Primary Merkel cell carcinoma of the salivary gland: a clinicopathologic study of four cases with a review of literature
Article information
Abstract
Background
Primary Merkel cell carcinoma of the salivary gland is currently not listed in the World Health Organization classification. However, cases of Merkel cell type neuroendocrine carcinomas of the salivary gland with perinuclear cytokeratin 20 positivity have been intermittently reported. We here investigated the clinicopathologic features of additional cases.
Methods
Data of four cases of Merkel cell type small cell neuroendocrine carcinoma of the salivary gland were retrieved. To confirm the tumors’ primary nature, clinical records and pathologic materials were reviewed. Optimal immunohistochemical staining was performed to support the diagnosis.
Results
All tumors were located in the parotid gland. Possibilities of metastasis were excluded in all cases through a meticulous clinicopathological review. Tumor histology was consistent with the diagnosis of small cell neuroendocrine carcinoma. Tumors’ immunohistochemical phenotypes were consistent with Merkel cell carcinoma, including Merkel cell polyomavirus large T antigen positivity in two of the four cases.
Conclusions
Merkel cell carcinomas can originate in salivary glands and are partly associated with Merkel cell polyomavirus infection as in cutaneous Merkel cell carcinomas.
INTRODUCTION
Small cell neuroendocrine carcinoma (SCNEC) is a high-grade neuroendocrine carcinoma (NEC) and can arise in several organs. It rarely originates in the salivary gland, accounting for <1% of salivary tumors [1]. SCNEC in the salivary gland deserves special attention as some of them have distinctive features compared to SCNEC of other sites, including diffuse perinuclear cytokeratin 20 (CK20) positivity [2]. A study showed that CK20-positive salivary SCNEC had significantly better prognosis than CK20-negative tumors [1]. Due to its histopathological resemblance to Merkel cell carcinoma (MCC), this group of CK20-positive SCNEC has been referred to by various names, including Merkel cell-like, Merkel cell variant, Merkel cell type SCNEC, or MCC [1-6].
MCC is a type of NEC mostly arising in the skin with characteristic features different from typical neuroendocrine tumors. The association with the oncogenic virus, Merkel cell polyomavirus (MCPyV), is the most remarkable finding of MCC. MCPyV is a type of polyomavirus identified from MCC tissue [7]. Subsequently, MCPyV infection has been observed in the healthy population with high prevalence, usually being asymptomatic [8-10]. Its oncogenic potential proved highly specific for cutaneous MCC, being detected in approximately 80% of all MCC [11,12]. The presence of MCPyV can be detected using immunohistochemical staining targeting MCPyV large or small T antigen. In contrast, ultraviolet (UV)-related mutations are believed to be a significant pathogenetic factor in the remaining 20% of all MCC, with UV mutations and MCPyV infections being mutually exclusive [13]. Additionally, MCC are distinguished from typical neuroendocrine tumors by the expression of several proteins, including CK20, CD5, terminal deoxynucleotidyl transferase (TdT), paired box 5 (PAX5), and SRY-box transcription factor 2 (SOX2) [6,14-16].
Although most MCC originate in the skin, they could arise in extracutaneous mucosal sites in the genitourinary tract, anorectal area, and head and neck areas [17-19]. MCC primarily located in the lymph node is regarded as metastasis from regressed occult cutaneous MCC [20]. Some researchers regarded salivary SCNEC of Merkel cell type as being a metastasis from occult cutaneous MCC on the basis of the presence of a UV signature mutation [21]. In this study, we investigated four cases of salivary gland Merkel cell type SCNEC and discussed the controversy associated with this tumor with a review of the literature.
MATERIALS AND METHODS
Case selection and review
Six cases of NEC of the salivary gland were retrieved from the database of the Department of Pathology of Asan Medical Center, Seoul, Korea, from 2000 to 2023. Two of them were excluded because one case was a large cell NEC, and the other showed positive immunostaining for thyroid transcription factor 1 (TTF-1) and enlarged subaortic lymph nodes, not completely excluding a metastasis of a regressed pulmonary small cell carcinoma. To confirm the absence of tumors from the outside of the salivary gland, a meticulous review of the remaining four cases for available clinical and radiological resources was performed. Two pathologists (G.H.K. and K.J.C.) performed a pathologic review to analyze tumor histology and immunohistochemistry (IHC) results.
