Association study of TYMS gene expression with TYMS and ENOSF1 genetic variants in neoadjuvant chemotherapy response of gastric cancer
Article information
Abstract
Background
The present research was designed to study the associations between genetic variants of TYMS and ENOSF1 genes with TYMS and ENOSF1 gene expression in neoadjuvant chemotherapy response among patients with gastric cancer.
Methods
Formalin-embedded and paraffin-fixed matched tumor and normal gastric cancer tissue samples from patients who received neoadjuvant 5-fluorouracil (5-FU) treatment were obtained. DNA and RNA were extracted for all samples. A 28-bp variable number tandem repeat (VNTR) at the 5' untranslated region of TYMS gene and rs2612091 and rs2741171 variants in the ENOSF1 gene were genotyped for normal tissue samples. The real-time polymerase chain reaction method was used to study the expression of ENOSF1 and TYMS genes in both normal and tumor tissues. Data were analyzed using REST 2000 and SPSS ver. 26.0 software programs.
Results
A significant association between TYMS 2R3R VNTR genotypes and 5-FU therapy was found (p = .032). The 3R3R and 2R2R genotypes were significantly associated with increased and decreased survival time, respectively (p = .003). The 3R3R genotype was significantly associated with TYMS overexpression (p < .001). Moreover, a significant association was found between the rs2612091 genotype and treatment outcome (p = .017).
Conclusions
This study highlights the impact of TYMS and ENOSF1 genes as predictive indicators for survival and response to 5-FU–based neoadjuvant chemotherapy in gastric cancer patients.
INTRODUCTION
Gastric cancer is the fifth most prevalent malignancy in the world and the fourth most lethal cancer type, with a 5-year survival rate less than 20.0% [1,2]. Nonmetastatic gastric and gastroesophageal adenocarcinomas in randomized clinical trials have shown a positive response to combinational treatments [3]. Advanced gastric malignancies can be treated with different cytotoxic substances, including platinum-based compounds, taxanes, fluoropyrimidines (FPs), and irinotecan [3]. The efficacy of chemotherapy, which plays a key role in treatment of both locally advanced and metastatic gastric cancers, is often hampered by the development of chemoresistance. Complex mechanisms are involved in chemoresistance in gastric cancer [4].
5-Fluorouracil (5-FU) is a frequently used FP in various malignancies [5]. The principal target of FPs is the thymidylate synthase (TS) enzyme, which plays a vital role in DNA synthesis. There are opposing associations between TS expression level and tumor vulnerability to FPs [5-7]. Genetic variants in the thymidylate synthetase (TYMS) gene, encoding the TS protein, have been used to predict drug toxicity risk [8]. Variations in the 5′ untranslated regions (5′-UTRs) of the TYMS gene have gained notable attention for their unique ability to affect the stability of TYMS mRNA and gene expression and TS levels [9]. In particular, there are significant associations of change in gene expression, response to 5-FU treatment, and toxic effects with a 28-bp variable number tandem repeat (VNTR) in the TYMS 5′-UTR [6,10-12]. Studies have revealed distinct connections between the 2R and 3R alleles of this VNTR in TYMS expression, 5-FU reactivity, and toxicity [10,13]. The 3R allele is associated with a significant increase in TYMS expression, potentially necessitating higher doses of 5-FU for effective treatment, resulting in a less favorable prognosis and reduced drug efficacy [10,12]. Specifically, the 3R/3R genotype displays greater TYMS expression than the 2R/2R genotype [10,12,14]. In contrast, the 2R/2R genotype has been linked with increased toxicity in patients receiving FP-based chemotherapy, resulting in significant adverse reactions [8,11,15].
Adjacent to the TYMS gene, the enolase superfamily member 1 (ENOSF1) gene seems to have dual roles as a protein-coding gene and an antisense transcript that modulates TYMS mRNA expression and protein levels [10,16-19]. The rs2612091 variant influences the expression of ENOSF1 mRNA and is associated with an increased risk of toxicity induced by capecitabine treatment [16,17].
