Previous issues
- Page Path
- HOME > Articles and issues > Previous issues
Reviews
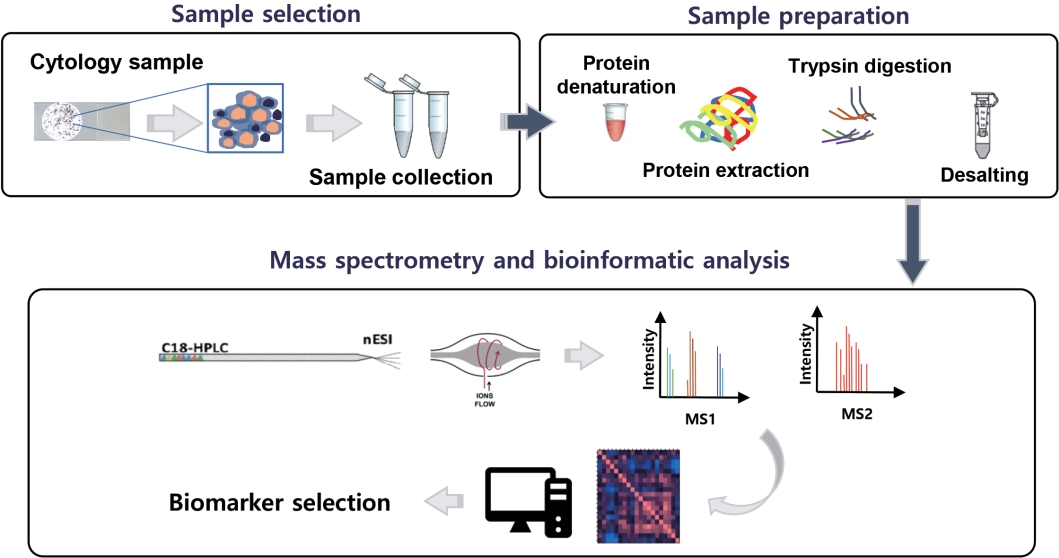
- The application of high-throughput proteomics in cytopathology
- Ilias P. Nikas, Han Suk Ryu
- J Pathol Transl Med. 2022;56(6):309-318. Published online November 9, 2022
- DOI: https://doi.org/10.4132/jptm.2022.08.30
- 7,189 View
- 154 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - High-throughput genomics and transcriptomics are often applied in routine pathology practice to facilitate cancer diagnosis, assess prognosis, and predict response to therapy. However, the proteins rather than nucleic acids are the functional molecules defining the cellular phenotype in health and disease, whereas genomic profiling cannot evaluate processes such as the RNA splicing or posttranslational modifications and gene expression does not necessarily correlate with protein expression. Proteomic applications have recently advanced, overcoming the issue of low depth, inconsistency, and suboptimal accuracy, also enabling the use of minimal patient-derived specimens. This review aims to present the recent evidence regarding the use of high-throughput proteomics in both exfoliative and fine-needle aspiration cytology. Most studies used mass spectrometry, as this is associated with high depth, sensitivity, and specificity, and aimed to complement the traditional cytomorphologic diagnosis, in addition to identify novel cancer biomarkers. Examples of diagnostic dilemmas subjected to proteomic analysis included the evaluation of indeterminate thyroid nodules or prediction of lymph node metastasis from thyroid cancer, also the differentiation between benign and malignant serous effusions, pancreatic cancer from autoimmune pancreatitis, non-neoplastic from malignant biliary strictures, and benign from malignant salivary gland tumors. A few cancer biomarkers—related to diverse cancers involving the breast, thyroid, bladder, lung, serous cavities, salivary glands, and bone marrow—were also discovered. Notably, residual liquid-based cytology samples were suitable for satisfactory and reproducible proteomic analysis. Proteomics could become another routine pathology platform in the near future, potentially by using validated multi-omics protocols.
-
Citations
Citations to this article as recorded by- Mass spectrometry-based proteomics of FFPE tissues: progress, limitations, and clinical translation barriers
Sara Abdulmohsen AlHammadi, Lamar Nabil Nagshabandi, Huzaifa Muhammad, Hatouf H. Sukkarieh, Ahmad Aljada
Clinical Proteomics.2025;[Epub] CrossRef - Identification of NIFTP-Specific mRNA Markers for Reliable Molecular Diagnosis of Thyroid Tumors
So-Yeon Lee, Jong-Lyul Park, Kwangsoon Kim, Ja Seong Bae, Jae-Yoon Kim, Seon-Young Kim, Chan Kwon Jung
Endocrine Pathology.2023; 34(3): 311. CrossRef
- Mass spectrometry-based proteomics of FFPE tissues: progress, limitations, and clinical translation barriers
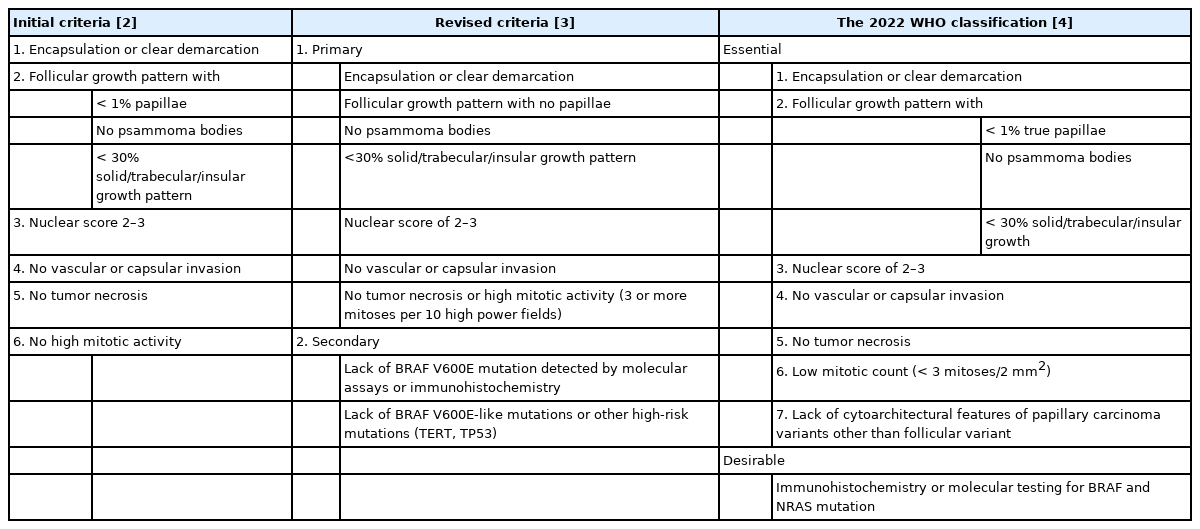
- Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: its updated diagnostic criteria, preoperative cytologic diagnoses and impact on the risk of malignancy
- Hee Young Na, So Yeon Park
- J Pathol Transl Med. 2022;56(6):319-325. Published online November 9, 2022
- DOI: https://doi.org/10.4132/jptm.2022.09.29
- 9,314 View
- 314 Download
- 9 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF - Due to the extremely indolent behavior, a subset of noninvasive encapsulated follicular variant papillary thyroid carcinomas has been classified as “noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP)” since 2016 and is no longer considered carcinoma. Since the introduction of this new terminology, changes and refinements have been made in diagnostic criteria. Initially, the incidence of NIFTP was estimated substantial. However, the reported incidence of NIFTP varies greatly among studies and regions, with higher incidence in North American and European countries than in Asian countries. Thus, the changes in the risk of malignancy (ROM) in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) differ inevitably among regions. Because more conservative surgery is recommended for NIFTPs, distinguishing NIFTPs from papillary thyroid carcinomas in preoperative fine-needle aspiration cytology became one of the major concerns. This review will provide comprehensive overview of updates on diagnostic criteria, actual incidence and preoperative cytologic diagnoses of NIFTP, and its impact on the ROM in TBSRTC.
