Search
- Page Path
- HOME > Search
Review Article
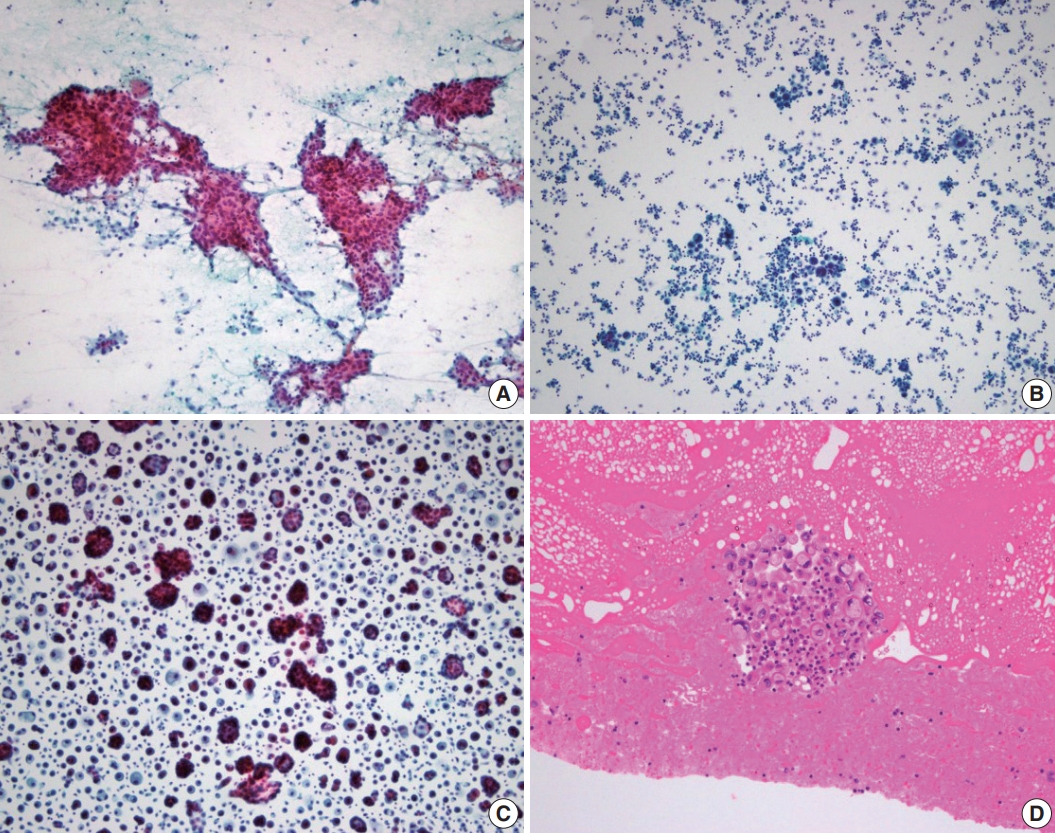
- Recent topics on thyroid cytopathology: reporting systems and ancillary studies
- Mitsuyoshi Hirokawa, Ayana Suzuki
- J Pathol Transl Med. 2025;59(4):214-224. Published online June 30, 2025
- DOI: https://doi.org/10.4132/jptm.2025.04.18
- 4,140 View
- 294 Download
-
 Abstract
Abstract
 PDF
PDF - As fine-needle aspiration techniques and diagnostic methodologies for thyroid nodules have continued to evolve and reporting systems have been updated accordingly, we need to be up to date with the latest information to achieve accurate diagnoses. However, the diagnostic approaches and therapeutic strategies for thyroid nodules vary across laboratories and institutions. Several differences exist between Western and Eastern practices regarding thyroid fine-needle aspiration. This review describes the reporting systems for thyroid cytopathology and ancillary studies. Updated reporting systems enhance the accuracy, consistency, and clarity of cytology reporting, leading to improved patient outcomes and management strategies. Although a single global reporting system is optimal, reporting systems tailored to each country is acceptable. In such cases, compatibility must be ensured to facilitate data sharing. Ancillary methods include liquid-based cytology, immunocytochemistry, biochemical measurements, flow cytometry, molecular testing, and artificial intelligence, all of which improve diagnostic accuracy. These methods continue to evolve, and cytopathologists should actively adopt the latest methods and information to achieve more accurate diagnoses. We believe this review will be useful to practitioners of routine thyroid cytology.