The rs2741171 variant (located downstream of TYMS and in the intronic region of ENOSF1) has been implicated in the development of hand-foot syndrome (HFS) [13]. Hence, we designed the current study to investigate associations of the 28-bp VNTR in the TYMS gene and of the rs2612091 and rs2741171 variants in the ENOSF1 gene and TYMS gene expression with 5-FU therapy in patients with gastric cancer.
MATERIALS AND METHODS
Participants
We recruited 100 matched tumor and normal formalin-fixed, paraffin-embedded (FFPE) samples from patients with gastric cancer who underwent gastrectomy at Imam Khomeini Hospital, Tehran, Iran, from 2012 to 2018. The cohort consisted of 70 male and 30 female participants, with an average age of 58.10 ± 11.43 years. All patients were treated with 5-FU before surgery. The FFPE blocks of all patients were examined by a pathologist, and matched tumor and normal tissues were punched. The evaluation of chemotherapy effectiveness was categorized based on the standards established by the College of American Pathologists [20]. This guideline classifies the treatment responses into four groups: comprehensive response (with a score of 0), nearly comprehensive response (with a score of 1), partial response (with a score of 2), and minimal or nonexistent response (with a score of 3). Patients were then classified into two groups of positive treatment response (with scores 0 to 2) and inadequate or absent response (with a score of 3).
DNA extraction and genotyping revisions
DNA of matched normal and tumor tissues were extracted using QIAamp DNA FFPE Tissue kit (Qiagen, Hilden, Germany). Genotyping of the 5'-UTR–TYMS VNTR variant was performed using previously reported primers [6,21]. Polymerase chain reaction (PCR) was conducted with a total volume of 22 μL as follows: 50 ng of genomic DNA, 5.0% dimethyl sulfoxide, 10 picomoles of each primer, and the 12 µl of 2× Hot Start PCR mix (Amplicon Co., Copenhagen, Denmark).
The PCR program was carried out with an initial denaturation at 95°C for 15 minutes, followed by 37 cycles of denaturation at 95°C for 1 minute, annealing at 64°C for 45 seconds, and extension at 72°C for 45 seconds and then a final extension step at 72°C for 10 minutes.
Genotyping
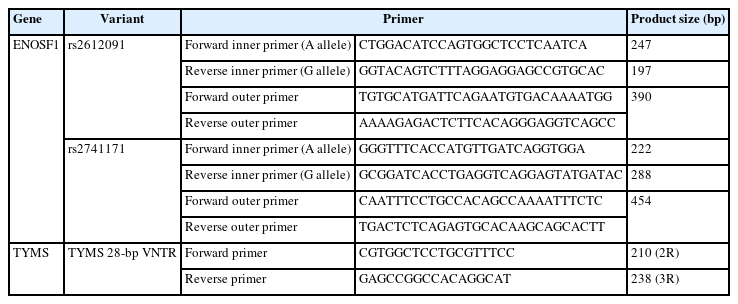
Specific primers were designed for genotyping of the VNTR at the 5′-UTR of the TYMS gene (Table 1). The 2R allele with a size of 210 bp and 3R allele with a size of 238 bp were identified using agarose gel electrophoresis. Tetra-ARMS PCR was used for genotyping of rs2612091 and rs2741171 variants in the ENOSF1 gene (Table 1). Tetra-ARMS PCR primers were designed using the Primer 1 online program (primer1.soton.ac.uk/primer1.html).
The PCR mixture reaction for tetra-ARMS PCR comprised between 50 and 100 ng of genomic DNA, 2× Hot start PCR Master Mix Blue (Amplicon Co.), and 5 pmol of each primer. The PCR conditions were 95ºC for 10 minutes; 40 cycles of 95ºC for 1 minute, 62ºC for 1 minute, and 72ºC for 1 minute; and a final extension for 10 minutes at 72ºC. Amplification products were examined, and genotypes were identified on a 3.0% agarose gel.
