Osteoclast-like Giant Cell Tumor of Parotid Gland with a Carcinomatous Component: A Case Report
Article information
Abstract
The giant cell tumor of the salivary gland is very rare, and 20 cases have been reported in the English-language literature. We report an additional case. A 57-year old man had noticed a mass in the right parotid area for several weeks. The diagnosis using aspiration cytology was a giant cell tumor possibly with a carcinomatous component. Superficial parotidectomy was carried out. The resected parotid gland contained a 1.8 cm-sized well-circumscribed brownish tumor. Histologically the tumor consisted of evenly distributed osteoclast-like giant cells, mononuclear cells and two small foci of a carcinomatous component. The osteoclast-like giant cells and mononuclear cells were positive for vimentin and CD68, and the carcinomatous component was positive for cytokeratin and epithelial membrane antigen. There was no metastatic lesion in the cervical lymph nodes. We believe this is the first case in Korea of an osteoclast-like giant cell tumor of the parotid gland.
The osteoclast-like giant cell tumor of the salivary gland (GCT-SG) is very rare and we found only 20 cases in the English-language literature.1-14 We report another case diagnosed by aspiration cytology. We believe this is the first case in Korea of an osteoclast-like GCT-SG.
CASE REPORT
A 57-year-old man had noticed a mass in the right parotid area for several weeks. The mass was solid and firm with no pain or tenderness. Liquid-based aspiration cytology was performed (Fig. 1). The cytologic findings were numerous scattered osteoclast-like multinucleated giant cells and isolated mononuclear cells. The osteoclast-like giant cells and most of the mononuclear cells had benign-looking nuclei, but a few mononuclear cells had slightly atypical nuclei with clumped chromatin and prominent nucleoli. There was a small cluster of slightly atypical epithelial-like cells. We considered the diagnosis to be a giant cell tumor possibly with a carcinomatous component.
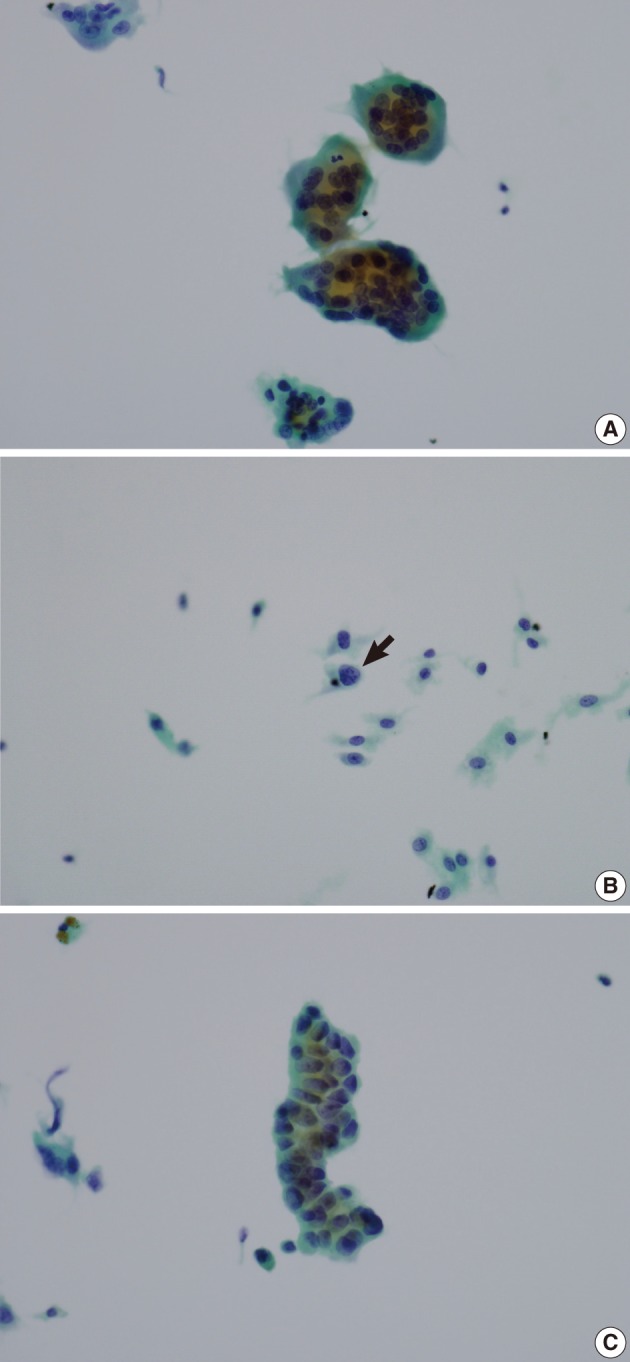
(A) Aspiration cytology of a parotid gland mass reveals many osteoclast-like giant cells with benign-looking nuclei. (B) Most of the scattered mononuclear cells have benign-looking vesicular nuclei, but a few cells (arrow) have enlarged nuclei with chromatin clumping. (C) There is a cluster of epithelial-like cells with slightly atypical nuclei.
