Search
- Page Path
- HOME > Search
Case Study
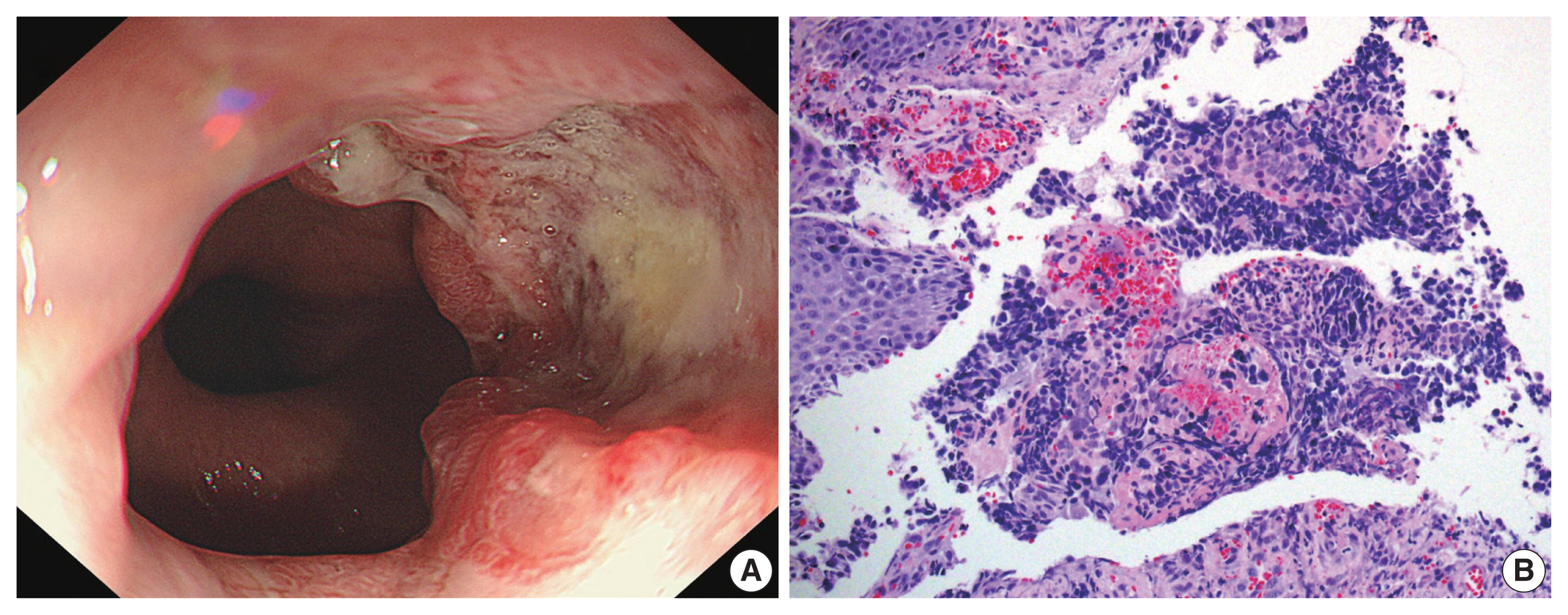
- A rare goblet cell adenocarcinoma arising from Barrett’s esophagus: the first reported case in the esophagus
- Chi Eun Oh, Sung Eun Kim, Sun-Ju Oh
- J Pathol Transl Med. 2024;58(2):81-86. Published online January 8, 2024
- DOI: https://doi.org/10.4132/jptm.2023.12.26
- 4,774 View
- 328 Download
-
 Abstract
Abstract
 PDF
PDF - Goblet cell adenocarcinoma (GCA) is a rare and distinctive amphicrine tumor comprised of goblet-like mucinous cells and neuroendocrine cells. It is believed to originate from pluripotent stem cells located at the base of crypts. GCA predominantly arises from the appendix, with a few reported cases in extra-appendiceal locations such as the colorectum, small intestine, and stomach. In this case report, we present a unique instance of a 64-year-old male who initially received a diagnosis of neuroendocrine carcinoma in the distal esophagus based on biopsy but, following resection, was subsequently re-diagnosed with GCA arising from Barrett’s esophagus.
