Diagnostic Difficulties in Fine Needle Aspiration of Benign Salivary Glandular Lesions
Article information
Abstract
Background
The diagnostic accuracy of fine needle aspiration cytology (FNAC) of salivary lesions is relatively high, but cytologic interpretation might be confusing if the sample is lacking typical cytologic features.
Methods
There were 77 cases of benign salivary lesions, consisting of pleomorphic adenoma (PA) in 61 cases, Warthin's tumor (WT) in 12 cases, and other benign lesions in 4 cases. The causes of the discrepancies between the FNAC and the histologic diagnoses were evaluated.
Results
Major discrepancies were noted in 4 of the 61 PA cases, and in 1 of 12 WT cases. The causes of the major discrepancies were a mislabeled site in 1 PA and 1 WT case, and an interpretation error in 3 PA cases. Minor discrepancies were more common in the WT cases (7 of 12 cases) than in the PA cases (11 of 61 cases). The causes of the minor discrepancies were a mislabeled site in 1 PA and 1 WT case, an inadequate sample in 7 PA and 2 WT cases, a lack of typical cytomorphology in 2 PA and 2 WT cases, and an interpretation error in 1 PA and 2 WT cases.
Conclusions
To increase the diagnostic accuracy in the benign salivary lesions, recognition of both characteristic and less typical cytomorphology is needed.
Fine needle aspiration cytology (FNAC) is widely used as a simple and safe diagnostic modality in salivary glandular lesions.1-3 The cytologic features of salivary lesions are relatively well defined, and the diagnostic accuracy of FNAC of salivary lesions is relatively high, and has been reported to be 87-100% in discrimination of benign from malignant lesions.4 However, the role of FANC in the diagnosis of salivary gland lesions is still controversial because correct tumor typing has been difficult5-7 and the diagnostic accuracy has been dependent on the quality and yield of the aspirate, as well as the expertise of the cytopathologist, because the salivary gland tumors constitute a heterogeneous group with extremely varied histopathologic features.6,8 Cytologic interpretation could be confusing if the salivary gland lesions were lacking the typical cytologic features, and the false positive or false negative rate of FNAC diagnosis were still high. The false positive rates of FNAC in pleomorphic adenoma (PA) and Warthin's tumor (WT) which were reported to be 9% and 8%, respectively, were relatively low,9 but the variegated cytomorphology of these tumors may lead to an error in interpretation.1,10 In some benign lesions such as monomorphic adenoma and oncocytoma, false positive rates were reported to be 53% and 18%, respectively.9
In this study, the authors analyzed the causes of the diagnostic discrepancies in the benign salivary glandular lesions based on a comparison of the FNAC smears and the histologic findings.
MATERIALS AND METHODS
There were 77 cases of histologically-proven benign salivary glandular lesions among 238 FNAC cases of salivary glandular lesions during the most recent 5 years at Pusan Paik Hospital. Of the 77 cases, PA was found in 61 cases, WT in 12 cases, monomorphic adenoma in 1 case, lipoma in 1 case and mucocele in 2 cases.
The diagnoses of FNAC and the subsequent operation were compared, and were categorized as concordant, minor discrepancy, or major discrepancy between them. The cases diagnosed simply as benign or other benign tumor were defined as minor discrepancy, and the cases diagnosed as atypical, malignant or metastasis as major discrepancy.
A review of the FNAC smears and the histologic sections was performed in the cases showing diagnostic discrepancies. The cytologic slides were routinely stained with Papanicolaou methods. The cytologic findings were reviewed based on the specimen adequacy and the presence or lack of typical cytomorphology. Evaluation of the causes of discrepancies was performed by comparing the histologic features and cytomorphology.