Review
- Biomarker testing of cytology specimens in personalized medicine for lung cancer patients
- Hyojin Kim, Jin-Haeng Chung
- J Pathol Transl Med. 2022;56(6):326-333. Published online November 9, 2022
- DOI: https://doi.org/10.4132/jptm.2022.10.17
- 6,871 View
- 177 Download
- 8 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - Every patient with advanced non–small cell lung cancer (NSCLC) should be tested for targetable driver mutations and gene arrangements that may open avenues for targeted therapy. As most patients with NSCLC in the advanced stage of the disease are not candidates for surgery, these tests have to be performed on small biopsies or cytology samples. A growing number of other genetic changes with targetable mutations may be treatable in the near future. To identify patients who might benefit from novel targeted therapy, relevant markers should be tested in an appropriate context. In addition, immunotherapy of lung cancer is guided by the status of programmed death-ligand 1 expression in tumor cells. The variety and versatility of cytological specimen preparations offer significant advantages for molecular testing; however, they frequently remain underused. Therefore, evaluating the utility and adequacy of cytologic specimens is important, not only from a lung cancer diagnosis, but also for the large number of ancillary studies that are necessary to provide appropriate clinical management. A large proportion of lung cancers is diagnosed by aspiration or exfoliative cytology specimens; thus, optimizing strategies to triage and best use the tissue for diagnosis and biomarker studies forms a critical component of lung cancer management. In this review, we discuss the opportunities and challenges of using cytologic specimens for biomarker testing of lung cancer and the role of cytopathology in the molecular era.
-
Citations
Citations to this article as recorded by- Proposal of real-world solutions for the implementation of predictive biomarker testing in patients with operable non-small cell lung cancer
Paul Hofman, Petros Christopoulos, Nicky D’Haene, John Gosney, Nicola Normanno, Ed Schuuring, Ming-Sound Tsao, Christine Quinn, Jayne Russell, Katherine E Keating, Fernando López-Ríos
Lung Cancer.2025; 201: 108107. CrossRef - Validation of ancillary procedures on formalin liquid fixed aspiration cytologic samples: from minimum to maximum
Orsolya Rideg, Tímea Dergez, Arnold Tóth, Tamás Tornóczky, Gábor Pavlovics, Endre Kálmán
American Journal of Clinical Pathology.2025; 164(6): 924. CrossRef - Molecular testing of cytology specimens: Issues in specimen adequacy and clinical utility
Ghulam Ghous, Komal Ijaz, Magda Esebua, Lester J. Layfield
Diagnostic Cytopathology.2024; 52(2): 123. CrossRef - The updated College of American Pathologists principles of analytic validation of immunohistochemical assays: A step forward for cytopathology
Sinchita Roy‐Chowdhuri
Cancer Cytopathology.2024; 132(9): 547. CrossRef - Best-Practice Biomarker Testing of Oesophago-Gastric Cancer in the UK: Expert Consensus Recommendations Developed Using a Modified Delphi
N.P. West, W. Mansoor, P. Taniere, E. Smyth, M. Rodriguez-Justo, A. Oniscu, P. Carter
Clinical Oncology.2024; 36(11): 701. CrossRef - Next step of molecular pathology: next-generation sequencing in cytology
Ricella Souza da Silva, Fernando Schmitt
Journal of Pathology and Translational Medicine.2024; 58(6): 291. CrossRef
- Proposal of real-world solutions for the implementation of predictive biomarker testing in patients with operable non-small cell lung cancer
Original Article
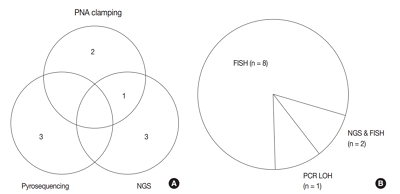
- Adjunctive markers for classification and diagnosis of central nervous system tumors: results of a multi-center neuropathological survey in Korea
- Yoon Jin Cha, Se Hoon Kim, Na Rae Kim
- J Pathol Transl Med. 2020;54(2):165-170. Published online February 20, 2020
- DOI: https://doi.org/10.4132/jptm.2020.02.04
- 8,429 View
- 225 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
The revised 4th 2016 World Health Organization (WHO) classification of tumors of the central nervous system (CNS) classification has adopted integrated diagnosis encompassing the histology and molecular features of CNS tumors. We aimed to investigate the immunohistochemistry, molecular testing, and testing methods for diagnosis of CNS tumors in pathological labs of tertiary centers in Korea, and evaluate the adequacy of tests for proper diagnosis in daily practice.