Immunohistochemistry
One representative formalin-fixed and paraffin-embedded tissue block was retrieved from each case, and 3-µm-thick sections were acquired. IHC staining was performed using Benchmark XT autostainer (Ventana Medical Systems, Tucson, AZ, USA) and OptiView DAB IHC Detection Kit (Ventana Medical Systems) following the manufacturer’s instructions. Detailed information of antibodies is provided in Table 1. All markers except p53 were assessed as positive or negative on the basis of the nuclear (TTF-1, MCPyV large T antigen, PAX5, retinoblastoma (Rb), p53, TdT, SOX2, and PTEN) or cytoplasmic (CK20, synaptophysin, chromogranin, CD56, and BRAF) staining, regardless of intensity or extent. The p53 expression was evaluated in four categories on the basis of the proportion and intensity of expression–negative, 1+ (weak), 2+ (moderate), and 3+ (strong).
RESULTS
Clinical features
The patients were three females and one male whose ages ranged from 59 to 78 years (mean, 69.7 years). All patients presented with a rapidly increasing palpable mass in the preauricular area, with accompanying pain in two of them. Computed tomography revealed enhancing mass in the parotid gland with a lobulating or partially infiltrating border (Fig. 1A). The following is the medical history of each patient: Case 1 has diabetes mellitus (DM) and rheumatoid arthritis treated with medication, case 2 has DM treated with medication, case 3 has DM treated with medication and experienced a cerebral stroke 5 months ago, and case 4 underwent a liver transplant due to hepatitis B-associated liver cirrhosis 1 year and 3 months ago. Each patient underwent total (cases 1, 2, and 4) or partial (case 3) parotidectomy. Additionally, cases 2, 3, and 4 underwent neck dissection. Notably, widespread lymph node metastases were identified in the unilateral cervical lymph nodes of case 3 (24 of 62 lymph nodes with the largest tumor dimension of 15 mm and extranodal extension) and case 4 (22 of 44 lymph nodes with the largest tumor dimension of 22 mm and extranodal extension). Adjuvant therapy was administered to two patients. Case 1 received radiotherapy as 66 Gy/25 fraction, whereas case 3 received concurrent chemoradiotherapy as 60 Gy/30 fraction with etoposide and