Review
- Standardization of the pathologic diagnosis of appendiceal mucinous neoplasms
- Dong-Wook Kang, Baek-hui Kim, Joon Mee Kim, Jihun Kim, Hee Jin Chang, Mee Soo Chang, Jin-Hee Sohn, Mee-Yon Cho, So-Young Jin, Hee Kyung Chang, Hye Seung Han, Jung Yeon Kim, Hee Sung Kim, Do Youn Park, Ha Young Park, So Jeong Lee, Wonae Lee, Hye Seung Lee, Yoo Na Kang, Younghee Choi
- J Pathol Transl Med. 2021;55(4):247-264. Published online July 8, 2021
- DOI: https://doi.org/10.4132/jptm.2021.05.28
- 21,318 View
- 1,118 Download
- 18 Web of Science
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Although the understanding of appendiceal mucinous neoplasms (AMNs) and their relationship with disseminated peritoneal mucinous disease have advanced, the diagnosis, classification, and treatment of AMNs are still confusing for pathologists and clinicians. The Gastrointestinal Pathology Study Group of the Korean Society of Pathologists (GPSG-KSP) proposed a multicenter study and held a workshop for the “Standardization of the Pathologic Diagnosis of the Appendiceal Mucinous Neoplasm” to overcome the controversy and potential conflicts. The present article is focused on the diagnostic criteria, terminologies, tumor grading, pathologic staging, biologic behavior, treatment, and prognosis of AMNs and disseminated peritoneal mucinous disease. In addition, GPSG-KSP proposes a checklist of standard data elements of appendiceal epithelial neoplasms to standardize pathologic diagnosis. We hope the present article will provide pathologists with updated knowledge on how to handle and diagnose AMNs and disseminated peritoneal mucinous disease.
-
Citations
Citations to this article as recorded by- Intrasplenic metastasis of appendiceal low-grade mucinous neoplasm – A case report and review of the literature
P. Meister, J. Rawitzer, M. Reschke, H.A. Baba, U. Neumann, M. Kaths
Current Problems in Cancer: Case Reports.2025; 18: 100364. CrossRef - Complete laparoscopic resection of giant appendiceal mucinous neoplasm, case report, and literature review
Shatha Awad Althobaiti, Rayan Z. Makeen, Abrar J. Filfilan, Ahmed Abdulaziz Hawsawi
Saudi Surgical Journal.2025; 13(1): 35. CrossRef - Survival Outcomes and Prognostic Factors in Metastatic Unresectable Appendiceal Adenocarcinoma Treated with Palliative Systemic Chemotherapy: A 10-Year Retrospective Analysis from Australia
Jirapat Wonglhow, Hui-Li Wong, Michael Michael, Alexander Heriot, Glen Guerra, Catherine Mitchell, Jeanne Tie
Cancers.2025; 17(20): 3297. CrossRef - Lower Gastrointestinal Bleeding Secondary to Appendiceal Mucinous Neoplasm: A Report of Two Cases and a Review of the Literature
Jesús Omar Soto Llanes, Samanta Kin Dosal Limón, Ana Jimena Iberri Jaime, Mario Zambrano Lara, Billy Jiménez Bobadilla
Cureus.2024;[Epub] CrossRef - Predicting Survival in Mucinous Adenocarcinoma of the Appendix: Demographics, Disease Presentation, and Treatment Methodology
Paul H. McClelland, Stephanie N. Gregory, Shirley K. Nah, Jonathan M. Hernandez, Jeremy L. Davis, Andrew M. Blakely
Annals of Surgical Oncology.2024; 31(9): 6237. CrossRef - Histoséminaire biopsies péritonéales tumorales. Néoplasies mucineuses appendiculaires
Peggy Dartigues
Annales de Pathologie.2024; 44(4): 274. CrossRef - Histoséminaire biopsies péritonéales tumorales. Cas no 2
Peggy Dartigues