RESULTS
Among the 77 cases of benign salivary glandular lesions, major diagnostic discrepancies between the FNAC and histologic diagnoses were noted in 4 of the 61 PA cases, and in 1 of the 12 WT cases, Minor diagnostic discrepancies were found in 7 of the 12 WT cases and in 11 of the 61 PA cases. One monomorphic adenoma case, one lipoma case and 2 cases of mucocele showed diagnostic concordances between the FNAC and histologic diagnoses.
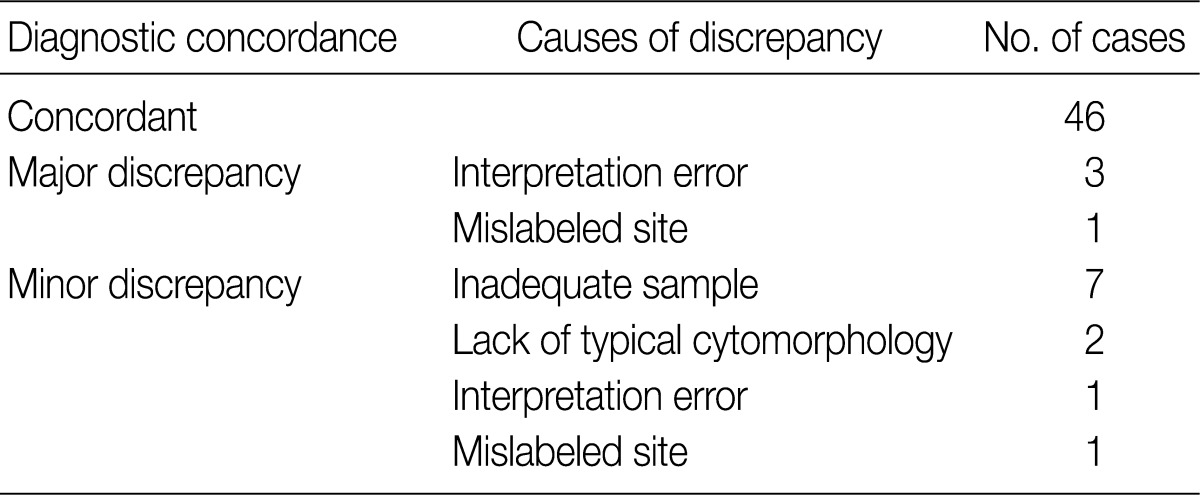
Of the 61 PA cases, 46 cases showed concordance between the FNAC and histologic diagnoses (Table 1). The cytologic diagnoses of 4 PA cases showing major discrepancies were as follows: mucoepidermoid carcinoma 1 case, malignant or atypical in 2 cases, and metastatic carcinoma 1 case the last due to mislabeling of the aspiration site as a lymph node. The case that was misdiagnosed as mucoepidermoid carcinoma revealed a low cellular yield and a few epithelial cell clusters that consisted of basaloid, squamoid and mucous cell-like cells, which were misinterpreted as mucoepidermoid carcinoma (Fig. 1A). Histologically, this case showed occasional squamous metaplasia and microcystic change (Fig. 1B). Another case that was overdiagnosed as malignant showed less conspicuous stromal tissue, and suspicious cytologic atypia. Histologically this tumor showed a spindle cellular mass with very scanty epithelial and stromal elements. One case, diagnosed as atypical, revealed low cellularity with a lack of both epithelial and stromal elements and suspicious nuclear atypia. Another case was diagnosed as metastatic carcinoma due to mislabeling as a lymph node instead of a salivary gland, and as a result, the smeared epithelial components were misinterpreted as metastasis.
