Differential Features of Microsatellite-Unstable Colorectal Carcinomas Depending on EPCAM Expression Status
Article information
Abstract
Background
Recent studies have revealed that a small subset of Lynch syndrome-associated colorectal carcinomas (CRCs) is caused by a germline EPCAM deletion-induced MSH2 epimutation. Based on the finding of this genetic alteration, we investigated the implications of EPCAM expression changes in microsatellite instability-high (MSI-H) CRCs.
Methods
Expression of EPCAM and DNA mismatch repair proteins was assessed by immunohistochemistry in 168 MSI-H CRCs. Using DNA samples of these tumors, MLH1 promoter methylation status was also determined by methylation-specific real-time polymerase chain reaction method (MethyLight).
Results
Among 168 MSI-H CRCs, complete loss (CL) and focal loss (FL) of EPCAM expression was observed in two (1.2%) and 22 (13.1%) cases, respectively. Both of the EPCAM-CL cases were found in MSH2-negative tumors without MLH1 promoter methylation. However, only nine of the 22 EPCAM-FL tumors had MSH2 deficiency. Of the 22 EPCAM-FL tumors, 13 showed MLH1 loss, and among them, nine cases were determined to have MLH1 methylation. EPCAM-FL was significantly associated with advanced stage (p=.043), distant metastasis (p=.003), poor differentiation (p=.001), and signet ring cell component (p=.004).
Conclusions
Loss of EPCAM expression is differentially associated with clinicopathological and molecular features, depending on the completeness of the loss, in MSI-H CRCs.
Lynch syndrome (formerly called hereditary nonpolyposis colorectal cancer) is an autosomal dominant disorder caused by a germline defect in the DNA mismatch repair (MMR) system. It is characterized by early development of malignancies, particularly colorectal carcinoma (CRC) and endometrial carcinoma.1 Defective MMR systems typically induce microsatellite instability (MSI), which results in frequent mutations in cancer-related genes, ultimately contributing to carcinogenesis.2 MSI is defined as an alteration in the length of DNA microsatellite repeat sequences and is accepted as one of the major molecular phenotypes in CRCs, including sporadic tumors as well as Lynch syndrome tumors. Sporadic CRCs that are microsatellite instability-high (MSI-H) account for approximately 10% to 15% of CRCs, and hereditary MSI-H CRCs (Lynch syndrome-associated CRCs) account for approximately 3% to 5% of CRCs in Western countries.3,4 It is well known that the major cause of a defective MMR system in Lynch syndrome is a germline mutation in one of the MMR genes, including MLH1, MSH2, PMS2, or MSH6, whereas nearly all sporadic MSI-H CRCs are thought to be caused by CpG island hypermethylation in the promoter region of MLH1.2,4
Although most Lynch syndrome-associated tumors have a molecular basis of an MMR deficiency caused by germline mutations in MMR genes, Ligtenberg et al.5 recently identified another mechanism of defective MMR in a small subset of Lynch syndrome families, characterized by MSH2 promoter methylation due to germline deletions in the 3' exons of the epithelial cell adhesion molecule (EPCAM; also known as TACSTD1) gene. This molecular alteration is also referred to as MSH2 "epimutation" on the basis of its unique heritable feature of epigenetic silencing.6 Various deletions in the 3' end of the EPCAM gene have been identified in Lynch syndrome tumors, and the molecular mechanism of the MSH2 epimutation has been successfully elucidated.7,8 Interestingly, recent investigations have suggested that the loss of EPCAM protein expression could be a supportive diagnostic marker in cases of Lynch syndrome because EPCAM loss is specifically found in Lynch syndrome patients carrying EPCAM germline deletions.9,10,11 Although these findings indicate that immunohistochemistry (IHC) for EPCAM could be a simple and useful method for the screening of germline EPCAM deletion-associated Lynch syndrome patients, the studies have some limitations in that only small sample sizes originating from a few Western races (Germans and Spaniards) were investigated by only two research groups. Additional studies using larger samples from diverse ethnic groups are necessary for the validation of the relationship between a loss of EPCAM expression and MSH2-deficient Lynch syndrome CRCs.
