 113. CrossRef - The immunocytochemical expression of VE‐1 (BRAF V600E‐related) antibody identifies the aggressive variants of papillary thyroid carcinoma on liquid‐based cytology
Patrizia Straccia, Chiara Brunelli, Esther D. Rossi, Paola Lanza, Maurizio Martini, Teresa Musarra, Celestino Pio Lombardi, Alfredo Pontecorvi, Guido Fadda
Cytopathology.2019; 30(5): 460. CrossRef - Utility of the BRAF p.V600E immunoperoxidase stain in FNA direct smears and cell block preparations from patients with thyroid carcinoma
Amber L. Smith, Michelle D. Williams, John Stewart, Wei‐Lien Wang, Savitri Krishnamurthy, Maria E. Cabanillas, Sinchita Roy‐Chowdhuri
Cancer Cytopathology.2018; 126(6): 406. CrossRef - Refinement of the criteria for ultrastructural peritubular capillary basement membrane multilayering in the diagnosis of chronic active/acute antibody-mediated rejection
Heounjeong Go, Sung Shin, Young Hoon Kim, Duck Jong Han, Yong Mee Cho
Transplant International.2017; 30(4): 398. CrossRef - Thyroid Fine-Needle Aspiration Cytology Practice in Korea
Yoon Jin Cha, Ju Yeon Pyo, SoonWon Hong, Jae Yeon Seok, Kyung-Ju Kim, Jee-Young Han, Jeong Mo Bae, Hyeong Ju Kwon, Yeejeong Kim, Kyueng-Whan Min, Soonae Oak, Sunhee Chang
Journal of Pathology and Translational Medicine.2017; 51(6): 521. CrossRef - Use of monoclonal antibodies to detect specific mutations in formalin-fixed, paraffin-embedded tissue sections
Zhenying Guo, Ricardo V. Lloyd
Human Pathology.2016; 53: 168. CrossRef
- Principles of Analytic Validation of Immunohistochemical Assays: Guideline Update
- Cytology Specimen Management, Triage and Standardized Reporting of Fine Needle Aspiration Biopsies of the Pancreas
- Won Jae Yoon, Martha Bishop Pitman
- J Pathol Transl Med. 2015;49(5):364-372. Published online August 10, 2015
- DOI: https://doi.org/10.4132/jptm.2015.07.19
- 13,832 View
- 144 Download
- 8 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF - The recent advances in pancreas cytology specimen sampling methods have enabled a specific cytologic diagnosis in most cases. Proper triage and processing of the cytologic specimen is pivotal in making a diagnosis due to the need for ancillary testing in addition to cytological evaluation, which is especially true in the diagnosis of pancreatic cysts. Newly proposed terminology for pancreaticobiliary cytology offers a standardized language for reporting that aims to improve communication among patient caregivers and provide for increased flexibility in patient management. This review focuses on these updates in pancreas cytology for the optimal evaluation of solid and cystic lesions of the pancreas.
-
Citations
Citations to this article as recorded by- Endoscopic ultrasound-guided tissue sampling: European Society of Gastrointestinal Endoscopy (ESGE) Technical and Technology Review
Antonio Facciorusso, Marianna Arvanitakis, Stefano Francesco Crinò, Carlo Fabbri, Adele Fornelli, John Leeds, Livia Archibugi, Silvia Carrara, Jahnvi Dhar, Paraskevas Gkolfakis, Beate Haugk, Julio Iglesias Garcia, Bertrand Napoleon, Ioannis S. Papanikolao
Endoscopy.2025; 57(04): 390. CrossRef - Imaging of pancreatic serous cystadenoma and common imitators
Camila Lopes Vendrami, Nancy A. Hammond, David J. Escobar, Zachary Zilber, Meaghan Dwyer, Courtney C. Moreno, Pardeep K. Mittal, Frank H. Miller
Abdominal Radiology.2024; 49(10): 3666. CrossRef - “Evolving Trends in Pancreatic Cystic Tumors: A 3-Decade Single-Center Experience With 1290 Resections”
Jorge Roldán, Jon M. Harrison, Motaz Qadan, Louisa Bolm, Taisuke Baba, William R. Brugge, Brenna W. Casey, Kumar Krishnan, Mari Mino-Kenudson, Martha B. Pitman, Avinash Kambadakone, Cristina R. Ferrone, Andrew L. Warshaw, Keith D. Lillemoe, Carlos Fernánd
Annals of Surgery.2023; 277(3): 491. CrossRef - Role of fluorescence confocal microscopy for rapid evaluation of EUS fine-needle biopsy sampling in pancreatic solid lesions
Serena Stigliano, Anna Crescenzi, Chiara Taffon, Francesco Covotta, Cesare Hassan, Giulio Antonelli, Martina Verri, Dario Biasutto, Roberto Mario Scarpa, Francesco Maria Di Matteo
Gastrointestinal Endoscopy.2021; 94(3): 562. CrossRef - Towards optimal pancreatic cyst fluid management: the need for standardisation
Giacomo Puppa, Yann Christinat, Thomas Alexander McKee
Gut.2019; 68(10): 1906. CrossRef - It is necessary to exam bottom and top slide smears of EUS-FNA for pancreatic cancer
Jong-chan Lee, Haeryoung Kim, Hyoung Woo Kim, Jongchan Lee, Kyu-hyun Paik, Jingu Kang, Jin-Hyeok Hwang, Jaihwan Kim
Hepatobiliary & Pancreatic Diseases International.2018; 17(6): 553. CrossRef - Rationale and feasibility of mucin expression profiling by qRT-PCR as diagnostic biomarkers in cytology specimens of pancreatic cancer
Milosz Wiktorowicz, Damian Mlynarski, Radoslaw Pach, Romana Tomaszewska, Jan Kulig, Piotr Richter, Marek Sierzega
Pancreatology.2018; 18(8): 977. CrossRef - Variations in cancer centers’ use of cytology for the diagnosis of unresectable pancreatic cancer in the National Cancer Data Base
Ted Gansler, Stacey A. Fedewa, Chun Chieh Lin, Ahmedin Jemal, Elizabeth M. Ward
Cancer Cytopathology.2016; 124(11): 791. CrossRef - Pancreatic Cytopathology
Jennifer A. Collins, Syed Z. Ali, Christopher J. VandenBussche
Surgical Pathology Clinics.2016; 9(4): 661. CrossRef
- Endoscopic ultrasound-guided tissue sampling: European Society of Gastrointestinal Endoscopy (ESGE) Technical and Technology Review
- The Utilization of Cytologic Fine-Needle Aspirates of Lung Cancer for Molecular Diagnostic Testing
- Michael H. Roh
- J Pathol Transl Med. 2015;49(4):300-309. Published online June 16, 2015
- DOI: https://doi.org/10.4132/jptm.2015.06.16
- 15,317 View
- 169 Download
- 34 Web of Science
- 32 Crossref
-
 Abstract
Abstract
 PDF
PDF - In this era of precision medicine, our understanding and knowledge of the molecular landscape associated with lung cancer pathogenesis continues to evolve. This information is being increasingly exploited to treat advanced stage lung cancer patients with tailored, targeted therapy. During the management of these patients, minimally invasive procedures to obtain samples for tissue diagnoses are desirable. Cytologic fine-needle aspirates are often utilized for this purpose and are important not only for rendering diagnoses to subtype patients’ lung cancers, but also for ascertaining molecular diagnostic information for treatment purposes. Thus, cytologic fine-needle aspirates must be utilized and triaged judiciously to achieve both objectives. In this review, strategies in utilizing fine-needle aspirates will be discussed in the context of our current understanding of the clinically actionable molecular aberrations underlying non-small cell lung cancer and the molecular assays applied to these samples in order to obtain treatment-relevant molecular diagnostic information.
