Microtubule-Associated Protein Tau, α-Tubulin and βIII-Tubulin Expression in Breast Cancer
Article information
Abstract
Background
The microtubule-associated protein Tau binds to both inner and outer surfaces of microtubules, leading to tubulin assembly and microtubule stabilization. The aim of this study was to evaluate the significance of Tau, α-tubulin, and βIII-tubulin expression in breast carcinoma and to assess their relationships with disease progression in the context of taxane treatment.
Methods
Immunohistochemical expressions of Tau, α-tubulin, and βIII-tubulin were assessed in 183 breast cancer cases. Expression was correlated with clinicopathologic parameters, disease progression and overall survival.
Results
Tau expression was correlated with lymph node metastasis and estrogen receptor (ER) positivity (p=.003 and p<.001, respectively). Loss of α-tubulin was significantly correlated with distant metastasis (p=.034). Loss of βIII-tubulin was correlated with lymph node metastasis and ER positivity (p=.004 and p<.001, respectively). In taxane-treated cases, Tau expression and loss of α-tubulin and βIII-tubulin expression were related to disease progression (p=.001, p=.028, and p=.030, respectively). Tau expression was associated with a worse survival rate in taxane-treated patients (p=.049).
Conclusions
Tau expression and loss of α-tubulin and βIII-tubulin expression were correlated with aggressive behavior in taxane-treated breast cancer. Further evaluation of Tau, α-tubulin and βIII-tubulin may be useful in predicting clinical behavior and seeking therapeutic measures in taxane-based chemotherapy for breast cancer.
Breast cancer is one of the most common malignancies and is the leading cause of cancer death among women.1 The heterogeneous nature of its histology, phenotype and clinical behavior leads to the need for targeted therapeutic agents. Tau, a microtubule-associated protein (MAP), binds to both the inner and outer surfaces of microtubules, leading to tubulin assembly and microtubule stabilization.2 Tubulin heterodimers consist of α- and β-tubulins, and the β-subunit binds taxane and causes suppression of both microtubular dynamics and cell proliferation.3 Overexpression of βIII-tubulin has been demonstrated in a variety of malignancies, including non-small-cell lung cancer and ovarian, gastric, prostate and breast cancers.4 Previous studies have implicated Tau and tubulin expression in breast cancer.5-11 The purpose of this study was to evaluate the expression of the MAP Tau, α-tubulin, and βIII-tubulin in breast cancer and correlate their expressions with known clinicopathologic parameters, disease progression and overall survival of patients. Furthermore, the relationships among Tau, α-tubulin, and βIII-tubulin expression and disease progression and overall survival of the patients were also evaluated in the context of taxane use as adjuvant chemotherapy.
MATERIALS AND METHODS
Clinical samples
A total of 183 primary breast carcinoma patients who had undergone surgical resection at St. Vincent's Hospital (Suwon, Korea) between January 1999 and August 2007 and had available follow-up data were included in this study. Neither chemotherapy nor radiotherapy was performed before surgery. Formalin-fixed paraffin-embedded (FFPE) tissue and clinical data were collected for each patient. All of the archival hematoxylin and eosin (H&E)-stained slides for each patient were reviewed. The nuclear grades and histologic grades were evaluated according to the Nottingham grading system.12 The tumor stages were evaluated according to the criteria of the 7th edition of the American Joint Committee on Cancer (AJCC). Medical records were collected and reviewed retrospectively. Disease progression was defined as local recurrence, distant metastasis or disease-related death. This study was approved by the Institutional Review Board of The Catholic University of Korea (VC12SISI0158).
Tissue microarray
Tissue microarrays (TMAs) were constructed from FFPE tissue blocks of 183 invasive carcinomas. The representative tumor site was chosen on H&E slides, and the site corresponding to the confirmed tumor site in the paraffin block was marked. Areas with necrosis, hemorrhage and artifacts were excluded. One selected area per each case was harvested using a 2-mm Quick-Ray tip-punch (Micro Digital Co., Seoul, Korea) and was transferred to a TMA mold with 60 pores and re-embedded with paraffin. TMA blocks were prepared as 4-µm-thick sections and were stained with H&E staining methods. The tissues were then examined to determine whether the appropriate tumor site had been selected.
