Unilateral Pigmented Extramammary Paget's Disease of the Axilla Associated with a Benign Mole: A Case Study and a Review of Literature
Article information
Abstract
Pigmented extramammary Paget's disease (PEMPD) is an uncommon intraepithelial adenocarcinoma and a rare variant of Paget's disease affecting skin that is rich in apocrine sweat glands such as the axilla, perianal region and vulva. It most commonly occurs in postmenopausal women and presents as a superficial pigmented scaly macule, mimicking a melanocytic lesion. The histological presentation is adenocarcinoma in situ with an increased number of melanocytes scattered between the Paget's cells. Therefore, PEMPD may be misdiagnosed as a melanocytic tumour both clinically and histologically. The tumour cells are usually positive for cytokeratin 7, epithelial membrane antigen, Cam 5.2, HER2, and mucicarmine stain while S100 and human melanoma black-45 highlight the processes of reactive dendritic cells. The association between Paget's cells and intratumoural reactive melanocytes is still unclear. We report our first case of PEMPD associated with an intradermal naevus involving the axilla in a 63-year-old woman.
Pigmented extramammary Paget's disease (PEMPD) is an uncommon intraepithelial adenocarcinoma and a rare variant of Paget's disease affecting areas with skin that is rich in apocrine sweat glands such as the axilla, perianal region and vulva. It most commonly occurs in postmenopausal women and presents as a superficial pigmented scaly macule, mimicking a melanocytic lesion. Histological examination shows adenocarcinoma in situ with an increased number of melanocytes scattered between the Paget's cells. Therefore, it is not uncommon for PEMPD to be clinically and histologically misdiagnosed as a melanocytic tumour. The tumour cells are usually positive for cytokeratin 7 (CK7), epithelial membrane antigen (EMA), Cam 5.2, HER2, and mucicarmine stain while S100, Melan A, and human melanoma black-45 (HMB-45) highlight the non-neoplastic dendritic cells.1,2,3,4,5,6 The association between Paget's cells and intratumoural reactive melanocytes is still unclear.
Herein we describe what is, to our knowledge, the first occurrence of unilateral PEMPD associated with an intradermal naevus involving the axilla of a 63-year-old female.
CASE REPORT
A 63-year-old Caucasian female was referred to the dermatology department due to a small, scaly, hyperpigmented lesion in the right axilla. The lesion had been asymptomatic for many years and was clinically thought to represent an irritated benign mole. The patient did not have a family history of breast cancer or report any nipple discharge or other breast changes. On clinical examination there was no evidence of lymphadenopathy or