In the present study, we investigated the expression statuses of EPCAM and MMR proteins using IHC in 168 MSI-H CRC tissues obtained from Korean patients. Promoter CpG island methylation status of the MLH1 gene was also determined for each sample. Association of alterations in EPCAM expression with various clinicopathological and molecular factors was analyzed.
MATERIALS AND METHODS
Study samples
Tissue collection and MSI analysis were conducted as previously described.12 Formalin-fixed, paraffin-embedded (FFPE) tissues of 168 MSI-H CRC cases were retrieved from the pathology archives of our hospitals. All tissue samples originated from patients who received curative surgery for CRC between 2004 and 2007, and were previously diagnosed as MSI-H through DNA testing using microsatellite markers recommended in the Bethesda guidelines (BAT-25, BAT-26, D5S346, D17S250, and D2S123).13 Microsatellite length alteration of two or more markers in a tumor determined MSI-H status. This study was approved by the Institutional Review Board (IRB No. H-1203-072-402).
Clinicopathological data
Collection and assessment of clinicopathological data were carried out as previously described.12 Clinical data including age, gender, tumor location, gross tumor type, tumor multiplicity, and TNM stage were collected from the medical records. Histopathological data, including lymphovascular invasion, perineural invasion, tumor grade (histological differentiation based on gland formation), extracellular mucin component, signet ring cell carcinoma component, and medullary carcinoma component, were evaluated by microscopic review of hematoxylin and eosin-stained tissue slides of the 168 MSI-H CRCs.
Immunohistochemistry
A tissue microarray (TMA) was constructed as previously described.12 Three different areas of tumor from each MSI-H CRC tissue were extracted as tissue cores for the construction of TMA blocks (Superbiochips Laboratories, Seoul, Korea). Immunostaining with antibodies against MLH1 (DAKO, Glostrup, Denmark), MSH2 (Invitrogen, Camarillo, CA, USA), PMS2 (Ventana Medical Systems, Tucson, AZ, USA), MSH6 (Ventana Medical Systems), and EPCAM (Ber-EP4 clone, Ventana Medical Systems) was performed on the TMA blocks. An automated IHC method was applied using the BenchMark XT immunostainer (Ventana Medical Systems) according to the manufacturer's protocol. The expression of MLH1/MSH2/PMS2/MSH6 proteins was interpreted as negative (loss) or positive (retained). Nuclear staining in tumor cells should be observed for the determination of positivity of MLH1/MSH2/PMS2/MSH6 proteins. EPCAM protein expression status was assessed to be negative (loss) or positive (retained). The normal expression pattern of the EPCAM protein is cytoplasmic and membranous staining. Complete loss of EPCAM expression (EPCAM-CL) was determined when negative staining was observed in 100% of tumor cells in all three tissue cores of an individual case. Focal loss of EPCAM expression (EPCAM-FL) was determined when negative staining was observed in 10% to 99% of tumor cells in all three tissue cores of an individual case. A case showing negative staining of EPCAM in less than 10% of tumor cells was determined to have retained EPCAM expression. All of the EPCAM-CL cases, which were initially determined by EPCAM IHC on TMA blocks, were confirmed by EPCAM IHC on the original FFPE tissue blocks (at least two representative tumor sections).
Genomic DNA extraction and bisulfite modification
DNA isolation from FFPE tissues and bisulfite modification of the extracted DNA were performed as previously described.14 Briefly, manually microdissected tumor tissues of the 168 MSI-H CRCs were digested in lysis buffer (proteinase K 3 mg/mL) and digestion solution (50 mM Tris, 1 mM EDTA, pH 8.0, and 1% Tween-20), and incubated at 55℃ overnight (up to 48 hours). The samples were then incubated at 95℃ to inactivate the proteinase K. The extracted genomic DNA was stored at -20℃ until use. Next, as a preparative stage for DNA methylation analysis, sodium bisulfite modification of genomic DNA was performed using the EZ DNA methylation kit (Zymo Research, Orange, CA, USA) according to the manufacturer's protocol.
