Primary Sigmoid Adenocarcinoma Metastasis to the Breast in a 28-Year-Old Female: A Case Study and a Review of Literature
Article information
Abstract
Metastasis to the breast from colorectal carcinoma is rare, only a few cases have been reported in the literature, and no cases have been reported in a young, 28-year-old patient. This report confirms the occurrence of the disease in a younger age group. The patient was referred to the Breast Clinic with a history of a gradually increasing lump in her right breast for two weeks' duration. On clinical examination, a 2-cm firm lump was noted in the upper inner quadrant of the right breast, which was clinically benign; however, histological examination of the breast core biopsy together with immunohistochemistry confirmed metastatic colorectal adenocarcinoma. The primary colorectal carcinoma was later confirmed to be a stage pT4N2M1 tumor, and the Duke stage was C1. Histology with immunohistochemistry is very important in the diagnosis of cases of this nature, but the clinical correlation should be taken into consideration at multidisciplinary team meetings to decide the final management of the patient.
Metastasis to the breast from extramammary sites is uncommon with fewer than 400 cases reported in the literature according to one study.1 Carcinoma in the contralateral breast and malignant melanoma are the most common primary cancers that metastasize to the breast, followed by lung, prostate, and stomach cancers.2,3 Metastasis from primary colorectal cancer is highly unusual and presents at a median age of 54 years.4
We report the first occurrence in our experience of a young, 28-year-old female who presented with a solitary breast lump from a concurrent colorectal primary tumor.
CASE REPORT
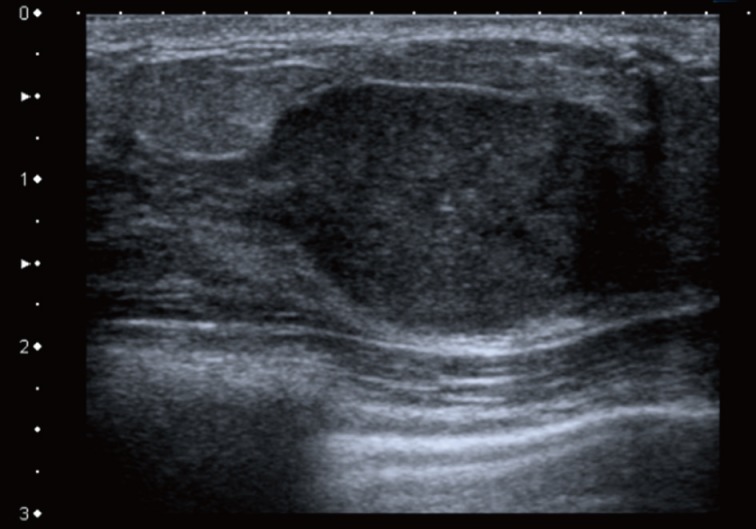
A 28-year-old Caucasian female presented to her general practitioner after she noticed a lump in her right breast for two weeks' duration. It was gradually increasing in size with no pain or nipple discharge. She had no previous breast problems or family history of breast cancer. On examination, a 2-cm firm lump was noted in the upper inner quadrant of her right breast, which was clinically benign. No lymph nodes were palpable. The ultrasound examination demonstrated a hypoechoic solid area with some calcification, consistent with a benign lesion (Fig. 1). A mammogram showed a partially well-defined nodule with intermediate type microcalcifications, which suggested suspicious morphology corresponding to the site of the primary carcinoma. Fine needle aspiration cytology showed low-grade atypia of probable benign nature set in a background of numerous polymorphs. Core biopsies were taken, and the histological examination revealed a malignant tumor made of cells showing prominent nuclear pleomorphism, significant mitotic activity, glandular structures with epithelial stratification, and focal cribriform formations. Some of the glands were dilated and contained necrotic debris within the lumen, or 'comedo-like necrosis.' These findings were consistent with a well to moderately differentiated adenocarcinoma (Fig. 2).