underlying malignancy in her axilla or either breast. The mammogram identified benign-appearing microcalcifications in the right breast without significant changes from previous screening images. The ultrasound scan showed features consistent with inflammatory changes, but no focal lesions or lymphadenopathy in the right axilla. Local excision of the lesion was performed and sent for histological examination. Macroscopically the skin biopsy measured 20×8×4 mm and incorporated a slightly raised brown nodule measuring 6 mm in maximum dimension.
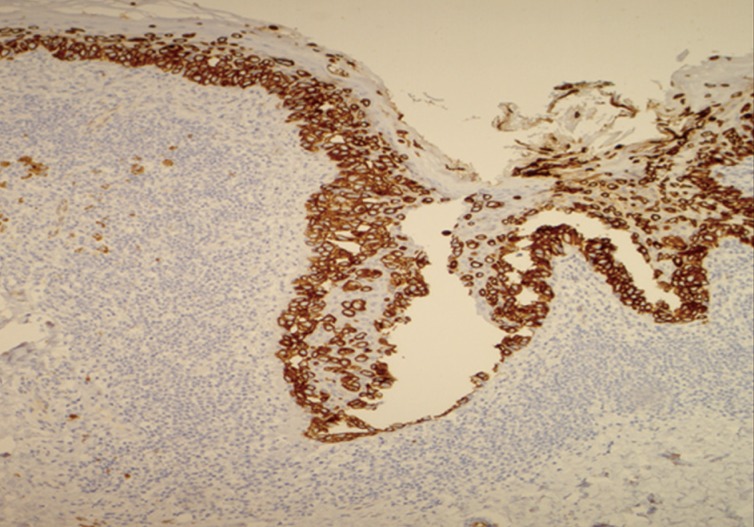
The histology of the excised lesion revealed a focus of Paget's disease, characterized by the presence of large round cells with abundant pale or granular/dusty cytoplasm, pleomorphic vesicular nuclei and prominent nucleoli (Paget's cells). These cells were arranged in small clusters or dispersed singly and occupy the whole thickness of the overlying epidermis (carcinoma in situ), which was focally hyperkeratotic (Fig. 1). There was tumour extension into the epithelium of the adnexa, consistent with a pattern of pagetoid spread, but no evidence of dermal invasion was noted (Fig. 2). No ectopic breast tissue was identified in the biopsy. In the mid dermis there was a small intradermal naevus and prominent evidence of chronic inflammation with deposits of melanophages (Figs. 3, 4). Immunohistochemistry was performed, where Paget's cells showed diffuse positive staining with Cam 5.2, EMA, CK7, and HER2 (Figs. 4, 5, 6). The Paget's cells were negative for p63, carcinoembryonic antigen (CEA), S100, Melan A, HMB-45, estrogen receptor, progesterone receptor, and CK20. There was a patchy reaction with gross cystic disease fluid protein 15 (GCDFP-15), but the mucicarmine stain was negative. HMB-45, a melanocytic marker stained many dendritic processes of benign melanocytes, which are scattered between the Paget's cells. Some of the Paget's cells showed a certain amount of melanin pigment in the cytoplasm that was highlighted by the HMB-45 staining (Fig. 7). A re-excision was carried out, as the tumour extended to the surgical margin. The subsequent histology examination revealed a residual focus of Paget's disease, with all margins clear of tumour.
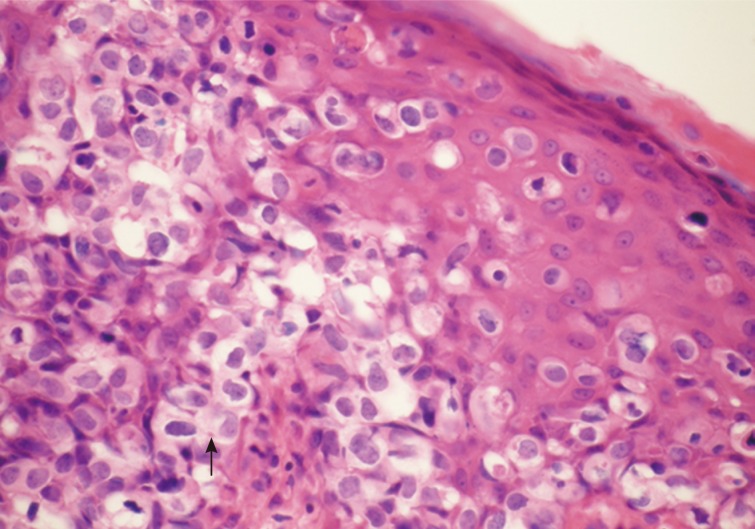
Pigmented extramammary Paget's disease. There is severe intraepidermal infiltration of pleomorphic cells with pale or granular cytoplasm and prominent nuclei (Paget's cells). They lie in vacuoles or form tiny clusters. Melanin pigment is seen in some tumour cells (arrow).
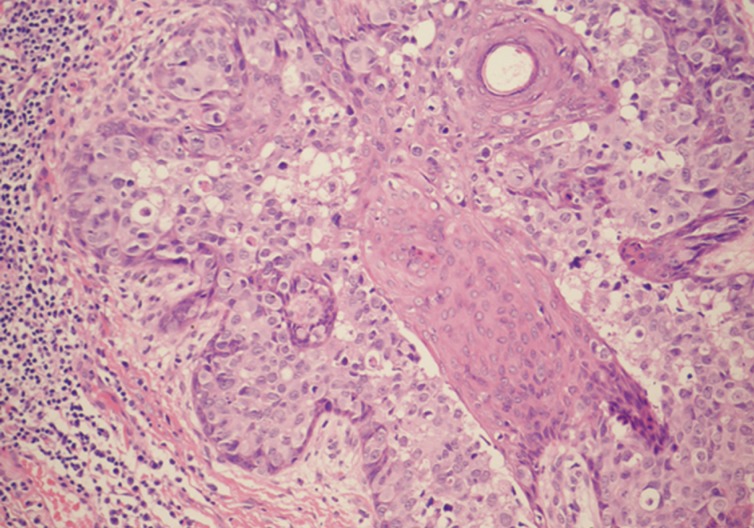
Pigmented extramammary Paget's disease. Spread of Paget's cells alongside the epithelium of the pilosebaceous unit, but without invasion in the adjacent dermis.