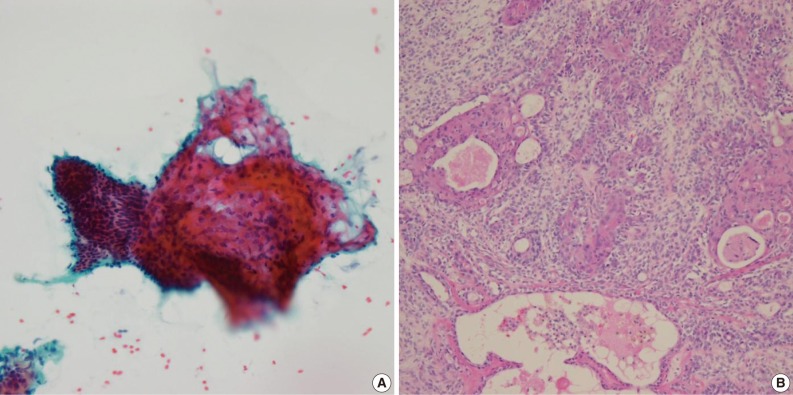
Cytologic and histologic findings of pleomorphic adenoma case misdiagnosed as mucoepidermoid carcinoma. The fine needle aspiration cytology smear shows a few cellular clusters with squamoid features (A), and histologically squamous metaplasia and microcystic changes are observed (B).
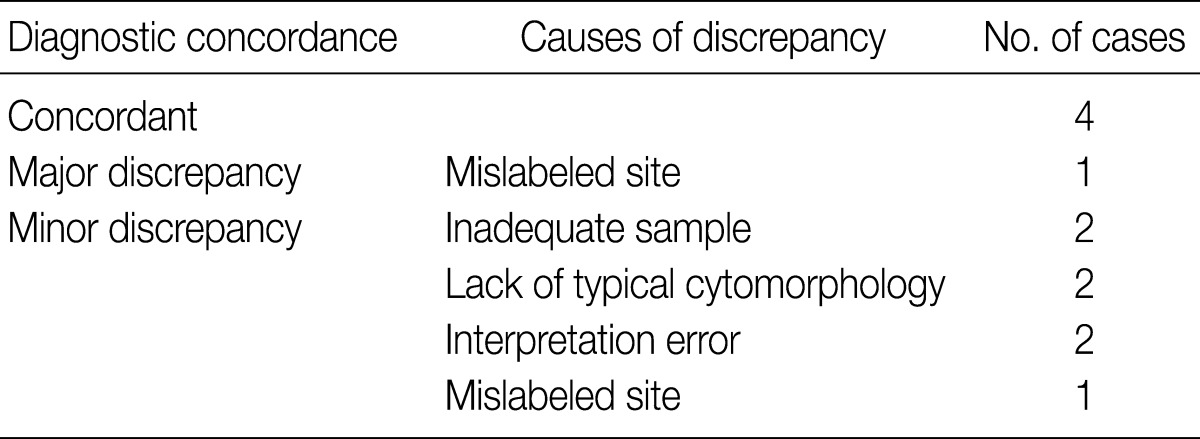
The cytologic diagnoses of the 11 PA cases showing minor discrepancies were simply benign in 7 cases, spindle cell lesion in 2 cases, suppurative inflammation in 1 case, and a benign condition in the 1 case that was mislabeled as a lymph node. The major causes of misinterpretation as simply a benign epithelial lesion or tumor was an inadequately sized sample. On review, a poor cellular yield and a small quantity of stromal components were noted. Two cases were misdiagnosed as a spindle cell tumor (ruling out schwannoma), and these cases showed predominant spindle cell populations cytologically and histologically. Another case that has been interpreted as ruling out myoepithelioma or monomorphic adenoma, showed degenerated epithelial or spindle cells on the cytologic smears, and the tumor revealed variegated histologic features with degenerative changes. One case that was misinterpreted as suppurative inflammation showed cell debris in a dirty background instead of the typical cytologic features suggesting pleomorphic adenoma. The cell debris in the dirty background was misinterpreted as degenerated inflammatory cells (Fig. 2).