Immunohistochemistry
Immunohistochemical staining was conducted on 4-µm sections of the TMA blocks. The paraffin sections were mounted on poly-L-lysine-coated glass slides, deparaffinized, and rehydrated in a graded series of ethanol, followed by antigen retrieval using a microwave. Endogenous peroxidase activity was blocked by treating the slides with 3% H2O2 in methanol for 10 minutes at room temperature. The primary antibodies were incubated overnight at 4℃. The following primary antibodies were used: Tau (1:200, #RB-1239-R7, Lab Vision, Fremont, CA, USA), α-tubulin (1:200, #RB-9281-R7, Lab Vision), βIII-tubulin (1:800, MU177-UC, BioGenex, San Ramon, CA, USA), estrogen receptor (ER; 1:300, 6F11, Novocastra, Newcastle upon Tyne, UK), progesterone receptor (PR; 1:600, 16, Novocastra), and HER2 (1:1,800, polyclonal, Dako, Glostrup, Denmark). Immunostaining was conducted using the rabbit or mouse DAKO ChemMate EnVision system and Peroxidase/DAB kit (Dako). The sections were then counterstained with Mayer's hematoxylin and dehydrated, cleared, and mounted. Normal breast epithelium was used as a positive control for Tau. Skin tissue was used as a positive control for α-tubulin and βIII-tubulin. Omission of the primary antibody served as a negative control.
The immunohistochemical staining and histology were examined independently by two pathologists. Evaluation was performed twice for each case without any knowledge of the specific diagnosis or prognosis. The cases with discrepant scores were discussed between pathologists to obtain a consensus. Immunoreactivity was interpreted in the cytoplasm for Tau, α-tubulin, and βIII-tubulin. The staining intensity was graded as follows: 0, negative; 1+, weak; 2+, moderate; and 3+, intense. Staining intensity of 0 or 1+ was interpreted negative, and tumors with 2+ or 3+ staining intensity were interpreted as positive.
Statistical analysis
Statistical analyses were performed using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA). The two-sided p-values were determined via chi-square tests and Fisher's exact test to evaluate the associations of expression of markers with clinicopathologic features of tumor and disease progression. Overall survival was analyzed using the Kaplan-Meier method with log-rank test. A value of p<.05 was considered significant for all of the analyses.
RESULTS
Patient characteristics
The mean age of the 183 patients was 51.5 years (range, 30 to 81 years). The median follow-up duration was 75.4 months (range, 2.3 to 179 months) after the primary surgery. One hundred forty-two (77.6%) patients were treated with adjuvant chemotherapy. Forty-five of those (31.7%) received taxane (docetaxel or paclitaxel)-containing regimens. Of the 183 total patients, 14 (7.7%) had local recurrence, 30 (16.4%) developed distant metastasis, and 19 (10.4%) died due to disease during follow-up.