-
Citations
Citations to this article as recorded by- The Bridge: Supernatant Derived From Cytological Sample Preparations
Sinchita Roy‐Chowdhuri
Cytopathology.2025; 36(3): 222. CrossRef - The American Cancer Society National Lung Cancer Roundtable strategic plan: Methods for improving turnaround time of comprehensive biomarker testing in non–small cell lung cancer
Sinchita Roy‐Chowdhuri, Haresh Mani, Adam H. Fox, Anne Tsao, Lynette M. Sholl, Farhood Farjah, Bruce E. Johnson, Raymond U. Osarogiagbon, M. Patricia Rivera, Gerard A. Silvestri, Robert A. Smith, Ignacio I. Wistuba
Cancer.2024; 130(24): 4200. CrossRef - Endobronchial ultrasound bronchoscopy Franseen fine needle biopsy tool versus standard fine needle aspiration needle: Impact on diagnosis and tissue adequacy
Matthew C. Aboudara, Timothy Saettele, Ossama Tawfik
Respiratory Medicine.2023; 208: 107131. CrossRef - Adequacy of small biopsy and cytology specimens for comprehensive genomic profiling of patients with non-small-cell lung cancer to determine eligibility for immune checkpoint inhibitor and targeted therapy
Erin Faber, Horiana Grosu, Sharjeel Sabir, Francis Anthony San Lucas, Bedia A Barkoh, Roland L Bassett, Rajyalakshmi Luthra, John Stewart, Sinchita Roy-Chowdhuri
Journal of Clinical Pathology.2022; 75(9): 612. CrossRef - Biomarker testing of cytology specimens in personalized medicine for lung cancer patients
Hyojin Kim, Jin-Haeng Chung
Journal of Pathology and Translational Medicine.2022; 56(6): 326. CrossRef - Formalin fixation for optimal concordance of programmed death‐ligand 1 immunostaining between cytologic and histologic specimens from patients with non–small cell lung cancer
Bregje M. Koomen, Jose van der Starre‐Gaal, Judith M. Vonk, Jan H. von der Thüsen, Jacqueline J. C. van der Meij, Kim Monkhorst, Stefan M. Willems, Wim Timens, Nils A. ’t Hart
Cancer Cytopathology.2021; 129(4): 304. CrossRef - Collection and Handling of Thoracic Small Biopsy and Cytology Specimens for Ancillary Studies: Guidelines from the College of American Pathologists (CAP)
Sinchita Roy-Chowdhuri
Journal of Molecular Pathology.2021; 2(1): 23. CrossRef - A decade of change: Trends in the practice of cytopathology at a tertiary care cancer centre
Peyman Dinarvand, Chinhua Liu, Sinchita Roy‐Chowdhuri
Cytopathology.2021; 32(5): 604. CrossRef - False‐negative programmed death‐ligand 1 immunostaining in ethanol‐fixed endobronchial ultrasound‐guided transbronchial needle aspiration specimens of non‐small‐cell lung cancer patients
Bregje M Koomen, Willem Vreuls, Mirthe de Boer, Emma J de Ruiter, Juergen Hoelters, Aryan Vink, Stefan M Willems
Histopathology.2021; 79(4): 480. CrossRef - Influence of preanalytical variables on performance of delta-like protein 3 (DLL3) predictive immunohistochemistry
Teodora Radonic, S. Duin, W. Vos, P. Kortman, Aeilko H. Zwinderman, Erik Thunnissen
Virchows Archiv.2021; 478(2): 293. CrossRef - Comparison of the SuperARMS and ARMS for detecting EGFR mutations in liquid-based cytology specimens from NSCLC patients
Wei Wu, Ziyang Cao, Wei Zhang, Liping Zhang, Likun Hou, Chunyan Wu
Diagnostic Pathology.2020;[Epub] CrossRef - Tumor cell representation by an improvised technique of fine-needle aspiration specimen acquisition and cell block preparation: Our experience in lung cancer cases in a peripheral center of eastern India
AnupKr Boler, Shreosee Roy, Arghya Bandyopadhyay, Abhishek Bandyopadhyay, MrinalKanti Ghosh
Journal of Cytology.2020; 37(2): 87. CrossRef - A new guideline from the College of American Pathologists to improve the adequacy of thoracic small specimens for ancillary studies
Sinchita Roy‐Chowdhuri
Cancer Cytopathology.2020; 128(10): 690. CrossRef - The utilization of cytologic and small biopsy samples for ancillary molecular testing
Michael H. Roh
Modern Pathology.2019; 32: 77. CrossRef - Diff-Quik Cytology Smears from Endobronchial Ultrasound Transbronchial Needle Aspiration Lymph Node Specimens as a Source of DNA for Next-Generation Sequencing Instead of Cell Blocks
David Fielding, Andrew J. Dalley, Farzad Bashirzadeh, Mahendra Singh, Lakshmy Nandakumar, Amy E. McCart Reed, Debra Black, Stephen Kazakoff, John V. Pearson, Katia Nones, Nicola Waddell, Sunil R. Lakhani, Peter T. Simpson
Respiration.2019; 97(6): 525. CrossRef - Molecular testing on endobronchial ultrasound (EBUS) fine needle aspirates (FNA): Impact of triage
Simon Sung, John P. Crapanzano, David DiBardino, David Swinarski, William A. Bulman, Anjali Saqi
Diagnostic Cytopathology.2018; 46(2): 122. CrossRef - Salvaging the supernatant: next generation cytopathology for solid tumor mutation profiling
Sinchita Roy-Chowdhuri, Meenakshi Mehrotra, Ana Maria Bolivar, Rashmi Kanagal-Shamanna, Bedia A. Barkoh, Brette Hannigan, Stephanie Zalles, Wenrui Ye, Dzifa Duose, Russell Broaddus, Gregg Staerkel, Ignacio Wistuba, L. Jeffrey Medeiros, Rajyalakshmi Luthra
Modern Pathology.2018; 31(7): 1036. CrossRef - Fluorescence in situ hybridization analysis on cytologic smears: An accurate and efficient method in the diagnosis of melanotic Xp11 translocation renal cancer
Alia Gupta, Mark Micale, Kurt D. Bernacki
Diagnostic Cytopathology.2018; 46(9): 786. CrossRef - Comprehensive Validation of Cytology Specimens for Next-Generation Sequencing and Clinical Practice Experience
Agnes Balla, Ken J. Hampel, Mukesh K. Sharma, Catherine E. Cottrell, Nikoletta Sidiropoulos
The Journal of Molecular Diagnostics.2018; 20(6): 812. CrossRef - Advances in Molecular Testing Techniques in Cytologic Specimens
Sinchita Roy-Chowdhuri
Surgical Pathology Clinics.2018; 11(3): 669. CrossRef - Next-generation molecular diagnosis: single-cell sequencing from bench to bedside
Wanjun Zhu, Xiao-Yan Zhang, Sadie L. Marjani, Jialing Zhang, Wengeng Zhang, Shixiu Wu, Xinghua Pan
Cellular and Molecular Life Sciences.2017; 74(5): 869. CrossRef - Big data from small samples: Informatics of next‐generation sequencing in cytopathology
Sinchita Roy‐Chowdhuri, Somak Roy, Sara E. Monaco, Mark J. Routbort, Liron Pantanowitz
Cancer Cytopathology.2017; 125(4): 236. CrossRef - Next‐generation sequencing of liquid‐based cytology non–small cell lung cancer samples
Jordan P. Reynolds, Yaolin Zhou, Maureen A. Jakubowski, Zhen Wang, Jennifer A. Brainard, Roger D. Klein, Carol F. Farver, Francisco A. Almeida, Yu‐Wei Cheng
Cancer Cytopathology.2017; 125(3): 178. CrossRef - Concurrent fine needle aspirations and core needle biopsies: a comparative study of substrates for next-generation sequencing in solid organ malignancies
Sinchita Roy-Chowdhuri, Hui Chen, Rajesh R Singh, Savitri Krishnamurthy, Keyur P Patel, Mark J Routbort, Jawad Manekia, Bedia A Barkoh, Hui Yao, Sharjeel Sabir, Russell R Broaddus, L Jeffrey Medeiros, Gregg Staerkel, John Stewart, Rajyalakshmi Luthra