-
Citations
Citations to this article as recorded by- Diagnosis of invasive encapsulated follicular variant papillary thyroid carcinoma by protein-based machine learning
Truong Phan-Xuan Nguyen, Minh-Khang Le, Sittiruk Roytrakul, Shanop Shuangshoti, Nakarin Kitkumthorn, Somboon Keelawat
Journal of Pathology and Translational Medicine.2025; 59(1): 39. CrossRef - Papillae, psammoma bodies, and/or many nuclear pseudoinclusions are helpful criteria but should not be required for a definitive cytologic diagnosis of papillary thyroid carcinoma: An institutional experience of 207 cases with surgical follow up
Tarik M. Elsheikh, Matthew Thomas, Jennifer Brainard, Jessica Di Marco, Erica Manosky, Bridgette Springer, Dawn Underwood, Deborah J. Chute
Cancer Cytopathology.2024; 132(6): 348. CrossRef - ThyroSeq overview on indeterminate thyroid nodules: An institutional experience
Sam Sirotnikov, Christopher C. Griffith, Daniel Lubin, Chao Zhang, Nabil F. Saba, Dehong Li, Amanda Kornfield, Amy Chen, Qiuying Shi
Diagnostic Cytopathology.2024; 52(7): 353. CrossRef - Oncocytic Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features: A Case Report
Kaveripakam Ajay Joseph, Sana Ahuja, Sufian Zaheer
Indian Journal of Surgical Oncology.2024; 15(S4): 606. CrossRef - Cytologic hallmarks and differential diagnosis of papillary thyroid carcinoma subtypes
Agnes Stephanie Harahap, Chan Kwon Jung
Journal of Pathology and Translational Medicine.2024; 58(6): 265. CrossRef - Preoperative evaluation of thyroid nodules – Diagnosis and management strategies
Tapoi Dana Antonia, Lambrescu Ioana Maria, Gheorghisan-Galateanu Ancuta-Augustina
Pathology - Research and Practice.2023; 246: 154516. CrossRef - Reevaluating diagnostic categories and associated malignancy risks in thyroid core needle biopsy
Chan Kwon Jung
Journal of Pathology and Translational Medicine.2023; 57(4): 208. CrossRef - Strategies for Treatment of Thyroid Cancer
Deepika Yadav, Pramod Kumar Sharma, Rishabha Malviya, Prem Shankar Mishra
Current Drug Targets.2023; 24(5): 406. CrossRef - Identification of NIFTP-Specific mRNA Markers for Reliable Molecular Diagnosis of Thyroid Tumors
So-Yeon Lee, Jong-Lyul Park, Kwangsoon Kim, Ja Seong Bae, Jae-Yoon Kim, Seon-Young Kim, Chan Kwon Jung
Endocrine Pathology.2023; 34(3): 311. CrossRef
- Diagnosis of invasive encapsulated follicular variant papillary thyroid carcinoma by protein-based machine learning
- Biomarker testing of cytology specimens in personalized medicine for lung cancer patients
- Hyojin Kim, Jin-Haeng Chung
- J Pathol Transl Med. 2022;56(6):326-333. Published online November 9, 2022
- DOI: https://doi.org/10.4132/jptm.2022.10.17
- 6,490 View
- 176 Download
- 8 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - Every patient with advanced non–small cell lung cancer (NSCLC) should be tested for targetable driver mutations and gene arrangements that may open avenues for targeted therapy. As most patients with NSCLC in the advanced stage of the disease are not candidates for surgery, these tests have to be performed on small biopsies or cytology samples. A growing number of other genetic changes with targetable mutations may be treatable in the near future. To identify patients who might benefit from novel targeted therapy, relevant markers should be tested in an appropriate context. In addition, immunotherapy of lung cancer is guided by the status of programmed death-ligand 1 expression in tumor cells. The variety and versatility of cytological specimen preparations offer significant advantages for molecular testing; however, they frequently remain underused. Therefore, evaluating the utility and adequacy of cytologic specimens is important, not only from a lung cancer diagnosis, but also for the large number of ancillary studies that are necessary to provide appropriate clinical management. A large proportion of lung cancers is diagnosed by aspiration or exfoliative cytology specimens; thus, optimizing strategies to triage and best use the tissue for diagnosis and biomarker studies forms a critical component of lung cancer management. In this review, we discuss the opportunities and challenges of using cytologic specimens for biomarker testing of lung cancer and the role of cytopathology in the molecular era.