Expression of Tau, α-tubulin, and βIII-tubulin
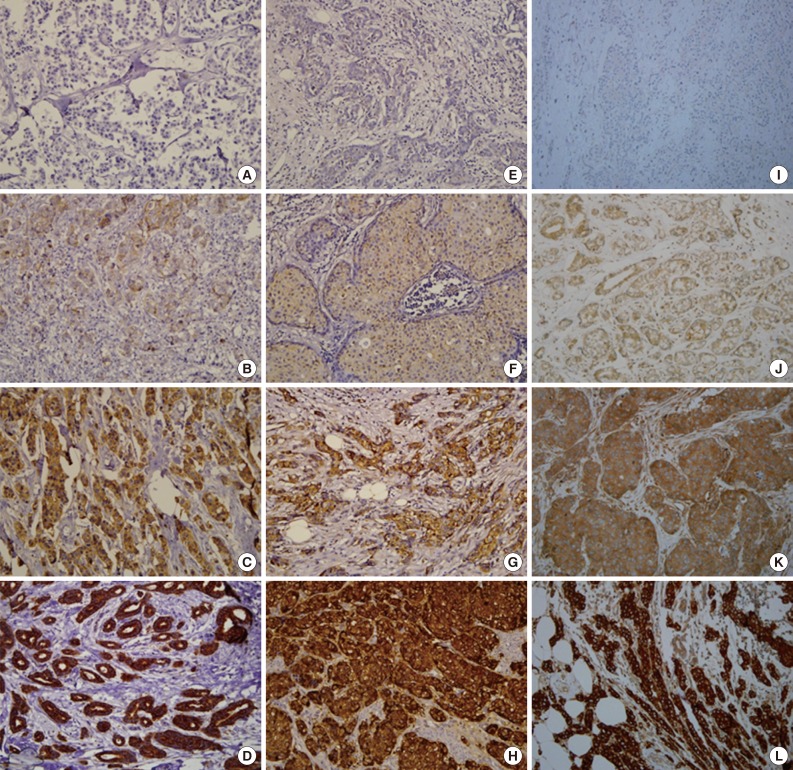
Normal breast epithelium expressed Tau with moderate (2+) staining. Occasional weak (1+) to moderate (2+) staining of α-tubulin and βIII-tubulin was observed in the normal breast epithelium. Of the 183 breast carcinoma cases, Tau, α-tubulin, and βIII-tubulin were expressed in 114 (62.3%), 54 (29.5%), and 111 (60.7%) cases, respectively. Representative examples of the immunohistochemical staining for each protein are presented in Fig. 1.
Relationships between Tau, α-tubulin, and βIII-tubulin and clinicopathologic factors
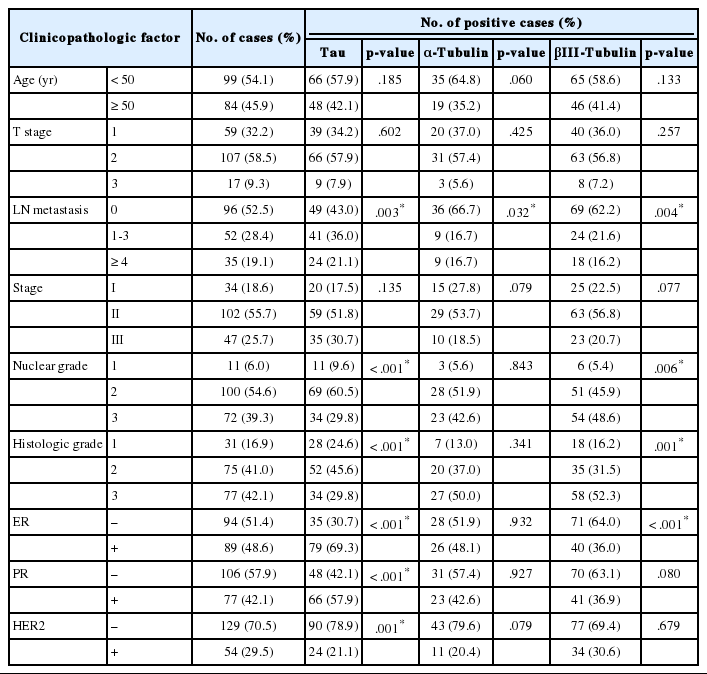
The correlations between Tau, α-tubulin, and βIII-tubulin and clinicopathological variables are presented in Table 1. Tauexpression was significantly associated with lymph node metastasis (p=.003). On the contrary, expressions of α-tubulin and βIII-tubulin were inversely correlated with lymph node metastasis (p=.032 and p<.001, respectively). Tau and βIII-tubulin expression was significantly associated with higher nuclear grade (p<.001 and p=.006, respectively) and higher histologic grade (p<.001 and p<.001, respectively). Tau expression was significantly increased in ER- and PR-positive cases (p<.001 and p<.001, respectively), and in HER-2-negative cases (p=.001). βIII-tubulin expression was significantly increased in ER-negative cases (p<.001).