cisplatin. In case 3, tumor recurrence involving the left neck and bilateral external iliac lymph nodes was observed, leading to additional systemic chemotherapy with etoposide and cisplatin. The average follow-up period was 19.4 months. Three patients were referred to local clinics within 1 year. No cases of patient mortality were observed until the last follow-up. Clinical data are presented in Table 2.
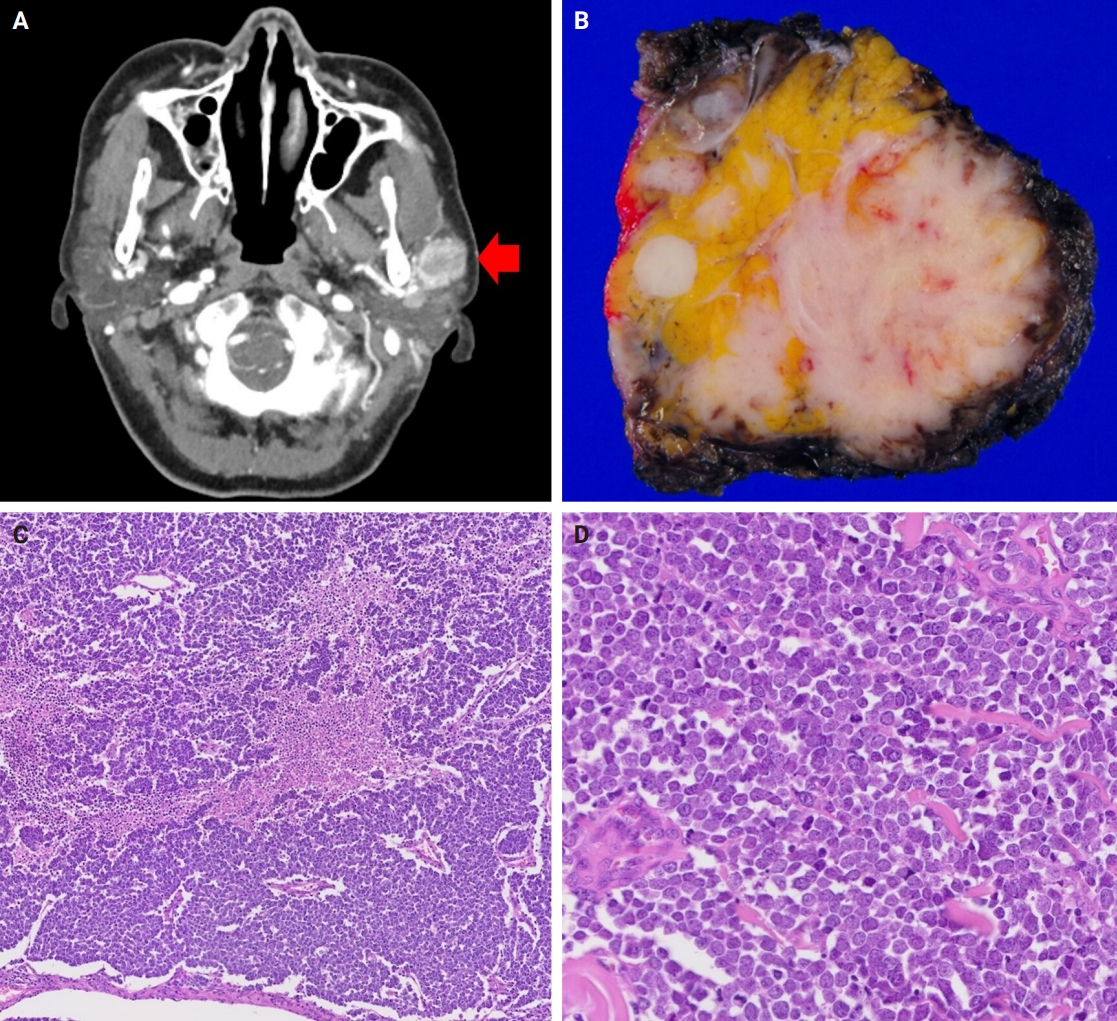
Radiologic, macroscopic, and microscopic images of the representative tumor (case 4). (A) Computed tomography image revealing an ill-defined mass in the left parotid gland (arrow). (B) Gross photography exhibiting an infiltrating tumor with a fleshy section. (C) Diverse histologic pattern with spotty necrosis. (D) Nuclei with salt and pepper chromatin, numerous mitoses, and apoptotic bodies.
Histology and IHC
Grossly, all tumors appeared as ill-demarcated solid masses involving the parotid gland with fleshy cut surface (Fig. 1B). The tumor size ranged from 1.3 to 4.6 cm (mean, 2.85 cm). Microscopic examination revealed infiltrating tumors composed of small monotonous tumor cells demonstrating diverse histological patterns within the tumor, including diffuse, nested, and trabecular patterns. Tumor cells exhibited hyperchromatic finely granular nuclei with nuclear molding and crush artifact. Significant mitotic activity was observed, with counts exceeding 20 per high-power field in some regions. Spotty necrosis and apoptotic debris were frequently observed (Fig. 1C, D). Results of IHC staining for MCC-associated markers are shown in Table 3. In all cases, neuroendocrine markers (synaptophysin, chromogranin, and CD56) were positive. CK20 showed diffuse expression with a perinuclear dot pattern in all cases. TTF-1 was negative in all cases (Fig. 2A, B). MCPyV large T antigen was identified in two cases (cases 3 and 4) (Fig. 2C, D). In both cases, more than 90% of cells showed nuclear staining with variable intensity. PAX5 was focally positive in one case (case 2). PTEN loss was observed in two cases (cases 1 and 3). Rb and p53 were positive in all cases with heterogeneous expression. TdT and BRAF were negative in all cases. SOX2 showed diffuse positive staining in all cases with strong intensity.