Methods
A survey, composed of eight questions concerning molecular testing for diagnosis of CNS tumors, was sent to 10 neuropathologists working in tertiary centers in Korea.
Results
For diagnosis of astrocytic and oligodendroglial tumors, all 10 centers performed isocitrate dehydrogenase mutations testing and 1p/19q loss of heterozygosity. For glioneuronal tumors, immunohistochemistry (IHC) assays for synaptophysin (n = 9), CD34 (n = 7), BRAF(VE1) (n = 5) were used. For embryonal tumors, particularly in medulloblastoma, four respondents used IHC panel (growth factor receptor bound protein 2-associated protein 1, filamin A, and yes-associated protein 1) for molecular subclassification. Regarding meningioma, all respondents performed Ki-67 IHC and five performed telomerase reverse transcriptase promoter mutation.
Conclusions
Most tertiary centers made proper diagnosis in line with 2016 WHO classification. As classification of CNS tumors has evolved to be more complex and more ancillary tests are required, these should be performed considering the effect of necessity and justification. -
Citations
Citations to this article as recorded by- Exploring the role of epidermal growth factor receptor variant III in meningeal tumors
Rashmi Rana, Vaishnavi Rathi, Kirti Chauhan, Kriti Jain, Satnam Singh Chhabra, Rajesh Acharya, Samir Kumar Kalra, Anshul Gupta, Sunila Jain, Nirmal Kumar Ganguly, Dharmendra Kumar Yadav, Timir Tripathi
PLOS ONE.2021; 16(9): e0255133. CrossRef
- Exploring the role of epidermal growth factor receptor variant III in meningeal tumors
Reviews
- Molecular Testing of Lung Cancers
- Hyo Sup Shim, Yoon-La Choi, Lucia Kim, Sunhee Chang, Wan-Seop Kim, Mee Sook Roh, Tae-Jung Kim, Seung Yeon Ha, Jin-Haeng Chung, Se Jin Jang, Geon Kook Lee
- J Pathol Transl Med. 2017;51(3):242-254. Published online April 21, 2017
- DOI: https://doi.org/10.4132/jptm.2017.04.10
- 18,212 View
- 617 Download
- 28 Web of Science
- 28 Crossref
-
 Abstract
Abstract
 PDF
PDF - Targeted therapies guided by molecular diagnostics have become a standard treatment of lung cancer. Epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements are currently used as the best predictive biomarkers for EGFR tyrosine kinase inhibitors and ALK inhibitors, respectively. Besides EGFR and ALK, the list of druggable genetic alterations has been growing, including ROS1 rearrangements, RET rearrangements, and MET alterations. In this situation, pathologists should carefully manage clinical samples for molecular testing and should do their best to quickly and accurately identify patients who will benefit from precision therapeutics. Here, we grouped molecular biomarkers of lung cancers into three categories—mutations, gene rearrangements, and amplifications—and propose expanded guidelines on molecular testing of lung cancers.
-
Citations
Citations to this article as recorded by- Association between PD-L1 expression with EGFR, ALK, and ROS1 driver oncogene mutations in non-small cell lung cancer
Dülger Onur, Yaylım İlhan, Öz Büge
Indian Journal of Pathology and Microbiology.2025; 68(1): 36. CrossRef - Evidence-based Approach to Transthoracic Needle Biopsy: Procedural Techniques, Risks, and Controversies
Shravan Sridhar, Hannah G. Ahn, Sayedomid Ebrahimzadeh, Felicia Tang, Brett Elicker
RadioGraphics.2025;[Epub] CrossRef - Enhancing Lung Cancer Care in Portugal: Bridging Gaps for Improved Patient Outcomes
Raquel Ramos, Conceição Souto Moura, Mariana Costa, Nuno Jorge Lamas, Renato Correia, Diogo Garcez, José Miguel Pereira, Carlos Sousa, Nuno Vale
Journal of Personalized Medicine.2024; 14(5): 446. CrossRef - Evolution of therapy for ALK-positive lung carcinomas: Application of third-generation ALK inhibitors in real clinical practice
A. F. Nasretdinov, A. V. Sultanbaev, Sh. I. Musin, K. V. Menshikov, R. T. Ayupov, A. A. Izmailov, G. A. Serebrennikov, V. E. Askarov, D. V. Feoktistov