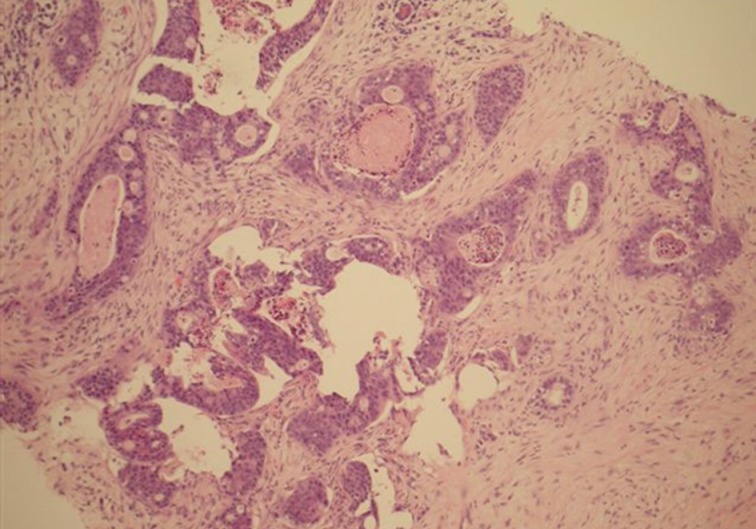
Breast core biopsy of the adenocarcinoma shows marked nuclear atypia, significant mitotic activity, cribriform formation and comedo-like necrosis within dilated glandular structures.
The main differential diagnosis included primary breast ductal carcinoma of nonspecific type and metastatic adenocarcinoma from other primary sites, including the colon, lung, upper gastrointestinal tract, hepato-pancreatobiliary sites, endometrium, and ovary. An immunohistochemical profile was performed, and the tumor was found to be strongly positive for CDX2 (Fig. 3) and cytokeratin (CK) 20 (Fig. 4). CK5/6 (myoepithelial marker) showed a patchy reaction, which suggested partial maintenance of the myoepithelial cells, findings that can mimic ductal carcinoma in situ (DCIS) of comedo type. The tumor cells were negative for CK7 (Fig. 5), HER2, gross cystic disease fluid protein 15, estrogen receptor, and progesterone receptor. The abovementioned immunoprofile confirmed the diagnosis of metastatic adenocarcinoma from a colorectal primary source. These findings excluded other primary tumors from the breast, lung, stomach, esophagus, endometrium, and ovary, which are usually positive for CK7 and negative for CK20 and CDX2. A detailed review of previous medical records was carried out and revealed that only ten days before presenting to the Breast Clinic, the patient underwent an urgent colonoscopy after she complained of severe weight loss, poor appetite, and diarrhea with blood and mucous. There was no family history of any bowel cancers. In addition, computed tomography scan exhibited a 3.6-cm metastatic deposit in the liver and a mass lesion in the recto-sigmoid junction with no evidence of lung deposits or enlarged lymph nodes. Histology of the colonic biopsies showed a moderately differentiated adenocarcinoma. This finding helped to reach a final diagnosis of metastatic adenocarcinoma to the breast from a primary sigmoid tumor.
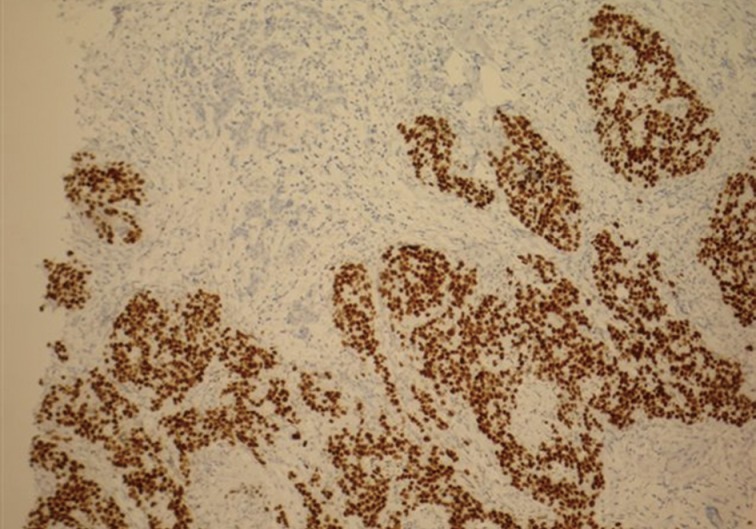
Breast core biopsy shows colorectal metastatic adenocarcinoma with diffuse positive nuclear staining for CDX2.