MLH1 methylation analysis
MLH1 methylation analysis was conducted as previously described.14 Briefly, a methylation-specific real-time polymerase chain reaction method (MethyLight assay) was used for the precise quantitative measurement of promoter CpG island methylation of the MLH1 gene in bisulfite-modified DNA samples from each of the 168 MSI-H CRCs. Primers and probes used for the MLH1-MethyLight assay were designed according to a previous study.15 Hypermethylation of a CpG island locus in the promoter of the MLH1 gene was defined when the percentage of the methylated reference value was >4.
Statistical analysis
SPSS ver. 20 (IBM SPSS Statistics, Armonk, NY, USA) was used for all statistical analyses. Comparisons of the categorical variables were analyzed using a chi-square test or Fisher's exact test. All p-values were two-sided, and statistical significance was established at p<.05.
RESULTS
EPCAM loss and clinicopathological features in MSI-H CRCs
Among the 168 MSI-H CRCs, the frequencies of EPCAM-CL and EPCAM-FL tumors were two (1.2%) and 22 (13.1%) cases, respectively. Completely negative EPCAM IHC was also observed in whole tumor sections of the two EPCAM-CL cases. The clinicopathological features of the 168 MSI-H CRCs according to EPCAM expression status are summarized in Table 1. Notably, EPCAM-FL tumors were significantly associated with higher stage (stage III/IV, p=.043), distant metastasis (p=.003), poor tumor differentiation (p=.001), and a signet ring cell carcinoma component (p=.004) (Table 1). In addition, although there was a lack of statistical significance, EPCAM-FL tumors demonstrated a tendency toward the presence of lymph node metastasis (pN1/pN2; p=.064) (Table 1).
EPCAM loss and molecular features in MSI-H CRCs