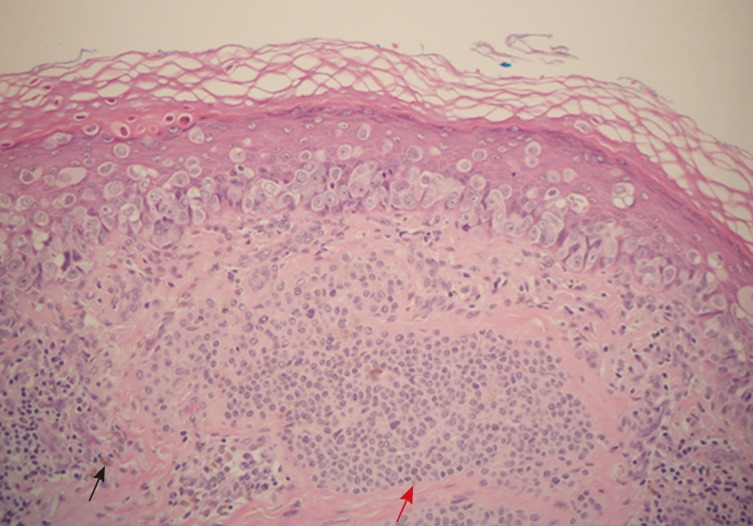
Pigmented extramammary Paget's disease. The red arrow shows the intradermal naevus and the black arrow indicates melanophages.
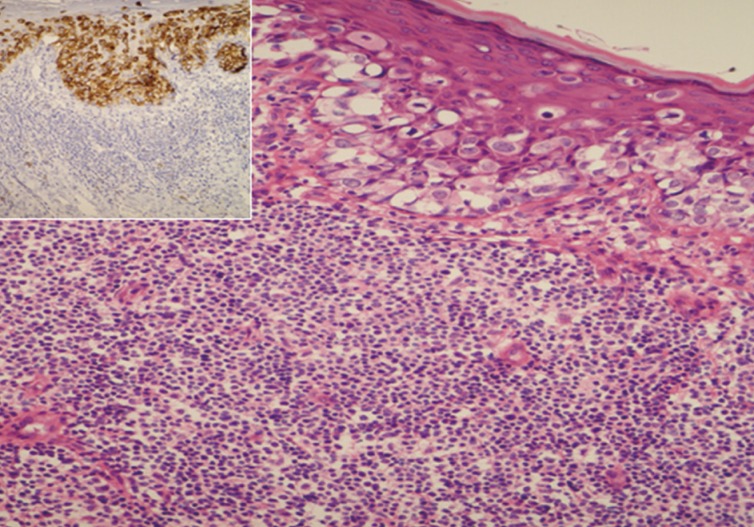
Pigmented extramammary Paget's disease. There is dense chronic inflammation in the mid dermis. Paget's cell are highlighted by a strong positive staining with epithelial membrane antigen (inset).
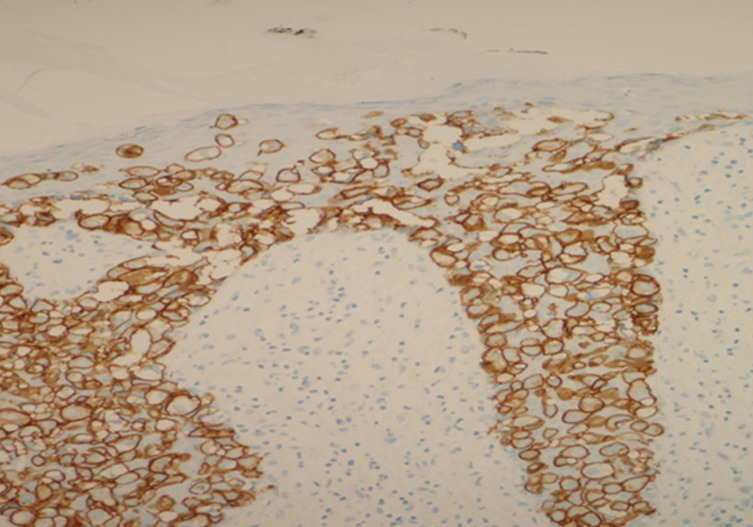
Pigmented extramammary Paget's disease. Paget's cells show strong complete membrane staining for HER2.