-
Citations
Citations to this article as recorded by- Proposal of real-world solutions for the implementation of predictive biomarker testing in patients with operable non-small cell lung cancer
Paul Hofman, Petros Christopoulos, Nicky D’Haene, John Gosney, Nicola Normanno, Ed Schuuring, Ming-Sound Tsao, Christine Quinn, Jayne Russell, Katherine E Keating, Fernando López-Ríos
Lung Cancer.2025; 201: 108107. CrossRef - Validation of ancillary procedures on formalin liquid fixed aspiration cytologic samples: from minimum to maximum
Orsolya Rideg, Tímea Dergez, Arnold Tóth, Tamás Tornóczky, Gábor Pavlovics, Endre Kálmán
American Journal of Clinical Pathology.2025;[Epub] CrossRef - Molecular testing of cytology specimens: Issues in specimen adequacy and clinical utility
Ghulam Ghous, Komal Ijaz, Magda Esebua, Lester J. Layfield
Diagnostic Cytopathology.2024; 52(2): 123. CrossRef - The updated College of American Pathologists principles of analytic validation of immunohistochemical assays: A step forward for cytopathology
Sinchita Roy‐Chowdhuri
Cancer Cytopathology.2024; 132(9): 547. CrossRef - Best-Practice Biomarker Testing of Oesophago-Gastric Cancer in the UK: Expert Consensus Recommendations Developed Using a Modified Delphi
N.P. West, W. Mansoor, P. Taniere, E. Smyth, M. Rodriguez-Justo, A. Oniscu, P. Carter
Clinical Oncology.2024; 36(11): 701. CrossRef - Next step of molecular pathology: next-generation sequencing in cytology
Ricella Souza da Silva, Fernando Schmitt
Journal of Pathology and Translational Medicine.2024; 58(6): 291. CrossRef
- Proposal of real-world solutions for the implementation of predictive biomarker testing in patients with operable non-small cell lung cancer
Original Articles
- Usefulness of BRAF VE1 immunohistochemistry in non–small cell lung cancers: a multi-institutional study by 15 pathologists in Korea
- Sunhee Chang, Yoon-La Choi, Hyo Sup Shim, Geon Kook Lee, Seung Yeon Ha
- J Pathol Transl Med. 2022;56(6):334-341. Published online October 27, 2022
- DOI: https://doi.org/10.4132/jptm.2022.08.22
- 7,429 View
- 163 Download
- 11 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Next-generation sequencing (NGS) is an approved test to select patients for BRAF V600E targeted therapy in Korea. However, the high cost, long turnaround times, and the need for sophisticated equipment and skilled personnel limit the use of NGS in daily practice. Immunohistochemistry (IHC) is a rapid and relatively inexpensive assay available in most laboratories. Therefore, in this study, we evaluate the usefulness of BRAF VE1 IHC in terms of predictive value and interobserver agreement in non–small cell lung cancers (NSCLCs).
Methods
A total of 30 cases with known BRAF mutation status were selected, including 20 cases of lung adenocarcinomas, six cases of colorectal adenocarcinomas, and four cases of papillary thyroid carcinomas. IHC for BRAF V600E was carried out using the VE1 antibody. Fifteen pathologists independently scored both the staining intensity and the percentage of tumor cell staining on whole slide images.
Results
In the lung adenocarcinoma subset, interobserver agreement for the percentage of tumor cell staining and staining intensity was good (percentage of tumor cell staining, intraclass correlation coefficient = 0.869; staining intensity, kappa = 0.849). The interobserver agreement for the interpretation using the cutoff of 40% was almost perfect in the entire study group and the lung adenocarcinoma subset (kappa = 0.815). Sensitivity, specificity, positive predictive value, and negative predictive value of BRAF VE1 IHC were 80.0%, 90.0%, 88.9%, and 81.8%, respectively.