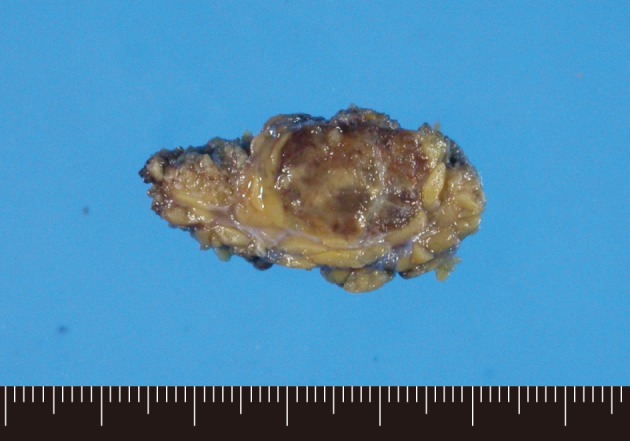
The resected parotid gland contained a 1.8 cm-sized well-circumscribed round tumor (Fig. 2). The cut surface was mottled with light and dark brown colors. There was no necrosis or hemorrhage. Light microscopic examination revealed a well-circumscribed but not encapsulated tumor (Fig. 3). The tumor consisted of evenly distributed multinucleated giant cells, which were morphologically indistinguishable from osteoclasts, and round or short-spindled mononuclear cells. At first sight the tumor appeared to be a giant cell tumor of bone, but a careful examination revealed that many mononuclear cells had hyperchromatic nuclei with clumped chromatin. Many mitotic figures were found, up to 30 per 10 high-power fields. In addition, there were two small foci of carcinomatous component. The carcinomatous component was very small, such that it disappeared in the additional sections. The nuclei of the carcinoma cells did not appear to be very malignant but the cells were arranged in a cribriform pattern so that they were recognized as malignant. It appeared that there was no transitional area between the giant cell tumor and the carcinomatous component. There was no metastatic lesion in the cervical lymph nodes.
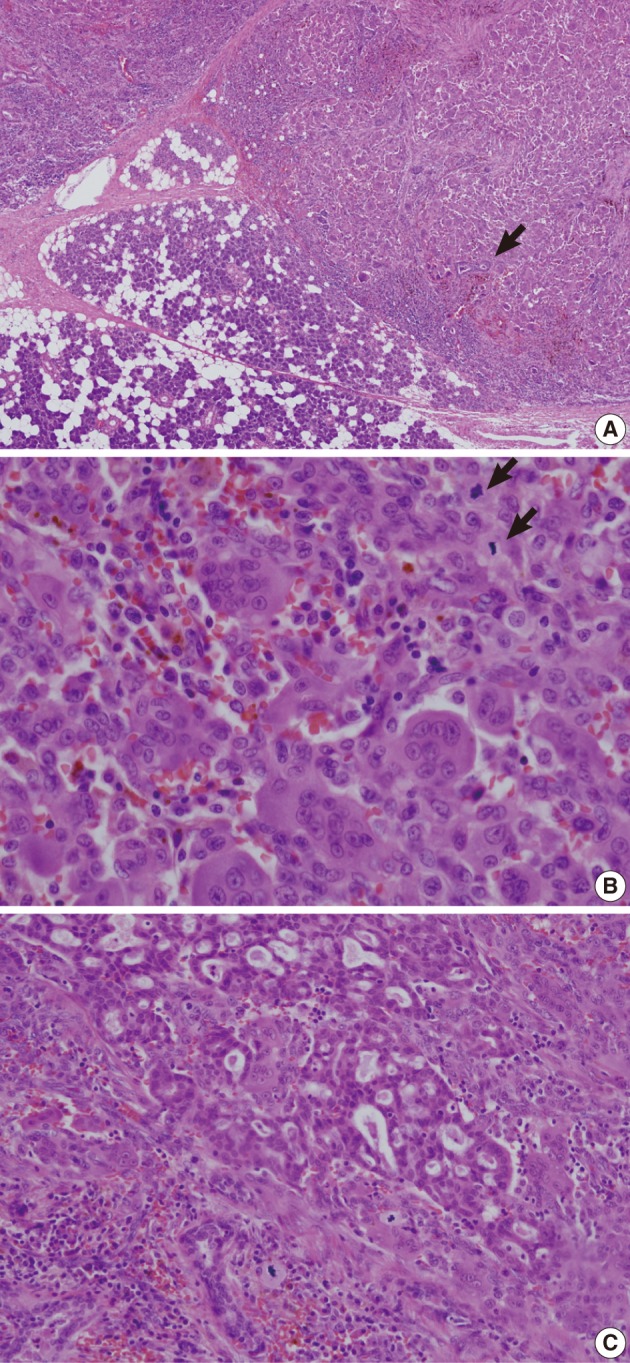
(A) A well-circumscribed but not encapsulated tumor is shown in the parotid gland. Several entrapped salivary ducts (arrow) are seen in the periphery of the tumor. (B) The tumor consists of osteoclast-like multinucleated giant cells and mononuclear cells. The mononuclear cells have round to oval vesicular nuclei with slightly coarse chromatin pattern. Mitotic figures (arrows) are found up to 30/10 high power field. (C) There is a small focus of carcinomatous component with cribriform arrangement of cancer cells.