Meditsinskiy sovet = Medical Council.2024; (10): 74. CrossRef - Cost-effectiveness of next-generation sequencing for advanced EGFR/ALK-negative non-small cell lung cancer
Dong-Won Kang, Sun-Kyeong Park, Sokbom Kang, Eui-Kyung Lee
Lung Cancer.2024; 197: 107970. CrossRef - miR-92a-3p regulates cisplatin-induced cancer cell death
Romain Larrue, Sandy Fellah, Nihad Boukrout, Corentin De Sousa, Julie Lemaire, Carolane Leboeuf, Marine Goujon, Michael Perrais, Bernard Mari, Christelle Cauffiez, Nicolas Pottier, Cynthia Van der Hauwaert
Cell Death & Disease.2023;[Epub] CrossRef - Diagnostic Approach of Lung Cancer: A Literature Review
Jesi Hana, Novia Nurul Faizah
Jurnal Respirasi.2023; 9(2): 141. CrossRef - Molecular Pathology of Lung Cancer
James J. Saller, Theresa A. Boyle
Cold Spring Harbor Perspectives in Medicine.2022; 12(3): a037812. CrossRef - Landscape of EGFR mutations in lung adenocarcinoma: a single institute experience with comparison of PANAMutyper testing and targeted next-generation sequencing
Jeonghyo Lee, Yeon Bi Han, Hyun Jung Kwon, Song Kook Lee, Hyojin Kim, Jin-Haeng Chung
Journal of Pathology and Translational Medicine.2022; 56(5): 249. CrossRef - Molecular biomarker testing for non–small cell lung cancer: consensus statement of the Korean Cardiopulmonary Pathology Study Group
Sunhee Chang, Hyo Sup Shim, Tae Jung Kim, Yoon-La Choi, Wan Seop Kim, Dong Hoon Shin, Lucia Kim, Heae Surng Park, Geon Kook Lee, Chang Hun Lee
Journal of Pathology and Translational Medicine.2021; 55(3): 181. CrossRef - TM4SF4 and LRRK2 Are Potential Therapeutic Targets in Lung and Breast Cancers through Outlier Analysis
Kyungsoo Jung, Joon-Seok Choi, Beom-Mo Koo, Yu Jin Kim, Ji-Young Song, Minjung Sung, Eun Sol Chang, Ka-Won Noh, Sungbin An, Mi-Sook Lee, Kyoung Song, Hannah Lee, Ryong Nam Kim, Young Kee Shin, Doo-Yi Oh, Yoon-La Choi
Cancer Research and Treatment.2021; 53(1): 9. CrossRef - The promises and challenges of early non‐small cell lung cancer detection: patient perceptions, low‐dose CT screening, bronchoscopy and biomarkers
Lukas Kalinke, Ricky Thakrar, Sam M. Janes
Molecular Oncology.2021; 15(10): 2544. CrossRef - Cost-effectiveness analyses of targeted therapy and immunotherapy for advanced non-small cell lung cancer in the United States: a systematic review
Anthony Yu, Eva Huang, Momoka Abe, Kang An, Sun-Kyeong Park, Chanhyun Park
Expert Review of Pharmacoeconomics & Outcomes Research.2021; 21(3): 381. CrossRef - The expanding capability and clinical relevance of molecular diagnostic technology to identify and evaluate EGFR mutations in advanced/metastatic NSCLC
Parth Shah, Jacob Sands, Nicola Normanno
Lung Cancer.2021; 160: 118. CrossRef - Testing for EGFR Mutations and ALK Rearrangements in Advanced Non-Small-Cell Lung Cancer: Considerations for Countries in Emerging Markets
Mercedes L Dalurzo, Alejandro Avilés-Salas, Fernando Augusto Soares, Yingyong Hou, Yuan Li, Anna Stroganova, Büge Öz, Arif Abdillah, Hui Wan, Yoon-La Choi
OncoTargets and Therapy.2021; Volume 14: 4671. CrossRef - Treatment of Patients With Non–Small-Cell Lung Cancer Harboring Rare Oncogenic Mutations
Melina E. Marmarelis, Corey J. Langer
Clinical Lung Cancer.2020; 21(5): 395. CrossRef - Detection of Targetable Genetic Alterations in Korean Lung Cancer Patients: A Comparison Study of Single-Gene Assays and Targeted Next-Generation Sequencing
Eunhyang Park, Hyo Sup Shim
Cancer Research and Treatment.2020; 52(2): 543. CrossRef - High prevalence of ROS1 gene rearrangement detected by FISH in EGFR and ALK negative lung adenocarcinoma
Yuyin Xu, Heng Chang, Lijing Wu, Xin Zhang, Ling Zhang, Jing Zhang, Yuan Li, Lei Shen, Xiaoli Zhu, Xiaoyan Zhou, Qianming Bai
Experimental and Molecular Pathology.2020; 117: 104548. CrossRef - An All-In-One Transcriptome-Based Assay to Identify Therapy-Guiding Genomic Aberrations in Nonsmall Cell Lung Cancer Patients
Jiacong Wei, Anna A. Rybczynska, Pei Meng, Martijn Terpstra, Ali Saber, Jantine Sietzema, Wim Timens, Ed Schuuring, T. Jeroen N. Hiltermann, Harry. J.M. Groen, Anthonie van der Wekken, Anke van den Berg, Klaas Kok