Modern Pathology.2017; 30(4): 499. CrossRef - Diagnosis of anaplastic lymphoma kinase rearrangement in cytological samples through a fluorescence in situ hybridization–based assay: Cytological smears versus cell blocks
Federica Zito Marino, Giulio Rossi, Matteo Brunelli, Maria Gabriella Malzone, Giuseppina Liguori, Giuseppe Bogina, Alessandro Morabito, Gaetano Rocco, Renato Franco, Gerardo Botti
Cancer Cytopathology.2017; 125(5): 303. CrossRef - Next‐generation sequencing of cytologic preparations: An analysis of quality metrics
David H. Hwang, Elizabeth P. Garcia, Matthew D. Ducar, Edmund S. Cibas, Lynette M. Sholl
Cancer Cytopathology.2017; 125(10): 786. CrossRef - Recent advances in the pathology and molecular genetics of lung cancer: A practical review for cytopathologists
Erika F. Rodriguez, Sara E. Monaco
Journal of the American Society of Cytopathology.2016; 5(5): 252. CrossRef - Utilization of ancillary studies in the cytologic diagnosis of respiratory lesions: The papanicolaou society of cytopathology consensus recommendations for respiratory cytology
Lester J. Layfield, Sinchita Roy‐Chowdhuri, Zubair Baloch, Hormoz Ehya, Kim Geisinger, Susan J. Hsiao, Oscar Lin, Neal I. Lindeman, Michael Roh, Fernando Schmitt, Nikoletta Sidiropoulos, Paul A. VanderLaan
Diagnostic Cytopathology.2016; 44(12): 1000. CrossRef - Inflammatory myofibroblastic tumour
Michael McDermott
Seminars in Diagnostic Pathology.2016; 33(6): 358. CrossRef - Preanalytic Variables in Cytology: Lessons Learned From Next-Generation Sequencing—The MD Anderson Experience
Sinchita Roy-Chowdhuri, John Stewart
Archives of Pathology & Laboratory Medicine.2016; 140(11): 1191. CrossRef - Biomarker Testing in Lung Carcinoma Cytology Specimens: A Perspective From Members of the Pulmonary Pathology Society
Sinchita Roy-Chowdhuri, Dara L. Aisner, Timothy Craig Allen, Mary Beth Beasley, Alain Borczuk, Philip T. Cagle, Vera Capelozzi, Sanja Dacic, Gilda da Cunha Santos, Lida P. Hariri, Keith M. Kerr, Sylvie Lantuejoul, Mari Mino-Kenudson, Andre Moreira, Kirtee
Archives of Pathology & Laboratory Medicine.2016; 140(11): 1267. CrossRef - Identification of a novel partner gene, KIAA1217, fused to RET: Functional characterization and inhibitor sensitivity of two isoforms in lung adenocarcinoma
Mi-Sook Lee, Ryong Nam Kim, Hoseok I, Doo-Yi Oh, Ji-Young Song, Ka-Won Noh, Yu-Jin Kim, Jung Wook Yang, Maruja E. Lira, Chang Hun Lee, Min Ki Lee, Yeoung Dae Kim, Mao Mao, Joungho Han, Jhingook Kim, Yoon-La Choi
Oncotarget.2016; 7(24): 36101. CrossRef
- The Bridge: Supernatant Derived From Cytological Sample Preparations
- Accuracy of Core Needle Biopsy Versus Fine Needle Aspiration Cytology for Diagnosing Salivary Gland Tumors
- In Hye Song, Joon Seon Song, Chang Ohk Sung, Jong-Lyel Roh, Seung-Ho Choi, Soon Yuhl Nam, Sang Yoon Kim, Jeong Hyun Lee, Jung Hwan Baek, Kyung-Ja Cho
- J Pathol Transl Med. 2015;49(2):136-143. Published online March 12, 2015
- DOI: https://doi.org/10.4132/jptm.2015.01.03
- 13,420 View
- 222 Download
- 75 Web of Science
- 76 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Core needle biopsy is a relatively new technique used to diagnose salivary gland lesions, and its role in comparison with fine needle aspiration cytology needs to be refined. Methods: We compared the results of 228 ultrasound-guided core needle biopsy and 371 fine needle aspiration procedures performed on major salivary gland tumors with their postoperative histological diagnoses. Results: Core needle biopsy resulted in significantly higher sensitivity and more accurate tumor subtyping, especially for malignant tumors, than fine needle aspiration. No patient developed major complications after core needle biopsy. Conclusions: We recommend ultrasoundguided core needle biopsy as the primary diagnostic tool for the preoperative evaluation of patients with salivary gland lesions, especially when malignancy is suspected. -
Citations
Citations to this article as recorded by- Frozen Section Analysis in Submandibular Gland Tumors: Optimizing Intraoperative Decision-Making
Amir Bolooki, Felix Johnson, Anna Stenzl, Zhaojun Zhu, Benedikt Gabriel Hofauer
Cancers.2025; 17(5): 895. CrossRef - The Myriad Spectrum of Salivary Gland Lesions: Cytohistological Correlation on Fine Needle Aspiration Cytology, Core Needle Biopsy, and Resections in a 5‐Year Single Institutional Experience of North India
Zachariah Chowdhury, Pallavi Majumdar, Sumeet Narain, Komal Lamba
Diagnostic Cytopathology.2025; 53(8): 391. CrossRef - Salivary Duct Carcinoma: A 12-Year Single Center Experience
Hyowon Ahn, Dongbin Ahn, Ji Hye Kwak
Korean Journal of Otorhinolaryngology-Head and Neck Surgery.2025; 68(6): 232. CrossRef - Giant Pleomorphic Adenoma of Submandibular Gland
Harendra Kumar, Qazi Saquib Rizwan, Mayank Gupta, Tarun Kumar
Indian Journal of Otolaryngology and Head & Neck Surgery.2024; 76(1): 1361. CrossRef - CT-guided core needle biopsies of head and neck tumors: a comprehensive monocenter analysis of safety and outcomes
Thomas Joseph Vogl, Heinrich Johannes Ketelsen, Scherwin Mahmoudi, Jan-Erik Scholtz, Vitali Koch, Leon David Grünewald, Peter Wild, Timo Stoever, Simon Bernatz
European Radiology.2024; 34(8): 5370. CrossRef - Indications for Submandibulectomy Within a 20-Year Period
Amir Bolooki, Anna Stenzl, Christopher Weusthof, Benedikt Hofauer
Ear, Nose & Throat Journal.2024;[Epub] CrossRef - Treatment of Warthin’s Tumors of the Parotid Gland With Radiofrequency Ablation: A Systematic Review of the Current Literature
Kenny Do, Eric Kawana, Sisi Tian, Jo-Lawrence Bigcas
Ear, Nose & Throat Journal.2024;[Epub] CrossRef - Evaluation of the anterior processes of the parotid gland: an ultrasonographic study
Tarık Ali Uğur, Hümeyra Tercanlı
Surgical and Radiologic Anatomy.2024; 46(6): 915. CrossRef - Salivary Gland Fine-Needle Aspiration
Federica Policardo, Antonino Mule’, Esther Diana Rossi
Surgical Pathology Clinics.2024; 17(3): 347. CrossRef - Implementation of the Milan System for Reporting Salivary Gland Cytology: A Two-Year Outcome Cytopathology Data of a Tertiary Care Center
Soudamini Mahapatra, Chinmaya Sundar Ray, Aparajita Mishra, Dileswari Pradhan
Cureus.2024;[Epub] CrossRef - Preoperative cytopathological investigatory aids in the diagnosis of salivary gland lesions
S Vidyalakshmi, K Shanmugasamy
Journal of Oral and Maxillofacial Pathology.2024; 28(2): 172. CrossRef - Machine Learning on Ultrasound Texture Analysis Data for Characterizing of Salivary Glandular Tumors: A Feasibility Study
Li-Jen Liao, Ping-Chia Cheng, Feng-Tsan Chan
Diagnostics.2024; 14(16): 1761. CrossRef - Association of Membranous Basal Cell Adenoma and Basal Cell Adenocarcinoma With Brooke-Spiegler Syndrome