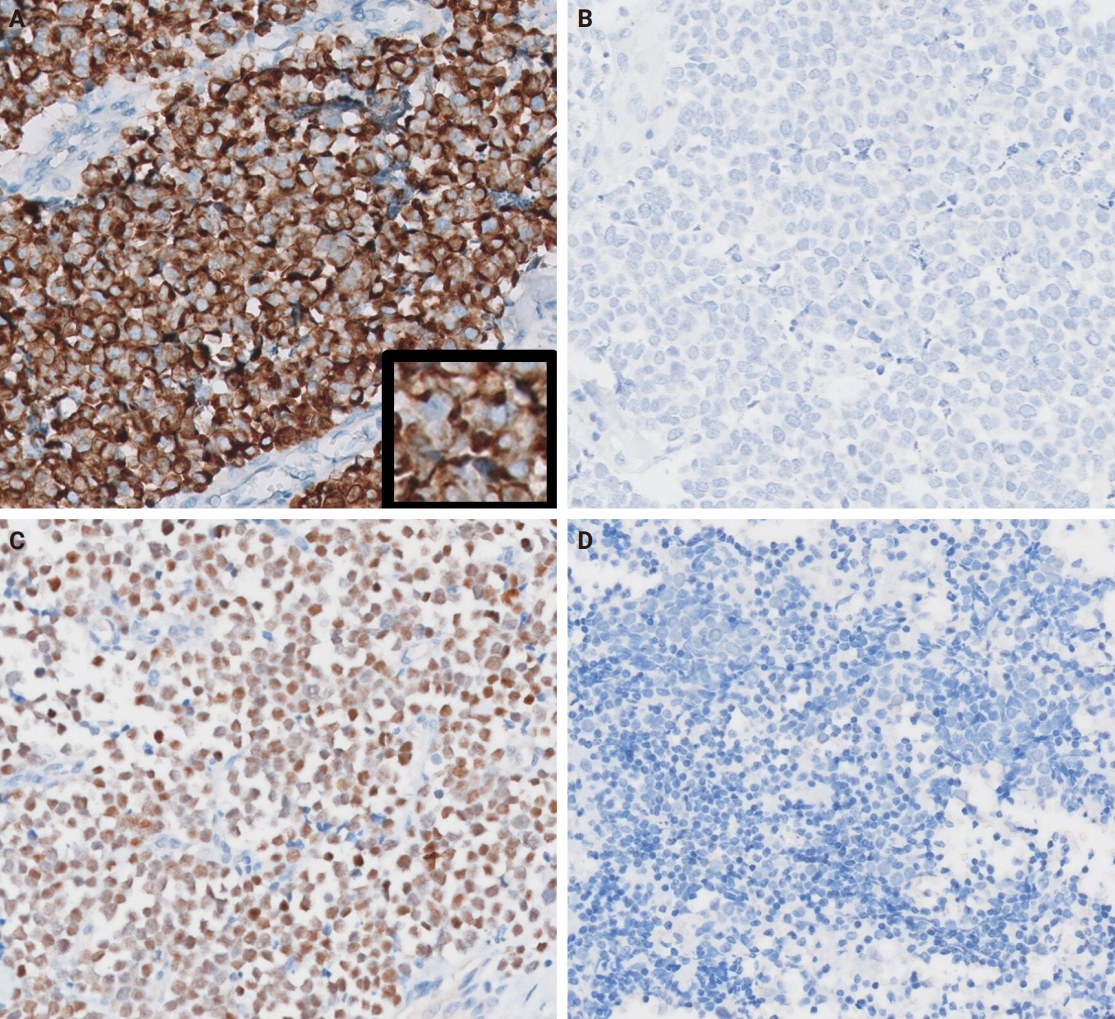
Representative immunohistochemistry images. (A) Diffuse expression of cytokeratin 20 with focal dot-like staining (inset) (case 3). (B) thyroid transcription factor 1 expression is absent (case 3). (C) Merkel cell polyomavirus large T antigen (MCPyV LTA)–positive case (case 4). (D) MCPyV LTA–negative case (case 2).
DISCUSSION
MCC is primarily of a cutaneous origin; however, extracutaneous cases, accounting for approximately 2% of all MCC, have been steadily reported with the salivary glands being the most common site [22]. In the literature, we could identify 45 cases of Merkel cell type (CK20-positive) NEC of the salivary gland (Table 4) [1,3-6,23-34]. Including our four cases, the mean age of the total 49 cases was 70.9 years, with a male predilection (65.3%). The majority of cases (46 cases, 93.9%) originated in the parotid gland, with three originating in the submandibular gland. MCPyV was detected in six of 15 cases tested. Immunosuppression and chronic inflammatory conditions including DM and rheumatic disorders contribute as risk factors for cutaneous MCCs [35,36]. Among our cases, three patients had DM (including one with concurrent rheumatoid arthritis), and one had a liver transplantation history. The underlying mechanisms generating a correlation between MCC and immunosuppression or chronic inflammatory disorders are not well-established; however, immunosuppression is linked with poor prognosis [37] and MCPyV negativity [38]. Although our patients demonstrated a relatively favorable prognosis, the small sample size and the relatively short follow-up duration were limitations. Out of a total 49 cases, 38 had documented survival status and follow-up duration. The survival duration ranged from 2 to 156 months, with a median of 36 months. This finding is better than the previously reported median survival of salivary SCNEC (25–28.5 months) [39,40], which is consistent with the previous finding that CK20-positive salivary SCNEC show better prognosis [1].