Cancers.2020; 12(10): 2843. CrossRef - Immunotherapy in EGFR-Mutant and ALK-Positive Lung Cancer
Alexander Gavralidis, Justin F. Gainor
The Cancer Journal.2020; 26(6): 517. CrossRef - Role of Immunocytochemistry in the Cytological Diagnosis of Pulmonary Tumors
Jasna Metovic, Luisella Righi, Luisa Delsedime, Marco Volante, Mauro Papotti
Acta Cytologica.2020; 64(1-2): 16. CrossRef - Molecular Diagnostic Assays and Clinicopathologic Implications of MET Exon 14 Skipping Mutation in Non–small-cell Lung Cancer
Eun Kyung Kim, Kyung A. Kim, Chang Young Lee, Sangwoo Kim, Sunhee Chang, Byoung Chul Cho, Hyo Sup Shim
Clinical Lung Cancer.2019; 20(1): e123. CrossRef - PD‐L1 expression in ROS1‐rearranged non‐small cell lung cancer: A study using simultaneous genotypic screening of EGFR, ALK, and ROS1
Jongmin Lee, Chan Kwon Park, Hyoung‐Kyu Yoon, Young Jo Sa, In Sook Woo, Hyo Rim Kim, Sue Youn Kim, Tae‐Jung Kim
Thoracic Cancer.2019; 10(1): 103. CrossRef - Human Leukocyte Antigen Class I and Programmed Death-Ligand 1 Coexpression Is an Independent Poor Prognostic Factor in Adenocarcinoma of the Lung
Yeon Bi Han, Hyun Jung Kwon, Soo Young Park, Eun-Sun Kim, Hyojin Kim, Jin-Haeng Chung
Journal of Pathology and Translational Medicine.2019; 53(2): 86. CrossRef - Molecular testing for advanced non-small cell lung cancer in Malaysia: Consensus statement from the College of Pathologists, Academy of Medicine Malaysia, the Malaysian Thoracic Society, and the Malaysian Oncological Society
Pathmanathan Rajadurai, Phaik Leng Cheah, Soon Hin How, Chong Kin Liam, Muhammad Azrif Ahmad Annuar, Norhayati Omar, Noriah Othman, Nurhayati Mohd Marzuki, Yong Kek Pang, Ros Suzanna Ahmad Bustamam, Lye Mun Tho
Lung Cancer.2019; 136: 65. CrossRef - Somatic mutations and immune checkpoint biomarkers
Brielle A. Parris, Eloise Shaw, Brendan Pang, Richie Soong, Kwun Fong, Ross A. Soo
Respirology.2019; 24(3): 215. CrossRef - Adverse Event Management in Patients with BRAF V600E-Mutant Non-Small Cell Lung Cancer Treated with Dabrafenib plus Trametinib
Anna Chalmers, Laura Cannon, Wallace Akerley
The Oncologist.2019; 24(7): 963. CrossRef - Genetic and clinicopathologic characteristics of lung adenocarcinoma with tumor spread through air spaces
Jae Seok Lee, Eun Kyung Kim, Moonsik Kim, Hyo Sup Shim
Lung Cancer.2018; 123: 121. CrossRef
- Association between PD-L1 expression with EGFR, ALK, and ROS1 driver oncogene mutations in non-small cell lung cancer
- The Utilization of Cytologic Fine-Needle Aspirates of Lung Cancer for Molecular Diagnostic Testing
- Michael H. Roh
- J Pathol Transl Med. 2015;49(4):300-309. Published online June 16, 2015
- DOI: https://doi.org/10.4132/jptm.2015.06.16
- 17,141 View
- 173 Download
- 35 Web of Science
- 34 Crossref
-
 Abstract
Abstract
 PDF
PDF - In this era of precision medicine, our understanding and knowledge of the molecular landscape associated with lung cancer pathogenesis continues to evolve. This information is being increasingly exploited to treat advanced stage lung cancer patients with tailored, targeted therapy. During the management of these patients, minimally invasive procedures to obtain samples for tissue diagnoses are desirable. Cytologic fine-needle aspirates are often utilized for this purpose and are important not only for rendering diagnoses to subtype patients’ lung cancers, but also for ascertaining molecular diagnostic information for treatment purposes. Thus, cytologic fine-needle aspirates must be utilized and triaged judiciously to achieve both objectives. In this review, strategies in utilizing fine-needle aspirates will be discussed in the context of our current understanding of the clinically actionable molecular aberrations underlying non-small cell lung cancer and the molecular assays applied to these samples in order to obtain treatment-relevant molecular diagnostic information.