Lexi Goehring, Debby Rampisela, Jordan L Pleitz
Cureus.2024;[Epub] CrossRef - A Retrospective 8‐Year Single Institutional Study in Germany Regarding Diagnosis, Treatment, and Outcome of Malignant Parotid Tumors
S. Andrianopoulou, L. S. Fiedler, B. M. Lippert, O. C. Bulut, Mohamed Rahouma
International Journal of Surgical Oncology.2024;[Epub] CrossRef - High Field MRI in Parotid Gland Tumors: A Diagnostic Algorithm
Chiara Gaudino, Andrea Cassoni, Martina Lucia Pisciotti, Resi Pucci, Chiara Veneroso, Cira Rosaria Tiziana Di Gioia, Francesca De Felice, Patrizia Pantano, Valentino Valentini
Cancers.2024; 17(1): 71. CrossRef - Nationwide Incidence Trends of Pediatric Parotid Malignancy in Korea and a Retrospective Analysis of Single-Institution Surgical Experience of Parotidectomy
Hyun Seong Kim, Seo Young Kim, Eun-Jae Chung, Seong Keun Kwon, Soon-Hyun Ahn, Yuh-Seog Jung, Jungirl Seok
Korean Society of Head and Neck Oncology.2024; 40(2): 7. CrossRef - The Usefulness of Ultrasound-Guided Core Needle Biopsy Compared to Fine Needle Aspiration in Pre-Operative Diagnosis of Cystic-Predominant Parotid Tumors
Youn Jin Cho, Young Rok Jo, Hyun Jun Hong, Hye Ran Lee
Korean Journal of Otorhinolaryngology-Head and Neck Surgery.2023; 66(8): 532. CrossRef - The Value of Ultrasound-guided Core Needle Biopsy in Differentiating Benign from Malignant Salivary Gland Lesions
Mohammad Ali Kazemi, Farzaneh Amini, Bita Kargar, Maryam Lotfi, Keyvan Aghazadeh, Hashem Sharifian, Behnaz Moradi, Javid Azadbakht
Indian Journal of Otolaryngology and Head & Neck Surgery.2023; 75(2): 266. CrossRef - Schnellschnittdiagnostik bei Tumoren des Trigonum submandibulare
S. Riemann, A. Knopf
HNO.2023; 71(3): 164. CrossRef - Myoepithelial Carcinoma Ex Pleomorphic Adenoma of the Submandibular Gland: A Case Report
Georgia Syrnioti, Antonia Syrnioti, Alharith Abdullah, Xuehui Lui, Ernesto Mendoza
Cureus.2023;[Epub] CrossRef - Intraductal Carcinoma: The Carcinoma In Situ of the Salivary Gland
Rhema Thomas, Tijjani Umar, Farzad Borumandi
Journal of Craniofacial Surgery.2023;[Epub] CrossRef - Fine-Needle Aspiration Cytology for Parotid Tumors
Masataka Taniuchi, Tetsuya Terada, Ryo Kawata
Life.2022; 12(11): 1897. CrossRef - Utility of the Milan System for Reporting Salivary Gland Cytology, with focus on the incidence and histologic correlates of atypia of undetermined significance (AUS) and salivary gland neoplasm of uncertain malignant potential (SUMP): A 3‐year institution
Christopher M. Cormier, Shweta Agarwal
Cancer Cytopathology.2022; 130(4): 303. CrossRef - Percutaneous CT-Guided Core Needle Biopsies of Head and Neck Masses: Review of 184 Cases at a Single Academic Institution, Common and Special Techniques, Diagnostic Yield, and Safety
R.W. Jordan, D.P. Shlapak, J.C. Benson, F.E. Diehn, D.K. Kim, V.T. Lehman, G.B. Liebo, A.A. Madhavan, J.M. Morris, P.P. Morris, J.T. Verdoorn, C.M. Carr
American Journal of Neuroradiology.2022; 43(1): 117. CrossRef - Nodular fasciitis of the submandibular gland
Ting Suen Wong, Richard Wei Chern Gan, Laszlo Karsai, Bun Yin Winson Wong
BMJ Case Reports.2022; 15(4): e245584. CrossRef - Validation of the Milan system for reporting salivary gland cytopathology: a single institution’s 10-year experience
Christopher Felicelli, Joseph Reznicek, Yevgen Chornenkyy, Lucy Jager, Daniel Johnson
Journal of the American Society of Cytopathology.2022; 11(5): 264. CrossRef - Application of the Milan system for reporting salivary gland cytopathology using cell blocks
Grégoire B. Morand, Raihanah Alsayegh, Alex M. Mlynarek, Marianne Plourde, Tiffany Mach, Marco A. Mascarella, Michael P. Hier, Livia Florianova, Marc P. Pusztaszeri
Virchows Archiv.2022; 481(4): 575. CrossRef - Comparisons among the Ultrasonography Prediction Model, Real-Time and Shear Wave Elastography in the Evaluation of Major Salivary Gland Tumors
Ping-Chia Cheng, Wu-Chia Lo, Chih-Ming Chang, Ming-Hsun Wen, Po-Wen Cheng, Li-Jen Liao
Diagnostics.2022; 12(10): 2488. CrossRef - A Novel Sonographic Scoring Model in the Prediction of Major Salivary Gland Tumors
Wu‐Chia Lo, Chih‐Ming Chang, Chi‐Te Wang, Po‐Wen Cheng, Li‐Jen Liao
The Laryngoscope.2021;[Epub] CrossRef - Assessing the diagnostic accuracy for pleomorphic adenoma and Warthin tumor by employing the Milan System for Reporting Salivary Gland Cytopathology: An international, multi‐institutional study
Derek B. Allison, Alexander P. Smith, Daniel An, James Adam Miller, Khurram Shafique, Sharon Song, Kartik Viswanathan, Elizabeth Eykman, Rema A. Rao, Austin Wiles, Güliz A. Barkan, Ritu Nayar, Guido Fadda, Celeste N. Powers, Esther Diana Rossi, Momin T. S
Cancer Cytopathology.2021; 129(1): 43. CrossRef - Magnetic resonance imaging of salivary gland tumours: Key findings for imaging characterisation
Davide Maraghelli, Michele Pietragalla, Cesare Cordopatri, Cosimo Nardi, Anna Julie Peired, Giandomenico Maggiore, Stefano Colagrande
European Journal of Radiology.2021; 139: 109716. CrossRef - The Milan System, from Its Introduction to Its Current Adoption in the Diagnosis of Salivary Gland Cytology
Esther Diana Rossi
Journal of Molecular Pathology.2021; 2(2): 114. CrossRef - Utility of the Milan system for reporting salivary gland cytopathology during rapid on‐site evaluation (ROSE) of salivary gland aspirates
Aanchal Kakkar, Mukin Kumar, Priyadarsani Subramanian, Arshad Zubair, Rajeev Kumar, Alok Thakar, Deepali Jain, Sandeep R. Mathur, Venkateswaran K. Iyer
Cytopathology.2021; 32(6): 779. CrossRef - Contribution of small tissue biopsy and flow cytometry to preoperative cytological categorization of salivary gland fine needle aspirates according to the Milan System: Single center experience on 287 cases
Tolga Bağlan, Serpil Dizbay Sak, Cevriye Cansız Ersöz, Koray Ceyhan
Diagnostic Cytopathology.2021; 49(4): 509. CrossRef - Is Milan for kids?: The Milan System for Reporting Salivary Gland Cytology in pediatric patients at an academic children's hospital with cytologic‐histologic correlation
Swati P. Satturwar, Maren Y. Fuller, Sara E. Monaco
Cancer Cytopathology.2021; 129(11): 884. CrossRef - Radiographic Interpretation in Oral Medicine and Hospital Dental Practice
Katherine France, Anwar A.A.Y. AlMuzaini, Mel Mupparapu
Dental Clinics of North America.2021; 65(3): 509. CrossRef - Carcinoma ex pleomorphic adenoma of major salivary glands: CT and MR imaging findings
Can Wang, Qiang Yu, Siyi Li, Jingjing Sun, Ling Zhu, Pingzhong Wang
Dentomaxillofacial Radiology.2021; 50(7): 20200485. CrossRef - Salivary gland carcinoma in children and adolescents: The EXPeRT/PARTNER diagnosis and treatment recommendations