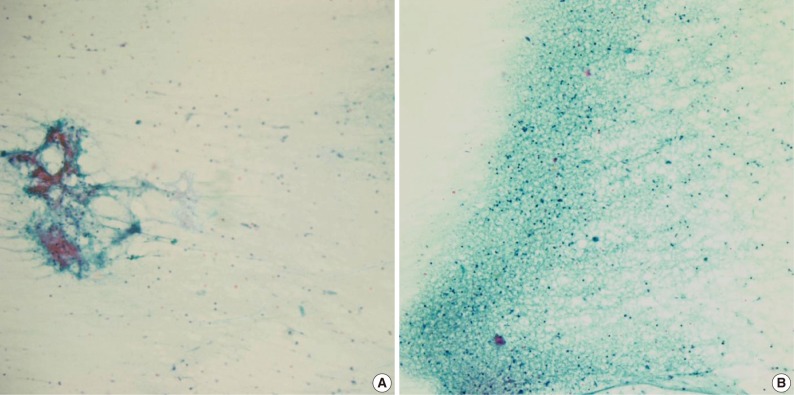
Cytologic findings of pleomorphic adenoma cases misdiagnosed as an inflammatory condition. The fine needle aspiration cytology smear displays low cellularity with a lack of three cellular components such as epithelial, myoepithelial and stromal origin (A), and the nuclear debris in the necrotic background that are misinterpreted as inflammatory cells (B).
Among the 12 WT cases, only 4 cases showed a diagnostic concordance between the FNAC diagnosis and histologic diagnosis (Table 2). In these 4 cases, oncocytic cell clusters and lymphocytes were observed in the necrotic background (Fig. 3A). Of the 12, 6 cases showed minor discrepancies, and 4 cases among the 6 were diagnosed as simply benign, 1 case as an inflammatory condition, and the other case as ruling out monomorphic adenoma. Two of these 4 cases, that were diagnosed as simply benign, were misdiagnosed due to inadequate aspirates. On review of the FNAC smears, lymphoid cells with rare epithelial or stromal components were noted. The other 2 of the 4 cases that were diagnosed as simply benign were misdiagnosed due to inadequate aspirates associated with cystic change in the WT. Aspirates from the cystic WT revealed a relatively poor cellular yield with lack of a oncocytic cell clusters and somewhat squamoid features (Fig. 3B), or revealed cell debris in the necrotic background with scanty spindle or squamoid cells mixed with the cell debris (Fig. 3C, D). One case that was diagnosed as an inflammatory condition, showed cell debris or variable sized or shaped, degenerated cells mixed with various inflammatory cells in the necrotic background, instead of epithelial cell clusters with oncocytic change or rich lymphoid cells, on review of the FNAC smears. The other case that was diagnosed as ruling out monomorphic adenoma was confirmed to be interpretation error. On cytologic-histologic correlation, diagnostic discrepancies in the WT cases were related to inadequate aspirates in 2 cases, were poor or rare epithelial cells associated with cystic change in 2 cases, and there were interpretation errors in 2 of the 6 cases. The remaining 2 cases were mislabeled as lymph nodes clinically, and one case was misdiagnosed as ruling out metastasis based on the presence of squamoid cells but it was described as not so atypical, and the other case was diagnosed as a benign condition, and the smeared cells were interpreted as benign follicular cells.