-
Citations
Citations to this article as recorded by- The Bridge: Supernatant Derived From Cytological Sample Preparations
Sinchita Roy‐Chowdhuri
Cytopathology.2025; 36(3): 222. CrossRef - Optimizing cytology and small biopsy specimen processing for ancillary studies: recommendations from the American Society of Cytopathology taskforce
Sinchita Roy-Chowdhuri, Christine N. Booth, Jonas J. Heymann, Elizabeth Jenkins, Joshua R. Menke, Sara E. Monaco, Ritu Nayar, Michiya Nishino, Roberto Ruiz-Cordero, Donna K. Russell, Anjali Saqi, Kaitlin E. Sundling, Michael J. Thrall, Vanda F. Torous, Ch
Journal of the American Society of Cytopathology.2025; 14(5): 285. CrossRef - Utility of Cell Block Preparation From CT-Guided FNAC with Immunohistochemistry in Diagnosing Primary Lung Carcinoma: A Prospective Study in A Tertiary Care Hospital
Subhargha Mandal, Abhishek Chowdhury, Riju Bhattacharyya, Palas Bhattacharyya
National Journal of Medical Research.2025; 15(04): 286. CrossRef - The American Cancer Society National Lung Cancer Roundtable strategic plan: Methods for improving turnaround time of comprehensive biomarker testing in non–small cell lung cancer
Sinchita Roy‐Chowdhuri, Haresh Mani, Adam H. Fox, Anne Tsao, Lynette M. Sholl, Farhood Farjah, Bruce E. Johnson, Raymond U. Osarogiagbon, M. Patricia Rivera, Gerard A. Silvestri, Robert A. Smith, Ignacio I. Wistuba
Cancer.2024; 130(24): 4200. CrossRef - Endobronchial ultrasound bronchoscopy Franseen fine needle biopsy tool versus standard fine needle aspiration needle: Impact on diagnosis and tissue adequacy
Matthew C. Aboudara, Timothy Saettele, Ossama Tawfik
Respiratory Medicine.2023; 208: 107131. CrossRef - Adequacy of small biopsy and cytology specimens for comprehensive genomic profiling of patients with non-small-cell lung cancer to determine eligibility for immune checkpoint inhibitor and targeted therapy
Erin Faber, Horiana Grosu, Sharjeel Sabir, Francis Anthony San Lucas, Bedia A Barkoh, Roland L Bassett, Rajyalakshmi Luthra, John Stewart, Sinchita Roy-Chowdhuri
Journal of Clinical Pathology.2022; 75(9): 612. CrossRef - Biomarker testing of cytology specimens in personalized medicine for lung cancer patients
Hyojin Kim, Jin-Haeng Chung
Journal of Pathology and Translational Medicine.2022; 56(6): 326. CrossRef - Formalin fixation for optimal concordance of programmed death‐ligand 1 immunostaining between cytologic and histologic specimens from patients with non–small cell lung cancer
Bregje M. Koomen, Jose van der Starre‐Gaal, Judith M. Vonk, Jan H. von der Thüsen, Jacqueline J. C. van der Meij, Kim Monkhorst, Stefan M. Willems, Wim Timens, Nils A. ’t Hart
Cancer Cytopathology.2021; 129(4): 304. CrossRef - Collection and Handling of Thoracic Small Biopsy and Cytology Specimens for Ancillary Studies: Guidelines from the College of American Pathologists (CAP)
Sinchita Roy-Chowdhuri
Journal of Molecular Pathology.2021; 2(1): 23. CrossRef - A decade of change: Trends in the practice of cytopathology at a tertiary care cancer centre
Peyman Dinarvand, Chinhua Liu, Sinchita Roy‐Chowdhuri
Cytopathology.2021; 32(5): 604. CrossRef - False‐negative programmed death‐ligand 1 immunostaining in ethanol‐fixed endobronchial ultrasound‐guided transbronchial needle aspiration specimens of non‐small‐cell lung cancer patients
Bregje M Koomen, Willem Vreuls, Mirthe de Boer, Emma J de Ruiter, Juergen Hoelters, Aryan Vink, Stefan M Willems