Annales de Pathologie.2024; 44(4): 245. CrossRef - A Case of Low-Grade Appendiceal Mucinous Neoplasm: The Role of Preoperative Imaging and Surgical Technique in Achieving Favorable Outcomes
Daniel A Meza-Martinez, Yeudiel Suro Santos, Samantha J Andrade-Ordoñez, Julio A Palomino-Payan, Brando J Fematt-Rodriguez
Cureus.2024;[Epub] CrossRef - Incidental Appendiceal Mucinous Neoplasm Found During Appendectomy in a 15-Year-Old Patient: A Case Report
Fernando Aguilar-Ruiz, Kevin Joseph Fuentes-Calvo, Sara Fernanda Arechavala-Lopez, Irving Fuentes-Calvo, Luis F Arias-Ruiz
Cureus.2024;[Epub] CrossRef - Uncovering the Hidden Threat: Ileocolic Intussusception in an Adult With Appendicular Tumor
Mrunal Panchal, Shishir Kumar, Khushboo Jha, Kaushik Saha, Abhijit Kundu
Cureus.2024;[Epub] CrossRef - Low-Grade Appendiceal Mucinous Neoplasm vs. Appendiceal Diverticulum: Distinction with Histomorphologic Features
Cevriye Cansiz Ersöz, Siyar Ersöz, Berna Savas, Arzu Ensari
Gastrointestinal Disorders.2024; 6(4): 905. CrossRef - Appendiceal perforation secondary to endometriosis with intestinal metaplasia: A case report
Minghua Wang, Jing Liu, Boxin Hu, Simin Wang, Ping Xie, Ping Li
Experimental and Therapeutic Medicine.2023;[Epub] CrossRef - Primary and secondary tumors of the peritoneum: key imaging features and differential diagnosis with surgical and pathological correlation
Javier Miguez González, Francesc Calaf Forn, Laura Pelegrí Martínez, Pilar Lozano Arranz, Rafael Oliveira Caiafa, Jordi Català Forteza, Lina Maria Palacio Arteaga, Ferrán Losa Gaspà, Isabel Ramos Bernadó, Pedro Barrios Sánchez, Juan Ramón Ayuso Colella
Insights into Imaging.2023;[Epub] CrossRef - Muzinöse Tumoren des Peritoneums
Anne Kristin Fischer, Andrea Tannapfel, Alexander Quaas
Die Chirurgie.2023; 94(10): 823. CrossRef - Landscape of Genetic Mutations in Appendiceal Cancers
Marian Constantin, Cristina Mătanie, Livia Petrescu, Alexandra Bolocan, Octavian Andronic, Coralia Bleotu, Mihaela Magdalena Mitache, Sorin Tudorache, Corneliu Ovidiu Vrancianu
Cancers.2023; 15(14): 3591. CrossRef - Delivery of an Incidental Appendiceal Mucinous Neoplasm
Madison Bowles, Jessica Y Ng, Hajir Nabi
Cureus.2022;[Epub] CrossRef - Unearthing novel fusions as therapeutic targets in solid tumors using targeted RNA sequencing
Sungbin An, Hyun Hee Koh, Eun Sol Chang, Juyoung Choi, Ji-Young Song, Mi-Sook Lee, Yoon-La Choi
Frontiers in Oncology.2022;[Epub] CrossRef
- Intrasplenic metastasis of appendiceal low-grade mucinous neoplasm – A case report and review of the literature
Original Articles
- Expressions of MIB-1, p53 and CEA in Endocervical Glandular Lesions.
- Mi Jin Kim, Young Gi Lee, Dong Sug Kim
- Korean J Pathol. 2001;35(1):41-47.
- 2,100 View
- 64 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Endocervical glandular lesions include glandular atypia (GA), endocervical glandular dysplasia (EGD), adenocarcinoma in situ (AIS), and invasive adenocarcinoma (IA). The diagnosis of malignant glandular lesions is occasionally difficult to distinguish from benign mimickers, and the morphologic features of EGD remain unsettled.
METHODS
Immunohistochemical stains for MIB-1, p53 and CEA were performed on 81 cases of paraffin-embedded endocervical glandular lesions including 22 IA, 15 AIS, 15 EGD, 13 GA, 8 microglandular hyperplasia (MGH) and 8 tubal metaplasia (TM).