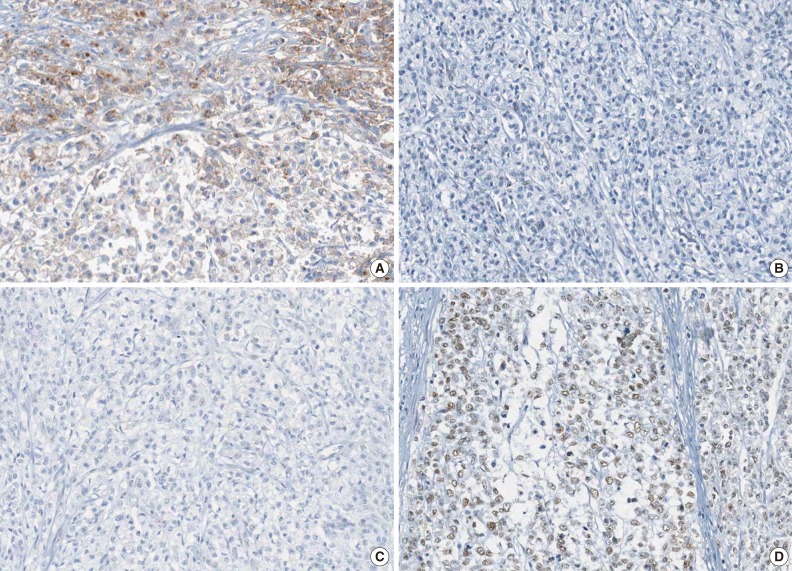
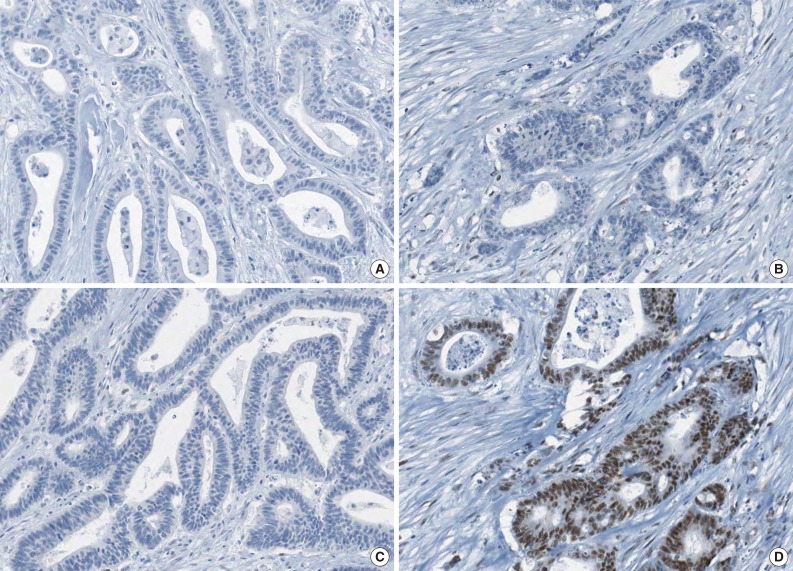
The molecular features of the 168 MSI-H CRCs, according to EPCAM expression status, are summarized in Table 2. Both of the EPCAM-CL tumors demonstrated a loss of MSH2/MSH6 expression, retained MLH1/PMS2 expression, and absence of MLH1 promoter methylation (Table 2, Fig. 1). In contrast, only 41% of EPCAM-FL tumors showed loss of MSH2 expression (nine of 22) (Table 2). Alternatively, a substantial number of EPCAM-FL tumors demonstrated loss of MLH1/PMS2 expression with retained MSH2 expression (13 of 22, 59%) (Table 2, Fig. 2). Among the 13 MLH1-negative EPCAM-FL tumors, nine cases showed MLH1 promoter methylation (Table 2).
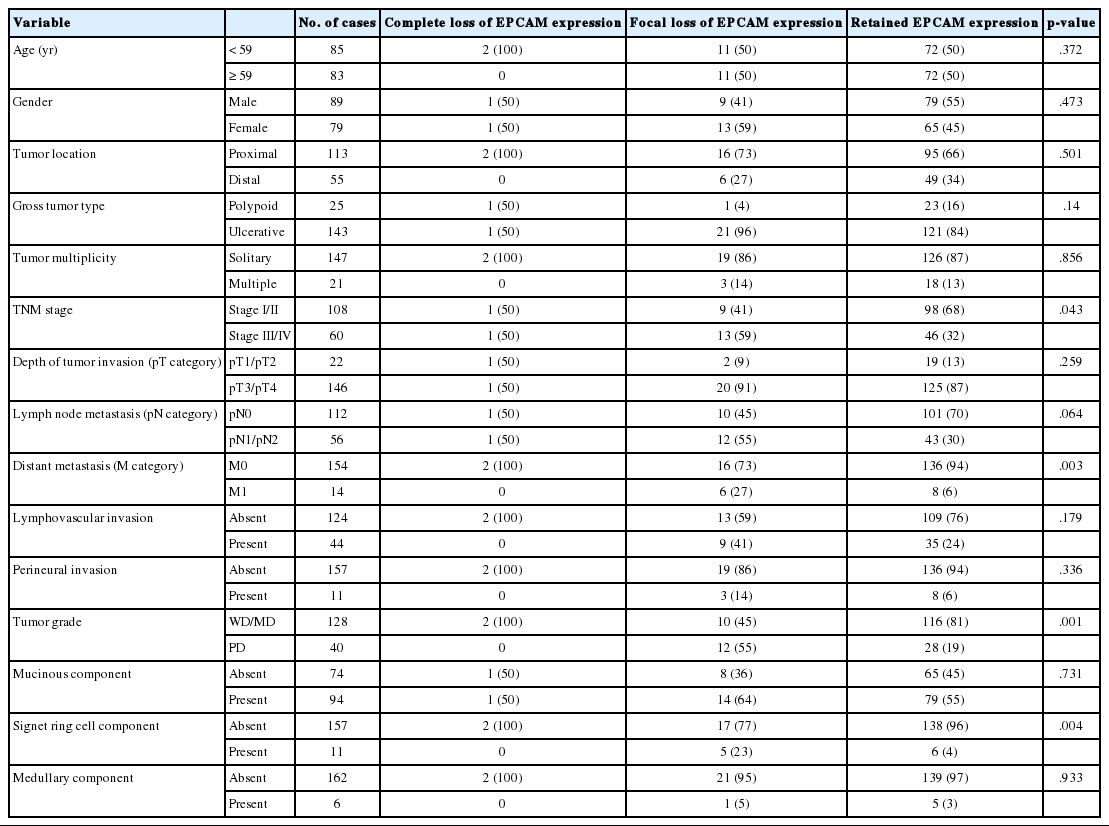
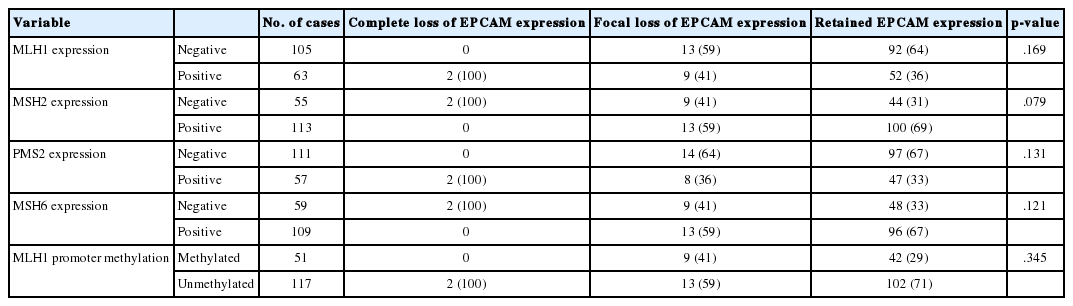
A representative case of microsatellite instability-high colorectal carcinoma demonstrating complete loss of EPCAM expression and MSH2 deficiency. Note the complete loss of EPCAM expression (A), loss of MSH2 expression (B), loss of MSH6 expression (C), and retained MLH1 expression (D).
DISCUSSION
EPCAM is a type I transmembrane glycoprotein that is functionally involved in the intercellular adhesion of epithelial cells. EPCAM is characterized by its specific expression in normal epithelial cells and epithelium-derived tumors across various tissue types. Therefore, EPCAM has been clinically explored as a putative diagnostic marker for the detection of circulating or metastasizing carcinoma cells in blood or body fluid of cancer patients and as a potential therapeutic target.16 In terms of pathological practice, IHC staining for EPCAM can be used as a supportive marker in the differentiation of adenocarcinoma from other mimicking tumors such