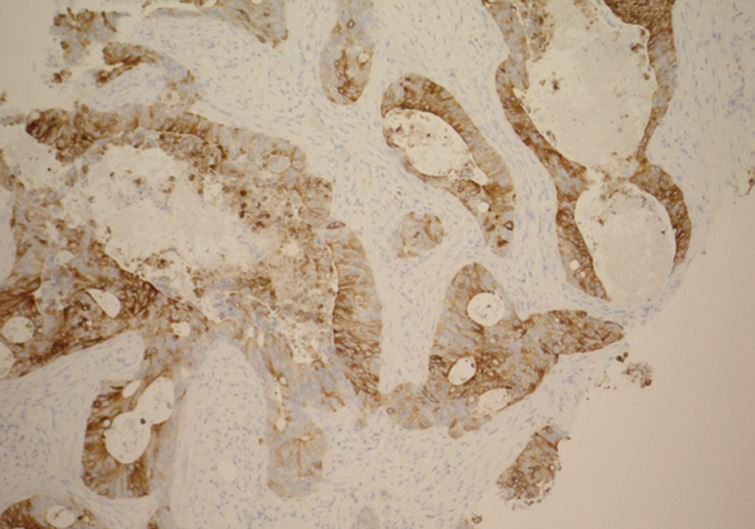
Breast core biopsy shows colorectal metastatic adenocarcinoma with diffuse positive staining for cytokeratin 20.
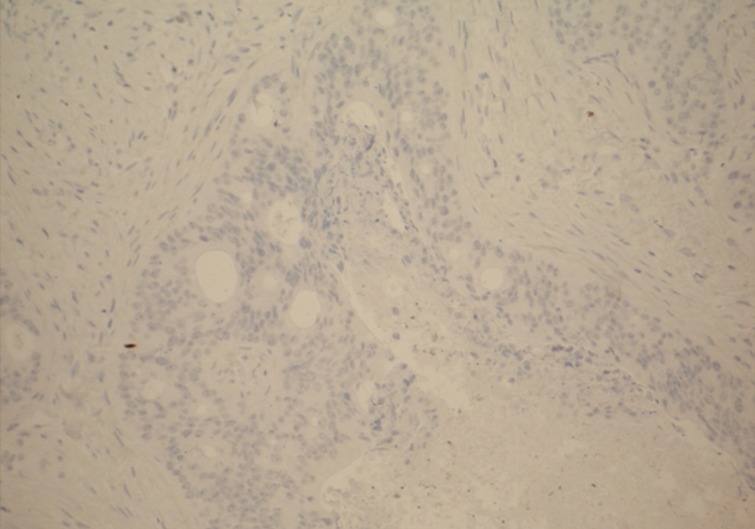
Breast core biopsy shows deposits of colorectal metastatic adenocarcinoma with a negative reaction for cytokeratin 7.
Treatment options for the patient were discussed at several multidisciplinary team (MDT) meetings, including breast, colorectal, and hepatobillary MDT meetings. The patient underwent anterior resection of the rectum. The primary lesion was revealed to be a perforated adenocarcinoma, with advanced clinical disease of pT4N2M1 and Dukes C1. The patient subsequently received chemotherapy; however, the breast and hepatic lesions were not removed due to the multi-focality nature of the disease.