RESULTS
The MIB-1 labelling index of IA was 59.68%, 69.53% for AIS, 26.60% for EGD, 16.03% for benign. p53 overexpression was noted in 4 (18%) cases of IA, 3 (20%) of AIS, but none of EGD and benign lesions. It was Interesting to note that one case of MGH showed p53 staining in low intensity. Diffuse strong cytoplasmic CEA positivity was present in all of IA and AIS, whereas seven (47%) of 15 EGD and 12 (41%) of 29 benign lesions showed focal cytoplasmic CEA positivity. There were significant differences in MIB-1 and CEA immunostainings among the adenocarcinoma, EGD, and benign glandular lesions. Adenocarcinoma was closely related to p53 overexpression, although occurring in a low percentage of the cases.
CONCLUSION
MIB-1 immunostaining can be useful in differentiating among endocervical adenocarcinoma, endocervical glandular dysplasia and benign glandular lesions. p53 overexpression might be helpful in the diagnosis of adenocarcinoma.
- Pathologic Analysis of Gallbladder Cancer by the Stage and Intestinal Metaplasia with the Diagnostic Significance of CEA and p53.
- Hee Jin Chang, Jung Il Suh
- Korean J Pathol. 1997;31(7):599-607.
- 2,077 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - Twenty cases of gallbladder cancers were examined using 5 mm stepwise tissue sections. We analyzed the clinicopathologic findings of the early (stage 1, II) and advanced carcinoma (stage III, IV, V) and those of carcinoma with or without metaplasia in the tumor. We also performed CEA and p53 immunohistochemical staining and compared their findings with those of normal mucosa and preneoplastic lesions. The results were as follow: 1) All of the early carcinomas (n=5) were incidentally diagnosed after the resection for the gallstone. They were compared to advanced carcinoma (n=15) in the absence of the lymphatic or angioinvasion, recurrence, metastasis and death. 2) Metaplastic and non-metaplastic carcinoma did not reveal any difference of the clinicopathologic findings except age distribution. 3) CEA and p53 were positive in preneoplastic and malignant lesions. The extent of staining was related to the degree of the atypia. From the above results, an early detection of gallbladder cancer is very important for the prognosis of the patients. Since preoperative diagnosis is difficult, thorough pathologic examination of routinely resected gallbladder is necessary for the early diagnosis. CEA and p53 immunohistochemical staining may be helpful in the differential diagnosis of non-neoplastic and neoplastic lesion of the gallbladder.
- Immunohistochemical Staining of Ovarian Tumors.
- Young Seak Kim, Yang Seok Chae, In Sun Kim, Seung Yong Paik
- Korean J Pathol. 1991;25(1):11-20.
- 2,823 View
- 58 Download
-
 Abstract
Abstract
 PDF
PDF - Forty-four ovairan tumors were immunohistochemically studied for the presence of broad-spectrum keratin, vimentin, desin, carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), and alpha 1-antitrypsin (AAT) in formalin-fixed, paraffin-embedded tissues. 1) Among the common epithelial tumors, all the serous carcinomas (4) expressed keratin and AAT, and one additionally CEA. Six mucinous carcinomas exhibited keratin-positivity in two. One endometrioid carcinoma coexpressed keratin and vimentin as well as AAT, but one clear cell carcinoma expressed only keratin. Keratin-and CEA-positivity in epithelial cell nests and vimentin-positivity in stromal cells were observed in two Brenner tumors. Two undifferentiated carcinomas showed keratin-positivity in one and focal CEA positivity in the other. 2) In sex cord-stromal tumors, four out of six granulsa cell tumors, all four thecomas and three fibromas expressed vimentin, and two granulosa cell tumors and two thecomas showed AAT-positivity. The others were negative. 3) Among germ cell tumors, four dysgerminomas showed focal vimentin-positive cells in two and diffuse staining for AAT. Seven endodermal sinus tumors expressed AAT in all. Additionally, AFP were positive in two and CEA in three out of them. One embryonal carcinoma expressed CEA, AAT and AFP. 4) In four metastatic carcinomas, three exhibited keratin-and CEA-positivity, whereas one exhibited keartin-and vimentin-positivity. All showed AAT-positivity. 5) There was no positive case for desmin among ovarian tumors.