Histopathology.2021; 79(4): 480. CrossRef - Influence of preanalytical variables on performance of delta-like protein 3 (DLL3) predictive immunohistochemistry
Teodora Radonic, S. Duin, W. Vos, P. Kortman, Aeilko H. Zwinderman, Erik Thunnissen
Virchows Archiv.2021; 478(2): 293. CrossRef - Comparison of the SuperARMS and ARMS for detecting EGFR mutations in liquid-based cytology specimens from NSCLC patients
Wei Wu, Ziyang Cao, Wei Zhang, Liping Zhang, Likun Hou, Chunyan Wu
Diagnostic Pathology.2020;[Epub] CrossRef - Tumor cell representation by an improvised technique of fine-needle aspiration specimen acquisition and cell block preparation: Our experience in lung cancer cases in a peripheral center of eastern India
AnupKr Boler, Shreosee Roy, Arghya Bandyopadhyay, Abhishek Bandyopadhyay, MrinalKanti Ghosh
Journal of Cytology.2020; 37(2): 87. CrossRef - A new guideline from the College of American Pathologists to improve the adequacy of thoracic small specimens for ancillary studies
Sinchita Roy‐Chowdhuri
Cancer Cytopathology.2020; 128(10): 690. CrossRef - The utilization of cytologic and small biopsy samples for ancillary molecular testing
Michael H. Roh
Modern Pathology.2019; 32: 77. CrossRef - Diff-Quik Cytology Smears from Endobronchial Ultrasound Transbronchial Needle Aspiration Lymph Node Specimens as a Source of DNA for Next-Generation Sequencing Instead of Cell Blocks
David Fielding, Andrew J. Dalley, Farzad Bashirzadeh, Mahendra Singh, Lakshmy Nandakumar, Amy E. McCart Reed, Debra Black, Stephen Kazakoff, John V. Pearson, Katia Nones, Nicola Waddell, Sunil R. Lakhani, Peter T. Simpson
Respiration.2019; 97(6): 525. CrossRef - Molecular testing on endobronchial ultrasound (EBUS) fine needle aspirates (FNA): Impact of triage
Simon Sung, John P. Crapanzano, David DiBardino, David Swinarski, William A. Bulman, Anjali Saqi
Diagnostic Cytopathology.2018; 46(2): 122. CrossRef - Salvaging the supernatant: next generation cytopathology for solid tumor mutation profiling
Sinchita Roy-Chowdhuri, Meenakshi Mehrotra, Ana Maria Bolivar, Rashmi Kanagal-Shamanna, Bedia A. Barkoh, Brette Hannigan, Stephanie Zalles, Wenrui Ye, Dzifa Duose, Russell Broaddus, Gregg Staerkel, Ignacio Wistuba, L. Jeffrey Medeiros, Rajyalakshmi Luthra
Modern Pathology.2018; 31(7): 1036. CrossRef - Fluorescence in situ hybridization analysis on cytologic smears: An accurate and efficient method in the diagnosis of melanotic Xp11 translocation renal cancer
Alia Gupta, Mark Micale, Kurt D. Bernacki
Diagnostic Cytopathology.2018; 46(9): 786. CrossRef - Comprehensive Validation of Cytology Specimens for Next-Generation Sequencing and Clinical Practice Experience
Agnes Balla, Ken J. Hampel, Mukesh K. Sharma, Catherine E. Cottrell, Nikoletta Sidiropoulos
The Journal of Molecular Diagnostics.2018; 20(6): 812. CrossRef - Advances in Molecular Testing Techniques in Cytologic Specimens
Sinchita Roy-Chowdhuri
Surgical Pathology Clinics.2018; 11(3): 669. CrossRef - Next-generation molecular diagnosis: single-cell sequencing from bench to bedside
Wanjun Zhu, Xiao-Yan Zhang, Sadie L. Marjani, Jialing Zhang, Wengeng Zhang, Shixiu Wu, Xinghua Pan
Cellular and Molecular Life Sciences.2017; 74(5): 869. CrossRef - Big data from small samples: Informatics of next‐generation sequencing in cytopathology
Sinchita Roy‐Chowdhuri, Somak Roy, Sara E. Monaco, Mark J. Routbort, Liron Pantanowitz