Conclusions
BRAF VE1 IHC could be a screening test for the detection of BRAF V600E mutation in NSCLC. However, further studies are needed to optimize the protocol and to establish and validate interpretation criteria for BRAF VE1 IHC. -
Citations
Citations to this article as recorded by- Dedifferentiated Leiomyosarcoma of the Uterine Corpus with Heterologous Component: Clinicopathological Analysis of Five Consecutive Cases from a Single Institution and Comprehensive Literature Review
Suyeon Kim, Hyunsik Bae, Hyun-Soo Kim
Diagnostics.2024; 14(2): 160. CrossRef - Differentiating BRAF V600E- and RAS-like alterations in encapsulated follicular patterned tumors through histologic features: a validation study
Chankyung Kim, Shipra Agarwal, Andrey Bychkov, Jen-Fan Hang, Agnes Stephanie Harahap, Mitsuyoshi Hirokawa, Kennichi Kakudo, Somboon Keelawat, Chih-Yi Liu, Zhiyan Liu, Truong Phan-Xuan Nguyen, Chanchal Rana, Huy Gia Vuong, Yun Zhu, Chan Kwon Jung
Virchows Archiv.2024; 484(4): 645. CrossRef - BRAF V600E Mutation of Non-Small Cell Lung Cancer in Korean Patients
Hyo Yeong Ahn, Chang Hun Lee, Min Ki Lee, Jung Seop Eom, Yeon Joo Jeong, Yeong Dae Kim, Jeong Su Cho, Jonggeun Lee, So Jeong Lee, Dong Hoon Shin, Ahrong Kim
Medicina.2023; 59(6): 1085. CrossRef - Reevaluating diagnostic categories and associated malignancy risks in thyroid core needle biopsy
Chan Kwon Jung
Journal of Pathology and Translational Medicine.2023; 57(4): 208. CrossRef
- Dedifferentiated Leiomyosarcoma of the Uterine Corpus with Heterologous Component: Clinicopathological Analysis of Five Consecutive Cases from a Single Institution and Comprehensive Literature Review
- A clinicopathologic and immunohistochemical study of primary and secondary breast angiosarcoma
- Evi Abada, Hyejeong Jang, Seongho Kim, Rouba Ali-Fehmi, Sudeshna Bandyopadhyay
- J Pathol Transl Med. 2022;56(6):342-353. Published online October 27, 2022
- DOI: https://doi.org/10.4132/jptm.2022.08.31
- 6,174 View
- 150 Download
- 7 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
We aimed to study the clinicopathologic and immunohistochemical (IHC) (CD117, c-Myc, and p53) characteristics, and overall survival of primary and secondary breast angiosarcoma (BAS).
Methods
This was a retrospective study of BAS cases diagnosed between 1997 and 2020 at our institution. Hematoxylin and eosin-stained slides were reviewed for tumor morphology, margin status, and lymph node metastasis. CD117, p53, D2-40, CD31, and c-Myc IHC stains were performed on 11 viable tissue blocks. Additional clinical information was obtained from the electronic medical records.
Results
Seventeen patients with BAS were identified. Of these, five (29%) were primary and 12 (71%) were secondary BAS, respectively. The median age at diagnosis for primary BAS was 36 years. The median age at diagnosis for secondary BAS was 67 years. The median time to secondary BAS development following radiotherapy was 6.5 years (range, 2 to 12 years). There was no significant difference between primary and secondary BAS in several histopathologic parameters examined, including histologic grade, necrosis, mitotic count, lymph node metastasis, and positive tumor margins. There was also no difference in CD117, p53, D2-40, CD31, and c-Myc expression by IHC between primary and secondary BAS. During a median followup of 21 months, primary BAS had two (40%) reported deaths and secondary BAS had three (25%) reported deaths. However, this difference in survival between both groups was not statistically significant (hazard ratio, 0.51; 95% confidence interval, 0.09 to 3.28; p = .450).
Conclusions
BAS is a rare and aggressive disease. No histologic, IHC (CD117, c-Myc, and p53), or survival differences were identified between primary and secondary BAS in this study. -
Citations
Citations to this article as recorded by- Angiosarcoma: a systematic review of biomarkers in diagnosis, prognosis, and therapeutic strategies
Huyen Thuc Tran Luong, Sofie Vercammen, Ario de Marco, Hilde de Rooster, Antonio Cosma
Frontiers in Oncology.2025;[Epub] CrossRef - Etiology, pathogenesis, and management of angiosarcoma associated with implants and foreign body: Clinical cases and research updates
Ramy Samargandi
Medicine.2024; 103(18): e37932. CrossRef - Ovarian angiosarcoma: A systematic review of literature and survival analysis
Shafi Rehman, Arya Harikrishna, Amisha Silwal, B.R. Sumie, Safdar Mohamed, Nisha Kolhe, Meghana Maddi, Linh Huynh, Jesus Gutierrez, Yoshita Rao Annepu, Ameer Mustafa Farrukh
Annals of Diagnostic Pathology.2024; 73: 152331. CrossRef - Neoadjuvant chemotherapy for radiation associated angiosarcoma (RAAS) of the breast: A retrospective single center study
Stijn J.C. van der Burg, Sophie J.M. Reijers, Anke Kuijpers, Lotte Heimans, Astrid N. Scholten, Rick L.M. Haas, Hester van Boven, Willemijn M. Kolff, Marie-Jeanne T.F.D. Vrancken Peeters, Martijn Kerst, Beatrijs A. Seinstra, Neeltje Steeghs, Winette T.A.