Case Report
- Parapelvic Renal Cyst (Pericalyceal Lymphangiectasis): A case report.
- Weon Seo Park, Je Geun Chi
- Korean J Pathol. 1994;28(2):210-212.
- 7,874 View
- 93 Download
-
 Abstract
Abstract
 PDF
PDF - Parapelvic renal cyst, also designated as pericalyceal lymphangiectasis, is an unusual lesion that is usually brought to light during surgery for ureteropelvic junction obstruction or recurrent pyelonephritis. Grossly, the renal pelvis is enveloped by a multilocular cystic mass filled with clear fluid. This lesion is confined to the peripelvic tissues and does not extend into the parenchyma, which, however, may show the effects of hydronephrosis or pyelonephritis. A 50-year-old man presented with hydronephrosis. An ultrasonography revealed hydronephro-sis of the left kidney. Intravenous pyelography and DMSA ("Tc-Dimercaptosuccinic acid) scan showed nonfunctioning kidney of the same side. Simple left nephrectomy was done. The renal pelvis was mildly dilated and a cyst was found buldging into the renal pelvis. The content was watery clear and the cyst was not connected to the renal pelvis or calyces. The cyst was round unilocular and lined by attenuated single layer of endothelial cells. The endothelial cells showed no reactivity to factor-VIII related antigen. With these findings, we concluded that this cystic lesion is basically lymphatic cyst and hydronephrosis was caused by the compression of pelvic out-flow of the kidney.
Original Articles
- Diagnostic Significance of the CEA, AgNORs and PCNA in the Gastric Dysplasia and Adenocarcinoma.
- Weon Cheol Han, Hyung Bae Moon
- Korean J Pathol. 1995;29(1):61-67.
- 2,007 View
- 15 Download
-
 Abstract
Abstract
 PDF
PDF - This study aimed to differentiate gastric mucosal lesions such as the inflammatory gastric mucosa, gastric dysplasia and adenocarcinoma, using the CEA(carcinoembryonic antigen), AgNORS(Nucleolar organizer regions) and PCNA(proliferating cell nuclear antigen) stains. The tissue samples were taken from 30 cases of inflammatory gastric mucosa (19 gastritis and 11 regenerative hyperplasia), 28 cases of gastric dysplasia (9 mild dysplasia, 10 moderate dysplasia and 9 severe dysplasia) and 21 cases of gastric adenocarcinoma. The CEA was expressed in 16 of 21 adenocarcinomas(76%), but in neither inflammatory nor dysplastic gastric mucosae. The mean number of AgNORs per nucleus was 1.54 in inflammatory gastric mucosa, 1.80 in gastric dysplasia, and 1.88 in adenocarcinoma. The number of AgNORs was increased in dysplasia and adenocarcinoma compared to the inflammatory gastric mucosa without statistical significance. The percentage of the PCN A positive cells was 35.2% in inflammatory gastric mucosa, 44.1 % in gastric dysplasia, and 69.0% in gastric adenocarcinoma. The positivity of the PCNA was significantly increased in adenocarcinoma compared to the inflammatory gastric mucosa and dysplasia. In conclusion, the frequency of the CEA positive staining was increased in the gastric adenocarcinoma, and so CEA stain will be able to provide an additive method for the differential diagnosis between severe dysplasia and adenocarcinoma of the stomach.
- Immunohistochemical Characteristics of Biliary Tract Carcinoma and Its Precancerous Lesions.
- Jiyoung Kim, Youngnyun Park, Hogeun Kim
- Korean J Pathol. 1998;32(11):985-992.