as mesothelioma.17 Interestingly, it has been suggested that EPCAM-positive tumor cells can be regarded as tumor-initiating cells in some tumor types, particularly in hepatocellular carcinoma.18,19 Moreover, it has also been reported that both EPCAM-positivity and -negativity are associated with advanced stage and/or poor survival in several malignancies.16 Collectively, elucidating the biological roles and clinicopathological implications of EPCAM expression alterations in various cancers represents an important research topic. Although it is understood that EPCAM expression is generally observed in the majority of CRCs, the characteristics of CRCs associated with a lack of EPCAM expression remain poorly studied. Several recent studies have provided interesting findings regarding the association between EPCAM loss and germline EPCAM deletions in a subset of MSH2-negative Lynch syndrome CRCs.9,10,11 Indeed, although germline EPCAM deletion has recently been established as one of the molecular causes of Lynch syndrome CRCs, there are still insufficient epidemiological and clinicopathological data about this unique molecular subtype of Lynch syndrome tumors throughout diverse countries and ethnic groups. In particular, investigations concerning EPCAM deletion and/or EPCAM loss in MSI-H CRC have not been conducted in Korean patients. Therefore, we investigated the correlation of EPCAM loss with various clinicopathological and molecular factors in a large series of MSI-H CRCs, including both Lynch syndrome-associated MSI-H CRCs as well as sporadic MSI-H CRCs. In the present study, we successfully collected and presented novel data regarding differential features of MSI-H CRCs depending on the EPCAM immunophenotype in Korean patients.