The Breast.2024; 78: 103825. CrossRef - Lymph node involvement in secondary breast angiosarcoma – a case presentation
Adriana Irina Ciuvică, Tiberiu Augustin Georgescu , Andrei Dennis Voichiţoiu , Angela Arsene , Luchian Marinescu , George Ionuţ Bucur , Livia Iordache , Nahedd Saba
Romanian Journal of Morphology and Embryology.2024; 65(3): 523. CrossRef - Primary ovarian angiosarcoma: Two case reports and review of literature
Ying Zhou, Yi-Wen Sun, Xiao-Yang Liu, Dan-Hua Shen
World Journal of Clinical Cases.2023; 11(21): 5122. CrossRef
- Angiosarcoma: a systematic review of biomarkers in diagnosis, prognosis, and therapeutic strategies
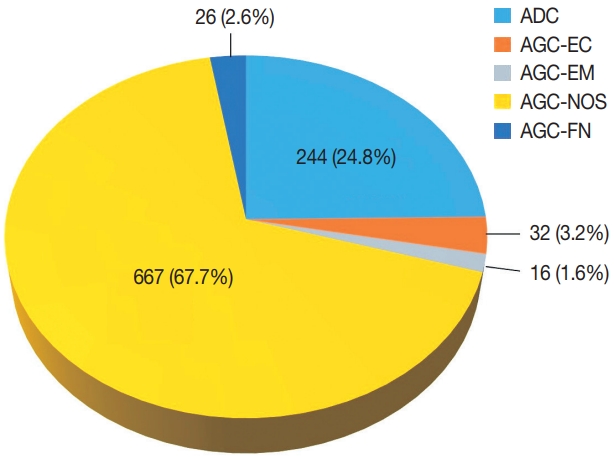
- Diagnostic distribution and pitfalls of glandular abnormalities in cervical cytology: a 25-year single-center study
- Jung-A Sung, Ilias P. Nikas, Haeryoung Kim, Han Suk Ryu, Cheol Lee
- J Pathol Transl Med. 2022;56(6):354-360. Published online November 9, 2022
- DOI: https://doi.org/10.4132/jptm.2022.09.05
- 8,298 View
- 154 Download
- 5 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Detection of glandular abnormalities in Papanicolaou (Pap) tests is challenging. This study aimed to review our institute’s experience interpreting such abnormalities, assess cytohistologic concordance, and identify cytomorphologic features associated with malignancy in follow-up histology.
Methods
Patients with cytologically-detected glandular lesions identified in our pathology records from 1995 to 2020 were included in this study.
Results
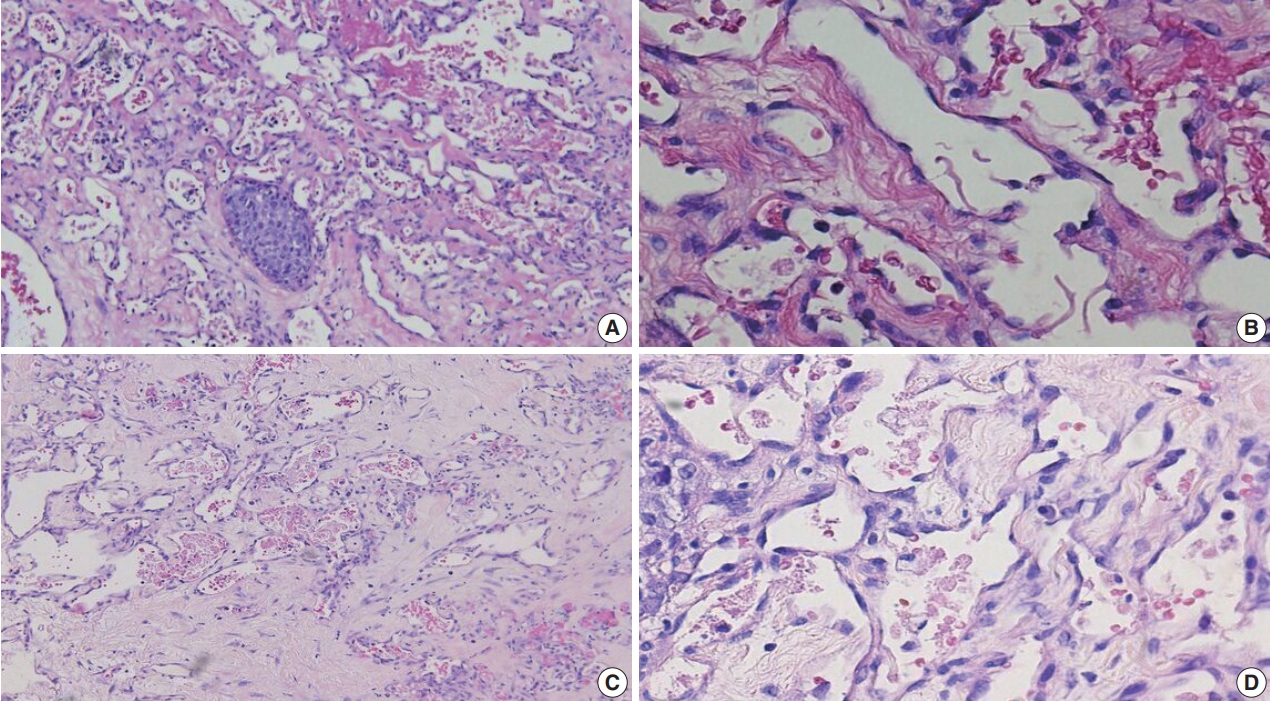
Of the 683,197 Pap tests performed, 985 (0.144%) exhibited glandular abnormalities, 657 of which had tissue follow-up available. One hundred eighty-eight cases were cytologically interpreted as adenocarcinoma and histologically diagnosed as malignant tumors of various origins. There were 213 cases reported as atypical glandular cells (AGC) and nine cases as adenocarcinoma in cytology, yet they were found to be benign in follow-up histology. In addition, 48 cases diagnosed with AGC and six with adenocarcinoma cytology were found to have cervical squamous lesions in follow-up histology, including four squamous cell carcinomas. Among the cytomorphological features examined, nuclear membrane irregularity, three-dimensional clusters, single-cell pattern, and presence of mitoses were associated with malignant histology in follow-up.