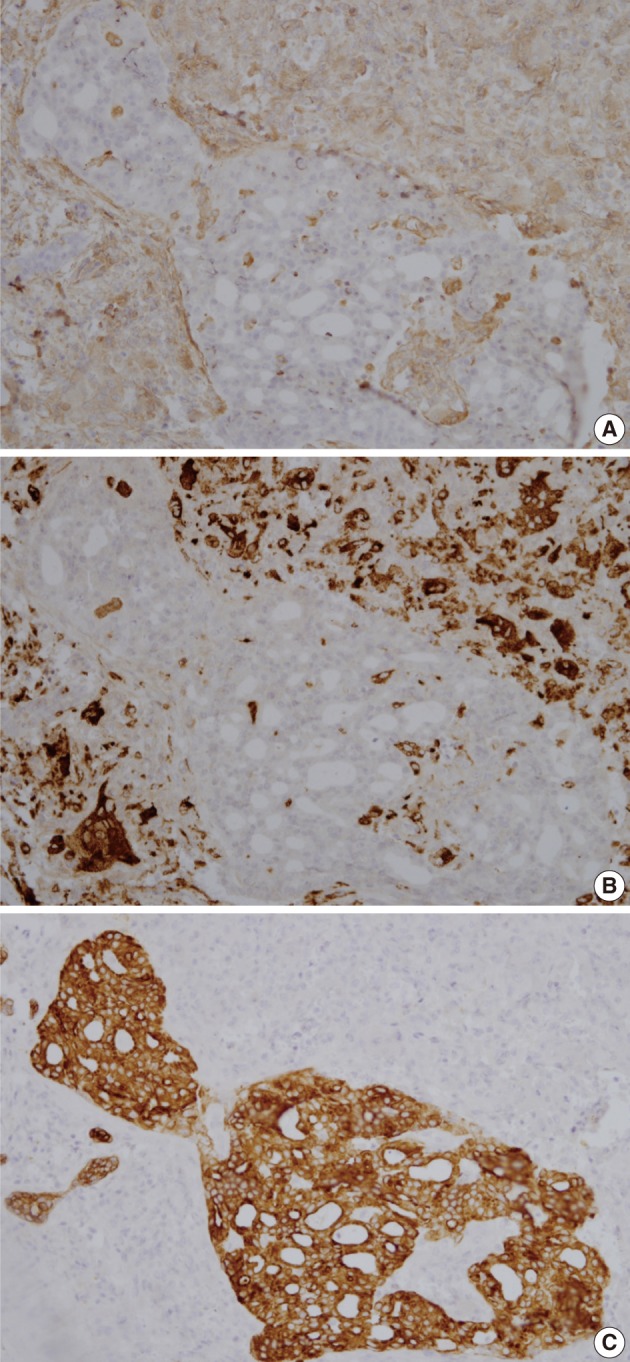
Immunohistochemically, osteoclast-like giant cells were diffusely positive for vimentin and CD68, and negative for cytokeratin and epithelial membrane antigen (Fig. 4). Mononuclear cells were diffusely positive for vimentin, partly positive for CD68, and negative for cytokeratin and epithelial membrane antigen. The carcinoma cells were positive for cytokeratin and epithelial membrane antigen, and negative for vimentin and CD68.
DISCUSSION
Giant cell tumors histologically mimicking giant cell tumors of bone may occur in the soft tissue and rarely in the visceral organs such as pancreas,15 thyroid,16 urinary bladder,17 and liver.18 These tumors are called osteoclast-type or osteoclast-like giant cell tumors because the giant cells seen in these tumors are morphologically similar to those seen in the giant cell tumor of bone. Of these, the giant cell tumor of soft tissue is similar to the giant cell tumor of bone both histologically and clinically. However, giant cell tumors of visceral organs usually contain a carcinomatous component and are clinically more aggressive than the giant cell tumor of bone.