Relationships between Tau, α-tubulin, and βIII-tubulin expression and disease progression
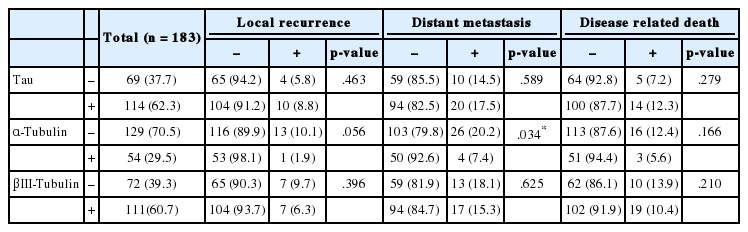
The correlations between Tau, α-tubulin, and βIII-tubulin and local recurrence of the tumor, distant metastasis or disease-related death are presented in Table 2. Local recurrence or disease-related death was not correlated with expression of any of these proteins. However, loss of α-tubulin expression was significantly associated with distant metastasis (p=.034).
Of the 142 patients who were treated by adjuvant chemotherapy, 45 (31.7%) received a taxane-containing regimen and 97 (68.3%) received a non-taxane-containing regimen. Disease progression was defined as local recurrence of the tumor, distant metastasis or disease-related death. The correlations between Tau, α-tubulin, and βIII-tubulin and disease progression according to the use of taxane are presented in Table 3. In the group treated with the taxane-containing regimen, Tau expression and loss of α-tubulin and βIII-tubulin expression were also significantly associated with disease progression (p=.001, p=.028, and p=.030, respectively). However, Tau, α-tubulin, and βIII-tubulin expression did not correlate with disease progression in the non-taxane-containing chemotherapy group (p=.943, p=.241, and p=.931, respectively).
Overall patient survival
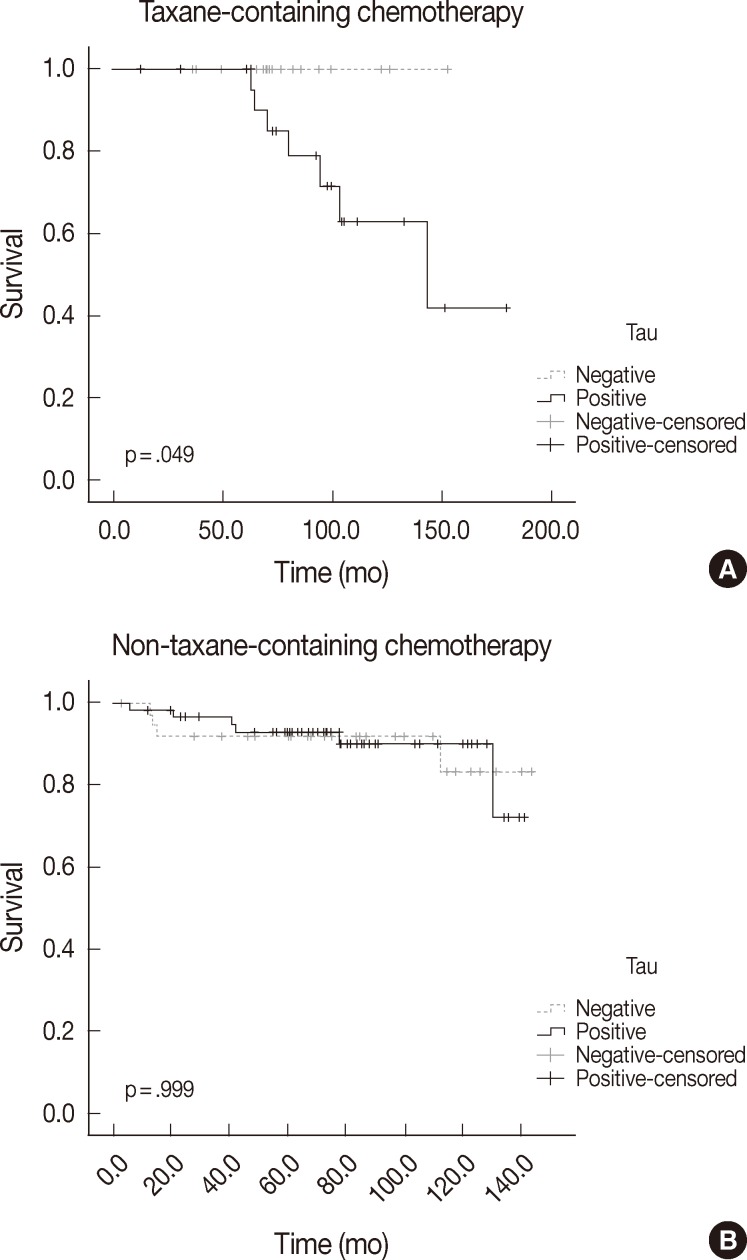
The differences in overall survival rate according to clinicopathologic and immunohistochemical parameters are summarized in Table 4. Lymph node metastasis and stage were the only factors that were significantly correlated with overall survival (p<.000 and p=.017, respectively). Tau, α-tubulin, and βIII-tubulin expression did not show any statistically significant relationship to overall survival in the whole paitent sample (p=.258, p=.169, and p=.325, respectively). In the group that received taxane-containing regimens, patients with Tau expression had a poor overall survival rate (p=.049). However, α-tubulin and βIII-tubulin expression did not show any statistically significant relationship to overall survival in this group (p=.066 and p=.396, respectively). Tau, α-tubulin, and βIII-tubulin expression did not relate to overall survival in patients treated with a non-taxane-containing regimen (p=.999, p=.578, and p=.785, respectively). The overall survival according to Tau expression and taxane treatment is depicted in Fig. 2.
DISCUSSION