Conclusions
This study showed our institute’s experience detecting glandular abnormalities in cervical cytology over a 25-year period, revealing the difficulty of this task. Nonetheless, the present study indicates that several cytological findings such as membrane irregularity, three-dimensional clusters, single-cell pattern, and evidence of proliferation could help distinguishing malignancy from a benign lesion. -
Citations
Citations to this article as recorded by- “Atypical Glandular Cells” on Cervical Cytology: Correlation Between Glandular Cell Component Volume and Histological Follow‐Up
Havva Gokce Terzioglu, Alessa Aragao, Julieta E. Barroeta
Diagnostic Cytopathology.2026; 54(2): 71. CrossRef - Expertise in Gynecological Pathology Impacts Diagnosis of Atypical Glandular Cell Category in Cervical Cytology
Havva Gökce Terzioglu, Alessa Aragao, Julieta E. Barroeta
Journal of Lower Genital Tract Disease.2025; 29(4): 297. CrossRef - Analysis of atypical glandular cells in ThinPrep Pap smear and follow-up histopathology
Tengfei Wang, Yinan Hua, Lina Liu, Bing Leng
Baylor University Medical Center Proceedings.2024; 37(3): 403. CrossRef
- “Atypical Glandular Cells” on Cervical Cytology: Correlation Between Glandular Cell Component Volume and Histological Follow‐Up
- Current status of cytopathology practice in Korea: impact of the coronavirus pandemic on cytopathology practice
- Soon Auck Hong, Haeyoen Jung, Sung Sun Kim, Min-Sun Jin, Jung-Soo Pyo, Ji Yun Jeong, Younghee Choi, Gyungyub Gong, Yosep Chong
- J Pathol Transl Med. 2022;56(6):361-369. Published online October 27, 2022
- DOI: https://doi.org/10.4132/jptm.2022.09.21
- 4,840 View
- 105 Download
- 7 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
The Continuous Quality Improvement program for cytopathology in 2020 was completed during the coronavirus pandemic. In this study, we report the result of the quality improvement program.
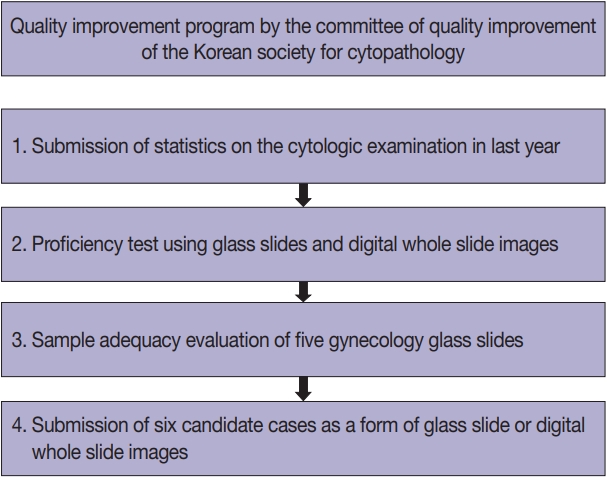
Methods
Data related to cytopathology practice from each institute were collected and processed at the web-based portal. The proficiency test was conducted using glass slides and whole-slide images (WSIs). Evaluation of the adequacy of gynecology (GYN) slides from each institution and submission of case glass slides and WSIs for the next quality improvement program were performed.
Results
A total of 214 institutions participated in the annual cytopathology survey in 2020. The number of entire cytopathology specimens was 8,220,650, a reduction of 19.0% from the 10,111,755 specimens evaluated in 2019. Notably, the number of respiratory cytopathology specimens, including sputum and bronchial washing/ brushing significantly decreased by 86.9% from 2019, which could be attributed to the global pandemic of coronavirus disease. The ratio of cases with atypical squamous cells to squamous intraepithelial lesions was 4.10. All participating institutions passed the proficiency test and the evaluation of adequacy of GYN slides.
Conclusions
Through the Continuous Quality Improvement program, the effect of coronavirus disease 2019 pandemic, manifesting with a reduction in the number of cytologic examinations, especially in respiratory-related specimen has been identified. The Continuous Quality Improvement Program of the Korean Society for Cytopathology can serve as the gold standard to evaluate the current status of cytopathology practice in Korea. -
Citations
Citations to this article as recorded by- A Study on the Workload of Cytotechnologists: Focus on Commercial Laboratories
Eun-Suk PARK
Korean Journal of Clinical Laboratory Science.2025; 57(2): 228. CrossRef - Integration of Digital Cytology in Quality Assurance Programs for Cytopathology
Yosep Chong, Maria Jesús Fernández Aceñero, Zaibo Li, Andrey Bychkov
Acta Cytologica.2025; : 1. CrossRef - National quality assurance program using digital cytopathology: a 5-year digital transformation experience by the Korean Society for Cytopathology
Yosep Chong, Hyeong Ju Kwon, Soon Auck Hong, Sung Soon Kim, Bo-Sung Kim, Younghee Choi, Yoon Jung Choi, Jung-Soo Pyo, Ji Yun Jeong, Soo Jin Jung, Hoon Kyu Oh, Seung-Sook Lee
Journal of Pathology and Translational Medicine.2025; 59(5): 320. CrossRef - Commercially Available Artificial Intelligence Solutions for Gynaecologic Cytology Screening and Their Integration Into Clinical Workflow
Yosep Chong, Andrey Bychkov
Cytopathology.2025;[Epub] CrossRef - Practice of Cytopathology in Korea: A 40‐Year Evolution Through Standardization, Digital Transformation, and Global Partnership
Yosep Chong, Ran Hong, Hyeong Ju Kwon, Haeryoung Kim, Lucia Kim, Soon Jae Kim, Yoon Jung Choi
Diagnostic Cytopathology.2025;[Epub] CrossRef - A stepwise approach to fine needle aspiration cytology of lymph nodes