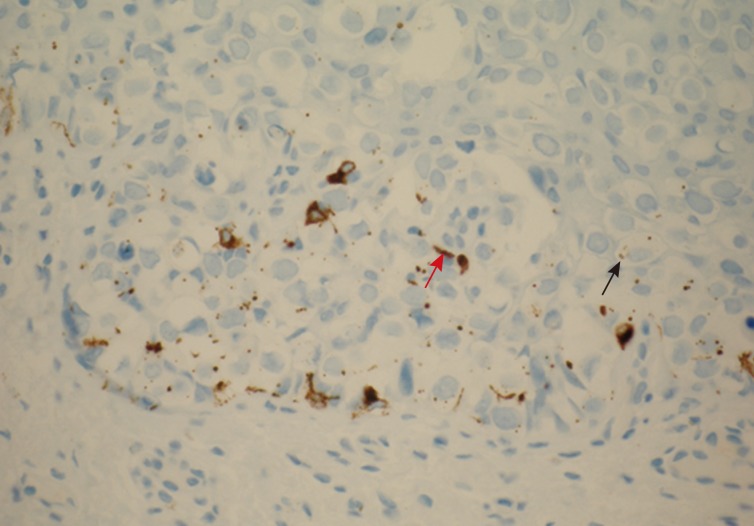
Pigmented extramammary Paget's disease. Human melanoma black-45 stains the non-neoplastic dendritic melanocytes within the epidermis (red arrow) and granular melanin pigment within the cytoplasm of Paget's cells (black arrow).
The patient is currently being followed up every six months, and has shown no signs of recurrence in the 30 months since the excision.
DISCUSSION
Paget's disease is an intraepidermal adenocarcinoma first described by Paget7 in 1874. Extramammary Paget's disease (EMPD) is an uncommon malignancy accounting for 6.5% of all cases of Paget's disease, and was first described by Crocker8 in 1889. It occurs in skin that is rich in apocrine sweat glands, with the anogenital region being the most commonly affected area. Other rare areas affected by EMPD include the axilla, vulva and even less frequently the external ear canal, truncal skin and eyelids.1,8 Unlike mammary Paget's disease, EMPD is less often associated with an underlying malignancy.9 A recent study showed that only 14% of patients with unilateral axillary EMPD had an underlying carcinoma.10
The pigmented variant of EMPD (PEMPD) is a very rare malignancy that mimics malignant melanoma and has been reported only a limited number of times in the literature.2,3,4,5,6 To our knowledge we report here the first case of PEMPD in the axilla associated with an intradermal naevus. Pigmentation of Paget's cells was first described clinically by Culberson and Horn11 in 1956. The hyperpigmentation in PEMPD results from epidermal colonization by numerous benign melanocytes scattered among tumour cells, accumulation of cytoplasmic melanin pigment in Paget's cells and the presence of melanophages in the dermis.12 In the current case, granular or dusty cytoplasm within the tumour cells was focally present and colonization of intervening dendritic melanocytes was not severe. Furthermore, in the mid dermis there was a mature-appearing melanocytic lesion consistent with an intradermal naevus as well as an intense lymphocytic inflammatory infiltrate with deposits of melanophages most similar to the appearance of regression in malignant melanoma. The association with a benign melanocytic lesion has not been described before in PEMPD. This association probably augmented the brown/dark colour of the skin nodule in our case.
The mechanism driving the increased number of reactive intraepidermal dendritic melanocytes and the relationship between Paget's cells and melanocytes remains unclear, although such reactive changes have also been reported in many other skin lesions such melanoacanthoma, seborrhoeic keratosis, basal cell carcinoma or pigmented Bowen's disease. The release of basic fibroblastic growth factor and other chemotactic agents from the tumour cells of pigmented epidermotropic breast cancer has been suggested to play a role in reactive proliferation of melanocytes and pigment transfer to tumour cells.12 This theory may also explain the presence of melanin pigment in the cytoplasm of some tumour cells and the subsequent clinical and histological pigmentation in PEMPD.