MAPs are a family of proteins that bind to and stabilize microtubules. Tau, one of the most extensively investigated MAPs, binds to both the inner and outer surfaces of microtubules and plays an important role in the bundling, spacing and assembling of microtubules.6 By sharing the same binding site as taxanes, Tau can compete with these drugs.13 Taxanes (e.g., paclitaxel and docetaxel) bind to tubulin and cause suppression of microtubular dynamics, which inhibits spindle formation and leads to cell cycle arrest during the G2/M phase.2
Several studies regarding the prognostic and predictive value of Tau expression have been reported in the treatment of breast cancer. Rouzier et al.6 evaluated Tau expression in 122 breast cancer patients who had received neoadjuvant chemotherapy of paclitaxel. Forty-four percent of Tau-negative tumors (28/64) had pathologic complete response (CR), compared with 17% of Tau-positive tumors (10/58) (p=.04). Tanaka et al.9 evaluated Tau expression in metastatic breast cancer patients. Sixty percent of Tau-negative expression showed favorable response (CR or partial response [PR]) to paclitaxel administration compared to 15% of Tau-positive expression. Shao et al.14 analyzed Tau expression in 54 advanced breast cancer patients who received first-line chemotherapy of paclitaxel and cisplatin. Tau expression was significantly associated with lower overall response rate (CR or PR) (p=.02). However, several studies have claimed contractor findings. Pentheroudakis et al.15 evaluated Tau expression in 274 high-risk early breast cancer patients treated with paclitaxel, but Tau mRNA status did not predict a clinical benefit from taxanes. Andre et al.7 reported that low Tau expression was associated with sensitivity to paclitaxel-containing preoperative chemotherapy in only ER-positive cases. Our results showed that Tau expression was significantly higher in the ER- and PR-positive groups (p<.001 and p<.001, respectively), supporting the relationship with Tau and hormone-related factors. In this study, the response to chemotherapy was evaluated in the form of disease progression. Tau expression was associated with aggressive tumor behavior (p=.001) in patients treated with taxane-containing regimen, suggesting that Tau-negative cases may benefit more from taxane-containing chemotherapy compared to Tau-positive cases. Tau expression was also associated with worse overall survival in patients treated with taxane-containing regimens (p=.049).