Yosep Chong, Gyeongsin Park, Hee Jeong Cha, Hyun-Jung Kim, Chang Suk Kang, Jamshid Abdul-Ghafar, Seung-Sook Lee
Journal of Pathology and Translational Medicine.2023; 57(4): 196. CrossRef - Diagnostic proficiency test using digital cytopathology and comparative assessment of whole slide images of cytologic samples for quality assurance program in Korea
Yosep Chong, Soon Auck Hong, Hoon Kyu Oh, Soo Jin Jung, Bo-Sung Kim, Ji Yun Jeong, Ho-Chang Lee, Gyungyub Gong
Journal of Pathology and Translational Medicine.2023; 57(5): 251. CrossRef
- A Study on the Workload of Cytotechnologists: Focus on Commercial Laboratories
- Development of quality assurance program for digital pathology by the Korean Society of Pathologists
- Yosep Chong, Jeong Mo Bae, Dong Wook Kang, Gwangil Kim, Hye Seung Han
- J Pathol Transl Med. 2022;56(6):370-382. Published online November 15, 2022
- DOI: https://doi.org/10.4132/jptm.2022.09.30
- 6,615 View
- 162 Download
- 5 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
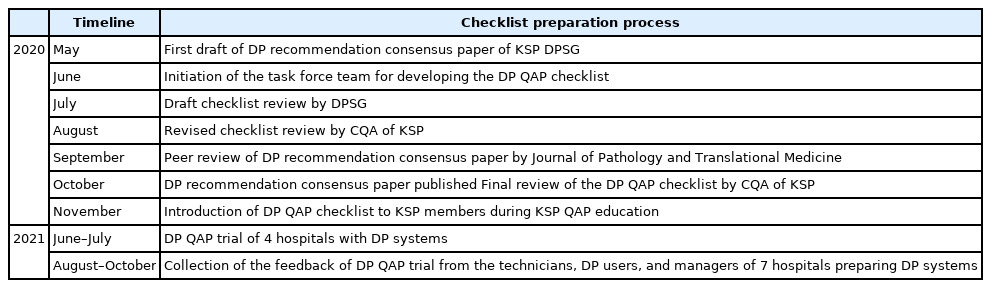
Digital pathology (DP) using whole slide imaging is a recently emerging game changer technology that can fundamentally change the way of working in pathology. The Digital Pathology Study Group (DPSG) of the Korean Society of Pathologists (KSP) published a consensus report on the recommendations for pathologic practice using DP. Accordingly, the need for the development and implementation of a quality assurance program (QAP) for DP has been raised.
Methods
To provide a standard baseline reference for internal and external QAP for DP, the members of the Committee of Quality Assurance of the KSP developed a checklist for the Redbook and a QAP trial for DP based on the prior DPSG consensus report. Four leading institutes participated in the QAP trial in the first year, and we gathered feedback from these institutes afterwards.
Results
The newly developed checklists of QAP for DP contain 39 items (216 score): eight items for quality control of DP systems; three for DP personnel; nine for hardware and software requirements for DP systems; 15 for validation, operation, and management of DP systems; and four for data security and personal information protection. Most participants in the QAP trial replied that continuous education on unfamiliar terminology and more practical experience is demanding.
Conclusions
The QAP for DP is essential for the safe implementation of DP in pathologic practice. Each laboratory should prepare an institutional QAP according to this checklist, and consecutive revision of the checklist with feedback from the QAP trial for DP needs to follow. -
Citations
Citations to this article as recorded by- An equivalency and efficiency study for one year digital pathology for clinical routine diagnostics in an accredited tertiary academic center
Viola Iwuajoku, Kübra Ekici, Anette Haas, Mohammed Zaid Khan, Azar Kazemi, Atsuko Kasajima, Claire Delbridge, Alexander Muckenhuber, Elisa Schmoeckel, Fabian Stögbauer, Christine Bollwein, Kristina Schwamborn, Katja Steiger, Carolin Mogler, Peter J. Schüf
Virchows Archiv.2025; 487(1): 3. CrossRef - Quality Assurance of the Whole Slide Image Evaluation in Digital Pathology: State of the Art and Development Results
Miklós Vincze, Béla Molnár, Miklós Kozlovszky
Electronics.2025; 14(10): 1943. CrossRef - Integration of Digital Cytology in Quality Assurance Programs for Cytopathology
Yosep Chong, Maria Jesús Fernández Aceñero, Zaibo Li, Andrey Bychkov
Acta Cytologica.2025; : 1. CrossRef - Diagnostic proficiency test using digital cytopathology and comparative assessment of whole slide images of cytologic samples for quality assurance program in Korea
Yosep Chong, Soon Auck Hong, Hoon Kyu Oh, Soo Jin Jung, Bo-Sung Kim, Ji Yun Jeong, Ho-Chang Lee, Gyungyub Gong
Journal of Pathology and Translational Medicine.2023; 57(5): 251. CrossRef
- An equivalency and efficiency study for one year digital pathology for clinical routine diagnostics in an accredited tertiary academic center
Newsletters
- What’s new in kidney tumor pathology 2022: WHO 5th edition updates
- Maria Tretiakova
- J Pathol Transl Med. 2022;56(6):383-384. Published online September 8, 2022
- DOI: https://doi.org/10.4132/jptm.2022.08.16
- 9,246 View
- 758 Download
- 8 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - The 5th edition WHO Classification of Urinary and Male Genital Tumours (2022) introduces significant changes relevant to daily practice, especially in the completely restructured renal cell tumor chapters. Herein we highlight the most important diagnostic updates of known kidney tumor types, new and molecularly defined entities and emerging/provisional entities.
