Search
- Page Path
- HOME > Search
Original Article
- Comparison of tissue-based and plasma-based testing for EGFR mutation in non–small cell lung cancer patients
- Yoon Kyung Kang, Dong Hoon Shin, Joon Young Park, Chung Su Hwang, Hyun Jung Lee, Jung Hee Lee, Jee Yeon Kim, JooYoung Na
- J Pathol Transl Med. 2025;59(1):60-67. Published online January 15, 2025
- DOI: https://doi.org/10.4132/jptm.2024.10.01
- 4,755 View
- 200 Download
-
 Abstract
Abstract
 PDF
PDF - Background
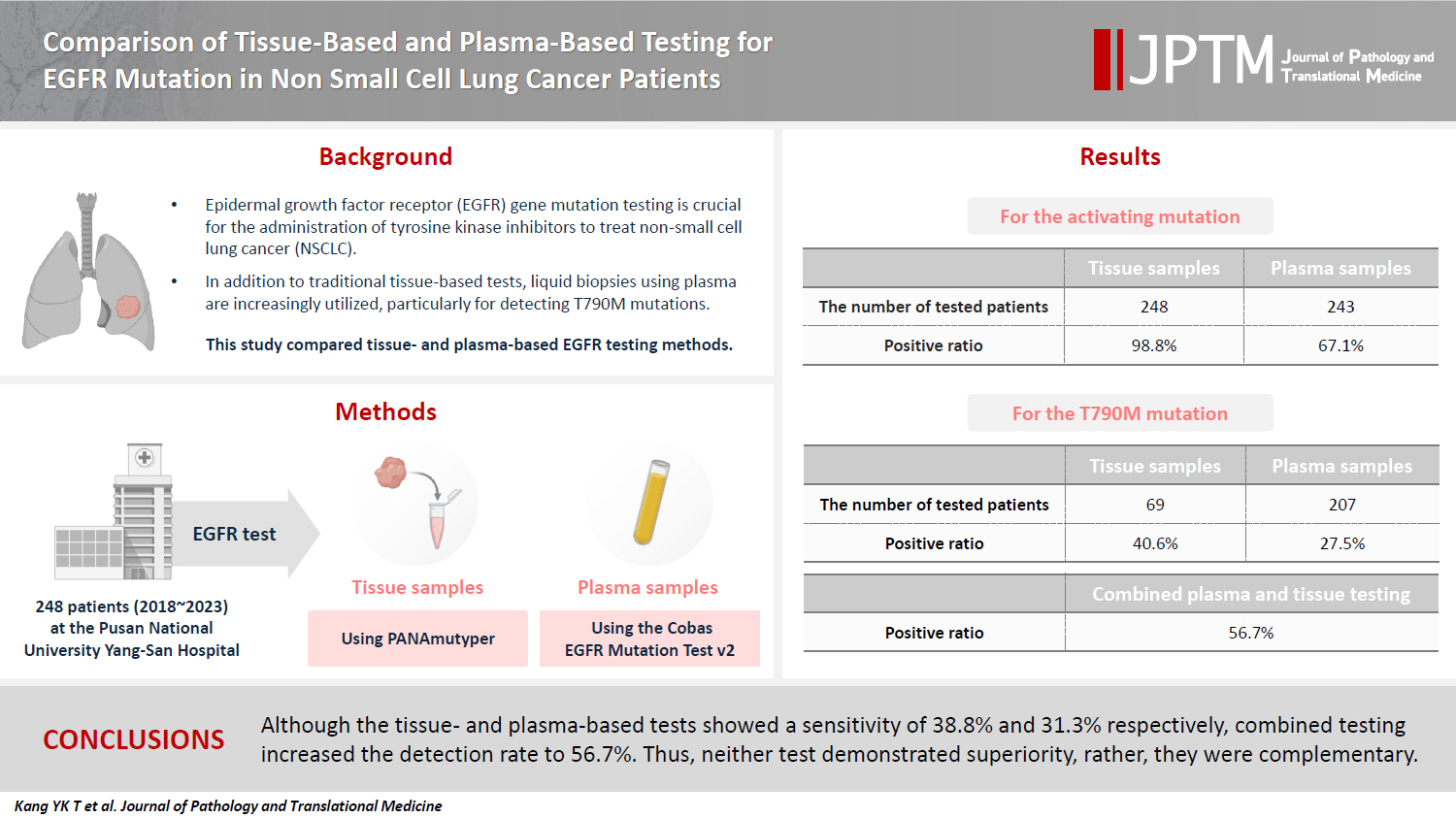
Epidermal growth factor receptor (EGFR) gene mutation testing is crucial for the administration of tyrosine kinase inhibitors to treat non–small cell lung cancer. In addition to traditional tissue-based tests, liquid biopsies using plasma are increasingly utilized, particularly for detecting T790M mutations. This study compared tissue- and plasma-based EGFR testing methods.