Aurore Surun, Dominik T. Schneider, Andrea Ferrari, Teresa Stachowicz‐Stencel, Jelena Rascon, Anna Synakiewicz, Abbas Agaimy, Kata Martinova, Denis Kachanov, Jelena Roganovic, Ewa Bien, Gianni Bisogno, Ines B. Brecht, Frédéric Kolb, Juliette Thariat, Anto
Pediatric Blood & Cancer.2021;[Epub] CrossRef - A Call for Universal Acceptance of the Milan System for Reporting Salivary Gland Cytopathology
Eric Barbarite, Sidharth V. Puram, Adeeb Derakhshan, Esther D. Rossi, William C. Faquin, Mark A. Varvares
The Laryngoscope.2020; 130(1): 80. CrossRef - Preoperative biopsy in parotid malignancies: Variation in use and impact on surgical margins
Liliya Benchetrit, Sina J. Torabi, Elliot Morse, Saral Mehra, Rahmatullah Rahmati, Heather A. Osborn, Benjamin L. Judson
The Laryngoscope.2020; 130(6): 1450. CrossRef - α‐Synuclein Real‐Time Quaking‐Induced Conversion in the Submandibular Glands of Parkinson's Disease Patients
Sireesha Manne, Naveen Kondru, Huajun Jin, Vellareddy Anantharam, Xuemei Huang, Arthi Kanthasamy, Anumantha G. Kanthasamy
Movement Disorders.2020; 35(2): 268. CrossRef - The Accessory Parotid Gland and its Clinical Significance
Mateusz A. Rosa, Dominik P. Łazarz, Jakub R. Pękala, Bendik Skinningsrud, Sigurd S. Lauritzen, Bernard Solewski, Przemysław A. Pękala, Jerzy A. Walocha, Krzysztof A. Tomaszewski
Journal of Craniofacial Surgery.2020; 31(3): 856. CrossRef - Comparison of core needle biopsy and fine‐needle aspiration in diagnosis of ma lignant salivary gland neoplasm: Systematic review and meta‐analysis
Jungheum Cho, Junghoon Kim, Ji Sung Lee, Choong Guen Chee, Youngjune Kim, Sang Il Choi
Head & Neck.2020; 42(10): 3041. CrossRef - The Milan system for reporting salivary gland cytopathology: The clinical impact so far. Considerations from theory to practice
Esther Diana Rossi, William C. Faquin
Cytopathology.2020; 31(3): 181. CrossRef - The role of core needle biopsy in parotid glands lesions with inconclusive fine needle aspiration
Farrokh Heidari, Firouzeh Heidari, Benyamin Rahmaty, Neda Jafari, Kayvan Aghazadeh, Saeed Sohrabpour, Ebrahim Karimi
American Journal of Otolaryngology.2020; 41(6): 102718. CrossRef - Role of Fine Needle Aspiration Cytology in the Diagnosis of Parotid Gland Tumors: Analysis of 193 Cases
Rahim Dhanani, Haissan Iftikhar, Muhammad Sohail Awan, Nida Zahid, Sehrish Nizar Ali Momin
International Archives of Otorhinolaryngology.2020; 24(04): e508. CrossRef - Cytohistological correlation of salivary gland tumours with emphasis on Milan system for reporting: A novel step towards internal quality assurance
Anandraj Vaithy.K, ATM Venkat Raghava, E S Keerthika Sri, K R Umadevi
IP Archives of Cytology and Histopathology Research.2020; 5(4): 283. CrossRef - Diagnosing Recently Defined and Uncommon Salivary Gland Lesions in Limited Cellularity Specimens: Cytomorphology and Ancillary Studies
Esther Diana Rossi, Zubair Baloch, William Faquin, Liron Pantanowitz
AJSP: Reviews and Reports.2020; 25(5): 210. CrossRef - Peripheral T Cell Lymphoma of Parotid Gland: A Diagnostic Challenge
J. G. Aishwarya, Satish Nair, C. N. Patil, Swarna Shivakumar, N. Shrivalli, Ashish Shah
Indian Journal of Otolaryngology and Head & Neck Surgery.2019; 71(S1): 533. CrossRef - Potential utility of core needle biopsy in the diagnosis of IgG4-related dacryoadenitis and sialadenitis
Kenichi Takano, Tsuyoshi Okuni, Keisuke Yamamoto, Ryuta Kamekura, Ryoto Yajima, Motohisa Yamamoto, Hiroki Takahashi, Tetsuo Himi
Modern Rheumatology.2019; 29(2): 393. CrossRef - Retrospective assessment of the effectiveness of the Milan system for reporting salivary gland cytology: A systematic review and meta‐analysis of published literature
Sahar J Farahani, Zubair Baloch
Diagnostic Cytopathology.2019; 47(2): 67. CrossRef - Values of fine‐needle aspiration cytology of parotid gland tumors: A review of 996 cases at a single institution
Manabu Suzuki, Ryo Kawata, Masaaki Higashino, Shuji Nishikawa, Tetsuya Terada, Shin‐Ichi Haginomori, Yoshitaka Kurisu, Yoshinobu Hirose
Head & Neck.2019; 41(2): 358. CrossRef - Positive Surgical Margins in Submandibular Malignancies: Facility and Practice Variation
Liliya Benchetrit, Elliot Morse, Benjamin L. Judson, Saral Mehra
Otolaryngology–Head and Neck Surgery.2019; 161(4): 620. CrossRef - The growth rate and the positive prediction of needle biopsy of clinically diagnosed Warthin’s tumor
Jungirl Seok, Woo-Jin Jeong, Soon-Hyun Ahn, Young Ho Jung
European Archives of Oto-Rhino-Laryngology.2019; 276(7): 2091. CrossRef - The Difference in the Clinical Features Between Carcinoma ex Pleomorphic Adenoma and Pleomorphic Adenoma
Jungirl Seok, Se Jin Hyun, Woo-Jin Jeong, Soon-Hyun Ahn, Hyojin Kim, Young Ho Jung
Ear, Nose & Throat Journal.2019; 98(8): 504. CrossRef - Fine‐needle aspiration cytology and radiological imaging in parotid gland tumours: Our experience in 103 patients
Clare Perkins, Edward Toll, Philip Reece
Clinical Otolaryngology.2019; 44(6): 1124. CrossRef - Ultrasound‐guided core needle biopsy in salivary glands: A meta‐analysis
Hee Joon Kim, Jong Seung Kim
The Laryngoscope.2018; 128(1): 118. CrossRef - Accuracy and effectiveness of ultrasound-guided core-needle biopsy in the diagnosis of focal lesions in the salivary glands
Jose Luis del Cura, Gloria Coronado, Rosa Zabala, Igone Korta, Ignacio López
European Radiology.2018; 28(7): 2934. CrossRef - The Milan System for Reporting Salivary Gland Cytopathology (MSRSGC): an ASC-IAC–sponsored system for reporting salivary gland fine-needle aspiration
Esther Diana Rossi, Zubair Baloch, Marc Pusztaszeri, William C. Faquin
Journal of the American Society of Cytopathology.2018; 7(3): 111. CrossRef - Routine and Advanced Ultrasound of Major Salivary Glands
Kunwar Suryaveer Singh Bhatia, Yuk-Ling Dai
Neuroimaging Clinics of North America.2018; 28(2): 273. CrossRef - The Milan System for Reporting Salivary Gland Cytopathology (MSRSGC): An ASC-IAC-Sponsored System for Reporting Salivary Gland Fine-Needle Aspiration
Esther Diana Rossi, Zubair W. Baloch, Marc Pusztaszeri, William C. Faquin
Acta Cytologica.2018; 62(3): 157. CrossRef - Evaluation and diagnosis of salivary gland neoplasms
Erica Jackson Mayland, Anna M. Pou
Operative Techniques in Otolaryngology-Head and Neck Surgery.2018; 29(3): 129. CrossRef - Feasibility and Safety of Multicenter Tissue and Biofluid Sampling for α-Synuclein in Parkinson’s Disease: The Systemic Synuclein Sampling Study (S4)
Lana M. Chahine, Thomas G. Beach, Nicholas Seedorff, Chelsea Caspell-Garcia, Christopher S. Coffey, Michael Brumm, Charles H. Adler, Geidy E. Serrano, Carly Linder, Sherri Mosovsky, Tatiana Foroud, Holly Riss, Dixie Ecklund, John Seibyl, Danna Jennings, V
Journal of Parkinson’s Disease.2018; 8(4): 517. CrossRef - Preoperative diagnostic of parotid gland neoplasms: fine-needle aspiration cytology or core needle biopsy?