RNA extraction and gene expression analysis
The total RNA from FFPE matched tumor and normal tissues of 100 patients was extracted using the Hybrid-R GeneAll Kit (GeneAll Co., Seoul, Korea). cDNA was synthesized using a commercial kit (Yekta Tajhiz Azma, Tehran, Iran). Then, quantitative real-time PCR (qPCR) analysis was performed using the Cyber Green method with previously published primers for TYMS gene expression [22]. In addition, specific primers were designed for the ENOSF1 gene expression analysis as follows: forward primer (5'-ACAGGCACTTCCAATTCCGA-3') and reverse primer (5'-AGAGCTGCTTCAACGTGTCA-3'). The GAPDH gene was used as the reference control with the following primer sequences: forward primer (5′-TCACCAGGGCTGCTTTTAAC-3′) and reverse primer (5′-GACAAGCTTCCCGTTCTCAG-3′).
For each assay, the total volume of the PCR reaction was 20 μL comprised of 2 μL of cDNA, 10 pmol of each primer, and 10 μL of Cyber Green 2× master mix (Amplicon Co.). The qPCR was performed using an initial denaturation step at 95°C for 15 minutes. This was followed by 40 cycles of denaturation at 95°C for 30 seconds, annealing at 60°C for 60 seconds, and extension at 72°C for 30 seconds. To ensure the robustness and reliability of the results, each sample underwent analysis in triplicate.
Statistical analysis
Data analysis was conducted using REST 2000 and SPSS ver. 26.0 software (IBM Corp., Armonk, NY, USA). The chi-square test, a two-sided independent t-test, and one-way ANOVA were applied to examine the relationships between variables. A significant difference was established at .05. Patient survival times were estimated using the log-rank test and Kaplan-Meier survival analysis. The Cox regression semi-parametric model assessed the mortality risk ratio across variant states. Changes in expression levels were measured using the 2-ΔΔCT method.
RESULTS
Patient characteristics
In this study of 100 patients with gastric cancer, 43 experienced resistance to chemotherapy. The average age of the patients was 58.10 ± 11.43 years 22 had grade 1 disease, 35 had grade 2, and 43 had grade 3. The stage of disease was 1 in 22 cases, 2 in 34, 3 in 39 cases, and 4 in five cases. Among the 57 patients who responded to treatment, 13 experienced complete remission (rated as score 0), 15 achieved near-complete remission (score 1), and 29 demonstrated a partial response (score 2). Over a maximum follow-up period of 5 years, 24 patients showed disease progression, 47 patients died, and 29 patients maintained a favorable condition.
Genotypic variations and chemotherapeutic response
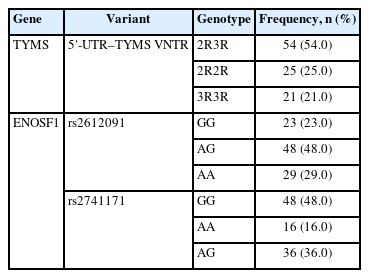
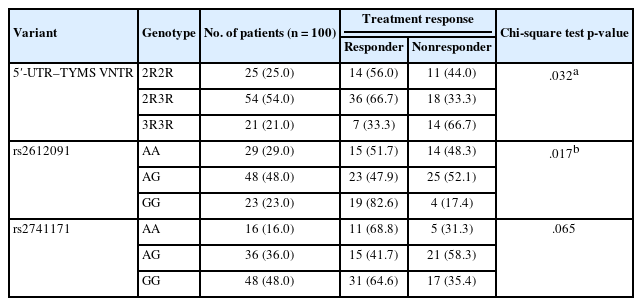
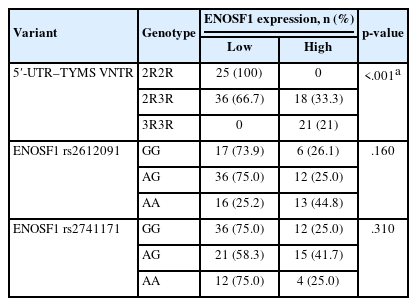
A significant association was observed between VNTR genotype and the effectiveness of neoadjuvant chemotherapy (p = .032) (Tables 2, 3). The 2R3R genotype was found more frequently in patients with a positive response to the treatment (66.7%), followed by the 2R2R genotype. Conversely, the 3R3R genotype was more common in those who did not respond to the treatment.