Methods
A total of 248 patients were tested for EGFR mutations using tissue and plasma samples from 2018 to 2023 at Pusan National University Yangsan Hospital. Tissue tests were performed using PANAmutyper, and plasma tests were performed using the Cobas EGFR Mutation Test v2.
Results
All 248 patients underwent tissue-based EGFR testing, and 245 (98.8%) showed positive results. Of the 408 plasma tests, 237 (58.1%) were positive. For the T790M mutation, tissue biopsies were performed 87 times in 69 patients, and 30 positive cases (38.6%) were detected. Plasma testing for the T790M mutation was conducted 333 times in 207 patients, yielding 62 positive results (18.6%). Of these, 57 (27.5%) were confirmed to have the mutation via plasma testing. Combined tissue and plasma tests for the T790M mutation were positive in nine patients (13.4%), while 17 (25.4%) were positive in tissue only and 12 (17.9%) in plasma only. This mutation was not detected in 28 patients (43.3%).
Conclusions
Although the tissue- and plasma-based tests showed a sensitivity of 37.3% and 32.8%, respectively, combined testing increased the detection rate to 56.7%. Thus, neither test demonstrated superiority, rather, they were complementary.
Review
- Provisional Guideline Recommendation for EGFR Gene Mutation Testing in Liquid Samples of Lung Cancer Patients: A Proposal by the Korean Cardiopulmonary Pathology Study Group
- Dong Hoon Shin, Hyo Sup Shim, Tae Jung Kim, Heae Surng Park, Yun La Choi, Wan Seop Kim, Lucia Kim, Sun Hee Chang, Joon Seon Song, Hyo jin Kim, Jung Ho Han, Chang Hun Lee, Geon Kook Lee, Se Jin Jang
- J Pathol Transl Med. 2019;53(3):153-158. Published online February 28, 2019
- DOI: https://doi.org/10.4132/jptm.2019.02.22
- 10,598 View
- 265 Download
- 7 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF - Liquid biopsy for detection of mutation from circulating tumor DNA is a new technology which is attractive in that it is non-invasive. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI) is an effective first line drug for advanced non-small cell lung cancer patients who harbor activating EGFR mutation. During the course of treatment, resistance against TKI arises which can be contributed to EGFR T790M mutation in about 50–60% of patients. Third generation TKI may overcome the resistance. In patients who cannot undergo tissue biopsy due to variable reasons, liquid biopsy is an excellent alternative for the detection of EGFR T790M mutation. However, this relatively novel method requires standardization and vigorous quality insurance. Thus, a standard set of guideline recommendations for liquid biopsy for EGFR mutation testing suitable for the Korean medical community is necessary. In this article, we propose a set of provisional guideline recommendations that was discussed and approved by the Cardiopulmonary Pathology Study Group of the Korean Society of Pathologists.
-
Citations
Citations to this article as recorded by- Comparison of tissue-based and plasma-based testing for EGFR mutation in non–small cell lung cancer patients
Yoon Kyung Kang, Dong Hoon Shin, Joon Young Park, Chung Su Hwang, Hyun Jung Lee, Jung Hee Lee, Jee Yeon Kim, JooYoung Na
Journal of Pathology and Translational Medicine.2025; 59(1): 60. CrossRef - Improving non-small-cell lung cancer survival through molecular characterization: Perspective of a multidisciplinary expert panel
M.G.O. Fernandes, A.S. Vilariça, B. Fernandes, C. Camacho, C. Saraiva, F. Estevinho, H. Novais e Bastos, J.M. Lopes, P. Fidalgo, P. Garrido, S. Alves, S. Silva, T. Sequeira, F. Barata
Pulmonology.2024; 30(1): 4. CrossRef - Unlocking the future of cancer diagnosis – promises and challenges of ctDNA-based liquid biopsies in non-small cell lung cancer
Chiara Reina, Berina Šabanović, Chiara Lazzari, Vanesa Gregorc, Christopher Heeschen
Translational Research.2024; 272: 41. CrossRef - Exosomes in Lung Cancer: Actors and Heralds of Tumor Development
Amaia Sandúa, Estibaliz Alegre, Álvaro González
Cancers.2021; 13(17): 4330. CrossRef - Molecular biomarker testing for non–small cell lung cancer: consensus statement of the Korean Cardiopulmonary Pathology Study Group
Sunhee Chang, Hyo Sup Shim, Tae Jung Kim, Yoon-La Choi, Wan Seop Kim, Dong Hoon Shin, Lucia Kim, Heae Surng Park, Geon Kook Lee, Chang Hun Lee
Journal of Pathology and Translational Medicine.2021; 55(3): 181. CrossRef - Current status and future perspectives of liquid biopsy in non-small cell lung cancer
Sunhee Chang, Jae Young Hur, Yoon-La Choi, Chang Hun Lee, Wan Seop Kim
Journal of Pathology and Translational Medicine.2020; 54(3): 204. CrossRef - Prevalence of T790M mutation among TKI-therapy resistant Lebanese lung cancer patients based on liquid biopsy analysis: a first report from a major tertiary care center
Hazem Assi, Arafat Tfayli, Nada Assaf, Sarah Abou Daya, Aram H. Bidikian, Dima Kawsarani, Puzant Fermanian, Ghazi Zaatari, Rami Mahfouz
Molecular Biology Reports.2019; 46(4): 3671. CrossRef
- Comparison of tissue-based and plasma-based testing for EGFR mutation in non–small cell lung cancer patients
Original Article
- Differential MicroRNA Expression between EGFR T790M and L858R Mutated Lung Cancer
- Ji Yeon Kim, Woo Jeong Lee, Ha Young Park, Ahrong Kim, Dong Hoon Shin, Chang Hun Lee
- J Pathol Transl Med. 2018;52(5):275-282. Published online August 16, 2018
- DOI: https://doi.org/10.4132/jptm.2018.07.29
- 8,334 View
- 129 Download
- 6 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
MicroRNAs (miRNAs) are short, non-coding RNAs that mediate post-transcriptional gene regulation. They are commonly deregulated in human malignancies, including non-small cell lung cancer (NSCLC). The aim of this study is to investigate miRNA expression in T790M-mutated NSCLC resistant to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors.