Tubulin heterodimers consist of α-tubulins and β-tubulins. α-Tubulin is also a structural component of the centrosome with γ-tubulin. Niu et al.16 reported that centrosomal α-tubulin and γ-tubulin were overexpressed in breast premalignant lesions and carcinoma, and that the expressions of α-tubulin and γ-tubulin positively correlated with the development of breast cancer. Banerjee reported elevated levels of α-tubulin in the paclitaxel-resistant cell line from MCF-7 breast cancer cells.17 However, there have not been many additional studies about the associations of α-tubulin expression and clinicopathologic features of breast cancer including disease progression. In our study, loss of α-tubulin expression was significantly associated with lymph node metastasis (p=.032) and distant metastasis (p=.034). In the taxane-containing chemotherapy group, loss of α-tubulin expression was significantly associated with disease progression rate (44.8% vs 12.5%, p=.028). On the other hand, α-tubulin did not show any correlation with disease progression in the non-taxane-containing chemotherapy group (p=.241). This suggests that α-tubulin expression can be used as a predictive marker for disease progression in taxane-containing chemotherapy. However, α-tubulin expression was not related to overall survival in patients treated with taxane-containing regimens (p=.066).
β-Tubulin, the other component of the tubulin heterodimer, is known to have eight different isoforms.5 Of these, βIII-tubulin plays a critical role in cell proliferation, differentiation and tumorigenesis due to its unique depolymerization activity.14 Overexpression of βIII-tubulin has been demonstrated in a variety of malignancies, including non-small-cell lung cancer and carcinoma of the ovary, stomach, pancreas, prostate and breast.4 Recent studies have reported the association of βIII-tubulin expression with response to taxane treatment. Yuan et al.18 investigated βIII-tubulin expression in advanced breast cancer with docetaxel therapy. They reported that there were significant differences between the effective rate (CR+PR) of patients with positive expression of βIII-tubulin (37.50%) and that of patients negative for expression (61.54%) (p<.01). On the contrary, Wang et al.4 reported that, in ER-negative tumors, high βIII-tubulin expression was significantly associated with lower residual cancer burden in all patients treated with neoadjuvant chemotherapy (p=.021) and in those treated with taxane (p=.038). In our study, βIII-tubulin expression was associated with higher nuclear and histologic grade (p=.006 and p=.001, respectively). Loss of βIII-tubulin was also associated with ER positivity (p<.001). In the taxane-containing chemotherapy group, loss of βIII-tubulin expression was significantly associated with a higher disease progression rate (52.9% vs 21.4%, p=.030). βIII-Tubulin did not show any correlation with disease progression in the non-taxane-containing chemotherapy group (p=.931). This suggests that loss of βIII-tubulin expression could be used as an indicator of chemoresistance in taxane-based chemotherapy. However, βIII-tubulin expression did not relate to overall survival in the taxane-containing chemotherapy group (p=.396).
This study is limited as it was conducted retrospectively on surgically removed breast cancer tissue. The patients included in this study had received diverse treatments, and the response to chemotherapy was only available in the form of disease progression and overall survival.
Our data show that Tau expression and loss of α-tubulin and βIII-tubulin expression are associated with aggressive behavior in breast cancer treated with taxane-containing chemotherapy. Furthermore, in the taxane-containing chemotherapy group, Tau expression in patients was significantly associated with worse overall survival rate. Further evaluation of Tau, α-tubulin, and βIII-tubulin may be useful in predicting clinical behavior and seeking therapeutic measures in taxane-based chemotherapy for breast cancer.
Acknowledgments
The authors wish to acknowledge the financial support of St. Vincent's Hospital, Research Institute of Medical Science (SVHR-2012-15).
Notes
No potential conflict of interest relevant to this article was reported.