Additionally, a significant association was found between the rs2612091 variant and the efficacy of treatment (p = .017), with the AG genotype more frequent in non-responders. However, no significant association was found between rs2741171 genotype and treatment outcome (p < .05).
Variant impact on survival outcomes
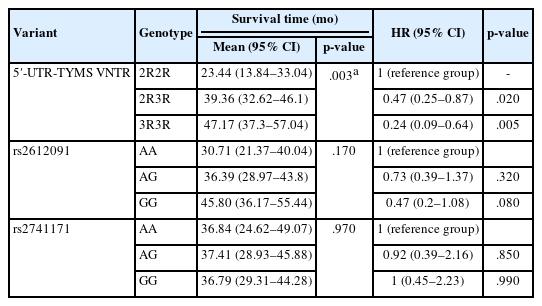
A significant association between overall survival and TYMS VNTR genotype was identified (p = .003). Those with the 3R3R genotype had longer survival times in contrast to those with the 2R3R genotype, who had the shortest survival time (Table 4, Fig. 1A). Mortality risk analysis using the Cox model showed that patients with the 3R3R or 2R3R genotype had reduced hazard ratio (HR) of 0.24 and 0.47, respectively, compared to those with the 2R2R genotype. Despite the lack of significance (p = .170), individuals carrying the GG or AA genotype of the rs2612091 variant showed longer and shorter survival, respectively, after treatment with 5-FU (Table 4, Fig. 1B).

Survival time analysis of patients with gastric cancer with different genetic variants after 5-fluorouracil treatment. (A) Graph of overall survival (OS) and variable number tandem repeat (VNTR) genotypes shows a significant relationship (p = .003). The longest survival time is related to the 3R3R genotype and the shortest survival time is related to the 2R3R genotype. (B) Graph of the association of OS and rs2612091. (C) Graph of the association of OS and rs2741171. TYMS, thymidylate synthetase; ENOSF1, enolase superfamily member 1.
Similarly, the Cox model analysis showed no significant difference in HR for mortality among patients with GG or AG genotype compared to those with the AA genotype (p > .05). For the rs2741171 variant, patients with AG genotype were associated with the longest survival after 5-FU treatment, whereas those with GG genotype had the shortest (Table 4, Fig. 1C). However, this observation was not statistically significant (p = .970).
Gene expression analysis
The TYMS gene was overexpressed in 61 tumor samples, while the remaining 39 did not reveal significant variations in gene expression. An association between TYMS gene expression and the 28-bp allele of the VNTR variant was found (p = .001). The TYMS gene expression was downregulated among samples with the 2R2R genotype and overexpressed among samples with the 3R3R genotype (Table 5). No statistical significance was found between TYMS gene expression and treatment response (p = .206).
Even though this result was not significant, the ENOSF1 gene was downregulated in most tumor tissue samples (69%) compared to adjacent normal tissue samples that showed overexpression (31.0%) (Table 5).
TYMS and ENOSF1 gene expression analysis
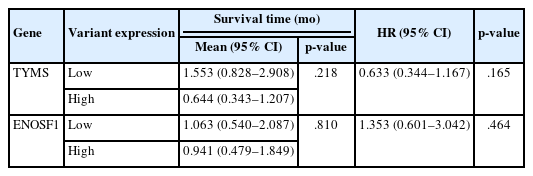
No significant association was found between survival time and TYMS or ENOSF1 gene expression (Table 6, Fig. 2). Furthermore, no significant association was detected between TYMS or ENOSF1 gene expression and mortality risk (Table 6, Fig. 2).