- 2,331 View
- 10 Download
-
 Abstract
Abstract
- Carcinomas of the biliary tract are known to be more common in East Asia than in Western countries, but their exact histopathological characteristics and tumorigenesis are not well elucidated. To examine the histological and immunohistochemical characteristics of the biliary tract carcinomas according to their anatomical sites and to elucidate their tumorigenesis, we performed histological review and immunohistochemical study in a total of 135 cases of biliary tract carcinomas; 24 intrahepatic bile duct, 34 gallbladder, 51 common bile duct, and 26 periampullary carcinomas. Precancerous lesions were associated with 5 (20.8%) cases of intrahepatic duct carcinomas (dysplasia 5), 7 (20.6%) cases of gallbladder carcinomas (adenoma 5, dysplasia 2), 10 (19.6%) cases of common bile duct carcinomas (adenoma 7, dysplasia 3), and 2(7.7%)cases of periampullary carcinomas (adenoma 2). Immunohistochemically, c-erbB-2 expression in gallbladder carcinoma (21/34, 62%) was significantly higher than that of intrahepatic (8/24, 33%). Ki-67 indices were higher in common bile duct carcinomas (19%) than those of intrahepatic bile duct (14%) or periampullary carcinomas (12%). Overexpression of p53 gene product in the periampullary carcinomas (20/22, 77%) was higher than that of intrahepatic (12/24, 50%) or common bile duct carcinoma (26/51, 51%). In the precancerous lesions the c-erbB-2 expression was present in 29% of the gallbladder, 20% of the intrahepatic, 10% of the common bile duct precancerous lesions and none of the 2 cases of adenomas in the periampullary region. The p53 overexpression in the precancerous lesions was frequent, ranging from 43% to 60%. These results suggest that a mechanism involving p53 gene mutation and c-erbB-2 gene activation is present in the tumorigenesis in a significant number of the biliary tract carcinomas and they may be the early events in the tumorigenesis of the biliary tract carcinomas.
- Immunohistochemical Studies on Localization of Carcinoembryonic Antigen and Epithelial Membrane Antigen in Adenoma and Well-differentiated Adenocarcinoma of the Stomach.
- Hye Soog Kim, Man Ha Huh, Sun Kyung Lee
- Korean J Pathol. 1989;23(1):36-42.
- 1,826 View
- 12 Download
-
 Abstract
Abstract
 PDF
PDF - This study was performed with the purpose of histochemical comparison of CEA and EMA localization between adenoma and well-differentiated adenocarcinoma of the stomach. The specimen was 12 lesions of adenoma and 15 foci of well-differentiated adenocarcinoma of the stomach. The markers in neoplastic tissue and neighbouring mucosa of the tumors were examined in paraffin sections using peroxidase-antiperoxidase method. The data obtained were evaluated statistically. The results were summarized as follows: 1) In 12 lesions of stomach adenoma, the positive reaction to CEA was seen in 3 lesions (20.0%), and to EMA in 10 lesions (83.3%). The positive rate of CEA in adenoma was lower than that of the neighbouring normal mucosa, but the positive rate of EMA was similar between the two. 2) In 15 foci of well-differentiated adenocarcinoma of the stomach, the positive reaction to CEA was seen in 13 foci (86.7%), and to EMA in 12 foci (80.0%). The positive rate of CEA in well-differentiated adenocarcinoma was higher than that of the neighbouring normal mucosa, while the positive rate of EMA was similar to each other. 3) Immunoreactivity to CEA in adenocarcinoma showed good positive correlation with the development of cuticular border of the neoplastic glands, while reactivity to EMA in adenocarcinoma was not related with the development of cuticular border. 4) The positive rate and intensity of CEA reaction in adenocarcinoma were higher than those in adenoma, but the positive rate and reactiveity of EMA were similar to those of adenoma. 5) The positive rate of CEA or EMA in the neighbouring mucosa of adenoma was not different compared with those in the neighbouring mucosa of adenocarcinoma. With the above results, it is concluded that adenoma and adenocarcinoma of the stomach may be different each other, biologically, and further more, it is presumable that adenoma may not be a premalignant lesions. It is considered that examination of CEA immunoreactivity may be helpful in differentiated of adenoma from well-differentiated adenocarcinoma, in most cases.

 E-submission
E-submission

 First
First Prev
Prev