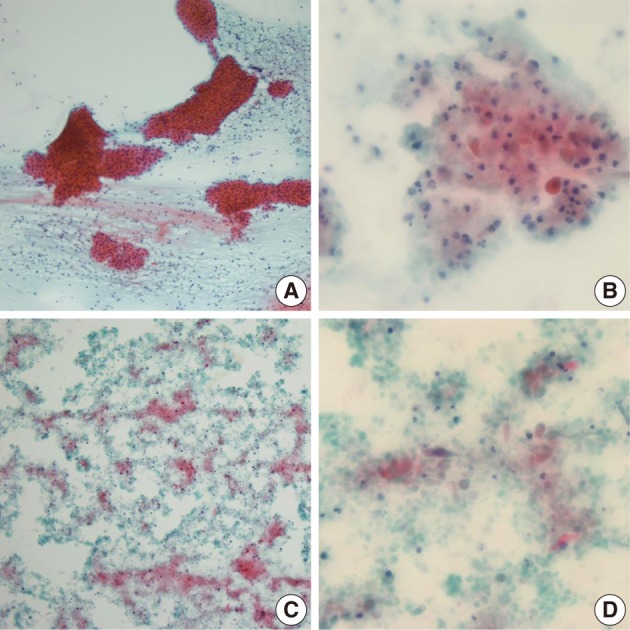
Cytologic findings of the Warthin's tumor cases showing diagnostic discrepancies. The typical cytologic features of Warthin's tumor consist of oncocytic cell clusters and mature lymphocytes in the necrotic background (A). A case, diagnosed as simply benign, shows degenerated or squamoid cells mixed with inflammatory cells instead of oncocytic cell clusters (B). Another Warthin's tumor case with cystic change reveals cell debris in the necrotic background (C), and scanty spindled or squamoid cells mixed with cell debris (D).
DISCUSSION
FANC was introduced as a diagnostic tool by Martin and Ellis11 in the 1930s,5 and FNAC of salivary glands was developed by Eneroth et al. between the 1950s and the 1960s.5 In the salivary gland lesions, FNAC is widely used as an initial diagnostic tool for further planning because it is inexpensive, rapid, well-tolerated, less invasive, and has been proven to have a high diagnostic accuracy.1,6,10,12-15
According to the literatures since 1994, sensitivity, specificity, and diagnostic accuracies of FNAC in the salivary glandular lesions has been reported as 70-100%, 91-100%, and 84-98.3%, respectively.1-6,9,10,13,14,16 Singh Nanda et al.5 reported that sensitivity, specificity, and positive and negative predictive values were 84.61%, 91.66%, 91.60%, and 85.00%, respectively, in benign salivary gland lesions, but 84.61%, 86.48%, 68.75%, and 94.11%, respectively, in malignant salivary gland lesions. There were no differences in sensitivity, but specificity and the positive predictive values were higher in the benign salivary gland lesions while the negative predictive value was higher in malignant lesions. Brennan et al.6 reported that there were few differences in the sensitivity and specificity of between initial FNAC and repeated FNAC as 70% and 93% and 84% and 95%, respectively.
A PA is the most common salivary gland neoplasm accounting for about 60% of all salivary gland tumor,17 thus PA should always be first considered when diagnosing the FNAC smears of a salivary gland.18 Histologically, PA shows a mixture of epithelial and mesenchymal elements. The epithelial components of a PA consist of cuboidal, basaloid, squamous, spindle, plasmacytoid and clear cells, and show trabeculae, ductal, cystic, and solid patterns. The mesenchymal components are mucoid, myxoid, cartilaginous, or hyalinized.19
In the aspirates from PA, various combinations of three main elements, myoepithelial cells, ductal cells, and chondromyxoid matrix were observed. The epithelial components consisted of myoepithelial cells and ductal cells forming sheets or scattered single cells. The myoepithelial cells were more conspicuous in the FNAC smears than in the histologic section, and they appeared as loose clusters or singly dispersed cells. The myoepithelial cells showed various types of cytomorphology such as plasmacytoid, spindled, stellate or clear cells.10,20 In air-dried preparations, the myoepithelial cells had a pale smudgy blue outline.18 The ductal cells were less conspicuous than the myoepithelial cells and presented as 3-dimensional clusters of cuboidal cells with round nuclei. In both myoepithelial cells and ductal cells, nucleoli were rarely seen. The chondromyxoid stroma of PA was generally cyanophilic and fibrillar in the Pap stains, and can obscure the epithelial component when mixed with the epithelial component. Infrequently, metaplastic squamous cells, cystic fluid, sebaceous cells, inflammatory cells, tyrosine crystals can be observed.10,20
The diagnostic accuracy of FNAC in PA has been reported to be 81.6% (40/49) by Paik et al.,3 84% (16/19) by Kim and Lee10 and 86.7% (26/30) by Zhang et al.4 In this study, the major diagnostic discrepancies between the FNAC and histologic diagnoses were noted in 4 (6.6%) of the 61 PA cases, and minor diagnostic discrepancies were found in 11 (18.0%) of the 61 PA cases. The diagnostic accuracy in this study was 75.4% (46/61), which was relatively low compared with the previous studies.