PEMPD may be misdiagnosed clinically and histologically as a melanocytic tumour such as malignant melanoma. Careful examination of the histomorphology, use of immunohistochemistry and special stains can help to establish the correct diagnosis. The axillary lesion in the current case was clinically diagnosed as an irritated benign mole; neither an increase in size or variable pigmentation was noted.
In PEMPD the neoplastic cells are localised in or above the basal layer, but not at the dermo-epidermal junction as found in melanoma in situ.13 Unlike PEMPD, superficial spreading melanoma is usually positive for S100, Melan A, and HMB-45 (melanocytic markers) and negative for CK7, CEA, HER2, Cam 5.2, and EMA. Mucicarrmine and GCDFP-15 are variably positive in Paget's cells. The nonpigmented variant of EMPD bears a similar immunoprofile to PEMPD. In our case, the neoplastic cells had infiltrated the full thickness of the epidermis and there was no evidence of cellular proliferation at the dermo-epidermal junction. PEMPD was confirmed by positive staining for Cam 5.2, CK7, EMA, HER2, and negative for S100, HMB-45. There was no evidence of reaction with mucicarmine and GCDFP-15. In addition HMB-45 was focally positive in reactive dendritic melanocytes, in the melanin granules within Paget's cells and in dermal melanophages.
PEMPD may also mimic other pathological conditions such as pigmented Bowen's disease, or squamous cell carcinoma in situ.14 The presence of intracellular mucin, signet cells, glandular structures and the absence of dyskeratotic cells are all relevant features that distinguish Paget's disease from Bowen's disease.1 In addition Paget's cells are usually positive for CK7, EMA, and CEA whilst these markers are negative in Bowen's disease.15
Simultaneous EMPD of the axilla and anogenital region is not rare; however unilateral axillary occurrences are extremely uncommon.16 In our case, the patient was diagnosed with a single unilateral lesion in the right axilla and had not developed any evidence of disease in any other location. EMPD is a slow growing tumour and its appearance is often modified by superimposed infections or repeated trauma with or without excoriation.1 The main presenting symptom in one third of axillary EMPD cases is pruritus.10
The prognosis in most patients with in situ EMPD is very good, however worse prognosis has been noted in the presence of dermal invasion.17 In our case there was focal neoplastic extension to the pilosebacious units, but without dermal invasion or any underlying malignancy.
The management of EMPD is essentially surgical, which in the case of the axillary site can lead to significant morbidity. Therefore, local excision is the favoured approach for noninvasive, locally confined disease.10 A recent study of seven cases of unilateral axillary EMPD showed no recurrence after local resection.10 In comparison, the recurrence rates for genital and perianal EMPD have been shown to be 32-50% and 50-70%, respectively.18 Our patient is now on long-term follow-up every six months, and has shown no signs of recurrence for 30 months.
In many cases the histological examination shows spread of EMPD beyond the visible lesion, and multiple surgical excisions are often required to control residual disease.19 The residual lesion in our case was surgically cleared in the second local excision following the identification of a positive margin on the first excision specimen. Intraoperative frozen sections have been used to improve the rates of complete excision. However, one study showed a 31% inaccuracy rate, which was comparable to the inaccuracy rate associated with the simple visual assessment of biopsy specimens.20 Other treatments such as photodynamic therapy have been used. Although the effectiveness this treatment for Paget's seems promising, the rarity of the disease and lack of comparative trials limit current understanding of the value of this therapy.
In conclusion, we report the first case of PEMPD associated with an intradermal naevus involving the axilla of a 63-year-old female. PEMPD of the axilla is a very rare finding, and generally has an indolent clinical course, presenting both clinical and histological diagnostic challenges. PEMPD is a potential diagnostic pitfall due to confusion with melanocytic tumours and therefore careful histology examination and use of immunohistochemistry as well as special stains is essential in establishing the accurate diagnosis. Wide local excision with clear surgical margins is the treatment of choice in PEMPD. The mechanism of Paget's cell pigmentation and reactive proliferation of intralesional melanocytes in PEMPD has been poorly explored. For this reason further studies are necessary to clarify the mechanism of hyperpigmentation and the relationship between reactive dendritic melanocytes, naevus cells, melanophages, and Paget's cells in PEMPD.
Notes
No potential conflict of interest relevant to this article was reported.