Reported cases of Merkel cell type neuroendocrine carcinoma of the salivary gland in the English literature
The Fifth World Health Organization classification of head and neck tumors presents that most salivary SCNEC carrying MCPyV or UV signature mutations are best classified as metastatic MCC [41]. However, no robust tools have been developed yet, which could be used for differentiating primary or metastatic MCC, if there have been regressed or occult skin tumors. Meticulous clinical and radiological investigations for exploring primary site appear to be the most critical for proper diagnosis. Nevertheless, as the skin is the most superficial part of the body, the absence of any skin tumors in patients’ histories is considered relatively reliable. MCPyV does not exclusively infect the skin cells but can be detected in systemic tissues, such as the saliva, aerodigestive tract, or liver [42]. It has been detected in extracutaneous MCC, including cases involving the maxillary sinus [18], stomach [43], and salivary glands [3-5]. UV mutation signatures are often cited as supporting evidence for a skin origin in tumors; however, they have also been observed in a range of extracutaneous tumors [44] including salivary squamous cell carcinoma cases [45,46]. Given that whole exome sequencing is the standard method for detecting UV signature mutations, its application was not feasible in our study due to the age of our specimens. To explore UV-related mutations, we instead employed IHC for PTEN and BRAF as surrogate markers, as mutations in these genes have been commonly associated with UV-induced damage [47,48]. Unfortunately, the IHC results did not reveal significant findings; specifically, we found no evidence of UV mutations in the MCPyV-negative cases. While previous studies have used UV mutation signatures to argue that cases of lung melanoma [49] and salivary squamous cell carcinoma [45] may represent occult cutaneous origin metastases, relying on UV mutation signatures as definitive evidence of a cutaneous origin remains challenging due to numerous variables [50]. That is, although certain DNA damage patterns are relatively strongly associated with UV exposure, it is difficult to completely rule out other causes of similar damage, and the lack of a standardized analytical approach can lead to variation in results depending on the specific genes analyzed and the threshold values applied. Therefore, after excluding other possible primary sites through clinical evaluation, it seems reasonable to consider salivary gland MCC as a primary salivary tumor.
Interestingly, MCC tumor cells show various lymphocytic markers, including TdT, PAX5, and CD5 [14,15], and it could pose a diagnostic pitfall when distinguishing MCCs from lymphocytic neoplasms. However, our cases were nearly all negative for TdT and PAX5, except for one case with trivial positivity for PAX5. Conversely, all cases showed diffuse positivity for SOX2, which is a Merkel cell differentiation regulator and an essential oncogene of MCC [51,52]. It can be used for distinguishing MCC from other skin tumors but not from extracutaneous NEC [16]. An essential pathogenetic mechanism of MCC is Rb protein inactivation [53]. However, loss of Rb expression is frequently observed in a minor portion of MCPyV-negative cases [16,54,55]. Although our cases consistently exhibited Rb and SOX2 positivity, it is less likely that this finding is specific for salivary gland Merkel cell type NEC. Overall, salivary gland Merkel type NEC has similar clinicopathological characteristics with cutaneous MCC.
In conclusion, we propose that MCC can arise in the salivary glands, in addition to the skin. After thoroughly ruling out the possibility of metastasis, these cases should be treated in a manner appropriate for a primary salivary gland malignancy. Salivary gland MCC exhibits a phenotype identical with cutaneous MCC, including MCPyV and SOX2 positivity. We expect that MCC would be listed as a primary salivary gland malignancy in the classification.
Notes
Ethics Statement
All procedures performed in the current study were approved by the Institutional Review Board of Asan Medical Center (approval No. 2023-1328) in accordance with the 1964 Helsinki Declaration and its later amendments. Formal written informed consent was not required with a waiver by the Institutional Review Board of Asan Medical Center.
Availability of Data and Material
1. The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
2. All data generated or analyzed during the study are included in this published article (and its supplementary information files).
3. The datasets generated or analyzed during the study are not publicly available due [REASON(S) WHY DATA ARE NOT PUBLIC] but are available from the corresponding author on reasonable request.
4. The datasets generated or analyzed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS]
5. Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Code Availability
Not applicable.
Author Contributions
Conceptualization: KJC. Data curation: GC, GHK. Formal analysis: GC, GHK. Investigation: GC, GHK. Methodology: KJC. Project administration: GC, KJC. Resources: JSS, HJL, YHJ, YSL, KJC. Supervision: KJC. Validation: JSS, HJL, KJC. Visualization: GC. Writing—original draft: GC. Writing—review & editing: JSS, HJL, KJC. Approval of final manuscript: all authors.
Conflicts of Interest
J.S.S., a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
No funding to declare.