In cases showing the typical three components of PA, the cytologic diagnosis can be easily performed,18,20 however, it is sometimes problematic. The cases showing dominant mucoid background and less cellular smear could be diagnosed as cystic lesions such as mucocele or mucoepidermoid carcinoma, and the cases showing cellular smears of exclusively epithelial or myoepithelial cells could be misinterpreted as monomorphic adenoma, or overdiagnosed as mucoepidermoid carcinoma or adenoid cystic carcinoma. The most helpful cytomorphologic features in distinguishing PA from malignancies are the presence of plasmacytoid myoepithelial cells and fibrillary stromal tissues. In adenoid cystic carcinoma cases, mainly dense clusters of basaloid cells and spherical hyaline globules have been observed.10,16,18,21 In cases of carcinoma ex PA, it is important to identify nuclear atypia.18,22 A neurogenic tumor should always be included in the differential diagnosis, and presence of metachromatic dense fibrillary materials is suggestive of PA.18
One case wrongly diagnosed as mucoepidermoid carcinoma revealed a low cellular yield and a few epithelial cell clusters containing squamoid and mucous cell-like cells. These cells might be related to squamous metaplasia and microcystic change based on the histological findings. However, on review of the FNAC slide, low cellularity, no obvious mucous or squamoid cells, and inconspicuous nuclear atypia were not compatible with the cytologic features of the mucoepidermoid carcinoma. In the 2 cases overdiagnosed as malignant or atypical, the cause of discrepancy was related to a lack of typical cytomorphology, and the FNAC smears showed low cellularity; however, the nuclear atypia was overestimated. Histologically, one of the cases revealed a spindle cellular mass with very scant epithelial elements. To avoid diagnostic pitfalls in PA cases, identification of the three main cytologic components is required.16,18 In one case that was misdiagnosed as metastatic carcinoma, it was due to mislabeling as a lymph node instead of a salivary gland; therefore, recognition of the clinical information and radiological findings is essential before examination of FNAC smears.
Of the 11 PA cases showing minor discrepancies, 7 cases were diagnosed as simply benign, and the major cause of the misinterpretations as a simply benign epithelial lesion or tumor was an inadequate sample. On cytologic review, a poor cellular yield and a small quantity of stromal components were associated. Two of the 11 cases were misdiagnosed as a spindle cell tumor (ruling out schwannoma), and these tumors showed predominant spindle cell populations histologically, but focal typical epithelial cells or stromal cells were observed on review of the FNAC smears. Another case, that was interpreted as ruling out myoepithelioma or monomorphic adenoma, showed degenerated epithelial or spindle cells, and histologically this case showed variegated histologic features with degenerative changes. In a case misinterpreted as suppurative inflammation, the small sized naked cells in the dirty background were misinterpreted as degenerated inflammatory cells.
In summary, the major causes showing diagnostic discrepancies in PA were poor cellularity, a lack of the three main cytologic components and the presence of a lack of typical cytomorphology related to degenerative change.