The GCT-SG is very rare and we found 20 cases in the English-language literature,1-14 with a total of 21 including our case. Seventeen cases occurred in the parotid gland, 3 cases in the submandibular gland4,8,9 and 1 case in the minor salivary gland.14 Most patients of GCT-SG were men and the male to female ratio was 17 : 4. The range of age was from 28 to 92, with the average age of 59. Twelve (57%) tumors contained a carcinomatous portion. In most cases, the carcinomatous portion was a salivary duct carcinoma, but in three cases, the carcinomatous portion was a carcinoma ex pleomorphic adenoma.1,7,9
In two cases, the tumor contained a pleomorphic adenoma instead of a carcinoma.8,11 Metastasis to the cervical lymph node was present in one case,12 in which the metastatic tumor originated from the salivary duct carcinoma component. Eight patients were monitored for 9 months or longer. Of the 8 patients, six had no evidence of disease at 9 months up to 6 years after surgery. Two patients died with clinical evidence of disseminated disease including pulmonary metastasis.2,6 Our case showed no evidence of recurrence or metastasis at 3 months after surgery.
GCT-SG morphologically differs from giant cell carcinoma or undifferentiated carcinoma with anaplastic giant cells. The giant cells of GCT-SG have bland-looking nuclei with homogenous chromatin distribution. In contrast, the giant cells in giant cell carcinoma or undifferentiated carcinoma with anaplastic giant cells are usually highly pleomorphic and hyperchromatic.
GCT-SG also morphologically differs from giant cell tumor of bone. Unlike giant cell tumor of bone, the nuclei of mononuclear cells in GCT-SG are not similar to those of giant cells and show at least some nuclear irregularity and hyperchromasia, although usually not as severe as those in undifferentiated carcinoma. In our case, the mononuclear tumor cells were very bland-looking, such that at first sight the tumor appeared to be a giant cell tumor of bone. In other cases, the nuclei of neoplastic cells were described as bland, uniform, moderately atypical or uniform to bizarre. In only two cases, the nuclei of neoplastic cells were described as anaplastic2 or highly malignant.12
Some authors believe that GCT-SG is a carcinoma and prefer the terminology of osteoclast-type giant cell carcinoma.10 We concur with this for the same reasons as they have suggested. First, GCT-SG usually contains a carcinomatous component, which cannot be explained by incidental coexistence. The carcinomatous component can be very small and focal, as it is in our case. It is possible that a small hidden focus of carcinomatous component may be found by a more diligent search in cases where the carcinomatous component was not reported to be present. Second, in contrast to giant cell tumor of bone, some of the mononuclear cells of GCT-SG expressed epithelial markers as well as histiocytic markers in several cases,2,3,10,12-14 although mononuclear cells in our case did not express epithelial markers. Third, the microsatellite pattern of the giant cell tumor component is more akin to the carcinomatous component and does not resemble giant cell tumor of bone.10
The osteoclast-like giant cells are thought to be non-neoplastic. The giant cells are consistently negative for epithelial markers, as they are in our case, but express CD68, a histiocytic marker, and may be reactive cells representing a host response to neoplasm.
In the World Health Organization (WHO) classification of tumors, the osteoclast-like giant cell tumor of the pancreas is described as a separate entity and is called undifferentiated carcinoma with osteoclast-like giant cells.19 In other organs, the osteoclast-like giant cell tumor is included in the undifferentiated carcinoma as an osteoclastic or a giant cell variant. According to the WHO classification of tumors, GCT-SG cannot be ascribed to any categories. We and some other authors10 do not support classifying this tumor under the category of undifferentiated carcinoma. We believe that GCT-SG has a stronger tendency of male predominance, less pleomorphic nuclei of tumor cells and more favorable prognosis than the undifferentiated carcinoma. The potential relationship of GCT-SG with salivary duct carcinoma and carcinoma ex pleomorphic adenoma, and whether the type and percent composition of the carcinomatous components determine the natural history need to be further evaluated.
We found two cases of GCT-SG diagnosed by fine needle aspiration.11,12 The cytologic findings were numerous osteoclast-like multinucleated giant cells and isolated or clustered malignant-appearing mononuclear cells. Cohesive sheets of large polygonal epithelial cells were seen in one of the two cases. In our case, there were many osteoclast-like giant cells and mononuclear cells but only one small cluster of slightly atypical epithelial cells. When needle aspiration reveals many osteoclast-like giant cells but few atypical cells, as it did in our case, giant cell granuloma should be considered in differential diagnoses. Giant cell granuloma is smaller than GCT-SG in tumor size. The osteoclast-like giant cells in giant cell granuloma are fewer in number and have fewer and smaller nuclei.7
Acknowledgments
We have heard that a similar case report has been accepted for publication in the Korean Journal of Pathology during the reviewing process. If this is the case, the present case would be the second case in Korea.
Notes
No potential conflict of interest relevant to this article was reported.