Cancer Cytopathology.2017; 125(4): 236. CrossRef - Next‐generation sequencing of liquid‐based cytology non–small cell lung cancer samples
Jordan P. Reynolds, Yaolin Zhou, Maureen A. Jakubowski, Zhen Wang, Jennifer A. Brainard, Roger D. Klein, Carol F. Farver, Francisco A. Almeida, Yu‐Wei Cheng
Cancer Cytopathology.2017; 125(3): 178. CrossRef - Concurrent fine needle aspirations and core needle biopsies: a comparative study of substrates for next-generation sequencing in solid organ malignancies
Sinchita Roy-Chowdhuri, Hui Chen, Rajesh R Singh, Savitri Krishnamurthy, Keyur P Patel, Mark J Routbort, Jawad Manekia, Bedia A Barkoh, Hui Yao, Sharjeel Sabir, Russell R Broaddus, L Jeffrey Medeiros, Gregg Staerkel, John Stewart, Rajyalakshmi Luthra
Modern Pathology.2017; 30(4): 499. CrossRef - Diagnosis of anaplastic lymphoma kinase rearrangement in cytological samples through a fluorescence in situ hybridization–based assay: Cytological smears versus cell blocks
Federica Zito Marino, Giulio Rossi, Matteo Brunelli, Maria Gabriella Malzone, Giuseppina Liguori, Giuseppe Bogina, Alessandro Morabito, Gaetano Rocco, Renato Franco, Gerardo Botti
Cancer Cytopathology.2017; 125(5): 303. CrossRef - Next‐generation sequencing of cytologic preparations: An analysis of quality metrics
David H. Hwang, Elizabeth P. Garcia, Matthew D. Ducar, Edmund S. Cibas, Lynette M. Sholl
Cancer Cytopathology.2017; 125(10): 786. CrossRef - Recent advances in the pathology and molecular genetics of lung cancer: A practical review for cytopathologists
Erika F. Rodriguez, Sara E. Monaco
Journal of the American Society of Cytopathology.2016; 5(5): 252. CrossRef - Utilization of ancillary studies in the cytologic diagnosis of respiratory lesions: The papanicolaou society of cytopathology consensus recommendations for respiratory cytology
Lester J. Layfield, Sinchita Roy‐Chowdhuri, Zubair Baloch, Hormoz Ehya, Kim Geisinger, Susan J. Hsiao, Oscar Lin, Neal I. Lindeman, Michael Roh, Fernando Schmitt, Nikoletta Sidiropoulos, Paul A. VanderLaan
Diagnostic Cytopathology.2016; 44(12): 1000. CrossRef - Inflammatory myofibroblastic tumour
Michael McDermott
Seminars in Diagnostic Pathology.2016; 33(6): 358. CrossRef - Preanalytic Variables in Cytology: Lessons Learned From Next-Generation Sequencing—The MD Anderson Experience
Sinchita Roy-Chowdhuri, John Stewart
Archives of Pathology & Laboratory Medicine.2016; 140(11): 1191. CrossRef - Biomarker Testing in Lung Carcinoma Cytology Specimens: A Perspective From Members of the Pulmonary Pathology Society
Sinchita Roy-Chowdhuri, Dara L. Aisner, Timothy Craig Allen, Mary Beth Beasley, Alain Borczuk, Philip T. Cagle, Vera Capelozzi, Sanja Dacic, Gilda da Cunha Santos, Lida P. Hariri, Keith M. Kerr, Sylvie Lantuejoul, Mari Mino-Kenudson, Andre Moreira, Kirtee
Archives of Pathology & Laboratory Medicine.2016; 140(11): 1267. CrossRef - Identification of a novel partner gene, KIAA1217, fused to RET: Functional characterization and inhibitor sensitivity of two isoforms in lung adenocarcinoma
Mi-Sook Lee, Ryong Nam Kim, Hoseok I, Doo-Yi Oh, Ji-Young Song, Ka-Won Noh, Yu-Jin Kim, Jung Wook Yang, Maruja E. Lira, Chang Hun Lee, Min Ki Lee, Yeoung Dae Kim, Mao Mao, Joungho Han, Jhingook Kim, Yoon-La Choi
Oncotarget.2016; 7(24): 36101. CrossRef
- The Bridge: Supernatant Derived From Cytological Sample Preparations

 E-submission
E-submission


 First
First Prev
Prev