Survival time analysis of gastric cancer patients after 5-fluorouracil treatment according to change in thymidylate synthetase (TYMS) gene expression. (A) Survival and gene expression graph of TYMS. (B) Survival and gene expression graph of enolase superfamily member 1 (ENOSF1). There was no correlation between expression changes of TYMS and ENOSF1 genes and overall survival (p = .218, p =.810).
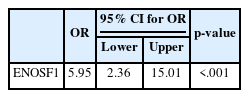
As shown in Table 7, there was an adverse significant association between TYMS and ENOSF1 gene expression (p = .001; odds ratio, 5.95; 95% confidence interval, 2.36 to 15.01), as sample tissues with overexpression of the TYMS gene showed reduced expression of the ENOSF1 gene in tumor tissues.
DISCUSSION
Genetic variants have a critical impact on gene expression in anti-tumor drug reactions, with a particular emphasis on FPs and their impact on gastric cancer [10,13,23-25].
TS, a key target of FPs, plays a crucial role in DNA synthesis and repair. Variations in the TYMS gene encoding the TS protein are linked with survival rates and toxicity levels in patients treated with 5-FU chemotherapy [26,27]. The ENOSF1 gene, closely related to and overlapping the TYMS, has also been identified as a potential factor in chemotherapy resistance, especially in 5-FU treatment [28,29]. This study meticulously investigated the 28-bp VNTR in the 5'-UTR of TYMS, along with the rs2612091 and rs2741171 variants in ENOSF1, in 100 gastric cancer patients undergoing 5-FU–based neoadjuvant chemotherapy. In addition, TYMS and ENOSF1 gene expression was measured on matched tumor and normal tissue samples.
A significant association was found between the TYMS 28-bp VNTR variant and neoadjuvant chemotherapy response (p = .032). The 2R3R genotype was more common in patients responding well to treatment than was the 3R3R genotype. In gastric cancer, TYMS overexpression has been detected in association with reduced 5-FU efficacy [11]. In addition, the 3R3R genotype is associated with TYMS overexpression and a poor response to 5-FU [11,30]. Similar patterns were observed in rectal cancer, where the 2R3R genotype is associated with better treatment outcomes [31]. In contrast, the 2R2R genotype may indicate a higher toxicity risk with FP treatment [8,11,15,26,32].
We also found a relationship between heightened toxicity and improved response to treatment in patients possessing the 2R2R genotype. Although the connection between the TYMS gene variant and treatment effectiveness is well-established, its association with survival time is uncertain. A previous study has reported significant disparity in survival time following 5-FU treatment between patients with the 3R3R and 2R2R genotypes (p = .003). However, other studies have reported that patients with the 3R2R genotype have a longer survival time than those with the 3R3R and 2R2R genotypes [33], with similar findings observed in lung cancer [34]. Nevertheless, some research did not identify noteworthy variations in survival time linked to TYMS 5'-UTR variants [32,34-38]. A significant relationship has been established between TYMS gene expression and the 28-bp VNTR variant (p < .001). The 2R2R genotype is associated with reduced TYMS expression, while the 3R3R genotype is linked to increased expression. This indicates the interaction between TYMS gene expression and VNTR alleles [11,32,39,40], emphasizing the predictive role of the 3R allele in intratumoral TYMS expression and 5-FU response. Patients with the 2R2R genotype, exhibiting lower levels of TYMS mRNA, tend to demonstrate a more favorable response to 5-FU [39]. However, reduced TYMS expression in normal tissues, particularly in lung and gastrointestinal cancers, increases the risk of 5-FU toxicity [11,32,40]. Nonetheless, the correlation between the 28-bp VNTR and TYMS expression remained inconclusive in certain studies [11,23,41]. Rosmarin et al. [17] underscored the influence of ENOSF1 on cell sensitivity to FPs, where the ENOSF1 rs2612091 G/G genotype in colorectal cancer is linked to shorter survival. That investigation also assessed the impact of the ENOSF1 rs2612091 variant on survival in advanced gastric cancer, indicating poorer outcomes with each additional G allele [42]. Furthermore, the G allele has been associated with increased capecitabine-related toxicity [17]. Different studies have explored adverse reactions to capecitabine, yielding mixed findings of the significance of these associations [9,28,43]. The involvement of the ENOSF1 c.742-227G>A variant in the development of FP-dependent HFS has also been acknowledged [44]. Palles et al. [45] have suggested modifying HFS management based on rs2612091 testing. The research has revealed a notable correlation between rs2612091 genotypes and the efficacy of 5-FU–based therapies (p = .017), with a greater frequency of non-responsiveness in individuals carrying the AG genotype. Nonetheless, the variance in survival duration following 5-FU treatment among genotypes was not significant (p = .170).