-
Citations
Citations to this article as recorded by- Thyroid-like Follicular Renal Cell Carcinoma: An Emerging Entity
Sarita Asotra, Himani Thakur, Kailash Chander Barwal
Archives of Medicine and Health Sciences.2025; 13(2): 283. CrossRef - Convolutional neural networks for the differentiation between benign and malignant renal tumors with a multicenter international computed tomography dataset
Michail E. Klontzas, Georgios Kalarakis, Emmanouil Koltsakis, Thomas Papathomas, Apostolos H. Karantanas, Antonios Tzortzakakis
Insights into Imaging.2024;[Epub] CrossRef - PI3K/AKT/mTOR Dysregulation and Reprogramming Metabolic Pathways in Renal Cancer: Crosstalk with the VHL/HIF Axis
Silviu Constantin Badoiu, Maria Greabu, Daniela Miricescu, Iulia-Ioana Stanescu-Spinu, Radu Ilinca, Daniela Gabriela Balan, Andra-Elena Balcangiu-Stroescu, Doina-Andrada Mihai, Ileana Adela Vacaroiu, Constantin Stefani, Viorel Jinga
International Journal of Molecular Sciences.2023; 24(9): 8391. CrossRef - Machine Learning Integrating 99mTc Sestamibi SPECT/CT and Radiomics Data Achieves Optimal Characterization of Renal Oncocytic Tumors
Michail E. Klontzas, Emmanouil Koltsakis, Georgios Kalarakis, Kiril Trpkov, Thomas Papathomas, Apostolos H. Karantanas, Antonios Tzortzakakis
Cancers.2023; 15(14): 3553. CrossRef - Serum Oxidative and Nitrosative Stress Markers in Clear Cell Renal Cell Carcinoma
Sabina Galiniak, Marek Biesiadecki, Mateusz Mołoń, Patrycja Olech, Krzysztof Balawender
Cancers.2023; 15(15): 3995. CrossRef - Redefining Renal Cell Carcinoma: A Molecular Perspective on Classification and Clinical Implications
Arjun Athreya Raghavan, Ian W Gibson, Robert Wightman, Piotr Czaykowski, Jeffrey Graham
European Medical Journal.2023; : 116. CrossRef
- Thyroid-like Follicular Renal Cell Carcinoma: An Emerging Entity
- What’s new in soft tissue and bone pathology 2022–updates from the WHO classification 5th edition
- Erica Y. Kao, Jose G. Mantilla
- J Pathol Transl Med. 2022;56(6):385-386. Published online November 15, 2022
- DOI: https://doi.org/10.4132/jptm.2022.10.18
- 11,929 View
- 627 Download
- 6 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - The 2020 release of the WHO Classification of Soft Tissue and Bone Tumors, 5th edition, contains several changes driven by new knowledge in the field. These include reclassification of some entities, refinement of risk classification systems, and the inclusion of novel disease processes, many of which are driven by recurrent gene fusions. The most notable changes are described here.
-
Citations
Citations to this article as recorded by- Chondroblastic Osteosarcoma in an Adolescent: Are Conventional Biopsy Techniques Just Scratching the Surface?
Neeraj Kumar Dhiman, Arjun Mahajan, Trupti Jain, Ajit Kumar Vishwakarma, Rahul Agrawal
Indian Journal of Otolaryngology and Head & Neck Surgery.2025; 77(2): 1023. CrossRef - Radiomics and Deep Learning Model for Benign and Malignant Soft Tissue Tumors Differentiation of Extremities and Trunk
Miaomiao Yang, Xiuming Zhang, Jiyang Jin
Academic Radiology.2025; 32(5): 2838. CrossRef - Conference on challenges in sarcoma (CCS) 2024: Expert opinions on non-evidence-based management aspects
Silvia Hofer, Chantal Pauli, Beata Bode, Sylvie Bonvalot, Christina Fotopoulou, Hans Gelderblom, Rick L Haas, Jendrik Hardes, Peter Hohenberger, Jens Jakob, Wolfgang G. Kunz, Andreas Leithner, Bernadette Liegl-Atzwanger, Lars Lindner, Aisha Miah, Peter Re
European Journal of Cancer.2025; 220: 115368. CrossRef - European standard clinical practice recommendations for children and adolescents with Rhabdomyosarcoma a joint EpSSG, CWS and ERN PaedCan project
Johannes H.M. Merks, Eva Brack, Martin Ebinger, Véronique Minard-Colin, Anne-Sophie Defachelles, Raquel Hladun, Timothy Rogers, Jörg Fuchs, Jan Godzinski, Sheila Terwisscha van Scheltinga, Gabriela Guillén Burrieza, Henry Mandeville, Beate Timmermann, Raq
EJC Paediatric Oncology.2025; 5: 100229. CrossRef - Management of a rapidly enlarging supraclavicular mass of unknown aetiology
Mateo Cukman, Karla Luzaic, Kristina Krstanovic, Sinisa Stevanovic
BMJ Case Reports.2024; 17(2): e255774. CrossRef - Pathogenetic and molecular classifications of soft tissue and bone tumors: A 2024 update
Andrei Ionut Patrichi, Simona Gurzu
Pathology - Research and Practice.2024; 260: 155406. CrossRef
- Chondroblastic Osteosarcoma in an Adolescent: Are Conventional Biopsy Techniques Just Scratching the Surface?

 E-submission
E-submission



 First
First Prev
Prev