Peter Zbären, Asterios Triantafyllou, Kenneth O. Devaney, Vincent Vander Poorten, Henrik Hellquist, Alessandra Rinaldo, Alfio Ferlito
European Archives of Oto-Rhino-Laryngology.2018; 275(11): 2609. CrossRef - A comparison study of the reporting systems for salivary gland fine needle aspirations: Are they really different?
Diana Montezuma, Sule Canberk, Ozlem Aydın, Mehmet Polat Dermirhas, André F. Vieira, Süha Goksel, Ümit İnce, Fernando Schmitt
Diagnostic Cytopathology.2018; 46(10): 859. CrossRef - Pediatric salivary gland carcinomas: Diagnostic and therapeutic management
Céleste Rebours, Vincent Couloigner, Louise Galmiche, Odile Casiraghi, Cécile Badoual, Sabah Boudjemaa, Anthony Chauvin, Monique Elmaleh, Brice Fresneau, Sylvie Fasola, Erea‐Noël Garabédian, Thierry Van Den Abeele, Daniel Orbach
The Laryngoscope.2017; 127(1): 140. CrossRef - Agreement between rapid on‐site evaluation and the final cytological diagnosis of salivary gland specimens
S. Wangsiricharoen, S. Lekawanvijit, S. Rangdaeng
Cytopathology.2017; 28(4): 321. CrossRef - Mesenchymal neoplasms of the head and neck: a cytopathologic analysis on fine needle aspiration
James Lee, Samia Kazmi, Christopher J. VandenBussche, Syed Z. Ali
Journal of the American Society of Cytopathology.2017; 6(3): 105. CrossRef - Clinical Results of Surgical Treatment in Parotid Tumors
Ahmet Kara
Journal of Otolaryngology-ENT Research.2017;[Epub] CrossRef - Parotid gland metastases of distant primary tumours: A diagnostic challenge
Achim M. Franzen, Thomas Günzel, Anja Lieder
Auris Nasus Larynx.2016; 43(2): 187. CrossRef - Modern Radiology in the Management of Head and Neck Cancer
G.J.C. Burkill, R.M. Evans, V.V. Raman, S.E.J. Connor
Clinical Oncology.2016; 28(7): 440. CrossRef - Fine‐needle aspiration and core needle biopsy: An update on 2 common minimally invasive tissue sampling modalities
Paul A. VanderLaan
Cancer Cytopathology.2016; 124(12): 862. CrossRef - Staging and follow-up of high-grade malignant salivary gland tumours: The role of traditional versus functional imaging approaches – A review
Nicole Freling, Flavio Crippa, Roberto Maroldi
Oral Oncology.2016; 60: 157. CrossRef - Biopsy of parotid masses: Review of current techniques
Sananda Haldar, Joseph D Sinnott, Kemal M Tekeli, Samuel S Turner, David C Howlett
World Journal of Radiology.2016; 8(5): 501. CrossRef - Review on the applications of ultrasonography in dentomaxillofacial region
Şehrazat Evirgen
World Journal of Radiology.2016; 8(1): 50. CrossRef - Comprehensive Cytomorphologic Analysis of Pulmonary Adenoid Cystic Carcinoma: Comparison to Small Cell Carcinoma and Non-pulmonary Adenoid Cystic Carcinoma
Seokhwi Kim, Jinah Chu, Hojoong Kim, Joungho Han
Journal of Pathology and Translational Medicine.2015; 49(6): 511. CrossRef
- Frozen Section Analysis in Submandibular Gland Tumors: Optimizing Intraoperative Decision-Making
- Incidence and Malignancy Rates of Diagnoses in the Bethesda System for Reporting Thyroid Aspiration Cytology: An Institutional Experience
- Ji Hye Park, Sun Och Yoon, Eun Ju Son, Hye Min Kim, Ji Hae Nahm, SoonWon Hong
- Korean J Pathol. 2014;48(2):133-139. Published online April 28, 2014
- DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.2.133
- 11,736 View
- 86 Download
- 39 Crossref
-
 Abstract
Abstract
 PDF
PDF Background The Bethesda System for Reporting Thyroid Cytopathology (BSRTC) uses six diagnostic categories to standardize communication of thyroid fine-needle aspiration (FNA) interpretations between clinicians and cytopathologists. Since several studies have questioned the diagnostic accuracy of this system, we examined its accuracy in our hospital.
Methods We calculated the incidences and malignancy rates of each diagnostic category in the BSRTC for 1,730 FNAs that were interpreted by four cytopathologists in Gangnam Severance Hospital between October 1, 2011, and December 31, 2011.
Results The diagnostic incidences of categories I-VI were as follows: 13.3%, 40.6%, 9.1%, 0.4%, 19.3%, and 17.3%, respectively. Similarly, the malignancy rates of these categories were as follows: 35.3%, 5.6%, 69.0%, 50.0%, 98.7%, and 98.9%, respectively. In categories II, V, and VI, there were no statistically significant differences in the ranges of the malignancy rates among the four cytopathologists. However, there were significant differences in the ranges for categories I and III.
Conclusions Our findings suggest that institutions that use the BSRTC should regularly update their diagnostic criteria. We also propose that institutions issue an annual report of incidences and malignancy rates to help other clinicians improve the case management of patients with thyroid nodules.
-
Citations
Citations to this article as recorded by- The Malignancy Rates of the Bethesda System for Reporting Thyroid Cytopathology: A 10-year Experience in a Single Asian Institute
Sarah I Liew, Nor S Ahmad, Navarasi R Gopal
World Journal of Endocrine Surgery.2025; 16(2): 42. CrossRef - Assessment of Thyroid Fine-Needle Aspirates Using 2023 Bethesda System
Niti Sureka, Charanjeet Ahluwalia, Sana Ahuja, Neha Kawatra Madan, Meetu Agrawal, Sunil Ranga
Acta Cytologica.2025; 69(3): 280. CrossRef - A Comprehensive Approach to the Thyroid Bethesda Category III (AUS) in the Transition Zone Between 2nd Edition and 3rd Edition of The Bethesda System for Reporting Thyroid Cytopathology: Subcategorization, Nuclear Scoring, and More