The rs2741171 variant downstream of TYMS and within ENOSF1 has shown no significant association with treatment response, patient survival, or ENOSF1 expression. Yang et al. reported that gastric cancer tumors exhibited twice the ENOSF1 expression levels of controls, identifying two peptide regions as potential diagnostic biomarkers [46,47]. In the present study, no substantial relationship was observed between the expression of ENOSF1 and either the response to treatment or the mean survival duration (p = .810).
Furthermore, the Cox model did not reveal a significant risk for mortality based on ENOSF1 expression levels. Previous research has supported the notion that ENOSF1 overexpression, functioning as a reverse TYMS, reduces TYMS expression through antisense RNA production and rTS-β protein synthesis to influence TYMS activity at post-transcriptional and post-translational levels [48,49].
In summary, we revealed a substantial association between the 28-bp TYMS VNTR and TYMS gene expression in the efficacy of neoadjuvant chemotherapy employing 5-FU in patients with gastric cancer. Those harboring the 2R3R genotype of TYMS showed superior treatment outcomes. Conversely, individuals bearing the 3R3R genotype showed elevated TYMS gene expression when juxtaposed with their counterparts possessing the 2R2R genotype. Nevertheless, an augmented response was discerned among subjects exhibiting a mixture of 3R and 2R alleles. This implies that the complex interaction between these two genetic repeats may be an unrecognized determinant influencing the response to 5-FU in the cohort under investigation. These findings suggest the need for analysis of interactions of other TYMS genetic variations with the 28-bp VNTR.
In addition, individuals with the AG genotype of the rs2612091 variant displayed a weaker response to neoadjuvant chemotherapy. However, this genetic variant did not demonstrate a significant correlation with overall survival rate or the expression level of the ENOSF1 gene.
The rs2741171 variant showed no apparent correlation with survival rate, neoadjuvant chemotherapy efficacy, or gene expression pattern in patients with gastric cancer. However, several limitations in this study should be considered. One major limitation is that a multivariate analysis was not performed but is crucial to adjust for confounding variables, such as age, sex, and other clinical factors, which may independently influence the outcomes. Without this analysis, it is difficult to assess the true relationships between the genetic variants and neoadjuvant chemotherapy response.
Additionally, the sample size in our study was relatively small, which can limit the statistical power and increase the risk of type II errors, and real associations might not be detected. Another limitation is that the study focused on a specific patient population, which may limit the generalizability of the findings to broader or more diverse groups. Moreover, environmental and lifestyle factors that were not considered may have impacted the observed outcomes. Therefore, future studies should include larger and more diverse populations, perform multivariate analyses, and consider additional factors such as gene-environment interactions to provide a more comprehensive understanding of the role of TYMS and ENOSF1 genetic variants in neoadjuvant chemotherapy response.
Notes
Ethics Statement
This study was approved by the ethics committee (ID IR.IAU.SRB.REC.1397.110 on October 23, 2017). A consent form was obtained from patients before using their samples in this study.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: KA, IS, SI, FA, ME. Data curation: KA, IS, SI, FA, ME. Formal analysis: KA, IS, SI, FA, ME. Writing—original draft: KA. Writing—review & editing: KA, IS, SI, FA, ME. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.