Methods
Six cases of resected NSCLC harboring the T790M mutation were examined. We performed miRNA time polymerase chain reaction (PCR) array profiling using EGFR T790M-mutated NSCLC and L858R-mutated NSCLC. Once identified, miRNAs that were differentially expressed between the two groups were validated by quantitative real-time polymerase chain reaction (qRT-PCR).
Results
miRNA PCR array profiling revealed three up-regulated miRNAs whose expression levels were altered 4.0-fold or more in the EGFR T790M mutation group than in the L858R group: miR-1 (fold change, 4.384), miR-196a (fold change, 4.138), and miR-124 (fold change, 4.132). The three differentially expressed miRNAs were validated by qRT-PCR, and they were found to be overexpressed in the T790M group relative to L858R group. In particular, expression levels of miR-1 and miR-124 were significantly higher in the T790M group (p-value of miR-1 = .004, miR-124 = .007, miR-196a = .096).
Conclusions
MiR-1, miR-124, and miR-196a are overexpressed in EGFR T790M mutated NSCLC. -
Citations
Citations to this article as recorded by- Whole exome sequencing and MicroRNA profiling of lung adenocarcinoma identified risk prediction features for tumors at stage I and its substages
Hao Ho, Sung-Liang Yu, Hsuan-Yu Chen, Shin-Sheng Yuan, Kang-Yi Su, Yi-Chiung Hsu, Chung-Ping Hsu, Cheng-Yen Chuang, Ya-Hsuan Chang, Yu-Cheng Li, Chiou-Ling Cheng, Gee-Chen Chang, Pan-Chyr Yang, Ker-Chau Li
Lung Cancer.2023; 184: 107352. CrossRef - Dynamic Evaluation of Circulating miRNA Profile in EGFR-Mutated NSCLC Patients Treated with EGFR-TKIs
Alessandro Leonetti, Mjriam Capula, Roberta Minari, Giulia Mazzaschi, Alessandro Gregori, Btissame El Hassouni, Filippo Papini, Paola Bordi, Michela Verzè, Amir Avan, Marcello Tiseo, Elisa Giovannetti
Cells.2021; 10(6): 1520. CrossRef - Generation of osimertinib-resistant cells from epidermal growth factor receptor L858R/T790M mutant non-small cell lung carcinoma cell line
Nalini Devi Verusingam, Yi-Chen Chen, Heng-Fu Lin, Chao-Yu Liu, Ming-Cheng Lee, Kai-Hsi Lu, Soon-Keng Cheong, Alan Han-Kiat Ong, Shih-Hwa Chiou, Mong-Lien Wang
Journal of the Chinese Medical Association.2021; 84(3): 248. CrossRef - Cell Behavior of Non-Small Cell Lung Cancer Is at EGFR and MicroRNAs Hands
Sarah Sayed Hassanein, Sherif Abdelaziz Ibrahim, Ahmed Lotfy Abdel-Mawgood
International Journal of Molecular Sciences.2021; 22(22): 12496. CrossRef - The Roles of MicroRNA in Lung Cancer
Kuan-Li Wu, Ying-Ming Tsai, Chi-Tun Lien, Po-Lin Kuo, Jen-Yu Hung
International Journal of Molecular Sciences.2019; 20(7): 1611. CrossRef
- Whole exome sequencing and MicroRNA profiling of lung adenocarcinoma identified risk prediction features for tumors at stage I and its substages

 E-submission
E-submission

 First
First Prev
Prev