A WT is the second common benign tumor of the salivary gland after PA,23 and a WT occurs mainly in the parotid gland bilaterally in about 10% of the cases, has a slight male dominance, and is related with cigarette smoking.12,20,23,24 Histologically, WT characteristically consists of cystic and solid areas. The cystic area is lined with two layers of tall and columnar oncocytic luminal cells and flattened or cuboidal basal cells, while the stroma consists of lymphoid tissues.12,20,25 Cytologically a WT shows variable cellularity, and consists of 3 components including epithelial cells, lymphocytes, and a fluidy background. Epithelial cells are oncocytic cells with a low nuclear/cytoplasmic (N/C) ratio, large bland nuclei, prominent nucleoli, abundant eosinophilic and granular cytoplasm, and a sharply demarcated cytoplasmic border. The epithelial cells present as monolayered sheets or as multilayered cohesive sheets with a 2 or 3 cell thickness. Most of the lymphocytes are small and mature, and dispersed single cells. A dirty background containing cell debris can be observed, and might show a mucoid substance or calcifications and crystals. Infrequently mast cells, siderophages, giant cells, sebaceous gland cells, metaplastic squamous cells, and spindle cells can be observed.15,20,26
In cases showing the three components, oncocytic epithelial cells or sheets, scattered lymphocytes, and a dirty background, the diagnosis of a WT can be made without difficulty. However, the above-mentioned three components are not characteristics of only WT.20 Differential diagnoses of WT include chronic sialadenitis, and a lymphoepithelial cyst, in which oncocytic epithelial cells and lymphocytes appear together.16,20 Oncocytoma and oncocytic carcinoma may also contain lymphocytes, but the oncocytic cell layers of these neoplasm are larger than that of oncocytic cells of WT, and has a thickness of 3 or more cells.15,20 The eosinophilic cells of acinic cell carcinoma may be confused with WT, but zymogen granules, cellular pleomorphism, and prominent nucleoli are seen in cases of acinic cell carcinoma.16,20 Intermediate squamoid cells of mucoepidermoid carcinoma, or metaplastic squamous or oncocytic cells of PA can also be confused with WT.10,15,16 In particular, one of the epithelial and lymphoid components has been predominant in WT, and diagnostic difficulties have frequently occurred in that situation.15
In this study, only 4 of the 12 WT cases showed diagnostic concordance between the FNAC diagnosis and histologic diagnosis. The diagnostic accuracy of WT was 33.3% (4/12), which was a relatively low concordance rate compared with the previous studies, which had 33-100% accuracies.3,10,15,27 Of the 12 WT cases, 6 showed minor discrepancies, and 4 cases of the 6 were diagnosed as simply benign, and the other 2 cases were diagnosed as an the inflammatory condition or ruling out monomorphic adenoma. The 2 cases that were diagnosed as simply benign were misdiagnosed due to inadequate aspirates. In both of the cases, the smeared cells were mostly lymphoid cells with rare epithelial or stromal components. Cystic change in a WT can occur, and aspirates from this cystic tumor in our cases revealed a relatively poor cellular yield and individual degenerated epithelial cells with an occasional squamoid feature, lacking oncocytic cells. Interpretation errors were noted in 2 cases, and one of the cases that was diagnosed as an inflammatory condition showed cell debris or a variable sized or shaped degenerated cells mixed with the inflammatory cells in the necrotic background instead of epithelial or oncocytic cells and rich lymphoid cells. The other case that was diagnosed as ruling out monomorphic adenoma was regarded as an interpretation error. On cytologic-histologic review, the diagnostic discrepancies in the WT cases were due to inadequate aspirates in 2 cases, poor or rare oncocytic cells associated with cystic change in 2 cases, and interpretation error in 2 cases. Another 2 cases were mislabeled as lymph nodes, clinically, and one of the cases was misdiagnosed as rule out metastasis based on the presence of squamoid cells, but the smeared epithelial cells were described as not so atypical, and the other case that was diagnosed as a benign condition had smeared cells that were interpreted as benign follicular lymphoid cells.
To increase the diagnostic accuracy in benign salivary glandular lesions, triple assessment consisting of cytologic features, clinical information, and radiologic findings is essential. Recognition of aspiration sites and the correlation with radiologic findings are important, and a detailed cytologic examination based on both typical and not typical cytologic features will be needed.
Notes
No potential conflict of interest relevant to this article was reported.