The EPCAM gene is located in the region immediately upstream of the MSH2 gene. Thus, deletions of the 3' end of the EPCAM gene in EPCAM-expressing cells can lead to transcriptional read-through and can induce promoter hypermethylation of the MSH2 gene.5 A previous study by Huth et al.10 reported that a loss of EPCAM expression in Lynch syndrome-associated CRC required biallelic deletions in the EPCAM gene and could be detected in the precancerous adenoma stage of EPCAM deletion carriers. A recent investigation by Musulen et al.11 also found high specificity of EPCAM protein loss for the detection of germline EPCAM deletion in MSH2-negative Lynch syndrome CRCs, and suggested including EPCAM IHC in routine diagnostic screening of Lynch syndrome. On the basis of these findings, MSH2 negativity of EPCAM-CL tumors in our study may be strongly indicative of germline EPCAM deletion-associated Lynch syndrome tumors. Although we did not conduct mutation analysis to determine EPCAM deletion status of our MSI-H CRC samples, our future investigation would additionally reveal the detailed relationship between EPCAM loss patterns and germline EPCAM deletions in CRCs.
The most remarkable finding in the present study was the correlation between the EPCAM-FL immunophenotype and aggressive pathological features in MSI-H CRC. EPCAM-FL tumors were significantly associated with stage III/IV, distant metastasis, poorly differentiated histology, and signet ring cell carcinoma component (Tables 1). More fundamentally, in terms of molecular background, among the 22 EPCAM-FL cancers, only nine cases were MSH2-deficient tumors. Another nine cases were determined to have MLH1 methylation. These molecular features imply that focal loss of EPCAM expression can occur in sporadic MSI-H CRCs in contrast with the EPCAM-CL immunophenotype. Because sporadic hypermethylated MSI-H CRCs were histologically associated with poor differentiation and a signet ring cell carcinoma component according to our previous study,20 the close correlation between these pathological features and EPCAM-FL tumors is a plausible finding. To elucidate the underlying molecular mechanism and molecular heterogeneity of the EPCAM-FL phenotype in CRCs, additional studies should be performed to identify genetic and epigenetic factors that affect EPCAM gene expression alterations.
In summary, the EPCAM-CL phenotype in MSI-H CRC is associated with MSH2 deficiency, and this finding strongly indicates the possibility that Lynch syndrome-associated CRCs have germline EPCAM deletion-associated MSH2 silencing. By contrast, a considerable portion of EPCAM-FL tumors demonstrated MLH1 methylation and aggressive pathological features. Further evaluations to clarify molecular mechanisms of complete loss and focal loss of EPCAM expression in CRCs are necessary, with an emphasis on their potential associations with genetic or epigenetic aberrations in the EPCAM gene.
Acknowledgments
This study was supported by The Korean Society of Pathologists Grant (2013), the National R&D Program for Cancer Control funded by the Ministry of Health and Welfare (MHW), Korea (0720540), the Priority Research Centers Program through the National Research Foundation (NRF) funded by the Ministry of Science, ICT and Future Planning (MSIP), Korea (2009-0093820), a grant from the Korea Health Technology R&D Project funded by the MHW (HI13C1804), and the NRF grant funded by the MSIP (2011-0030049).
Notes
No potential conflict of interest relevant to this article was reported.