Merve Bagıs, Nuray Can, Necdet Sut, Ebru Tastekin, Ezgi Genc Erdogan, Buket Yilmaz Bulbul, Yavuz Atakan Sezer, Osman Kula, Elif Mercan Demirtas, Inci Usta
Endocrine Pathology.2024; 35(1): 51. CrossRef - Evaluation of the Efficacy of Thyroid Imaging Reporting and Data Systems Classification in Risk Stratification and in the Management of Thyroid Swelling by Comparing It With Fine-Needle Aspiration Cytology and Histopathological Examination
Abhishek K Saw, Zenith H Kerketta, Khushboo Rani, Krishna Murari, Kritika Srivastava, Ajay Kumar, Sunny LNU, Anish Baxla, Nabu Kumar, Nusrat Noor
Cureus.2024;[Epub] CrossRef - A COMPARATIVE STUDY BETWEEN CONVENTIONAL METHOD AND THE BETHESDA SYSTEM FOR REPORTING THYROID CYTOPATHOLOGY
Pooja Mangal, Arti Gupta
GLOBAL JOURNAL FOR RESEARCH ANALYSIS.2023; : 67. CrossRef - Study of Fine Needle Aspiration Cytology (FNAC) of Thyroid Gland According to the Bethesda System
Keval A Patel, Garima Anandani, Bhawana S Sharma, Riddhi A Parmar
Cureus.2023;[Epub] CrossRef - Correlation of Thyroid Fine Needle Aspiration Biopsy With Histopathological Results
Cemalettin Durgun
Cureus.2023;[Epub] CrossRef - The Bethesda System for Reporting Thyroid Cytopathology: Validating at Tribhuvan University Teaching Hospital
Kunjan Acharya, Shreya Shrivastav, Prashant Triipathi, Bigyan Raj Gyawali, Bijaya Kharel, Dharma Kanta Baskota, Pallavi Sinha
International Archives of Otorhinolaryngology.2022; 26(01): e097. CrossRef - Validating the ‘CUT score’ risk stratification tool for indeterminate thyroid nodules using the Bethesda system for reporting thyroid cytopathology
Sapir Pinhas, Idit Tessler, Luba Pasherstnik Bizer, Khaled khalilia, Meir Warman, Meital Adi, Doron Halperin, Oded Cohen
European Archives of Oto-Rhino-Laryngology.2022; 279(1): 383. CrossRef - ANALYSIS OF FINE NEEDLE ASPIRATIONS OF THE THYROID: CYTOLOGICAL-HISTOPATHOLOGICAL CORRELATION AND OUTCOMES OF THE BETHESDA SYSTEM
Ayca TAN
SDÜ Tıp Fakültesi Dergisi.2022; 29(2): 213. CrossRef - Reproducibility of Cytomorphological Diagnosis and Assessment of Risk of Malignancy of Thyroid Nodules Based on the Bethesda System for Reporting Thyroid Cytopathology
Sasmita Panda, Mamita Nayak, Lucy Pattanayak, Paresh Kumar Behera, Sagarika Samantaray, Sashibhusan Dash
Journal of Microscopy and Ultrastructure.2022; 10(4): 174. CrossRef - Comparative analysis of cytomorphology of thyroid lesion on conventional cytology versus liquid-based cytology and categorize the lesions according to The Bethesda System for Reporting Thyroid Cytopathology
M Qamar Alam, Pinki Pandey, Megha Ralli, Jitendra Pratap Singh Chauhan, Roopak Aggarwal, Vineet Chaturvedi, Asttha Kapoor, Kapil Trivedi, Savita Agarwal
Journal of Cancer Research and Therapeutics.2022; 18(Suppl 2): S259. CrossRef - Thyroid cytology in Pakistan: An institutional audit of the atypia of undetermined significance/follicular lesion of undetermined significance category
Saira Fatima, Rabia Qureshi, Sumbul Imran, Romana Idrees, Zubair Ahmad, Naila Kayani, Arsalan Ahmed
Cytopathology.2021; 32(2): 205. CrossRef - Outcomes of the Bethesda system for reporting thyroid cytopathology: Real‐life experience
Galit Avior, Or Dagan, Isaac Shochat, Yulia Frenkel, Idit Tessler, Alona Meir, Anat Jaffe, Oded Cohen
Clinical Endocrinology.2021; 94(3): 521. CrossRef - National differences in cost analysis of Afirma Genomic sequencing classifier
Ohad Ronen, Maya Oichman
Clinical Endocrinology.2021; 94(4): 717. CrossRef - Thyroid malignancy rates according to the Bethesda reporting system in Israel - A multicenter study
Ory Madgar, Galit Avior, Isaac Shochat, Ben-Zion Joshua, Lior Baraf, Yuval Avidor, Avi khafif, Niddal Assadi, Eran E. Alon
European Journal of Surgical Oncology.2021; 47(6): 1370. CrossRef - Application of the Bethesda system for reporting thyroid cytopathology for classification of thyroid nodules: A clinical and cytopathological characteristics in Bhutanese population
Sonam Choden, Chimi Wangmo, Sushna Maharjan
Diagnostic Cytopathology.2021; 49(11): 1179. CrossRef - Malignancy rates in thyroid nodules classified as Bethesda categories III and IV; a subcontinent perspective
Adnan Zahid, Waqas Shafiq, Khawaja Shehryar Nasir, Asif Loya, Syed Abbas Raza, Sara Sohail, Umal Azmat
Journal of Clinical & Translational Endocrinology.2021; 23: 100250. CrossRef - The combination of ACR‐Thyroid Imaging Reporting and Data system and The Bethesda System for Reporting Thyroid Cytopathology in the evaluation of thyroid nodules—An institutional experience
Shanmugasundaram Sakthisankari, Sreenivasan Vidhyalakshmi, Sivanandam Shanthakumari, Balalakshmoji Devanand, Udayasankar Nagul
Cytopathology.2021; 32(4): 472. CrossRef - Ultrasound-guided fine needle aspiration cytology and ultrasound examination of thyroid nodules in the UAE: A comparison
Suhail Al-Salam, Charu Sharma, Maysam T. Abu Sa’a, Bachar Afandi, Khaled M. Aldahmani, Alia Al Dhaheri, Hayat Yahya, Duha Al Naqbi, Esraa Al Zuraiqi, Baraa Kamal Mohamed, Shamsa Ahmed Almansoori, Meera Al Zaabi, Aysha Al Derei, Amal Al Shamsi, Juma Al Kaa
PLOS ONE.2021; 16(4): e0247807. CrossRef - Incidence, Clinical Characteristics, and Histopathological Results of Atypia of Undermined Significance in a Tertiary Center in UAE
Maha Osman Shangab, Azza Abdulaziz Khalifa, Fatheya Al Awadi, Mouza Alsharhan, Alaaeldin Bashier
Dubai Diabetes and Endocrinology Journal.2021; 27(1): 1. CrossRef - McGill Thyroid Nodule Score in Differentiating Thyroid Nodules in Total Thyroidectomy Cases of Indeterminate Nodules