DISCUSSION
Rectal carcinomas are very uncommon in a younger age group.2 To our knowledge, there is not a single case report of a secondary breast metastasis from simultaneous primary rectal cancer in a 28-year-old patient. The incidence of concurrent primary colorectal cancer with secondary breast metastasis is very low, with only three cases reported so far.4 These cancers usually occur more commonly in an older age group, generally presenting at a very late stage and as such are associated with a poor prognosis. The peak age incidence is reported to be in the fifth decade (median age, 54.3 years; range, 32 to 86 years).4
The occurrence of breast metastases from extramammary sites is 0.5% to 6%. Such tumors are even rarer from colonic adenocarcinomas with just 19 cases reported in the literature.2 The incidence is 6.6% to 7% in autopsy studies.2 The majority of these malignancies occur in women: only 5% to 8% occur in men.5
Recent medical and surgical interventions have improved the survival rate in colorectal carcinoma. Distant metastasis is usually associated with either recurrent or advanced disease,6 and the most common sites for metastasis are local lymph nodes, the liver, and lungs.7
Secondary breast neoplasms are difficult to identify. They present as palpable, discrete, rapidly growing small nodules, most commonly in the upper outer quadrant of the breast.6,8 On mammogram, they have specific features and can mimic benign lesions. Their appearance ranges from well to poorly circumscribed lesions that are not associated with microcalcifications.5,9 In our case, microcalcification was seen within the lesion.
It is important to distinguish primary from metastatic breast disease in order to offer the patient appropriate treatment. An ultrasound-guided core needle biopsy of the lesion is the standard of care for establishing an accurate diagnosis, and hence full clinical details at the time of histology examination are paramount.10 The differentiation between primary breast carcinoma and colorectal metastatic adenocarcinoma can be difficult on routine hematoxylin and eosin staining. On the other hand, the presence of an unusual pattern of tumor morphology, which can be quite different from primary breast carcinoma, and an absence of in situ ductal carcinoma in the surrounding breast indicates a metastatic lesion. Back-to-back glands along with a cribriform-like formation may occur in colorectal adenocarcinoma, but such lesions usually show epithelial stratification, higher cellular atypia, mitotic activity, and polymorphs within the lumen. A cribriform breast carcinoma usually has low-grade cellular pleomorphism and insignificant mitotic activity. Intestinal type mucosa with goblet cells is a helpful feature, but unfortunately this was not seen in our case. The impression of a 'foreign type' tumor, which is not seen in day-to-day practice, should raise suspicions of metastasis. Immunohistochemical studies help establish the histological type as well as the tumor origin. In our case, the final diagnosis was achieved by using a small panel of immunohistochemistry. The majority of primary breast cancers are CK7 positive and CK20 negative, whereas metastatic colonic adenocarcinomas are CK7 negative and CK20 positive.11 The strong nuclear positivity with CDX2 is highly sensitive and specific for colonic cancers.12 In addition, estrogen and progesterone receptors are usually negative in metastatic breast cancers. A patchy reaction for CK5/6 and comedo-like necrosis can mimic DCIS, as was described in our case. Careful examination of tumor histomorphology features such as epithelial stratification, high nuclear atypia, significant mitotic activity, and positive reactions for CK20 and CDX2 can help to overcome this difficulty.
Metastatic carcinomas in the breast are associated with a poor prognosis with a survival rate of less than 12 months from the time of breast tumor diagnosis.5,13 Patient management is complex and depends upon multiple factors, including extent of the primary tumor, lymph node status, distant metastasis, age, and other comorbidities. Proper clinical correlation and immunohistochemistry helps to reach the final correct diagnosis in the majority of cases. These tools play a vital part in patient management and can help avoid unnecessary mastectomies. With limited treatment options available, surgical resection of the primary lesion with chemotherapy at an early stage can improve the prognosis.
Acknowledgments
The authors would like to thank Miss C. Kavanagh (Department of histopathology Royal Oldham Hospital) for her contribution to this paper. No financial support was required for the preparation of this paper.
Notes
No potential conflict of interest relevant to this article was reported.