Hadi A Al-Hakami, Reem Al-Mohammadi, Rami Al-Mutairi, Haya Al-Subaie, Mohammed A Al Garni
Indian Journal of Surgical Oncology.2020; 11(2): 268. CrossRef - The Bethesda System for Reporting Thyroid Cytopathology: A Cytohistological Study
Bakiarathana Anand, Anita Ramdas, Marie Moses Ambroise, Nirmal P. Kumar
Journal of Thyroid Research.2020; 2020: 1. CrossRef - Differences in cytopathologist thyroid nodule malignancy rate
Ohad Ronen, Hector Cohen, Eyal Sela, Mor Abu
Cytopathology.2020; 31(4): 315. CrossRef - Thyroid Multimodal Ultrasound Evaluation—Impact on Presurgical Diagnosis of Intermediate Cytology Cases
Andreea Borlea, Dana Stoian, Laura Cotoi, Ioan Sporea, Fulger Lazar, Ioana Mozos
Applied Sciences.2020; 10(10): 3439. CrossRef - Fine-needle aspiration cytology of nodular thyroid lesions: A 1-year experience of the thyroid cytopathology in a large regional and a University Hospital, with histological correlation
Kaumudi Konkay, Radhika Kottu, Mutheeswaraiah Yootla, Narendra Hulikal
Thyroid Research and Practice.2019; 16(2): 60. CrossRef - Review of a single institution's fine needle aspiration results for thyroid nodules: Initial observations and lessons for the future
Ohad Ronen, Hector Cohen, Mor Abu
Cytopathology.2019; 30(5): 468. CrossRef - Strain Elastography as a Valuable Diagnosis Tool in Intermediate Cytology (Bethesda III) Thyroid Nodules
Dana Stoian, Florin Borcan, Izabella Petre, Ioana Mozos, Flore Varcus, Viviana Ivan, Andreea Cioca, Adrian Apostol, Cristina Adriana Dehelean
Diagnostics.2019; 9(3): 119. CrossRef - Improvement of diagnostic performance of pathologists by reducing the number of pathologists responsible for thyroid fine needle aspiration cytology: An institutional experience
Jae Yeon Seok, Jungsuk An, Hyun Yee Cho
Diagnostic Cytopathology.2018; 46(7): 561. CrossRef - Bethesda Classification and Cytohistological Correlation of Thyroid Nodules in a Brazilian Thyroid Disease Center
Kassia B. Reuters, Maria C.O.C. Mamone, Elsa S. Ikejiri, Cleber P. Camacho, Claudia C.D. Nakabashi, Carolina C.P.S. Janovsky, Ji H. Yang, Danielle M. Andreoni, Rosalia Padovani, Rui M.B. Maciel, Felipe A.B. Vanderlei, Rosa P.M. Biscolla
European Thyroid Journal.2018; 7(3): 133. CrossRef - The impact of rapid on‐site evaluation on thyroid fine‐needle aspiration biopsy: A 2‐year cancer center institutional experience
Ricardo G. Pastorello, Camila Destefani, Pedro H. Pinto, Caroline H. Credidio, Rafael X. Reis, Thiago de A. Rodrigues, Maryane C. de Toledo, Louise De Brot, Felipe de A. Costa, Antonio G. do Nascimento, Clóvis A. L. Pinto, Mauro A. Saieg
Cancer Cytopathology.2018; 126(10): 846. CrossRef - The Use of the Bethesda System for Reporting Thyroid Cytopathology in Korea: A Nationwide Multicenter Survey by the Korean Society of Endocrine Pathologists
Mimi Kim, Hyo Jin Park, Hye Sook Min, Hyeong Ju Kwon, Chan Kwon Jung, Seoung Wan Chae, Hyun Ju Yoo, Yoo Duk Choi, Mi Ja Lee, Jeong Ja Kwak, Dong Eun Song, Dong Hoon Kim, Hye Kyung Lee, Ji Yeon Kim, Sook Hee Hong, Jang Sihn Sohn, Hyun Seung Lee, So Yeon Pa
Journal of Pathology and Translational Medicine.2017; 51(4): 410. CrossRef - Thyroid FNA cytology in Asian practice—Active surveillance for indeterminate thyroid nodules reduces overtreatment of thyroid carcinomas
K. Kakudo, M. Higuchi, M. Hirokawa, S. Satoh, C. K. Jung, A. Bychkov
Cytopathology.2017; 28(6): 455. CrossRef - Thyroid Fine-Needle Aspiration Cytology Practice in Korea
Yoon Jin Cha, Ju Yeon Pyo, SoonWon Hong, Jae Yeon Seok, Kyung-Ju Kim, Jee-Young Han, Jeong Mo Bae, Hyeong Ju Kwon, Yeejeong Kim, Kyueng-Whan Min, Soonae Oak, Sunhee Chang
Journal of Pathology and Translational Medicine.2017; 51(6): 521. CrossRef - Bethesda System for Reporting Thyroid Cytopathology: A three-year study at a tertiary care referral center in Saudi Arabia
Mohamed Abdulaziz Al Dawish, Asirvatham Alwin Robert, Aljuboury Muna, Alkharashi Eyad, Abdullah Al Ghamdi, Khalid Al Hajeri, Mohammed A Thabet, Rim Braham
World Journal of Clinical Oncology.2017; 8(2): 151. CrossRef - A meta‐analytic review of the Bethesda System for Reporting Thyroid Cytopathology: Has the rate of malignancy in indeterminate lesions been underestimated?
Patrizia Straccia, Esther Diana Rossi, Tommaso Bizzarro, Chiara Brunelli, Federica Cianfrini, Domenico Damiani, Guido Fadda
Cancer Cytopathology.2015; 123(12): 713. CrossRef - Value of TIRADS, BSRTC and FNA-BRAFV600E mutation analysis in differentiating high-risk thyroid nodules
Yu-zhi Zhang, Ting Xu, Dai Cui, Xiao Li, Qing Yao, Hai-yan Gong, Xiao-yun Liu, Huan-huan Chen, Lin Jiang, Xin-hua Ye, Zhi-hong Zhang, Mei-ping Shen, Yu Duan, Tao Yang, Xiao-hong Wu
Scientific Reports.2015;[Epub] CrossRef - A study of malignancy rates in different diagnostic categories of the Bethesda system for reporting thyroid cytopathology: An institutional experience
P. Arul, C. Akshatha, Suresh Masilamani
Biomedical Journal.2015; 38(6): 517. CrossRef - Diagnostic accuracy of Bethesda system for reporting thyroid cytopathology: an institutional perspective
Samreen Naz, Atif Hashmi, Amna khurshid, Naveen Faridi, Muhammad Edhi, Anwar Kamal, Mehmood Khan
International Archives of Medicine.2014; 7(1): 46. CrossRef
- The Malignancy Rates of the Bethesda System for Reporting Thyroid Cytopathology: A 10-year Experience in a Single Asian Institute

 E-submission
E-submission

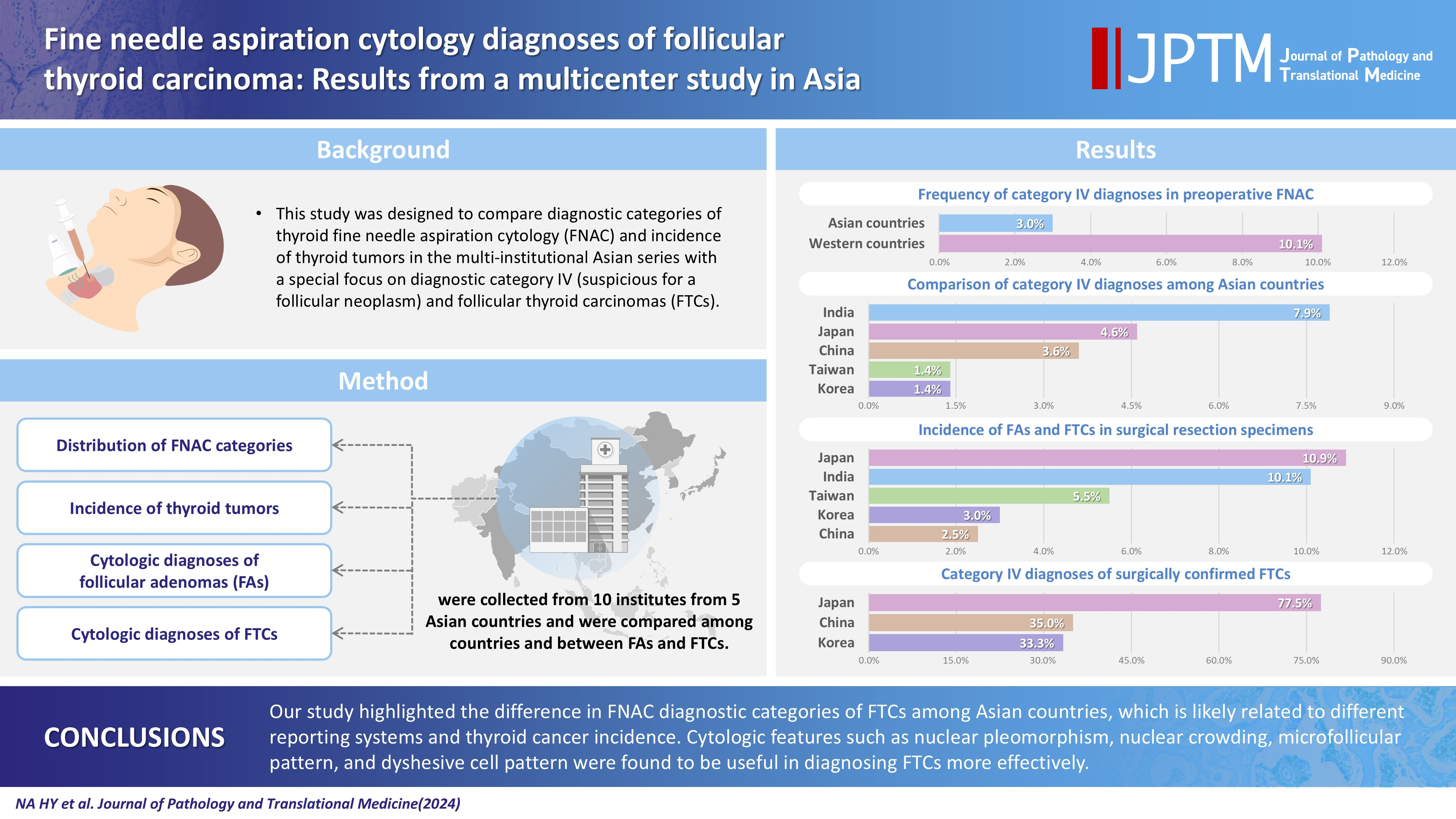

 First
First Prev
Prev



